Abstract
NGAL (human neutrophil gelatinase-associated lipocalin) and its mouse analogue 24p3 are members of the lipocalin family of small secreted proteins. These proteins are up-regulated in a number of pathological conditions, including cancers, and may function as transporters of essential factors. Although previous publications have suggested that 24p3 has pro-apoptotic functions, other data are more suggestive of a survival function. The current study was designed to determine whether NGAL is pro- or anti-apoptotic. Apoptosis induced in human adenocarcinoma A549 cells by the 5-lipoxygenase-activating-protein inhibitor MK886, or several celecoxib-derived PDK1 (phosphoinositide-dependent kinase 1) inhibitors that are devoid of cyclo-oxygenase-2 inhibitory activity, was accompanied by a dose- and time-dependent increase of NGAL mRNA levels, as was reported previously with 24p3. A similar induction of NGAL mRNA was observed in human breast cancer MCF7 cells treated with MK886, indicating this was not a cell-specific effect. Treatment of A549 cells with up to 150 μg/106 cells of purified recombinant NGAL protein had no effect on viability, whereas antisera against the full-length NGAL protein induced apoptosis in these cells. The stable overexpression of NGAL in A549 cells had no effect on proliferation or viability. However, the cell death induced by a PDK1 inhibitor was reduced by 50% in NGAL-overexpressing cells. Decreasing NGAL mRNA and protein expression with siRNA (small interfering RNA) in A549 cells increased the toxicity of a PDK1 inhibitor by approx. 45%. These data indicate that, although the induction of NGAL correlates with apoptosis, this induction represents a survival response. Because NGAL is a secreted protein, it may play an extracellular role in cell defence against toxicants and/or facilitate the survival of the remaining cells.
Keywords: apoptosis, neutrophil gelatinase-associated lipocalin (NGAL), survival factor, 24p3 protein
Abbreviations: COX-2, cyclo-oxygenase 2; DAPI, 4,6-diamidino-2-phenylindole; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IGF, insulin-like growth factor; IL, interleukin; MMP-9, matrix metalloproteinase 9; NGAL, neutrophil gelatinase-associated lipocalin; PDK1, phosphoinositide-dependent kinase-1; PI, propidium iodide; RT, reverse transcriptase; siRNA, small interfering RNA; TGF-α, transforming growth factor α
INTRODUCTION
Lipocalins are a diverse family of over 20 small soluble, and often secreted, proteins. They have a large degree of diversity at the sequence level (only 20% identity), although most share three conserved motifs. These proteins are defined as lipocalins largely on the basis of their three-dimensional structure, which comprises a single eight-stranded, continuously hydrogen-bonded anti-parallel β-barrel. This antiparallel structure forms an enclosing cavity that is thought to bind a wide variety of low-molecular-mass molecules, including retinoids, arachidonic acid, various steroids and iron (reviewed in [1,2]). Thus lipocalins are generally considered as transporters with several different functions. These functions include the regulation of immune responses, modulation of cell growth and metabolism, transportation of iron and prostaglandin synthesis [1].
NGAL (neutrophil gelatinase-associated lipocalin) was first purified from human neutrophils because of its association with gelatinase, although this association appears to be functionally irrelevant [3]. NGAL exists as a 25 kDa monomer, 46 kDa disulphide-linked homodimer and a 135 kDa disulphide-linked heterodimer with neutrophil gelatinase [3]. NGAL is thought to be an acute phase protein [4] whose expression is up-regulated in humans, typically in epithelial cells, under diverse inflammatory conditions including appendicitis, inflammatory bowel disease and diverticulitis [5]. Elevated NGAL expression is also observed in human cancers, such as breast [6], colorectal [5], pancreatic [7] and ovarian [8] cancer. However, there is little information regarding the functions of NGAL. Several studies have suggested that NGAL is a potent bacteriostatic agent by sequestering iron [9,10]. Another study [11] indicated NGAL is capable of protecting MMP-9 (matrix metalloproteinase 9) from degradation by interacting with this protein. It has also been reported that NGAL can protect against acute ischaemic renal injury [12]. Overall, the full functional repertoire of NGAL remains to be exposed.
As a secreted binding protein, NGAL may play a role in transport or signalling with survival/growth factors. Recently, 24p3, a mouse homologue of NGAL (NGAL and 24p3 are 62% identical in amino acid sequence [13]), was suggested to play a crucial role in IL (interleukin)-3 deprivation [14] and MK886 [a FLAP (5-lipoxygenase-activating protein) inhibitor]-induced apoptosis [15] in murine pro-lymphoid progenitor FL5.12 cells. In addition, 24p3 is expressed at high levels in the mouse mammary gland and uterus, where it is proposed to induce apoptosis, thereby allowing selected cells to survive involution [16,17]. However, although these studies showed an association of 24p3 with apoptosis, a cause–effect relationship was not established, and other work indicates that this lipocalin is not universally involved in growth-factor-withdrawal apoptosis [18].
To clarify the overall functions of NGAL, the present study was designed to determine whether this lipocalin is pro- or anti-apoptotic. Our previous work demonstrated that the murine 24p3 was profoundly induced by MK886 [15]. The present study demonstrates that a similar induction of the analogous human protein, NGAL, is seen in human lung non-small cell cancer A549 cells and breast cancer MCF7 cells treated with MK886. Induction of NGAL was also evident with a series of celecoxib-derived agents that induce apoptosis, but lack the ability to inhibit COX-2 (cyclo-oxygenase-2). Rather, these agents, as well as MK886, appear to act through an inhibition of PDK1 (phosphoinositide-dependent kinase 1) ([19], and C.-S. Chen, unpublished work). Although the induction of NGAL was temporally correlated with apoptosis, this appears to be a response to, not a cause of, cell death. Specifically, exogenously added NGAL protein was non-toxic, and the overexpression of NGAL provided some protection from PDK-1 inhibitor-induced apoptosis, whereas the down-regulation of NGAL resulted in an increased toxicity to these xenobiotics, and polyclonal antisera against full-length NGAL induced apoptosis. Overall, these data suggest that NGAL plays a role in cell survival, and can provide protection from the apoptosis induced by PDK1 inhibitors.
MATERIALS AND METHODS
Cell culture
Human lung adenocarcinoma A549 cells obtained from the American Type Culture Collection (Manassas, VA, U.S.A.) and human breast cancer MCF7 cells obtained from Dr Kimberly Kline (University of Texas at Austin; these cells were originally obtained from Dr Suzanne Fuque, University of Texas Health Sciences Center, San Antonio, TX, U.S.A.) were used. A549 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% (v/v) fetal bovine serum (Atlanta Biologicals, Norcross, GA, U.S.A.) and 50 μg/ml gentamicin. MCF-7 cells were cultured in minimum essential medium with Earle's balanced salts supplemented with 10% fetal bovine serum plus 2 mM glutamine, 100 μg/ml streptomycin, 100 units/ml penicillin, 1× (v/v) non-essential amino acids and 10 mM Hepes. Cells were passaged at 70% confluence using 1 mM EDTA/0.025% trypsin for 3–5 min.
Reagents and treatments
MK886 was obtained from Biomol Research Laboratories (Plymouth Meeting, PA, U.S.A.). OSU03013 and OSU03012 (celecoxib-derived PDK1 inhibitors that are devoid of COX-2 inhibitory activity) were synthesized as described in [19]. Recombinant NGAL protein was a gift from Dr Roland Strong (Fred Hutchinson Cancer Research Center, Seattle, WA, U.S.A.). Polyclonal rabbit anti-NGAL antiserum was generated by Sigma-Genosys (The Woodlands, TX, U.S.A.) using recombinant NGAL protein. Anti-FLAG antibodies were purchased from Sigma (St Louis, MO, U.S.A.). All chemicals were dissolved in DMSO. Exponentially growing cells were used for all experiments. Before treatment, A549 and MCF7 cells were plated and allowed to adhere overnight. All treatments, including appropriate vehicle controls, were added directly to the culture medium.
Measurements of apoptosis
Apoptosis of A549 cells was detected by several methods: (1) the sub-G1 population of cells was determined by flow cytometry in ethanol-fixed cells stained with PI (propidium iodide), as described in [20]; (2) caspase-3 cleavage was determined by Western blotting, as described below; (3) histone-associated DNA fragments were measured using the Cell Death Detection ELISA kit following the manufacturer's instructions (Roche Applied Science, Indianapolis, IN, U.S.A.); and (4) nuclear condensation was visualized by staining cells with DAPI (4,6-diamidino-2-phenylindole). For the ELISA assay, lysates from treated and untreated cells were added into a 96-well plate that was previously coated with streptavidin, and incubated with biotinylated anti-histone and anti-DNA antibodies for 2 h at room temperature (≈24 °C). Results were obtained as the ΔA405–490 nm and expressed as the change relative to controls, indicating the relative enrichment of nucleosomes in the cytoplasm. For DAPI staining, cells were exposed to 2 μg/ml DAPI in 100% methanol for 15 min. The stained cells were then visualized with a fluorescence microscope, and photos were taken. Cells with condensed chromatin or fragmented nuclei were considered as being apoptotic.
Cell viability
Cell viability was measured using the Promega CellTiter Aqueous One Solution Cell Proliferation Assay kit (Madison, WI, U.S.A.) according to the manufacturer's recommended protocol. The assay depends on the reduction of a tetrazolium compound MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulphophenyl)-2H-tetrazolium] to formazan. The absorption at 490 nm is directly proportional to the number of living cells. Briefly, treated or untreated cells were incubated with the reaction solution for 1–2 h at 37 °C. The absorption at 490 nm was measured using a microplate reader. The results are presented as the percentage of controls.
RNA isolation and Northern blot analysis
Total RNA from cells was isolated using the RNeasy mini kit following the manufacturer's protocol (Qiagen, Valencia, CA, U.S.A.). Total RNA (15 μg) was separated in a 1% denaturing formaldhyde–agarose gel and transferred to a nylon membrane (Schleicher & Schuell, Keene, NH, U.S.A.) with 20×SSC (3 M NaCl/0.3 M sodium citrate). Membranes were then cross-linked using a UV Stratalinker 2400 (Stratagene, La Jolla, CA, U.S.A.) and air-dried. NGAL cDNA was synthesized by RT-PCR using primers designed according to the sequence of the NGAL gene. cDNA was radiolabelled with [α-32P]dCTP using random primers following the manufacturer's instructions (Invitrogen, Carlsbad, CA, U.S.A.). The radiolabelled cDNA was purified on Sephadex G-50 columns (Bio-Rad, Hercules, CA, U.S.A.) and used as the probe. Membranes were probed with the 32P-labelled NGAL cDNA in a hybridization buffer (Stratagene) overnight at 68 °C. Following washing with 1×SSC and 0.1% (w/v) SDS, membranes were autoradiographed with an intensifying screen. Band intensities were determined using UN-SCAN-IT software (Silk Scientific, Orem, UT, U.S.A.) on scanned images. A GAPDH (glyceraldehyde-3-phosphate dehydrogenase) cDNA probe was used to normalize RNA loading.
Plasmid construction and generation of stable NGAL-overexpressing cell lines
To construct the NGAL expression plasmid, the full-length human NGAL cDNA was synthesized by RT (reverse transcriptase)-PCR using the primer designed according to the sequence of the NGAL gene. When used, a FLAG-tagged sequence was added on to the C-terminus of the NGAL gene. The amplified NGAL cDNA or FLAG-tagged NGAL cDNA was then subcloned into the mammalian expression vector pcDNA3.1/HisC (Invitrogen) in the sense orientation. The identity and orientation of this construct were confirmed by DNA sequencing.
A549 cells were transfected with 2 μg of DNA of pcDNA3.1/HisC/NGAL using the Lipofectamine™ reagent (Invitrogen) according to the manufacturer's protocol. pcDNA3.1/HisC DNA was used as a control. A549 cells stably expressing the pcDNA3.1/HisC/NGAL construct were selected in a medium containing 100 μg/ml G418 (Geneticin) for at least 14 days. The expression of NGAL was determined by Western blotting using either anti-FLAG antibodies or NGAL antiserum.
siRNA (small interfering RNA) gene silencing
The siRNA sequence used for targeted silencing of NGAL gene was synthesized using the Silencer siRNA Construction Kit (Ambion, Austin, TX, U.S.A.) according to the manufacturer's instructions. The NGAL siRNA oligonucleotides selected were (sense strand is given): NGAL#1, 5′-GGGAAUGCAAUUCUCAGAGTT-3′; NGAL#2, 5′-GGACUUUUGUUCCAGGUUGTT-3′; and NGAL#3, 5′-GGGAGUACUUCAAGAUCACTT-3′. An siRNA targeted to no known gene (Ambion) was used as a negative control. The synthetic double-stranded siRNA oligonucleotides were then delivered into A549 cells using different doses of Oligofectamine transfection reagent (Invitrogen) according to the manufacturer's recommended protocol. Reduction in NGAL gene expression by NGAL siRNA was measured by real-time RT-PCR 72 h post-transfection, and also by assessing the amount of NGAL protein secreted into the medium at this time.
Real-time RT-PCR analysis
Total RNA from siRNA-transfected A549 cells was isolated using the RNAqueous® MAG-96 kit (Ambion). The purified, DNase-treated RNA was reverse-transcribed with random decamers using the RETROscript® Kit (Ambion). Gene expression levels were determined by real-time RT-PCR using SuperTaq™ Real Time reagents (Ambion) on the ABI Prism 7900 SDS (Applied Biosystems, Foster City, CA, U.S.A.). The NGAL data were collected using a primer set (forward, GTAGGCCTGGCAGGGAATG; reverse, GGAACAAAAGTCCTGATCCAGTAGTC), in combination with SYBR® Green technology (Invitrogen).
18 S rRNA was amplified [forward, TTGACTCAACACGGGAAACCT; reverse, AGAAAGAGCTATCAATCTGTCAATCCT; probe, 5′-VIC-ACCCGGCCCGGACACGGA-TAMRA-3′(‘VIC’ and ‘TAMRA’ are standard fluorescent dyes)] as an endogenous control to adjust for well-to-well variances in the amount of starting template. The values were normalized to a sample transfected with the Silencer™ Negative Control #1 siRNA (Ambion).
Western blotting
Proteins in the culture media (30 μl) or cell lysate (100 μg) were separated by SDS/PAGE (12% gels). The separated proteins were electrophoretically transferred on to Immobilon™ transfer membranes (Millipore, Bedford, MA, U.S.A.) and incubated with a blocking solution, 5% dry milk in TBST [25 mM Tris/HCl (pH 7.6)/200 mM NaCl/0.15% (v/v) Tween 20], for 1 h at room temperature. NGAL protein levels were measured by immunoblotting using rabbit polyclonal antiserum specific for corresponding proteins at 1:500 or 1:1000 dilutions. Blots were washed for three 15 min periods at room temperature with TBST, and then incubated for 1 h with secondary goat anti-rabbit, peroxidase-linked antibodies (1:5000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.) in a blocking solution. Blots were then washed (3×15 min). Bands were visualized by ECL (enhanced chemiluminescence) according to the manufacturer's protocol (Amersham, Piscataway, NJ, U.S.A.).
Statistics
Data were analysed using ANOVA followed by the Newman–Keuls test analyses. Data are presented as the means±S.E.M. P values less than 0.05 were considered significant.
RESULTS
Induction of apoptosis
To investigate the relationship between apoptosis and the levels of NGAL expression, the abilities of MK886, OSU03012 and OSU03013 to induce apoptosis in A549 cells were first assessed. Flow cytometry of cells stained with PI revealed a prominent dose-dependent increase in the sub-G1 peak, indicative of apoptosis after treatment with OSU03012 for 16 h (Figure 1A). Caspase-3 was also activated at 16 h, as shown by caspase-3 cleavage to the active 17 kDa fragment (Figure 1B). Using the Cell Death Detection ELISA kit, MK886 induced apoptosis at 24 h in A549 cells in a dose-dependent manner (Figure 1C). Following DAPI staining, apoptosis was also evident at 16 h with 7.5 μM OSU03013 (Figure 1D). These results are consistent with the previously reported ability of the PDK1 inhibitors to induce apoptosis in prostate cancer cells [19], and of MK886 to induce this form of death in various cell lines [21–24].
Figure 1. Induction of apoptosis in A549 cells by MK886 and OSU03012.
(A) Apoptosis was measured by flow cytometry with PI staining after treatment with OSU03012 at the indicated doses for 16 h. The sub-G1 population was considered as apoptotic cells. Data are the means±S.E.M. for three independent experiments. *Significantly different from 0 μM OSU03012 (P<0.05). (B) A representative Western blot of caspase 3 cleavage after treatment with OSU03012 at the indicated dose for 16 h. (C) Apoptosis was assessed by the Cell Death ELISA after treatment with MK886 for 24 h. Data are expressed as the means±S.E.M. for three independent experiments. *Significantly different from 0 μM MK886 (P<0.05). (D) DAPI staining of A549 cells after treatment with 7.5 μM OSU03013 for 16 h.
The induction of NGAL expression by MK886
Our previous work showed that 24p3, a mouse analogue of NGAL, is an MK886-inducible gene [15]. Although NGAL is analogous, it is not identical to 24p3. Furthermore, in contrast with 24p3, which is virtually undetectable in murine proB cells under basal conditions [15], NGAL mRNA is expressed basally at readily detectable levels in human lung adenocarcinoma A549 cells (Figure 2). It was necessary, therefore, to determine whether the expression of NGAL could be induced by MK886. The expression of NGAL mRNA was detected by Northern blotting using NGAL cDNA as a probe. Expression was induced in A549 cells in a time- and dose-dependent manner by treatment with MK886 (Figure 2). An increase in NGAL mRNA levels was evident with 30 μM MK886, and a further increase with 40 μM at 16 h. The earliest detectable increase in NGAL mRNA above control levels using 30 μM MK886 was after 16 h. This increase was maintained to at least 24 h. Similar results were observed in human breast cancer MCF7 cells, although basal NGAL expression was not evident, and somewhat higher doses of MK886 were needed in this cell line, both to induce mRNA (Figure 3) and to cause cell death (results not shown).
Figure 2. Induction of NGAL mRNA by MK886 in A549 cells.
The dose–response experiment (A) was assessed at 16 h. The time–response experiment (B) was performed with 30 μM MK886. The blots were scanned and normalized to GAPDH for determination of intensity levels relative to 0 μM MK886 (A) or 4 h DMSO (B). The blots shown are representative of three independent experiments.
Figure 3. Induction of NGAL mRNA in MCF7 cells.
The dose–response experiment (A) was assessed at 16 h. The time–response experiment (B) was performed with 60 μM MK886. The blots shown are representative of three independent experiments.
Induction of NGAL mRNA expression by other agents
To determine whether the induction of NGAL expression was specific to MK886 or was a more general response to inducers of apoptosis, we analysed NGAL mRNA expression in cells treated with several additional apoptosis-inducing xenobiotics by Northern blotting. Two PDK1 inhibitors that induce apoptosis [19] were tested in A549 cells. Both compounds clearly increased NGAL mRNA levels at 16 h (Figure 4A). In addition, two vitamin E analogues that induce apoptosis in MCF7 cells [25] also induced NGAL mRNA expression in this cell line (Figure 4B). Thus NGAL expression is induced in response to a number of distinct xenobiotics that induce apoptosis.
Figure 4. Induction of NGAL.
(A) Northern blot analyses of NGAL expression in A549 cells treated for 16 h with OSU03012 (10 μM) and OSU13013 (7.5 μM), which induce apoptosis. GAPDH was used as a loading control. The results shown are representative of three independent experiments. (B) The expression of NGAL mRNA 16 h after treatment of MCF7 cells with vitamin E succinate or α-TEA [2,5,7,8-tetramethyl-2R(4R,8R,12-trimethyltridecyl)chroman-6-yloxyacetic acid].
NGAL protein and apoptosis
The up-regulation of NGAL expression in response to treatment of two different human cell lines with xenobiotics that induce apoptosis, the correlation of this induction with the extent of apoptosis, and the previous correlations with the homologous murine 24p3 [15] raised the possibility that NGAL is involved in cell death signalling. To test this idea, A549 cells were treated with purified recombinant NGAL at several concentrations up to 150 μg/106 cells. No effects on proliferation or cell viability were observed up to 72 h (results not shown). Thus exogenous NGAL protein alone, which would mimic the secreted protein in culture, was unable to trigger cell death pathways.
Up-regulation of NGAL reduced cell sensitivity to apoptosis
To test further the involvement of NGAL in cell survival, several A549 cell clones stably overexpressing NGAL were generated. The levels of NGAL protein secreted into the medium after 48 h from two clones (1A and 2B; the latter is with a FLAG tag) are shown in Figure 5(A). As compared with the vector-transfected cells, the mobility of FLAG-tagged NGAL proteins was slightly slower than endogenous NGAL. Endogenous NGAL protein can also be seen in the 2B lane. NGAL protein was measurable in a cell extract from the overexpressing, but not the vector-transfected, cells (Figure 5B). Both a control transfectant and the two NGAL-overexpressing clones were treated for 20 h with 10 μM OSU03012. Using the CellTiter AQ assay (Figure 5C), it was found that the NGAL-1A and NGAL-2B cells proliferated at 82±11% and 84±6% of untreated controls respectively, whereas the proliferation of cells stably transfected with control vectors was 43±12% of vehicle-treated controls (P<0.05). Analysis by flow cytometry showed that the sub-G1 population of NGAL-1A and NGAL-2B was 32% and 48% of that of cells stably transfected with control vectors after treatment with 10 μM OSU03012 for 20 h (Figure 5D). Similar data were obtained by cell death ELISA assay (Figure 5E). Thus overexpression of NGAL protects cells from apoptosis induced by these compounds.
Figure 5. Overexpression of NGAL protects cells from apoptosis.
(A) Culture medium (30 μl) from A549 cells stably transfected with pcDNA3.1/Hisc/NGAL was analysed for NGAL content after 48 h by Western blotting using either NGAL antisera or FLAG antibodies. Clone 1A was of NGAL alone, whereas 2B contained NGAL with a FLAG tag. (B) Extracts from A549 cells stably transfected with pcDNA3.1/Hisc/NGAL were analysed after 48 h for NGAL content by Western blotting using NGAL antisera (40 μg of protein per lane). (C–E) Cells stably overexpressing NGAL were treated with 10 μM OSU03012 for 20 h and then analysed either for cell proliferation (C) using a Cell Proliferation Assay kit or apoptosis by flow cytometry with PI staining (D) or ELISA assay (E). Sub-G1 populations were considered as apoptotic cells. Data are the means±S.E.M. for three independent experiments. *Significantly different from controls (P<0.05).
NGAL antibodies and apoptosis
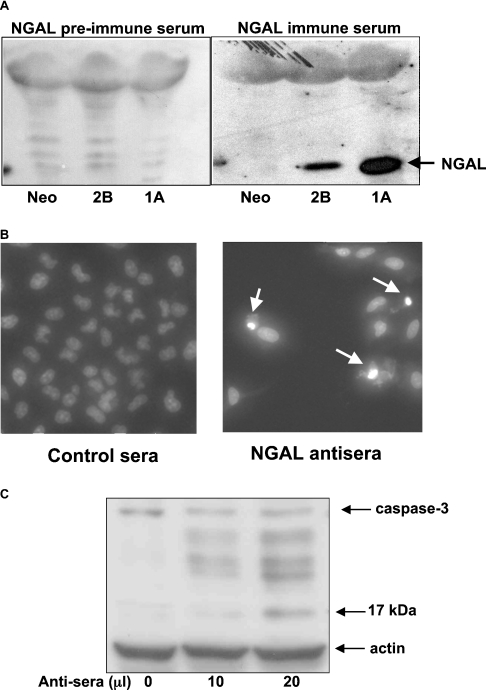
To determine whether blocking NGAL had any effects, A549 cells were incubated with the antisera against the full-length NGAL antibody. The specificity of this antibody is shown in Figure 6(A). Media from A549 cells showed only minor non-specific bands when treated with control antisera. However, the NGAL antisera recognized this protein without any significant cross-reactivity with other proteins in the media. The NGAL antisera were highly toxic to A549 cells, with a large decrease in total cell number, an increase in condensed nuclei (Figure 6B) and the cleavage of caspase-3 shown, indicative of apoptosis (Figure 6C).
Figure 6. Induction of apoptosis by NGAL antibodies.
(A) The Western blot shows the specificity of NGAL antisera using culture medium from A549 cells stably transfected with pcDNA3.1/Hisc/NGAL or vector (Neo) control. (B) DAPI staining shows a decrease in total cell number, and an increased number of condensed nuclei (shown by the arrows) indicative of apoptosis 24 h after A549 cells were treated with either of the polyclonal NGAL antisera as compared with control antisera. (C) Cell lysate from A549 cells treated with an indicated dose of polyclonal NGAL antisera was analysed for the cleavage of caspase 3 by Western blotting using antibodies recognizing both intact caspase 3 and its cleaved fragments. Actin was used as a loading control.
Down-regulation of NGAL increased cell sensitivity to the toxicity of OSU03012
The hypothesis that NGAL exerts protective activities was tested further by down-regulating the expression of this gene with siRNA. A549 cells were transfected with three different siRNAs (25 nM) targeting the NGAL mRNA sequence. Using real-time RT-PCR, reductions in NGAL mRNA of 63, 79 and 30% were achieved at 72 h with siRNA#1, #2 and #3 respectively (Figure 7A). With densitometry, a 65% decrease in NGAL protein in the media was evident with siRNA #2 compared with both controls (Figure 7A, inset). Thus NGAL siRNA #2 was used for further studies. The siRNA transfection procedure alone at 25 nM siRNA had no effect on cell proliferation from 72–80 h after transfection, as assessed by the Promega proliferation assay (results not shown). However, the toxicity of 10 μM OSU03012 over 8 h was increased, with the negative control siRNA directed against no known gene. This is shown by the 55% decline in proliferation relative to non-transfected cells (Figure 7B). This was not due to effects on NGAL protein (Figure 7A), but may be due to increased permeability of the cells from the transfection reagents (a common problem with siRNA) leading to increased drug uptake. Nevertheless, OSU03012 was even more toxic in the presence of siRNA #2, as shown by a further significant decrease in proliferation (Figure 7B). This indicates that the loss of NGAL is detrimental to these cells.
Figure 7. The effect of gene silencing on NGAL expression and on cell proliferation after treatment with a PDK1 inhibitor.
(A) The effects of gene silencing by NGAL siRNAs on NGAL mRNA and protein. A549 cells were transfected with 25 nM NGAL siRNAs by Oligolipofectamine reagent, as described in the Materials and methods section. After 72 h, the expression of NGAL was detected by real-time RT-PCR. Values are presented as means±S.E.M. of siRNA controls (n=3). NGAL protein secreted into the media after 72 h treatment with 25 nM of siNGAL#2 was examined by Western blotting using NGAL antiserum (inset). (B) Cell proliferation was determined by CellTiter AQ Cell Proliferation Assay. A549 cells were treated with 10 μM OSU03012 for 8 h, 72 h post-transfection with 25 nM NGAL siRNA#2. Data are presented as the percentages of negative control siRNA cells, and are the means±S.E.M. for three independent experiments. *Significantly different from cells transfected with negative control (Neg. Cont.) siRNA (P<0.05).
DISCUSSION
NGAL is induced in different cell lines and tissues by a range of stimuli, including IL-1 in A549 cells [26] and IGF (insulin-like growth factor) or TGF-α (transforming growth factor α) in primary human keratinocytes [27]. The present results demonstrate that induction also occurs in two human epithelial cell lines (A549 and MCF7) in response to several apoptosis-inducing xenobiotics. Interestingly, these NGAL-inducing agents are related to lipoxygenase or COX-2 inhibitors, suggesting some similarities in their apoptotic mechanisms. This potential link is strengthened by the finding that NGAL is highly induced in inflammatory colorectal diseases and in several cancers [28], including colorectal cancer [5], the cancer where COX-2 inhibitors have shown some efficacy [29,30]. Furthermore, all of the agents that induce NGAL also inhibit protein kinase B/Akt ([19], and Z. Tong and J. P. Kehrer, unpublished work), and all are toxic to tumour cells, but relatively non-toxic to normal cells ([25], and Z. Tong and J. P. Kehrer, unpublished work). This raises the interesting question of whether the induction of NGAL is simply a marker for such differential toxicity, or is perhaps involved functionally.
The precise biological roles of NGAL are not fully known, although a number of functions have been postulated. For example, elevated NGAL expression in certain human neoplastic diseases [6,7,31] and its association with MMP-9 [11] suggest a role in tumour progression. Up-regulated NGAL expression in the colon epithelium is associated with a variety of inflammatory conditions [5], and its induction by IL-1β in A549 cells [26] demonstrates an involvement in immunomodulation. Like the induction of the murine 24p3 by MK886, the induction of NGAL in A549 cells by this chemical correlated with apoptosis. This correlation has led to the conclusion that 24p3, and by inference NGAL, is pro-apoptotic. However, the current data strongly suggest that NGAL plays a role in cell survival. Thus the induction of NGAL in response to apoptotic stressors may be a compensatory response that involves cell defence pathways. This conclusion is supported by the findings that: (1) purified recombinant NGAL protein was completely non-toxic; (2) antibodies against full-length NGAL were highly toxic; (3) decreasing NGAL expression by siRNA resulted in significantly less cell proliferation following xenobiotic treatment; and (4) the overexpression of NGAL significantly reduced cell death by apoptosis-inducing agents related to COX-2 and lipoxygenase inhibitors.
Additional support for the survival activity of NGAL comes from Sorensen et al. [27], who reported that NGAL was strongly induced by growth factors including IGF and TGF-α in primary human keratinocytes. This induction is believed to play a role in wound healing. Furthermore, NGAL can protect against acute ischaemic renal injury [12], and down-regulation of ExFABP, a related chicken lipocalin, by transient transfection with antisense ExFABP induces apoptosis in chicken embryo chondrocytes [32]. Finally, there is a positive correlation between NGAL expression and proliferation (S-phase fraction) in human breast tumours [6]. Overall, the available data provide some substantial evidence that the synthesis and secretion of NGAL may be important for survival of several distinct cell types.
This conclusion regarding the survival function of NGAL differs from that reached with the mouse analogue 24p3 [12,13]. The reasons for this difference are not clear. Our previous interpretation [15] was based on the correlation between expression and apoptosis and the diminished toxicity seen in FL5.12 cells stably expressing antisense to 24p3. However, if 24p3 is a survival factor, this latter procedure would only yield highly resistant cells through the clonal selection process. Excess 24p3 could also be perceived as pro-apoptotic if it excessively sequesters growth factors; a process that would be detrimental to highly growth-factor-dependent cells, such as FL5.12.
The mechanisms underlying the toxicity of the antisera against the complete NGAL protein are not clear. Simply preventing the uptake of growth factors is unlikely to be the sole explanation because A549 cells survive, and even proliferate, for lengthy times in the absence of serum. In contrast with the polyclonal rabbit antisera, a commercial mouse monoclonal NGAL antibody obtained from the Antibody Shop (Gentofte, Denmark) was non-toxic (Z. Tong and J. P. Kehrer, unpublished work). This may be because it recognizes a different epitope and/or was generated using only a portion of the NGAL protein (amino acid residues 21–198). It is also possible that binding to NGAL on cell surface receptors is critical to elicit toxicity.
Extracellular stimuli are able to activate both apoptotic and anti-apoptotic pathways. For instance, UV irradiation of primary human keratinocytes at a dose sufficient to induce apoptosis activates PI3K (phosphoinositide 3-kinase)/Akt survival pathways, and the blockage of the PI3K/Akt pathway by LY294002 enhances UV-induced apoptosis [33]. Similar activation of both pro-survival and pro-apoptotic pathways has been reported in RN46A neuronal cells stimulated with hypoxia [34] and in prostate and breast cancer cells, and fibroblast cells treated to induce apoptosis with serum deprivation, etoposide or the mitochondrial toxin BMD188 [35]. Therefore the balance between pro- and anti-apoptosis signals determines cell fate after exposure to toxic reagents. This concept has been extended by suggesting that, in the presence of apoptotic stimuli, surviving cells become more resistant to apoptosis so that not too many cells kill themselves [36]. The induction of a secreted protein like NGAL, which occurs before substantial apoptosis ensues, may be part of such a survival response. Thus NGAL may be involved not only in the maintenance of cell growth, but also in a general defence against toxic stimuli.
Lipocalins are secreted proteins that are capable of binding small lipophilic substances [1]. Studies on the structure of NGAL have not revealed the preferred ligand, but have shown that it binds N-formyl-Met-Leu-Phe, a neutrophil chemoattractant, as well as other lipophilic mediators of inflammation such as leukotrienes and lipopolysaccharide [37]. NGAL is also able to bind MMP-9 and protect it from degradation [11]. In addition, NGAL exhibits a very high affinity for bacterial catecholate-type ferric siderophores, and can interfere with siderophore-mediated iron acquisition [10]. It is possible that NGAL protects cells from toxic stress through an interaction with certain proteins, thereby modulating the functions of those proteins. Other possible mechanisms underlying the survival effect of NGAL may be an ability of this protein to act as a scavenger for unwanted endogenous or exogenous compounds (such as the xenobiotics used in the present study). In support of this notion, Lechner et al. [38] reported that human tear lipocalin (lcn-1) is induced by oxidative stress, including H2O2 and FeSO4 in NT2 cells, and it acts as a scavenger of potentially harmful lipid peroxidation products. In conjunction with their binding activity, lipocalins are generally considered as transporters of a variety of small molecules [1]. NGAL has been identified as an iron-transporting protein [2]. It is well known that the delivery of iron to cells is crucial for cell growth and development. Therefore the functions of NGAL in the support of cell survival may occur through the transport of iron, retinal or other molecules that make cells more resistant to toxic stress.
In summary, NGAL is induced by a number of apoptosis-inducing xenobiotics. Down-regulation of NGAL expression by siRNAs decreases further the inhibition of proliferation induced by a PDK1 inhibitor, while overexpression of NGAL reduced cell death induced by this compound. In addition, purified NGAL protein has no cytotoxic effects, whereas an antiserum against full-length NGAL is toxic. Thus, although the induction of NGAL expression correlates with the induction of apoptosis, it would appear that NGAL is a survival factor involved in cellular defences against toxic stimuli. Down-regulation of NGAL expression in certain tumour cells may be a possible approach to improving the response of cancer cells to treatment.
Acknowledgments
This work was supported by RO1 grants from the National Institutes of Health CA83701 (J. P. K.) and CA94829 (C.-S. C.). The University of Texas at Austin's Center for Molecular and Cellular Toxicology also provided assistance. Support was also received through NIEHS Center Grant ES07784. J. P. K. is the Gustavus and Louise Pfeiffer Professor of Toxicology.
References
- 1.Flower D. R. The lipocalin protein family: structure and function. Biochem. J. 1996;318:1–14. doi: 10.1042/bj3180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang J., Goetz D., Li J. Y., Wang W., Mori K., Setlik D., Du T., Erdjument-Bromage H., Tempst P., Strong R., Barasch J. An iron delivery pathway mediated by a lipocalin. Mol. Cell. 2002;10:1045–1056. doi: 10.1016/s1097-2765(02)00710-4. [DOI] [PubMed] [Google Scholar]
- 3.Kjeldsen L., Johnsen A. H., Sengelov H., Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J. Biol. Chem. 1993;268:10425–10432. [PubMed] [Google Scholar]
- 4.Nilsen-Hamilton M., Liu Q., Ryon J., Bendickson L., Lepont P., Chang Q. Tissue involution and the acute phase response. Ann. N. Y. Acad. Sci. 2003;995:94–108. doi: 10.1111/j.1749-6632.2003.tb03213.x. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen B. S., Borregaard N., Bundgaard J. R., Timshel S., Sehested M., Kjeldsen L. Induction of NGAL synthesis in epithelial cells of human colorectal neoplasia and inflammatory bowel diseases. Gut. 1996;38:414–420. doi: 10.1136/gut.38.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stoesz S. P., Friedl A., Haag J. D., Lindstrom M. J., Clark G. M., Gould M. N. Heterogeneous expression of the lipocalin NGAL in primary breast cancers. Int. J. Cancer. 1998;79:565–572. doi: 10.1002/(sici)1097-0215(19981218)79:6<565::aid-ijc3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 7.Furutani M., Arii S., Mizumoto M., Kato M., Imamura M. Identification of a neutrophil gelatinase-associated lipocalin mRNA in human pancreatic cancers using a modified signal sequence trap method. Cancer Lett. 1998;122:209–214. doi: 10.1016/s0304-3835(97)00391-1. [DOI] [PubMed] [Google Scholar]
- 8.Bartsch S., Tschesche H. Cloning and expression of human neutrophil lipocalin cDNA derived from bone marrow and ovarian cancer cells. FEBS Lett. 1995;357:255–259. doi: 10.1016/0014-5793(94)01303-i. [DOI] [PubMed] [Google Scholar]
- 9.Flo T. H., Smith K. D., Sato S., Rodriguez D. J., Holmes M. A., Strong R. K., Akira S., Aderem A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature (London) 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 10.Goetz D. H., Holmes M. A., Borregaard N., Bluhm M. E., Raymond K. N., Strong R. K. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol. Cell. 2002;10:1033–1043. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- 11.Yan L., Borregaard N., Kjeldsen L., Moses M. A. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J. Biol. Chem. 2001;276:37258–37265. doi: 10.1074/jbc.M106089200. [DOI] [PubMed] [Google Scholar]
- 12.Mishra J., Mori K., Ma Q., Kelly C., Yang J., Mitsnefes M., Barasch J., Devarajan P. Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J. Am. Soc. Nephrol. 2004;15:3073–3082. doi: 10.1097/01.ASN.0000145013.44578.45. [DOI] [PubMed] [Google Scholar]
- 13.Kjeldsen L., Cowland J. B., Borregaard N. Human neutrophil gelatinase-associated lipocalin and homologous proteins in rat and mouse. Biochim. Biophys. Acta. 2000;1482:272–283. doi: 10.1016/s0167-4838(00)00152-7. [DOI] [PubMed] [Google Scholar]
- 14.Devireddy L. R., Teodoro J. G., Richard F. A., Green M. R. Induction of apoptosis by a secreted lipocalin that is transcriptionally regulated by IL-3 deprivation. Science. 2001;293:829–834. doi: 10.1126/science.1061075. [DOI] [PubMed] [Google Scholar]
- 15.Tong Z., Wu X., Kehrer J. P. Increased expression of the lipocalin 24p3 as an apoptotic mechanism for MK886. Biochem. J. 2003;372:203–210. doi: 10.1042/BJ20021696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bong J. J., Seol M. B., Kim H. H., Han O., Back K., Baik M. The 24p3 gene is induced during involution of the mammary gland and induces apoptosis of mammary epithelial cells. Mol. Cells. 2004;17:29–34. [PubMed] [Google Scholar]
- 17.Ryon J., Bendickson L., Nilsen-Hamilton M. High expression in involuting reproductive tissues of uterocalin/24p3, a lipocalin and acute phase protein. Biochem. J. 2002;367:271–277. doi: 10.1042/BJ20020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamezaki K., Shimoda K., Numata A., Aoki K., Kato K., Takase K., Nakajima H., Ihara K., Haro T., Ishikawa F., et al. The lipocalin 24p3, which is an essential molecule in IL-3 withdrawal-induced apoptosis, is not involved in the G-CSF withdrawal-induced apoptosis. Eur. J. Haematol. 2003;71:412–417. doi: 10.1046/j.0902-4441.2003.00160.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhu J., Huang J. W., Tseng P. H., Yang Y. T., Fowble J., Shiau C. W., Shaw Y. J., Kulp S. K., Chen C. S. From the cyclooxygenase-2 inhibitor celecoxib to a novel class of 3-phosphoinositide-dependent protein kinase-1 inhibitors. Cancer Res. 2004;64:4309–4318. doi: 10.1158/0008-5472.CAN-03-4063. [DOI] [PubMed] [Google Scholar]
- 20.Mortenson M. M., Schlieman M. G., Virudachalam S., Bold R. J. Effects of the proteasome inhibitor bortezomib alone and in combination with chemotherapy in the A549 non-small-cell lung cancer cell line. Cancer Chemother. Pharmacol. 2004;54:343–353. doi: 10.1007/s00280-004-0811-4. [DOI] [PubMed] [Google Scholar]
- 21.Anderson K. M., Seed T., Plate J. M., Jajeh A., Meng J., Harris J. E. Selective inhibitors of 5-lipoxygenase reduce CML blast cell proliferation and induce limited differentiation and apoptosis. Leuk. Res. 1995;19:789–801. doi: 10.1016/0145-2126(95)00043-7. [DOI] [PubMed] [Google Scholar]
- 22.Anderson K. M., Seed T., Jajeh A., Dudeja P., Byun T., Meng J., Ou D., Bonomi P., Harris J. E. An in vivo inhibitor of 5-lipoxygenase, MK886, at micromolar concentration induces apoptosis in U937 and CML cells. Anticancer Res. 1996;16:2589–2599. [PubMed] [Google Scholar]
- 23.Datta K., Biswal S. S., Xu J., Towndrow K. M., Feng X., Kehrer J. P. A relationship between 5-lipoxygenase-activating protein and bcl-xL expression in murine pro-B lymphocytic FL5.12 cells. J. Biol. Chem. 1998;273:28163–28169. doi: 10.1074/jbc.273.43.28163. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh J., Myers C. E. Inhibition of arachidonate 5-lipoxygenase triggers massive apoptosis in human prostate cancer cells. Proc. Natl. Acad. Sci. U.S.A. 1998;95:13182–13187. doi: 10.1073/pnas.95.22.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson K., Simmons-Menchaca M., Lawson K. A., Atkinson J., Sanders B. G., Kline K. Differential response of human ovarian cancer cells to induction of apoptosis by vitamin E Succinate and vitamin E analogue, alpha-TEA. Cancer Res. 2004;64:4263–4269. doi: 10.1158/0008-5472.CAN-03-2327. [DOI] [PubMed] [Google Scholar]
- 26.Cowland J. B., Sorensen O. E., Sehested M., Borregaard N. Neutrophil gelatinase-associated lipocalin is up-regulated in human epithelial cells by IL-1 beta, but not by TNF-alpha. J. Immunol. 2003;171:6630–6639. doi: 10.4049/jimmunol.171.12.6630. [DOI] [PubMed] [Google Scholar]
- 27.Sorensen O. E., Cowland J. B., Theilgaard-Monch K., Liu L., Ganz T., Borregaard N. Wound healing and expression of antimicrobial peptides/polypeptides in human keratinocytes, a consequence of common growth factors. J. Immunol. 2003;170:5583–5589. doi: 10.4049/jimmunol.170.11.5583. [DOI] [PubMed] [Google Scholar]
- 28.Bratt T. Lipocalins and cancer. Biochim. Biophys. Acta. 2000;1482:318–326. doi: 10.1016/s0167-4838(00)00154-0. [DOI] [PubMed] [Google Scholar]
- 29.Fife R. S., Stott B., Carr R. E. Effects of a selective cyclooxygenase-2 inhibitor on cancer cells in vitro. Cancer Biol. Ther. 2004;3:228–232. doi: 10.4161/cbt.3.2.692. [DOI] [PubMed] [Google Scholar]
- 30.Steinbach G., Lynch P. M., Phillips R. K., Wallace M. H., Hawk E., Gordon G. B., Wakabayashi N., Saunders B., Shen Y., Fujimura T., Su L. K., Levin B. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N. Engl. J. Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 31.Friedl A., Stoesz S. P., Buckley P., Gould M. N. Neutrophil gelatinase-associated lipocalin in normal and neoplastic human tissues. Cell type-specific pattern of expression. Histochem. J. 1999;31:433–441. doi: 10.1023/a:1003708808934. [DOI] [PubMed] [Google Scholar]
- 32.Di Marco E., Sessarego N., Zerega B., Cancedda R., Cancedda F. D. Inhibition of cell proliferation and induction of apoptosis by ExFABP gene targeting. J. Cell. Physiol. 2003;196:464–473. doi: 10.1002/jcp.10310. [DOI] [PubMed] [Google Scholar]
- 33.Wang H. Q., Quan T., He T., Franke T. F., Voorhees J. J., Fisher G. J. Epidermal growth factor receptor-dependent, NF-kappaB-independent activation of the phosphatidylinositol 3-kinase/Akt pathway inhibits ultraviolet irradiation-induced caspases-3, -8, and -9 in human keratinocytes. J. Biol. Chem. 2003;278:45737–45745. doi: 10.1074/jbc.M300574200. [DOI] [PubMed] [Google Scholar]
- 34.Zhang S. X., Gozal D., Sachleben L. R., Jr, Rane M., Klein J. B., Gozal E. Hypoxia induces an autocrine-paracrine survival pathway via platelet-derived growth factor (PDGF)-B/PDGF-beta receptor/phosphatidylinositol 3-kinase/Akt signaling in RN46A neuronal cells. FASEB J. 2003;17:1709–1711. doi: 10.1096/fj.02-1111fje. [DOI] [PubMed] [Google Scholar]
- 35.Liu J. W., Chandra D., Rudd M. D., Butler A. P., Pallotta V., Brown D., Coffer P. J., Tang D. G. Induction of prosurvival molecules by apoptotic stimuli: involvement of FOXO3a and ROS. Oncogene. 2005;24:2020–2031. doi: 10.1038/sj.onc.1208385. [DOI] [PubMed] [Google Scholar]
- 36.LeGrand E. K. An adaptationist view of apoptosis. Q. Rev. Biol. 1997;72:135–147. doi: 10.1086/419763. [DOI] [PubMed] [Google Scholar]
- 37.Goetz D. H., Willie S. T., Armen R. S., Bratt T., Borregaard N., Strong R. K. Ligand preference inferred from the structure of neutrophil gelatinase associated lipocalin. Biochemistry. 2000;39:1935–1941. doi: 10.1021/bi992215v. [DOI] [PubMed] [Google Scholar]
- 38.Lechner M., Wojnar P., Redl B. Human tear lipocalin acts as an oxidative-stress-induced scavenger of potentially harmful lipid peroxidation products in a cell culture system. Biochem. J. 2001;356:129–135. doi: 10.1042/0264-6021:3560129. [DOI] [PMC free article] [PubMed] [Google Scholar]