Abstract
Objective:
In this retrospective review, we evaluated the advantages and disadvantages of LADG for patients of heavier weight with early gastric cancer.
Summary Background Data:
LADG has been used to treat early gastric cancer. We and others have reported less operative blood loss, less pain, early recovery of bowel activity, early restart of oral intake, and a shorter hospital stay with LADG compared with a conventional open method. There is, however, little information on the advantages of LADG for obese patients with early gastric cancer.
Methods:
Between January 1996 and March 2002, 76 patients with preoperatively diagnosed early gastric carcinoma underwent LADG in our department. We classified these patients into 2 groups on the basis of body mass index (BMI). Nineteen patients had a high-BMI (≥ 24.2 kg/m2), and 57 patients had a normal-BMI (<24.2 kg/m2). We collected data by retrospectively reviewing the medical charts.
Results:
Extension of the minilaparotomic incision or conversion to laparotomy was needed in 6 (32%) of the 19 patients in the high-BMI group, whereas only 3 (5%) of 57 patients in the normal-BMI group required either. In the high-BMI group, Roux-en-Y anastomosis rather than Billroth I anastomosis was adopted more often than in the normal-BMI group, due to the difficulty of the reconstruction (58% versus 4%, P = 0.001). Significantly longer operative time (370 ± 61 minutes versus 317 ± 58 minutes, P = 0.015) and prolonged recovery of bowel activity (3.5 ± 1.0 days versus 2.6 ± 1.0 days, P = 0.007) were observed in the patients in the high-BMI group.
Conclusions:
In the current study, LADG in patients of heavier weight was accompanied by more technical difficulties, and the disadvantages of longer operative time and delayed recovery of bowel activity was observed in patients of heavier weight. Heavier weight appears to be an ominous factor in the successful completion of LADG and should be considered in the decision to use LADG. There are still benefits of a decreased incidence of serious wound and hernia complications in successful cases.
To clarify the influence of obesity on laparoscopy-assisted distal gastrectomy (LADG) for early gastric cancer, 76 patients were classified into 2 groups on the basis of body mass index (BMI). More technical difficulties, longer operative time, and delayed recovery of bowel activity were observed in patients of heavier weight who underwent LADG.
Gastric cancer is one of the most common neoplasms in Japan. Favorable surgical results are attributable to early diagnosis and standardization of surgical procedures.1–7 Since the benefits of laparoscopic cholecystectomy were first reported,8,9 laparoscopic surgical techniques have been adopted for the treatment of cancers of several organs, primarily because laparoscopic surgery is less invasive than the conventional open surgical method. Laparoscopy-assisted distal gastrectomy (LADG) has been used to treat early gastric cancers not requiring extensive lymph node dissection. The advantages of this procedure, when compared with the conventional open method, include less operative blood loss, less pain, earlier recovery of bowel activity, earlier resumption of oral intake, and a shorter hospital stay.10–16 LADG can result in an adequate gastrectomy and achievement of appropriate lymph node dissection. However, operative time is longer, and the procedures are considered to be technically complicated when compared with the open method.
There is a high morbidity rate during and after surgery in obese patients.17 Although it is thought that the quick recovery after laparoscopic surgery would benefit obese patients, few data exist supporting this conclusion. In this study, we reviewed the outcomes of our patients of heavier weight with early gastric cancer after LADG.
PATIENTS AND METHODS
Patients
Between January 1996 and March 2002, 79 patients with preoperatively diagnosed, early gastric carcinoma underwent LADG in our institute. We excluded from the study 3 patients who had synchronous operations for colonic cancers. We performed LADG when the lesion was located in the antrum or the body of the stomach. Since the extent of lymph node dissection was limited in LADG, we excluded lesions that we preoperatively assumed to be infiltrating the deep submucosal layer and would require standard radical lymph node dissection (D2 as described in the general rules for the Gastric Study in Surgery and Pathology in Japan18).7 We did not consider histologic type or tumor size. Patients with mucosal lesions suitable for endoscopic mucosal resection were also excluded.19 We preoperatively assessed the depth of invasion using upper gastrointestinal radiography, endoscopy, and endoscopic ultrasonography.1,6
Patients were classified into 2 groups on the basis of their body mass index (BMI). Patients in the high-BMI group had a BMI ≥ 24.2kg/m2, and the patients in the normal group had a BMI < 24.2 kg/m2.
Surgical Procedures
We described our LADG procedure in our previous report.10 Briefly, the patient was placed in a supine position under general anesthesia. A subumbilical Hasson trocar was inserted via the open surgical method, and 4 trocars were introduced under laparoscopic guidance. Under pneumoperitoneum of 8–12 mm Hg, the greater omentum was divided using laparoscopic coagulating shears (Ultrashears; Tyco Health Care, Norwalk, CT). The left gastroepiploic vessels were dissected at the point before the first branch, and the right gastroepiploic vein and artery were divided individually at their root. The common hepatic and splenic arteries were prepared along the upper border of the pancreas by lifting the stomach. The right gastric vessels were divided in the hepatoduodenal ligament. The lesser omentum was opened, the lymph nodes around the celiac axis were dissected, and the left gastric vein and artery were divided individually by double-clipping (Fig. 1). The perigastric lymph nodes were dissected along the upper lesser curvature up to the esophagocardial junction. We performed dissection of the lymph nodes along the left gastric artery, common hepatic artery, and celiac axis in addition to D118 lymph node dissection. The trocar incision in the epigastrium was extended to 5 cm vertically, and the ring drape for laparoscopic surgery and a moving window (Omni Tract System; Manson, St. Paul, MN) were inserted. The stomach was resected through the window, and standard Billroth I anastomosis was chosen. When Billroth I was technically impossible, Roux-en-Y reconstruction was performed. Conversion to laparotomy was necessary when appropriate lymph node dissection could not be accomplished laparoscopically. A chief senior surgeon decided during surgery if conversion to laparotomy was needed. Following the laparoscopic procedures, if resection of the stomach or anastomosis through a 5-cm epigastric incision was difficult, the epigastric incision was lengthened to 7 cm or more.

FIGURE 1. Lymph node dissection around the celiac axis. Arrow shows the stump of the left gastric artery.
Postoperative Clinical Course
To alleviate postoperative pain, all patients received continuous epidural injection of local anesthetic (1% mepivacaine) of 3–4 mL/h for 48 hours after surgery.
Statistical Analysis
We obtained the following information from the medical charts: age, sex, concurrent illness, tumor characteristics, operative time, estimated blood loss, number of dissected lymph nodes, volume of anesthetic agents, time to first flatus, time to resumed oral intake, length of fever, white blood cell count, serum C-reactive protein level, postoperative complications, and length of hospital stay. These values were expressed as mean ± SD and compared between 2 groups of patients using either the Student t test or the χ2 test. Significance of the correlation of each set of values was determined by Spearman rank correlation.20 A two-sided P value < 0.05 was statistically significant. We performed these analyses using the StatView software program (Abacus Concepts, Berkeley, CA).
RESULTS
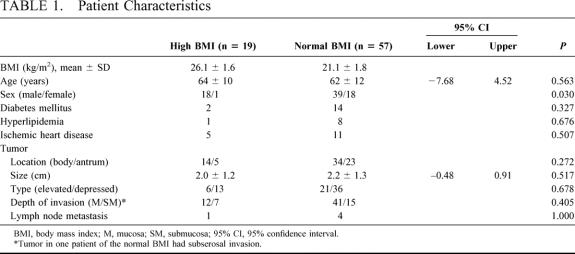
Between January 1996 and May 1999, LADG was performed on 29 (73%) of 40 patients with early gastric carcinoma for which LADG was indicated potentially. Between June 1999 and March 2002, 47 patients with early gastric carcinoma who were candidates were treated with the procedure. During the entire study period, we assigned 19 patients to the high-BMI group (BMI: 26.1 ± 1.6 kg/m2, 24.2–30.0 kg/m2) and 57 patients were to the normal-BMI group (BMI: 21.1 ± 1.8 kg/m2, 16.8–23.9 kg/m2). Age, concurrent illness, tumor size, tumor location, tumor type, and cancer staging were similar between both patient groups (Table 1). The male to female ratio was significantly higher in the high-BMI group than in the normal group.
TABLE 1. Patient Characteristics
The surgical outcome of all 76 patients is given in Table 2. The procedure was converted to laparotomy in 5 patients (6.6%). There was no significant difference in the number of conversions performed between the 2 BMI groups (2 patients [3.5%] in the normal-BMI group and 3 patients [15.8%] in the high-BMI group, P = 0.096). The reason for conversion to laparotomy in the normal-BMI group was uncontrollable bleeding. One patient experienced bleeding at the lymphadenectomy site around the celiac axis, and the other from the incidentally injured liver. The 3 patients of the high-BMI group in whom we converted LADG to laparotomy (BMI = 27.3, 26.8, and 25.6 kg/m2, respectively) had well-developed fat tissue involving the omentum, thereby preventing appropriate lymph node dissection. These 3 patients underwent surgery between June 1999 and March 2002, but not on the initial learning curve before May 1999.
TABLE 2. Surgical Outcome of All 76 Patients

Further extension of the epigastric incision to 7 cm was needed for anastomosis in 4 patients (3 in the high-BMI group and 1 in the normal-BMI group). Open conversion and extension of the incision were required more frequently in the high-BMI group than in the normal-BMI group (P = 0.006). Roux-en-Y reconstruction was performed in 2 patients (3.5%) in the normal-BMI group and 6 (36.8%) in the high-BMI group because of the difficulty of gastroduodenostomy (P = 0.001).
Pathologic examination revealed no cancer at the surgical margins in all patients, and there was no significant difference in the number of dissected lymph nodes between the 2 BMI groups. Lymph node metastasis was observed in 1 patient in the high-BMI group and in 4 patients in the normal-BMI group.
The comparison of patients who underwent successful LADG is presented in Table 3. The total volume of anesthetic agents used during surgery was similar between both BMI groups. In the incidence of major postoperative complications, there was no significant difference between the groups. Leakage at the site of gastroduodenostomy occurred in 3 patients, intraabdominal abscess in 5 patients, and postoperative bleeding requiring additional surgery occurred in 1 patient taking warfarin. There was no significant difference in estimated blood loss between both groups.
TABLE 3. Successful LADG: Surgical Outcome and Postoperative Course (n = 67)
Operative time was significantly longer in the high-BMI group than in the normal group (370 ± 61 minutes versus 317 ± 58 minutes, P = 0.015). Time to first flatus after surgery was significantly longer in the high-BMI patients than in the normal patients (3.5 ± 1.0 days versus 2.6 ± 1.0 days, P = 0.007). There was a significant correlation between time to first flatus and operative time (P = 0.038).
Patients who underwent LADG began walking the day after surgery. Oral intake was resumed on the fourth postoperative day in patients without major complications in both groups. There was no significant difference between the 2 groups in the duration of fever after surgery, white blood cell count, or the level of serum C-reactive protein of the first and seventh postoperative days. The length of postoperative hospital stay was not significantly different between the groups either.
Neither recurrence nor cancer death occurred in any patients who underwent LADG during a follow-up period of 1–75 months (median = 24 months, mean = 29 months). After LADG, no patients suffered from either ileus or incisional hernia.
DISCUSSION
Previous studies have indicated that there are many advantages to LADG for early gastric cancer patients compared with a conventional open method.10,13 Surgeons consider obesity a contraindication for laparoscopic surgery because of difficulty entering the abdominal cavity and limited visualization of surgical fields. There have been few studies investigating the feasibility and safety of laparoscopic surgery for cancer in obese patients. It was reported that laparoscopic surgery in obese women with endometrial cancer raised the risk of conversion to laparotomy. However, the incidence of operative and postoperative complications, amount of operative blood loss, operative time, and the length of hospital stay were similar between obese and thin patients who underwent laparoscopic surgery.21,22
We defined a body mass index ≧24.2 as the high-BMI group, which is smaller compared with the western criteria of obesity.23,24 The high-BMI group in the present study should be defined as heavier weight rather than obesity. We experienced difficulty performing LADG in patients in the high-BMI group because of insufficient visualization of the abdominal cavity by the well-developed omentum. Pneumoperitoneum in the upper abdomen of male patients of heavier weight is limited, compared with that in the pelvic cavity of multiparous female patients.21,22 The present results may be attributable to the male-to-female ratio, which was significantly higher in the high-BMI group. Higher insufflation pressure (18–20 mm Hg)21 or the extreme rotation of the operative table may improve visualization of the abdominal cavity. To reduce the incidence of extension of the incision for the reconstruction, a new device for laparoscopic surgery needs to be evolved.
Recovery of bowel activity in the high-BMI group was significantly delayed compared with the time in the normal-BMI group. Time to first flatus is an objective and reliable indicator for predicting the time to resumption of oral intake. The reason for the delayed recovery of bowel activity in patients of heavier weight is unclear in this study. It is unknown whether obese people generally experience impaired bowel movement.25 On the basis of our study, we conclude that diabetes mellitus, which influences autonomic nerve function,26,27 does not account for the delay because the incidence of diabetes mellitus was not significantly different between the 2 BMI groups. We also ruled out lasting constipating effects of anesthetic agents as a cause for the delay because the total volumes of inspiratory and narcotic agents administered during and after surgery in the 2 groups were not significantly different.
We observed a significant correlation between operative time and time to first flatus in this study. We speculate that operative stress on the intestine, which could not be estimated in the present study, may have been more intense in patients of heavier weight due to longer surgical time. However, direct manipulation of the intestine was minimal during LADG. Roux-en-Y reconstruction was required more often after LADG in patients of heavier weight than in normal patients, contributing to a longer operative time. We suggest that the Roux-en-Y reconstruction may also have affected the time to first flatus. The ease with which a Billroth I reconstruction is done for gastric cancer is influenced by anatomy that patients present rather than the lesions for which a laparoscopy-assisted operation is adapted. As our experience of LADG, it may be the same reason that Roux-en-Y procedure would be the more typical experience for a non-Japanese surgeon doing gastrectomy.
Despite the disadvantage of the delayed recovery of bowel activity in the short-term period after surgery in patients of heavier weight, we have not observed any patients suffering from ileus or incisional hernia after LADG. We speculate that minimal manipulation of the intestine and the small incision required for LADG may prevent adhesion of the intestine and incisional hernia.
We could not estimate pain control after surgery because of the standardized postoperative care using continuous epidural injection of local anesthetics in our department. However, this kind of postoperative pain control shortened the time to first flatus (mean 2.6 days in patients with normal BMI) when compared with another report.13 Considering the small incision needed for laparoscopic surgery, obese patients who underwent LADG may have had less postoperative pain as well as less scarring.
The length of hospital stay could not be estimated correctly because of health care practices in Japan. In contrast to the new emphasis on early patient discharge in western countries, the length of hospital stay in Japan is still determined by agreement between the patient and the attending physician. Hospitalization in Japan is not as expensive as in the West, and Japanese medical insurance is structured quite differently from that in western countries. These factors make it difficult to accurately compare between Japan and western countries the time to resumption of full activity and work following laparoscopic surgery, as well as the length of hospital stay.
The feasibility and safety of LADG for patients of heavier weight have not been thoroughly evaluated in the present study because we did not compare patients of heavier weight who underwent LADG with such patients who had a conventional open method. A randomized control study with a longer follow-up period is needed to assess whether laparoscopic surgery is superior to laparotomy for treating obese patients with early gastric cancer. This retrospective study is preliminary but informative, suggesting that LADG in patients of heavier weight is accompanied by more surgical difficulties compared with patients with a normal BMI. In addition, when compared with patients with a healthy body weight, patients of heavier weight experienced longer operative times and delayed recovery of bowel activity, which may lead to delayed resumption of oral intake and return to full activity and work and a prolonged hospital stay. When physicians consider LADG for patients of heavier weight with early gastric cancer, BMI should be taken into account. There are still benefits of a decreased incidence of serious wound and hernia complications in successful cases.
Footnotes
Reprints: Hirokazu Noshiro, Department of Surgery and Oncology, Graduate School of Medical Sciences, Kyushu University, 3-1-1 Maidashi, Higashi-ku, Fukuoka 812–8582, Japan. E-mail: noshiros@surg1.med.kyushu-u.ac.jp.
REFERENCES
- 1.Akahoshi K, Chijiiwa K, Sasaki I, et al. Pre-operative TN staging of gastric cancer using a 15 Mhz ultrasound miniprobe. Br J Radiol. 1997;70:703–707. [DOI] [PubMed] [Google Scholar]
- 2.Fukutomi H, Sakita T. Analysis of early gastric cancer cases collected from major hospitals and institutes in Japan. Jpn J Clin Oncol. 1984;14:169–179. [PubMed] [Google Scholar]
- 3.Iriyama K, Asakawa T, Koike H, et al. Is extensive lymphadenectomy necessary for surgical treatment of intramucosal carcinoma of the stomach? Arch Surg. 1989;124:309–311. [DOI] [PubMed] [Google Scholar]
- 4.Kitaoka H, Yoshikawa K, Hirota T, et al. Surgical treatment of early gastric cancer. Jpn J Clin Oncol. 1984;14:283–293. [PubMed] [Google Scholar]
- 5.Korenaga D, Haraguchi M, Tsujitani S, et al. Clinicopathological features of mucosal carcinoma of the stomach with lymph node metastasis in eleven patients. Br J Surg. 1986;73:431–433. [DOI] [PubMed] [Google Scholar]
- 6.Yanai H, Matsumoto Y, Harada T, et al. Endoscopic ultrasonography and endoscopy for staging depth of invasion in early gastric cancer: a pilot study. Gastrointest Endosc. 1997;46:212–216. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura K, Morisaki T, Sugitani A, et al. An early gastric carcinoma treatment strategy based on analysis of lymph node metastasis. Cancer. 1999;85:1500–1505. [PubMed] [Google Scholar]
- 8.McIntyre RC, Zoeter MA, Weil KC, et al. A comparison of outcome and cost of open vs. laparoscopic cholecystectomy. J Laparoendosc Surg. 1992;2:143–148. [DOI] [PubMed] [Google Scholar]
- 9.Peters JH, Ellison EC, Innes JT, et al. Safety and efficacy of laparoscopic cholecystectomy. A prospective analysis of 100 initial patients. Ann Surg. 1991;213:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimizu S, Uchiyama A, Mizumoto K, et al. Laparoscopically assisted distal gastrectomy for early gastric cancer: is it superior to open surgery? Surg Endosc. 2000;14:27–31. [DOI] [PubMed] [Google Scholar]
- 11.Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146–148. [PubMed] [Google Scholar]
- 12.Nagai Y, Tanimura H, Takifuji K, et al. Laparoscope-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1995;5:281–287. [PubMed] [Google Scholar]
- 13.Adachi Y, Shiraishi N, Shiromizu A, et al. Laparoscopy-assisted Billroth I gastrectomy compared with conventional open gastrectomy. Arch Surg. 2000;135:806–810. [DOI] [PubMed] [Google Scholar]
- 14.Kitano S, Shimoda K, Miyahara M, et al. Laparoscopic approaches in the management of patients with early gastric carcinomas. Surg Laparosc Endosc. 1995;5:359–362. [PubMed] [Google Scholar]
- 15.Watson DI, Devitt PG, Game PA. Laparoscopic Billroth II gastrectomy for early gastric cancer. Br J Surg. 1995;82:661–662. [DOI] [PubMed] [Google Scholar]
- 16.Adachi Y, Suematsu T, Shiraishi N, et al. Quality of life after laparoscopy-assisted Billroth I gastrectomy. Ann Surg. 1999;229:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdel-Moneim RI. The hazards of surgery in the obese. Int Surg. 1985;70:101–103. [PubMed] [Google Scholar]
- 18.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma. Tokyo: Kanehara; 1998. [DOI] [PubMed] [Google Scholar]
- 19.Takekoshi T, Baba Y, Ota H, et al. Endoscopic resection of early gastric carcinoma: results of a retrospective analysis of 308 cases. Endoscopy. 1994;26:352–358. [DOI] [PubMed] [Google Scholar]
- 20.Snedecor GW, Cochran WG. Statistical methods. Ames, IA: Iowa State University Press; 1980. [Google Scholar]
- 21.Eltabbakh GH, Piver MS, Hempling RE, et al. Laparoscopic surgery in obese women. Obstet Gynecol. 1999;94:704–708. [DOI] [PubMed] [Google Scholar]
- 22.Eltabbakh GH, Shamonki MI, Moody JM, et al. Hysterectomy for obese women with endometrial cancer: laparoscopy or laparotomy? Gynecol Oncol. 2000;78:329–335. [DOI] [PubMed] [Google Scholar]
- 23.Van Italie TB. Health implications of overweight and obesity in the United States. Ann Intern Med. 1985;103:983–938. [DOI] [PubMed] [Google Scholar]
- 24.Hurd WW, Ohl DA. Blunt trocar laparoscopy. Fertil Steril. 1994;61:1177–1180. [PubMed] [Google Scholar]
- 25.Wisen O, Hellstrom PM. Gastrointestinal motility in obesity. J Intern Med. 1995;237:411–418. [DOI] [PubMed] [Google Scholar]
- 26.Annese V, Bassotti G, Caruso N, et al. Gastrointestinal motor dysfunction, symptoms, and neuropathy in noninsulin-dependent (type 2) diabetes mellitus. J Clin Gastroenterol. 1999;29:171–177. [DOI] [PubMed] [Google Scholar]
- 27.Verne GN, Sninsky CA. Diabetes and the gastrointestinal tract. Gastroenterol Clin North Am. 1998;27:861–874. [DOI] [PubMed] [Google Scholar]