Abstract
Zebrafish is gaining popularity in behavioral neuroscience in general and in alcohol research in particular. Alcohol is known to affect numerous molecular mechanisms depending on dose and administration regimen. Prominent among these mechanisms are several neurotransmitter systems. Here we analyze the responses of the dopaminergic and serotoninergic neurotransmitter systems of zebrafish to acute alcohol treatment (1 h long exposure of adult fish to 0.00%, 0.25%, 0.50%, or 1.00% ethyl alcohol) by testing the concentration of dopamine, its metabolite DOPAC, and serotonin and its metabolite 5-HIAA from whole brain extracts. We utilize a sensitive HPLC method and describe significant alcohol induced changes in zebrafish for the first time. We show that dopamine significantly increased in a quasi-linear dose dependent manner, DOPAC showed a smaller apparent increase which was non-significant, while both serotonin and 5-HIAA showed a significant increase only in the highest acute dose group. We discuss the methodological novelty of our work and theorize about the implications of the neurotransmitter level changes from a behavioral perspective.
Keywords: Alcohol, Alcoholism, Dopamine, DOPAC, HPLC, Serotonin, 5-HIAA, Zebrafish
1. Introduction
Alcohol (EtOH, Ethyl Alcohol, Ethanol) abuse and alcoholism are prevalent diseases that have a significant negative impact on the patient and society [1]. However, our understanding of the biological mechanisms of these disorders is still limited, preventing adequate therapies [2,3]. Animal models have been proposed to facilitate investigation in this direction [4]. Zebrafish, a freshwater tropical fish, has been suggested as a potential laboratory study species for such research [5]. This species was first chosen as a laboratory model organism about 30 years ago for the analysis of vertebrate embryonic development (for review see ref. [6]). Since then zebrafish has become an important study organism for numerous biological phenomena and human diseases [6]. Zebrafish is increasingly recognized as a good study species for the analysis of behavior and brain function in general (e.g. [7-12] and references therein) and of alcohol induced behavioral changes in particular [5,13-16]. Alcohol is known to influence a large number of neurobiological mechanisms, and, for example, has been shown to alter the functioning of all major neurotransmitter systems (for a recent review see ref. [17]) including the dopaminergic [18-20] and the serotoninergic systems (e.g. [21-23]). However, neurochemical analyses have not been performed in this context using zebrafish.
Immunohistochemical techniques have been successfully employed in the analysis of the development and anatomy of neurotransmitter systems in zebrafish (e.g. [24-28]). These approaches utilized antibodies against particular enzymes or receptors involved in the synthesis or the binding of certain neurotransmitters of zebrafish. However, these indirect approaches are limited in terms of their ability to precisely quantify neurotransmitter amounts (levels or concentrations) in the brain. Briefly, this latter question has not been addressed in zebrafish despite the fact that such studies have been regularly conducted using high precision liquid chromatography (HPLC) in, for example, laboratory rodents [29]. A possible explanation for the paucity of HPLC neurotransmitter analyses in the zebrafish brain is that compared to the brain of other laboratory vertebrate species e.g. rat, mice, monkey and human, the zebrafish brain is small (about 2 mg in a fully mature adult) and thus the amount of neurotransmitter one needs to detect is low. Nevertheless, a most recent study [30] suggested that HPLC analysis is indeed possible in zebrafish.
Alcoholism and alcohol abuse are known to affect aminergic neurons and lead to abnormalities in the levels of aminergic neurotransmitters (e.g. dopamine and serotonin), which then results in significant changes in behavior (e.g. [19] and references therein). Given that acute alcohol treatment has been shown to lead to significant behavioral changes in zebrafish [5,15,16,31] we decided to investigate whether changes in aminergic neurotransmitters may be detectable in this species too. For example, intermediate doses of alcohol (0.25-0.50%, v/v) when administered acutely were shown to increase locomotory activity as well as aggression [5]. Shoaling, a form of social behavior also known as group preference, was impaired by increasing doses of acute alcohol [15], responses to a predator, its computer animated image, or a predator model were also found enhanced by intermediate and impaired by higher doses of acute alcohol (e.g. [5,15,16]). Some of these acute alcohol administration induced behavioral changes may correlate with alterations in the way neurotransmitter systems function in zebrafish. However, such analyses have not been performed perhaps due to methodological limitations.
In the current study, we present a highly sensitive one-step HPLC method for the analysis of dopamine, serotonin, and their metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and 5-hydroxyindoleacetic acid (5-HIAA) in the zebrafish brain. Using this method we study the temporal changes in the levels of neurochemicals in the zebrafish brain from the start of acute alcohol exposure up to 80 min, and also conduct a dose response analysis after 60 min-long acute alcohol exposure. We hope to demonstrate that our method is capable of detecting acute alcohol induced neurotransmitter level changes in zebrafish, a finding that would open the door to future systematic analyses of numerous neurotransmitter systems and of the potential correlated responses between behavior and neurotransmitters in zebrafish.
2. Materials and methods
2.1. Animals and housing
Adult, 90-day-old, zebrafish (Danio rerio, 50-50% males and females) of the AB strain bred in our facility (University of Toronto Mississauga Vivarium, Mississauga, ON, Canada) were used. The progenitors of our fish were obtained from the Zebrafish International Research Centre (ZIRC) (Eugene, Oregon). Experimental subjects were of the second generation of these founder fish. All fish used in the current study were bred, raised, and housed in the same manner and in the same vivarium room. AB is one of the most frequently studied zebrafish strains used in forward genetic (mutagenesis) studies (e.g. [32]) and shows homozygosity at 84% of its loci [33]. One reason for this frequent use is that a large number of genetic markers have been developed for this strain. Another reason is that the high homozygosity level ensures stability of the genetic characteristics of this strain across generations.
The experimental fish were raised and housed in 3 l transparent acrylic tanks (15 fish per tank) that were part of a zebrafish rack system (Aquaneering Inc., San Diego, CA, USA). The system had multistage filtration that contained a mechanical filter, a fluidized glass bed biological filter, an activated carbon filter, and a fluorescent UV light sterilizing unit. Every day 10% of the water was replaced with fresh system water (deionized water supplemented with 60 mg/l Instant Ocean Sea Salt [Big Al’s Pet Store, Mississauga, Ontario, CA]). The water temperature was maintained at 27 °C. Illumination was provided by fluorescent light tubes from the ceiling with lights turned on at 08:00 h and off at 19:00 h. Until their age of 10 days postfertilization developing fish were fed Larval AP 100 (particle size below 100 μm, ZeiglerBros, Inc., Gardners, PA, USA) and subsequently nauplii of brine shrimp (Artemia salina) until their age of 3 weeks. Older fish received a mixture of dried fish food (4 parts of Nelson Silver Cup, Aquaneering Inc., San Diego, CA, USA) and powered spirulina (1 part, Jehmco Inc., Lambertville, NJ, USA).
In previous behavioral studies acute alcohol treatment was employed by immersing zebrafish in alcohol solutions for 1 h [5,15,16,34]. This length of exposure was chosen based upon studies that showed maximal alcohol blood, brain concentrations and/or plateauing of behavioral changes after this length of exposure ([5] and references therein, [13]). In order to confirm the appropriate length of acute alcohol exposure, we investigated the temporal kinetics of alcohol effects on dopamine, serotonin and their metabolites in the zebrafish brain as follows. Experimental zebrafish were incubated in five 8 l tanks (12 fish per tank) in the presence of the highest concentration of alcohol (1.00%, v/v) employed in this study for 0, 20, 40, 60 and 80 min, respectively. After the corresponding time of alcohol exposure, the fish were removed from the tanks and their brains were dissected. We pooled two brains within treatment groups (12 fish per group) for HPLC analysis, and thus for statistical calculations the sample size (n) equaled 6.
Our results showed that after 40 min in alcohol, the alcohol induced changes in neurochemicals reached a plateau. Thus, we chose the 60 min time point for our subsequent dose response analysis. In this latter study experimental zebrafish were incubated in four 8 l tanks (16 fish per tank) in the presence of one of the following alcohol concentrations: 0.00% (control), 0.25%, 0.50% or 1.00% (v/v), a between-subject experimental design. After a 1 h long acute alcohol exposure, the fish were removed from the alcohol dosing tanks and their brains were dissected and processed as described below. For HPLC analyses we again pooled two brains within treatment groups so for statistical calculations the sample size (n) equaled 8. The alcohol treatment and brain processing order of fish were randomized across concentration groups.
2.2. HPLC analysis
After acute alcohol treatment, the fish were decapitated rapidly and their brains were dissected on ice under a dissecting microscope. The brains were frozen in a microcentrifuge tube (2 brains per tube) at -80 °C. For HPLC sample preparation, the tubes were taken out of the freezer and the brains were suspended in artificial cerebrospinal fluid (ACSF, Harvard, 10 μl/2 brains). The brains were sonicated for 4 times (2 s pulse each time) on ice. 2 μl of the sonicate from each sample was analyzed for protein content by BioRad protein assay reagent (BioRad, Hercules, CA, United States). To each tube, 1 μl of stabilizer (0.2N perchloric acid and 1.0 M ascorbic acid) was added, and the sonicates were centrifuged at 10,000 rpm for 10 min at 4 °C. The supernatant was collected carefully and stored at -80 °C until use.
HPLC analysis for the dopamine, 3,4-dihydroxyphenylacetic acid, serotonin and 5-hydroxyindoleacetic acid of the supernatants was carried out using a BAS 460 MICROBORE-HPLC system with electrochemical detection (Bio-analytical Systems Inc., West Lafayette, IN, USA) together with a Uniget C-18 reverse phase microbore column as the stationary phase (BASi, Cat no. 8949). The mobile phase consisted of buffer [0.1 M monochloro acetic acid, 0.5 mM Na-EDTA, 0.15 g/L Na-octylsulfonate and 10 nM sodium chloride, pH 3.1 (all chemicals were from Sigma)], acetonitrile and tetrahydrofuran (all solvents from Fisher Scientific) at a ratio 94:3.5:0.7. The flow rate was 1.0 ml/min and the working electrode (Uniget 3 mm glassy carbon, BAS P/N MF-1003) was set at 550 mV vs. Ag/Ag/Cl reference electrode. Detection gain was 1.0 nA, filter was 0.2 Hz and detection limit was set at 20 nA. 5 μl of the sample supernatant was directly injected into the HPLC for analysis. Standard dopamine, DOPAC, serotonin and 5-HIAA (all from Sigma) were used to quantify and identify the peaks on the chromatographs. The retention times for dopamine, DOPAC, serotonin and 5-HIAA were approximately 3.7, 5.5, 6.5 and 16.2 min, respectively, under the set conditions. The detection limits for dopamine, DOPAC 5-HIAA and serotonin were determined by running the known concentrations of dopamine, DOPAC, 5-HIAA and serotonin separately in the HPLC system under the set condition. For this purpose, standard solutions of 1 mg per ml was made with pure dopamine, DOPAC, 5-HIAA and serotonin and diluted accordingly to get the desired concentrations of the stock solutions for running in HPLC. The sensitivity was selected at the concentration at which we got the signal to noise ratio of 3:1.
2.3. Statistical analysis
One-way variance analysis (ANOVA) was performed to investigate the effect of length of alcohol exposure and in a subsequent study the effect of different alcohol concentrations (both studies used a between-subject design). In case of a significant main effect, post hoc Tukey Honestly Significant Difference (HSD) tests were performed to study differences between time points or dose groups.
3. Results
3.1. Detection threshold for dopamine, serotonin and their metabolites in the zebrafish brain
The highly sensitive microbore-HPLC system employed here provided strong and clear signals for the neurotransmitters dopamine, serotonin, and for their metabolites in zebrafish brain lysates in one run. A typical chromatogram of the HPLC analysis is shown in Fig. 1. The lowest levels of dopamine, DOPAC, serotonin and 5-HIAA we could assay by this method were 0.1, 0.1, 0.3 and 0.5 pg per 5 μl sample (brain extract), respectively. The calibration curve was linear over the range 1-400 pg on-column. Although we have pooled two brains per point in the current study, it is notable that the peaks were robust and given the detection thresholds we found, we conclude that a single brain or, in fact, a part of the zebrafish brain could have been sufficient for the analysis.
Fig. 1.

Chromatogram of DOPAC, dopamine, serotonin and 5-HIAA separated by the microbore HPLC column. Panel A: Standard DOPAC (50 pg), dopamine (150 pg), serotonin (100 pg) and 5-HIAA (50 pg). Panel B: DOPAC, dopamine, serotonin and 5-HIAA separated from zebrafish brain lysate. Observe the temporal positioning of signals between the standards and tissue sample obtained neurochemicals. Also note the differences in amplitudes of the signals.
3.2. Temporal kinetics of acute alcohol effects on the dopamine, serotonin and their metabolites in the zebrafish brain
We have analyzed the temporal kinetics of acute effects of 1% alcohol on the aminergic system of zebrafish brain. It was observed that the levels of dopamine, serotonin and their metabolites increased with time of exposure and after 40 min the levels of neurochemicals did not appreciably increase any further up to the last time point (80 min from start of acute alcohol treatment) examined (Fig. 2). Statistical analysis confirmed this observation. ANOVA showed a significant time effect for all neurochemicals tested: dopamine F(4, 29) = 29.493, p < 0.0001; DOPAC F(4, 29) = 18.740, p < 0.0001; serotonin F(4, 29) = 25.273, p < 0.0001; 5-HIAA F(4, 29) = 13.026, p < 0.0001. Tukey HSD tests confirmed our observations and showed that the first two time points (0 and 20 min) did not significantly differ from each other (p > 0.05), the last three time points (40, 60 and 80 min) did not significantly (p > 0.05) differ from each other, and the first two time points significantly (p < 0.05) differed from the last three time points. Briefly these findings are in line with prior reports in which the concentration of alcohol in the brain was analyzed and was found to reach a steady level after approximately 60 min of acute alcohol exposure [13]. Hence, in our subsequent dose response analysis, the acute alcohol treatment consisted of a 60 min alcohol exposure (also as in previous behavioral studies, e.g. [5,15,16]) after which the tissue samples were taken and the HPLC analysis was performed.
Fig. 2.

Temporal changes in the concentration of dopamine, DOPAC, serotonin, and 5-HIAA in zebrafish brain after exposure to 1.00% alcohol. Mean ± S.E.M. are shown. Sample sizes (n) = 6 for each time point analyzed. Note that this is a between-subject design. Also note that neurochemical levels significantly increase up to the 40th min of acute alcohol exposure but further significant increases are not seen in fish that were tested at later time points. For details of methodology and statistical analyses see Sections 2 and 3.
3.3. Effects of acute alcohol exposure on levels of dopamine, serotonin and their metabolites in the zebrafish brain
The dopamine level in the untreated AB zebrafish brain (0% alcohol) was 4.18 ± 0.28 ng per mg of brain protein, a value that is consistent with what has been reported most recently by López Patiño et al. [30]. Alcohol treatment significantly (ANOVA F(3, 31) = 24.138, p < 0.0001) elevated brain dopamine levels in a dose dependent manner (Fig. 3). Tukey HSD test confirmed this finding and showed that all concentration groups significantly (p < 0.05) differed from one another. The dose response trajectory found for DOPAC suggested an apparent elevation of DOPAC levels after acute exposure to the lowest alcohol dose (0.25%), a response that appeared to remain at the same level in the acute dose groups treated with higher concentration of alcohol (Fig. 4). However, the apparent increase of DOPAC levels induced by acute alcohol treatment turned out to be non-significant (ANOVA F(3, 31) = 2.109, p > 0.10).
Fig. 3.
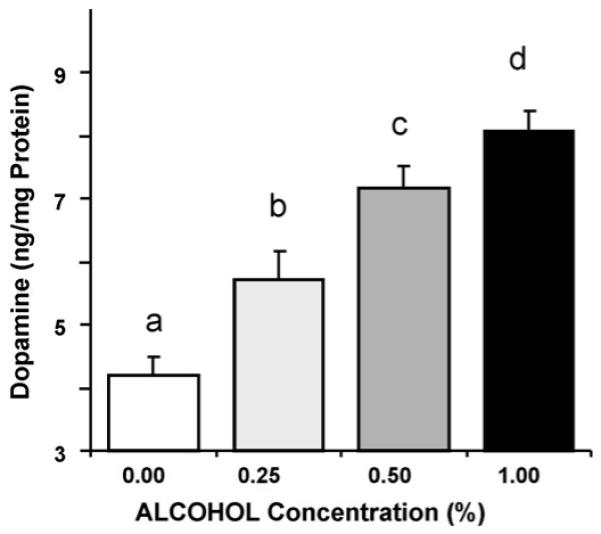
Acute, 1-h long, alcohol exposure increases dopamine levels (expressed as ng dopamine per mg total protein in the sample) in a dose dependent manner in zebrafish. Mean ± S.E.M. are shown. Sample size (n) = 8. The letter above each bar represents the results of Tukey HSD test: bars not sharing a letter designation are significantly different from each other, i.e. p < 0.05. Observe the apparently linear dose response in dopamine levels and note that each dose group is significantly different from the other. For details of methodology and statistical analyses see Sections2 and 3.
Fig. 4.

Acute, 1-h long, alcohol exposure apparently increases levels of DOPAC (expressed as ng DOPAC per mg total protein in the sample) in zebrafish. Mean ± S.E.M. are shown. Sample size (n) = 8. Observe the apparent response to the lowest dose of alcohol and that the response does not seem to increase at higher doses but note that the differences are non-significant. For details of methodology and statistical analyses see Sections 2 and 3.
Effects of alcohol on the levels of serotonin and of the serotonin metabolite, 5-HIAA, are shown in Figs. 5 and 6. Serotonin levels increased slowly with increasing dose of alcohol and the strongest elevation was noticeable only at the 1.00% alcohol concentration. ANOVA found the alcohol concentration effect significant (F(3, 30) = 3.021, p < 0.05), and Tukey HSD confirmed the above observation and showed that only the highest alcohol dose (1.00%) group differed significantly (p < 0.05) from the control (0.00%). Acute alcohol induced changes in the levels of 5-HIAA were less robust at lower doses of alcohol but a surge of 5-HIAA was seen in the brains of fish exposed to the highest acute dose, 1.00% alcohol. ANOVA confirmed a significant alcohol dose effect (F(3, 31) = 5.844, p < 0.01) and Tukey HSD showed that the highest dose group (1.00%) significantly (p < 0.05) differed from all other treatment groups but these other groups did not significantly (p > 0.05) differ from each other.
Fig. 5.

Acute, 1-h long, alcohol exposure increases serotonin levels (expressed as ng serotonin per mg total protein in the sample) in zebrafish. Mean ± S.E.M. are shown. Sample size (n) = 8. The letter above each bar represents the results of Tukey HSD test: bars not sharing a letter designation are significantly different from each other, i.e. p < 0.05. Observe the apparent dose dependent increases in serotonin levels but note that only the highest dose group differs significantly from the control. For details of methodology and statistical analyses see Sections 2 and 3.
Fig. 6.

Acute, 1-h long, alcohol exposure increases 5-HIAA levels (expressed as ng 5-HIAA per mg total protein in the sample) in zebrafish. Mean ± S.E.M. are shown. Sample size (n) = 8. The letter above each bar represents the results of Tukey HSD test: bars not sharing a letter designation are significantly different from each other, i.e. p < 0.05. Observe the robust increase in 5-HIAA at the highest acute dose and note that this dose group significantly differs from all other dose groups including the control. For details of methodology and statistical analyses see Sections 2 and 3.
4. Discussion
Aminergic neurotransmitters are mediators of numerous important functions in the brain and abnormalities in their levels have been implicated in several diseases of the central nervous system in humans. For example, the dopaminergic and serotoninergic systems have been shown to play fundamental roles in such varied diseases as schizophrenia (e.g. [35,36]), depression (e.g. [37,38]), Parkinson’s disease (e.g. [39,40]), and even in Alzheimer’s disease (e.g. [41,42]). Also importantly, the dopaminergic and serotoninergic neurotransmitter systems have been shown to play roles in alcoholism and alcohol abuse (e.g. Lovinger, 1999; Lovinger and Zhou, 1994; [18,21,43-45]). Zebrafish serotonergic and domaninergic systems have similarities to the respective mammalian systems, and thus zebrafish has been suggested as a model organism for the analysis of human disorders associated with dysfunction of these neurotransmitter systems (e.g. [46-48]). The HPLC method used here for the analysis of dopamine, serotonin and their metabolites has proven to be highly sensitive and could detect significant acute alcohol exposure induced changes, a notable finding due to translational relevance. Briefly, HPLC is now added to the already impressive arsenal of genetic and other tools available for zebrafish researchers.
There are several methods available for quantification of the amount of dopamine, serotonin and their metabolites in a single run (as opposed to separate analyses for each neurochemical as conducted e.g. in ref. [30]) from brain homogenates. However, many of these methods suffer from limitations. For example, some authors attempted to isolate the neurochemicals first from tissue homogenates by absorption on a resin followed by separation on an HPLC column [49,50]. Others utilized pre-column derivatization leading to increased error variation in the assay [29,51,52]. Our current method has a substantial advantage over these above techniques: there is no need for tissue extraction using resins or pre-column derivatization. Direct injection of the sonicated homogenates is sufficient for obtaining the results. The direct injection methodology we employed here has been successfully utilized by others but the detection limit and linearity in our system appear higher than in these previous studies. For example, linearity was found within the narrow range of 10-150 pg in other systems [53] but in our case it was between 1 and 400 pg, a substantially broader range, which then translates to more precise measurements across broader neurotransmitter level changes. Sensitivity was found to be between 3.8 and 7.5 pg (e.g. [53]), or between 0.8 and 9 pg in other studies [54] but it was substantially better in our system, ranging from 0.1 to 0.5 pg depending on neurochemical. The superiority of our method is due to the highly sensitive microbore HPLC technique we employed, a methodology that is usually utilized in the analysis of microdialysates [55,56]. We propose that our method will be appropriate for the monitoring of the effects of numerous drugs or novel chemicals that affect a range of neurotransmitter systems in the zebrafish brain.
In the current study we focused our attention to acute alcohol exposure induced changes in the dopaminergic and serotoninergic systems. Our results, in general, indicated a significant increase of neurochemicals in response to increasing doses of acute alcohol administration. Dopamine levels rose linearly with increasing alcohol concentrations employed, while the dopamine metabolite DOPAC showed a modest and non-significant increase that was apparent even after the lowest alcohol dose (a dose response trajectory that is now replicated and found significant in a follow up study by Gerlai et al. [31]). The dose dependently increased dopamine responses are in line with the known rewarding properties of alcohol (e.g. [17]). Briefly, presynaptic release of dopamine is believed to underlie the reinforcing effects of alcohol [57]. Interestingly, acute alcohol treatment has been found to lead to a linear dose dependent color reaction in zebrafish [5]: increasing doses of alcohol made the fish brighter colored. Notably, such color changes are observed under normal conditions during courtship, when the fish spawn, or during agonistic encounters. Thus, previously, it was speculated [5] that perhaps the color changes reflected the hedonistic, rewarding, aspects of acute alcohol exposure, a conclusion now supported by our neurochemical analysis. Furthermore, alcohol was previously found to reduce shoaling responses (the preference to swim close to conspecifics, a form of aggregation behavior) in a dose dependent manner in AB zebrafish [15] and in an outbred zebrafish population too [5]. Our finding of increased dopamine levels after acute alcohol exposure is notable in this context because shoaling, i.e. the sight of conspecifics, has been shown to have rewarding properties ([58]; also see refs. [59,60]). Briefly, the rewarding properties of alcohol may compete with the rewarding aspects of the sight of conspecifics in zebrafish, a hypothesis that is in line with studies using other species including non-human and human primates in which alcohol was found to reduce affiliative social interaction (Shively et al., 2002) [61].
Interestingly, the serotoninergic responses did not follow the linear dose response seen in dopamine. The serotonin, as well as its metabolite, 5-HIAA, was significantly increased in zebrafish only by the highest (1.00%) alcohol dose. Acute alcohol has been shown to enhance the release of serotonin in both the rat (Langen et al., 2002; Thielen et al., 2002; Yan, 1999) [62-64] and the human brain [22] and thus our findings appear to translate well to the mammalian situation. The behavioral function of the serotonin level increases is debated but an association between alcohol abuse and serotonin deficiency has been suggested and serotonin’s role in alcohol craving has been hypothesized (Ciccocioppo, 1999) [65].
Although speculating about the potential behavioral relevance of the found neurochemical changes in zebrafish is tempting and some of these speculations may be correct, it must be acknowledged that alcohol acts through numerous neurotransmitters, second messenger systems and other molecular targets. Thus, our speculations are only correlative, and conclusions about causality cannot be drawn at this point. Briefly, one would need to systematically assay and/or manipulate all possible neurotransmitter systems and also attempt a comprehensive exploration of other molecular changes, perhaps at the gene expression level, before one can understand the mechanistic details of alcohol’s actions in the brain of zebrafish. Nevertheless, our current paper demonstrates that researchers now have yet another tool on their belt with which such questions may be addressed in zebrafish.
Acknowledgements
We would like to thank Rajesh Krishnannair for his technical help with fish maintenance. Supported by NIH/NIAAA (1R01AA015325-01A2 grant to RG).
References
- [1].Harwood HJ, Fountain D, Livermore G. Economic costs of alcohol abuse and alcoholism. Recent Dev Alcohol. 1998;14:307–30. doi: 10.1007/0-306-47148-5_14. [DOI] [PubMed] [Google Scholar]
- [2].O’Brien CP, Eckardt MJ, Linnoila MI. Pharmacotherapy of alcoholism. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: the fourth generation of progress. Raven Press, Ltd.; New York: 1995. pp. 1745–55. [Google Scholar]
- [3].Lawrence AJ. Therapeutics for alcoholism: what’s the future? Drug Alcohol Rev. 2007;26:3–8. doi: 10.1080/09595230601036937. [DOI] [PubMed] [Google Scholar]
- [4].Browman KE, Crabbe JC. Alcohol and genetics: new animal models. Mol Med Today. 1999;5:310–8. doi: 10.1016/s1357-4310(99)01480-x. [DOI] [PubMed] [Google Scholar]
- [5].Gerlai R, Lahav M, Guo S, Rosenthal A. Drinks like a fish: Zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol Biochem Behav. 2000;67:773–82. doi: 10.1016/s0091-3057(00)00422-6. [DOI] [PubMed] [Google Scholar]
- [6].Grunwald DJ, Eisen JS. Timeline: headwaters of the zebrafish-emergence of a new model vertebrate. Nat Rev Genet. 2002;3:717–24. doi: 10.1038/nrg892. [DOI] [PubMed] [Google Scholar]
- [7].Bass SLS, Gerlai R. Zebrafish (Danio rerio) responds differentially to stimulus fish: the effects of sympatric and allopatric predators and harmless fish. Behav Brain Res. 2008;186:107–17. doi: 10.1016/j.bbr.2007.07.037. [DOI] [PubMed] [Google Scholar]
- [8].Blaser R, Gerlai R. Behavioral phenotyping in Zebrafish: comparison of three behavioral quantification methods. Behav Res Methods. 2006;38:456–69. doi: 10.3758/bf03192800. [DOI] [PubMed] [Google Scholar]
- [9].Miller NY, Gerlai R. Oscillations in shoal cohesion in zebrafish (Danio rerio) Behav Brain Res. 2008;193:148–51. doi: 10.1016/j.bbr.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Miller N, Gerlai R. Quantification of shoaling behaviour in Zebrafish (Danio rerio) Behav Brain Res. 2007;184:157–66. doi: 10.1016/j.bbr.2007.07.007. [DOI] [PubMed] [Google Scholar]
- [11].Speedie N, Gerlai R. Alarm substance induced behavioral responses in zebrafish (Danio rerio) Behav Brain Res. 2008;188:168–77. doi: 10.1016/j.bbr.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sison M, Cawker J, Buske C, Gerlai R. Fishing for genes of vertebrate behavior: Zebra fish as an upcoming model system. Lab Anim. 2006;35:33–9. doi: 10.1038/laban0506-33. [DOI] [PubMed] [Google Scholar]
- [13].Dlugos CA, Rabin RA. Ethanol effects on three strains of zebrafish: model system for genetic investigations. Pharm Biochem Behav. 2003;74:471–80. doi: 10.1016/s0091-3057(02)01026-2. [DOI] [PubMed] [Google Scholar]
- [14].Fernandes Y, Gerlai R. Long-term behavioral changes in response to early developmental exposure to ethanol in zebrafish. Alcohol Exp Clin Res. doi: 10.1111/j.1530-0277.2008.00874.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gerlai R, Ahmad F, Prajapati S. Differences in acute alcohol-induced behavioral responses among zebrafish populations. Alcohol Clin Exp Res. 2008;32:1763–73. doi: 10.1111/j.1530-0277.2008.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gerlai R, Lee V, Blaser R. Effects of acute and chronic ethanol exposure on the behavior of adult zebrafish (Danio rerio) Pharmacol Biochem Behav. 2006;85:752–61. doi: 10.1016/j.pbb.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Thielen RJ, Engleman EA, Rodd ZA, Murphy JM, Lumeng L, Li TK, et al. Ethanol drinking and deprivation alter dopaminergic and serotonergic function in the nucleus accumbens of alcoholpreferring rats. J Pharmacol Exp Ther. 2004;309:216–25. doi: 10.1124/jpet.103.059790. [DOI] [PubMed] [Google Scholar]
- [20].Sari Y, Bell RL, Zhou FC. Effects of chronic alcohol and repeated deprivations on dopamine D1 and D2 receptor levels in the extended amygdala of inbred alcohol-preferring rats. Alcohol Clin Exp Res. 2006;30:46–56. doi: 10.1111/j.1530-0277.2006.00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rodd-Henricks ZA, McKinzie DL, Edmundson VE, Dagon CL, Murphy JM, McBride WJ, et al. Effects of 5-HT(3) receptor antagonists on daily alcohol intake under acquisition, maintenance, and relapse conditions in alcohol-preferring (P) rats. Alcohol. 2000;21:73–85. doi: 10.1016/s0741-8329(00)00083-5. [DOI] [PubMed] [Google Scholar]
- [22].Lovinger DM. Serotonin’s role in alcohol’s effects on the brain. Alcohol Health Res World. 1997;21:114–20. [PMC free article] [PubMed] [Google Scholar]
- [23].Crabbe JC, Phillips TJ, Feller DJ, Hen R, Wenger CD, Lessov CN, et al. Elevated alcohol consumption in null mutant mice lacking 5-HT1B serotonin receptors. Nat Genet. 1996;14:98–101. doi: 10.1038/ng0996-98. [DOI] [PubMed] [Google Scholar]
- [24].Delgado L, Schmachtenberg O. Immunohistochemical localization of GABA, GAD65, and the receptor subunits GABA(Aalpha1) and GABA (B1) in the Zebrafish cerebellum. Cerebellum. 2008;7:444–50. doi: 10.1007/s12311-008-0047-7. [DOI] [PubMed] [Google Scholar]
- [25].Edwards JG, Greig A, Sakata Y, Elkin D, Michel WC. Cholinergic innervation of the zebrafish olfactory bulb. J Comp Neurol. 2007;504:631–45. doi: 10.1002/cne.21480. [DOI] [PubMed] [Google Scholar]
- [26].McLean DL, Fetcho JR. Relationship of tyrosine hydroxylase and serotonin immunoreactivity to sensorimotor circuitry in larval zebrafish. J Comp Neurol. 2004;480:57–71. doi: 10.1002/cne.20281. [DOI] [PubMed] [Google Scholar]
- [27].McLean DL, Fetcho JR. Ontogeny and innervation patterns of dopaminergic, noradrenergic, and serotonergic neurons in larval zebrafish. J Comp Neurol. 2004;480:38–56. doi: 10.1002/cne.20280. [DOI] [PubMed] [Google Scholar]
- [28].Rink E, Guo S. The too few mutant selectively affects subgroups of monoaminergic neurons in the zebrafish forebrain. Neuroscience. 2004;127:147–54. doi: 10.1016/j.neuroscience.2004.05.004. [DOI] [PubMed] [Google Scholar]
- [29].Yoshitake T, Yoshitake S, Fujino K, Nohta H, Yamaguchi M, Kehr J. High-sensitive liquid chromatographic method for determination of neuronal release of serotonin, noradrenaline and dopamine monitored by microdialysis in the rat prefrontal cortex. J Neurosci Methods. 2004;140:163–8. doi: 10.1016/j.jneumeth.2004.04.041. [DOI] [PubMed] [Google Scholar]
- [30].López Patiño MA, Yu L, Yamamoto BK, Zhdanova IV. Gender differences in zebrafish responses to cocaine withdrawal. Physiol Behav. 2008;95:36–47. doi: 10.1016/j.physbeh.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gerlai R, Chatterjee D, Pereira T, Sawashima T, Krishnannair R. Acute and chronic alcohol dose: population differences in behavior and neurochemistry of zebrafish. Genes Brain Behav. doi: 10.1111/j.1601-183X.2009.00488.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lockwood B, Bjerke S, Kobayashi K, Guo S. Acute effects of alcohol on larval zebrafish: a genetic system for large-scale screening. Pharmacol Biochem Behav. 2004;77:647–54. doi: 10.1016/j.pbb.2004.01.003. [DOI] [PubMed] [Google Scholar]
- [33].Guryev V, Koudijs MJ, Berezikov E, Johnson SL, Plasterk RH, van Eeden FJ, et al. Genetic variation in the zebrafish. Genome Res. 2006;16:491–7. doi: 10.1101/gr.4791006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gerlai R. Zebra fish: an uncharted behavior genetic model. Behav Genet. 2003;33:461–8. doi: 10.1023/a:1025762314250. [DOI] [PubMed] [Google Scholar]
- [35].Murray RM, Lappin J, Di Forti M. Schizophrenia: from developmental deviance to dopamine dysregulation. Eur Neuropsychopharmacol Suppl. 2008;3:S129–134. doi: 10.1016/j.euroneuro.2008.04.002. [DOI] [PubMed] [Google Scholar]
- [36].Horacek J, Bubenikova-Valesova V, Kopecek M, Palenicek T, Dockery C, Mohr P, et al. Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs. 2006;20:389–409. doi: 10.2165/00023210-200620050-00004. [DOI] [PubMed] [Google Scholar]
- [37].Stein DJ. Depression, anhedonia, and psychomotor symptoms: the role of dopaminergic neurocircuitry. CNS Spectr. 2008;13:561–5. doi: 10.1017/s1092852900016837. [DOI] [PubMed] [Google Scholar]
- [38].Belmaker RH. The future of depression psychopharmacology. CNS Spectr. 2008;13:682–7. doi: 10.1017/s1092852900013766. [DOI] [PubMed] [Google Scholar]
- [39].Ferrari-Toninelli G, Bonini SA, Cenini G, Maccarinelli G, Grilli M, Uberti D, et al. Dopamine receptor agonists for protection and repair in Parkinson’s disease. Curr Top Med Chem. 2008;8:1089–99. doi: 10.2174/156802608785161402. [DOI] [PubMed] [Google Scholar]
- [40].Lemke MR. Depressive symptoms in Parkinson’s disease. Eur J Neurol. 2008;15(Suppl 11):21–5. doi: 10.1111/j.1468-1331.2008.02058.x. [DOI] [PubMed] [Google Scholar]
- [41].Lanari A, Amenta F, Silvestrelli G, Tomassoni D, Parnetti L. Neurotransmitter deficits in behavioural and psychological symptoms of Alzheimer’s disease. Mech Ageing Dev. 2006;127:158–65. doi: 10.1016/j.mad.2005.09.016. [DOI] [PubMed] [Google Scholar]
- [42].Geldenhuys WJ, Van der Schyf CJ. Serotonin 5-HT6 receptor antagonists for the treatment of Alzheimer’s disease. Curr Top Med Chem. 2008;8:1035–48. doi: 10.2174/156802608785161420. [DOI] [PubMed] [Google Scholar]
- [43].Spanagel R, Weiss F. The dopamine hypothesis of reward: past and current status. Trends Neurosci. 1999;22:521–7. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- [44].Lovinger DM. 5-HT3 receptors and the neural actions of alcohols: an increasingly exciting topic. Neuroche Int. 1999;35:125–30. doi: 10.1016/s0197-0186(99)00054-6. [DOI] [PubMed] [Google Scholar]
- [45].Lovinger DM, Zhou Q. Alcohols potentiate ion current mediated by recombinant 5-HT3RA receptors expressed in a mammalian cell line. Neuropharmacology. 1994;33:1567–72. doi: 10.1016/0028-3908(94)90131-7. [DOI] [PubMed] [Google Scholar]
- [46].Flinn L, Bretaud S, Lo C, Ingham PW, Bandmann O. Zebrafish as a new animal model for movement disorders. Neurochemistry. 2008;106:1991–7. doi: 10.1111/j.1471-4159.2008.05463.x. [DOI] [PubMed] [Google Scholar]
- [47].Boehmler W, Obrecht-Pflumio S, Canfield V, Thisse C, Thisse B, Levenson R. Evolution and expression of D2 and D3 dopamine receptor genes in zebrafish. Dev Dyn. 2004;230:481–93. doi: 10.1002/dvdy.20075. [DOI] [PubMed] [Google Scholar]
- [48].Panula P, Sallinen V, Sundvik M, Kolehmainen J, Torkko V, Tiittula A, et al. Modulatory neurotransmitter systems and behavior: towards zebrafish models of neurodegenerative diseases. Zebrafish. 2006;3:235–47. doi: 10.1089/zeb.2006.3.235. [DOI] [PubMed] [Google Scholar]
- [49].Warsh JJ, Chiu A, Godse DD. Simultaneous determination of norepinephrine, dopamine and serotonin in rat brain regions by ion-pair liquid chromatography on octyl silane columns and amperometric detection. J Chromatogr. 1982;228:131–41. doi: 10.1016/s0378-4347(00)80426-0. [DOI] [PubMed] [Google Scholar]
- [50].Westerink BH, Mulder TB. Determination of picomole amounts of dopamine, noradrenaline 3,4-dihydroxyphenylalanine 3,4-dihydroxyphenylacetic acid, homovanillic acid, and 5-hydroxyindolacetic acid in nervous tissue after one-step purification on Sephadex G-10, using high-performance liquid chromatography with a novel type of electrochemical detection. J Neurochem. 1981;36:1449–62. doi: 10.1111/j.1471-4159.1981.tb00586.x. [DOI] [PubMed] [Google Scholar]
- [51].Yamaguchi M, Yoshitake T, Fujino K, Kawano K, Kehr J, Ishida J. Determination of norepinephrine in microdialysis samples by microbore column liquid chromatography with fluorescence detection following derivatization with benzylamine. Anal Biochem. 1999;270:296–302. doi: 10.1006/abio.1999.4079. [DOI] [PubMed] [Google Scholar]
- [52].Fujino K, Yoshitake T, Kehr J, Nohta H, Yamaguchi M. Simultaneous determination of 5-hydroxyindoles and catechols by high-performance liquid chromatography with fluorescence detection following derivatization with benzylamine and 1,2-diphenylethylenediamine. J Chromatogr A. 2003;1012:169–77. doi: 10.1016/s0021-9673(03)01180-4. [DOI] [PubMed] [Google Scholar]
- [53].Chi JD, Odontiadis J, Franklin M. Simultaneous determination of catecholamines in rat brain tissue by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1999;731:361–7. doi: 10.1016/s0378-4347(99)00255-8. [DOI] [PubMed] [Google Scholar]
- [54].Alvarez JC, Bothua D, Collignon I, Advenier C, Spreux-Varoquaux O. Simultaneous measurement of dopamine, serotonin, their metabolites and tryptophan in mouse brain homogenates by high-performance liquid chromatography with dual coulometric detection. Biomed Chromatogr. 1999;13:293–8. doi: 10.1002/(SICI)1099-0801(199906)13:4<293::AID-BMC863>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- [55].Afonso V, Grella SL, Chatterjee D, Fleming AS. Previous maternal experience affects accumbal dopaminergic responses to pup stimuli. Brain Res. 2008;1198:115–23. doi: 10.1016/j.brainres.2007.12.042. [DOI] [PubMed] [Google Scholar]
- [56].Gonzales RA, Weiss F. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci. 1998;18:10663–71. doi: 10.1523/JNEUROSCI.18-24-10663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103:121–46. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- [58].Al-Imari L, Gerlai R. Sight of conspecifics as reward in associative learning in zebrafish (Danio rerio) Behav Brain Res. 2008;189:216–9. doi: 10.1016/j.bbr.2007.12.007. [DOI] [PubMed] [Google Scholar]
- [59].Saverino C, Gerlai R. The social zebrafish: behavioral responses to conspecific, heterospecific, and computer animated fish. Behav Brain Res. 2008;191:77–87. doi: 10.1016/j.bbr.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Pather S, Gerlai R. Shuttle box learning in zebrafish (Danio rerio) Behav Brain Res. 2009;196:323–7. doi: 10.1016/j.bbr.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Shively CA, Grant KA, Register TC. Effects of long-term moderate alcohol consumption on agonistic and affiliative behavior of socially housed female cynomolgus monkeys (Macaca fascicularis) Psychopharmacology (Berl) 2002;165:1–8. doi: 10.1007/s00213-002-1223-y. [DOI] [PubMed] [Google Scholar]
- [62].Langen B, Dietze S, Fink H. Acute effect of ethanol on anxiety and 5-HT in the prefrontal cortex of rats. Alcohol. 2002;27:135–41. doi: 10.1016/s0741-8329(02)00219-7. [DOI] [PubMed] [Google Scholar]
- [63].Thielen RJ, Bare DJ, McBride WJ, Lumeng L, Li TK. Ethanol-stimulated serotonin release in the ventral hippocampus: an absence of rapid tolerance for the alcohol-preferring P rat and insensitivity in the alcohol-nonpreferring NP rat. Pharmacol Biochem Behav. 2002;71:111–7. doi: 10.1016/s0091-3057(01)00633-5. [DOI] [PubMed] [Google Scholar]
- [64].Yan QS. Extracellular dopamine and serotonin after ethanol monitored with 5-minute microdialysis. Alcohol. 1999;19:1–7. doi: 10.1016/s0741-8329(99)00006-3. [DOI] [PubMed] [Google Scholar]
- [65].Ciccocioppo R. The role of serotonin in craving: from basic research to human studies. Alcohol. 1999;34:244–53. doi: 10.1093/alcalc/34.2.244. [DOI] [PubMed] [Google Scholar]


