Abstract
Interleukin 2 (IL-2) was one of the first cytokines to be discovered. However, the complex role of IL-2 and its receptor in the regulation of immune responses is only now emerging. This review explores the various signals triggered by IL-2 and discusses their translation into biological function. A model is outlined that accommodates the seemingly contradictory functions of IL-2, and explains how one cytokine can be an essential T-cell growth and differentiation factor and yet also be indispensable to maintain peripheral tolerance.
Keywords: adaptive immunity, immune regulation, immune stimulation, interleukin 2, T lymphocyte
Introduction
Interleukin 2 (IL-2) and its receptor (IL-2R) were the first cytokine and cytokine receptor to be cloned, more than 20 years ago (Leonard et al, 1984; Nikaido et al, 1984; Taniguchi et al, 1983). The first function attributed to IL-2 was a potent capacity to enhance in vitro T-cell proliferation and differentiation (Gillis et al, 1978; Gillis & Smith, 1977; Morgan et al, 1976; Smith, 1988), and it was therefore originally named T-cell growth factor (TCGF). In line with its in vitro function, IL-2 was also assumed to have a crucial role in vivo during antigen-driven clonal expansion of T cells. As IL-2 is mainly produced by activated T cells and, in particular, by activated CD4+ T-helper cells, at least part of their ‘helper' function for CD8+ T cells was attributed to IL-2 (Keene & Forman, 1982). Subsequent to these initial descriptions of the function of IL-2, numerous studies have highlighted many more seemingly contradictory functions of this cytokine (Fig 1). With respect to immune-enhancing functions, IL-2 has a role in supporting proliferation (Bamford et al, 1994; Gillis et al, 1978; Gillis & Smith, 1977; Morgan et al, 1976; Smith, 1988; Smith et al, 1980) and survival (Blattman et al, 2003) of T cells, and differentiation of naive T cells into effector and memory cells (Cho et al, 2007; Kamimura & Bevan, 2007; Ke et al, 1998). Recent evidence indicates that IL-2 is also an important factor that allows the generation of memory T cells, which are able to undergo secondary expansion when they re-encounter an antigen (Bachmann et al, 2007; Williams et al, 2006). Furthermore, IL-2 has the ability to overcome the proliferation block of anergic cells generated in vitro (Powell et al, 1998; Schwartz, 1996) and, in certain situations, also in vivo (Kündig et al, 1996).
Figure 1.
In vivo and in vitro immunoregulatory and immunostimulatory functions of interleukin 2 (IL-2). AICD, activation-induced cell death; TREG, regulatory T cell.
In opposition to these immune-enhancing functions, IL-2 can promote activation-induced cell death (AICD) of T cells (Dai et al, 1999; Lenardo, 1991; Refaeli et al, 1998; Zheng et al, 1998) and was therefore implicated in downregulating antigen-specific T-cell numbers after the clonal expansion phase of an immune response (Khoruts et al, 1998; Kneitz et al, 1995; Ku et al, 2000; Refaeli et al, 1998; Van Parijs et al, 1997).
IL-2 also has anti-inflammatory properties, as do other pro-inflammatory cytokines, such as interferon-γ (IFNγ; Bachmann & Kopf, 2002). In a similar process to IFNγ—which exerts anti-inflammatory properties by suppressing T-helper 17 cells—IL-2 can constrain IL-17 production (Laurence et al, 2007), and exert its immunosuppressive function by stimulating the generation and homeostasis of regulatory T cells (TREG). Indeed, IL-2 is a non-redundant factor for the in vivo homeostasis of TREG, which constitute a fundamental part of immunological self-tolerance and immune regulation (D'Cruz & Klein, 2005; Fontenot et al, 2005; Klebb et al, 1996; Papiernik et al, 1998; Suzuki et al, 1999; Wolf et al, 2001).
The aim of this review is to summarize and integrate the diverse functions of IL-2 with a particular emphasis on its in vivo roles as assessed in experimental murine models.
IL-2 and IL-15: similar receptors, different function
The high-affinity receptor for IL-2 is a heterotrimeric membrane protein complex consisting of an IL-2-specific α-subunit (IL-2Rα, CD25), a β-subunit (IL-2Rβ, CD122) and the common cytokine receptor γ-chain (γc, CD132). The IL-2Rα chain alone binds to IL-2 with low affinity (dissociation constant (Kd) = ∼10−8M) and has a short cytoplasmic tail that is not involved in recruitment of cytoplasmic signal transduction molecules. In combination with the IL-2Rβ and γc chains, the affinity of the receptor for IL-2 is increased by three orders of magnitude (Kd = ∼10−11M). In addition, the cytoplasmic domains of the IL-2Rβ and γc chains are responsible for signal transduction through activation of the Janus kinase 3 (JAK3)/signal transducer and activator of transcription 5 (STAT5) and AKT-dependent signalling pathways, and the mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K) pathways, respectively (reviewed in Kovanen & Leonard, 2004; Ma et al, 2006; Waldmann, 2006). The receptor for IL-15 has a similar structure to that of IL-2R: they share the IL-2/15Rβ (CD122) and γc (CD132) chains. It is the receptor α-chains that are unique for the respective receptors and that confer specificity. However, unlike the IL-2Rα chain, which binds to IL-2 with low affinity (10−8M), the IL-15Rα chain alone binds to IL-15 with high affinity (10−11M), and is therefore well suited to capture IL-15 efficiently and to present it to other cells (see below). Because the signal transduction of both receptors is mediated through the cytoplasmic domains of the IL-2/15Rβ and γc chains, the receptors share JAK and STAT signalling pathways, and trigger phosphorylation of lymphocyte-specific protein tyrosine kinase (lck) and spleen tyrosine kinase (syk), which are members of the src family of protein tyrosine kinases. Both also induce expression of the anti-apoptotic B-cell lymphoma 2 (Bcl-2) protein, and stimulate the PI3K–AKT and ras/raf MAPK pathways. This eventually leads to activation of c-fos-containing and c-jun-containing transcription-factor complexes (Miyazaki et al, 1995).
The signal transduction pathways shared between IL-2R and IL-15R might imply that they have similar functions and responses. Indeed, several functions are elicited by both cytokines: induction of T-cell proliferation, involvement in the differentiation of cytotoxic T lymphocytes, and generation, activation and persistence of natural killer (NK) cells, as well as stimulation of B-cell proliferation and immunoglobulin synthesis (reviewed in Waldmann et al, 2001). However, in vivo studies with knockout mice that lack IL-2, IL-15 or components of their respective receptors have shown that IL-2 and IL-15 also mediate a series of specific and non-overlapping functions. Mice that are deficient in IL-2 or in components of IL-2R exhibit a phenotype of pronounced and uncontrolled lymphoproliferation (Sadlack et al, 1993; Schorle et al, 1991; Suzuki et al, 1995; Willerford et al, 1995), which is linked to autoimmune disease, indicating that IL-2/IL-2R signalling is a crucial component of self-tolerance. IL-2/IL-2R signalling is required for the homeostasis of Treg (D'Cruz & Klein, 2005; Fontenot et al, 2005; Klebb et al, 1996; Papiernik et al, 1998; Suzuki et al, 1999; Wolf et al, 2001), which in turn are crucial guards for maintaining self-tolerance and preventing immunopathology through the active modulation of adaptive immune responses—in particular, by inhibiting proliferation and differentiation of self-reactive T cells. Mice (and humans) that lack Treg due to genetic defects—such as mutations in, or deletions of, the forkhead box P3 transcription factor (Foxp3), which is instructive for the development of Treg—exhibit a phenotype similar to IL-2-deficient or IL-2R-deficient mice, which supports the view that IL-2 is crucial for the presence of Treg (Brunkow et al, 2001; Fontenot et al, 2003). An additional role of IL-2 in controlling self-reactive T cells—and perhaps in downregulating T-cell responses—is mediated by its ability to promote AICD in T cells. AICD is induced in lymphocytes after repeated stimulation, and results from co-expression of the death receptor Fas (CD95) and its ligand FasL (CD95L; Brunner et al, 1995; Dhein et al, 1995; Ju et al, 1995; Krammer, 2000), as well as from repression of the anti-apoptotic protein FLIP (Refaeli et al, 1998). AICD is promoted in the presence of, and is dependent on, IL-2, as IL-2-deficient (Kneitz et al, 1995) and IL-2Rα-deficient T cells are resistant to it (Van Parijs et al, 1998). However, an important role for AICD in the downregulation of immune responses in vivo has been difficult to demonstrate. In fact, during many immune responses, T-cell frequencies decline after the antigen has largely been eliminated (and the T cells are therefore no longer activated by antigen). Even during chronic viral infections, IL-2 has been shown to enhance T-cell responses rather than blunt them (Bachmann et al, 2007; Blattman et al, 2003). Therefore, IL-2/IL-2R signalling is crucially involved in peripheral tolerance and immune regulation, whereas no comparable role can be attributed to IL-15. Instead, IL-15 seems to have a dominant role in the homeostasis of memory CD8+ T cells, NK cells, NK T cells and subsets of intraepithelial lymphocytes (reviewed in Ma et al, 2006; Waldmann, 2006).
How do these two closely related cytokine receptors mediate specific and different signals in vivo? Several aspects contribute to the differences in the in vivo functions of IL-2 and IL-15: differential expression of the high-affinity cytokine receptors, distinct interactions with the respective receptors and some variability in signalling pathways. The α-chains of the IL-2 and IL-15 receptors are expressed in different cell types: IL-2Rα is expressed in activated T and B cells, whereas IL-15Rα is expressed in activated monocytes and dendritic cells (reviewed in Ma et al, 2006; Waldmann, 2006). IL-2 is secreted and upregulates expression of the IL-2Rα chain, binds to and stabilizes the trimeric complex IL-2Rα/IL-2/15Rβ/γc, and signals through JAK1/JAK3. By contrast, IL-15 is only secreted in small quantities and is mainly retained on the membrane bound to the IL-15Rα chain (Burkett et al, 2004). The membrane-bound IL-15/IL-15Rα complex can be recycled through endosomal vesicles and re-expressed on the cell surface, thereby allowing prolonged IL-15 presentation on activated dendritic cells and monocytes (Dubois et al, 2002). In contrast to IL-2—which binds in its soluble form to trimeric high-affinity receptors on the target cells—membrane-bound IL-15/IL-15Rα complexes bind to IL-2/15Rβ/γc receptors on the target cells even in the absence of IL-15Rα-chain expression. Therefore, IL-15 signalling requires coordinated expression of IL-15 and IL-15Rα on the signal-providing cell (dendritic cell or monocyte), and is dependent on cell–cell contact between the signal-providing cell and the signal-receiving cell (NK cell or memory CD8+ T cell; Burkett et al, 2004; Sandau et al, 2004). There are also indications that some distinct signalling pathways are involved in inducing proliferation through IL-2 or IL-15; while IL-15 induces proliferation by 12 kDa FK506-binding protein (FKBP12)-mediated activation of p70 S6 kinase, FKBP12 is not required for IL-2-induced proliferation (Dubois et al, 2003).
The temporal regulation of IL-2Rα-chain compared with IL-15Rα-chain expression might also be an important parameter that could directly translate into different signal-transcription kinetics. Indeed, IL-2 induces sustained PI3K/protein kinase B (PKB) signalling, whereas IL-15 induces only a transient signal, which correlates with sustained compared with transient expression of the respective receptors (Cornish et al, 2006). Furthermore, it is conceivable that many of the available IL-2/15Rβ/γc chains are pulled into a complex with the IL-2Rα chain, rendering these cells particularly sensitive to IL-2 but less so to IL-15. Therefore, activated T cells that transiently express IL-2Rα, or TREG that constitutively express IL-2Rα, might preferentially receive signals through IL-2R that trigger their proliferation, differentiation and maintenance. By contrast, in steady-state conditions or after resolution of an immune response, memory T cells no longer express the IL-2Rα chain but have elevated levels of IL-2/15Rβ/γc chains instead, making them responsive to IL-15/IL-15Rα presented by dendritic cells or monocytes. This leads to a proliferative signal that allows the homoeostatic division, and therefore maintenance, of memory T-cell populations.
In vivo role of IL-2 in T-cell responses
IL-2 was believed initially to be required for the in vivo proliferation of T cells owing to its ‘classic' function as a growth factor in vitro. Mice deficient in IL-2 or its receptor components were used to test this assumption, and, on many occasions, T-cell expansion after infection or immunization seemed to be reduced only marginally, if at all. Infection of IL-2-deficient mice with lymphocytic choriomeningitis virus (LCMV), vaccinia virus (Bachmann et al, 1995; Kündig et al, 1993) or vesicular stomatitis virus (VSV) (D'Souza & Lefrancois, 2003; D'Souza et al, 2002) showed normal expansion of CD8+ T cells, and even peptide immunization led to normal expansion of CD8+ T cells (Kramer et al, 1994), although some studies have shown reduced expansion and effector function of CD8+ T cells after LCMV infection (Cousens et al, 1995; Utermohlen et al, 1994). Therefore, in many situations, IL-2 is a redundant growth factor in vivo. Furthermore, IL-15 can partly compensate for a lack of IL-2 in mediating T-cell proliferation, at least in vitro (Van Parjis et al, 1997). However, when the functionality of CD8+ T cells derived from IL-2-deficient mice was assessed, varying degrees of impairment were observed. The effector function of virus-specific CD8+ T cells after infection with vaccinia virus or LCMV seemed to be impaired only marginally, if at all (Bachmann et al, 1995; Kündig et al, 1993); in marked contrast, CD8+ T cells that were primed by peptide immunization were dysfunctional (Kramer et al, 1994). These discrepancies could be the result of the differential induction of innate immune responses by infection as opposed to peptide immunization; while the former are potent inducers of innate responses, the latter show only limited activation of innate immunity, depending on the adjuvant used. Therefore, the plethora of cytokines induced upon infection might deliver differentiation factors, such as type 1 IFNs, IL-12 or IL-15, which could promote the development of effector functions in the absence of IL-2. As IL-2-deficient and IL-2Rα-deficient mice develop a lymphoproliferative disease and autoimmunity owing to the lack of TREG, one might question the suitability of these models to determine quantitatively the role of IL-2 in regulating T-cell responses. It is likely that the proliferation of non-specific T cells to high levels might create an environment that also affects antigen-specific T-cell responses. Moreover, the lack of TREG potentially favours the expansion of antigen-specific T cells. Because of this, it was important to re-address the role of IL-2 in the generation and maintenance of antigen-specific T-cell responses in healthy hosts with a normal TREG population, and in the absence of ongoing lymphoproliferative disease. Several experiments were therefore conducted in which IL-2-deficient or IL-2Rα-deficient T cells that express transgenic (tg) T-cell receptors (TCRs) were adoptively transferred into hosts, and their responses to antigen stimulation were assessed in vivo. TCR tg T cells were either activated in vitro and then transferred into a lymphopenic RAG1-deficient host, or T-cell activation was performed directly in vivo. In both systems, IL-2-deficient or IL-2Rα-deficient T cells behaved similarly to control T cells with respect to the maintenance of cell numbers and secondary expansion after in vivo antigen challenge (Dai et al, 2000). One potential caveat to studies in which T cells are transferred into severely lymphopenic hosts is the non-specific activation and proliferation of the transferred T cells, which might affect the generation and maintenance of memory T cells. In subsequent studies, TCR tg CD8+ T cells (wild type, IL-2-deficient or IL-2Rα-deficient) were transferred into normal hosts and their responses were compared after VSV infection. Initial cycling of the CD8+ T cells was IL-2-independent, and CD8+ T cells expanded normally in the absence of IL-2 or IL-2Rα expression in secondary lymphoid organs. However, the accumulation of CD8+ T cells and their effector functions in peripheral organs were reduced, implying a role for IL-2 in homing to peripheral organs and/or in the accumulation and differentiation of effector cells within these organs (D'Souza & Lefrancois, 2003; D'Souza et al, 2002).
Another model used to investigate the role of IL-2 (and IL-15) in the induction and maintenance of T-cell responses in a normal environment is an IL-2/15Rβ-deficient mouse that expresses a tg form of IL-2/15Rβ exclusively in the thymus (Malek et al, 2000, 2001). In this model, peripheral T cells are non-responsive to IL-2 or IL-15, but the mice develop normal TREG and do not show signs of abnormal lymphoproliferation or autoimmunity (Malek et al, 2002). T cells obtained from these tg mice showed a ∼50% reduction in proliferation and a defect in acquisition of effector functions after in vitro activation (Malek et al, 2001). However, primary and secondary in vivo T-cell responses after infection with vaccinia virus, allogeneic skin grafting or in vivo anti-CD3 stimulation were relatively normal, indicating that both IL-2 and IL-15 are essentially dispensable for the in vivo expansion and differentiation of effector cells (Yu et al, 2003).
A new mouse model has been recently generated that allows the comparative analysis of IL-2Rα-deficient and IL-2Rα-sufficient CD8+ T cells in a normal host environment without the use of adoptive transfer of TCR tg T cells. In this model, mixed bone-marrow chimeric mice are generated in which 50% of the haematopoietic system is derived from normal bone marrow and 50% is derived from the bone marrow of IL-2Rα-deficient mice. Therefore, 50% of the CD8+ T cells are IL-2Rα-deficient and cannot receive IL-2 signals, whereas the other 50% are normal and can receive them. These chimeric mice are healthy and do not develop a lymphoproliferative disorder or autoimmune disease. Two independent studies have analysed the CD8+ T-cell response in these chimeric mice after LCMV infection, and have reached similar conclusions (Bachmann et al, 2007; Williams et al, 2006): the expansion, contraction and memory maintenance of LCMV-specific CD8+ T cells was generally similar between IL-2Rα-deficient and IL-2Rα-sufficient CD8+ T cells, although a fivefold reduction in numbers—but not in frequencies—was observed in one study (Bachmann et al, 2007). IL-2Rα-deficient and IL-2Rα-sufficient LCMV-specific CD8+ T cells from secondary lymphoid organs, as well as from peripheral tissue, were also similar with respect to their effector functions, such as IFNγ and tumour necrosis factor-α (TNFα) secretion (Bachmann et al, 2007). Importantly, although the frequencies of IL-2Rα-deficient and IL-2Rα-sufficient LCMV-specific memory CD8+ T cells were comparable, the IL-2Rα-deficient LCMV-specific CD8+ T cells showed a marked impairment in their ability to undergo secondary expansion. This defect could be rescued if IL-2R signals were provided during the priming phase by administration of IL-2/anti-IL-2 monoclonal antibody complexes, which can signal through low-affinity IL-2R in the absence of IL-2Rα (Boyman et al, 2006; Williams et al, 2006). It therefore seems that IL-2 signals perceived during the priming period of CD8+ T cells are essential for the programming of proliferation-competent memory CD8+ T cells. However, impaired secondary proliferation could also be partly rescued with the same approach if the IL-2R signal was provided during the challenge phase.
These findings in CD8+ T cells are in contrast to a recent report on the role of IL-2 signalling in CD4+ T cells. IL-2R signalling seems to be a prerequisite for effective generation and maintenance of memory CD4+ T cells (Dooms et al, 2007). However, autocrine IL-2 production by CD4+ T cells does not seem to influence the extent of primary expansion, as adoptively transferred IL-2-deficient TCR tg CD4+ T cells expanded to levels similar to those of their wild-type counterparts (Khoruts et al, 1998).
Additional studies in the mixed bone-marrow chimaeras showed that, although CD8+ T-cell responses were efficiently induced after acute infection with live LCMV, immunization with non-replicating virus-like particles induced poor expansion of IL-2Rα-deficient CD8+ T cells. This implies that, depending on the nature of the antigen and, perhaps, the level of innate immune activation or the duration of antigen availability, primary expansion or survival of activated cells can be largely IL-2-dependent or IL-2-independent (Bachmann et al, 2007).
As IL-2 is implicated in promoting AICD of T cells that are specific for self-antigens (reviewed in Abbas, 2003; Schimpl et al, 2002), it is conceivable that it might also have a role in downregulating T-cell responses in the setting of chronic infections where antigens persist for prolonged periods of time. This possibility was also addressed in the mixed bone-marrow chimaeras. If these mice were infected with an LCMV strain that induces chronic infection, the IL-2Rα-deficient LCMV-specific CD8+ T cells were rapidly deleted after an initial expansion, whereas IL-2Rα-sufficient LCMV-specific CD8+ T cells were physically maintained (Bachmann et al, 2007). These results are supported by the previous finding that the injection of exogenous IL-2 during chronic infection sustained, rather than abrogated, T-cell responses (Blattman et al, 2003). Both results imply that IL-2 signals sustain CD8+ T cells during chronic infection, rather than promote AICD as might have been predicted. In fact, during chronic infection, LCMV-specific CD8+ T-cell responses rapidly cycle (Agnellini et al, 2007), and IL-2 might be required for the survival of at least some of these rapidly proliferating cells. Furthermore, in chronic viral infections, the virus-specific T-cell population is constantly replenished by the recruitment of new naive T cells (Vezys et al, 2006), and it is possible that IL-2 might have an important role in this process.
Recent data obtained after administration of antibody-potentiated IL-2 to naive mice indicate that strong IL-2 signals without deliberate immunization—and, hence, without specific TCR-triggered T-cell activation—induce the activation of T cells and their differentiation into functional central memory T cells (Cho et al, 2007; Kamimura & Bevan, 2007).
From these results, a picture emerges in which the diverse functions of IL-2 in the regulation of immune responses are dictated by the antigen kinetics of immunization or infection in relation to the dynamics of the T-cell response (Fig 2), the timing of IL-2 signals in relation to the immune response (Fig 3) and the extent of the activation of the innate immune system.
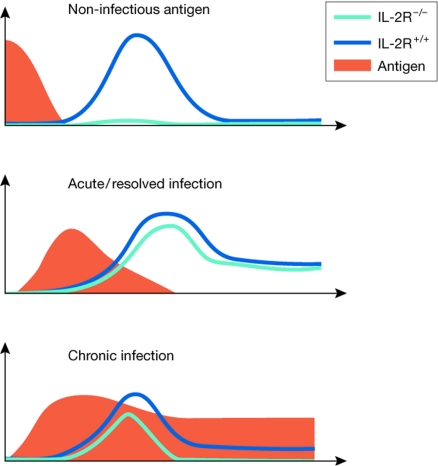
Figure 2.
Antigen kinetics and role of interleukin 2 in CD8+ T-cell dynamics. Non-infectious antigens (such as non-replicating virus-like particles) require IL-2 signals for optimal expansion of CD8+ T cells (Bachmann et al, 2007). Acute/resolved viral infections, such as lymphocytic choriomeningitis virus (LCMV) or vesicular stomatitis virus induce IL-2-independent primary CD8+ T-cell responses (Bachmann et al, 2007; D'Souza & Lefrancois, 2003; D'Souza et al, 2002; Williams et al, 2006). During chronic viral infections (such as high doses of LCMV or murine cytomegalovirus) virus-specific CD8+ T cells require IL-2 signals for their maintenance (Bachmann et al, 2007). The antigen load and availability are shown in red, whereas the antigen-specific CD8+ T-cell responses are shown in green (IL-2Rα−/−) or blue (wild type). IL-2, interleukin 2.
Figure 3.
The timing of interleukin 2 signals determines their impact on CD8+ T-cell dynamics. Although IL-2 is dispensable for initiation of early cell cycling after T-cell receptor triggering, if it is provided to T cells during the early priming phase (phase 1), the differentiation, accumulation and memory formation of the T cells is improved. Also, when IL-2 is provided just after the peak expansion of T cells—when antigen is cleared and the T-cell frequencies decline (phase 3)—it can reduce the extent of the T-cell contraction (Blattman et al, 2003) and IL-2 promotes the survival of memory cells. By contrast, if IL-2 is provided during the expansion phase of a T-cell response (phase 2)—when T cells are activated and antigen is present—it is detrimental for the T cells as they will die by activation-induced cell death (Blattman et al, 2003). IL-2, interleukin 2.
IL-2, T-cell help, co-stimulation and anergic T cells
Multiple parameters affect the strength and duration of T-cell responses. Two factors, however, are particularly important: the presence of specific antigen (signal 1) and co-stimulation (signal 2). T cells that are primed in the absence of co-stimulation—that is, those that receive signal 1 in the absence of signal 2—are rendered anergic (Kündig et al, 1996; Schwartz, 2003). Such anergic T cells are characterized by an inability to produce IL-2 or to proliferate on antigenic stimulation, which is a phenotype that can be rescued by the addition of IL-2 (Powell et al, 1998; Schwartz, 1996). These characteristics are similar to those of T cells that have been primed in the absence of IL-2, which also show a profound impairment in their ability to undergo secondary expansion after re-encounter with antigen (Bachmann et al, 2007; Williams et al, 2006) that can be at least partly rescued by the addition of IL-2. Epigenetic modification of the IL-2 promoter on CD28 signalling might be the basis of the similarity of the two phenotypes. CD28 co-stimulation induces stable histone acetylation and loss of cytosine methylation of the IL-2 promoter, rendering it accessible to DNA-binding proteins (Thomas et al, 2005). Therefore, co-stimulation imprints the ability to produce IL-2 into the genome of T cells. The presence of IL-2 during priming might sustain this promoter modification, rendering the primed T cells fully proliferation competent.
It is interesting to note that cytotoxic T cells induced in the absence of helper T cells can exhibit a similar phenotype to T cells primed in the absence of co-stimulation or IL-2 signalling, as they are also proliferation-incompetent in several experimental systems (Bachmann et al, 2004, 2005; Janssen et al, 2003, 2005; Shedlock & Shen, 2003; Sun & Bevan, 2003) and show increased DNA methylation at the IL-2 promoter (Northrop et al, 2006). Because the functional deficits of memory CD8+ T cells primed in the absence of CD28, IL-2 signalling or T-cell help are remarkably similar, it would be straightforward to propose that a lack of sufficient IL-2 is the underlying cause for all three phenotypes.
Concluding remarks
IL-2 is a cytokine that exhibits an impressive number of different functions largely dictated by the biological context in which it operates. It is pivotal for cellular activation, important for primary T-cell responses and essential for secondary T-cell responses. In addition, IL-2 has the key function of downregulating immune responses. Although IL-2 specifically promotes T-cell activation and proliferation of only those cells that have been stimulated by cognate antigenic interaction, downregulation of T-cell responses occurs non-specifically by facilitating a separate population of TREG. As a consequence, a lack of IL-2 results in reduced antigen-specific T-cell responses as well as generalized non-specific T-cell activation. Harnessing our increased understanding of the biology of IL-2 might allow more selective targeting of specific IL-2 functions in the future.

Martin F. Bachmann

Annette Oxenius
Acknowledgments
We apologize to all those authors whose work could not be cited owing to space constraints and the large number of studies performed in this field. A.O. is a European Molecular Biology Organization (EMBO) Young Investigator and is supported by the Swiss National Science Foundation, the Vontobel Foundation, the Gebert-Ruef-Foundation, the Horten Foundation and United Bank of Switzerland AG on behalf of a client.
References
- Abbas AK (2003) The control of T cell activation vs. tolerance. Autoimmun Rev 2: 115–118 [DOI] [PubMed] [Google Scholar]
- Agnellini P, Wolint P, Rehr M, Cahenzli J, Karrer U, Oxenius A (2007) Impaired NFAT nuclear translocation results in split exhaustion of virus-specific CD8+ T cell functions during chronic viral infection. Proc Natl Acad Sci USA 104: 4565–4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann MF, Kopf M (2002) Balancing protective immunity and immunopathology. Curr Opin Immunol 14: 413–419 [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Schorle H, Kuhn R, Muller W, Hengartner H, Zinkernagel RM, Horak I (1995) Antiviral immune responses in mice deficient for both interleukin-2 and interleukin-4. J Virol 69: 4842–4846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann MF, Schwarz K, Wolint P, Meijerink E, Martin S, Manolova V, Oxenius A (2004) Cutting edge: distinct roles for T help and CD40/CD40 ligand in regulating differentiation of proliferation-competent memory CD8+ T cells. J Immunol 173: 2217–2221 [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Wolint P, Schwarz K, Oxenius A (2005) Recall proliferation potential of memory CD8+ T cells and antiviral protection. J Immunol 175: 4677–4685 [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Wolint P, Walton S, Schwarz K, Oxenius A (2007) Differential role of IL-2R signaling for CD8(+) T cell responses in acute and chronic viral infections. Eur J Immunol 37: 1502–1512 [DOI] [PubMed] [Google Scholar]
- Bamford RN, Grant AJ, Burton JD, Peters C, Kurys G, Goldman CK, Brennan J, Roessler E, Waldmann TA (1994) The interleukin (IL) 2 receptor beta chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci USA 91: 4940–4944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R (2003) Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med 9: 540–547 [DOI] [PubMed] [Google Scholar]
- Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J (2006) Selective stimulation of T cell subsets with antibody–cytokine immune complexes. Science 311: 1924–1927 [DOI] [PubMed] [Google Scholar]
- Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F (2001) Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet 27: 68–73 [DOI] [PubMed] [Google Scholar]
- Brunner T et al. (1995) Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature 373: 441–444 [DOI] [PubMed] [Google Scholar]
- Burkett PR, Koka R, Chien M, Chai S, Boone DL, Ma A (2004) Coordinate expression and trans presentation of interleukin (IL)-15Rα and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J Exp Med 200: 825–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH, Boyman O, Kim HO, Hahm B, Rubinstein MP, Ramsey C, Kim DM, Surh CD, Sprent J (2007) An intense form of homeostatic proliferation of naive CD8+ cells driven by IL-2. J Exp Med 204: 1787–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish GH, Sinclair LV, Cantrell DA (2006) Differential regulation of T-cell growth by IL-2 and IL-15. Blood 108: 600–608 [DOI] [PubMed] [Google Scholar]
- Cousens LP, Orange JS, Biron CA (1995) Endogenous IL-2 contributes to T cell expansion and IFN-γ production during lymphocytic choriomeningitis virus infection. J Immunol 155: 5690–5699 [PubMed] [Google Scholar]
- D'Cruz LM, Klein L (2005) Development and function of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin 2 signaling. Nat Immunol 6: 1152–1159 [DOI] [PubMed] [Google Scholar]
- D'Souza WN, Lefrancois L (2003) IL-2 is not required for the initiation of CD8 T cell cycling but sustains expansion. J Immunol 171: 5727–5735 [DOI] [PubMed] [Google Scholar]
- D'Souza WN, Schluns KS, Masopust D, Lefrancois L (2002) Essential role for IL-2 in the regulation of antiviral extralymphoid CD8 T cell responses. J Immunol 168: 5566–5572 [DOI] [PubMed] [Google Scholar]
- Dai Z, Arakelov A, Wagener M, Konieczny BT, Lakkis FG (1999) The role of the common cytokine receptor gamma-chain in regulating IL-2-dependent, activation-induced CD8+ T cell death. J Immunol 163: 3131–3137 [PubMed] [Google Scholar]
- Dai Z, Konieczny BT, Lakkis FG (2000) The dual role of IL-2 in the generation and maintenance of CD8+ memory T cells. J Immunol 165: 3031–3036 [DOI] [PubMed] [Google Scholar]
- Dhein J, Walczak H, Baumler C, Debatin KM, Krammer PH (1995) Autocrine T-cell suicide mediated by APO-1/(Fas/CD95). Nature 373: 438–441 [DOI] [PubMed] [Google Scholar]
- Dooms H, Wolslegel K, Lin P, Abbas AK (2007) Interleukin-2 enhances CD4+ T cell memory by promoting the generation of IL-7R α-expressing cells. J Exp Med 204: 547–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois S, Mariner J, Waldmann TA, Tagaya Y (2002) IL-15Rα recycles and presents IL-15 in trans to neighboring cells. Immunity 17: 537–547 [DOI] [PubMed] [Google Scholar]
- Dubois S, Shou W, Haneline LS, Fleischer S, Waldmann TA, Muller JR (2003) Distinct pathways involving the FK506-binding proteins 12 and 12.6 underlie IL-2-versus IL-15-mediated proliferation of T cells. Proc Natl Acad Sci USA 100: 14169–14174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY (2003) Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 4: 330–336 [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY (2005) A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol 6: 1142–1151 [DOI] [PubMed] [Google Scholar]
- Gillis S, Smith KA (1977) Long term culture of tumour-specific cytotoxic T cells. Nature 268: 154–156 [DOI] [PubMed] [Google Scholar]
- Gillis S, Baker PE, Ruscetti FW, Smith KA (1978) Long-term culture of human antigen-specific cytotoxic T-cell lines. J Exp Med 148: 1093–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP (2003) CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421: 852–856 [DOI] [PubMed] [Google Scholar]
- Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP (2005) CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature 434: 88–93 [DOI] [PubMed] [Google Scholar]
- Ju ST, Panka DJ, Cui H, Ettinger R, el-Khatib M, Sherr DH, Stanger BZ, Marshak-Rothstein A (1995) Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature 373: 444–448 [DOI] [PubMed] [Google Scholar]
- Kamimura D, Bevan MJ (2007) Naive CD8+ T cells differentiate into protective memory-like cells after IL-2 anti IL-2 complex treatment in vivo. J Exp Med 204: 1803–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Y, Ma H, Kapp JA (1998) Antigen is required for the activation of effector activities, whereas interleukin 2 is required for the maintenance of memory in ovalbumin-specific, CD8+ cytotoxic T lymphocytes. J Exp Med 187: 49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene JA, Forman J (1982) Helper activity is required for the in vivo generation of cytotoxic T lymphocytes. J Exp Med 155: 768–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoruts A, Mondino A, Pape KA, Reiner SL, Jenkins MK (1998) A natural immunological adjuvant enhances T cell clonal expansion through a CD28-dependent, interleukin (IL)-2-independent mechanism. J Exp Med 187: 225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebb G, Autenrieth IB, Haber H, Gillert E, Sadlack B, Smith KA, Horak I (1996) Interleukin-2 is indispensable for development of immunological self-tolerance. Clin Immunol Immunopathol 81: 282–286 [DOI] [PubMed] [Google Scholar]
- Kneitz B, Herrmann T, Yonehara S, Schimpl A (1995) Normal clonal expansion but impaired Fas-mediated cell death and anergy induction in interleukin-2-deficient mice. Eur J Immunol 25: 2572–2577 [DOI] [PubMed] [Google Scholar]
- Kovanen PE, Leonard WJ (2004) Cytokines and immunodeficiency diseases: critical roles of the γ(c)-dependent cytokines interleukins 2, 4, 7, 9, 15, and 21, and their signaling pathways. Immunol Rev 202: 67–83 [DOI] [PubMed] [Google Scholar]
- Kramer S, Mamalaki C, Horak I, Schimpl A, Kioussis D, Hung T (1994) Thymic selection and peptide-induced activation of T cell receptor-transgenic CD8 T cells in interleukin-2-deficient mice. Eur J Immunol 24: 2317–2322 [DOI] [PubMed] [Google Scholar]
- Krammer PH (2000) CD95's deadly mission in the immune system. Nature 407: 789–795 [DOI] [PubMed] [Google Scholar]
- Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P (2000) Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science 288: 675–678 [DOI] [PubMed] [Google Scholar]
- Kündig T, Schorle H, Bachmann MF, Hengartner H, Zinkernagel RM, Horak I (1993) Immune responses in interleukin-2 deficient mice. Science 262: 1059–1061 [DOI] [PubMed] [Google Scholar]
- Kündig TM, Shahinian A, Kawai K, Mittrücker HW, Sebzda E, Bachmann MF, Mak TW, Ohashi PS (1996) Duration of TCR stimulation determines costimulatory requirement of T cells. Immunity 5: 41–52 [DOI] [PubMed] [Google Scholar]
- Laurence A et al. (2007) Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity 26: 371–381 [DOI] [PubMed] [Google Scholar]
- Lenardo MJ (1991) Interleukin-2 programs mouse αβ T lymphocytes for apoptosis. Nature 353: 858–861 [DOI] [PubMed] [Google Scholar]
- Leonard WJ et al. (1984) Molecular cloning and expression of cDNAs for the human interleukin-2 receptor. Nature 311: 626–631 [DOI] [PubMed] [Google Scholar]
- Ma A, Koka R, Burkett P (2006) Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol 24: 657–679 [DOI] [PubMed] [Google Scholar]
- Malek TR, Porter BO, Codias EK, Scibelli P, Yu A (2000) Normal lymphoid homeostasis and lack of lethal autoimmunity in mice containing mature T cells with severely impaired IL-2 receptors. J Immunol 164: 2905–2914 [DOI] [PubMed] [Google Scholar]
- Malek TR, Yu A, Scibelli P, Lichtenheld MG, Codias EK (2001) Broad programming by IL-2 receptor signaling for extended growth to multiple cytokines and functional maturation of antigen-activated T cells. J Immunol 166: 1675–1683 [DOI] [PubMed] [Google Scholar]
- Malek TR, Yu A, Vincek V, Scibelli P, Kong L (2002) CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rβ-deficient mice. Implications for the nonredundant function of IL-2. Immunity 17: 167–178 [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Liu ZJ, Kawahara A, Minami Y, Yamada K, Tsujimoto Y, Barsoumian EL, Permutter RM, Taniguchi T (1995) Three distinct IL-2 signaling pathways mediated by bcl-2, c-myc, and lck cooperate in hematopoietic cell proliferation. Cell 81: 223–231 [DOI] [PubMed] [Google Scholar]
- Morgan DA, Ruscetti FW, Gallo RC (1976) Selective in vitro growth of T lymphocytes from normal human bone marrow. Science 193: 1007–1009 [DOI] [PubMed] [Google Scholar]
- Nikaido T, Shimizu A, Ishida N, Sabe H, Teshigawara K, Maeda M, Uchiyama T, Yodoi J, Honjo T (1984) Molecular cloning of cDNA encoding human interleukin-2 receptor. Nature 311: 631–635 [DOI] [PubMed] [Google Scholar]
- Northrop JK, Thomas RM, Wells AD, Shen H (2006) Epigenetic remodeling of the IL-2 and IFN-γ loci in memory CD8 T cells is influenced by CD4 T cells. J Immunol 177: 1062–1069 [DOI] [PubMed] [Google Scholar]
- Papiernik M, de Moraes ML, Pontoux C, Vasseur F, Penit C (1998) Regulatory CD4 T cells: expression of IL-2R α chain, resistance to clonal deletion and IL-2 dependency. Int Immunol 10: 371–378 [DOI] [PubMed] [Google Scholar]
- Powell JD, Ragheb JA, Kitagawa-Sakakida S, Schwartz RH (1998) Molecular regulation of interleukin-2 expression by CD28 co-stimulation and anergy. Immunol Rev 165: 287–300 [DOI] [PubMed] [Google Scholar]
- Refaeli Y, Van Parijs L, London CA, Tschopp J, Abbas AK (1998) Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity 8: 615–623 [DOI] [PubMed] [Google Scholar]
- Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I (1993) Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell 75: 253–261 [DOI] [PubMed] [Google Scholar]
- Sandau MM, Schluns KS, Lefrancois L, Jameson SC (2004) Cutting edge: transpresentation of IL-15 by bone marrow-derived cells necessitates expression of IL-15 and IL-15R α by the same cells. J Immunol 173: 6537–6541 [DOI] [PubMed] [Google Scholar]
- Schimpl A, Berberich I, Kneitz B, Kramer S, Santner-Nanan B, Wagner S, Wolf M, Hunig T (2002) IL-2 and autoimmune disease. Cytokine Growth Factor Rev 13: 369–378 [DOI] [PubMed] [Google Scholar]
- Schorle H, Holtschke T, Hunig T, Schimpl A, Horak I (1991) Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature 352: 621–624 [DOI] [PubMed] [Google Scholar]
- Schwartz RH (1996) Models of T cell anergy: is there a common molecular mechanism? J Exp Med 184: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RH (2003) T cell anergy. Annu Rev Immunol 21: 305–334 [DOI] [PubMed] [Google Scholar]
- Shedlock DJ, Shen H (2003) Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 300: 337–339 [DOI] [PubMed] [Google Scholar]
- Smith KA (1988) Interleukin-2: inception, impact, and implications. Science 240: 1169–1176 [DOI] [PubMed] [Google Scholar]
- Smith KA, Gilbride KJ, Favata MF (1980) Lymphocyte activating factor promotes T-cell growth factor production by cloned murine lymphoma cells. Nature 287: 853–855 [DOI] [PubMed] [Google Scholar]
- Sun JC, Bevan MJ (2003) Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300: 339–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H et al. (1995) Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor β. Science 268: 1472–1476 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Zhou YW, Kato M, Mak TW, Nakashima I (1999) Normal regulatory α/β T cells effectively eliminate abnormally activated T cells lacking the interleukin 2 receptor β in vivo. J Exp Med 190: 1561–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T, Matsui H, Fujita T, Takaoka C, Kashima N, Yoshimoto R, Hamuro J (1983) Structure and expression of a cloned cDNA for human interleukin-2. Nature 302: 305–310 [DOI] [PubMed] [Google Scholar]
- Thomas RM, Gao L, Wells AD (2005) Signals from CD28 induce stable epigenetic modification of the IL-2 promoter. J Immunol 174: 4639–4646 [DOI] [PubMed] [Google Scholar]
- Utermohlen O, Tarnok A, Bonig L, Lehmann-Grube F (1994) T lymphocyte-mediated antiviral immune responses in mice are diminished by treatment with monoclonal antibody directed against the interleukin-2 receptor. Eur J Immunol 24: 3093–3099 [DOI] [PubMed] [Google Scholar]
- Van Parijs L, Biuckians A, Ibragimov A, Alt FW, Willerford DM, Abbas AK (1997) Functional responses and apoptosis of CD25 (IL-2R α)-deficient T cells expressing a transgenic antigen receptor. J Immunol 158: 3738–3745 [PubMed] [Google Scholar]
- Van Parijs L, Biuckians A, Abbas AK (1998) Functional roles of Fas and Bcl-2-regulated apoptosis of T lymphocytes. J Immunol 160: 2065–2071 [PubMed] [Google Scholar]
- Vezys V, Masopust D, Kemball CC, Barber DL, O'Mara LA, Larsen CP, Pearson TC, Ahmed R, Lukacher AE (2006) Continuous recruitment of naive T cells contributes to heterogeneity of antiviral CD8 T cells during persistent infection. J Exp Med 203: 2263–2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann TA (2006) The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol 6: 595–601 [DOI] [PubMed] [Google Scholar]
- Waldmann TA, Dubois S, Tagaya Y (2001) Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity 14: 105–110 [PubMed] [Google Scholar]
- Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW (1995) Interleukin-2 receptor α chain regulates the size and content of the peripheral lymphoid compartment. Immunity 3: 521–530 [DOI] [PubMed] [Google Scholar]
- Williams MA, Tyznik AJ, Bevan MJ (2006) Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature 441: 890–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M, Schimpl A, Hunig T (2001) Control of T cell hyperactivation in IL-2-deficient mice by CD4(+)CD25(−) and CD4(+)CD25(+) T cells: evidence for two distinct regulatory mechanisms. Eur J Immunol 31: 1637–1645 [DOI] [PubMed] [Google Scholar]
- Yu A, Zhou J, Marten N, Bergmann CC, Mammolenti M, Levy RB, Malek TR (2003) Efficient induction of primary and secondary T cell-dependent immune responses in vivo in the absence of functional IL-2 and IL-15 receptors. J Immunol 170: 236–242 [DOI] [PubMed] [Google Scholar]
- Zheng L, Trageser CL, Willerford DM, Lenardo MJ (1998) T cell growth cytokines cause the superinduction of molecules mediating antigen-induced T lymphocyte death. J Immunol 160: 763–769 [PubMed] [Google Scholar]