Abstract
Background
Hyperkalemia has been associated with higher mortality in long-term hemodialysis (HD) patients. There are little data concerning the relationship between dietary potassium intake and outcome.
Study design
Mortality-predictability of dietary potassium intake from reported food items, estimated from the Block Food Frequency Questionnaire (FFQ) at the start of the cohort, were examined in a 5-year (2001–06) cohort of 224 HD patients in Southern California using Cox proportional hazards regression.
Setting and Participants
224 long-term hemodialysis patients from 8 DaVita dialysis clinics.
Predictors
Dietary potassium intake ranking using Block FFQ
Outcomes
5-year survival
Results
HD patients with higher potassium intakes had greater dietary energy, protein and phosphorus intakes and higher predialysis serum potassium and phosphorus. Greater dietary potassium intake was associated with significantly increased death hazard ratios (HR) in the unadjusted models and after incremental adjustments for case-mix, nutritional factors (including 3-month averaged predialysis serum creatinine, potassium and phosphorus, body mass index, normalized protein nitrogen appearance, and energy, protein and phosphorus intake) and inflammatory markers. The HR (95% confidence intervals) of death across the 3 higher quartiles of dietary potassium intake in the fully adjusted model (compared to the lowest quartile) were 1.4 (0.6–3.0), 2.2 (0.9–5.4) and 2.4 (1.1–7.5), respectively (p for trend: 0.03). Restricted cubic spline analyses confirmed the incremental mortality-predictability of higher potassium intake.
Limitations
FFQs may underestimate individual potassium intake and should be used to rank dietary intake across population.
Conclusions
Higher dietary potassium intake is associated with increased death risk in long-term HD patients, even after adjustments for serum potassium and dietary protein, energy and phosphorus intake and nutritional and inflammatory markers. The potential role of dietary potassium in the high mortality rate of HD patients warrants clinical trials.
Keywords: Dietary potassium, food frequency questionnaire, mortality, hemodialysis
The number of new patients with advanced chronic kidney disease (CKD) undergoing maintenance dialysis is increasing worldwide.(1, 2) However, the life expectancy of these patients is still much lower than that of the general population. The causes for the excess all-cause mortality are not clear.(3, 4) Disorders of potassium metabolism are common in CKD patients and especially in those undergoing chronic hemodialysis (HD) treatment.(5, 6) As kidney function decreases, the ability to maintain serum potassium in the physiologically normal range is jeopardized.(7–11) One of the many roles for dialysis treatment is to regulate serum and body potassium levels. Indeed in long-term HD patients, hyperkalemia has been associated with higher mortality.(5, 6, 12, 13) Hence, persons with CKD are advised to ingest less than 1500 mg (<76 mmol) of potassium per day according to diverse sources, including the “patient information” of UpToDate (13a).
Notwithstanding the epidemiological data in HD patients relating adverse health effects to high serum potassium and considering the importance of limiting the amount of potassium in the diet of in these patients, there are little data concerning the association of the quantity of dietary potassium intake with outcome. Whereas serum potassium may vary erratically as a result of many factors (thrice weekly HD treatments, residual renal function, gastrointestinal disorders such as diarrhea, variation in blood sugar or insulin, tissue degradation during infection, etc), assessment of averaged dietary potassium may be a better surrogate of overall potassium load. However, it is not easy to determine the independent effects of dietary potassium on survival, because other dietary components that may influence clinical outcome, such as protein or phosphorus intake, tend to covary with potassium intake. In this study we examined the independent relationship of potassium intake with mortality in a cohort of 224 long-term HD patients who were followed for up to 5 years, in whom other nutritional and inflammatory measures, including circulating cytokines, and body composition, were also assessed. We hypothesized that higher dietary potassium intake is independently associated with increased death risk in maintenance HD patients.
Methods
Study Design and Population
We studied prevalent HD patients who participated in the NIH-funded NIED (Nutritional and Inflammatory Evaluation in Dialysis) Study.(14–18) The original patient cohort was derived from a pool of approximately 1,300 HD outpatients in eight DaVita Inc. chronic dialysis facilities in the South Bay Los Angeles area (the NIED Study website [www.NIEDStudy.org] contains more details). Inclusion criteria were outpatients who had been undergoing HD for at least 8 weeks, were 18 years or older and who signed a local Institutional Review Board approved consent form. Patients with an anticipated life expectancy of less than 6 months (for example, due to metastatic malignancy or advanced HIV/AIDS disease) were excluded. From October 1, 2001, through December 31, 2006, 893 HD patients from the eight DaVita dialysis clinics in the Los Angeles South Bay area signed the informed consent form and underwent the periodic evaluations. Of these, we examined the first recruited 224 patients who enrolled in the NIED Study between October 1, 2001 and March 31, 2002 and whose dietary intake were examined via food questionnaires. The medical chart of each HD patient was thoroughly reviewed by a collaborating physician, and data pertaining to underlying kidney disease and comorbid conditions were extracted. A modified version of the Charlson comorbidity index, i.e. without the age and kidney disease components, was used to assess the severity of comorbidities.(19, 20) The survival of these 224 HD patients was followed up to 63 months, i.e., until December 31, 2006.
Anthropometric Measures and Body Composition
Anthropometric measurements were performed by DaVita dietitians while patients undergoing HD treatments or within 5–20 min after termination of the treatment. Body weight was measured at the end of the HD treatment. Biceps and triceps skinfold thicknesses were measured with Lange calipers (Cambridge Scientific Instruments, www.cambridgescientific.com) as previously described. (21, 22)
Body fat and fat-free body mass were measured by near infrared interactance (NIR) at the same time as the anthropometric measurements.(23, 24) A commercial near-infrared interactance sensor with a coefficient of variation of 0.5% for total body fat measurements (portable Futrex 6100, www.futrex.com) was used as described elsewhere.(24)
Laboratory Tests
Pre-HD blood samples for NIED Study measurements were obtained at the time of quarterly blood tests in the participating DaVita clinics. The single-pool Kt/V also included post-HD blood sampled to estimate the weekly dialysis dose. All routine laboratory measurements including serum potassium were performed in the DaVita Laboratories using automated methods. In this study, 3-month averaged values of routine laboratory measures were used. In the first 110 recruited patients from 4 DaVita clinics, enrolled in October 2001, all blood measures during the entire 4th calendar quarter of 2001 were averaged into a single value. In the remaining 114 patients, recruited in January 2002 from the other 4 clinics, similar averaging was performed during the 1st quarter of 2002. The quarterly averaged serum potassium value for each patient was calculated using all reported potassium measures (from 3 monthly to 6 or more bi-weekly values).
Serum high sensitivity C-reactive protein (CRP) was measured by a turbidometric immunoassay,(25, 26) and interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α) were measured with immunoassay kits based on a solid-phase sandwich ELISA.(27, 28). Plasma total homocysteine concentrations were determined by high-performance liquid chromatography, and serum transthyretin (prealbumin) was measured by immunoprecipitin analysis in the Harbor-UCLA Clinical Laboratories.(4)
Dietary Intakes via Food Frequency Questionnaire
In order to estimate the dietary intake of participating subjects over 6 to 12 months at the start of the cohort, the Block Food Frequency Questionnaire (FFQ) was administered once during the enrollment calendar quarter, i.e., the last quarter of 2001 and the first quarter of 2002 for the first 110 and remaining 114 patients, respectively, to correspond the period of 3-month averaging of laboratory data. The full-length, Block FFQ was originally developed by Gladys Block, PhD, at the National Cancer Institute, and has since been updated and improved.(29, 30) Different versions of this questionnaire have been extensively studied and validated in different population.(29–31) The Block 98 version (developed by Block Dietary Data Systems [Berkeley, CA] and distributed by NutritionQuest [www.Nutritionquest.com]) is an 8-page paper and pencil form that can be completed at home or during outpatient visits, such as HD sessions, in about 20 to 40 minutes. The FFQ includes more than 150 multiple-choice questions based on 107 food items. The first five questions are general inquiries concerning types of fruits, vegetables, cereal, and fat or oil that are ingested. Seventeen subsequent questions are about intakes of vitamins and minerals or herbal supplements. The next 130 items are detailed questions about food intake habits and asks how often a person consumes around 100 common foods. Each of these questions has two sets of multiple-choice answers, i.e., the frequency of intake of the given food item with up to nine options from never to every day; and the quantity (portions or servings) of the ingested food and with four levels. At the end, there are nine additional questions about the general impressions and opinions of the responder with regard to the whole questionnaire. Block FFQ has been validated for most nutrients including potassium using multiple diet records.(30, 32)
In this study, a group of trained research assistants and dietitians supervised the FFQ administration while the HD patients were undergoing routine HD treatment in their dialysis clinics. The completed FFQ booklets were reviewed immediately after they were returned, and if any question remained unanswered, the FFQ was returned to the patient with the request to attempt to answer the blank questions. All completed FFQs were scanned by an optical mark reader scanner, and the results were analyzed using software specifically designed for Block FFQ based on food ingredient data from the United States Department of Agriculture.
It is important to note that FFQs, as compared to real-time dietary recording or diary techniques, tend to underestimate nutrient intake, but this underestimation is. usually non-differential.(30, 32–34) Hence, nutrient intakes calculated from FFQs correlate with those from dietary diaries and recalls.(30, 32, 34) Estimated nutrient intakes from FFQs should therefore be reported and analyzed as either ranked values, i.e., quartiles, percentiles, etc., or as ratios of two different nutrients (e.g. phosphorus per protein ratio),(45) rather than absolute values per individuals.
Statistical Methods
Pearson's correlation coefficient (r) was used for analysis of linear associations. A restricted cubic splines graph was utilized as an exploratory data analysis strategy to illustrate systematic relations between dietary potassium and mortality. This method also served to examine the non-linear associations as continuous mortality predictors as an alternative to potential inappropriate assumptions concerning linearity.(35) Thereafter, to calculate the relative risks of death, hazard ratios (HR) were obtained using Cox proportional hazard models after controlling for the relevant covariates. Kaplan-Meier analyses were employed to assess the differences in surviving proportions between quartiles of potassium intake.
We performed incremental levels of multivariate adjustment: (A) Case-mix variables included age, gender, race/ethnicity, diabetes, dialysis vintage, insurance (Medicare vs. others), marital status, modified Charlson comorbidity score, dialysis dose (Kt/V), phosphorus binder intake and residual renal function. (B) Dietary intake variables included energy, protein, and phosphorus intake as estimated from FFQ. (C) Malnutrition inflammation complex syndrome (MICS) variables included serum phosphorus, albumin, creatinine, bicarbonate, calcium, ferritin, blood hemoglobin, white blood count and lymphocyte percentage; prescribed erythropoietin and active vitamin D doses; normalized protein nitrogen appearance (nPNA), also known as normalized protein catabolic rate (nPCR); and body mass index. (D) Additional adjustment was made for three inflammatory markers (CRP, IL-6, and TNFα). Descriptive and multivariate statistics were carried out with the statistical software Stata version 10 (Stata Corp, www.stata.com).
Results
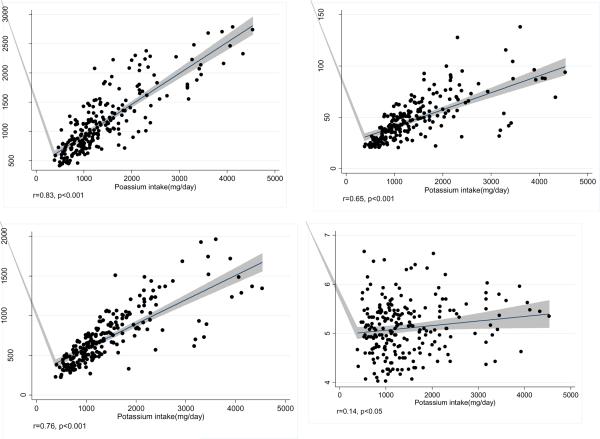
Baseline demographic and clinical characteristics and laboratory values of the 224 HD patients are shown in Table 1. They were similar to the rest of the 893 participants of the NIED Study (data not shown). The patients' mean age (±SD) was 55.0±13.8 years; 48% of patients were women (n=107), 54% (n=120) Hispanic, and 25% (n=57) African-American. We categorized participants into quartiles depending on their dietary potassium intake with 56 patients in each quartile. Table 1 also shows the foregoing demographic, clinical and laboratory measures in each quartile of potassium intake. There was no significant difference in the prevalence of diabetes mellitus and other comorbidities according to the Charlson comorbidity score. There were no remarkable differences across the four quartiles with regard to measures of body size or composition, anthropometry, or biochemical measurements measures including inflammatory markers. Hence, in our analyses, we did not normalize dietary potassium for measures of body size or composition. The top 10 sources of dietary potassium in our study population included beef, chicken, Mexican food (burritos, tamales, enchiladas, etc), hamburgers, legumes, fresh fruits, real fruit juices, fried potatoes, cheeseburgers, and canned fruits. In correlation analyses, potassium intake was positively correlated with the dietary energy, protein and phosphorus intake, and also marginally (r=0.14, p<0.05) with predialysis serum potassium. Figure 1 demonstrates the positive linear associations between potassium intake and the intakes of energy, protein and phosphorus.
Table 1.
Baseline characteristics and mortality, according to the quartile of dietary potassium intake
| Variables | All Patients (n=224) | Dietary potassium intake | ||||
|---|---|---|---|---|---|---|
| Q1 (n=56) | Q2 (n=56) | Q3 (n=56) | Q4 (n=56) | P for trend | ||
| Demography | ||||||
| Age, years | 55±14 | 53±13 | 58±11 | 55±14 | 55±16 | 0.5 |
| Women, % | 47.8 | 60.7 | 44.6 | 42.9 | 42.9 | 0.06 |
| Race: % African-American | 25.4 | 17.9 | 26.8 | 28.6 | 28.6 | 0.2 |
| Ethnicity: % Hispanic | 53.6 | 58.9 | 50.0 | 53.6 | 51.8 | 0.6 |
| Primary insurance: % Medicare | 49.5 | 46.4 | 41.8 | 61.5 | 50.0 | 0.4 |
| Diabetes mellitus, % | 60.1 | 46.4 | 67.9 | 60.7 | 65.4 | 0.3 |
| Marital status: % married | 48.8 | 50.9 | 50.9 | 38.4 | 54.9 | 0.9 |
| Charlson comorbidity score, (±SD) | 2.07±1.44 | 1.77±1.51 | 2.26±1.32 | 1.94±1.46 | 2.28±1.42 | 0.1 |
| HD vintage < 6 months | 22% | 23% | 21% | 20% | 22% | 0.6 |
| Five-year Mortality (%) | 36 | 21 | 37 | 36 | 50 | <0.001 |
| Estimated daily potassium intake (mg/d)** | 1,875±1,089 | 879±161 | 1,342±109 | 1,852±217 | 3,440±969 | <0.001 |
| Body composition | ||||||
| BMI, kg/m2 | 27.17±6.75 | 27.11±7.10 | 26.83±6.35 | 27.13±6.09 | 27.61±7.53 | 0.7 |
| Triceps skinfold, mm | 17.9±9.2 | 18.8±8.8 | 17. ±9.6 | 17.8±9.8 | 17.8±8.9 | 0.6 |
| Biceps skinfold, mm | 10.4±9.4 | 11.9±12.3 | 9.8±9.6 | 9.4±6.6 | 10.4±7.6 | 0.6 |
| Mid-arm muscle circumference, cm | 20.72±5.22 | 21.65±5.40 | 20.59±5.17 | 20.48±5.79 | 20.14±4.44 | 0.09 |
| NIR measured body fat, % | 27.20±10.29 | 28.72±9.73 | 26.87±10.44 | 26.73±9.93 | 26.40±11.18 | 0.3 |
| HD treatment measures | ||||||
| Vintage, months | 34.8±29.5 | 36.4±34.3 | 33.8±30.9 | 35.2±25.7 | 33.7±27.0 | 0.9 |
| Dose, spKt/Vurea | 1.58±0.30 | 1.62±0.28 | 1.55±0.31 | 1.60±0.28 | 1.58±0.32 | 0.3 |
| Erythropoietin dose, 1,000 U/week | 14.6±11.3 | 13.3±11.1 | 12.9±7.5 | 16.9±13.9 | 15.2±11.5 | 0.1 |
| Serum or plasma measurements | ||||||
| Albumin, g/dl | 3.86±0.34 | 3.85±0.29 | 3.87±0.35 | 3.88±0.29 | 3.81±0.42 | 0.9 |
| Transthyretin (prealbumin), mg/dl | 28.2±9.1 | 28.4±8.0 | 27.0±8.8 | 30.5±10.0 | 27.1±9.3 | 0.9 |
| Creatinine, mg/dl | 10.7±3.4 | 11.1±2.9 | 9.9±3.1 | 10.9±3.7 | 10.8±3.7 | 0.5 |
| Calcium, mg/dl | 9.27±0.66 | 9.27±0.66 | 9.43±0.58 | 9.26±0.9 | 9.14±0.69 | 0.07 |
| Iron, microgram/dl | 65.2±26.6 | 64.5±24.9 | 65.4±25.9 | 60.3±25.3 | 70.8±29.7 | 0.6 |
| Phosphorus, mg/dl | 5.8±1.4 | 5.6±1.3 | 5.6±1.3 | 6.0±1.5 | 6.07±1.58 | 0.1 |
| Potassium, mg/dl | 5.1±0.5 | 5.1±0.5 | 5.0±0.5 | 5.1±0.5 | 5.2±0.5 | 0.06 |
| Ferritin, ng/ml | 649±487 | 592±401 | 744±611 | 664±472 | 599±440 | 0.8 |
| Bicarbonate, mg/dl | 21.8±2.5 | 22.0±2.6 | 21.5±2.6 | 21.7±2.5 | 22.0±2.4 | 0.6 |
| Total homocysteine, μmol/l | 24.1±9.3 | 25.1±9.9 | 22.3±9.1 | 24.4±9.3 | 24.5±8.9 | 0.1 |
| C-reactive protein, mg/l | 6.3±7.9 | 6.4±12.3 | 6.3±5.3 | 5.5±5.8 | 6.9±5.9 | 0.1 |
| IL-6, pg/ml | 24.6±69.1 | 25.6±63.0 | 31.5±98.6 | 13.6±17.1 | 27.7±71.5 | 0.8 |
| TNF-α, pg/ml | 7.84±5.09 | 8.30±5.63 | 6.56±2.36 | 8.45±5.31 | 8.08±6.17 | 0.7 |
| Blood hemoglobin, g/dl | 11.87±0.98 | 11.76±1.00 | 11.94±0.91 | 11.90±0.91 | 11.89±1.11 | 0.4 |
| WBC count, ×1,000 cells/μl | 7.22±1.98 | 7.26±2.02 | 7.30±2.21 | 6.89±1.49 | 7.46±2.13 | 0.8 |
| Lymphocytes, % of total WBC | 21.1±7.1 | 20.8±7.9 | 20.8±6.4 | 21.4±7.5 | 21.6±6.8 | 0.3 |
All values are presented as mean ± SD or percentages.
Conversion factors for units: albumin in g/dL to g/L, ×10; creatinine in mg/dL to micromole/L, ×88.4; calcium in mg/dL to mmol/L, ×0.2495; iron in micrograms/dL to micromoles/L, ×0.179; phosphorus in mg/dL to mmol/L, ×0.3229; total homocysteine μmol/l to mg/L, ×0.135; hemoglobin in g/dL to g/L, ×10. No conversion necessary for ferritin in ng/mL and micrograms/L and WBC in 10^3/microliter and 10^9/l.
* p values for triceps and biceps skin fold thicknesses, dialysis dose (vintage), ferritin, erythropoietin dose, CRP, IL-6, and TNF-α are based on the logarithmic values of these measures.
FFQ tends to underestimate daily nutrient intake proportionately including daily potassium intake. Abbreviations: BMI, body mass index; HD, hemodialysis; NIR, near-infrared [interactance]; Q, quartile; spKt/Vurea, single-pool Kt/Vurea, WBC, white blood cell; IL-6, interleukin 6; TNF-α , tumor necrosis factor α.
Figure 1.
Scatter plots with regression lines reflecting the correlations between potassium intake with calorie (upper, left), protein (upper right) and phosphorus intakes (lower left) and baseline predialysis serum potassium (lower right). Shaded areas reflect the 95% confidence intervals.
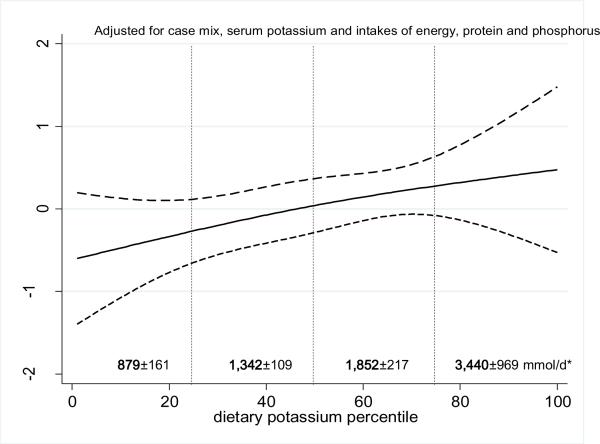
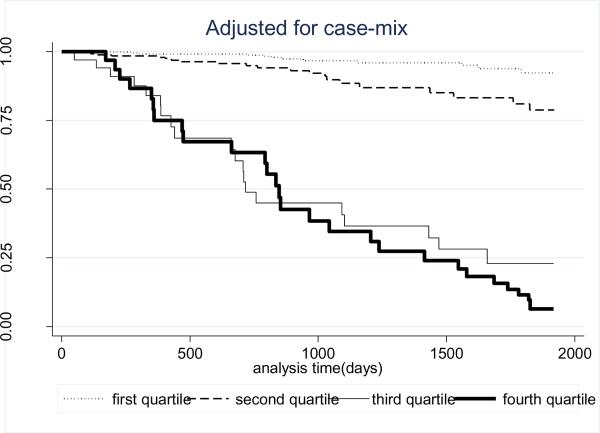
Over the 5 years that the cohort 81 (36%) patients died. Figure 2 shows the cubic splines graph illustrating the multivariate adjusted association between estimated dietary potassium intake (in percentiles) and 5-year mortality in 224 HD patients using a parsimonious model with reduced number of adjustors. A rather robust trend towards increased death risk was observed in the HD patients with higher dietary potassium intakes. The lowest quartile of dietary potassium appeared to be associated with the greatest survival. Figure 3 shows the Kaplan-Meier analyzed proportion of surviving patients across the four quartiles of dietary potassium intake, indicating that the highest dietary potassium quartiles were associated with the worse survival. The estimated death hazard ratios are shown in Table 2. The highest quartile of dietary potassium intake was associated with significantly increased death risk [hazard ratio (95% CI)] in the unadjusted [2.9(1.5–5.6)], case-mix adjusted [2.4 (1.2–4.1)], case-mix plus nutritional status adjusted [2.4(1.1–7.5)] and case-mix plus nutritional status and inflammatory markers adjusted analyses [2.4(1.1–7.5)].
Figure.2.
Spline model with 95% CI and 2 degree of freedom reflecting adjusted mortality predictability of potassium intake, expressed as a percentile of the average dietary potassium intake in the 224 maintenance hemodialysis patients (from Oct. 2001 to Jan. 2007). A parsimonious model with a limited number of adjusters (case mix, serum potassium and intakes of energy, protein and phosphorus) has been examined here. Estimated daily potassium intake in mmoles (mean+/−SD) is shown for each quartile (Q). Estimates is based on a food frequency questionnaire, which tends to underestimate daily nutrient intake proportionately, including daily potassium intake.
Figure 3.
Kaplan-Meier proportion of surviving HD patients after 5 years of observation according to the quartiles of potassium intake in 224 HD patients,adjusted for case-mix.Case-mix variables: age, gender, race/ethnicity, diabetes mellitus, dialysis vintage, primary insurance, marital status, Charlson co-morbidity score, dialysis dose (Kt/V), intake of phosphorus binders and residual renal function.
Table 2.
5-year mortality according to quartiles of dietary potassium intake
| Variables | Q1 (n=56) | Q2 (n=56) | Q3 (n=56) | Q4 (n=56) | P for trend |
|---|---|---|---|---|---|
| Unadjusted | 1.00 | 2.34 (1.15,4.76) [p=0.01] | 2.28 (1.11,4.68) [p=0.02] | 2.86 (1.45,5.63) [p=0.00] | 0.009 |
| Case-mix1 adjusted | 1.00 | 1.88 (0.88,3.98) [p=0.10] | 2.25 (1.06,4.78) [p=0.03] | 2.35 (1.15,4.80) [p=0.01] | 0.008 |
| Case-mix + nutritional status2 adjusted | 1.00 | 1.56 (0.70,3.49) [p=0.27] | 1.73 (0.71,4.17) [p=0.22] | 2.42 (1.09,7.46) [p=0.02] | 0.08 |
| Case-mix + nutrition + inflammation3 adjusted | 1.00 | 1.35 (0.60,3.04) [p=0.46] | 2.22 (0.91,5.43) [p=0.08] | 2.40 (1.07,7.49) [p=0.04] | 0.03 |
Values shown are Hazard ratios (HR) and 95% confidence interval (CI) in 224 maintenance hemodialysis patients in the time period from Oct. 2001 to Jan. 2007.
Case-mix variables include age, gender, race/ethnicity, diabetes, dialysis vintage, insurance (medicare), marital status, modified Charlson comorbidity score, dialysis dose (Kt/V), intake of sevelamer HCl or calcium-based binders and residual renal function.
Nutritional status includes 3-month average predialysis serum creatinine, potassium & phosphorus, BMI, nPNA, and energy, protein and phosphorus intake
Inflammation includes serum CRP, IL-6 & TNFα
Abbreviation: Q, quartile
Discussion
Using a frequently utilized and previously validated FFQ to estimate daily nutrient intake for ranking 224 HD patients according to their dietary potassium intake, we found that higher dietary potassium was correlated with higher 5-year mortality. Although there are several reports of an association between the predialysis serum potassium and outcomes in CKD patients,(6, 12, 13, 37) to our knowledge this is the first investigation in HD patients to report the association of dietary potassium intake with mortality. The incremental mortality-predictability of dietary potassium in HD patients may have important clinical and dietary implications in the management of CKD patients.
The FFQs are widely used in the field of Nutritional Epidemiology to estimate food intake-associated relative risk for disease conditions because of the ease with which they can be administered and with which the information on food intake can be entered into computerized data bases. Moreover, FFQs can provide comparative long-term estimates of dietary intake of certain foods and nutrients across large populations.(38) However, the Food and Nutrition Board cautions that FFQ data may not be accurate enough to assess the absolute amounts of the dietary intakes at individual (person) level because of three limitations: (1) Lack of direct quantitative assessment of individual amounts of nutrients consumed each day; (2) Inability to cover all possible food items that people may ingest; and (3) Inclusion of diverse varieties of a given food under one single food item question, leading to potential failure to capture significant differences among different food subtypes.(38)
In the present study, it was our intent to assess the relative amounts of potassium ingested by HD patients. Hence, we made the assumption that the FFQs accurately assessed to which dietary potassium quartile or percentile a HD patient belonged. However, the calculated absolute potassium intake shown in Table 1 may be underestimates. Assessment of dietary potassium by FFQ in HD patients may be considered a more valid tool for estimating graded exposure to dietary potassium load as compared to serum potassium per se. The weekly to monthly measured pre-HD serum potassium value may significantly misrepresent dietary potassium load; serum potassium can fluctuate during a single day in response to intake of sporadic nutrients such as carbohydrates or throughout the dialysis cycle.(39) The dose and schedule of hemodialysis treatments as well as the degree of residual renal function, net tissue breakdown (e.g. due to infections) and acid-base status may also affect the predialysis potassium level.(40) Moreover, in the HD patient, serum potassium concentrations will also fluctuate in response to net intestinal potassium absorption or excretion such as during diarrhea.(40) Thus, predialysis serum potassium can provide only a short-term estimate. Indeed, it is pertinent that in our current study, the pre-HD serum potassium correlated to a low degree with the calculated dietary potassium intake. Although dietary potassium intake does affect serum potassium in HD patients, perhaps this relationship is only strong when the potassium intake is very low or very high.
The results of our study indicating that potassium intake is directly associated with survival are in concordance with other studies.(6, 12, 13) These studies evaluated the impact of the baseline serum potassium level on survival in chronic dialysis patients. Predialysis serum potassium was shown to be an independent prognostic associate of death. The risk of death increased with higher serum potassium values. On the other hand in the study by Saran et al (37) hyperkalemia above 6.0 mEq/L failed to achieve statistical significance with respect to the relative risk of mortality. Poor adherence to dietary and medical advice is quite common in adult chronic dialysis patients.(41) In many societies and patients, limitations of fiscal resources as well as cultural factors make dietary adaptation difficult.(42, 43)
Some limitations should be considered in interpreting our findings. First is the limited sample size, which prevents a more detailed analysis of the association of potassium intake and cardiovascular or cause-specific mortality or of other types of subgroup analyses, although death causes of dialysis patients in the 2746 death reports may not be accurate. Second is the selection bias during enrollment. However, because mortality in our cohort was less than that in the base population, it might be argued that selection bias was in a direction that would lead to bias toward the null; therefore, without this bias, our results might have been even stronger. Third is the lack of information regarding the dialysate potassium concentration (the so-called K bath); however, even though we have previously examined the mortality-predictability of K bath in a large national cohort,(12) the selection of K bath is usually secondary to serum potassium level and, hence, subject to substantial confounding by indication. Fourth, the FFQ has been applied only one time in the beginning of the study, but dietary potassium habits may have changed subsequently. Fifth, although it is validated for potassium intake in the healthy population(30, 32), the Block FFQ has not been validated in dialysis patients, who may differ in their food intakes from dialysis to nondialysis days(44). Furthermore, FFQ may underestimate the amount of daily protein (38) and potassium intake.(30, 36) However, our findings are based on ranked (quartiles and percentiles) data rather than absolute amounts of dietary intakes per individuals.
An important strength of our study is the long period of follow-up (63 months). Other strengths are the comprehensive laboratory evaluations with 3-month averaging at the same time when FFQ was administered, the concomitant assessments of body composition, and the detailed evaluation of the clinical and comorbid states of the patients. Our cohort has been extensively characterized for markers of inflammation, nutritional status and body composition. The availability of these measures allowed us to show that potassium intake is associated with mortality risk independent of influences from other known nutritional or inflammatory markers. A unique feature of this study is its novelty in assessing potassium intake measured by FFQ, which is a validated method to estimate long-term usual dietary intake at a population basis and is arguably a more valid tool for estimating overall body potassium exposure or burden as compared to an instantaneous serum potassium measurement.
In conclusion, higher dietary potassium intake is associated with increased death risk in HD patients, even after adjustments for serum potassium and dietary protein, energy and phosphorus intake. Taken together, these results support the importance of dietary interventions on survival in CKD patients and suggest a potential role for dietary potassium in the high mortality rate for dialysis patients. Future randomized controlled studies are needed to confirm these findings.
Acknowledgement
The authors are thankful to Ms. Stephanie Griffith and Dr. Victor Goh at Harbor-UCLA GCRC Core Laboratories for the management of blood samples and measuring inflammatory markers. They are also indebted to hard-working collaborating dietitians in 8 DaVita dialysis facilities in Los Angeles South Bay area and DaVita teammates in these facilities.
Support: This study was supported by National Institutes of Health, National Institute of Diabetes, Digestive and Kidney Disease grants R21-DK61162 and K23-DK061162 (to Dr Kalantar-Zadeh). Additional sources of funding include research grants from DaVita Clinical Research (DCR), a philanthropic grant from Mr Harold Simmons (to Dr Kalantar-Zadeh), and a General Clinical Research Center (GCRC) grant (M01-RR00425) from the National Centers for Research Resources, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The abstract of this study was accepted for oral presentation at the annual meeting of the American Society of Nephrology, Oct 27–30, 2009, San Diego, CA.
Financial Disclosure:The authors declare that they have no relevant financial interests.
References
- 1.Port FK. Morbidity and mortality in dialysis patients. Kidney Int. 1994;46:1728–37. doi: 10.1038/ki.1994.475. [DOI] [PubMed] [Google Scholar]
- 2.Iseki K, Kawazoe N, Osawa A, Fukiyama K. Survival analysis of dialysis patients in Okinawa, Japan (1971–1990) Kidney Int. 1993;43:404–9. doi: 10.1038/ki.1993.59. [DOI] [PubMed] [Google Scholar]
- 3.Berl T, Henrich W. Kidney-heart interactions: epidemiology, pathogenesis, and treatment. Clin J Am Soc Nephrol. 2006;1:8–18. doi: 10.2215/CJN.00730805. [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 5.Lowrie EG, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis. 1990;15:458–82. doi: 10.1016/s0272-6386(12)70364-5. [DOI] [PubMed] [Google Scholar]
- 6.Iseki K, Uehara H, Nishime K, Tokuyama K, Yoshihara K, Kinjo K, Shiohira Y, Fukiyama K. Impact of the initial levels of laboratory variables on survival in chronic dialysis patients. Am J Kidney Dis. 1996;28:541–8. doi: 10.1016/s0272-6386(96)90465-5. [DOI] [PubMed] [Google Scholar]
- 7.Gonick HC, Kleeman CR, Rubini ME, Maxwell MH. Functional impairment in chronic renal disease. 3. Studies of potassium excretion. Am J Med Sci. 1971;261:281–90. doi: 10.1097/00000441-197105000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Hayes CP, Jr., Robinson RR. Fecal Potassium Excretion in Patients on Chronic Intermittent Hemodialysis. Trans Am Soc Artif Intern Organs. 1965;11:242–6. doi: 10.1097/00002480-196504000-00046. [DOI] [PubMed] [Google Scholar]
- 9.Hayes CP, Jr., McLeod ME, Robinson RR. An extravenal mechanism for the maintenance of potassium balance in severe chronic renal failure. Trans Assoc Am Physicians. 1967;80:207–16. [PubMed] [Google Scholar]
- 10.Kopple JD, Coburn JW. Metabolic studies of low protein diets in uremia. I. Nitrogen and potassium. Medicine (Baltimore) 1973;52:583–95. doi: 10.1097/00005792-197311000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Schrier RW, Regal EM. Influence of aldosterone on sodium, water and potassium metabolism in chronic renal disease. Kidney Int. 1972;1:156–68. doi: 10.1038/ki.1972.23. [DOI] [PubMed] [Google Scholar]
- 12.Kovesdy CP, Regidor DL, Mehrotra R, Jing J, McAllister CJ, Greenland S, Kopple JD, Kalantar-Zadeh K. Serum and dialysate potassium concentrations and survival in hemodialysis patients. Clin J Am Soc Nephrol. 2007;2:999–1007. doi: 10.2215/CJN.04451206. [DOI] [PubMed] [Google Scholar]
- 13.Unruh ML, Evans IV, Fink NE, Powe NR, Meyer KB. Skipped treatments, markers of nutritional nonadherence, and survival among incident hemodialysis patients. Am J Kidney Dis. 2005;46:1107–16. doi: 10.1053/j.ajkd.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 13a.Kidney Disease. Available at www.utdol.com; Accessed ****
- 14.Bross R, Zitterkoph J, Pithia J, Benner D, Rambod M, Kovesdy CP, Kopple JD, Kalantar-Zadeh K. Association of serum total iron-binding capacity and its changes over time with nutritional and clinical outcomes in hemodialysis patients. Am J Nephrol. 2009;29:571–81. doi: 10.1159/000191470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colman S, Bross R, Benner D, Chow J, Braglia A, Arzaghi J, Dennis J, Martinez L, Baldo DB, Agarwal V, Trundnowski T, Zitterkoph J, Martinez B, Khawar OS, Kalantar-Zadeh K. The Nutritional and Inflammatory Evaluation in Dialysis patients (NIED) study: overview of the NIED study and the role of dietitians. J Ren Nutr. 2005;15:231–43. doi: 10.1053/j.jrn.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Rambod M, Kovesdy CP, Kalantar-Zadeh K. Malnutrition-Inflammation Score for risk stratification of patients with CKD: is it the promised gold standard? Nat Clin Pract Nephrol. 2008;4:354–5. doi: 10.1038/ncpneph0834. [DOI] [PubMed] [Google Scholar]
- 17.Rambod M, Bross R, Zitterkoph J, Benner D, Pithia J, Colman S, Kovesdy CP, Kopple JD, Kalantar-Zadeh K. Association of Malnutrition-Inflammation Score with quality of life and mortality in hemodialysis patients: a 5-year prospective cohort study. Am J Kidney Dis. 2009;53:298–309. doi: 10.1053/j.ajkd.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shantouf R, Kovesdy CP, Kim Y, Ahmadi N, Luna A, Luna C, Rambod M, Nissenson AR, Budoff MJ, Kalantar-Zadeh K. Association of serum alkaline phosphatase with coronary artery calcification in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:1106–14. doi: 10.2215/CJN.06091108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehrotra R, Kermah D, Fried L, Kalantar-Zadeh K, Khawar O, Norris K, Nissenson A. Chronic peritoneal dialysis in the United States: declining utilization despite improving outcomes. J Am Soc Nephrol. 2007;18:2781–8. doi: 10.1681/ASN.2006101130. [DOI] [PubMed] [Google Scholar]
- 20.Beddhu S, Bruns FJ, Saul M, Seddon P, Zeidel ML. A simple comorbidity scale predicts clinical outcomes and costs in dialysis patients. Am J Med. 2000;108:609–13. doi: 10.1016/s0002-9343(00)00371-5. [DOI] [PubMed] [Google Scholar]
- 21.Nelson EE, Hong CD, Pesce AL, Peterson DW, Singh S, Pollak VE. Anthropometric norms for the dialysis population. Am J Kidney Dis. 1990;16:32–7. doi: 10.1016/s0272-6386(12)80782-7. [DOI] [PubMed] [Google Scholar]
- 22.Williams AJ, McArley A. Body composition, treatment time, and outcome in hemodialysis patients. J Ren Nutr. 1999;9:157–62. doi: 10.1016/s1051-2276(99)90056-0. [DOI] [PubMed] [Google Scholar]
- 23.Kalantar-Zadeh K, Dunne E, Nixon K, Kahn K, Lee GH, Kleiner M, Luft FC. Near infra-red interactance for nutritional assessment of dialysis patients. Nephrol Dial Transplant. 1999;14:169–75. doi: 10.1093/ndt/14.1.169. [DOI] [PubMed] [Google Scholar]
- 24.Kalantar-Zadeh K, Kuwae N, Wu DY, Shantouf RS, Fouque D, Anker SD, Block G, Kopple JD. Associations of body fat and its changes over time with quality of life and prospective mortality in hemodialysis patients. Am J Clin Nutr. 2006;83:202–10. doi: 10.1093/ajcn/83.2.202. [DOI] [PubMed] [Google Scholar]
- 25.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–65. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 26.Erbagci AB, Tarakcioglu M, Aksoy M, Kocabas R, Nacak M, Aynacioglu AS, Sivrikoz C. Diagnostic value of CRP and Lp(a) in coronary heart disease. Acta Cardiol. 2002;57:197–204. doi: 10.2143/AC.57.3.2005389. [DOI] [PubMed] [Google Scholar]
- 27.Pecoits-Filho R, Barany P, Lindholm B, Heimburger O, Stenvinkel P. Interleukin-6 is an independent predictor of mortality in patients starting dialysis treatment. Nephrol Dial Transplant. 2002;17:1684–8. doi: 10.1093/ndt/17.9.1684. [DOI] [PubMed] [Google Scholar]
- 28.Beutler B, Cerami A. The biology of cachectin/TNF--a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–55. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- 29.Block G, Thompson FE, Hartman AM, Larkin FA, Guire KE. Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. J Am Diet Assoc. 1992;92:686–93. [PubMed] [Google Scholar]
- 30.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43:1327–35. doi: 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- 31.Klein RF, Friedman-Campbell M, Tocco RV. History taking and substance abuse counseling with the pregnant patient. Clin Obstet Gynecol. 1993;36:338–46. doi: 10.1097/00003081-199306000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Block G, Hartman AM, Naughton D. A reduced dietary questionnaire: development and validation. Epidemiology. 1990;1:58–64. doi: 10.1097/00001648-199001000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Block G, Wakimoto P, Jensen C, Mandel S, Green RR. Validation of a food frequency questionnaire for Hispanics. Prev Chronic Dis. 2006;3:A77. [PMC free article] [PubMed] [Google Scholar]
- 34.Day N, McKeown N, Wong M, Welch A, Bingham S. Epidemiological assessment of diet: a comparison of a 7-day diary with a food frequency questionnaire using urinary markers of nitrogen, potassium and sodium. Int J Epidemiol. 2001;30:309–17. doi: 10.1093/ije/30.2.309. [DOI] [PubMed] [Google Scholar]
- 35.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–61. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 36.Brunner E, Stallone D, Juneja M, Bingham S, Marmot M. Dietary assessment in Whitehall II: comparison of 7 d diet diary and food-frequency questionnaire and validity against biomarkers. Br J Nutr. 2001;86:405–14. doi: 10.1079/bjn2001414. [DOI] [PubMed] [Google Scholar]
- 37.Saran R, Bragg-Gresham JL, Rayner HC, Goodkin DA, Keen ML, Van Dijk PC, Kurokawa K, Piera L, Saito A, Fukuhara S, Young EW, Held PJ, Port FK. Nonadherence in hemodialysis: associations with mortality, hospitalization, and practice patterns in the DOPPS. Kidney Int. 2003;64:254–62. doi: 10.1046/j.1523-1755.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- 38.Kalantar-Zadeh K, Kopple JD, Deepak S, Block D, Block G. Food intake characteristics of hemodialysis patients as obtained by food frequency questionnaire. J Ren Nutr. 2002;12:17–31. doi: 10.1053/jren.2002.29598. [DOI] [PubMed] [Google Scholar]
- 39.Kooienga L. Phosphorus balance with daily dialysis. Semin Dial. 2007;20:342–5. doi: 10.1111/j.1525-139X.2007.00304.x. [DOI] [PubMed] [Google Scholar]
- 40.Kaveh K, Kimmel PL. Compliance in hemodialysis patients: multidimensional measures in search of a gold standard. Am J Kidney Dis. 2001;37:244–66. doi: 10.1053/ajkd.2001.21286. [DOI] [PubMed] [Google Scholar]
- 41.Wolcott DL, Maida CA, Diamond R, Nissenson AR. Treatment compliance in end-stage renal disease patients on dialysis. Am J Nephrol. 1986;6:329–38. doi: 10.1159/000167186. [DOI] [PubMed] [Google Scholar]
- 42.Foley RN, Parfrey PS, Hefferton D, Singh I, Simms A, Barrett BJ. Advance prediction of early death in patients starting maintenance dialysis. Am J Kidney Dis. 1994;23:836–45. doi: 10.1016/s0272-6386(12)80137-5. [DOI] [PubMed] [Google Scholar]
- 43.Delmez JA, Slatopolsky E. Hyperphosphatemia: its consequences and treatment in patients with chronic renal disease. Am J Kidney Dis. 1992;19:303–17. doi: 10.1016/s0272-6386(12)80446-x. [DOI] [PubMed] [Google Scholar]
- 44.Kloppenburg WD, Stegeman CA, Hooyschuur M, van der Ven J, de Jong PE, Huisman RM. Assessing dialysis adequacy and dietary intake in the individual hemodialysis patient. Kidney Int. 1999;55:1961–9. doi: 10.1046/j.1523-1755.1999.00412.x. [DOI] [PubMed] [Google Scholar]
- 45.Noori N, Kalantar-Zadeh K, Kovesdy CP, Bross R, Benner D, Kopple JD. Association of dietary phosphorus intake and phosphorus to protein ratio with mortality in hemodialysis patients. Clin J Am Soc Nephrol. 2010 doi: 10.2215/CJN.08601209. [in press] (doi: 10.2215/CJN.08601209) [DOI] [PMC free article] [PubMed] [Google Scholar]