Abstract
Memory CD8 T cells, essential for defense against intracellular pathogens, are heterogeneous with respect to phenotype and function. Constitutively lytic effector memory cells primarily reside in nonlymphoid tissues, whereas secondary lymphoid tissues contain functionally quiescent central memory cells. However, the mechanism by which functionally distinct memory populations are maintained is unknown. In this study, we show that resting CD8 memory cells modified their functional abilities upon entry into nonlymphoid tissues, as exemplified by the induction of granzyme B and lytic activity. Contemporaneously, the costimulator CD27 was down-regulated. These findings hold important implications for memory cell lineage development and tissue-specific immunity.
Seminal studies provide evidence for the existence of two subsets of memory CD8+ T cells, central memory cells (TCM)3 which express the receptors CCR7 and CD62L, allowing efficient homing to lymph nodes, and effector memory cells (TEM) which lack these homing receptors (1, 2) and therefore reside mainly in nonlymphoid tissues (3, 4). TCM cells and TEM cells also differ in both their proliferative ability and effector functions with TEM having a lower proliferative capacity than TCM (5, 6). TCM cells lack immediate effector function whereas CD8 TEM cells express molecules necessary for lytic activity such as perforin and granzyme B and display direct ex vivo effector function (1, 2, 4). More recently, it has become evident that some memory CD8 T cells express a mixed CD62L− CCR7+ phenotype that differs from the phenotypes of classical TEM and TCM cells, implying further heterogeneity within the memory lineage (7). In addition it has been demonstrated that virus-specific intraepithelial lymphocytes in the gut resemble neither TCM nor TEM CD8 T cells isolated from spleen or blood suggesting that anatomic location may play a role in local memory differentiation (8). Furthermore, the lack of expression of CD27, a member of the TNF receptor family, has emerged as an indicator of constitutive effector function in human and mouse memory CD8 T cells (1, 9–11).
In terms of function, it has been shown that for CD8 T cells, nonlymphoid derived memory cells exhibit constitutive effector levels of lytic activity whereas splenic memory cells, despite a CD62L- phenotype, are poorly lytic directly ex vivo (4). The basis for this dichotomy is unknown but memory CD8 T cells have the ability to traffic between lymphoid and nonlymphoid tissues as a part of a common blood-borne pool (12–14). Thus, functional differences between memory T cells in distinct locations could be due to the existence of subsets of effector memory cells that preferentially home to specific tissues or alternatively due to the modulation of functional activities upon entry of blood-borne memory cells into nonlymphoid tissues. Therefore, we undertook studies to assess whether entry of nonlytic memory cells into nonlymphoid tissues resulted in functional modifications. Our results indicate that memory cell phenotype and function are plastic and are dynamically modulated as a result of migration.
Materials and Methods
Mice and infections
C57BL/6J (Ptprcb = CD45.2) mice were purchased from The Jackson Laboratory and C57BL/6 (Ptprca = CD45.1) mice from Charles River through the NCI program. B6.SJL-Ptprca Pepcb/BoyJ-Tg(TcraTcrb)1100Mjb/J-B6.129S7-Rag1tm1Mom (CD45.1 OT-I-RAG−/−) were bred at University of Connecticut Health Center. Mice were infected i.v. with VSV-ova (1 × 106 pfu) or 1 × 103 cfu of LM-ova (15, 16).
Isolation of T cell subsets and adoptive transfer
Lymphocytes were isolated from lymphoid and nonlymphoid tissues as previously described (4). In some experiments, spleen cells were isolated using the same technique as that employed for isolation of nonlymphoid cells. This isolation protocol did not result in alterations of the expression levels of CD27 or CD62L (data not shown). CD27high/CD62Lhigh, CD27high/CD62Llow, and CD27low/CD62Llow populations were purified by sorting using a FACSVantage SE (BD Biosciences). The purity of sorted samples ranged between 91% and 99% and cell populations were adoptively transferred i.v.
Analysis of intracellular granzyme B expression
For intracellular detection of granzyme B, cells were stained with antihuman granzyme B or an isotype control for 45 min at RT.
Measurement of cytolytic activity
Cytolytic activity was measured using [51Cr]sodium chromate-labeled EL4 cells (an H-2b thymoma) with or without the addition of 10 µg/ml of the VSV-N protein-derived peptide RGYVYQGL. Serial dilutions of sorted effector cells were incubated in 96-well round-bottom microtiter plates with 2.5 × 103 target cells for 5 h at 37°C. Percent specific lysis was calculated as: 100 × [(cpm released with effectors – cpm released alone)]/[(cpm released by detergent – cpm released alone)].
Results and Discussion
Preferential expression of granzyme B by nonlymphoid memory CD8 T cells correlates with CD27 levels
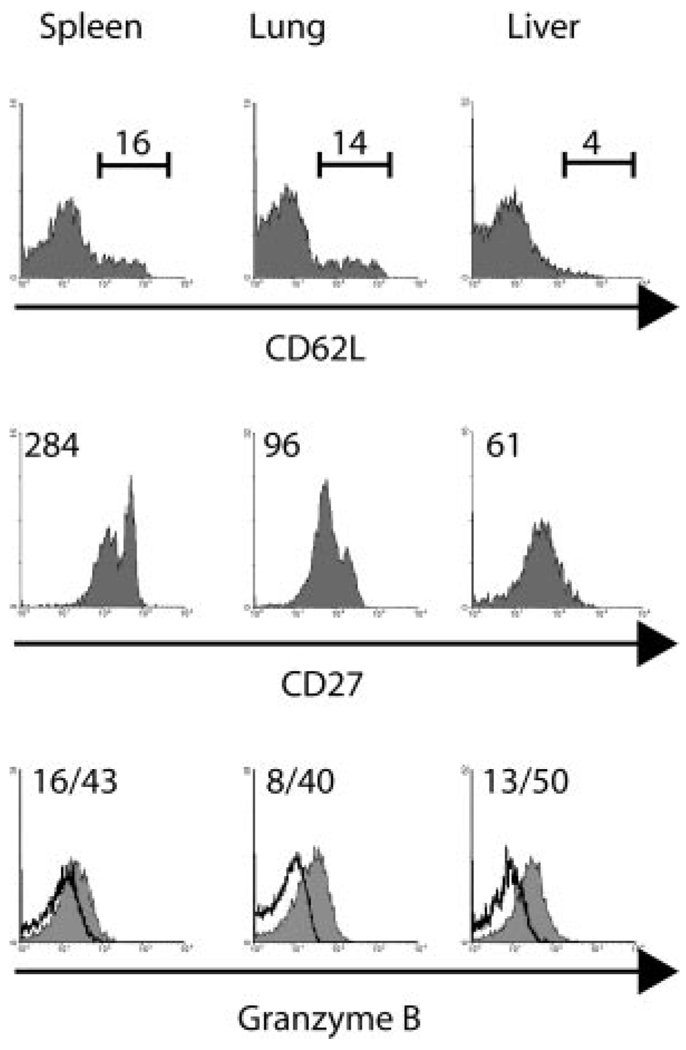
To determine whether antimicrobial CD8 memory T cells were phenotypically and functionally distinct in different tissues, we compared expression levels of CD27, CD62L, and granzyme B (Fig. 1). Granzyme B is involved in lymphocyte-mediated killing of target cells by CTL (17, 18). We have previously shown that unlike tissue-resident cells, splenic memory cells are poorly lytic indicating that there is not a strict correlation between CD62L expression and lytic activity (4). Therefore, we employed CD27 expression in conjunction with CD62L expression to further dissect memory cell subsets, since studies have shown that the CD27low CD8+ T cell subset is primarily composed of cells preferentially expressing perforin and exhibiting CTL activity (1, 10, 19). More recently, it has been shown that influenza virus-specific CD27low T cells are present in the lung after secondary infection and that the loss of CD27 coincides with high granzyme B expression (10). To generate memory cells, CD45.1 OT-I cells were transferred into B6 CD45.2 mice that were then infected i.v. with LM-ova (15, 16), rested for 46 days and secondarily infected with VSV-ova (20). Lymphocytes were isolated 70 days later from the spleen, lung, and liver and the phenotype and granzyme B expression of the Ag-specific T cells was examined. At this time point, small populations of memory CD8 T cells in the spleen and lung expressed CD62L, whereas liver memory cells generally lacked this receptor (Fig. 1). CD27 levels were heterogeneous, with distinct CD27high and CD27low memory cell subsets in the spleen and lung whereas liver memory cells were largely CD27low. Interestingly, granzyme B expression was lowest in splenic memory cells and was substantially higher in lung and liver memory cells (Fig. 1). These data are also consistent with the recent demonstration that CD27 down-regulation correlates with the expression of cytotoxic effector molecules such as granzyme B (10).
FIGURE 1.
Phenotype and granzyme B expression of splenic and nonlymphoid memory CD8 T cells. 1 × 103 CD45.1 OT-I-RAG−/− splenocytes were transferred into CD45.2 mice and 1 day later the mice were infected with 1 × 103 cfu of LM-ova i.v. and 46 days post infection re-infected with 1 × 106 pfu VSV-ova i.v. 70 days later the level of CD62L, CD27 and GrB expression of the OT-I memory CD8 T cells was measured by flow cytometry. Values indicate the mean fluorescent intensity (MFI) of staining for the population. For GrB staining the MFI is shown for the isotype control and test, respectively. Data are representative of 3–4 mice analyzed from two experiments.
Granzyme B is up-regulated on CD8 TCM cells after entry into nonlymphoid tissues
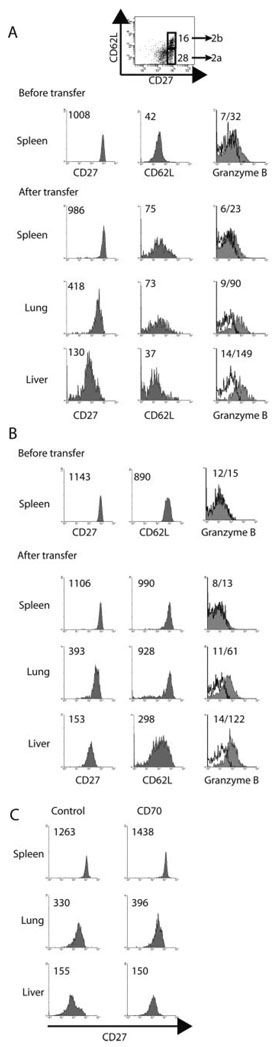
To determine whether memory cells altered their phenotype upon entry into nonlymphoid tissues, we transferred sorted CD62Llow/CD27high memory cells from the spleen to naive mice and analyzed the transferred cells 4 days later. These cells expressed very low levels of granzyme B before transfer (Fig. 2A). Splenic memory cells after transfer retained high levels of CD27 (mean fluorescense intensity (MFI) 986) and low levels of CD62L (MFI 75) and granzyme B (MFI 23). However, upon entry into nonlymphoid tissues CD27 expression was down-regulated in the lung (MFI 418) and even more so in the liver (MFI 130), whereas CD62L expression remained low. Also, granzyme B expression was induced in the transferred cells in both the lung (MFI 90) and the liver (MFI 149) (Fig. 2A). We also followed the fate of transferred CD62Lhigh/CD27high cells (TCM). Before transfer, this population did not express detectable granzyme B (Fig. 2B). After transfer, CD27 expression remained unchanged in the spleen (MFI 1106), but was substantially down-regulated in the lung (MFI 393) and liver (MFI 153). CD62L expression remained high in the spleen and lung but was down-regulated to some extent in the liver. Granzyme B expression was considerably up-regulated in both the lung (MFI 61) and liver (MFI 122) (Fig. 2B). Thus, TCM can give rise to functional TEM. It should be noted that in all of the transfer experiments, recovery of transferred cells was 3–10% of total input cell number (data not shown), in agreement with estimates of naive and memory cell survival after transfer (21), suggesting that the results obtained were not due to minor contaminants that preferentially survived and migrated to specific tissues.
FIGURE 2.
Splenic CD8 TCM down-regulate CD27 and acquire granzyme B after entry into nonlymphoid tissues. OT-I transferred CD45.2 mice were infected with 1 × 103 CFU of LM-ova i.v. 48 days later mice were re-infected with 1 × 106 PFU VSV-ova i.v., and 97 days later, splenocytes were isolated, enriched for CD8 T cells, donor cells were sorted into either CD62Llow/CD27high cells (A) or CD62Lhigh/CD27high cells (B) and transferred to naive CD45.2 mice (1×106 and 5×105, respectively). Four days later lymphocytes were isolated from the spleen, lung, and liver, and the level of CD27, CD62L, and granzyme B expression by the transferred OT-I T cells was determined. C, OT-I transferred mice were infected with LM-ova i.v.; 46 days later mice were infected with VSV-ova i.v., and 58 days later splenocytes were isolated, enriched for CD8 T cells, donor cells were sorted, and 8.5 × 105 CD27high cells were transferred to naive CD45.2 mice. On days 0, 1, and 2, mice were treated with either 250 µg of anti-CD70 (FR70) or control rat IgG. Four days later, lymphocytes were isolated from the spleen, lung and liver, and the level of CD27 was determined on the transferred OT-I T cells. Values indicate the MFI of staining for the population. For GrB staining the MFI is shown for the isotype control and test, respectively. Data are from one representative animal of 1–4 mice analyzed from four experiments (n = 9).
T cells down-modulate CD27 after interaction with the CD27 ligand CD70 in vitro and in vivo (22, 23). To analyze whether the observed down-regulation of CD27 was due to CD70 engagement, we transferred CD27high splenic memory cells and simultaneously administered a blocking anti-CD70 mAb (24). Four days posttransfer lymphocytes were isolated from the spleen, lung, and liver and CD27 expression was examined (Fig. 2C). In both the lung and the liver, the level of CD27 on transferred memory cells was down-regulated compared with the cells in the spleen and blocking CD70 had no effect on CD27 levels. The amount of anti-CD70 mAb employed effectively blocked the primary CD8 T cell response to LM infection indicating the mAb was active (data not shown). These results suggested that down-modulation of CD27 expression on migrating memory cells was not CD70-mediated but was the result of as yet unidentified factors acting to reduce CD27 levels.
Splenic memory cells acquire lytic activity after entry into nonlymphoid tissues
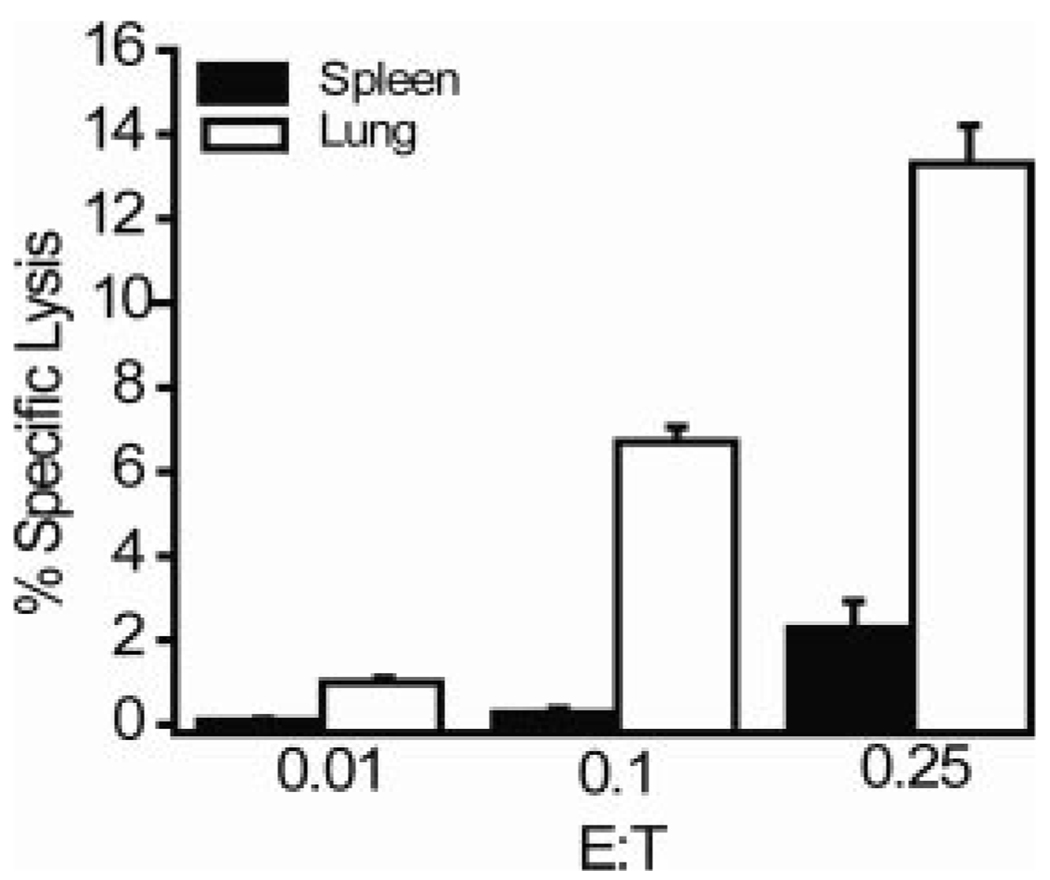
The presence of granzyme B is not an absolute indicator of the ability of a CD8 T cell to mediate target cell lysis since other molecules are also required for CTL activity. Therefore, we measured the direct lytic activity of transferred splenic memory CD8 T cells. Enriched CD8 T cells from spleens of mice previously infected with VSV were adoptively transferred into naive mice and 4 days later sorted splenic and lung tetramer+ CD8 T cells were assayed for their CTL capacity. Transferred memory cells isolated from the spleen exhibited low levels of lytic activity (Fig. 3), as did the starting population before transfer (data not shown). In contrast, memory cells obtained from the lung exhibited substantial levels of lytic activity, inducing 10–15% specific lysis at a very low E:T ratio (0.25:1) (Fig. 3). Thus, the induction of granzyme B expression correlated with up-regulation of cytolytic activity in migrating memory CD8 T cells.
FIGURE 3.
Splenic CD8 TM acquire lytic activity after entry into nonlymphoid tissues. CD45.2 B6 mice were infected with 1 × 105 PFU of VSV-Indiana, rested for 71 days and infected with 1 × 105 VSV-New Jersey. Approximately 9 mo after the secondary infection, CD8 T cells were enriched from the spleens and transferred to CD45.1 B6 mice; 4 days posttransfer tetramer+ CD8 T cells were sorted from the spleen and lungs and set up in a CTL assay. Experiments were performed twice with similar results, and the data are the mean of three mice from one experiment.
Nonlymphoid TEM cells modulate CD27 expression but retain granzyme B following transfer
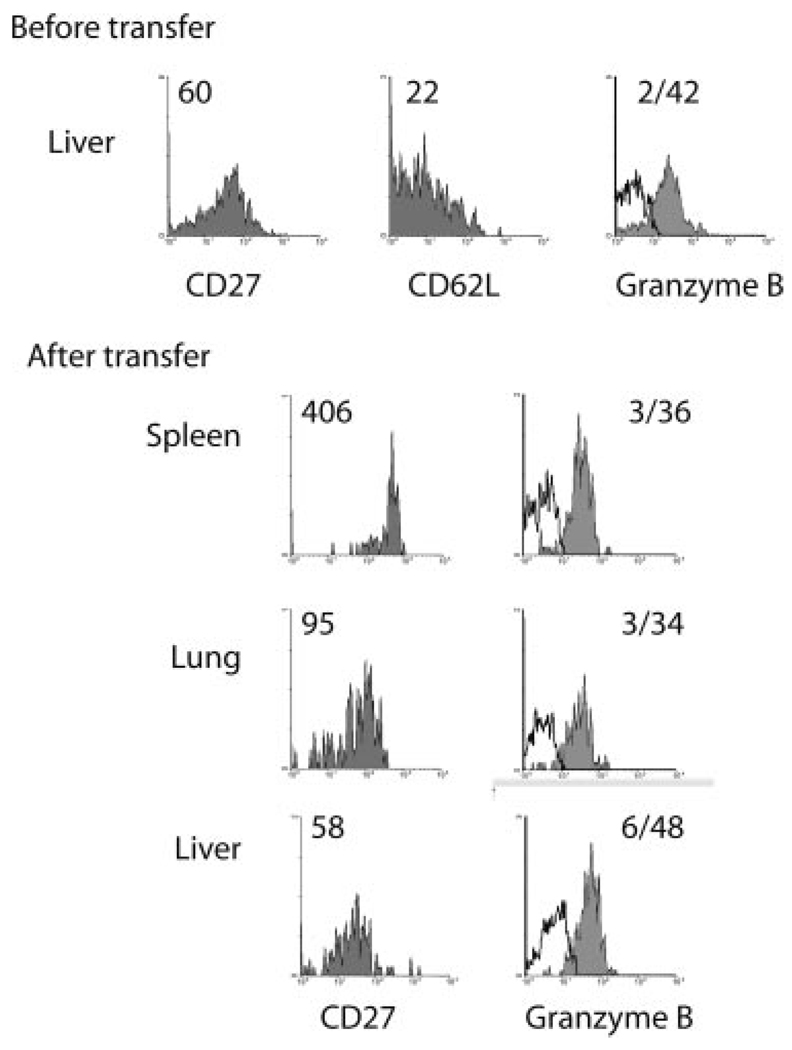
Our results indicated that TCM cells could convert to functional TEM cells upon entry into nonlymphoid tissue. However, whether this phenomenon represented a “revolving door” through which TEM cells exiting from nonlymphoid tissues reverted to TCM-like cells was unknown. To test this possibility, CD62Llow/CD27lowGrBhigh memory CD8 T cells isolated from the liver were transferred to naive animals and 4 days later were analyzed. Interestingly, transferred CD62Llow/CD27low liver memory cells isolated from the spleen exhibited heightened CD27 levels as compared with their nonlymphoid counterparts in the lung and liver where CD27 expression was similar to that of the cells before transfer (Fig. 4). However, granzyme B levels remained high in transferred memory cells isolated from all tissues. Thus, in this case CD27 expression levels did not apparently correlate with lytic activity. Although at present we do not have an explanation for this finding, given the observed heterogeneity in CD27 expression (Fig. 1), it is perhaps likely that CD27 expression levels may not precisely correlate with granzyme B expression. It is also possible that a period longer than 4 days is required to observe down-regulation of lytic molecules such as granzyme B and this may depend on protein half-life. Alternatively, under normal circumstances lytic memory cells may not exit nonlymphoid tissues in large numbers. Nevertheless, the results again indicated the short-term mutability of the memory cell population with respect to phenotype.
FIGURE 4.
Nonlymphoid TEM cells modulate CD27 expression but retain granzyme B following transfer. OT-I transferred CD45.2 B6 mice were infected with LM-ova and 48 days later were infected with VSV-ova i.v.; 97 days later, CD62Llow liver memory T cells were sorted and 1 × 105 OT-I cells were transferred to naive CD45.2 B6 recipients, and 4 days posttransfer CD27, CD62L, and granzyme B expression of OT-I cells from the spleen, lung, and liver was determined. Values indicate the MFI of staining for the population. For GrB staining, the MFI is shown for the isotype control and test, respectively. Data are from one representative animal of two mice analyzed from two experiments.
Our data indicated that CD27 but not CD62L levels correlated in most cases with increased levels of granzyme B. Furthermore, CD62Llow/CD27low/GrBhigh memory CD8 T cells appeared to represent terminally differentiated cells whereas both CD62Llow/CD27high/GrBlow and CD62Lhigh/CD27high/GrBneg memory CD8 T cells were functionally flexible. Although it has been suggested that CD27 is lost from CD8 T cells only after repetitive antigenic stimulation (10), we found that down-regulation of CD27 can occur in the absence of Ag, as shown by adoptive transfer of OT-I memory CD8 T cells into naive hosts. However, all memory CD8 T cells we examined expressed CD27 at some level leaving open the possibility that further loss of CD27 may occur following repetitive antigenic stimulation. In addition to modulation of CD27 levels, migration of nonlytic memory CD8 T cells to nonlymphoid tissues triggered the induction of the cytolytic effector molecule, granzyme B and resulted in the induction of lytic activity. These data indicated that memory T cell phenotype and function are not fixed and are dependent on location. Although it remains unclear whether there is a subset of TEM cells that preferentially home to nonlymphoid tissues, we would predict that this is not the case as all populations of purified memory T cells homed to the different tissues and up-regulated granzyme B expression. Since cells entering both the lung and liver were functionally modulated, common factors involved in effector molecule regulation must exist in at least these tissues, if not others. It should be noted that our studies utilized secondary memory cells, which may exhibit distinct characteristics as compared with primary memory CD8 T cells. Our original experiments of mouse TCM and TEM studied primary and secondary memory cells (4) and as reiterated here, showed that splenic primary and secondary memory CD8 T cells contain at least a subpopulation of cells with a TEM phenotype that display negligible levels of lytic activity. Recent reports indicate that lytic activity, granzyme B expression and protective capacity of memory CD8 T cells increase with the number of booster immunizations (25, 26). Nonetheless, a subset of TCM is apparent after multiple immunizations, particularly when the lack of CD62L expression is not used as criteria to identify TEM. The length of time after immunization is another important criteria to consider when assessing memory CD8 T cells since phenotype and perhaps function can change over time as a result of population dynamics (26–28). In any case, our data favor the hypothesis that one consequence of traversing the endothelium to enter parenchymal tissues is induction of lytic activity. This phenomenon may be regulated by T cell interactions with chemokines and adhesion molecules that are necessary for extravasation of memory T cells from blood vessels to extralymphoid sites. A pathway such as this could represent a regulatory mechanism for ensuring optimal activity of memory T cells in the appropriate locale.
Acknowledgments
We thank Diane Gran for FACS sorting.
Footnotes
This work was supported by National Institutes of Health Grant DK45260.
Abbreviations used in this paper: TCM, central memory T cells; B6, C57BL/6; LM-ova, Listeria monocytogenes expressing ova; VSV-ova, vesicular stomatitis virus encoding ovalbumin; TEM, effector memory T cells; MFI, mean fluorescence intensity.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, van Lier RA. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 3.Warnock RA, Askari S, Butcher EC, von Andrian UH. Molecular mechanisms of lymphocyte homing to peripheral lymph nodes. J. Exp. Med. 1998;187:205–216. doi: 10.1084/jem.187.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masopust D, Vezys V, Marzo AL, Lefrançois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 5.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 6.Marzo AL, Klonowski KD, Le Bon A, Borrow P, Tough DF, Lefrancois L. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat. Immunol. 2005;6:793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Unsoeld H, Pircher H. Complex memory T-cell phenotypes revealed by coexpression of CD62L and CCR7. J. Virol. 2005;79:4510–4513. doi: 10.1128/JVI.79.7.4510-4513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masopust D, Vezys V, Wherry J, Barber DL, Ahmed R. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J. Immunol. 2006;176:2079–2083. doi: 10.4049/jimmunol.176.4.2079. [DOI] [PubMed] [Google Scholar]
- 9.Tomiyama H, Takata H, Matsuda T, Takiguchi M. Phenotypic classification of human CD8+ T cells reflecting their function: inverse correlation between quantitative expression of CD27 and cytotoxic effector function. Eur. J. Immunol. 2004;34:999–1010. doi: 10.1002/eji.200324478. [DOI] [PubMed] [Google Scholar]
- 10.Baars PA, Sierro S, Arens R, Tesselaar K, Hooibrink B, Klenerman P, van Lier RA. Properties of murine (CD8+) CD27− T cells. Eur. J. Immunol. 2005;35:3131–3141. doi: 10.1002/eji.200425770. [DOI] [PubMed] [Google Scholar]
- 11.de Bree GJ, van Leeuwen EM, Out TA, Jansen HM, Jonkers RE, van Lier RA. Selective accumulation of differentiated CD8+ T cells specific for respiratory viruses in the human lung. J. Exp. Med. 2005;202:1433–1442. doi: 10.1084/jem.20051365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masopust D, Vezys V, Usherwood EJ, Cauley LS, Olson S, Marzo AL, Ward RL, Woodland DL, Lefrançois L. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J. Immunol. 2004;172:4875–4882. doi: 10.4049/jimmunol.172.8.4875. [DOI] [PubMed] [Google Scholar]
- 13.Klonowski KD, Williams KJ, Marzo AL, Blair DA, Lingenheld EG, Lefrançois L. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 2004;20:551–562. doi: 10.1016/s1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- 14.Zammit DJ, Turner DL, Klonowski KD, Lefrançois L, Cauley LS. Residual antigen presentation after influenza virus infection affects CD8 T cell activation and migration. Immunity. 2006;24:439–449. doi: 10.1016/j.immuni.2006.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pope C, Kim S-K, Marzo A, Masopust D, Williams K, Jiang J, Shen H, Lefrançois L. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J. Immunol. 2001;166:3402–3409. doi: 10.4049/jimmunol.166.5.3402. [DOI] [PubMed] [Google Scholar]
- 16.Foulds KE, Zenewicz LA, Shedlock DJ, Jiang J, Troy AE, Shen H. Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. J. Immunol. 2002;168:1528–1532. doi: 10.4049/jimmunol.168.4.1528. [DOI] [PubMed] [Google Scholar]
- 17.Barry M, Bleackley RC. Cytotoxic T lymphocytes: all roads lead to death. Nat. Rev. Immunol. 2002;2:401–409. doi: 10.1038/nri819. [DOI] [PubMed] [Google Scholar]
- 18.Russell JH, Ley TJ. Lymphocyte-mediated cytotoxicity. Annu. Rev. Immunol. 2002;20:323–370. doi: 10.1146/annurev.immunol.20.100201.131730. [DOI] [PubMed] [Google Scholar]
- 19.Hintzen RQ, de Jong R, Lens SM, van Lier RA. CD27: marker and mediator of T-cell activation. Immunol. Today. 1994;15:307–311. doi: 10.1016/0167-5699(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 20.Kim SK, Reed DS, Olson S, Schnell MJ, RoseJ JK, Morton PA, Lefrançois L. Generation of mucosal cytotoxic T cells against soluble protein by tissue-specific environmental and costimulatory signals. Proc. Natl. Acad. Sci. USA. 1998;95:10814–10819. doi: 10.1073/pnas.95.18.10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hataye J, Moon JJ, Khoruts A, Reilly C, Jenkins MK. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science. 2006;312:114–116. doi: 10.1126/science.1124228. [DOI] [PubMed] [Google Scholar]
- 22.Hintzen RQ, Lens SM, Lammers K, Kuiper H, Beckmann MP, van Lier RA. Engagement of CD27 with its ligand CD70 provides a second signal for T cell activation. J. Immunol. 1995;154:2612–2623. [PubMed] [Google Scholar]
- 23.Arens R, Tesselaar K, Baars PA, van Schijndel GM, Hendriks J, Pals ST, Krimpenfort P, Borst J, van Oers MH, van Lier RA. Constitutive CD27/CD70 interaction induces expansion of effector-type T cells and results in IFNγ-mediated B cell depletion. Immunity. 2001;15:801–812. doi: 10.1016/s1074-7613(01)00236-9. [DOI] [PubMed] [Google Scholar]
- 24.Oshima H, Nakano H, Nohara C, Kobata T, Nakajima A, Jenkins NA, Gilbert DJ, Copeland NG, Muto T, Yagita H, Okumura K. Characterization of murine CD70 by molecular cloning and mAb. Int. Immunol. 1998;10:517–526. doi: 10.1093/intimm/10.4.517. [DOI] [PubMed] [Google Scholar]
- 25.Masopust D, Ha SJ, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J. Immunol. 2006;177:831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- 26.Jabbari A, Harty JT. Secondary memory CD8+ T cells are more protective but slower to acquire a central-memory phenotype. J. Exp. Med. 2006;203:919–932. doi: 10.1084/jem.20052237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lefrançois L. Development, trafficking, and function of memory T-cell subsets. Immunological Rev. 2006;211:93–103. doi: 10.1111/j.0105-2896.2006.00393.x. [DOI] [PubMed] [Google Scholar]
- 28.Lefrançois L, Marzo AL. The descent of memory T-cell subsets. Nat. Rev. Immunol. 2006;6:618–623. doi: 10.1038/nri1866. [DOI] [PubMed] [Google Scholar]