Abstract
A striking variety of glycosylation occur in the Golgi complex in a protein-specific manner, but how this diversity and specificity are achieved remains unclear. Here we show that stacked fragments (units) of the Golgi complex dispersed in Drosophila imaginal disk cells are functionally diverse. The UDP-sugar transporter FRINGE-CONNECTION (FRC) is localized to a subset of the Golgi units distinct from those harboring SULFATELESS (SFL), which modifies glucosaminoglycans (GAGs), and from those harboring the protease RHOMBOID (RHO), which processes the glycoprotein SPITZ (SPI). Whereas the glycosylation and function of NOTCH are affected in imaginal disks of frc mutants, those of SPI and of GAG core proteins are not, even though FRC transports a broad range of glycosylation substrates, suggesting that Golgi units containing FRC and those containing SFL or RHO are functionally separable. Distinct Golgi units containing FRC and RHO in embryos could also be separated biochemically by immunoisolation techniques. We also show that Tn-antigen glycan is localized only in a subset of the Golgi units distributed basally in a polarized cell. We propose that the different localizations among distinct Golgi units of molecules involved in glycosylation underlie the diversity of glycan modification.
Keywords: fringe connection, glycosylation, posttranslational modification
The pattern of glycosylation is extremely diverse, yet is highly specific to each protein. How can this specificity (and diversity) be achieved? There are >300 glycosylenzymes in humans and >100 in Drosophila, but is their enzymatic specificity sufficient to explain the precise modification of all substrates? One possible mechanism that might also contribute to the specific (and diverse) pattern of glycosylation would be the localization/compartmentalization of glycosylenzymes.
The Golgi complex, where protein glycosylation takes place, has been regarded as a single functional unit, consisting of cis-, medial-, and transcisternae in mammalian cells. However, the three-dimensional reconstruction of electron microscopic images of the mammalian Golgi structure has suggested the existence of more than one Golgi stack, with the individual stacks being connected into a ribbon by tubules bridging equivalent cisternae (1). Furthermore, during mitosis, the Golgi cisternae of mammalian cells become fragmented without their disassembly (2, 3). In Drosophila, Golgi cisternae are stacked but are not connected to form a ribbon at the embryonic and pupal stages even during interphase (4, 5), although there has been no evidence to date to indicate functional differences among the Golgi fragments.
We previously reported a Drosophila UDP-sugar transporter, FRINGE CONNECTION (FRC), that transports a broad range of UDP-sugars that can be used for the synthesis of various glycans, including N-linked types, GAGs, and mucin types (6, 7). Interestingly, despite its broad specificity, loss-of-function studies have revealed that FRC is selectively required for Notch glycosylation, but not for GAG synthesis. This observation prompted us to study at FRC localization; in this study, we found that FRC is localized only to a subset of Golgi fragments in Drosophila disks and embryos.
Here, we showed that FRC, SFL (8), a glycosylenzyme of GAGs, and RHO (9, 10), a processing enzyme of SPI glycoprotein, are localized to distinct Golgi fragments, which we refer to as “Golgi units,” in Drosophila cells. No frc mutants exhibited any defects in the glycosylation and function of SPI in addition to those of GAG core proteins. Moreover, we biochemically separated distinct Golgi units containing FRC and RHO by immunoisolation technique. Our study clearly shows that there are functionally distinct Golgi units in a Drosophila cell.
Materials and Methods
Fly Strains and Clonal Analysis. Canton-S flies were used as wild-type flies; arm-Gal4, UAS-spiMYC, and UAS-Star lines were kindly provided by J. R. Lee, S. Urban, C. F. Garvey, and M. Freeman (Laboratory of Molecular Biology, Medical Research Council, Cambridge, U.K.). Expression of FRC-MYC was achieved by crossing arm-Gal4 and UAS-frcMYC lines. The frc and ttv mutant mosaic clones were generated with the FLP-FRT system. First-instar larvae from a cross of yw hs-flp; FRT2A frcRY34/TM3G or ptc-lacZ/CyO; FRT2A frcRY34/TM3G or dll-lacZ/CyO; FRT2A frcRY34/TM3G with w; FRT2A ubi-GFP or from a cross of yw; FRT42D ttv524/CyO act-GFP (kindly provided by T. Tabata, University of Tokyo, Tokyo) with yw hs-flp; FRT42D ubi-GFP/CyO were incubated for 1 h at 37°C. Imaginal wing and eye disks were dissected from third-instar larvae and stained as described below.
Immunostaining. The 3G10 antibody (1:100 dilution; Seikagaku), which reacts with heparan sulfate, was used to detect GAGs by immunostaining as described (11). Rabbit polyclonal antibodies to dGM130 and to SFL were generated by injection with the synthetic peptides EIKENTEQPSHNHAGDHLNC and LGSRPIPQWLKDDLSTGT, respectively. Eye and wing disks dissected from third-instar larvae were fixed for 1 h with 4% paraformaldehyde in PBS containing 0.04% Triton X-100. Embryos were fixed as described (6). The disks were then incubated first for 30 min at room temperature in PBS containing 0.1% Triton X-100 and 0.1% BSA and then over-night at 4°C with Biotin-conjugated PNA (1:20 dilution; Seikagaku) or the following primary antibodies: mouse anti-120-kDa protein (1:200; Calbiochem), rabbit anti-dGM130 (1:200), rabbit anti-dSYX16 (1:500; kindly provided by H. Xu, Hospital for Sick Children, Toronto), rat anti-c-MYC (1:200; Cosmobio), rabbit anti-RHO (1:500; kindly provided by E. Bier, University of California, San Diego), rat anti-ELAV [7E8A10, 1:20; Developmental Studies Hybridoma Bank (DSHB)], mouse monoclonal antibody 22C10 (1:20; DSHB), rabbit anti-BAR (1:100; kindly provided by T. Kojima and K. Saigo, Universityof Tokyo, Tokyo), rabbit anti-SFL (1:100), mouse anti-WG (4D4, 1:100; DSHB), mouse anti-βgalactosidase (1:100, Promega), rabbit anti-GFP (1:500; MBL, Nagoya, Japan) and mouse anti-Tn antigen (HB-Tn1, 1:20; Dako). After three 20-min washes with PBS containing Triton X-100 and BSA, the disks were incubated for 2 h at room temperature with appropriate secondary antibodies: Cy3-conjugated anti-rat IgG (1:200; Kirkegaard & Perry Laboratories), Cy3-conjugated anti-rabbit IgG (1:200; Amersham Pharmacia), Cy3-conjugated anti-mouse IgG (Amersham Pharmacia), Cy5-conjugated anti-mouse IgG (1:200; Amersham Pharmacia), Cy5-conjugated anti-rabbit IgG (1:200; Amersham Pharmacia), Alexa Fluor 488-conjugated anti-rabbit IgG (1:200; Molecular Probes), Alexa Fluor 488-conjugated anti-rat IgG (1:200; Molecular Probes), Alexa Fluor 488-conjugated anti-mouse IgM (1:200; Molecular Probes), Alexa Fluor 488-conjugated streptavidin (1:200; Molecular Probes), or biotin-conjugated anti-rabbit IgG (1:200; The Jackson Laboratories). The biotin-conjugated secondary antibodies were detected with Alexa Fluor 555-conjugated streptavidin (1:200; Molecular Probes). An Alexa Fluor 488 TSA kit (Molecular Probes) was used for the detection of SFL and RHO.
Image Capture. Imaginal disks and embryos were imaged by using an Olympus FV-500 (Olympus) with a ×20 Olympus UPlanApo objective [numerical aperture (NA), 0.85], or ×100 Olympus UPlanApo oil immersion objective (NA, 1.35). The Alexa 488-conjugated antibodies and GFP molecules were excited at 488 nm by a multiargon laser and imaged by using a 505-525 bandpass filter. The Cy3-conjugated antibodies and Alexa 555-conjugated streptavidin were excited at 543 nm by a HeNe-G laser and imaged by using a 560-600 bandpass filter. The Cy5-conjugated antibodies were excited with at 633 nm by a HeNe-R laser and imaged by using a long-pass 660 filter. Captured images were processed by using photoshop (Adobe Systems, San Jose, CA).
Analysis of SPI-MYC Glycosylation. GFP-negative larvae were selected from the progeny of UAS-Star; frcRY34/TM3, ubi-GFP females crossed with arm-Gal4 UAS-spiMYC; frcRY34/TM3, ubi-GFP males. About 100 larvae were homogenized in SDS sample buffer and heated at 100°C for 5 min. Half of each homogenate was treated first for 32 h at 37°C with neuraminidase (Roche Molecular Biochemicals), which removes sialic acid sugars, and then for 28 h at 37°C with a mixture of O-glycosidases (Roche Molecular Biochemicals) and PNGase F (New England Biolabs), which remove both high-mannose glycans and complex N-linked glycans. Both halves of each homogenate were then subjected to SDS/PAGE and blot analysis with either WGA or antibodies to MYC as described (6, 10).
Immunoisolation of the Golgi Units. Embryos were collected at 0-12 h after egg laying, decorionated, and stored at -80°C. A total of 0.1 g of frozen embryos was homogenized in lysis buffer (50 mM Tris·Cl, pH 7.5/150 mM KCl/5 mM MgCl2) with 1 mM EGTA and Complete EDTA-free protease inhibitors (Roche Diagnostics) using pestle A (five strokes; loose-fitting), followed by pestle B (10 strokes; tight-fitting) in a Dounce homogenizer placed on ice. The extract was centrifuged at 1,000 × g at 4°C for 5 min. The debris and nuclei were pelleted down. The supernatant was carefully pipetted out without disturbing the pellet and incubated with magnetic beads (sheep anti-mouse IgG beads; Dynal) coated covalently with either the monoclonal antibodies to MYC (9E10; Santa Cruz Biotechnology), hemagglutinin (HA) (2C16; MBL), or normal mouse IgG (The Jackson Laboratory) at 4°C for 1 h. Unbound material was separated from the beads by using a magnet. The bead fraction was washed for 5 min five times and boiled in SDS/PAGE sample buffer. After SDS/PAGE, immunoblotting was performed as previously described (6). Anti-MYC (A14, Santa Cruz Biotechnology) and anti-HA (Novus) polyclonal antibodies were used as the primary antibodies, and horseradish peroxidase-conjugated anti-rabbit IgG antibody (The Jackson Laboratory) was used as the secondary antibody. Super Signal (Pierce) was used for detection. For quantitation, the signal intensity of each band was measured by using LAS-1000 plus (Fuji) and normalized to that of corresponding input. The ratio of band intensities in each immunoisolate was calculated by these normalized intensities.
Results
The Golgi Complex in Drosophila Disk Cells Is a Stack of Cis-, Medial-, and Transcisternae. The Golgi complex is a stack of cis-, medial-, and transcisternae in mammalian cells. On the other hand, Golgi markers often do not overlap with each other in Saccharomyces cerevisiae, in which the Golgi cisternae are not stacked but disassembled (12). The Golgi cisternae of Drosophila are stacked but are not connected to form a ribbon at the embryonic and pupal stages even during interphase. To determine whether Drosophila imaginal disk cells have assembled or disassembled Golgi cisternae, we compared the localizations of the cis-cisternal marker dGM130 (5), the transcisternal marker dSYNTAXIN16 (dSYX16) (13), and the Golgi-tethered 120-kDa protein (4), which is commonly used to detect the Golgi complex in Drosophila. The 120-kDa protein was identified (M.A. and S.G., unpublished data) by immunoaffinity purification and protein sequencing as a Drosophila homolog of the vertebrate 160-kDa medial Golgi sialoglycoprotein (MG160) (14), which resides uniformly in the medial-cisternae of the Golgi apparatus in vertebrate cells. An antibody specific for the 120-kDa protein also stained numerous Golgi fragments in imaginal disk cells (Fig. 1 B and E), consistent with previous observations (5). More than 80% of immunoreactivity for the 120-kDa protein was colocalized with both dGM130 and dSYX16 (Fig. 1 A-F and supporting information, which is published on the PNAS web site), suggesting that 120-kDa protein-positive fragments of the Golgi complex indeed comprise assembled cisternae; these fragments will be referred to as “Golgi units” hereafter. The distributions of the 120-kDa protein, dGM130, and peanut agglutinin (PNA), another transcisternal marker, also showed that the markers are closely apposed but not identical, suggesting that the Golgi units are polarized (Fig. 1 G-I). Interestingly, most of the PNA-positive transcisternae were oriented toward the basal side of the cell, within the Golgi complex, whereas most of the GM130-positive cis-cisternae were oriented toward the apical side of the cell. The cis-to-trans polarity of each Golgi unit thus appears to be correlated with the apico-basal polarity of the disk cells (see supporting information).
Fig. 1.
Stacking of cis- to transcisternal markers in Golgi fragments of Drosophila imaginal disk cells. (A-F) Colocalization of the 120-kDa protein (red in B, C, E, and F) with dGM130 (cyan in A and C) and dSYX16 (cyan in D and F) in wing disk cells, as revealed by immunostaining. (G-I) Triple staining with anti-dGM130 (blue), anti-120-kDa protein (red), and peanut agglutinin (green), revealing stacking of the three cisternal markers. Top, apical; bottom, basal. (Scale bars, 5 μm.)
frc-Null Mutant Larvae Also Manifest a Highly Selective Phenotype. We have previously shown that Drosophila mutant larvae defective in the UDP-sugar transporter FRC manifest a highly selective phenotype: the lack of NOTCH glycosylation in the presence of normal GAG synthesis (6). This limited phenotype was unexpected, given that FRC exhibits a broad specificity for UDP sugars used in the synthesis of various glycans including N-linked types, GAGs, and mucin types. However, given that the frcR29 allele studied previously (6) is hypomorphic, we examined whether the selective glycosylation defect might be a consequence of partial loss of FRC activity. With the use of imprecise excision, we generated a new allele, frcRY34, the presence of which results in the death of most larvae during the second-instar stage, much earlier than the death induced by frcR29. Real-time PCR analysis revealed that the amount of frc transcripts in the second-instar larvae of frcRY34 or frcR29 mutants was 4.2 and 24.4% of that in the wild type, respectively (data not shown). About 1 kb of the gene, including the transcription initiation site, was deleted in the frcRY34 allele (data not shown). Together, these observations suggest that frcRY34 is essentially a null allele.
Clonal cells of the frcRY34 mutant exhibited normal levels of GAGs, as detected by immunostaining with the 3G10 antibody, whereas the amount of GAGs was reduced in clones of tout-velu (ttv) mutant cells (see supporting information), as shown in ref. 11. Given that GAGs are required for signaling by HEDGEHOG (HH), WINGLESS (WG), and DECAPENTAPLEGIC (DPP), we examined the expression of corresponding target genes [patched (ptc) for HH signaling and Dll for WG and DPP signaling] in the wing disks of the frcRY34 mutant. Expression of ptc and that of Dll in the ventral compartment of the wing disks were unaffected in the mutant clones (see supporting information), suggestive of normal GAG function.
Given that NOTCH glycosylation by FRINGE (FNG) (15-17), a fucose-specific β1,3-N-acetylglucosaminyltransferase, requires FRC activity, we examined NOTCH glycosylation in the frcRY34 mutant. The frcRY34 mutant clones in the dorsal compartment, but not those in the ventral compartment, of the wing disks induced wg expression at their borders (see supporting information), as previously observed with fng mutant clones (18), suggesting that NOTCH glycosylation is impaired in the frcRY34 mutant. The ectopic expression of WG induced by the frcRY34 mutant clones is likely responsible for the observed induction of Dll expression in the dorsal compartment.
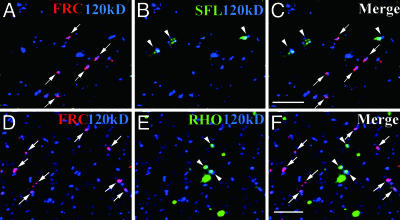
FRC Is Localized to a Subset of Golgi Units Distinct from Those Harboring SFL. To determine why the loss of a UDP-sugar transporter with a broad specificity selectively affects NOTCH glycosylation, we first examined the subcellular localization of FRC. FRC tagged with the MYC epitope was expressed in imaginal disks under the control of the arm-Gal4 driver. The Gal4-induced expression of FRC-MYC rescued the frc mutant phenotype (data not shown), suggesting that FRC-MYC is functional and properly localized. Immunostaining of imaginal disks of wild-type larvae expressing FRC-MYC with antibodies to MYC and to the 120-kDa protein revealed that FRC was localized to only a small subset of Golgi units (Fig. 2A). This differential immunostaining of different Golgi units is not likely to be due to differential penetration of the antibodies or cripticity of the epitopes. The penetration of antibodies would not vary within the cell, because the Golgi units were distributed evenly throughout the cell, not in a biased manner. Moreover, it is unlikely that degradation of the epitopes during the immunostaining experiments due to contaminating proteases might alter the cripticity of the epitopes in different Golgi units, as the percentage of different Golgi units among the anti-120-kDa-positive Golgi units was statistically constant in several independent experiments. Thus, we hypothesized that the Golgi units might be functionally heterogeneous, and that those containing FRC might modify some proteins, including NOTCH, but not others.
Fig. 2.
Localization of FRC to Golgi units distinct from those containing SFL or RHO in imaginal disk cells. (A-C) Distinct localizations of FRC and SFL in Golgi units. Wing disks of wild-type larvae expressing MYC epitope-tagged FRC were stained with anti-MYC (red, arrows), anti-SFL (green, arrowheads), and anti-120-kDa protein (blue). The SFL signal was almost completely absent in sfl mutant embryos (data not shown), suggesting that the antibodies are specific for SFL. (D-F) Distinct localizations of FRC and RHO in Golgi units. Eye disks of wild-type larvae expressing FRC-MYC were stained with anti-MYC (red, arrows), anti-RHO (green, arrowheads), and anti-120-kDa protein. Some RHO staining did not overlap with that of the 120-kDa protein, as described (19). (Scale bars, 5 μm.)
To test this hypothesis, we compared the localizations of various molecules involved in protein modification in the Golgi complex with that of FRC. We found that SFL was also restricted to a subset of Golgi units, but that its distribution did not overlap with that of FRC (Fig. 2 A-C and supporting information). This differential localization of SFL and FRC might thus explain our observation that frc mutant clones in wing disks do not show any defect in GAG synthesis by SFL.
The Golgi Units Harboring FRC and RHO Are Functionally Different. The SPI-processing enzyme RHO was also localized to a subset of Golgi units distinct from those containing FRC (Fig. 2 D-F), in addition to its presence in other compartments as described (19). This result indicated the existence of at least two types of Golgi units, those containing RHO and those containing FRC. To determine whether these two types of Golgi units differ functionally, we examined the glycosylation state and function of SPI in frc mutants.
Given that the extent of NOTCH glycosylation, as detected by wheat germ agglutinin (WGA), is markedly reduced in frc mutants compared with that in the wild-type background (6), we examined whether the WGA-reactive glycan of SPI is also affected by frc mutation. We expressed MYC epitope-tagged SPI in the wild type or the frcRY34 mutant. SPI-MYC was then precipitated from larval homogenates with antibodies to MYC and was examined for its glycosylation by SDS/PAGE and subsequent blot analysis with WGA. The reactivity of the SPI glycan with WGA was similar in the frc mutant and in the wild type (Fig. 3A). We further examined whether the frcRY34 mutation affects the SPI glycan by mobility shift analysis. The electrophoretic mobility of glycosylated SPI from the wild type was also similar to that from the frc mutant (Fig. 3B). Deglycosylation of SPI by neuraminidase, peptide-N-glycosidase (PNGase) F, and O-glycanases also increased its mobility to the same extent in wild-type and frc mutant larvae (Fig. 3B), suggesting that the core protein was not affected by the frc mutation. Together, these results indicate that the function of FRC is not necessary for formation of the SPI glycan.
Fig. 3.
Glycosylation state and function of SPI are not affected by frc mutation. (A and B) SPI-MYC was precipitated from homogenates of wild-type (WT) or frcRY34 mutant larvae and subjected to SDS/PAGE followed by WGA blot analysis (A) or immunoblot analysis with anti-MYC (B). In B, homogenates were treated (+) or not (-) with PNGase F, O-glycosidase, and neuraminidase after precipitation of SPI-MYC. (C-F) Photoreceptor cells of the eye disks of third-instar larvae. Eye disks of wild-type (C) or frcR29 (D) larvae were stained with anti-ELAV (green), and those of the wild type (E) or a frcRY34 mutant clone, which is negatively marked with green fluorescent protein (GFP, green) and outlined by dashed lines in F, were stained with anti-BAR (red), monoclonal antibody 22C10 (blue), or anti-ELAV (green in E), as indicated. (C and D) rho and spi mutants hamper the differentiation of ELAV-positive photoreceptors, except for R8. Observation of more than one ELAV-positive photoreceptors in each ommatidium (outlined by dashed line) indicated that the function of RHO and SPI were normal. (E and F) BAR-positive R1 and R6 photoreceptors (red) were induced in the mutant clone but their locations were irregular. (G and H) Legs of the wild type (G) and a frcR29 mutant that died in the pupal case (H). Bracts (arrows) formed normally in the frc mutant. (I and J) Wing veins of rhove1,frc+/rhove1,frc+ (I) and rhove1,frcRY34/rhove1,frc+ (J) adult flies. The frcRY34 mutation did not affect the partial loss of the distal regions of veins 3 to 5 apparent in the rhove1 homozygote. (Scale bars, 5 μmin C-F, 10 μmin G, and 500 μmin I.)
We next evaluated SPI function by examining developmental processes such as photoreceptor recruitment (20) and bract formation (21), both of which require SPI activation. During eye development, although SPI is not necessary for the primary induction of the photoreceptor R8, it is required for the subsequent recruitment of R1 to R7. Given that photoreceptors R1 to R8 express ELAV (22) (Fig. 3C) and that R1 and R6 express BAR (23) (Fig. 3E), we examined the expression of these proteins in frc mutants. In mutants harboring the hypomorphic allele frcR29, all photoreceptors were normally induced (Fig. 3D), although their direction was irregular as seen in fringe (24) or Notch (25) mutants. Similar results were obtained by clonal analysis of frcRY34 mutants (Fig. 3F). SPI function in photoreceptor recruitment thus did not appear to be impaired in the frc mutants. The frcR29 mutant also formed normal bracts on malformed legs (Fig. 3H). We tested for genetic interaction between rho and frc mutations in wing vein formation. The rhove1 mutant is viable but shows partial loss of L3-5 veins (26) (Fig. 3I). This phenotype was also apparent in rhove1, frcRY34/rhove1, frc+ flies (Fig. 3J), suggesting that FRC does not affect RHO function. From these results, we conclude that the function of the RHO-SPI pathway is not affected by frc mutation.
The Golgi Units Harboring FRC and RHO Are Biochemically Separable. To confirm that the Golgi units containing FRC and those containing RHO are distinct, we examined whether these Golgi units could be selectively isolated by using antibodies to MYC (for MYC-tagged FRC) or HA (for HA-tagged RHO). Because it was very difficult to collect enough of the imaginal disks, we switched our starting material to embryos, and first examined whether FRC and RHO were also localized to distinct Golgi units in embryos. FRC-MYC and RHO-HA were coexpressed in the embryos by the arm-Gal4 driver, and immunostaining with antibodies to MYC and to HA revealed that the Golgi units containing FRC-MYC (45.4% of total Golgi units) and those containing RHO-HA (43.0% of total Golgi units) were largely distinct: only 11.6% of total Golgi units were positive for both FRC-MYC and RHO-HA (Fig. 4 A and D). We then attempted immunoisolation from embryonic lysates by using either antibody to MYC or HA and examined how much FRC-MYC and RHO-HA were coisolated in each immunoisolate. When FRC-MYC was immunoisolated with an antibody to MYC, the recovery of FRC-MYC was 5.7 times greater than that of RHO-HA. Moreover, when RHO-HA was immunoisolated with an antibody to HA, the recovery of RHO-HA was 18.3 times greater than that of FRC-MYC (Fig. 4 G and J). The immunoblot analysis of these immunoisolates with the anti-120-kDa antibody confirmed that the Golgi units were concentrated in these immunoisolates (data not shown). Immunoisolation with control IgG gave only a negligible signal in both experiments. These results support the notion that FRC-MYC-containing fraction is distinct and could be separated from RHO-HA-containing fraction.
Fig. 4.
FRC-FNG and RHO are localized to distinct Golgi units in embryos. (A-F) FRC-MYC and RHO-HA (A and D), FRC-MYC and FNG-HA (B and E), or RHO-HA and FNG-MYC (C and F) were expressed in embryonic epidermal cells by arm-Gal4 driver. Cells were stained with anti-120kD protein (green), anti-MYC (red), and anti-HA (blue). (A and D) FRC (red) and RHO (blue) were localized to distinct Golgi units (green). (B, C, E, and F) FRC (red) and FNG (blue) were colocalized to the same Golgi units (arrows in B and E), whereas RHO (blue) and FNG (red) were localized to the distinct Golgi units (C and F). (Scale bar, 5 μm.) (G-K) Biochemical separation of FRC- and RHO-positive Golgi units. (G-I) FRC-MYC and RHO-HA (G), FRC-MYC and FNG-HA (H), or RHO-HA and FNG-MYC (I) were expressed by arm-Gal4 driver. Golgi were immunoisolated with either anti-MYC or anti-HA and subjected to SDS/PAGE followed by immunoblot analyses with anti-MYC and anti-HA. (J and K) Ratios of FRC-MYC and RHO-HA (n = 4, J), FRC-MYC and FNG-HA (n = 4, K), or RHO-HA and FNG-MYC (n = 4, K) in each immunoisolate are shown.
We further examined whether these distinct Golgi units contain different constituents. FRINGE (FNG) is one of the candidate molecules that may be colocalized with FRC. Therefore, we examined expression of ectopically expressed FNG in RHO- and FRC-containing immunoisolates. We found that expression of FNG in FRC-containing immunoisolates was 26 times greater than in RHO-containing immunoisolates, supporting the idea that FNG is localized in the FRC-positive Golgi units rather than the RHO-positive Golgi units (Fig. 4 H, I, and K). We also confirmed by immunostaining analysis (Fig. 4 B, C, E, and F) that FNG was colocalized mostly with FRC (88.1% of the FNG-positive Golgi units), but not with RHO (16.6% of the FNG-positive Golgi units), by immunostaining analysis.
Tn-Antigen Glycan Is Localized to a Subset of the Golgi Units Distributed Basally. Our data suggest that different Golgi units perform different functions, a notion that is also supported by the observation that Tn antigen (O-linked N-acetylgalactosamine) was detected in only a subset of Golgi units in imaginal eye disk cells (Fig. 5 A-C). In addition, we found that most of these Tn antigen-positive Golgi units were distributed in the basal region of the disk cells (Fig. 5D), suggesting that the differential distribution of Golgi units might contribute to the apicobasal polarity of glycan distribution.
Fig. 5.
Synthesis of Tn antigen is restricted to a subset of Golgi units. (A-C) The undifferentiated cells of eye disks before progression of the morphogenetic furrow are shown in vertical view (A: top, apical; bottom, basal) and in horizontal views (B, apical side; C, basal side). Immunostaining revealed that Tn antigen (cyan) was mostly restricted to the basally located Golgi units stained by anti-dGM130 (red). Arrows indicate Tn antigen localized in the Golgi units. (Scale bars, 5 μm.) (D) Percentages of Tn antigen-positive (cyan) and Tn antigen-negative (red) Golgi units at various horizontal levels of imaginal disk cells. Numbers of the Golgi units counted at each surface are 301 (0.0-μm depth), 221 (1.5 μm), 158 (3.0 μm), 233 (4.5 μm), 156 (6.0 μm), 152 (7.5 μm), and 120 (9.0 μm).
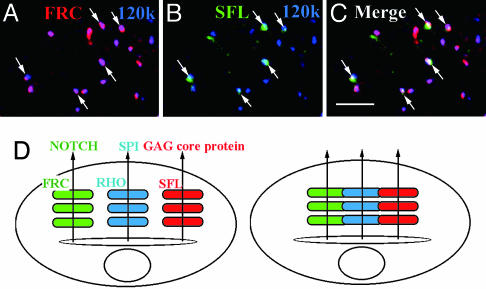
SFL Is Colocalized with FRC at the Embryonic Stage. In contrast to the larval stage, FRC is required for GAG synthesis at the early embryonic stage (6, 7). To determine why the FRC requirement for GAG synthesis differs between the embryonic and larval stages, we stained embryos expressing FRC-MYC with antibodies to SFL and to MYC. SFL was found to be colocalized with FRC (Fig. 6 A-C and supporting information), likely explaining the importance of FRC for GAG synthesis at the embryonic stage. In addition, this embryonic requirement of FRC for GAG synthesis excludes the possibility that the selective defects in NOTCH and not in GAG synthesis observed in frc mutant larvae are caused by the selective FRC-dependent transport of a subset of UDP-sugars used only for glycosylation of NOTCH but not for GAGs synthesis.
Fig. 6.
Colocalization of FRC and SFL in Drosophila embryos and schematic models of the Golgi complex in Drosophila and mammalian cells. (A-C) A Drosophila embryo expressing FRC-MYC at stage 14 immunostained with anti-MYC (red), anti-120-kDa protein (blue), and anti-SFL (green). Arrows indicate colocalization of FRC and SFL. (Scale bar, 5 μm.) (D) Distinct Golgi units are dispersed in Drosophila cells (Left) but are connected in mammalian cells (Right). Different units contain different sets of molecules involved in posttranslational modifications such as glycosylation and proteolytic processing. Secretory or membrane proteins such as NOTCH, SPI, and GAG core proteins may be transported to appropriate Golgi units in Drosophila cells.
Discussion
Our results provide evidence for the existence of functionally distinct Golgi units in Drosophila cells (Fig. 6D). Such functional heterogeneity of Golgi units is likely responsible for the diversity of protein glycosylation. At least two types of Golgi units containing either FRC or SFL were shown to be present in larval disk cells. Two distinct sets of proteins, exemplified by NOTCH and GAG core proteins, might thus be selectively transported to FRC- or SFL-containing Golgi units, respectively, where they undergo glycosylation by different sets of molecules.
The variety of Golgi units might be established by separate transport of secretory proteins and glycosylenzymes from the endoplasmic reticulum (ER) to the distinct Golgi units. In yeast, glycosylphosphatidylinositol (GPI)-anchored proteins exit the ER in vesicles distinct from those containing other secretory protein (27). Given that the GAG core protein DALLY in Drosophila is anchored to the membrane by GPI (28), it is possible that DALLY and NOTCH are loaded into distinct vesicles as they exit the ER.
Combinations of glycosylenzymes and transporters, such as SFL and FRC, contained in Golgi units of Drosophila differ not only between embryos and larval disk cells but also among cell types. For example, we found that FRC was localized to all Golgi units in salivary gland cells at the larval stage (data not shown). It was also recently shown that all of the Golgi complexes dispersed in oocytes may have the ability to process the GURKEN precursor protein, which is usually cleaved in a subset of the Golgi complexes residing in the dorso-anterior region (29). The Golgi units may thus be altered in a manner dependent on development, cell type, and signaling processes.
The functional diversity of Golgi units also might contribute to the polarized distribution of glycans along the apicobasal axis of cells. We found that Tn antigen is synthesized in the basal Golgi units of larval disk cells. Furthermore, certain types of glycans are distributed along the apicobasal axis of pupal ommatidia (H.Y. and S.G., unpublished data). These glycans might thus be synthesized differentially in the Golgi units that are asymmetrically distributed along the apicobasal axis and then be secreted at either the apical or basal cell surface.
Whereas Golgi units are dispersed throughout Drosophila cells, the Golgi complex in mammalian cells is thought to be a single entity that is located in the pericentriolar region through its association with the microtubule-organizing center in interphase and which is fragmented at the onset of mitosis. The Golgi fragments apparent in mammalian cells during mitosis are highly similar to the Golgi units of Drosophila cells in both electron (2, 5) and confocal (3) microscopic images. The mammalian Golgi complex during interphase may therefore be comprised of functionally distinct units that are associated with the microtubule-organizing center and connected with each other (Fig. 6D).
Supplementary Material
Acknowledgments
We thank E. Bier, M. Freeman, C. F. Garvey, T. Kojima, J. R. Lee, K. Saigo, S. Urban, H. Xu, Bloomington Stock Center, and Developmental Studies Hybridoma Bank for fly strains or antibodies, as well as M. Freeman, P. A. Lawrence, J. R. Lee, T. Taguchi, S. Urban, and members of the S.G. laboratory for helpful discussions and critical comments. This work was started in the Laboratory of Molecular Biology (Medical Research Council, Cambridge, U.K.) and continued at Mitsubishi-Kagaku Institute of Life Sciences; it was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and the Japan Science and Technology Agency (to S.G.).
Abbreviation: HA, hemagglutinin.
References
- 1.Ladinsky, M. S., Mastronarde, D. N., McIntosh, J. R., Howell, K. E. & Staehelin, L. A. (1999) J. Cell Biol. 144, 1135-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lucocq, J. M., Pryde, J. G., Berger, E. G. & Warren, G. (1987) J. Cell Biol. 104, 865-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shima, D. T., Haldar, K., Pepperkok, R., Watson, R. & Warren, G. (1997) J. Cell Biol. 137, 1211-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanley, H., Botas, J. & Malhotra, V. (1997) Proc. Natl. Acad. Sci. USA 94, 14467-14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kondylis, V., Goulding, S. E., Dunne, J. C. & Rabouille, C. (2001) Mol. Biol. Cell 12, 2308-2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goto, S., Taniguchi, M., Muraoka, M., Toyoda, H., Sado, Y., Kawakita, M. & Hayashi, S. (2001) Nat. Cell Biol. 3, 816-822. [DOI] [PubMed] [Google Scholar]
- 7.Selva, E. M., Hong, K., Baeg, G. H., Beverley, S. M., Turco, S. J., Perrimon, N. & Hacker, U. (2001) Nat. Cell Biol. 3, 809-815. [DOI] [PubMed] [Google Scholar]
- 8.Lin, X. & Perrimon, N. (1999) Nature 400, 281-284. [DOI] [PubMed] [Google Scholar]
- 9.Lee, J. R., Urban, S., Garvey, C. F. & Freeman, M. (2001) Cell 107, 161-171. [DOI] [PubMed] [Google Scholar]
- 10.Urban, S., Lee, J. R. & Freeman, M. (2001) Cell 107, 173-182. [DOI] [PubMed] [Google Scholar]
- 11.Takei, Y., Ozawa, Y., Sato, M., Watanabe, A. & Tabata, T. (2004) Development (Cambridge, U.K.) 131, 73-82. [DOI] [PubMed] [Google Scholar]
- 12.Rossanese, O. W., Soderholm, J., Bevis, B. J., Sears, I. B., O'Connor, J., Williamson, E. K. & Glick, B. S. (1999) J. Cell Biol. 145, 69-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu, H., Boulianne, G. L. & Trimble, W. S. (2002) J. Cell Sci. 115, 4447-4455. [DOI] [PubMed] [Google Scholar]
- 14.Gonatas, J. O., Mezitis, S. G., Stieber, A., Fleischer, B. & Gonatas, N. K. (1989) J. Biol. Chem. 264, 646-653. [PubMed] [Google Scholar]
- 15.Bruckner, K., Perez, L., Clausen, H. & Cohen, S. (2000) Nature 406, 411-415. [DOI] [PubMed] [Google Scholar]
- 16.Hicks, C., Johnston, S. H., diSibio, G., Collazo, A., Vogt, T. F. & Weinmaster, G. (2000) Nat. Cell Biol. 2, 515-520. [DOI] [PubMed] [Google Scholar]
- 17.Moloney, D. J., Panin, V. M., Johnston, S. H., Chen, J., Shao, L., Wilson, R., Wang, Y., Stanley, P., Irvine, K. D., Haltiwanger, R. S. & Vogt, T. F. (2000) Nature 406, 369-375. [DOI] [PubMed] [Google Scholar]
- 18.Kim, J., Irvine, K. D. & Carroll, S. B. (1995) Cell 82, 795-802. [DOI] [PubMed] [Google Scholar]
- 19.Sturtevant, M. A., Roark, M., O'Neill, J. W., Biehs, B., Colley, N. & Bier, E. (1996) Dev. Biol. 174, 298-309. [DOI] [PubMed] [Google Scholar]
- 20.Tio, M. & Moses, K. (1997) Development (Cambridge, U.K.) 124, 343-351. [DOI] [PubMed] [Google Scholar]
- 21.del Alamo, D., Terriente, J. & Diaz-Benjumea, F. J. (2002) Development (Cambridge, U.K.) 129, 1975-1982. [DOI] [PubMed] [Google Scholar]
- 22.Robinow, S. & White, K. (1991) J. Neurobiol. 22, 443-461. [DOI] [PubMed] [Google Scholar]
- 23.Higashijima, S., Kojima, T., Michiue, T., Ishimaru, S., Emori, Y. & Saigo, K. (1992) Genes Dev. 6, 50-60. [DOI] [PubMed] [Google Scholar]
- 24.Cho, K. O. & Choi, K. W. (1998) Nature 396, 272-276. [DOI] [PubMed] [Google Scholar]
- 25.Dominguez, M. & de Celis, J. F. (1998) Nature 396, 276-278. [DOI] [PubMed] [Google Scholar]
- 26.Sturtevant, M. A., Roark, M. & Bier, E. (1993) Genes Dev. 7, 961-973. [DOI] [PubMed] [Google Scholar]
- 27.Muniz, M., Morsomme, P. & Riezman, H. (2001) Cell 104, 313-320. [DOI] [PubMed] [Google Scholar]
- 28.Nakato, H., Futch, T. A. & Selleck, S. B. (1995) Development (Cambridge, U.K.) 121, 3687-3702. [DOI] [PubMed] [Google Scholar]
- 29.Herpers, B. & Rabouille, C. (2004) Mol. Biol. Cell 15, 5306-5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.