Abstract
This article provides an overview of a symposium held as part of the proceedings at the 10th European Academy of Neurology Congress in Helsinki, Finland, on 2 July 2024. Migraine is a common neurological disease and a leading cause of disability worldwide. Anti-calcitonin gene-related peptide (CGRP) therapies are the first to be specifically developed for migraine prevention and are recommended as a first-line option by the American Headache Society and European Headache Federation. Data on the effectiveness of anti-CGRP therapies are now available from clinical trials and real-world studies, and this article briefly reviews these data and discusses what they mean for people with migraine, and how healthcare professionals can take the conversation back to their clinics.
Keywords: Anti-CGRP monoclonal antibodies, Chronic migraine, Headache frequency, Migraine, Migraine prevention, Migraine progression, Patient-reported outcomes, Quality of life, Real-world evidence
Key Summary Points
| Migraine is a chronic disease with episodic attacks. A migraine attack is not just a headache. Migraine attacks are made up of several stages comprising a constellation of disabling and impactful symptoms |
| Migraine is the second leading cause of disability in women aged 15–49 years worldwide. Disability worsens with increases in monthly headache and/or migraine days |
| Anti-calcitonin gene-related peptide (CGRP) monoclonal antibodies (mAbs) are the first therapies developed specifically for migraine prevention |
| Evidence of the efficacy of anti-CGRP mAb therapies for migraine prevention is available from both clinical trials and real-world studies, and switching anti-CGRP mAb therapy can be effective in people who have previously experienced treatment failure with an anti-CGRP mAb |
| Current treatment strategies and reimbursement/insurance rely on headache frequency alone, meaning that patients may experience delays in accessing optimised treatments, leading to worsening of their disease |
| Patient-centricity is key in treatment planning and improves adherence; people with migraine should be involved in the process of selecting treatments and establishing goals |
Introduction
Migraine is a prevalent, disabling and sometimes progressive disease affecting more than 1 billion people globally [1]. It is the second leading cause of disability-adjusted life-years in women aged 15–49 years and of people aged 20–59 years (with the leading causes being gynaecological disorders and stroke, respectively) [1, 2]. Migraine-associated disability occurs both during and between attacks [3, 4]. Migraine can be classified as episodic or chronic based on headache frequency; the diagnostic criteria for chronic migraine (CM) include headache that occurs on ≥ 15 days/month for > 3 months, with headache ≥ 8 days/month exhibiting migraine features or responding to triptans or ergot-derivative medications [5]. Approximately 3% of people with episodic migraine (EM) convert to CM annually using the traditional definition of progression of crossing the threshold of 15 monthly headache days (MHDs), intensifying the overall burden of disease [6, 7]. Progression or migraine disease worsening can be conceptualised in many ways and can be associated with many undesirable outcomes [8]. People with migraine also experience stigma, which is associated with disability and lower quality of life, depression and anxiety, less care-seeking and poorer treatment outcomes [9, 10]. In the realm of migraine prophylaxis, the advent of anti-calcitonin gene-related peptide (CGRP) therapies marks a pioneering development, as these neuropeptides are the first pharmacological agents specifically tailored for migraine prevention. This contrasts with the historical reliance on traditional oral preventive medications initially formulated for other indications that were repurposed for migraine, such as beta blockers, flunarizine, antidepressants, anti-epileptics and onabotulinumtoxinA [11–13]. The introduction of anti-CGRP treatments heralds a new era in migraine management, promising a targeted intervention for a condition that has long challenged the medical community and people with migraine alike [14].
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Migraine Burden
The impact of migraine ripples throughout a person’s life, family and community, having consequences on personal, academic, occupational, financial and social levels, as well as affecting the people around the person with migraine [4]. Migraine is a leading cause of years lived with disability in young to middle-aged people, affecting their key years for completing education, building careers and establishing families [1]. Migraine burden and impact generally worsen with increases in MHD frequency and can be seen in worse disability, impact, interictal burden and quality of life, as well as higher rates of comorbidities [15]. In the CaMEO study, a US population-based survey involving 13,061 respondents who provided insights into the influence of migraine on their professional lives, 4271 (32.7%) respondents reported that migraine adversely affected various aspects of their careers [4]. Notably, respondents with CM were significantly more likely to report career-related repercussions than respondents with EM, although impact increased with MHD frequency from low- to middle- to high-frequency EM followed by CM [4]. Beyond the professional sphere, the impact of migraine extended into personal relationships: 60.6% of people with CM believed that their romantic partnerships would be better if headaches were no longer a concern, while 56.0% felt that their relationship with their children would similarly improve in the absence of headaches [4]. Rates of perceived impacts increased with MHD frequency, but some participants, even in the lowest-frequency strata, reported negative impacts [4]. Similar general and interictal migraine impacts on work, school, personal relationships, stigma and hiding were seen in the Eurolight study, which sampled from the adult population of ten countries in the European Union (EU) [3].
In the OVERCOME study, which surveyed 59,001 people with migraine in the USA, 31.7% experienced migraine-related stigma (as measured by the Migraine-Related Stigma questionnaire) often or very often, and more frequent headache days were associated with a higher likelihood of stigma, although stigma was more strongly related to negative outcomes such as poor quality of life and high interictal burden than MHD frequency [9]. In the Eurolight study, a third (32.9%) of participants with migraine were reluctant to tell people about their headaches, approximately 10% reported that their families and friends did not understand their headaches and almost 12% reported that their employers and colleagues did not understand the impact of headaches/migraine [3]. People who experience migraine stigma often exhibit a higher degree of disability, as indicated by their Migraine Disability Assessment (MIDAS) score. Additionally, stigma is associated with a greater interictal burden, the anxiety, avoidance, planning and impairment between migraine attacks due to persistent symptoms and/or fear of the impacts of future attacks and lower migraine-specific quality of life [9, 10].
Migraine Progression
Migraine can be a progressive disease, and a range of risk factors is associated with an increased risk of progressing from EM to CM, including higher frequency of MHDs, non-optimised acute treatment, depression and frequent acute medication use [16–18]. Migraine has a dynamic course with a natural variability in MHDs [19] and a complex interaction between EM and CM; it has been reported that about 3% of patients progress from EM to CM annually [6, 7, 18] and that 26% of patients experience regression from CM to EM over a 2-year period [20]. In the context of acute medication overuse and migraine disease progression, a paradoxical scenario unfolds for many patients. Despite the escalated intake of acute headache medications, some people experience a counterintuitive increase in the frequency of MHDs. This phenomenon engenders a vicious cycle in which the increased frequency and severity of migraine attacks leads to an increase in days of acute headache medication use, which in turn may precipitate a secondary headache disorder known as medication-overuse headache [5]. In addition, acute medications may be used even without the existence of a headache or migraine attack, potentially as an attempt to avoid an attack, as seen in rates of acute medication overuse in patients with EM who do not meet the International Classification of Headache Disorders criteria for medication-overuse headache [5, 21].
Disease progression is associated with increased disability and impact on health-related quality of life. This progression is also associated with comorbidities such as depression, anxiety, other chronic pain conditions and obesity [17]. In fact, the risk of progression from EM to CM associated with depression was found to increase with increased depression severity [22]. Amplified economic and societal strain due to utilisation of healthcare resources and loss of productivity is associated with increasing migraine frequency, even if the number of MHDs does not cross the 15-day threshold [4, 17, 23, 24]. As such, there has been a recent shift towards expanding the definition of migraine progression across the spectrum of migraine, considering increases in migraine or headache days that do not cross the 15-day threshold as well as considering increasing disability, worsening quality of life and an increase in comorbidities for people across the migraine spectrum [8, 25–27].
Migraine Prevention
Preventive treatments, including both medications and non-medication therapies, are strategic interventions in the vicious cycle of migraine worsening, offering the potential to reduce migraine frequency, severity and impact, and to lower the use of acute medications [28–32]. By mitigating the frequency and intensity of migraine attacks, these treatments play a crucial role in reversing migraine progression [33–37], providing a pathway to improved outcomes and health-related quality of life [5, 32, 38–41]. Optimising migraine treatment is, therefore, important for long-term disease management. Treatment optimisation may include acute and preventive therapies, as well as pharmacological and non-pharmacological interventions such as lifestyle changes (e.g., exercise, stress management), biobehavioural therapies (e.g., biofeedback, cognitive behavioural therapy and relaxation therapies) and neurostimulation to reduce attack frequency and symptoms [17, 42].
Unmet Therapeutic Prevention Needs in Migraine
Despite their potential benefit, preventive therapies are underused among those who are candidates for such therapies [43, 44]. Data from the OVERCOME EU migraine cohort show that of 20,756 participants with migraine, 73.9% eligible for preventive treatment reported that they did not currently use it [44]. According to European Headache Federation guidance, people with migraine who are affected by their migraine for ≥ 2 days per month despite optimised acute medication should be considered for preventive treatment, although factors such as the severity and duration of migraine attacks should also be taken into account [45]. The American Headache Society (AHS) 2021 consensus statement guidance on migraine preventive treatment is similar, recommending prevention for people with migraine ≥ 2 days per month with severe disability, with ≥ 4 days per month with at least moderate disability or ≥ 6 days per month with no requirement for any degree of disability [12].
Anti-CGRP Monoclonal Antibodies for Migraine Prevention
Anti-CGRP monoclonal antibodies (mAbs) are an effective migraine preventive treatment class of drugs [46–52]. Erenumab, galcanezumab and fremanezumab (subcutaneously administered) have all demonstrated onset of efficacy within the first week of treatment [46, 47, 52]. In the PROMISE-1 and -2 trials, intravenously administered eptinezumab demonstrated efficacy, with a lower proportion of patients with migraine on day 1 in both EM and CM compared with those receiving placebo [48–50]. The RELIEF trial, a randomised, double-blind, acute migraine trial in adults who also qualified for preventive treatment (primary end points were time to freedom from headache pain and time to absence of most bothersome symptom), demonstrated that eptinezumab begins to work within hours after infusion, and participants who received eptinezumab had statistically significantly shorter time to freedom from headache pain and time to absence of most bothersome symptom compared with those receiving placebo [51].
A post hoc analysis of the Phase IIIb DELIVER trial (n = 890) evaluated the sustained response to eptinezumab and assessed the potential for response in participants who were initially non-responders (defined as those with a migraine responder rate [MRR] of < 30% from baseline during weeks 1–12). The end points were the proportion of participants with ≥ 50% or ≥ 75% reduction in monthly migraine days and maintenance and shifts in MRRs from weeks 1–12 to weeks 13–24 [53]. The data demonstrated that in a proportion of these participants, the time to treatment effect varied: about one-third of those who demonstrated < 30% MRR in the first 12 weeks following infusion then achieved ≥ 30% MRR after their second dose in weeks 13–24 [53]. These data support that two infusions of eptinezumab may be required before any evaluation of treatment and that sustained reductions in headache frequency could be observed up to 24 weeks [53].
In the same eptinezumab study, it was observed that sustained reductions in headache frequency may allow participants to transition from the most debilitating headache frequency categories, such as CM and high-frequency EM, to lower-frequency levels, with some participants reporting complete absence of migraine attacks or headaches [53]. At baseline, no participants reported fewer than four MHDs; however, after the initial dose of eptinezumab, 23% of pooled participants who were randomised to receive either 100 mg or 300 mg eptinezumab intravenously reported fewer than four MHDs, increasing to 47% by weeks 61–72. The percentage of those treated with eptinezumab who experienced ≥ 8 MHDs decreased from 90% at baseline to 27% after the final dose.
While many healthcare providers traditionally use ≥ 50% MMR as the threshold for defining clinically meaningful benefit, emerging data suggest that this threshold may not fully capture the nuances of treatment effect, especially for those who initially fall into the category of < 30% MMR after the first dose [54]. In addition, an observational study in the USA that assessed 94 participants’ experiences of being treated with eptinezumab for CM demonstrated that 56% reported that their confidence in their overall well-being was higher or much higher after starting treatment [55]. As such, there is increasing interest in re-evaluating the criteria for treatment success to include a more comprehensive assessment of participants’ improvement. This shift in perspective aims to ‘raise the bar’ for treatment success, ensuring clinical outcomes align more closely with the needs of people with migraine and the multifaceted nature of migraine management. In a study of participants who received erenumab at a dose of 70 mg or 140 mg every 4 weeks, 17.0% and 20.9%, respectively, achieved ≥ 75% MRR compared with 7.8% who received placebo over 12 weeks [56]. In the same study, 4.3% and 2.7% of participants who received 70 mg or 140 mg of erenumab, respectively, achieved 100% MRR compared with 0.4% who received placebo over 12 weeks [56]. Participants who achieved ≥ 75% and 100% MRR also demonstrated a greater improvement in headache impact and disability [56]. Similarly, among the PROMISE-2 cohort of participants with CM who received eptinezumab and achieved ≥ 75% MRR (29.9%; n = 211/706) over weeks 1–12, a post hoc analysis demonstrated that these participants experienced improved health-related quality of life and reduced disability (defined by the Patient Global Impression of Change scale and 6-item Headache Impact Test [HIT-6]), with 76/211 (36.0%) reporting that their headache had little to no life impact at week 12 [57]. It is important to note that there are currently no head-to-head comparisons between any of these anti-CGRP mAbs.
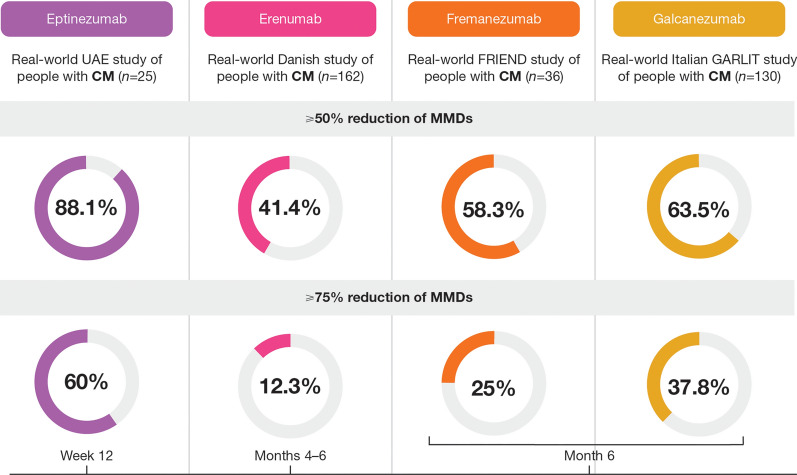
With the approval of anti-CGRP treatments by regulatory bodies, real-world data on the effectiveness of anti-CGRP mAbs are now emerging (Fig. 1) [34, 58–60]. In a real-world Danish study of 162 participants with CM who received erenumab, 41.4% and 12.3% achieved ≥ 50% and ≥ 75% MRR, respectively, by week 24 [59]. In an Italian study of 130 participants with CM who received galcanezumab, 63.5% and 37.8% achieved ≥ 50% and ≥ 75% MRR, respectively, by month 6 [60]. In an update to this study, of 148 participants with CM, 60.5% and 38.1% achieved ≥ 50% and ≥ 75% MRR, respectively, by month 12 [61]. A study conducted in the United Arab Emirates involving 25 participants with CM who were administered eptinezumab demonstrated potential for higher outcomes: 88.0% and 60.0% achieved ≥ 50% and ≥ 75% MRR, respectively, by month 6, although this would need to be investigated in a larger cohort of participants [34].
Fig. 1.
Real-world evidence of the effectiveness of anti-CGRP monoclonal antibodies in patients with CM [34, 58–60]. Please note that these were independent studies and that methodological differences between studies preclude head-to-head comparisons. CGRP calcitonin gene-related peptide, CM chronic migraine, MMD monthly migraine day, UAE United Arab Emirates
Anti-CGRP mAbs have also demonstrated maintained effectiveness [37, 62, 63]. Studies on galcanezumab and fremanezumab have demonstrated that 80% of participants who continued treatment for 12 months achieved and maintained ≥ 50% MRR [62]. In a real-world study, erenumab demonstrated long-term effectiveness in a cohort of participants with ‘difficult to treat’ or treatment-resistant CM: 97/177 (54.8%) participants reported significant improvement (≥ 30% in patient-related outcome measures or quality-of-life questionnaires) and chose to continue treatment after the end of the study for a median duration of 25 months [63]. Long-term patient-reported outcomes also improved with treatment with erenumab: HIT-6 and MIDAS scores improved by 20% and 87% from baseline to months 25–30, respectively [63]. Participants (n = 94), 89% of whom reported previous subcutaneous anti-CGRP mAb use and 82% of whom reported previous onabotulinumtoxinA use, who received eptinezumab for ≥ 6 months also reported improvement in satisfaction with several elements of daily living, as 69% of participants reported that their ability to participate in their social or family life was higher or much higher, and 68% reported that their ability to perform their usual daily activities was higher or much higher [37].
Anti-CGRP mAbs have also demonstrated improved tolerability compared with older preventive therapies [64]. Real-world studies of erenumab, galcanezumab, fremanezumab and eptinezumab have indicated similar adverse-event profiles and identified no new safety signals from those observed in clinical trials [34, 59, 65, 66]. In a real-world study of 162 participants aged > 65 years who were treated with erenumab, galcanezumab or fremanezumab, 41 (25.3%) participants experienced adverse effects, all of which were considered to be mild [67]. At month 3, the most commonly reported adverse effect was constipation (9.9%; n = 16/162), and two instances of hypertension were reported [67].
Reimbursement criteria in some countries require treatment to be temporarily discontinued after a set time and then resumed. For example, the Italian Medicines Agency requires treatment discontinuation after 1 year followed by drug withdrawal of ≥ 1 month [68]. Such recommendations are based on expert opinion rather than scientific evidence and, as such, prospective, randomised studies that investigate the impact of treatment discontinuation are warranted [69]. A sub-group of 154 participants enrolled in the Italian EARLY (erenumab) and GARLIT (galcanezumab) studies was followed during a 3-month drug discontinuation; of the 107 participants with CM, 35.5% experienced ≥ 50% MRR by month 3 of treatment discontinuation compared with 60.6% during the last month of anti-CGRP treatment [68]. Treatment discontinuation led to relapse to CM: 39/84 (46.4%) participants who had reverted from CM to EM during treatment relapsed to CM by month 3 of treatment discontinuation [68]. However, the efficacy of treatment returned after resumption, when monthly migraine days returned to a level similar to the period before treatment discontinuation [70].
Treatment with anti-CGRP mAbs should not be discontinued altogether after treatment failure, as switching within the class can be effective [71, 72]. Some people (n = 5/25) with migraine who discontinued treatments targeting the CGRP receptor due to non-response have experienced successful treatment response when switched to an anti-CGRP treatment that targets the CGRP ligand, such as galcanezumab or fremanezumab [71]. In a small, real-world study, 13/18 (72.2%) participants who switched from galcanezumab to eptinezumab experienced additional or compounded reductions in monthly migraine days on top of improvements gained from their initial prescription, although it should be noted that head-to-head comparisons between these two treatments are not available [72].
Anti-CGRP Treatment-Emergent Adverse Events
The most common treatment-emergent adverse events across all four currently available anti-CGRP mAbs include hypersensitivity reactions and injection site reaction and pain [28–30, 73]. Specific common adverse events for erenumab and galcanezumab include constipation and pruritus [29, 30], and those for fremanezumab include injection site pain, induration, erythema and pruritus [28]. Eptinezumab is associated with nasopharyngitis and fatigue [73]. The US Food and Drug Administration approved safety labelling changes for erenumab to include warnings for constipation, hypertension and Raynaud’s phenomenon, and recently issued a safety labelling change for all CGRP-targeting therapies [74].
Patient-Centricity and Treatment Success
The definition of treatment success may differ between healthcare professionals and people with migraine. For example, current clinical guidelines and reimbursement regulations are typically based on the achievement of defined thresholds. The AHS consensus statement considers preventive treatment success to include a 50% reduction in the frequency of days with headache or migraine and a reduction in migraine-related disability [12]. The German Society for Neurology guidelines for treatment success include a ≥ 50% reduction in average monthly migraine days over a treatment period of 3 months for those with EM and a ≥ 30% reduction for those with CM [75]. These goals may not reflect patient values, patient preferences or direct patient-reported outcome measures, and may act as an obstacle to patient satisfaction and treatment adherence [45]. As a result, people with migraine should be involved in the process of selecting treatment and establishing individual treatment goals, expectations and limitations of any treatment, as optimised medication communication is central to a positive working alliance [12, 76, 77]. This is an iterative process, and treatment plans should be reassessed with goals being reached, modified and reset over time as appropriate. It is important that the available data are contextualised for people with migraine and that, with the emergence of new evidence on the efficacy and safety of anti-CGRP mAbs, we raise the bar for treatment success.
Conclusions
Migraine is not just a disease where people experience headaches; rather, it is a chronic disease made up of attacks comprising a constellation of disabling symptoms over multiple phases that can have a large negative impact on health-related quality of life as well as on family, friends and work colleagues, academic and occupational achievements, financial well-being and internalised stigma [1]. Although various preventive therapy options are available in guidelines and consensus statement recommendations, including medication, neuromodulation, biobehavioural therapies and lifestyle modification, CGRP-targeted preventive therapy remains highly underused [12]. Evidence from both clinical trials and real-world data demonstrate that treatment with anti-CGRP mAbs improves multiple outcomes for people with migraine, including reduced headache and migraine frequency, reduced severity duration and impact of remaining migraine attacks and headaches, reduced acute medication use, improved health-related quality of life and reduced disability [37, 53, 56, 57]. Evidence also shows that if an individual experiences treatment failure with an anti-CGRP mAb, they can be switched to another as they could experience a better treatment outcome with another drug within the same class [71, 72]. People with migraine should be central in the shared decision-making process of their care, as goal management is a collaborative and ongoing iterative process. Finally, while there is a growing wealth of data demonstrating the effectiveness of anti-CGRP mAb therapies, there are still a number of questions that require further study, including their effectiveness in subtypes of migraine, identifying predictors of response, long-term efficacy and safety, and combination therapy.
Acknowledgments
Medical Writing and Editorial Assistance
Amy Irvine, PhD, and Adam Paton, BA, of Caudex, an IPG Health Company, provided medical writing and editing support for this manuscript, which was funded by H. Lundbeck A/S in accordance with Good Publication Practice guidelines.
Author Contributions
Dawn C. Buse, Jan Versijpt and Hans-Christoph Diener contributed to the preparation of this manuscript, commented on previous versions of the manuscript and read and approved the final manuscript.
Funding
Educational financial support for the symposium and the Neurology and Therapy journal Rapid Service Fee was provided by H. Lundbeck A/S.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Declarations
Conflict of Interest
Dawn C. Buse has been a consultant for AbbVie, Amgen, Biohaven, Collegium, Lilly, Lundbeck, Pfizer, Teva and Theranica. Jan Versijpt received personal fees and non-financial support from Medtronic, Pfizer and Teva; personal fees from Lundbeck and Novartis; and grants and non-financial support from Allergan/AbbVie. Hans-Christoph Diener received honoraria for participation in clinical trials, advisory boards and presentations for AbbVie, Lilly, Lundbeck, Novartis, Orion Pharma, Pfizer, Teva and WebMD.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Footnotes
Prior Presentation: This article is based on a symposium session at the 10th Congress of the European Academy of Neurology, Helsinki, Finland, 2 July 2024.
References
- 1.GBD 2021 Nervous System Disorders Collaborators. Global, regional, and national burden of disorders affecting the nervous system, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024;23(4):344–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steiner TJ, Stovner LJ, Jensen R, Uluduz D, Katsarava Z, on behalf of Lifting The Burden: the Global Campaign against Headache. Migraine remains second among the world’s causes of disability, and first among young women: findings from GBD2019. J Headache Pain. 2020;21(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lampl C, Thomas H, Tassorelli C, et al. Headache, depression and anxiety: associations in the Eurolight project. J Headache Pain. 2016;17:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buse DC, Fanning KM, Reed ML, et al. Life with migraine: effects on relationships, career, and finances from the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study. Headache. 2019;59(8):1286–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Headache Classification Committee of the International Headache Society (HIS). The International Classification of Headache Disorders, 3rd Edition. Cephalalgia. 2018;38(1):1–211. [DOI] [PubMed] [Google Scholar]
- 6.May A, Schulte LH. Chronic migraine: risk factors, mechanisms and treatment. Nat Rev Neurol. 2016;12(8):455–64. [DOI] [PubMed] [Google Scholar]
- 7.Bigal ME, Serrano D, Buse D, Scher A, Stewart WF, Lipton RB. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache. 2008;48(8):1157–68. [DOI] [PubMed] [Google Scholar]
- 8.Buse DC, Muenzel EJ, Zagar AJ, et al. Rates and risk factors for migraine progression using multiple definitions of progression: results of the longitudinal OVERCOME (US) study. Headache. 2025;65(4):589–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shapiro RE, Nicholson RA, Seng EK, et al. Migraine-related stigma and its relationship to disability, interictal burden, and quality of life. Results of the OVERCOME (US) study. Neurology. 2024;102(3):e208074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seng EK, Shapiro RE, Buse DC, Robbins MS, Lipton RB, Parker A. The unique role of stigma in migraine-related disability and quality of life. Headache. 2022;62(10):1354–64. [DOI] [PubMed] [Google Scholar]
- 11.Martelletti P, Edvinsson L, Ashina M. Shaping the future of migraine targeting calcitonin-gene-related-peptide with the disease-modifying migraine drugs (DMMDs). J Headache Pain. 2019;20(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ailani J, Burch RC, Robbins MS, on behalf of the Board of Directors of the American Headache Society. The American Headache Society Consensus Statement: update on integrating new migraine treatments into clinical practice. Headache. 2021;61(7):1021–39. [DOI] [PubMed] [Google Scholar]
- 13.Evers S, Áfra J, Frese A, et al. EFNS guideline on the drug treatment of migraine—revised report of an EFNS task force. Eur J Neurol. 2009;16(9):968–81. [DOI] [PubMed] [Google Scholar]
- 14.Do TP, Guo S, Ashina M. Therapeutic novelties in migraine: new drugs, new hope? J Headache Pain. 2019;20(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buse DC, Reed ML, Fanning KM, Bostic RC, Lipton RB. Demographics, headache features, and comorbidity profiles in relation to headache frequency in people with migraine: results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2020;60(10):2340–56. [DOI] [PubMed] [Google Scholar]
- 16.Xu J, Kong F, Buse DC. Predictors of episodic migraine transformation to chronic migraine: a systematic review and meta-analysis of observational cohort studies. Cephalalgia. 2020;40(5):503–16. [DOI] [PubMed] [Google Scholar]
- 17.Lipton RB, Buse DC, Nahas SJ, et al. Risk factors for migraine disease progression: a narrative review for a patient-centered approach. J Neurol. 2023;270(12):5692–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buse DC, Greisman JD, Baigi K, Lipton RB. Migraine progression: a systematic review. Headache. 2019;59(3):306–38. [DOI] [PubMed] [Google Scholar]
- 19.Serrano D, Lipton RB, Scher AI, et al. Fluctuations in episodic and chronic migraine status over the course of 1 year: implications for diagnosis, treatment and clinical trial design. J Headache Pain. 2017;18(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manack A, Buse DC, Serrano D, Turkel CC, Lipton RB. Rates, predictors, and consequences of remission from chronic migraine to episodic migraine. Neurology. 2011;76(8):711–8. [DOI] [PubMed] [Google Scholar]
- 21.Schwedt TJ, Buse DC, Argoff CE, et al. Medication overuse and headache burden: results from the CaMEO study. Neurol Clin Pract. 2021;11(3):216–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashina S, Serrano D, Lipton RB, et al. Depression and risk of transformation of episodic to chronic migraine. J Headache Pain. 2012;13(8):615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torres-Ferrús M, Quintana M, Fernandez-Morales J, Alvarez-Sabin J, Pozo-Rosich P. When does chronic migraine strike? A clinical comparison of migraine according to the headache days suffered per month. Cephalalgia. 2017;37(2):104–13. [DOI] [PubMed] [Google Scholar]
- 24.Buse DC, Scher AI, Dodick DW, et al. Impact of migraine on the family: perspectives of people with migraine and their spouse/domestic partner in the CaMEO study. Mayo Clin Proc. 2016;91(5):596–611. [DOI] [PubMed] [Google Scholar]
- 25.Ishii R, Schwedt TJ, Dumkrieger G, et al. Chronic versus episodic migraine: the 15-day threshold does not adequately reflect substantial differences in disability across the full spectrum of headache frequency. Headache. 2021;61(7):992–1003. [DOI] [PubMed] [Google Scholar]
- 26.Buse D, Muenzel J, Zagar A, et al. Expanding the definition of migraine progression: empirical evidence from the longitudinal OVERCOME (US) study. Headache. 2024;64(Suppl 1):74–5 (Abstract P-338). [Google Scholar]
- 27.Cammarota F, De Icco R, Vaghi G, et al. High-frequency episodic migraine: time for its recognition as a migraine subtype? Cephalalgia. 2024;44(10):3331024241291578. [DOI] [PubMed] [Google Scholar]
- 28.Ferrari MD, Diener HC, Ning X, et al. Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): a randomised, double-blind, placebo-controlled, phase 3b trial. Lancet. 2019;394(10203):1030–40. [DOI] [PubMed] [Google Scholar]
- 29.Reuter U, Goadsby PJ, Lanteri-Minet M, et al. Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: a randomised, double-blind, placebo-controlled, phase 3b study. Lancet. 2018;392(10161):2280–7. [DOI] [PubMed] [Google Scholar]
- 30.Mulleners WM, Kim B-K, Lainez MJA, et al. Safety and efficacy of galcanezumab in patients for whom previous migraine preventive medication from two to four categories had failed (CONQUER): a multicentre, randomised, double-blind, placebo-controlled, phase 3b trial. Lancet Neurol. 2020;19(10):814–25. [DOI] [PubMed] [Google Scholar]
- 31.Ashina M, Lanteri-Minet M, Pozo-Rosich P, et al. Safety and efficacy of eptinezumab for migraine prevention in patients with two-to-four previous preventive treatment failures (DELIVER): a multi-arm, randomised, double-blind, placebo-controlled, phase 3b trial. Lancet Neurol. 2022;21(7):597–607. [DOI] [PubMed] [Google Scholar]
- 32.Ashina M, Tepper SJ, Gendolla A, et al. Long-term effectiveness of eptinezumab in patients with migraine and prior preventive treatment failures: extension of a randomized controlled trial. J Headache Pain. 2023;24(1):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krymchantowski AV, Jevoux C, Krymchantowski AG, Silva-Néto RP. Monoclonal antibodies for chronic migraine and medication overuse headache: a real-world study. Front Neurol. 2023;14:1129439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bader Y, Suliman R, Harb M, Santos V, Al Qaisi I, Alsaadi T. Effectiveness and safety of eptinezumab in episodic and chronic migraine headache in the UAE: a retrospective study. Neurol Ther. 2023;12(5):1683–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao YJ, Ong JJY, Sonu SK, et al. A real-world prospective observational study of eptinezumab in Asian patients with migraine. Headache. 2024;64(7):810–24. [DOI] [PubMed] [Google Scholar]
- 36.Scheffler A, Wenzel P, Bendig M, et al. Effectiveness and tolerability of eptinezumab in treating patients with migraine resistant to conventional preventive medications and CGRP (receptor) antibodies: a multicentre retrospective real-world analysis from Germany. J Headache Pain. 2024;25(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Argoff C, Herzog SP, Smith RM, et al. Real-world effectiveness and satisfaction with intravenous eptinezumab treatment in patients with chronic migraine: REVIEW, an observational, multi-site, US-based study. J Headache Pain. 2024;25(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tepper SJ, Ailani J, Ford JH, et al. Effects of galcanezumab on health-related quality of life and disability in patients with previous failure of 2–4 migraine preventive medication categories: results from a phase IIIb randomized, placebo-controlled, multicenter clinical trial (CONQUER). Clin Drug Investig. 2022;42(3):263–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spierings ELH, Ning X, Ramirez Campos V, Cohen JM, Barash S, Buse DC. Improvements in quality of life and work productivity with up to 6 months of fremanezumab treatment in patients with episodic and chronic migraine and documented inadequate response to 2 to 4 classes of migraine-preventive medications in the phase 3b FOCUS study. Headache. 2021;61(9):1376–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ornello R, Casalena A, Frattale I, et al. Conversion from chronic to episodic migraine in patients treated with erenumab: real-life data from an Italian region. J Headache Pain. 2020;21(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marmura MJ, Diener H-C, Cowan RP, et al. Preventive migraine treatment with eptinezumab reduced acute headache medication and headache frequency to below diagnostic thresholds in patients with chronic migraine and medication-overuse headache. Headache. 2021;61(9):1421–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lipton RB, Fanning KM, Serrano D, Reed ML, Cady R, Buse DC. Ineffective acute treatment of episodic migraine is associated with new-onset chronic migraine. Neurology. 2015;84(7):688–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lipton RB, Buse DC, Serrano D, Holland S, Reed ML. Examination of unmet treatment needs among persons with episodic migraine: results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2013;53(8):1300–11. [DOI] [PubMed] [Google Scholar]
- 44.Pascual J, Panni T, Dell’Agnello G, Gonderten S, Novick D, Evers S. Preventive treatment patterns and treatment satisfaction in migraine: results of the OVERCOME (EU) study. J Headache Pain. 2023;24(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eigenbrodt AK, Ashina H, Khan S, et al. Diagnosis and management of migraine in ten steps. Nat Rev Neurol. 2021;17(8):501–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwedt T, Reuter U, Tepper S, et al. Early onset of efficacy with erenumab in patients with episodic and chronic migraine. J Headache Pain. 2018;19(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goadsby PJ, Dodick DW, Martinez JM, et al. Onset of efficacy and duration of response of galcanezumab for the prevention of episodic migraine: a post-hoc analysis. J Neurol Neurosurg Psychiatry. 2019;90(8):939–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lipton RB, Goadsby PJ, Smith J, et al. Efficacy and safety of eptinezumab in patients with chronic migraine. PROMISE-2. Neurology. 2020;94(13):e1365–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dodick DW, Gottschalk C, Cady R, Hirman J, Smith J, Snapinn S. Eptinezumab demonstrated efficacy in sustained prevention of episodic and chronic migraine beginning on day 1 after dosing. Headache. 2020;60(10):2220–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ashina M, Saper J, Cady R, et al. Eptinezumab in episodic migraine: a randomized, double-blind, placebo-controlled study (PROMISE-1). Cephalalgia. 2020;40(3):241–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ailani J, McAllister P, Winner PK, et al. Rapid resolution of migraine symptoms after initiating the preventive treatment eptinezumab during a migraine attack: results from the randomized RELIEF trial. BMC Neurol. 2022;22(1):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winner PK, Spierings ELH, Yeung PP, et al. Early onset of efficacy with fremanezumab for the preventive treatment of chronic migraine. Headache. 2019;59(10):1743–52. [DOI] [PubMed] [Google Scholar]
- 53.Ashina M, Lipton RB, Ailani J, et al. Responder rates with eptinezumab over 24 weeks in patients with prior preventive migraine treatment failures: post hoc analysis of the DELIVER randomized clinical trial. Eur J Neurol. 2024;31(2):e16131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Houts CR, Wirth RJ, McGinley JS, Cady R, Lipton RB. Determining thresholds for meaningful change for the Headache Impact Test (HIT-6) total and item-specific scores in chronic migraine. Headache. 2020;60(9):2003–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Argoff C, Khan FA, Herzog SP, et al. Real-world evidence of the effectiveness and satisfaction with eptinezumab treatment in patients with chronic migraine. Poster P165. 65th Annual Scientific Meeting of the American Headache Society, Austin, 15–18 June 2023.
- 56.Brandes JL, Diener H-C, Dolezil D, et al. The spectrum of response to erenumab in patients with chronic migraine and subgroup analysis of patients achieving ≥50%, ≥75%, and 100% response. Cephalalgia. 2020;40(1):28–38. [DOI] [PubMed] [Google Scholar]
- 57.Lipton RB, Charleston L IV, Tassorelli C, Brevig T, Hirman J, Cady R. Patient-reported outcomes, health-related quality of life, and acute medication use in patients with a ≥75% response to eptinezumab: subgroup pooled analysis of the PROMISE trials. J Headache Pain. 2022;23(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barbanti P, Egeo G, Aurilia C, et al. Fremanezumab in the prevention of high-frequency episodic and chronic migraine: a 12-week, multicenter, real-life, cohort study (the FRIEND study). J Headache Pain. 2022;23(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cullum CK, Do TP, Ashina M, et al. Real-world long-term efficacy and safety of erenumab in adults with chronic migraine: a 52-week, single-center, prospective, observational study. J Headache Pain. 2022;23(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vernieri F, Altamura C, Brunelli N, et al. Galcanezumab for the prevention of high frequency episodic and chronic migraine in real life in Italy: a multicenter prospective cohort study (the GARLIT study). J Headache Pain. 2021;22(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vernieri F, Brunelli N, Marcosano M, et al. Maintenance of response and predictive factors of 1-year GalcanezumAb treatment in real-life migraine patients in Italy: the multicenter prospective cohort GARLIT study. Eur J Neurol. 2023;30(1):224–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ray JC, Dalic L, Baker J, Cheng S, Hutton EJ, Matharu M. Twelve-month efficacy of CGRP monoclonal antibodies and predictive value of short-term response: results of an Australian multicentre study. BMJ Neurol Open. 2024;6(1): e000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Troy E, Shrukalla AA, Buture A, et al. Medium-term real-world data for erenumab in 177 treatment resistant or difficult to treat chronic migraine patients: persistence and patient reported outcome measures after 17–30 months. J Headache Pain. 2023;24(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lampl C, MaassenVanDenBrink A, Deligianni CI, et al. The comparative effectiveness of migraine preventive drugs: a systematic review and network meta-analysis. J Headache Pain. 2023;24(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pavelic AR, Wöber C, Riederer F, Zebenholzer K. Monoclonal antibodies against calcitonin gene-related peptide for migraine prophylaxis: a systematic review of real-world data. Cells. 2022;12(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ashina M, Mitsikostas DD, Amin FM, et al. Real-world effectiveness of fremanezumab for the preventive treatment of migraine: interim analysis of the pan-European, prospective, observational, phase 4 PEARL study. Cephalalgia. 2023;43(11):3331024231214987. [DOI] [PubMed] [Google Scholar]
- 67.Muñoz-Vendrell A, Campoy S, Caronna E, et al. Effectiveness and safety of anti-CGRP monoclonal antibodies in patients over 65 years: a real-life multicentre analysis of 162 patients. J Headache Pain. 2023;24(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vernieri F, Brunelli N, Messina R, et al. Discontinuing monoclonal antibodies targeting CGRP pathway after one-year treatment: an observational longitudinal cohort study. J Headache Pain. 2021;22(1):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Al-Hassany L, Lyons HS, Boucherie DM, et al. The sense of stopping migraine prophylaxis. J Headache Pain. 2023;24(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Raffaelli B, Terhart M, Mecklenburg J, et al. Resumption of migraine preventive treatment with CGRP(-receptor) antibodies after a 3-month drug holiday: a real-world experience. J Headache Pain. 2022;23(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Overeem LH, Peikert A, Hofacker MD, et al. Effect of antibody switch in non-responders to a CGRP receptor antibody treatment in migraine: a multi-center retrospective cohort study. Cephalalgia. 2022;42(4–5):291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Suliman R, Santos V, Al Qaisi I, et al. Effectiveness of switching CGRP monoclonal antibodies in non-responder patients in the UAE: a retrospective study. Neurol Int. 2024;16(1):274–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ashina M. Migraine. N Engl J Med. 2020;383(19):1866–76. [DOI] [PubMed] [Google Scholar]
- 74.US Food & Drug Administration. Drug safety-related labeling changes (SrLC): ERENUMAB-AOOE. 2025. https://www.accessdata.fda.gov/scripts/cder/safetylabelingchanges/index.cfm?event=searchdetail.page&DrugNameID=1894#. Accessed 7 May 2025.
- 75.Diener H-C, Förderreuther S, Kropp P. Treatment of migraine attacks and preventive treatment of migraine. 2022. https://ihs-headache.org/wp-content/uploads/2023/06/DMKG_Treatment-of-migraine-attacks-and-preventive-treatment-of-migraine-2022.pdf. Accessed 11 June 2024.
- 76.Mangrum R, Gerstein MT, Hall CJ III, et al. Priority acute and preventive migraine treatment benefits: results of the Migraine Clinical Outcome Assessment System (MiCOAS) qualitative study of people living with migraine. Headache. 2023;63(7):953–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Urtecho M, Wagner B, Wang Z, et al. A qualitative evidence synthesis of patient perspectives on migraine treatment features and outcomes. Headache. 2023;63(2):185–201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.