Abstract
 Several 6-C-substituted 2-acetamido-2-deoxy-β-d-glucopyranosides (β-d-GlcNAc monosaccharides 1a–3a and 1,4-linked disaccharides 1b–3b) were studied by solution NMR spectroscopy. Conformational analysis of the (6S)- and (6R)–C-methyl-substituted β-d-GlcNAc monosaccharides indicates that the stereodefined methyl groups impose predictable conformational biases on the exocyclic C-5–C-6 bond, as determined by 1H-1H and 13C–1H coupling constants. Variable-temperature NMR experiments in methanol-d4 were performed to determine ΔΔH and ΔΔS values derived from the two lowest energy conformers. These indicate that while the influence of 6-C-methyl substitution on conformational enthalpy is in accord with the classic principles of steric interactions, conformational preference in solution can also be strongly affected by other factors such as solvent-solute interactions and solvent reorganization.
Several 6-C-substituted 2-acetamido-2-deoxy-β-d-glucopyranosides (β-d-GlcNAc monosaccharides 1a–3a and 1,4-linked disaccharides 1b–3b) were studied by solution NMR spectroscopy. Conformational analysis of the (6S)- and (6R)–C-methyl-substituted β-d-GlcNAc monosaccharides indicates that the stereodefined methyl groups impose predictable conformational biases on the exocyclic C-5–C-6 bond, as determined by 1H-1H and 13C–1H coupling constants. Variable-temperature NMR experiments in methanol-d4 were performed to determine ΔΔH and ΔΔS values derived from the two lowest energy conformers. These indicate that while the influence of 6-C-methyl substitution on conformational enthalpy is in accord with the classic principles of steric interactions, conformational preference in solution can also be strongly affected by other factors such as solvent-solute interactions and solvent reorganization.
Introduction
Protein–carbohydrate and carbohydrate–carbohydrate interactions play essential roles in the recognition of cell surfaces and polysaccharides in the extracellular matrix.1 For pyranosidic carbohydrates, the exocyclic C-5 hydroxymethyl substituent has particular significance and often provides key interactions at biological interfaces.2,3 The exocyclic C-5–C-6 bond is known to be torsionally flexible;3 however, a number of systems recognize or require specific orientations. For example, the polymorphic forms of the crystalline polysaccharide chitin (β-1,4-linked poly-N-acetyl-d-glucosamine) are likely determined by hydrogen bonding between interstrand O-6 hydroxyl groups, which are in turn directed by the C-5 hydroxymethyl conformations.4-6 There is also strong evidence that the O-6 hydroxyl is intimately involved in stabilizing the interaction between chitin and various chitinases.7 Understanding the factors which affect the conformation of the C-5–C-6 bond may reveal insights for designing interfaces with selective recognition properties, or for directing the supramolecular architecture of polysaccharides such as chitin.
The conformation of this exocyclic bond can be rationally biased by introducing a small but sterically demanding methyl group at the (6S)- or (6R)-position (see Figure 1). This conformational director destabilizes staggered rotamers via 1,3-diaxial-like interactions, which increases steric repulsion energies by 1–3 kcal/mol.8 The steric director approach has been used to probe the effect of conformational bias on protein-carbohydrate binding and enzymatic hydrolysis, using 6-C-substituted glucoand galactopyranosides9 and related 1,6-linked oligosaccharide derivatives.10
FIGURE 1.
Staggered gt, tg, and gg conformations of 6-C-substituted β-d-GlcNAc derivatives 1–3.
Here we describe the conformational analysis of 6-C-monodeuterated and 6-C-methyl-substituted 2-acetamido-2-deoxy-β-d-glucopyranosyl (β-d-GlcNAc) monosaccharides (1a–3a) and their corresponding 1,4-linked disaccharides (1b–3b), using vicinal coupling constants from variable-temperature nuclear magnetic resonance (VT-NMR) spectroscopy. A previous analysis of these compounds at 298 K in methanol-d4 has established that the stereodefined 6-C-methyl group imposes a strong conformational bias on the C-5–C-6 bond, with predictable outcomes for the lowest energy conformations.11 In this article, further evaluation of 3JH,H coupling constants over a wide temperature range (229–320 K) permits these conformational preferences to be described in thermodynamic terms with use of three-state conformer models. Parametrized Karplus analyses of this type allow us to estimate relative differences in enthalpy, which is pertinent for understanding conformational effects in crystal polymorphism and ligand-receptor binding. With respect to the latter, it is interesting to note that aminoglycoside antibiotics in the gentamicin family including Geneticin (G418) possess 6-C-methyl-substituted glucosamines (see Figure 2).12,13 A recent X-ray crystal structure of G418 complexed to an RNA fragment suggests that the (6R)-C-methyl group may destabilize a specific G–C base pair.13 Conformational analysis of these units may contribute toward structure–activity studies of gentamicin analogues, whose medicinal utility is hampered by the evolution of drug-resistant bacterial strains.14
FIGURE 2.

(6R)-C-Methyl-substituted glucosamine in Geneticin (G418).
SCHEME 1.
Synthesis of 6-C-Substituted Monosaccharidesa
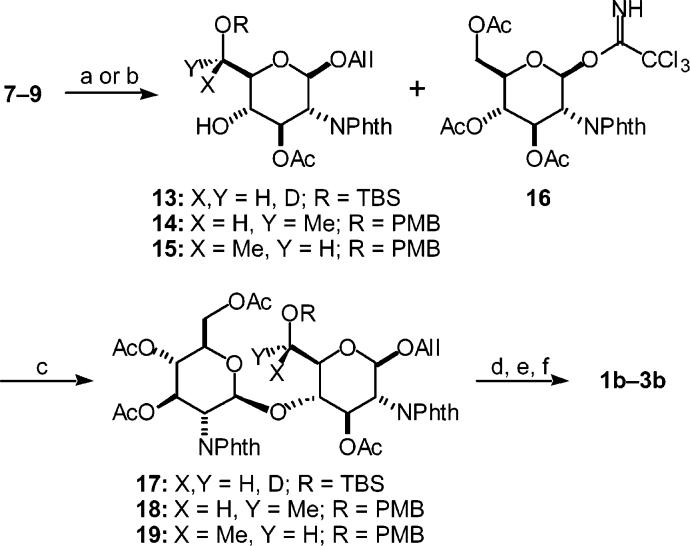
a Reagents and conditions: (a) (i) AcOH, THF:H2O, 45 °C, (ii) TBS-Cl, Et3N, imidazole, CH2Cl2:THF (93%, two steps); (b) (i) dihydropyran, PPTS, CH2Cl2, (ii) n-Bu4NF, THF (76%, two steps); (c) 7: (i) (COCl)2, DMSO, CH2Cl2, −78 °C, Et3N, 0 °C (62%), (ii) NaBD4, CH2Cl2:MeOH, −10 °C (6S:6R 2:1), (iii) p-TsOH, MeOH (46%, two steps); (d) 8: (i) (COCl)2, DMSO, CH2Cl2, −78 °C, Et3N, 0 °C, (ii) AlMe3, CuCN, THF, −55 °C to rt (6S:6R 6:1), (iii) p-TsOH, MeOH (25%, three steps); (e) 9: (i) and (ii) same as (d), (iii) (COCl)2, DMSO, CH2Cl2, −78 °C; Et3N, 0 °C (81%), (iv) ZnCl2, i-Bu2AlH, THF, −78 °C (6S:6R 1:6), (v) p-TsOH, MeOH (44%, two steps); (f) (i) (CH2NH2)2, n-BuOH, 100 °C, (ii) Ac2O, pyridine (10: 74%, 11: 87%, 12: 93%, two steps); (g) NaOMe, MeOH:CH2Cl2 (1:1, v/v) (1a: 71%, 1b: 98%, 1c: 100%). Selected acronyms: All = allyl, Phth = phthalimido, PMP = p-methoxyphenyl, TBS = tert- butyldimethylsilyl, THP = tetrahydropyranyl.
Results and Discussion
Synthesis
Multigram quantities of protected glucosamine derivative 4 were prepared from glucosamine hydrochloride according to literature procedures (see Scheme 1).15 Reductive cleavage of the 4,6-O-p-methoxybenzylidene acetal with borane and Bu2BOTf to the corresponding 4-O-p-methoxybenzyl (PMB) ether16 were problematic due to the reactivity of the allyl ether at 0 °C;17 however, the 4,6-diol could be regioselectively protected as 6-O-tert-butyldimethylsilyl (TBS) ether 5, then converted to 4-O-tetrahydropyranyl (THP)-protected primary alcohol 6 in 71% overall yield from 4.
Compound 6, which served as the common intermediate for the 6-C-substituted β-d-GlcNAc derivatives, was oxidized to the corresponding aldehyde with Swern conditions.18 This was reduced with NaBD4 and deprotected at O-4 to yield 6-C-d-substituted diol 7 as a 7:3 6S:6R mixture of diastereomers, with 6S- or 6R-stereo-chemical assignments based on 3J5,6 coupling constants of the corresponding 4,6-O-isopropylidene acetal derivative (3J5,6R = 5.2 Hz, 3J5,6S = 10.2 Hz). Monodeuteration simplifies the coupling of H-5 to H-6R and H-6S to two-spin systems, making conformational analysis straightforward. Next, the aldehyde was reacted with methyl Grignard reagents, which have been shown to add to similar substrates in good yields and stereoselectivity; 9 unfortunately, the aldehyde proved to be a remarkably poor electrophile. After a broad survey of nucleophiles and reaction conditions (see Table 1 for selected conditions), we were able to achieve methylation with 6:1 6S: 6R stereoselectivity using AlMe3 in the presence of a stoichiometric amount of CuCN in THF at low temperatures, in 40% yield over two steps from 6.19
TABLE 1.
Stereoselectivity of Methyl Addition to Glucosamine-Derived Aldehydea
 |
| entry | reagent (equiv) | R | additive (equiv)b | solvent | yield,c % | 8:9 (6S:6R)d |
|---|---|---|---|---|---|---|
| 1 | MeMgI (2) | THP | THF | 25 | 4:1 | |
| 2 | AlMe3 (6) | THP | CH2Cl2 | 60 | 4:1 | |
| 3 | AlMe3 (4) | Bn | CH2Cl2 | 60 | 3:1 | |
| 4 | AlMe3 (4) | MEM | CH2Cl2 | 0 | ||
| 5 | AlMe3 (5) | THP | CuCN (0.2) | THF | 54 | 5:1 |
| 6 | AlMe3 (5) | THP | CuCN (1) | THF | 40 | 6:1 |
See Experimental Section for standard reaction conditions.
Equivalents in parentheses relative to aldehyde.
Combined yield after methylation and THP deprotection.
In the case of R = THP, both diastereomers yielded mixtures of 6S and 6R epimers.
The 4-O-THP group had an important role in the reaction outcome. Replacing this group with benzyl ether caused the stereoselectivity to drop to a 3:1 6S:6R ratio, whereas the 4-O-methoxyethoxymethyl (MEM)-protected aldehyde was unreactive to AlMe3. The chiral THP group did not drastically influence methylation stereochemistry, as both diastereomers yielded the 6S-epimer as the major product.
Separation of the C-6 diastereomers was achieved upon cleavage of the THP group and recrystallization of the (6S)-C-methyl β-d-GlcNAc precursor 8. Assignments of C-6 stereochemistry were confirmed by vicinal coupling constant analysis of the corresponding 4,6-O-anisylidene acetals (from 8: 3J5,6 = 5.7 Hz; 9: 3J5,6 = 9.2 Hz). Larger quantities of the (6R)-C-methyl derivative 9 were obtained by oxidation of the diastereomeric 6-C-methyl adducts and reduction of the corresponding methyl ketone under chelate-controlled conditions with use of iBu2AlH in the presence of ZnCl2 with 6:1 6R:6S stereo-selectivity,20 followed by THP cleavage and careful separation by silica gel chromatography. It is worth mentioning that bulky reducing agents such as NaAl(OCH2CH2OCH3)2H2, LiAl(sBu)3H, or LiAl(OtBu)3H did not deliver the desired 6R epimer with high selectivities, whereas several other conditions produced the 6S-epimer preferentially. Diols 7–9 were then transformed into β-d-GlcNAc derivatives 10–12 in excellent yields by ethylenediamine-mediated cleavage of the phthalimide group and peracetylation. Last, methanolysis afforded the desired 6-C-substituted β-d-GlcNAc monosaccharides 1a–3a in quantitative yields.
SCHEME 2.
Synthesis of 6-C-Substituted Disaccharidesa
a Reagents and conditions: (a) 13: TBS–Cl, Et3N, imidazole, CH2Cl2:THF (89%); (b) 14, 15: (i) p-MeOC6H4CH(OMe)2, camphorsulfonic acid, 4A mol sieves, toluene, 90 °C, (ii) NaBH3CN, HCl, THF:Et2O, 4A mol sieves, −30 °C (14: 43%, 15: 27%, two steps); (c) 16 (2 equiv), TMSOTf, 4A mol sieves, CH2Cl2, −30 °C (17: 51%, 18: 98%, 19: 78%); (d) 1b: n-Bu4NF, THF; (e) 2b, 3b: DDQ, t-BuOH, pH 7 buffer, CH2Cl2; (f) (i) (CH2NH2)2, n-BuOH, 100 °C, (ii) Ac2O, pyridine, (iii) NaOMe, MeOH:CH2Cl2 (1b: 99%, 2b: 79%, 3b: 71%, four steps). Selected acronyms: All = allyl, Phth = phthalimido, PMB = p-methoxybenzyl, TBS=tert-butyldimethylsilyl.
To determine the relative influence of a neighboring glycosidic unit at C-4 on sidechain conformations, disaccharides 1b–3b were also synthesized (see Scheme 2). Monodeuterated diol 7 was protected as 6-O-TBS ether 13, whereas 6-C-methyl-substituted diols 8 and 9 were protected as 4,6-O-p-methoxybenzylidene acetals, and converted to their respective 6-O-PMB ethers 14 and 15 by reductive cleavage under acidic conditions.21 These alcohols were coupled straightforwardly with trichloroacetimidate 1622 to produce the protected β-1,4-linked disaccharides 17–19 in excellent yields. Global deprotection, peracetylation, and methanolysis afforded the desired 6-C-substituted β-d-GlcNAc disaccharides 1b–3b in high overall yields.
Conformational Analysis
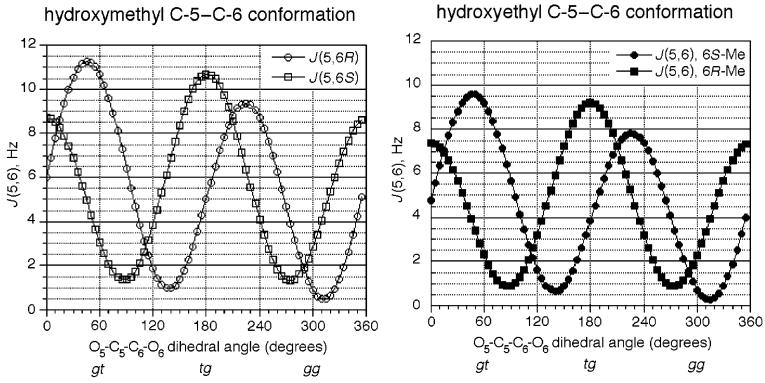
The influence of C-6 substituents on side chain conformations in methanol-d4 was evaluated primarily by 3JH,H coupling constant analysis, using empirically parametrized Karplus equations developed by Altona and co-workers.23 Conformational analyses of the hydroxymethyl C-5–C-6 bond in pyranosides are typically based on Karplus curves parametrized for 1,2-dialkoxypropanes. These produce dihedral angles as a function of two coupling constants 3J5,6R and 3J5,6S, which reflect the weighted average of staggered conformations.3 In the case of 6-C-methyl-substituted pyranosides, the hydroxyethyl C-5–C-6 bond must be considered as a 1,2-dialkoxybutane, which produces a Karplus relationship with a significantly reduced amplitude (see Figure 3). Coupling constant analysis of hydroxyethyl C-5–C-6 conformations rests on a single 3J5,6 value, and requires additional data to produce a unique dihedral angle solution. To this end, geminal and vicinal 13C–1H coupling constants (2JC,H and 3JC,H) obtained from proton-coupled 13C NMR spectra were employed as supporting constraints. Empirical measurements compiled by Serianni24 and Murata25 provide a set of limiting 3JC,H values complementary to the parametrized Karplus equations, and can be useful for defining rotamers with significant contributions to the time-averaged conformations.
FIGURE 3.
Parametrized Karplus relationships describing C-5-C-6 conformations as a function of 3J5,6, based on the empirical formulations of Haasnoot et al.23 C-5 hydroxymethyl and C-5 hydroxyethyl conformations were evaluated by using Karplus curves parametrized for 1,2-dialkoxypropanes3 (left) and 1,2-dialkoxybutanes (right), respectively.
Conformational analysis about the C-5–C-6 bond was performed based on the relative populations of the three staggered conformers gt, tg, and gg (see Figure 1).26 Individual rotamers of 6-C-methyl β-d-GlcNAc derivatives (2a,b and 3a,b) were correlated with 3JH,H values derived from the Karplus curves at the relative minima determined by semiempirical methods (see below), supplemented by “large” and “small” 2JC,H and 3JC,H coupling constants as defined by Serianni24 and Murata.25 Despite their limited precision, JC,H couplings allow each staggered conformer to be defined by a unique set of coupling constant parameters (see Table 2).27 Last, we note that the ring protons of all β-d-GlcNAc derivatives in this study have 3JH,H values consistent with stable chair conformations, permitting conformational analysis to be modeled on a diamond lattice framework.28
TABLE 2.
Assignment of Selected JH,H and JC,H Values to Staggered Conformers about the Exocyclic C-5–C-6 Bond
| 3JH–5,H–6a | 2JC–6,H–5b | 3JC–7,H–5c | |
|---|---|---|---|
| (6S)-C-CH3 substitution (2a,b) | |||
| gtd | 9.2 | L | S |
| tgd | 2.6 | L | L |
| ggd | 0.7 | S | S |
| (6R)-C-CH3 substitution (3a,b) | |||
| gte | 2.3 | L | L |
| tge | 9.1 | L | S |
| gge | 1.4 | S | S |
3JH,H values (in Hz) are derived from Karplus relationships parametrized for 1,2-dialkoxybutanes (Figure 3), with a standard deviation of 0.5 Hz.23
Correlation of 2JC,H values with the dihedral angle defined by H-6 and O-5 suggests “L” (−4 to −6 Hz) and “S” (0 to +2 Hz) values.24,25
Correlation of 3JC,H values with the dihedral angle defined by H-7 and C-5 suggests “L” (+5 to +7 Hz) and “S” (+1 to +3 Hz) values.24,25
gt, tg, and gg defined by O5–C5–C6–O6 dihedral angles of +60°, +170°, and −60°, respectively.
gt, tg, and gg defined by O5–C5–C6–O6 dihedral angles of +60°, +175°, and −70°, respectively.
Coupling constant analysis of compounds 1–3 at 298 K was presented in an earlier communication11 and is briefly summarized here. In accord with previous reports,29 the C-5C–6 bond of monodeuterated β-d-GlcNAc derivatives 1a and 1b preferred the gg and gt conformers over the tg conformer, which is destabilized by 1,3-diaxial-like interactions between O-4 and O-6 (see Figure 1). By comparison, the C-5–C-6 bond in (6S)-C-methyl β-d-GlcNAc derivatives 2a and 2b had a clear preference for the gg conformation over gt or tg, whereas that of (6R)-C-methyl β-d-GlcNAc derivatives 3a and 3b preferred gt over tg or gg (see Table 3 for 3J5,6 and selected JC,H values). It is noteworthy that the 3J5,6 values of disaccharides 2b and 3b at 298 K indicate a stronger preference for their lowest energy conformations (gg and gt, respectively) than their monosaccharides 2a and 3a. At first glance, this suggests that the neighboring C-4 glycosidic unit has a greater steric interaction with the C-6 methyl group than the C-4 hydroxyl, but a more detailed examination reveals this not to be the case (see below).
TABLE 3.
Selected JH,H and JC,H Coupling Constants for 6-C-Substituted β-d-GlcNAc Mono- and Disaccharides at 298 Ka
| compd | 3JH–5,H–6b | 2JC–6,H–5c | 3JC–7,H–5c |
|---|---|---|---|
| 1a | 6.2, 2.6 | ||
| 1bd | 4.9, 2.0 | ||
| 2a | 1.8 | 2.9 | 1.6 |
| 2be | 1.5 | 2.3 | 1.2 |
| 3a | 3.9 | 4.2 | 3.4 |
| 3bf | 2.4 | 4.5 | 3.8 |
1H NMR spectra were acquired at 600 MHz in CD3OD unless otherwise noted. Proton-coupled 13C NMR spectra were acquired at 500 MHz in CD3OD unless otherwise noted.
In Hz (±0.25 Hz).
In Hz (±0.3 Hz).
1H NMR in CD3OD:D2O (3:1, v/v).
13C NMR in CD3OD:D2O (5:3, v/v).
13C NMR in CD3OD:D2O (5:1, v/v).
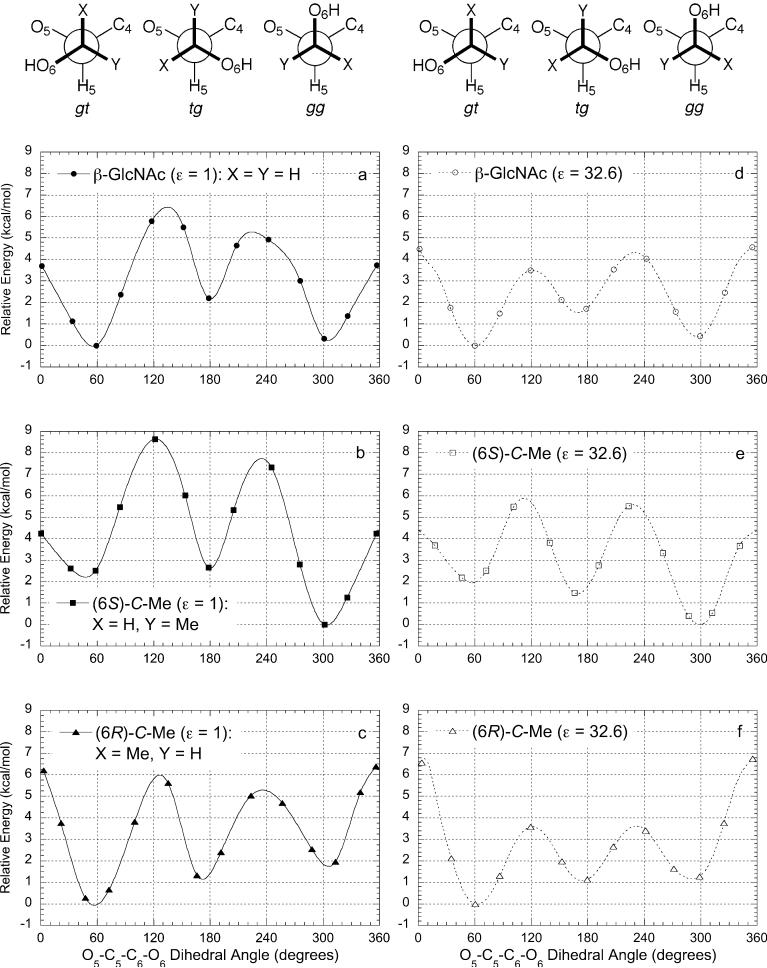
To obtain quantitative estimates of the relative conformational energies, VT-NMR studies were conducted in methanol-d4 between 230 and 320 K and applied toward three-conformer models. The latter proved to be a challenge in the case of the (6S)- and (6R)-C-methyl-substituted compounds: a first-order analysis based on experimentally measured interaction energies8 indicated a difference of less than 0.5 kcal/mol between the minor staggered conformers. This was confirmed by molecular mechanics (AMBER) calculations of conformational energies as a function of dihedral angle about the C-5–C-6 bond of methyl β-d-GlcNAc derivatives, in the gas phase (ε = 1; see Figure 4a-c) or in a dielectric continuum representing methanol (ε = 32.6; see Figure 4d-f).30 The most stable conformations of the 6-C-methyl-substituted compounds were favored by more than 1 kcal/mol over the next lowest energy conformer; however, the relative energies of the minor conformers were less clearly defined. In the low-dielectric simulations, energy differences between minor conformers were 0.5 kcal/mol or less, with rotational barriers on the order of 4–6 kcal/mol. Both the energy differences and rotational barriers were reduced upon increasing the media dielectric constant; in the case of the (6S)-C-methyl derivative, the relative minima were switched. We thus elected to carry out conformational analyses using a three-conformer model under two limiting assumptions: one with the two minor isomers being isoenergetic, the other with a free-energy difference of 0.5 kcal/mol between minor isomers. These assumptions necessarily limited the precision of the analyses, but could otherwise enable a reliable assessment of conformational stability in thermodynamic terms.
FIGURE4.
Potential energy curves for 6-C-substituted β-d-GlcNAc derivatives as a function of dihedral angle. Gas-phase conformational energies were calculated by using molecular mechanics (Amber) with ε = 1 (a–c); conformations in methanol were simulated by using ε = 32.6 (d–f).
Distributions of staggered conformers about the C-5–C-6 bonds were calculated by using the Karplus relationships defined in Figure 3, with dihedral angles for gt, tg, and gg based on the minima calculated for methyl β-dGlcNAc derivatives in methanol (see Table 4). For the 6-C-monodeuterated derivatives 1a and 1b, an unrestricted three-conformer model could be derived from two 3J5,6 values, with dihedral angles for gt, tg, and gg defined at +60°, +170°, and −65°, respectively (see Figure 4d).26 The negative tg values are artifacts of the parametrization; nevertheless, the population ratios of gg vs gt are expected to be slightly more accurate than those derived from earlier analyses, whose populations are based on dihedral angles of +60°, +180°, and −60°.3,29 For the 6-C-methyl derivatives 2a,b and 3a,b, three-conformer models were based on a single 3J5,6 value using the limiting assumptions above, with dihedral angles for gt, tg, and gg defined at +60°, +170°, and −60° for 2a,b and at +60°, +175°, and −70° for 3a,b (see Figure 4e,f).
TABLE 4.
3J5,6 Coupling Constants, Conformer Distributions, and Relative Free Energy Differences As Determined by VT-NMR Spectroscopy
| compd | Ta | J5,6Sb | J5,6Rb | gt | tg | gg | −ΔΔGc |
|---|---|---|---|---|---|---|---|
| 1a | 239 | 1.76 | 6.15 | 55 | −12 | 57 | 0.02 (0.06) |
| 258 | 2.20 | 6.15 | 53 | −6 | 53 | 0.00 (0.07) | |
| 278 | 2.20 | 5.72 | 49 | −6 | 57 | 0.09 (0.08) | |
| 302 | 2.64 | 6.16 | 52 | −1 | 49 | −0.04 (0.09) | |
| 320 | 2.63 | 6.16 | 52 | −1 | 49 | −0.04 (0.09) | |
| 1bd | 239 | 1.66 | 4.61 | 38 | −12 | 74 | 0.31 (0.06) |
| 248 | 1.72 | 4.77 | 40 | −11 | 71 | 0.29 (0.07) | |
| 258 | 2.05 | 4.65 | 38 | −7 | 69 | 0.32 (0.07) | |
| 278 | 1.97 | 4.65 | 38 | −8 | 70 | 0.34 (0.08) | |
| 302 | 2.04 | 4.93 | 41 | −8 | 67 | 0.30 (0.08) | |
| 320 | 2.04 | 4.93 | 41 | −8 | 67 | 0.32 (0.09) | |
| 2ae (tg = gt) | 230 | 1.48 | 7.5 | 7.5 | 85 | 1.11 (0.20) | |
| 250 | 1.63 | 9 | 9 | 82 | 1.10 (0.18) | ||
| 277 | 1.78 | 10 | 10 | 80 | 1.12 (0.18) | ||
| 298 | 1.83 | 11 | 11 | 78 | 1.17 (0.18) | ||
| 319 | 2.08 | 13 | 13 | 74 | 1.08 (0.17) | ||
| 2af (tg > gt) | 230 | 1.48 | 7 | 21 | 72 | 0.57 (0.23) | |
| 250 | 1.63 | 8.5 | 23 | 68.5 | 0.54 (0.21) | ||
| 277 | 1.78 | 10 | 25 | 65 | 0.53 (0.21) | ||
| 298 | 1.83 | 10.5 | 25 | 64.5 | 0.57 (0.22) | ||
| 319 | 2.08 | 13 | 29 | 58 | 0.44 (0.21) | ||
| 2be (tg = gt) | 230 | 1.41 | 7 | 7 | 86 | 1.16 (0.22) | |
| 247 | 1.41 | 7 | 7 | 86 | 1.25 (0.24) | ||
| 258 | 1.40 | 6.5 | 6.5 | 87 | 1.31 (0.25) | ||
| 278 | 1.54 | 8 | 8 | 84 | 1.29 (0.23) | ||
| 302 | 1.53 | 8 | 8 | 84 | 1.41 (0.25) | ||
| 320 | 1.60 | 8.5 | 8.5 | 83 | 1.44 (0.24) | ||
| 2bf (tg > gt) | 230 | 1.41 | 6.5 | 19 | 74.5 | 0.63 (0.25) | |
| 247 | 1.41 | 6.5 | 18 | 75.5 | 0.71 (0.27) | ||
| 258 | 1.40 | 6.5 | 17 | 76.5 | 0.77 (0.28) | ||
| 278 | 1.54 | 8 | 19.5 | 72.5 | 0.73 (0.25) | ||
| 302 | 1.53 | 8 | 18 | 74 | 0.85 (0.28) | ||
| 320 | 1.60 | 8.5 | 19 | 72.5 | 0.86 (0.27) | ||
| 3ae (tg = gg) | 229 | 2.60 | 90 | 5 | 5 | 1.31 (0.85) | |
| 233 | 2.59 | 90 | 5 | 5 | 1.35 (0.95) | ||
| 269 | 3.19 | 70 | 15 | 15 | 0.82 (0.24) | ||
| 278 | 3.49 | 60 | 20 | 20 | 0.60 (0.20) | ||
| 302 | 3.93 | 45 | 27.5 | 27.5 | 0.29 (0.20) | ||
| 320 | 4.18 | 36 | 32 | 32 | 0.08 (0.22) | ||
| 3af (tg > gg) | 229 | 2.60 | 94 | 4.5 | 1.5 | 1.37 (0.84) | |
| 233 | 2.59 | 94 | 4.5 | 1.5 | 1.41 (0.94) | ||
| 269 | 3.19 | 81 | 14 | 5 | 0.94 (0.21) | ||
| 278 | 3.49 | 74 | 18.5 | 7.5 | 0.77 (0.17) | ||
| 302 | 3.93 | 63.5 | 25.5 | 11 | 0.55 (0.15) | ||
| 320 | 4.18 | 57 | 29.5 | 13.5 | 0.42 (0.15) | ||
| 3be (tg = gg) | 230 | 2.05 | |||||
| 248 | 2.14 | ||||||
| 258 | 2.18 | ||||||
| 278 | 2.33 | 99 | 0.5 | 0.5 | 2.9g | ||
| 302 | 2.43 | 96 | 2 | 2 | 2.3g | ||
| 320 | 2.56 | 91 | 4.5 | 4.5 | 1.9g |
Calibrated temperatures in K.
Coupling constants in Hz (±0.25 Hz).
Free-energy difference between the two lowest energy conformations, in kcal/mol (uncertainties in parentheses).
Conformational analysis performed in 3:1 CD3OD:D2O.
Conformer populations calculated assuming minor conformers are equal in energy.
Conformer populations calculated assuming minor conformers have a free-energy difference of 0.5 kcal/mol.
The accuracy of these values is affected by the lower limit set by the parametrized Karplus equation. See Experimental Section for further details.
In all cases, the 6-C-methyl-substituted derivatives favored the sterically least encumbered conformations, regardless of temperature (gg for 2a,b and gt for 3a,b). The population ratios of the two lowest energy conformers were used to determine relative free-energy differences (−ΔΔG) based on each three-conformer model (see Table 4). In the case of (6S)-C-methyl monosaccharide 2a, −ΔΔG values for the lower limiting case were found to be on the order of 0.5 kcal/mol, whereas those for the upper limiting case were on the order of 1.1 kcal/mol. A similar situation was observed for (6S)-C-methyl disaccharide 2b, albeit with slightly greater free-energy differences. For (6R)-C-methyl monosaccharide 3a, the −ΔΔG values were less affected by the choice of three-conformer model but more affected by temperature, with values ranging between 0.3 and 1.4 kcal/mol. Last, in the case of (6R)-C-methyl disaccharide 3b, the preference for the lowest energy conformation was overwhelming (−ΔΔG > 1.9 kcal/mol), to the extent that the accuracy of thermodynamic analysis was limited by the parametrized Karplus equation itself. It should be noted that the uncertainty of ΔΔG increases rapidly as the J5,6 value approaches the lower limit set by the parametrized Karplus equation (2.3 Hz for 3a,b). Furthermore, in some cases the J5,6 value lay below this limit, which suggests that the preferred orientation of the C-5–C-6 bond in disaccharide 3b is distorted away from a staggered gt conformation, as opposed to the model (6R)-C-methyl monosaccharide used for Figure 4f.
Linear free-energy relationships were determined for all compounds except 3b (see Figure 5), whose J5,6 values coincided with the lower limit imposed by the Karplus equation parametrized for 1,2-dialkoxybutanes. Least-squares analyses of the corresponding van't Hoff plots yielded approximate ΔΔH and ΔΔS values (see Table 5), which revealed additional insights into the forces influencing the conformational preference of the exocyclic C-5–C-6 bond. First, the −ΔΔH values for the monosaccharides (1a, 2a) are comparable to or greater than those for the corresponding disaccharides (1b, 2b). This indicates that the presence of the O-4 glycoside does not have a significant steric influence on the conformation of the C-5–C-6 bond; indeed, in the case of 2b the enthalpic difference is much less than that predicted on the basis of steric interactions alone (see above). Second, the −ΔΔH value of (6R)-C-methyl β-d-GlcNAc 3a is much larger than can be accounted for based on increased 1,3-diaxial-like interactions. It should be noted that the same is also true for the relative free energy difference of disaccharide 3b.
FIGURE 5.
Van't Hoff plots describing conformational preferences of the C-5–C-6 bond for 6-C-substituted β-d-GlcNAc derivatives. (a) gg vs gt for 6-C-d-substituted β-d-GlcNAc monosaccharide 1a (filled circles) and disaccharide 1b (open squares). (b) gg vs tg for (6S)-C-methyl-substituted β-d-GlcNAc monosaccharide 2a, with tg = gt (filled circles) or tg > gt (open squares). (c) gg vs tg for (6S)-C-methyl-substituted β-d-GlcNAc disaccharide 2b, with tg = gt (filled circles) or tg > gt (open squares). (d) gt vs tg for (6R)-C-methyl-substituted β-d-GlcNAc 3a, with tg = gg (filled circles) or tg > gg (open squares).
TABLE 5.
Thermodynamic Values Describing Conformational Preferences about the C-5–C-6 Bonda
| ΔΔGrtb | ΔΔHb | ΔΔSc | |
|---|---|---|---|
| 1a: gg vs gt | +0.04 (0.09) | −0.21 (0.23) | −0.72 (0.84) |
| 1b: gg vs gtd | −0.30 (0.08) | −0.27 (0.08) | 0.14 (0.30) |
| 2a: gg vs tg (tg = gt) | −1.17 (0.18) | −1.07 (0.12) | 0.16 (0.46) |
| 2a: gg vs tg (tg > gt) | −0.57 (0.22) | −0.78 (0.15) | −0.92 (0.56) |
| 2b: gg vs tg (tg = gt) | −1.41 (0.25) | −0.50 (0.12) | 2.97 (0.44) |
| 2b: gg vs tg (tg > gt) | −0.85 (0.28) | −0.09 (0.13) | 2.45 (0.50) |
| 3a: gt vs tg (tg = gg) | −0.29 (0.20) | −4.62 (0.18) | −14.26 (0.66) |
| 3a: gt vs tg (tg > gg) | −0.55 (0.15) | −4.01 (0.19) | −11.41 (0.72) |
Thermodynamic values were derived from 1H NMR coupling constants obtained at 600 MHz in CD3OD from 229 to 320 K. Values for ΔΔH and ΔΔS were obtained from a linear leastsquares fit of the data plotted in Figure 5. Uncertainties (in parentheses) for ΔΔG (rt = 298 or 302 K) were derived by using indeterminate errors from 3JH,H measurements (±0.25 Hz); uncertainties for ΔΔH and ΔΔS were obtained from linear regression analysis and do not include propagation of indeterminate error.
In kcal/mol.
In cal·mol/K.
Compound was dissolved in 3:1 CD3OD:D2O.
Our observations indicate that the C-5–C-6 conformations are influenced by solvation effects.31 These can be described in terms of solvent-solute interactions and solvent reorganization, i.e., the restructuring of solvent molecules around the cavity occupied by the solute. Solvent–solute interactions can affect conformational preferences if the quality of key polar interactions is sensitive to local environmental factors, whereas solvent reorganization can account for large changes in solvation enthalpy and entropy in rough proportion to the solvent-exposed surface area of the solute.32 Recent simulations on the solvation energies of hydrocarbons in water as a function of conformation (cavity size) indicate that changes in solvation free energies are dominated by entropic changes in solvent reorganization.33
Both solvent-solute interactions and solvent reorganization may have a significant influence on the relative changes in conformational enthalpies and entropies for 6-C-methyl-substituted derivatives 2a,b (gg vs tg,gt) and 3a,b (gt vs tg,gg). Solvation entropy should in principle favor the minor tg conformer, because the consolidation of polar and nonpolar groups reduces the size of the solvophobic cavity. However, the protruding 6-C-methyl group increases the size of the solvophobic pocket and is likely to have an adverse effect on solvation reorganization. Furthermore, solvation enthalpy disfavors the tg conformer, because fewer well-defined hydrogen bonds between solvent molecules around the solute cavity and the hydroxyl groups at C-4 and C-6 are possible.
The conformational preferences of the 6-C-methyl-substituted β-d-GlcNAc derivatives can now be evaluated in the context of both steric interactions and solvent effects. In the case of (6S)-C-methyl-substituted monosaccharide 2a, the minor changes in entropy indicate that the preference for gg over the higher energy conformers is due mostly to local steric interactions, with solvation having little influence. In the case of (6S)-C-methyl-substituted disaccharide 2b, ΔΔH is decreased and accompanied by a rise in ΔΔS, indicating significant changes in solvation energy. In particular, the positive change in entropy implies that the minor conformations require greater solvent reorganization. Last, in the case of (6R)-C-methyl-substituted monosaccharide 3a, the preference for the gt conformer is clearly strengthened by additional solvent-solute interactions. The addition of a bound solvent molecule to the gt conformer is accompanied by a large and negative change in entropy.
In summary, a thermodynamic analysis of the conformational preferences of the C-5–C-6 bond in 6-C-substituted β-d-GlcNAc derivatives reveals the complex balance of forces which influence their solution conformations. Overall, our results support the application of classical steric interactions to predict preferred conformations in polar solvents at ambient temperatures, and confirm the validity of the steric director concept. However, solvent–solute interactions and solvent reorganization can have a considerable impact on conformational enthalpies and entropies, and may significantly alter the conformational behavior of compounds with very similar structures.
Experimental Section
NMR Conformational Analysis
Chemical shifts for 1H and 13C NMR spectra are reported (in parts per million) relative to CDCl3 (δ 7.24 and 77.0 ppm, respectively) or CD3-OD (δ 3.30 and 49.0 ppm, respectively).
Staggered conformer populations for 6-C-d-substituted compounds were calculated by using a three-conformer model (gt = +60°; tg = +170°; gg = −70°) based on the relative minima calculated for methyl β-d-GlcNAc in methanol (see Figure 4d) and Karplus relationships parametrized for 1,2-dialkoxypro-panes (see Figure 3). Populations were derived with eqs 1-3:
| (1) |
| (2) |
| (3) |
Conformer populations for (6S)-C-CH3-substituted compounds were calculated by using three-conformer models with limiting assumptions described in the main text, based on Karplus relationships parametrized for 1,2-dialkoxybutanes (see Figure 3). For the case in which the two minor conformers are equal in energy (tg = gt), populations were derived with eqs 4 and 5:
| (4) |
| (5) |
For the case in which the two minor conformers differ in energy by 0.5 kcal/mol (tg > gt), populations were derived with eqs 6-8:
| (6) |
| (7) |
| (8) |
Conformer populations for (6R)-C-CH3-substituted compounds were calculated in a similar manner. For the case in which the two minor conformers are equal in energy (tg = gg), populations were derived with eqs 9 and 10:
| (9) |
| (10) |
For the case in which the two minor conformers differ in energy by 0.5 kcal/mol (tg > gg), populations were derived with eqs 11-13:
| (11) |
| (12) |
| (13) |
Allyl 3-O-Acetyl-2-deoxy-4,6-O-(p-methoxybenzylidene)-2-phthalimido-β-d-glucopyranoside (4)
A solution of allyl-2-deoxy-2-phthalimido-3,4,6-tri-O-acetyl-β-d-glucopyrano-side11 (10.2 g, 21.5 mmol) in CH2Cl2 (130 mL) was treated with NaOMe (8 mL, 1 M solution in MeOH) at −5 °C. The reaction was stirred for 2 h at 0 °C, and then poured onto a column of activated Dowex 50X-W H+ ion-exchange resin. Elution with spectral grade MeOH (500 mL) followed by solvent evaporation affords the crude triol in 93% yield (6.98 g) as a white solid.
The intermediate triol and activated 4A molecular sieves were suspended in toluene (80 mL) and treated with p-anisaldehyde dimethylacetal (4.70 mL, 29.7 mmol) and camphorsulfonic acid (426 mg, 1.83 mmol). The reaction mixture was stirred at 90 °C for 20 h, diluted with EtOAc, filtered over Celite, and quenched with water. A basic aqueous workup (EtOAc) followed by recrystallization (EtOAc/hexanes, 60/140 mL) afforded the desired acetal as a white solid in 88% yield (8.13 g).
The acetal intermediate (8.00 g, 17.1 mmol) was dissolved in pyridine (40 mL) and treated with Ac2O (40 mL) at 0 °C. The reaction mixture was stirred at room temperature for 24 h, quenched with EtOH (100 mL), and concentrated to dryness. The crude residue was recrystallized (EtOAc/hexanes, 10/100 mL) to afford 4 as yellow needles in 68% yield (5.93 g). [α]d−20 (c 1, CHCl3); IR (thin film) ν 2877, 1777, 1742, 1717, 1615, 1386, 1251, 1226, 1101, 1034, 991, 722 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.83 (m, 2 H), 7.71 (m, 2 H), 7.36 (d, 2 H, J = 8.7 Hz), 6.86 (d, 2 H,, J = 8.7 Hz), 5.86 (dd, 1 H, J = 8.9, 10.4 Hz), 5.68 (m, 1 H), 5.48 (s, 1 H), 5.45 (d, 1 H, J = 8.5 Hz), 5.12 (dm, 1 H, J = 17.2 Hz), 5.03 (dm, 1 H, J = 10.4 Hz), 4.37 (dd, 1 H, J = 4.3 Hz, 10.2 Hz), 4.30 (dd, 1 H,, J = 8.5 Hz), 4.26 (ddt, 1 H, J = 5.0, 12.9, 1.5 Hz), 4.02 (ddt, 1 H, J = 6.2, 12.9, 1.3 Hz), 3.82 (t, 1 H, J = 9.8 Hz), 3.78 (s, 3 H), 3.75 (t, 1 H, J = 9.0 Hz), 3.72 (m, 1 H), 1.86 (s, 3 H); 13C NMR (75 MHz, CDCl3) δ 170.0, 167.5, 160.1, 134.1, 133.2, 131.4, 129.4, 127.5, 123.4, 117.7, 113.5, 101.5, 97.7, 79.1, 70.2, 69.7, 68.5, 66.1, 55.3, 55.2, 20.5; HRMS (EI) calcd for C27H27NO9 [M + H]+ 510.1764, found 510.1753.
Allyl 3-O-Acetyl-6-O-(tert-butyldimethylsilyl)-2-deoxy-2-phthalimido-β-d-glucopyranoside (5)
A solution of 4 (7.27 g, 14.3 mmol) in AcOH/THF/H2O (100 mL, 8:1:1 v/v/v) was heated to 45 °C for 5 h, evaporated to dryness, and azeotroped with toluene. Upon basic aqueous workup (CHCl3), the solid residue was washed with cold hexanes to afford the intermediate diol in 97% yield. The crude diol (5.40 g, 13.8 mmol) was dissolved in anhydrous THF/CH2Cl2 (80 mL, 1:3 v/v) and treated with Et3N (3.26 mL, 23.4 mmol), imidazole (94 mg, 1.38 mmol), then TBSCl (3.12 g, 20.7 mmol). The reaction mixture was stirred at room temperature for 16 h, then diluted with CH2Cl2 (100 mL) and quenched with saturated aqueous NaHCO3 solution (100 mL). Product extraction (CH2Cl2) was followed by 5% CuSO4 aqueous solution (50 mL), water (50 mL), and brine (50 mL) washes. The crude residue was purified by flash chromatography on silica gel (EtOAc/hexanes, 1:2 v/v) to afford 5 in 96% yield (6.72 g). [α]d−9(c 1, CHCl3); IR (thin film) ν 3481, 2930, 2857, 1778, 1747, 1718, 1387, 1231, 1114, 1067, 1040, 837, 779, 722 cm-1; 1H NMR (300 MHz, CDCl3) δ 7.78 (m, 2 H), 7.66 (m, 2 H), 5.66 (m, 1 H), 5.61 (dd, 1 H, J = 8.8, 10.7 Hz), 5.37 (d, 1 H, J = 8.5 Hz), 5.06 (dm, 1 H, J = 17.3 Hz), 4.97 (dm, 1 H, J = 10.4 Hz), 4.20 (ddt, 1 H, J = 5.0, 12.9, 1.5 Hz), 4.15 (dd, 1 H, J = 8.5, 10.7 Hz), 3.99 (ddt, 1 H, J = 6.2, 12.9, 1.3 Hz), 3.90 (dd, 2 H, J = 4.9, 11.7 Hz), 3.71 (t, 1 H, J = 9.8 Hz), 3.57 (dt, 1 H, J = 4.9, 9.8 Hz), 3.44 (br s, 1 H), 1.84 (s, 3 H), 0.87, 0.86, 0.85 (3 s, 9 H), 0.07, 0.06 (2 s, 6 H); 13C NMR (75 MHz, CDCl3) δ 170.9, 167.7, 134.1, 133.4, 131.4, 123.3, 117.3, 96.8, 74.6, 73.5, 71.7, 69.7, 64.1, 54.5, 25.7, 20.5, 18.1, −5.5, −5.6; HRMS (ESI) calcd for C25H35NO8Si [M + Na]+ 528.2030, found 528.2021.
Allyl 3-O-Acetyl-2-deoxy-2-phthalimido-4-O-tetrahydropyranyl-β-d-glucopyranoside (6)
A solution of 5 (6.72 g, 13.3 mmol) in CH2Cl2 (65 mL) was treated with 3,4-dihydro-2H-pyran (12.1 mL, 0.133 mol) and PPTS (84.6 mg, 0.332 mmol). The reaction was stirred at room temperature for 20 h, then diluted with CH2Cl2 (65 mL) and quenched with saturated NaHCO3 solution (60 mL). Product extraction (CH2Cl2) and standard aqueous workup followed. The crude residue was dissolved without further purification in anhydrous THF (70 mL) and treated with n-Bu4NF (20 mL, 1 M solution in THF). The reaction mixture was stirred at room temperature for 1 h, then filtered over a silica gel plug (EtOAc). After solvent evaporation, the crude residue was coevaporated with CHCl3 (thrice), then washed with cold hexanes (3 × 50 mL) to afford primary alcohol 6 as a mixture of diastereoisomers in 76% yield (4.78 g) over two steps. 1H NMR (300 MHz, CDCl3) δ 7.82 (m, 2 H), 7.70 (m, 2 H), 5.73 (dd, 1 H, J = 8.6, 10.6 Hz), 5.69 (m, 1 H), 5.42 (d, 1 H, J = 8.5 Hz), 5.12 (dm, 1 H, J = 17.2 Hz), 5.04 (dm, 1 H, J = 10.4 Hz), 4.66 and 4.56 (dd, 1 H, J = 3.0, 4.0 Hz), 4.24 (ddt, 1 H, J = 5.3, 12.9, 1.4 Hz), 4.21 (dd, 1 H, J = 8.5, 10.6 Hz), 4.04 (ddt, 1 H, J = 6.0, 12.9, 1.3 Hz), 3.93 (ddd, 1 H, J = 2.6, 5.0, 11.1 Hz), 3.86 (t, 1 H, J = 9.2 Hz), 3.76 (m, 2 H), 3.62 (ddd, 1 H, J = 2.6, 3.9, 4.6 Hz), 3.42 (ddd, J = 3.9, 5.9, 11.1 Hz), 2.00 (dd, 1 H, J = 5.0, 5.9 Hz), 1.89 and 1.87 (2 s, 3H), 1.80–1.30 (6 H); 13C NMR (75 MHz, CDCl3) δ 169.9, 169.4, 167.5, 133.9, 133.2, 133.2, 130.9, 123.0, 116.9, 116.9, 102.0, 100.1, 96.8, 96.7, 75.5, 75.1, 74.6, 74.5, 72.9, 71.6, 69.6, 69.4, 65.0, 62.4, 61.2, 60.9, 54.6, 30.8, 30.8, 24.8, 24.5, 20.7, 20.3, 20.1, 19.1; HRMS (ESI) calcd for C24H29NO9 [M + Na]+ 498.1740, found 498.1746.
Allyl 3-O-Acetyl-2-deoxy-(6R/6S)-C-2H-2-phthalimido-β-d-glucopyranoside (7)
Primary alcohol 6 (200 mg, 0.421 mmol) was oxidized by the usual Swern procedure to afford the corresponding aldehyde (124 mg, 0.262 mmol, 62% yield) after purification by flash chromatography on silica gel (Et2O). A solution of aldehyde (114 mg, 0.241 mmol) in MeOH/CH2-Cl2 (5 mL, 3:2 v/v) was treated with NaBD4 (30.0 mg, 0.717 mmol) at −10 °C. The reaction mixture was stirred for 1 h. Excess reagent was destroyed with acetone (5 mL) and the reaction quenched with saturated NH4Cl solution (12 mL). Aqueous workup (EtOAc) and purification by flash column chromatography on silica gel (30–50% EtOAc gradient in hexanes) afforded 4-O-THP-protected monodeuterated intermediate alcohol in 69% isolated yield.
The monodeuterated intermediate (79.1 mg, 0.166 mmol) was dissolved in spectral grade MeOH (5 mL) and treated with p-toluenesulfonic acid (31.6 mg, 0.166 mmol). The reaction mixture was stirred for1hat room temperature, then diluted with CHCl3 (12 mL) and quenched at 0 °C with saturated NaHCO3 solution (12 mL). Aqueous workup (CHCl3) and solvents removal afforded compound 7 in 67% yield (45.5 mg) in pure form without further purification. 1H NMR (300 MHz, CDCl3) δ 7.84 (m, 2 H), 7.72 (m, 2 H), 5.69 (m, 1 H), 5.63 (dd, 1 H, J = 8.8, 10.7 Hz), 5.39 (d, 1 H, J = 8.4 Hz), 5.11 (dm, 1 H, J = 17.2 Hz), 5.03 (dm, 1 H, J = 10.4 Hz), 4.25 (ddt, 1 H, J = 4.1, 12.9, 1.4 Hz), 4.24 (ddt, 1 H, J = 6.1, 12.9, 1.3 Hz), 4.23 (dd, 1 H, J = 8.4, 10.7 Hz), 3.97–3.74 (m, 2 H), 3.60 (ddd, 1H, J = 3.9, 4.7, 9.8 Hz), 3.20 (br s, 1 H), 2.25 (br s, 1 H), 1.87 (s, 3 H); 13C NMR (75 MHz, CDCl3) δ 171.3, 167.8, 134.2, 133.4, 131.4, 123.5, 117.6, 97.2, 75.4, 73.6, 70.2, 67.0, 62.2, 61.9, 54.7, 20.6; HRMS (EI) calcd for C19H20DNO8 [M + H]+ 393.1408, found 393.1409.
Allyl 3-O-Acetyl-2-deoxy-(6S)-C-methyl-2-phthalimido-β-d-glucopyranoside (8)
A solution of oxalyl chloride (0.55 mL, 6.31 mmol) in CH2Cl2 (6.6 mL) at −78 °C was treated with a solution of DMSO (0.89 mL, 12.6 mmol) in CH2Cl2 (1.8 mL). The reagents were stirred for 15 min. A solution of primary alcohol 6 (1 g, 2.10 mmol) in CH2Cl2 (6 mL) was added over 20 min via syringe pump. After being stirred for 30 min, the reaction mixture was treated with Et3N (2.64 mL, 18.9 mmol) at −78 °C, allowed to reach 0 °C over 20 min, then diluted in C6H6/Et2O (20 mL, 4:1 v/v) and quenched with saturated NH4-Cl solution (18 mL). Upon standard aqueous workup, the residue was azeotroped with toluene (thrice) to afford the desired aldehyde in quantitative crude yield.
A suspension of crude aldehyde (996 mg, 2.10 mmol) and CuCN (188 mg, 2.10 mmol) in anhydrous THF (20 mL) was stirred at room temperature for 10 min, cooled to −55 °C, then treated with AlMe3 (5.26 mL, 2 M solution in hexanes) and allowed to reach room temperature overnight. The reaction mixture was diluted with Et2O (20 mL) and quenched with saturated Na/K-tartrate/NH4Cl (24 mL, 1:1 v/v) solution at 0 °C. The biphasic mixture was stirred for 30 min or until the aqueous phase turned blue. Aqueous workup (EtOAc) followed by flash chromatography on silica gel (30–50% EtOAc gradient in hexanes) afforded the desired methyl adduct as a 6:1 mixture of (6S):(6R) diastereomers in 40% isolated yield (402 mg) over two steps.
A solution of methyl adduct (0.40 g, 0.821 mmol) in spectral grade MeOH (8 mL) was treated with p-toluenesulfonic acid (156 mg, 0.821 mmol). The reaction mixture was stirred at room temperature for 1 h, then diluted with CHCl3 (12 mL) and quenched with saturated NaHCO3 solution (24 mL) at 0 °C. Aqueous workup (CHCl3) afforded a 6:1 mixture of (6S): (6R) diols as judged by 1H NMR spectroscopy. The crude residue was dissolved in anhydrous THF (1 mL), and triturated with cold hexanes (10 mL) to afford (6S)-C-methyl epimer 8 as a white solid in 63% isolated yield (209 mg). [α]d+1(c 0.9, CHCl3); IR (thin film) ν 3474, 2924, 1777, 1747, 1716, 1493, 1452, 1387, 1232, 1032, 755 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.83 (m, 2 H), 7.71 (m, 2 H), 5.71 (m, 1 H), 5.62 (dd, 1 H, J = 9.0, 10.5 Hz), 5.39 (d, 1 H, J = 8.5 Hz), 5.12 (dm, 1 H, J = 17.2 Hz), 5.05 (dm, 1 H, J = 10.4 Hz), 4.22 (ddm, 1 H, J = 4.1, 12.9 Hz), 4.21 (dd, 1 H, J = 8.5, 10.5 Hz), 4.16 (br q, 1 H, J = 6.6 Hz), 4.05 (ddt, 1 H, J = 6.3, 12.9, 1.5 Hz), 3.92 (br t, 1 H, J = 9.8 Hz), 3.37 (dd, 1 H, J = 1.7, 9.8 Hz), 3.19 (br s, 1 H), 2.22 (br s, 1 H), 1.91 (s, 3 H), 1.34 (d, 3 H); 13C NMR (75 MHz, CDCl3): δ 171.3, 165.0, 134.2, 133.5, 131.5, 123.5, 117.7, 97.3, 77.6, 73.9, 70.3, 69.5, 65.6, 54.7, 20.7, 19.7; HRMS (ESI) calcd for C20H23NO8 [M + H]+ 405.1424, found 405.1427.
Allyl 3-O-Acetyl-2-deoxy-(6R)-C-methyl-2-phthalimido-β-d-glucopyranoside (9)
Primary alcohol 6 (1.0 g, 2.10 mmol) was oxidized by the usual Swern procedure to the corresponding aldehyde. Subsequent treatment of the crude aldehyde with AlMe3 (5.3 mL,2 M in hexanes) in the presence of CuCN (188 mg, 2.10 mmol) in anhydrous THF (20 mL) afforded the corresponding secondary alcohol (521 mg, 61% yield over two steps) according to the above-described procedure. A solution of oxalyl chloride (0.74 mL, 8.5 mmol) in CH2-Cl2 (8.5 mL) at −78 °C was treated with a solution of DMSO (1.2 mL, 17 mmol) in CH2Cl2 (2.4 mL). The reagents were stirred for 25 min. A solution of secondary alcohol (0.5 g, 1.0 mmol) in CH2Cl2 (3 mL) was added over 20 min via syringe pump. After 20 min, the reaction solution was treated with Et3N (2.8 mL, 20.2 mmol) at −78 °C, allowed to reach 0 °C over 20 min, then diluted with C6H6/Et2O (25 mL, 4:1 v/v) and quenched with saturated NH4Cl solution (24 mL). Aqueous workup (CH2Cl2) followed by flash chromatography on silica gel (10–40% EtOAc gradient in hexanes) afforded intermediate methyl ketone in 81% yield (404 mg) as a mixture of diastereomers.
A solution of methyl ketone (0.32 g, 0.66 mmol) in anhydrous THF (12 mL) was treated with ZnCl2 (0.84 mL, 1 M solution in THF) and cooled to −78 °C. After 10 min, i-Bu2AlH (2.65 mL, 1 M solution in hexanes) was added dropwise. The reaction mixture was stirred for 30 min at −78 °C, then quenched with EtOAc (20 mL) and treated with Na2SO4·10H2O (4 g). The suspension was stirred at room temperature for 1 h, diluted with Et2O (50 mL), filtered over Celite, and evaporated to dryness. The crude residue was dissolved in MeOH (5 mL) and treated with p-toluenesulfonic acid (125 mg, 0.66 mmol) according to the procedure described above to afford a 6:1 mixture of (6R):(6S)-C-methyl epimers as judged by 1H NMR spectroscopy. The desired (6R)-C-methyl diol 9 was separated by flash chromatography on silica gel (10–20% acetone gradient in toluene) in 44% yield over two steps (119 mg). [α]d +2 (c 1, CHCl3); IR (thin film) ν 3474, 2935, 1777, 1747, 1716, 1468, 1387, 1231, 1033, 722 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.84 (m, 2 H), 7.71 (m, 2 H), 5.71 (m, 1 H), 5.63 (dd, 1 H, J = 8.8, 10.7 Hz), 5.38 (d, 1 H, J = 8.4 Hz), 5.12 (dm, 1 H, J = 17.2 Hz), 5.06 (dm, 1 H, J = 10.4 Hz), 4.22 (dd, 1 H, J = 8.4, 10.7 Hz), 4.21 (ddt, 1 H, J = 5.3, 12.9, 1.4 Hz), 4.08 (q, 1 H, J = 6.2 Hz), 4.05 (ddm, 1 H, J = 6.1, 12.9, 1.5 Hz), 3.78 (t, 1 H, J = 9.5 Hz), 3.40 (dd, 1 H, J = 6.4, 9.5 Hz), 2.70 (br s, 2 H), 1.92 (s, 3 H), 1.34 (d, 3 H, J = 6.2 Hz); 13C NMR (75 MHz, CDCl3)δ 171.4, 165.0, 134.3, 133.5, 131.5, 123.6, 117.7, 97.2, 77.2, 73.6, 73.5, 70.3, 70.2, 54.6, 20.7, 19.4; HRMS (ESI) calcd for C20H23NO8 [M + H]+ 406.1502, found 405.1427, calcd [M + Na]+ 428.1321, found 428.1331.
General Procedure for Phthalimide Cleavage and Acetylation (10–12)
In a typical procedure, a solution of monosaccharide 9 (72.7 mg, 0.179 mmol) in n-BuOH (5 mL) was treated with ethylenediamine (0.96 mL, 14.3 mmol). The reaction mixture was stirred at 100 °C in a pressure tube for 20 h, then concentrated to dryness, coevaporated with toluene (thrice), and dried under high vacuum for 1 h. The crude amine was dissolved in pyridine (4.5 mL) and treated with Ac2O (3 mL) at 0 °C. The reaction mixture was stirred at room temperature for 20 h, then quenched with EtOH (24 mL) at 0 °C and concentrated to dryness. Purification by flash column chromatography on silica gel (1.25–5% EtOH gradient in CHCl3) afforded peracetylated monosaccharide 12 in 93% isolated yield over two steps (66.7 mg). Monosaccharides 7 (21.2 mg, 53.9 μmol) and 8 (42.4 mg, 0.105 mmol) were converted to peracetates 10 (15.5 mg, 74% yield over two steps) and 11 (36.7 mg, 87% yield over two steps) by the same procedure.
Allyl 2-N-acetyl-2-deoxy-(6R/6S)-C-2H-3,4,6-tri-O-acetyl-β-d-glucopyranoside (10):
1H NMR (300 MHz, CDCl3) δ 5.94 (m, 1 H), 5.48 (br d, 1 H, J = 8.5 Hz), 5.27 (dd, 1 H, J = 9.5, 10.3 Hz), 5.25 (dm, 1 H, J = 17.2 Hz), 5.17 (dm, 1 H, J = 10.4 Hz), 5.04 (t, 1 H, J = 9.9 Hz), 4.69 (d, 1 H, J = 8.2 Hz), 4.31 (ddm, 1 H, J = 5.0, 12.9 Hz), 4.21 (d, 0.7 H, J = 4.3 Hz), 4.13 (d, 0.3 H, J = 2.4 Hz), 4.06 (ddm, 1 H, J = 6.9, 12.9 Hz), 3.85 (dt, 1 H, J = 10.3, 8.2 Hz), 3.66 (dd, 1 H, J = 3.4, 9.9 Hz), 2.06 (s, 3 H), 2.01 (s, 3 H), 2.00 (s, 3 H), 1.92 (s, 3 H); 13C NMR (75 MHz, CDCl3) δ 170.8, 170.7, 170.1, 169.4, 133.5, 117.8, 99.6, 72.3, 71.7, 69.9, 68.7, 54.7, 23.3, 20.7, 20.6, 20.6; HRMS (ESI) calcd for C17H24DNO9 [M + Na]+ 411.1490, found 411.1499.
Allyl 2-N-acetyl-2-deoxy-(6S)-C-methyl-3,4,6-tri-O-acetyl-β-d-glucopyranoside (11):
[α]d −21 (c 0.5, CHCl3); IR (thin film) ν 3318, 2937, 2877, 1747, 1666, 1537, 1432, 1375, 1305, 1253, 1146, 1054 cm−1; 1H NMR (300 MHz, CDCl3) δ 5.85 (m, 1 H), 5.47 (br d, 1 H, J = 8.7 Hz), 5.27 (dd, 1 H, J = 9.6, 10.5 Hz), 5.23 (dm, 1 H, J = 17.2 Hz), 5.16 (dm, 1 H, J = 10.4 Hz), 5.05 (t, 1 H, J = 9.9 Hz), 5.02 (dq, 1 H, J = 2.1, 6.6 Hz), 4.70 (d,1 H, J = 8.4 Hz), 4.33 (ddt, 1 H, J = 5.2, 12.9, 1.5 Hz), 4.10 (ddt, 1 H, J = 6.4, 12.9, 1.2 Hz), 3.81 (q, 1 H, J = 9.5 Hz), 3.43 (dd, 1 H, J = 2.1, 9.9 Hz), 2.02 (s, 3 H), 1.98 (s, 3 H), 1.95 (s, 3 H), 1.90 (s, 3 H), 1.28 (d, 3 H, J = 6.6 Hz); 13C NMR (75 MHz, CDCl3) δ 170.8, 170.6, 170.2, 169.3, 133.6, 117.8, 99.9, 74.7, 72.5, 70.0, 68.3, 66.1, 55.1, 23.3, 21.0, 20.7, 20.5, 15.7; HRMS (ESI) calcd for C18H27NO9 [M + Na]+ 424.1584, found 424.1587.
Allyl 2-N-acetyl-2-deoxy-(6R)-C-methyl-3,4,6-tri-O-acetyl-β-d-glucopyranoside (12):
[α]d +1(c 1, CHCl3); IR (thin film) ν 3297, 3090, 2950, 2877, 1741, 1665, 1552, 1432, 1375, 1305, 1258, 1237, 1144, 1072, 1041 cm−1; 1H NMR (300 MHz, CDCl3) δ 5.85 (br d, 1 H, J = 8.7 Hz), 5.82 (m, 1 H), 5.21 (dm, 1 H, J) 17.2 Hz), 5.17 (dd, 1 H, J = 9.5, 10.5 Hz), 5.14 (dm, 1 H, J) 10.4 Hz), 4.91 (t, 1 H, J = 10.1 Hz), 4.88 (dq, 1 H, J = 2.0, 6.6 Hz), 4.59 (d, 1 H, J = 8.4 Hz), 4.27 (ddt, 1 H, J = 5.0, 12.9, 1.5 Hz), 4.05 (ddt, 1 H, J = 6.4, 12.9, 1.0 Hz), 3.88 (q, 1 H, J) 9.6 Hz), 3.58 (dd, 1 H, J = 2.0, 10.1 Hz), 1.99 (s, 6 H), 1.97 (s, 3 H), 1.89 (s, 3 H), 1.22 (d, 3 H, J = 6.6 Hz); 13C NMR (75 MHz, CDCl3) δ 170.8, 170.2, 170.1, 169.5, 133.6, 117.6, 99.5, 74.4, 72.7, 69.6, 69.3, 69.0, 54.3, 23.2, 21.1, 20.6, 20.6, 13.5; HRMS (ESI) calcd for C18H27NO9 [M + H]+ 402.1764, found 402.1781.
Typical Deacetylation Procedure (1a–3a)
A solution of monosaccharide 10 (9.0 mg, 23.2 μmol) in CH2Cl2/MeOH (2 mL, 1:1 v/v) was treated with NaOMe (8.5 μL, 1 M in MeOH) at 0 °C. The reaction mixture was stirred at room temperature for 20 h, diluted with MeOH (10 mL), and neutralized with activated acidic Dowex 50X-W H+ ion-exchange resin (25 mg). The resin beads were filtered off and thoroughly rinsed with MeOH (3 × 15 mL). The filtrate was evaporated to dryness, affording monosaccharide 1a as a white solid in 71% yield (4.3 mg). Similarly, monosaccharides 11 (57.0 mg, 0.142 mmol) and 12 (66.7 mg, 0.166 mmol) afforded polyols 2a (38.2 mg, 98% yield) and 3a (46.1 mg, quantitative yield), respectively, with the same procedure.
Allyl 2-N-acetyl-2-deoxy-(6R/6S)-C-2H-β-d-glucopyranoside (1a):
1H NMR (500 MHz, CD3OD) δ 5.89 (m, 1 H, vinylic H-β), 5.27 (dm, 1 H, J = 17.3 Hz, cis-vinylic H-γ), 5.13 (dm, 1 H, J = 10.5 Hz, trans-vinylic H-γ′), 4.44 (d, 1 H, J1,2 ) 8.4 Hz, H-1), 4.34 (ddm, 1 H, J = 4.8, 13.3 Hz, allylic H-α), 4.07 (ddm, 1 H, J = 5.7, 13.3 Hz, allylic H-α′), 3.86 (d, 0.3 H, J5,6S = 2.6 Hz, H-6S), 3.67 (m, 1.7 H, H-2 and H-6R), 3.45 (dd, 1 H, J3,4 = 8.7 Hz, J2,3 = 10.3 Hz, H-3), 3.31 (buried, 1 H, H-4), 3.25 (dd, 1 H, J5,6R = 6.2 Hz, J4,5 = 9.7 Hz, H-5), 1.97 (s, 3 H, COCH3); 13C NMR (125 MHz, CD3OD) δ 173.9, 135.7, 117.1, 102.0, 78.1, 76.2, 72.3, 70.8, 62.7, 57.5, 23.1; HRMS (EI) calcd for C11H18DNO6 [M + H]+ 263.1353, found 263.1354.
Allyl 2-N-acetyl-2-deoxy-(6S)-S-methyl-β-d-glucopyranoside (2a):
1H NMR (500 MHz, CD3OD) δ 5.90 (m, 1 H, vinylic H-β), 5.26 (dm, 1 H, J = 17.2 Hz, cis-vinylic H-γ), 5.14 (dm, 1 H, J = 10.5 Hz, trans-vinylic H-γ′), 4.43 (d, 1 H, J1,2 = 8.4 Hz, H-1), 4.30 (ddt, 1 H, J = 5.0, 13.3, 1.6 Hz, allylic H-α), 4.05–4.10 (m, 2 H, allylic H-α′ and H-6), 3.68 (dd, 1 H, J1,2 = 8.4 Hz, J2,3 = 10.3 Hz, H-2), 3.56 (dd, 1 H, J3,4 = 8.9 Hz, J4,5) 9.6 Hz, H-4), 3.45 (dd, 1 H, J3,4 = 8.9 Hz, J2,3 = 10.3 Hz, H-3), 3.01 (dd, 1 H, J5,6 = 1.8 Hz, J4,5) 9.6 Hz, H-5), 1.98 (s, 3 H, COCH3), 1.28 (d, 3 H, J6,7 = 6.6 Hz, CH3); 13C NMR (125 MHz, CD3OD) δ 173.9, 135.8, 117.1, 102.2, 79.6, 76.4, 71.8, 70.9, 65.9, 57.5, 23.1, 20.1; HRMS (ESI) calcd for C12H21NO6 [M + Na]+ 298.1267, found 298.1265.
Allyl 2-N-acetyl-2-deoxy-(6R)-C-methyl-β-d-glucopyranoside (3a):
1H NMR (500 MHz, CD3OD) δ 5.89 (m, 1 H, vinylic H-β), 5.27 (dm, 1 H, J = 17.3 Hz, cis-vinylic H-γ), 5.13 (dm, 1 H, J = 10.5 Hz, trans-vinylic H-γ′), 4.42 (d, 1 H, J1,2 = 8.4 Hz, H-1), 4.32 (ddt, 1 H, J = 4.9, 13.3, 1.6 Hz, allylic H-α), 4.07 (ddt, 1 H, J = 5.8, 13.3, 1.4 Hz, allylic H-α′), 4.05 (dq, 1 H, J5,6 = 3.9 Hz, J6,7 = 6.5 Hz, H-6), 3.66 (dd, 1 H, J1,2 = 8.4 Hz, J2,3 = 10.3 Hz, H-2), 3.44 (dd, 1 H, J3,4 = 8.7 Hz, J2,3 = 10.3 Hz, H-3), 3.30 (dd, 1 H, J3,4 = 8.7 Hz, J4,5 = 9.7 Hz, H-4), 3.20 (dd, 1 H, J5,6 = 3.9 Hz, J4,5 = 9.7 Hz, H-5), 1.97 (s, 3 H, COCH3), 1.24 (d, 3 H, J6,7 = 6.5 Hz, CH3); 13C NMR (125 MHz, CD3OD) δ 173.9, 135.8, 117.1, 102.1, 79.6, 76.3, 74.4, 70.8, 69.0, 57.4, 23.1, 17.7; HRMS (ESI) calcd for C12H21NO6 [M + Na]+ 298.1267, found 298.1262.
Allyl 3-O-Acetyl-6-O-(tert-butyldimethylsilyl)-2-deoxy(6R/6S)-C-2H-2-phthalimido-β-d-glucopyranoside (13)
A solution of 7 (30.5 mg, 0.078 mmol) in CH2Cl2/THF (750 μL, 3:1 v/v) was treated with Et3N (18.4 μL, 0.132 mmol), imidazole (1.3 mg, 0.019 mmol), then TBSCl (41.0 mg, 0.272 mmol). The reaction mixture was stirred at room temperature for 20 h, then diluted with CH2Cl2 (50 mL) and quenched with saturated NaHCO3 solution (12 mL). Product extraction (CH2Cl2) was followed by 5% CuSO4 aqueous solution (50 mL), water (50 mL), and brine (50 mL) washes. The crude residue was purified by flash column chromatography on silica gel (10–40% EtOAc gradient in hexanes) to afford acceptor 13 in 89% yield (35.1 mg). 1H NMR (300 MHz, CDCl3) δ 7.82 (m, 2 H), 7.70 (m, 2 H), 5.69 (m, 1 H), 5.63 (dd, 1 H, J = 8.7, 10.7 Hz), 5.38 (d, 1 H, J = 8.4 Hz), 5.09 (dm, 1 H, J = 17.2 Hz), 5.02 (dm, 1 H, J = 10.4 Hz), 4.22 (ddt, 1 H, J = 5.0, 12.9, 1.5 Hz), 4.20 (dd, 1 H, J = 8.4, 10.7 Hz), 4.01 (ddt, 1 H, J = 6.2, 12.9, 1.4 Hz), 3.95 (d, 0.7 H, J = 4.9 Hz), 3.87 (d, 0.3 H, J = 5.9 Hz), 3.78 (br t, 1 H, J = 9.5 Hz), 3.58 (dd, 1 H, J = 5.4, 9.5 Hz), 3.35 (br s, 1 H), 1.89 (s, 3 H), 0.90 (s, 9 H), 0.10, 0.09 (2 s, 6 H); 13C NMR (75 MHz, CDCl3) δ 171.0, 167.8, 134.1, 133.5, 131.5, 123.4, 117.5, 97.0, 74.1, 73.5, 72.4, 69.9, 64.6, 54.6, 25.8, 20.6, 18.2, −5.4, −5.5; HRMS (EI) calcd for C25H34DNO8Si [M + H]+ 507.2273, found 507.2276.
Allyl 3-O-Acetyl-2-deoxy-6-O-(p-methoxybenzyl)-(6S)-C-methyl-2-phthalimido-β-d-glucopyranoside (14)
Diol 8 (179 mg, 0.441 mmol) and 4A molecular sieves were suspended in toluene (5 mL) and treated with p-anisaldehyde dimethylacetal (1.4 mL, 8.83 mmol) and camphorsulfonic acid (25.6 mg, 0.110 mmol). The reaction mixture was stirred at 90 °C for 3 h, then diluted with EtOAc (50 mL) and filtered over Celite. Basic aqueous workup (EtOAc) followed by flash chromatography on silica gel (10–40% EtOAc gradient in hexanes, 0.2% Et3N) afforded (6S)-C-Me-substituted acetal as a white solid in 71% yield (165 mg).
A suspension of the acetal (99 mg, 0.19 mmol) and 4A molecular sieves in anhydrous THF (7 mL) was cooled to −30 °C and treated with NaBH3CN (83.2 mg, 1.32 mmol). HCl (2 mL, ca. 1 M solution in Et2O) was added dropwise to the reaction mixture, and repeated every 30 min over 1.5 h reaction time. The reaction mixture was diluted with Et2O, filtered over Celite, then quenched with a 0.5 M HCl aqueous solution. Basic aqueous workup (EtOAc) followed by flash chromatography on silica gel (10–50% EtOAc gradient in hexanes) afforded acceptor 14 as a white solid in 61% yield (60.8 mg). [α]d −13 (c 0.5, CHCl3); IR (thin film) ν 3475, 2917, 1777, 174, 1718, 1613, 1514, 1387, 1246, 1044, 722 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.82 (m, 2 H), 7.69 (m, 2 H), 7.27 (d, 2 H, J = 8.7 Hz), 6.87 (d, 2 H, J = 8.7 Hz), 5.69 (m, 1 H), 5.66 (dd, 1 H, J = 9.0, 10.7 Hz), 5.34 (d, 1 H, J = 8.4 Hz), 5.09 (dm, 1 H, J = 17.2 Hz), 5.02 (dm, 1 H, J = 10.4 Hz), 4.63 (d, 1 H, J = 11.5 Hz), 4.43 (d, 1 H, J = 11.5 Hz), 4.22 (m, 1 H), 4.22 (dd, 1 H, J = 8.4, 10.7 Hz), 4.01 (m, 1 H), 3.97–3.89 (m, 2 H), 3.79 (s, 3 H), 3.50 (dd, 1 H, J = 3.2, 9.7 Hz), 2.84 (d, 1 H, J = 2.7 Hz), 1.90 (s, 3 H), 1.32 (d, 3 H, J = 6.4 Hz); 13C NMR (125 MHz, CDCl3) δ 170.9, 159.4, 134.1, 133.6, 131.5, 129.9, 129.6, 123.5, 117.5, 113.9, 97.3, 75.4, 73.6, 72.5, 70.9, 69.9, 69.4, 55.3, 54.6, 20.7, 14.6; HRMS (EI) calcd for C28H31NO9 [M + H]+ 526.2077, found 526.2066.
Allyl 3-O-Acetyl-2-deoxy-6-O-(p-methoxybenzyl)-(6R)-C-methyl-2-phthalimido-β-d-glucopyranoside (15)
(6R)-C-Me-substituted acetal (220 mg) was obtained in 76% isolated yield from diol 9 (225 mg, 0.555 mmol) by the above-described procedure. The acetal intermediate (45.0 mg, 85.9 μmol) was treated with NaBH3CN (3 × 32.7 mg, 1.56 mmol) and HCl (5 mL, ca. 1 M solution in Et2O) over a 6-h period according to the above-described procedure. Alcohol 15 was isolated in 35% yield (15.9 mg), along with unreacted starting material (14.4 mg, 32%). [α]d +53 (c 0.45, CHCl3); IR (thin film) ν 3852, 3752, 2936, 1777, 1746, 1718, 1616, 1513, 1387, 1228, 1043, 721 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.85 (m, 2 H), 7.73 (m, 2 H), 7.27 (d, 2 H, J = 8.5 Hz), 6.90 (d, 2 H, J = 8.5 Hz), 5.75 (m, 1 H), 5.65 (dd, 1 H, J = 8.7, 10.6 Hz), 5.42 (d, 1 H, J = 8.4 Hz), 5.15 (dm, 1 H, J = 17.2 Hz), 5.09 (dm, 1 H, J = 10.4 Hz), 4.68 (d, 1 H, J = 11.1 Hz), 4.44 (d, 1 H, J = 11.1 Hz), 4.25 (ddt, 1 H, J = 5.3, 12.9, 1.0 Hz), 4.22 (dd, 1 H, J = 8.4, 10.6 Hz), 4.07 (ddt, 1 H, J = 6.2, 12.9, 1.0 Hz), 3.87 (dq, 1 H, J = 6.0, 7.3 Hz), 3.82 (s, 3 H), 3.78 (dd, 1 H, J = 8.7, 9.3 Hz), 3.43 (dd, 1 H, J = 7.3, 9.3 Hz), 1.92 (s, 3 H), 1.41 (d, 3 H, J = 6.0 Hz); 13C NMR (75 MHz, CDCl3) δ 170.5, 167.8, 159.5, 134.1, 133.5, 131.5, 129.6, 129.1, 123.4, 117.6, 114.0, 97.0, 77.6, 76.2, 73.3, 73.2, 70.4, 70.1, 55.2, 54.5, 20.6, 16.5; HRMS (EI) calcd for C28H31NO9 [M + H]+ 526.2077, found 526.2057.
2-Deoxy-2-phthalimido-3,4,6-tri-O-acetyl-α/β-d-glucopyranosyl Trichloroacetimidate (16)17
A solution of tetra-O-acetyl-2-deoxy-2-phthalimido-β-d-glucopyranoside (1.5 g, 3.14 mmol) in anhydrous DMF (10 mL) was treated with hydrazine acetate (0.68 g, 7.41 mmol). The reaction mixture was stirred at room temperature for 3 h, then cooled to 0 °C and quenched with saturated NaHCO3 solution (36 mL). Standard aqueous workup (CHCl3) and purification over a silica gel plug (30–50% EtOAc gradient in hexanes, 0.2% Et3N) afforded the corresponding lactol in 35% yield. The lactol (471 mg, 1.09 mmol) was azeotroped with toluene (thrice), dried under high vacuum overnight, and dissolved in CH2Cl2 (10 mL). The solution was treated with 4A molecular sieves, CCl3-CN (3.25 mL. 32.4 mmol), then Cs2CO3 (106 mg, 0.324 mmol). The reaction mixture was stirred at room temperature for 3 h, diluted with CH2Cl2 (50 mL), filtered over Celite, then quenched with saturated NaHCO3 solution (50 mL). Standard aqueous workup (CH2Cl2) followed by flash chromatography over silica gel (25–50% EtOAc gradient in hexanes, 0.2% Et3N) afforded imidate donor 16 in 74% isolated yield (461.5 mg). 1H NMR (300 MHz, CDCl3) δ 8.64 (s, 1 H), 7.84 (m, 2 H), 7.72 (m, 2 H), 6.61 (d, 1 H, J = 9.0 Hz), 5.90 (dd, 1 H, J = 9.2, 10.7 Hz), 5.26 (dd, 1 H, J = 9.2, 10.1 Hz), 4.61 (dd, 1 H, J = 9.0, 10.7 Hz), 4.37 (dd, 1 H, J = 4.3, 12.4 Hz), 4.18 (dd, 1 H, J = 2.2, 12.4 Hz), 4.05 (ddd, 1 H, J = 2.2, 4.3, 10.1 Hz), 2.10 (s, 3 H), 2.05 (s, 3 H), 1.88 (s, 3 H).
Glycosidic Coupling (17–19)
In a typical procedure, both imidate donor 16 (134.1 mg, 0.23 mmol) and acceptor 14 (60.8 mg, 0.11 mmol) were azeotroped with toluene (thrice), dried under high vacuum for 45 min, then dissolved in CH2Cl2 (2 mL) and treated with 4A activated molecular sieves. The suspension was stirred at room temperature for 15 min then cooled to −30 °C and treated with TMSOTf (64 μL, 0.5 M solution in CH2Cl2). The reaction mixture was stirred at −30 °C for 2 h, then quenched with Et3N (24 μL, 0.17 mmol), filtered over Celite, and concentrated to dryness. The crude residue was purified by flash column chromatography on silica gel (25–60% EtOAc gradient in hexanes) to afford disaccharide 18 in 98% isolated yield (107 mg). Similarly, acceptors 13 (40.0 mg, 0.079 mmol) and 15 (84.0 mg, 0.16 mmol) were respectively converted into disaccharides 17 (37.1 mg, 51% isolated yield) and 19 (118.3 mg, 78% isolated yield) with the same procedure.
Allyl 3-O-acetyl-6-O-(tert-butyldimethylsilyl)-2-deoxy-4-O-(2-deoxy-2-phthalimido-3,4,6-tri-O-acetyl-β-d-glucopyranosyl)-(6R/6S)-C-2H-2-phthalimido-β-d-glucopyrano-side (17):
1H NMR (300 MHz, CDCl3) δ 7.87–7.64 (m, 8 H), 5.74 (dd, 1 H, J = 9.0, 10.5 Hz), 5.63 (dd, 1 H, J = 9.0, 10.6 Hz), 5.63 (m, 1 H), 5.48 (d, 1 H, J = 8.4 Hz), 5.24 (d, 1 H, J = 8.4 Hz), 5.08 (dd, 1 H, J = 9.0, 10.1 Hz), 5.02 (dm, 1 H, J = 17.2 Hz), 4.97 (dm, 1 H, J = 10.4 Hz), 4.42 (dd, 1 H, J = 4.6, 12.3 Hz), 4.19 (dd, 1 H, J = 8.4, 10.5 Hz), 4.11 (ddt, 1 H, J = 5.0, 12.9, 1.4 Hz), 4.10 (dd, 1 H, J = 8.4, 10.6 Hz), 4.04 (dd, 1 H, J = 2.7, 12.3 Hz), 4.00 (t, 1 H, J = 9.9 Hz), 3.92 (ddt, 1 H, J = 6.3, 12.9, 1.3 Hz), 3.78 (ddd, 1 H, J = 2.7, 4.6, 10.1 Hz), 3.62 (d, 0.3 H, J = 1.5 Hz), 3.40 (d, 0.7 H, J = 3.5 Hz), 3.31 (dd, 1 H, J = 2.5, 9.9 Hz), 2.06 (s, 3 H), 1.98 (s, 3 H), 1.89 (s, 3 H), 1.79 (s, 3 H), 0.90 (s, 9 H), 0.05 and 0.04 (2 s, 6 H).
Allyl 3-O-acetyl-2-deoxy-4-O-(2-deoxy-2-phthalimido-3,4,6-tri-O-acetyl-β-d-glucopyranosyl)-6-O-(p-methoxybenzyl)-(6S)-C-methyl-2-phthalimido-β-d-glucopyrano-side (18):
[α]d −12 (c 1, CHCl3); IR (thin film) ν 2950, 1734, 1717, 1684, 1653, 1559, 1540, 1507, 1457, 1046, 668 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.85-7.68 (m, 8 H), 7.41 (d, 2 H, J = 8.7 Hz), 6.91 (d, 2 H, J = 8.7 Hz), 5.68 (m, 1 H), 5.61 (dd, 1 H, J = 9.2, 10.7 Hz), 5.59 (dd, 1 H, J = 9.0, 10.7 Hz), 5.22 (d, 1H, J = 8.5 Hz), 5.16 (d, 1 H, J = 8.4 Hz), 5.05 (dm, 1 H, J = 17.2 Hz), 4.99 (dd, 1 H, J = 9.2, 10.4 Hz), 4.98 (dm, 1 H, J = 10.1 Hz), 4.67 (d, 1 H, J = 11.3 Hz), 4.41 (d, 1 H, J = 11.3 Hz), 4.22 (dd, 1 H, J = 6.5, 12.3 Hz), 4.20 (t, 1 H, J = 9.0 Hz), 4.18 (dd, 1 H, J = 8.4, 10.7 Hz), 4.17 (m, 1 H), 4.11 (dd, 1 H, J = 8.5, 10.7 Hz), 3.98 (ddt, 1 H, J = 6.4, 12.9, 1.6 Hz), 3.86 (dd, 1H, J = 2.0, 12.3 Hz), 3.82 (s, 3 H), 3.65 (dq, 1 H, J = 2.1, 6.4 Hz), 3.24 (dd, 1 H, J = 2.1, 9.8 Hz), 2.90 (ddd, 1 H, J = 2.0, 6.5, 10.4 Hz), 2.03 (s, 3 H), 1.97 (s, 3 H), 1.88 (s, 3 H), 1.79 (s, 3 H), 1.19 (d, 3 H, J = 6.4 Hz); 13C NMR (75 MHz, CDCl3) δ 170.5, 170.2, 169.4, 167.5, 159.0, 134.4, 134.1, 133.6, 131.4, 129.0, 123.6, 123.4, 117.6, 113.7, 97.1, 95.9, 73.4, 71.4, 71.2, 70.6, 69.9, 69.6, 68.4, 61.3, 55.2, 55.0, 54.9, 20.6, 20.5, 20.5, 20.4, 15.2; HRMS (ESI) calcd for C48H50N2O18 [M + Na]+ 965.2956, found 965.2959.
Allyl 3-O-acetyl-2-deoxy-4-O-(2-deoxy-2-phthalimido-3,4,6-tri-O-acetyl-β-d-glucopyranosyl)-6-O-(p-methoxybenzyl)-(6R)-C-methyl-2-phthalimido-β-d-glucopyrano-side (19):
[α]d +7 (c 1, CHCl3); IR (thin film) ν 2943, 1778, 1749, 1718, 1612, 1514, 1498, 1387, 1228, 1144, 1046, 722 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.81 (m, 4 H), 7.69 (m, 4 H), 7.03 (d, 2 H, J = 8.7 Hz), 6.80 (d, 2 H, J = 8.7 Hz), 5.75 (dd, 1 H, J = 7.9, 10.7 Hz), 5.67 (dd, 1 H, J = 9.2, 10.4 Hz), 5.64 (m, 1 H), 5.45 (d, 1 H, J = 8.4 Hz), 5.24 (d, 1 H, J = 8.5 Hz), 5.12 (t, 1 H, J = 9.3 Hz), 5.02 (dm, 1 H, J = 17.2 Hz), 4.96 (dm, 1 H, J = 10.4 Hz), 4.38 (dd, 1 H, J = 4.0, 12.3 Hz), 4.21 (dd, 1 H, J = 8.4, 10.7 Hz), 4.15 (ddt, 1 H, J = 5.0, 13.0, 1.4 Hz), 4.12 (dd, 1 H, J = 8.5, 10.4 Hz), 4.09 (d, 2 H, J = 11.6 Hz), 4.01 (dd, 1 H, J = 2.1, 12.3 Hz), 3.94 (ddt, 1 H, J = 6.4, 13.0, 1.6 Hz), 3.78 (s, 3 H), 3.76–3.70 (m, 3 H), 3.52 (dq, 1 H, J = 1.0, 6.6 Hz), 2.04 (s, 3 H), 1.96 (s, 3 H), 1.91 (s, 3 H), 1.80 (s, 3 H), 0.96 (d, 3 H, J = 6.6 Hz); 13C NMR (125 MHz, CDCl3) δ 170.5, 170.1, 169.8, 169.3, 167.7, 159.0, 134.4, 134.1, 133.5, 131.3, 130.5, 128.8, 123.6, 123.4, 117.6, 113.6, 96.9, 96.7, 76.5, 75.2, 73.4, 71.9, 71.3, 70.6, 70.4, 69.8, 68.3, 61.4, 55.2, 54.8, 20.6, 20.6, 20.5, 20.3, 14.2; HRMS (ESI) calcd for C48H50N2O18 [M + Na]+ 965.2956, found 965.2944.
Allyl N-Acetyl-4-O-(N-acetyl-2-deoxy-β-d-glucopyranosyl)-2-deoxy-(6R/6S)-C-2H-β-d-glucopyranoside (1b)
A solution of disaccharide 17 (37.5 mg, 40.6 μmol) in anhydrous THF was treated with TBAF (183 μL, 1 M solution in THF). The reaction mixture was stirred at room temperature for 20 h, then evaporated to dryness. The crude residue was coevaporated with CHCl3 (thrice), dissolved in n-BuOH (5 mL), and treated with ethylenediamine (0.44 mL, 6.6 mmol) according to the above-described procedure. The resulting free amine intermediate was dissolved in pyridine (3 mL) and treated with Ac2O (2 mL) also according to the above-described procedure. Column chromatography on silica gel (1.25–5% EtOH gradient in CHCl3) afforded the desired peracetylated C-6 monodeuterated disaccharide intermediate (27.2 mg, 99% over three steps): [α]d +4(c 1, CHCl3); IR (thin film) ν 3289, 2920, 1745, 1661, 1541, 1372, 1233, 1047 cm−1; 1H NMR (500 MHz, CDCl3) δ 6.28 (d, 1 H, J = 9.1 Hz), 5.97 (d, 1 H, J = 9.5 Hz), 5.81 (m, 1 H), 5.22 (dm, 1 H, J = 17.2 Hz), 5.18 (dd, 1 H, J = 9.4, 10.3 Hz), 5.15 (dm, 1 H, J = 10.4 Hz), 5.11 (dd, 1 H, J = 8.2, 9.7 Hz), 5.01 (t, 1 H, J = 9.6 Hz), 4.59 (d, 1 H, J = 8.2 Hz), 4.50 (d,1H, J = 7.8 Hz), 4.34 (dd, 1 H, J = 4.3, 12.3 Hz), 4.27 (ddt, 1H, J = 5.0, 13.1, 1.5 Hz), 4.23 (d, 1 H, J = 5.6 Hz), 4.01 (ddt, 1 H, J = 6.1, 13.1, 1.4 Hz), 4.00 (dd, 1 H, J = 2.4, 12.3 Hz), 3.88–3.79 (m, 2 H), 3.72 (t, 1 H, J = 8.9 Hz), 3.65 (ddd, 1 H, J = 2.4, 4.3, 9.6 Hz), 3.61 (dd, 1 H, J = 5.6, 8.9 Hz), 2.10 (s, 3 H), 2.05 (s, 3 H), 2.03 (s, 3 H), 1.98 (s, 3 H), 1.97 (s, 3 H), 1.94 (s, 3 H), 1.91 (s, 3 H); 13C NMR (125 MHz, CDCl3) δ 171.0, 170.8, 170.7, 170.6, 170.3, 169.3, 133.5, 117.6, 101.1, 99.9, 99.7, 76.1, 72.7, 72.4, 72.3, 71.7, 69.7, 68.1, 62.3, 61.7, 54.7, 53.4, 23.2, 20.9, 20.7, 20.6.
A solution of C-6 monodeuterated disaccharide peracetate (25.4 mg, 37.6 μmol) in CH2Cl2/MeOH (3 mL, 1:1 v/v) was treated with NaOMe (25 μL, 1 M in MeOH) according to the above-described procedure to afford monodeuterated disaccharide 1b as a white solid in quantitative yield (17.5 mg). 1H NMR (600 MHz, CD3OD:D2O, v/v 3:1) δ 5.88 (m, 1 H, vinylic H-β), 5.27 (dm, 1 H, J = 17.3 Hz, vinylic H-γ), 5.17 (dm, 1 H, J = 10.6 Hz, vinylic H-γ′), 4.52 (d, 1 H, J1′,2′ = 8.5 Hz, H-1′), 4.46 (d, 1 H, J1,2 = 8.3 Hz, H-1), 4.31 (ddt, 1 H, J = 4.9, 13.3, 1.6 Hz, allylic H-α), 4.08 (ddt, 1 H, J = 5.9, 13.3, 1.4 Hz, allylic H-α′), 3.91 (dd, 1 H, J5′,6′a = 2.2 Hz, J6′a,6′b = 12.1 Hz, H-6′a), 3.80 (d, 0.3 H, J5,6S = 2.0 Hz, H-6S), 3.74 (dd, 1 H, J1,2 = 8.3 Hz, J2,3 = 10.4 Hz, H-2), 3.73 (dd, 1 H, J1′,2′ = 8.5 Hz, J2′,3′ = 10.4 Hz, H-2′), 3.67 (dd, 1 H, J5′,6′b = 6.3 Hz, J6′a,6′b = 12.1 Hz, H-6′b), 3.64 (dd, 1 H, J3,4 = 8.3 Hz, J2,3 = 10.4 Hz, H-3), 3.63 (d, 0.7 H, J5,6R = 4.9 Hz, H-6R), 3.55 (dd, 1 H, J3,4 = 8.3 Hz, J4,5 = 9.7 Hz, H-4), 3.48 (dd, 1 H, J3′,4′ = 8.5 Hz, J2′,3′ = 10.4 Hz, H-3′), 3.41 (ddd, 1 H, J5′,6′a = 2.2 Hz, J5′,6′b = 6.3 Hz, J4′,5′) 9.9 Hz, H-5′), 3.36 (m, 2 H, H-4′ and H-5), 2.04 (s, 3 H, COCH3), 1.99 (s, 3 H, COCH3); 13C NMR (75 MHz, CD3OD/D2O, 4:1 v/v) δ 174.4, 174.2, 135.3, 117.7, 103.2, 101.8, 81.4, 78.0, 76.3, 75.6, 74.2, 71.8, 71.1, 62.4, 61.3, 57.2, 56.5, 23.2, 23.1; HRMS (ESI) calcd C19H31DN2O11 [M + Na]+ 488.1967, found 488.1975.
6-C-Methyl-Substituted Disaccharides (2b, 3b)
A solution of disaccharide 18 (91.8 mg, 97.4 μmol) in CH2Cl2 (5.5 mL) was treated with pH 7 buffer (0.41 mL), t-BuOH (0.19 mL, 1.95 mmol), and DDQ (71 mg, 0.31 mmol) at 0 °C. The heterogeneous solution was stirred at room temperature for 20 h, then diluted with CH2Cl2 (15 mL) and quenched with saturated NaHCO3 solution (12 mL) at 0 °C. Standard aqueous workup (CH2Cl2) followed by flash column chromatography on silica gel (25–70% EtOAc gradient in hexanes) afforded the free 6-OH disaccharide intermediate in 80% isolated yield (63.8 mg).
According to the previously described procedure, a solution of the disaccharide intermediate (50.4 mg, 61.2 μmol) in n-BuOH (5.5 mL) was treated with ethylenediamine (0.65 mL, 9.80 mmol) affording the free amine intermediate, which was then dissolved in pyridine (3 mL) and treated with Ac2O (2 mL). Column chromatography on silica gel (1.25–5% EtOH gradient in CHCl3) afforded the desired (6S)-C-CH3-substituted peracetylated disaccharide intermediate (42.8 mg, quantitative yield over two steps): [α]D –53 (c 1, CHCl3); IR (thin film) ν 3347, 2940, 1745, 1664, 1532, 1431, 1375, 1231, 1150, 1048 cm−1; 1H NMR (500 MHz, CDCl3) δ 6.19 (d, 1 H, J = 9.6 Hz), 5.83 (m, 1 H), 5.46 (d, 1 H, J = 9.5 Hz), 5.31 (dq, 1 H, J = 1.1, 6.6 Hz), 5.23 (dq, 1 H, J = 17.4, 1.6 Hz), 5.18 (dm, 1 H, J = 10.4, 1.6 Hz), 5.03 (t, 1 H, J = 9.6 Hz), 5.01 (dd, 1 H, J = 10.7, 8.8 Hz), 4.91 (t, 1 H, J = 10.2 Hz), 4.39 (d, 1 H, J = 8.4 Hz), 4.34 (dd, 1 H, J = 4.1, 12.4 Hz), 4.30 (ddt, 1 H, J = 11.7, 5.2, 1.6 Hz), 4.16 (q, 1 H, J = 8.4 Hz), 4.09 (ddt, 1 H, J = 6.4, 11.7, 1.6 Hz), 4.09 (q, 1 H, J = 8.4 Hz), 4.04 (d, 1 H, J = 8.4 Hz), 3.98 (dd, 1 H, J = 2.3, 12.4 Hz), 3.54 (t, 1 H, J = 9.6 Hz), 3.51 (m, 1 H), 3.27 (dd, 1 H, J = 8.6, 9.6 Hz), 2.16 (s, 3 H), 2.05 (s, 3 H), 1.99 (s, 3 H), 1.98 (s, 3 H), 1.97 (s, 3 H), 1.96 (s, 3 H), 192 (s, 3 H), 1.30 (d, 3 H); 13C NMR (125 MHz, CDCl3) δ 172.0, 171.0, 170.8, 170.6, 170.1, 169.9, 169.2, 133.6, 117.9, 102.6, 100.4, 77.4, 76.1, 73.2, 72.8, 72.0, 70.0, 67.8, 66.7, 61.6, 54.0, 53.5, 23.3, 23.0, 21.6, 20.7, 20.6, 20.6, 20.5, 16.6; HRMS (ESI) calcd for C30H44N2O16 [M + H]+ 689.2769, found 689.2742.
Disaccharide 19 (117.6 mg, 0.125 mmol) was converted to the corresponding (6R)-C-CH3-substituted peracetate intermediate in a similar fashion (63.0 mg, 74% yield over three steps): [α]D –36 (c 1, CHCl3); IR (thin film) ν 3341, 2940, 1744, 1687, 1666, 1537, 1431, 1372, 1233, 1151, 1047 cm−1; 1H NMR (500 MHz, CDCl3) δ 6.25 (d, 1 H, J = 8.2 Hz), 6.09 (d, 1 H, J = 9.4 Hz), 5.82 (m, 1 H), 5.35 (t, 1 H, J = 10.0 Hz), 5.22 (dq, 1H, J = 17.2, 1.5 Hz), 5.14 (m, 2 H), 5.05 (t, 1 H, J = 9.5 Hz), 5.01 (t, 1 H, J = 8.1 Hz), 4.79 (d, 1 H, J = 8.2 Hz), 4.42 (d, 1 H, J = 7.7 Hz), 4.34 (dd, 1 H, J = 4.8, 12.4 Hz), 4.26 (ddt, 1 H, J = 4.8, 13.2, 1.5 Hz), 4.05 (ddt, 1 H, J = 6.1, 13.2, 1.5 Hz), 4.01 (t, 1 H, J = 8.6 Hz), 4.00 (dd, 1 H, J = 2.1, 12.4 Hz), 3.70 (m, 1 H), 3.67 (q, 1 H, J = 8.2 Hz), 3.60 (q, 1 H, J = 7.7 Hz), 3.53 (dd, 1 H, J = 2.9, 12 Hz), 2.03 (s, 3 H), 2.01 (s, 6 H), 1.97–1.96 (2 s, 6 H), 1.93 (s, 3 H), 1.89 (s, 3 H), 1.26 (d, 3 H, J = 6.5 Hz); 13C NMR (125 MHz, CDCl3) δ 171.2, 170.6, 170.6, 170.4, 170.3, 169.5, 133.7, 117.3, 100.1, 99.8, 76.2, 75.4, 72.6, 71.9, 71.7, 69.4, 68.9, 68.4, 61.9, 55.4, 53.1, 23.2, 23.1, 21.2, 20.7, 20.6, 20.6, 13.8; HRMS (ESI) calcd for C30H44N2O16 [M + H]+ 689.2769, found 689.2739.
A solution of (6S)-C-CH3-substituted disaccharide peracetate (65.0 mg, 94.4 μmol) in CH2Cl2/MeOH (6 mL, 1:1 v/v) was treated with NaOMe (60 μL, 1 M in MeOH) according to the above-described procedure, affording disaccharide 2b as a white solid in 99% yield (45.0 mg). Similarly, (6R)-C-CH3-substituted disaccharide peracetate (63.0 mg, 91.5 μmol) afforded disaccharide 3b (43.0 mg, 96% yield).
Allyl N-acetyl-4-O-(N-acetyl-2-deoxy-β-d-glucopyranosyl)-2-deoxy-(6S)-C-methyl-β-d-glucopyranoside (2b):
1H NMR (600 MHz, CD3OD) δ 5.89 (m, 1 H, vinylic H-β), 5.26 (dq, 1 H, J = 1.6, 17.3 Hz, cis-vinylic H-γ), 5.14 (dq, 1 H, J = 10.5, 1.6 Hz, trans-vinylic H-γ′), 4.50 (d, 1 H, J1′,2′ = 8.4 Hz, H-1′), 4.40 (d, 1 H, J1,2 = 8.5 Hz, H-1), 4.29 (ddt, 1 H, J = 5.0, 13.3, 1.5 Hz, allylic H-α), 4.07 (ddt, 1 H, J = 5.8, 13.3, 1.5 Hz, allylic H-α′), 3.91 (dq, 1 H, J5,6 = 1.5 Hz, J6,7 = 6.6 Hz, H-6), 3.90 (dd, 1 H, J5′,6′a = 2.3 Hz, J6′a,6′b = 11.8 Hz, H-6′a), 3.74 (dd, 2 H, J1,2 = J1′,2′ = 8.5 Hz, J2,3 = J2′,3′ = 10.2 Hz, H-2 and H-2′), 3.65 (dd, 1 H, J5′,6′b = 6.1 Hz, J6′a,6′b = 11.8 Hz, H-6′b), 3.64 (dd, 1 H, J3,4 = 8.6 Hz, J4,5 = 9.4 Hz, H-4), 3.59 (dd, 1 H, J3,4 = 8.6 Hz, J2,3 = 10.2 Hz, H-3), 3.42 (dd, 1 H, J3′,4′ = 8.4 Hz, J2′,3′ = 10.2 Hz, H-3′), 3.35 (dm, 1 H, J4′,5′ = 9.3 Hz, H-5′), 3.34 (t, 1 H, J3′,4′ = J4′,5′ = 8.8 Hz, H-4′), 3.08 (dd, 1 H, J5,6 = 1.5 Hz, J4,5 = 9.4 Hz, H-5), 1.99 (s, 3 H, COCH3), 1.96 (s, 3 H, COCH3), 1.29 (d, 3 H, J6,7 = 6.6 Hz, CH3); 13C NMR (125 MHz, CD3OD/D2O, v/v 5:3) δ 174.7 (CO), 174.7 (CO), 134.9 (C-β), 118.4 (C-γ), 103.1 (C-1′), 101.6 (C-1), 81.4 (C-4), 77.8 (C-5), 77.5 (C-5′), 75.1 (C-3′), 74.0 (C-3), 71.4 (C-4′), 71.3 (C-α), 64.9 (C-6), 62.1 (C-6′), 56.9, 56.3 (C-2/2′), 23.2, 22.9 (NHAc), 19.9 (CH3); HRMS (ESI) calcd for C20H34N2O11 [M + Na]+ 501.2060, found 501.2063.
Allyl N-acetyl-4-O-(N-acetyl-2-deoxy-β-d-glucopyranosyl)-2-deoxy-(6R)-C-methyl-β-d-glucopyranoside (3b):
1H NMR (600 MHz, CD3OD) δ 5.88 (m, 1 H, vinylic H-β), 5.27 (dq, 1 H, J = 17.3, 1.8 Hz, cis-vinylic H-γ), 5.13 (dq, 1 H, J = 10.5, 1.8 Hz, trans-vinylic H-γ′), 4.50 (d, 1 H, J1′,2′ = 8.4 Hz, H-1′), 4.40 (d, 1 H, J1,2 = 8.4 Hz, H-1), 4.34 (ddt, 1 H, J = 4.8, 13.4, 1.8 Hz, allylic H-α), 4.08 (ddt, 1 H, J = 5.8, 13.4, 1.8 Hz, allylic H-α′), 4.02 (dq, 1 H, J5,6 = 2.4 Hz, J6,7 = 6.5 Hz, H-6), 3.91 (dd, 1 H, J5′,6′a = 2.3 Hz, J6′a,6′b = 11.8 Hz, H-6′a), 3.72 (dd, 1 H, J1,2 = 8.4 Hz, J2,3 = 10.2 Hz, H-2), 3.65 (dd, 1 H, J1′,2′ = 8.4 Hz, J2′,3′ = 10.4 Hz, H-2′), 3.62 (dd, 1 H, J5′,6′b = 6.7 Hz, J6′a,6′b = 11.8 Hz, H-6′b), 3.60 (dd, 1 H, J3,4 = 7.9 Hz, J2,3 = 10.2 Hz, H-3), 3.48 (dd, 1 H, J3′,4′ = 8.6 Hz, J2′,3′ = 10.4 Hz, H-3′), 3.39 (dd, 1 H, J5,6 = 2.4 Hz, J4,5 = 9.7 Hz, H-5), 3.35 (dm, 1 H, J4′,5′ = 9.8 Hz, H-5′), 3.33 (dd, 1 H, J3,4 = 7.9 Hz, J4,5 = 9.7 Hz, H-4), 3.28 (dd, 1 H, J3′,4′ = 8.6 Hz, J4′,5′ = 9.8 Hz, H-4′), 2.01 (s, 3 H, COCH3), 1.96 (s, 3 H, COCH3), 1.22 (d, 3 H, J6,7 = 6.5 Hz, CH3); 13C NMR (125 MHz, CD3OD/D2O, v/v 5:1) δ 174.5 (CO), 174.3 (CO), 135.3 (C-β), 117.8 (C-γ), 103.0 (C-1′), 101.9 (C-1), 83.3 (C-4), 78.4 (C-5), 77.9 (C-5′), 75.3 (C-3′), 74.6 (C-3), 71.8 (C-4′), 71.0 (C-α), 66.8 (C-6), 62.4 (C-6′), 57.3 (C-2′), 56.4 (C-2), 23.3, 23.2 (NHAc), 16.3 (CH3); HRMS (ESI) calcd for C20H34N2O11 [M + Na]+ 501.2060, found 501.2060.
Supplementary Material
Acknowledgment
This work was supported by the American Chemical Society Petroleum Research Foundation (33341-G4, 36069-AC1), the American Cancer Society (IRG-58-006-41), the American Heart Association Midwest Affiliates (30399Z), and the National Science Foundation (CHE-0243496). J.A. thanks the American Heart Association Midwest Affiliates for financial support in the form of a predoctoral fellowship (0110248Z). Support from the Purdue Cancer Center is also gratefully acknowledged. We thank Dr. Klaas Hallenga, Dr. Edwin Rivera, and Mr. Scott A. Bradley of the Purdue Interdepartmental NMR Facility and Dr. Karl V. Wood of the Purdue Mass Spectrometry Center for their assistance, and Prof. Paul G. Wenthold for helpful discussions on error analysis.
Footnotes
Supporting Information Available: Spectroscopic data for title compounds 1a–3b and synthetic intermediates 4–19, including 1H, 13C, {1H}-coupled-13C, DQF-COSY, and HMQC NMR spectra. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Hecht SM, editor. Bioorganic Chemistry: Carbohydrates. Oxford University Press; Oxford, UK: 1999. [Google Scholar]
- 2.Adelhorst K, Bock K. Acta Chem. Scand. 1992;46:1114–1121. doi: 10.3891/acta.chem.scand.46-1114. [DOI] [PubMed] [Google Scholar]
- 3.Bock K, Duus JØ. J. Carbohydr. Chem. 1994;13:513–543. [Google Scholar]
- 4.Blackwell J. In: Methods in Enzymology. Wood WA, Kellogg ST, editors. Vol. 161. Academic Press; San Diego, CA: 1988. pp. 435–442. [Google Scholar]
- 5.Minke R, Blackwell J. J. Mol. Biol. 1978;120:167–181. doi: 10.1016/0022-2836(78)90063-3. [DOI] [PubMed] [Google Scholar]
- 6.Gardner KH, Blackwell J. Biopolymers. 1975;14:1581–1595. doi: 10.1002/bip.1975.360140804. [DOI] [PubMed] [Google Scholar]
- 7.van Aalten DMF, Komander D, Synstad B, Gaseidnes S, Peter MG, Eijsink VGH. Proc. Natl. Acad. Sci. U.S.A. 2001;98:8979–8984. doi: 10.1073/pnas.151103798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Eliel EL, Gilbert EC. J. Chem. Am. Soc. 1969;91:5487–5495. [Google Scholar]; (b) Corey EJ, Feiner NF. J. Org. Chem. 1980;45:765–780. [Google Scholar]; (c) Eliel EL, Wilen SH, Mander LN. Stereochemistry of Organic Compounds. Wiley; New York: 1994. Chapter 10 and references therein. [Google Scholar]
- 9.(a) Lemieux RU, Wong TC, Thøgerson H. Can. J. Chem. 1982;60:81–86. [Google Scholar]; (b) Lough C, Hindsgaul O, Lemieux RU. Carbohydr. Res. 1983;120:43–53. doi: 10.1016/0008-6215(83)88005-7. [DOI] [PubMed] [Google Scholar]
- 10.(a) Lindh I, Hindsgaul O. J. Am. Chem. Soc. 1991;113:216–223. [Google Scholar]; (b) Sabesan S, Neira S, Davidson F, Duus JØ, Bock K. J. Am. Chem. Soc. 1994;116:1616–1634. [Google Scholar]; (c) Spohr U, Le N, Ling C-C, Lemieux RU. Can J. Chem. 2001;79:238–255. [Google Scholar]
- 11.Achkar J, Sanchez-Larraza I, Wei A. Carbohydr. Res. 2002;337:83–86. doi: 10.1016/s0008-6215(01)00295-6. [DOI] [PubMed] [Google Scholar]
- 12.Berdy J, Pauncz JK, Vajna ZM, Horvath G, Gyimesi J, Koczka I. J. Antibiot. 1977;30:945–954. doi: 10.7164/antibiotics.30.945. [DOI] [PubMed] [Google Scholar]
- 13.Vicens Q, Westhof E. J. Mol. Biol. 2003;326:1175–1188. doi: 10.1016/s0022-2836(02)01435-3. [DOI] [PubMed] [Google Scholar]
- 14.Wright GD, Davies J. Trends Microbiol. 1997;5:234–240. doi: 10.1016/S0966-842X(97)01033-0. [DOI] [PubMed] [Google Scholar]
- 15.(a) Kiso M, Anderson L. Carbohydr. Res. 1985;136:309–323. doi: 10.1016/0008-6215(85)85205-8. [DOI] [PubMed] [Google Scholar]; (b) El-Sokkary RI, Silwanis BA, Nashed MA, Paulsen H. Carbohydr. Res. 1990;203:319–323. [Google Scholar]; (c) Hernández-Torres JM, Liew S-T, Achkar J, Wei A. Synthesis. 2002:487–490. [Google Scholar]
- 16.Jiang L, Chan T-H. Tetrahedron Lett. 1998;39:355–358. [Google Scholar]
- 17.Hernández-Torres JM, Achkar J, Wei A. J. Org. Chem. 2004;69:7206–7211. doi: 10.1021/jo048999m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mancuso AJ, Huang S-L, Swern D. J. Org. Chem. 1978;43:2480–2482. [Google Scholar]
- 19.Flemming S, Kabbara J, Nickisch K, Westermann J, Mohr J. Synlett. 1995:183–185. [Google Scholar]
- 20.Frenette R, Monette M, Bernstein MA, Young RN, Verhoeven TR. J. Org. Chem. 1991;56:3083–3089. [Google Scholar]
- 21.Johansson R, Samuelsson B. J. Chem. Soc., Chem. Commun. 1984:201–202. [Google Scholar]
- 22.Grundler G, Schmidt RR, Michel J. Carbohydr. Res. 19841985;135:203–218. [Google Scholar]
- 23.Haasnoot CAG, de Leeuw FAAM, Altona C. Tetrahedron. 1980;36:2783–2792. [Google Scholar]
- 24.Podlasek CA, Wu J, Stripe WA, Brondo PB, Serianni AS. J. Am. Chem. Soc. 1995;117:8635–8644. [Google Scholar]
- 25.Matsumori N, Kanedo D, Murata M, Nakamura H, Tachibana K. J. Org. Chem. 1999;64:866–876. doi: 10.1021/jo981810k. [DOI] [PubMed] [Google Scholar]
- 26.Rockwell GD, Grindley TB. J. Am. Chem. Soc. 1998;120:10953–10963. Conformational analysis of the C5-C6 bond is more accurate when conformers are assumed to adopt slightly nonstaggered geometries. See. [Google Scholar]
- 27.(a) Spoormaker T, de Bie MJA. Recl. Trav. Chim. 1978;97:85–87. It has been shown that 13C–1H coupling constants can be used to support parametrized Karplus relationships for quantifying the relative populations of staggered conformers, particularly for C–O–C–H couplings. However, the amount of empirical data available to support such relationships for C–C–C–H couplings is presently limited. Also technical limitations arise when collecting 2,3JC,H values at low temperatures on natural abundance compounds. See: [Google Scholar]; (b) Spoormaker T, de Bie MJA. Recl. Trav. Chim. Pays-Bas. 1979;98:380–388. [Google Scholar]; (c) Spoormaker T, de Bie MJA. Recl. Trav. Chim. Pays-Bas. 1980;99:154–160. [Google Scholar]; (d) Mulloy B, Frenkiel TA, Davies DB. Carbohydr. Res. 1988;184:39–46. doi: 10.1016/0008-6215(88)80004-1. [DOI] [PubMed] [Google Scholar]; (e) Tvaroska I, Gajdos J. Carbohydr. Res. 1995;271:151–162. [Google Scholar]; (f) Bose B, Zhao S, Stenutz R, Cloran F, Bondo PB, Bondo G, Hertz B, Carmichael I, Serianni AS. J. Am. Chem. Soc. 1998;120:11158–11173. [Google Scholar]
- 28.(a) Prelog V. Pure Appl. Chem. 1964;9:119–130. [Google Scholar]; (b) Prelog V. Angew. Chem., Int. Ed. Engl. 1989;28:1147–1152. [Google Scholar]; (c) Wei A, Haudrechy A, Audin C, Jun H-S, Haudrechy-Bretel N, Kishi Y. J. Org. Chem. 1995;60:2160–2169. and references therein. [Google Scholar]
- 29.(a) Perkins SJ, Johnson LN, Phillips DC, Dwek RA. Carbohydr. Res. 1977;59:19–34. [Google Scholar]; (b) Nishida Y, Hori H, Ohrui H, Meguro H. Carbohydr. Res. 1987;170:106–111. [Google Scholar]
- 30.Kirschner KN, Woods RJ. Proc. Natl. Acad. Sci. U.S.A. 2002;98:10541–10545. doi: 10.1073/pnas.191362798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.(a) Lemieux RU, Pavia AA, Martin JC, Watanabe KA. Can. J. Chem. 1969;47:4427–4439. Others have commented on the influence of solvation on conformational equilibria: [Google Scholar]; (b) Praly J-P, Lemieux RU. Can. J. Chem. 1987;65:213–223. [Google Scholar]; (c) Gregoire F, Wei SH, Streed RW, Brameld KA, Fort D, Hanely LJ, Walls JD, Goddard WA, Roberts JD. J. Am. Chem. Soc. 1998;120:7537–7543. [Google Scholar]
- 32.Cabani S, Gianni P, Mollica V, Lepori L. J. Solution Chem. 1981;10:563–595. [Google Scholar]
- 33.Gallichio E, Kubo MM, Levy RM. J. Phys. Chem. B. 2000;104:6271–6285. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.