Abstract
Yeast cells respond to stress by mediating condition-specific gene expression changes and by mounting a common response to many stresses, called the environmental stress response (ESR). Giaever et al. previously revealed poor correlation between genes whose expression changes in response to acute stress and genes required to survive that stress, raising question about the role of stress-activated gene expression. Here we show that gene expression changes triggered by a single dose of stress are not required to survive that stimulus but rather serve a protective role against future stress. We characterized the increased resistance to severe stress in yeast preexposed to mild stress. This acquired stress resistance is dependent on protein synthesis during mild-stress treatment and requires the “general-stress” transcription factors Msn2p and/or Msn4p that regulate induction of many ESR genes. However, neither protein synthesis nor Msn2/4p is required for basal tolerance of a single dose of stress, despite the substantial expression changes triggered by each condition. Using microarrays, we show that Msn2p and Msn4p play nonredundant and condition-specific roles in gene-expression regulation, arguing against a generic general-stress function. This work highlights the importance of condition-specific responses in acquired stress resistance and provides new insights into the role of the ESR.
INTRODUCTION
Cells living in variable environments must adapt to sudden environmental changes that can stress the cellular system. Most natural environments are inherently variable over space and time, and as such environmental stresses can occur across gradients, in combinations, and in rapid succession. The ability to survive successive stress treatments and to prepare for severe stress after early signs of a problem presents a significant selective advantage for creatures living in natural environments.
Acquired stress resistance (sometimes called the “adaptive response”) is a phenomenon in which cells exposed to a mild dose of one stress can subsequently survive an otherwise lethal dose of the same or a second stress. The phenomenon has been documented in diverse organisms, including bacteria, plants, fungi, and humans, and in many cases requires a transcriptional response to the mild stress treatment (for example, Hahn et al., 1989; Talalay and Fahey, 2001; Chinnusamy et al., 2004; Durrant and Dong, 2004; Charng et al., 2006; Hecker et al., 2007; Jeong et al., 2006; Zhao et al., 2006; Kensler et al., 2007; Matsumoto et al., 2007).
Acquired stress resistance is also well documented in budding yeast, Saccharomyces cerevisiae (Mitchel and Morrison, 1982, 1983; Blomberg et al., 1988; Barnes et al., 1990; Jamieson, 1992; Flattery-O'Brien et al., 1993; Davies et al., 1995; Lewis et al., 1995; Swan and Watson, 1999; Chi and Arneborg, 2000; Pereira et al., 2001; Kandror et al., 2004; Palhano et al., 2004). Cross-stress protection between pairs of different stresses was proposed to depend on a large gene expression response triggered by stress, called the environmental stress response (ESR; Martinez-Pastor et al., 1996; Schmitt and McEntee, 1996; Gasch et al., 2000; Causton et al., 2001). This response consists of ∼300 induced genes and 600 repressed genes whose functions are related to stress defense and protein synthesis, respectively. Many of the genes induced in the ESR are regulated in part by two paralogous transcription factors, Msn2p and Msn4p (Gasch et al., 2000; Causton et al., 2001). These proteins are assumed to play largely redundant roles in “general” stress protection, because single deletion of either gene provides little to no phenotype in stress sensitivity (Estruch and Carlson, 1993; Martinez-Pastor et al., 1996; Schmitt and McEntee, 1996). Nuclear localization of the factors, and hence induction of their target genes, is activated by a wide range of stressors and is thought to protect cells against the offending stress and provide cross-stress protection against other stresses (Martinez-Pastor et al., 1996; Schmitt and McEntee, 1996; Gorner et al., 1998, 2002). However, numerous lines of evidence indicate that, although Msn2p and Msn4p targets are induced by a panel of different conditions, many of the individual genes are not required to survive the original stress (Gasch, 2002a). More broadly, global studies show a poor correlation between stress-activated gene expression changes and the importance of the genes' function in surviving that stress (Giaever et al., 2002). These observations suggest that gene expression changes triggered by stress play another role besides survival of the original stress stimulus.
Here we show that stress-activated gene expression changes, including those regulated by Msn2p and Msn4p, are not required to survive a single stress treatment but rather play a critical role in acquired stress resistance. Although cells lacking MSN2 and/or MSN4 (MSN2/4) displayed normal basal levels of stress tolerance, both the single and double mutants showed significant defects in acquired resistance to most stresses examined. Interestingly, the defect in acquired resistance varied for each mutant and depending on the mild stress treatment. We therefore characterized genomic expression in cells lacking either MSN2 or MSN4 or both factors. We show that the two proteins play nonredundant roles in gene-expression regulation that vary by gene and by condition. Together, these results highlight the importance of condition-specific gene expression responses in acquired stress resistance.
MATERIALS AND METHODS
Acquired Stress Resistance Experiments
All data shown are for strains in the BY4741 background (Mat a his3Δ1 leu2Δ0 met15Δ0 ura3Δ0), described in Supplemental Table S1. Strains lacking MSN2 or MSN4 were generated in the W303 (Mat a leu2Δ3112 trp1Δ1 can1Δ100 ura3Δ1 ade2Δ1 his3Δ11,15) and RM11-1a (Mat a leu2Δ0 ura3Δ0) backgrounds as described in Supplemental Table S1, and a W303 double deletion strain was obtained from the lab of M. Carlson. Cultures were grown in YPD (1% yeast extract, 2% peptone, 2% glucose) for at least 12 h to an optical density (OD600) of 0.3. Each culture was split into two cultures: one received a single dose of mild (“primary”) stress, and the other served as a mock control to which no stress was added. Primary stresses included 0.2 mM H2O2, 0.7 M NaCl, 5% or 10% ethanol, growth to different phases, or a shift from 30 to 37°C (for which cells were collected by centrifugation and resuspended in YPD prewarmed to 37°C). Mock-treated cells were handled identically except that carrier solution without drug (or medium preheated to 30°C in the case of heat shock) was added.
Resistance to a panel of severe (“secondary”) stresses was scored at various times after primary-stress addition. An aliquot of unstressed cells was collected immediately before primary-stress addition. At 15, 30, 60, and 120 min later, an aliquot of cells was collected by brief centrifugation and resuspended in YPD at OD600 0.6. Cells were then diluted 3× in 96-well plates containing YPD alone or YPD plus one of 11 doses of each secondary stress ranging from 0 to 5 mM H2O2, 0 to 15% ethanol, and 0 to 3 M NaCl; cells were also exposed to elevated temperatures ranging from 37 to 57°C using the gradient function of a thermocycler (Eppendorf, Fremont, CA). Cells were exposed to H2O2, ethanol, and NaCl secondary stresses for 2 h at 30°C with shaking in 96-well plates or for 10 min at high temperatures. For postdiauxic phase experiments, cells were grown to OD600 2.7, diluted to OD600 0.6, and immediately added to the secondary stresses. For stationary phase experiments, cells were grown for 6 d at 30°C with shaking and then diluted to an OD600 of 0.6 in spent medium and added to the secondary stresses. A 200-fold dilution of each culture was spotted on YPD agar plates and grown for 48 h, after which viability at each dose of stress was scored by visual inspection using a four-point scale to score 100%, 50–100%, 10–50%, or 0% survival compared with the no-secondary-stress control. An overall survival score was calculated as the sum of scores over 11 doses of stress; each score was normalized to the maximum dose of secondary stress survived relative to the mock-treated cultures. Cycloheximide and thiolutin experiments were done as above, except that 10 μg/ml cycloheximide or thiolutin was added to the culture 20 or 5 min, respectively, before primary-stress addition and/or concurrent with secondary stress. A mock-treated culture received inhibitor treatment but no primary stress.
Microarray and Quantitative PCR Experiments
Cell collection, lysis, and total RNA isolation were performed as previously described (Gasch, 2002b). Sample labeling was done according to described protocols (Gasch, 2002b) using cyanine dyes (Flownamics, Madison WI), Superscript III (Invitrogen, Carlsbad, CA), and amino-allyl-dUTP (Ambion, Austin, TX). Microarrays were spotted in house using 70mer oligonucleotides representing each of the yeast ORFs (Qiagen, Chatsworth, CA). Arrays were scanned using a scanning laser (GenePix 4000B) from Molecular Devices (Sunnyvale, CA). Microarray data were normalized using the method of Lyne et al. (2003). All microarray data are available in the GEO database under accession number GSE8335.
The response of BY4741, msn2Δ, msn4Δ, and the double-deletion strain to heat shock or NaCl was characterized by microarray analysis. Cells were grown for two or three doublings in YPD at 30°C to early-log phase, and a sample of each culture was collected as the unstressed reference. Cells were collected at 45 min after addition of 0.7 M NaCl or 15 min after cells were briefly centrifuged and resuspended in 37°C medium. RNA collected from each stressed culture was compared with RNA from the unstressed culture of the same strain. Five biological replicates were performed. Real-time quantitative PCR (qPCR) reactions were performed using Sybr green Jumpstart Taq (Sigma Aldrich, St. Louis, MO) and an Applied Biosystems 7500 detector (Foster City, CA). The average of technical replicates was normalized to the internal control transcript, ERV25, which does not show stress-dependent changes in expression.
For Figure 5, three microarray time courses were conducted in duplicate for wild-type cells. Cells were grown as above, exposed to 0.7 M NaCl and collected for microarray analysis before stress and at 15, 30, 45, and 60 min after application of NaCl. After the 60-min time point, cells were collected by centrifugation, resuspended in fresh YPD medium, and either allowed to grow for 30 min (YPD control) or exposed to 0.5 mM H2O2 stress for the second microarray time course. The third time course followed cells that were grown in YPD and then exposed directly to 0.5 mM H2O2 without pretreatment with NaCl; cells were collected at 10, 20, 30, and 40 min after the addition of H2O2. For all microarray experiments, the sample collected after stress addition was compared with the unstressed control. A third series of time courses was performed on cells lacking MSN2, as described above.
Figure 5.
Cells with acquired stress resistance show an altered expression response to stress. Cells were exposed to 0.5 mM H2O2, alone or after 60 min pretreatment with 0.7M NaCl. Microarrays were conducted at 10, 20, 30, and 40 min after H2O2 treatments or 15, 30, 45, and 60 min after NaCl addition. The average log2 expression is shown for (A) 316 genes with acclimated H2O2-responsive repression in wild-type cells pretreated with NaCl; (B) 288 genes with acclimated H2O2-responsive induction in wild-type cells pretreated with NaCl; and (C) 64 Yap1p targets (Gasch et al., 2000) in wild-type (top panels) and msn2Δ cells (bottom panels).
Statistics and Microarray Data Analysis
Genes dependent on Msn2p or Msn4p or both were identified as those with a statistically significant defect in induction compared with wild-type cells (q < 0.05; Storey and Tibshirani, 2003) or with an induction defect in at least 90% of the unpaired microarray comparisons across strains plus a ≥1.5× weaker average induction compared with wild type. These genes were classified as described in Figure 4 based on an average expression change that was ≥1.5× different between indicated strains.
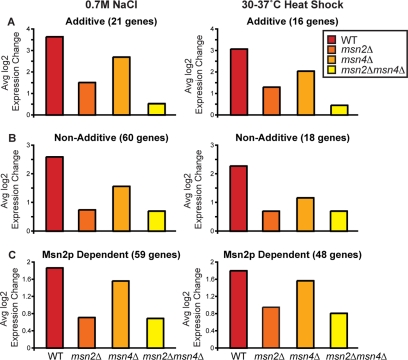
Figure 4.
Genes show different dependencies on Msn2p versus Msn4p, depending on the stress. Gene expression was characterized using DNA microarrays in wild-type and mutant cells 45 min after treatment with 0.7M NaCl or 15 min after a 30–37°C heat shock. The average log2 expression change is shown for genes dependent on Msn2p and/or Msn4p in response to NaCl (left) or heat shock (right): (A) 21 genes and 16 genes that showed a greater expression defect in the msn2Δmsn4Δ strain than either single mutant responding to NaCl treatment or heat shock, respectively; (B) 60 genes and 18 genes that showed an induction defect in both msn2Δ and msn4Δ strains responding to NaCl treatment or heat shock, respectively, but for which no additive defect was observed in the double mutant; and (C) 59 genes and 48 genes that showed a significant defect only in the msn2Δ and msn2Δmsn4Δ strains responding to NaCl treatment and heat shock, respectively.
For the acclimation experiments, H2O2-dependent gene expression changes were defined as those with >1.5× change in expression in at least three time points from the replicate H2O2-alone time courses, applied to data in which at least 80% of the data were present. Acclimated expression changes in cells treated with H2O2 after NaCl exposure were defined as those with a >1.5-fold weaker expression response to H2O2 in at least three time points out of the pooled wild-type time courses or two time points in the msn2Δ time course. Gene clustering was done in Cluster 3.0 (http://bonsai.ims/u-tokyo.ac.jp/∼mdehoon/software) using hierarchical clustering and uncentered Pearson correlation as the metric (Eisen et al., 1998). Enrichment of gene functional categories was performed using the hypergeometric distribution in Excel (Microsoft, Redmond, WA) or the program Funspec (Robinson et al., 2002) with Bonferroni-corrected p values < 0.01 taken as significant.
RESULTS
Characterization of Acquired Stress Resistance in Wild-Type Cells
We first set out to systematically characterize acquired stress resistance in wild-type cells using our assay. We measured the ability of cells pretreated with mild (primary) stress to survive a panel of severe (secondary) doses of stress. Cells were exposed to a single dose of primary stress for varying times and then removed from primary stress and exposed to 11 doses of each of four secondary stresses (NaCl, H2O2, ethanol, and extreme heat) and a no-stress control. A scoring system measured the fraction of primary stress-treated cells that survived the 11 doses of each secondary stress, compared with a mock-treated culture. Each score was adjusted to reflect the fold-increase in maximum dose of stress survived by the cells in each experiment (see Materials and Methods).
Acquired stress resistance was common, although not universal for all stresses (Figure 1), consistent with previous reports (Jamieson, 1992; Flattery-O'Brien et al., 1993; Lewis et al., 1995; Palhano et al., 2004). With the exception of ethanol (data not shown), all of the primary stresses provoked resistance to a more severe dose of the same stressor (referred to as “same-stress” resistance). Mild treatment with these stresses also provided “cross-stress” protection to at least one other stress: heat shock and growth to stationary phase provoked resistance to all four of the secondary stresses tested, in agreement with previous results (Hall, 1983; Werner-Washburne et al., 1993; Lewis et al., 1995), whereas primary NaCl or H2O2 treatment provided protection against severe doses of both of these conditions. The only exception to these trends was mild ethanol treatment, which provided no protection against any stress (data not shown). This peculiarity is apparently specific to the S288c lab strain, because wild yeast strains can readily acquire stress resistance after mild ethanol treatment (J. C. Painter, J. A. Lewis, and A. P. Gasch, unpublished data).
Figure 1.
Wild-type cells acquire resistance to severe stress after exposure to mild stress. Cell survival of severe (secondary) stress was scored at various times after exposure to a mild (primary) dose of stress. The fold-increase in maximum secondary-stress dose survived compared with a mock-treated culture is shown at 15, 30, 60, and 120 min of primary stress exposure. Primary stresses included (A) 0.7M NaCl, (B) 0.2 mM H2O2, (C) a 30°C to 37°C heat shock, or (D) growth to different phases. Increased thermotolerance is indicated as maximum °C survived. Plots represent the average and SE of the mean (SEM) of at least triplicate experiments, except for growth-phase experiments, which show the average and range of duplicates. Asterisks represent resistance that was significantly different from mock-treated cells in ≥2 time points (p < 0.01, t test).
Comparing the levels and dynamics of acquired stress resistance revealed important differences in the response to each stress combination. In particular, numerous lines of evidence suggest that same-stress resistance occurs via distinct mechanism(s) compared with cross-stress protection. First, cells exposed to a mild heat shock acquired resistance to a higher dose of the same stress faster (i.e., within a shorter exposure time to mild stress) than they acquired cross-stress resistance (Figure 1C). Second, the dose of primary stress had different effects on same- versus cross-stress resistance (Supplemental Figure S1). For example, cells exposed to one of three different primary doses of NaCl stress (0.5, 0.7, or 0.9 M) acquired roughly equivalent levels of resistance to secondary NaCl stress but showed a dose-dependent response in cross-stress protection against H2O2 stress (Supplemental Figure S1). Finally, acquired resistance to severe NaCl or H2O2 stress after a mild heat shock (cross-stress protection) was dependent on Msn2/4p, whereas resistance to severe heat after a mild heat shock (same-stress protection) was not (see below). These results are consistent with the model that cross-stress protection occurs via a distinct response to the original stressor.
Nascent Protein Synthesis Is Necessary for Acquired Stress Resistance But Not Basal Stress Tolerance
The dynamics of acquired stress protection roughly correlated with that of gene expression changes triggered by each primary stress (data not shown), suggesting that nascent transcription and/or protein synthesis is required. Indeed, we found that resistance to severe H2O2 or NaCl after mild treatment with either stress was abolished or diminished if cycloheximide was given concurrent with the primary stress, but not if given in combination with the secondary stress (Figure 2). Treatment of cells with a high dose of thiolutin, at which both transcription and protein synthesis are halted (Jimenez et al., 1973), also abolished acquired resistance after primary NaCl treatment (data not shown). Therefore, normal acquisition of secondary-stress resistance requires protein synthesis during exposure to primary stress but not secondary stress treatment.
Figure 2.
Protein synthesis is required for acquired stress resistance. Acquired stress resistance was measured as described in Figure 1 after 60 min treatment with (A) NaCl or (B) H2O2 primary stress, in the presence or absence of cycloheximide. Cycloheximide-treated cells were exposed to 10 μg/ml inhibitor for 20 min before and throughout primary-stress and/or secondary-stress exposures. Each plot shows the average and SEM of triplicate experiments. Conditions that provided acquired stress resistance compared with mock-treated cells are marked with an asterisk (p < 0.01).
Surprisingly, however, neither protein synthesis nor gene expression was required for basal tolerance of a single stress treatment. Cells pretreated with cycloheximide or thiolutin showed no significant difference in basal tolerance of NaCl, H2O2, ethanol, or extreme heat in our assay, over a range of stress doses, compared with untreated cells (Supplemental Figure S2). This result confirms a long-standing observation that basal thermotolerance is not diminished in the presence of cycloheximide (Hall, 1983). Thus, the extensive remodeling of genomic expression triggered by acute stress treatment is not required to survive that treatment, but is required to survive subsequent stress exposure.
Acquired Stress Resistance, But Not Basal Stress Tolerance, Requires the Transcription Factors Msn2p and Msn4p
We next characterized acquired stress resistance in cells lacking either one or both of the “general-stress” transcription factors Msn2p and Msn4p, previously proposed to participate in the adaptive response (Estruch and Carlson, 1993; Martinez-Pastor et al., 1996; Schmitt and McEntee, 1996). Consistent with prior studies (Estruch and Carlson, 1993; Martinez-Pastor et al., 1996), we found that cells lacking either factor displayed wild-type levels of basal stress tolerance over a range of stress doses in both liquid and solid media, with the exception of ethanol tolerance (Supplemental Figures S3 and S4). Martinez-Pastor et al. (1996) previously reported sensitivity of the msn2Δmsn4Δ double mutant to very extreme doses of stress at which few wild-type cells survive. We did not capture any sensitivity in our assay after short-term stress exposure (Supplemental Figures S3 and S4). These phenotypes are not specific to the S288c background, because mutants in the W303 background or the vineyard isolate RM11-1a had the same phenotypes (data not shown). Thus, Msn2p and Msn4p are not required for acute stress tolerance over a range of heat, NaCl, and H2O2 treatments.
In contrast, the mutant strains displayed a major defect in acquired stress resistance triggered by these stresses (Figure 3). Interestingly, the defect was different depending on the primary stress and the mutant. For example, msn2Δ and msn4Δ cells treated with a mild heat shock showed reduced tolerance of secondary NaCl or H2O2 stress but not severe temperatures (Figure 3 and Supplemental Table S2). The defect was exacerbated in a strain lacking both MSN2 and MSN4, indicating that the proteins play partially overlapping roles in response to heat shock. The single mutants treated with mild NaCl treatment also displayed a defect in acquired resistance to severe NaCl or H2O2, although the defect was greater in the msn2Δ mutant compared with the msn4Δ strain. Similar defects were observed in the W303 mutant strains investigated (data not shown). In contrast to primary NaCl and heat stress, cells exposed to mild H2O2 treatment (Figure 3) or cells grown to stationary phase (Supplemental Table 2) acquired significant resistance to severe NaCl or H2O2 treatment largely independently of Msn2p or Msn4p. Thus, the factors contribute to acquired stress resistance triggered by some, but not all, primary stresses.
Figure 3.
Strains lacking Msn2p or Msn4p show defects in acquired stress resistance. Cells were exposed to primary and secondary stresses as described in Figure 1. The fold-increase in maximum dose of secondary stress survived 60 min after exposure to 30–37°C heat shift, 0.7 M NaCl, or 0.2 mM H2O2 primary stress is shown for wild-type and mutant strains, as indicated by the key. Resistance to (A) NaCl or (B) H2O2 secondary stress is shown after each primary-stress treatment indicated on the x-axis. Plots show the average and SEM of at least triplicate experiments.
Msn2p and Msn4p Play Condition-specific and Nonredundant Regulatory Roles
To identify the role of Msn2p and Msn4p in primary stress response, we characterized gene expression in wild-type cells and cells lacking MSN2, MSN4, or both factors as cells responded to NaCl or heat shock (see Materials and Methods for details). We identified 140 genes and 82 genes whose induction was dependent on one or both factors in response to NaCl or heat shock, respectively. Only 49 genes were affected by MSN2 and/or MSN4 (MSN2/MSN4) deletion under both conditions, revealing only partial overlap in the Msn2/4p targets identified in the two experiments.
Genes fell into different classes according to their dependence on Msn2p versus Msn4p (Figure 4), and this was validated by qPCR (Supplemental Figure S5). Few, if any, genes showed a clear expression defect in the double-deletion but not the single-deletion strains, a pattern expected if the mutants were acting entirely redundantly. Furthermore, only 20% of the Msn2/4p targets identified in each experiment showed an additive induction defect in the double-deletion strain. For example, DDR1 and STF2 showed weaker induction in the msn2Δ and msn4Δ mutants compared with wild type and an exacerbated defect in the msn2Δmsn4Δ double mutant responding to both stresses (Supplemental Figure S5). This observation reveals that Msn2p and Msn4p are partially redundant for induction of these genes. The remaining 80% of targets did not show an additive phenotype in the double-deletion strain. In fact, many of these genes required both Msn2p and Msn4p for full induction (Figure 4B and Discussion). In general, Msn2p played a larger role than Msn4p in regulating gene expression, evidenced by both the number of genes dependent only on Msn2p (40–60% of targets, depending on the stress) and the greater contribution of Msn2p to the induction of genes dependent on both factors. Thus, the regulatory role of Msn2p versus Msn4p is distinct for different genes, confirming previous reports at the single-gene level (Schmitt and McEntee, 1996; Treger et al., 1998; Amoros and Estruch, 2001) and is nonredundant in the majority of cases.
Interestingly, the dependence on Msn2p versus Msn4p varied by stress, revealing condition-specific differences in Msn2/4p action. First, there were significantly more Msn2/4p targets triggered by NaCl stress (140 genes) than heat shock (82 genes). This was confirmed at several genes by qPCR, such as ICY1, YDR379C-A, and YLR356W, whose induction was Msn2/4p dependent in response to NaCl but independent of Msn2/4p after heat shock (see Discussion). Second, many of the genes regulated by Msn2/4p in response to both stresses were classified differently depending on the stress, revealing condition-specific regulation at individual genes (Supplemental Table S3). For example, expression of HSP12 showed an additive dependence on Msn2p and Msn4p in response to heat shock (consistent with previous studies [Treger et al., 1998]) but was dependent only on Msn2p in response to NaCl stress. Third, Msn4p played a larger role in response to NaCl than heat shock: more genes were affected by MSN4 deletion alone in response to NaCl (76/140, or 54% of targets) than heat shock (25/82, only 30% of targets). qPCR results suggest there may be additional NaCl-affected genes with a subtle defect in the msn4Δ strain that was below the original cutoff used in the microarray analysis (Supplemental Figure S5). Furthermore, Msn4p contributed more to the induction of genes with an additive defect in response to NaCl treatment. Thus, the precise roles of Msn2p and Msn4p clearly vary by condition.
Primary-Stress Treatment Alters the Gene Expression Response to Secondary Stress
To characterize the effects of acquired stress resistance on gene expression, we followed genomic expression in cells exposed to severe H2O2 treatment, with and without mild NaCl pretreatment. The response to H2O2 was significantly altered if cells had been pretreated with NaCl (Figure 5). Expression of nearly 777 genes was induced and 626 genes was repressed in untreated cells exposed to H2O2 (see Materials and Methods). Of these, nearly 40% of induced genes and 50% of repressed genes showed smaller expression changes in response to H2O2 if cells had been preexposed to NaCl (Figure 5). The smaller changes in expression indicate that the H2O2 response of these genes was acclimated in cells previously stressed by NaCl. Notably, more than 60% of these genes were already altered in expression during the NaCl pretreatment. The genes that displayed acclimated expression changes after H2O2 treatment were strongly enriched for genes in the ESR (p < 10−12). In contrast, many genes did not show acclimation in cells pretreated with NaCl, including targets of the H2O2-activated transcription factor Yap1 (Figure 5C).
Unlike wild-type cells, in which nearly half of the H2O2-triggered expression changes were acclimated in NaCl-pretreated cells, there were significantly fewer acclimated expression changes in the msn2Δ strain responding to the same conditions. Of the genes that showed acclimation, the majority was repressed by H2O2 treatment (Figure 5A, bottom). The smaller number of acclimated expression changes in the msn2Δ strain correlates with the observed defect in acquired stress resistance under these conditions. Thus, the acclimated expression seen in wild-type cells is a response specific to cells with acquired stress resistance and not simply a consequence of multiple stress treatments.
DISCUSSION
Cells respond to acute stress by dramatically altering genomic expression; however, few (1–3%) of the genes whose expression is affected are required to survive that stress treatment (Giaever et al., 2002). Our results suggest that the primary function of these gene expression changes is to provide protection against impending stress. Strains lacking the transcription factors Msn2p and/or Msn4p have a major defect in acquired stress resistance but no observed sensitivity to a single dose of acute stress. The defect in acquired, but not basal, stress resistance correlates with a defect in gene expression in the msn2/4 mutants responding to primary stress (Figure 4), again suggesting that the stress-activated expression changes contribute to acquired instead of basal stress tolerance. Consistently, nascent protein synthesis is not required to survive the acute stresses studied here but is critical for acquisition of normal levels of subsequent stress resistance. Finally, cells that have already acquired stress resistance show smaller differences in stress-activated gene expression, at a specific subset of genes heavily enriched for ESR genes (Figure 5). Thus, the observed expression changes provide a preparative role for impending stress rather than a protective role against the original stimulus.
Given the time required for nascent transcription and translation, it is understandable that cells would not rely on time-consuming syntheses to survive sudden exposure to fungicidal stress. Even for fungistatic stresses, such as shift to a suboptimal carbon source (for which induction of key metabolism genes is required for survival, e.g., galactose utilization genes), the vast majority of expression changes are not required for survival, at least in the short-term (Giaever et al., 2002). Natural environments are highly variable, and stressful environmental changes can occur in combination and in close succession. Furthermore, many stresses may accumulate incrementally over temporal gradients. The ability to prepare for impending stress would provide an important advantage for microbes competing in the wild. The advantage of such a response has likely contributed to the extensive conservation of stress-activated gene expression changes in fungi and other microbes (Gasch et al., 2000; Chen et al., 2003; Gasch, 2007).
Cross-stress protection had been attributed to a general-stress activation of Msn2/4p and initiation of the ESR (Martinez-Pastor et al., 1996; Schmitt and McEntee, 1996; Gorner et al., 1998; Navarro-Avino et al., 1999; Gasch et al., 2000; Causton et al., 2001). However, our results argue that acquired stress resistance cannot be explained by simple activation of the ESR, but rather is determined by condition-specific responses. First, although all conditions examined here activate the ESR (including ethanol treatment, data not shown), cross-stress protection is not universal. Second, cross-stress protection shows different dependencies on Msn2p versus Msn4p depending on the stress combination. Finally, survival of the identical secondary stresses (severe NaCl and H2O2) occurs via distinct regulatory systems depending on the mild stress, indicating that the mechanism of protection is established by the primary stress treatment. Together, these results argue against the model that Msn2/4p give rise to a general-stress response that universally underlies cross-stress protection (Martinez-Pastor et al., 1996; Schmitt and McEntee, 1996).
Instead, our work supports the model that Msn2p and Msn4p play largely nonredundant, condition-specific roles in providing future stress protection. Both the acquired-stress phenotypes (Figure 3) and the gene expression defects (Figure 4) indicate that Msn2p and Msn4p play distinct roles in response to different stresses. We found no genes where Msn2p and Msn4p play entirely redundant roles, and few (∼20%) of Msn2/4p targets showed evidence of partial redundancy in Msn2p versus Msn4p regulation. Instead, many genes required both Msn2p and Msn4p, acting nonredundantly, for full induction. There were clear differences in gene targets as well as the relative contributions of Msn2p and Msn4p at those genes, depending on the stress to which cells were exposed. Thus, Msn2p and Msn4p have much more specific roles in stress defense than previously appreciated.
A number of possible models could explain the condition-specific involvement of Msn2/4p. First, it has been shown previously that other transcription factors, including Hsf1p in response to heat shock (Treger et al., 1998; Amoros and Estruch, 2001; Grably et al., 2002), can activate Msn2/4p targets in response to specific stresses, thus reducing the requirement for Msn2/4p under those conditions. The involvement of Hsf1p is not sufficient to explain our results, as ∼75% of genes dependent on Msn2/4p in response to NaCl but not heat shock (including those validated by qPCR) do not contain an upstream Hsf1p-binding element, are not bound by Hsf1p during heat shock, and are not known Hsf1p targets (Hahn et al., 2004 and data not shown). Although Hsf1p does not fully explain our observation, the activity of additional transcription factors is almost certainly an important factor in determining the stress-specific involvement of Msn2/4p (Gasch, 2002a). An alternate hypothesis is that Msn2/4p are activated differently in response to different stresses. Both factors display different phosphorylation profiles in response to different conditions (Garreau et al., 2000), although the effects of these differences are not known. It is possible that differential phosphorylation affects the regulatory activity, binding affinity, or cofactor interactions of one or both factors.
There are clear differences in the contribution of Msn2p versus Msn4p to gene induction at individual promoters; however, the mechanism of this difference is unclear. The factors bind the same recognition sequence (CCCCT) through DNA binding domains that are 72% identical (Estruch and Carlson, 1993; Martinez-Pastor et al., 1996; Schmitt and McEntee, 1996); the relative binding affinities of the factors are not known. Promoter analysis of the gene classes identified here showed significant enrichment of upstream STREs for all classes, but no other differences in promoter architecture that suggest possible mechanisms of differential Msn4p versus Msn2p involvement (data not shown). Interestingly, MSN4 itself is induced in response to a variety of stresses (whereas MSN2 is preferentially induced during NaCl treatment; Gasch et al., 2000 and this study), suggesting that Msn4p may act in the maintenance or amplification of gene induction through positive feedback. Induction of a substantial number of targets requires both Msn2p and Msn4p, raising the possibility that the factors act cooperatively, either simultaneously or sequentially, at these promoters.
This study shows that the mechanism of acquired stress resistance, and cross-stress protection in particular, is clearly more complex than previously appreciated. Future aims will be directed at identifying the individual genes that contribute to acquired resistance of specific stresses, as well as the finer features of their condition-specific regulation.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Marian Carlson (Columbia University) for graciously providing the W303 msn2Δmsn4Δ strain, Jessica Will for experimental assistance, the UW-Madison Gene Expression Center for equipment usage, and members of the Gasch lab for critical discussions. This work was supported by a Beckman Young Investigator Award to A.P.G., and D.B.B. was supported by National Institutes of Health Predoctoral Training Grant 5T32GM07133.
Abbreviations used:
- ESR
environmental stress response
- H2O2
hydrogen peroxide
- NaCl
sodium chloride
- Msn2/4p
Msn2p and/or Msn4p.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-07-0680) on August 27, 2008.
REFERENCES
- Amoros M., Estruch F. Hsf1p and Msn2/4p cooperate in the expression of Saccharomyces cerevisiae genes HSP26 and HSP104 in a gene- and stress type-dependent manner. Mol. Microbiol. 2001;39:1523–1532. doi: 10.1046/j.1365-2958.2001.02339.x. [DOI] [PubMed] [Google Scholar]
- Barnes C. A., Johnston G. C., Singer R. A. Thermotolerance is independent of induction of the full spectrum of heat shock proteins and of cell cycle blockage in the yeast Saccharomyces cerevisiae. J. Bacteriol. 1990;172:4352–4358. doi: 10.1128/jb.172.8.4352-4358.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg A., Larsson C., Gustafsson L. Microcalorimetric monitoring of growth of Saccharomyces cerevisiae: osmotolerance in relation to physiological state. J. Bacteriol. 1988;170:4562–4568. doi: 10.1128/jb.170.10.4562-4568.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causton H. C., Ren B., Koh S. S., Harbison C. T., Kanin E., Jennings E. G., Lee T. I., True H. L., Lander E. S., Young R. A. Remodeling of yeast genome expression in response to environmental changes. Mol. Biol. Cell. 2001;12:323–337. doi: 10.1091/mbc.12.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charng Y. Y., Liu H. C., Liu N. Y., Hsu F. C., Ko S. S. Arabidopsis Hsa32, a novel heat shock protein, is essential for acquired thermotolerance during long recovery after acclimation. Plant Physiol. 2006;140:1297–1305. doi: 10.1104/pp.105.074898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Toone W. M., Mata J., Lyne R., Burns G., Kivinen K., Brazma A., Jones N., Bahler J. Global transcriptional responses of fission yeast to environmental stress. Mol. Biol. Cell. 2003;14:214–229. doi: 10.1091/mbc.E02-08-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Z., Arneborg N. Saccharomyces cerevisiae strains with different degrees of ethanol tolerance exhibit different adaptive responses to produced ethanol. J. Industrial Microbiol. Biotechnol. 2000;24:75–78. [Google Scholar]
- Chinnusamy V., Schumaker K., Zhu J. K. Molecular genetic perspectives on cross-talk and specificity in abiotic stress signalling in plants. J. Exp. Botany. 2004;55:225–236. doi: 10.1093/jxb/erh005. [DOI] [PubMed] [Google Scholar]
- Davies J. M., Lowry C. V., Davies K. J. Transient adaptation to oxidative stress in yeast. Arch. Biochem. Biophys. 1995;317:1–6. doi: 10.1006/abbi.1995.1128. [DOI] [PubMed] [Google Scholar]
- Durrant W. E., Dong X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004;42:185–209. doi: 10.1146/annurev.phyto.42.040803.140421. [DOI] [PubMed] [Google Scholar]
- Eisen M. B., Spellman P. T., Brown P. O., Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estruch F., Carlson M. Two homologous zinc finger genes identified by multicopy suppression in a SNF1 protein kinase mutant of Saccharomyces cerevisiae. Mol. Cell. Biol. 1993;13:3872–3881. doi: 10.1128/mcb.13.7.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flattery-O'Brien J., Collinson L. P., Dawes I. W. Saccharomyces cerevisiae has an inducible response to menadione which differs from that to hydrogen peroxide. J. Gen. Microbiol. 1993;139:501–507. doi: 10.1099/00221287-139-3-501. [DOI] [PubMed] [Google Scholar]
- Garreau H., Hasan R. N., Renault G., Estruch F., Boy-Marcotte E., Jacquet M. Hyperphosphorylation of Msn2p and Msn4p in response to heat shock and the diauxic shift is inhibited by cAMP in Saccharomyces cerevisiae. Microbiology. 2000;146(Pt 9):2113–2120. doi: 10.1099/00221287-146-9-2113. [DOI] [PubMed] [Google Scholar]
- Gasch A. P. The Environmental stress response: a common yeast response to environmental stresses. In: Hohmann S., Mager P., editors. Yeast Stress Responses. Vol. 1. Heidelberg: Springer-Verlag; 2002a. pp. 11–70. [Google Scholar]
- Gasch A. P. Yeast genomic expression studies using DNA microarrays. In: Guthrie C., Fink G. R., editors. Methods in Enzymology. Vol. 350. San Diego: Academic Press; 2002b. pp. 393–414. [DOI] [PubMed] [Google Scholar]
- Gasch A. P. Comparative genomics of the environmental stress response in ascomycete fungi. Yeast. 2007;24:961–976. doi: 10.1002/yea.1512. [DOI] [PubMed] [Google Scholar]
- Gasch A. P., Spellman P. T., Kao C. M., Carmel-Harel O., Eisen M. B., Storz G., Botstein D., Brown P. O. Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell. 2000;11:4241–4257. doi: 10.1091/mbc.11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever G., et al. Functional profiling of the Saccharomyces cerevisiae genome. Nature. 2002;418:387–391. doi: 10.1038/nature00935. [DOI] [PubMed] [Google Scholar]
- Gorner W., Durchschlag E., Martinez-Pastor M. T., Estruch F., Ammerer G., Hamilton B., Ruis H., Schuller C. Nuclear localization of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Genes Dev. 1998;12:586–597. doi: 10.1101/gad.12.4.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorner W., Durchschlag E., Wolf J., Brown E. L., Ammerer G., Ruis H., Schuller C. Acute glucose starvation activates the nuclear localization signal of a stress-specific yeast transcription factor. EMBO J. 2002;21:135–144. doi: 10.1093/emboj/21.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grably M. R., Stanhill A., Tell O., Engelberg D. HSF and Msn2/4p can exclusively or cooperatively activate the yeast HSP104 gene. Mol. Microbiol. 2002;44:21–35. doi: 10.1046/j.1365-2958.2002.02860.x. [DOI] [PubMed] [Google Scholar]
- Hahn G. M., Ning S. C., Elizaga M., Kapp D. S., Anderson R. L. A comparison of thermal responses of human and rodent cells. Int. J. Radiat. Biol. 1989;56:817–825. doi: 10.1080/09553008914552101. [DOI] [PubMed] [Google Scholar]
- Hahn J. S., Hu Z., Thiele D. J., Iyer V. R. Genome-wide analysis of the biology of stress responses through heat shock transcription factor. Mol. Cell. Biol. 2004;24:5249–5256. doi: 10.1128/MCB.24.12.5249-5256.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. G. Yeast thermotolerance does not require protein synthesis. J. Bacteriol. 1983;156:1363–1365. doi: 10.1128/jb.156.3.1363-1365.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker M., Pane-Farre J., Volker U. SigB-dependent general stress response in Bacillus subtilis and related gram-positive bacteria. Annu. Rev. Microbiol. 2007;61:215–236. doi: 10.1146/annurev.micro.61.080706.093445. [DOI] [PubMed] [Google Scholar]
- Jamieson D. J. Saccharomyces cerevisiae has distinct adaptive responses to both hydrogen peroxide and menadione. J. Bacteriol. 1992;174:6678–6681. doi: 10.1128/jb.174.20.6678-6681.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong W. S., Jun M., Kong A. N. Nrf2, a potential molecular target for cancer chemoprevention by natural compounds. Antioxidants Redox Signal. 2006;8:99–106. doi: 10.1089/ars.2006.8.99. [DOI] [PubMed] [Google Scholar]
- Jimenez A., Tipper D. J., Davies J. Mode of action of thiolutin, an inhibitor of macromolecular synthesis in. Saccharomyces cerevisiae. Antimicrobial Agents Chemother. 1973;3:729–738. doi: 10.1128/aac.3.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandror O., Bretschneider N., Kreydin E., Cavalieri D., Goldberg A. L. Yeast adapt to near-freezing temperatures by STRE/Msn2,4-dependent induction of trehalose synthesis and certain molecular chaperones. Mol. Cell. 2004;13:771–781. doi: 10.1016/s1097-2765(04)00148-0. [DOI] [PubMed] [Google Scholar]
- Kensler T. W., Wakabayashi N., Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- Lewis J. G., Learmonth R. P., Watson K. Induction of heat, freezing and salt tolerance by heat and salt shock in Saccharomyces cerevisiae. Microbiology. 1995;141(Pt 3):687–694. doi: 10.1099/13500872-141-3-687. [DOI] [PubMed] [Google Scholar]
- Lyne R., Burns G., Mata J., Penkett C. J., Rustici G., Chen D., Langford C., Vetrie D., Bahler J. Whole-genome microarrays of fission yeast: characteristics, accuracy, reproducibility, and processing of array data. BMC Genom. 2003;4:27. doi: 10.1186/1471-2164-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Pastor M. T., Marchler G., Schuller C., Marchler-Bauer A., Ruis H., Estruch F. The Saccharomyces cerevisiae zinc finger proteins Msn2p and Msn4p are required for transcriptional induction through the stress response element (STRE) EMBO J. 1996;15:2227–2235. [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H., Hamada N., Takahashi A., Kobayashi Y., Ohnishi T. Vanguards of paradigm shift in radiation biology: radiation-induced adaptive and bystander responses. J. Radiat. Res. 2007;48:97–106. doi: 10.1269/jrr.06090. [DOI] [PubMed] [Google Scholar]
- Mitchel R. E., Morrison D. P. Heat-shock induction of ionizing radiation resistance in Saccharomyces cerevisiae, and correlation with stationary growth phase. Radiat. Res. 1982;90:284–291. [PubMed] [Google Scholar]
- Mitchel R. E., Morrison D. P. Heat-shock induction of ultraviolet light resistance in Saccharomyces cerevisiae. Radiat. Res. 1983;96:95–99. [PubMed] [Google Scholar]
- Navarro-Avino J. P., Prasad R., Miralles V. J., Benito R. M., Serrano R. A proposal for nomenclature of aldehyde dehydrogenases in Saccharomyces cerevisiae and characterization of the stress-inducible ALD2 and ALD3 genes. Yeast. 1999;15:829–842. doi: 10.1002/(SICI)1097-0061(199907)15:10A<829::AID-YEA423>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Palhano F. L., Orlando M. T., Fernandes P. M. Induction of baroresistance by hydrogen peroxide, ethanol and cold-shock in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 2004;233:139–145. doi: 10.1016/j.femsle.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Pereira M. D., Eleutherio E. C., Panek A. D. Acquisition of tolerance against oxidative damage in Saccharomyces cerevisiae. BMC Microbiol. 2001;1:11. doi: 10.1186/1471-2180-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M. D., Grigull J., Mohammad N., Hughes T. R. FunSpec: a web-based cluster interpreter for yeast. BMC Bioinform. 2002;3:35. doi: 10.1186/1471-2105-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A. P., McEntee K. Msn2p, a zinc finger DNA-binding protein, is the transcriptional activator of the multistress response in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 1996;93:5777–5782. doi: 10.1073/pnas.93.12.5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey J. D., Tibshirani R. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. USA. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan T. M., Watson K. Stress tolerance in a yeast lipid mutant: membrane lipids influence tolerance to heat and ethanol independently of heat shock proteins and trehalose. Can. J. Microbiol. 1999;45:472–479. doi: 10.1139/w99-033. [DOI] [PubMed] [Google Scholar]
- Talalay P., Fahey J. W. Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. J. Nutr. 2001;131:3027S–3033S. doi: 10.1093/jn/131.11.3027S. [DOI] [PubMed] [Google Scholar]
- Treger J. M., Schmitt A. P., Simon J. R., McEntee K. Transcriptional factor mutations reveal regulatory complexities of heat shock and newly identified stress genes in Saccharomyces cerevisiae. J. Biol. Chem. 1998;273:26875–26879. doi: 10.1074/jbc.273.41.26875. [DOI] [PubMed] [Google Scholar]
- Werner-Washburne M., Braun E., Johnston G. C., Singer R. A. Stationary phase in the yeast Saccharomyces cerevisiae. Microbiol. Rev. 1993;57:383–401. doi: 10.1128/mr.57.2.383-401.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Sapolsky R. M., Steinberg G. K. Interrupting reperfusion as a stroke therapy: ischemic postconditioning reduces infarct size after focal ischemia in rats. J. Cereb. Blood Flow Metab. 2006;26:1114–1121. doi: 10.1038/sj.jcbfm.9600348. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.