Abstract
Superdormant spores of Bacillus subtilis and Bacillus megaterium were isolated in 4 to 12% yields following germination with high nutrient levels that activated one or two germinant receptors. These superdormant spores did not germinate with the initial nutrients or those that stimulated other germinant receptors, and the superdormant spores' defect was not genetic. The superdormant spores did, however, germinate with Ca2+-dipicolinic acid or dodecylamine. Although these superdormant spores did not germinate with high levels of nutrients that activated one or two nutrient germinant receptors, they germinated with nutrient mixtures that activated more receptors, and using high levels of nutrient mixtures activating more germinant receptors decreased superdormant spore yields. The use of moderate nutrient levels to isolate superdormant spores increased their yields; the resultant spores germinated poorly with the initial moderate nutrient concentrations, but they germinated well with high nutrient concentrations. These findings suggest that the levels of superdormant spores in populations depend on the germination conditions used, with fewer superdormant spores isolated when better germination conditions are used. These findings further suggest that superdormant spores require an increased signal for triggering spore germination compared to most spores in populations. One factor determining whether a spore is superdormant is its level of germinant receptors, since spore populations with higher levels of germinant receptors yielded lower levels of superdormant spores. A second important factor may be heat activation of spore populations, since yields of superdormant spores from non-heat-activated spore populations were higher than those from optimally activated spores.
Spores of various Bacillus species are formed in sporulation and are metabolically dormant and very resistant to environmental stress factors (21, 37). While such spores can remain in this dormant, resistant state for long periods, they can return to life rapidly through the process of germination, during which the spore's dormancy and extreme resistance are lost (36). Spore germination has long been of intrinsic interest, and continues to attract applied interest, because (i) spores of a number of Bacillus species are major agents of food spoilage and food-borne disease and (ii) spores of Bacillus anthracis are a major bioterrorism agent. Since spores are much easier to kill after they have germinated, it would be advantageous to trigger germination of spores in foods or the environment and then readily inactivate the much less resistant germinated spores. However, this simple strategy has been largely nullified because germination of spore populations is heterogeneous, with some spores, often called superdormant spores, germinating extremely slowly and potentially coming back to life long after treatments are applied to inactivate germinated spores (8, 9, 16). The concern over superdormant spores in populations also affects decisions such as how long individuals exposed to B. anthracis spores should continue to take antibiotics, since spores could remain dormant in an individual for long periods and then germinate and cause disease (3, 11).
In many species, spore germination can be increased by a prior activation step, generally a sublethal heat treatment, although the changes taking place during heat activation are not known (16). Spore germination in Bacillus species is normally triggered by nutrients such as glucose, amino acids, or purine ribosides (27, 36). These agents bind to germinant receptors located in the spore's inner membrane that are specific for particular nutrients. In Bacillus subtilis, the GerA receptor responds to l-alanine or l-valine, while the GerB and GerK receptors act cooperatively to respond to a mixture of l-asparagine (or l-alanine), d-glucose, d-fructose and K+ ions (AGFK [or Ala-GFK]) (1, 27, 36). There are even more functional germinant receptors in Bacillus megaterium spores, and these respond to d-glucose, l-proline, l-leucine, l-valine, or even salts, such as KBr (6). Glucose appears to trigger germination of B. megaterium spores through either of two germinant receptors, GerU or GerVB, while l-proline triggers germination through only the GerVB receptor, and KBr germination is greatly decreased by the loss of either GerU or GerVB (6). Nutrient binding to the germinant receptors triggers the release of small molecules from the spore core, most notably the huge depot (∼10% of spore dry weight) of pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA]) present in spores predominantly as a 1:1 diluted chelate with Ca2+ (Ca-DPA) (35, 36). Ca-DPA release then triggers the activation of one of two redundant cortex lytic enzymes (CLEs) that degrade the spore's peptidoglycan cortex, and cortex degradation completes spore germination and allows progression into outgrowth and then vegetative growth (27, 33, 36).
Spore germination can also be triggered by nonnutrient agents, including Ca-DPA and cationic surfactants (27, 33, 36). With B. subtilis spores, Ca-DPA triggers germination by activating one particular CLE, termed CwlJ, and bypasses the spore's germinant receptors. Germination by the cationic surfactant dodecylamine also bypasses the germinant receptors, and this agent appears to release small molecules including Ca-DPA from the spore core either by opening a normal channel in the spore's inner membrane for Ca-DPA and other small molecules or by creating such a channel (31, 38, 39).
Almost all work on the specifics of the germination of spores of Bacillus species has focused on the majority of spores in populations, and little detailed attention has been paid to that minority of spores that either fail to germinate or germinate extremely slowly. However, it is these latter spores that are most important in unraveling the cause of superdormancy and perhaps suggesting a means to germinate and thus easily inactivate such superdormant spores. Consequently, we have undertaken the task of isolating superdormant spores from spore populations, using buoyant density centrifugation to separate dormant spores from germinated spores. The properties of these purified superdormant spores were then studied, and this information has suggested some reason(s) for spore superdormancy.
MATERIALS AND METHODS
Bacillus strains used and spore preparation.
The B. megaterium strain used in this work was B. megaterium QM B1551 (ATCC 12872) (wild type). The B. subtilis strains used were as follows: (i) PS533 (wild type) carrying plasmid pUB110 encoding resistance to kanamycin (34); (ii) FB22, in which the GerA receptor is absent due to insertion of a spectinomycin resistance cassette in the gerA operon (1, 24); the operon encoding the GerB receptor also carries a mutation in the gerBA cistron such that the mutant GerB receptor, termed GerB*, responds to l-alanine or l-asparagine alone, and this response is stimulated by d-glucose; (iii) FB87 lacking both the GerB and GerK receptors and resistant to chloramphenicol and macrolide antibiotics (25); (iv) FB10 carrying a mutation in the gerBB cistron such that the GerB* receptor responds to l-alanine or l-asparagine alone, and this response is stimulated by d-glucose (1, 24); (v) PS3415 containing the gerB* operon from strain FB10 under the control of the very strong, forespore-specific sspB promoter, such that GerB* levels are elevated 200- to 400-fold in spores (4, 24); (vi) PS3502 that is identical to strain PS3415 but in which the gerB* operon is under the control of the strong sspD promoter and GerB* levels in spores are elevated ∼20-fold (4, 24); (vii) PS3665 with the gerBB* mutation from strain FB10 and lacking the GerA and GerK receptors (1); and (viii) PS3710 with the gerBA* mutation from strain FB22 and lacking the GerA and GerK receptors (1).
Spores of B. subtilis strains were prepared at 37°C on 2× Schaeffer's glucose medium plates without antibiotics and harvested, purified, and stored at 4°C protected from light as described previously (22). B. megaterium spores were prepared at 30°C in liquid supplemented nutrient broth medium without antibiotics and harvested, purified and stored at 4°C protected from light as described previously (22). All spores used in this work were free (>98%) of growing or sporulating cells, germinated spores, and cell debris, as determined by observation in a phase-contrast microscope.
Spore germination.
Unless otherwise noted, spores were germinated at an optical density at 600 nm (OD600) of ∼1 shortly after heat activation (75°C for 30 min and 60°C for 15 min for spores of B. subtilis and B. megaterium, respectively) of spores at an OD600 of ≥10 in water and cooling on ice. These heat activation temperatures were optimal for the spores of these two species (see Results). Unless otherwise noted, solutions used for germination were as follows: (i) glucose (10 mM d-glucose-25 mM KPO4 buffer [pH 7.4]); (ii) alanine (10 mM l-alanine-25 mM Tris-HCl buffer [pH 7.4]); (iii) valine (10 mM l-valine-25 mM Tris-HCl buffer [pH 7.4]); (iv) proline (5 mM l-proline-25 mM Tris-HCl buffer [pH 7.4]); (v) KBr (50 mM KBr-25 mM KPO4 buffer [pH 7.4]); (vi) AGFK (12 mM l-asparagine-13 mM d-glucose-13 mM d-fructose-12.5 mM KPO4 buffer [pH 7.4]); (vii) asparagine-glucose (5 mM l-asparagine-10 mM d-glucose in 25 mM Tris-HCl buffer [pH 8.4]); (viii) alanine-glucose (5 mM l-alanine-10 mM d-glucose in 25 mM Tris-HCl buffer [pH 8.4]); (ix) asparagine (6 mM l-asparagine in 25 mM KPO4 buffer [pH 7.4]); (x) dodecylamine (1.2 mM dodecylamine-25 mM KPO4 buffer [pH 7.4]); (xi) Ca-DPA (60 mM Ca-DPA [pH 7.4]-2.5 mM Tris-HCl buffer [pH 7.5]); and (xii) either LB medium (23) or 10× LB medium. In some experiments several of these germination solutions were combined, and in others, the concentrations of nutrient germinants, but not the buffers, were decreased to give moderate concentrations as follows: valine, 300 μM l-valine; AGFK, 3 mM l-asparagine-3.3 mM d-glucose-3.3 mM d-fructose-3.1 mM KPO4 buffer (pH 7.4); glucose, 200 μM d-glucose; asparagine-glucose, 300 μM l-asparagine-10 mM d-glucose; and proline, 250 μM l-proline. Germination of B. subtilis and B. megaterium spores with all agents except Ca-DPA and dodecylamine was at 37°C or 30°C, respectively. Germination of B. subtilis and B. megaterium spores with dodecylamine was at 45° and 37°C, respectively, and Ca-DPA germination was at 30°C. Germination with Ca-DPA and dodecylamine used spores that had not been heat activated (25, 31).
Spore germination was routinely followed by monitoring the OD600 of spore suspensions that falls by ∼55% during complete spore germination due to changes in the spore core's refractive index upon Ca-DPA release, and the further water uptake and the swelling of the core as germination are completed (27, 36). The approximate percentages of spores that had completed germination in these experiments were assessed by either phase-contrast microscopy or flow cytometry after staining with a nucleic acid stain that stains the core of only spores that have fully germinated (2). In dodecylamine germination, DPA release was measured to assess spore germination by monitoring the OD270 of the supernatant fluid from 1 ml of culture (31), with spores at an initial OD600 of 1.5. To assess Ca-DPA germination, ∼100 spores were examined by phase-contrast microscopy at the end of germination experiments, since the phase-bright dormant spores become phase dark upon completion of spore germination.
The different methods used to monitor spore germination measure somewhat different aspects of the overall process. An assay of DPA release measures this event alone, while phase-contrast microscopy assesses the completion of germination, including cortex lysis and full spore core swelling; flow cytometry using a nucleic acid stain also measures the completion of spore germination. However, loss of OD600 is due in part (∼50%) to the decrease in the core's refractive index following release of Ca-DPA and its replacement by water, with the core swelling and further water uptake that accompanies cortex hydrolysis causing the remainder of the decrease in OD600 (32, 36). Since there can be significant loss in OD600 during germination even if the cortex is not hydrolyzed (32), it was important to always estimate the degree of completion of spore germination by using either phase-contrast microscopy or flow cytometry.
Isolation of superdormant spores.
For isolation of superdormant spores, germination was routinely done with 0.2 to 1 liters of spores at an OD600 of 1 as described above. After ∼45 min (experiment with 10× LB medium; when spore outgrowth began) or ∼2 h (all other experiments), the culture was harvested by centrifugation, and the pellet was washed with 5 to 10 ml water, recentrifuged, and suspended in 200 to 600 μl of 20% Nycodenz (Sigma, St. Louis, MO). Aliquots of the suspension (∼100 μl) were layered on a solution of 50% Nycodenz in 2-ml ultracentrifuge tubes, and the tubes were centrifuged for 45 min at 13,000 × g. Under these conditions, dormant spores pellet, and germinated spores float (7, 19). The dormant spores were removed, washed with water to remove Nycodenz, and resuspended in water, the OD600 values were determined, and the spores were heat activated again, since heat activation is reversible (16), and germinated again as was done initially. The germinating culture was again harvested, dormant spores were isolated by buoyant density centrifugation as described above, and the final dormant spore pellet was washed several times with water, suspended in 1 ml of water, and stored at 4°C. Since heat activation is reversible (16), germination of isolated superdormant spores was routinely preceded by heat activation.
Spore viability.
Spore viability was assessed by spotting appropriate dilutions of heat-shocked spore suspensions at an OD600 of 1 on LB medium plates (23) with the appropriate antibiotics and by incubating the plates at 30°C (B. megaterium) or 37°C (B. subtilis) for 16 to 24 h and counting colonies. Initial dormant B. megaterium and B. subtilis spore suspensions at an OD600 of 1 had 5 × 107 and 1.2 × 108 CFU/ml, respectively.
RESULTS
Isolation of superdormant spores.
For isolation of superdormant spores, we took advantage of the fact that dormant spores have a very high core wet density, and thus pellet during centrifugation in a buoyant density medium in which germinated spores float (7, 19). Wild-type B. subtilis (strain PS533) and B. megaterium spores were heat activated and germinated for long periods of time with high levels of the nutrient germinants l-valine (B. subtilis) and d-glucose (B. megaterium) that give high rates and extents of spore germination, the remaining dormant spores were isolated by buoyant density centrifugation, and the process was repeated. This procedure yielded 3 to 4% of initial spores as forms that appeared dormant in the phase-contrast microscope and had normal DPA levels (Table 1 and data not shown; also, see below). For the purposes of this work, the spores isolated in this manner will be termed superdormant, although as is perhaps not surprising (8, 9), yields of superdormant spores were dependent on the nature of the nutrient germinants used for their isolation (see below). The putative superdormant spores isolated as described above germinated poorly if at all with the germinants used to isolate them, in comparison with the rapid and largely complete germination of the initial spore populations with these germinants (Fig. 1A and C). Superdormant spores were also isolated from B. subtilis PS533 spores, using AGFK to trigger spore germination through cooperative action between the GerB and -K receptors (1, 27, 36). The yield of superdormant spores isolated by germination with AGFK was significantly higher than with valine (Table 1), but these spores still germinated poorly with AGFK (Fig. 1B).
TABLE 1.
Yields of superdormant spores with different nutrient germinantsa
| Species/strain | Germinante | Yield (%) |
|---|---|---|
| B. subtilis PS533 (wild type) | Valineb | 3.8c |
| AGFK | 12 | |
| 10× LB medium | 0.7 | |
| AGFK plus valineb | 0.6 | |
| Moderate valineb,d | 58 | |
| B. subtilis FB22 (ΔgerA gerBA*) | Asparagine-glucose | 8.7c |
| 10 mM glucose plus low asparagined | 63 | |
| B. subtilis FB87 (ΔgerB ΔgerK) | Valineb | 14.5 |
| B. subtilis PS3665 (ΔgerA gerBB* ΔgerK) | Asparagine | 5.7 |
| B. subtilis PS3710 (ΔgerA gerBA* ΔgerK) | Asparagine | 10.6 |
| B. megaterium (wild type) | Glucose | 3.5c |
| 10× LB medium | 0.5 | |
| Moderate glucosed | 38 |
Superdormant spores of various strains were prepared using various nutrients, and the yields of superdormant spores were determined, as described in Materials and Methods.
l-Valine was used in these isolations instead of l-alanine to avoid problems due to inhibition of germination because of generation of inhibitory d-alanine by alanine racemase (5).
These values were determined in triplicate with less than 20% variation in the values obtained.
Isolation of these superdormant spores began with 100 ml of germinating spores at an OD600 of 1.
Normal and moderate nutrient germinant levels are given in Materials and Methods.
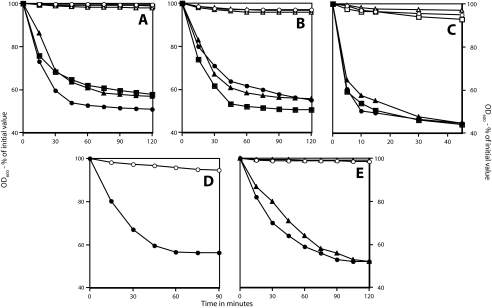
FIG. 1.
Germination of superdormant and initial spore preparations from various strains isolated using high levels of nutrients that target one or two germinant receptors. Superdormant spores of B. subtilis strain PS533 (wild type) (A and B), B. megaterium (C), and B. subtilis strains FB87 (ΔgerB ΔgerK) and FB22 (ΔgerA gerBA*) (D and E, respectively) were isolated by germination with valine (A and D), AGFK (B), glucose (C), or asparagine-glucose (E), as described in Materials and Methods. These spores were then germinated, spore germination was monitored by following the OD600 of the culture, and the extent of spore germination at the end of the experiment was determined as described in Materials and Methods. Closed symbols indicate the initial spores used; open symbols represent the isolated superdormant spores. (A) Circles, germination with alanine; triangles, germination with valine; and squares, germination with AGFK. (B) Circles, germination with AGFK; triangles, germination with valine; and squares, germination with alanine. (C) Circles, germination with glucose; triangles, germination with KBr; and squares, germination with proline. (D) Circles, germination with valine. (E) Circles, germination with asparagine-glucose; triangles, germination with alanine-glucose. The appropriate percentages of germinated spores in the various germinations were as follows. (A) Open symbols, <5%; •, 90%; ▴ and ▪, 80%. (B) Open symbols, <10%; closed symbols, 80 to 90%. (C) Open symbols, 10%; closed symbols, >90%. (D) ○, 10%; •, 80%. (E) ○ and ▵, <5%; • and ▴, 85%.
Superdormant spores were also isolated from B. subtilis spores that lacked various germinant receptors, including the following: FB87 spores that lack the GerB and -K receptors; FB22 spores that lack the GerA receptor and contain the modified GerB* receptor, allowing these spores to germinate with asparagine or alanine alone (24); and PS3665 and PS3710 spores that carry the gerBB* and gerBA* mutations, respectively, and lack the GerA and -K receptors. The isolation of superdormant spores from strain FB87 spores used valine as the germinant; for FB22 spores, a mixture of asparagine and glucose was used, as glucose stimulates spore germination with the GerB* receptor through cooperative action with the GerK germinant receptor; and asparagine alone was used to isolate PS3665 and PS3710 superdormant spores (1, 24). These germinant receptor-deficient spores gave 6 to 16% superdormant spores (Table 1), significantly higher yields than those obtained from spores that retained their normal complement of germinant receptors. Again, these superdormant spores exhibited little or no germination with the germinants used for their isolation (Fig. 1D and E; also data not shown). For all the superdormant spores isolated as described above, increasing levels of nutrient germinants to 50 mM for l-valine, 100 mM for d-glucose, or 30 mM for l-asparagine still did not trigger their germination (data not shown).
Germination of superdormant spores with nutrient germinants not used in their isolation.
While superdormant spores that did not germinate with germinants targeting one or two germinant receptors were readily isolated, an obvious question was whether these superdormant spores were also defective in germination with other germinants recognized by the initially targeted germinant receptor or with germinants recognized by other germinant receptors. The answer to this question was clearly yes. Superdormant B. subtilis PS533 spores isolated by germination with valine were defective in germination with alanine that also utilizes the GerA germinant receptor, as well as in AGFK germination that utilizes the GerB and -K germinant receptors (Fig. 1A). Similarly, the superdormant PS533 spores isolated by germination with AGFK not only did not germinate with AGFK but also germinated poorly with alanine or valine (Fig. 1B). The superdormant PS533 spores isolated by germination with valine or AGFK also exhibited poorer germination with LB medium than did the initial dormant spores (Fig. 2A), even though LB medium contains nutrients that can trigger spore germination by any of the B. subtilis spore's three functional germinant receptors, at least to some degree (12, 25). Superdormant FB22 spores isolated by germination with asparagine-glucose also did not germinate with alanine-glucose and exhibited less germination with LB medium than did PS533 dormant spores isolated by germination with valine (Fig. 1E and 2A). In addition, superdormant B. megaterium spores isolated by germination with glucose exhibited only minimal germination with proline, KBr, valine, or even LB medium, all of which gave rapid germination of the initial dormant spores (Fig. 1C and 2B; also data not shown).
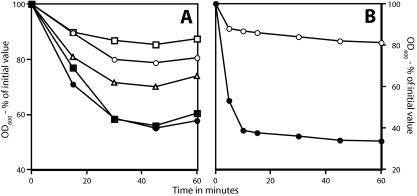
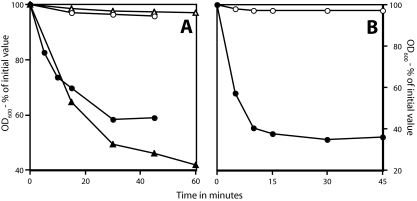
FIG. 2.
Germination of superdormant and initial spores with LB medium. Spores of B. subtilis strains PS533 (wild type; ○, •, and ▵) and FB22 (ΔgerA gerBA*) (squares) (A) and B. megaterium spores (wild type; circles) (B), either initial (• and ▪) or superdormant (○, ▵, and □), isolated by germination with valine (PS533; ○), AGFK (▵), asparagine-glucose (FB22; □), or glucose (B. megaterium; ○) were germinated with LB medium, and the OD600 of the culture was measured to follow spore germination. The extents of germination at the end of the incubations were as follows. (A) ○, 25%; ▵, 50%; □, 15%; •, 95%; ▪, 90%. (B) ○, 25%; •, 95%. The rise in OD600 toward the end of the germinations in panel A is due to the spore outgrowth that takes place in this complete medium.
Viability and germination of superdormant spores with nonnutrient germinants.
One possible explanation for the lack of germination of the superdormant spores described above is that these spores have severe damage to an essential component of the germination apparatus such that they are incapable of germinating. Indeed, the apparent viabilities of the superdormant B. subtilis FB22 and PS533 spores and the B. megaterium spores isolated by germination with asparagine-glucose, valine, or glucose were only 10 to 20% of the initial dormant spores, respectively, on LB medium plates (Table 2).
TABLE 2.
Apparent viability of superdormant spores prepared with different nutrient germinantsb
| Species/strain | Germinant | Apparent viability (%)a
|
|
|---|---|---|---|
| Without Ca-DPA | With Ca-DPA | ||
| B. subtilis PS533 (wild type) | Valine | 23 | 95 |
| B. subtilis FB22 (ΔgerA gerBA*) | Asparagine-glucose | 13 | 97 |
| B. megaterium (wild type) | Glucose | 9 | 75 |
Apparent viability is expressed relative to that of initial wild-type spores without Ca-DPA treatment.
Superdormant spores were isolated using normal nutrient germinant levels, as described in Materials and Methods, and apparent spore viability was assessed on LB plates relative to the OD600 values of spore suspensions, as described in Materials and Methods.
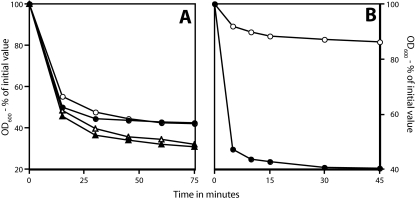
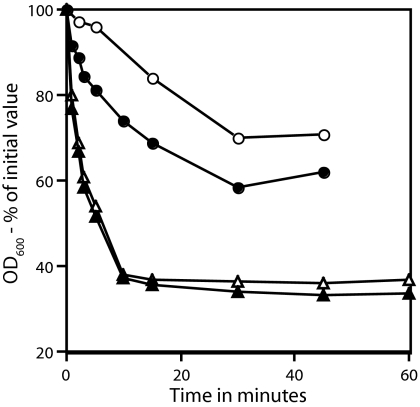
While the low apparent viability of the superdormant spores was consistent with a defect in these spores, it was possible that these spores were, in fact, fully viable but could not germinate efficiently with nutrient germinants. In addition to nutrient germinants, there are several nonnutrient germinants for spores of Bacillus species that can bypass at least part of the spore's normal germination apparatus, the germinant receptors in particular (1, 36). Ca-DPA appears to act as a germinant by directly stimulating cortex phosphatidylglycerol hydrolysis by the CLE CwlJ, while the cationic surfactant dodecylamine triggers Ca-DPA release directly from the spore core (33, 36). Strikingly, germination of the superdormant B. subtilis and B. megaterium spores with Ca-DPA and dodecylamine was indistinguishable from that of wild-type spores (Fig. 3A to D). In addition, prior Ca-DPA treatment raised the viability of superdormant spores to close to that of wild-type spores (Table 2). One other notable finding in the work using dodecylamine to trigger spore germination was that the superdormant spores had the same DPA content as the original dormant spores (Fig. 3C and D).
FIG. 3.
Germination of superdormant and initial spores with Ca-DPA (A and B) or dodecylamine (C and D). Spores of B. subtilis strains PS533 (wild type) and FB22 (ΔgerA gerBA*) (A and C) or B. megaterium (B and D) were germinated with either Ca-DPA or dodecylamine, and germination was followed as described in Materials and Methods. The superdormant PS533, FB22, and B. megaterium spores were isolated by germination with valine, asparagine-glucose, or glucose, respectively. (A and C) ○ and •, PS533 spores; ▵ and ▴, FB22 spores. (B and D) ○ and •, B. megaterium spores. In all panels, ○ and ▵ indicate superdormant spores, and • and ▴ represent initial dormant spores. It was notable in the experiments using dodecylamine as a germinant that both initial and superdormant spores had essentially identical (within 15%) DPA levels as determined by the amount of OD270-absorbing material released from spores (data not shown).
The germination defect in superdormant spores is not due to a genetic change.
The finding that the viability and germination of the superdormant B. megaterium and B. subtilis spores were at wild-type levels if spores were treated with Ca-DPA allowed us to test the possibility that the germination defect in the superdormant spores was due to a genetic change. Superdormant B. subtilis PS533 spores (1 ml) isolated after valine germination and B. megaterium spores isolated after glucose germination were germinated for 60 min at an OD600 of 1 with Ca-DPA, and an appropriate dilution was spread onto LB medium plates. After incubation for ∼24 h to allow colonies to appear, 10 separate single colonies were isolated, each was sporulated on an agar plate, the spores were harvested and purified, and their germination with valine (B. subtilis) or glucose (B. megaterium) was tested. Strikingly, the spores from all 10 separate colonies from the Ca-DPA-treated superdormant spores germinated essentially identically to the initial dormant spore population (data not shown). Thus, the superdormancy of at least the great majority of the spores of B. megaterium and B. subtilis is not due to a genetic change in the superdormant spores.
Germination of superdormant spores with mixtures of germinants.
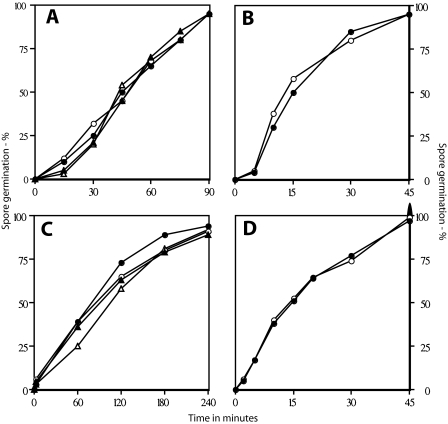
The recovery of the superdormant spores by germination with Ca-DPA and the evidence that they had not accumulated some genetic damage suggested that these superdormant spores might be simply unable to respond properly to signals generated by stimulation of one or at most two germinant receptors; perhaps these spores would respond better to a mixture of germinants that can trigger spore germination through even more germinant receptors. Indeed, superdormant B. subtilis PS533 spores isolated by germination with valine or AGFK germinated rapidly and almost completely with mixtures of valine and AGFK or alanine and AGFK (Fig. 4A). These superdormant spores also germinated moderately well with LB medium as noted above (Fig. 2A) and even better with 10× LB medium (Fig. 5; also data not shown). Superdormant B. megaterium spores isolated after germination with glucose also germinated somewhat with a mixture of glucose, proline, and KBr, although not as well as the initial dormant spores (Fig. 4B), and germinated as well as the initial dormant spores did with 10× LB medium (Fig. 5).
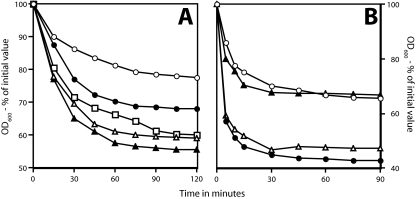
FIG. 4.
Germination of initial and superdormant spores with germinant mixtures. Spores of B. subtilis PS533 (wild type) (A) or B. megaterium (wild type) (B) were isolated by germination with valine (B. subtilis) or glucose (B. megaterium). The initial (• and ▴) and superdormant (○ and ▵) spores were then germinated with valine plus AGFK (panel A, ○ and •) or alanine plus AGFK (panel A, ▵ and ▴) and glucose, KBr, and proline (B), and spore germination was followed by monitoring the OD600 of the culture. The extents of spore germination were 80% (○ and •) and 90% (▵ and ▴) (A) and 20% (○) and 95% (•) (B).
FIG. 5.
Germination of initial and superdormant spores with 10× LB medium. B. subtilis PS533 (wild type; ○ and •) and B. megaterium (wild type; ▵ and ▴) spores, either initial spore preparations (• and ▴) or superdormant spores isolated by germination with l-valine (○) or d-glucose (▵), were germinated with 10× LB medium; spore germination was followed by measuring the OD600, and the extent of germination was measured, as described in Materials and Methods. The extents of spore germination at the end of incubations were 60% (○), 75% (•), and 90% (▵ and ▴). The increase in OD600 in the B. subtilis cultures is due to spore outgrowth in this complete medium.
One prediction from the results noted above was that if wild-type B. subtilis spores were germinated with either 10× LB medium or a mixture of l-valine and AGFK and B. megaterium spores were germinated with 10× LB medium, yields of superdormant spores would be much lower than those obtained with single germinants. Indeed, yields of superdormant spores following germination with 10× LB medium (B. megaterium and B. subtilis spores) or valine plus AGFK (B. subtilis spores) were 5- to 10-fold lower than when nutrients targeting a single germinant receptor were used (Table 1). However, superdormant spores isolated using the complex rich medium or germinant mixture still germinated extremely poorly with the germinants used in their isolation, even in 10× LB medium (Fig. 6A and B).
FIG. 6.
Germination of initial spores and superdormant spores isolated by germination with nutrient mixtures. Spores of B. subtilis PS533 (wild type) (A) or B. megaterium either initial (• and ▴) or superdormant (○ and ▵) spores (B) isolated by germination with 10× LB medium (○) or AGFK plus valine (▵) were germinated with 10× LB medium (○ and •) or AGFK plus valine (▵ and ▴). The progress of spore germination was assessed by monitoring the OD600 of cultures, and the extents of germination were determined, as described in Materials and Methods. The extents of spore germination in the various cultures were <5% (○ and ▵) and 95% (• and ▴) (A) and <5% (○) and 95% (•) (B). The increase in OD600 in the initial B. subtilis spores germinating in 10× LB medium is due to the outgrowth in this complete medium.
Isolation and characterization of dormant spores remaining after germination with moderate germinant levels.
The data given above indicated that yields of superdormant spores were lower when better nutrient germinants or germinant mixtures were used to isolate these spores. This observation further suggested that if superdormant spores were isolated using moderate germinant concentrations, then the yields of the superdormant spores should be higher. Indeed, when superdormant spores were isolated by germination with concentrations of valine or AGFK (B. subtilis PS533), asparagine-glucose (B. subtilis FB22), or glucose (B. megaterium) giving 20 to 60% germination, the yields of superdormant spores increased 5- to 10-fold (Table 1). The superdormant spores isolated by these moderate nutrient concentrations now germinated minimally, if at all, with these same nutrient concentrations, in contrast to the initial dormant spores (Table 3). However, these superdormant spores germinated almost as well as the initial dormant spores with high concentrations of these respective nutrients (Table 3).
TABLE 3.
Germination of initial and superdormant spores prepared with moderate germinant concentrationsa
| Spore used | SD spore prepn | Germination in | Spore germination (%/2 h)
|
|
|---|---|---|---|---|
| SD | Initial | |||
| B. subtilis wt | 300 μM l-val | 300 μM l-val | 5 | 50 |
| B. subtilis wt | 300 μM l-val | 5 mM l-val | 75 | 90 |
| B. subtilis wt | 1/4 AGFK | 1/4 AGFK | <5 | 60 |
| B. subtilis wt | 1/4 AGFK | AGFK | 80 | 90 |
| B. subtilis gerA gerBA* | 200 μM l-asn | 200 μM l-asn | 5 | 60 |
| B. subtilis gerA gerBA* | 200 μM l-asn | 5 mM l-asn | 70 | 90 |
| B. megaterium | 200 μM d-glucose | 200 μM d-glucose | <5 | 70 |
| B. megaterium | 200 μM d-glucose | 10 mM d-glucose | 80 | 90 |
Superdormant spores of B. subtilis wild-type (PS533) and gerA gerBA* (FB22) strains and B. megaterium were prepared with moderate germinant concentrations, initial and superdormant spores were germinated with either the moderate germinant concentration or the high normal germinant concentration, and extents of spore germination after 2 h of incubation were determined, as described in Materials and Methods. Note that 10 mM d-glucose was present in all germinations with l-asparagine. SD, superdormant; wt, wild type; l-val, l-valine; l-asn, l-asparagine.
The superdormant B. subtilis spores isolated with a moderate concentration of l-valine also germinated more poorly than did the initial spores with a low concentration of AGFK, although both types of spores germinated relatively similarly with the normal high AGFK concentration (Table 4). Similarly, superdormant B. megaterium spores isolated with a low concentration of d-glucose germinated poorly with a low concentration of l-proline, although they germinated almost as well as the initial spores with the normal high proline concentration (Table 4).
TABLE 4.
Germination of initial and superdormant spores prepared with moderate germinant concentrations by germinants targeting different germinant receptorsa
| Spore used | SD spore prepn | Germination in | Spore germination (%/2 h)
|
|
|---|---|---|---|---|
| SD | Initial | |||
| B. subtilis wt | 300 μM l-val | 1/4 AGFK | <3 | 40 |
| B. subtilis wt | 300 μM l-val | AGFK | 65 | 80 |
| B. megaterium | 200 μM d-glucose | 250 μM l-pro | <5 | 40 |
| B. megaterium | 200 μM d-glucose | 10 mM l-pro | 80 | 90 |
Superdormant spores of B. subtilis wild-type (PS533) and B. megaterium were prepared with moderate germinant concentrations, initial and superdormant spores were germinated with either a moderate or the normal high germinant concentration targeting a different germinant receptor(s) than that targeted in superdormant spore isolation, and extents of spore germination after 2 h of incubation were determined, as described in Materials and Methods. SD, superdormant; wt, wild type; l-val, l-valine; l-pro, l-proline.
Effect of germinant receptor levels on yields of superdormant spores.
There are a number of possible explanations for the variation in the yields of superdormant spores, depending on the type of germinant receptor stimulated or the nutrient germinant levels used for superdormant spore isolation. However, a simple hypothesis is that a major determinant of the yields of superdormant spores when a particular germinant receptor is stimulated is the level of that particular receptor, as well as any heterogeneity in receptor numbers between individual spores. This is made even more likely by the observations that (i) the level of at least the GerB receptor in B. subtilis spores is quite low, averaging ≤25 molecules per spore (26), and (ii) increasing average numbers of GerB* or GerA receptors significantly increase rates of spore germination with these germinant receptors' ligands (1, 4). To test whether changes in levels of germinant receptors have effects on the yields of superdormant spores, we used spores of three isogenic B. subtilis strains, FB10, PS3502, and PS3415, in which levels of the GerB* receptor are wild type (FB10), elevated ∼20-fold (PS3502), and elevated 200- to 400-fold (PS3415) (26), and isolated superdormant spores by germination with asparagine. Strikingly, the yields of superdormant spores went down markedly as the levels of the GerB* receptor in spores increased (Table 5).
TABLE 5.
Effect of germinant receptor levels on yields of superdormant sporesa
| Bacillus sp. strain used | Relative GerB* level in sporesb | Yield of superdormant spores (%) |
|---|---|---|
| B. subtilis FB10 (gerBB*) | 1 | 1.9 |
| B. subtilis PS3502 (PsspD::gerBB*) | 20 | 0.2 |
| B. subtilis PS3415 (PsspB::gerBB*) | 200-400 | <0.01 |
Spores of various strains were germinated with asparagine, superdormant spores were isolated, and their yields were determined, as described in Materials and Methods.
Values for relative levels of GerB* in spores of these strains are from the literature (26).
Effect of heat activation on yields of superdormant spores.
Another factor that almost certainly will affect levels of superdormant spores is the degree of spore activation, generally by a sublethal heat treatment, since nonactivated spores often germinate extremely poorly (16). Indeed, B. subtilis spores that were not heat activated germinated more poorly with l-valine or AGFK than did heat-activated spores, with a 30-min treatment at 75°C giving maximal germination (Fig. 7A; also data not shown). Similar results were obtained with B. megaterium spores for glucose and proline germination, although 15 min at 60°C gave optimal germination (Fig. 7B; also data not shown). As predicted from these results, yields of superdormant spores were much higher from B. megaterium and B. subtilis spores that were not heat activated than from optimally activated spores (Table 6).
FIG. 7.
Effect of heat activation at various temperatures on germination of B. subtilis (A) and B. megaterium (B) spores. Spores of B. subtilis PS533 (wild type) and B. megaterium were either not heat activated (○) or heat activated for 30 min at 60°C (•), 70°C (▵), 75°C (▴), or 80°C (□) and germinated with l-valine (B. subtilis) or for 15 min at 60°C (•), 65°C (▵), or 70°C (▴) and germinated with d-glucose (B. megaterium). Germination was followed, and the extents of germination were determined, as described in Materials and Methods. The percentages of germinated spores at the end of the various germinations were 40% (○), 50% (•), 70% (▵), 85% (▴), and 60% (□) (A) and 60% (○), 95% (•), 85% (▵), and 65% (▴) (B).
TABLE 6.
Effect of heat activation on yields of superdormant sporesa
| Bacillus sp. strain used | Germinant used | Yield of superdormant spores (%) with:
|
|
|---|---|---|---|
| No activation | Heat activation | ||
| B. megaterium wild type | d-Glucose | 36 | 5 |
| B. megaterium wild type | l-Proline | 47 | 5.5 |
| B. subtilis wild type | AGFK | 40 | 13 |
| B. subtilis wild type | l-Valine | 18 | 4 |
Spores of various species with or without optimal heat activation were germinated with various agents, superdormant spores were isolated, and their yields were determined, as described in Materials and Methods.
DISCUSSION
The results in this communication provide a number of new insights into the causes of superdormancy in spores of Bacillus species and the process of spore germination itself. Clearly, the definition of a superdormant spore is a relative one and is dependent on the germinant used for isolation of these spores, as well as the germinant receptor(s) used to recognize the germinants used. Thus, low levels of nutrients gave higher yields of superdormant spores than did high nutrient levels, and nutrient mixtures that target multiple germinant receptors gave lower yields of superdormant spores than those obtained with germinants targeting only one or two germinant receptors. In all cases, however, the isolated superdormant spores responded poorly not only to the nutrient concentrations used to isolate the superdormant spores, but also to nutrients that targeted other germinant receptors.
The observations summarized above should be considered in light of a number of several others, including the following: (i) high levels of nutrients targeting one or more germinant receptors gave higher extents of spore germination than did low levels of the same nutrients, and (ii) mixtures of nutrients effectively triggered the germination of superdormant spores isolated by germination with simple nutrient germinants. Together, these observations suggest that spores in populations are a heterogenous mix of individuals with different capacities to respond to nutrient germinants. Some spores germinate with low nutrient concentrations, while others respond only to high concentrations. For some spores, stimulation of a single germinant receptor is sufficient to trigger germination, while others require activation of multiple germinant receptors.
What is the cause of this huge variation in ability of individual spores in a population to germinate with different levels of germinants or utilize one or only multiple germinant receptors? An obvious possibility is that this is because of natural stochastic variation in levels or activities of an essential component of the spore germination apparatus. At present, four such essential components for B. subtilis spore germination are known, as follows: (i) one or both of the redundant CLEs, CwlJ and SleB; (ii) the channels that allow DPA release in stage I of germination that may be composed of SpoVA proteins (28, 38, 39); (iii) the GerD protein that greatly stimulates Bacillus sp. spore germination with agents that target the germinant receptors (29, 30); and (iv) the germinant receptors. It appears unlikely that the culprit is the CLEs, since superdormant spores germinated normally with exogenous Ca-DPA, and this agent triggers germination by activating CwlJ (36). In addition, CwlJ alone is sufficient for hydrolysis of the spore cortex (24, 33, 36), and the OD600 loss by superdormant spores when they are germinated with the germinants used for their isolation was so low that these spores cannot even be releasing significant DPA, since ∼50% of the fall in OD600 during spore germination of Bacillus sp. spores is due to DPA release (13, 32, 33). It also seems very unlikely that a defect in DPA channels causes the heterogeneity in germination, because (i) DPA release in Ca-DPA germination of superdormant spores was identical to that in the initial dormant spores, since both completed germination at the same rate; (ii) dodecylamine germination was also identical for both the superdormant spores and the initial dormant spores, and dodecylamine germination may proceed via activation of the normal DPA channel in spores (36); and (iii) the level of SpoVA proteins in spores appears to be relatively high at ∼15,000 molecules/spore, based on levels of SpoVAD (38).
In contrast to the two possibilities discussed above, the third one, that variations in GerD levels are responsible for heterogeneity in spore germination, with low GerD levels giving rise to superdormant spores, seems more likely. Loss of GerD increases the heterogeneity in nutrient-triggered spore germination, most likely by greatly increasing the lag time between the addition of nutrients and the initiation of Ca-DPA release (29). Although how GerD modulates the length of the lag time in nutrient germination is not known, GerD has no effects on spore germination with either Ca-DPA or dodecylamine, consistent with the normal germination of superdormant spores with these two agents. However, one GerD property that is less consistent with variations in GerD levels being the cause of germination heterogeneity is that there are on average ∼2,000 GerD molecules/spore (30). This number may be too high for stochastic variation in GerD levels to be significant. Unfortunately, there have been, as of yet, no studies of the effects of variation in GerD levels below those in wild-type spores on nutrient germination rates.
The fourth possibility noted above is that it is the variation in levels of the spores' germinant receptors that causes the heterogeneity in spore germination rates. This is consistent with (i) the likely low average numbers of germinant receptors per spore noted above, (ii) the marked decrease in the yields of superdormant spores when the GerB* receptor was overexpressed, and (iii) the increases in spore germination rates with germinants that target the GerA or GerB* receptors when these receptors are overexpressed (4). Perhaps there is significant stochastic variation in the normally small average numbers of germinant receptors/spore (∼25 for the GerB receptor based on the average numbers of GerBA protein/spore) (26) that leads to a significant percentage of spores with very small numbers of receptors, and these spores germinate poorly with nutrient germinants. Indeed, there is often significant stochastic variation in numbers of low abundance proteins in bacteria, due to variations in either transcription or translation of poorly expressed genes (14, 20). In addition, average numbers of various germinant receptors may be even lower than those determined by Western blot analysis of single receptor proteins, since there is evidence that receptors may be oligomers of individual receptor proteins (26). It also appears likely that different receptors interact to some degree, perhaps physically (6, 12), and such receptor aggregation could amplify signals from large numbers of germinant receptors and cause decreased signals from small numbers of germinant receptors, much as is the case for the aggregates of the chemotaxis receptors in bacteria (10, 15). The fact that different germinant receptors may aggregate and that this aggregate may amplify germination signals is consistent with the generally higher yields of superdormant spores from spores of strains that lack one or more germinant receptors.
While it is attractive to imagine that variations in the levels of germinant receptors cause the germination heterogeneity in spore populations, with spores with low receptor levels being less responsive to germinant signals and thus more likely to be superdormant, it is not clear why this should be the case. Is it the low levels of germinant receptors that are the cause, perhaps because of an insufficient germination signal, or is a higher threshold for this signal needed to elicit germination in some spores in a population, those most likely to be in the superdormant category? Unfortunately, we do not know how ligand binding to germinant receptors triggers spore germination. It has been suggested that signals from different germinant receptors are collected by a signal integrator that triggers spore germination, when the total signal rises over some threshold (1). However, there is no direct evidence for the existence of such an integrator, although its existence, even if hypothetical, explains much of the cooperative behavior of many of the spores' germinant receptors (1). It is also noteworthy that some works examining the germination of individual spores of a number of Bacillus species have indicated that the heterogeneity in germination is almost exclusively in the lag period between the addition of a nutrient germinant and the initiation of rapid DPA release (5; D. Chen, L. Peng, P. Setlow, and Y.-Q. Li, unpublished results). However, the identities of events taking place in this lag period and the factors determining its length are not known.
An additional factor that determines the level of superdormant spores in populations is the degree of spore activation (16), with heat activation used in the current work. Non-heat-activated spores gave much higher yields of superdormant spores than did optimally activated populations, with yields of superdormant spores from non-heat-activated spores being three- to eightfold higher. While we do not know the molecular changes in spores caused by heat activation, this treatment seems likely to act only via the germinant receptor pathway for spore germination, since neither Ca-DPA nor dodecylamine germination is stimulated by heat activation (16, 23, 29). Thus, levels of superdormant spores in populations may be determined by the number of germinant receptors per spore and the accessibility and responsiveness of these receptors, with the latter two perhaps greatly increased in some way by heat activation.
Whatever the cause of the heterogeneity of the rates and the extents of the germination of spores of Bacillus species, it is likely that this is an important adaptation to the spore formers' environment. Having all spores in a population germinate at the same rate and to the same extent would make it simple to study spore germination in the laboratory but, in the environment, would likely place spore populations at risk, since all spores might germinate in what are actually not favorable conditions. Having many spores in a population that germinate much slower than the majority provides insurance against such an undesirable outcome, thus making the survival of the population more likely. It is also possible that the elucidation of the cause(s) of heterogeneity in spore germination may give us deeper insight into the mechanism of spore germination itself.
The presence of generally small percentages of superdormant forms in populations of spores of Bacillus species is also another example of phenotypic variation in isogenic bacterial populations. Other examples of this phenomenon include antibiotic-resistant persister cells and competence development in B. subtilis (17, 18). In all of these cases, the small fractions of phenotypically different cells appear to be genetically identical to the population as a whole, and the epigenetic adaptations giving rise to the different cells have obvious survival advantage. It may be most illuminating in the future to compare the mechanisms used to generate these different phenotypically variable states.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (GM19698) (to P.S.).
B. Setlow assisted on some experiments.
Footnotes
Published ahead of print on 9 January 2009.
REFERENCES
- 1.Atluri, S., K. Ragkousi, D. E. Cortezzo, and P. Setlow. 2006. Cooperativity between different nutrient receptors in germination of spores of Bacillus subtilis and reduction of this cooperativity by alterations in the GerB receptor. J. Bacteriol. 18828-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black, E. P., K. Koziol-Dube, D. Guan, J. Wei, B. Setlow, D. E. Cortezzo, D. G. Hoover, and P. Setlow. 2005. Factors influencing the germination of Bacillus subtilis spores via the activation of nutrient receptors by high pressure. Appl. Environ. Microbiol. 715879-5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brookmeyer, R., E. Johnson, and R. Bollinger. 2003. Modeling the optimum duration of antibiotic prophylaxis in an anthrax outbreak. Proc. Natl. Acad. Sci. USA 10010129-10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cabrera-Martinez, R.-M., F. Tovar-Rojo, V. Ramana Vepachedu, and P. Setlow. 2003. Effects of overexpression of nutrient receptors on germination of spores of Bacillus subtilis. J. Bacteriol. 1852457-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, D., S.-S. Huang, and Y.-Q. Li. 2006. Real-time detection of kinetic germination and heterogeneity of single Bacillus spores in aqueous solution by confocal laser tweezers Raman spectroscopy. Anal. Chem. 786936-6941. [DOI] [PubMed] [Google Scholar]
- 6.Christie, G., and C. R. Lowe. 2007. Role of chromosomal and plasmid-borne receptor homologues in the response of Bacillus megaterium QM B1551 spores to germinants. J. Bacteriol. 1894375-4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman, W. H., D. Chen, Y.-Q. Li, A. E. Cowan, and P. Setlow. 2007. How moist heat kills spores of Bacillus subtilis. J. Bacteriol. 1898458-8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gould, G. W. 1969. Spore germination, p. 397-444. In G. W. Gould and A. Hurst (ed.), The bacterial spore. Academic Press, New York, NY.
- 9.Gould, G. W. 1970. Germination and the problem of dormancy. J. Appl. Bacteriol. 3334-49. [DOI] [PubMed] [Google Scholar]
- 10.Hazelbauer, G. L., J. J. Falke, and J. S. Parkinson. 2008. Bacterial chemoreceptors: high-performance signaling in networked arrays. Trends Biochem. Sci. 339-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heine, H. S., J. Bassett, L. Miller, J. M. Hartings, B. E. Irvine, M. L. Pitt, D. Fritz, S. L. Norris, and W. R. Byrne. 2007. Determination of antibiotic efficacy against Bacillus anthracis in a mouse aerosol challenge model. Antimicrob. Agents Chemother. 511373-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Igarashi, T., and P. Setlow. 2005. Interaction between individual protein components of the GerA and GerB nutrient receptors that trigger germination of Bacillus subtilis spores. J. Bacteriol. 1872513-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishikawa, S., K. Yamane, and J. Sekiguchi. 1998. Regulation and characterization of a newly deduced cell hydrolase gene (cwlJ) which affects germination of Bacillus subtilis spores. J. Bacteriol. 1801375-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaern, M., T. C. Elston, W. J. Blake, and J. J. Collins. 2005. Stochasticity in gene expression: from theories to phenotypes. Nat. Rev. Genet. 6451-464. [DOI] [PubMed] [Google Scholar]
- 15.Kentner, D., and V. Sourjik. 2007. Spatial organization of the bacterial chemotaxis system. Curr. Opin. Microbiol. 9619-624. [DOI] [PubMed] [Google Scholar]
- 16.Keynan, A., and Z. Evenchick. 1969. Activation, p. 359-396. In G. W. Gould and A. Hurst (ed.), The bacterial spore. Academic Press: New York, NY.
- 17.Leisner, M., K. Stingl, E. Frey, and B. Maier. 2008. Stochastic switching to competence. Curr. Opin. Microbiol. 11553-559. [DOI] [PubMed] [Google Scholar]
- 18.Lewis, K. 2007. Persister cells, dormancy and infectious disease. Nat. Rev. Microbiol. 548-56. [DOI] [PubMed] [Google Scholar]
- 19.Lindsay, J. A., T. C. Beaman, and P. Gerhardt. 1985. Protoplast water content of bacterial spores determined by buoyant density sedimentation. J. Bacteriol. 163735-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Losick, R., and C. Desplan. 2008. Stochasticity and cell fate. Science 32065-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicholson, W. L., N. Munakata, G. Horneck, H. J. Melosh, and P. Setlow. 2000. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 64548-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination and outgrowth, p. 391-450. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley and Sons, Chichester, England.
- 23.Paidhungat, M., B. Setlow, A. Driks, and P. Setlow. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J. Bacteriol. 1825505-5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paidhungat, M., and P. Setlow. 1999. Isolation and characterization of mutations in Bacillus subtilis that allow spore germination in the novel germinant d-alanine. J. Bacteriol. 1813341-3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paidhungat, M., and P. Setlow. 2000. Role of Ger-proteins in nutrient and non-nutrient triggering of spore germination in Bacillus subtilis. J. Bacteriol. 1822513-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paidhungat, M., and P. Setlow. 2001. Localization of a germinant receptor protein (GerBA) to the inner membrane of Bacillus subtilis spores. J. Bacteriol. 1833982-3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paidhungat, M., and P. Setlow. 2002. Spore germination and outgrowth, p. 537-548. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its relatives: from genes to cells. American Society for Microbiology, Washington, DC.
- 28.Paredes-Sabja, D., B. Setlow, P. Setlow, and M. R. Sarker. 2008. Characterization of Clostridium perfringens spores that lack SpoVA proteins and dipicolinic acid. J. Bacteriol. 1904648-4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pelczar, P. L., T. Igarashi, B. Setlow, and P. Setlow. 2007. The role of GerD in the germination of Bacillus subtilis spores. J. Bacteriol. 1891090-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pelczar, P. L., and P. Setlow. 2008. Localization of the germination protein GerD to the inner membrane in Bacillus subtilis spores. J. Bacteriol. 1905635-5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Setlow, B., A. E. Cowan, and P. Setlow. 2003. Germination of spores of Bacillus subtilis with dodecylamine. J. Appl. Microbiol. 95637-648. [DOI] [PubMed] [Google Scholar]
- 32.Setlow, B., E. Melly, and P. Setlow. 2001. Properties of spores of Bacillus subtilis blocked at an intermediate stage of spore germination. J. Bacteriol. 1834894-4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Setlow, B., L. Peng, C. A. Loshon, Y.-Q. Li, G. Christie, and P. Setlow. Characterization of the germination of Bacillus megaterium spores lacking enzymes that degrade the spore cortex. J. Appl. Microbiol., in press. [DOI] [PubMed]
- 34.Setlow, B., and P. Setlow. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J. Bacteriol. 1783486-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Setlow, B., P. G. Wahome, and P. Setlow. 2008. Release of small molecules during germination of spores of Bacillus species. J. Bacteriol. 1904759-4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Setlow, P. 2003. Spore germination. Curr. Opin. Microbiol. 6550-556. [DOI] [PubMed] [Google Scholar]
- 37.Setlow, P. 2006. Spores of Bacillus subtilis: their resistance to radiation, heat and chemicals. J. Appl. Microbiol. 101514-525. [DOI] [PubMed] [Google Scholar]
- 38.Vepachedu, V. R., and P. Setlow. 2005. Localization of SpoVAD to the inner membrane of spores of Bacillus subtilis. J. Bacteriol. 1875677-5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vepachedu, V. R., and P. Setlow. 2007. Role of SpoVA proteins in the release of dipicolinic acid during germination of Bacillus subtilis spores triggered by dodecylamine or lysozyme. J. Bacteriol. 1891565-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]