Abstract
siglecs are a family of sialic-acid binding immunoglobulin-like lectins mostly expressed by cells of the immune system that have the potential to interact with sialylated glycans expressed not only on host cells but also on certain pathogens. Campylobacter jejuni is a common pathogen of humans that expresses surface lipooligosaccharides (LOS) that can be modified with ganglioside-like terminal structures in the core oligosaccharides. In this study, we examined the interaction of 10 siglecs with LOS purified from four different C. jejuni isolates expressing GM1-like, GD1a-like, GD3-like, and GT1a-like oligosaccharides. Of all siglecs examined, only Siglec-7 exhibited specific, sialic acid-dependent interactions with C. jejuni LOS in solid-phase binding assays. Binding was especially prominent with LOS from the HS:19(GM1+ GT1a+) isolate, with weaker binding with LOS from the HS:19(GD3+) isolate. Binding of Siglec-7 was also observed with intact bacteria expressing these LOS structures. Specific binding of HS:19(GM1+ GT1a+) bacteria was demonstrated with Siglec-7 expressed on transfected Chinese hamster ovary cells and with peripheral blood leukocytes, among which HS:19(GM1+ GT1a+) bacteria bound selectively to both natural killer cells and monocytes which naturally express Siglec-7. These results raise the possibility that, in addition to their role in generating autoimmune antibody responses, C. jejuni LOS could interact with Siglec-7 expressed by leukocytes, modulate the host-pathogen interaction, and contribute to the clinical outcome and the development of secondary complications such as Guillain-Barré syndrome.
Sialic acid-binding immunoglobulin (Ig)-like lectins (siglecs) are a family of type I membrane proteins containing a homologous N-terminal V-set Ig-like domain that binds sialylated glycoconjugates and various numbers of C2-set Ig-like domains (10). In humans, 11 siglecs have been defined (reviewed by Crocker in reference 9). Apart from myelin-associated glycoprotein (MAG)/Siglec-4, they are all expressed differentially on various leukocyte subsets of the hematopoietic and immune systems. The CD33-related siglecs are a distinct subgroup characterized by striking differences in gene repertoires among mammalian species (1). In humans, there are eight CD33-related siglecs which share ∼50 to 80% sequence similarity and have two conserved tyrosine-based motifs in their cytoplasmic tails, including a membrane-proximal immune receptor tyrosine-based inhibitory motif (ITIM) and a membrane-distal ITIM-like motif. It has been demonstrated recently that these tyrosine-based motifs are important for the CD33-related siglec-dependent inhibitory signaling via the recruitment and activation of the protein tyrosine phosphatases SHP-1 and SHP-2 (4, 5).
Where studied, siglecs show striking differences in glycan binding specificities, which in the cases of Siglec-7 and -9, are determined by a six-amino-acid stretch within the C-C′ β-strand loops of their respective V-set Ig-like domains (40). Although most siglecs are masked at the cell surface by cis interactions with sialic acids on the same cell membrane, unmasking can occur following exposure of the cells to sialidase or in some cases following cellular activation (33). Even masked siglecs have been shown to be capable of interacting with ligands presented on other surfaces in trans, especially if the trans ligands are presented in a high-affinity or high-avidity format (8). It has been proposed that the recognition of “self” sialoglycoconjugates by siglecs could regulate cellular activation during inflammatory and immune responses (12), and support for this model has been obtained from studies of CD22/Siglec-2 on B cells (25) and Siglec-7 on NK cells (29). However, given that certain pathogens can also express sialic acids that are important for pathogenicity, it is possible that the interaction between a pathogen's sialoglycoconjugates and siglecs could modulate the immune response, possibly to the advantage of the pathogen.
Campylobacter jejuni is a human pathogen commonly responsible for gastroenteritic infections. In addition to causing uncomplicated enteritis, C. jejuni strains with sialylated lipooligosaccharides (LOS) are a major causative trigger of the postinfectious autoimmune neuropathies termed the Guillain-Barré syndrome (18). The basis for this association lies in the structural identity between glycans borne by the core oligosaccharide of C. jejuni LOS and the peripheral nerve gangliosides. This identity results in aberrant targeting of the immune response from microbe to nerve, a phenomenon referred to as molecular mimicry (41). C. jejuni can express a variety of different sialyloligosaccharides in its core oligosaccharide, including mimics of gangliosides GM1, GD1a, GD3, and GT1a (37). The pattern of terminal glycosylation is determined by genetic variation in the LOS biosynthetic loci that include genes encoding CMP-NeuAc synthetase and a bifunctional sialyltransferase, CstII, that can transfer sialic acid to galactose in α2,3 linkage as well as to sialic acid itself in α2,8 linkage. Through this variation, a wide variety of ganglioside-mimicking C. jejuni strains have been identified (14).
The aim of this study was to investigate whether ganglioside-like structures presented on the LOS of C. jejuni could be recognized by siglecs and hence implicated in the interactions between pathogens and leukocytes. We demonstrate that Siglec-7, which is naturally expressed on monocytes and natural killer (NK) cells, was the only siglec of 10 tested that was able to mediate specific, sialic acid-dependent interactions both as a soluble recombinant protein and at the cell surface. These findings raise the possibility that, in addition to being important for generating autoimmune antibody responses, C. jejuni LOS may interact with leukocyte-expressed Siglec-7 and modulate host inflammatory and immune responses.
MATERIALS AND METHODS
Reagents.
Unless otherwise specified, all reagents and chemicals were purchased from Sigma-Aldrich (Poole, United Kingdom). Vibrio cholerae sialidase (specific for α2,3, α2,6, and α2,8 sialic acid linkages) was obtained from Calbiochem (Nottingham, United Kingdom). Fluorescein isothiocyanate (FITC)-conjugated rabbit anti-mouse IgF(ab′)2 was obtained from Dako (Ely, United Kingdom). The phycoerythrin-conjugated peanut lectin was purchased from Vector Laboratories (Peterborough, United Kingdom). The panel of Siglec-Fc proteins (20) was generated using stably transfected Chinese hamster ovary (CHO) cell lines cultured in serum-free X-VIVO 10 medium (Cambrex Bio Science, Wokingham, United Kingdom). Each Siglec-Fc fusion protein used contained the entire extracellular region, except for sialoadhesin/Siglec-1 (Sn), which had the first three domains only (11). The concentration of each siglec in the tissue culture supernatants was measured by enzyme-linked immunosorbent assay, using human IgG as a standard. The mouse anti-human Siglec-7 monoclonal antibody (MAb) S7.7A was previously described as a blocking MAb (29) and was used as purified IgG. A mouse anti-human Sn MAb, 7D2, was used as a nonbinding isotype-matched control antibody (16).
Bacteria and cells.
The C. jejuni serostrains and isolates with structurally defined core oligosaccharides that were used are shown in Fig. 1A. The HS:19 (formerly O:19) and OH4382 and OH4384 isolates of the HS:19 serostrain (3) were provided by J. Penner and D. Woodward (31, 32). The HS:3 serostrain was provided by A. P. Moran, Galway, Ireland, and contains no known ganglioside-mimicking core oligosaccharide structures (2). Isolate HS:19 has been referred to previously as HS:19(GM1+ GD1a+), isolate OH4384 as HS:19(GM1+ GT1a+), and isolate OH4382 as HS:19(GD3+) (7). For clarity, the isolates in this study are referred to by their original names. It should be noted that the HS typing system is based on antigens of capsular origin, rather than the LOS antigens (22). Bacteria were grown on blood agar plates in a microaerobic atmosphere and harvested into distilled water after 48 h of growth. Bacteria were killed by heating at 60°C for 1 h. LOS were isolated by hot phenol-water extraction, quantified and analyzed for purity by silver staining after sodium dodecyl sulfate-polyacrylamide gel electrophoresis and thin-layer chromatography, resuspended in distilled water, and stored at −20°C (15). For bacterial adhesion assays and fluorescence microscopy experiments, C. jejuni strains were labeled for 1 h at room temperature with FITC (Molecular Probes, Paisley, United Kingdom). After extensive washes in phosphate-buffered saline (PBS) with 0.1% bovine serum albumin (BSA), the bacterial pellet was resuspended in the appropriate assay buffer.
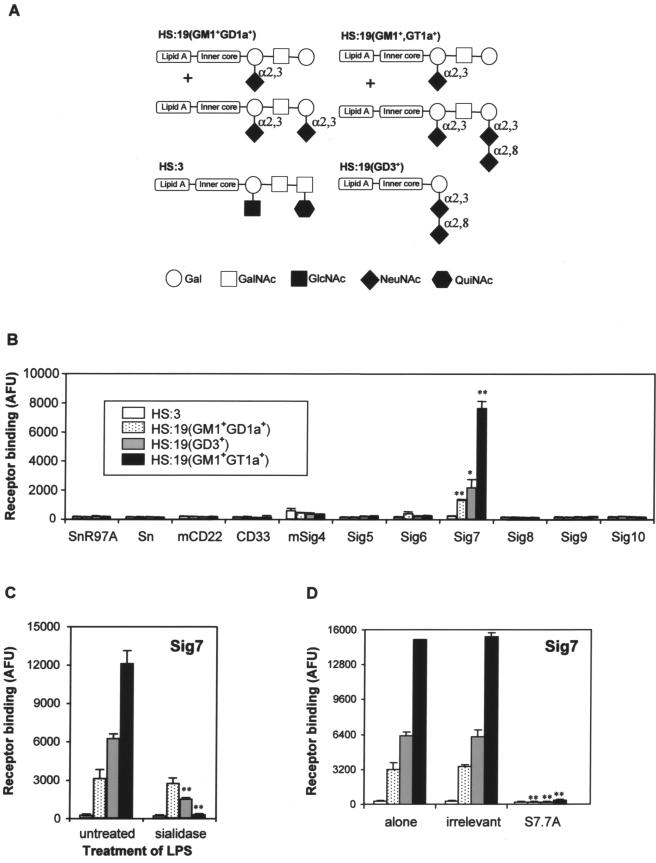
FIG. 1.
Sialic acid-dependent binding of Siglec-7 to purified LOS from C. jejuni. (A) Schematic representation of C. jejuni LOS structures used in this study. The sialic acid linkages are indicated. (B) Human and murine (m) siglec-Fc proteins were precomplexed with anti-human IgG-alkaline phosphatase and added to plastic microwells coated with LOS purified from the indicated C. jejuni strains. SnR97A is a mutated form of Sn-Fc that is unable to bind sialic acids and was used as a negative control. Relative binding is given in arbitrary fluorescence units (AFU) determined following the addition of a fluorescein diphosphate substrate. *, P < 0.05; **, P < 0.01 (both apply to binding of HS19 isolates versus binding of HS3). (C) Plastic microwells were coated with LOS from the strains indicated in panel B and treated with V. cholerae sialidase before the addition of precomplexed Siglec-7-Fc. **, P < 0.01 for binding of sialidase-treated bacteria versus untreated bacteria. LPS, lipopolysaccharide. (D) Either an irrelevant MAb (7D2 anti-Sn) or anti-Siglec-7 MAb S7.7A was added to the precomplexed Siglec-7 prior to its addition to the indicated LOS. **, P < 0.01 for binding of bacteria in the presence of S7.7A versus binding of bacteria alone. (B through D) Data from one experiment representative of three independent experiments performed.
Wild-type CHO cells (CHO-WT) and Siglec-7-expressing CHO cells (CHO-Sig7) (30) were cultured in RPMI medium supplemented with 10% fetal calf serum, penicillin (100 U/ml), and streptomycin (100 μg/ml). Human peripheral blood mononuclear cells (PBMCs) were isolated from the blood of healthy donors by Ficoll-Paque (Amersham Biosciences, Little Chalfont, United Kingdom) density gradient centrifugation. Purified NK cells were isolated from PBMCs by negative selection using a MACS NK cell isolation kit and an autoMACS (Miltenyi Biotech, Bisley, United Kingdom).
Siglec-Fc binding assay.
Siglec-Fc proteins (10 μg/ml) were complexed with alkaline phosphatase-conjugated anti-human IgG Ab (1:1,000) for 1 h at room temperature. Control MAb 7D2 or blocking anti-Siglec-7 MAb 7.7A was added to the precomplexed Fc proteins 15 min before the incubation with LOS. Ninety-six-well plates were coated with C. jejuni-derived LOS (10 μg/ml in PBS), incubated overnight at 4°C, and treated or not with 0.4 U/ml of sialidase for 1 h at 37°C; the plates were then washed in PBS plus 0.1% BSA and incubated for 1 h at room temperature with precomplexed Siglec-Fc. Unbound Fc proteins were then washed, and 100 μl per well of 10 μM fluorescein diphosphate (Molecular Probes) was added. After incubation at 37°C for 15 to 30 min, fluorescence was measured using a fluorescence plate reader (Cytofluor; PerSeptive Biosystems). For Siglec-Fc binding on living or heat-inactivated bacteria, precomplexed Siglec-Fc proteins were mixed with 5 × 107 bacteria and incubated for 1 h at 4°C. After washes in PBS-0.1% BSA, 100 μl per well of 10 μM fluorescein diphosphate was added to the plates, and the plates were incubated at 37°C for 15 to 30 min. After centrifugation, supernatants were transferred to new 96-well plates, and fluorescence readings were performed as described above.
Fluorescence microscopy.
CHO-WT and CHO-Sig7 cells were cultured overnight on glass coverslips (BDH, Lutterworth, United Kingdom). Cells were treated or not with sialidase for 1 h at 37°C, washed, and incubated for 1 h at 4°C with FITC-labeled bacteria at a 10:1 ratio of bacteria to cells. Unbound bacteria were gently washed off, and cells were fixed with 4% paraformaldehyde. Images were acquired using an AxioVision imaging system (Imaging Associates, Bicester, United Kingdom) and a Zeiss Axioskop immunofluorescence microscope equipped with a Zeiss 20× objective (Jena, Germany).
Flow cytometry.
All incubations were carried out on ice. Cells were incubated with primary MAbs (10 μg/ml) for 30 min, followed by FITC-conjugated rabbit F(ab′)2 anti-mouse Ig for 30 min, and then analyzed with a FACSCalibur (Becton Dickinson, Oxford, United Kingdom). For bacterial binding assays, CHO-WT, CHO-Sig7, PBMCs, or purified NK cells were treated or not with 0.4 U/ml of sialidase for 30 min at 37°C, washed in PBS-0.1% BSA, and incubated for 1 h at 4°C with FITC-labeled bacteria at a ratio of 10 bacteria per cell. Bacteria were either left untreated or pretreated with 0.4 U/ml sialidase, as described above for LOS treatment. In some experiments, control MAb 7D2 or anti-Siglec-7 blocking MAb 7.7A was added to the cells 15 min before the incubation with bacteria. Bacterial adhesion to cells was assessed by flow cytometry as described previously (20). Side- and forward-scatter properties were used to distinguish cells (cell gate) from free bacteria, and 10,000 events were collected from the cell gate. Results were expressed as a bacterial binding index, calculated by multiplying the percentage of FITC-positive cells in the cell gate by the mean fluorescence intensity of FITC-positive cells within the cell gate.
Statistics.
Values show the means ± standard deviations of triplicate results from single representative experiments. For all data shown, three independent experiments were routinely carried out, unless indicated otherwise. Student's t test was used to determine the level of statistical significance.
RESULTS
Selective recognition by Siglec-7 of sialylated lipopolysaccharide from C. jejuni.
A panel of murine and human siglec-Fc proteins was used in a precomplexed, high-avidity format to measure binding to plastic-coated LOS isolated from different C. jejuni serostrains and isolates, including the HS:3 (nonsialylated) and HS:19(GM1+ GD1a+) serostrains and the HS:19(GD3+) and HS:19(GM1+ GT1a+) isolates (Fig. 1A and B). HS:19 strains have been well characterized previously and shown to be associated with Guillain-Barré syndrome (3). Out of 10 siglecs screened, namely, Sn/Siglec-1, CD22/Siglec-2, CD33/Siglec-3, MAG/Siglec-4, and Siglec-5, -6, -7, -8, -9, and -10, clear binding was observed only with Siglec-7. This siglec bound strongly to HS:19(GM1+ GT1a+) LOS, less strongly to HS:19(GD3+) LOS, and only weakly to HS:19(GM1+ GD1a+) LOS. No binding was seen with the nonsialylated HS:3 LOS. Sialidase treatment of LOS samples abolished Siglec-7 recognition of HS:19(GM1+ GT1a+) and HS:19(GD3+), demonstrating that these interactions were sialic acid dependent but had no effect on HS:19(GM1+ GD1a+) (Fig. 1C). In addition, preincubation of Siglec-7-Fc with anti-Siglec-7 S7.7A, a blocking MAb, completely abrogated its binding to all LOS species compared with that of a control antibody (Fig. 1D).
Recognition of intact C. jejuni by siglecs.
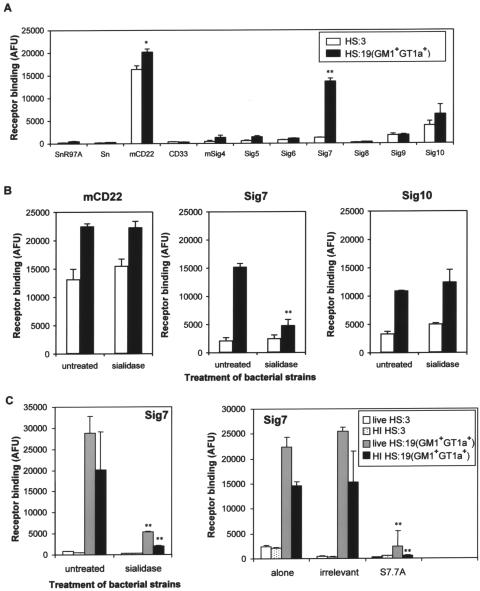
The same panel of siglec-Fc proteins was then evaluated with a binding assay using the intact heat-inactivated bacteria of HS:3 and HS:19(GM1+ GT1a+) C. jejuni strains. Consistent with the LOS binding patterns seen in Fig. 1B, Siglec-7 bound strongly to C. jejuni HS:19(GM1+ GT1a+) bacteria (Fig. 2A). Interestingly, mouse CD22 and, to a lesser extent, human Siglec-10 were able to bind both HS:3 and HS:19(GM1+ GT1a+) C. jejuni strains (Fig. 2A), but this was not affected by sialidase treatment of the bacteria (Fig. 2B). In contrast, Siglec-7 binding to HS:19(GM1+ GT1a+) bacteria was abrogated by sialidase treatment (Fig. 2B and C, left panel). Siglec-7 bound equally well or better to live HS:19(GM1+ GT1a+) than to heat-inactivated bacteria (Fig. 2C), and binding to living and heat-inactivated HS:19(GM1+ GT1a+) was similarly blocked by sialidase treatment of the bacteria (Fig. 2C, left panel) or by pretreating Siglec-7-Fc complexes with anti-Siglec-7 S7.7A blocking MAb (Fig. 2C, right panel). Therefore, heat-inactivated bacteria were considered a suitable substitute for live bacteria in subsequent experiments.
FIG. 2.
Sialic acid-dependent binding of Siglec-7 to heat-inactivated and live C. jejuni bacteria. (A) Heat-inactivated HS:3 and HS:19(GM1+ GT1a+) strains were added to precomplexed siglec-Fc proteins in suspension, and binding was measured using a fluorescent substrate. Relative binding is given in arbitrary fluorescence units (AFU). (B) Binding assays were performed with either untreated or sialidase-treated HS:3 or HS:19(GM1+ GT1a+) strains using CD22-Fc, Siglec-7-Fc or Siglec-10-Fc. (C) The binding properties of live or heat-inactivated (HI) C. jejuni bacteria to Siglec-7-Fc were compared either without or with sialidase treatment of bacteria (left panel) or following the preincubation of precomplexed Siglec-7-Fc with anti-Siglec-7 MAb S7.7A or an irrelevant MAb (right panel). Left panel, **, P < 0.01 for binding of sialidase-treated bacteria versus untreated bacteria. Right panel, **, P < 0.01 for binding of bacteria in the presence of S7.7A versus binding of bacteria alone. Panels A and B show data from one experiment representative of three independent experiments performed. Panel C shows data from a single experiment.
Siglec-7-expressing CHO cells bind to C. jejuni HS:19(GM1+ GT1a+) bacteria in a sialic acid- and Siglec-7-dependent manner.
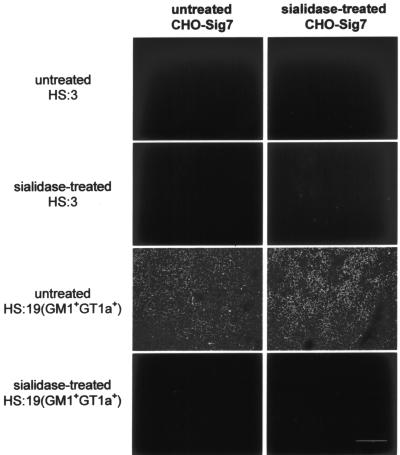
To test whether the results observed with precomplexed soluble Siglec-7-Fc proteins could be reproduced using cell surface-expressed Siglec-7, we used CHO cells stably transfected with human Siglec-7 cDNA (CHO-Sig7) and compared the binding with that of parental CHO cells (CHO-WT). When cells were examined by immunofluorescence microscopy, no binding of the HS:19(GM1+ GT1a+) C. jejuni isolate was observed with CHO-WT cells (data not shown), whereas high levels of binding were seen with CHO-Sig7 cells, and this was abrogated following treatment of the bacteria with sialidase (Fig. 3). A previous study showed that Siglec-7 can be masked at the cell surface and unmasked following sialidase treatment (29). Consistent with this finding, sialidase treatment of CHO-Sig7 led to a small increase in binding to bacteria (Fig. 3). In contrast to the HS:19(GM1+ GT1a+) strain, no binding of the untreated or sialidase-treated HS:3 strain to CHO-Sig7 was observed (Fig. 3).
FIG. 3.
Binding of Siglec-7 expressed on CHO cells to C. jejuni bacteria. HS:3 and HS:19(GM1+ GT1a+) strains were FITC labeled and used in a binding assay with untreated or sialidase-treated CHO-Sig7 cells. Binding of bacteria was assessed by fluorescence microscopy. Bar, 100 μm.
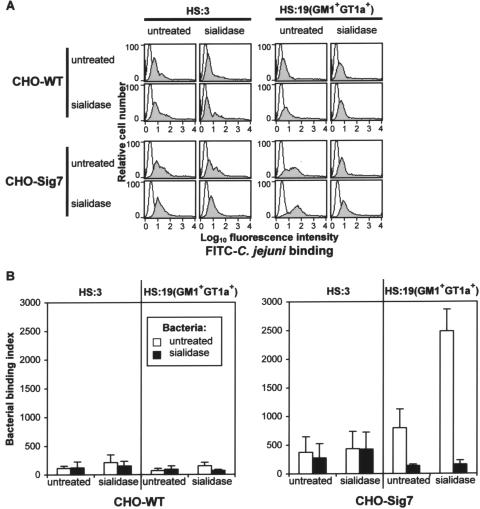
In order to quantify the level of bacterial binding, a flow cytometry assay in which CHO cells were incubated with FITC-labeled bacteria in suspension was developed (Fig. 4). Very low levels of HS:3 bacteria bound to CHO-WT and CHO-Sig7 cells, irrespective of whether bacteria or cells were treated with sialidase (Fig. 4A and B). In contrast, the HS:19(GM1+ GT1a+) isolate showed enhanced binding to CHO-Sig7 cells that was increased further with sialidase-treated cells that expressly unmasked Siglec-7 (Fig. 4A and B). Binding of the HS:19(GM1+ GT1a+) isolate to CHO-Sig7 cells was greatly reduced following sialidase treatment of bacteria (Fig. 4A and B) or following pretreatment of CHO cells with anti-Siglec-7 MAb S7.7A (data not shown). Taken together, these results show that Siglec-7-expressing cells recognize HS:19(GM1+ GT1a+) C. jejuni in a sialic acid- and Siglec-7-dependent manner.
FIG. 4.
Flow cytometric analyses of binding interactions between CHO cells expressing Siglec-7 and C. jejuni bacteria. Untreated or sialidase-treated HS:3 and HS:19(GM1+ GT1a+) strains were FITC labeled and used in a binding assay with untreated or sialidase-treated CHO-WT cells and CHO-Sig7 cells. (A) Fluorescence-activated cell sorter histograms showing binding of bacteria (gray histograms) are compared with histograms of cells incubated in the absence of bacteria (open histograms). (B) Quantification of bacterial binding to CHO-WT and CHO-Sig7 cells by flow cytometry. Data show means ± 1 standard deviation of binding indices derived from three independent experiments.
Siglec-7-expressing NK cells recognize C. jejuni HS:19(GM1+ GT1a+) bacteria in a sialic acid-dependent manner.
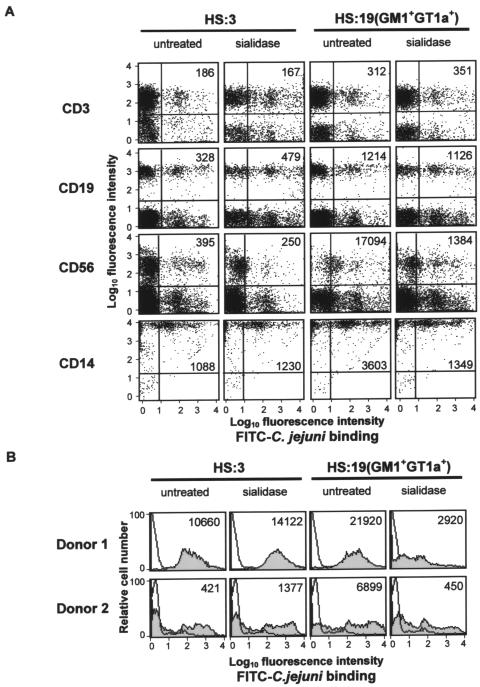
Human Siglec-7 is expressed strongly on human peripheral blood NK cells and at lower levels on monocytes but is absent from B cells and most T cells (29). To test whether the HS:19(GM1+ GT1a+) isolate could be recognized by these cells, human PBMCs were incubated with untreated or sialidase-treated FITC-labeled bacteria. The HS:3 serostrain was included as a control for the nonspecific effects of sialidase treatment on binding to PBMCs. Following staining with anti-CD3, -CD19, -CD56, or -CD14 MAbs to detect T cells, B cells, NK cells, or monocytes, respectively, flow cytometry analysis was performed, and the percentages of each cell type bound to bacteria and their mean fluorescence intensities were used to calculate their bacterial binding indices (see Materials and Methods). As shown in Fig. 5A, different levels of binding of the HS:3 and HS:19(GM1+ GT1a+) isolates were seen with CD3+ T cells and CD19+ B cells, but this binding was not reduced following sialidase treatment of the bacteria. In contrast, clear evidence for sialidase-sensitive binding of the HS:19(GM1+ GT1a+) isolate was observed with both NK cells and monocytes, whereas the control HS:3 strain bound at lower levels and was unaffected by sialidase treatment (Fig. 5A). Binding to NK cells was especially strong and showed a 13-fold overall reduction following sialidase treatment (Fig. 5A).
FIG. 5.
Siglec-7-expressing NK cells and monocytes recognize C. jejuni HS:19(GM1+ GT1a+) bacteria in a sialic acid-dependent manner. Untreated or sialidase-treated HS:3 and HS:19(GM1+ GT1a+) strains were FITC labeled and incubated with human PBMCs (A) or purified NK cells (B). (A) Binding of bacteria was assessed by flow cytometry following labeling with the indicated MAbs: CD3, T cells; CD19, B cells; CD56, NK cells; CD14, monocytes. Values show the bacterial binding indices for each gated population of cells. (B) Binding of bacteria to purified NK cells from two different human donors. Open histograms show background fluorescence in the absence of added bacteria, and shaded histograms show binding signals obtained in the presence of bacteria. Values show the bacterial binding indices for each population of cells.
The strong, sialidase-sensitive binding of HS:19(GM1+ GT1a+) bacteria to NK cells was confirmed using purified NK cells from two different donors (Fig. 5B). Different levels of HS:3 binding were observed, but in contrast to the HS:19(GM1+ GT1a+) binding, there was no reduction following sialidase treatment of the bacteria (Fig. 5B). In conclusion, HS:19(GM1+ GT1a+) bacteria showed sialidase-sensitive binding to NK cells and monocytes, which naturally express Siglec-7, but not to lymphocyte populations, which are Siglec-7 negative.
DISCUSSION
The goal of this study was to determine whether sialylated LOS derived from pathogenic strains of C. jejuni could be recognized by siglecs. The C. jejuni typing system referred to here is based on HS antigens of capsular origin (22), and many such typed strains also contain ganglioside-like structures in their LOS. Using a large panel of recombinant proteins, Siglec-7 was shown to mediate sialic acid-dependent binding to LOS extracted from HS:19(GM1+ GT1a+) or HS:19(GD3+) isolates of C. jejuni. Specific sialic acid-dependent binding to HS:19(GM1+ GT1a+) bacteria was also observed using recombinant soluble and cell surface-expressed forms of Siglec-7. Finally, we showed that human peripheral blood NK cells and monocytes could mediate sialic acid-dependent binding of C. jejuni HS:19(GM1+ GT1a+) bacteria. Until now, a major focus for understanding the pathophysiological significance of C. jejuni LOS sialylation has been in the context of host mimicry and the induction of antiganglioside antibodies that are thought to give rise to Guillain-Barré syndrome (18, 37). Our demonstration that Siglec-7 can also interact with certain isolates of C. jejuni raises the possibility that sialylation of LOS has broader significance and may be involved in modulating host immune and inflammatory responses following C. jejuni infection via siglec-dependent interactions.
Siglec-7 contains three Ig-like extracellular domains, and sialic acid recognition is mediated by the N-terminal V-set Ig-like domain. Siglec-7 is unusual among the CD33-related siglecs in its strong preference for α2,8-linked disialylated glycans and internally branched α2,6-linked sialic acids over terminal α2,6- or α2,3-linked sialic acids (6, 40). The α2,8 disialic acid structure is represented in both the GD3-like and GT1a-like LOS preparations that showed sialic acid-dependent binding to Siglec-7 and is therefore likely to be responsible for the binding observed (Fig. 1A). Siglec-9 is highly related to Siglec-7, yet it shows strikingly different binding preferences for sialylated glycans, including GD1a oligosaccharide, which is poorly recognized by Siglec-7 (40). It was surprising, therefore, that the LOS containing a GD1a-like structure was not recognized by Siglec-9 or by other siglecs such as Sn or MAG, which also bind well to this structure (34). GM1 has so far not been shown to be recognized by any siglec, and GD1a and GM1 are reported to be present in a ratio of about 1:1 in the HS:19(GM1+ GD1a+) strain (7). Therefore, it is possible that, under the conditions used, the density of GD1a-like LOS was too low to support binding or that steric hindrance by core sugars within the LOS prevented interaction with these siglecs. Similar conclusions concerning steric hindrance were reached in a previous study of siglec binding to the Neisseria meningitidis LOS that presents α2,3-linked sialic acid on a lacto-N-neotetraose capping structure. N. meningitidis LOS was recognized only by Sn and Siglec-5, whereas several other siglecs were expected to bind this LOS based on their binding specificities, defined with short oligosaccharide ligands (20). In the present study, the strong binding of Siglec-7 to LOS from the HS:19(GM1+ GT1a+) strain could be due to a higher affinity of Siglec-7 for the GT1a structure than of Siglec-9 for the GD1a structure, or the ratio of GT1a to GM1 in this strain may be more favorable for Siglec-7 binding. In addition, we cannot rule out the possibility that Siglec-7 has a low affinity for GM1 that leads to additive binding with GT1a; this could also explain why LOS from the HS:19(GM1+ GD1a+) strain still showed low-level sialidase-resistant, anti-Siglec-7-inhibitable binding (Fig. 1C and D), since the internal sialic acid on GM1 is resistant to sialidase treatment but could contribute to weak interactions with the sialic acid-binding site of Siglec-7.
C. jejuni can cause severe gastroenteritis in humans that is accompanied by a cellular inflammatory response involving an influx of both neutrophils and monocytes (reviewed in reference 38). Although neutrophils are thought to kill complement-opsonized bacteria efficiently following phagocytosis (23), this does not appear to be the case with monocytes and macrophages, in which even nonopsonized C. jejuni organisms are rapidly phagocytosed and then survive for several days (17, 24, 35, 36). Survival in macrophages could be important for the dissemination of bacteria within the gut and systemically to the joints in relation to the reactive arthritis observed in a small proportion of C. jejuni-infected patients (23). Infected macrophages elicit a strong cytokine response that is likely to exacerbate the inflammatory response, and they may also undergo caspase-independent apoptosis (21, 35). Since ligation of CD33-related siglecs has been shown to modulate both cytokine production and apoptosis (17, 35), it would be interesting to determine if bacterially mediated ligation of Siglec-7 during monocyte infection has any influence on these key cellular processes. Similar to all human CD33-related siglecs, Siglec-7 contains an ITIM and an ITIM-like motif that can be tyrosine phosphorylated and recruit SHP-1 and SHP-2 (4, 19, 39). Several studies have shown that ligation of Siglec-7 can modulate cellular activation functions, including the inhibition of NK cell cytotoxicity (13, 29), inhibition of T-cell receptor signaling in transfected Jurkat cells (19), inhibition of serotonin secretion in transfected rat basophil leukemic cells (4), and inhibition of Ca2+ flux in transfected U937 monocytic cell lines (39).
In addition to monocytes, Siglec-7 is also expressed on monocyte-derived macrophages and dendritic cells (28). Little is known about the role of dendritic cells in the resolution of C. jejuni infections, but Siglec-7-dependent interactions between LOS and dendritic cells could potentially modulate the function of these key cells that link the innate and adaptive immune systems. CD33-related siglecs have also been shown to mediate endocytosis (27, 28), and Siglec-7 could therefore contribute to the uptake process by macrophages and other cell types. Cellular receptors that mediate the uptake of nonopsonized bacteria have not been characterized, and it is of interest that, in the present study, we could demonstrate sialic acid-dependent binding to monocytes and NK cells. This indicates that despite the masking of Siglec-7 on NK cells, demonstrated previously using glycopolymers (29), the presentation of ganglioside-like structures on C. jejuni, such as GT1a, can overcome cis inhibition and promote cellular interactions. NK cells are an important source of cytokines, such as gamma interferon, and have been shown to contribute to host protection in experimental shigella infections of mice (26); however, their potential role in campylobacter infections has not been studied.
In conclusion, we provide the first evidence that the sialylated LOS of C. jejuni are recognized by Siglec-7 and that this can lead to an enhanced, sialic acid-dependent association with Siglec-7-positive cells, including monocytes and NK cells. It will be of considerable interest in the future to investigate whether this interaction modulates the host immune response, either to the benefit of the pathogen or, alternatively, to the benefit of the host.
Acknowledgments
We thank Claire Jones, Kevin Lock, Gavin Nicoll, and Jiquan Zhang for the Siglec-Fc fusion proteins and anti-Siglec-7 MAb.
This work was supported by a Wellcome Trust Senior Fellowship (GR047677MA), awarded to P.R.C., and a Wellcome Trust Programme grant, awarded to H.J.W.
Editor: V. J. DiRita
REFERENCES
- 1.Angata, T., E. H. Margulies, E. D. Green, and A. Varki. 2004. Large-scale sequencing of the CD33-related Siglec gene cluster in five mammalian species reveals rapid evolution by multiple mechanisms. Proc. Natl. Acad. Sci. USA 101:13251-13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aspinall, G. O., C. M. Lynch, H. Pang, R. T. Shaver, and A. P. Moran. 1995. Chemical structures of the core region of Campylobacter jejuni O:3 lipopolysaccharide and an associated polysaccharide. Eur. J. Biochem. 231:570-578. [PubMed] [Google Scholar]
- 3.Aspinall, G. O., A. G. McDonald, H. Pang, L. A. Kurjanczyk, and J. L. Penner. 1994. Lipopolysaccharides of Campylobacter jejuni serotype O:19: structures of core oligosaccharide regions from the serostrain and two bacterial isolates from patients with the Guillain-Barre syndrome. Biochemistry 33:241-249. [DOI] [PubMed] [Google Scholar]
- 4.Avril, T., H. Floyd, F. Lopez, E. Vivier, and P. R. Crocker. 2004. The membrane-proximal immunoreceptor tyrosine-based inhibitory motif is critical for the inhibitory signaling mediated by Siglecs-7 and -9, CD33-related Siglecs expressed on human monocytes and NK cells. J. Immunol. 173:6841-6849. [DOI] [PubMed] [Google Scholar]
- 5.Avril, T., S. D. Freeman, H. Attrill, R. G. Clarke, and P. R. Crocker. 2005. Siglec-5 (CD170) can mediate inhibitory signaling in the absence of immunoreceptor tyrosine-based inhibitory motif phosphorylation. J. Biol. Chem. 280:19843-19851. [DOI] [PubMed] [Google Scholar]
- 6.Blixt, O., B. E. Collins, I. M. van den Nieuwenhof, P. R. Crocker, and J. C. Paulson. 2003. Sialoside specificity of the siglec family assessed using novel multivalent probes: identification of potent inhibitors of myelin-associated glycoprotein. J. Biol. Chem. 278:31007-31019. [DOI] [PubMed] [Google Scholar]
- 7.Bowes, T., E. R. Wagner, J. Boffey, D. Nicholl, L. Cochrane, M. Benboubetra, J. Conner, K. Furukawa, K. Furukawa, and H. J. Willison. 2002. Tolerance to self gangliosides is the major factor restricting the antibody response to lipopolysaccharide core oligosaccharides in Campylobacter jejuni strains associated with Guillain-Barr é syndrome. Infect. Immun. 70:5008-5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins, B. E., O. Blixt, A. R. DeSieno, N. Bovin, J. D. Marth, and J. C. Paulson. 2004. Masking of CD22 by cis ligands does not prevent redistribution of CD22 to sites of cell contact. Proc. Natl. Acad. Sci. USA 101:6104-6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crocker, P. R. 2005. Siglecs in innate immunity. Curr. Opin. Pharmacol. 5:431-437. [DOI] [PubMed] [Google Scholar]
- 10.Crocker, P. R., E. A. Clark, M. Filbin, S. Gordon, Y. Jones, J. H. Kehrl, S. Kelm, N. Le Douarin, L. Powell, J. Roder, R. L. Schnaar, D. C. Sgroi, K. Stamenkovic, R. Schauer, M. Schachner, T. K. van den Berg, P. A. van der Merwe, S. M. Watt, and A. Varki. 1998. Siglecs: a family of sialic-acid binding lectins. Glycobiology 8:v. [DOI] [PubMed]
- 11.Crocker, P. R., S. Freeman, S. Gordon, and S. Kelm. 1995. Sialoadhesin binds preferentially to cells of the granulocytic lineage. J. Clin. Investig. 95:635-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crocker, P. R., and A. Varki. 2001. Siglecs, sialic acids and innate immunity. Trends Immunol. 22:337-342. [DOI] [PubMed] [Google Scholar]
- 13.Falco, M., R. Biassoni, C. Bottino, M. Vitale, S. Sivori, R. Augugliaro, L. Moretta, and A. Moretta. 1999. Identification and molecular cloning of p75/AIRM1, a novel member of the sialoadhesin family that functions as an inhibitory receptor in human natural killer cells. J. Exp. Med. 190:793-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Godschalk, P. C., A. P. Heikema, M. Gilbert, T. Komagamine, C. W. Ang, J. Glerum, D. Brochu, J. Li, N. Yuki, B. C. Jacobs, A. van Belkum, and H. P. Endtz. 2004. The crucial role of Campylobacter jejuni genes in anti-ganglioside antibody induction in Guillain-Barre syndrome. J. Clin. Investig. 114:1659-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodyear, C. S., G. M. O'Hanlon, J. J. Plomp, E. R. Wagner, I. Morrison, J. Veitch, L. Cochrane, R. W. Bullens, P. C. Molenaar, J. Conner, and H. J. Willison. 1999. Monoclonal antibodies raised against Guillain-Barre syndrome-associated Campylobacter jejuni lipopolysaccharides react with neuronal gangliosides and paralyze muscle-nerve preparations. J. Clin. Investig. 104:697-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartnell, A., J. Steel, H. Turley, M. Jones, D. G. Jackson, and P. R. Crocker. 2001. Characterization of human sialoadhesin, a sialic acid binding receptor expressed by resident and inflammatory macrophage populations. Blood 97:288-296. [DOI] [PubMed] [Google Scholar]
- 17.Hickey, T. E., G. Majam, and P. Guerry. 2005. Intracellular survival of Campylobacter jejuni in human monocytic cells and induction of apoptotic death by cytholethal distending toxin. Infect. Immun. 73:5194-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes, R. A., and D. R. Cornblath. 2005. Guillain-Barre syndrome. Lancet 366:1653-1666. [DOI] [PubMed] [Google Scholar]
- 19.Ikehara, Y., S. K. Ikehara, and J. C. Paulson. 2004. Negative regulation of T cell receptor signaling by Siglec-7 (p70/AIRM) and Siglec-9. J. Biol. Chem. 279:43117-43125. [DOI] [PubMed] [Google Scholar]
- 20.Jones, C., M. Virji, and P. R. Crocker. 2003. Recognition of sialylated meningococcal lipopolysaccharide by siglecs expressed on myeloid cells leads to enhanced bacterial uptake. Mol. Microbiol. 49:1213-1225. [DOI] [PubMed] [Google Scholar]
- 21.Jones, M. A., S. Tötemeyer, D. J. Maskell, C. E. Bryant, and P. A. Barrow. 2003. Induction of proinflammatory responses in the human monocytic cell line THP-1 by Campylobacter jejuni. Infect. Immun. 71:2626-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karlyshev, A. V., D. Linton, N. A. Gregson, and B. W. Wren. 2002. A novel paralogous gene family involved in phase-variable flagella-mediated motility in Campylobacter jejuni. Microbiology 148:473-480. [DOI] [PubMed] [Google Scholar]
- 23.Ketley, J. M. 1997. Pathogenesis of enteric infection by Campylobacter. Microbiology 143:5-21. [DOI] [PubMed] [Google Scholar]
- 24.Kiehlbauch, J. A., R. A. Albach, L. L. Baum, and K.-P. Chang. 1985. Phagocytosis of Campylobacter jejuni and its intracellular survival in mononuclear phagocytes. Infect. Immun. 48:446-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanoue, A., F. D. Batista, M. Stewart, and M. S. Neuberger. 2002. Interaction of CD22 with alpha2,6-linked sialoglycoconjugates: innate recognition of self to dampen B cell autoreactivity? Eur. J. Immunol. 32:348-355. [DOI] [PubMed] [Google Scholar]
- 26.Le-Barillec, K., J. G. Magalhaes, E. Corcuff, A. Thuizat, P. J. Sansonetti, A. Phalipon, and J. P. Di Santo. 2005. Roles for T and NK cells in the innate immune response to Shigella flexneri. J. Immunol. 175:1735-1740. [DOI] [PubMed] [Google Scholar]
- 27.Linenberger, M. L. 2005. CD33-directed therapy with gemtuzumab ozogamicin in acute myeloid leukemia: progress in understanding cytotoxicity and potential mechanisms of drug resistance. Leukemia 19:176-182. [DOI] [PubMed] [Google Scholar]
- 28.Lock, K., J. Zhang, J. Lu, S. H. Lee, and P. R. Crocker. 2004. Expression of CD33-related siglecs on human mononuclear phagocytes, monocyte-derived dendritic cells and plasmacytoid dendritic cells. Immunobiology 209:199-207. [DOI] [PubMed] [Google Scholar]
- 29.Nicoll, G., T. Avril, K. Lock, K. Furukawa, N. Bovin, and P. R. Crocker. 2003. Ganglioside GD3 expression on target cells can modulate NK cell cytotoxicity via siglec-7-dependent and -independent mechanisms. Eur. J. Immunol. 33:1642-1648. [DOI] [PubMed] [Google Scholar]
- 30.Nicoll, G., J. Ni, D. Liu, P. Klenerman, J. Munday, S. Dubock, M. G. Mattei, and P. R. Crocker. 1999. Identification and characterization of a novel siglec, siglec-7, expressed by human natural killer cells and monocytes. J. Biol. Chem. 274:34089-34095. [DOI] [PubMed] [Google Scholar]
- 31.Penner, J. L., and G. O. Aspinall. 1997. Diversity of lipopolysaccharide structures in Campylobacter jejuni. J. Infect. Dis. 176:S135-S138. [DOI] [PubMed] [Google Scholar]
- 32.Prendergast, M. M., and A. P. Moran. 2000. Lipopolysaccharides in the development of the Guillain-Barre syndrome and Miller Fisher syndrome forms of acute inflammatory peripheral neuropathies. J. Endotoxin Res. 6:341-359. [PubMed] [Google Scholar]
- 33.Razi, N., and A. Varki. 1999. Cryptic sialic acid binding lectins on human blood leukocytes can be unmasked by sialidase treatment or cellular activation. Glycobiology 9:1225-1234. [DOI] [PubMed] [Google Scholar]
- 34.Schnaar, R. L., B. E. Collins, L. P. Wright, M. Kiso, M. B. Tropak, J. C. Roder, and P. R. Crocker. 1998. Myelin-associated glycoprotein binding to gangliosides. Structural specificity and functional implications. Ann. N. Y. Acad. Sci. 845:92-105. [DOI] [PubMed] [Google Scholar]
- 35.Siegesmund, A. M., M. E. Konkel, J. D. Klena, and P. F. Mixter. 2004. Campylobacter jejuni infection of differentiated THP-1 macrophages results in interleukin 1 beta release and caspase-1-independent apoptosis. Microbiology 150:561-569. [DOI] [PubMed] [Google Scholar]
- 36.Wassenaar, T. M., M. Engelskirchen, S. Park, and A. Lastovica. 1997. Differential uptake and killing potential of Campylobacter jejuni by human peripheral monocytes/macrophages. Med. Microbiol. Immunol. 186:139-144. [DOI] [PubMed] [Google Scholar]
- 37.Willison, H. J., and N. Yuki. 2002. Peripheral neuropathies and anti-glycolipid antibodies. Brain 125:2591-2625. [DOI] [PubMed] [Google Scholar]
- 38.Wooldridge, K. G., and J. M. Ketley. 1997. Campylobacter-host cell interactions. Trends Microbiol. 5:96-102. [DOI] [PubMed] [Google Scholar]
- 39.Yamaji, T., M. Mitsuki, T. Teranishi, and Y. Hashimoto. 2005. Characterization of inhibitory signaling motifs of the natural killer cell receptor Siglec-7: attenuated recruitment of phosphatases by the receptor is attributed to two amino acids in the motifs. Glycobiology 15:667-676. [DOI] [PubMed] [Google Scholar]
- 40.Yamaji, T., T. Teranishi, M. S. Alphey, P. R. Crocker, and Y. Hashimoto. 2002. A small region of the natural killer cell receptor, Siglec-7, is responsible for its preferred binding to alpha 2,8-disialyl and branched alpha 2,6-sialyl residues. A comparison with Siglec-9. J. Biol. Chem. 277:6324-6332. [DOI] [PubMed] [Google Scholar]
- 41.Yuki, N. 2005. Carbohydrate mimicry: a new paradigm of autoimmune diseases. Curr. Opin. Immunol. 17:577-582. [DOI] [PubMed] [Google Scholar]