Abstract
Rationale: Laryngopharyngeal reflux (LPR) affects up to 20% of Western populations. Although individual morbidity is usually moderate, treatment costs are high and there are associations with other diseases, including laryngeal cancer. To date, there have been no studies of the mucosal immune response to this common inflammatory disease.
Objectives: To determine the mucosal immune response to LPR.
Methods: We performed a prospective immunologic study of laryngeal biopsies from patients with LPR and control subjects (n = 12 and 11, respectively), and of primary laryngeal epithelial cells in vitro.
Measurements and Main Results: Quantitative multiple-color immunofluorescence, using antibodies for lymphocytes (CD4, CD8, CD3, CD79, CD161), granulocytes (CD68, EMBP), monocytic cells (CD68, major histocompatibility complex [MHC] class II), and classical and nonclassical MHC (I, II, β2-microglobulin, CD1d). Univariate and multivariate analysis and colocalization measurements were applied. There was an increase in percentage area of mucosal CD8+ cells in the epithelium (P < 0.005), whereas other leukocyte and granulocyte antigens were unchanged. Although epithelial MHC class I and II expression was unchanged by reflux, expression of the nonclassical MHC molecule CD1d increased (P < 0.05, luminal layers). In vitro, laryngeal epithelial cells constitutively expressed CD1d. CD1d and MHC I expression were inversely related in all subjects, in a pattern which appears to be unique to the upper airway. Colocalization of natural killer T (NKT) cells with CD1d increased in patients (P < 0.01).
Conclusions: These data indicate a role for the CD1d–NKT cell axis in response to LPR in humans. This represents a useful target for novel diagnostics and treatments in this common condition.
Keywords: laryngopharyngeal reflux, CD1d, natural killer T cells, epithelial cells
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Laryngopharyngeal reflux affects up to 20% of the United States population. Although morbidity is low, treatment costs are high, and it is associated with other diseases, including mucosal cancer.
What This Study Adds to the Field
The nonclassical major histocompatibility complex molecule CD1d appears to be involved in the response to laryngopharyngeal reflux. This finding suggests novel approaches to the diagnosis and treatment of this common condition.
The larynx represents the crossroads between the gastrointestinal and respiratory systems, and is the junction of the IgA-dominated upper and IgG-dominated lower airways. It is the narrowest point of the airway and a point of great airway turbulence, leading to the deposition of high densities of inhaled, and sometimes ingested, challenges. It also has a bacterial flora distinct from that of other parts of the respiratory tract (1), and there is evidence supporting a distinct immunologic role for the larynx.
Work by our group and others has demonstrated a high density of immunologically active cells in the laryngeal mucosa of animals (2–6) and humans (7–10). There are occasional highly organized lymphoid collections, similar to Peyer's patches (larynx-associated lymphoid tissue) (2, 7), although, as with the bowel (11), more than 90% of immune cells are diffusely distributed throughout the epithelium and lamina propria (8, 9). Environmental challenges alter laryngeal immunology: exposure of rat larynxes to viruses increases numbers of mucosal dendritic cells (4), whereas we have shown previously that the laryngeal mucosa of smokers contains more CD4+ T cells (9). Also, patients who have had a laryngectomy suffer increased chest and other infections, which could be partly due to the loss of laryngeal immune responses (12).
Laryngopharyngeal reflux (LPR; synonyms are posterior laryngitis, extraesophageal reflux) is common, affecting an estimated 20% of the adult population in the United States (13). On the basis of U.K. audit figures (unpublished data), 4% of the £500 million ($1 billion) spent on proton pump inhibitors annually by the National Health Service (i.e., £20 million, $40 million), is used to treat LPR. The main clinical features are mucosal inflammation and edema, but symptoms as diverse as globus pharyngeus and chronic cough have been linked to this disease. Furthermore, a recent case-control study found an odds ratio of 2.1 for the development of laryngeal cancer in persons with reflux (14). We found significantly less carbonic anhydrase type III in the laryngeal mucosa of patients with reflux, contributing to the lower pH environment necessary for pepsin-mediated damage (15). In a pig laryngeal ex vivo model, such damage could be seen as epithelial “holes” (16). However, to date, there have been no reports of the changes in immunologically active cells in the laryngeal mucosa of this common chronic inflammatory condition.
The first aim of this study was to examine changes in the expression of immunologically relevant cell subsets and antigen-presenting molecules within the laryngeal mucosa as a result of LPR. The second aim was to use these changes to develop hypotheses about the immunologic function of the laryngeal mucosa. Some of the results of these studies have been previously reported in the form of an abstract (17).
METHODS
Patients and Control Subjects
Patients and control subjects were obtained from nonconsecutive consenting persons presenting to the Center for Voice Disorders, Wake Forest University Baptist Medical Center, North Carolina, between August 2004 and April 2005. Demographics of patients and control subjects are shown in Table 1.
TABLE 1.
DEMOGRAPHIC CHARACTERISTICS OF PATIENTS AND CONTROL SUBJECTS
| Control | LPR | |
|---|---|---|
| Total recruited | 11 | 12 |
| Female | 5 | 10 |
| Non-white | 1 | 2 |
| Median BMI (range) | 28 (22–40) | 28 (23–35) |
| Median age, yr (range) | 40 (30–57) | 49 (45–60) |
| Median RSI (range) | 3 (0–7) | 24 (21–30) |
Definition of abbreviations: BMI = body mass index; LPR = laryngopharyngeal reflux; RSI = reflux symptom index.
Both groups had relatively high median BMIs. See online supplement Methods for further details of diagnostic criteria.
Biopsies of posterior (nonvibrating edge) vocal cord epithelium were taken using transnasal esophagoscopy under topical anesthesia (17) and immediately snap frozen in isopentane which had been precooled over liquid nitrogen. Specimens were mounted on cork discs in OCT (Sakura, Torrance, CA) and stored at −80°C.
Quantitative Multiple-Color Immunofluorescence Histology
Five-micrometer frozen tissue sections were subjected to two- or three-step multiple-color immunofluorescence as previously described (8–10, 18–20). See Table E1 of the online supplement for details of antibodies used. Sections were mounted, multiple fields at ×20 magnification were digitized, and grayscale images captured on a Leica DMRA microscope using a Hamamatsu Orca-ER camera and Q-Fluoro software (Leica, Milton Keynes, UK). Percentage areas of positive pixel staining were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD; http://rsb.info.nih.gov), and mean fluorescence intensity of staining across the basal to apical layers of the epithelium was measured using Image ProPlus software (Media Cybernetics, Silver Spring, MD). See online supplement for further details of quantitative image analysis.
In Vitro Staining of Laryngeal Epithelial Cells
Laryngeal epithelial cells were isolated from biopsies of normal patients undergoing routine surgery for nonlaryngeal disease, and cultured in serum-free medium using methods previously described (19). Cytospins of epithelial cells at first passage were subjected to dual-color immunofluorescence as described above and in the online supplement, using a mouse anti-human monoclonal antibody to CD1d (see Table E1).
Colocalization Method
The levels of colocalization of CD1d, CD3, and CD161 were calculated as described previously (20) using ImageJ. Briefly, preferential colocalization of molecules (and therefore fluorochromes) was determined by comparisons of the observed distributions of colors with those predicted from a null hypothesis that each element of positive staining was randomly and independently distributed (the expected levels of colocalization). The data were log10 transformed to obtain a normal distribution, and the mean observed and expected values were calculated for the reflux and control groups.
Statistical Analysis
Average pixel area for each fluorochrome was calculated for each biopsy using macros developed by our group (20). Analysis of log10(n + x) transformed data used analysis of variance (ANOVA) and t tests (SPSS version 12.0 for Windows; SPSS, Inc., Chicago, IL). For line analysis, paired t tests were used to compare intensities of antigen expression between epithelium and lamina propria. Where standard deviations were unequal, central tendency was expressed using a geometric mean and a paired t test was performed on log10-transformed data. The observed levels of colocalization of fluorochromes were compared with the expected levels using a two-tailed, paired Student's t test.
RESULTS
To investigate the immunologic consequences of LPR, we first studied the composition and location of the infiltrating cells in the laryngeal mucosa of the patient and control samples. No differences in numbers of B cells between patients with LPR and control subjects were found, confirming our earlier observation that B cells are almost entirely confined to the lamina propria (9). Similarly, there was no change in the expression of neutrophil, eosinophil, or monocytic lineage markers with reflux.
Next, the distribution of CD8+ lymphocytes was compared. There was a highly significant increase in overall CD8+ staining in the laryngeal epithelium of patients with reflux (P < 0.005; Figure 1A). When the epithelium was then stratified into luminal and basal layers, the ratios of areas of expression of CD8 in the luminal and basal epithelial layers was significantly higher in patients compared with control subjects (P < 0.0001; Figure 1B), suggesting that the response to LPR may involve accumulation of CD8+ T cells in the luminal epithelial layer.
Figure 1.
(A) Log10(n + 2) average percentage pixel area staining of CD8 in patients with laryngopharyngeal reflux (LPR) and control subjects. Bars indicate geometric means. Epithelial CD8+ cells increased in reflux (P < 0.005). (B) The ratio of expression of average luminal to basal epithelial CD8 staining in patients with LPR and control subjects. Bars indicate geometric means. Patients with LPR had significantly greater expression of CD8 in the luminal epithelial layer (P < 0.0001). (C) Two-color immunofluorescence image of laryngeal mucosa of a control showing CD8+ T-cells (red) concentrated in basal layer of the epithelium (EP), the area with the highest epithelial cell expression of MHC Class I (green). LP = lamina propria; white line = basement membrane.
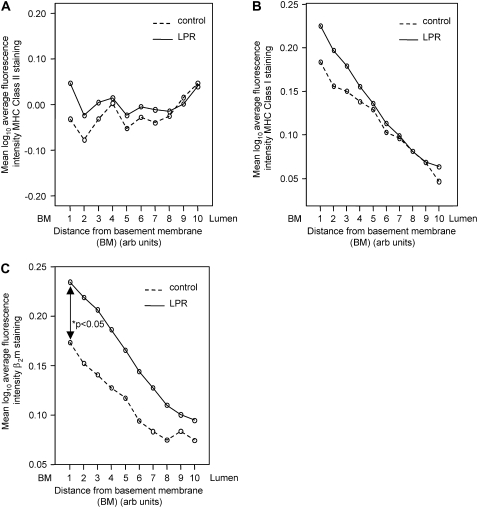
Next, we investigated whether changes in the expression of the antigen-presenting major histocompatibility complex (MHC) class I and class II molecules were associated with LPR. In these experiments, as described previously (10), the thickness of the epithelium was divided into 10 equal distances (deciles), and changes across these layers from basal to luminal levels were used to assess the intensity of expression of a given molecule. In concordance with our previous results, MHC class II molecules were expressed consistently throughout all layers of the epithelium in the healthy control subjects. This pattern was preserved in patients with LPR and there was no statistically significant difference in MHC class II expression between the control and the patient group (Figure 2A).
Figure 2.
Average log10 fluorescence intensity of expression of (A) major histocompatibility complex (MHC) class II, (B) MHC class I, and (C) β2-microglobulin (β2m) across the laryngeal epithelium of patients with laryngopharyngeal reflux (LPR) and control subjects. Intensity of expression has been measured at each of 10 levels for each group, with basal layers to the left and luminal layers to the right. Patients with LPR had increased β2m expression near the basement membrane (P < 0.05).
In keeping with previous reports, the expression of MHC class I molecules was polarized, with more antigen present in the deeper layers and gradually decreasing in abundance toward the luminal side in control samples. The staining pattern in patients with LPR was identical, and the level of expression did not show any differences between control and LPR larynxes (Figure 2B). The expression of β2-microglobulin (β2m), the molecule associated with classical MHC class I molecules, showed the same polarized behavior, with levels decreasing toward the lumen. However, as shown in Figure 2C, comparison of individual points between the two groups showed a trend to increased β2m levels in the LPR samples, and this difference reached statistical significance in the deepest layers of the epithelium (P < 0.05; Figure 2C). This suggests that not all β2m protein may be complexed with classical class I molecules in patients with reflux, raising the possibility that one of the nonclassical class I molecules could be associated with β2m in laryngeal epithelial cells. We explored whether the difference between patterns of MHC class I and β2m expression might be due to the expression of CD1d in the larynx.
CD1d was present on epithelial cells with a membranous expression pattern both in the healthy control individuals and the patients. For both groups, there was significantly more CD1d expression in the superficial (luminal) layers (P < 0.001 multiple range test; Figure 3A) decreasing toward more basal deciles. These results indicate a topographic switching of expression of MHC class IhiCD1dlo epithelial cells in the basal layers, gradually transitioning to the MHC class IloCD1dhi phenotype seen in the luminal layers (Figures 3 A and 3B). In LPR samples, there was an overall trend (ANOVA F = 0.4, P = 0.2) toward an increased CD1d expression, which reached significance for the most superficial levels (P = 0.023; Figure 3A)
Figure 3.
CD1d expression by human laryngeal epithelium. (A) Average log10 fluorescence intensity of expression of CD1d. Expression was measured in the same way as for Figure 2, with basal layers to the left and luminal to the right. Patients with laryngopharyngeal reflux (LPR) exhibited greater expression of CD1d toward the luminal edge of the epithelium (P = 0.023). (B) Three-color immunofluorescence image of laryngeal mucosa of a patient with LPR showing topographic switching between MHC class I/β2m (basal layers, green) and CD1d (luminal layers, blue). CD161+ cells (red) may be seen colocalizing with CD1d (white arrow). White line denotes basement membrane (BM), EPI = epithelium; LP = lamina propria.
To explore whether laryngeal epithelial cells express CD1d constitutively, or if the presence of this antigen-presenting molecule is regulated by environmental factors in situ, we studied the presence of CD1d on cultured epithelial cells. Cytospins of epithelial cells, derived from healthy donors at first passage, were stained for the presence of CD1d using the same antibody as above. As shown in Figure 4, all cells expressed CD1d in a predominantly membranous pattern, indicating that bacterial products are not necessary to induce the transcription/translation of this protein (Figure 4).
Figure 4.
Cytospins of cultured normal human primary laryngeal epithelial cells stained for CD1d. Green = CD1d, blue = nuclei. There is constitutive expression of CD1d (green) in a membranous pattern.
CD1d internalizes, processes, and presents glycolipid antigens to adjacent T cells (21), but also presents antigens to a small subset of T lymphocytes called natural killer T (NKT) cells (22–24). Therefore, we proceeded to measure the expression of NKT cell markers in our subjects. Our choice of antigens for identification of NKT cells was limited by the need to have different immunoglobulin isotype–specific primary and secondary antibodies for each molecule in any given combination, and by the importance of colocalizing these cells with CD1d. Thus, we defined NKT cells as CD3+CD161+ lymphocytes. Because CD161 is predominantly present on natural killer (NK) cells, we compared the prevalence and location of NK cells (CD161+CD3− lymphocytes) with NKT cells, which also express the T-cell marker CD3. In these experiments, NK cells exhibited a significantly reduced tendency to associate with CD1d in the laryngeal mucosa (P < 0.01). The majority of CD161+ cells colocalizing with CD1d were CD3+ T cells (P < 0.01; Figure 5), indicating the possibility that NKT cells were likely to interact with CD1d in the mucosa. In turn, this suggests antigen recognition by NKT cells at this location. Finally, whereas the number of CD3+ CD161+ NKT cells did not change in patients with reflux, there was a marked increase in the number of CD161−CD3+ T cells accumulating in the luminal, CD1dhi layers of the epithelium, which corroborates our finding that patients with reflux had significantly increased CD8+ T-lymphocyte staining in the luminal layer of the laryngeal epithelium (Figure 1B).
Figure 5.
Colocalization studies for CD161, CD3, and CD1d in laryngeal epithelium in control subjects (open and hatched bars for observed and expected results, respectively) and patients with laryngopharyngeal reflux (LPR) (solid and cross-hatched bars for observed and expected results, respectively). The graph shows the observed and expected areas of colocalization. Error bars represent standard errors. *P < 0.01.
DISCUSSION
LPR is the most frequently encountered chronic inflammatory condition of the larynx. Despite its high prevalence, and the high associated health care costs, the immunology of this disease state remains to be fully elucidated. The aim of this study was to examine changes in the expression of the main antigen-presenting molecules present on the epithelial cells and the composition and frequency of immunologically relevant cells within the mucosa in LPR. Therefore, we performed a prospective, blinded, controlled, quantitative study on the expression of selected immunologically relevant cell surface molecules in vivo, using biopsies of laryngeal mucosa from patients with this common chronic inflammatory disease and matched control subjects.
The main differences in the immunologic infiltrate between healthy control subjects and patients with LPR consisted of an increase in the numbers of CD8+ cells. CD8+ TCRαβ+ T cells classically respond to antigen associated with MHC class I, which was consistent with our observation that these cells localized to the deeper, MHC class Ihi layers of the epithelium in control subjects. However, in patients with reflux, the expression of CD1d was increased toward the outer layer of the epithelium to a greater degree than in control subjects. Next, we investigated if changes in antigen-presenting molecules could give clues toward the mechanisms of the observed increase in CD8 staining toward the luminal epithelial layer. Consistent with our previous studies (10), MHC class I levels diminished significantly across the epithelium from basal to luminal surfaces. A similar decrease was seen in the expression of β2m, which forms complexes with the MHC class I heavy chain and a short peptide antigen. However, comparison between healthy control subjects and patients with LPR demonstrated a relative elevation in β2m production in the patient group. This suggested the existence of β2m protein in a form not complexed with classical class I molecules and led us to test the hypothesis that nonclassical MHC molecule expression by laryngeal epithelial cells is involved in the mucosal response to refluxate.
A number of nonclassical MHC molecules, including HLA-G, HLA-E, and various CD1 isoforms, associate with β2m (25). However, HLA-G expression has only been unequivocally demonstrated in extravillous cytotrophoblasts, whereas the formation of HLA-E– β2m complexes requires the presence of leader peptides derived from classical class I molecules. Thus, the presence of β2m-associated HLA-E on laryngeal epithelial cells (LEC), especially toward the luminal surface, was unlikely. At the same time, the expression of the group 2, β2m-independent CD1 molecule, CD1d, has been described on various epithelial structures (26, 27), including the mucosa of the alimentary tract, prompting us to investigate the expression of this molecule. Our results show the expression of CD1d in the laryngeal epithelium with a unique topographic switching of expression of MHC class IhiCD1dlo epithelial cells in the basal layers gradually transitioning to the MHC class IloCD1dhi phenotype, as they move toward the lumen. This would suggest that, in LPR, there may be a change in the type of antigen presentation occurring in response to refluxate.
Although a similar pattern of CD1d expression has been reported on keratinocytes, with higher abundance of the protein toward the skin surface (28), this is related to the terminal maturation of these cells in the stratum corneum, paralleled with increasing involucrin levels (29). The authors proposed that this switching is a paraphenomenon associated with aging and cell death. However, laryngeal epithelial cells, by contrast, remain viable throughout, with live cells in contact with luminal challenges, including refluxate. Meanwhile, MHC class I was expressed at higher levels toward the stratum basale and declined toward the granulosum in skin (30), which mirrors our findings of classical MHC class I expression in the larynx.
In murine intestinal epithelium, it has been observed that CD1d is most prevalent apically and laterally within crypts (27), and this is also the case in human small intestinal epithelium (31, 32). We performed a limited study of human small intestine that confirmed this (data not shown). The relationship between MHC class I and CD1d expression we have observed in the laryngeal mucosa may be restricted to the upper airway, or to squamous mucosal epithelia generally. This is supported by our observation that MHC class I expression also diminishes progressively toward the luminal surface of human tonsillar epithelium (10). We therefore hypothesize that the observed switch in antigen-presenting molecules, with reduced MHC class I expression and the prevalence of CD1d at the lumen in laryngeal epithelium, has an important functional role, such as the induction and/or maintenance of tolerance to incident antigens. In addition, of all tested antigen-presentation pathways, only this CD1d-mediated antigen-presentation pathway showed significant changes in LPR.
NKT cells, CD3+ T lymphocytes that recognize glycolipid antigens presented by CD1d, are characterized by the expression of a limited TCRα/β receptor repertoire (22, 33) and the ability to produce cytokines very rapidly after TCR ligation (34). They play a role in protection against bacterial infections, the elimination of malignant cells, and the maintenance of self-tolerance. Given the observation of strong epithelial expression of CD1d, which is known to present certain bacterial antigens, the size of the laryngeal “bacteriome” (1), and the exposure of this epithelium to incident antigens and microbes, NKT cells may be functionally relevant at this site. Limitations of currently available anti-TCR antibodies and CD1d tetramer technologies did not allow us to investigate NKT cell numbers in the larynx in vivo directly using a single cell surface molecule (35, 36). Thus, we used CD161 as an indirect marker in our studies and where this colocalized with CD3 on lymphocytes, cells were classified as NKT cells. This approach has its limitations. Although NKT cells were initially described as TCRα/β+ T cells expressing NKR-P1 (the murine equivalent of CD161), this definition is no longer used. It is now clear that a subset of CD1d-reactive NKT cells does not express CD161, whereas a large fraction of CD161+ T lymphocytes express a diverse TCR repertoire.
Whether these latter cells respond to CD1d-restricted antigens by secreting the same cytokines remains to be determined. Furthermore, CD161 is almost universally expressed by NK cells. Nonetheless, studying the coexpression of CD161+CD3+ in the larynx allowed us to investigate the likelihood of NKT cell contact with the CD1d present on epithelial cells. The absence of positive association of CD161+CD3− cells, NK cells, with CD1d suggested little functional interaction between the two in vivo, consistent with the lack of CD1d-reactive T-cell receptor on NK cells. By contrast, CD161+CD3+ cells significantly colocalized with CD1d, strongly suggesting that at least some of these were CD1d-restricted NKT cells. Taken together, the results indicate a physiologic switch from immunologic surveillance mediated by classical MHC class I antigen complexes recognized by CD8+ T cells near the basement membrane, to nonclassical MHC class I molecules presenting antigens to NKT cells, and possibly CD1d-restricted CD8+ T cells and NKT cells nearer the lumen. Furthermore, elevated CD1d expression by epithelial cells in patients with LPR indicates a regulation of this pathway by acid refluxate. There is some evidence that CD1d-responsive CD8+ cells may be involved in the pathogenesis of inflammatory bowel disease in humans (37). NK cells also express CD8 at low levels, and it is possible that because the antibody used to detect CD8 was raised against the α chain of the TCR, some of the positive staining observed was attributed to the presence of NK cells. Further studies of the interaction between antigens presented by CD1d on laryngeal epithelial cells and CD8+ T cells in the presence or absence of refluxate seem justified to provide further insights into the immunology of the upper airways.
In summary, we have observed a novel expression pattern whereby MHC class IhiCD1dlo epithelial cells in the basal layers gradually transition to MHC class IloCD1dhi epithelial cells in the luminal layers. Epithelial cells in culture constitutively expressed CD1d. These data suggest functional separation of luminal and basal layers in a manner not observed in nonsquamous mucosal surfaces, including the small intestine, a known site for the induction of mucosal tolerance. CD161+CD3+ (NKT) cells significantly colocalize to the CD1d layers, as do CD8+ T cells in reflux. These cells, CD8+ T cells, and CD1d expression by superficial epithelial cells were all increased in the mucosa of patients with reflux. We therefore hypothesize that the graded change from classical to nonclassical MHC class I expression between the layers of the laryngeal epithelium is crucial in the maintenance of the balance between upper airway tolerance and appropriate inflammatory responses. Selective regulation of the CD1d–NKT cell axis during the chronic inflammatory challenge of LPR appears to play a central role in the immunopathology of this condition. We propose, first, that this interaction is central to the pathogenesis of airway disease and, second, that the larynx is an important and accessible target for novel diagnostic and therapeutic strategies in a range of airway diseases.
Supplementary Material
Acknowledgments
The authors thank Mr. Crawford Johnston, Wake Forest University, Winston-Salem, NC, for database help and cocoordinating biopsy collection and transfer. Dr. Mark O. Lively, Wake Forest University, assisted with local organization of the study. The advice of Dr. Tristan Cogan and Dr. Sarah Ayling, both University of Bristol, was invaluable in preparing the manuscript.
L.E.N.R. was supported by a Courtenay-Cowlin Fellowship (University of Bristol); M.A.B. was supported by a Research Clinical Leave Fellowship from the Wellcome Trust.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200706-895OC on March 6, 2008
Conflict of Interest Statement: L.E.N.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. L.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.G.-O. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.F.I. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.R.S. received an honorarium from Pfizer to present a scientific review lecture at the 24th World Buiatrics Congress (Nice 2006). N.J. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.A.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.A.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Gillett S, Rees L, Cogan T, Birchall M, Bailey M. Characterisation of the bacterial flora of the larynx [abstract]. Clin Otolaryngol 2006;31:583. [Google Scholar]
- 2.Gorti GK, Birchall MA, Haverson K, Macchiarini P, Bailey M. A preclinical model for laryngeal transplantation: anatomy and mucosal immunology of the porcine larynx. Transplantation 1999;68:1638–1642. [DOI] [PubMed] [Google Scholar]
- 3.Jecker P, McWilliam A, Marsh A, Holt PG, Mann WJ, Pabst R, Westermann J. Acute laryngotracheitis in the rat induced by Sendai virus: the influx of six different types of immunocompetent cells into the laryngeal mucosa differs strongly between the subglottic and the glottic compartment. Laryngoscope 2001;111:1645–1651. [DOI] [PubMed] [Google Scholar]
- 4.Jecker P, Mann WJ, McWilliam AS, Holt PG. Dendritic cell influx differs between the subglottic and glottic mucosae during acute laryngotracheitis induced by a broad spectrum of stimuli. Ann Otol Rhinol Laryngol 2002;111:567–572. [DOI] [PubMed] [Google Scholar]
- 5.Wang EC, Damrose EJ, Mendelsohn AH, Nelson SD, Shintaku IP, Ye M, Berke GS, Blackwell KE. Distribution of class I and II human leukocyte antigens in the larynx. Otolaryngol Head Neck Surg 2006;134:280–287. [DOI] [PubMed] [Google Scholar]
- 6.Barker EV, Haverson K, Stokes CR, Birchall M, Bailey M. The larynx as an immunological organ. Clin Exp Immunol 2006;143:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kracke A, Hiller AS, Tschernig T, Kasper M, Kleemann WJ, Troger HD, Pabst R. Larynx-associated lymphoid tissue (LALT) in young children. Anat Rec 1997;248:413–420. [DOI] [PubMed] [Google Scholar]
- 8.Rees LE, Ayoub O, Birchall MA, Bailey M. Differential major histocompatibility complex class II locus expression on human laryngeal epithelium. Clin Exp Immunol 2003;134:497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rees LEN, Jones PH, Ayoub O, Gunasekaran S, Rajkumar K, Stokes CR, Haverson K, Bailey M, Birchall MA. Smoking influences the immunological architecture of the human larynx. Clin Immunol 2006;118:342–347. [DOI] [PubMed] [Google Scholar]
- 10.Hobbs CGL, Rees LEN, Heyderman RS, Birchall MA, Bailey M. Quantitative differences in major histocompatibility complex class I expression in human tonsillar and laryngeal epithelium. Clin Exp Immunol 2006;145:365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gullberg E, Soderholm JD. Peyer's patches and M cells as potential sites of the inflammatory onset in Crohn's disease. Ann N Y Acad Sci 2006;1072:218–232. [DOI] [PubMed] [Google Scholar]
- 12.Fontana GA, Pantaleo T, Lavorini F, Mutolo D, Polli G, Pistolesi M. Coughing in laryngectomized patients. Am J Respir Crit Care Med 1999;160:1578–1584. [DOI] [PubMed] [Google Scholar]
- 13.Koufman JA, Amin MR, Panetti M. Prevalence of reflux in 113 consecutive patients with laryngeal and voice disorders. Otolaryngol Head Neck Surg 2000;123:385–388. [Published erratum appears in Otolaryngol Head Neck Surg 2001;124:104.] [DOI] [PubMed] [Google Scholar]
- 14.Vaezi MF, Qadeer MA, Lopez R, Colabianchi N. Laryngeal cancer and gastroesophageal reflux disease: a case-control study. Am J Med 2006;119:768–776. [DOI] [PubMed] [Google Scholar]
- 15.Johnston N, Dettmar PW, Postma GN, Lively MO, Belafsky PC, Birchall M, Koufman JA. Effect of pepsin on laryngeal stress protein (sep70, sep53, and hsp70) response: role in laryngopharyngeal reflux disease. Ann Otol Rhinol Laryngol 2006;115:47–58. [DOI] [PubMed] [Google Scholar]
- 16.Bulmer D. Damage and protection of the laryngeal mucosa in reflux disease [Ph.D. dissertation]. Newcastle-upon-Tyne, UK: University of Newcastle-upon-Tyne; 2003.
- 17.Birchall M, Hobbs C, Phillips A, Bailey M, Rees LEN, Johnston N, Koufman J. MHC molecule expression in laryngeal epithelium of patients with laryngopharyngeal reflux compared to normal subjects. Clin Otolaryngol 2005;30:584. [Google Scholar]
- 18.Belafsky PC, Postma GN, Daniel E, Koufman JA. Transnasal esophagoscopy. Otolaryngol Head Neck Surg 2001;125:588–589. [DOI] [PubMed] [Google Scholar]
- 19.Haverson K, Singha S, Stokes CR, Bailey M. Professional and non-professional antigen-presenting cells in the porcine small intestine. Immunology 2000;101:492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rees LEN, Sipaul F, Gunasekaran S, Birchall MA, Bailey M. The isolation and characterisation of primary human laryngeal epithelial cells. Mol Immunol 2006;43:725–730. [DOI] [PubMed] [Google Scholar]
- 21.Inman C, Rees LEN, Barker EV, Haverson K, Stokes CR, Bailey M. Validation of computer-assisted, pixel-based analysis of multiple-colour immunofluorescence histology. J Immunol Methods 2005;302:156–167. [DOI] [PubMed] [Google Scholar]
- 22.Somnay-Wadgaonkar K, Nusrat A, Kim HS, Canchis WP, Balk SP, Colgan SP, Blumberg RS. Immunolocalization of CD1d in human intestinal epithelial cells and identification of a beta2-microglobulin-associated form. Int Immunol 1999;11:383–392. [DOI] [PubMed] [Google Scholar]
- 23.Porcelli SA, Modlin RL. CD1 and the expanding universe of T cell antigens. J Immunol 1995;155:3709–3710. [PubMed] [Google Scholar]
- 24.Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol 1997;15:535–562. [DOI] [PubMed] [Google Scholar]
- 25.Van Kaer L. NKT cells: T lymphocytes with innate effector functions. Curr Opin Immunol 2007;19:354–364. [DOI] [PubMed] [Google Scholar]
- 26.Braud VM, Allan DS, McMichael AJ. Functions of nonclassical MHC and non-MHC-encoded class I molecules. Curr Opin Immunol 1999;11:100–108. [DOI] [PubMed] [Google Scholar]
- 27.Beckman EM, Porcelli SA, Morita CT, Behar SM, Furlong ST, Brenner MB. Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature 1994;372:691–694. [DOI] [PubMed] [Google Scholar]
- 28.Bleicher PA, Balk SP, Hagen SJ, Blumberg RS, Flotte TJ, Terhorst C. Expression of murine CD1 on gastrointestinal epithelium. Science 1990;250:679–682. [DOI] [PubMed] [Google Scholar]
- 29.Bonish B, Jullien D, Dutronc Y, Huang BB, Modlin R, Spada FM, Porcelli SA, Nickoloff BJ. Overexpression of CD1d by keratinocytes in psoriasis and CD1d-dependent IFN-gamma production by NK-T cells. J Immunol 2000;165:4076–4085. [DOI] [PubMed] [Google Scholar]
- 30.Fishelevich R, Malanina A, Luzina I, Atamas S, Smyth MJ, Porcelli SA, Gaspari AA. Ceramide-dependent regulation of human epidermal keratinocyte CD1d expression during terminal differentiation. J Immunol 2006;176:2590–2599. [DOI] [PubMed] [Google Scholar]
- 31.Gielen V, Schmitt D, Thivolet J. HLA class I antigen (heavy and light chain) expression by Langerhans cells and keratinocytes of the normal human epidermis: ultrastructural quantitation using immunogold labelling procedure. Arch Dermatol Res 1988;280:131–136. [DOI] [PubMed] [Google Scholar]
- 32.Balk SP, Burke S, Polischuk JE, Frantz ME, Yang L, Porcelli S, Colgan SP, Blumberg RS. Beta 2-microglobulin-independent MHC class Ib molecule expressed by human intestinal epithelium. Science 1994;265:259–262. [DOI] [PubMed] [Google Scholar]
- 33.Brigl M, van den Elzen P, Chen X, Meyers JH, Wu D, Wong CH, Reddington F, Illarianov PA, Besra GS, Brenner MB, et al. Conserved and heterogeneous lipid antigen specificities of CD1d-restricted NKT cell receptors. J Immunol 2006;176:3625–3634. [DOI] [PubMed] [Google Scholar]
- 34.Allez M, Brimnes J, Dotan I, Mayer L. Expansion of CD8+ T cells with regulatory function after interaction with intestinal epithelial cells. Gastroenterology 2002;123:1516–1526. [DOI] [PubMed] [Google Scholar]
- 35.Stetson DB, Mohrs M, Reinhardt RL, Baron JL, Wang ZE, Gapin L, Kronenberg M, Locksley RM. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med 2003;198:1069–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Terabe M, Swann J, Ambrosino E, Sinha P, Takaku S, Hayakawa Y, Godfrey DI, Ostrand-Rosenberg S, Smyth MJ, Berzofsky JA. A nonclassical non-Valpha14Jalpha18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. J Exp Med 2005;202:1627–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Godfrey DI, McConville MJ, Pellicci DG. Chewing the fat on natural killer T cell development. J Exp Med 2006;203:2229–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.