Abstract
Because the cornea is optimized for refraction, it relies on supporting tissues for moistening and nutrition and in particular for immune protection. Its main support tissue is the conjunctiva, in addition to the lacrimal gland, the latter which provides soluble mediators via the tear film. The cornea and conjunctiva constitute a moist mucosal surface and there is increasing evidence that apart from innate defence mechanisms, also lymphoid cells contribute to the normal homeostasis of the corneal surface. A Medline-based literature search was performed in order to review the existing literature on the existence, composition and functions of mucosa-associated lymphoid tissue (MALT) at the ocular surface for corneal protection. The existence of lymphoid cells at the ocular surface and appendage has been known for many years, but for a long time they were believed erroneously to be inflammatory cells. More recent research has shown that in addition to the known presence of lymphoid cells in the lacrimal gland, they also form MALT in the conjunctiva as conjunctiva-associated lymphoid tissue (CALT) and in the lacrimal drainage system as lacrimal drainage-associated lymphoid tissue (LDALT). Together this constitutes an eye-associated lymphoid tissue (EALT), which is a new component of the mucosal immune system of the body. When the topographical distribution of CALT is projected onto the ocular surface, it overlies the cornea during eye closure and is hence in a suitable position to assist the corneal immune protection during blinking and overnight. It can detect corneal antigens and prime respective effector cells, or distribute protective factors as secretory IgA.
Keywords: conjunctiva, cornea, defence, eye-associated lymphoid tissue (EALT), mucosal immunity
Introduction
The cornea is the most important structure of the ocular surface for the maintenance of visual function. Its anatomy is optimized for clarity in order to perform maximal light transmission, and for consistency of dimensions and radius in order to perform a stable refraction. The cornea consists mainly (apart from the epithelia at both sides) of a relatively stiff piece of dense connective tissue (stroma) built up by tightly arranged lamellae of collagen bundles (Bron et al. 1997). The cornea contains relatively few cells in the stroma, and most of these are so-called keratocytes (fibrocytes); there are no lymphoid cells or other apparent protective elements, apart from some dendritic cells and their precursors (Hamrah et al. 2003). For a number of functions such as moistening and nutrition, and also for the purpose of defence, the cornea therefore depends largely on its major support tissue – the conjunctiva – in addition to soluble factors that are provided via the tear film by the lacrimal gland. It is increasingly recognized that the conjunctiva is also involved in the local production of factors such as secretory IgA, the production of which was formerly believed to be exclusively located in the lacrimal gland (Knop et al. 2003).
Unlike the cornea, the conjunctiva is composed of a loose connective tissue layer (lamina propria) beneath the epithelium; it also has a much larger surface. The lamina propria is richly vascularized and contains numerous different cell types for innate defence (e.g. macrophages, occasional neutrophilic granulocytes, mast cells) and specific immune protection (e.g. lymphocytes, plasma cells, dendritic cells). Because both organs together represent a moist mucosal surface, it is important to consider the principles of mucosal immunology in order to understand their defence mechanisms.
It is also important to consider the topography of the conjunctiva because it has a distinct morphology in different locations with regard to factors such as epithelial cell morphology, goblet cell density and the distribution of lymphoid tissue, as we will see later. Different topographical classifications are used, and sometimes only the bulbar and palpebral conjunctiva are distinguished clinically. In our work, a differentiated nomenclature is used according to established textbooks (Ruskell, 1995a; Bron et al. 1997), distinguishing as many as six different zones, starting from the lid margin; these are termed the marginal, tarsal, orbital, fornical, bulbar and limbal conjunctiva (Fig. 1).
Fig. 1.
Schematic topography of the conjunctival zones. The mucosa of the ocular surface is formed by the conjunctiva and cornea. The conjunctiva is the major support tissue for the preservation of the optical function of the cornea. It has different topographical zones, with a distinct morphology (i.e. from the lid margin: marginal, tarsal, orbital, fornical, bulbar and limbal). For immune protection it is equipped (unlike the cornea) with resident lymphoid cells belonging to the mucosal immune system of the body; the figure shows lymphocytes in black and plasma cells in blue.
The ocular surface is a part of the mucosal immune system
Mucosal organs represent a special moist compartment of the body's surface that is equipped with a diverse array of defence mechanisms in order to avoid microbial colonization, as is also true for the ocular surface (Allansmith, 1979; Lemp & Blackman, 1981; Chandler & Gillette, 1983; Jensen & Gluud, 1985; Smolin, 1985; Bron & Seal, 1986; McClellan, 1997; Haynes et al. 1998; Sack et al. 2001; Zierhut et al. 2002). Besides the innate defence mechanisms, lymphoid cells are becoming increasingly recognized as playing an important part in the preservation of mucosal integrity. Lymphoid cells form a ‘mucosa-associated lymphoid tissue’ (MALT) in these organs, which together constitute a ‘common mucosal immune system’ (Mestecky et al. 1978; Bienenstock et al. 1978).
MALT represents an accepted component in organs such as the intestine, respiratory system or genital tract. However, its presence at the normal human ocular surface is not fully recognized as yet; for example, the supply of the ocular surface with protective immunoglobulins is usually attributed to the lacrimal gland. Until relatively recently, immunohistological evidence for the presence of IgA-positive plasma cells and its transepithelial transporter molecule was controversial at the ocular surface, although the presence of plasma cells was always confirmed by histological studies. In the conjunctiva, for example, lymphoid cells occur in most parts but they are interspersed as a thin, discontinuous and inconspicuous layer into the epithelium and connective tissue of the lamina propria. This may be one reason why these cells were often overlooked by previous investigators. They have either not received much attention or they were assumed to be inflammatory infiltrations and not recognized as part of the mucosal immune system until the advent of mucosal immunology in other parts of the body.
Our investigations focus on the presence and organization of lymphoid tissue at the human ocular surface and appendage, and its still undefined role in the health and pathology of the ocular surface. This paper provides a review of the literature on lymphoid tissue at the ocular surface and appendage, as well as giving our own findings of an eye-associated lymphoid tissue (EALT) that represents a new component of the mucosal immune system. In particular, we discuss how we discuss how this system can provide immune protection for the cornea that is itself free of lymphoid cells.
MALT is divided into two forms, an ‘organized’ lymphoid tissue where lymphocytes are organized into lymphoid follicles, and an extensive so-called ‘diffuse’ lymphoid tissue (for reviews see Kraehenbuhl & Neutra, 1992; Hein, 1999). The different mucosal organs are connected with each other via the regulated migration of lymphoid cells (recirculation) and hence form an interconnected mucosal immune system (for a review see Knop & Knop, 2004b). The follicular organized form of MALT represents the afferent arm of mucosal immunity where antigens are taken up from the environment by the specialized overlying follicle-associated epithelium (FAE) (Bockman & Cooper, 1973). Antigens are then presented to lymphocytes by antigen-presenting cells, leading to lymphocyte activation, proliferation and eventual differentiation into effector cells of the B- or T-lineage (Brandtzaeg & Farstad, 1999), which leave the tissues via afferent lymphatic vessels and finally re-enter the bloodstream.
The diffuse form of lymphoid tissue, in contrast, is perceived as the efferent arm of mucosal immunity and is populated, mainly via post-capillary blood vessels, by the arising effector cells that are diffusely interspersed along most mucous membranes and their associated glands. Lymphoid cells reside in the connective tissue as lamina propria lymphocytes (LPL) and plasma cells or inside the basal layers of the epithelium as intraepithelial lymphocytes (IEL). CD8-positive suppressor/cytotoxic effector cells are usually more frequent, especially in the epithelium, than CD4-positive T-helper cells that regulate the differentiation of other cell types.
Plasma cells in mucosal tissues contribute to secretory immunity, one of the main immune effector mechanisms, by the production of polymeric immunoglobulins (mainly IgA). These are transported through the overlying epithelium with the help of an epithelial transporter molecule (secretory component, SC) and build up a protective layer at the mucosal surface (Brandtzaeg et al. 2001). Accepted secretory effector sites are the mucosal surfaces and associated glands of the digestive tract, urogenital tract, airways, etc. (Tourville et al. 1969). Of the ocular tissues, only the lacrimal gland was included here until recently (Franklin et al. 1973; Allansmith et al. 1976b; Wieczorek et al. 1988; Sullivan, 1999).
Lymphatic cells at the ocular surface and appendage
Lymphatic cells at the ocular surface and appendage of humans and animals have been known since the earliest histological investigations (as reviewed by Virchow, 1910) but their normal status and function were a continuing matter of controversy.
The lacrimal gland is an accepted part of the immune system
The lacrimal gland has been an accepted part of the immune system since IgA was detected as the predominant immunoglobulin in human tear fluid (Chodirker & Tomasi, 1963; Kühl, 1971). IgA and its transporter SC were identified in the normal human lacrimal gland by immunomorphological techniques (Franklin et al. 1973; Allansmith et al. 1976b; Wieczorek et al. 1988) but only inconsistently in the human conjunctiva (Allansmith et al. 1976a). In the rat (Gudmundsson et al. 1985; Hann et al. 1988) and mouse (McGee & Franklin, 1984), IgA and SC were only observed in the lacrimal gland but not in the conjunctiva. These observations seemed to support the primary importance of the lacrimal gland for ocular surface immune protection (Gudmundsson et al. 1985). But unlike in humans, the rat conjunctiva has a virtual absence of diffuse lymphoid cells including plasma cells from the conjunctiva (Chodosh et al. 1998b). In the rabbit, which resembles the human situation more closely, IgA-positive plasma cells were found in the lamina propria and SC in the epithelial cells of both the normal lacrimal gland and the conjunctiva (Franklin et al. 1979).
Conjunctiva-associated lymphoid tissue (CALT)
CALT occurs in most animal species
Conjunctival lymphoid follicles have been described in various species. However, very little lymphoid tissue is found in rodents such as rat and mouse (McMaster et al. 1967; Setzer et al. 1987; Chodosh et al. 1998b), which do not show lymphoid follicles under normal conditions and have very few diffusely interspersed lymphoid cells. The regular occurrence of follicles, most of which are secondary ones with a bright germinal centre indicating lymphoid cell proliferation after antigen contact, were found in the conjunctiva of species such as the guinea-pig (McMaster et al. 1967; Dwyer et al. 1983), rabbit (Axelrod & Chandler, 1979; Franklin & Remus, 1984; Knop & Knop, 1996a), turkey (Fix & Arp, 1989), chicken (Fix & Arp, 1991) and monkey (Ruskell, 1995b). These results were later confirmed and extended to several other species by (Chodosh et al. 1998a,b). In fact, conjunctival lymphoid follicles tend to form solitary nodules in most species.
Diffuse lymphoid tissue is rarely mentioned in animals, although some authors report the regular presence of IgA and plasma cells, and its transporter molecule, in the overlying epithelium, and focus on the importance of conjunctival follicles for the generation of secretory immunity (Axelrod & Chandler, 1979; Franklin et al. 1979; Franklin & Remus, 1984) in the rabbit eye. In other species, however, especially in the rat conjunctiva, diffuse lymphatic cells are rare and components of the secretory immune system are reported to be absent (Gudmundsson et al. 1985).
The contribution of Gordon Ruskell to the analysis of CALT in animals
Gordon Ruskell, who was an active member of the Association for Eye Research (AER) for many decades, acted as chairman for one of our first international oral papers at the AER congress in Bad Honneff in 1990, and over the years became a colleague and friend who was always generous in sharing the wealth of his experience.
Apart from all his other interests, and the great contribution he made to our understanding of the eye and appendage, Gordon Ruskell developed an interest in the conjunctival follicles of the cynomolgus monkey in the early 1990s, and found important evidence for CALT in a primate species. He first reported these structures at the AER congress in Granada 1993, in order to stimulate research into this topic. This reflected an early common interest, because we had just received a grant to perform research into this topic in humans. Gordon Ruskell later applied his masterly knowledge of ocular anatomy and morphological techniques to perform an elegant and precise light and electron microscopic study (Ruskell, 1995b). As a dedicated anatomist, he started with a consideration of the conjunctival topography, and reported that only the peripheral tarsal and orbital regions of the upper and lower palpebral conjunctiva contained lymph follicles with an average diameter of 0.1–0.7 mm. They were more numerous in the upper lid than the lower lid, and appeared connected by large lymphatic vessels resulting in chains of follicles. The solitary primary and secondary follicles were associated with high endothelial venules (HEV), displaying evidence consistent with lymphocyte extravasation. Lymphocytes, reticulocytes, macrophages (including tingible body macrophages), putative follicular dendritic cells and plasmacytes were identified in the follicles. Mitoses were found in secondary follicles, mostly confined to the germinal centres, and they were numerous in many. These follicles had a regular FAE, which contained characteristic antigen-transporting M cells. Gordon Ruskell also noted that the underlying basal lamina was fragmented. In a later publication he showed that these lymphoid follicles were equipped with a sensory innervation pointing to a potential neuroimmunological regulation (Ruskell & VanderWerf, 1997).
As anecdotal evidence of Gordon Ruskell's unsurpassed British genius for understatement, humour and politeness, we remember how when we tried to congratulate him on the excellence of his work during a break at the 1996 JERMOV congress in Montpellier, he merely laughed and speculated that we were saying the same to all the delegates. Then, asking us to excuse his manners, he took a large bite from his hamburger and carried on eating.
In later years we worked together in the Section of Anatomy of the European Association for Vision and Eye Research (EVER), and co-organized special interest symposia on CALT (1999) and on the functional anatomy of the ocular surface (2002) at EVER meetings.
Evidence for CALT in humans
Lymphoid follicles have been the subject of research for many decades, and several authors have described their presence in the human conjunctiva. In a histological study on human autopsy specimens, Osterlind (1944) routinely found lymphoid follicles (or ‘nodules’). They were not present in the newborn, then became most numerous just before puberty, and slowly decreased in number until old age. Most of them were secondary follicles, oval in shape and with an average diameter of 0.3 mm, as also described by Kessing (1968). In another histological study on conjunctival leucocytes, Allansmith et al. (1978). also noted the presence of primary follicles in about half of the normal biopsy specimens studied. Immunohistochemical results on follicles were inconsistent, however; some did not observe them at all (Belfort & Mendes, 1979; Bhan et al. 1982) or observed them only rarely (Sacks et al. 1986), and concluded that they represented inflammatory lesions resulting from infiltration of inflammatory cells; others, by contrast, reported the presence of follicles in all fornical tissues investigated (Dua et al. 1994). One study on secondary lymphoid follicles in the fornix found them in only 31% of cases, and hence concluded that MALT is only acquired in response to antigenic stimulation (Wotherspoon et al. 1994).
Diffusely interspersed ‘free’ lymphoid cells, including many plasma cells, were observed by Osterlind (1944) in most of his postnatal specimens, reaching maximal development in the peritarsal and orbital conjunctiva, but he described their presence as due to ‘lymphoid infiltration’. Although these cells regularly occurred in otherwise normal specimens, they were still regarded as a sign of subclinical or clinical inflammation (‘conjunctivitis chronica simplex’). Kessing (1968) also mentioned free lymphoid cells as a regular characteristic of the lamina propria in a study on the distribution of conjunctival mucus crypts and goblet cells. Allansmith et al. (1976b) confirmed the regular presence of plasma cells and lymphocytes in the human conjunctiva in a comparative study of conjunctiva and lacrimal glands. In keeping with received wisdom, they were still termed ‘inflammatory cells’ even though they occurred in every normal tissue (Allansmith et al. 1978).
Early immunohistological studies using sheep erythrocytes and complement complex as markers for T and B cells reported that the normal conjunctiva does not contain lymphocytes or plasma cells (Belfort & Mendes, 1979), whereas others found some lymphoid cells in the lamina propria and dendritic Langerhans cells in the epithelium (Bhan et al. 1982). Further studies supported the predominance of T cells over B cells and of CD8- over CD4-positive T cells, especially in the conjunctival epithelium (Sacks et al. 1986; Dua et al. 1994; Wotherspoon et al. 1994; Hingorani et al. 1997). This resembled other lymphoid organs (Kraehenbuhl & Neutra, 1992) and led to the interpretation that, in the conjunctiva, immune reactions may be down-regulated in order to avoid inflammation. Lymphocytes bearing the human mucosa lymphocyte antigen (HML-1) were found in all investigated conjunctival tissues (Dua et al. 1994; Hingorani et al. 1997).
However, the topographical distribution of conjunctival lymphoid tissue remained controversial. The more recent studies, which relied mainly on small clinical biopsies, described the highest expression either in the epibulbar zone (Dua et al. 1994; Hingorani et al. 1997) or in the fornix (Sacks et al. 1986), and some authors restricted their investigations to fornical tissue (Wotherspoon et al. 1994), despite the reported predominance in the tarso-orbital position, in studies that used wholemount preparations in the human (Osterlind, 1944; Kessing, 1968) or in the monkey (Ruskell, 1995b).
Lymphoid tissue at the ocular surface and appendage
To summarize, when we began our investigations there was still much disagreement about the regular presence of conjunctival lymphoid follicles, of a diffuse lymphoid tissue and of the components of the secretory immune system (IgA and SC) that are important for the diagnosis of a normal lymphoid tissue. The same was true regarding the topographical distribution of lymphoid tissue at the human ocular surface. Furthermore, most of our knowledge about lymphoid tissue in the lacrimal drainage system came from histological studies from the 19th century which indicated the presence of lymphoid cells (see Virchow, 1910).
Therefore, we investigated a large number of complete and macroscopically normal human tissues from body donors at our Department of Anatomy. These included normal human conjunctival sacs (53), lacrimal drainage systems (53) and lacrimal glands (nine) from an aged population (at the middle of the seventh decade, on average). The tissues were studied using different methods. Inspection of stained and optically cleared conjunctival whole mounts with a stereo magnifier was performed to give information on topography and morphometry. Sections of paraffin-embedded and cryo-preserved tissues were analysed by histology and immunohistology to enable us to identify the tissue structure and cell types. Ultrastructural analysis of cellular details was performed by transmission and scanning electron microscopy. However, when the results were first submitted for publication in 1997 they were rejected because they contradicted the standard textboook descriptions of the subject. Continued research over the years has nevertheless verified the presence of a normal ocular mucosal immune system on structural and molecular biological levels.
Typical diffuse lymphoid tissue is a regular component of the human ocular surface and contributes to the secretory immune system
Diffusely scattered lymphatic cells were found in every normal human conjunctiva (Knop & Knop, 1996a; Knop & Knop, 1997a; Knop & Knop, 2000); they appeared as a carpet-like layer of inhomogeneous density in the observation of stained and cleared translucent whole mounts (Fig. 2). The highest density was observed in the tarsal and orbital conjunctiva, diminishing towards the fornix and further towards the bulbar conjunctiva, where only few cells were detectable (Knop & Knop, 1999a). Histological methods revealed that the diffusely scattered lymphoid cells were, with the exception of IEL, confined to the narrow subepithelial lamina propria and hence appeared as a layer-like structure (Figs 2 and 3). This lymphoid layer consisted mainly of lymphocytes and plasma cells and had an inhomogeneous cell density and extension (one to several cells thick, Fig. 3). Immunohistochemistry confirmed that most lymphocytes were CD3-positive T lymphocytes, whereas CD20-positive B lymphocytes were rare and restricted to the follicular accumulations. Among abundant small vessels, high endothelial venules (Fig. 3) also occurred here (Knop & Knop, 1998a,b). Most of the plasma cells stained intensely for IgA (Fig. 4). The transporter-protein SC was regularly present in the upper part of the normal conjunctival epithelium and was characteristically diminished in the follicle-associated epithelium. A diffuse lymphoid tissue and lymphoid follicles were also found associated with the tarsal crypt system of the conjunctiva (Knop & Knop, 1997a,2002c).
Fig. 2.
Translucent whole-mount and section of a normal human conjunctiva. In a haematoxylin-stained and cleared whole-mount of the normal human conjunctiva (A), the different amount of lymphoid tissue occurs as different darkness of staining. A layer of diffuse lymphoid tissue of varying density is universally present. Lymphoid follicles occasionally occur and lymphoid cells are also associated with the conjunctival crypt system. This consists of the tarsal tubular crypts of Henle (arrowhead) and the interwoven crypt furrows of Stieda at the tarsal margin and lid margin. A respective haematoxylin–eosin-stained paraffin section (B) of the tarso-orbital zone shows lymphoid cells in the subepithelial lamina propria, either diffusely interspersed or organized into a follicle; scale bar = 1 mm.
Fig. 3.
Diffuse lymphoid tissue. The diffuse lymphoid tissue is seen in a haematoxylin–eosin-stained paraffin section (A) and in transmission electron micrographs (B–D) of the normal human conjunctiva. It consists, in addition to some intraepithelial lymphocytes (iel in A, B) in the epithelium (ep), mainly of lymphocytes (lym in A, D), and of plasma cells (pc in A, C, D) that form a layer-like arrangement in the lamina propria (lp). Numerous small vessels, including occasional high endothelial venules (hev in A, D) with lymphocytes in the wall and in the periphery (arrow), regularly occur; scale bar = 10 µm.
Fig. 4.
The secretory immune system is expressed with similar characteristics in the ocular surface and appendage. A diffuse lymphoid tissue composed of lymphocytes (arrowheads) and plasma cells (arrows) is similarly found between the secretory acini of the lacrimal gland (A–C), in the conjunctiva (D, E) and in the lacrimal drainage system (F, G). The components of the secretory immune system (IgA-positive plasma cells and its transporter SC in the overlying epithelium) are also not only observed in the lacrimal gland (B, C) as previously thought, but equally expressed in the mucosa of the conjunctiva (D, E) and in the lacrimal drainage system (F, G) as seen by immunohistochemistry. This indicates a local production of specific protective mucosal immunoglobulins in all parts of the ocular surface and appendage; scale bars in A–E = 10 µm; F, G = 100 µm.
In the upstream lacrimal gland we found the previously described mixture of plasma cells and lymphocytes in the loose connective tissue between the secretory acini that can, in the light of the present knowledge on mucosal immunity, also be characterized as a diffuse lymphoid tissue. Immunohistology revealed evidence for the expression of IgA protein in the periacinar plasma cells and for its transporter SC in the acinar epithelium. As a novel observation, we found that the diffuse lymphoid tissue with IgA-positive plasma cells in the lamina propria and its transporter molecule in the overlying epithelium is continuous from the periacinar tissue of the lacrimal gland along the lacrimal excretory ducts into the conjunctiva, and all along the conjunctival mucosa into the lacrimal drainage system (Fig. 4) down towards the nose (Knop & Knop, 2002a; 2003). Diffuse lymphoid tissue, with similar characteristics – including the presence of mucosa-specific lymphocytes (HML-1-positive) and MHC-2-positive dendritic cells – was present in the lacrimal drainage system (Knop & Knop, 2001; Knop & Knop, 2002a). It was continuous with that of the conjunctiva via the lacrimal canaliculi.
Lymphoid follicles with typical characteristics occur in the normal human conjunctiva and lacrimal drainage system
Lymphoid follicles occurred in the majority (60%) of complete human conjunctival tissues with a right/left symmetry in both eyes in most (86%) donors (Knop & Knop, 2000). Follicle diameters ranged from 0.1 to 1.25 mm with an average of about 0.25 mm (Knop & Knop, 1999b) and the follicle shape was usually lenticular and only slightly prominent over the conjunctival surface (Fig. 5). Like the diffuse lymphoid tissue, follicles were mainly observed in the tarsal and orbital conjunctiva of the upper and lower lids, with fewer follicles in the fornix. In the bulbar conjunctiva, follicles were rarely seen. Immunohistochemistry confirmed that the follicles were accumulations of B cells (Fig. 5) accompanied by diffusely scattered T cells in the periphery. The overlying epithelium showed typical specializations, including flattened epithelial cells, absence of goblet cells, an increase in intraepithelial lymphocytes and a decreased amount of IgA and its transporter SC (Knop & Knop, 2000), similar to FAE elsewhere in the mucosal immune system (Parsons et al. 1991).
Fig. 5.
Lymphoid follicles. Follicles in the conjunctiva are usually relatively flat and lenticular, but still consist of accumulations of B lymphocytes as seen by immunostaining (A). Those in the lacrimal drainage system are more roundish (B–D). They occasionally show a bright germinal centre (gc and interrupted circular line in B) with a lymphocyte corona (c) and an overlying subepithelial dome areal. The apical epithelium has characteristics of a follicle-associated epithelium (fae). In the parafollicular zone high endothelial venules (arrowheads in B) occur. T cells (C) are distributed around the central cell mass of B cells (D); scale bar in A–E = 10 µm; F, G = 100 µm.
HEV for a regulated migration of lymphoid cells were always present in the parafollicular zone and also in the diffuse tissue (Knop & Knop, 1998b). They showed the typical ultrastructural morphology (Fig. 3D) of these specialized post-capillary venules with roundish endothelial cells encircled by layers of pericytes, and they contained lymphoid cells in their wall and in the periphery (Knop & Knop, 1998a), indicating their transit from the bloodstream into the lymphoid tissue.
Follicles with similar characteristics and HEV were also observed in a majority (56%) of the complete lacrimal drainage systems (Knop & Knop, 2002b), with a right/left symmetry in 78% of individuals (Knop & Knop, 2001). Their average diameter (0.5 mm) was larger and the shape more roundish (Fig. 5) compared with the conjunctiva, reflecting a larger space in the lamina propria in this location. Ultrastructural investigation revealed characteristics of antigen-transporting M cells in the FAE. Follicles were more frequent in the larger cavernous parts of the lacrimal drainage system (lacrimal sac and nasolacrimal duct), but also occurred occasionally in the terminal part to the lacrimal canaliculi (Knop & Knop, 2001). Apart from occasional smaller lymphocyte accumulations, no obvious lymphoid follicles were found in the lacrimal gland.
Discussion
These results in complete normal human ocular tissues (lacrimal gland, conjunctiva and lacrimal drainage system) show the presence of a regular MALT, confirmed using histological, immunohistological, electron microscopic and molecular biological methods. They support previous findings of the presence of human conjunctival lymphoid cells that were restricted to histological investigations (Virchow, 1910; Osterlind, 1944; Kessing, 1968), to the investigation of small biopsies (Sacks et al. 1986; Dua et al. 1994; Hingorani et al. 1997) or to specimens from selected fornical zones (Wotherspoon et al. 1994). In addition, we were able to clarify its topographical distribution in the conjunctiva by the use of complete human ocular tissues and to show the continuity of the MALT from the lacrimal gland over the conjunctiva into the lacrimal drainage system as a basis to recognize this mucosal immune system as an EALT. It was also possible to show the regular presence of IgA and SC proteins by improved immunohistochemical methods and to verify the respective mRNA in conjunctiva, similar to lacrimal gland tissues. This provides evidence that the conjunctiva plays a role in specific immune defence by local production of secretory IgA.
Lymphoid follicles are a normal component of human ocular MALT
Lymphoid follicles occur in a majority of human conjunctival tissues, as reported by several studies that show them in varying frequencies from 50% (Allansmith et al. 1978) or 60% (Knop & Knop, 2000) to 100% of cases (Dua et al. 1994), and their number depends on age (Osterlind, 1944). Only biopsy-based studies that did not primarily set out to look for follicles reported them as absent or scarce (Belfort & Mendes, 1979; Bhan et al. 1982; Sacks et al. 1986; Hingorani et al. 1997). A study that was restricted to fornical zones and only considered secondary follicles reported them in 30% of cases but did not state the total number of follicles. Because human conjunctival follicles occur in every young individual, and an involution is reported with progressing age (Osterlind, 1944), these findings confirm conjunctival follicles as a normal component of the human conjunctiva, even if they do not occur in every aged specimen. Similar numbers are reported in the lacrimal drainage system in about 40–50% of cases (Knop & Knop, 2001; Paulsen et al. 2002) or in about 60% in a study that only investigated complete lacrimal drainage systems containing all tissue from the lacrimal canaliculi down to the nasolacrimal duct (Knop & Knop, 2002b).
Conjunctival follicles are usually relatively small in diameter (∼0.3 mm) and lenticular in shape (Osterlind, 1944; Knop & Knop, 2000), rather than roundish as generally found in the lacrimal drainage system. This is probably a result of the small space available inside the conjunctival lamina propria compared with the larger connective tissue of the lacrimal drainage system. T cells were not always distinctly demarcated in the parafollicular areas but were frequently more diffusely distributed throughout the parafollicular space. Regardless, follicles show typical structural characteristics, with an FAE (Knop & Knop, 2000) and occasionally bright germinal centre in the human conjunctiva (Wotherspoon et al. 1994) and lacrimal drainage system (Knop & Knop, 2000; 2001; Paulsen et al. 2000; Paulsen et al. 2002). Follicles in the lacrimal drainage system are larger than in the conjunctiva (about 0.5 mm). Indications for cells whose structure resembles antigen-transporting M cells have so far only been observed in the FAE of the lacrimal sac (Knop & Knop, 2001).
Ocular lymphoid tissue has specialized vessels (HEV) (Knop & Knop, 1998b) for the regulated migration of lymphoid cells that show a typical ultrastructure (Knop & Knop, 1998a) and express adhesion molecules (Haynes et al. 1999). The ocular mucosal immune system is therefore connected to the exchange of lymphoid cells with the other organs of the mucosal immune system and hence integrated into the MALT system.
Diffuse lymphoid tissue is universally present at the human ocular surface and appendage
Complete and normal human tissues enabled us to verify the general presence of interspersed lymphoid cells and to diagnose them as the diffuse type of lymphoid tissue. This extends from the lacrimal gland throughout the conjunctiva into the lacrimal drainage system down to the nose. This finding of a diffuse lymphoid effector tissue that unites the conjunctiva and the ocular appendage appears to be the first description of such (Knop & Knop, 1996a,2002a; 2003).
The detection of a constitutive presence of lymphoid tissue with typical characteristics inside the lacrimal drainage system also appears to be the first such description (Knop & Knop, 1996b; Knop & Knop, 1997b), and we have since been able to include it in the mucosal immune system as ‘lacrimal drainage-associated lymphoid tissue’ (LDALT) (Knop & Knop, 2001) according to the established immunological nomenclature. These results are supported by other findings of lymphoid tissue in this area (Paulsen et al. 2000; Paulsen et al. 2002) and of previous isolated findings of IgA (Perra et al. 1995; Sirigu et al. 2000).
The presence of plasma cells at the normal ocular surface and lacrimal drainage system was controversial for many years. Although frequently revealed using histological methods (Osterlind, 1944; Kessing, 1968; Allansmith et al. 1976b; 1978; Wotherspoon et al. 1994; Hingorani et al. 1997), they were not reliably found using immunohistochemistry because some found many (Bhan et al. 1982) whereas others observed few (Allansmith et al. 1976a) or none at all (Sacks et al. 1986). Similarly, the presence of the IgA transporter molecule SC was only reliably found in the lacrimal and accessory lacrimal glands (Franklin et al. 1973; Allansmith et al. 1976a; Allansmith & Gillette, 1980) and either not at all (Allansmith & Gillette, 1980; Allansmith et al. 1976a) or inconsistently (Cohen & Allansmith, 1981) in the conjunctival epithelium. In contrast, in the similarly structured rabbit conjunctiva the physiological presence of SC is established and even used as an epithelial differentiation marker (Liu et al. 1981). These inconsistencies in the early immunohistological detection of IgA and SC were possibly due to technical problems, because in studies with modern detection systems both proteins were reliably found in the conjunctiva (Knop & Knop, 2000) and lacrimal drainage system (Knop & Knop, 2001). Recently, the respective mRNA could also be verified by RT-PCR (Knop et al. 2003; Knop & Knop, 2004c). These results clearly characterize the conjunctiva and lacrimal drainage system as components of the secretory immune system similar to the lacrimal gland, which can locally produce secretory IgA and do not solely depend on the IgA supply from the lacrimal gland via tear flow.
The mucosal immune system of the ocular surface and adnexa forms an EALT
From our own investigations, together with previous reports in the literature, several lines of evidence suggest that the ocular mucosal tissues form a functional immunological unit for ocular surface immune protection (Fig. 6).
Fig. 6.
Eye-associated lymphoid tissue (EALT) at the ocular surface and appendage. EALT consists of lymphoid tissue that is continuous (blue tissue outline) from the lacrimal gland throughout the conjunctiva (as CALT) into the lacrimal drainage system (as LDALT). It is functionally connected by the flow of tears over the surfaces (yellow arrows over conjunctiva and lacrimal drainage system). It is also connected, via specialized blood vessels that provide regulated inflow and via lymph vessels that provide outflow, to the recirculation of lymphoid cells in the body. Follicles in CALT and LDALT allow the detection of ocular surface antigens and the population (large interrupted arrows) of the same and other organs, as, for example, the lacrimal gland, with specific effector cells (e.g. plasma cells).
First, a diffuse lymphoid tissue is recognized as continuous from the lacrimal gland along its excretory ducts into the conjunctiva and further into the lacrimal drainage system. It acts as an immunological effector site with lymphocytes and plasma cells for cellular and secretory immunity. Secondly, all three organs (lacrimal gland, conjunctiva and lacrimal drainage system) are connected via the flow of tears and hence conceivably share the same protective and aggressive factors, which necessarily ties their immune function together. Finally, we have identified specialized vessels (HEV) in the conjunctiva and lacrimal drainage system that were previously unknown and represent the basis for a regulated lymphocyte migration and exchange between the ocular tissues themselves and with the other organs of the mucosal immune system. Similar mechanisms must also take place in the lacrimal gland, despite the absence of obvious HEV, because the gland contains a selective accumulation of IgA-positive plasma cells.
Therefore, the conjunctiva, together with the lacrimal gland and the lacrimal drainage system, forms a functional unit for ocular surface immune protection that should be termed, according to the existing immunological nomenclature, an eye-assocated lymphoid tissue or EALT. It appears to have special importance for corneal immunity owing e.g. to its topographical localization, which corresponds to the corneal position during eye closure.
Through such a system that contains lymphoid follicles for antigen detection and production of effector cells (in the conjunctiva and lacrimal drainage system) it is possible that ocular antigens can be detected locally in order to provide the ocular tissues with respective effector cells and especially the lacrimal gland with specific plasma cells. This may be important, although the intestinal mucosa is assumed to have a generally dominant role in mucosal immunity, because it appears unlikely that all antigens that occur at the ocular surface are also present in the intestine.
The topography of EALT in the conjunctiva is congruent with the position of the cornea
The topography of lymphoid tissue may reflect the distribution and amount of antigen. It is most strongly expressed in the tarso-orbital region of the conjunctiva, where an accumulation of foreign matter is found (Zuckerman, 1966), and it may occur inside the epithelial infoldings representing the conjunctival crypts (Knop & Knop, 2002c).
The tarso-orbital predominance of lymphoid tissue found in the human tissue whole-mounts (Fig. 7) does not fit (?) with results from biopsies that seemed to indicate a predominance of lymphoid tissue either in the bulbar (Dua et al. 1994; Hingorani et al. 1997) or fornical (Sacks et al. 1986; Wotherspoon et al. 1994) conjunctiva.
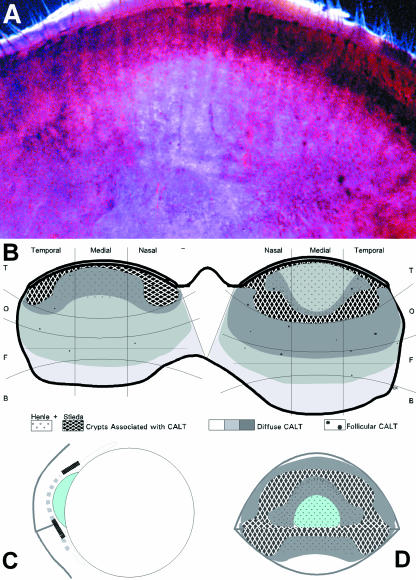
Fig. 7.
EALT is in an ideal topographical position to support corneal immune protection. In a haematoxylin-stained and cleared whole-mount of the normal human conjunctiva the different amount of lymphoid tissue occurs as different darkness of staining (A). The tarsal and beginning orbital conjunctiva of the upper lid are seen; the lid margin is to the top. The distribution of the lymphoid tissue in the upper and lower lid is also indicated in a schematic drawing (B) showing the main expression of lymphoid tissue in the tarso-orbital conjunctiva; increasing density of diffuse lymphoid tissue is indicated by darker shades. A schematic cross-section (C) and frontal view (D) with projection of the conjunctival lymphoid tissue on the ocular surface show that CALT co-localizes with the position of the cornea during eye closure. The tarsal conjunctiva, with a local minimum of lymphoid cells in the upper mid-tarsal area, contains numerous tubular crypts of Henle (small circles in B, D, and interrupted line in C) that contribute to immune protection.
These biopsy-based results can probably be explained by problems of exact localization of a small clinical biopsy compared with a tissue whole-mount, but may also be caused by the occasionally imprecise classification of the conjunctival zones. The orbital conjunctiva (Fig. 1), for example, is often not considered as a separate zone located between the tarsal and fornical conjunctiva; sometimes it is counted as belonging to the fornical zone. It is also difficult to judge macroscopically how far the fornical zone extends onto the bulbus. Therefore, ‘fornical’ biopsies may in fact contain orbital tissue and ‘epibulbar’ biopsies may already contain fornical tissue, which both leads to an erroneously high cell count of lymphoid cells. By contrast, we found a local minimum in the midtarsal region, which may explain the low reported density of ‘tarsal’ lymphoid cells in at least one biopsy-based study (Hingorani et al. 1997), although the tarso-orbital zone in general contains numerous lymphoid cells as observed in whole-mount tissues (Knop & Knop, 2001). Although there is a local minimum of diffuse lymphoid tissue in the upper mid-tarsal conjunctiva which overlies the central cornea in the closed eye, this region is equipped with numerous tubular crypts of Henle (Fig. 7B–D). These are associated with frequent plasma cells and show an active production of secretory IgA (Knop & Knop, 2002c) and its supply to the ocular, and in this case also the corneal, surface.
The clearly observed predominance of lymphoid tissue in the tarso-orbital conjunctiva, mainly in the upper but also in the lower lid, is supported by other studies that used conjunctival whole-mount tissues from the human (Osterlind, 1944; Kessing, 1968) or from other primate species such as the monkey (Ruskell, 1995b). This distribution applies to all components of CALT as the diffuse lymphoid cells, those associated with the tarsal conjunctival crypt system and also to the lymphoid follicles (Knop & Knop, 1997a; 2000).
A role for EALT in corneal immune protection
If the topographical location of the conjunctival lymphoid tissue is projected onto the ocular surface (Fig. 7), it can be detected that it corresponds to the position of the cornea during eye closure when it is moved slightly upwards. EALT, in the tarso-orbital regions of the conjunctiva, is then in the position to support the immune protection of the cornea that is itself largely free of lymphoid cells. It may act during blinking as an ‘immunological windscreen-wiper’ and during sleep as an ‘immunological cushion’.
The immunological support of EALT for the cornea may be two-fold. In the efferent immune function, EALT can provide the cornea with innate and specific antibacterial peptides and proteins, including secretory IgA (Knop et al. 2003), that are not produced in the cornea. Furthermore, the presence of a resident EALT may explain how the cornea can be provided with factors and cells that were observed in the closed-eye model of the tear film (Sack et al. 2000). During eye closure there is an up-regulated level of homeostasis of the pro-inflammatory factors from mononuclear cells (Sack et al. 2002) that can only reach the tear film through the conjunctival mucosa, and of anti-inflammatory factors of mucosal origin (Sack et al. 2004), which serves to prevent microbial growth in the moist chamber of the closed-eye tear film. In the afferent immune function, by contrast, the direct contact of conjunctival EALT with the corneal surface may also suggest that it can assist the cornea in the detection of corneal antigens and in the generation of an appropriate immune response. Its role in corneal transplantation immunology, when the graft is in direct contact with the overlying conjunctival lymphoid tissue, is insufficiently understood because the rejection of corneal grafts seems to be mediated mainly by corneal dendritic cells (Hamrah et al. 2003) that travel to the regional lymph nodes (Yamagami et al. 2002) in order to initiate the immune rejection. The function of EALT, however, in accordance with MALT in other organs, appears to be the active generation of immune tolerance against suitable antigens at the ocular surface rather than inflammatory immune reactions as occur in transplant rejection.
The main function of the mucosal immune system may possibly be the generation of tolerance (McGhee et al. 1999) against the multitude of non-pathological antigens that occur at the mucosal surfaces in general and especially at the ocular surface, which is directly exposed to the environment. The mucosal immune system has the task of maintaining a delicate balance between the generation of inflammatory immune defences that could potentially harm the mucosal surface and the generation of immune tolerance, which prevents such problems. The importance of local immune regulation for corneal integrity is indicated by the generation of different types of effector T-cells, as e.g. a bias on either the Th1- or the Th2-type T-helper cells in corneal Pseudomonas aeruginosa infection. If the ocular immune system is biased towards a Th1 immune response, serious inflammatory damage to the cornea occurs, whereas a prevailing Th2 response results in milder alterations (Hazlett, 2004).
If the generation of immune tolerance is impaired due to damage to the surface epithelium leading to production of inflammatory cytokines (as described in inflammatory ocular surface disease, including dry eye syndrome: Pflugfelder et al. 1999), the uncontrolled access of antigens can result, and the cells of the lymphoid system may be deregulated (Knop et al. 2004). A resulting inflammatory immune response, with the production of further inflammatory cytokines and proteases, can lead to corneal destruction (Pflugfelder et al. 1999; Li et al. 2001). The elevation of inflammatory cytokines and proteases is reported in inflammatory disease of the intestine (MacDonald et al. 1999), and also in ocular surface disease (Barton et al. 1998; Stern et al. 1998; Afonso et al. 1999; Garrana et al. 1999; Meller et al. 2000; Pflugfelder et al. 2000; Li et al. 2001; Dursun et al. 2002). As far as deregulation is concerned, cells of the physiological protective mucosal immune system can also be involved in inflammatory ocular surface disease (Stern et al. 2002; Knop & Knop, 2002a; Knop & Knop, 2004a), as found in other mucosal organs.
Acknowledgments
This research was supported by research grants from the Sandoz Stiftung für Therapeutische Forschung, the Gesellschaft der Freunde der Medizinischen Hochschule Hannover, the Arbeitskreis Trockenes Auge im Berufsverband der Augenärzte Deutschlands and the Deutsche Forschungsgemeinschaft, DFG, and was twice awarded the Sicca Prize for Dry Eye Research by the Arbeitskreis Trockenes Auge des Berufsverbandes der Augenärzte Deutschlands.
References
- Afonso AA, Sobrin L, Monroy DC, Selzer M, Lokeshwar B, Pflugfelder SC. Tear fluid gelatinase B activity correlates with IL-1alpha concentration and fluorescein clearance in ocular rosacea. Invest. Ophthalmol. Vis. Sci. 1999;40:2506–2512. [PubMed] [Google Scholar]
- Allansmith MR, Hahn GS, Simon MA. Tissue, tear, and serum IgE concentrations in vernal conjunctivitis. Am. J. Ophthalmol. 1976a;81:506–511. doi: 10.1016/0002-9394(76)90310-x. [DOI] [PubMed] [Google Scholar]
- Allansmith MR, Kajiyama G, Abelson MB, Simon MA. Plasma cell content of main and accessory lacrimal glands and conjunctiva. Am. J. Ophthalmol. 1976b;82:819–826. doi: 10.1016/0002-9394(76)90056-8. [DOI] [PubMed] [Google Scholar]
- Allansmith MR, Greiner JV, Baird RS. Number of inflammatory cells in the normal conjunctiva. Am. J. Ophthalmol. 1978;86:250–259. doi: 10.1016/s0002-9394(14)76821-7. [DOI] [PubMed] [Google Scholar]
- Allansmith MR. Defense of the ocular surface. Int. Ophthalmol. Clin. 1979;19:93–109. [PubMed] [Google Scholar]
- Allansmith MR, Gillette TE. Secretory component in human ocular tissues. Am. J. Ophthalmol. 1980;89:353–361. doi: 10.1016/0002-9394(80)90004-5. [DOI] [PubMed] [Google Scholar]
- Axelrod AJ, Chandler JW. Morphologic characteristics of conjunctival lymphoid tissue in the rabbit. In: Silverstein AM, Connor GR, editors. Proceedings of the Second International Symposium on the Immunology and Immunopathology of the Eye; Masson Publishing; New York. 1979. pp. 292–301. [Google Scholar]
- Barton K, Nava A, Monroy DC, Pflugfelder SC. Cytokines and tear function in ocular surface disease. Adv. Exp. Med. Biol. 1998;438:461–469. doi: 10.1007/978-1-4615-5359-5_64. [DOI] [PubMed] [Google Scholar]
- Belfort R, Jr, Mendes NF. T- and B-lymphocytes in the human conjunctiva and lacrimal gland. In: Silverstein AM, O'Connor RG, editors. Immunology and Immunopathology of the Eye. New York: Masson Publishing; 1979. pp. 287–291. [Google Scholar]
- Bhan AK, Fujikawa LS, Foster CS. T-cell subsets and Langerhans cells in normal and diseased conjunctiva. Am. J. Ophthalmol. 1982;94:205–212. doi: 10.1016/0002-9394(82)90076-9. [DOI] [PubMed] [Google Scholar]
- Bienenstock J, McDermott M, Befus D, O'Neill M. A common mucosal immunologic system involving the bronchus, breast and bowel. Adv. Exp. Med. Biol. 1978;107:53–59. doi: 10.1007/978-1-4684-3369-2_7. [DOI] [PubMed] [Google Scholar]
- Bockman DE, Cooper MD. Pinocytosis by epithelium associated with lymphoid follicles in the bursa of Fabricius, appendix, and Peyer's patches. An electron microscopic study. Am. J. Anat. 1973;136:455–477. doi: 10.1002/aja.1001360406. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P, Farstad IN. The human mucosal B-cell system. In: Ogra PL, Mestecky J, Lamm ME, Strober W, McGhee JR, Bienenstock J, editors. Handbook of Mucosal Immunology. Vol. 27. San Diego: Academic Press; 1999. pp. 439–468. [Google Scholar]
- Brandtzaeg P, Baekkevold ES, Morton HC. From B to A the mucosal way. Nat. Immunol. 2001;2:1093–1094. doi: 10.1038/ni1201-1093. [DOI] [PubMed] [Google Scholar]
- Bron AJ, Seal DV. The defences of the ocular surface. Trans. Ophthalmol. Soc. UK. 1986;105:18–25. [PubMed] [Google Scholar]
- Bron AJ, Tripathi DM, Tripathi BJ. Wolff's Anatomy of the Eye and Orbit. London: Chapman & Hall Medical; 1997. [Google Scholar]
- Chandler JW, Gillette TE. Immunologic defense mechanisms of the ocular surface. Ophthalmology. 1983;90:585–591. doi: 10.1016/s0161-6420(83)34510-3. [DOI] [PubMed] [Google Scholar]
- Chodirker W, Tomasi TB. Gamma globulins. Quantitative relationships in human serum and non-vascular fluids. Science. 1963;142:1080–1081. doi: 10.1126/science.142.3595.1080. [DOI] [PubMed] [Google Scholar]
- Chodosh J, Nordquist RE, Kennedy RC. Anatomy of mammalian conjunctival lymphoepithelium. Adv. Exp. Med. Biol. 1998a;438:557–565. doi: 10.1007/978-1-4615-5359-5_79. [DOI] [PubMed] [Google Scholar]
- Chodosh J, Nordquist RE, Kennedy RC. Comparative anatomy of mammalian conjunctival lymphoid tissue: a putative mucosal immune site. Dev. Comp. Immunol. 1998b;22:621–630. doi: 10.1016/s0145-305x(98)00022-6. [DOI] [PubMed] [Google Scholar]
- Cohen EJ, Allansmith MR. Fixation techniques for secretory component in human lacrimal gland and conjunctiva. Am. J. Ophthalmol. 1981;91:789–793. doi: 10.1016/0002-9394(81)90014-3. [DOI] [PubMed] [Google Scholar]
- Dua HS, Gomes JA, Jindal VK, et al. Mucosa specific lymphocytes in the human conjunctiva, corneoscleral limbus and lacrimal gland. Curr. Eye Res. 1994;13:87–93. doi: 10.3109/02713689409042401. [DOI] [PubMed] [Google Scholar]
- Dursun D, Wang M, Monroy D, et al. Experimentally induced dry eye produces ocular surface inflammation and epithelial disease. Adv. Exp. Med. Biol. 2002;506:647–655. doi: 10.1007/978-1-4615-0717-8_91. [DOI] [PubMed] [Google Scholar]
- Dwyer RS, Darougar S, Monnickendam MA. Unusual features in the conjunctiva and cornea of the normal guinea-pig: clinical and histological studies. Br. J. Ophthalmol. 1983;67:737–741. doi: 10.1136/bjo.67.11.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fix AS, Arp LH. Conjunctiva-associated lymphoid tissue (CALT) in normal and Bordetella avium-infected turkeys. Vet. Pathol. 1989;26:222–230. doi: 10.1177/030098588902600306. [DOI] [PubMed] [Google Scholar]
- Fix AS, Arp LH. Morphologic characterization of conjunctiva-associated lymphoid tissue in chickens. Am. J. Vet. Res. 1991;52:1852–1859. [PubMed] [Google Scholar]
- Franklin RM, Kenyon KR, Tomasi TB., Jr Immunohistologic studies of human lacrimal gland: localization of immunoglobulins, secretory component and lactoferrin. J. Immunol. 1973;110:984–992. [Google Scholar]
- Franklin RM, Prendergast RA, Silverstein AM. Secretory immune system of rabbit ocular adnexa. Invest. Ophthalmol. Vis. Sci. 1979;18:1093–1096. [PubMed] [Google Scholar]
- Franklin RM, Remus LE. Conjunctival-associated lymphoid tissue: evidence for a role in the secretory immune system. Invest. Ophthalmol. Vis. Sci. 1984;25:181–187. [PubMed] [Google Scholar]
- Garrana RM, Zieske JD, Assouline M, Gipson IK. Matrix metalloproteinases in epithelia from human recurrent corneal erosion. Invest. Ophthalmol. Vis. Sci. 1999;40:1266–1270. [PubMed] [Google Scholar]
- Gudmundsson OG, Sullivan DA, Bloch KJ, Allansmith MR. The ocular secretory immune system of the rat. Exp. Eye Res. 1985;40:231–238. doi: 10.1016/0014-4835(85)90008-9. [DOI] [PubMed] [Google Scholar]
- Hamrah P, Liu Y, Zhang Q, Dana MR. The corneal stroma is endowed with a significant number of resident dendritic cells. Invest. Ophthalmol. Vis. Sci. 2003;44:581–589. doi: 10.1167/iovs.02-0838. [DOI] [PubMed] [Google Scholar]
- Hann LE, Allansmith MR, Sullivan DA. Impact of aging and gender on the Ig-containing cell profile of the lacrimal gland. Acta Ophthalmol. Copenhagen. 1988;66:87–92. doi: 10.1111/j.1755-3768.1988.tb08540.x. [DOI] [PubMed] [Google Scholar]
- Haynes RJ, Tighe PJ, Dua HS. Innate defence of the eye by antimicrobial defensin peptides [letter] [see comments] Lancet. 1998;352:451–452. doi: 10.1016/s0140-6736(05)79185-6. [DOI] [PubMed] [Google Scholar]
- Haynes RJ, Tighe PJ, Scott RA, Singh DH. Human conjunctiva contains high endothelial venules that express lymphocyte homing receptors. Exp. Eye Res. 1999;69:397–403. doi: 10.1006/exer.1999.0712. [DOI] [PubMed] [Google Scholar]
- Hazlett LD. Corneal response to Pseudomonas aeruginosa infection. Prog. Retin. Eye Res. 2004;23:1–30. doi: 10.1016/j.preteyeres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Hein WR. Organization of mucosal lymphoid tissue. In: Kraehenbuhl JR, Neutra MR, editors. Defense of Mucosal Surfaces: Pathogenesis, Immunity and Vaccines. Berlin: Springer Verlag; 1999. pp. 1–15. [Google Scholar]
- Hingorani M, Metz D, Lightman SL. Characterisation of the normal conjunctival leukocyte population. Exp. Eye Res. 1997;64:905–912. doi: 10.1006/exer.1996.0280. [DOI] [PubMed] [Google Scholar]
- Jensen OL, Gluud BS. Bacterial growth in the conjunctival sac and the local defense of the outer eye. Acta Ophthalmol. Suppl. 1985;173:80–82. doi: 10.1111/j.1755-3768.1985.tb06849.x. [DOI] [PubMed] [Google Scholar]
- Kessing SV. Mucous gland system of the conjunctiva. A quantitative normal anatomical study. Acta Ophthalmol. Copenhagen Suppl. 1968;95:1–133. [PubMed] [Google Scholar]
- Knop E, Knop N. MALT tissue of the conjunctiva and nasolacrimal system in the rabbit and human. Vision Res. 1996a;36:S60. [Google Scholar]
- Knop N, Knop E. The lacrimal sac in the rabbit and human is associated with MALT. Vision Res. 1996b;36:S195. [Google Scholar]
- Knop E, Knop N. The mucosa associated lymphoid tissue of the human conjunctiva consists of three components: solitary follicles, crypt associated MALT and a lymphoid layer. Invest. Ophthalmol. Vis. Sci. 1997a;38:S125. [Google Scholar]
- Knop N, Knop E. The MALT tissue of the ocular surface is continued inside the lacrimal sac in the rabbit and human. Invest. Ophthalmol. Vis. Sci. 1997b;38:S126. [Google Scholar]
- Knop E, Knop N. Fine structure of high endothelial venules in the human conjunctiva. Ophthalmic Res. 1998a;30:169. [Google Scholar]
- Knop E, Knop N. High endothelial venules are a normal component of lymphoid tissue in the human conjunctiva and lacrimal sac. Invest. Ophthalmol. Vis. Sci. 1998b;39:S548. [Google Scholar]
- Knop E, Knop N. Conjunctiva-associated lymphoid tissue (CALT) in the human eye – Components and topographical distribution. Ophthalmic Res. 1999a;31(Suppl):156. [Google Scholar]
- Knop E, Knop N. Conjunctiva-associated lymphoid tissue (CALT) in the human eye – Morphometric analysis of lymphoid follicles. Ophthalmic Res. 1999b;31:63. [Google Scholar]
- Knop N, Knop E. Conjunctiva-associated lymphoid tissue in the human eye. Invest. Ophthalmol. Vis. Sci. 2000;41:1270–1279. [PubMed] [Google Scholar]
- Knop E, Knop N. Lacrimal drainage associated lymphoid tissue (LDALT): A part of the human mucosal immune system. Invest. Ophthalmol. Vis. Sci. 2001;42:566–574. [PubMed] [Google Scholar]
- Knop E, Knop N. A functional unit for ocular surface immune defense formed by the lacrimal gland, conjunctiva and lacrimal drainage system. Adv. Exp. Med. Biol. 2002a;506:835–844. doi: 10.1007/978-1-4615-0717-8_118. [DOI] [PubMed] [Google Scholar]
- Knop E, Knop N. Human lacrimal drainage-associated lymphoid tissue (LDALT) belongs to the common mucosal immune system. Adv. Exp. Med. Biol. 2002b;506:861–866. doi: 10.1007/978-1-4615-0717-8_121. [DOI] [PubMed] [Google Scholar]
- Knop N, Knop E. The crypt system of the human conjunctiva. Adv. Exp. Med. Biol. 2002c;506:867–872. doi: 10.1007/978-1-4615-0717-8_122. [DOI] [PubMed] [Google Scholar]
- Knop E, Claus P, Knop N. Eye-associated lymphoid tissue (EALT): RT-PCR verifies the presence of mRNA for IgA and its transporter (secretory component) in the normal human conjunctiva. Invest. Ophthalmol. Vis. Sci. 2003;44:S3801. [Google Scholar]
- Knop E, Knop N. [Eye-associated lymphoid tissue (EALT) is continuously spread throughout the ocular surface from the lacrimal gland to the lacrimal drainage system] Ophthalmologe. 2003;100:929–942. doi: 10.1007/s00347-003-0936-6. [DOI] [PubMed] [Google Scholar]
- Knop E, Knop N. Eye associated lymphoid tissue (EALT) and the ocular surface. Proceedings of the 5th International Symposium on Ocular Pharmacology and Therapy; Medimond; Bologna. 2004a. pp. 91–98. [Google Scholar]
- Knop E, Knop N. Lymphocyte homing in the mucosal immune system to the eye-associated lymphoid tissue (EALT) In: Zierhut M, Sullivan DA, Stern ME, editors. Immunology of the Ocular Surface and Tearfilm. Vol. 2. Amsterdam: Swets and Zeitlinger; 2004b. pp. 35–72. [Google Scholar]
- Knop E, Knop N. The normal human eye-associated lymphoid tissue (EALT) regularly produces an accessory molecule (J-chain) of the secretory immune system. Invest. Ophthalmol. Vis. Sci. 2004c;45:1483. [Google Scholar]
- Knop E, Knop N, Pleyer U. Clinical aspects of MALT. In: Pleyer U, Mondino B, editors. Uveitis and Immunological Disorders. Berlin: Springer Verlag; 2004. pp. 67–89. [Google Scholar]
- Kraehenbuhl JP, Neutra MR. Molecular and cellular basis of immune protection of mucosal surfaces. Physiol. Rev. 1992;72:853–879. doi: 10.1152/physrev.1992.72.4.853. [DOI] [PubMed] [Google Scholar]
- Kühl W. [Immunoglobulin content of human tears] Albrecht Von Graefes Arch. Klin Exp. Ophthalmol. 1971;182:76. doi: 10.1007/BF00406519. [DOI] [PubMed] [Google Scholar]
- Lemp MA, Blackman HJ. Ocular surface defense mechanisms. Ann. Ophthalmol. 1981;13:61–63. [PubMed] [Google Scholar]
- Li DQ, Lokeshwar BL, Solomon A, Monroy D, Ji Z, Pflugfelder SC. Regulation of MMP-9 production by human corneal epithelial cells. Exp. Eye Res. 2001;73:449–459. doi: 10.1006/exer.2001.1054. [DOI] [PubMed] [Google Scholar]
- Liu SH, Tagawa Y, Prendergast RA, Franklin RM, Silverstein AM. Secretory component of IgA: a marker for differentiation of ocular epithelium. Invest. Ophthalmol. Vis. Sci.xs. 1981;20:100–109. [PubMed] [Google Scholar]
- MacDonald TT, Bajaj-Elliott M, Pender SL. T cells orchestrate intestinal mucosal shape and integrity. Immunol. Today. 1999;20:505–510. doi: 10.1016/s0167-5699(99)01536-4. [DOI] [PubMed] [Google Scholar]
- McClellan KA. Mucosal defense of the outer eye. Surv. Ophthalmol. 1997;42:233–246. doi: 10.1016/s0039-6257(97)00090-8. [DOI] [PubMed] [Google Scholar]
- McGee DW, Franklin RM. Lymphocyte migration into the lacrimal gland is random. Cell Immunol. 1984;86:75–82. doi: 10.1016/0008-8749(84)90360-5. [DOI] [PubMed] [Google Scholar]
- McGhee JR, Lamm ME, Strober W. Mucosal immune responses. An overview. In: Ogra PL, Mestecky J, Lamm ME, Strober W, McGhee JR, Bienenstock J, editors. Handbook of Mucosal Immunology. San Diego: Academic Press; 1999. pp. 485–506. [Google Scholar]
- McMaster PR, Aronson SB, Bedford MJ. Mechanisms of the host response in the eye. IV. The anterior eye in germ-free animals. Arch. Ophthalmol. 1967;77:392–399. doi: 10.1001/archopht.1967.00980020394019. [DOI] [PubMed] [Google Scholar]
- Meller D, Li DQ, Tseng SC. Regulation of collagenase, stromelysin, and gelatinase B in human conjunctival and conjunctivochalasis fibroblasts by interleukin-1beta and tumor necrosis factor-alpha. Invest. Ophthalmol. Vis. Sci. 2000;41:2922–2929. [PubMed] [Google Scholar]
- Mestecky J, McGhee JR, Michalek SM, Arnold RR, Crago SS, Babb JL. Concept of the local and common mucosal immune response. Adv. Exp. Med. Biol. 1978;107:185–192. doi: 10.1007/978-1-4684-3369-2_22. [DOI] [PubMed] [Google Scholar]
- Osterlind G. An investigation into the presence of lymphatic tissue in the human conjunctiva, and its biological and clinical importance. Acta Ophthalmol. Copenhagen Suppl. 1944;23:1–79. [Google Scholar]
- Parsons KR, Bland AP, Hall GA. Follicle associated epithelium of the gut associated lymphoid tissue of cattle. Vet. Pathol. 1991;28:22–29. doi: 10.1177/030098589102800104. [DOI] [PubMed] [Google Scholar]
- Paulsen FP, Paulsen JI, Thale AB, Tillmann BN. Mucosa-associated lymphoid tissue in human efferent tear ducts. Virchows Arch. 2000;437:185–189. doi: 10.1007/s004280000248. [DOI] [PubMed] [Google Scholar]
- Paulsen FP, Paulsen JI, Thale AB, Schaudig U, Tillmann BN. Organized mucosa-associated lymphoid tissue in human naso-lacrimal ducts. Adv. Exp. Med. Biol. 2002;506:873–876. doi: 10.1007/978-1-4615-0717-8_123. [DOI] [PubMed] [Google Scholar]
- Perra MT, Serra A, Sirigu P, Turno F. A histochemical and immunohistochemical study of certain defense mechanisms in the human lacrimal sac epithelium. Arch. Histol. Cytol. 1995;58:517–522. doi: 10.1679/aohc.58.517. [DOI] [PubMed] [Google Scholar]
- Pflugfelder SC, Jones D, Ji Z, Afonso A, Monroy D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjogren's syndrome keratoconjunctivitis sicca. Curr. Eye Res. 1999;19:201–211. doi: 10.1076/ceyr.19.3.201.5309. [DOI] [PubMed] [Google Scholar]
- Pflugfelder SC, Solomon A, Stern ME. The diagnosis and management of dry eye: a twenty-five-year review. Cornea. 2000;19:644–649. doi: 10.1097/00003226-200009000-00009. [DOI] [PubMed] [Google Scholar]
- Ruskell GL. Accessory Visual Apparatus. In: Williams PL, editor. Gray's Anatomy. 38. London: Churchill-Livingstone; 1995a. pp. 1353–1367. [Google Scholar]
- Ruskell GL. Organization and cytology of lymphoid tissue in the cynomolgus monkey conjunctiva. Anat. Rec. 1995b;243:153–164. doi: 10.1002/ar.1092430202. [DOI] [PubMed] [Google Scholar]
- Ruskell GL, VanderWerf F. Sensory innervation of conjunctival lymph follicles in cynomolgus monkeys. Invest. Ophthalmol. Vis. Sci. 1997;38:884–892. [PubMed] [Google Scholar]
- Sack RA, Beaton A, Sathe S, Morris C, Willcox M, Bogart B. Towards a closed eye model of the pre-ocular tear layer. Prog. Retin. Eye Res. 2000;19:649–668. doi: 10.1016/s1350-9462(00)00006-9. [DOI] [PubMed] [Google Scholar]
- Sack RA, Nunes I, Beaton A, Morris C. Host-defense mechanism of the ocular surfaces. Biosci. Rep. 2001;21:463–480. doi: 10.1023/a:1017943826684. [DOI] [PubMed] [Google Scholar]
- Sack RA, Sathe S, Beaton AR, Bogart B, Lew G. Changes in the diurnal pattern of the distribution of gelatinases and associated proteins in normal and pathological tear fluids: evidence that the PMN cell is a major source of MMP activity in tear fluid. Adv. Exp. Med. Biol. 2002;506:539–545. doi: 10.1007/978-1-4615-0717-8_76. [DOI] [PubMed] [Google Scholar]
- Sack RA, Sathe S, Beaton A, et al. Is the cystatin-like domain of TSL functionally active in external ocular infections and during the normal diurnal cycle? Exp. Eye Res. 2004;78:371–378. doi: 10.1016/s0014-4835(03)00198-2. [DOI] [PubMed] [Google Scholar]
- Sacks EH, Wieczorek R, Jakobiec FA, Knowles DM. Lymphocytic subpopulations in the normal human conjunctiva. A monoclonal antibody study. Ophthalmology. 1986;93:1276–1283. doi: 10.1016/s0161-6420(86)33580-2. [DOI] [PubMed] [Google Scholar]
- Setzer PY, Nichols BA, Dawson CR. Unusual structure of rat conjunctival epithelium. Light and electron microscopy. Invest. Ophthalmol. Vis. Sci. 1987;28:531–537. [PubMed] [Google Scholar]
- Sirigu P, Maxia C, Puxeddu R, Zucca I, Piras F, Perra MT. The presence of a local immune system in the upper blind and lower part of the human nasolacrimal duct. Arch. Histol. Cytol. 2000;63:431–439. doi: 10.1679/aohc.63.431. [DOI] [PubMed] [Google Scholar]
- Smolin G. The defence mechanism of the outer eye. Trans. Ophthalmol. Soc. UK. 1985;104:363–366. [PubMed] [Google Scholar]
- Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC. A unified theory of the role of the ocular surface in dry eye. Adv. Exp. Med. Biol. 1998;438:643–651. doi: 10.1007/978-1-4615-5359-5_91. [DOI] [PubMed] [Google Scholar]
- Stern ME, Gao J, Schwalb TA, et al. Conjunctival T-cell subpopulations in Sjogren's and non-Sjogren's patients with dry eye. Invest. Ophthalmol. Vis. Sci. 2002;43:2609–2614. [PubMed] [Google Scholar]
- Sullivan DA. Ocular mucosal immunity. In: Ogra PL, Mestecky J, Lamm ME, Strober W, McGhee J, Bienenstock J, editors. Handbook of Mucosal Immunology. London: Academic Press; 1999. pp. 1241–1281. [Google Scholar]
- Tourville DR, Adler RH, Bienenstock J, Tomasi TBJ. The human secretory immunoglobulin system: immunohistological localization of gamma A, secretory ‘piece’, and lactoferrin in normal human tissues. J. Exp. Med. 1969;129:411–429. doi: 10.1084/jem.129.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virchow H. Mikroskopische Anatomie der äusseren Augenhaut und des Lidapparates. In: saemisch T, editor. Graefe-Saemisch Handbuch der Gesamten Augenheilkunde, Band 1, 1Abteilung, Kapitel II. Leipzig: Verlag W. Engelmann; 1910. p. 431. [Google Scholar]
- Wieczorek R, Jakobiec FA, Sacks EH, Knowles DM. The immunoarchitecture of the normal human lacrimal gland. Relevancy for understanding pathologic conditions. Ophthalmology. 1988;95:100–109. doi: 10.1016/s0161-6420(88)33228-8. [DOI] [PubMed] [Google Scholar]
- Wotherspoon AC, Hardman L, Isaacson PG. Mucosa-associated lymphoid tissue (MALT) in the human conjunctiva. J. Pathol. 1994;174:33–37. doi: 10.1002/path.1711740106. [DOI] [PubMed] [Google Scholar]
- Yamagami S, Dana MR, Tsuru T. Draining lymph nodes play an essential role in alloimmunity generated in response to high-risk corneal transplantation. Cornea. 2002;21:405–409. doi: 10.1097/00003226-200205000-00014. [DOI] [PubMed] [Google Scholar]
- Zierhut M, Dana MR, Stern ME, Sullivan DA. Immunology of the lacrimal gland and ocular tear film. Trends Immunol. 2002;23:333–335. doi: 10.1016/s1471-4906(02)02228-7. [DOI] [PubMed] [Google Scholar]
- Zuckerman BD. Conjunctival pigmentation due to cosmetics. Am. J. Ophthalmol. 1966;62:672–676. doi: 10.1016/0002-9394(66)92191-x. [DOI] [PubMed] [Google Scholar]