ABSTRACT
Background and objectives: The purpose of this study was to describe common radiographic patterns that may be useful in predicting the diagnosis of rhinocerebral mucormycosis. Methods: We retrospectively evaluated the imaging and clinical data of four males and one female, 3 to 72 years old, with rhinocerebral mucormycosis. Results: All the patients presented with sinusitis and ophthalmological symptoms. Most of the patients (80%) had isointense lesions relative to brain in T1-weighted images. The signal intensity in T2-weighted images was more variable, with only one (20%) patient showing hyperintensity. A pattern of anatomic involvement affecting the nasal cavity, maxillary sinus, orbit, and ethmoid cells was consistently observed in all five patients (100%). Our series demonstrated a mortality rate of 60%. Conclusion: Progressive and rapid involvement of the cavernous sinus, vascular structures and intracranial contents is the usual evolution of rhinocerebral mucormycosis. In the context of immunosupression, a pattern of nasal cavity, maxillary sinus, ethmoid cells, and orbit inflammatory lesions should prompt the diagnosis of mucormycosis. Multiplanar magnetic resonance imaging shows anatomic involvement, helping in surgery planning. However, the prognosis is grave despite radical surgery and antifungals.
Keywords: Rhinocerebral mucormycosis, imaging findings, MRI, neuroradiology
Rhinocerebral mucormycosis is an acute, fulminant, and often lethal opportunistic infection typically affecting diabetic or immunocompromised patients.1 It is caused by one of the members of the mucoraceal family, including Absidia, Mucor, and Rhizopus.2 Clinically, presenting symptoms are nonspecific including headache, low-grade fever, facial swelling, and orbital or paranasal sinus syndrome. After infection of the nasal cavity and paranasal sinuses, the fungi cause a necrotizing vasculitis that extends rapidly into deep face, orbits, cranial cavity, and brain through skull base partitions and foramina.2 When limited involvement of the paranasal sinuses is present, survival rates are between 50% and 80%.3 However, when brain invasion has occurred, mortality is greater than 80%. Because of its lethal nature, it must be recognized early and treated aggressively. We retrospectively reviewed the neuroimaging findings in a series of five patients with rhinocerebral mucormycosis to establish common radiographic patterns that may be useful in predicting the diagnosis of this infection.
MATERIALS AND METHODS
We evaluated the imaging and clinical data of four males and one female, 3 to 72 years old, with mucormycosis of the craniofacial areas. Patients were selected for study if the diagnosis of mucormycosis was established by means of biopsy, culture, or autopsy, and computed tomography (CT) scans or magnetic resonance (MR) images were available for review. All the patients were immunosuppressed. Two had diabetes mellitus, and four had hematologic conditions and concomitant immunocompromised states. All patients had MR imaging with a 1.5-T system. Both T1- and T2-weighted images were obtained as well as T1-weighted images after intravenous injection of gadopentetate dimeglumine (0.1 mmol/kg). Four patients had CT scans available for review.
Images were evaluated for density, signal intensity, and contrast enhancement characteristics. The CT density was evaluated in non-enhanced images and compared with muscle/brain. The MR signal intensity was compared with gray matter on the T1- and T2-weighted images (Table 1). Gadolinium enhancement was graded on a scale from none to marked. All studies were reviewed by two neuroradiologists (DAH, ABD), and the anatomic structures involved by the infection were defined by consensus. Clinical information about the presentation, management, and evolution of disease was obtained from medical history in all cases.
Table 1.
Imaging Findings of Rhinocerebral Mucormycosis
| Case Number | Sex/Age | Predisposing Condition | CT Density | T1 Signal | T2 Signal | MR Enhancement | Compromise |
|---|---|---|---|---|---|---|---|
| CT, computed tomography; MR, magnetic resonance imaging; M, male; NC, nasal cavity; MS, maxillary sinus; ES, ethmoid sinus; O, orbit; SS, sphenoid sinus; ASB, anterior skull base; P, pachymeningeal (dural); L, leptomeningeal; CS, cavernous sinus; MCF, middle cranial fossa; CA, carotid artery; BI, brain infarction; F, female; 5, fifth cranial nerve and perineural brainstem invasion. | |||||||
| 1 | M/13 | Blastic anemia, neutropenia | Isodense | Isointense | Hypointense* | Absent | NC, MS, ES, O, SS, ASB, P, L |
| 2 | M/72 | Autoimmune hemolytic anemia | Isodense | Isointense | Isointense | Mild | NC, MS, ES, O, CS |
| 3 | M/72 | Diabetes | Hyperdense | Isointense | Hypointense | Marked | NC, MS, ES, O, SS, MCF, CS, CA, BI |
| 4 | F/16 | Leukemia | Isodense | Isointense | Isointense | Marked | NC, MS, ES, O, SS, ASB, P |
| 5 | M/45 | Diabetes | N/A | Hypointense | Hyperintense | Absent | NC, MS, ES, O, MCF, CS, CA, 5, BI |
Heterogenous but predominantly hypointense.
RESULTS
Clinical Presentation
All the patients presented with sinusitis and ophthalmological symptoms. Three patients (60%) had clinical symptoms of cavernous sinus involvement including diplopia/ophthalmoplegia and facial pain/numbness.
Computed Tomography Findings
Of the four patients who had CT scans available for review, 3 (75%) had isodense to muscle/brain lesions. Only one patient (25%) had hyperdense lesions relative to muscle/brain in the noninvasive portion suggesting secondary obstructive changes (inspissated secretions).
Magnetic Resonance Imaging Signal Intensity
Most of the patients (80%) had isointense lesions relative to brain in T1-weighted images. The signal intensity in T2-weighted images was more variable, with only one (20%) patient showing hyperintensity. The rest of the lesions were either hypointense or isointense in long retention time images (Table 1).
Enhancement Pattern
One patient (20%) didn't have enhancement of his inflammatory process after the administration of gadolinium. Two patients (40%) had variable enhancement, with mixed non-enhancing and marked enhancing portions of their inflammatory lesions. One patient (20%) had mild enhancement and the remaining patient (20%) had no enhancement at all. Dural enhancement was observed in two patients (60%) and mixed leptomeningeal and pachymeningeal enhancement was present in another patient (20%).
Anatomic Involvement
A pattern of anatomic involvement affecting the nasal cavity, maxillary sinus, orbit, and ethmoid cells was consistently observed in all five patients (100%; Figs. 1–6). Additionally, there was variable involvement of sphenoid sinus, cavernous sinus, carotid artery, skull base floor, and intracranial structures as described in Table 1.
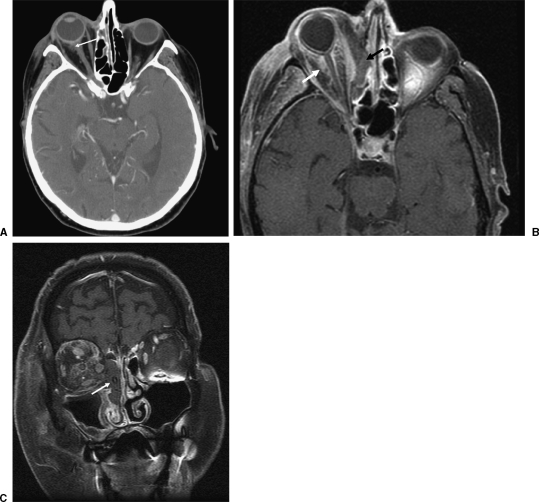
Figure 1.
A 72-year-old male diabetic patient presented with right eye pain and proptosis. (A) Computed tomography axial image shows subtle increased density in intraconal fat in the right orbit (arrow), representing early inflammatory process. (B, C) T1 fat-saturated images obtained after the administration of gadolinium the same day demonstrate progression of infection, with orbital invasion and severe compromise of left nasal cavity and ethmoid cells (arrows). Fast interval change is suggestive of rhinocerebral mucormycosis.
Figure 2.
A 72-year-old male diabetic patient presented with right eye pain and proptosis. (A) Magnetic resonance imaging (MRI) control after right orbital exenteration. Despite amphotericin treatment and surgical debriding, cavernous sinus compromise and abnormal signal in contact with the carotid wall (arrow) was noted after 3 months. (B) Interval progression with carotid wall invasion (arrow) was noted during follow up. (C) Catheter angiography shows linear and irregular filling of the cavernous portion of the internal carotid artery (arrow) confirming MRI findings. Vascular invasion is a frequent feature of mucormycosis infection.
Figure 3.

A 16-year-old female patient with leukemia in remission presented with facial and ocular pain. Computed tomography coronal image shows an inflammatory process involving nasal cavity, ethmoid cells, maxillary sinus, and orbit in the left side (arrows). Combined involvement of the previously mentioned anatomic structures should raise suspicion of the diagnosis of invasive fungal sinusitis.
Figure 4.
A 16-year-old female patient with leukemia in remission presented with facial and ocular pain, status post right orbital exenteration and debriding. (A) Magnetic resonance imaging fat-saturated post gadolinium T1 coronal image shows an inflammatory lesion involving the cribriform plate (arrow). (B) Interval change is noted, with increasing thickness of dural enhancement (arrow), corresponding to intracranial extension, which is a frequent finding in mucormycosis infection.
Figure 5.
A 45-year-old diabetic patient presented with orbital symptoms and fifth cranial nerve palsy. (A) Magnetic resonance imaging (MRI) fat-saturated T1 post gadolinium coronal image shows lack of enhancement of the left cavernous sinus (arrow), corresponding to mucormycosis invasion. (B) MRI post gadolinium T1-weighted axial image shows perineural spread through the left fifth cranial nerve with brainstem and middle cerebellar peduncle invasion (arrows). (C) MR diffusion-weighted image shows restricted diffusion (arrow). (D) MR fat-saturated T1 post gadolinium coronal image shows interval progression in invasion of the cavernous sinus and carotid artery (arrow). Perineural (V3) spread through the foramen ovale is also noted (arrowhead). (E) MR angiography time of flight shows thinning of the left cavernous internal carotid artery and MCA branches (arrows). (F) MR flair axial image shows left hemispheric infarcts (arrows).
Figure 6.
A 13-year-old male patient with blastic anemia and neutropenia. (A) Computed tomography coronal image shows an extensive inflammatory process involving the nasal cavity, ethmoid cells, maxillary, frontal sinus, and orbit in the left, which may raise the suspicion of invasive fungal infection (arrows). Facial soft tissues are also involved (arrowheads). (B) Magnetic resonance fat-saturated T1-weighted axial image shows pial/arachnoid enhancement corresponding to leptomeningeal mucormycosis spread (arrows). Soft tissue compromise is again noted (arrowheads).
Clinical Evolution
Orbital exenteration, ethmoidectomy, medial maxillectomy, and debridement of the nasal vault were performed in all patients. More extensive debridement of necrotic tissue was performed as required in each particular case according to surgical findings (Figs. 7, 8). All patients received amphotericin-B locally and parenterally. Two patients (40%) recovered, while three patients (60%) expired.
Figure 7.
Intraoperative photo of hard palate prior to maxillectomy, taken ~6 hours after presentation. On initial examination this lesion (arrow) was a subcentimeter area of pallor on the left, at the junction between the hard and soft palates.
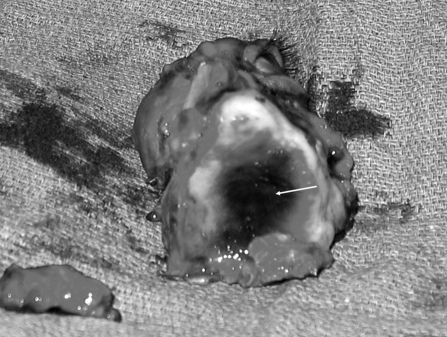
Figure 8.
Intraoperative photo of left maxillectomy and orbital exenteration, inferior view. Note the extent of necrosis of the palate (arrow).
DISCUSSION
Mucormycosis, also known as zygomycosis and phycomycosis, was first described by Paulltauf in 1885.4 Phycomycetes are ubiquitous fungi occurring in soil, air, skin, body orifices, manure, spoiled food, and dust.5,6 Inoculation occurs by inhalation, when spores reach the nasal cavity and/or nasopharynx. The fungus may then spread to the paranasal sinuses and subsequently to the orbit, meninges, and brain by direct extension.7 Orbital involvement results from spread through the nasolacrimal duct and medial orbital wall. Such invasion is facilitated by the thinness of the lamina papyracea, congenital dehiscence often present along the medial wall, and the perforations of the medial wall by arteries and veins.8,9 Mucormycosis invades the walls of the blood vessels resulting in vascular occlusion, thrombosis, and infarction, as well as dissemination to the central nervous system from the primary focus.5,10,11 Spread to the brain may occur via the orbital apex, orbital vessels, or via the cribriform plate.12 Generally, the presenting symptoms are low-grade fever, cephalgia, sinusitis, facial swelling, orbital apex syndrome with blurred vision, and cranial palsies from cavernous sinus involvement in an immunocompromised patient.2,13,14,15 Early visual loss would favor the diagnosis of rhino-orbital-cerebral mucormycosis over bacterial cavernous sinus thrombosis in which blindness is a much later finding.16
We found that MRI signal intensity of mucormycosis lesions tends to be isointense or hypointense in all sequences. After the administration of gadolinium the lesions had variable enhancement patterns ranging from homogeneous to heterogenous or non-enhancing at all. We think that contrast-enhanced T1-weighted images are helpful in delineating the intracranial spread when meningeal enhancement is present as well as in identifying invasion of the cavernous portion of the internal carotid artery by the disease. This had been previously described by Mohindra and associates17 who showed that MRI can detect cavernous sinus invasion and vascular complications such as ischemia.
An aggressive sinonasal and orbital inflammatory process was observed in all of our five patients. This behavior was also reported previously by Mnif and colleagues,18 who suggested that the association between orbital cellulitis and sinusitis in the context of immunosuppression should prompt the diagnosis of mucormycosis. However, the differential diagnosis should consider other fungi, Graves' disease, carotid cavernous fistula and thrombosis, bacterial cellulites, inflammatory pseudotumor, and paranasal sinus tumor. The aggressive clinical features of the disease can suggest the diagnosis of fungal infection, but the final diagnosis depends on the anatomopathological demonstration of fungal tissue invasion (Fig. 9). Tissue cultures may be positive, allowing the identification of specific species.
Figure 9.
Histologic sections of periorbital tissue with multiple, broad nonseptate hyphae (arrows) surrounded by inflammatory infiltrate (hematoxylin & eosin, 400 ×).
Our series demonstrated a mortality rate of 60%, in agreement with other series which show up to 50% mortality without intracranial manifestation, and up to 90% mortality if there is intracranial involvement.19,20 The survival rates in those with invasive mucormycosis reflect the variance in the ability to reverse the underlying predisposing cause. Immunocompromise can vary from diabetic ketoacidosis to leukemia and bone marrow failure. Up to 80% of diabetic patients survive whereas fewer than 50% of nondiabetic patients survive.21 In our series, two out of the three deaths were due to nondiabetic conditions, namely aplastic anemia and acute lymphocytic leukemia.
These mortality rates exemplify the aggressive nature of this disease process. Mucormycosis has earned its reputation as the most acutely fatal fungal infection known to humans. It is rapidly growing, and within 24 hours, cultures of mucor can grow to the top of the culture plate. It is important that if the diagnosis is suspected, the suspicion be acted on immediately. As seen in Figs. 7 and 8, the area of necrosis in the patient shown had greatly expanded in less than 6 hours.
CONCLUSION
Progressive and rapid involvement of the cavernous sinus, vascular structures, and intracranial contents is the usual evolution of rhinocerebral mucormycosis. Multimodality imaging is helpful in prompting an early diagnosis when a pattern of nasal cavity, maxillary sinus, ethmoid cells, and orbit inflammatory process is present, especially when iso- or hypointense lesions are observed. Multiplanar MRI shows anatomic involvement, which helps in surgery planning. However, the prognosis is grave despite radical surgery and antifungals.
REFERENCES
- Rumboldt Z, Castillo M. Indolent intracranial mucormycosis: case report. AJNR Am J Neuroradiol. 2002;23:932–934. [PMC free article] [PubMed] [Google Scholar]
- Chan L L, Singh S, Jones D, et al. Imaging of mucormycosis skull base osteomyelitis. AJNR Am J Neuroradiol. 2000;21:828–831. [PMC free article] [PubMed] [Google Scholar]
- Anselmo-Lima W T, Lopes R P, Valera F C, et al. Invasive fungal rhinosinusitis in immunocompromised patients. Rhinology. 2004;42:141–144. [PubMed] [Google Scholar]
- Paulltauf A. Mycosis mucorina. Virchows Arch. 1885;102:543. [Google Scholar]
- Naussbaum E S, Holl W A. Rhinocerebral mucormycosis: changing patterns of disease. Surg Neurol. 1994;41:152–156. doi: 10.1016/0090-3019(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Hopkins M A, Treloar D M. Mucormycosis in diabetes. Am J Crit Care. 1997;6:363–367. [PubMed] [Google Scholar]
- Kohn R, Helper R. Management of limited rhino-orbital mucormycosis without exenteration. Ophthalmology. 1985;92:1440–1443. doi: 10.1016/s0161-6420(85)33844-7. [DOI] [PubMed] [Google Scholar]
- Abramson E, Wilson D, Arky R A. Rhinocerbral phycomycosis in association with diabetic ketoacidosis. Ann Intern Med. 1967;66:735–742. doi: 10.7326/0003-4819-66-4-735. [DOI] [PubMed] [Google Scholar]
- Rangel-Guerra R A, Martinez H R, Saenz C, et al. Rhinocerebral and systemic mucormycosis: clinical experience with 36 cases. J Neurol Sci. 1996;143:19–30. doi: 10.1016/s0022-510x(96)00148-7. [DOI] [PubMed] [Google Scholar]
- Thajeb P, Thajeb T, Dai D. Fatal strokes in patients with rhino-orbito-cerebral mucormycosis and associated vasculopathy. Scand J Infect Dis. 2004;36:643–648. doi: 10.1080/00365540410020794. [DOI] [PubMed] [Google Scholar]
- Ochi J W, Harris J P, Feldman J I, et al. Rhinocerebral mucormycosis: results of aggressive surgical debridement and amphotericin B. Laryngoscope. 1988;98:1339–1342. doi: 10.1288/00005537-198812000-00011. [DOI] [PubMed] [Google Scholar]
- Sheman D D. Orbital Anatomy and Its Clinical Applications. Philadelphia, PA: Lippincott-Raven; 1992. pp. 1–26.
- Gamba J L, Woodruff W W, Djang W T, et al. Craniofacial mucormycosis: assessment with CT. Radiology. 1986;160:207–212. doi: 10.1148/radiology.160.1.3715034. [DOI] [PubMed] [Google Scholar]
- Terk M R, Underwood D J, Zee C, et al. MR imaging in rhinocerebral and intracranial mucormycosis with CT and pathologic correlation. Magn Reson Imaging. 1992;10:81–87. doi: 10.1016/0730-725x(92)90376-b. [DOI] [PubMed] [Google Scholar]
- Harril W C, Stewart M G, Lee A G, et al. Chronic rhinocerebral mucormycosis. Laryngoscope. 1996;106:1292–1297. doi: 10.1097/00005537-199610000-00024. [DOI] [PubMed] [Google Scholar]
- Johnson E Van, Kline L B, Julian B A, et al. Bilateral cavernous sinus thrombosis due to mucormycosis. Arch Ophthalmol. 1988;106:1089–1092. doi: 10.1001/archopht.1988.01060140245034. [DOI] [PubMed] [Google Scholar]
- Mohindra S, Mohindra S, Gupta R, et al. Rhinocerebral mucormycosis: the disease spectrum in 27 patients. Mycoses. 2007;50:290–296. doi: 10.1111/j.1439-0507.2007.01364.x. [DOI] [PubMed] [Google Scholar]
- Mnif N, Hmaied E, Oueslati S, et al. Imaging of rhinocerebral mucormycosis. J Radiol. 2005;86:1017–1020. doi: 10.1016/s0221-0363(05)81485-4. [DOI] [PubMed] [Google Scholar]
- Naussbaum E S, Holl W A. Rhinocerebral mucormycosis: changing patterns of disease. Surg Neurol. 1994;41:152–156. doi: 10.1016/0090-3019(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Yohai R A, Bullock J D, Aziz A A, et al. Survival factors in rhino-orbital-cerebral mucormycosis. Surv Ophthalmol. 1994;39:3–22. doi: 10.1016/s0039-6257(05)80041-4. [DOI] [PubMed] [Google Scholar]
- Gwaltney J M., Jr Acute community-acquired sinusitis. Clin Infect Dis. 1996;23:1209–1255. doi: 10.1093/clinids/23.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]