Abstract
Objective:
To assess long-term survival and prognostic factors in a large series of patients with bile duct cancer.
Summary Background Data:
The incidence of bile duct cancer is low but increasing. Determinants of survival vary in the literature, due to a lack of sufficient numbers of patients in most series.
Methods:
We studied 564 consecutive patients with bile duct cancer operated upon between 1973 and 2004. Patients were divided into intrahepatic, perihilar, and distal groups. Principle outcome measures were complications, 30-day mortality, and survival.
Results:
Of the 564 patients, 44 (8%) had intrahepatic, 281 (50%) had perihilar, and 239 (42%) had distal tumors. Approximately half (294, 52%) were treated before 1995, while 270 (48%) were treated thereafter. The perioperative mortality rate was 4%. In log-rank analyses, survival was higher in the later time period (P = 0.002), in patients with intrahepatic disease (P = 0.001), with negative resection margins (P < 0.001), with well/moderately differentiated tumors (P < 0.001), and those with negative lymph nodal status (P < 0.001). In multivariate analysis, negative margins (P < 0.001), tumor differentiation (P < 0.001), and negative nodal status (P < 0.001), but not tumor diameter, were significant independent prognostic factors. In R0-resected patients, lymph node status (P < 0.001), but not tumor diameter, histology, or differentiation, further predicted survival. The median survivals for R0-resected intrahepatic, perihilar, and distal tumors were 80, 30, and 25 months, respectively, and the 5-year survivals were 63%, 30%, and 27%, respectively.
Conclusion:
R0 resection remains the best chance for long-term survival, and lymph node status is the most important prognostic factor following R0 resection.
Review of 564 consecutive patients with bile duct cancer operated upon between 1973 and 2004 revealed that R0 surgical resection remains the best chance for long-term survival, and lymph node status is the most important prognostic factor following R0 resection.
The incidence of bile duct cancer is increasing worldwide, currently accounting for 3% of all gastrointestinal cancers.1,2 In the United States, there are approximately 5000 new cases each year.3 These tumors can occur anywhere along the biliary tree from the most proximal peripheral intrahepatic ducts to the distal intraduodenal bile duct. We have previously reported a classification system dividing tumors into 3 groups: intrahepatic, perihilar, and distal tumors.4 Using this three-tiered classification system, we present a detailed analysis of our recent cholangiocarcinoma experience at our institution, together with that of the previous 2 decades, for a total of 546 patients operated upon since 1973.
PATIENTS AND METHODS
With approval by the Johns Hopkins Institutional Review Board, the records of all patients with histologically confirmed bile duct cancer undergoing surgical exploration at the Johns Hopkins Hospital from 1973 until 2004 were retrospectively reviewed. Recorded data included demographics, medical history, presenting symptoms, and radiographic and clinical tumor data. Patients with bile duct cancer treated nonoperatively and those who underwent liver transplantation as primary therapy were excluded.
Patients were classified into 3 groups according to the location of the primary lesion: 1) intrahepatic, 2) perihilar, and 3) distal. Intrahepatic tumors were defined as confined to the liver, and not involving the extrahepatic biliary tree. Perihilar tumors were defined as those involving or requiring resection of the hepatic duct bifurcation, and were typically located in the extrahepatic biliary tree proximal to the origin of the cystic duct. Distal tumors were extrahepatic lesions located in the peripancreatic region. R0 resections were defined as those leaving behind no gross or microscopic tumor, R1 resections as those with microscopically positive margins, and R2 resections as those where not all gross tumor was removed. A palliation was defined as an operation in which no major resection was attempted and interventions focused on biopsy and bypass. An early time period was defined as January 1973 to December 1995, and the later time period from January 1996 to March 2004.
χ2 tests were used to compare categorical variables, and one-way analysis of variance followed by Scheffé tests to compare continuous variables among the patients with the 3 tumor locations. Kaplan-Meier curves were plotted and log-rank tests used to compare time from operation to death. Cox proportional hazards models were used to calculate adjusted and unadjusted hazard ratios (HRs) for margin status, lymph node status, tumor diameter, differentiation, and time period. For intrahepatic tumors, there was only one small (<2 cm) tumor. Therefore, tumor diameter was not used as a predictor in Cox analysis of intrahepatic tumors. All P values were two-sided, and P < 0.05 was considered significant.
RESULTS
Patients and Tumors
During the 31-year period of this study, 564 patients underwent surgical exploration for bile duct cancer. Forty-four (7.8%) of the patients had intrahepatic tumors, 281 (50%) had perihilar tumors, and 239 (42%) had distal lesions. Patient and tumor characteristics are presented in Tables 1 and 2. Overall, the median age was 65 years and the patients were predominantly male whites. However, patients with intrahepatic lesions were significantly more likely to be younger (P < 0.001) and female (P = 0.006), compared with patients with perihilar and distal cancers. Biliary stones, inflammatory bowel disease, and primary sclerosing cholangitis were concomitant diagnoses in 17%, 3.1%, and 2.2% of patients, respectively. The most common symptom attributable to bile duct cancer in the entire series was jaundice, but compared with the perihilar and distal groups, patients with intrahepatic cancers were more likely to present with abdominal pain (P < 0.001) and less likely to present with weight loss (P = 0.03) or jaundice (P < 0.001).
TABLE 1. Patient Characteristics, Operation Dates, Associated Diseases, Presenting Symptoms, and Preoperative Laboratory Data by Tumor Location
TABLE 2. Tumor Histology, Differentiation, Tumor Diameter, Microscopic Margins, and Lymph Node Involvement by Tumor Location
As shown in Table 2, histologic evaluation of the tumors revealed that nearly all (96.6%) were adenocarcinoma. The tumor diameter for all patients ranged from 0.1 cm to 19 cm. Median tumor diameter (25th–75th percentile [in cm]) for intrahepatic tumors was 5.5 (4.2–10.0), which was larger than the diameter of perihilar (2.5 [1.5–3.5]) or distal (2.0 [1.5–2.5]) tumors (P < 0.001). An R0 status was achieved in 46% of all resections but was less likely in perihilar cases (19%) than in intrahepatic (45%), or distal cases (78%) (P < 0.001). Resected lymph nodes were positive for bile duct cancer in 47% of patients overall. A larger proportion of distal cancers (60%) were associated with positive lymph nodes compared with perihilar (28%) and intrahepatic (29%) cancers (P < 0.001). However, harvesting of lymph nodes during resection of intrahepatic bile duct cancers was not routine.
Operative Procedures
Of the 564 patients, 430 (76%) underwent resection and 134 (24%) underwent palliation (Table 3). In general, resectable intrahepatic tumors were treated with hepatic resection, usually without lymph node dissection. Perihilar lesions were resected via excision of the extrahepatic biliary tree with lymph node dissection, with or without hepatic resection including the caudate. Distal cancers were resected with a pancreaticoduodenectomy or, for small tumors just distal to the cystic duct, with excision of the extrahepatic biliary tree with lymph node dissection.
TABLE 3. Operative Procedures, Postoperative Complications, and Median Survival by Tumor Location
Morbidity and Mortality
The overall complication rate for the entire population was 35%. As shown in Table 3, the most common complications were superficial wound infection (13.1%), abscess (7.5%), sepsis (6.3%), pancreatic leak (5.3%), delayed gastric emptying (5.3%), and biliary leak (4.0%). For most complications, the incidence did not depend on the location of the primary tumor, with 3 exceptions. First, delayed gastric emptying was markedly more common after resection of distal lesions (typically via a pancreaticoduodenectomy) (P < 0.001). Second, the incidence of sepsis was lowest in the distal group and highest in the perihilar group (P = 0.004). Third, the rate of biliary leak was highest in the perihilar group, while the rate of pancreatic leak was highest in the distal group (P = < 0.001).
The perioperative mortality rate for all groups was 4.0%, and for the intrahepatic, perihilar, and distal groups was 4.5%, 5.4%, and 3.0%, respectively (P = 0.41).
Survival
The mean follow-up for all patients and for survivors (censored patients) was 29 and 76 months, respectively. The 5-year overall and R0 survival for all 564 patients with bile duct cancer was 18% and 30%, and the median survival was 15 and 28 months, respectively. For patients in the intrahepatic, perihilar, and distal groups, the 5-year survival was 40%, 10%, and 23% and the median survival was 28, 13, and 18 months, respectively (P < 0.001 by log-rank test) (Fig. 1). Perihilar patients had worse survival: by comparison, the HRs (95% confidence intervals [CIs]) for intrahepatic and distal patients were 0.47 (0.31–0.72) and 0.66 (0.54–0.80), respectively. Figure 2 (top) shows that the 5-year survival after R0 resection of intrahepatic, perihilar, and distal tumors was 63%, 30%, and 27%, and the median survival was 80, 30, and 25 months, respectively.
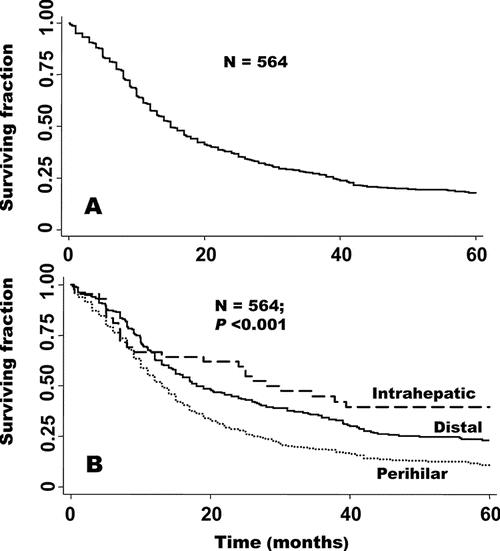
FIGURE 1. Overall survival for the entire group (A) and by tumor location (B).
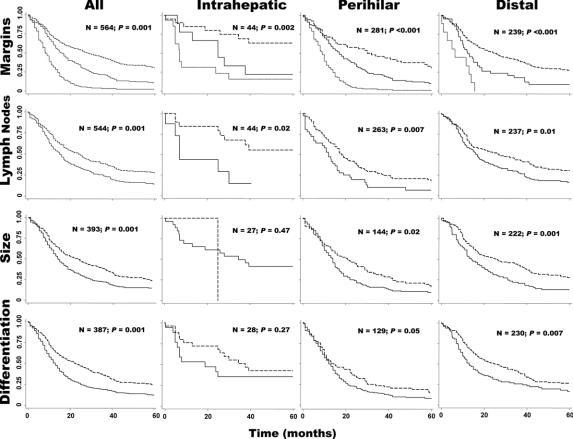
FIGURE 2. Overview of survival analyses by tumor location and pathology. All graphs depict surviving fraction on the y-axis. For analysis of margins, dashed line represents R0 resections, solid line R1/R2 resections, and dotted line palliations. For analysis of lymph nodes, dashed lines represent negative status and solid lines positive status. For analysis of tumor diameter, dashed lines represent tumor diameter ≤2 cm, and solid lines >2 cm. For analysis of differentiation, dashed lines represent well/moderately differentiated tumors and solid lines poor/unknown lesions.
Figure 2 and Table 4 provide global views of the survival of all patients and of patients in the intrahepatic, perihilar, and distal groups, separated by the status of the resection margins (R0, R1/2, or palliated), lymph node status (positive or negative for cancer), tumor diameter (≤ or >2 cm), and degree of differentiation (well/moderately or poorly/unknown). For all patients combined, log-rank analyses showed that negative margin status (P < 0.001), negative lymph node status (P < 0.001), small tumor diameter (P < 0.001), and higher degree of differentiation (P < 0.001) predicted higher survival. When these 4 prognostic variables were examined by location, Kaplan-Meier analysis revealed that negative margin status and negative lymph node status were associated with increased survival for each location. For perihilar and distal tumors, but not for intrahepatic tumors, tumor diameter <2 cm and higher degree of differentiation significantly predicted improved survival (Fig. 2). The null results for intrahepatic cancers, however, may reflect the small size of this group. According to multivariate analyses (adjusted results in Table 4), negative margins of resection (P < 0.001), negative lymph node status (P < 0.001), and differentiation (P < 0.001) predicated improved survival among all patients (all 3 tumor locations). When the patients were separated by group, however, only margin status predicted improved survival for all 3 tumor locations, while negative lymph node status predicted improved survival for perihilar and distal tumors, and tumor diameter and degree of differentiation were significantly associated only with distal tumors (Table 4).
TABLE 4. Hazard Ratios and 95% Confidence Intervals for Factors Predicting Survival in Cholangiocarcinoma Patients, Stratified by Tumor Location
Because the status of resection margins was one of the most robust predictors of survival in the above analyses and the only parameter that the surgeon has some control over, all patients with negative margins of resection were studied to learn which factors further influenced survival. In this cohort of resection margin-negative patients, only lymph node status was predictive of survival (HR = 1.73; 95% CI, 1.26–2.39; P < 0.001). By contrast, tumor diameter (P = 0.39), degree of differentiation (P = 0.08), and administration of adjuvant therapy (chemotherapy versus radiation or both; P = 0.84) were not significant predictors of survival in log-rank or Cox regression analyses.
Of the 173 patients who underwent resection of a perihilar tumor, 36 underwent concomitant partial hepatectomy and had longer median survival (26 months) than those perihilar patients who did not undergo partial hepatic resection (19 months), but this difference was not significant (P = 0.17). Similarly, the margin-negative rate was higher in patients undergoing hepatic resection (36%) compared with those not undergoing hepatic resection (28%), but this difference was not statistically significant (P = 0.37).
The analysis of the effect of adjuvant therapy on survival was problematic because of the wide variance of adjuvant regimens over the 31 years spanned by this series. Of 514 informative patients, 161 (29%) received chemotherapy. Of those patients who received chemotherapy, fewer were in the perihilar group (17%) compared with those in either the distal (40%) or intrahepatic (39%) group (P < 0.001). Nearly half of all patients (47%) received radiation therapy, with no difference among the 3 groups. There was no statistically significant difference in survival between those who received and those who did not receive chemotherapy and/or radiotherapy, either in the entire series or in any of the 3 anatomic groups analyzed. Furthermore, chemotherapy and/or radiotherapy did not significantly change survival in subgroups of patients who had R0 or R1/R2 surgical resections.
Trends in Management and Outcomes
To study the relationship between time period of treatment and survival, we chose a cutoff of December 31,1995 (the last accrual date of a 1996-reported series of the first 294 patients treated at our hospital4). Patients explored before this date were grouped into the earlier time period, and patients explored after this date were grouped into the later time period. Approximately half (294, 52%) were treated in the earlier period and 270 (48%) were treated in the later period.
The proportion of patients with perihilar bile duct cancers undergoing a partial hepatectomy for resection of their disease was higher during the later time period compared with the early time period (35% vs. 13%, respectively; P < 0.001). Similarly, although caudate resection did not typically accompany resection of a perihilar cancer at our institution during the early period, within the later period significantly more perihilar resections were performed with concomitant caudate resections during the years 2000 to 2004 compared with the years 1996 to 1999 (P < 0.05).
During the later period, the negative margin rate of resected perihilar tumors increased from 25% to 39%. Consistent with this change in management, the survival of all patients operated on in the more recent period was higher compared with the earlier period: 5-year survival and median survival were 22% and 18 months for the later period, and 14% and 14 months, respectively, for the earlier period (Fig. 3; HR = 0.75; 95% CI, 0.62–0.90; P = 0.002). In addition, the R0 rate during the later time period (58%) was nearly double that of the early period (34%) (P < 0.001). However, when the patients were subdivided by location of tumor, this difference was significant only for intrahepatic cases (P = 0.02) but not for perihilar (P = 0.22) or distal (P = 0.91) cases. Further analysis revealed that the proportion of tumor locations changed through time. Intrahepatic, perihilar, and distal tumors constituted 7%, 66%, and 27% of the tumors in the earlier time period, whereas the corresponding numbers in the later period were 9%, 32%, and 59%, respectively. Therefore, improved prognosis in the later time period could in part be attributed to a lower percentage of perihilar tumors, which had the worst prognosis. After adjustment for location, survival was still improved in the later period, but this difference was not significant (HR = 0.86; 95% CI, 0.70–1.05; P = 0.13).
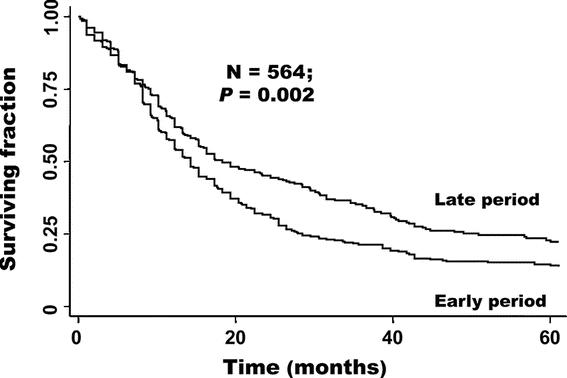
FIGURE 3. Survival according to time period. Early period (1973–1995) versus late period (1996–2004).
DISCUSSION
We have reported our 31-year experience with 564 patients operated upon for bile duct cancer at a single institution. This report updates the report of our experience through 1995,4 doubling the number of cases and demonstrating a prolonged survival in the later period compared with the early period. Using the previously reported three-tiered classification system, we identified that the perihilar group had a significantly shorter survival than either the intrahepatic group or the distal group. We have confirmed the importance of resection-margin status for survival, and further identified that in those patients who do have negative margins, the next most important prognosticator is lymph node status.
The likely reason the perihilar group had a shorter survival than the intrahepatic and distal groups is that the perihilar group had a higher proportion of positive margins. This reflected an earlier philosophy at our institution and elsewhere1,5,6 that increased morbidity and mortality associated with aggressive liver resections for hilar cancers potentially outweighed benefits derived from obtaining negative margins. However, it is now widely recognized and demonstrated by this study and others that margin status is one of the most robust predictors of long-term survival. This paradigm shift, the recognition that more aggressive attempts to achieve negative margins, including hepatic resection as needed, has recently yielded considerably improved 5-year survival rates for perihilar cancer.7 At our institution, for example, the rate of negative margins had doubled in this series compared with our earlier experience.4 As a result, the trend in our series has been toward longer survival in those patients undergoing concomitant hepatic resection. However, those patients undergoing hepatic resection are also more likely to have larger tumors. A comparison of recent series, including 5-year survival and perioperative mortally rates, is shown in Table 5. 5,11–29
TABLE 5. Comparison of Recent Series
It is widely recognized that intrahepatic bile duct cancers have a different epidemiology than extrahepatic tumors.8 Patients with intrahepatic tumors in our series had the best survival of the 3 groups, consistent with the findings of some series,5 but not others, including one series of cholangiocarcinomas of all locations that used the same Hopkins classification system.9 Despite having a longer survival time, intrahepatic patients had a lower rate of negative margins compared with distal lesion. Possible explanations for this apparent discrepancy include different tumor biology and the fact that the age of patients with intrahepatic tumors was significantly younger compared with patients with extrahepatic tumors (P < 0.001, Table 1). In addition, regarding the intrahepatic group in general, it should also be noted that, because the this group was by far the smallest of the 3 groups evaluated, relatively wider margins of error for any given parameter may be expected. Based on our experience and review of the literature, there are insufficient data to clearly support lymphadenectomy during resection of intrahepatic tumors. Therefore, as mentioned in Results, the harvesting of lymph nodes was not routinely performed during resection of intrahepatic tumors in our series.
The evaluation of patients with bile duct cancer changed dramatically during the 31-year period of this study. For example, visceral angiography, common in the first 2 decades of the study, was in the third decade largely replaced by computerized tomography (CT) angiography or magnetic resonance angiography. Nearly all patients after the 1970s underwent CT, and in the last decade many of these CT scans were performed with 0.5-mm intervals and three-dimensional reconstructions, reducing reliance on percutaneous transhepatic cholangiography or endoscopic retrograde cholangiopancreatography. Magnetic resonance cholangiopancreatography studies have furthered this shift over the last decade. The use of preoperative stenting also evolved over the time period of study. The use of preoperative stenting, although no longer done routinely, was used throughout the 3 study decades, in decompressing obstructed biliary trees and in patients with significant malnutrition, biliary sepsis, or other medical problems requiring recovery before an elective resection.10
Similarly, proportion of patients in each of the 3 anatomic groups changed significantly in the recent period compared with the early period. Although the proportion of patients with intrahepatic tumors was relatively constant, a smaller proportion of patients had perihilar tumors and a larger proportion had distal tumors (Table 1). This change was likely a result of changes in referral patterns over time.
CONCLUSION
At our institution, the 5-year survival after resection of bile duct cancer has significantly improved. We conclude from the results of this experience and from the current literature that achieving R0 status, with concomitant liver resection as necessary, is the most important variable associated with outcome and long-term survival. Among patients who have had an R0 resection, lymph node status is likely the next most important prognostic factor.
Footnotes
Reprints: Richard D. Schulick, MD, FACS, Department of Surgery, Rm. 442, Cancer Research Building, 1650 Orleans Street, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore, MD, 21231-1000. E-mail: rschulick@jhmi.edu.
REFERENCES
- 1.Vauthey JN, Blumgart LH. Recent advances in the management of cholangiocarcinomas. Semin Liver Dis. 1994;14:109–114. [DOI] [PubMed] [Google Scholar]
- 2.Kuwayti K, Baggenstoss AH, Stauffer MH, et al. Carcinoma of the major intrahepatic and the extrahepatic bile ducts exclusive of the papilla of Vater. Surg Gynecol Obstet. 1957;104:357–366. [PubMed] [Google Scholar]
- 3.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115–125. [DOI] [PubMed] [Google Scholar]
- 4.Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma: a spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463–473 ; discussion 473–475. [DOI] [PMC free article] [PubMed]
- 5.Madariaga JR, Iwatsuki S, Todo S, et al. Liver resection for hilar and peripheral cholangiocarcinomas: a study of 62 cases. Ann Surg. 1998;227:70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boerma EJ. Research into the results of resection of hilar bile duct cancer. Surgery. 1990;108:572–580. [PubMed] [Google Scholar]
- 7.Kosuge T, Yamamoto J, Shimada K, et al. Improved surgical results for hilar cholangiocarcinoma with procedures including major hepatic resection. Ann Surg. 1999;230:663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazaridis KN, Gores GJ. Cholangiocarcinoma. Gastroenterology. 2005;128:1655–1667. [DOI] [PubMed] [Google Scholar]
- 9.Kelley ST, Bloomston M, Serafini F, et al. Cholangiocarcinoma: advocate an aggressive operative approach with adjuvant chemotherapy. Am Surg. 2004;70:743–748 ; discussion 748–749. [PubMed]
- 10.Lillemoe KD, Cameron JL. Surgery for hilar cholangiocarcinoma: the Johns Hopkins approach. J Hepatobiliary Pancreat Surg. 2000;7:115–121. [DOI] [PubMed] [Google Scholar]
- 11.Pichlmayr R, Lamesch P, Weimann A, et al. Surgical treatment of cholangiocellular carcinoma. World J Surg. 1995;19:83–88. [DOI] [PubMed] [Google Scholar]
- 12.Jan YY, Jeng LB, Hwang TL, et al. Factors influencing survival after hepatectomy for peripheral cholangiocarcinoma. Hepatogastroenterology. 1996;43:614–619. [PubMed] [Google Scholar]
- 13.Casavilla FA, Marsh JW, Iwatsuki S, et al. Hepatic resection and transplantation for peripheral cholangiocarcinoma. J Am Coll Surg. 1997;185:429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valverde A, Bonhomme N, Farges O, et al. Resection of intrahepatic cholangiocarcinoma: a Western experience. J Hepatobiliary Pancreat Surg. 1999;6:122–127. [DOI] [PubMed] [Google Scholar]
- 15.Inoue K, Makuuchi M, Takayama T, et al. Long-term survival and prognostic factors in the surgical treatment of mass-forming type cholangiocarcinoma. Surgery. 2000;127:498–505. [DOI] [PubMed] [Google Scholar]
- 16.Weber SM, Jarnagin WR, Klimstra D, et al. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg. 2001;193:384–391. [DOI] [PubMed] [Google Scholar]
- 17.Sugiura Y, Nakamura S, Iida S, et al. Extensive resection of the bile ducts combined with liver resection for cancer of the main hepatic duct junction: a cooperative study of the Keio Bile Duct Cancer Study Group. Surgery. 1994;115:445–451. [PubMed] [Google Scholar]
- 18.Su CH, Tsay SH, Wu CC, et al. Factors influencing postoperative morbidity, mortality, and survival after resection for hilar cholangiocarcinoma. Ann Surg. 1996;223:384–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagino M, Nimura Y, Kamiya J, et al. Segmental liver resections for hilar cholangiocarcinoma. Hepatogastroenterology. 1998;45:7–13. [PubMed] [Google Scholar]
- 20.Miyazaki M, Ito H, Nakagawa K, et al. Aggressive surgical approaches to hilar cholangiocarcinoma: hepatic or local resection? Surgery. 1998;123:131–136. [PubMed] [Google Scholar]
- 21.Neuhaus P, Jonas S, Bechstein WO, et al. Extended resections for hilar cholangiocarcinoma. Ann Surg. 1999;230:808–818 ; discussion 819. [DOI] [PMC free article] [PubMed]
- 22.Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–517 ; discussion 517–519. [DOI] [PMC free article] [PubMed]
- 23.Kondo S, Hirano S, Ambo Y, et al. Forty consecutive resections of hilar cholangiocarcinoma with no postoperative mortality and no positive ductal margins: results of a prospective study. Ann Surg. 2004;240:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rea DJ, Munoz-Juarez M, Farnell MB, et al. Major hepatic resection for hilar cholangiocarcinoma: analysis of 46 patients. Arch Surg. 2004;139:514–523 ; discussion 523–525. [DOI] [PubMed]
- 25.Nishio H, Nagino M, Nimura Y. Surgical management of hilar cholangiocarcinoma: the Nagoya experience. Hepatobiliary Pancreat Surg. 2006;7:259–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dinant S, Gerhards MF, Rauws EA, et al. Improved outcome of resection of hilar cholangiocarcinoma (Klatskin tumor). Ann Surg Oncol. 2006;13:872–880. [DOI] [PubMed] [Google Scholar]
- 27.Abdel Wahab M, Fathy O, Elghwalby N, et al. Resectability and prognostic factors after resection of hilar cholangiocarcinoma. Hepatogastroenterology. 2006;53:5–10. [PubMed] [Google Scholar]
- 28.Bortolasi L, Burgart LJ, Tsiotos GG, et al. Adenocarcinoma of the distal bile duct: a clinicopathologic outcome analysis after curative resection. Dig Surg. 2000;17:36–41. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida T, Matsumoto T, Sasaki A, et al. Prognostic factors after pancreatoduodenectomy with extended lymphadenectomy for distal bile duct cancer. Arch Surg. 2002;137:69–73. [DOI] [PubMed] [Google Scholar]
- 30.Lieser MJ, Barry MK, Rowland C, et al. Surgical management of intrahepatic cholangiocarcinoma: a 31-year experience. J Hepatobiliary Pancreat Surg. 1998;5:41–47. [DOI] [PubMed] [Google Scholar]