Abstract
Platelet endothelial cell adhesion molecule (PECAM)-1 has been previously implicated in endothelial cell migration; additionally, anti-PECAM-1 antibodies have been shown to inhibit in vivo angiogenesis. Studies were therefore performed with PECAM-1-null mice to further define the involvement of PECAM-1 in blood vessel formation. Vascularization of subcutaneous Matrigel implants as well as tumor angiogenesis were both inhibited in PECAM-1-null mice. Reciprocal bone marrow transplants that involved both wild-type and PECAM-1-deficient mice revealed that the impaired angiogenic response resulted from a loss of endothelial, but not leukocyte, PECAM-1. In vitro wound migration and single-cell motility by PECAM-1-null endothelial cells were also compromised. In addition, filopodia formation, a feature of motile cells, was inhibited in PECAM-1-null endothelial cells as well as in human endothelial cells treated with either anti-PECAM-1 antibody or PECAM-1 siRNA. Furthermore, the expression of PECAM-1 promoted filopodia formation and increased the protein expression levels of Cdc42, a Rho GTPase that is known to promote the formation of filopodia. In the developing retinal vasculature, numerous, long filamentous filopodia, emanating from endothelial cells at the tips of angiogenic sprouts, were observed in wild-type animals, but to a lesser extent in the PECAM-1-null mice. Together, these data further establish the involvement of endothelial PECAM-1 in angiogenesis and suggest that, in vivo, PECAM-1 may stimulate endothelial cell motility by promoting the formation of filopodia.
Platelet endothelial cell adhesion molecule-1 (PECAM-1) is a 130-kd transmembrane glycoprotein member of the Ig superfamily expressed on endothelial cells (ECs) as well as leukocytes and platelets.1 Although it was originally recognized as a protein capable of binding interactions with itself and possibly other non-PECAM-1 molecules,2,3,4,5,6 there is now a significant body of evidence that PECAM-1 also participates in signaling cascades.7,8 Of note, PECAM-1 has two tyrosine residues (Y663 and Y686) that each fall within a conserved signaling sequence known as the immunoreceptor tyrosine-based inhibitory motif.9,10 Phosphorylation of these tyrosine residues enables the binding of Src homology (SH)-2 domain containing molecules, including the SHP-2 tyrosine phosphatase.11,12,13,14 Consistent with an involvement in blood vessel formation,15,16,17 PECAM-1 has been implicated in the adhesive and signaling events required for endothelial cell migration,17,18 a process critical to angiogenesis. These studies of human ECs, and cellular transfectants expressing human PECAM-1, have suggested that PECAM-1 may promote endothelial cell motility by recruiting SHP-2 to the cell membrane, which induces the turnover of focal adhesions.18
The finding that anti-PECAM-1 antibodies impair vessel formation supports a role for PECAM-1 during in vivo angiogenesis.15,16,17 Mice deficient in the expression of PECAM-1 are viable, suggesting that vascular development in the absence of PECAM-1 is sufficient to allow for adequate embryogenesis.19 However, subsequent studies have shown that the loss of PECAM-1 results in decreased neutrophil recruitment in response to interleukin-120,21 and in other inflammatory settings,22,23 enhanced susceptibility to endotoxic shock,24 increased endothelial sensitivity to apoptotic stress,25 and impaired alveolarization.26 To date, however, in vivo angiogenesis has been investigated in these animals in only a limited number of reports.27,28 Studies were therefore performed in PECAM-1-null mice to more fully define the formation of vessels in several animal models, as well as the functional activity of ECs isolated from wild-type and PECAM-null mice.
We found that vascularization of subcutaneous Matrigel implants, as well as tumor angiogenesis, were inhibited in PECAM-1-null mice. Reciprocal bone marrow transplants involving wild-type and PECAM-1-deficient mice revealed that the impaired angiogenic response resulted from a loss of endothelial PECAM-1 and not leukocyte PECAM-1. In subsequent studies of ECs isolated from these animals, we found that in vitro cell migration was significantly compromised in the ECs isolated from PECAM-1-deficient mice. Further, a feature of an actively motile cell includes the presence of cellular protrusions known as filopodia,29,30 which mediate several functions required for cell migration. The formation of filopodia was impaired in PECAM-1-null ECs, and in human umbilical vein endothelial cells (HUVEC) treated with anti-PECAM-1 antibody or in which PECAM-1 expression had been knocked down by siRNA. In addition, expression of PECAM-1 in cellular transfectants promoted filopodia formation. The expression of PECAM-1 increased the protein levels of Cdc42, a Rho GTPase known to promote the formation of filopodia.31,32,33,34,35 These in vitro data were consistent with the finding that reduced numbers of endothelial filopodial extensions were detected in PECAM-1-null mice during postnatal vascularization of the retina in the developing murine eye. Together, these data further establish the involvement of endothelial PECAM-1 in the formation of vessels and suggest that, in vivo, PECAM-1 may stimulate endothelial cell motility by promoting the formation of filopodia.
Materials and Methods
Reagents and Chemicals
All reagents and chemicals were obtained from Sigma (St. Louis, MO) unless otherwise specified.
Antibodies
The following antibodies against murine surface receptors were used: mAb 390, rat anti-PECAM-115,16; rat anti-ICAM-136; and rat anti-ICAM-2 (Southern Biotech, Birmingham, AL). The following antibodies were also used: Cdc42 and VE-cadherin (Santa Cruz Biotechnology, Santa Cruz, CA); Rac1 and RhoA (BD Transduction Laboratories, San Jose, CA); and GAPDH (Chemicon/Millipore, Temecula, CA). Cell surface antibody binding was determined by flow cytometry using previously described procedures.18
Cell Lines
The H5V murine endothelial line,37 B16 murine melanoma line (obtained from the ATCC), and ID8-VEGF tumor line38 were cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum, penicillin/streptomycin, and 2 mmol/L l-glutamine, with insulin also added to the medium for the culturing of the ID8-VEGF cells. Lung ECs were isolated from wild-type and PECAM-1-null mice using published protocols.39 Mouse ECs and HUVEC (Clonetics, San Diego, CA) were cultured on gelatin in medium 199 containing 12.7% fetal bovine serum, 75 μg/ml endothelial growth factor, 100 μg/ml heparin and 2 mmol/L glutamine. Mouse ECs and HUVEC were used between passages 2 and 6. The human mesothelioma cell line, REN,18 was cultured in RPMI medium supplemented with 10% fetal bovine serum, penicillin/streptomycin, and 2 mmol/L l-glutamine. REN cell transfectants stably expressing human PECAM-1 (REN-HP) were cultured in the same media with G418 (0.5 g/L; Gibco BRL, Grand Island, NY).
Animals
The Institutional Animal Care and Utilization Committees at both the Wistar Institute and the University of Pennsylvania School of Medicine approved all animal care procedures. PECAM-1-null mice that had been backcrossed for >10 generations onto a C57BL/6 background22 were the kind gift of Dr. Joseph Madri (Yale University) through Dr. Steven Albelda (University of Pennsylvania). Wild-type mice, also on a C57BL/6 background, were obtained from Taconic (Germantown, NY).
Matrigel Neovascularization Model
Wild-type and PECAM-1-null mice in the C57BL/6 background were injected subcutaneously with 0.3 ml of Matrigel (Chemicon/Millipore) containing 1 × 106 B16 melanoma cells to induce the growth of vessels into the gel. After 5 to 7 days, the animals were sacrificed. The gels were then harvested and either processed for hemoglobin analysis as a measure of the vascularization of the gels or fixed in 10% formalin for paraffin sections and hematoxylin and eosin staining. Tissue adjacent to as well as distant from the gel were also harvested and processed for silver staining as previously described.40
Tumor Growth and Angiogenesis
A total of 2 × 106 B16 melanoma or ID8-VEGF ovarian tumor cells in a total volume of 50 μl were injected subcutaneously into the flanks of wild-type and PECAM-1-null mice. After 14 days (B16 melanoma) or 9 weeks (ID8-VEGF ovarian tumor), the mice were sacrificed and the tumors harvested, weighed, and processed for staining. For quantitation of angiogenesis, frozen sections of comparably sized tumors were stained with ICAM-2 antibody to identify murine blood vessels. To assess the tumor angiogenic response, serial sections were obtained from different levels within the tumor separated by ∼100 μm. A total of four to eight levels per tumor and four to six 40X fields per level were analyzed. Computer-assisted image analysis (Image-Pro Plus program, Media Cybernetics, Silver Spring, MD) was then used to determine the percentage of tumor occupied by vessels.
Generation of Bone Marrow Chimeric Animals
Bone marrow chimeric mice were generated as previously described.41 Briefly, to generate recipient animals, 6-week-old wild-type or PECAM-1 null mice were irradiated with 1000 rads from a Cs-137 irradiation source. Within 24 hours after irradiation, donor marrow was obtained from the femur and tibia of non-irradiated mice, and 5 × 106 cells were injected via the tail vein into the irradiated recipient mice. Experiments were subsequently conducted 4 to 6 weeks after transplantation. Flow cytometry analysis of leukocytes, using an anti-mouse PECAM-1 antibody, confirmed the phenotype of each chimeric mouse.
Immunohistochemical Staining
Immunohistochemistry was performed using a commercially available kit according to the manufacturer’s instructions (ABC Immunodetection kit, Vector Laboratories, Burlingame, CA). Briefly, 6-μm-thick sections were prepared by cryostat, transferred to glass slides, and fixed in ice-cold acetone and rinsed in phosphate-buffered saline (PBS). The sections were then treated with 0.5% H2O2 in PBS for 30 minutes, and then blocked with 0.5% bovine serum albumin for 30 minutes. The primary antibody was applied for 1 hour, washed, and then incubated with biotinylated secondary antibody for 60 minutes. The reaction was developed with an avidin-biotin complex reaction and the sections lightly counterstained with hematoxylin.
In Vitro Cell Proliferation
ECs were cultured for 24 hours in 96-well plates (4000 cells/well) and the number of viable cells determined using the Promega CellTiter 96 AQueous non-radioactive cell proliferation assay (Madison WI).
In Vitro Cell Death Detection
For the studies of apoptosis, confluent cells were exposed for 5 hours to serum-free medium or complete medium with or without antibody. Apoptosis was then assessed using the APOPercentage apoptosis assay (Biocolor Ltd, Belfast, N. Ireland).
In Vitro Wound-Induced Migration Assay
Endothelial cell wounding was performed as previously described.18 Twenty thousand murine ECs (primary or H5V cells) were added to 24-well tissue culture plates and allowed to grow to confluence. Linear (primary ECs) or circular (H5V cells) defects were then scratched into the monolayer. The wounded culture was washed with PBS and then incubated for 24 hours in medium (with 1% serum) with antibodies (100 μg/ml) included for studies with H5V cells. Images were obtained immediately after wounding and then again 24 hours later. The distance migrated by cells at the wound edge (primary ECs) or change in wound area (H5V cells) were determined using computer-assisted image analysis. For each condition, three to five wounds were analyzed. The data are presented as distance migrated (primary ECs) or as change in wound area expressed as a percentage of control (H5V cells).
In Vitro Matrigel Invasion/Migration Assay
Matrigel-coated Transwell inserts (Costar; 8-μm pore filter) were prepared by twice adding 100 μl of Matrigel (250 μg/ml) to the Transwell and allowing the Matrigel to dry at 37°C in a non-humidified oven for 24 hours. Murine ECs were labeled overnight with [3H]thymidine and resuspended to a concentration of 200,000 cells/ml in low serum media (5% serum), with antibodies (100 μg/ml) included for studies with H5V cells. The resulting cell suspensions (500 μl) were then placed in Transwell filter inserts, which in turn were placed in 12-well plates containing 20% serum media and incubated for 8 (H5V cells) or 18 (primary EC) hours at 37°C in 5% CO2. The cells migrated through the Matrigel, passed through the pores of the filter, and adhered on the lower surface of the filter. After incubation the wells were removed, washed, and the top surface of the filter wiped with a cotton swab. The filters were then carefully cut out, placed in scintillation fluid, and counted in a β-counter. The data are presented as percent cells migrated (primary ECs) or as cells migrated expressed as a percentage of control (H5V).
Assessing the Morphology of Plated Cells
Mouse ECs, HUVEC, or REN cells resuspended in complete medium were seeded into four-well Lab-Tek chamber slides (Nunc, Thermo Fischer Scientific, Rochester, NY), that were uncoated (REN cells) or coated with 1% gelatin (ECs). Subsequently, the cells were allowed to spread for predetermined times at 37°C, fixed with 2% paraformaldehyde for 5 minutes and then stained with 0.1% toluidine blue. Images were then captured and analyzed by computer-assisted image analysis.
siRNA-Mediated Knockdown of Protein Expression
siRNA oligos specific for PECAM-1 were obtained from Applied Biosystems/Ambion (Austin, TX). To knock down protein expression, cells were grown to 80% confluence in 12-well dishes and then transduced at 37°C 5% CO2 for 6 hours with 0.1 μmol/L siRNA according to the manufacturer’s (Santa Cruz) instructions.
Visualization and Quantification of Retinal Vasculature
The vasculature of the developing mouse retina was processed and visualized using published procedures.42 Briefly, mouse eyes were fixed in 4% paraformaldehyde in PBS at 4°C overnight and then washed with PBS. Retinas were dissected, permeabilized in PBS with 1% bovine serum albumin and 0.5% Triton X-100 at 4°C overnight, rinsed in PBS, washed twice in PBlec (PBS, pH 6.8, 1% Triton-X100, 0.1 mmol/L CaCl2, 0.1 mmol/L MgCl2, 0.1 mmol/L MnCl2), and incubated in biotinylated isolectin B4 (Bandeiraea simplicifolia, Sigma-Aldrich), 20 μg/ml in PBlec at 4°C overnight. After five washes in PBS, samples were incubated with fluorescein avidin D (Vector Laboratories, Burlingame, CA) diluted 1:100 in PBS, 0.5% bovine serum albumin, and 0.25% Triton X-100 at 4°C for 6 hours. After washing and a brief postfixation in paraformaldehyde, the retinas were flat-mounted using Vectashield Hard Set (H-1400, Vector Laboratories) and then analyzed using fluorescence microscopy (Olympus IX70) and the Metamorph imaging system (Molecular Devices, Downingtown, PA).
Western Blotting
Total cell lysates were loaded in equal protein amounts (10 μg) determined by BCA (Pierce, Rockford, IL). Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Novex; Invitrogen) followed by transfer onto nitrocellulose membranes using the iBlot dry blotting system (Invitrogen), which employs semidry electrotransfer. Membranes were washed in 1X TTBS for 2 to 3 minutes, blocked with 5% blotting grade blocker solution from Bio-Rad Laboratories (Hercules, CA), and incubated with the primary antibody in 2% bovine serum albumin for 1 hour at room temperature. Unbound antibodies were washed off with TTBS before membranes were incubated with horseradish peroxidase-labeled species-specific secondary antibodies for 1 hour at room temperature. After again washing the membranes with PBS, bound antibody signals were detected by enhanced chemiluminescence substrate and documented on X-ray film. The chemiluminescent signals were quantified by densitometry (ImageQuant, Amersham, Piscataway, NJ) and normalized to the housekeeping protein GAPDH.
Statistical Analyses
Differences among groups were analyzed using one-way analysis of variance. Results are presented as means ± SE. When statistically significant differences were found (P < 0.05), individual comparisons were made using the Bonferroni/Dunn test.
Results
Reduced Vascularization of Matrigel Implants in PECAM-1-Null Mice
To confirm the results of previous antibody studies implicating PECAM-1 in vessel formation, in vivo murine angiogenesis was studied in mice deficient in the expression of PECAM-1.19 Initial studies were done with a model in which vessels develop over 5 to 7 days around and within subcutaneously implanted Matrigel plugs containing B16 tumor cells as a source of angiogenic growth factors (Figure 1). After 5 days, a “blush” of vessel proliferation was seen surrounding plugs in the wild-type animals that was not evident in the PECAM-1-null mice (Figure 1, A and B). Further histological analysis of extracted implants demonstrated that the vascularization of implants from PECAM-1-null animals was strikingly less than that observed for the wild-type mice (Figure 1, C and D). Consistent with this, vascularization of the plugs, as assessed by hemoglobin concentration, was significantly reduced (50%) in the PECAM-1 knockout mice compared with wild-type animals (Figure 1E). Silver staining of vessels in the skin was also done to assess the morphology of vessels invading the Matrigel plugs (Figure 2). Vessels remote from the Matrigel plugs in both animal strains were similar in appearance and size (Figure 2, A and B). In contrast, the vessels adjacent to the Matrigel implants were much more dilated and tortuous in the wild-type mice, compared with those of the PECAM-1-deficient animals (Figure 2, C and D). This suggests that the absence of PECAM-1 may disrupt and alter in vivo angiogenesis.
Figure 1.
Neovascularization of Matrigel implants in wild-type and PECAM-1-null mice. Shown are images of subcutaneous Matrigel implants (asterisks) containing B16 melanoma cells as a source of angiogenic factors harvested after 5 days from wild-type (A) and PECAM-1-null (PECAM KO) (B) animals. A “blush” of vessel proliferation (white arrow) was evident around the implants from the wild-type animal that was not present in the PECAM-1-null mice. H&E staining demonstrated the presence of many more vessels (black arrows) invading the gels in the wild-type (C) mice compared with the gels from the PECAM-1-deficient mice (D). The vascularization of the gels, as assessed by hemoglobin concentration (E), was significantly reduced in PECAM-1-null mice compared with wild-type mice. Data are presented as means ± SE (n = 10, *P < 0.01).
Figure 2.
Morphology of vessels remote from and adjacent to Matrigel implants in wild-type and PECAM-1-null mice. Skin tissue was harvested from wild-type (A and C) and PECAM-1-null (PECAM-1 KO) mice (B and D) from areas remote from (A and B) and adjacent to (C and D) the Matrigel implants and the vasculature visualized by silver staining. In areas remote from the Matrigel plugs the morphology and pattern of the vasculature were similar in wild-type and PECAM-1null mice (A and B, red arrows), while the vessels adjacent to and invading the gels in PECAM-1-null mice were less dilated and tortuous than the comparable vessels in wild-type animals (C and D, white arrows).
Reduced Tumor Growth and Angiogenesis in PECAM-1-Null Mice
To extend the findings of the Matrigel studies, the subcutaneous growth and associated tumor angiogenesis of an ovarian tumor line overexpressing VEGF (ID8) and the B16 melanoma line were investigated in PECAM-1-null mice (Figure 3). When compared with the growth observed in wild-type mice, it was noted that the growth of both of these tumors was significantly reduced (40 to 50%) in the PECAM-1-deficient animals (Figure 3, A–D). The vessel density of comparably sized tumors obtained from wild-type and mutant mice was subsequently determined using ICAM-2 staining to identify murine vessels (Figure 3, E and F). Using computer-assisted image analysis, the vessel density was found to be significantly reduced for both tumors grown in the PECAM-1-null mice. This suggests that the inhibition of tumor growth in the PECAM-1-null mice resulted from a reduced angiogenic response.
Figure 3.
Tumor growth and angiogenesis in wild-type and PECAM-1-null mice. The growth of the ID8-VEGF ovarian tumor line (A and C) and a B16 melanoma line (B and D), as accessed by tumor weight, was significantly inhibited in the PECAM-1-null (PECAM-1 KO) mice (ID8, n = 10, *P < 0.002; B16, n = 22, **P = 0.05). The vessel densities in tumors of comparable sizes, as assessed by percentage of the tumor occupied by vessels (E and F), were significantly reduced in the PECAM-1-null mice (ID8, n = 10, *P < 0.02; B16, n = 8, **P < 0.008). Data are presented as means ± SE.
Murine Angiogenesis Involves Endothelial PECAM-1
Recruited leukocytes, tissue macrophages, and circulating bone marrow-derived progenitor endothelial cells have been identified as cellular participants during in vivo angiogenesis and thus, as PECAM-1 expressing cells, they may also contribute to the participation of PECAM-1 in this process.43,44,45,46,47,48 To identify the possible involvement of PECAM-1 expressed on leukocytes, macrophages, and/or circulating endothelial progenitor cells in vessel formation, bone marrow chimeric animals were generated to selectively propagate bone marrow-derived wild-type vascular cells against a background of PECAM-1-deficient endothelium. This approach was used successfully to distinguish the importance of endothelial versus platelet PECAM-1 in hemostasis.41 Experiments were done in which the following chimeric (donor-recipient) mice were generated: wild-type into wild-type (WTBM-WTEC; wild-type control), wild-type into PECAM-1 knockout (WTBM-KOEC) and PECAM-1 knockout into PECAM-1 knockout (KOBM-KOEC; knockout control). Flow cytometry analysis confirmed that the blood leukocytes from the WTBM-WTEC and WTBM-KOEC mice expressed PECAM-1, while the leukocytes from the KOBM-KOEC animals were devoid of PECAM-1 (Figure 4, A–C). The vascularization of subcutaneously implanted Matrigel plugs was subsequently studied (Figure 4D). The angiogenic responses in the WTBM-KOEC and KOBM-KOEC mice were very similar but were significantly reduced compared with the WTBM-WTEC mice. The levels of inhibition in the WTBM-KOEC and KOBM-KOEC chimeric animals (50%) were comparable with what was observed with the PECAM-1-null mice (Figure 1). The failure of wild-type, PECAM-1-positive leukocytes and/or bone marrow-derived endothelial progenitor cells to restore the wild-type phenotype in the knockout animals is consistent with the endothelial involvement of PECAM-1 during in vivo angiogenesis.
Figure 4.
Neovascularization of Matrigel implants in bone marrow chimeric mice. Vascularization of subcutaneous Matrigel implants was studied in the following chimeric (donor-recipient) mice: wild-type into wild-type (WTBM-WTEC), wild-type into PECAM-1-null (WTBM-KOEC), PECAM-1-null into PECAM-1-null (KOBM-KOEC). Flow cytometry analysis confirmed the successful engraftment of the mice (A--C). The angiogenic responses (assessed by hemoglobin concentration) WTBM-KOEC KOBM-KOEC were very similar but were significantly reduced compared with the WTBM-WTEC (D). Data are presented as means ± SE (n = 8, *P < 0.05).
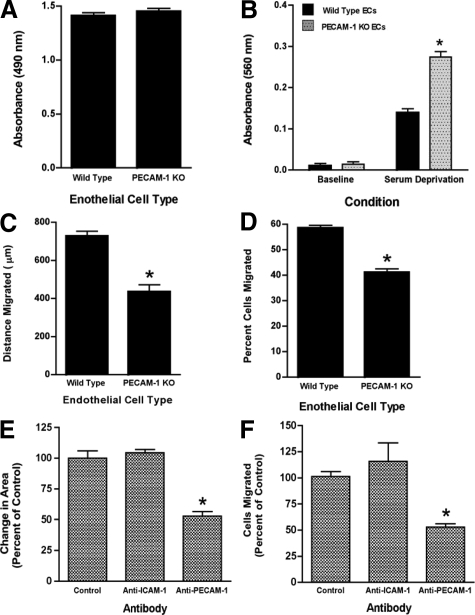
Reduced Cell Migration by PECAM-1-Null ECs
The studies in the bone marrow chimeric animals indicate that the loss of endothelial PECAM-1 function inhibits in vivo angiogenesis. To further investigate this, the activity of endothelial cells isolated from wild-type and PECAM-1-null animals was studied. Cell proliferation and levels of apoptosis (in the absence of stress) in wild-type and PECAM-1-deficient ECs were comparable (Figure 5, A and B). However, it was found that wound-induced migration, as well as the migration of single cells through matrix-coated filters, were significantly less in PECAM-1-null ECs compared with wild-type ECs (Figure 5, C and D). These findings are consistent with previous studies, which have demonstrated that expression of PECAM-1 in cellular transfectants increases cell motility17,18 and suggest that inhibition of vessel formation observed in PECAM-1-null mice is due in part to impairment in PECAM-1-dependent cell motility. Also consistent with these data is the finding that (acute) inhibition of murine PECAM-1 function with an anti-PECAM-1 antibody inhibits the cell migration of a murine endothelial cell line (Figure 5, E and F).
Figure 5.
In vitro function of murine ECs isolated from wild-type and PECAM-1-null mice. A: The proliferation of wild-type and PECAM1-null (PECAM-1 KO) ECs cultured for 24 hours in the presence of serum was assessed using a colorimetric assay and measurement of the reaction mixture at 490 nm. The proliferative responses of the two cell types were comparable (n = 4). B: Apoptosis was assessed after 5 hours in the presence or absence of serum. PECAM-1 KO ECs were more susceptible to apoptosis induced by serum deprivation (n = 15, *P < 0.001, compared with wild-type ECs). C: Linear defects were made in confluent cell monolayers and closure of the wounds after 24 hours was assessed by computer-assisted image analysis. Wound-induced migration was decreased in the PECAM-1-null ECs (n = 8, *P < 0.001). D: The percentage of cells migrating through Matrigel-coated Transwell filters was reduced in the PECAM-1-deficient ECs (n = 4, *P < 0.001). E and F: Anti-mouse PECAM-1 antibody inhibited the closure of circular wounds in confluent monolayers of H5V murine ECs (n = 4, *P < 0.0002, compared with control) as well as the migration of these cells through Matrigel-coated filters (n = 3–4, * P < 0.05, compared with control). Data are presented as means ± SE.
Loss of PECAM-1 Inhibits the Formation of Filopodia by ECs, while Filopodia Formation Is Stimulated by the Expression of PECAM-1
The phenotype of a motile cell is characterized morphologically by the presence of cellular protrusions such as filopodia and lamellipodia.29,30 Given the involvement of PECAM-1 in the motility of ECs, we analyzed the initial morphology of wild-type and PECAM-1-deficient murine ECs plated on gelatin as they transitioned over 2 to 3 hours from adherent rounded cells to flattened spread cells to polarized cells with various cellular protrusions. Within 40 minutes after plating, the majority of wild-type ECs (nearly 70%) displayed various filopodial extensions, while most (>90%) of the PECAM-1-null cells still demonstrated a rounded or flattened morphology (Figure 6, A–C). The absence of PECAM-1, however, did not prevent the eventual formation of filopodia, as after 90 minutes, the null cells did begin to form filopodia (data not shown). We also observed that the morphology of HUVEC in which PECAM-1 expression had been suppressed by ∼90%, using siRNA treatment, was notable for few cells with long cellular extensions, compared with the cells that were treated with control siRNA (Figure 7, A–D). To confirm these findings from murine and human ECs, we investigated the effect of expressing human PECAM-1 in the REN cell human mesothelioma line (which does not express PECAM-1).18 The expression of human PECAM-1 in REN cells resulted in filopodia that were significantly longer than those that formed in the control cells. The mean filopodial length was 27.4 μm for REN-HP cells compared with 15.1 for the REN cells (n = 60, P < 0.0001; data are presented as SE). Thus while nearly 80% of the filopodia in the control cells were less than 20 μm in length, the majority (67%) of filopodia in the REN cell transfectants were longer than 20 μm, with more than 16% extending beyond 40 μm (Figure 8, A–C). Taken together these data indicate that PECAM-1 increases the rate and/or promotes the efficiency of filopodia formation in ECs.
Figure 6.
Filopodia formation by wild-type and PECAM-1-null ECs. Shown are wild-type (A) and PECAM-1-null (PECAM-1 KO) ECs (B) 40 minutes after plating on gelatin. At this time point, the wild-type ECs have quickly transitioned to a morphology in which the majority of cells have filopodia (A1-3, arrows). In contrast, the PECAM-1 KO ECs at 40 minutes have attached and spread, but few display filopodia (B1–3). The formation of filopodia was assessed by determining the percentage of cells with filopodial extensions (C) and was found to be significantly reduced in the PECAM-1-null ECs. Data are presented as means ± SE (n = 4, *P < 0.0001).
Figure 7.
Filopodia formation by HUVEC treated with PECAM-1 siRNA. HUVEC plated on gelatin were transduced with nonspecific (A) or PECAM-1 (B) siRNA and then viewed after 72 hours (at the point of maximal PECAM-1 suppression). Cells with filopodia were numerous in the HUVEC treated with control siRNA (A1 and 2, black arrows), while most of the HUVEC exposed to PECAM-1 siRNA lacked filopodial extensions (B1 and 2, white arrows). The formation of filopodia was assessed by determining the percentage of cells with filopodial extensions (C) and was found to be very significantly reduced in the cells treated with PECAM-1 siRNA (n = 5, *P < 0.0001). Data are presented as means ± SE. Western blot analysis is displayed from cell lysates of HUVEC treated with nonspecific or PECAM-1 siRNA (D). PECAM-1 expression after PECAM-1 siRNA treatment was reduced to 12% of the expression in nonspecific siRNA controls as assessed by densitometry. Staining for β-actin demonstrated equal loading (data not shown).
Figure 8.
Filopodia formation by REN cells and REN cells expressing human PECAM-1. Shown are REN cells (A) and REN-HP cells (B). Filopodia were short and rudimentary in the REN cells (A, black arrows), but were increased in length in cells (B, white arrows). The frequency distribution of the lengths of the filopodia (< 20 μm, 20–40 μm, and >400 μm) was plotted (C) and revealed that unlike the REN cells, the majority of filopodia emanating from the REN-HP cells were greater than 20 μm and frequently extended beyond 40 μm (n = 60).
Anti-PECAM-1 Antibodies Inhibit Filopodia Formation by HUVEC
PECAM-1 has been reported to participate in binding interactions both with itself (homophilic adhesion) and with several non-PECAM-1 molecules (heterophilic adhesion), including heparan-containing proteoglycans.2,3,4,5,6 To determine whether PECAM-1-dependent ligand interactions might play a role in the ability of PECAM-1 to promote endothelial filopodia formation, we studied the effects of two functionally distinct antibodies: mAb 37, which blocks heterophilic binding; and mAb 62, which blocks both homophilic and heterophilic bindings.49 We found that the initial formation of filopodia by HUVEC was suppressed by both antibodies but not by control IgG (Table 1). Given the functional properties of the antibodies (mAb 37 only blocks heterophilic binding) and the fact that these studies were done with non-confluent cells, these data suggest that endothelial cell filopodia formation may be mediated by PECAM-1-dependent heterophilic binding to matrix proteins.
Table 1.
Effect of Anti-PECAM-1 Antibody on the Formation of Filopodia by HUVEC
HUVEC were plated on gelatin in the presence of IgG, mAb 37, or mAb 62 (100 μg/ml), and the percentage of cells with filopodia was determined after 1 hour. Compared with IgG, both antibodies decreased the percentage of cells with filopodia (n = 5–7, *P < 0.01). Data are presented as means ± SE.
P < 0.01.
Expression of PECAM-1 Increases Cdc42 Levels
Rho GTPases, including Rho, Rac, and Cdc42, act as molecular switches that regulate signal transduction pathways by cycling between a GDP-bound inactive form and a GTP-bound active form.31,32,33,34,35 Although they influence a wide range of biochemical processes, their best-defined activity is in the regulation of actin dynamics: Rho A causes the formation of stress fibers; Rac1 results in the formation of lamellipodia and membrane ruffling; and Cdc42 leads to the formation of filopodia.31,32,33,34,35 Given the finding that the expression of PECAM-1 promotes filopodia formation, we sought to determine the expression level of Cdc42 in cells expressing PECAM-1. We observed that the loss of PECAM-1 in murine ECs was associated with decreased Cdc42 protein expression (Figure 9, A and B), while the presence of PECAM-1 in REN cells up-regulated the expression of Cdc42 (Figure 9, C and D). In contrast, the expression of RhoA and Rac1 appeared to be independent of changes in the levels of PECAM-1.
Figure 9.
PECAM-1 expression and Cdc42 levels. Cell lysates from wild-type and PECAM-1-null (PECAM-1 KO) ECs (A and B), and from REN cells and REN-HP cells (C and D) were immunoblotted with antibodies against PECAM-1, Cdc42, Rac1, RhoA, VE-cadherin, and GAPDH. The loss of PECAM-1 in murine ECs decreased Cdc42 protein expression (A), while the presence of PECAM-1 in REN-HP cells increased the expression of Cdc42 (C). Densitometric analysis was performed (B and D). The data were normalized to GAPDH and expressed for the PECAM-1 KO ECs as fold change compared with wild-type ECs (n = 2) and for the REN-HP cells as fold change compared with REN cells (n = 3). Data are presented as means ± SE.
Decreased Endothelial Tip Cell Filopodia in PECAM-1-Null Mice during Vascularization of the Developing Retina
The development of the retinal vasculature of mice is completed after birth and provides an excellent model for studying postnatal physiological angiogenesis.50,51 It involves the initial formation of a superficial vascular plexus (week 1) across the surface of the retina that subsequently sprout vessels that penetrate into the retina to give rise to the deep retinal vascular plexuses (weeks 2–4). The developing superficial vascular plexus, as it advances from the central retinal artery across the retinal surface, provides an in vivo system for studying ECs located at the tips of angiogenic sprouts and the filopodia emanating from these tip cells. We therefore analyzed the retinal vasculature from 5-day-old wild-type and PECAM-1-null mice that were stained with fluorescein isothiocyanate-labeled isolectin-B. The density of the developing retinal vasculature of the PECAM-1-null mice was significantly less than that of the wild-type animals (Figure 10, A and B). For PECAM-1-null mice the number of retinal vessel branch points/mm2 and closed capillary loops/mm2 were decreased, while the mean retinal area (μm2) enclosed within a closed capillary loop was increased, data that are all consistent with a less dense vascular plexus (Table 2). Further, in the retina of wild-type mice, numerous angiogenic sprouts are observed at the advancing edge of the vascular plexus, with multiple long filamentous filopodia extending from the ECs at the tips of each sprout (Figure 10, C and E). In contrast, the angiogenic sprouts of the retinal vasculature from the PECAM-1-deficient mice tended to appear more blunt ended and the filopodial extensions emanating from the sprouts were fewer in number and shorter in length (Figure 10, D and F). These data provide further (in vivo) evidence of the involvement of PECAM-1 in the formation of endothelial cell filopodia.
Figure 10.
Postnatal retinal vascularization. Shown is the leading edge of the developing retinal vasculature, stained by fluorescein isothiocyanate-labeled isolectin-B in wild-type (A, C, E), and PECAM-1-null mice (B, D, F). The density and complexity of the vascular plexus were greater in the wild-type (A) compared with the PECAM-1-deficient mice (B) (see Table 2). Long filamentous filopodia extending from the endothelial tip cells of the angiogenic sprouts were observed in the wild-type mice (C and E, white arrows), while the angiogenic sprouts of the retinal vasculature from the PECAM-1-null mice (D and F, red arrows) were more blunt ended and displayed few of the filamentous filopodial projections.
Table 2.
Quantitation of the Vascularization of the Developing Postnatal Mouse Retina
| Mouse strain | Number of branch points/mm2 | Number of capillary loops/mm2 | Mean retinal area enclosed within a capillary loop (μm2) |
|---|---|---|---|
| Wild-type | 349 ± 31 | 373 ± 22 | 2133 ± 76 |
| PECAM-1-null | 221 ± 11* | 235 ± 13* | 3686 ± 186* |
The number of retinal vessel branch points/mm2 (n = 6–8) and closed capillary loops/mm2 (n = 6–8), as well as the mean retinal area (μm2) enclosed within a closed capillary loop (n = 9–12), were determined for wild-type and PECAM-1-null mice (
P < 0.0001).
Discussion
In this report, studies were done with PECAM-1-null mice to further define the involvement of PECAM-1 during in vivo angiogenesis. We found that vascularization of subcutaneous Matrigel implants, as well as tumor angiogenesis, were inhibited in PECAM-1-null mice. Reciprocal bone marrow transplants involving wild-type and PECAM-1-deficient mice revealed that the impaired angiogenic response resulted from a loss of endothelial and not leukocyte PECAM-1. In subsequent studies of ECs isolated from these animals, we found that in vitro wound and single cell migration were significantly compromised in the ECs isolated from PECAM-1-deficient mice. The formation of filopodia was also impaired in PECAM-1-null ECs and in HUVEC treated with anti-PECAM-1 antibody or in which PECAM-1 expression had been suppressed by siRNA. In addition, expression of PECAM-1 in cellular transfectants promoted filopodia formation. Consistent with these data, were the findings that the protein levels of Cdc42, a Rho GTPase known to promote the formation of filopodia, were increased by the expression of PECAM-1. In the developing retinal vasculature, the ECs at the tips of angiogenic sprouts in wild-type animals displayed long filamentous filopodia, while filopodial extensions emanating from the endothelial tip cells in the PECAM-1-null animals were fewer in number and shorter in length. Together, these data further establish the involvement of endothelial PECAM-1 in the formation of vessels and suggest that in vivo, PECAM-1 may stimulate endothelial cell motility by promoting the formation of filopodia.
A role for PECAM-1 during in vivo angiogenesis was initially established by studies demonstrating that anti-PECAM-1 antibody treatment inhibited corneal neovascularization induced by angiogenic implants,15 vascularization of subcutaneous Matrigel plugs,15 and tumor angiogenesis.15,16,17 The availability of PECAM-1-null mice19 has provided the opportunity to further explore the activity of PECAM-1 during in vivo angiogenesis. These PECAM-1-deficient mice are viable, suggesting that vascular development in the absence of PECAM-1 is sufficient to allow for adequate embryogenesis. As a result, only a limited number of studies to date have investigated the formation of blood vessels in PECAM-1-null mice. We found that the vascularization of subcutaneous Matrigel implants (Figure 1) and two subcutaneous tumors (Figure 3) were reduced in PECAM-1-deficient mice. These findings are consistent with that of Solowiej and associates who noted an impaired angiogenic response in a model of foreign body-induced chronic inflammation.27 Together, these data point to a role for PECAM-1 in pathological angiogenesis. Of note, while the vessels invading the Matrigel implants in the wild-type animals were large and tortuous, the invading vessels in the PECAM-1-deficient mice were smaller in caliber and less irregular (Figure 2). These PECAM-1-null vessels were also less leaky (data not shown). This suggests that the loss of PECAM-1, independent of any impairment in endothelial motility (see below), leads to a “normalization” of blood vessels. It has been proposed that antiangiogenic agents may enhance the tumoricidal actions of cytotoxic drugs by “normalizing” the tumor vasculature in a way that allows for sustained drug delivery.52 This raises the possibility that PECAM-1 antagonists may serve as effective adjuvant cancer agents (see below).
We also noted that the initial postnatal vascularization of the murine retina was impaired in the PECAM-1-null mice (Figure 10). These data confirm the findings of DiMaio et al who have also studied postnatal retinal vasculature development in PECAM-1-deficient mice.28 The vascular pattern and density, however, were similar in wild-type and PECAM-1-null adult mice (data not shown), suggesting that the loss of PECAM-1 delays but does not prevent the eventual normal development of the retinal vasculature. These findings are similar to what was observed for murine postnatal lung development, where lung alveolarization was delayed in the PECAM-1-deficient mice (due to presumed impairments in angiogenesis) but had substantially recovered by the time the mice reached adulthood.26 These data further implicate PECAM-1 in postnatal vascular developmental processes such as those that might be occurring in the eyes and lungs. However, in a model of dermal wound healing we have found that the rate of wound closure and the extent of wound angiogenesis were similar in wild-type and PECAM-1-null-mice or in mice treated with control IgG or anti-PECAM-1 antibody (data not shown). These data are consistent with the finding that anti-human PECAM-1 antibody treatment did not inhibit wound angiogenesis in human skins grafted onto SCID mice.53 This suggests that the involvement of PECAM-1 in blood vessel formation may depend on the tissue context and angiogenic stimulus.
PECAM-1 is expressed not only on ECs but also on bone marrow-derived cells such as recruited leukocytes, tissue macrophages, and circulating endothelial progenitor cells, which also participate in angiogenesis.43,44,45,46,47,48 The absence of PECAM-1 on these bone marrow-derived cells, in addition to the loss of PECAM-1 on ECs, could contribute to the altered angiogenic phenotype of the PECAM-1-deficient mice. The inability of wild-type PECAM-1-expressing leukocytes and/or bone marrow-derived endothelial progenitor cells to restore the wild-type phenotype in the PECAM-1-null animals (WTBM-KOEC) provides strong evidence for the endothelial involvement of PECAM-1 during in vivo angiogenesis (Figure 4).
We have previously reported that anti-human PECAM-1 antibody inhibits the cell migration of HUVEC,17 while expression of human PECAM-1 in non-PECAM-1-expressing cell lines promotes cell motility.17,18 Our finding that the loss or antibody antagonism of murine PECAM-1 inhibits the motility of murine ECs (Figure 5) is therefore consistent with the results of these earlier studies of human PECAM-1. The data presented here are also in agreement with studies of murine kidney ECs, which demonstrated that the absence of PECAM-1 caused these cells to be less migratory.54
The mechanism of PECAM-1’s involvement in endothelial motility has been the subject of previous investigations.18 These studies have suggested that the angiogenic activation of ECs results in PECAM-1 tyrosine phosphorylation and the binding of the SHP-2 phosphatase to PECAM-1. This interaction results in the recruitment of SHP-2 to the cell membrane, where it mediates the dephosphorylation of focal adhesion proteins such as paxillin and vinculin. These dephosphorylation events in turn stimulate the disassembly and turnover of focal adhesions and thus promote endothelial cell motility. The data presented in this report suggest that PECAM-1 may enhance endothelial motility by also promoting the formation of filopodia, a feature of actively motile cells.29,30 As noted above, we found that the loss of PECAM-1 in murine ECs or HUVEC inhibited the initial formation of filopodial protrusions, while the expression of PECAM-1 in a non-PECAM-1 cell line stimulated filopodia formation (Figures 6–8). Our data are consistent with the finding that PECAM-1-null platelets were impaired in their capacity to extend filopodia.55 However, an earlier report by Gratizinger and associates found that re-expression of human PECAM-1 in a transformed, PECAM-1-null, mouse endothelial cell line suppressed the formation of filopodia.56 The reasons for the differences between our study and this earlier report are unclear, but may reflect differences in the cell lines that were used.
Further evidence for the involvement of PECAM-1 in the formation of filopodia comes from the fact that ECs at the tips of the developing retinal vasculature in the PECAM-1-deficient mice were notable for a paucity of long filopodial projections (Figure 10). With respect to this observation, we would note that Dimaio et al in their study of retinal vascular development did not detect a significant difference between wild-type and PECAM-1-null mice in the number of endothelial tip cell filopodia in the developing retina.28 The reasons for this difference between our study and theirs are not clear, but may be due in part to different methods used to stain the vasculature.
Filopodia probe and sense for chemicals in the pericellular environment, act as hubs for signal transduction, and mediate attachment to the extracellular matrix, processes that are all integral to directed cell migration.29,30 It is therefore not surprising that a molecule such PECAM-1 that stimulates the formation of filopodia also promotes EC motility. We have also observed that PECAM-1 not only promotes filopodia formation but concentrates at the tips of filopodial protrusions (data not shown), a finding that has been reported for other cell adhesion and surface molecules such as VEGF receptor, cadherins, and integrins.42,57 The specific mechanisms by which PECAM-1 enhances the formation of filopodia remain to be determined but are the subject of ongoing investigation. We have, however, found that two anti-PECAM-1 antibodies that block PECAM-1-dependent heterophilic binding also inhibit filopodia formation by subconfluent HUVEC (Table 1). This suggests that PECAM-1-dependent heterophilic ligand interactions2,6 with constituents of the extracellular matrix may be involved in the stimulation of filopodia formation by PECAM-1, although the existence of these interactions has been questioned.4 Small GTPases of the Rho superfamily (RhoA, Rac1, and Cdc42) have been linked to actin cytoskeletal remodeling and morphological changes involved in cell motility, with Cdc42 implicated in the formation of filopodia.31,32,33,34,35 Our data indicating that the protein levels of Cdc42 are increased in cells expressing PECAM-1 (Figure 9) are therefore consistent with a role for PECAM-1 in the formation of filopodia. The mechanisms by which PECAM-1 regulates Cdc42 expression are currently being investigated.
The findings of this study (Figure 3), as well as that of previous reports using antibody inhibition of PECAM-1,16,17 raise the possibility of PECAM-1 as a potential target for cancer therapy.58 Not only does the loss or inhibition of PECAM-1 function block tumor growth and angiogenesis, it also may act to normalize pathological vessels (Figure 2), an activity that facilitates the efficacy of antitumor agents.52 Anti-PECAM-1 therapy is likely to be well tolerated, since the absence of PECAM-1 does not result in a lethal vascular phenotype,19 deleterious effects have not been noted in animals treated with anti-PECAM-1 antibodies,15,16,17 and the absence of PECAM-1 function does not appear to prevent or inhibit dermal wound healing (see above). However, given its potential role in protecting against endotoxic and apoptotic stresses24,25 and as a mediator of leukocyte recruitment,20,21,22,23 human clinical trials will be required to establish the ultimate safety of therapy targeted against PECAM-1. In this regard, further studies with the goal of specifically defining the role of PECAM-1 as a facilitator of endothelial cell motility will be very important.
Footnotes
Address reprint requests to Horace M. DeLisser, M.D., Pulmonary, Allergy and Critical Care Division, SVM-Hill Pavilion, Room 410B, 380 South University Avenue, Philadelphia, PA 19104-3945. E-mail: delisser@mail.med.upenn.edu.
Supported by Department of Defense grant PR043482 (to H.M.D.) and National Institutes of Health grant HL079090 (to H.M.D).
References
- Newman PJ. The biology of PECAM-1. J Clin Invest. 1997;99:3–8. doi: 10.1172/JCI119129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisser HM, Yan HC, Newman PJ, Muller WA, Buck CA, Albelda SM. Platelet/endothelial cell adhesion molecule-1 (CD31)-mediated cellular aggregation involves cell surface glycosaminoglycans. J Biol Chem. 1993;268:16037–16046. [PubMed] [Google Scholar]
- Sun J, Williams J, Yan HC, Amin KM, Albelda SM, DeLisser HM. Platelet endothelial cell adhesion molecule-1 (PECAM-1) homophilic adhesion is mediated by immunoglobulin-like domains 1 and 2 and depends on the cytoplasmic domain and the level of surface expression. J Biol Chem. 1996;271:18561–18570. doi: 10.1074/jbc.271.31.18561. [DOI] [PubMed] [Google Scholar]
- Sun Q, Paddock C, Visentin GP, Zukowski MM, Muller WA, Newman PJ. Cell surface glycosaminoglycans do not serve as ligands for PECAM-1. J Biol Chem. 1998;273:11483–11490. doi: 10.1074/jbc.273.19.11483. [DOI] [PubMed] [Google Scholar]
- Deaglio S, Morra M, Mallone R, Ausiello CM, Prager E, Garbarino G, Dianzani U, Stockinger H, Malavasi F. Human CD38 (ADP-ribosyl cyclase) is a counter-receptor of CD31, an Ig superfamily member. J Immunol. 1998;160:395–402. [PubMed] [Google Scholar]
- Coombe DR, Stevenson SM, Kinnear BF, Gandhi NS, Mancera RL, Osmond RI, Kett WC. Platelet endothelial cell adhesion molecule 1 (PECAM-1) and its interactions with glycosaminoglycans: 2. Biochemical analyses Biochemistry. 2008;47:4863–4875. doi: 10.1021/bi7024595. [DOI] [PubMed] [Google Scholar]
- Newman PJ, Newman DK. Arterioscler. Thromb Vasc Biol. 2003;23:953–964. doi: 10.1161/01.ATV.0000071347.69358.D9. [DOI] [PubMed] [Google Scholar]
- Ilan N, Madri JA. Curr Opin Cell Biol. 2003;15:515–524. doi: 10.1016/s0955-0674(03)00100-5. [DOI] [PubMed] [Google Scholar]
- Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290:84–89. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- Billadeau DD, Leibson PJ. ITAMs versus ITIMs: striking a balance during cell regulation. J Clin Invest. 2002;109:161–168. doi: 10.1172/JCI14843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DE, Ward CM, Wang R, Newman PJ. The protein-tyrosine phosphatase SHP-2 binds platelet/endothelial cell adhesion molecule-1 (PECAM-1) and forms a distinct signaling complex during platelet aggregation. Evidence for a mechanistic link between PECAM-1- and integrin-mediated cellular signaling. J Biol Chem. 1997;272:6986–6993. doi: 10.1074/jbc.272.11.6986. [DOI] [PubMed] [Google Scholar]
- Lu TT, Barreuther M, Davis S, Madri JA. Platelet endothelial cell adhesion molecule-1 is phosphorylatable by c-Src, binds Src-Src homology 2 domain, and exhibits immunoreceptor tyrosine-based activation motif-like properties. J Biol Chem. 1997;272:14442–14446. doi: 10.1074/jbc.272.22.14442. [DOI] [PubMed] [Google Scholar]
- Cao MY, Huber M, Beauchemin N, Famiglietti J, Albelda SM, Veillette A. Regulation of mouse PECAM-1 tyrosine phosphorylation by the Src and Csk families of protein-tyrosine kinases. J Biol Chem. 1998;273:15765–15772. doi: 10.1074/jbc.273.25.15765. [DOI] [PubMed] [Google Scholar]
- Sagawa K, Kimura T, Swieter M, Siraganian RP. The protein-tyrosine phosphatase SHP-2 associates with tyrosine-phosphylated adhesion molecule PECAM-1 (CD31). J Biol Chem. 1997;272:31086–31091. doi: 10.1074/jbc.272.49.31086. [DOI] [PubMed] [Google Scholar]
- DeLisser HM, Christofidou-Solomidou M, Strieter RM, Burdick MD, Robinson CS, Wexler RS, Kerr JS, Garlanda C, Merwin JR, Madri JA, Albelda SM. Involvement of endothelial PECAM-1/CD31 in angiogenesis. Am J Pathol. 1997;151:671–677. [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Christofidou-Solomidou M, Garlanda C, DeLisser HM. Antibody against murine PECAM-1 inhibits tumor angiogenesis in mice. Angiogenesis. 1999;3:181–188. doi: 10.1023/a:1009092107382. [DOI] [PubMed] [Google Scholar]
- Cao G, O'Brien CD, Zhou Z, Sanders SM, Greenbaum JN, Makrigiannakis A, DeLisser HM. Involvement of human PECAM-1 in angiogenesis and in vitro endothelial cell migration. Am J Physiol Cell Physiol. 2002;282:C1181–C1190. doi: 10.1152/ajpcell.00524.2001. [DOI] [PubMed] [Google Scholar]
- O'Brien CD, Cao G, Makrigiannakis A, DeLisser HM. Role of immunoreceptor tyrosine-based inhibitory motifs of PECAM-1-dependent cell migration. Am J Physiol Cell Physiol. 2004;287:C1103–C1113. doi: 10.1152/ajpcell.00573.2003. [DOI] [PubMed] [Google Scholar]
- Duncan GS, Andrew DP, Takimoto H, Kaufman SA, Yoshida H, Spellberg J, Luis de la Pompa J, Elia A, Wakeham A, Karan-Tamir B, Muller WA, Senaldi G, Zukowski MM, Mak TW. Genetic evidence for functional redundancy of platelet/endothelial cell adhesion molecule-1 (PECAM-1): CD31-deficient mice reveal PECAM-1-dependent and PECAM-1-independent functions. J Immunol. 1999;162:3022–3030. [PubMed] [Google Scholar]
- Thompson RD, Noble KE, Larb KY, Dewar A, Duncan GS, Mak TW, Nourshargh S. Platelet-endothelial cell adhesion molecule-1 (PECAM-1)-deficient mice demonstrate a transient and cytokine-specific role for PECAM-1 in leukocyte migration through the perivascular basement membrane. Blood. 2001;97:1854–1860. doi: 10.1182/blood.v97.6.1854. [DOI] [PubMed] [Google Scholar]
- Woodfin A, Reichel CA, Khandoga A, Corada M, Voisin MB, Scheiermann C, Haskard DO, Dejana E, Krombach F, Nourshargh S. JAM-A mediates neutrophil transmigration in a stimulus-specific manner in vivo: evidence for sequential roles for JAM-A and PECAM-1 in neutrophil transmigration. Blood. 2007;110:1848–1856. doi: 10.1182/blood-2006-09-047431. [DOI] [PubMed] [Google Scholar]
- Albelda SM, Lau KC, Chien P, Huang ZY, Arguiris E, Bohen A, Sun J, Billet JA, Christofidou-Solomidou M, Indik ZK, Schreiber AD. Role for platelet-endothelial cell adhesion molecule-1 in macrophage Fcgamma receptor function. Am J Respir Cell Mol Biol. 2004;31:246–255. doi: 10.1165/rcmb.2003-0404OC. [DOI] [PubMed] [Google Scholar]
- Schenkel AR, Chew TW, Muller WA. Platelet endothelial cell adhesion molecule deficiency or blockade significantly reduces leukocyte emigration in a majority of mouse strains. J Immunol. 2004;173:6403–6408. doi: 10.4049/jimmunol.173.10.6403. [DOI] [PubMed] [Google Scholar]
- Carrithers M, Tandon S, Canosa S, Michaud M, Graesser D, Madri JA. Enhanced susceptibility to endotoxic shock and impaired STAT3 signaling in CD31-deficient mice. Am J Pathol. 2005;166:185–196. doi: 10.1016/S0002-9440(10)62243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas M, Stapleton M, Bergom C, Mattson DL, Newman DK, Newman PJ. Endothelial cell PECAM-1 confers protection against endotoxic shock. Am J Physiol Heart Circ Physiol. 2005;288:H159–H164. doi: 10.1152/ajpheart.00500.2004. [DOI] [PubMed] [Google Scholar]
- DeLisser HM, Helmke BP, Cao G, Egan PM, Taichman D, Fehrenbach M, Zaman A, Cui Z, Mohan GS, Baldwin HS, Davies PF, Savani RC. Loss of PECAM-1 function impairs alveolarization. J Biol Chem. 2006;281:8724–8731. doi: 10.1074/jbc.M511798200. [DOI] [PubMed] [Google Scholar]
- Solowiej A, Biswas P, Graesser D, Madri JA. Lack of platelet endothelial cell adhesion molecule-1 attenuates foreign body inflammation because of decreased angiogenesis. Am J Pathol. 2003;162:953–962. doi: 10.1016/S0002-9440(10)63890-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimaio TA, Wang S, Huang Q, Scheef EA, Sorenson CM, Sheibani N. Attenuation of retinal vascular development and neovascularization in PECAM-1-deficient mice. Dev Biol. 2008;315:72–88. doi: 10.1016/j.ydbio.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila PK, Lappalainen P. Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol. 2008;9:446–454. doi: 10.1038/nrm2406. [DOI] [PubMed] [Google Scholar]
- Gupton SL, Gertler FB. Filopodia: the fingers that do the walking. Sci STKE. 2007;400:re5. doi: 10.1126/stke.4002007re5. [DOI] [PubMed] [Google Scholar]
- Schmitz AA, Govek EE, Böttner B, Van Aelst L. Rho GTPases: signaling, migration, and invasion. Exp Cell Res. 2000;261:1–12. doi: 10.1006/excr.2000.5049. [DOI] [PubMed] [Google Scholar]
- Raftopoulou M, Hall A. Cell migration: rho GTPases lead the way. Dev Biol. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- Wennerberg K, Der CJ. Rho-family GTPases: it’s not only Rac and Rho (and I like it). J Cell Sci. 2004;117:1301–1312. doi: 10.1242/jcs.01118. [DOI] [PubMed] [Google Scholar]
- Cerione RA. Cdc42: new roads to travel. Trends Cell Biol. 2004;14:127–132. doi: 10.1016/j.tcb.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Sinha S, Yang W. Cellular signaling for activation of Rho GTPase Cdc42. Cell Signal. 2008;20:1927–1934. doi: 10.1016/j.cellsig.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Doerschuk CM, Quinlan WM, Doyle NA, Bullard DC, Vestweber D, Jones ML, Takei F, Ward PA, Beaudet AL. The role of P-selectin and ICAM-1 in acute lung injury as determined using blocking antibodies and mutant mice. J Immunol. 1996;157:4609–4614. [PubMed] [Google Scholar]
- Garlanda C, Parravicini C, Sironi M, De Rossi M, Wainstok de Calmanovici R, Carozzi F, Bussolino F, Colotta F, Mantovani A, Vecchi A. Progressive growth in immunodeficient mice and host cell recruitment by mouse endothelial cells transformed by polyoma middle-sized T antigen: implications for the pathogenesis of opportunistic vascular tumors. Proc Natl Acad Sci USA. 1994;91:7291–7295. doi: 10.1073/pnas.91.15.7291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Yang N, Garcia JR, Mohamed A, Benencia F, Rubin SC, Allman D, Coukos G. Generation of a syngeneic mouse model to study the effects of vascular endothelial growth factor in ovarian carcinoma. Am J Pathol. 2002;161:2295–2309. doi: 10.1016/s0002-9440(10)64505-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrenbach ML, Cao G, Williams JT, Finklestein JM, Zhu J-X, Delisser HM. Isolation of murine lung endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2009;296:L1096–L1103. doi: 10.1152/ajplung.90613.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald DM. Endothelial gaps and permeability of venules in rat tracheas exposed to inflammatory stimuli. Am J Physiol Lung Cell Mol Physiol. 1994;266:L61–L83. doi: 10.1152/ajplung.1994.266.1.L61. [DOI] [PubMed] [Google Scholar]
- Mahooti S, Graesser D, Patil S, Newman P, Duncan G, Mak T, Madri JA. PECAM-1 (CD31) expression modulates bleeding time in vivo. Am J Pathol. 2000;157:75–81. doi: 10.1016/S0002-9440(10)64519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schruefer R, Lutze N, Schymeinsky J, Walzog B. Human neutrophils promote angiogenesis by a paracrine feedforward mechanism involving endothelial interleukin-8. Am J Physiol Heart Circ Physiol. 2005;288:H1186–H1192. doi: 10.1152/ajpheart.00237.2004. [DOI] [PubMed] [Google Scholar]
- Chavakis T, Cines DB, Rhee JS, Liang OD, Schubert U, Hammes HP, Higazi AA, Nawroth PP, Preissner KT, Bdeir K. Regulation of neovascularization by human neutrophil peptides (alpha-defensins): a link between inflammation and angiogenesis. FASEB J. 2004;18:1306–1308. doi: 10.1096/fj.03-1009fje. [DOI] [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Crowther M, Brown NJ, Bishop ET, Lewis CE. Microenvironmental influence on macrophage regulation of angiogenesis in wounds and malignant tumors. J Leukoc Biol. 2001;70:478–490. [PubMed] [Google Scholar]
- Coukos G, Benencia F, Buckanovich RJ, Conejo-Garcia JR. The role of dendritic cell precursors in tumour vasculogenesis. Br J Cancer. 2005;92:1182–1187. doi: 10.1038/sj.bjc.6602476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conejo-Garcia JR, Buckanovich RJ, Benencia F, Courreges MC, Rubin SC, Carroll RG, Coukos G. Vascular leukocytes contribute to tumor vascularization. Blood. 2005;105:679–681. doi: 10.1182/blood-2004-05-1906. [DOI] [PubMed] [Google Scholar]
- Nakada MT, Amin K, Christofidou-Solomidou M, O'Brien CD, Sun J, Gurubhagavatula I, Heavner GA, Taylor AH, Paddock C, Sun QH, Zehnder JL, Newman PJ, Albelda SM, DeLisser HM. Antibodies against the first Ig-like domain of human platelet endothelial cell adhesion molecule-1 (PECAM-1) that inhibit PECAM-1-dependent homophilic adhesion block in vivo neutrophil recruitment. J Immunol. 2000;164:452–462. doi: 10.4049/jimmunol.164.1.452. [DOI] [PubMed] [Google Scholar]
- Dorrell MI, Friedlander M. Mechanisms of endothelial cell guidance and vascular patterning in the developing mouse retina. Prog Retin Eye Res. 2006;25:277–295. doi: 10.1016/j.preteyeres.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Fruttiger M. Development of the retinal vasculature. Angiogenesis. 2007;10:77–88. doi: 10.1007/s10456-007-9065-1. [DOI] [PubMed] [Google Scholar]
- Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- Matsumura T, Wolff K, Petzelbauer P. Endothelial cell tube formation depends on cadherin 5 and CD31 interactions with filamentous actin. J Immunol. 1997;158:3408–3416. [PubMed] [Google Scholar]
- Kondo S, Scheef EA, Sheibani N, Sorenson CM. PECAM-1 isoform-specific regulation of kidney endothelial cell migration and capillary morphogenesis. Am J Physiol Cell Physiol. 2007;292:C2070–C2083. doi: 10.1152/ajpcell.00489.2006. [DOI] [PubMed] [Google Scholar]
- Wee JL, Jackson DE. The Ig-ITIM superfamily member PECAM-1 regulates the “outside-in” signaling properties of integrin alpha(IIb)beta3 in platelets. Blood. 2005;106:3816–3823. doi: 10.1182/blood-2005-03-0911. [DOI] [PubMed] [Google Scholar]
- Gratzinger D, Canosa S, Engelhardt B, Madri JA. Platelet endothelial cell adhesion molecule-1 modulates endothelial cell motility through the small G-protein Rho. FASEB J. 2003;17:1458–1469. doi: 10.1096/fj.02-1040com. [DOI] [PubMed] [Google Scholar]
- Tanoue T, Takeichi M. Mammalian Fat1 cadherin regulates actin dynamics and cell-cell contact. J Cell Biol. 2004;165:517–528. doi: 10.1083/jcb.200403006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisser HM. Targeting PECAM-1 for anti-cancer therapy. Cancer Biol Ther. 2007;6:121–122. doi: 10.4161/cbt.6.1.3827. [DOI] [PubMed] [Google Scholar]