Abstract
Electrically evoked release of [3H]-noradrenaline ([3H]-NA) or [3H]-5-hydroxytryptamine ([3H]-5-HT) in slices of human and the rat neocortex was used to characterize presynaptic opioid receptors.
Release of [3H]-NA in rat neocortical slices was reduced only by the μ-receptor agonist DAMGO (pIC50: 7.27, CI95: [7.22, 7.32]; Imax: 77.6±1.6%; antagonized by naloxone: pA2: 8.88, CI95: [8.78, 8.98]).
Release of [3H]-NA in human neocortical slices was unaffected by DAMGO, but inhibited by the δ-receptor agonist DPDPE (Imax: 25.7±2.2%) and the κ-receptor agonist U-50,488H (19.7±2.7% inhibition at 1 μM). Both effects were antagonized by naltrindole (1 μM).
Release of [3H]-5-HT in rat neocortical slices, was inhibited by DAMGO (10 μM) and U-50,488H (1 and 10 μM) only in the presence of the 5-HT receptor antagonist methiotepin (1 μM).
Release of [3H]-5-HT in human neocortical slices was unaffected by DPDPE, but U-50,488H (Imax: 40.8±8.3%; antagonized by 0.1 μM norbinaltorphimine) and DAMGO (16.4±3.9% inhibition at 1 μM; antagonized by 0.1 μM naloxone) acted inhibitory.
Release of [3H]-5-HT in human neocortical slices was reduced by nociceptin/orphanin (0.1 and 1 μM). These effects were antagonized by the ORL1 antagonist J-113397 (1-[(3R,4R)-1-cyclo-octylmethyl-3-hydroxymethyl-4-piperidyl]-3-ethyl-1,3-dihydro-2H-benzimidazol-2-one; 0.1 μM).
This study provides evidence for significant species differences in opioid receptor-mediated modulation of NA and 5-HT-release in human vs rat neocortex. In rats, μ-opioid receptors modulate NA release, but 5-HT release is only weakly affected by μ- and κ-opioids. In contrast, NA release in human neocortex is modulated via δ-opioid receptors, but 5-HT release mainly via κ-opioid receptors. In addition also the ORL1 receptor seems to be involved in 5-HT release modulation.
Keywords: DAMGO; DPDPE; human neocortical slices; [3H]-5-hydroxytryptamine release; [3H]-noradrenaline release; Presynaptic opioid receptors; rat neocortical slices; U-50,488H
Introduction
Opioid receptors belong to the large super family of seven transmembrane-spanning G protein-coupled receptors and to date four opioid receptors have been cloned: the MOP, or μ-opioid receptors, the KOP, or κ-opioid receptors, the DOP, or δ-opioid receptors and the NOP receptors, also called ORL1 or nociceptin/orphanin FQ (N/OFQ) receptors (for review see Waldhoer et al., 2004). Please note, that in the remaining part of the present paper only the μ-, κ-, δ- and ORL1-receptor terminology will be used (see also http://www.iuphar.org).
Opioid receptors occur both on the cell bodies and the axon terminals of neurons and it is well established that at least part of the effects of opioids on, for instance, nociception, behaviour, motor and vegetative functions are mediated by their inhibitory effects on neurotransmitter release via presynaptic opioid receptors (Mulder & Schoffelmeer, 1993). As there are selective agonists and antagonists available for each receptor type, much effort has been made in the past to characterize the particular opioid receptor subtype(s) involved in the modulation of the release of specific neurotransmitters in different regions of the CNS of various rodent species (e.g. Jackisch et al., 1988; Jackisch, 1991; Schoffelmeer et al., 1992b; Mulder & Schoffelmeer, 1993). For instance, as opioid peptides show an overlapping localization in various CNS regions with noradrenaline (NA) or serotonin (5-HT) (Khachaturian & Watson, 1982; Murakami et al., 1989), the opioid receptors involved in the modulation of NA and 5-HT release were studied. As a consequence it is now well established that NA release is inhibited via μ-receptors in the rat neocortex (Hagan & Hughes, 1984; Werling et al., 1987), via μ-, κ- and δ-receptors in the guinea-pig neocortex and hippocampus (Werling et al., 1987; 1989), and via κ-receptors in the rabbit hippocampus (Jackisch et al., 1986b) and cortex (Limberger et al., 1986; 1988b). Studies on the involvement of opioid receptors in the presynaptic modulation of 5-HT release in the rat neocortex are less frequent and have yielded conflicting results. For instance, no effects of opioid drugs on 5-HT release have been observed in earlier studies (Hagan & Hughes, 1984), whereas newer publications claim an involvement of μ- and κ-receptors (Sbrenna et al., 2000).
On the other hand, only little information on the role of presynaptic opioid receptors in the human CNS is available. We have shown, for instance, in fresh neocortical tissue specimens from humans that the release of acetylcholine (ACh) is inhibited by opioid drugs via both κ- and δ-opioid receptors (Feuerstein et al., 1996; 1998). With regard to the release of NA in fresh neocortical tissue from humans, no data are available about the role of μ-, δ- and κ-opioid receptors, although we provided evidence that nociceptin/orphanin Q via ORL1 receptors acted in an inhibitory fashion (Rominger et al., 2002). However, to our knowledge, opioid receptor-mediated inhibition of 5-HT release in the human neocortex (including ORL1 receptor mediated effects) has not been studied so far.
In this context it is noteworthy that it seems rather difficult to extrapolate data from the rodent to the human brain, as it is well known – especially in the field of presynaptic opioid receptors – that different opioid receptor types may modulate a given transmitter in various brain regions of the same species and, moreover, that these opioid receptor types may be different in another species, despite the same brain region and neurotransmitter (see Jackisch et al., 1986a; Lapchak et al., 1989; Jackisch, 1991). Therefore it was the aim of the present study to characterize for the first time in the human neocortex, the opioid receptor types involved in the regulation of the release of NA and 5-HT, that is, of two CNS neurotransmitters with a well-known role in the perception and modulation of pain. In addition, similar experiments were also performed on slices of the rat neocortex in order to compare the effects of opioids in both species and, moreover, to clarify the contradictory results of opioid drugs on rat neocortical 5-HT release.
Methods
Tissue preparation
Fresh specimens of human neocortex were obtained during surgical access to remove epileptic (25 patients) or brain tumour tissue (3 patients). The specimens were taken from the temporal (23), occipital (3) or frontal (2) lobe of either the right or left hemisphere. Fifteen of the patients were female, 13 were male. Their ages varied from 3 to 64 years (average age 33.6 years, 8 patients were less than 20 years old). The procedure was approved by the local Ethical Committee of the University of Freiburg and the patients themselves (or, in case of children, their parents), who were informed about the purpose of the investigation and signed a declaration of consent. The surgically removed tissue was immediately immersed into ice-cold, oxygenated (modified) Krebs–Henseleit buffer (KHB, composition (in mM): NaCl 118, KCl 4.8, CaCl2 1.3, MgSO4 1.2, NaHCO3 25, KH2PO4 1.2, glucose 10, Na2-EDTA 0.03, ascorbic acid 0.6; saturated with 95% O2/5% CO2; pH 7.4). Tissue blocks of pure grey matter (about 8 × 3 mm) were carefully dissected from the underlying white matter, and cut into 350 μM thick slices using a McIlwain tissue chopper (Saur, Reutlingen, Germany). Tissue specimens were discarded if visibly infiltrated by haemorrhage or tumour tissue. Moreover, microscopic infiltration of tissue by malignant growth could also be detected retrospectively, as a significantly lower stimulation-evoked tritium efflux was obtained from such slices (Feuerstein et al., 1990); consequently, data from slices in which the evoked overflow was more than two standard deviations lower than the mean evoked overflow of other human brain slices (see Table 1) were excluded from further evaluation.
Table 1.
Tissue accumulation of tritium and electrically evoked overflow of tritium during the first stimulation period (S1) in rat and human neocortical slices preincubated with either [3H]-NA or [3H]-5-HT
| Preincubation of slices with | Species (N) | [3H]-accumulation (pmoles/slice) | Evoked overflow of [3H] at S1 (% of tissue-3H) |
|---|---|---|---|
| [3H]-NA |
Human (6) |
0.49±0.09++ |
1.03±0.16+ |
| |
Rat (5) |
0.99±0.11 |
1.56±0.07 |
| [3H]-5-HT |
Human (8) |
0.89±0.13 |
1.10±0.08 |
| Rat (5) | 1.15±0.09 | 1.03±0.02 |
Human (number of individual patients N=6–8; number of slices n=103, or 136) and rat (number of individual rats N=5; number of slices n=100, or 84) neocortical slices were preincubated with [3H]-NA, or [3H]-5-HT, respectively, (0.1 μM, each; the latter in the presence of nomifensin (1 μM)) and then superfused in the presence of desipramine (1 μM), or 6-nitroquipazine and nomifensin (1 μM, each), respectively. Overflow of tritium was evoked by electrical fields using the following conditions: for NA release experiments: 4 (rat neocortex), or 8 (human neocortex) pulses at 100 Hz, 2 ms, 34 mA; for 5-HT release experiments: 2 × 4 (rat neocortex), or 2 × 8 (human neocortex) pulses at 100 Hz, 2 ms, 65 mA. Statistics:
P<0.05;
P<0.01 vs the corresponding rat values (for power analysis: see text).
Neocortical slices were also prepared from male rats (Wistar; 200–300 g); they were, however, not studied simultaneously with human brain slices. These rats, maintained according to institutional policies and guidelines, were sacrificed under CO2 anaesthesia by cervical dislocation. Their brains were rapidly removed and placed in ice-cold and oxygenated KHB. Slices from the parieto-occipital cortex (350 μM thick and about similar in size as human neocortical slices) were prepared as described above.
Evoked release of [3H]-NA and [3H]-5-HT
The slices were preincubated at 37°C for 45 min (NA release experiments), or 60 min (5-HT release experiments) in KHB containing [3H]-NA (0.1 μM), or [3H]-5-HT (0.1 μM), respectively. For [3H]-5-HT release experiments, the preincubation medium was supplemented with nomifensin (1 μM), in order to avoid false labelling of dopaminergic axon terminals with [3H]-5-HT (see Discussion). Following incubation, the slices were rinsed, transferred to superfusion chambers (volume 0.1 ml), and superfused at a rate of 1.2 ml min−1 with buffer routinely supplemented with desipramine (1 μM) in experiments measuring [3H]-NA release, or with 6-nitroquipazine (1 μM) and nomifensin (1 μM) in experiments measuring [3H]-5-HT release, respectively. Collection of 2-min superfusate fractions began after 30-min (NA release), or 45-min (5-HT release), respectively. During superfusion, the slices were stimulated electrically under conditions supposed to prevent effects of endogenously released transmitters or modulators on the evoked NA or 5-HT release, respectively (see Discussion). The following stimulation conditions were used: NA release experiments: 4 (rat neocortex), or 8 (human neocortex) pulses, 100 Hz, 2 ms, 34 mA; 5-HT release experiments: 2 × 4 (rat neocortex), or 2 × 8 (human neocortex) pulses, 100 Hz, 2 ms, 65 mA. Field stimulation was applied after 34 (S1), 52 (S2) and 70 (S3) minutes (NA release experiments), or after 49 (S1), 67 (S2) and 85 (S3) minutes (5-HT release experiments). Drugs to be investigated were added to the medium from 8 min before S2 and S3 onwards, with concentrations increasing from S2 to S3. The drug concentrations used did not change basal tritium outflow (data not shown) except for high concentrations of U-50,488H, which enhanced basal [3H] efflux in 5-HT release experiments in both rat and human neocortical slices (see Results). At the end of the experiment, slices were dissolved in 0.1 ml Solvable™ (Perkin Elmer, Rodgau, Germany), and the radioactivity of slices and superfusate fractions was determined by liquid scintillation spectrometry.
In some experiments on human and rat neocortical slices preincubated with [3H]-NA, the overflow of [3H] was also induced by high K+-concentrations in the medium (20 mM K+ for 2 min after 66 [S1] and 80 min [S2] of superfusion; during high K+-stimulation, the Na+ concentrations were reduced correspondingly). During superfusion 1 μM desipramine was routinely present; moreover, these experiments were performed both in the absence and the presence of 0.3 μM TTX throughout superfusion.
Calculations and statistics
The tritium outflow was calculated as a fraction of the tritium content in the slice at the onset of the corresponding collection period (fractional rate). The effects of drugs on basal tritium outflow were evaluated as described previously (Jehle et al., 2000). The respective stimulation-evoked tritium overflow at S1, S2 and S3, expressed as a percentage of the tritium content of the slice at the onset of the respective stimulation period, was calculated by subtracting basal outflow, which was assumed to decline linearly from 2 min before stimulation to the 2-min period 6 min after the onset of the stimulation. The effects of drugs on the stimulation-evoked overflow were estimated by calculating the ratio Sn/S1 of the evoked [3H]-NA and [3H]-5-HT release at the respective stimulation periods. All drug effects were normalized by dividing each individual Sn/S1-ratio by the mean ratio of the corresponding controls (no drug addition before Sn).
As in experiments on human tissue (in contrast to those on rat neocortex), data were obtained from different neocortical brain regions of patients (which by themselves also differed in their age and sex) we first tested whether the significance of drug effects in the experiments (factor 1) were influenced by the factor 2 (=‘individual patient'). For both [3H]NA and [3H]5-HT release experiments we found that the significance of drug effects (evaluated in a 1-way-ANOVA) was not changed, if in addition of the factor 1 (‘drug effect') also the factor 2 (‘individual patient') was considered in 2-way ANOVA. For instance, in experiments on [3H]-NA release from human tissue, 4 different drug treatments (DPDPE 0.1, 1 and 10 μM, U-50,488H 1 μM) inhibited NA release in neocortical slices of a total of 4 patients. In the 1-way-ANOVA of these data (which does not consider factor 2) factor 1 explains 64.6% of the total sum of squares (RSquare=0.646, significance of drug effects P<0.0001). When factor 2 (‘individual patient') is considered in addition (i.e. in a 2-way ANOVA of the same set of data) the RSquare value increases to only 0.687 and the significance of drug effects remains unchanged (P<0.0001), whereas the contribution of factor 2 (individual patients) to the total sum of squares is negligible (P=0.067). Similarly, in experiments on [3H]-5-HT release in human neocortical tissue, eight different drug treatments (DAMGO 1 μM, nociceptin 0.1 and 1 μM, U-50,488H 0.1, 1, 3, 10 and 33 μM) inhibited 5-HT release in neocortical slices of a total of 8 patients. The following values were obtained: 1-way ANOVA: RSquare=0.451, significance of drug effects P<0.0001; 2-way ANOVA (including factor 2): RSquare 0.497, significance of drug effects P<0.0001, significance of factor 2 (individual patients) P=0.061. Nevertheless, although the factor ‘individual patient' appears to be negligible considering the P-values given above, we admit that it cannot be completely discounted.
The same sets of data were also used to check the influence of individual factors of the patients on the significance of drug effects on [3H]-NA release (or [3H]-5-HT release, respectively) in human neocortical tissue. For the factor ‘brain region' (independent of the factors ‘age' and ‘sex') the two-way ANOVA yielded a RSquare of 0.687 (or 0.468, respectively) and the contribution of factor 2 (brain region) to the total sum of squares was negligible (P=0.027 or 0.091, respectively), as these P-values have to be seen in the light of highly significant effects of drug treatments (P<0.0001). Nevertheless, although the factor ‘brain region' appears to be negligible considering the P-values given above, it is evident that it cannot be completely discounted.
Moreover, also the factors ‘sex' and ‘age' did not significantly contribute to the significance of drug effects ([3H]-NA release (or [3H]5-HT release, respectively): factor ‘sex': P=0.748 (or 0.070, respectively); factor ‘age': P=0.068 (or 0.061, respectively). Nevertheless, although in experiments on [3H]-NA and [3H]-5-HT release in human neocortical tissue the influence of the factor ‘patient' (including the factors ‘brain region', ‘sex' and ‘age') on the effects of opioid drugs seems to be negligible, we admit that these factors cannot be completely disregarded.
The statistical tests given above also imply that data from several slices of an individual patient should not be regarded as ‘repeated measures' of the same sample. As the factor ‘patient' (or patient characteristics, like ‘age', ‘sex', or ‘brain region') had almost no influence on the effects of drugs, information would be lost if only the mean values instead of individual slice data of a given patient were considered for statistical analysis. Therefore, as in the rat, data on ‘drug effects' obtained from experiments on human tissue are shown as arithmetical means±standard error of means (s.e.m.), or as means with 95% confidence intervals (CI95) to assess the statistical significance of differences (Gardner & Altman, 1986; Altman, 1991) from a number (n) of individual slice values regardless of the factor ‘patient'.
On the other hand it should be noted that 2-way ANOVAs of those data from human tissue, which concern either the tissue accumulation (pmoles/slice) of [3H]-NA and [3H]-5-HT, respectively, or the evoked overflow of [3H] at S1 (in % of tissue-3H) from these slices, were significantly affected (P<0.0001, for both) by the factor ‘individual patient'. Hence, the significance of differences between rats and humans shown in Table 1 should be interpreted with caution.
For estimation of the pIC50 value of DAMGO (in the absence of naloxone) and of the Imax of DAMGO (in the presence of naloxone) on [3H]-NA release in the rat neocortex from the concentration–effect curve, individual Sx/S1 data (normalized) were evaluated by non-linear regression analysis as described previously (Feuerstein & Limberger, 1999). The apparent pA2 of naloxone was then calculated using the formula of Furchgott (Furchgott, 1972):  , with pKd being the negative logarithm of the dissociation constant of DAMGO in the absence of naloxone (0.1 μM), pKd* in its presence and ‘lg[nalox]' the logarithm of the molar antagonist concentration (=−7). All data were analysed using parametric analysis of variance (ANOVA) or by the Kruskal–Wallis test (non parametric ANOVA). When appropriate, ANOVA was followed by 2 × 2 comparisons based on the Newman–Keuls test (parametric) or on the multiple comparisons test of Dunn (non parametric). T-tests were also used where appropriate (Winer, 1971).
, with pKd being the negative logarithm of the dissociation constant of DAMGO in the absence of naloxone (0.1 μM), pKd* in its presence and ‘lg[nalox]' the logarithm of the molar antagonist concentration (=−7). All data were analysed using parametric analysis of variance (ANOVA) or by the Kruskal–Wallis test (non parametric ANOVA). When appropriate, ANOVA was followed by 2 × 2 comparisons based on the Newman–Keuls test (parametric) or on the multiple comparisons test of Dunn (non parametric). T-tests were also used where appropriate (Winer, 1971).
Finally, power analyses were performed to answer the question, whether a given sample size in particular experimental situations enabled our statistical tests to detect meaningful effects. Our statistical null hypothesis was always, that there is no relevant difference between the groups to be compared (e.g. control group and a certain treatment group). ‘Relevant' was defined as a pharmacologically meaningful difference d between treatment means and control means. Under this assumption, the error probability to wrongly decline the null hypothesis should be lower than α=0.05 and the power to detect a reasonable distance (>20%) from the null hypothesis (i.e.: 1–β) should be at least 0.80, i.e. β=0.2. For these power analyses, we assumed normal distribution of our data and applied the standard normal distribution (u-distribution) for the calculation of β with  and σ calculated from the standard deviations of the two data groups to be compared with sample sizes n1 and n2. Following this computation, values of (1−β) >0.80 indicate, that our statistical test were able to detect meaningful effects. It should be noted, that for power analysis the number of patients (N) was used together with the corresponding variances.
and σ calculated from the standard deviations of the two data groups to be compared with sample sizes n1 and n2. Following this computation, values of (1−β) >0.80 indicate, that our statistical test were able to detect meaningful effects. It should be noted, that for power analysis the number of patients (N) was used together with the corresponding variances.
Drugs
Substances commercially purchased included DAMGO ([D-Ala2,N-MePhe4,Gly-ol5]enkephalin) and DPDPE ([D-Pen2,D-Pen5]enkephalin), Bachem Biochemica; Heidelberg, Germany; desipramine, methiotepin, naltrindole, 6-nitroquipazine, N/OFQ, Tocris Cookson, Biotrend Chemikalien GmbH, Köln, Germany; nor-binaltorphimine, tetrodotoxin, and U-50,488H (trans-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)-cyclohexyl]-benzeneacetamide), Sigma Aldrich GmbH; Taufkirchen, Germany; [3H]-5-hydroxytryptamine (27.1 Ci mmol−1), and [3H]-noradrenaline (56.4 Ci mmol−1), Perkin Elmer, Rodgau, Germany; idazoxan, Reckitt & Colman; Hull, United Kingdom; naloxone, Research Biochemicals International; Natick, USA; nomifensin, Aventis, Strasburg, France; UK-14,304 (5-bromo-6-(2-imidazolin-2-ylamino)-quinoxaline-tartrate), Pfizer; Sandwich, United Kingdom; J-113397 (1-[(3R,4R)-1-cyclo-octylmethyl-3-hydroxymethyl-4-piperidyl]-3-ethyl-1,3-dihydro-2H-benzimidazol-2-one) was prepared by custom synthesis (Pfizer, Cambridge, U.K.) Stock solutions of drugs and peptides were prepared in distilled water (or ethanol: J-113397).
Results
Basic properties of neurotransmitter release models used
Preincubation of human neocortical slices with either [3H]-NA or [3H]-5-HT led to a tissue accumulation of these neurotransmitters which was lower than in the corresponding rat neocortical slices, despite a similar size of the slices (Table 1). Power analysis of these data yielded (1−β)-values of 0.947, or only 0.314, for [3H]-NA-, or [3H]-5-HT-accumulation respectively, underlining that only the marked difference in [3H]-NA-accumulation, but not the minimal difference in [3H]-5-HT-accumulation, could be detected with a sufficiently high probability (e.g. 94.7%) as significant (P<0.05) in between human and rat tissue (but see Methods, Calculations and Statistics).
Electrical field stimulation of these slices during superfusion induced an overflow of tritium during the first stimulation period (S1), which amounted to the values shown in Table 1. It is evident that in human neocortical slices the evoked overflow of [3H] in % of tissue accumulation of tritium was smaller than in rat neocortical slices, despite a higher number of electrical pulses (8 vs 4 pulses). In contrast, species differences in the evoked overflow at S1 were not observed in neocortical slices preincubated with [3H]-5-HT. Power analysis of these data yielded (1 – β)-values of 0.809, or only 0.102 for the evoked release of 3[H]-NA, or [3H]-5-HT respectively, underling that only the evoked release of [3H]-NA, but not that of [3H]-5-HT, differed significantly (P<0.01) in between human and rat tissue (but see Methods, Calculations and Statistics).
The presence of opioid receptor antagonists throughout superfusion did not affect the evoked overflow of tritium in slices preincubated with [3H]-NA. There was, however, one exception: naloxone (0.1 μM) significantly reduced the evoked overflow of [3H] in human neocortical slices preincubated with [3H]-5-HT by about 25% (data not shown). Please note that the antagonists present throughout superfusion were chosen in agreement with the main opioid receptor types observed in the corresponding tissue (see below).
Figure 1a and Table 2 show that the evoked [3H] overflow from human neocortical slices preincubated with [3H]-NA was Ca2+-dependent and tetrodotoxin (TTX) sensitive. Moreover, using 4 or 8 pulses at 100 Hz in experiments on rat or human neocortical slices, respectively, the evoked release of [3H]-NA was not enhanced by the α2 adrenoceptor antagonist idazoxan. On the other hand, idazoxan completely (rat neocortex), or partially (human neocortex) antagonized the inhibitory effect of the α2-adrenoceptor agonist UK-14,304.
Figure 1.
Time course of 3H-outflow from human neocortical slices preincubated with either 0.1 μM [3H]-NA (a), or 0.1 μM [3H]-5-HT (b): effects of Ca2+-free medium and tetrodotoxin (TTX, 0.3 μM). During superfusion ((a): in the presence of desipramine, 1 μM; (b): in the presence of 6-NQ and nomifensin, 1 μM each), overflow of [3H] was evoked by two electrical field stimulations ((a): 8 pulses at 100 Hz, 2 ms, 34 mA and (b): 2 × 8 pulses at 100 Hz, 2 ms, 65 mA) as indicated by the black squares (S1, S2) in the lowest plots. Ca2+-free or TTX-containing media were added 8 min before S2, as shown by the horizontal bars; n=4–5 for each curve.
Table 2.
Properties of electrically evoked overflow of tritium in rat and human neocortical slices preincubated with either [3H]-NA: effects of Ca2+-free medium, tetrodotoxin and α2-adrenoceptor agonists and antagonists
| Drug before Sn | Rat neocortex | Human neocortex |
|---|---|---|
| Ca2+-free medium |
ND |
15.28±4.61 (n=5)*** |
| Tetrodotoxin (0.3 μM) |
ND |
9.84±3.08 (n=5)*** |
| UK-14,304 (0. 1 μM) |
13.40±1.47 (n=5)*** |
11.06±3.430 (n=3)*** |
| Idazoxan (1 μM) |
106.66±1.30 (n=6) |
96.59±2.94 (n=8) |
| UK-14,304 (0.1 μM) (idazoxan, 1 μM, throughout) | 98.92±1.81 (n=5)+++ | 55.6±11.07 (n=3)***,+++ |
Rat or human neocortical slices were preincubated with [3H]-NA and then superfused continuously in the presence of desipramine (1 μM); in addition to desipramine, idazoxan (1 μM) was present throughout superfusion in part of the experiments. Overflow of tritium was evoked up to three times (S1–S3) by electrical fields using the following conditions: 4 (rat neocortex), or 8 (human neocortex) pulses at 100 Hz, 2 ms, 34 mA (for tissue accumulation of [3H]-NA and the evoked overflow of [3H] at S1 see Table 1). Drugs to be tested were added to the superfusion medium from 8 min before Sn onwards, their effects are expressed in % of the corresponding controls (no drug addition before Sn). Statistics:
P<0.001 vs controls;
P<0.001 vs UK-14,304 alone; ND, not determined, but see discussion.
Also in human neocortical slices preincubated with [3H]-5-HT, the absence of Ca2+ or the presence of TTX (0.3 μM) in the medium strongly reduced the evoked overflow of [3H] (see Figure 1b: evoked overflow of [3H] in % of controls: absence of Ca2+: 14.7±4.3 [n=5; P<0.001]; presence of TTX: 28.1±6.3 [n=5; P<0.001]). Similar observations were also made in rat neocortical slices preincubated with [3H]-5-HT (evoked overflow of [3H] in % of controls: absence of Ca2+: 21.3±3.8 [n=13; P<0.01]; presence of TTX: 27.3±5.9 [n=13; P<0.01]). However, despite the application of a low number of pulses at a high frequency (2 × 8 pulses at 100 Hz), the evoked overflow of [3H] from both human and rat neocortical slices preincubated with [3H]-5-HT was significantly increased in the presence of the nonselective 5-HT receptor antagonist methiotepin (1 μM; in % of controls: human neocortex: 127.1±4.7 [n=5; P<0.001]; rat neocortex: 116.9±2.9 [n=12; P<0.05]).
Rat neocortical slices: effects of opioid drugs on evoked [3H]-NA release
Neither the κ-opioid receptor agonist U-50,488H nor the δ-opioid receptor agonist DPDPE significantly affected the electrically-evoked release of [3H]-NA in rat neocortical slices (effects in % of corresponding controls: 0.1 μM U-50,488H: 100.0±2.8 [n=10, n.s.]; 1 μM U-50,488H: 93.4±3.1 [n=10, n.s.]; 0.1 μM DPDPE: 0.1 μM: 98.8±1.3 [n=10, n.s.]; 1 μM DPDPE: 93.8±2.5 [n=10, n.s.]). In contrast, the release of [3H]-NA was potently reduced in a concentration-dependent manner by the μ-opioid receptor agonist DAMGO (Figure 2). The maximal inhibitory effect (Imax) amounted to 77.6±1.6% inhibition (i.e. to 22.4±1.6% of corresponding controls) and the pIC50 value to 7.27 (CI95: [7.22, 7.32]). The concentration response curve of DAMGO was shifted to the right in the presence of naloxone (0.1 μM) throughout superfusion. The pA2 value of naloxone, as estimated by nonlinear regression analysis of the data points (assuming the same Imax value as in the absence of naloxone) amounted to 8.88 (CI95: [8.78, 8.98]). None of the agonists used had an effect on basal [3H]-outflow in rat neocortical slices.
Figure 2.
Effects of DAMGO on electrically-evoked overflow of [3H] in rat neocortical slices preincubated with 0.1 μM [3H]-NA. Following preincubation the slices were superfused either in presence of desipramine (1 μM) alone, or in the additional presence of naloxone (0.1 μM). During superfusion, the overflow of [3H] was evoked by three electrical field stimulations (S1, S2, S3; 4 pulses at 100 Hz, 2 ms, 34 mA). DAMGO was added in increasing concentrations 8 min before S2 or S3, respectively, as shown on the abscissa. Effects of DAMGO on the evoked overflow of [3H] are shown as Sn/S1 ratios expressed as percentage of the corresponding untreated controls; means±s.e.m.; n=8–18, per drug concentration.
DAMGO also inhibited K+-evoked overflow of [3H] from rat neocortical slices preincubated with [3H]-NA (although less potently than that induced by electrical stimulation) and this effect was unchanged in the presence of TTX throughout superfusion (effects of 1 μM DAMGO in % of controls: 56.0±4.8, n=13, P<0.001 [absence of TTX], or: 56.0±4.2, n=8, P<0.001 [presence of 0.3 μM TTX throughout superfusion]).
Human neocortical slices: effects of opioid drugs on evoked [3H]-NA release
The main results are summarized in Figure 3: it is evident that DAMGO did not significantly reduce the electrically-evoked release of [3H]-NA in human neocortical slices. Although U-50,488H showed a weak inhibitory effect at a concentration of 1 μM (80.3±2.7% of controls) in human neocortical tissue, this effect was antagonized in the presence of the δ-opioid receptor antagonist naltrindole. In contrast, the δ-opioid receptor agonist DPDPE significantly reduced the evoked release of [3H]-NA in human neocortical slices already at 0.1 μM; at 10 μM DPDPE the inhibition amounted to 25.7±2.2% (i.e. to 74.3±2.2% of controls). These inhibitory effects of DPDPE were completely antagonized in the presence of naltrindole (1 μM) throughout superfusion. None of the agonists used had an effect on basal [3H]-outflow in human neocortical slices. Power analysis of these data yielded the following (1−β)-values: U-50,488H 1 μM: 0.88; DPDPE 0.1, 1 and 10 μM: 0.67, 0.85 and 0.99, respectively.
Figure 3.
Effects of DAMGO, U-50,488H and DPDPE on electrically evoked overflow of [3H] in human neocortical slices preincubated with 0.1 μM [3H]-NA. Following preincubation the slices were superfused either in presence of desipramine (1 μM) alone, or in the additional presence of naltrindole (1 μM). During superfusion, overflow of [3H] was evoked by three electrical field stimulations (S1, S2, S3; 8 pulses at 100 Hz, 2 ms, 34 mA). The opioid receptor agonists were added in increasing concentrations 8 min before S2 or S3, respectively, as shown on the abscissa. Effects of drugs on the evoked overflow of [3H] are shown as Sn/S1 ratios expressed as percentage of the corresponding untreated controls (dotted line). Significance of differences vs corresponding controls: **P<0.01, ***P<0.001; vs absence of naltrindole: +P<0.05, +++P<0.001; means±s.e.m.; n=7–10, per drug concentration (data from 3 to 4 patients; for power analysis: see text).
DPDPE also potently inhibited the overflow of [3H] induced by K+-depolarization (20 mM for 2 min) from human neocortical slices preincubated with [3H]-NA (effects of 1 μM DPDPE in % of controls: 73.64±3.40, n=22, P<0.001). This effect was not influenced by the presence of 0.3 μM TTX throughout superfusion (effects of 1 μM DPDPE in % of controls: 75.79±4.65, n=14, P<0.001).
Rat neocortical slices: effects of opioid drugs on evoked [3H]-5-HT release
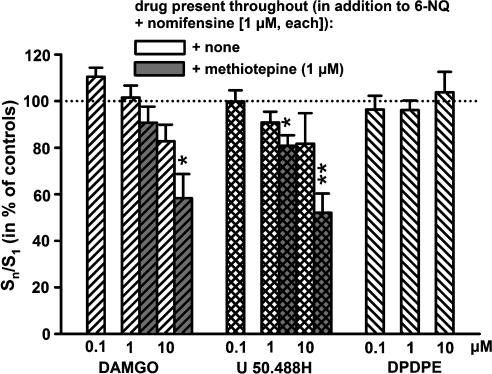
As evident from Figure 4, neither DAMGO, nor U-50,488H, nor DPDPE in concentrations up to 10 μM, showed significant inhibitory effects on the electrically-evoked release of [3H]-5-HT in rat neocortical slices when only 6-nitroquipazine and nomifensin (1 μM, each) were present throughout superfusion. Significant inhibitory effects of U-50,488H and DAMGO were observed, however, in the additional presence of the unselective 5-HT antagonist methiotepin (1 μM; DPDPE was not tested under these conditions; Figure 4). Whereas DAMGO and DPDPE did not affect the basal [3H]-outflow, U-50,488H caused a significant increase of basal tritium outflow at the highest concentration used (10 μM; 133.0±3.8% of controls, P<0.001).
Figure 4.
Effects of DAMGO, U-50,488H and DPDPE on electrically evoked overflow of [3H] in rat neocortical slices preincubated with 0.1 μM [3H]-5-HT (in the presence of 1 μM nomifensin). Following preincubation, the slices were superfused either in the presence of 6-nitroquipazine and nomifensin (1 μM, each), or in the additional presence of methiotepin (1 μM). During superfusion, overflow of [3H] was evoked by three electrical field stimulations (S1, S2, S3; 2 × 4 pulses at 100 Hz, 2 ms, 65 mA). The opioid receptor agonists were added in increasing concentrations 8 min before S2 or S3, respectively, as shown on the abscissa. Effects of drugs on the evoked overflow of [3H] are shown as Sn/S1 ratios expressed as percentage of the corresponding untreated controls (dotted line); means±s.e.m.; n=5–15 per drug concentration and condition. In the absence of methiotepin throughout, none of the drug effects differed significantly from the corresponding controls; significance of drug effects (in presence of methiotepin) vs corresponding controls: *P<0.05; **P<0.01.
Human neocortical slices: effects of opioid drugs on evoked [3H]-5-HT release
Results are presented in Figures 5 and 6. In human neocortical slices, as observed in the rat, DPDPE did not significantly affect the electrically-evoked release of [3H]-5-HT. DAMGO also exhibited only a slight inhibitory effect at 1 μM which was no longer significant in the presence of naloxone (0.1 μM) or the selective κ-opioid receptor antagonist nBNI (0.01 μM). Power analysis of the data shown in Figure 5 yielded the following value for (1 – β): DAMGO 1 μM: 0.86. On the other hand the κ-opioid receptor agonist U-50,488H showed distinct and concentration-dependent effects already at 0.1 μM (Figure 6). It should be noted, however, that – as in rat neocortical slices – high concentrations of U-50,488H also significantly enhanced basal tritium outflow (10 μM U-50,488H: 133.3±10.3 %; 33 μM U-50,488H: 395.4±21.8% of controls) in slices of the human neocortex. Figure 6 also shows that the concentration response curve of U-50,3488H was significantly shifted to the right in the presence of the κ-opioid receptor antagonist nBNI (0.01 μM). Power analysis of the data shown in Figure 6 yielded the following values for (1 – β): U-50,488H 0.1, 1 and 10 μM: 0.83, 0.97 and 1.00, respectively.
Figure 5.
Effects of DAMGO and DPDPE on electrically evoked overflow of [3H] in human neocortical slices preincubated with 0.1 μM [3H]-5-HT (in the presence of 1 μM nomifensin). Following preincubation, the slices were superfused either in presence of 6-NQ and nomifensin (1 μM, each, hatched columns), or in the additional presence of nBNI (0.01 μM, grey columns) or of naloxone (0.1 μM, black columns) throughout superfusion. During superfusion, overflow of [3H] was evoked by three electrical field stimulations (S1, S2, S3; 2 × 8 pulses at 100 Hz, 2 ms, 65 mA). The opioid receptor agonists were added in increasing concentrations 8 min before S2 or S3, respectively, as shown on the abscissa. Effects of drugs on the evoked overflow of [3H] are shown as Sn/S1 ratios expressed as percentage of the corresponding untreated controls (dotted line). Significance of effects vs corresponding controls: **P<0.01; means±s.e.m.; n=9–22 per drug concentration (data from 2 to 3 patients; for power analysis: see text).
Figure 6.
Effects of U-50,488H on electrically evoked overflow of [3H] in human neocortical slices preincubated with 0.1 μM [3H]-5-HT (in the presence of 1 μM nomifensin). Following preincubation, the slices were superfused either in presence of 6-NQ and nomifensin (1 μM, each, filled circles), or in the additional presence of nBNI (0.01 μM, open circles) throughout superfusion. During superfusion, overflow of [3H] was evoked by three electrical field stimulations (S1, S2, S3; 2 × 8 pulses at 100 Hz, 2 ms, 65 mA). U-50,488H was added in increasing concentrations 8 min before S2 or S3, respectively, as shown on the abscissa. Effects of U-50,488H (in absence or presence of nBNI) on the evoked overflow of [3H] are shown as Sn/S1 ratios expressed as percentage of the corresponding untreated controls (dotted line). Significance of effects vs corresponding controls: *P<0.05, **P<0.01; ***P<0.001; vs U-50,488H alone: #P<0.05, ###P<0.001; means±s.e.m.; n=7–22 per drug concentration (data from 2 to 6 patients; for power analysis: see text).
Human and rat neocortical slices: effects of ORL1 receptor ligands on evoked [3H]-5-HT release
The electrically-evoked overflow of [3H] from human neocortical slices preincubated with [3H]-5-HT was significantly inhibited by the ORL1 agonist nociceptin/orphanin (N/OFQ) at 0.1 and 1 μM; these effects were antagonized or reduced in the presence of the selective ORL1 antagonist J-113397 at a concentration of 0.1 μM (Figure 7); in the presence of J-113397 the effects of N/OFQ (0.1 and 1 μM) did not differ significantly from the corresponding controls. Interestingly, the presence of the ORL1 antagonist throughout superfusion also slightly increased (P<0.05) the evoked overflow of [3H] at S1 (in the absence of 0.1 μM J-113397 the evoked overflow at S1 amounted to: 1.13±0.06% of tissue-3H [n=43]; in the presence of J-113397 to: 1.34±0.07% of tissue-3H [n= 39]). Power analysis of the data shown in Figure 7 yielded the following values for (1 – β): N/OFQ 0.1 and 1 μM: 0.87 and 0.88, respectively.
Figure 7.
Effects of nociceptin (N/OFQ) in the absence or presence of the ORL1 antagonist J-113397 (0.1 μM) on the electrically evoked overflow of [3H] from human neocortical slices preincubated with [3H]-5-HT (in the presence of 1 μM nomifensin). Following preincubation, the slices were superfused either in presence of 6-NQ and nomifensin (1 μM, each, gray columns), or in the additional presence of J-113397 (0.1 μM, black columns) throughout superfusion. During superfusion, overflow of [3H] was evoked by three electrical field stimulations (S1, S2, S3; 2 × 8 pulses at 100 Hz, 2 ms, 65 mA). N/OFQ was added in increasing concentrations 8 min before S2 or S3, respectively, as shown on the abscissa. Effects of N/OFQ (in absence or presence of J-113397) on the evoked overflow of [3H] are shown as Sn/S1 ratios expressed as percentage of the corresponding untreated controls (dotted line). Significance of effects vs corresponding controls: ***P<0.001; significance of effects vs absence of J-113397: ++P<0.01; means±s.e.m.; n=12–22 per drug concentration (data from three patients; for power analysis: see text).
Also in slices of the rat neocortex preincubated with [3H]-5-HT, N/OFQ significantly reduced the evoked [3H]-overflow (effects in % of controls: 0.1 μM N/OFQ: 52.0±2.7 [n=10; P<0.001]; 1 μM N/OFQ: 57.9±4.1 [n=10; P<0.001]). In this tissue, however, no experiments were performed in the presence of the ORL1 antagonist J-113397.
Discussion
The model
In 5-HT release experiments, slices of neocortical tissue from rats or humans were preincubated with 0.1 μM [3H]-5-HT in order to label vesicular stores in serotonergic axon terminals. However, as there is a known affinity of 5-HT to the dopamine transporter (Lichtensteiger et al., 1967; Shaskan & Snyder, 1970; Ternaux et al., 1977) we had to consider that an uptake of [3H]-5-HT into dopaminergic axon terminals might occur (‘false labelling' (Feuerstein et al., 1986; Lupp et al., 1992)). As the tritium content of cortical slices preincubated with [3H]-5-HT in the presence of nomifensin (1 μM), a blocker of the dopamine reuptake, was significantly lower than in its absence (data not shown), nomifensin was present consistently in 5-HT release assays throughout the duration of the experiment, including the preincubation period with [3H]-5-HT. It should be noted, however, that nomifensin (1 μM) also inhibits the reuptake of NA, thereby possibly increasing the inhibitory tone of endogenously released NA at presynaptic α2-adrenoceptors on serotonergic axon terminals (Jackisch et al., 1999).
In contrast, during preincubation of neocortical tissue with 0.1 μM [3H]-NA, false labelling of serotonergic axon terminals seems to be less probable due to significantly lower affinity (Km) and Vmax values of NA at the (human) 5-HT transporter, as compared to the corresponding values at the NA transporter (Paczkowski et al., 1996; James & Bryan-Lluka, 1997; Tatsumi et al., 1997). Moreover, also false labelling of dopaminergic nerve endings in the neocortex with [3H]-NA also seems of minor importance in view of the low density of dopaminergic axons terminals in neocortical tissue.
Using these conditions of preincubation, it seems to emerge from Table 1, that – despite a similar size of the brain slices – tissue accumulation of both [3H]-NA and [3H]-5-HT was lower in slices from human as compared to rat neocortex (Table 1). With regard to the evoked release of [3H] (in % of tissue accumulation of [3H]), at least that of slices preincubated with [3H]-NA was also lower in human as compared to rat neocortical slices, even despite a higher number of electrical pulses. Moreover power analysis of the data (see Methods and Results) supports the probability to detect significant differences. However, as outlined in Methods (Calculations and Statistics), the factor ‘individual patient' (i.e. differences in brain region, age and sex of individual patients) significantly affected both tissue accumulation and evoked overflow of the tritiated neurotransmitters (S1 values). Hence, the conclusion that the density of noradrenergic or serotonergic axon terminals in human neocortical tissue is lower than in the rat neocortex seems premature, albeit possible. On the other hand, regarding these differences, a lower viability of human neocortical slices due to differences in sample handling can be excluded, as fresh human tissue was always used following a short time delay after surgery, which was similar to that used in the experiments on rat tissue. Also, in view of a long preincubation (45–60 min) and preperfusion (30–45 min) period of the slices, effects of residual anaesthetics originating from the surgery seems unlikely.
Concerning the mechanism of the electrically evoked overflow of [3H], the present study shows that it was Ca2+-dependent and TTX sensitive in human neocortical tissue preincubated with both [3H]-NA or [3H]-5-HT, respectively (Table 2 and text). Similar observations were made previously by different groups in both rat and human neocortex tissue preincubated with [3H]-NA (Taube et al., 1977; Feuerstein et al., 1990), or [3H]-5-HT, respectively (Schlicker et al., 1985; Raiteri et al., 1990; Siniscalchi et al., 1999; Sbrenna et al., 2000). Hence, it may be assumed that the electrically evoked overflow of [3H] from such slices represents action potential-induced, exocytotic release of NA or 5-HT, respectively, from noradrenergic and serotonergic axon terminals in rat and human neocortex.
A general problem of the electrically-evoked release technique applied in this study is the possible interference of endogenously released transmitters with the effects of exogenously added agonists, possibly via common signal transduction mechanisms. In view of the present study it is important to know that the α2-autoreceptor mechanism on noradrenergic axon terminals (Mulder et al., 1978; Schoffelmeer & Mulder, 1983; Mulder et al., 1987; Feuerstein et al., 1990) has been shown to interact with the activation of opioid receptors (Limberger et al., 1986; 1988a, 1988b; Schoffelmeer et al., 1986a, 1986b; Jackisch et al., 1986b). In a similar manner endogenously released 5-HT acting via 5-HT1B-autoreceptors in rat and 5-HT1D-autoreceptors in human neocortex (Galzin et al., 1992; Maura et al., 1993; Fink et al., 1995) might also interfere with exogenous agonists. Finally, as the release of 5-HT has been shown to be modulated via α2-adrenoceptors in both rat and human brain tissue (for references see Feuerstein et al., 1993), even endogenously released NA activating these α2-heteroreceptors might interact with opioid receptor stimulation on serotonergic axon terminals. Therefore, in the present experiments we used stimulation conditions (‘pseudo one pulse' (POP) conditions; Singer, 1988; Jackisch et al., 1999), which largely avoid interferences with endogenously released transmitters during ongoing stimulation. The data from the present experiments show that at least the release of [3H]-NA was free of autoinhibition under the present stimulation conditions both in rat and human neocortex, as the α2-adrenoceptor antagonist idazoxan did not enhance the evoked [3H]-NA release (Table 2). In contrast, the evoked release of [3H]-5-HT appeared still to be subjected to autoinhibition despite the application of POP stimulation conditions, as in both tissues the (unselective) 5-HT1 antagonist methiotepin acted in a facilitatory manner.
Presynaptic opioid receptors on noradrenergic terminals in rat and human neocortex
In several previous studies (Hagan & Hughes, 1984; Schoffelmeer et al., 1986a; Mulder et al., 1987; Werling et al., 1987) it has been shown that the electrically evoked release of [3H]-NA in rat neocortical slices was inhibited only by DAMGO, a highly selective μ-agonist (Handa et al., 1981; Gillan & Kosterlitz, 1982; Paterson et al., 1983; Kosterlitz, 1985), suggesting the presence of μ-opioid receptors on noradrenergic axon terminals (Mulder et al., 1987) in this tissue. Very similar observations were made in the present investigation: from the three opioid receptor selective agonists tested, only DAMGO strongly inhibited the evoked release of NA in rat neocortex slices with an Emax of 77.6±1.6% and a pIC50 value of 7.27 (Figure 2), values which closely correspond to data from the literature (Schoffelmeer et al., 1992a). Moreover, the effect of DAMGO was antagonized by the preferential μ-opioid receptor antagonist naloxone (Figure 2; Sawynok et al., 1979; Pasternak et al., 1980; Goldstein & Naidu, 1989), with a pA2 value of 8.88 also comparable to the literature (Mulder et al., 1991).
In contrast to the rat neocortex, however, DAMGO was without any effect on the evoked release of [3H]-NA in human neocortical slices (Figure 3). This observation suggests the absence of μ-opioid receptors on noradrenergic axon terminals in the human neocortex and underlines a possibly important species difference. Moreover, whereas κ-opioid receptors were found to modulate acetylcholine release from cholinergic neurons in human neocortex tissue (Feuerstein et al., 1996); their occurrence on noradrenergic neurons in this tissue can be excluded: the κ-agonist U-50,488H acted only slightly inhibitory at 1 μM (but not at 10 μM) and its effect was antagonized by the highly selective δ-antagonist naltrindole (Figure 3; Portoghese et al., 1988; Rogers et al., 1990).
On the other hand, the present data show that the selective δ-opioid receptor agonist DPDPE (Mosberg et al., 1983; Cotton et al., 1985; Hirning et al., 1985; Clark et al., 1986) clearly reduced the electrically evoked release of [3H]-NA in human neocortical tissue, although the inhibition was rather weak (Figure 3; Imax about 26%). Moreover, this inhibition was completely blocked (Figure 3) by the selective δ-opioid receptor antagonist naltrindole (Portoghese et al., 1988; Rogers et al., 1990). From these findings we conclude that in the human neocortex the δ-opioid receptors, rather than μ-opioid receptors (as in the rat), are involved in the modulation of NA release, a conclusion that is also supported by the outcome of the power analyses. In support of this conclusion, we further observed that DPDPE (1 μM) also inhibited the K+-evoked release of [3H]-NA both in the absence and presence of the Na+ channel blocker tetrodotoxin (0.3 μM), that is, also during inhibition of action potential propagation. Thus, in contrast to the δ-opioid receptors, which modulate acetylcholine release in the human neocortex (Feuerstein et al., 1998), those modulating the release of NA in this tissue are most probably located directly on (or near) the axon terminals of noradrenergic neurons themselves and not on interneurons.
In this context it should also be emphasized that in the human neocortex δ-opioid receptors seem to play an important role in the regulation of transmitter release, as – as we have shown previously (Feuerstein et al., 1996; 1998) – the release of acetylcholine in this tissue was significantly affected by δ-opioid receptor activation. This observation is surprising, as the density of δ-opioid ligand binding sites has been found to be very low in the human CNS (Pfeiffer et al., 1982) in contrast to much higher values for μ- and κ-binding sites (Cross et al., 1987). On the other hand, even though the overall density of δ-opioid receptors was found to be very low, its highest density was observed in the temporal cortex (Blackburn et al., 1988), that is, the cortical region mainly used in the present experiments.
Presynaptic opioid receptors on serotonergic terminals in rat and human neocortex
In the presence of 6-nitroquipazine and nomifensin none of the opioid agonists studied (DAMGO, U-50,488H and DPDPE) showed significant inhibitory effects on the electrically evoked release of 5-HT in rat neocortical slices, although both DAMGO and U-50,488H exhibited a slight tendency to inhibit (Figure 4). These findings are in agreement with those from Hagan & Hughes (1984), but they contrast to significant inhibitory effects of μ- and κ-opioid receptor agonists described by another group (Sbrenna et al., 2000). These contradictory results might be caused (1) by differences in the experimental conditions: both Hagan & Hughes (1984) and ourselves used electrical field stimulation, whereas Sbrenna et al. (2000) used K+-stimulation to evoke the release of 5-HT. (2) On the other hand, as discussed above, endogenously released agonists (e.g. 5-HT, NA) might interact with the signal transduction of presynaptic opioid receptors, especially under stimulation conditions (as in the present study), which were not completely free of autoinhibition (see above). For these reasons, we reinvestigated the effects of DAMGO and U-50,488H in the presence of methiotepin (Figure 4), that is, in the presence of 5-HT1B autoreceptor blockade. Under these conditions both the μ-agonist DAMGO and the κ-agonist U-50,488H acted clearly as inhibitors, supporting the conclusion of Sbrenna et al. (2000), that in the neocortex of rats the evoked release of 5-HT is modulated by both μ-and κ-opioid receptor activation.
The evoked release of 5-HT in human neocortical slices was only weakly affected by the μ-opioid receptor agonist DAMGO (significant only at 1 μM), whereas the δ-agonist DPDPE showed no effect at all (Figure 5): these data do not support the occurrence of presynaptic μ- and δ-opioidreceptors, respectively, on serotonergic neurons in this tissue. On the other hand, 5-HT release in human neocortex was clearly inhibited by the specific κ-opioid receptor agonist U-50,488H (Vonvoigtlander et al., 1983) already at concentrations as low as 0.1 μM; an Imax of about 41% was reached at 33 μM. It should be noted that the inhibitory effects of U-50,488H on the evoked release of [3H]-5-HT were (1) already detectable in the absence of effects on the basal [3H]-outflow (see text) and (2) that they were antagonized by a low concentration (10 nM) of the selective κ-antagonist norbinaltorphimine (nBNI; Birch et al., 1987). Therefore it can be concluded, that the release of 5-HT in human neocortex tissue is modulated by κ-opioid receptors, a conclusion which is also supported by the outcome of the power analyses. This role of κ-opioid receptors in the regulation of both acetylcholine (Feuerstein et al., 1996) and 5-HT release (present study) in the human neocortex is also in agreement with the observation that this receptor type occurs at a high density in this tissue (Cross et al., 1987).
In the rat neocortex, evidence for the occurrence of ORL1 receptors on serotonergic axon terminals has been provided previously (Siniscalchi et al., 1999; Schlicker & Morari, 2000). The present study supports these findings for the rat neocortex and further shows that these receptors also modulate the release of 5-HT in the human neocortex (Figure 7), a conclusion which is also supported by the outcome of the power analyses: N/OFQ (nociceptin) significantly inhibited the evoked release from slices preincubated with [3H]-5-HT; moreover, these effects were antagonized by the specific ORL1 receptor antagonist J-113,397 (Kawamoto et al., 1999). Interestingly, the effects of N/OFQ, although relatively weak (about 25% inhibition at 1 μM N/OFQ), were in the same range as those on the evoked release of NA in the human neocortex (Rominger et al., 2002), but smaller than in the rat neocortex (Siniscalchi et al., 1999; Schlicker & Morari, 2000). In view of the rather small effects (see above) of other presynaptic opioid receptors (i.e. the μ-, δ-, κ-receptors), it may be concluded that presynaptic ORL1 receptors also play an important role in the modulation of both NA and 5-HT release in the human neocortex.
Possible clinical significance of the present findings
(1) In view of the striking species differences in the opioid receptor types involved, it seems hazardous to project observations on the effects of subtype selective opioid drugs from rats to the clinical situation in man.
(2) Somehow surprisingly, presynaptic μ-opioid receptors appear not to be involved in NA- and 5-HT-mediated pain modulation in the human neocortex. This observation, however, does not exclude their possible role (i) in other parts of the human pain processing system, or (ii) at postsynaptic receptors, which were not studied in the present investigation.
(3) It has been suggested that the sedative effect of opioid drugs on cortical activity is mediated via κ-receptors (Goodman & Snyder, 1982) and that activation of serotonergic neurons from the raphé nuclei lead to an overall activation of the neocortex (Loubinoux et al., 2005). Therefore, the present finding, that serotonergic transmission in the human neocortex is inhibited via κ-opioid receptors might, at least in part, explain the sedative effect of opioid drugs.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (Ja 244/5-1).
Abbreviations
- 5-HT
5-hydroxytryptamine
- 6-NQ
6-nitroquipazine
- CI95
95% confidence interval
- DAMGO
[D-Ala2,N-MePhe4,Gly-ol5]enkephalin
- DPDPE
[D-Pen2,D-Pen5]enkephalin
- EDTA
ethylenediamine tetra acetate
- J-113397 (1-[(3R
4R)-1-cyclo-octylmethyl-3-hydroxymethyl-4-piperidyl]-3-ethyl-1,3-dihydro-2H-benzimidazol-2-one
- NA
noradrenaline
- nBNI
norbinaltorphimine
- ORL1
opioid receptor-like 1
- Sn
stimulation period n
- TTX
tetrodotoxin
- U-50,488H, trans-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)-cyclohexyl]-benzeneacetamide; UK-14,304
5-bromo-6-(2-imidazoline-2-ylamino)-quinoxaline-tartrate
References
- ALTMAN D.G. Statistics in medical journals: developments in the 1980s. Stat. Med. 1991;10:1897–1913. doi: 10.1002/sim.4780101206. [DOI] [PubMed] [Google Scholar]
- BIRCH P.J., HAYES A.G., SHEEHAN M.J., TYERS M.B. Norbinaltorphimine: antagonist profile at kappa opioid receptors. Eur. J. Pharmacol. 1987;144:405–408. doi: 10.1016/0014-2999(87)90397-9. [DOI] [PubMed] [Google Scholar]
- BLACKBURN T.P., CROSS A.J., HILLE C., SLATER P. Autoradiographic localization of delta opiate receptors in rat and human brain. Neuroscience. 1988;27:497–506. doi: 10.1016/0306-4522(88)90283-7. [DOI] [PubMed] [Google Scholar]
- CLARK J.A., ITZHAK Y., HRUBY V.J., YAMAMURA H.I., PASTERNAK G.W. [D-Pen2,D-Pen5]enkephalin (DPDPE): a delta-selective enkephalin with low affinity for mu 1 opiate binding sites. Eur. J. Pharmacol. 1986;128:303–304. doi: 10.1016/0014-2999(86)90784-3. [DOI] [PubMed] [Google Scholar]
- COTTON R., KOSTERLITZ H.W., PATERSON S.J., RANCE M.J., TRAYNOR J.R. The use of [3H]-[D-Pen2,D-Pen5]enkephalin as a highly selective ligand for the delta-binding site. Br. J. Pharmacol. 1985;84:927–932. doi: 10.1111/j.1476-5381.1985.tb17387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROSS A.J., HILLE C., SLATER P. Subtraction autoradiography of opiate receptor subtypes in human brain. Brain. Res. 1987;418:343–348. doi: 10.1016/0006-8993(87)90101-6. [DOI] [PubMed] [Google Scholar]
- FEUERSTEIN T.J., ALBRECHT C., WESSLER I., ZENTNER J., JACKISCH R. delta 1-Opioid receptor-mediated control of acetylcholine (ACh) release in human neocortex slices. Int. J. Dev. Neurosci. 1998;16:795–802. doi: 10.1016/s0736-5748(98)00086-0. [DOI] [PubMed] [Google Scholar]
- FEUERSTEIN T.J., DOOLEY D.J., SEEGER W. Inhibition of norepinephrine and acetylcholine release from human neocortex by omega-conotoxin GVIA. J. Pharmacol. Exp. Ther. 1990;252:778–785. [PubMed] [Google Scholar]
- FEUERSTEIN T.J., GLEICHAUF O., PECKYS D., LANDWEHRMEYER G.B., SCHEREMET R., JACKISCH R. Opioid receptor-mediated control of acetylcholine release in human neocortex tissue. Naunyn Schmiedebergs Arch. Pharmacol. 1996;354:586–592. doi: 10.1007/BF00170832. [DOI] [PubMed] [Google Scholar]
- FEUERSTEIN T.J., HERTTING G., LUPP A., NEUFANG B. False labelling of dopaminergic terminals in the rabbit caudate nucleus: uptake and release of [3H]-5-hydroxytryptamine. Br. J. Pharmacol. 1986;88:677–684. doi: 10.1111/j.1476-5381.1986.tb10250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEUERSTEIN T.J., LIMBERGER N. Mathematical analysis of the control of neurotransmitter release by presynaptic receptors as a supplement to experimental data. Naunyn Schmiedebergs Arch. Pharmacol. 1999;359:345–359. doi: 10.1007/pl00005361. [DOI] [PubMed] [Google Scholar]
- FEUERSTEIN T.J., MUTSCHLER A., LUPP A., VAN VELTHOVEN V., SCHLICKER E., GÖTHERT M. Endogenous noradrenaline activates alpha 2-adrenoceptors on serotonergic nerve endings in human and rat neocortex. J. Neurochem. 1993;61:474–480. doi: 10.1111/j.1471-4159.1993.tb02148.x. [DOI] [PubMed] [Google Scholar]
- FINK K., ZENTNER J., GÖTHERT M. Subclassification of presynaptic 5-HT autoreceptors in the human cerebral cortex as 5-HT1D receptors. Naunyn Schmiedebergs Arch. Pharmacol. 1995;352:451–454. doi: 10.1007/BF00172785. [DOI] [PubMed] [Google Scholar]
- FURCHGOTT R.F.The classification of adrenoceptors (adrenergic receptors). An evaluation from the standpoint of receptor theory Catecholamines Handbook of Experimental Pharmacology 1972Berlin, Heidelberg, New York: Springer; 283–335.eds. Blaschko, H. & Muscholl, E. pp [Google Scholar]
- GALZIN A.M., POIRIER M.F., LISTA A., CHODKIEWICZ J.P., BLIER P., RAMDINE R., LOO H., ROUX F.X., REDONDO A., LANGER S.Z. Characterization of the 5-hydroxytryptamine receptor modulating the release of 5-[3H]hydroxytryptamine in slices of the human neocortex. J. Neurochem. 1992;59:1293–1301. doi: 10.1111/j.1471-4159.1992.tb08440.x. [DOI] [PubMed] [Google Scholar]
- GARDNER M.J., ALTMAN D.G. Confidence intervals rather than P values: estimation rather than hypothesis testing. Br. Med. J. (Clin. Res. Ed). 1986;292:746–750. doi: 10.1136/bmj.292.6522.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILLAN M.G., KOSTERLITZ H.W. Spectrum of the mu, delta- and kappa-binding sites in homogenates of rat brain. Br. J. Pharmacol. 1982;77:461–469. doi: 10.1111/j.1476-5381.1982.tb09319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDSTEIN A., NAIDU A. Multiple opioid receptors: ligand selectivity profiles and binding site signatures. Mol. Pharmacol. 1989;36:265–272. [PubMed] [Google Scholar]
- GOODMAN R.R., SNYDER S.H. Kappa opiate receptors localized by autoradiography to deep layers of cerebral cortex: relation to sedative effects. Proc. Natl. Acad. Sci. U.S.A. 1982;79:5703–5707. doi: 10.1073/pnas.79.18.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGAN R.M., HUGHES I.E. Opioid receptor sub-types involved in the control of transmitter release in cortex of the brain of the rat. Neuropharmacology. 1984;23:491–495. doi: 10.1016/0028-3908(84)90020-0. [DOI] [PubMed] [Google Scholar]
- HANDA B.K., LAND A.C., LORD J.A., MORGAN B.A., RANCE M.J., SMITH C.F. Analogues of beta-LPH61-64 possessing selective agonist activity at mu-opiate receptors. Eur. J. Pharmacol. 1981;70:531–540. doi: 10.1016/0014-2999(81)90364-2. [DOI] [PubMed] [Google Scholar]
- HIRNING L.D., MOSBERG H.I., HURST R., HRUBY V.J., BURKS T.F., PORRECA F. Studies in vitro with ICI 174,864, [D-Pen2, D-Pen5]-enkephalin (DPDPE) and [D-Ala2, NMePhe4, Gly-ol]-enkephalin (DAGO) Neuropeptides. 1985;5:383–386. doi: 10.1016/0143-4179(85)90034-4. [DOI] [PubMed] [Google Scholar]
- JACKISCH R.Regulation of neurotransmitter release by opiates and opioid peptides in the central nervous system Presynaptic Regulation of Neurotransmitter Release: A Handbook 1991Tel Aviv: Freund; 551–592.eds. Feigenbaum, J. & Hanani, M. pp [Google Scholar]
- JACKISCH R., GEPPERT M., BRENNER A.S., ILLES P. Presynaptic opioid receptors modulating acetylcholine release in the hippocampus of the rabbit. Naunyn Schmiedebergs Arch. Pharmacol. 1986a;332:156–162. doi: 10.1007/BF00511406. [DOI] [PubMed] [Google Scholar]
- JACKISCH R., GEPPERT M., ILLES P. Characterization of opioid receptors modulating noradrenaline release in the hippocampus of the rabbit. J. Neurochem. 1986b;46:1802–1810. doi: 10.1111/j.1471-4159.1986.tb08499.x. [DOI] [PubMed] [Google Scholar]
- JACKISCH R., GEPPERT M., LUPP A., HUANG H.Y., ILLES P.Types of opioid receptors modulating neurotransmitter releaser in discrete brain regions Regulatory Roles of Opioid Peptides 1988Weinheim: Verlag Chemie; 240–258.eds. Illes, P. & Farsang, C. pp [Google Scholar]
- JACKISCH R., HAAF A., JELTSCH H., LAZARUS C., KELCHE C., CASSEL J.C. Modulation of 5-hydroxytryptamine release in hippocampal slices of rats: effects of fimbria-fornix lesions on 5-HT1B-autoreceptor and alpha2-heteroreceptor function. Brain Res. Bull. 1999;48:49–59. doi: 10.1016/s0361-9230(98)00145-2. [DOI] [PubMed] [Google Scholar]
- JAMES K.M., BRYAN-LLUKA L.J. Efflux studies allow further characterisation of the noradrenaline and 5-hydroxytryptamine transporters in rat lungs. Naunyn Schmiedebergs Arch. Pharmacol. 1997;356:126–133. doi: 10.1007/pl00005019. [DOI] [PubMed] [Google Scholar]
- JEHLE T., BAUER J., BLAUTH E., HUMMEL A., DARSTEIN M., FREIMAN T.M., FEUERSTEIN T.J. Effects of riluzole on electrically evoked neurotransmitter release. Br. J. Pharmacol. 2000;130:1227–1234. doi: 10.1038/sj.bjp.0703424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWAMOTO H., OZAKI S., ITOH Y., MIYAJI M., ARAI S., NAKASHIMA H., KATO T., OHTA H., IWASAWA Y. Discovery of the first potent and selective small molecule opioid receptor-like (ORL1) antagonist: 1-[(3R,4R)-1-cyclooctylmethyl-3- hydroxymethyl-4-piperidyl]-3-ethyl-1, 3-dihydro-2H-benzimidazol-2-one (J-113397) J. Med. Chem. 1999;42:5061–5063. doi: 10.1021/jm990517p. [DOI] [PubMed] [Google Scholar]
- KHACHATURIAN H., WATSON S.J. Some perspectives on monoamine-opioid peptide interaction in rat central nervous system. Brain. Res. Bull. 1982;9:441–462. doi: 10.1016/0361-9230(82)90154-x. [DOI] [PubMed] [Google Scholar]
- KOSTERLITZ H.W. Has morphine a physiological function in the animal kingdom? [news] Nature. 1985;317:671–672. doi: 10.1038/317671a0. [DOI] [PubMed] [Google Scholar]
- LAPCHAK P.A., ARAUJO D.M., COLLIER B. Regulation of endogenous acetylcholine release from mammalian brain slices by opiate receptors: hippocampus, striatum and cerebral cortex of guinea-pig and rat. Neuroscience. 1989;31:313–325. doi: 10.1016/0306-4522(89)90376-x. [DOI] [PubMed] [Google Scholar]
- LICHTENSTEIGER W., MUTZNER U., LANGEMANN H. Uptake of 5-hydroxytryptamine and 5-hydroxytryptophan by neurons of the central nervous system normally containing catecholamines. J. Neurochem. 1967;14:489–497. doi: 10.1111/j.1471-4159.1967.tb09548.x. [DOI] [PubMed] [Google Scholar]
- LIMBERGER N., SINGER E.A., STARKE K. Only activated but not non-activated presynaptic alpha 2-autoreceptors interfere with neighbouring presynaptic receptor mechanisms. Naunyn Schmiedeberg's Arch. Pharmacol. 1988a;338:62–67. doi: 10.1007/BF00168813. [DOI] [PubMed] [Google Scholar]
- LIMBERGER N., SPÄTH L., HÖLTING T., STARKE K. Mutual interaction between presynaptic alpha 2-adrenoceptors and opioid kappa-receptors at the noradrenergic axons of rabbit brain cortex. Naunyn Schmiedebergs Arch. Pharmacol. 1986;334:166–171. doi: 10.1007/BF00505817. [DOI] [PubMed] [Google Scholar]
- LIMBERGER N., SPÄTH L., STARKE K. Presynaptic alpha 2-adrenoceptor, opioid kappa-receptor and adenosine A1-receptor interactions on noradrenaline release in rabbit brain cortex. Naunyn Schmiedebergs Arch. Pharmacol. 1988b;338:53–61. doi: 10.1007/BF00168812. [DOI] [PubMed] [Google Scholar]
- LOUBINOUX I., TOMBARI D., PARIENTE J., GERDELAT-MAS A., FRANCERIES X., CASSOL E., RASCOL O., PASTOR J., CHOLLET F. Modulation of behavior and cortical motor activity in healthy subjects by a chronic administration of a serotonin enhancer. Neuroimage. 2005;27:299–313. doi: 10.1016/j.neuroimage.2004.12.023. [DOI] [PubMed] [Google Scholar]
- LUPP A., BÄR K.I., LÜCKING C.H., FEUERSTEIN T.J. Different effects of serotonin (5-HT) uptake blockers in caudate nucleus and hippocampus of the rabbit: role of monoamine oxidase in dopaminergic terminals. Psychopharmacology. 1992;106:118–126. doi: 10.1007/BF02253598. [DOI] [PubMed] [Google Scholar]
- MAURA G., THELLUNG S., ANDRIOLI G.C., RUELLE A., RAITERI M. Release-regulating serotonin 5-HT1D autoreceptors in human cerebral cortex. J. Neurochem. 1993;60:1179–1182. doi: 10.1111/j.1471-4159.1993.tb03274.x. [DOI] [PubMed] [Google Scholar]
- MOSBERG H.I., HURST R., HRUBY V.J., GEE K., YAMAMURA H.I., GALLIGAN J.J., BURKS T.F. Bis-penicillamine enkephalins possess highly improved specificity toward delta opioid receptors. Proc. Natl. Acad. Sci. U.S.A. 1983;80:5871–5874. doi: 10.1073/pnas.80.19.5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULDER A.H., BURGER D.M., WARDEH G., HOGENBOOM F., FRANKHUYZEN A.L. Pharmacological profile of various kappa-agonists at kappa-, mu- and delta-opioid receptors mediating presynaptic inhibition of neurotransmitter release in the rat brain. Br. J. Pharmacol. 1991;102:518–522. doi: 10.1111/j.1476-5381.1991.tb12203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULDER A.H., DE LANGEN C.D., DE REGT V., HOGENBOOM F. Alpha-receptor-mediated modulation of 3H-noradrenaline release from rat brain cortex synaptosomes. Naunyn Schmiedebergs Arch. Pharmacol. 1978;303:193–196. doi: 10.1007/BF00508068. [DOI] [PubMed] [Google Scholar]
- MULDER A.H., SCHOFFELMEER A.N.M.Multiple opioid receptors and presynaptic modulation of neurotransmitter release in the brain Handbook of Experimental Pharmacology 1993Berlin, Heidelberg: Springer-Verlag; 125–144.ed. Herz, A. pp [Google Scholar]
- MULDER A.H., HOGENBOOM F., WARDEH G., SCHOFFELMEER A.N. Morphine and enkephalins potently inhibit [3H]noradrenaline release from rat brain cortex synaptosomes: further evidence for a presynaptic localization of mu-opioid receptors. J. Neurochem. 1987;48:1043–1047. doi: 10.1111/j.1471-4159.1987.tb05624.x. [DOI] [PubMed] [Google Scholar]
- MURAKAMI S., OKAMURA H., PELLETIER G., IBATA Y. Differential colocalization of neuropeptide Y- and methionine- enkephalin-Arg6-Gly7-Leu8-like immunoreactivity in catecholaminergic neurons in the rat brain stem. J. Comp. Neurol. 1989;281:532–544. doi: 10.1002/cne.902810404. [DOI] [PubMed] [Google Scholar]
- PACZKOWSKI N.J., VUOCOLO H.E., BRYAN-LLUKA L.J. Conclusive evidence for distinct transporters for 5-hydroxytryptamine and noradrenaline in pulmonary endothelial cells of the rat. Naunyn Schmiedebergs Arch. Pharmacol. 1996;353:423–430. doi: 10.1007/BF00261439. [DOI] [PubMed] [Google Scholar]
- PASTERNAK G.W., CHILDERS S.R., SNYDER S.H. Naloxazone, a long-acting opiate antagonist: effects on analgesia in intact animals and on opiate receptor binding in vitro. J. Pharmacol. Exp. Ther. 1980;214:455–462. [PubMed] [Google Scholar]
- PATERSON S.J., ROBSON L.E., KOSTERLITZ H.W. Classification of opioid receptors. [Review] Br. Med. Bull. 1983;39:31–36. doi: 10.1093/oxfordjournals.bmb.a071787. [DOI] [PubMed] [Google Scholar]
- PFEIFFER A., PASI A., MEHRAEIN P., HERZ A. Opiate receptor binding sites in human brain. Brain. Res. 1982;248:87–96. doi: 10.1016/0006-8993(82)91150-7. [DOI] [PubMed] [Google Scholar]
- PORTOGHESE P.S., SULTANA M., TAKEMORI A.E. Naltrindole, a highly selective and potent non-peptide delta opioid receptor antagonist. Eur. J. Pharmacol. 1988;146:185–186. doi: 10.1016/0014-2999(88)90502-x. [DOI] [PubMed] [Google Scholar]
- RAITERI M., MAURA G., FOLGHERA S., CAVAZZANI P., ANDRIOLI G.C., SCHLICKER E., SCHALNUS R., GOTHERT M. Modulation of 5-hydroxytryptamine release by presynaptic inhibitory alpha 2-adrenoceptors in the human cerebral cortex. Naunyn Schmiedebergs Arch. Pharmacol. 1990;342:508–512. doi: 10.1007/BF00169037. [DOI] [PubMed] [Google Scholar]
- ROGERS H., HAYES A.G., BIRCH P.J., TRAYNOR J.R., LAWRENCE A.J. The selectivity of the opioid antagonist, naltrindole, for delta-opioid receptors. J. Pharm. Pharmacol. 1990;42:358–359. doi: 10.1111/j.2042-7158.1990.tb05428.x. [DOI] [PubMed] [Google Scholar]
- ROMINGER A., FÖRSTER S., ZENTNER J., DOOLEY D.J., MCKNIGHT A.T., FEUERSTEIN T.J., JACKISCH R., VLASKOVSKA M. Comparison of the ORL1 receptor-mediated inhibition of noradrenaline release in human and rat neocortical slices. Br. J. Pharmacol. 2002;135:800–806. doi: 10.1038/sj.bjp.0704523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAWYNOK J., PINSKY C., LABELLA F.S. On the specificity of naloxone as an opiate antagonist. Life Sci. 1979;25:1621–1632. doi: 10.1016/0024-3205(79)90403-x. [DOI] [PubMed] [Google Scholar]
- SBRENNA S., MARTI M., MORARI M., CALO G., GUERRINI R., BEANI L., BIANCHI C. Modulation of 5-hydroxytryptamine efflux from rat cortical synaptosomes by opioids and nociceptin. Br. J. Pharmacol. 2000;130:425–433. doi: 10.1038/sj.bjp.0703321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLICKER E., BRANDT F., CLASSEN K., GOTHERT M. Serotonin release in human cerebral cortex and its modulation via serotonin receptors. Brain. Res. 1985;331:337–341. doi: 10.1016/0006-8993(85)91559-8. [DOI] [PubMed] [Google Scholar]
- SCHLICKER E., MORARI M. Nociceptin/orphanin FQ and neurotransmitter release in the central nervous system. Peptides. 2000;21:1023–1029. doi: 10.1016/s0196-9781(00)00233-3. [DOI] [PubMed] [Google Scholar]
- SCHOFFELMEER A.N., DE VRIES T.J., HOGENBOOM F., HRUBY V.J., PORTOGHESE P.S., MULDER A.H. Opioid receptor antagonists discriminate between presynaptic mu and delta receptors and the adenylate cyclase-coupled opioid receptor complex in the brain. J. Pharmacol. Exp. Ther. 1992a;263:20–24. [PubMed] [Google Scholar]
- SCHOFFELMEER A.N., MULDER A.H. Differential control of Ca2+-dependent [3H]noradrenaline release from rat brain slices through presynaptic opiate receptors and alpha-adrenoceptors. Eur. J. Pharmacol. 1983;87:449–458. doi: 10.1016/0014-2999(83)90084-5. [DOI] [PubMed] [Google Scholar]
- SCHOFFELMEER A.N., PUTTERS J., MULDER A.H. Activation of presynaptic alpha 2-adrenoceptors attenuates the inhibitory effect of mu-opioid receptor agonists on noradrenaline release from brain slices. Naunyn Schmiedebergs Arch. Pharmacol. 1986a;333:377–380. doi: 10.1007/BF00500012. [DOI] [PubMed] [Google Scholar]
- SCHOFFELMEER A.N., VAN VLIET B.J., DE VRIES T.J., HEIJNA M.H., MULDER A.H. Regulation of brain neurotransmitter release and of adenylate cyclase activity by opioid receptors. Biochem. Soc. Trans. 1992b;20:449–453. doi: 10.1042/bst0200449. [DOI] [PubMed] [Google Scholar]
- SCHOFFELMEER A.N., WIERENGA E.A., MULDER A.H. Role of adenylate cyclase in presynaptic alpha 2-adrenoceptor- and mu-opioid receptor-mediated inhibition of [3H]noradrenaline release from rat brain cortex slices. J. Neurochem. 1986b;46:1711–1717. doi: 10.1111/j.1471-4159.1986.tb08488.x. [DOI] [PubMed] [Google Scholar]
- SHASKAN E.G., SNYDER S.H. Kinetics of serotonin accumulation into slices from rat brain: relationship to catecholamine uptake. J. Pharmacol. Exp. Ther. 1970;175:404–418. [PubMed] [Google Scholar]
- SINGER E.A. Transmitter release from brain slices elicited by single pulses: a powerful method to study presynaptic mechanisms. Trends. Pharmacol. Sci. 1988;9:274–276. doi: 10.1016/0165-6147(88)90004-1. [DOI] [PubMed] [Google Scholar]
- SINISCALCHI A., RODI D., BEANI L., BIANCHI C. Inhibitory effect of nociceptin on [3H]-5-HT release from rat cerebral cortex slices. Br. J. Pharmacol. 1999;128:119–123. doi: 10.1038/sj.bjp.0702793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TATSUMI M., GROSHAN K., BLAKELY R.D., RICHELSON E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur. J. Pharmacol. 1997;340:249–258. doi: 10.1016/s0014-2999(97)01393-9. [DOI] [PubMed] [Google Scholar]
- TAUBE H.D., STARKE K., BOROWSKI E. Presynaptic receptor systems on the noradrenergic neurones of rat brain. Naunyn Schmiedebergs Arch. Pharmacol. 1977;299:123–141. doi: 10.1007/BF00498554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERNAUX J.P., HERY F., BOURGOIN S., ADRIEN J., GLOWINSKI J., HAMON M. The topographical distribution of serotoninergic terminals in the neostriatum of the rat and the caudate nucleus of the cat. Brain. Res. 1977;121:311–326. doi: 10.1016/0006-8993(77)90154-8. [DOI] [PubMed] [Google Scholar]
- VONVOIGTLANDER P.F., LAHTI R.A., LUDENS J.H. U-50,488: a selective and structurally novel non-Mu (kappa) opioid agonist. J. Pharmacol. Exp. Ther. 1983;224:7–12. [PubMed] [Google Scholar]
- WALDHOER M., BARTLETT S.E., WHISTLER J.L. Opioid receptors. Annu. Rev. Biochem. 2004;73:953–990. doi: 10.1146/annurev.biochem.73.011303.073940. [DOI] [PubMed] [Google Scholar]
- WERLING L.L., BROWN S.R., COX B.M. Opioid receptor regulation of the release of norepinephrine in brain. Neuropharmacology. 1987;26:987–996. doi: 10.1016/0028-3908(87)90077-3. [DOI] [PubMed] [Google Scholar]
- WERLING L.L., MCMAHON P.N., PORTOGHESE P.S., TAKEMORI A.E., COX B.M. Selective opioid antagonist effects on opioid-induced inhibition of release of norepinephrine in guinea pig cortex. Neuropharmacology. 1989;28:103–107. doi: 10.1016/0028-3908(89)90044-0. [DOI] [PubMed] [Google Scholar]
- WINER B.J. Statistical Principles in Experimental Design. New York: McGraw-Hill; 1971. [Google Scholar]