Abstract
Arabidopsis RD21 is a cysteine protease of the papain family. Unlike other members of the papain family, RD21 has a C-terminal extension sequence composed of two domains, a 2-kD proline-rich domain and a 10-kD domain homologous to animal epithelin/granulin family proteins. The RD21 protein was accumulated as 38- and 33-kD proteins in Arabidopsis leaves. An immunoblot showed that the 38-kD protein had the granulin domain, whereas the 33-kD protein did not. A pulse-chase experiment with Bright-Yellow 2 transformant cells expressing RD21 showed that RD21 was synthesized as a 57-kD precursor and was then slowly processed to make the 33-kD mature protein via the 38-kD intermediate. After a 12-h chase, the 38-kD intermediate was still detected in the cells. These results indicate that the N-terminal propeptide was first removed from the 57-kD precursor, and the C-terminal granulin domain was then slowly removed to yield the 33-kD mature protein. Subcellular fractionation of the Bright-Yellow 2 transformant showed that the intermediate and mature forms of RD21 were localized in the vacuoles. Under the acidic conditions of the vacuolar interior, the intermediate was found to be easily aggregated. The intermediate and the mature protein were accumulated in association with leaf senescence. Taken together, these results indicate that the intermediate of RD21 was accumulated in the vacuoles as an aggregate, and then slowly matured to make a soluble protease by removing the granulin domain during leaf senescence.
Senescence of plant tissues is associated with a series of biochemical and physiological changes. These include a degradation of cellular materials and disintegration of organelles. Vacuoles are a main compartment for degradation of cellular materials such as proteins, nucleic acids, and oligosaccharides (Matile, 1975). To degrade the macromolecules, the vacuoles accumulate various hydrolytic enzymes during senescence. Vacuolar proteins, including hydrolytic enzymes, are synthesized on the rough endoplasmic reticulum and are transported to the vacuoles. These proteins are often synthesized as a larger precursor, including an N-terminal propeptide (NTPP) and/or a C-terminal propeptide (CTPP). NTPPs and CTPPs are post-translationally processed just after arriving at the vacuoles to make the respective mature proteins. Vacuolar processing enzyme (VPE) has been shown to be an enzyme responsible for maturation of various vacuolar proteins within the vacuoles (Hara-Nishimura et al., 1993a, 1993b; Okamoto and Minamikawa, 1995; Hara-Nishimura, 1998; Yamada et al., 1999). Three VPE homologs of Arabidopsis have been identified: αVPE and γVPE in vegetative tissues and βVPE in storage tissues (Kinoshita et al., 1995a, 1995b, 1999).
NTPPs and CTPPs of vacuolar proteins are known to function as a vacuolar targeting signal and/or a modulator of enzyme activities. The barley (Hordeum vulgare) aleurain (Rogers et al., 1985) and sweet potato (Ipomoea batatas) sporamin (Murakami et al., 1986) have a vacuolar targeting signal, Asn-Pro-Ile-Arg (NPIR), in their NTPPs (Matsuoka and Nakamura, 1991, 1999; Chrispeels and Raikhel, 1992). The NPIR sequence is recognized by a vacuolar sorting receptor such as pea (Pisum sativum) BP-80 (Paris et al., 1997; Humair et al., 2001), pumpkin (Cucurbita maxima) PV72 (Shimada et al., 1997), and Arabidopsis AtELP (Ahmed et al., 2000). On the other hand, NTPPs of human cathepsin B (P07858) and cathepsin S (M90696) function as an autoinhibitory domain that covers the active site within the molecule (Turk et al., 1996; Maubach et al., 1997). The removal of the NTPPs is coupled with an activation of these enzymes (Mach et al., 1993).
An rd21 gene encoding a papain family protease, RD21 (D13043), was found to be up-regulated during dehydration of Arabidopsis plants (Koizumi et al., 1993). Papain family proteases have NTPP that includes an ERFNIN motif, which is conserved in rat cathepsin H (Y00708), cathepsin L (Y00697), and human cathepsin S (Bromme et al., 1993; Karrer et al., 1993). The NTPPs seem to function as an autoinhibitory domain of papain family proteases. In contrast to no CTPP in most members of papain family, RD21 has a long extension sequence at the C terminus. Such an extension sequence has been found in 14 members of papain family (Gietl et al., 2000), including rice (Oryza sativa) oryzains α (D90406) and β (D90407; Watanabe et al., 1991), maize (Zea mays) CPPIC (AB020961; Yamada et al., 2000), Sandersonia aurantiaca PRT15 (AF133838), and Arabidopsis XBCP3 (AF388175).
The C-terminal extension sequences are composed of two domains, a Pro-rich domain and a domain that has a high homology to animal proteins of the epithelin/granulin family (Bhandari et al., 1992; Bateman and Bennett, 1998). The epithelins and granulins are approximately 6-kD proteins that stimulate or inhibit the growth of animal cells (Bateman and Bennett, 1998). The crystal structure of carp granulin 1 has seven disulfide bonds and four stacked β hairpins (Hrabal et al., 1996). Compared with the animal granulins, plant granulins have an approximately 4-kD insertion with two Cys residues, which might cause a structural difference between plant and animal granulins. Plant granulins are a subclass of the papain family with a single granulin domain in the C-terminal region. On the other hand, animal granulins are synthesized as a larger precursor containing seven-tandem repeats of granulin domain, and the precursor is processed to make multiple mature granulins (Bhandari et al., 1992).
In this study, we show a unique maturation mechanism of RD21 in the vacuoles of senescing leaves. An intermediate of RD21 was accumulated as an aggregate in the vacuoles, and slowly matured during leaf senescence.
RESULTS
Arabidopsis Leaves Accumulate Not Only the Mature Protein of RD21 but Also the Intermediate
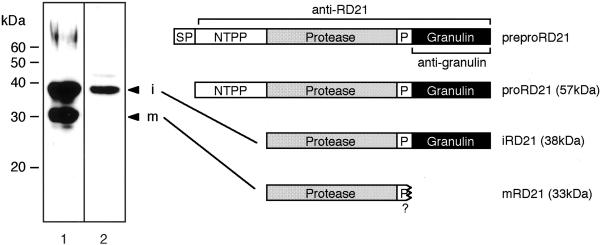
Arabidopsis RD21 is a Cys protease that belongs to papain family. The proprotein precursor of RD21 (proRD21) has a Pro-rich domain and a granulin domain, although precursors of most members of papain family do not have them (Fig. 1). The rd21 gene has been shown to be up-regulated during dehydration (Koizumi et al., 1993). To clarify an accumulation of RD21 at the protein level, we raised antibodies against proRD21 and the granulin domain (Fig. 1). An immunoblot analysis of Arabidopsis leaves showed that anti-RD21 antibodies specifically recognized 38- and 33-kD proteins (Fig. 1, lane 1), and anti-granulin antibodies recognized the 38-kD protein but not the 33-kD protein (lane 2). This indicated that the 38-kD protein corresponded to an RD21 intermediate (iRD21) that had the granulin domain, and the 33-kD protein corresponded to a mature protein (mRD21) without the granulin domain. It should be noted that no 10-kD free granulin protein was detected in the leaves. The calculated molecular masses, 35,375 D for iRD21 and 23,538 D for mRD21, are much lower than the 38 kD and 33 kD, respectively, that were estimated by SDS-PAGE. Such a discrepancy was reported with other papain family proteases (Schmid et al., 1998; Yamada et al., 2000). The reduction of mobility on an SDS gel may be caused by the nature of papain family proteases that are resistant to binding by SDS (Nelson, 1971). The RD21 precursor has two sites of Asn-linked glycosylation in the polypeptide (Koizumi et al., 1993). However, no reduction of molecular masses of iRD21 was observed after digestion with N-glycosidase F (data not shown). This indicates that RD21 has no Asn-linked oligosaccharide.
Figure 1.
An intermediate and a mature protein of RD21 are found in the Arabidopsis leaves. The precursor of RD21 is composed of five regions: a SP, an NTPP, a protease domain, a P, and a granulin domain. Antibodies against the proRD21 (anti-RD21) or the granulin domain (anti-granulin) were raised. The total protein from the leaves of 35-d-old Arabidopsis plants was subjected to SDS-PAGE followed by immunoblot analysis with these antibodies. Anti-RD21 antibodies recognized a 38-kD intermediate (iRD21) and a 33-kD mature protein (mRD21) of RD21 (lane 1). Anti-granulin antibodies recognized iRD21 but did not recognize mRD21 (lane 2). preproRD21, A preproprotein precursor of RD21; proRD21, proprotein precursor of RD21; i, iRD21; m, mRD21; SP, signal peptide; P, Pro-rich domain. The molecular mass of each marker protein is given on the left in kilodaltons.
Developmental Changes in the Level of the RD21 Proteins in Senescing Leaves and in Growing Seedlings
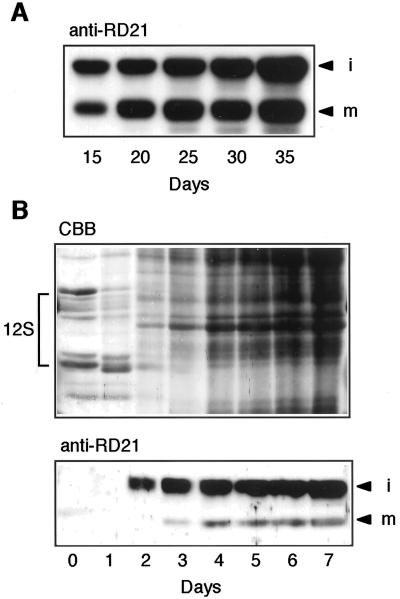
Recently, the rd21 gene was reported to be a senescence-associated gene (Weaver et al., 1998; Kinoshita et al., 1999). We examined an accumulation pattern of RD21 in Arabidopsis leaves during senescence. Figure 2A shows an immunoblot of the first and second rosette leaves with anti-RD21 antibodies. iRD21 and mRD21 were found in fully expanded leaves of 15-d-old plants and then the amounts of both proteins increased in parallel with degradation of chlorophyll in senescing leaves of 20- to 35-d-old plants. The result indicates that the RD21 proteins were accumulated in senescing leaves in association with up-regulation of the gene.
Figure 2.
Developmental changes in the levels of iRD21 and mRD21 in Arabidopsis leaves during senescence and in seedlings after seed germination. A, Total protein (10 μg) from the first and second rosette leaves of 15-, 20-, 25-, 30-, and 35-d-old Arabidopsis plants was subjected to SDS-PAGE followed by immunoblot analysis with anti-RD21 antibodies (anti-RD21). B, Ten dry seeds and 10 seedlings at 1 to 7 d after germination were homogenized in 200 μL of SDS-PAGE sample buffer. Each extract (10 μL) was subjected to SDS-PAGE and subsequent staining with Coomassie Blue (top, CBB) or immunoblot analysis with anti-RD21 antibodies (bottom, anti-RD21). i, iRD21; m, mRD21; 12S, 12S globulin, a seed storage protein.
Figure 2B shows an accumulation pattern of RD21 in seedlings after seed germination. The accumulation of iRD21 was first observed 2 d after seed germination (Fig. 2B, bottom). By contrast, the degradation of seed storage proteins started immediately after seed imbibition (Fig. 2B, top). There is no correlation between the RD21 accumulation and the degradation of storage proteins. This suggests that RD21 might not be responsible for mobilization of seed storage proteins. Interestingly, iRD21 levels were much higher than mRD21 levels in seedlings (Fig. 2B, bottom). The relatively high amounts of iRD21 compared with mRD21 in seedlings was in contrast to similar levels of iRD21 and mRD21 in senescing leaves (Fig. 2A).
Two-Step Maturation of RD21 Involves Removal of NTPP and Subsequent Slow Removal of CTPP
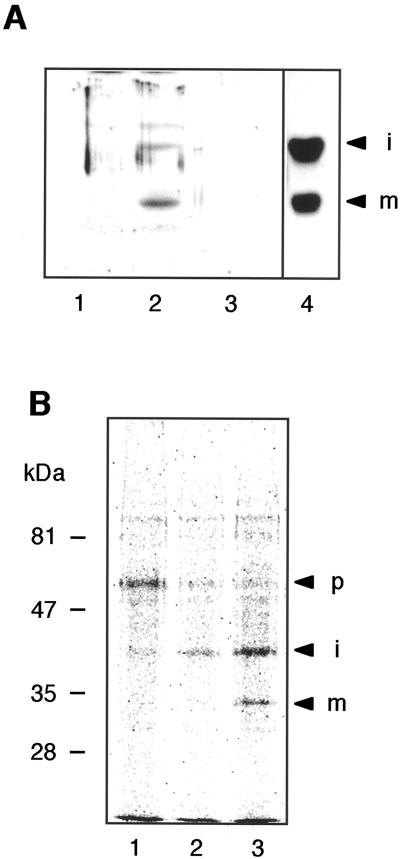
The next issue to be resolved was the maturation process of RD21. Figure 3A shows an immunoblot of transformant Bright-Yellow 2 (BY2) cells expressing the RD21 protein (BY2/RD21) with anti-RD21 antibodies. The 38- and 33-kD proteins were detected in the transformant cells (lane 2) but not in the culture medium (lane 3). They showed the same mobility on the blot as the respective proteins from the rosette leaves of 20-d-old Arabidopsis plants (lane 4). This indicated that RD21 was processed and accumulated in the transformant cells as in the Arabidopsis leaves.
Figure 3.
A stepwise conversion of the 57-kD proRD21 into the 33-kD mRD21 via the 38-kD iRD21. A, The suspension culture of the transformant BY2/RD21 was separated into the cells and the medium. Each 10 μg of total protein from the cells (lane 2) and the medium (lane 3) was subjected to SDS-PAGE followed by immunoblot analysis with anti-RD21 antibodies. The total protein (10 μg) from cells of nontransformant BY2 (lane 1) and the rosette leaves of 20-d-old Arabidopsis plants (lane 4) are also shown on the blot. B, The transformant BY2/RD21 cells were pulse-labeled with [35S] Met and Cys for 1 h (lane 1), and then were incubated with unlabeled Met and Cys for 4 and 12 h (lanes 2 and 3, respectively). The labeled RD21-related proteins were immunoprecipitated with anti-RD21 antibodies. The immunoprecipitates were subjected to SDS-PAGE and were then analyzed with a BAS3000 system. p, proRD21; i, iRD21; m, mRD21. The molecular mass of each marker protein is given on the left in kilodaltons.
To clarify the maturation process of RD21, we performed a pulse-chase experiment with the transformant BY2/RD21 cells. Figure 3B shows the pulse-chase-labeled RD21-related proteins in BY2/RD21. A single 57-kD band corresponding to proRD21 was detected after a 2-h pulse. After a 4-h chase, proRD21 disappeared and iRD21 appeared. mRD21 appeared after a 12-h chase. The result suggested a stepwise maturation of RD21: The first step involves the conversion of proRD21 into iRD21 by removal of the NTPP and the second step involves the conversion of iRD21 into mRD21 by removal of the C-terminal granulin domain. The maturation of RD21 was too slow to detect mRD21 after the 4-h chase. iRD21 was still detected in the cells after 12-h chase. This indicated that the second step of the maturation was limited.
The Intermediate of RD21 Is Localized in the Vacuoles Together with the Mature Protein
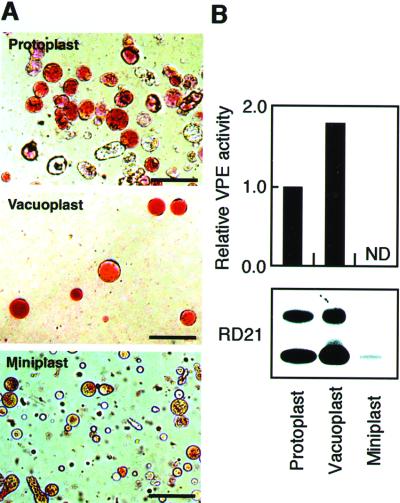
An immunoblot of the transformant BY2/RD21 showed that iRD21 and mRD21 are not secreted from the cells (Fig. 3A, lane 3). This raised the question of the subcellular localization of iRD21 and mRD21. To answer the question, we separated the protoplasts of the transformant BY2/RD21 cells into the vacuoplasts and the miniplasts. A vacuoplast is composed of a vacuole surrounded with a plasma membrane, whereas a miniplast is composed of a protoplast lacking vacuoles. The protoplasts, the vacuoplasts, and the miniplasts were stained with neutral red and were inspected with a light field microscope (Fig. 4A). The vacuoplasts were uniformly stained with neutral red, whereas the miniplasts contained few acid components. To evaluate the fractionation in greater detail, we measured an activity of a vacuolar marker enzyme, VPE (Kinoshita et al., 1999). Figure 4B (top) shows that the marker enzyme activity was found in the vacuoplasts but not in the miniplasts. This indicated that there was little cross-contamination between the vacuoplasts and the miniplasts. An immunoblot with anti-RD21 antibodies showed that iRD21 and mRD21 were detected in the vacuoplasts but not in the miniplasts (Fig. 4B, bottom). These results indicate that iRD21 was localized in the vacuoles together with mRD21.
Figure 4.
iRD21 and mRD21 are localized in the vacuoles. A, Protoplasts from the transformant BY2/RD21 cells were separated into the vacuoplasts and the miniplasts. Each fraction was stained with neutral red. Bars = 50 μm. B, Each fraction derived from approximately 1 × 106 cells was used for measuring an activity of a vacuolar marker enzyme, VPE (top), and for immunoblot with anti-RD21 antibodies (bottom). ND, Not detected.
The Intermediate of RD21 Forms an Aggregate in the Vacuoles
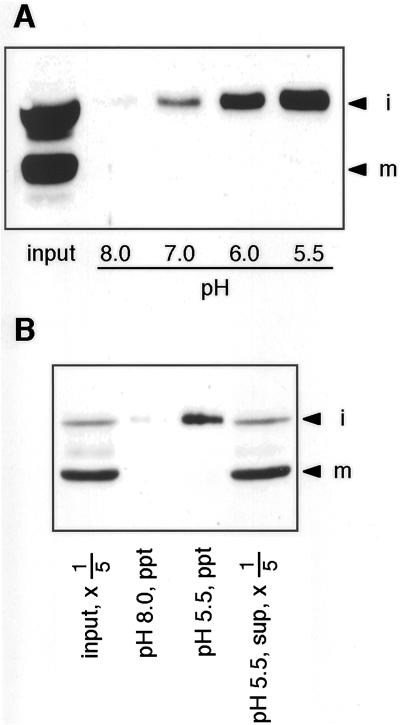
The next question is why iRD21 is not quickly converted into mRD21 in the vacuoles. To answer the question, we investigated the nature of iRD21 under the acidic conditions of the vacuolar interior. The extract from the leaves of 35-d-old Arabidopsis plants was incubated at pH 8.0, 7.0, 6.0, and 5.5. Figure 5A shows that iRD21 was recovered in the precipitate fraction at acidic pHs. By contrast, mRD21 was always soluble. This indicated that iRD21 formed a large aggregate in acidic solution. There was the possibility that iRD21 formed an artificial aggregate with cytosolic proteins. To exclude the possibility, we performed the same experiment with the vacuoplast proteins from the transformant BY2/RD21 cells. Figure 5B shows that iRD21 was also precipitated at the acidic pH, although mRD21 was recovered in the supernatant. These results suggested that most of iRD21 forms an aggregate in the vacuoles rather than the soluble form. The estimated pIs (4.65 of iRD21 and 4.68 of mRD21) revealed that the formation of the aggregate of iRD21 is not due to the isoelectric precipitation at the acidic pH. The aggregation of iRD21 is caused by the granulin domain because iRD21 is insoluble and mRD21 is soluble at the acidic pH.
Figure 5.
iRD21 forms an aggregate under the acidic conditions. A, The extract (20 μg of protein) from the leaves of 35-d-old Arabidopsis plants was incubated at pH 8.0, 7.0, 6.0, or 5.5. The formed insoluble aggregates were subjected to SDS-PAGE followed by immunoblot analysis with anti-RD21 antibodies. The extract (20 μg of protein) before incubation is also shown on the blot (input). B, The extract (100 μL) from the vacuoplasts of the transformant BY2/RD21 cells was incubated at pH 8.0 or 5.5. The formed insoluble aggregates (ppt) and 20 μL of supernatant (sup, × 1/5) were subjected to SDS-PAGE followed by immunoblot analysis with anti-RD21 antibodies. The extract (20 μL) before incubation is also shown on the blot (input, × 1/5). i, iRD21; m, mRD21.
Maturation of RD21 Is Mediated by a Soluble Protease(s) in Arabidopsis Leaves
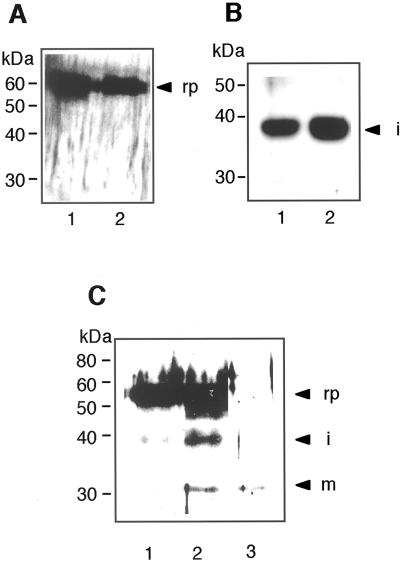
The above results raised the question of how the RD21 precursor was converted into the mature form. To answer it, we performed an in vitro processing experiment using the recombinant proRD21 that was expressed in insect cells. The determined N-terminal amino acid sequence of the recombinant proRD21 showed that the signal peptide was cleaved off. With the purified recombinant proRD21, we examined a possibility of a self-catalytic conversion of proRD21 into iRD21. Figure 6A shows that proRD21 was not self-catalytically converted into iRD21 after incubation at pH 8.0 or 5.5 for 16 h. We further examined the possibility of a self-catalytic conversion of iRD21 into mRD21 with the iRD21 that was accumulated in the 2-d-old young seedlings, which accumulated no mRD21 (Fig. 2B). Figure 6B shows that iRD21 was not self-catalytically converted into mRD21 after incubation at pH 8.0 or 5.5 for 16 h. These results suggested that some processing enzyme(s) are required for maturation of RD21.
Figure 6.
In vitro processing of RD21. A, The recombinant proRD21 was incubated alone at pH 8.0 (lane 1) or pH 5.5 (lane 2) for 16 h, and was then subjected to SDS-PAGE followed by immunoblot analysis with anti-RD21 antibodies. B, iRD21 was extracted from 2-d-old Arabidopsis seedlings. iRD21 was incubated at pH 8.0 (lane 1) or pH 5.5 (lane 2) for 16 h, and was then subjected to SDS-PAGE followed by immunoblot analysis. C, The extract from the Arabidopsis leaves was prepared and used as a processing enzyme source. The recombinant proRD21 was incubated with the leaf extract at pH 5.5 for 16 h, and was then subjected to SDS-PAGE followed by immunoblot analysis (lane 2). The recombinant proRD21 (lane 1) and the leaf extract used as an enzyme source (lane 3) are also shown on the blot. rp, Recombinant proRD21; i, iRD21; m, mRD21. The molecular mass of each marker protein is given on the left in kilodaltons.
In the next step, we performed an in vitro processing experiment with a crude processing enzyme. The extract from the Arabidopsis leaves was prepared and used as a processing enzyme source. Figure 6C shows that the conversion of the recombinant proRD21 into iRD21 was observed after incubation at pH 5.5 for 16 h (lane 2). The blot (lane 3) shows no detectable iRD21 in the same amount of the crude enzyme used for the experiment. The result indicates that proRD21 was converted into iRD21 by the action of a processing enzyme(s) in the leaf extract. Previously, we reported αVPE and γVPE increased in the level during leaf senescence (Kinoshita et al., 1999). However, the Arabidopsis αvpe, γvpe double mutant accumulated iRD21 and mRD21 in the leaves (data not shown). Thus, VPEs might not be involved in the maturation of RD21.
DISCUSSION
Our results show that proRD21 is converted into the mature form by the sequential removal of the NTPP and the CTPP. RD21 has the catalytic triad that was conserved among members of papain family (Kamphuis et al., 1985). The NTPP might function as an autoinhibitory domain that masks the catalytic site, as occurs with cathepsin B and cathepsin S (Turk et al., 1996; Maubach et al., 1997). Thus, the removal of the NTPP of proRD21 might expose the catalytic site of the protease. However, the resultant iRD21 forms an aggregate in the vacuoles that may prevent protease activity. Further removal of the CTPP from iRD21 is required to produce soluble mRD21 in the vacuoles.
The pulse-chase experiment with the transformant BY2/RD21 indicated that the removal of the CTPP from iRD21 occurs very slowly. This is also supported by the observation that a large amount of iRD21 was accumulated in Arabidopsis leaves. The slow conversion of iRD21 into mRD21 might be due to the formation of an aggregate of iRD21. A processing enzyme could not easily access to the iRD21 molecules to convert them into mRD21. iRD21 with the CTPP (the granulin domain) is insoluble, whereas mRD21 lacking the domain is soluble. Therefore, the granulin domain mediates formation of an aggregate to prevent the efficient maturation of iRD21.
The granulin-mediated aggregation of iRD21 might involve a novel system for a regulated protease activation. A granulin domain is also found in two papain family proteases, rice oryzain α and maize CPPIC. Oryzain α with a protease activity is isolated as a 23.5-kD protein lacking a granulin domain (Watanabe et al., 1991). Maize CPPIC with no protease activity is isolated as a 40-kD protein having a granulin domain (Yamada et al., 1998). The 40-kD CPPIC has been shown to be activated by an addition of SDS (Yamada et al., 2001). It is possible that the inactive CPPIC forms an aggregate, which can be solubilized and activated by the action of SDS. These results imply that the aggregate of iRD21 is inactive and the soluble mRD21 is active in the vacuoles. It is possible that the iRD21 aggregate functions as a stock of active protease molecules to prepare cell death during leaf senescence and to prepare cell death of young seedlings by environmental stresses and wound.
The animal granulins are secreted from cells and act as growth factors. It remains to be elucidated whether plant granulins are also growth factors. However, we could not detect the granulin molecule derived from the RD21 precursors in Arabidopsis leaves. Thus, the level of the granulin might be very low if it is in the tissues.
The seedlings of Arabidopsis accumulated much more of iRD21 than mRD21. Instead, mRD21 was extensively accumulated in senescent leaves. These results suggest that RD21 has a role in degradation of cellular proteins during leaf senescence, rather than in the degradation of seed storage proteins after seed germination. Schmid et al. (1999) reported that a papain family protease (Cys-EP) plays a role in the degradation of cellular materials during cell death of the endosperm of castor bean (Ricinus communis), but not in the degradation of storage proteins. Cys-EP is accumulated as a proprotein precursor (pro-Cys-EP) in a membrane-bound organelle, ricinosome, but not in the vacuoles. Pro-Cys-EP is self-catalytically converted into the mature active enzyme at acidic pH (Schmid et al., 2001). In contrast to RD21, the proenzyme has no granulin domain at the C terminus. The maturation mechanism of the castor bean enzyme is different from that of Arabidopsis RD21. The other two papain family proteases with granulin domain that might be related to cell death were registered in the database, S. aurantiaca PRT15 (J.R. Eason and T.T. Bucknell, unpublished data) and Arabidopsis XBCP3 (E.P. Beers and C. Zhao, unpublished data).
Recently, we reported that the RD21 precursor is specifically accumulated in approximately 0.5-μm diameter × approximately 5-μm long bodies in the epidermal cells of healthy Arabidopsis seedlings (Hayashi et al., 2001). We designated them the endoplasmic reticulum (ER) bodies because they are directly derived from the ER. When the seedlings are stressed with a concentrated salt solution, leading to death of the epidermal cells, the ER bodies start to fuse with each other and with the vacuoles, thereby mediating the delivery of the precursor directly to the vacuoles. In the vacuoles, the inactive enzyme should be converted to the active one. Thus, the ER bodies appear to be a novel proteinase-storing system that assists in cell death under the stressed conditions.
MATERIALS AND METHODS
Growth Conditions of Arabidopsis Plants
Arabidopsis (ecotype Columbia) was grown essentially as described previously by Kinoshita et al. (1999), except that the culture medium contained Murashige and Skoog medium, 2.5 mm MES [2-(N-morpholino)ethanesulfonic acid]-KOH (pH 5.7), and no Suc, vitamins, or myoinositol. After a 4-d incubation at 4°C to break seed dormancy, the seeds were germinated and grown at 22°C under continuous light (100 μE s−1 m−2). Seedlings and the first and second rosette leaves from the plants were used for the experiments.
Preparation of Specific Antiserum
An expressed sequence tag clone encoding RD21 (103I15T7) was obtained from the Arabidopsis Biological Resource Center. A cDNA encoding the proRD21 domain or the granulin domain was inserted into the pET32 vector (Novagen, Madison, WI). The fusion proteins with a His-tag were synthesized in Escherichia coli BL21(DE3) cells and were purified with a Ni2+ column. Rabbits were immunized with each fusion protein as described previously (Inoue et al., 1995). The antibodies exhibited a high specificity against the RD21-related proteins (Fig. 1) and were of sufficiently high titer to detect 1 ng of the RD21-related proteins in the crude extract (data not shown).
Immunoblot Analysis
The seedlings and leaves of Arabidopsis and BY2 cells were homogenized on ice in 10 mm Tris-HCl (pH 8.0) and 0.1% (w/v) SDS before centrifugation to obtain soluble total proteins. The proteins were subjected to SDS-PAGE and were transferred electrophoretically to a nylon membrane (0.22 μm, Millipore Japan, Tokyo). The membrane blot was incubated with anti-RD21 antibodies (diluted 1,000-fold) or anti-granulin antibodies (diluted 500-fold) in a solution of 50 mm Tris-HCl (pH 7.5), 0.15 m NaCl, 0.05% (v/v) Tween 20, and 3% (w/v) skim milk. Alkaline phosphatase-conjugated goat antibodies against rabbit IgG (diluted 2,000-fold; Cappel, West Chester, PA) or horseradish peroxidase-conjugated goat antibodies against rabbit IgG (diluted 2,000-fold; Amersham Pharmacia Biotech Japan, Tokyo) were used as secondary antibodies.
Transformation of BY2 Cells
The cDNA encoding preproRD21 was inserted into a T-DNA binary vector pBI121HmRV as described previously (Kinoshita et al., 1999). The plasmid vector was introduced into Agrobacterium tumefaciens (strain EHA101) by the electroporation method. Tobacco (Nicotiana tabacum) BY2 cells were transformed with the gene via A. tumefaciens according to the method of Matsuoka and Nakamura (1991) to produce a transformant BY2/RD21 cells.
Preparation of Vacuoplast and Miniplast from BY2 Cells
The vacuoplasts and the miniplasts were isolated from protoplasts of the transformant BY2/RD21 cells essentially as described by Sonobe (1990). To obtain protoplasts, the BY2/RD21 cells (40 g) at 6 d after inoculation were incubated with 30 mL of solution of 1% (w/v) cellulase Onozuka-RS (Yakult, Tokyo), 0.1% (w/v) pectolyase Y-23 (Seishin, Tokyo), 25 mm Tris-MES (pH 5.5), and 0.45 m mannitol for 2 h at 30°C. The resultant protoplasts were collected by centrifugation at 700g for 10 min and were resuspended in 30 mL of 30% (v/v) Percoll (Amersham Pharmacia Biotech Japan), 25 mm Tris-MES (pH 7.0), 20 mm MgCl2, and 0.45 m mannitol. Two milliliters of 25 mm Tris-MES (pH 5.5) and 0.45 m mannitol was added to the top of the Percoll suspension before it was centrifuged at 10,000g for 1 h using a swing rotor (SW28.1, Beckman Instruments, Palo Alto, CA). The precipitate was composed of miniplasts—the protoplasts with no vacuoles. The upper fraction included vacuoplasts—the vacuoles surrounded by plasma membranes.
In Vivo Labeling and Immunoprecipitation
The transformant BY2/RD21 cells at 4 d after inoculation were harvested by centrifugation at 800g for 1 min and were resuspended in the culture medium (3-fold volume of the packed cell volume). The cell suspension (3 mL) was pulse-labeled for 1 h with 240 μCi [35S] Met and Cys (Pro-mix l-[35S] in vitro cell labeling mix, Amersham Pharmacia Biotech Japan). To chase the label, unlabeled l-Met and l-Cys were added to the suspension at a final concentration of 2 mm as described by Matsuoka et al. (1990). After a chase period of 0, 4, or 12 h, each 1-mL suspension was centrifuged to collect the labeled cells. After washing with 10 mm Tris-HCl (pH 7.5), the cells were homogenized with 200 μL of 10 mm Tris-HCl (pH 7.5) and were centrifuged to obtain the extracts. To 100 μL of total extract, 13 μL of 10% (w/v) SDS was added and boiled for 5 min. To the denatured sample solution, 13 μL of 20% (v/v) Triton X-100, 869 μL of Tris-buffered saline, and 5 μL of anti-RD21 antibodies were added and incubated at 4°C for 16 h. Protein A Sepharose CL-4B (Amersham Pharmacia Biotech Japan) was added to the sample solution and incubated at room temperature for 3 h. The antigen-antibody complex trapped by protein A Sepharose was washed and subjected to SDS-PAGE. The labeled RD21-related proteins were detected with a BAS3000 system (Fuji, Tokyo).
Expression of ProRD21 in Insect Cells
We used the baculovirus expression system (BD PharMingen, San Diego) that includes a host cell of Sf21 derived from Spodoptera frugiperda, a transfer vector (pAcGP67), and an expression vector (BaculoGold linearized baculovirus DNA). pAcGP67 has a nucleotide sequence encoding an N-terminal signal sequence for secretion (Stewart et al., 1991). A cDNA encoding a His-tag followed by proRD21 was introduced into pAcGP67. Isolation and amplification of the recombinant virus and expression of the recombinant proRD21 in the cells were done according to the manufacturer's directions. The secreted proRD21 was purified with a Ni2+ resin column and was dissolved in 50 mm Tris-HCl (pH 8.0) to use for the in vitro processing experiment.
In Vitro Processing of RD21
The leaves of 35-d-old Arabidopsis plants were homogenized in 143 mm sodium acetate (pH 5.5), and the leaf extract was used as a processing enzyme source. The recombinant proRD21 (10 ng) was incubated at room temperature for 16 h with or without the leaf extract (0.5 μg of protein) and was then subjected to SDS-PAGE followed by immunoblot with anti-RD21 antibodies.
Acid Precipitation of the Intermediate of RD21
The leaves of 35-d-old Arabidopsis plants were homogenized in 10 mm Tris-HCl (pH 8.0) and centrifuged at 100,000g for 1 h. Each 20 μL (20 μg of protein) of the supernatant was incubated in 80 μL of 100 mm Tris-HCl (pH 8.0 or 7.0) or 100 mm sodium acetate (pH 6.0 or 5.5). The insoluble aggregates that formed were collected by centrifugation at 15,000g for 10 min and were then subjected to SDS-PAGE followed by immunoblot with anti-RD21 antibodies.
Alternatively, the vacuoplasts from the transformant BY2/RD21 cells were homogenized and centrifuged as described above. To each 100 μL of the supernatant, 10 μL of 1 m Tris-HCl (pH 8.0) or 1 m sodium acetate (pH 5.5) was added. The formed insoluble aggregates were subjected to immunoblot as described above.
ACKNOWLEDGMENTS
We are grateful to Mamiko Ohtake (National Institute for Basic Biology) for growing Arabidopsis plants, to Miwa Kuroyanagi (National Institute for Basic Biology) for her skillful technique for baculovirus manipulation, and to Yumiko Makino (National Institute for Basic Biology) for helpful support with peptide sequencing.
Footnotes
This work was supported by Grants-in-Aid for “Research for the Future Program” from the Japan Society for the Promotion of Science (grant no. JSPS–RFTF96L60407), for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (nos. 10182102, 12138205, and 12304049), and by a postdoctoral fellowship from the Japan Society for the Promotion of Science (to K.Y.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010551.
LITERATURE CITED
- Ahmed SU, Rojo E, Kovalentina V, Venkataraman S, Dombrowski JE, Matsuoka K, Raikhel NV. The plant vacuolar sorting receptor AtELP is involved in transport of NH2-terminal propeptide-containing vacuolar proteins in Arabidopsis thaliana. J Cell Biol. 2000;149:1335–1344. doi: 10.1083/jcb.149.7.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman A, Bennett HPJ. Granulins: the structure and function of an emerging family of growth factors. J Endocrinol. 1998;158:145–151. doi: 10.1677/joe.0.1580145. [DOI] [PubMed] [Google Scholar]
- Bhandari V, Palfree RGE, Bateman A. Isolation and sequence of the granulin precursor cDNA from human bone marrow reveals tandem cysteine-rich granulin domains. Proc Natl Acad Sci USA. 1992;89:1715–1719. doi: 10.1073/pnas.89.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromme D, Bonneau PR, Lachance P, Wiederanders B, Kirschke H, Peters C, Thomas DY, Storer AC, Vernet T. Functional expression of human cathepsin S in Saccharomyces cerevisiae: purification and characterization of the recombinant enzyme. J Biol Chem. 1993;268:4832–4838. [PubMed] [Google Scholar]
- Chrispeels MJ, Raikhel N. Short peptide domains target proteins to plant vacuoles. Cell. 1992;68:613–616. doi: 10.1016/0092-8674(92)90134-x. [DOI] [PubMed] [Google Scholar]
- Gietl C, Schmid M, Simpson D. Ricinosomes and aleurain-containing vacuoles (ACVs): protease-storing organelles. In: Robinson DG, Rogers JC, editors. Vacuolar Compartments: Annual Plant Reviews. Vol. 5. Sheffield, UK: Sheffield Academic Press; 2000. pp. 90–111. [Google Scholar]
- Hara-Nishimura I. Asparaginyl endopeptidase. In: Barrett AJ, Rawlings ND, Woessner JF, editors. Handbook of Proteolytic Enzymes. London: Academic Press; 1998. pp. 746–749. [Google Scholar]
- Hara-Nishimura I, Takeuchi Y, Inoue K, Nishimura M. Vesicle transport and processing of the precursor to 2S albumin in pumpkin. Plant J. 1993a;4:793–800. doi: 10.1046/j.1365-313x.1993.04050793.x. [DOI] [PubMed] [Google Scholar]
- Hara-Nishimura I, Takeuchi Y, Nishimura M. Molecular characterization of a vacuolar processing enzyme related to a putative cysteine proteinase of Schistosoma mansoni. Plant Cell. 1993b;5:1651–1659. doi: 10.1105/tpc.5.11.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Yamada K, Shimada T, Matsushima R, Nishizawa N, Nishimura M, Hara-Nishimura I. A proteinase-storing body that prepares for cell death or stress in the epidermal cells of Arabidopsis. Plant Cell Physiol. 2001;42:894–899. doi: 10.1093/pcp/pce144. [DOI] [PubMed] [Google Scholar]
- Hrabal R, Chen Z, James S, Bennett HP, Ni F. The hairpin stack fold, a novel protein architecture for a new family of protein growth factors. Nat Struct Biol. 1996;3:747–752. doi: 10.1038/nsb0996-747. [DOI] [PubMed] [Google Scholar]
- Humair D, Felipe DH, Neuhaus JM, Paris N. Demonstration in yeast of the function of BP-80, a putative plant vacuolar sorting receptor. Plant Cell. 2001;13:781–792. doi: 10.1105/tpc.13.4.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Motozaki A, Takeuchi Y, Nishimura M, Hara-Nishimura I. Molecular characterization of proteins in protein-body membrane that disappear most rapidly during transformation of protein bodies into vacuoles. Plant J. 1995;7:235–243. doi: 10.1046/j.1365-313x.1995.7020235.x. [DOI] [PubMed] [Google Scholar]
- Kamphuis IG, Drenth J, Barker EN. Thiol proteases: comparative studies based on the high-resolution structures of papain and actinidin, and on amino acid sequence information for cathepsins B and H, and stem bromelain. J Mol Biol. 1985;182:317–329. doi: 10.1016/0022-2836(85)90348-1. [DOI] [PubMed] [Google Scholar]
- Karrer KM, Peiffer SL, DiTomas ME. Two distinct gene subfamilies within the family of cysteine protease genes. Proc Natl Acad Sci USA. 1993;90:3063–3067. doi: 10.1073/pnas.90.7.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Nishimura M, Hara-Nishimura I. Homologues of a vacuolar processing enzyme that are expressed in different organs in Arabidopsis thaliana. Plant Mol Biol. 1995a;29:81–89. doi: 10.1007/BF00019120. [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Nishimura M, Hara-Nishimura I. The sequence and expression of the γ-VPE gene, one member of a family of three genes for vacuolar processing enzymes in Arabidopsis thaliana. Plant Cell Physiol. 1995b;36:1555–1562. [PubMed] [Google Scholar]
- Kinoshita T, Yamada K, Hiraiwa N, Nishimura M, Hara-Nishimura I. Vacuolar processing enzyme is up-regulated in the lytic vacuoles of vegetative tissues during senescence and under various stressed conditions. Plant J. 1999;19:43–53. doi: 10.1046/j.1365-313x.1999.00497.x. [DOI] [PubMed] [Google Scholar]
- Koizumi M, Yamaguchi-Shinozaki K, Tsuji H, Shinozaki K. Structure and expression of two genes that encode distinct drought-inducible cysteine proteinases in Arabidopsis thaliana. Gene. 1993;129:175–182. doi: 10.1016/0378-1119(93)90266-6. [DOI] [PubMed] [Google Scholar]
- Mach L, Schwihla H, Stuwe K, Rowan AD, Mort JS, Glossl J. Activation of procathepsin B in human hepatoma cells: the conversion into the mature enzyme relies on the action of cathepsin B itself. Biochem J. 1993;293:437–442. doi: 10.1042/bj2930437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matile P. Cell Biology Monographs. Vol. 1. Wien, Germany: Springer-Verlag; 1975. The lytic compartment of plant cells. [Google Scholar]
- Matsuoka K, Matsumoto S, Hattori T, Machida Y, Nakamura K. Vacuolar targeting and post-translational processing of the precursor to the sweet potato tuberous root storage protein in heterologous plant cells. J Biol Chem. 1990;265:19750–19757. [PubMed] [Google Scholar]
- Matsuoka K, Nakamura K. Propeptide of a precursor to a plant vacuolar protein required for vacuolar targeting. Proc Natl Acad Sci USA. 1991;88:834–838. doi: 10.1073/pnas.88.3.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka K, Nakamura K. Large alkyl side-chains of isoleucine and leucine in the NPIRL region constitute the core of the vacuolar sorting determinant of sporamin precursor. Plant Mol Biol. 1999;41:825–835. doi: 10.1023/a:1006357413084. [DOI] [PubMed] [Google Scholar]
- Maubach G, Schilling K, Rommerskirch W, Wenz I, Schultz JE, Weber E, Wiederanders B. The inhibition of cathepsin S by its propeptide-specificity and mechanism of action. Eur J Biochem. 1997;250:745–750. doi: 10.1111/j.1432-1033.1997.00745.x. [DOI] [PubMed] [Google Scholar]
- Murakami S, Hattori T, Nakamura K. Structural differences in full-length cDNAs for two classes of sporamin, the major soluble protein of sweet potato tuberous roots. Plant Mol Biol. 1986;7:343–355. doi: 10.1007/BF00032564. [DOI] [PubMed] [Google Scholar]
- Nelson CA. The binding of detergents to proteins: I. The maximum amount of dodecyl sulfate bound to proteins and the resistance to binding of several proteins. J Biol Chem. 1971;246:3895–3901. [PubMed] [Google Scholar]
- Okamoto T, Minamikawa T. Purification of a processing enzyme (VmPE-1) that is involved in post-translational processing of a plant cysteine endopeptidase (SH-EP) Eur J Biochem. 1995;231:300–305. doi: 10.1111/j.1432-1033.1995.tb20700.x. [DOI] [PubMed] [Google Scholar]
- Paris N, Rogers AW, Jiang L, Kirsch T, Beevers L, Phillips TE, Rogers JC. Molecular cloning and further characterization of a probable plant vacuolar sorting receptor. Plant Physiol. 1997;115:29–39. doi: 10.1104/pp.115.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JC, Dean D, Heck GR. Aleurain: a barley thiol protease closely related to mammalian cathepsin H. Proc Natl Acad Sci USA. 1985;82:6512–6516. doi: 10.1073/pnas.82.19.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M, Simpson D, Gietl C. Programmed cell death in castor bean endosperm is associated with the accumulation and release of a cysteine endopeptidase from ricinosomes. Proc Natl Acad Sci USA. 1999;96:14159–14164. doi: 10.1073/pnas.96.24.14159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M, Simpson D, Kalousek F, Gietl C. A cysteine endopeptidase with a C-terminal KDEL motif isolated from castor bean endosperm is a marker enzyme for the ricinosome, a putative lytic compartment. Planta. 1998;206:466–475. doi: 10.1007/s004250050423. [DOI] [PubMed] [Google Scholar]
- Schmid M, Simpson DJ, Sarioglu H, Lottspeich F, Gietl C. The ricinosomes of senescing plant tissue bud from the endoplasmic reticulum. Proc Natl Acad Sci USA. 2001;98:5353–5358. doi: 10.1073/pnas.061038298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Kuroyanagi M, Nishimura M, Hara-Nishimura I. A pumpkin 72-kDa membrane protein of precursor accumulating vesicles has characteristics of a vacuolar sorting receptor. Plant Cell Physiol. 1997;38:1414–1420. doi: 10.1093/oxfordjournals.pcp.a029138. [DOI] [PubMed] [Google Scholar]
- Sonobe S. Cytochalasin B enhances cytokinetic cleavage in miniprotoplasts isolated from cultured tobacco cells. Protoplasma. 1990;155:239–242. [Google Scholar]
- Stewart L, Hirst M, Ferber ML, Merryweather A, Cayley PJ, Possee R. Construction of an improved baculovirus insecticide containing an insect-specific toxin gene. Nature. 1991;352:85–88. doi: 10.1038/352085a0. [DOI] [PubMed] [Google Scholar]
- Turk D, Podobnik M, Kuhelj R, Dolinar M, Turk V. Crystal structures of human procathepsin B at 3.2 and 3.3 Angstroms resolution reveal an interaction motif between a papain-like cysteine protease and its propeptide. FEBS Lett. 1996;384:211–214. doi: 10.1016/0014-5793(96)00309-2. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Abe K, Emori Y, Hosoyama H, Arai S. Molecular cloning and gibberellin-induced expression of multiple cysteine proteinases of rice seeds (oryzains) J Biol Chem. 1991;266:16897–16902. [PubMed] [Google Scholar]
- Weaver LM, Gan S, Quirino B, Amasino RM. A comparison of the expression patterns of several senescence-associated genes in response to stress and hormone treatment. Plant Mol Biol. 1998;37:455–469. doi: 10.1023/a:1005934428906. [DOI] [PubMed] [Google Scholar]
- Yamada K, Shimada T, Kondo M, Nishimura M, Hara-Nishimura I. Multiple functional proteins are produced by cleaving Asn-Gln bonds of a single precursor by vacuolar processing enzyme. J Biol Chem. 1999;274:2563–2570. doi: 10.1074/jbc.274.4.2563. [DOI] [PubMed] [Google Scholar]
- Yamada T, Kondo A, Ohta H, Masuda T, Shimada H, Takamiya K. Isolation of the protease component of maize cysteine protease-cystatin complex: release of cystatin is not crucial for the activation of the cysteine protease. Plant Cell Physiol. 2001;42:710–716. doi: 10.1093/pcp/pce089. [DOI] [PubMed] [Google Scholar]
- Yamada T, Ohta H, Masuda T, Ikeda M, Tomita N, Ozawa A, Shioi Y, Takamiya K. Purification of a novel type of SDS-dependent protease in maize using a monoclonal antibody. Plant Cell Physiol. 1998;39:106–114. doi: 10.1093/oxfordjournals.pcp.a029281. [DOI] [PubMed] [Google Scholar]
- Yamada T, Ohta H, Shinohara A, Iwamatsu A, Shimada H, Tsuchiya T, Masuda T, Takamiya K. A cysteine protease from maize isolated in a complex with cystatin. Plant Cell Physiol. 2000;41:185–191. doi: 10.1093/pcp/41.2.185. [DOI] [PubMed] [Google Scholar]