Summary
Increased public health awareness and expanded low-dose computed tomography (CT) utilization, accelerated by the COVID-19 pandemic, have elevated detection rates of pulmonary ground-glass nodules (GGNs). Patients with multiple primary lung cancer (MPLC) often present with multiple GGNs, posing challenges for precise treatment and prognostic assessment. Current therapies including stereotactic body radiation therapy (SBRT), chemotherapy, and immunotherapy face efficacy and safety limitations. While video-assisted thoracic surgery (VATS) is the primary treatment for high-risk GGNs, the sole reliance on surgery may cause excessive loss of lung function. Image-guided thermal ablation techniques can effectively treat smaller lesions with lung preservation. This review explores molecular mechanisms of ablation, VATS-ablation synergy, and the potential value of this approach in combination with immunotherapy. The clinical application prospects, including advancements in navigation techniques and equipment, are also discussed. Overall, this hybrid surgical strategy represents a promising option for patients with multiple lesions, minimizing lung function loss and the psychological burden.
Subject areas: Surgery, Medical procedure, Cancer
Graphical abstract
Surgery; Medical procedure; Cancer
Introduction
Currently, lung cancer still has the highest incidence among malignant tumors in the world and is the leading cause of tumor-related deaths.1 As public health awareness has increased in recent years and with the spread of COVID-19, computed tomography (CT) scanning has been widely used in the population, leading to a gradual increase in the rate of detection of primary lung cancer characterized by ground-glass nodules (GGNs), especially multiple primary lung cancer (MPLC), which is characterized by multiple GGNs.
MPLC refers to the simultaneous or sequential occurrence of two or more primary malignant tumors in the same patient’s lungs. MPLC can also be classified into synchronous multiple primary lung cancer (sMPLC) and heterochronous multiple primary lung cancer (hMPLC) according to the time interval between the occurrence of different foci, histological characteristics, and other characteristics. sMPLC accounts for approximately 2.6%–7.9% of cases in patients undergoing surgery for non-small cell lung cancer.2,3,4,5,6 According to the Martini-Melamed (M-M) criteria,7 sMPLC refers to (1) Multiple isolated tumors occurring at the same time, which may be of the same or different pathological types. (2) In cases of identical pathology, the tumors are generally distributed across different lung segments, lobes, or sides of the lung, and the individual tumors are of an in situ origin and without evidence of extrapulmonary metastasis or lymphatic spread.8 In clinical practice, an important imaging characteristic of MPLC is the presence of multiple GGNs.
According to relevant statistics, approximately 50% of GGN patients have multiple GGNs.9,10,11 Although the academic community regards lung cancers with GGN characteristics as a less active subtype of lung adenocarcinoma,12 13% of cases of GGN progression are still detected within a 10-year follow-up.13 In contrast, in postsurgical patients with MPLC featuring multiple GGNs, the presence of one residual GGN is followed by the subsequent growth of other residual GGNs in 41% of patients.14 The ability to effectively manage these residual GGNs is critical to the prognosis of MPLC patients.15,16,17 The aforementioned patients with multiple MPLC characterized by multiple GGNs are different from patients with single nodules in terms of clinicopathological features and treatment,18 but their precise treatment is also one of the difficulties in the current field of lung cancer research.
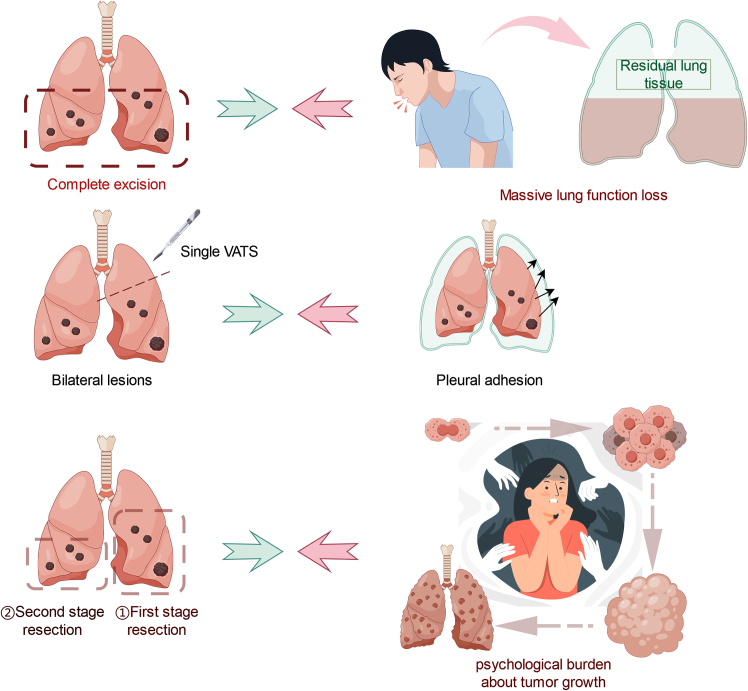
Although many types of treatments are available for patients with MPLC, these strategies have limitations in clinical practice (Figure 1). To date, insufficient evidence has been reported for the effectiveness of stereotactic body radiation therapy (SBRT) in the treatment and prediction of the prognosis of patients with MPLC.19,20 Nevertheless, radiological dose delivery and real-time image guidance are difficult to perform simultaneously. Moreover, chemotherapy can scarcely eliminate all cancer cells, and the systemic side effects should not be ignored. Targeted therapy plays a significant role in the comprehensive treatment of lung cancer, but obtaining pathological results for multiple lesions from patients with MPLC is still difficult.21,22 The pathological results for the multiple lesions may not reveal the same mutation even when the samples are obtained via percutaneous biopsy. Furthermore, more evidence is still needed to demonstrate the effectiveness in immunotherapy (PD-1/PD-L1) against MPLC.23
Figure 1.
Dilemma regarding the current major treatments for MPLC
Currently, video-assisted thoracic surgery (VATS) remains the main option for the treatment of high-risk GGNs diagnosed via non-invasive techniques, such as imaging24; Nonetheless, if surgery is used as the only option for the management of all GGNs, an unnecessary loss of lung function may occur. How to eliminate satellite lesions effectively from MPLC and reduce the psychological burden on patients while preserving lung function as much as possible is an essential issue in clinical practice.25,26,27 Several studies have confirmed that image-guided thermal ablation (IGTA) (including radiofrequency ablation [RFA], microwave ablation [MWA], and laser ablation) and cryoablation are effective methods for treating GGNs with smaller diameters,28,29,30,31,32,33,34,35,36 as thermal ablation (e.g., RFA, MWA, and laser ablation) induces cell death via hyperthermia, whereas cryoablation uses hypothermia. However, the application value of VATS combined with hybrid ablation surgery for MPLC and the relevant medical-industrial integration value still need to be further investigated.
In this review, we introduce tumor ablation techniques and their associated molecular mechanisms. In addition, we discuss the background of the emergence, value of the combination, and prospects for the development of ablation techniques combined with VATS hybrid surgery.
Background on the emergence of VATS combined with thermal ablation surgery
VATS alone has limitations in current applications
In clinical practice, surgically resected lesions predominantly are tumor lesions with the following characteristics: more solid components, larger size, vascular or tracheal penetration, abnormal lymph node enlargement in the drainage area, pleural traction, etc., and significant signs of malignancy.
Surgical management has undergone remarkable progress in the twentieth century. The gold standard of extent resection has evolved from pneumonectomy to lobectomy or even sublobar resection. For patients with GGNs, wedge resection is generally preferred. Nevertheless, the incisional approach has evolved from rib-spreading thoracotomy to 3-port VATS, uniportal VATS, or even robotic-assisted thoracic surgery. Surgical treatments are performed in a minimally invasive fashion.37
However, for patients with multiple GGNs, premature surgical resection with VATS is associated with a series of contradictions (Figure 2).
-
(1)
First, there is a contradiction between the extensive early resection of multiple GGNs and the preservation of postoperative lung function. The pathological types of GGNs are divided into alveolar epithelial atypical adenomatous hyperplasia (AAH), adenocarcinoma in situ (AIS), microinvasive lung adenocarcinoma (MIA), and invasive adenocarcinoma (IAC).38,39,40,41,42 For preinvasive lesions (lesions prior to IAC) in patients with MPLC, extensive early surgical resection does not significantly improve overall survival compared with elective surgery after 3–4 months of follow up. However, the premature surgical resection of a large number of nodules can cause early organ damage, a loss of lung function, a decrease in a patient’s postoperative quality of life, and a risk of perioperative complications.18.
-
(2)
Second, there is a contradiction between bilateral multiple primary lesions and the unilateral extent to which the VATS procedure can be performed at one time. For patients with multiple bilateral MPLC nodules, thoracoscopic surgery cannot be used to resect multiple bilateral lung nodules within a short period. In addition, the number of surgical resections is limited because extensive and significant pleural adhesions can occur after surgery.
-
(3)
Finally, the contradiction between staged surgery for multiple bilateral nodules and the psychological burden caused by lesion progression must be addressed. Elderly patients or patients with underlying diseases have a poorer baseline function and consequently cannot tolerate the simultaneous resection of multiple MPLC nodules. However, resection of only some GGN lesions is associated with a greater psychological burden due to fear among tumor patients, and second-stage surgery is more difficult.25,26,27 Therefore, for MPLCs with different pathological types, distant anatomical locations, and many nodules, a combination of therapeutic strategies is needed in practice.
Figure 2.
Limitations of using thoracoscopy alone to manage multiple primary lung nodules
Thermal ablation and VATS have good concurrent indications and efficacy in MPLCs
Currently, the main nonsurgical treatment modalities for MPLC include chemotherapy, immunotherapy, SBRT, and ablation therapy (Table 1). Among them, chemotherapy and immunotherapy are important forms of adjuvant and neoadjuvant therapy and are mainly applied to control residual tumor cells after surgery or to downstage lesions before surgery. However, chemotherapy and immunotherapy can induce systemic side effects and cannot radically eliminate tumor cells43; thus, these treatments are not suitable for combination with surgery. In contrast, SBRT has only local side effects and can control local lesions to a certain extent; however, SBRT equipment requires specific radiation shielding, which conventional surgical platforms find difficult to accommodate. Therefore, SBRT is not suitable for combination with thoracoscopic surgery. Ablation therapy can locally eradicate lesions, and conventional bedside CT or electromagnetic navigation can meet the localization requirements. Additionally, VATS can be used to observe the lesion treatment status after ablation therapy and simultaneously assist in managing short-term complications of ablation therapy. Therefore, when combined with VATS, ablation therapy has good applicability in the management of MPLC. Notably, this article primarily focuses on preoperative CT/ENB-guided ablation (targeting lesions inaccessible during VATS) rather than intraoperative ablation (performed under direct visualization via VATS), as the latter may allow for complete resection of lesions during the surgical procedure itself. This distinction emphasizes the complementary role of preoperative ablation in managing lesions that cannot be addressed through standard VATS resection, thereby preserving lung function while ensuring oncological efficacy.
Table 1.
Comparison of nonsurgical treatments for lung cancer
| Nonsurgical treatment | Capacity for eradication | Side effects | Combination with surgical treatment |
|---|---|---|---|
| Chemotherapy | No | Systemic side effects | Pre- or postoperative, or intrapleural perfusion hyperthermia |
| Immunotherapy | No | Systemic side effects | Pre- or postoperative |
| CT/ENB-guided ablation therapy | Yes | Local sideeffects | Can be performed concurrently with surgery (pre- or postoperative ablation) |
| Stereotactic body directed radiotherapy (SBRT) | Yes | Local side effects | Independent of surgery |
| Target therapy | No | Systemic side effects | Pre- or postoperative, need for pathology gene detection. |
Ablative therapy has shown noninferiority in terms of the prognosis of stage I non-small cell lung cancer (NSCLC). In a meta-analysis including 792 patients, it was shown that there was no significant difference between ablative therapy and surgery in terms of overall survival (OS) and tumor-specific survival (CSS) from 1 to 5 years, or disease-free survival from 3 to 5 years.44,45,46,47,48,49,50,51 The advantages of combining ablative therapy include its minimal to no effect on lung function compared with that of conventional surgical treatment and its lower costs associated with both single treatment and cumulative treatment for stage I NSCLC over a period of 1 year.52
Introduction to the principles and molecular mechanisms of thermal ablation and cryoablation
Unlike surgical resection, which cuts into the tissue surrounding the tumor, direct destruction of the tumor tissue by ablation causes a more significant immune response.53,54,55,56,57,58,59,60,61,62 Hence, hybrid surgery may provide a better postoperative antitumor effect. Knowing the molecular mechanism of ablation may help in understanding the principles of hybrid surgery.
Mechanism of ablative techniques
Ablation is a type of energy-dependent therapy, the main mechanism of which is the precise and minimally invasive induction of irreversible damage or coagulative necrosis of tumor cells in focal tissues through the biological effects of hyperthermia (such as RFA, MWA, and laser ablation) or hypothermia (such as cryoablation), which subsequently causes local inflammatory responses.63,64
Currently, the thermal ablation techniques applied to lung tumors include RFA, MWA, cryoablation, and laser ablation. RFA, MWA, and cryoablation are the main techniques used. Table 2 compares the detailed differences among these three ablation techniques.
Table 2.
Comparison of the advantages, disadvantages and molecular mechanisms of ablation techniques
| Technology type | Principle | Advantages | Disadvantages | Molecular mechanisms in the central region | Molecular mechanisms of transitional regions |
|---|---|---|---|---|---|
| RFA | Radio frequency alternating current (60°C–100°C) |
|
|
|
|
| MWA | Microwave electromagnetic field (60°C–150°C) |
|
|
||
| Laser Ablation | Photonic-to-thermal energy conversion (the temperature cannot be determined) |
|
|
||
| Cryoablation | Heat absorption by gasification (−140°C to 20°C–40°C, argon–helium, or −196°C–80°C liquid nitrogen) |
|
|
|
|
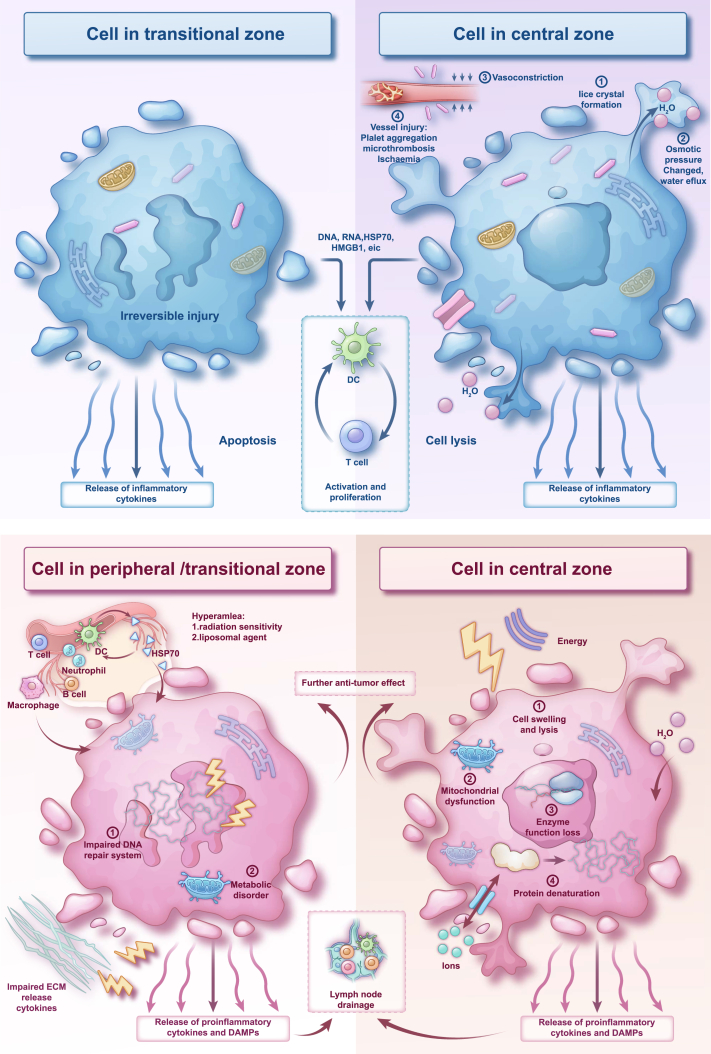
The general effect of thermal ablation or cryoablation on tumor cells can be divided into three regions67: the central region, the migrating region, and the surrounding unaffected tissue (Figure 3). The central region is the area around the ablation needle where coagulative necrosis directly occurs; the migrating region shows apoptosis or reversible cellular damage, mainly due to heat transfer from the central region; and the peripheral tissues are those in the periphery of the migrating zone that are not affected by the energy.
Figure 3.
Mechanisms of thermal ablation and cryoablation
While the figure depicts shared mechanisms, the specific temperature ranges and immune effects vary by technique (see Table 2).
Depending on the ablation technique applied, the cellular molecular mechanisms differ, but the overall mechanisms are similar.
The molecular mechanisms of the central region are classified into two categories: direct and indirect.
Thermal ablation-induced changes in cell membrane integrity are the central, direct cause of hyperthermia-induced cell death, including alterations in cell membrane fluidity and permeability, which in turn cause dysfunction of the intracellular microfilament/microtubule system and further lead to abnormalities in facilitated diffusion and active transport. Metabolite deposition and abnormalities in intracellular fluid homeostasis ultimately cause cell lysis.68 In addition, mitochondrial dysfunction is closely related to hyperthermia-induced cellular damage. Other possible mechanisms of intracellular damage due to hyperthermia include the disruption of RNA synthesis, lysosomal enzyme leakage, and Golgi damage.68,69
In addition to these direct causes, indirect or delayed cellular damage, including apoptosis, vascular injury, ischemic or ischaemia-reperfusion damage, and the release of lysosomal activators and cytokines, can occur after ablative therapy. These effects promote antitumor immunity and help with the overall effect of local treatment.
In the migrating region, a predominantly inflammatory cell infiltrate occurs, and these cells include neutrophils, macrophages, dendritic cells, NK cells, and specific T and B cells,56,57,60 which are present in distant tissues and the circulation while comprising the postablation microenvironment. The possible main reasons for this infiltration are as follows.
-
(1)
Damage-associated molecular patterns (DAMPs) caused by programmed cell death RNA, DNA, and heat shock proteins (HSPs), especially HSP70 and high-mobility group protein B1 (HMGB1),55,70 cause intrinsic immunity that triggers acquired immunity.
-
(2)
The extracellular matrix surrounding the tumor contains hyaluronic acid, collagen, and endothelial cells and releases proinflammatory cytokines,55 which in turn activate the inflammatory cytokines interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), IL-1β, and IL-8 and vascular adhesion molecules.
Ablative techniques activate specific immune systems in hybrid surgery
Radiofrequency ablation, microwave ablation, and cryoablation have different mechanisms of immune response, which may have different synergistic effects with surgery.
After radiofrequency ablation, the increase in HSP70 specificity plays a key role in stimulating the antitumor immune response. In animal experiments, HSPs derived from tumor cells or virus-infected cells can induce antigen-specific immunity.71,72 There is also evidence from clinical studies that HSPs are significantly elevated in the serum of patients after radiofrequency ablation, and the degree of elevation of serum HSP70 correlates with increased survival.58
Nevertheless, attention has also been given to the decreased number of CD4+ CD25+ FOXP3+ regulatory T cells in the post-ablative tumor microenvironment after radiofrequency ablation,53 which implies that peripheral tissues have decreased immune tolerance to tumor antigens and that this decreased tolerance results in stronger antitumor cellular and humoral immunity. Some clinical studies have shown that the number of tumor-specific T cells in peripheral tissues is increased in patients after RFA, and there is a correlation between these cell counts and the time of tumor-free survival (TFS).54,56
In these patients, the immune response may play a role in controlling other residual GGNs, increasing overall survival after hybrid surgery.
Like RFA, which is a form of energy ablation, MWA disrupts the homeostasis of a tumor cell’s internal environment by causing intracellular dipolar molecules to rotate and thus generate thermal energy; however, MWA is more efficient, with a greater range of action (up to 2 cm), and produces faster results.
However, compared with RFA, MWA more weakly stimulates local inflammation and activates intrinsic and acquired antitumor immunity, producing lower levels of the cytokines IL-1, IL-6, and HSP7061,62 because the higher temperature may destroy tumor-specific antigens. Recent studies have shown that MWA activates antitumor immunity by remodeling the immune microenvironment of tumour-draining lymph nodes (TdLNs), with the initial 4 days after ablation representing a critical window for immune activation.73 Emerging evidence further indicates that combining MWA with Flt3L, a dendritic cell-mobilizing agent, significantly increases the efficacy of PD-1 blockade therapy, suggesting a potential translational direction in ablation immunotherapy research.74
Cryoablation technology is currently less commonly used in China, mainly because of its relatively high cost. The mechanism of direct cellular damage in the region of lethal hypothermia (−20 to −40°C) by cryoablation differs from that of thermal ablation, in which the extracellular fluid water freezes earlier than the intracellular fluid water does,75 and the local microvasculature of the tumor is severely damaged, causing ischemic damage in the target area.
In nonlethal hypothermia, the activation of cellular autophagy and a postablation immune response play significant roles.76 As thermal ablation techniques (RFA and MWA) destroy the tumor-specific antigens left behind through thermal denaturation, thus weakening the postablation immune response, the preservation of the immunogenicity of tumor-specific antigens by cryoablation results in increased plasma inflammatory cytokine levels59,77 and increased postablation antigen accumulation in dendritic cells (animal models).78 From this perspective, cryoablation might be the future of ablative techniques in hybrid surgery.
Decision-making in hybrid surgery
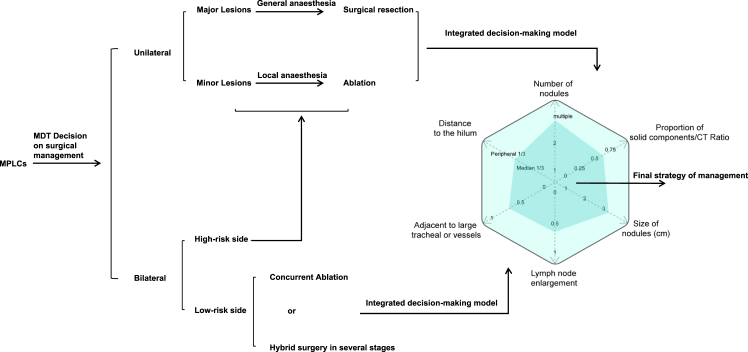
The technique for decision-making in hybrid surgery is shown in Figure 4. For patients with MPLCs, a multidisciplinary team (MDT) makes the decision regarding surgical management. For patients with bilateral MPLCs, thoracic surgeons assess the risk associated with the lesions. We prefer the use of an integrated decision-making model to determine whether concurrent ablation or staged hybrid surgery should be applied to the low-risk side, whereas the high-risk side will follow the standard approach used for unilateral lesions. For unilateral lesions, major lesions are surgically resected, whereas minor lesions are treated with ablation. The type of lesion is determined by six key factors within the integrated decision-making model. The larger the area of the hexagon representing these factors is, the more likely surgical resection is to be favored. Conversely, smaller areas tend to favor ablation treatment. We must emphasize that synchronous hybrid surgery for bilateral lesions demands a rigorous multidisciplinary evaluation. Only low-risk contralateral nodules may safely undergo combined ablation and unilateral VATS. Inadequately assessed bilateral hybrid interventions risk precipitating bilateral pneumonectomy-like complications, despite isolated reports of such practices in earlier studies.79,80
Figure 4.
Decision-making model for hybrid surgery
Represents our institutional protocol based on the MDT consensus and prior studies (Computed tomography-guided radiofrequency ablation combined with video-assisted thoracoscopic surgery for multiple pulmonary nodules: A retrospective study from the National Cancer Center in China). It is not yet validated prospectively but aligns with the NCCN guidelines for multifocal NSCLC.
Surgical planning for hybrid surgery
VATS, as the treatment of choice for early-stage NSCLC, is still highly valuable in clinical practice. Therefore, we used surgery to treat the major lesions and combined surgery with thermal ablation to treat the minor lesions while retaining the advantages of anatomical surgical resection for pathological biopsy and lymph node dissection to achieve the “3M” goals of resolving as many lesions as possible at one time (“more lesions eliminated”), reserving as much lung function as possible (“more lung function reserved”), and preserving the possibility of receiving further treatment (“more chance for further treatment”).
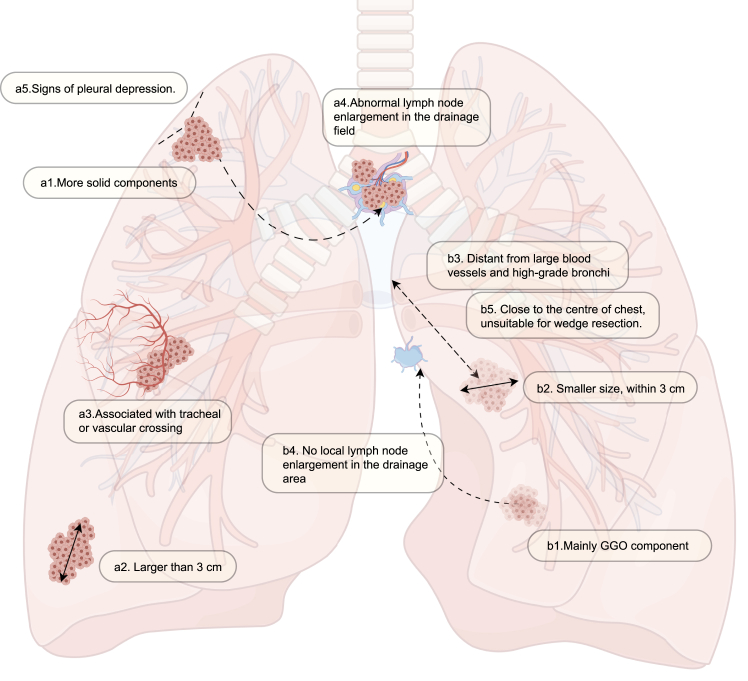
In clinical practice, we refer to the rules of thumb listed in the following text for the classification of major and minor lesions (Figure 5). The characteristics of the major lesions were as follows: (a1) multiple solid components; (a2) large size, usually larger than 3 cm81; (a3) association with tracheal and vascular crossing; (a4) abnormal lymph node enlargement in the drainage field; and (a5) signs of pleural depression.
Figure 5.
Surgical planning strategy for hybrid surgery
The characteristics of the minor lesions are as follows (Figure 5): (b1) predominantly ground-glass density GGO (Ground-Glass Opacity) components; (b2) smaller size, usually within 3 cm; (b3) distance from large blood vessels and high-grade bronchi; (b4) no local lymph node enlargement in the drainage area; and (b5) a tumor location close to the center of the chest, which makes it unsuitable for wedge resection.
VATS combined with ablation hybrid surgery: Technical route
The technical course of the combined procedure is as follows MDT decision-making on the operation, preoperative planning, preoperative anesthesia and disinfection, CT-guided puncture of the target lesion/electromagnetic navigation bronchoscopy (ENB)-guided approach to the target lesion, ablation, monitoring and adjustments during the ablation process, assessment of the postablation efficacy, change in position and resterilization, thoracoscopic puncture, resection of the planned site and assessment of the efficacy of the ablation, closure of the thoracic cavity, postoperative management, and CT follow-up of the efficacy of the procedure. The sequences of surgical resection and ablation are switchable; however, ablation before surgical resection is preferable because surgical treatment can address possible complications after surgical resection. Hybrid surgery can be performed with only general anesthesia or local anesthesia followed by general anesthesia.
Preoperative preparation
Patient indications were assessed by medical history, physical examination, and recent imaging outcomes. MDT evaluation was recommended, involving shared decision making (SDM) with the patient to formulate a final diagnosis and treatment opinion if necessary.82,83 The critical imaging evidence for developing a preoperative plan is a high-resolution contrast-enhanced CT of the chest that is completed within 1 month, which is important for defining the lung tumor size, morphology, internal structure, location, and adjacency. Lesions adjacent to major blood vessels (with a potential heat-sink effect) or critical nerves carry risks of incomplete ablation or adjacent tissue injury. A multidisciplinary evaluation by a team of ablation specialists is strongly recommended to assess procedural feasibility and safety. Notably, the following contraindications for hybrid surgery are areas of concern84:
Absolute contraindications—(1) platelet count <50 × 109/L and (2) serious bleeding tendencies and coagulation disorders that cannot be improved in the short term (prothrombin time >18 s, prothrombin time activity <40%).
Relative contraindications—(1) severe cachexia and cardiopulmonary insufficiency; (2) significant infectious lesions on puncture routes; (3) severe COPD, emphysema, or pulmonary fibrosis; (4) severe pulmonary arterial hypertension; (5) patients using mechanical ventilation (ventilators) or an implanted cardiac pacemaker; and (6) patients with a psychotic episode.
Preoperative anesthesia and sterilization
Complex local anesthesia, including ropivacaine and lidocaine, with the use of a mask airway, is recommended according to the patient’s condition for CT-guided percutaneous ablation.84 As for ENB-guided endobronchial ablation, tracheal intubation and intravenous anesthesia are recommended.79,80,85 In principle, both general anesthesia and local anesthesia are applicable for ablation procedures, but each has distinct advantages and disadvantages. General anesthesia offers better patient comfort; however, it carries a higher risk of pneumothorax and may result in impaired lung expansion during ablation. Local anesthesia allows for patient cooperation, a shorter procedure time, and avoids intubation, but is associated with significant patient discomfort.
Minor lesion ablation
Ablation plan:
the ablation plan before surgery contains four elements. (1) use of the gross tumor region (GTR), which depends on the imaging-defined lesion area determined by preoperative high-resolution contrast-enhanced CT, to determine the location, size, morphology, and relationship with neighboring organs of the lesion.86 For ENB-guided ablation, computed tomography three-dimensional reconstruction is needed. (2) Confirmation of the puncture point and body surface positioning (using a sheet of paper with a metal grid while CT scanning). For ENB-guided ablation, confirmation of the tracheal route is needed. (3) Determination of the puncture path, i.e., the puncture path and its distance from the puncture point to the lesion. (4) Determination of the power and time for ablation according to the size of the lesion and the portion of the solid component, and use the preset parameters if there are no special circumstances.81 For ENB-guided ablation, computed tomography three-dimensional reconstruction is needed to plan the entry path of the ablation antenna.
Puncture target area (GTR)
after the patient is anesthetized and disinfected, according to the GTR of the presurgical ablation plan, from the puncture point on the body surface, the predetermined depth of puncture is gradually reached according to the puncture pathway, followed by the use of intrasurgical CT to observe whether the ablation needle reaches the GTR of the predetermined target area. For ENB-guided ablation, the ablation antenna reaches the target area through the guidance of ENB.
Ablation of GTR tissue
different ablation modes are used according to the size and number of tumors, such as (1) single GTR in one ablation session; (2) multiple GTR in one ablation session; and (3) multiple ablation needles/antennas and multiple GTRs in one ablation session. Specific ablation parameters are used according to the device type.
Monitoring and adjustment during the ablation process
CT is mainly used to monitor the “halo” sign after ablation, and when the boundary of the ablated GGO is 5–10 mm larger than that of the original GTR, the ablation is completed, and the needle/antenna is withdrawn.86 During the ablation process, it is also necessary to monitor whether the ablation needle is off-target, whether to adjust the power of the ablation needle, and whether there are intraoperative complications, such as bleeding, pneumothorax, etc., and to immediately switch to television-guided thoracoscopic surgery (VATS) if necessary. During the ablation process, the heart rate, blood pressure, and level of blood oxygen should be routinely monitored, including the patient’s respiration and bleeding, and symptomatic treatment should be given if necessary.
Position change and re-sterilization
The surgical team transfers the patient to the operating table. All procedures were performed under general anesthesia with double-lumen endotracheal intubation for selective lung ventilation.87 According to the patient’s intended surgical site, the patient can assume the lateral position, and according to the scope of sterilization for thoracoscopic surgery: anteriorly and posteriorly up to the midline, up to the clavicle and 1/3 of the upper arm, and down past the rib margins, sterilization with 0.5% PVP iodine should be performed, followed by the laying of a surgical towel (in the lateral position).
VATS major lesion resection
Anatomical lobectomy, sublobar resection, or wedge resection under thoracoscopic video display. First, the surgeon can confirm whether the minor lesions are completely ablated and whether there are complications such as bleeding or pneumothorax. The major lesion is subsequently resected via the following procedure. First, the anatomical structure around the lesion is clarified, the pulmonary vessels are bluntly separated, and the pulmonary vessels are ligated via an endoscopic knotter or cut with an endoscopic vascular suture cutter. The peribronchial lymph nodes are subsequently cleared. Surgeons should electrocoagulate small arterioles for hemostasis, using an endoscopic titanium staple to clamp the bronchial artery. After the bronchus is completely free, surgeons will select the surface to be severed, insert the endoscopic bronchial stump closure device, and cut off the bronchus. The pathology specimen of the main lesion is then placed into a specimen bag and removed through a small incision. Electrocoagulation or argon gas hemostasis is performed, the thoracic cavity is flushed, ventilation of both lungs is applied, the lungs are inflated to check whether there is any air leakage from the bronchial stumps, and the operation is concluded by placement of a drainage tube.
Summarized perspectives on VATS combined with ablation
Some institutions have implemented the strategy of primary and minor lesion classification in the treatment of synchronous multiple primary lung cancer (sMPLC), and VATS combined with ablation surgery has been effective in the treatment of GGO-like lesions with a predominantly ground-glass component or lesions that do not have lymph nodes but have a certain risk of malignancy, as well as some nonnodular lesions, across numerous different pathologies or pathological stages. VATS ablation has been shown to be effective for several different pathological types or stages of GGO-like lesions with predominantly ground-glass opacities or for lesions without lymph node enlargement but with a certain risk of malignancy; VATS ablation is also effective for some nonnodular lesions, mainly in terms of more comprehensive management of GGO-like lesions and greater preservation of lung function after the procedure. Table 3 lists references concerning ablation combined with VATS hybrid surgery.
Table 3.
Studies describing ablation combined with VATS hybrid surgery
| Authors | Number of cases | Number of nodules/tumors | Number of nodules removed by VATS | Number of ablated nodules | Number and proportion of complications | References |
|---|---|---|---|---|---|---|
| Qu R et al. | 11 | 37 | 16 | 21 | 1 (pneumothorax) | Qu et al.80 |
| Bao F et al. | 10 | 23 | 13 | 10 | 4 (air leakage∗ 1, haemoptysis∗ 2, lung infection∗ 1) | Bao et al.88 |
| Zeng C et al. | 57 | 216 | 120 | 96 | 10 (pneumothorax∗ 2, subcutaneous emphysema∗ 2, persistent cough∗ 6) | Zeng et al.85 |
| Jiang N et al. | 1 (single case report) | 4 | 3 | 1 | 0 | Jiang et al.79 |
| Cui X et al. | 91 | More than 182 | 91 | More than 91 | 49 (pneumothorax∗ 31 requiring close drainage∗ 7 (7.69%), pleural effusion∗ 6, hydropneumothorax∗ 5) | Cui et al.89 |
| Liu B et al. | 48 | 87 | 67 | 20 | 3 (air leak∗ 1, chylothorax∗ 1, massive pleural effusion∗ 1) | Liu et al.90 |
Regarding research on VATS combined with ablation hybrid surgery for lung cancers (Table 4), the main open-access ongoing clinical studies are registered by Chuan Huang et al. (Beijing Hospital), Jiayuan Sun et al. (Shanghai Chest Hospital), and Hecheng Li et al. (Ruijin Hospital) and are aimed to investigate the feasibility, perioperative safety, postoperative quality of life, and oncological efficacy of VATS combined with ablation for simultaneous multiple primary lung cancers. In addition to specific studies of VATS combined with ablation hybrid surgery for lung tumors, Jason A. (Beth Israel Deaconess Medical Center), Philippe L. Pereira, Hermann Aeber (University Hospital Tuebingen), Damian E. Dupuy (Meditronics), and Jennifer M. Kennedy (University Hospital Tuebingen) have conducted studies on the feasibility, perioperative safety, postoperative quality of life, and oncological efficacy of hybrid surgery for simultaneous or multiple primary lung cancers. These clinical studies are also informative for determining the procedural safety and prognostic benefits of combining VATS with lung ablation techniques for the treatment of lung tumors.
Table 4.
Clinical trials on VATS combined with ablation hybrid surgery
| PI | research period | Number of samples/cases | Main observational indicators | Subobservable indicators | Unique inclusion and exclusion criteria for projects | Shared inclusion criteria | Shared exclusion criteria | Reporting unit | Research numbers |
|---|---|---|---|---|---|---|---|---|---|
| Chuan Huang | 2022.6.1–2024.12.31 | 50 | Surgical feasibility, perioperative safety (complications), postoperative quality of life, oncological outcomes | – | ∗∗Inclusion Criteria: Age ≥65 years old; |
1. Age ≥18 years old. 2. Clinical diagnosis of synchronous MPLC according to the Martini-Melamed criteria; 3. patient can tolerate hybrid surgery according to MDT assessment 4. Lung tumor is resectable 5. Subjects voluntarily participate in the study and sign the written informed consent form at. |
1. bronchoscopy cannot reach the minor lesion 2. Large blood vessels are within 2 mm or less from the contralateral minor lesion. 3. Patient cannot tolerate bronchoscopy 4. Any comorbidity that the investigator feels would interfere with the safety of the subject or the evaluation of study objectives 5. Pregnant or breast feeding. 6. Pacemaker, implantable cardioverter, or another electronic implantable device |
Beijing Hospital | ChiCTR2300069053 |
| Jiayuan Sun | 2020.7–2025.12 | 30 | Objective response rate (ORR) based on mRECIST criteria, assessed up to 1 year | PFS,OS,postoperative complication | ∗∗Inclusion Criteria: (1) Ablation Lesion A is accessible/adjacent to bronchi and the size is ≤3 cm according to HRCT | Shanghai Chest Hospital | NCT04730453 | ||
| Hecheng Li | 2023.2–2025.12 | 172(86 + 86) | Perioperative complication rate | ORR,Perioperative surgery-related indicators, lung function, quality of life EORTC QLQ-LC29,economic cost,DFS |
∗∗Inclusion Criteria: (1) Age ≥18 years and ≤80 years; (2) Bilateral simultaneous MPLC diagnosed by HRCT; (3) At least one minor lesion (6 mm ≤ diameter ≤20 mm, CTR <0.5) is located on the contralateral side of the major lesion and requires treatment after MDT discussion. (4) The ipsilateral minor lesion can be concurrently subjected to sublung lobectomy (5) ECOG PS score of 0–1 ∗∗Exclusion criteria: (1) Large blood vessels are present within 2 mm from the contralateral minor lesion. |
Ruijin Hospital | NCT05662553 | ||
| Walter J. Scott (Criteria not publicly available) | 2002.6–2004.2 | 20 | – | – | ∗∗Inclusion Criteria: (1) Diagnosis of malignant non-small cell lung cancer (NSCLC). (2) Intraoperative needle biopsy confirmation allowed.(3)All tissue to be treated by radiofrequency tumor ablation must be completely contained within boundaries of planned lung resection | Fox Chase Cancer Center | NCT00039507 | ||
| Jason A Beattie (Surgical pathology is only used to assess the effectiveness of ablation) | 2022.6.1–2025.6.30 | 10 | (1) Rate of planned ablations(Day 1) (2) Pathological changes in the tumor tissue: The % necrosis, % viable tumor, and % stroma/inflammation present in the targeted nodules after ablation(4 weeks). |
(1) Histological changes in lung tissue outside the zone of predicted ablation: The % necrosis and of % stroma/inflammation will be described. (2) Immune-histochemical changes in the tumor tissue: Immune-histochemical evaluation including TTF-1, Napsin-A, p40, or other immune-histochemical assessments will be performed if needed on a per case basis. |
∗∗Inclusion Criteria: (1) Subject with Stage I - II primary lung cancer (Solitary nodules up to 3 cm) as defined by previous pathology or ROSE. (2) Pathological proof of target nodule/tumor type and malignancy. (3) Target nodule/tumor which can be accessed via bronchoscopy and confirmed location with cone beam CT scan intraoperatively ∗∗Exclusion criteria: (1) Target nodule <1.0 cm. (2) Prior radiation or neo adjuvant chemotherapy of the target nodule/tumor. (3) Patients in other therapeutic lung cancer studies. |
Beth Israel Deaconess Medical Center | NCT05281237 | ||
| Philippe L Pereira, Hermann Aebert (Surgical pathology is only used to assess the effectiveness of ablation) | 2004.4–2006.5 | 9 | Rate of incompletely treated tumors | Pathologic tissue changes, rate of major and minor complications | ∗∗Inclusion Criteria: (1) Maximum of 3 lung tumors. (2) Maximum tumor size less than 5 cm. (3) Must be able to receive standard surgery ∗∗Exclusion Criteria: (1) Pathological coagulation tests positive. (2) Maximum tumor size more than 5 cm. (3) Bilateral minor lung cancer with more than 3 tumors. |
University Hospital Tuebingen | NCT00610844 |
Prospects
Prospects for the application of hybrid surgery
Prospects for the use of hybrid surgery are currently focused on 3 main areas: the optimization of intraoperative ablation techniques, advances in intraoperative guidance techniques, and immune-combination therapy with hybrid procedures.
Upgrading of ablation techniques
Lung tumor ablation technology has gradually improved and developed. According to the characteristics of the ablation medium, this technology has now gradually developed into radiofrequency ablation, microwave ablation, and cryoablation, which have not yet been widely applied. In addition, with the further development of medical-industrial interdisciplinary fields, high-intensity focused ultrasound (HIFU) has also shown potential value in lung tumor treatment in vitro and in animal experiments.91 Photodynamic therapy (PDT), an important branch of laser ablation, can sensitize special nanoparticles to chemotherapeutic drugs through a photochemical reaction, thus impairing glycolysis process in tumor cells and enhancing the killing effect on tumor tissues. However, PDT has been used less often in lung tumors, and its effect and prognosis need to be systematically studied.92,93,94 As emerging ablation methods, reversible electroporation (RE) and irreversible electroporation (IRE) are considered to have important clinical potential because of their nonthermal effects and their ability to increase cell permeability in combination with cytotoxic drugs. However, owing to their high cost, these modalities are mostly used for unresectable local lesions that thermal ablation cannot eliminate as well. The difficulty of conducting clinical cohort studies involving these methods remains high.95,96,97
Guiding technique progress
In terms of navigation strategies for lung tumor ablation, intraoperative navigation via CT or magnetic resonance imaging (MRI) is widely used in clinical practice. EBUS (endobronchial ultrasonography) intraoperative navigation is usually employed for the ablation of more dangerous tumors adjacent to the hilum, heart, and great vessels. A few institutions have applied more advanced three-dimensional imaging combined with ENB for intraoperative navigation shows potential for technical refinement. With the development of fusion imaging technology, the combination of multiple imaging signals to build guidance paths may become a trend, and the integration of augmented reality (AR) and virtual reality (VR) devices in surgery warrants exploration. Robot-assisted surgeries, such as da Vinci (Intuitive Surgical, Inc.), can also be combined with RFA for hybrid surgery.
Immunotherapy for hybrid surgery
In recent years, the PD-L1 antibody atezolizumab has been reported to have a significant inhibitory effect on residual nodules in the lung region after RFA in clinical practice. However, no significant effect of the PD-L1 antibody atezolizumab has been observed for other tumors in the non-RFA ablation region. The practical application of the MWA technique has been reported in combination with camrelizumab.98,99 With the development of chimeric antigen receptor (CAR) technology, CAR-T cell systemic therapy was initially accepted for nonsolid tumors. Further research on CAR-T cell therapy in lung cancer has revealed that the altered tumor microenvironment after microwave ablation significantly enhances the antitumor effect of AXL-specific (AXL, Anexelekto, a kind of Receptor Tyrosine Kinase) CAR-T cells,100 suggesting that thermal ablation technology may have a synergistic effect with chimeric antigen therapy.101
The advancement of nanomaterial technology has significantly propelled the development of ablation combined with immunotherapy. As exemplified by the work of Xu, M et al., a multifunctional nanoplatform (UCILA) was ingeniously designed to integrate temperature monitoring with photothermal therapy. This platform simultaneously enables the high-resolution visualization of the tumor vasculature and real-time tracking of the metabolic distribution of UCILA in vivo. In animal studies, the synergistic application of UCILA-mediated photothermal ablation and CAR-NK immunotherapy produced a statistically significant improvement in survival rates, highlighting its dual capability for precise thermal dose regulation and systemic immune activation.102 Additionally, Jiang, Y et al. produced a nanoplatform (APNA) that promoted T cell infiltration through a photothermal effect.103 In the context of non-conventional ablation modalities, recent studies have shown that the hypoxia-tailored drug-loaded nanoplatform ADMOFs, designed for the hypoxic microenvironment following high-intensity focused ultrasound (HIFU) ablation, not only overcomes hypoxia-associated resistance to DOX (Doxorubicin) but also achieves a tumor volume inhibition rate of 77%.104 Numerous advancements in nanomaterial technology may provide valuable insights into tumor ablation immunotherapy.
Prospects of basic research on hybrid surgery
Currently, the main basic research in hybrid surgery focuses on thermal ablation technology, which is mainly divided into biomedical directions related to tumor necrosis, apoptosis, and tumor immunity and engineering directions related to the distribution of the ablation thermal field, temperature field simulation, ablation antenna design, and temperature measurement technology.
In recent years, in the biomedical field, research on tumor thermal ablation has been progressing rapidly, and the academic community has a deeper understanding of the molecular mechanism and therapeutic synergistic effect of tumor thermal ablation.
However, in the field of engineering, much room for the development of a theoretical system of lung tissue thermal ablation technology still exists. (1) The idea of a multisource conformal thermal field was proposed by Phasukkit et al.105 through a finite element analysis of three-source microwave antennae and was initially verified in isolated bovine liver by Hoffmann et al.106 However, at present, there is no relevant research on the translation of these theoretical viewpoints into clinical practice, and such applications have not yet appeared in relevant studies. (2) The development of water-circulating internal cooling antennas effectively solves the problem of high rod temperatures, enabling the implementation of high-power, long-term, high-energy-level ablation, and the morphology of the ablation zone tends to be more spherical. However, relevant evidence for its clinical application and cost-effectiveness analysis needs to be obtained. (3) Owing to the complexity of the structure of human body tissues and individual variability, there is still considerable uncertainty in the solution of the heat transfer process of complicated biological tissues. Some scholars have carried out a series of studies on the change in the heat transfer properties of human tissues at different temperatures and the effect of the vascular tree on heat transfer,107 which provide a preliminary viewpoint on related issues. However, there are still some discrepancies between the simulation and experimental results of temperature field simulations, which require systematic cooperation between clinical and device engineering. (4) In terms of thermometry, invasive thermometry for tumors is relatively dangerous and inaccurate; however, currently, clinical thermometry technology is not available. The development of the current technology available for clinical ablation poses some technical difficulties that must be overcome. Zhou Zhuhuang et al.108 summarized the shortcomings of traditional ultrasound thermometry technology, such as the need for motion compensation. Because of the problems encountered with traditional thermometry technology, Camilo Correa-Gallego et al.109 reported that ultrasound and elastography can provide an accurate gross estimation of ablation zone size; however, these methods cannot be used to predict the degree of cellular injury and may cause the ultimate size of the ablation area to be significantly underestimated. This issue limits the application of ultrasonic temperature measurement technology. Indeed, the application of newer iterations of thermometry in ablation devices and comparative studies on the level and quality of ablation treatment and patient prognosis require the full cooperation of clinicians and technical engineers.
Summary
The widespread adoption of chest LDCT, accelerated by COVID-19 screening protocols, has improved early lung cancer detection but concurrently increased psychosocial burdens from overdiagnosed pulmonary nodules. While conventional VATS anatomical lobectomy effectively treats early-stage malignancies, its associated pulmonary functional loss limits utility for multifocal lesions, particularly MPLCs. Hybrid VATS-ablation surgery addresses this dilemma by combining resection of dominant GGNs with concurrent ablation of satellite high-risk lesions. Early institutional studies demonstrate preserved lung function and oncological efficacy, supported by emerging evidence of synergies with CAR-T therapy and medical-industrial integration opportunities. This integrated approach leverages minimally invasive precision to balance curative intent and organ preservation, offering a tailored approach for multifocal disease. Nevertheless, clinical adoption requires resolution of interdisciplinary coordination challenges, standardization of ablation protocols, and validation through multicenter trials with extended follow up. Further exploration of ablation-induced immune modulation and techno-economic analyses will solidify its role in precision thoracic oncology.
Limitations of the study
Current evidence on VATS-ablation hybrid surgery has several limitations. (1) The number and range limits of hybrid surgery for managing pulmonary nodules need to be further explored in practical clinical applications. (2) The coordination between different specialized departments (interventional, thoracic surgery, etc.) needs to be improved, and technical collaboration is necessary. (3) Patients’ acceptance of the strategy of hybrid surgical treatment needs to be improved through medical education. (4) Currently, there is a lack of large-scale, multicentre prospective clinical studies confirming the efficacy of hybrid surgery. (5) There is a lack of long-term (10–20 years) clinical follow-up data for patients who have undergone hybrid surgery. (6) There are many manufacturers of ablation therapy equipment, differences in equipment standards and performance pose major challenges to the promotion of this technology, and there is a need to establish recognized treatment norms and standards. (7) Systematic basic research is relatively lacking, and research on the ablation field distribution and postablation immune mechanisms needs further development.
Acknowledgments
The authors acknowledge Home for Researchers (www.home-for-researchers.com) for providing technical support in figure illustration during this study. This work was supported by the National Natural Science Foundation of China (82203154), the Capital’s Funds for Health Improvement and Research (2024-2-4027), National Key R&D Program of China (2022YFC2407404), the National High Level Hospital Clinical Research Funding (CFA202502013, CFA202503003), the Cooperation Fund of CHCAMS (CFA202502013, CFA202503003).
Author contributions
R.X., study conception, study design, data analysis and interpretation, statistical analysis, manuscript preparation, and manuscript editing. G.Z., study design, data analysis and interpretation, statistical analysis, manuscript preparation, and manuscript editing. N.R., Y.C., S.W., and C.Y. data acquisition. Q.X., Fe.T., L.Z., and J.H., funding acquisition supervision, and manuscript review.
Declaration of interests
The authors declare competing interests.
Contributor Information
Fengwei Tan, Email: tanfengwei@126.com.
Liang Zhao, Email: drzhaoliang@126.com.
Jie He, Email: prof.jiehe@gmail.com.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Chang Y.L., Wu C.T., Lee Y.C. Surgical treatment of synchronous multiple primary lung cancers: experience of 92 patients. J. Thorac. Cardiovasc. Surg. 2007;134:630–637. doi: 10.1016/j.jtcvs.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Nakata M., Sawada S., Yamashita M., Saeki H., Kurita A., Takashima S., Tanemoto K. Surgical treatments for multiple primary adenocarcinoma of the lung. Ann. Thorac. Surg. 2004;78:1194–1199. doi: 10.1016/j.athoracsur.2004.03.102. [DOI] [PubMed] [Google Scholar]
- 4.Rostad H., Strand T.E., Naalsund A., Norstein J. Resected synchronous primary malignant lung tumors: a population-based study. Ann. Thorac. Surg. 2008;85:204–209. doi: 10.1016/j.athoracsur.2007.07.091. [DOI] [PubMed] [Google Scholar]
- 5.Trousse D., Barlesi F., Loundou A., Tasei A.M., Doddoli C., Giudicelli R., Astoul P., Fuentes P., Thomas P. Synchronous multiple primary lung cancer: an increasing clinical occurrence requiring multidisciplinary management. J. Thorac. Cardiovasc. Surg. 2007;133:1193–1200. doi: 10.1016/j.jtcvs.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Yu Y.C., Hsu P.K., Yeh Y.C., Huang C.S., Hsieh C.C., Chou T.Y., Hsu H.S., Wu Y.C., Huang B.S., Hsu W.H. Surgical results of synchronous multiple primary lung cancers: similar to the stage-matched solitary primary lung cancers? Ann. Thorac. Surg. 2013;96:1966–1974. doi: 10.1016/j.athoracsur.2013.04.142. [DOI] [PubMed] [Google Scholar]
- 7.Chen C., Huang X., Peng M., Liu W., Yu F., Wang X. Multiple primary lung cancer: a rising challenge. J. Thorac. Dis. 2019;11:S523–S536. doi: 10.21037/jtd.2019.01.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martini N., Melamed M.R. Multiple primary lung cancers. J. Thorac. Cardiovasc. Surg. 1975;70:606–612. [PubMed] [Google Scholar]
- 9.Li Q., Xiao T., Li J., Niu Y., Zhang G. The diagnosis and management of multiple ground-glass nodules in the lung. Eur. J. Med. Res. 2024;29:305. doi: 10.1186/s40001-024-01904-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazzone P.J., Lam L. Evaluating the Patient With a Pulmonary Nodule: A Review. JAMA. 2022;327:264–273. doi: 10.1001/jama.2021.24287. [DOI] [PubMed] [Google Scholar]
- 11.McWilliams A., Tammemagi M.C., Mayo J.R., Roberts H., Liu G., Soghrati K., Yasufuku K., Martel S., Laberge F., Gingras M., et al. Probability of cancer in pulmonary nodules detected on first screening CT. N. Engl. J. Med. 2013;369:910–919. doi: 10.1056/NEJMoa1214726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y., Fu F., Chen H. Management of Ground-Glass Opacities in the Lung Cancer Spectrum. Ann. Thorac. Surg. 2020;110:1796–1804. doi: 10.1016/j.athoracsur.2020.04.094. [DOI] [PubMed] [Google Scholar]
- 13.Lee H.W., Jin K.N., Lee J.K., Kim D.K., Chung H.S., Heo E.Y., Choi S.H. Long-Term Follow-Up of Ground-Glass Nodules After 5 Years of Stability. J. Thorac. Oncol. 2019;14:1370–1377. doi: 10.1016/j.jtho.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Sato Y., Fujimoto D., Morimoto T., Uehara K., Nagata K., Sakanoue I., Hamakawa H., Takahashi Y., Imai Y., Tomii K. Natural history and clinical characteristics of multiple pulmonary nodules with ground glass opacity. Respirology. 2017;22:1615–1621. doi: 10.1111/resp.13089. [DOI] [PubMed] [Google Scholar]
- 15.Detterbeck F.C., Marom E.M., Arenberg D.A., Franklin W.A., Nicholson A.G., Travis W.D., Girard N., Mazzone P.J., Donington J.S., Tanoue L.T., et al. The IASLC Lung Cancer Staging Project: Background Data and Proposals for the Application of TNM Staging Rules to Lung Cancer Presenting as Multiple Nodules with Ground Glass or Lepidic Features or a Pneumonic Type of Involvement in the Forthcoming Eighth Edition of the TNM Classification. J. Thorac. Oncol. 2016;11:666–680. doi: 10.1016/j.jtho.2015.12.113. [DOI] [PubMed] [Google Scholar]
- 16.Liu M., He W.X., Song N., Yang Y., Zhang P., Jiang G.N. Discrepancy of epidermal growth factor receptor mutation in lung adenocarcinoma presenting as multiple ground-glass opacities. Eur. J. Cardio. Thorac. Surg. 2016;50:909–913. doi: 10.1093/ejcts/ezw113. [DOI] [PubMed] [Google Scholar]
- 17.Shimada Y., Saji H., Otani K., Maehara S., Maeda J., Yoshida K., Kato Y., Hagiwara M., Kakihana M., Kajiwara N., et al. Survival of a surgical series of lung cancer patients with synchronous multiple ground-glass opacities, and the management of their residual lesions. Lung Cancer. 2015;88:174–180. doi: 10.1016/j.lungcan.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Wang X., Wu M., Shen H., Nie Y., Zhang K., Wei Z., Wang Z., Yang F., Chen K. Comparison of Clinical and Pathological Characteristics Between Extremely Multiple GGNs and Single GGNs. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.725475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Creach K.M., Bradley J.D., Mahasittiwat P., Robinson C.G. Stereotactic body radiation therapy in the treatment of multiple primary lung cancers. Radiother. Oncol. 2012;104:19–22. doi: 10.1016/j.radonc.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Dong B., Chen R., Zhu X., Wu Q., Jin J., Wang W., Zhu Y., Jiang H., Bi N., Wang X., et al. Comparison of stereotactic body radiation therapy versus surgery for multiple primary lung cancers after prior radical resection: A multicenter retrospective study. Clin. Transl. Radiat. Oncol. 2023;40 doi: 10.1016/j.ctro.2023.100601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin K.C., Ko W.C., Tsai Y.D., Chang C.Y., Yang Y.H., Huang Y.S., Chang Y.C. Hemorrhage risk prediction after computed tomography-guided lung biopsy: Clinical parameters and quantitative pulmonary vascular analysis. J. Formos. Med. Assoc. 2025;124:79–86. doi: 10.1016/j.jfma.2024.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Yang Y., Ma J., Peng Z., Zhou X., Du N., Zhang W., Yan Z. Pneumothorax and pulmonary hemorrhage after C-arm cone-beam computed tomography-guided percutaneous transthoracic lung biopsy: incidence, clinical significance, and correlation. BMC Pulm. Med. 2024;24:33. doi: 10.1186/s12890-023-02822-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng B., Li C., Li J., Gong L., Liang P., Chen Y., Zhan S., Xiong S., Zhong R., Liang H., et al. The activity and immune dynamics of PD-1 inhibition on high-risk pulmonary ground glass opacity lesions: insights from a single-arm, phase II trial. Signal Transduct. Target. Ther. 2024;9:93. doi: 10.1038/s41392-024-01799-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duma N., Santana-Davila R., Molina J.R. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc. 2019;94:1623–1640. doi: 10.1016/j.mayocp.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Andersson E., Dai Ydrefelt Y., Johannesson M., Lundbäck M., Mannila M., Persson M., Swahn E., Bolejko A. Surveillance of indeterminate pulmonary nodules detected with CT in a Swedish population-based study (SCAPIS): psychosocial consequences and impact on health-related quality of life-a multicentre prospective cross-sectional study. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2021-048721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith Z., Barnett S.A., Gorelik A., Pascoe D.M., Manser R.L. Strategies for the Management of Solitary Pulmonary Nodules: A Survey of Patient Preferences. Ann. Thorac. Surg. 2022;113:1670–1675. doi: 10.1016/j.athoracsur.2021.04.094. [DOI] [PubMed] [Google Scholar]
- 27.Xiao R., Huang Y., Meng S., Liu X., Zhao X., Wang J., Li X. A cross-sectional study of psychological burden in Chinese patients with pulmonary nodules: Prevalence and impact on the management of nodules. Thorac. Cancer. 2021;12:3150–3156. doi: 10.1111/1759-7714.14165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dupuy D.E., Fernando H.C., Hillman S., Ng T., Tan A.D., Sharma A., Rilling W.S., Hong K., Putnam J.B. Radiofrequency ablation of stage IA non-small cell lung cancer in medically inoperable patients: Results from the American College of Surgeons Oncology Group Z4033 (Alliance) trial. Cancer. 2015;121:3491–3498. doi: 10.1002/cncr.29507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bi N., Shedden K., Zheng X., Kong F.M.S. Comparison of the Effectiveness of Radiofrequency Ablation With Stereotactic Body Radiation Therapy in Inoperable Stage I Non-Small Cell Lung Cancer: A Systemic Review and Pooled Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2016;95:1378–1390. doi: 10.1016/j.ijrobp.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bilal H., Mahmood S., Rajashanker B., Shah R. Is radiofrequency ablation more effective than stereotactic ablative radiotherapy in patients with early stage medically inoperable non-small cell lung cancer? Interact. Cardiovasc. Thorac. Surg. 2012;15:258–265. doi: 10.1093/icvts/ivs179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang H., Chiu Y.C., Lee S.W., Yen C.C., Pai J.T., Lee C.Y., Wu Y.L., Lee C.M., Hsieh Y.S. Novel light delivery method for performing transbronchial photodynamic therapy ablation to treat peripheral lung cancer: A pilot study. Photodiagnosis Photodyn. Ther. 2022;40 doi: 10.1016/j.pdpdt.2022.103063. [DOI] [PubMed] [Google Scholar]
- 32.Kinoshita T., Effat A., Gregor A., Inage T., Ishiwata T., Motooka Y., Ujiie H., Wilson B.C., Zheng G., Weersink R., et al. A Novel Laser Fiberscope for Simultaneous Imaging and Phototherapy of Peripheral Lung Cancer. Chest. 2019;156:571–578. doi: 10.1016/j.chest.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberg C., Puls R., Hegenscheid K., Kuehn J., Bollman T., Westerholt A., Weigel C., Hosten N. Laser ablation of metastatic lesions of the lung: long-term outcome. AJR Am. J. Roentgenol. 2009;192:785–792. doi: 10.2214/ajr.08.1425. [DOI] [PubMed] [Google Scholar]
- 34.Liu S., Liang B., Li Y., Xu J., Qian W., Lin M., Xu M., Niu L. CT-Guided Percutaneous Cryoablation in Patients with Lung Nodules Mainly Composed of Ground-Glass Opacities. J. Vasc. Interv. Radiol. 2022;33:942–948. doi: 10.1016/j.jvir.2022.04.021. [DOI] [PubMed] [Google Scholar]
- 35.Mabud T.S., Swilling D., Guichet P., Zhu Y., Manduca S., Patel B., Azour L., Taslakian B., Garay S.M., Moore W. Pulmonary Cryoablation Outcomes in Octogenarians and Nonagenarians with Primary Lung Cancer. J. Vasc. Interv. Radiol. 2023;34:2006–2011. doi: 10.1016/j.jvir.2023.07.022. [DOI] [PubMed] [Google Scholar]
- 36.Sänger J.A., Graur A., Tahir I., Price M.C., Keane F.K., Lanuti M., Sharma A., Fintelmann F.J. Outcomes following cryoablation of stage IA non-small cell lung cancer in patients with and without interstitial lung disease: A retrospective single-center cohort study. Lung Cancer. 2023;181 doi: 10.1016/j.lungcan.2023.107231. [DOI] [PubMed] [Google Scholar]
- 37.Abbas A.E. Surgical Management of Lung Cancer: History, Evolution, and Modern Advances. Curr. Oncol. Rep. 2018;20:98. doi: 10.1007/s11912-018-0741-7. [DOI] [PubMed] [Google Scholar]
- 38.Detterbeck F.C., Bolejack V., Arenberg D.A., Crowley J., Donington J.S., Franklin W.A., Girard N., Marom E.M., Mazzone P.J., Nicholson A.G., et al. The IASLC Lung Cancer Staging Project: Background Data and Proposals for the Classification of Lung Cancer with Separate Tumor Nodules in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016;11:681–692. doi: 10.1016/j.jtho.2015.12.114. [DOI] [PubMed] [Google Scholar]
- 39.Gao J.W., Rizzo S., Ma L.H., Qiu X.Y., Warth A., Seki N., Hasegawa M., Zou J.W., Li Q., Femia M., et al. Pulmonary ground-glass opacity: computed tomography features, histopathology and molecular pathology. Transl. Lung Cancer Res. 2017;6:68–75. doi: 10.21037/tlcr.2017.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobayashi Y., Mitsudomi T., Sakao Y., Yatabe Y. Genetic features of pulmonary adenocarcinoma presenting with ground-glass nodules: the differences between nodules with and without growth. Ann. Oncol. 2015;26:156–161. doi: 10.1093/annonc/mdu505. [DOI] [PubMed] [Google Scholar]
- 41.Lee H.Y., Choi Y.L., Lee K.S., Han J., Zo J.I., Shim Y.M., Moon J.W. Pure ground-glass opacity neoplastic lung nodules: histopathology, imaging, and management. AJR Am. J. Roentgenol. 2014;202:W224–W233. doi: 10.2214/ajr.13.11819. [DOI] [PubMed] [Google Scholar]
- 42.Travis W.D., Brambilla E., Burke A.P., Marx A., Nicholson A.G. Introduction to The 2015 World Health Organization Classification of Tumors of the Lung, Pleura, Thymus, and Heart. J. Thorac. Oncol. 2015;10:1240–1242. doi: 10.1097/jto.0000000000000663. [DOI] [PubMed] [Google Scholar]
- 43.Evens A., Cyriac G., Jeong D., Araujo C., Gage K., Rose T. A Pictorial Essay of Immunotherapy: Complications that Internists Will See, Whether They Like it or Not. Am. J. Med. 2019;132:808–815. doi: 10.1016/j.amjmed.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 44.Chan M.V., Huo Y.R., Cao C., Ridley L. Survival outcomes for surgical resection versus CT-guided percutaneous ablation for stage I non-small cell lung cancer (NSCLC): a systematic review and meta-analysis. Eur. Radiol. 2021;31:5421–5433. doi: 10.1007/s00330-020-07634-7. [DOI] [PubMed] [Google Scholar]
- 45.Hu H., Zhai B., Liu R., Chi J.C. Microwave Ablation Versus Wedge Resection for Stage I Non-small Cell Lung Cancer Adjacent to the Pericardium: Propensity Score Analyses of Long-term Outcomes. Cardiovasc. Interv. Radiol. 2021;44:237–246. doi: 10.1007/s00270-020-02601-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maxwell C.M., Ng C., Fernando H.C. Stereotactic Body Radiation Therapy Versus Ablation Versus Surgery for Early-Stage Lung Cancer in High-Risk Patients. Thorac. Surg. Clin. 2023;33:179–187. doi: 10.1016/j.thorsurg.2023.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Safi S., Rauch G., op den Winkel J., Kunz J., Schneider T., Bischof M., Heussel C.P., Huber P.E., Herth F.J.F., Dienemann H., Hoffmann H. Sublobar Resection, Radiofrequency Ablation or Radiotherapy in Stage I Non-Small Cell Lung Cancer. Respiration. 2015;89:550–557. doi: 10.1159/000381555. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y., Liu B., Cao P., Wang W., Wang W., Chang H., Li D., Li X., Zhao X., Li Y. Comparison between computed tomography-guided percutaneous microwave ablation and thoracoscopic lobectomy for stage I non-small cell lung cancer. Thorac. Cancer. 2018;9:1376–1382. doi: 10.1111/1759-7714.12842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yao W., Lu M., Fan W., Huang J., Gu Y., Gao F., Wang Y., Li J., Zhu Z. Comparison between microwave ablation and lobectomy for stage I non-small cell lung cancer: a propensity score analysis. Int. J. Hyperthermia. 2018;34:1329–1336. doi: 10.1080/02656736.2018.1434901. [DOI] [PubMed] [Google Scholar]
- 50.Zemlyak A., Moore W.H., Bilfinger T.V. Comparison of survival after sublobar resections and ablative therapies for stage I non-small cell lung cancer. J. Am. Coll. Surg. 2010;211:68–72. doi: 10.1016/j.jamcollsurg.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 51.Zeng C., Lu J., Tian Y., Fu X. Thermal Ablation Versus Wedge Resection for Stage I Non-small Cell Lung Cancer Based on the Eighth Edition of the TNM Classification: A Population Study of the US SEER Database. Front. Oncol. 2020;10 doi: 10.3389/fonc.2020.571684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kwan S.W., Mortell K.E., Hippe D.S., Brunner M.C. An Economic Analysis of Sublobar Resection versus Thermal Ablation for Early-Stage Non–Small-Cell Lung Cancer. J. Vasc. Interv. Radiol. 2014;25:1558–1565. doi: 10.1016/j.jvir.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 53.Fietta A.M., Morosini M., Passadore I., Cascina A., Draghi P., Dore R., Rossi S., Pozzi E., Meloni F. Systemic inflammatory response and downmodulation of peripheral CD25+Foxp3+ T-regulatory cells in patients undergoing radiofrequency thermal ablation for lung cancer. Hum. Immunol. 2009;70:477–486. doi: 10.1016/j.humimm.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 54.Hiroishi K., Eguchi J., Baba T., Shimazaki T., Ishii S., Hiraide A., Sakaki M., Doi H., Uozumi S., Omori R., et al. Strong CD8(+) T-cell responses against tumor-associated antigens prolong the recurrence-free interval after tumor treatment in patients with hepatocellular carcinoma. J. Gastroenterol. 2010;45:451–458. doi: 10.1007/s00535-009-0155-2. [DOI] [PubMed] [Google Scholar]
- 55.Sabel M.S. Cryo-immunology: a review of the literature and proposed mechanisms for stimulatory versus suppressive immune responses. Cryobiology. 2009;58:1–11. doi: 10.1016/j.cryobiol.2008.10.126. [DOI] [PubMed] [Google Scholar]
- 56.Wissniowski T.T., Hänsler J., Neureiter D., Frieser M., Schaber S., Esslinger B., Voll R., Strobel D., Hahn E.G., Schuppan D. Activation of tumor-specific T lymphocytes by radio-frequency ablation of the VX2 hepatoma in rabbits. Cancer Res. 2003;63:6496–6500. [PubMed] [Google Scholar]
- 57.Zerbini A., Pilli M., Laccabue D., Pelosi G., Molinari A., Negri E., Cerioni S., Fagnoni F., Soliani P., Ferrari C., Missale G. Radiofrequency thermal ablation for hepatocellular carcinoma stimulates autologous NK-cell response. Gastroenterology. 2010;138:1931–1942. doi: 10.1053/j.gastro.2009.12.051. [DOI] [PubMed] [Google Scholar]
- 58.Haen S.P., Gouttefangeas C., Schmidt D., Boss A., Clasen S., von Herbay A., Kosan B., Aebert H., Pereira P.L., Rammensee H.G. Elevated serum levels of heat shock protein 70 can be detected after radiofrequency ablation. Cell Stress Chaperones. 2011;16:495–504. doi: 10.1007/s12192-011-0261-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Erinjeri J.P., Thomas C.T., Samoilia A., Fleisher M., Gonen M., Sofocleous C.T., Thornton R.H., Siegelbaum R.H., Covey A.M., Brody L.A., et al. Image-guided thermal ablation of tumors increases the plasma level of interleukin-6 and interleukin-10. J. Vasc. Interv. Radiol. 2013;24:1105–1112. doi: 10.1016/j.jvir.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dromi S.A., Walsh M.P., Herby S., Traughber B., Xie J., Sharma K.V., Sekhar K.P., Luk A., Liewehr D.J., Dreher M.R., et al. Radiofrequency ablation induces antigen-presenting cell infiltration and amplification of weak tumor-induced immunity. Radiology. 2009;251:58–66. doi: 10.1148/radiol.2511072175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahmad F., Gravante G., Bhardwaj N., Strickland A., Basit R., West K., Sorge R., Dennison A.R., Lloyd D.M. Changes in interleukin-1β and 6 after hepatic microwave tissue ablation compared with radiofrequency, cryotherapy and surgical resections. Am. J. Surg. 2010;200:500–506. doi: 10.1016/j.amjsurg.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 62.Ahmad F., Gravante G., Bhardwaj N., Strickland A., Basit R., West K., Sorge R., Dennison A.R., Lloyd D.M. Renal effects of microwave ablation compared with radiofrequency, cryotherapy and surgical resection at different volumes of the liver treated. Liver Int. 2010;30:1305–1314. doi: 10.1111/j.1478-3231.2010.02290.x. [DOI] [PubMed] [Google Scholar]
- 63.Ahmed M., Solbiati L., Brace C.L., Breen D.J., Callstrom M.R., Charboneau J.W., Chen M.H., Choi B.I., de Baère T., Dodd G.D., 3rd, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. Radiology. 2014;273:241–260. doi: 10.1148/radiol.14132958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bailey C.W., Sydnor M.K., Jr. Current State of Tumor Ablation Therapies. Dig. Dis. Sci. 2019;64:951–958. doi: 10.1007/s10620-019-05514-9. [DOI] [PubMed] [Google Scholar]
- 65.Ritz J.P., Lehmann K.S., Mols A., Frericks B., Knappe V., Buhr H.J., Holmer C. Laser-induced thermotherapy for lung tissue--evaluation of two different internally cooled application systems for clinical use. Lasers Med. Sci. 2008;23:195–202. doi: 10.1007/s10103-007-0472-8. [DOI] [PubMed] [Google Scholar]
- 66.Lerner E.C., Edwards R.M., Wilkinson D.S., Fecci P.E. Laser ablation: Heating up the anti-tumor response in the intracranial compartment. Adv. Drug Deliv. Rev. 2022;185 doi: 10.1016/j.addr.2022.114311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahmed M., Brace C.L., Lee F.T., Jr., Goldberg S.N. Principles of and advances in percutaneous ablation. Radiology. 2011;258:351–369. doi: 10.1148/radiol.10081634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fajardo L.F., Egbert B., Marmor J., Hahn G.M. Effects of hyperthermia in a malignant tumor. Cancer. 1980;45:613–623. doi: 10.1002/1097-0142(19800201)45:3<613::aid-cncr2820450331>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 69.Nikfarjam M., Muralidharan V., Christophi C. Mechanisms of focal heat destruction of liver tumors. J. Surg. Res. 2005;127:208–223. doi: 10.1016/j.jss.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 70.den Brok M.H.M.G.M., Sutmuller R.P.M., van der Voort R., Bennink E.J., Figdor C.G., Ruers T.J.M., Adema G.J. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res. 2004;64:4024–4029. doi: 10.1158/0008-5472.Can-03-3949. [DOI] [PubMed] [Google Scholar]
- 71.Arnold D., Faath S., Rammensee H., Schild H. Cross-priming of minor histocompatibility antigen-specific cytotoxic T cells upon immunization with the heat shock protein gp96. J. Exp. Med. 1995;182:885–889. doi: 10.1084/jem.182.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu. Rev. Immunol. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- 73.Sang J., Liu P., Wang M., Xu F., Ma J., Wei Z., Ye X. Dynamic Changes in the Immune Microenvironment in Tumor-Draining Lymph Nodes of a Lewis Lung Cancer Mouse Model After Microwave Ablation. J. Inflamm. Res. 2024;17:4175–4186. doi: 10.2147/jir.S462650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang M., Sang J., Xu F., Wang S., Liu P., Ma J., Chen Z., Xie Q., Wei Z., Ye X. Microwave Ablation Combined with Flt3L Provokes Tumor-Specific Memory CD8(+) T Cells-Mediated Antitumor Immunity in Response to PD-1 Blockade. Adv. Sci. 2025;12 doi: 10.1002/advs.202413181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Briard J.G., Poisson J.S., Turner T.R., Capicciotti C.J., Acker J.P., Ben R.N. Small molecule ice recrystallization inhibitors mitigate red blood cell lysis during freezing, transient warming and thawing. Sci. Rep. 2016;6 doi: 10.1038/srep23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hanai A., Yang W.L., Ravikumar T.S. Induction of apoptosis in human colon carcinoma cells HT29 by sublethal cryo-injury: mediation by cytochrome c release. Int. J. Cancer. 2001;93:526–533. doi: 10.1002/ijc.1359. [DOI] [PubMed] [Google Scholar]
- 77.Chapman W.C., Debelak J.P., Wright Pinson C., Washington M.K., Atkinson J.B., Venkatakrishnan A., Blackwell T.S., Christman J.W. Hepatic cryoablation, but not radiofrequency ablation, results in lung inflammation. Ann. Surg. 2000;231:752–761. doi: 10.1097/00000658-200005000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.den Brok M.H.M.G.M., Sutmuller R.P.M., Nierkens S., Bennink E.J., Frielink C., Toonen L.W.J., Boerman O.C., Figdor C.G., Ruers T.J.M., Adema G.J. Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br. J. Cancer. 2006;95:896–905. doi: 10.1038/sj.bjc.6603341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jiang N., Zhang L., Hao Y., Wu X., Zhao Y., Cong B., Peng C. Combination of electromagnetic navigation bronchoscopy-guided microwave ablation and thoracoscopic resection: An alternative for treatment of multiple pulmonary nodules. Thorac. Cancer. 2020;11:1728–1733. doi: 10.1111/1759-7714.13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qu R., Tu D., Hu S., Wang Q., Ping W., Hao Z., Cai Y., Zhang N., Wang J., Fu X. Electromagnetic Navigation Bronchoscopy-Guided Microwave Ablation Combined With Uniportal Video-Assisted Thoracoscopic Surgery for Multiple Ground Glass Opacities. Ann. Thorac. Surg. 2022;113:1307–1315. doi: 10.1016/j.athoracsur.2021.04.061. [DOI] [PubMed] [Google Scholar]
- 81.Ye X., Fan W., Wang Z., Wang J., Wang H., Niu L., Fang Y., Gu S., Liu L., Liu B., et al. Clinical practice guidelines on image-guided thermal ablation of primary and metastatic lung tumors (2022 edition) J. Cancer Res. Ther. 2022;18:1213–1230. doi: 10.4103/jcrt.jcrt_880_22. [DOI] [PubMed] [Google Scholar]
- 82.Austin C.A., Mohottige D., Sudore R.L., Smith A.K., Hanson L.C. Tools to Promote Shared Decision Making in Serious Illness: A Systematic Review. JAMA Intern. Med. 2015;175:1213–1221. doi: 10.1001/jamainternmed.2015.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Geerse O.P., Stegmann M.E., Kerstjens H.A.M., Hiltermann T.J.N., Bakitas M., Zimmermann C., Deal A.M., Brandenbarg D., Berger M.Y., Berendsen A.J. Effects of Shared Decision Making on Distress and Health Care Utilization Among Patients With Lung Cancer: A Systematic Review. J. Pain Symptom Manage. 2018;56:975–987.e5. doi: 10.1016/j.jpainsymman.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 84.Ye X., Fan W., Wang Z., Wang J., Wang H., Wang J., Wang C., Niu L., Fang Y., Gu S., et al. Expert consensus on thermal ablation therapy of pulmonary subsolid nodules (2021 Edition) J. Cancer Res. Ther. 2021;17:1141–1156. doi: 10.4103/jcrt.jcrt_1485_21. [DOI] [PubMed] [Google Scholar]
- 85.Zeng C., Fu X., Yuan Z., Hu S., Wang X., Ping W., Cai Y., Wang J. Application of electromagnetic navigation bronchoscopy-guided microwave ablation in multiple pulmonary nodules: a single-centre study. Eur. J. Cardio. Thorac. Surg. 2022;62 doi: 10.1093/ejcts/ezac071. [DOI] [PubMed] [Google Scholar]
- 86.Liu B., Ye X., Fan W., Zhi X., Ma H., Wang J., Wang P., Wang Z., Wang H., Wang X., et al. Expert consensus on the multidisciplinary diagnosis and treatment of multiple ground glass nodule-like lung cancer (2024 Edition) J. Cancer Res. Ther. 2024;20:1109–1123. doi: 10.4103/jcrt.jcrt_563_24. [DOI] [PubMed] [Google Scholar]
- 87.Xie D., Wang H., Fei K., Chen C., Zhao D., Zhou X., Xie B., Jiang L., Chen Q., Song N., et al. Single-port video-assisted thoracic surgery in 1063 cases: a single-institution experience. Eur. J. Cardio. Thorac. Surg. 2016;49(Suppl 1):i31–i36. doi: 10.1093/ejcts/ezv408. [DOI] [PubMed] [Google Scholar]
- 88.Bao F., Yu F., Wang R., Chen C., Zhang Y., Lin B., Wang Y., Hao X., Gu Z., Fang W. Electromagnetic bronchoscopy guided microwave ablation for early stage lung cancer presenting as ground glass nodule. Transl. Lung Cancer Res. 2021;10:3759–3770. doi: 10.21037/tlcr-21-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cui X., Zhao J., Lu R., Sui Y., Shao C., Zhang Z., Chen J. Microwave ablation after VATS in patients with multiple pulmonary nodules. J. Cancer Res. Ther. 2024;20:2029–2034. doi: 10.4103/jcrt.jcrt_898_24. [DOI] [PubMed] [Google Scholar]
- 90.Liu B., Zhang Y., Su L., Wang R. Treatment options for pulmonary multifocal ground glass opacity type adenocarcinoma: Surgery combine thermal ablation? J. Interv. Med. 2020;3:180–183. doi: 10.1016/j.jimed.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wolfram F., Boltze C., Schubert H., Bischoff S., Lesser T.G. Effect of lung flooding and high-intensity focused ultrasound on lung tumours: an experimental study in an ex vivo human cancer model and simulated in vivo tumours in pigs. Eur. J. Med. Res. 2014;19:1. doi: 10.1186/2047-783x-19-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Crous A., Abrahamse H. Photodynamic therapy of lung cancer, where are we? Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.932098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Freitag L. PDT in early central lung cancer. Thorax. 2007;62:374–375. doi: 10.1136/thx.2006.067892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huang J., Zhuang C., Chen J., Chen X., Li X., Zhang T., Wang B., Feng Q., Zheng X., Gong M., et al. Targeted Drug/Gene/Photodynamic Therapy via a Stimuli-Responsive Dendritic-Polymer-Based Nanococktail for Treatment of EGFR-TKI-Resistant Non-Small-Cell Lung Cancer. Adv. Mater. 2022;34 doi: 10.1002/adma.202201516. [DOI] [PubMed] [Google Scholar]
- 95.Go E.J., Yang H., Chon H.J., Yang D., Ryu W., Kim D.H., Han D.K., Kim C., Park W. Combination of Irreversible Electroporation and STING Agonist for Effective Cancer Immunotherapy. Cancers (Basel) 2020;12 doi: 10.3390/cancers12113123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Song Z.Q., Xu X.H., Pan Z.H., Yao C.G., Zhou Q.H. Mechanisms for steep pulse irreversible electroporation technology to kill human large cell lung cancer cells L9981. Int. J. Clin. Exp. Med. 2014;7:2386–2394. [PMC free article] [PubMed] [Google Scholar]
- 97.Tasu J.P., Tougeron D., Rols M.P. Irreversible electroporation and electrochemotherapy in oncology: State of the art. Diagn. Interv. Imaging. 2022;103:499–509. doi: 10.1016/j.diii.2022.09.009. [DOI] [PubMed] [Google Scholar]
- 98.Huang Y., Wang J., Hu Y., Cao P., Wang G., Cai H., Wang M., Yang X., Wei Z., Ye X. Microwave ablation plus camrelizumab monotherapy or combination therapy in non-small cell lung cancer. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.938827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wei Z., Yang X., Ye X., Huang G., Li W., Han X., Wang J., Meng M., Ni Y., Zou Z., Wen Q. Camrelizumab combined with microwave ablation improves the objective response rate in advanced non-small cell lung cancer. J. Cancer Res. Ther. 2019;15:1629–1634. doi: 10.4103/jcrt.JCRT_990_19. [DOI] [PubMed] [Google Scholar]
- 100.Cao B., Liu M., Wang L., Zhu K., Cai M., Chen X., Feng Y., Yang S., Fu S., Zhi C., et al. Remodelling of tumour microenvironment by microwave ablation potentiates immunotherapy of AXL-specific CAR T cells against non-small cell lung cancer. Nat. Commun. 2022;13:6203. doi: 10.1038/s41467-022-33968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Duan X., Wang M., Han X., Ren J., Huang G., Ju S., Zhang Q. Combined use of microwave ablation and cell immunotherapy induces nonspecific immunity of hepatocellular carcinoma model mice. Cell Cycle. 2020;19:3595–3607. doi: 10.1080/15384101.2020.1853942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu M., Xue B., Wang Y., Wang D., Gao D., Yang S., Zhao Q., Zhou C., Ruan S., Yuan Z. Temperature-Feedback Nanoplatform for NIR-II Penta-Modal Imaging-Guided Synergistic Photothermal Therapy and CAR-NK Immunotherapy of Lung Cancer. Small. 2021;17 doi: 10.1002/smll.202101397. [DOI] [PubMed] [Google Scholar]
- 103.Jiang Y., Huang J., Xu C., Pu K. Activatable polymer nanoagonist for second near-infrared photothermal immunotherapy of cancer. Nat. Commun. 2021;12:742. doi: 10.1038/s41467-021-21047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sun T., Li J., Zhou Y., Zeng C., Luo C., Luo X., Li H. Metal-Organic Framework-Mediated Synergistic Hypoxia-Activated Chemo-Immunotherapy Induced by High Intensity Focused Ultrasound for Enhanced Cancer Theranostics. Small. 2024;20 doi: 10.1002/smll.202306338. [DOI] [PubMed] [Google Scholar]
- 105.Phasukkit P., Tungjitkusolmun S., Sangworasil M. Finite-element analysis and in vitro experiments of placement configurations using triple antennas in microwave hepatic ablation. IEEE Trans. Biomed. Eng. 2009;56:2564–2572. doi: 10.1109/tbme.2009.2027128. [DOI] [PubMed] [Google Scholar]
- 106.Hoffmann R., Rempp H., Erhard L., Blumenstock G., Pereira P.L., Claussen C.D., Clasen S. Comparison of four microwave ablation devices: an experimental study in ex vivo bovine liver. Radiology. 2013;268:89–97. doi: 10.1148/radiol.13121127. [DOI] [PubMed] [Google Scholar]
- 107.Nan Q., Lu Y., Liu Y., Zeng Y. IEEE; 2010. Large Blood Vessel Effect on Thermal Ablation with a Water-Cooled Microwave Antenna; pp. 936–938. [Google Scholar]
- 108.Li S., Zhou Z., Wu S., Wu W. A Review of Quantitative Ultrasound-Based Approaches to Thermometry and Ablation Zone Identification Over the Past Decade. Ultrason. Imaging. 2022;44:213–228. doi: 10.1177/01617346221120069. [DOI] [PubMed] [Google Scholar]
- 109.Correa-Gallego C., Karkar A.M., Monette S., Ezell P.C., Jarnagin W.R., Kingham T.P. Intraoperative ultrasound and tissue elastography measurements do not predict the size of hepatic microwave ablations. Acad. Radiol. 2014;21:72–78. doi: 10.1016/j.acra.2013.09.022. [DOI] [PubMed] [Google Scholar]