Abstract
CD4+ T helper cells are well known for their role in providing critical signals during priming of cytotoxic CD8+ T lymphocyte (CTL) responses in vivo. T help is required for the generation of primary CTL responses as well as in promoting protective CD8+ memory T cell development1. However, the role of CD4 help in the control of CTL responses at the effector stage is unknown. Here, we show that fully helped effector CTLs are not themselves self-sufficient for entry into the infected tissue, but rely on the CD4+ T cells to provide the necessary cue. CD4+ T helper cells control the migration of CTL indirectly through the secretion of IFN-γ and induction of local chemokine secretion in the infected tissue. Our results reveal a previously unappreciated role of CD4 help in mobilizing effector CTL to the peripheral sites of infection where they help to eliminate infected cells.
Elimination of invading pathogens often requires coordinated effort by effector lymphocytes for containment and clearance. Successful defense against intracellular pathogens requires neutralizing antibodies and CTL responses, both of which largely depend on CD4+ T cell help. Whereas generation of primary CD8+ T cell responses to non-inflammatory antigens2–4 and certain virus infections, such as herpes simplex virus (HSV)5, require CD4+ T cell help, primary CTL responses to acute infection with Listeria monocytogenes and lymphocytic choriomeningitis virus can occur in the absence of CD4+ T cells. Instead, the latter type of infections require CD4 help in promoting memory CTL development6–8. The critical role of CD4 help in the priming and maintenance of CTL responses is well characterized; however, whether CD4+ T cells help at the stages following CTL differentiation have not been described and is currently unknown.
To explore the role of CD4 help in effector CTL responses, we employed a physiological model of local virus infection that enables tracking of antigen-specific CD8+ T cells. HSV-2 infects humans through sexual contact and causes genital herpes. When inoculated into the vaginal cavity, HSV-2 replicates predominantly in the mucosal epithelial cells and establishes latency in the innervating neurons. Since the viral infection is localized, the genital herpes model enabled us to dissect the role of CD4 help in CTL migration to the site of infection. To avoid neurovirulence associated with WT HSV-2, without compromising the ability to prime robust innate and adaptive immunity, we employed thymidine-kinase (TK) defective HSV-2 (TK− HSV-2)9. Upon TK− HSV-2 infection, both CD4+ and CD8+ T cells are primed in the local draining lymph nodes10, and both total (Supplementary Fig. 1a) and virus-specific (Supplementary Fig. 1b and c) effector T cells migrate into the vaginal mucosa starting with CD4+ T cells around day 3–4 followed by CD8+ T cells on day 4–5. Notably, migration of virus-specific CD8+ T cells to the infection site was highly dependent on the presence of CD4+ T cells, evidenced by the failure of CD8+ T cells to migrate to the local tissue in mice that were either CD4-deficient, or were depleted of CD4+ T cells (Supplementary Fig. 2a). However, because primary CTL expansion following HSV-1 infection has been reported to depend on CD4+ T cells5 through their ability to license dendritic cells11, we examined the total number of congenically marked (CD45.1+) HSV-gB specific TCR transgenic T cells (gBT-I)12 generated in CD4−/− and CD4-depeleted mice. Consistent with previous reports5, 11, gBT-I responses in various tissues after local HSV-2 infection also depended largely on the presence of CD4+ T cells (Supplementary Fig. 2b–d).
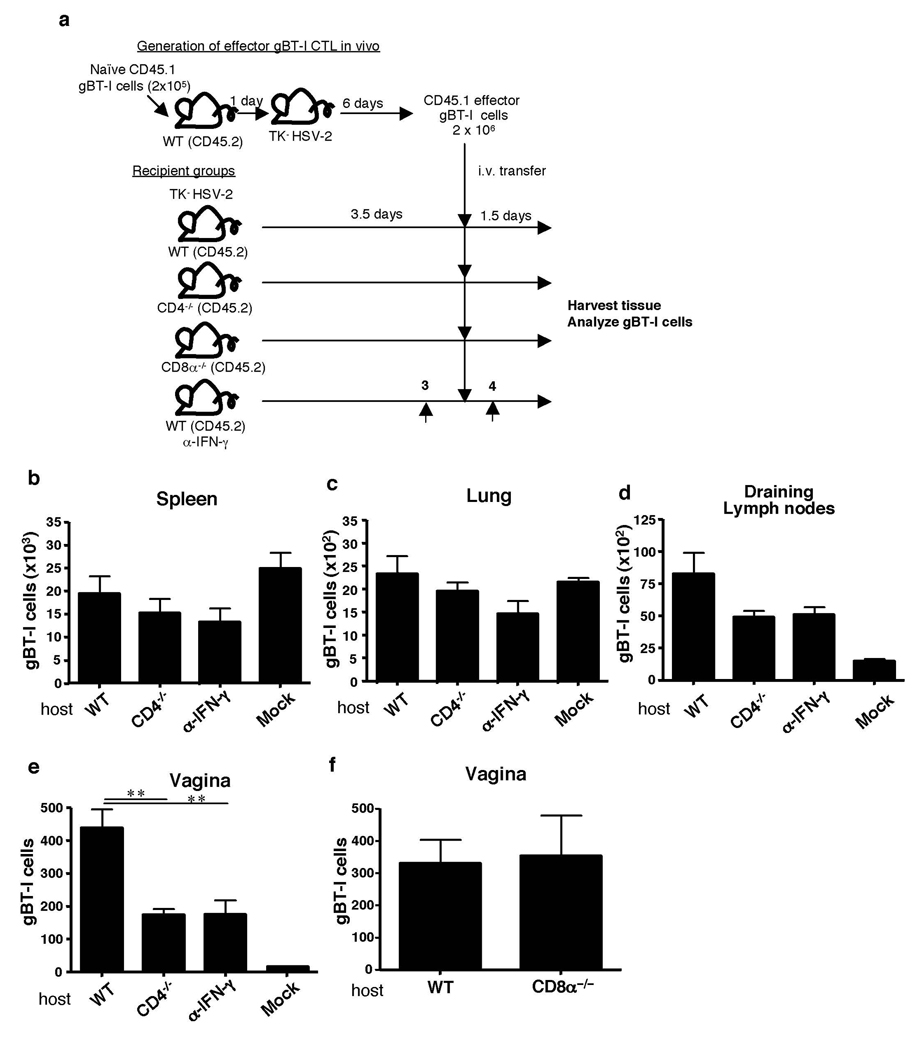
To determine the mechanism by which CD4+ T cells license CTL migration, fully “helped” CD8+ effector T cells were first generated in WT hosts (Fig. 1a). A physiological number (2 × 105 cells/mouse13) of gBT-I cells were transferred into naïve WT mice. Subsequently, these mice were infected with TK− HSV-2 and effector CD8+ T cells were isolated (Supplementary Fig. 3 a and b) and transferred into recipient mice that had been infected with TK− HSV-2 3.5 days earlier. It is well known that effector CTLs migrate to various lymphoid and non-lymphoid organs including the lung, liver and intestine14, 15. Accordingly, effector CD8+ T cells were found in lymphoid and peripheral organs irrespective of the infection status of the host (Fig. 1b–d). This homeostatic distribution pattern did not depend on the presence of CD4+ T cells. In stark contrast, while the fully helped effector CTLs migrated into the infected vaginal tissue in the WT hosts, their ability to do so was significantly impaired in the absence of CD4+ T cells (Fig. 1e and Supplemental Fig. 3c). Similar results were obtained using different time course (Supplementary Fig. 4). In contrast, adoptively transferred fully helped gBT-I T cells were able to migrate to the HSV-infected vagina in CD8-deficient host (Fig. 1f), indicating that CD4, but not CD8, T cells are required for licensing CTL entry into the vaginal mucosa. Tregs have been shown to facilitate early protective responses to local HSV-2 infection by allowing a timely entry of immune cells into infected tissue16. To examine whether effector or Foxp3+ CD4+ T cells account for the mobilization of CTL into the infected vaginal mucosa, either total or Foxp3− HSV-primed CD4+ T cells were adoptively transferred into HSV-infected CD4−/− hosts. Analysis of migration of helped gBT-I cells in such animals revealed that effector CD4+ T cells were equally capable of CTL mobilization into the infected tissue compared to total CD4+ T cells (including effectors and Tregs) (Supplementary Fig. 5). These data indicated that while Tregs are capable of facilitating effector lymphocyte entry16, effector CD4+ T cells alone mediate CTL recruitment and can override the requirement for Tregs. Collectively, our results revealed that, while homeostatic migration of effector CTLs occurs independently of CD4+ T help, fully differentiated CTLs are not self-sufficient for accelerated recruitment to the infected tissue during a viral infection. This situation is reminiscent of the requirement for “pioneering” CD4+ T cells for entry by pathogenic CD4+ T cells in the central nervous system17. Further, our data uncovered a previously unknown function of T helper cells in mobilizing CD8+ T cell recruitment to the site of infection.
Figure 1. CD4 help is required for CTL migration into the vaginal mucosa after HSV-2 infection.
(a) Naïve congenic 2 × 105 gBT-I cells were transferred into WT mice. Six days after TK− HSV-2 vaginal infection, 2 × 106 effector gBT-I cells isolated from these mice were transferred into secondary hosts (some treated with anti-IFN-γ Ab) infected with TK− HSV-2 3.5 days earlier. Numbers of gBT-I cells in the indicated tissues (b–f) were assessed 5 days post infection. The data are pooled from two independent experiments and presented as mean ± SEM (n = 5 – 6 mice per group). **, p < 0.01.
To address the mechanism by which T help enables CTL recruitment to the site of viral replication, we focused on one of the major effector functions of CD4+ Th1 cells, namely, the secretion of IFN-γ. First, we asked whether CD4–catalyzed CTL recruitment was mediated by the action of IFN-γ. To this end, CTL migration to the infected tissue in WT and IFN-γR−/− mice was compared. CD8+ T cell recruitment to the vagina was significantly reduced in IFN-γR−/− mice (Supplemental Fig. 6a), despite the fact that these mice generated comparable levels of HSV-2-specific CD4+ and CD8+ T cell responses (Supplemental Fig. 6b, c). Vaginal infection with HSV-2 generates two waves of IFN-γ secretion; the first wave of IFN-γ is secreted by NK cells at 2 d p.i., while CD4+ T cells produce IFN-γ starting at 4 d p.i. (Supplementary Fig. 7a)18. To specifically examine the requirement for CD4+ T cell-secreted IFN-γ in CTL recruitment, WT mice were infected with TK− HSV-2, and IFN-γ neutralizing Ab was given at 3 and 4 days post infection. With this regimen, NK-secreted IFN-γ is not affected, while CD4-secreted IFN-γ is blocked. Fully helped CD8+ T cells, when injected into IFN-γ neutralized mice, were significantly impaired in their migration to the infected tissue (Fig. 1e). In addition, NK depletion throughout the course of HSV infection did not alter the recruitment of effector CTL to the vaginal mucosa in either WT or CD4−/− hosts (Supplementary Fig. 8). These results, combined with the fact that CD4+ T cells are required for migration of CTLs, indicated that CTL recruitment primarily, if not exclusively, depends on IFN-γ produced by CD4+ T cells.
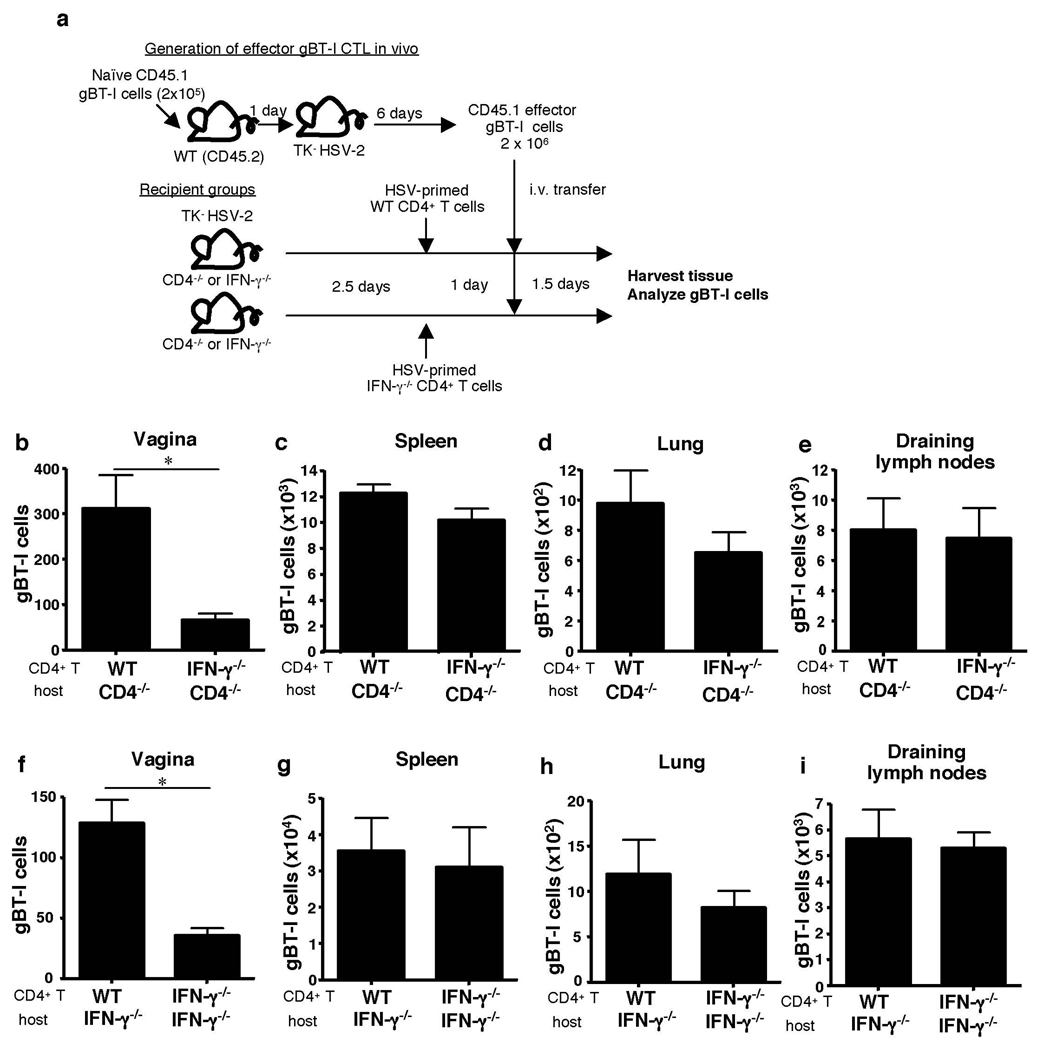
To determine whether CD4+ T cells indeed provide the IFN-γ required to pioneer the vaginal mucosa for CTL entry, we tested both sufficiency and requirement for CD4-derived IFN-γ in this process. First, to examine the requirement for CD4-derived IFN-γ, migration of fully helped gBT-I cells was assessed in CD4−/− mice reconstituted with either WT or IFN-γ−/− HSV-primed CD4+ T cells (Fig. 2a). Only the WT, but not IFN-γ−/−, effector CD4+ T cells were able to rescue CTL migration to the site of infection (Fig. 2b). These data indicated that IFN-γ secretion from CD4+ T cells is required for CTL entry into the vaginal mucosa following HSV-2 infection. Second, sufficiency of CD4-derived IFN-γ in CTL migration was examined by reconstituting IFN-γ−/− mice with either WT or IFN-γ−/− HSV-primed CD4+ T cells. Notably, migration of gBT-I effector cells were facilitated by the presence of WT CD4+ T cells, whereas IFN-γ−/− CD4+ T cells were only able to promote basal level of CTL recruitment (Fig. 2f). Interestingly, effector CD4+ T cell entry into the vaginal tissue also required IFN-γ secretion and responsiveness to IFN-γ by the CD4+ T cells themselves (Supplementary Fig. 9), suggesting an autocrine-mediated conditioning of CD4+ T cells for access to the vaginal mucosa. To address whether IFN-γ secretion by CD4 T cells within the vagina is required for CTL entry, we took advantage of the fact that CD4+ T cell entry occurs prior to day 3 post infection whereas CTL entry is delayed until day 5 post infection (Supplementary Fig. 1). Selective blockade of CD4-secreted IFN-γ (Supplementary Fig. 7a) on days 3 and 4 of infection still allowed effector CD4+ T cells to enter the vaginal tissue (Supplementary Fig. 10). However, this treatment resulted in a significant reduction in CTL migration (Fig. 1e). These data collectively indicated that CD4+ T cells that have migrated to the vagina must still produce IFN-γ in order to enable CTL entry to the site. Taken together, our data provide evidence that CD4+ T cell-derived IFN-γ is both necessary and sufficient in enabling CTL entry to the site of infection.
Figure 2. CD4 T cell-secreted IFN-γ mediates CTL entry into infected vaginal tissue.
(a) HSV-primed WT or IFN-γ−/− CD4+ T cells (107) were adoptively transferred into CD4−/− (b–e) or IFNγ−/− mice (f–i) at 2.5 days post HSV-2 ivag infection. One day later, fully helped congenic effector gBT-I (2 × 106) cells were transferred into these mice. Five days after infection, the number of gBT-I cells in the indicated tissues was analyzed (b–i). Results are mean ± SEM (n = 4) and are representative of four independent experiments. Statistics were determined by unpaired two-tailed t-test. *, p < 0.05. **, p < 0.01.
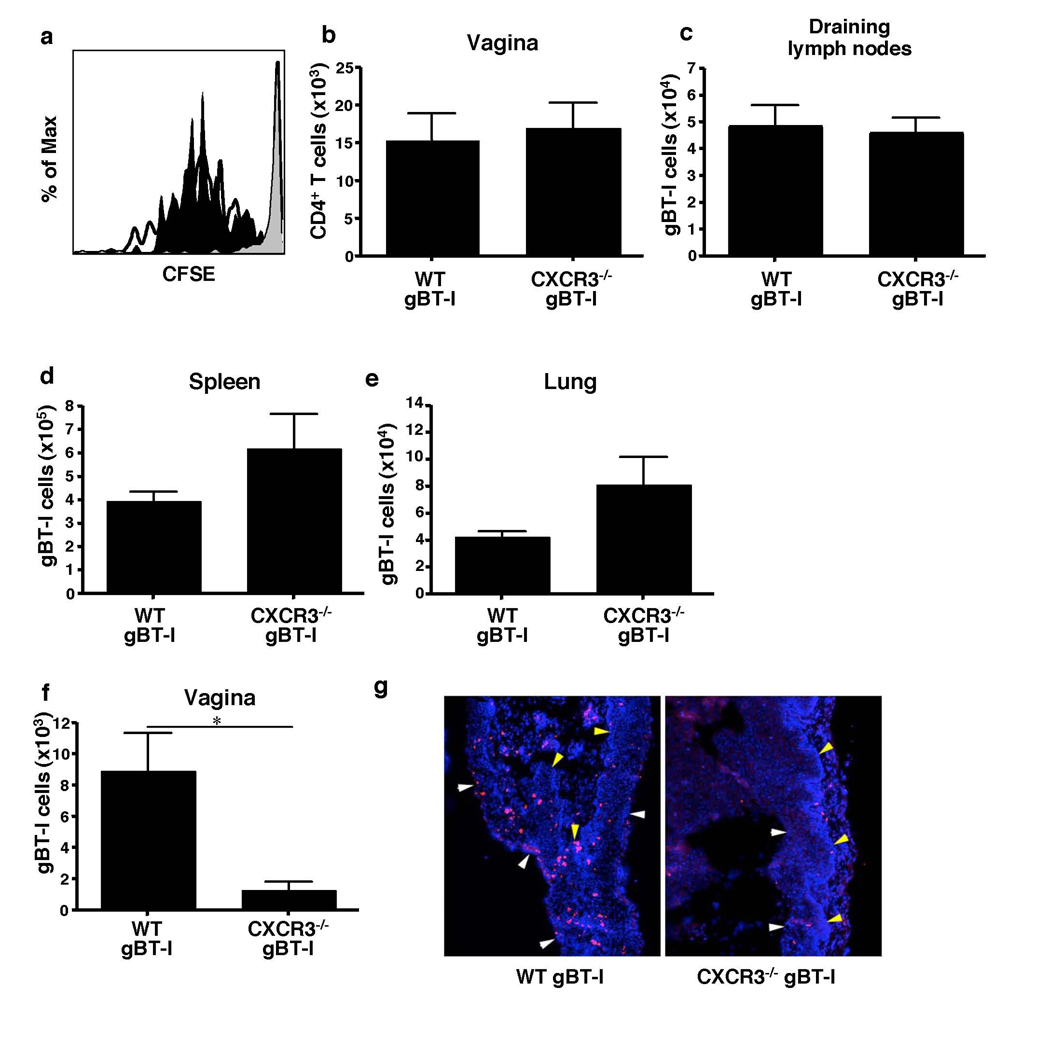
Next, the requirement for IFN-γ-inducible chemokines in CTL recruitment was assessed. To this end, congenically marked gBT-I transgenic mice were crossed to CXCR3-deficient mice incapable of responding to IFN-γ-inducible chemokines, CXCL9 and CXCL10. Physiologically relevant numbers (105 (Fig. 2) or 103 (Supplementary Fig. 11)) of purified CD45.1+ CXCR3−/− gBT-I cells were transferred into WT hosts and their proliferation and migration was measured 6 days after HSV-2 infection. CXCR3−/− gBT-I cells igrated and proliferated normally in the draining lymph nodes of WT hosts (Fig. 3a). In addition, both WT and CXCR3−/− gBT-I cells accumulated in the draining lymph nodes (Fig. 3c and Supplementary Fig. 11b), and migrated into the spleen and peripheral tissues (Fig. 3d, e and Supplementary Fig. 11c, d). In stark contrast, and despite the presence of abundant CD4+ Th1 cells in the vaginal mucosa (Fig. 3b), CXCR3 knockout CTLs failed to migrate into the virally infected vaginal tissue (Fig. 3f and Supplementary Fig. 11a). Moreover, the small numbers of CXCR3−/− gBT-I cells that managed to migrate into the vaginal tissue were mostly excluded from the epithelial layer (Fig. 3g), the primary site of virus infection and replication10. Therefore, these data indicated that effector CTL migration into the infected tissue requires CXCR3, whose ligand expression is induced by IFN-γ produced by CD4+ T cells.
Figure 3. CTL recruitment to the infected tissue depends on CXCR3.
Recipient mice were reconstituted with 105 WT or CXCR3−/− gBT-I cells. (a) At 3.5 days post infection, division of WT (blank), CXCR3−/− (filled), and uninfected (gray) gBT-I cells in the draining lymph nodes was analyzed. Numbers of total CD4+ T cells within the vagina (b) and gBT-I cells in the indicated tissues (c–f) were analyzed on day 6 post infection. (g) Vaginal tissues 7 days after infection were stained for gBT-I cells (red) and nuclei (blue). Arrows; basement membrane, yellow; vaginal lumen, white. Results are mean ± SEM (n = 4). Data are representative of three separate experiments. *, p < 0.05.
We next asked whether the CD4+ T cells directly provide help to CD8+ T cells, or whether CD4+ T cells control the recruitment of CD8+ T cells indirectly through modifying the local tissue environment. To address the former possibility, we examined the necessity of CD4+ T cells for CD8+ T cells to express and respond through CXCR3. To this end, gBT-I cells were primed in either WT or CD4-deficient hosts by intravaginal infection with TK− HSV-2 and expression of CXCR3 was assessed. While the majority of naïve CD8+ T cells expressed low to undetectable levels of CXCR3, effector gBT-I cells expressed high levels of CXCR3, regardless of the absence of CD4+ T cells (Supplementary Fig. 12a). Further, both helped and helpless CTLs responded to and migrated towards CXCL9 and CXCL10 comparably (Supplementary Fig. 12b). These data revealed that CD4 help is not required for the functional expression of CXCR3 by the effector CD8+ T cells. Therefore, CD4+ T cell help is not provided directly to CD8+ T cells, but is likely mediated through modification of the local microenvironment.
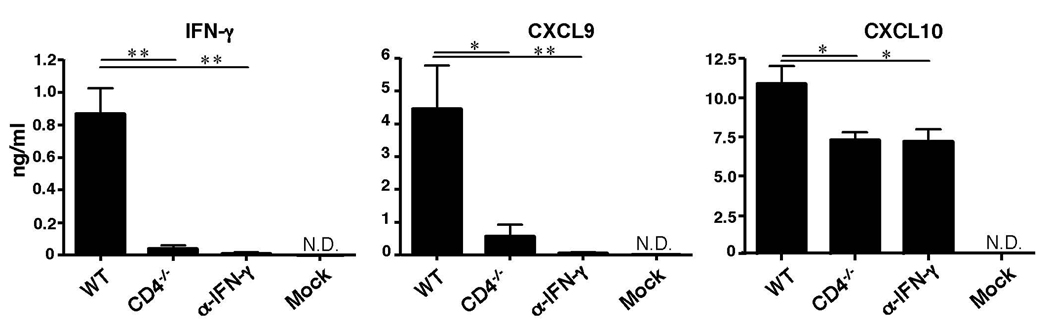
In an effort to understand the mechanism by which CD4+ T helper cells indirectly enable migration of CD8+ T cells to the site of infection, we examined the production of CD4-dependent cytokines and chemokines at the local mucosa. As expected, IFN-γ levels 4 days after infection were diminished in CD4 deficient mice within the vaginal lumen (Fig. 4) and within the lamina propria (Supplementary Fig. 7b). In the absence of CD4+ T cells or in mice injected with IFN-γ blocking Ab starting at day 3 post infection, secretion of CXCL9 was diminished and a significant reduction in the levels of CXCL10 was observed (Fig. 4). Thus, cumulative levels of CXCR3 ligands are significantly reduced in the vaginal tissue in the absence of CD4 or IFN-γ. Because CXCL9 and CXCL10 are also induced by type I IFNs, and because we detected a reduced but significant recruitment of residual CTL to the vaginal tissue in the absence of CD4+ T cells or IFN-γ (Figure 1 & Figure 2), we next assessed the importance of type I IFNs in lymphocyte recruitment following HSV-2 infection. Despite normal priming19, CD4+ T cell recruitment to the vagina was significantly reduced in the absence of IFN-αβR (Supplementary Fig. 13b). Consequently, IFN-γ, CXCL9 and CXCL10 secretion, and subsequent recruitment of CTL were significantly decreased in IFN-αβR−/− mice (Supplementary Fig. 13c). Notably, neutralization of IFN-γ in IFN-αβR−/− mice completely abolished CXCL9 and CXCL10 secretion and eliminated the residual CTL recruitment. These data indicated that in the absence of CD4+ T cells or IFN-γ, type I IFNs secreted at the site of infection lead to the production of CXCL10, leading to recruitment of a minor population of CTL to the vagina. However, CD4+ T cells play a dominant role in guiding effector CTLs to the site of infection. Collectively, these data indicate that CD4+ T cells mobilize CTLs to the sites of infection through licensing the local tissue environment - by inducing expression of chemokines necessary for CTLs to enter from systemic circulation into the site of infection. We speculate that the source of such chemokines is likely the infected vaginal epithelial cells, as high levels of mRNA for both CXCL9 and CXCL10 are detected within the epithelial layer by day 4 post infection (data not shown).
Figure 4. Secretion of CTL-recruiting chemokines in the infected tissue depends on CD4 T help.
Four days after HSV-2 infection, vaginal wash was collected from WT, CD4−/− mice or mice that were treated with anti-IFN-γ Ab on day 3 p.i. (as in Fig. 1a). Cytokine and chemokine concentrations were determined by Luminex bead assay. Data were pooled from two independent experiments (n = 6) and represented as mean ± SEM. *, p < 0.05; **, p < 0.01 (one-way ANOVA followed by Tukey’s post-hoc multiple comparison).
Our study reveals that, in addition to playing a key role in priming of CD8+ T cells and in promoting memory CD8+ T cell development1, CD4+ T cells serve as gate-keepers of CTL entry into the infected tissues. CD4+ T cells orchestrate this through the secretion of IFN-γ and turning on the expression of chemokines CXCL9 and CXCL10 in situ, which enable the CXCR3+ effector CTL population to migrate from the peripheral blood into the infected areas. Both of these chemokines have been shown to be important for CTL recruitment and defense against HSV-2 infection20. These findings reveal that fully differentiated CTLs are self-insufficient for entry into infected peripheral tissues, and that an additional layer of regulation is provided by CD4+ T cells to carry out their effector functions. The multiple stages in which CD4 help is required for CTL responses - during priming, memory and now effector phases - likely reflect the need to restrict the cytotoxic activity to the site of infection. In contrast, effector CTL migration into other mucosal tissues such as the lung and intestine15 does not require either CD4 help or inflammation. Thus, certain organs are “permissible” for effector CTL migration, while other organs (e.g., vagina) are “restricted” and rely on CD4 help and inflammatory chemokines to recruit memory of effector T cells only when they are needed.
Many pathogens invade the host through establishing local infection before spreading to other organs. The recruitment of the effector lymphocytes to the initial site of microbial entry represents a key challenge in ensuring the protection of the host. Our findings imply that vaccines that target CTL immunity must incorporate T helper epitopes to allow their migration to the site of infection once the pathogen invades the host. In addition, treatment of CTL-mediated organ-specific autoimmune diseases might benefit from selective depletion of CD4+ T cells or IFN-γ from the local tissue, so as to block further recruitment of pathogenic CTL populations. Future studies to understand the key players involved in CD4+ T cell mediated recruitment of effector CTL will help our ability to design effective interventions for treatment of infectious diseases, autoimmunity and cancer.
Methods Summary
Adoptive transfer of naïve CD8+ T cells and generation of effector gBT-I cells
Indicated numbers of naïve gBT-I transgenic CD8+ T cells from the spleens of CD45.1+gBT-I or CD45.1+CXCR3−/− gBT-I mice were adoptively transferred into recipient mice. In some experiments, the donor cells were labeled with 0.05 µM CFSE (Invitrogen) before transfer. CD4+ T cells from recipient mice were depleted by injection of 200 µg of GK1.5 antibody (> 99.5 % depletion). Some recipient mice were injected with neutralizing antibody against IFN-γ (i.p., 1 mg XMG1.2). Depo-Proveratreated 6–8 weeks-old female recipient mice were infected intravaginally (ivag) with 106 PFU of 186TKΔKpn (TK− HSV-2)9 or with uninfected Vero lysate (mock infection) control as described previously10. Effector CTLs were isolated from the spleen at 6 days post infection, and transferred into day 4 HSV-2-infected recipients. The numbers of CD45.1+ gBT-I cells in the primary or secondary hosts were analyzed by flow cytometry.
Flow cytometry
Single suspensions were prepared from each experimental group using modified protocol as described21. To analyze chemokine receptor expression, cell suspensions were stained with an anti-CXCR3 antibody (220803). The H-2Kb-gB498-505 tetramer used here was prepared by NIH tetramer core facility. Samples were acquired on a FACSCaliber (BD Bioscience) and analyzed with FlowJo software (TreeStar).
Cytokine and chemokine measurement in the vaginal wash
The vaginal wash was collected using a standard method21. The amount of cytokine/chemokine in the vaginal wash was measured using a multiplex Luminex beads assay (Millipore) or ELISA according to manufacturers’ instructions.
Supplementary Material
Acknowledgments
We thank Drs. F. R. Carbone and W. R. Heath for the gBT-I transgenic mouse, Drs. R. Medzhitov and J. M. Thompson for critical reading of the manuscript, and Dr. N. Iijima for technical assistance. This work is supported by NIH grants to A. I. AI054359 and AI062428. Y. N. was a Japan Society for the Promotion of Science fellow. A. I. is a recipient of the Burroughs Wellcome Investigators in Pathogenesis of Infectious Disease.
Footnotes
‘Supplementary Information accompanies the paper on www.nature.com/nature.’
Author Contributions
Experiments were conceived and designed by Y.N. and A.I. Experiments were performed by Y.N. Data were analyzed by Y.N. and A. I. Paper was written by Y.N. and A.I. C.G. and B. L. provided CXCR3 knockout mice.
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions.
References
- 1.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 2.Bennett SR, et al. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 3.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 4.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 5.Jennings SR, Bonneau RH, Smith PM, Wolcott RM, Chervenak R. CD4-positive T lymphocytes are required for the generation of the primary but not the secondary CD8-positive cytolytic T lymphocyte response to herpes simplex virus in C57BL/6 mice. Cell Immunol. 1991;133:234–252. doi: 10.1016/0008-8749(91)90194-g. [DOI] [PubMed] [Google Scholar]
- 6.Janssen EM, et al. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 7.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 8.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones CA, Taylor TJ, Knipe DM. Biological properties of herpes simplex virus 2 replication-defective mutant strains in a murine nasal infection model. Virology. 2000;278:137–150. doi: 10.1006/viro.2000.0628. [DOI] [PubMed] [Google Scholar]
- 10.Zhao X, et al. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J Exp Med. 2003;197:153–162. doi: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith CM, et al. Cognate CD4(+) T cell licensing of dendritic cells in CD8(+) T cell immunity. Nat Immunol. 2004;5:1143–1148. doi: 10.1038/ni1129. [DOI] [PubMed] [Google Scholar]
- 12.Mueller SN, Heath W, McLain JD, Carbone FR, Jones CM. Characterization of two TCR transgenic mouse lines specific for herpes simplex virus. Immunol Cell Biol. 2002;80:156–163. doi: 10.1046/j.1440-1711.2002.01071.x. [DOI] [PubMed] [Google Scholar]
- 13.Stock AT, et al. Optimization of TCR transgenic T cells for in vivo tracking of immune responses. Immunol Cell Biol. 2007;85:394–396. doi: 10.1038/sj.icb.7100076. [DOI] [PubMed] [Google Scholar]
- 14.Marshall DR, et al. Measuring the diaspora for virus-specific CD8+ T cells. Proc Natl Acad Sci U S A. 2001;98:6313–6318. doi: 10.1073/pnas.101132698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 16.Lund JM, Hsing L, Pham TT, Rudensky AY. Coordination of early protective immunity to viral infection by regulatory T cells. Science. 2008;320:1220–1224. doi: 10.1126/science.1155209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reboldi A, et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10:514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 18.Milligan GN, Bernstein DI. Interferon-gamma enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology. 1997;229:259–268. doi: 10.1006/viro.1997.8441. [DOI] [PubMed] [Google Scholar]
- 19.Iijima N, et al. Dendritic cells and B cells maximize mucosal Th1 memory response to herpes simplex virus. J Exp Med. 2008;205:3041–3052. doi: 10.1084/jem.20082039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thapa M, Welner RS, Pelayo R, Carr DJ. CXCL9 and CXCL10 expression are critical for control of genital herpes simplex virus type 2 infection through mobilization of HSV-specific CTL and NK cells to the nervous system. J Immunol. 2008;180:1098–1106. doi: 10.4049/jimmunol.180.2.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iijima N, Linehan MM, Saeland S, Iwasaki A. Vaginal epithelial dendritic cells renew from bone marrow precursors. Proc Natl Acad Sci U S A. 2007;104:19061–19066. doi: 10.1073/pnas.0707179104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hancock WW, et al. Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J Exp Med. 2000;192:1515–1520. doi: 10.1084/jem.192.10.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato A, Iwasaki A. Induction of antiviral immunity requires Toll-like receptor signaling in both stromal and dendritic cell compartments. Proc Natl Acad Sci U S A. 2004;101:16274–16279. doi: 10.1073/pnas.0406268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fontenot JD, et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 25.Iwasaki A, Kelsall BL. Localization of distinct Peyer's patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3alpha, MIP-3beta, and secondary lymphoid organ chemokine. J Exp Med. 2000;191:1381–1394. doi: 10.1084/jem.191.8.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.