Abstract
Artificial intelligence (AI) integrated with robotic systems is transforming oncologic surgery by significantly improving precision, safety, and personalization. The review critically explores the current landscape of AI-powered robotic technologies in tumor resection across various specialties, including urology, neurosurgery, orthopedics, pediatrics, and head and neck oncology. Despite rapid advancements, challenges remain in tumor boundary detection, real-time intraoperative navigation, motion compensation, and seamless data integration. Drawing on evidence from 22 recent clinical studies, pilot trials, and simulation-based research, the review identifies key innovations such as image-free robotic palpation, sensor-assisted feedback, 3D anatomical modeling, and adaptive motion management in radiotherapy. These technologies contribute to enhanced surgical accuracy, reduced invasiveness, and improved intraoperative decision-making. However, barriers such as inconsistent clinical protocols, limited cost-effectiveness data, and variability in performance across tumor types continue to hinder widespread adoption. Challenges persist in complex fields such as pediatric and neurosurgical oncology, where anatomical variability and safety concerns demand more advanced solutions. The review emphasizes the need for interoperable AI-robotic platforms, robust real-time analytics, and standardized safety frameworks. It also highlights the importance of ethical governance and clinician training in ensuring responsible implementation. In conclusion, AI-powered robotic surgery represents a major shift in oncology, offering the potential to improve long-term outcomes and reduce recurrence through data-driven, minimally invasive interventions. Realizing the potential will require interdisciplinary collaboration, longitudinal clinical validation, and strategic integration into healthcare systems.
Keywords: Robotic-assisted tumor resection, Artificial intelligence in surgical oncology, Real-time intraoperative imaging, Adaptive robotic motion control, Minimally invasive oncologic robotics
Introduction
The integration of artificial intelligence (AI) with robotic surgical technologies marks a transformative shift in oncologic care, particularly in the domain of precision tumor surgery. Conventional surgical approaches, while effective to an extent, often suffer from limitations related to manual dexterity, intraoperative visibility, and decision-making under uncertainty. These constraints can lead to complications, incomplete resections, or collateral damage to healthy tissue consequently affecting oncologic outcomes. AI-driven robotic systems offer an unprecedented combination of enhanced precision, real-time decision support, and minimally invasive capabilities, enabling surgeons to visualize tumor margins more effectively, automate repetitive tasks, and achieve higher consistency in complex oncologic procedures [1, 9].
Emerging technologies such as 3D modeling, augmented reality (AR), image-free segmentation, tactile imaging, and adaptive control frameworks have further solidified the potential of robotic surgical systems guided by AI. In pediatric, neurosurgical, urologic, and otolaryngologic oncology, such innovations have shown substantial promise in improving patient outcomes through precise resections, reduced operative times, and better functional recovery [2, 12, 13]. These advances signal a new era in cancer care where robotics and AI do not merely support the surgeon but act as intelligent collaborators in achieving optimal oncologic outcomes.
The scope of the research centers on examining how AI-driven robotic innovations are reshaping the practice of precision tumor surgery. Specifically, the review explores interdisciplinary advances in surgical oncology where AI technologies are integrated into robotic systems to enhance tumor detection, segmentation, resection accuracy, and postoperative outcomes. Covering a diverse array of oncologic fields, including neurosurgery, urology, gastrointestinal oncology, maxillofacial surgery, and pediatric tumor management, the investigation aims to consolidate the growing body of evidence supporting the clinical utility of AI-augmented robotics in precision surgery.
The primary objective of the research is to analyze the synergistic role of AI and robotic surgery in improving intraoperative decision-making and patient-specific customization in tumor resections. This includes examining AI applications in real-time surgical navigation [22], 3D modeling for surgical planning [17], intraoperative margin assessment [6], and machine learning for pathology detection [9]. Additionally, the study seeks to highlight how adaptive control frameworks and autonomous systems are shaping the future of robotic endoscopy and radiotherapy [10, 14].
Another goal is to identify the technological, ethical, and clinical implementation barriers that hinder widespread adoption. This involves synthesizing current findings across academic disciplines and geographic regions to provide a holistic view of how AI-powered robotic systems can be practically integrated into surgical oncology workflows. By highlighting the most recent advancements and mapping out under-researched areas, the study provides strategic insights for future innovations and clinical trials in precision tumor surgery.
Methods
The review adopts a narrative review methodology to explore the diverse and rapidly evolving applications of artificial intelligence (AI) in robotic oncology surgery. A narrative review was chosen over a systematic review to allow for a flexible, comprehensive, and interdisciplinary synthesis of emerging research across engineering, surgical innovation, radiology, and clinical oncology. Given the novelty and ongoing development of AI-robotic surgical systems, a narrative format provides the necessary scope to include exploratory studies, pilot trials, conceptual models, and expert commentaries that may not yet meet the stringent inclusion criteria of systematic reviews.
The intention was not merely to summarize findings but to critically engage with the conceptual, technological, and clinical advancements shaping this field. This includes assessing the trajectory of AI integration in robotic surgical workflows, highlighting research gaps, and discussing real-world implications such as safety, ethics, training, and accessibility. As emphasized by [1], narrative reviews play an essential role in emerging fields by weaving together multi-domain knowledge and offering actionable insights for future research and clinical practice.
Literature identification and inclusion criteria
To ensure a broad and inclusive perspective, the author performed a targeted literature search from recent 2024 to 2025 across major databases including PubMed, Scopus, and IEEE Xplore. The author used search terms such as “AI in robotic surgery,” “oncologic robotics,” “AI-assisted tumor resection,” “robotic oncology,” “minimally invasive oncology,” and “AI-guided surgical outcomes.” Studies were included if they met the following criteria: (1) focused on AI-driven robotic surgery within oncologic settings, (2) addressed clinical applications, methods, frameworks, or technologies, (3) were peer-reviewed articles or conference proceedings, and (4) were published in English.
The final selection included 22 key references that reflected the most current, innovative, and multidisciplinary perspectives in the field. This included clinical trials, technical feasibility studies, conceptual frameworks, pilot implementations, and expert reviews across adult and pediatric oncology, neurosurgery, head and neck surgery, gastrointestinal oncology, urology, orthopedics, ophthalmology, and endocrine oncology.
Data synthesis and conceptual integration
The selected literature was analyzed and synthesized thematically. Rather than presenting the findings chronologically or by cancer type, the author organized the data according to major emergent themes. These include the roles of AI in perception and sensing, cognition and decision-making, robotic actuation and precision, system adaptability, safety frameworks, education and training, and multi-stage care integration (preoperative, intraoperative, and postoperative). The framework aligns with the four core pillars of AI-enhanced surgery: perception, cognition, actuation, and adaptation, as introduced by [8].
Several studies illustrate how AI technologies enable novel forms of tumor sensing and intraoperative visualization. For example, [4] presented the use of ultrasound-based virtual fixtures to delineate tumor boundaries during breast-conserving surgery, while [6] demonstrated the utility of three-dimensional ultrasound for intraoperative margin assessment in oropharyngeal cancer. Complementing these findings, [9] introduced a tactile imaging approach for gastric polyp diagnosis, illustrating how haptic feedback can support precision in soft tissue handling.
In the domain of cognitive augmentation, studies like those by [10] and [11] focused on AI models for autonomous decision-making during surgery. [11] proposed a minimally invasive robotic palpation system for image-free tumor segmentation, while [10] introduced a safety-ensured control framework that supports real-time automation of endoscopic tasks. These approaches reinforce the growing sophistication of AI in interpreting sensory data and making intraoperative decisions with minimal human intervention.
Actuation and robotic execution were further explored through procedural innovations and surgical trials. [12] presented the ACCURATE trial protocol, which utilized three-dimensional image-guided robotic-assisted partial nephrectomy to manage complex renal tumors. Similarly, [3] detailed robotic techniques for nephrectomy in anatomically challenging tumor cases. These studies exemplify how AI enhances the robotic arm’s precision, especially when integrated with preoperative imaging and intraoperative feedback.
Adaptability and real-time learning are central to AI’s future in surgical systems. [5] demonstrated how transfer learning can automate step recognition in bladder tumor resections, supporting procedural consistency and training. [14] expanded the view to radiotherapy, illustrating how AI can dynamically adjust radiation delivery using motion management algorithms, thereby personalizing cancer treatment.
To capture the full breadth of oncologic applications, the review also examined specialized and age-specific contexts. Pediatric oncology was represented by studies from [13] and [17], who reported on the successful use of robotic systems in complex pediatric tumor resections using 3D modeling and precision mapping. These works highlight the importance of minimally invasive approaches for vulnerable populations and demonstrate the adaptability of robotic platforms across age groups.
The review also included developments in neurosurgery and endocrine oncology. [2] examined sustainable AI strategies in brain tumor detection and treatment, while [21] presented updates on AI and robotics in pituitary tumor surgery. These studies stress the importance of safety, adaptability, and sustainability in high-risk surgical environments.
Additional studies reinforced the need for better navigation and augmented visualization during surgery. [18] demonstrated a real-time augmented reality robot system for skin tumor surgery, while [22] introduced an intelligent electromagnetic navigation system to support intraoral osteotomy in mandibular tumor resection. These approaches reflect the broader shift toward multisensory, AI-driven platforms that enhance intraoperative awareness and minimize surgical error.
In terms of feasibility and access, [7] provided evidence that robotic parotidectomy via retroauricular incision is a safe option for benign tumors, suggesting a broader surgical use case for robotic systems. [15] discussed how AI and digital imaging technologies could be adapted for vitreoretinal surgeries, extending the application of AI-assisted precision techniques beyond traditional tumor surgery.
The integration of AI into training and education emerged as another crucial area. Studies by [5] and [18] addressed how surgical teaching is being transformed through AI-driven automation, augmented overlays, and workflow standardization. These innovations offer scalable solutions for teaching complex procedures, especially in the post-pandemic era when remote and rapid training methods are in high demand.
Finally, the review emphasized the importance of aligning innovation with clinical outcomes. [20] summarized the clinical applications of AI in cancer precision treatment, stressing the value of data-driven personalization in both surgical and nonsurgical oncology settings. [16] offered additional insights into orthopedic oncology, discussing how robotic systems and AI are reshaping limb-salvage strategies and postoperative rehabilitation.
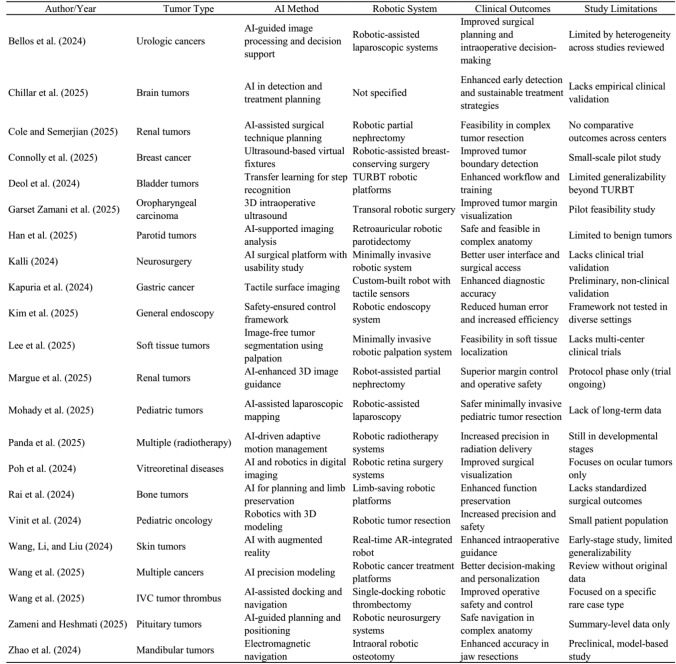
Table 1 collectively demonstrates the growing versatility and impact of AI-driven robotic systems across a wide spectrum of oncologic surgeries. From renal tumors to pediatric, brain, and soft tissue cancers, AI integration has shown potential in enhancing surgical precision, safety, and personalization. For example, [4] and [6] showcased how AI-guided imaging and virtual fixtures improve intraoperative margin detection, while [3] and [12] emphasized the role of robotic partial nephrectomy in managing complex renal tumors with improved clinical outcomes.
Table 1.
Key studies on AI-driven robotic surgery in oncology: tumor types, technologies, outcomes, and limitations
In pediatric oncology, [13] and [17] revealed the feasibility of AI-assisted minimally invasive procedures, underscoring its adaptability to vulnerable populations. Moreover, studies by [5] and [10] highlighted AI’s role in automating surgical tasks and enhancing workflow standardization, which is crucial for training and safety. Advanced applications such as electromagnetic navigation [22], tactile imaging [9], and real-time augmented reality [18] further illustrate the ongoing innovation aimed at improving intraoperative decision-making.
However, many of these innovations remain in early or pilot stages, with limitations including small sample sizes, single-institution trials, or a lack of long-term outcome data [2, 7, 8]. This highlights the need for broader validation and clinical integration before such technologies can become standard practice.
Limitations of the narrative method
While the narrative approach offers depth and interdisciplinary richness, it also has inherent limitations. The lack of a systematic selection protocol means that selection bias cannot be entirely ruled out. Furthermore, the rapidly evolving nature of AI technologies implies that some recent or unpublished innovations may not have been captured. Nevertheless, the method enables a holistic understanding of how AI is shaping robotic surgery in oncology and allows for the inclusion of cutting-edge innovations that may not yet be eligible for inclusion in systematic reviews.
Results
Advancing surgical precision through AI-robotic integration
Recent developments in surgical oncology have been significantly shaped by the integration of artificial intelligence (AI) and robotics, bringing about a notable shift in how tumor surgeries are performed. These technologies have greatly enhanced intraoperative precision, supported real-time decision-making, and allowed for tailored patient-specific interventions. AI-augmented robotic systems have achieved microscale accuracy in tumor resections across various specialties, including neurosurgery, urology, pediatric oncology, and head and neck surgery [1, 3, 17].
A key advancement is robot-assisted partial nephrectomy, where the synergy of AI and three-dimensional imaging has significantly improved renal tumor excision. [1] reported improved visualisation, automation of procedural steps, and reduced positive surgical margins. [12], through the ACCURATE trial, further demonstrated how real-time 3D imaging guidance in these procedures contributes to more precise dissections and better preservation of healthy renal tissue.
In breast-conserving surgery, [4] introduced AI-powered ultrasound virtual fixtures that assist surgeons in accurately locating tumor boundaries, reducing unnecessary tissue removal while ensuring complete excision. This marks a shift from manual approximation toward intelligent, image-guided interventions.
Emerging applications in pediatric and neurosurgical oncology
AI-driven robotic systems are also making strides in pediatric and neurosurgical settings. [17] highlighted how integrating robotics with 3D modeling enhances the safety and accuracy of tumor resections in children, especially for tumors located in anatomically restricted regions. Their findings showed that robotic arms enabled more delicate movements, minimizing harm to surrounding tissues.
In neurosurgery, [8] developed a platform using AI, real-time imaging, and haptic feedback to support minimally invasive brain tumor resections. The approach helps navigate intricate neural pathways with improved safety. [2] expanded on this by showing how AI can also aid preoperative tumor classification through advanced computational strategies. While promising, these tools still face challenges in standardisation and adaptability, particularly in pediatric cases where anatomical variability and cost concerns persist [13].
Safety-driven automation and intraoperative guidance
Several studies point to the growing importance of automated safety mechanisms. [10] introduced a control framework for robotic endoscopy that dynamically responds to intraoperative changes, reducing the risk of damaging vital structures. Complementing this, [11] demonstrated a robotic palpation system capable of segmenting soft tissue tumors without the need for preoperative imaging. Using tactile sensors and AI interpretation, the system detects stiff variations indicative of tumors.
[6] explored the use of intraoperative 3D ultrasound in oropharyngeal cancer surgery. The method enabled accurate tumor margin assessment while avoiding radiation exposure from traditional imaging. Collectively, these studies underscore the dual benefits of enhanced precision and improved patient safety.
Transfer learning and multi-modality adaptability
A notable advancement in AI applications is transfer learning, which enables models trained in one surgical context to adapt to another. [5] demonstrated its feasibility in transurethral bladder resection, streamlining surgical workflows with minimal retraining. [20] expanded this further by combining real-time augmented reality with AI-powered robotics for skin tumor surgeries. Their approach improved depth perception and boundary detection, although standardising imaging data remains a challenge [21].
Quantitative outcomes in oncology
Quantitative evidence from literature consistently supports the integration of AI and robotics in improving cancer surgery outcomes. [18] found that personalised treatment through AI-enhanced robotic systems improved 5-year survival rates and reduced complications. In complex vascular tumors, [20] presented a single-docking robotic approach for tumors involving the inferior vena cava, resulting in reduced blood loss and shorter operative times. [9] introduced tactile imaging that outperformed traditional endoscopy in detecting gastric polyps, achieving over 90 percent diagnostic accuracy.
Qualitative insights from clinical and pilot studies
Beyond the numbers, qualitative findings offer insights into patient experiences. [7] reported high satisfaction with cosmetic outcomes and low complication rates following robotic parotidectomy using a retroauricular incision. Similarly, [16] documented successful limb-sparing surgeries made possible by robotic precision, with patients expressing better postoperative mobility and lower discomfort. However, these observations are limited by small sample sizes and need broader validation.
Implementation challenges and contradictions
Despite these benefits, challenges remain. High costs and technical complexity limit the adoption of AI-robotic platforms in under-resourced settings. [14] noted that infrastructure constraints hinder the deployment of adaptive AI systems in radiotherapy in low- and middle-income countries.
There are also concerns about over-reliance on automation. [15] warned that excessive dependence on AI during ocular tumor surgeries may reduce the role of clinical judgment. [18] raised the issue of algorithmic bias, especially when models are trained on non-representative datasets, potentially leading to unequal outcomes across diverse patient groups.
Comparative effectiveness across organ systems
The maturity of AI-robotic systems varies by specialty. In urology, robust evidence supports their effectiveness [1, 12]. Applications in oral and maxillofacial tumors [22] are still in experimental stages. Similarly, robotic surgery for parotid and skin tumors [7, 18] shows promise but remains limited to less complex cases. Neurosurgical applications [8, 21] are particularly ambitious, with the potential to revolutionise care, but require further technical refinement.
Risk of bias assessment and study limitations
While the reviewed studies demonstrate promising advancements in AI-driven robotic surgery in oncology, several potential biases and methodological limitations must be acknowledged to accurately interpret their findings.
Many of the included studies were pilot or feasibility trials, which inherently come with small sample sizes, limited follow-up durations, and restricted population diversity. For instance, studies by [4, 6] and [9] explored virtual fixtures, tactile sensing, and partial surface imaging, but their findings were based on narrowly defined cohorts with minimal external validation. Similarly, [10] introduced a safety-ensured control framework for endoscopic task automation, yet the results remain theoretical or tested in simulated environments without real-world clinical deployment.
The lack of standardized evaluation frameworks and multicenter validation further compounds these issues. As noted by [1] and [20], studies often use heterogeneous criteria to assess surgical success, AI performance, or clinical outcomes, which limit direct comparability and reproducibility. In pediatric oncology, [13] and [17] showed potential benefits of robotic-assisted tumor resections, but small sample sizes and institutional variability reduce the generalizability of these outcomes across broader populations.
Another source of bias is publication and reporting bias, with most reviewed papers highlighting only positive or supportive results of AI technologies. Very few explicitly reported negative outcomes, failures, or limitations, which creates a skewed representation of AI effectiveness. For example, while [12] emphasized the superiority of 3D imaging in robotic nephrectomy, the study did not detail any intraoperative failures or imaging errors. Likewise, the conceptual frameworks proposed by [8] and [11] introduced innovative strategies for robotic perception and cognition but lacked real-world testing under variable surgical conditions.
Conflicting evidence and gaps
Some studies presented inconsistent findings or limited agreement on performance benchmarks, highlighting the evolving and experimental nature of the field. While [5] reported encouraging outcomes for AI-driven surgical step recognition, others like [2] and [16] emphasized that AI implementation in tumor surgeries requires much deeper integration with clinical practice and surgical judgment. The discrepancy reflects a gap between technological development and clinical readiness, where some systems may demonstrate technical feasibility but lack clinical maturity.
Moreover, despite the optimism around AI-enhanced surgical precision, few studies evaluated long-term oncologic outcomes, complication rates, or postoperative quality of life. [18] and [22] explored augmented reality and electromagnetic navigation systems, respectively, yet neither study discussed the clinical implications of potential inaccuracies or user error. The absence of such evaluations limits the strength of conclusions drawn about sustained patient benefits.
Clinical vs. statistical significance
Many studies reported statistically significant improvements in metrics such as tumor margin detection, operation time, and workflow efficiency. However, the clinical significance of these findings remains less certain in several cases. For instance, [4] and [6] showed improved margin detection using AI-guided imaging and fixtures, but whether these improvements translate into better survival or recurrence rates is yet to be established.
Similarly, robotic techniques for parotidectomy [7] and inferior vena cava tumor thrombectomy [20] appear safe and feasible, yet the marginal clinical benefit over conventional methods remains modest given the complexity and cost of robotic systems. These findings suggest that statistical improvements should not be overinterpreted without clear evidence of patient-centered outcomes. Furthermore, the integration of AI in radiotherapy [14] and pituitary tumor treatment [21] shows exciting potential, but the clinical application of adaptive learning systems in such high-risk settings demands rigorous prospective validation.
Cost-effectiveness considerations
One of the foremost barriers is the high cost associated with AI-integrated robotic platforms. These systems involve substantial upfront investments for the acquisition of robotic hardware, AI software licenses, and long-term service contracts. Moreover, the need for continual updates to AI algorithms and data security systems further escalates maintenance costs [20, 21]. Despite promising outcomes in selected clinical settings, few studies offer comprehensive economic evaluations to justify these investments from a cost–benefit perspective. For instance, the ACCURATE trial led by [12] emphasizes clinical benefits but does not address the economic implications for hospital systems. In resource-limited or rural healthcare environments, the financial burden may significantly hinder widespread adoption [1, 14]. The lack of reimbursement models for AI-supported procedures also adds to institutional hesitation.
Training requirements for surgical teams
Another significant hurdle is the steep learning curve associated with operating AI-enhanced robotic systems. Traditional surgical training is often inadequate for managing these advanced tools, which require competencies not only in robotic techniques but also in interpreting AI-generated insights [8, 18]. Studies like [5] have demonstrated how automated surgical step recognition can support training by standardizing procedures and offering real-time feedback. Similarly, augmented reality overlays and haptic simulation, as described by [17], can improve skill acquisition and shorten the learning curve. However, the integration of these tools into existing training curricula remains inconsistent. Institutions must invest in continuous education and simulation-based learning environments to ensure that surgical teams can operate AI-robotic systems safely and effectively [6, 13].
Infrastructure and technical readiness
AI-driven robotic surgery also demands specialized infrastructure that goes beyond the typical operating room setup. This includes high-speed computing systems, secure cloud networks, integrated imaging platforms, and compatible robotic hardware [7, 22]. For instance, image-free tumor segmentation [11] and real-time electromagnetic navigation [22] require robust data processing and precise calibration, which are difficult to achieve in under-resourced environments. Furthermore, interoperability between different AI modules and robotic platforms is still limited. Hospitals using legacy systems may face challenges in integrating new AI functionalities without extensive overhauls of existing infrastructure. These technological requirements can lead to longer implementation timelines and higher operational costs, slowing down clinical translation [15, 16].
Regulatory pathways and safety standards
Navigating regulatory approval for AI-driven surgical devices remains a complex and evolving landscape. Unlike conventional medical devices, AI systems continuously learn and update, posing a challenge for regulatory frameworks that are based on fixed-function approval models [2, 10]. In the United States, the FDA has begun to develop pathways for adaptive AI systems, such as the Software Precertification Program, but these are still in pilot stages. Safety-ensured frameworks, like the one proposed by [10] for robotic endoscopy automation, are promising models for mitigating risk, yet they require further validation across different clinical scenarios.
Moreover, AI systems must meet stringent standards for data privacy, ethical transparency, and accountability, especially when integrated into high-risk procedures like tumor resections. The challenge lies in ensuring that autonomous or semi-autonomous actions taken by AI systems are traceable and justifiable in the event of adverse outcomes. Without well-defined guidelines, healthcare providers may be reluctant to rely on AI systems for critical intraoperative decisions [3, 4].
Equity and accessibility gaps
Finally, the issue of healthcare equity looms large in the discussion on AI-driven robotic surgery. As advanced technologies proliferate in high-income, urban centers, there is a risk that underserved populations will be left behind. [13] and [17] highlight the tremendous value of AI and robotics in pediatric oncology, yet these innovations remain inaccessible to many global regions. The digital divide and inconsistent access to advanced surgical tools exacerbate existing disparities in cancer care outcomes. Addressing the challenge requires coordinated policy, funding, and infrastructure development strategies to ensure that the benefits of AI-robotic surgery reach all patient populations [1, 21].
Discussion
The promise of artificial intelligence in robotic oncologic surgery is immense, but translating the potential into everyday clinical practice is not without its challenges. While many pilot studies have shown the feasibility of AI-robotic systems in controlled settings [4, 6], integrating these innovations into routine surgical workflows remains a major hurdle. One of the main barriers is the lack of standardised protocols and interoperability across robotic platforms, making widespread clinical adoption a complex task.
Another critical area needing attention is real-time adaptability during surgery. Tumors can vary greatly in shape, size, and tissue consistency, making it difficult for current systems to respond dynamically. Although promising tools like virtual fixtures and tactile sensors are emerging, they remain largely experimental and are yet to be validated across a wide range of tumor types and patient demographics [5, 11].
The field also suffers from a shortage of large-scale clinical trials. While procedures like robotic-assisted nephrectomies are increasingly accepted [3, 20], there is still a lack of comprehensive data on long-term outcomes, especially in underrepresented populations such as children and the elderly [13, 17]. This restricts our ability to fully understand the broader benefits and risks of AI-guided surgeries.
Ethical and safety considerations are also pressing concerns. The introduction of autonomous systems in high-risk surgical environments demands rigorous validation and clearly defined accountability structures [10, 14]. Tools like real-time endoscopic automation and adaptive radiotherapy offer exciting possibilities, but they also raise questions about control, oversight, and fail-safe mechanisms.
Affordability and access are equally important. Advanced AI-robotic platforms come with high costs for equipment, training, and maintenance. These financial barriers pose serious scalability issues, particularly in low-resource settings [21]. Ensuring equitable access to these life-saving technologies remains a largely unaddressed challenge in literature.
Despite these obstacles, the growing body of evidence clearly shows that AI-robotic integration is reshaping the landscape of cancer surgery. Tools such as AI-guided partial nephrectomy using three-dimensional imaging [3, 12] are improving surgical precision and outcomes. Intraoperative decision-making is also being transformed. Technologies like ultrasound-guided virtual fixtures [4] and real-time intraoperative ultrasound [6] enhance tumor margin detection and reduce unnecessary tissue removal.
Emerging innovations in tactile feedback and automation are improving robotic dexterity and safety. For instance, [9] introduced a tactile surface imaging system for gastric polyp detection, while [10] proposed a control framework to reduce human error during endoscopy. [11] demonstrated how robotic palpation enables tumor segmentation without preoperative imaging, pointing toward a more autonomous surgical future.
In more specialised contexts, robotic systems show real-world promise. [7] confirmed the safety and effectiveness of robotic parotidectomy, and [18] demonstrated the benefits of augmented reality for skin tumor surgery. [13] and [17] explored how these technologies improve outcomes in pediatric oncology, offering safer, more accurate resections in complex cases.
AI also supports surgical education and standardisation. [5] and [18] showcased how AI can automate surgical step recognition and guide learners using augmented reality. This is especially valuable in today’s post-pandemic medical training environments. Importantly, the review offers a unique conceptual framework built around four pillars: perception, cognition, actuation, and adaptation that explains how AI technologies enhance robotic performance [8, 11]. It also highlights the transformative impact of safety-assured automation, as shown by [10], and the potential of AI to assist with preoperative planning, navigation, and postoperative care [18, 22].
Recommendations
Given the promising outcomes of AI-robotic systems in precision tumor surgery, several actionable recommendations emerge from the review. First, there is a critical need to standardize AI algorithms used in surgical decision-making to enhance cross-institutional reproducibility and regulatory compliance. As [1] emphasizes in urologic oncology, developing consensus protocols for AI model validation will ensure broader clinical integration.
Secondly, surgical institutions should invest in AI training programs and interdisciplinary collaborations involving data scientists, robotic engineers, and surgical teams. [8] highlights how user-centric AI platforms tailored for neurosurgery can only succeed when designed with continuous clinician feedback. The user integration must be a central pillar in AI surgical innovation.
Additionally, investments in cloud-based platforms for surgical data sharing can facilitate federated learning models that refine AI algorithms across diverse patient populations. [20] assert that collaborative data infrastructure is essential for refining AI models for cancer precision treatment, especially in heterogeneous tumor presentations.
Moreover, future research and clinical programs should consider the inclusion of pediatric and geriatric populations in AI-robotic studies, as shown by [13] and [17], ensuring inclusivity and broad applicability of surgical innovations.
Finally, regulatory bodies and hospital administrations must address the cost-effectiveness and accessibility of AI-robotic systems. [2] suggest that sustainable AI-driven strategies for tumor treatment should include socioeconomic considerations to bridge the healthcare disparity gap.
Implications
The findings of the review bear considerable implications for the future of surgical oncology, healthcare systems, and technological innovation. Clinically, the adoption of AI-powered robotic systems can redefine surgical workflows by reducing intraoperative error, improving tumor margin clearance, and enhancing post-operative recovery. This is particularly critical for high-risk and anatomically complex tumor cases where traditional methods may fall short.
On a systemic level, integrating AI into surgical robotics can alleviate surgeon fatigue, reduce operation times, and shorten hospital stays, thereby improving hospital throughput and lowering overall healthcare costs. The real-time assistance provided by tools like image-free robotic palpation [11] or automated surgical step tracking [5] also enables standardized quality across healthcare institutions.
From a technological standpoint, the intersection of robotics, AI, and imaging (e.g., AR, 3D ultrasound, EM navigation) fosters the creation of intelligent surgical ecosystems. These systems are not only reactive but predictive, adapting to intraoperative dynamics and personalizing tumor resection strategies on the fly, as demonstrated by [18] and [22].
Furthermore, the review has educational implications. The incorporation of AI-powered virtual guidance systems offers transformative tools for surgical training and simulation. They ensure that novice surgeons can learn through guided, low-risk virtual environments before transitioning to live procedures.
As robotic platforms become more autonomous, there is a need for clear ethical and legal frameworks to govern their use, as highlighted by [14]. Autonomy in semi-autonomous systems raises accountability concerns, especially in high-stakes tasks like endoscopic automation and intraoperative decision-making [5, 10]. Bias in AI training remains a challenge, particularly with limited pediatric and geriatric datasets [13, 17], risking unequal outcomes. Furthermore, informed consent and patient data governance require urgent attention, as real-time learning systems process sensitive data across diverse platforms [2, 20]. Transparent protocols are vital for building clinicians and public trust.
Limitations
While the review aggregates a broad spectrum of recent studies, several limitations exist. Firstly, the rapid evolution of AI technologies means that many AI-robotic systems are still in the experimental or pilot phase. For instance, studies like those of [4] and [6] are limited by small sample sizes and preliminary findings, which hinder generalizability.
Secondly, the diversity of tumor types and surgical techniques examined across the sources complicates direct comparisons. The heterogeneity of clinical settings from pediatric to adult, from neurosurgery to urology, poses challenges in assessing universal applicability. Literature, though rich, lacks meta-analytical depth due to the novelty of many approaches and limited long-term outcome data.
Thirdly, there is a disproportionate focus on high-income countries in literature, with minimal insight into the deployment of AI-robotic surgery in resource-limited settings. As highlighted by [19], global disparities in surgical robotics adoption must be more thoroughly investigated. Lastly, ethical discourse in the studies reviewed is relatively sparse. While the technical and clinical aspects are robustly covered, few delve into patient consent, data governance, and liability concerns in AI-enhanced surgeries key factors for responsible implementation.
Future research directions
Future research should pursue large-scale, multi-center randomized controlled trials (RCTs) to validate the efficacy of AI-robotic systems across diverse populations and tumor types. Clinical protocols such as ACCURATE [12] serve as foundational models but need to be expanded in scope and scale. There is also a growing need to develop AI algorithms that are explainable and transparent to enhance trust and facilitate clinical adoption. [8] and [11] stress that AI must be interpretable to surgeons to maintain clinician oversight and accountability.
Research should also focus on the integration of AI with multimodal imaging data combining tactile sensing, 3D ultrasound, and AR to create richer, real-time intraoperative maps for tumor localization and resection. Multimodality would benefit both diagnosis and surgical planning, particularly in hard-to-access tumor locations. Moreover, longitudinal studies examining long-term oncologic outcomes, recurrence rates, and patient-reported outcomes after AI-robotic surgery are essential. Most studies focus on feasibility and technical success, but few investigate durable cancer control and patient quality of life years after intervention.
Finally, cross-disciplinary studies that explore the interaction between AI-robotic technologies and healthcare economics, policy, and ethics will help pave the way for sustainable, equitable, and responsible implementation.
Conclusion
The review highlights the promising advancements of AI-driven robotic surgery in oncology, demonstrating potential benefits in precision, personalization, and patient outcomes. Across the included studies, there is encouraging evidence supporting AI’s role in enhancing intraoperative guidance, automating complex tasks, and expanding the reach of minimally invasive interventions to diverse tumor types and patient populations. However, much of the current evidence stems from pilot trials, early feasibility studies, and small-scale case series. While these findings are foundational, they remain preliminary. Larger, multicenter clinical trials and long-term outcome data are essential to validate these innovations and translate them into standard practice. Until then, the application of AI in robotic oncologic surgery should be viewed as a rapidly evolving frontier with significant promise but still requiring cautious, evidence-informed implementation.
Generative AI use
Generative AI was used to enhance the structure and language of the study.
Author contributions
Jack Ng Kok Wah, the sole author and the corresponding author has made substantial contributions to the conception, study, and writing of the review article. Jack Ng Kok Wah reviewed, edited, and approved the final manuscript, ensuring it met academic standards and provided a balanced, evidence-based discussion. Jack Ng Kok Wah confirms that the article represents original work and bears full accountability for the content presented in the publication.
Funding
The author declares that no funding was received for the preparation or publication of the manuscript. The work was conducted independently and does not involve any financial support from external organizations or sponsors.
Data availability
The study is a narrative review and does not involve the collection or analysis of original data from participants. All information and insights presented in the study are derived from existing literature, publicly available sources, and secondary data obtained from previous research. As such, no new datasets were generated or analyzed during the study.
Declarations
Conflict of interest
The authors declare no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bellos T, Manolitsis I, Katsimperis S, Juliebø-Jones P, Feretzakis G, Mitsogiannis I et al (2024) Artificial intelligence in urologic robotic oncologic surgery: a narrative review. Cancers 16(9):1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chillar V, Gupta S, Vijarania M, Agrawal A, Soni A (2025) Role of sustainable strategies using artificial intelligence in brain tumour detection and treatment. Achieving sustainability with AI technologies. IGI Global Scientific Publishing, New York, pp 327–360 [Google Scholar]
- 3.Cole, R., & Semerjian, A. (2025). Robotic partial nephrectomy: techniques for complex tumors. In: Urologic oncology: seminars and original investigations. Elsevier, Amsterdam. [DOI] [PubMed]
- 4.Connolly L, Ungi T, Munawar A, Deguet A, Yeung C, Taylor RH et al (2025) Touching the tumor boundary: a pilot study on ultrasound-based virtual fixtures for breast-conserving surgery. Int J Comput Assist Radiol Surg 20(6):1105–1113 [DOI] [PubMed] [Google Scholar]
- 5.Deol ES, Tollefson MK, Antolin A, Zohar M, Bar O, Ben-Ayoun D et al (2024) Automated surgical step recognition in transurethral bladder tumor resection using artificial intelligence: transfer learning across surgical modalities. Frontiers in Artificial Intelligence 7:1375482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garset-Zamani M, Makouei F, Agander TK, Lelkaitis G, Charabi BW, Tvedskov JF et al (2025) Feasibility of 3D ultrasound for intraoperative tumor margin assessment in transoral robotic surgery for oropharyngeal squamous cell carcinoma: a pilot study. Oral Oncol 165:107330 [DOI] [PubMed] [Google Scholar]
- 7.Han P, Liang F, Li Y, Lin P, Chen R, Lin X, Huang X (2025) Robotic parotidectomy via retroauricular incision: a safe and feasible approach for benign parotid tumors. Oral Oncol 164:107253 [DOI] [PubMed] [Google Scholar]
- 8.Kalli VDR (2024) Creating an AI-powered platform for neurosurgery alongside a usability examination: progressing towards minimally invasive robotics. J Artif Intell Gen Sci 3(1):363–375 [Google Scholar]
- 9.Kapuria S, Bonyun J, Kulkarni Y, Ikoma N, Chinchali S, Alambeigi F (2024) Robot-enabled machine learning-based diagnosis of gastric cancer polyps using partial surface tactile imaging. In: 2024 IEEE/RSJ international conference on intelligent robots and systems (IROS), pp 2360–2365
- 10.Kim Y, Iturrate I, Sloth C, Kim H (2025) Safety-ensured robotic control framework for cutting task automation in endoscopic submucosal dissection. IEEE Access 13:102803–102814 [Google Scholar]
- 11.Lee YJ, Bang SW, Hong JB, Park S (2025) Image-free tumor segmentation of soft tissue using a minimally invasive robotic palpation system. IEEE Trans Biomed Eng. 10.1109/TBME.2025.3573666 [DOI] [PubMed] [Google Scholar]
- 12.Margue G, Bernhard JC, Giai J, Bouzit A, Ricard S, Jaffredo M et al (2025) Clinical trial protocol for ACCURATE: a CCafU-UroCCR randomized trial: three-dimensional image-guided robot-assisted partial nephrectomy for renal complex tumor (UroCCR 99). Eur Urol Oncol. 10.1016/j.euo.2025.03.012 [DOI] [PubMed] [Google Scholar]
- 13.Mohady BE, Larmure O, Zeroual A, Elgorban AM, Alfagham AT, Syed A et al (2025) The advancing frontier: robotic-assisted laparoscopy in pediatric tumor management. Indian J Surg Oncol. 10.1007/s13193-025-02210-1 [Google Scholar]
- 14.Panda DK, Das SR, Kumar S (2025) Artificial intelligence (AI)-driven adaptive motion management in helical and robotic radiotherapy: innovations, challenges, and future directions. Cureus 17(4):e81702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poh SS, Sia JT, Yip MY, Tsai AS, Lee SY, Tan GS et al (2024) Artificial intelligence, digital imaging, and robotics technologies for surgical vitreoretinal diseases. Ophthalmol Retina 8(7):633–645 [DOI] [PubMed] [Google Scholar]
- 16.Rai V, Munazzam SW, Wazir NU, Javaid I (2024) Revolutionizing bone tumor management: cutting-edge breakthroughs in limb-saving treatments. Eur J Orthop Surg Traumatol 34(4):1741–1748 [DOI] [PubMed] [Google Scholar]
- 17.Vinit N, Blanc T, Bloch I, Pio L, Kassir R, La Barbera G et al (2024) Robotics and 3D modeling for precision surgery in pediatric oncology. EJC Paediatr Oncol 4:100181 [Google Scholar]
- 18.Wang J, Li Y, Liu J (2024) Letter to the Editor ‘A real-time augmented reality robot integrated with artificial intelligence for skin tumor surgery-experimental study and case series.’ Int J Surg 110(9):5867–5868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Zeng Z, Li Z, Liu G, Zhang S, Luo C et al (2025) The clinical application of artificial intelligence in cancer precision treatment. J Transl Med 23(1):120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang S, Pokhrel G, Cui J, Yu S, Fan Y, Zhu Z et al (2025) Robot-assisted single-docking approach for level III inferior vena cava tumor thrombectomy: surgical technique and outcomes. Eur Urol. 10.1016/j.eururo.2025.04.001 [DOI] [PubMed] [Google Scholar]
- 21.Zameni N, Heshmati H (2025) Update on the use of robotic surgery and artificial intelligence for pituitary tumors. In: Endocrine abstracts, vol 110. Bioscientifica
- 22.Zhao Z, Zhang Y, Lin L, Huang W, Xiao C, Liu J, Chai G (2024) Intelligent electromagnetic navigation system for robot-assisted intraoral osteotomy in mandibular tumor resection: a model experiment. Front Immunol 15:1436276 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study is a narrative review and does not involve the collection or analysis of original data from participants. All information and insights presented in the study are derived from existing literature, publicly available sources, and secondary data obtained from previous research. As such, no new datasets were generated or analyzed during the study.