Abstract
Photodynamic therapy (PDT) uses non-toxic photosensitizers and harmless visible light in combination with oxygen to produce cytotoxic reactive oxygen species that kill malignant cells by apoptosis and/or necrosis, shut down the tumour microvasculature and stimulate the host immune system. In contrast to surgery, radiotherapy and chemotherapy that are mostly immunosuppressive, PDT causes acute inflammation, expression of heat-shock proteins, invasion and infiltration of the tumour by leukocytes, and might increase the presentation of tumour-derived antigens to T cells.
The principle of photodynamic therapy (PDT) was first proposed over 100 years ago1. A recent review in Nature Reviews Cancer by Rakesh Jain and colleagues described some of the historical milestones in the development of PDT as a cancer treatment2. Many of the photosensitizers (PSs) that have been studied since PDT was first proposed are based on a porphyrin-like nucleus3. PSs function as catalysts when they absorb visible light and then convert molecular oxygen to a range of highly reactive oxygen species (ROS). The ROS that are produced during PDT have been shown to destroy tumours by multifactorial mechanisms4,5 (FIG. 1). PDT has a direct affect on cancer cells, producing cell death by necrosis and/or apoptosis6. PDT also has an affect on the tumour vasculature, whereby illumination and ROS production causes the shutdown of vessels and subsequently deprives the tumour of oxygen and nutrients7,8. Finally, PDT also has a significant effect on the immune system9–11, which can be either immunostimulatory or immunosuppressive.
Figure 1. The mechanism of action on tumours in photodynamic therapy.
The photosensitizer (PS) absorbs light and an electron moves to the first short-lived excited singlet state. This is followed by intersystem crossing, in which the excited electron changes its spin and produces a longer-lived triplet state. The PS triplet transfers energy to ground-state triplet oxygen, which produces reactive singlet oxygen (1O2). 1O2 can directly kill tumour cells by the induction of necrosis and/or apoptosis, can cause destruction of tumour vasculature and produces an acute inflammatory response that attracts leukocytes such as dendritic cells and neutrophils.
Most of the commonly used cancer therapies are immunosuppressive. Chemotherapy and ionizing radiation delivered at doses sufficient to destroy tumours are known to be toxic to the bone marrow, which is the source of all cells of the immune system, and neutropaenia and other forms of myelosuppression are often the dose-limiting toxicity of these therapies. However, it should be noted that low doses of either ionizing radiation12,13 or chemotherapy14 can have immunostimulatory effects, including the induction of heat-shock proteins15. Less well known is the fact that major surgery can also have an immunosuppressive effect that leads to a significant diminution of lymphocyte and natural killer (NK) cell function16. The ideal cancer therapy would not only destroy the primary tumour, but at the same time trigger the immune system to recognize, track down and destroy any remaining tumour cells, be they at or near the site of the primary tumour or distant micrometastases. PDT, in common with some other local cancer therapies such as cryotherapy17 and hyperthermia18, might have these desirable properties.
The importance of the immune system in the host response against cancer has been studied for many years, but immunotherapy is only accepted as a treatment option in a few cases. More than 700 cases of spontaneous regression in advanced tumours in patients have been reported19, including malignant melanoma, hepatocellular carcinoma, lung metastases after destruction of the primary renal cell carcinoma and Hodgkin disease. Moreover, such spontaneous regressions normally occur following an infection.
Cancer immunotherapy (even if unrecognized as such) has a long history. The Egyptians noted that surgical opening of the tumour site could produce tumour regression, one would assume through the generation of infection and activation of the immune system20. Over 100 years ago a surgeon from New York, William Coley21, discovered that some infections could produce tumour regression, and he created a ‘vaccine’ based initially on erysipelas-causing bacteria22. The bacillus Calmette–Guerin (BCG) vaccine derived from Mycobacterium bovis has been used to prevent tuberculosis since 1921, and has been applied for immunostimulation in neoplasia since the 1960s. The most effective use of this treatment is for superficial bladder cancer23.
Since these early studies, groundbreaking discoveries in immunology have identified the roles of lymphocyte classes and subclasses24, dendritic cells and antigen presentation25, interleukins (IL) and other cytokines26, and tumour-associated antigens and major histocompatibility complex (MHC) molecules27 in mediating the anti-tumour immune response. However, most cancers avoid or escape immune control28,29, and death from metastatic cancer is still the most likely result. In this Review we discuss the effect of PDT on the anti-tumour immune response, and the role of PDT in stimulating and suppressing both the innate immune response and adaptive immune response. We also summarize the available data on combinations of PDT with other immunostimulatory therapies.
Effects of PDT on tumour cells
Many effects of PDT on cancer cells that are grown in tissue culture have been reported that, if replicated in vivo, would make activation of the immune system probable after PDT treatment in patients. The combination of PSs with their activating light causes an unusual mixture of apoptotic and necrotic cell death6, which is different from most conventional cytotoxic agents that usually only trigger apoptotic cell death. The balance between apoptosis and necrosis after PDT in vitro depends on several parameters, including the total PDT dose (PDT dose is the product of PS concentration and light fluence), the intracellular localization of the PS, the fluence rate, the oxygen concentration and the cell type4. There is an extensive body of literature that examines the pathways of apoptosis that are induced after PDT in both normal and tumour cells in tissue culture, such as signalling pathways30,31, mitochondrial events4 and mediators of apoptosis32. The occurrence of apoptosis after PDT in tumours in vivo has also been shown33–35, but there have been no studies looking at in vivo clearance mechanisms of apoptotic cells in tumours after PDT.
Many studies have examined the relationship between the mode of tumour cell death (by methods other than PDT) and the efficiency of induction of the immune response, both in vitro and in vivo36,37. Although some reports show that apoptotic tumour cells are more effective than necrotic tumour cells at inducing an immune response38,39, there are other reports that show that modes of cancer therapy that predominantly induce necrosis are actually better at activating the immune system than methods that predominantly induce apoptosis40,41. In the case of necrosis, cytosolic constituents spill into the extracellular space through the damaged plasma membrane and provoke a robust inflammatory response. These products are safely isolated by the intact membranes that initially persist in apoptotic cells, which are phagocytosed by macrophages. The acute inflammation that is caused by PDT-induced necrosis might potentiate immunity by attracting host leukocytes into the tumour and increasing antigen presentation (FIG. 2).
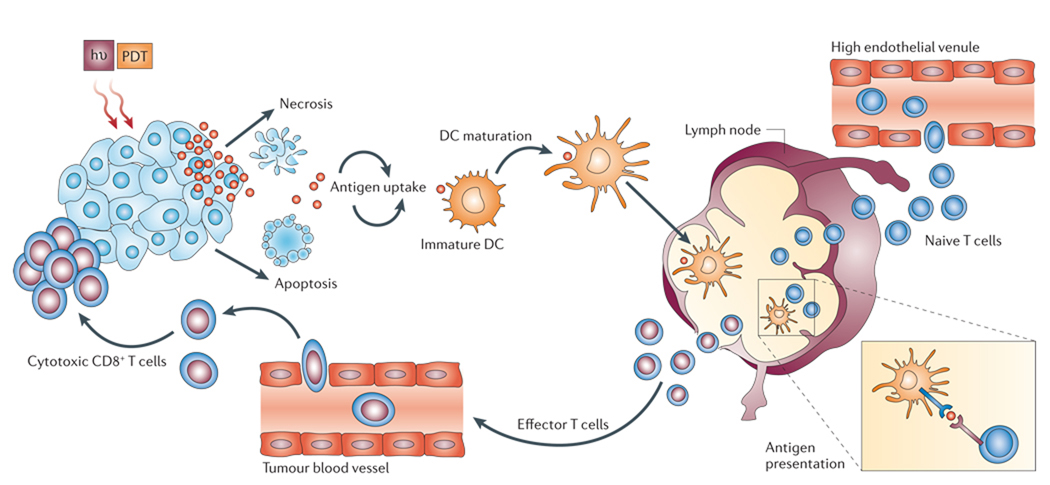
Figure 2. Photodynamic therapy induces activation of antigen-specific T cells.
When light (hν) is delivered to a photosensitizer (PS)-loaded tumour it induces both apoptotic and necrotic cell death. These cells are phagocytosed by dendritic cells (DCs) that have accumultated owing to the acute inflammatory response which is triggered by photodynamic therapy (PDT). DCs mature after stimulation by cytokines, which are released at the site of inflammation, and home to the regional lymph nodes where they present antigens to the T lymphocytes. Activated T lymphocytes become effector T cells and, attracted by chemokines, migrate to the tumour and kill the tumour cells.
One of the most important cellular factors induced by PDT and released from necrotic tumour cells is extracellular heat-shock protein 70 (HSP70) (FIG. 3). HSP70 is effectively induced after stress and, when it remains intracellular, it chaperones unfolded proteins and prevents cell death by inhibiting the aggregation of cellular proteins42. These properties not only enable intracellular HSP70 to inhibit tumour cell death by apoptosis, but also promote the formation of stable complexes with cytoplasmic tumour antigens. These antigens can then either be expressed at the cell surface or escape intact from dying necrotic cells to interact with antigen-presenting cells (APCs) and stimulate an anti-tumour immune response43. Extracellular HSP70 binds to high-affinity receptors on the surface of the APCs, which leads to the activation and maturation of dendritic cells (DCs), a process that enables the cross-presentation of the peptide antigen cargo of HSP70 by the APCs to CD8+ cytotoxic T cells44.
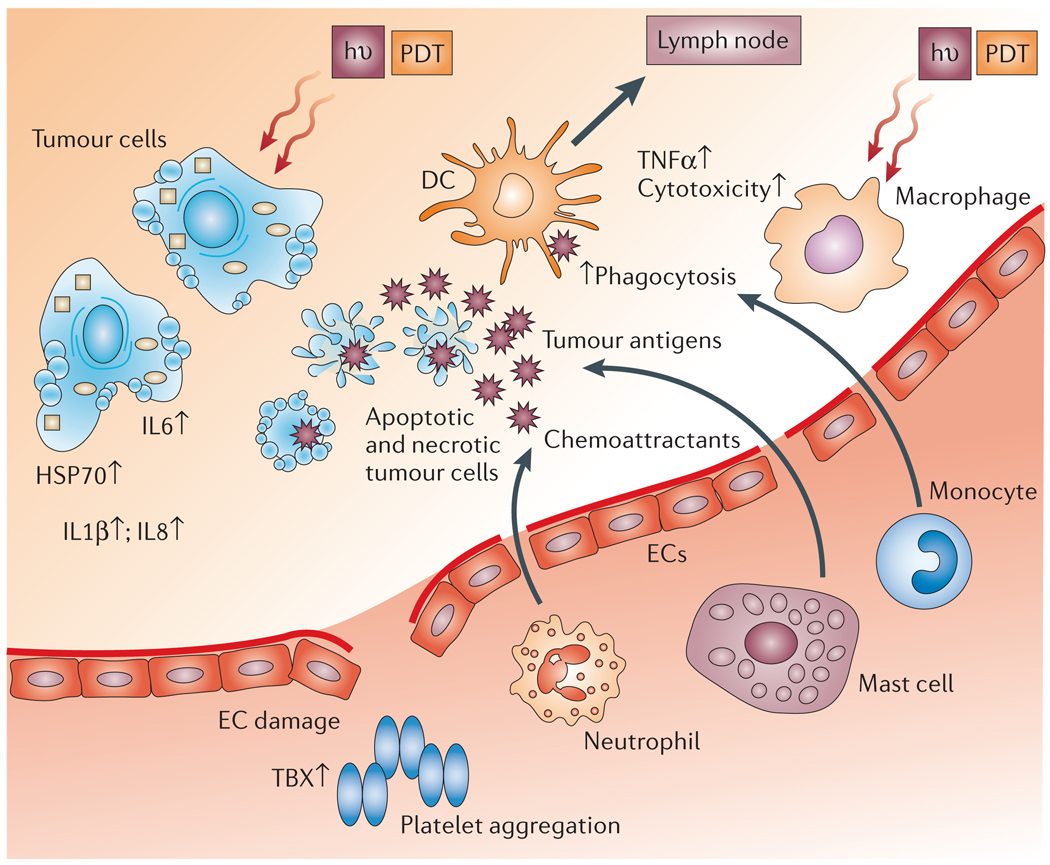
Figure 3. Consequences of photodynamic therapy-induced inflammation.
Damage to endothelial cells (ECs) activates a casade of events that lead to local inflammation, vessel dilatation and platelet aggregation. Much of this is caused by the release of thromboxane (TBX), cytokines such as interleukin 1β (IL1β), IL6 and IL8, the production of tumour-necrosis factor-α (TNFα), and infiltration of the treated tumour by cells of the immune system. Necrotic and apoptotic tumour cells express heat-shock proteins (HSPs) and provide antigens to dendritic cells (DCs) that migrate to lymph nodes. hν, light; PDT, photodynamic therapy.
Levels of HSP70 mRNA were increased with PDT mediated by three PSs (mono-l-aspartyl chlorin e6, tin etiopurpurin and Photofrin), but only mono-l-aspartyl chlorin e6 and tin etiopurpurin increased HSP70 protein levels in mouse tumour cells in vitro and in vivo45. Foster and co-workers46 used fluorescence imaging to show that the PS m-tetrahydroxyphenylchlorin (mTHPC) mediated the induction of HSP70 in EMT6 cells that had undergone PDT. These cells were stably transfected with a plasmid that contained the gene which encodes green fluorescent protein (GFP) under the control of an HSP70 promoter, and they could see increased GFP expression after PDT in both an in vitro and in vivo tumour model. Verwanger et al. used a cDNA microarray to find the highest expression level of various genes after PDT in vitro. HSP70 showed the highest increase in expression (12-fold), followed by the immediate early genes FOS and JUN47. In addition, a recent paper43 reported that 15–25% of total cellular HSP70 became exposed at the cell surface almost instantly after the treatment of cells with Photofrin-based PDT, and a large proportion of this was released within 1 hour of PDT at a cytotoxic dose. In addition to HSP70, there have been reports that PDT induces the expression of other heat-shock protein family members such as HSP47 (REF. 48) and HSP60 (REF. 49), as well as other stress-inducible proteins such as glucose-regulated protein 78 (GRP78) (REF. 50), GRP94 (REF. 43) and haem oxygenase51. The release of HSP-bound tumour antigens that can easily be taken up by APC from PDT-induced necrotic tumour cells might explain the efficiency of PDT in stimulating an immune response against tumours.
At a glance
Photodynamic therapy (PDT) uses non-toxic dyes and harmless visible light in combination with oxygen to produce highly reactive oxygen species that kill cells.
In addition to destroying tumour tissue by a process that can produce cellular necrosis and the expression of stress proteins, PDT produces an acute inflammation, and attracts leukocytes to treated tumours.
PDT might increase the immunogenicity of dead tumour cells by exposing or creating new antigens, and by inducing heat-shock proteins that increase the efficiency of antigen cross-presentation to form more effective tumour-specific cytotoxic T cells.
The pro-inflammatory effects of PDT might increase dendritic-cell migration, antigen uptake and maturation.
PDT can produce tumour cures and long-lasting tumour-specific immunity (memory), as has been shown by the rejection of tumours on rechallenge in certain mouse and rat models.
PDT has been combined with a range of immunostimulatory therapies, including microbial adjuvants, to increase the anti-tumour immunity produced by PDT alone.
There are only a few reports of the immunostimulatory effects of PDT in humans, but increasing recognition of the effect should lead to further work and possibly to improved patient outcome.
Effects of PDT on immune cells
There are reports based on data from in vitro studies that PDT can have an effect on monocyte/macrophage and lymphocyte cell lineages. Lymphocytes are usually easily killed by PDT52, and activated lymphocytes are especially susceptible53. This finding has led to PDT being proposed as a treatment for graft versus host disease54, some forms of autoimmune disease55 and cutaneous T-cell lymphoma56. On the other hand, macrophages can be activated by low, sublethal doses of PDT57. Reports show that PDT-treated macrophages secrete tumour-necrosis factor-α (TNFα)58. When a mixture of macrophages and lymphocytes undergoes PDT, lysophosphatidyl choline is released from lymphocytes and this molecule induces the expression of β-galactosidase in B lymphocytes and, together with NEU1 sialidase from T lymphocytes, these enzymes modify the vitamin D3 binding protein in bovine serum to yield a potent macrophage-activating factor (MAF)59,60. The production of this MAF also occurs in mice, where it is derived from the analogous vitamin D3 binding protein in mouse serum60. Evidence also indicates that macrophages can show preferential cytotoxicity to tumour cells that have been treated with a sublethal dose of PDT61. Another report62 showed that although the tumoricidal function of peritoneal macrophages that were removed from mice after PDT was unaltered, there was a reduction in NK cell function.
Cytokine release and inflammation after PDT
PDT produces an acute inflammatory response whether it is delivered to normal tissue or to tumours (FIG. 3). Inflammatory cytokines and chemokines have been detected in the serum of mice that have received PDT directed at a subcutaneous tumour or to an area of normal skin. These include IL6 in particular and macrophage inflammatory protein 1 (MIP1) and MIP2 (REF. 63). Increased levels of IL1β, IL6, IL8 and IL10 were detected in patients after surgery and PDT for mesothelioma64. The sources of these inflammatory mediators can be many of the various cell types that are present in tumours. For instance, malignant cells themselves, tumour endothelial cells and tumour-infiltrating leukocytes, but not fibroblasts, have been shown to produce members of the class of inflammatory mediators known as prostaglandins65,66. The release of thromboxane from endothelial cells after PDT is partly responsible for the vascular shutdown67. This induction of acute inflammation is important in triggering the immune response, as it shares some similarities with the type of danger signal provided by the host inflammatory response to the microbial invasion of healthy tissue. The tumour environment is more aptly described as a state of chronic inflammation, as opposed to a state of acute inflammation68.
Transcription factors and cytokine production
The acute inflammation that is observed after PDT is likely to be caused by the expression of two transcription factors, nuclear factor κB (NFκB) and activator protein 1 (AP1). Both factors participate in the transcriptional activation of genes that encode immunoregulatory and proinflammatory proteins69, and are known to be activated by cellular oxidative stress70,71. Photofrin-mediated PDT produced NFκB translocation in murine L1210 leukaemia cells under PDT conditions that resulted in approximately 20% cell survival72. However, Kick et al. found that HeLa cells that were treated with Photofrin-mediated PDT showed an increase in IL6 expression caused by the activation of AP1, not NFκB73. Colon carcinoma HCT116 cells that were treated with pyropheophorbide, a methyl ester, and red light, led to IκB processing and two distinct waves of NFκB activation; first by promoting the internalization of surface IL1 receptors, and then by ceramide generation74,75. HL60 cells that were transfected with a construct containing 5 NFκB sites of the HIV type-1 terminal repeat, cloned upstream of the luciferase gene, showed increased luciferase activity after benzoporphyrin derivative (BPD)-mediated PDT76. NFκB activation is the most important mediator of acute inflammation, and its induction after PDT in vitro confirms the observation that PDT induces acute inflammation in vivo.
COX2 and prostaglandin synthesis
Expression of the enzyme cyclooxygenase 2 (COX2, also known as prostaglandin endoperoxide synthase 2 (PTGS2)) is regulated by NFκB, and produces the inflammatory mediators known as the eicosanoids (including prostaglandin E2 (PGE2) and leukotrienes). PDT was found to cause prolonged expression of COX2 in PDT-treated mouse cancer cells and tumours in vivo, along with increased PGE2 synthesis77. Although PGE2 is pro-inflammatory, it is usually thought to have immunosuppressive effects78. PGE2 levels were attenuated in cells that were coincubated with the COX2 inhibitor NS-398. Moreover, systemic administration of NS-398 decreased the PDT-induced expression of both PGE2 and vascular endothelial growth factor in BA mouse mammary tumours, and increased the number of cures. COX2 inhibitors do not sensitize cancer cells to PDT-mediated killing per se, but can be used to potentiate the anti-tumour effectiveness of PDT when they are given after illumination. This anti-tumour effect is probably caused by the inhibition of angiogenesis, which is necessary for tumour regrowth79. Volanti et al. found that COX2 expression was mainly the result of NFκB activation, but the mechanism of activation differed in two cell lines80. In T24 bladder carcinoma cells, NFκB activation occurs through a protein kinase C-α (PKCα)- and phosphatidylinositol-3-kinase (PI3K)-dependent activation of the IκB kinase complex, whereas in HeLa cells, NFκB activation is mediated by PKC- and PI3K-independent pathways. Interestingly, hypericin-mediated PDT81 led to the increased expression of COX2 and PGE2, except that this time activation of the p38 mitogen activated protein kinase (MAPK) pathway was implicated (as shown by the MAPK inhibitor PD169316), as opposed to the activation of NFκB found in other systems. Overexpression of p38 MAPK also increased cellular resistance to PDT-induced apoptosis, but this effect was independent of COX2. Further work is necessary to understand the precise role of COX2 and eicosanoids in the PDT-induced immune response against tumours.
Neutrophil recruitment and the production of IL6
Kick et al.73 compared IL6 mRNA production after PDT or UVB treatment. PDT-induced IL6 protein levels were higher and were detectable earlier than after treatment with UVB. PDT-induced IL6 expression was mediated by AP1, and was independent of PKC activity, NFκB or the multiple cytokine- and second messenger-responsive element in the IL6 promoter.
Using a BALB/c mouse model, Gollnick and colleagues82 showed that PDT delivered to normal and tumour tissue caused marked changes in the expression of IL6 and IL10, but not TNFα. This group63 also found that 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a (HPPH)-mediated PDT caused neutrophil migration into the treated tumour area owing to a transient and local increase in the expression of the chemokine MIP2 (the murine equivalent of IL8), and the increased expression of the adhesion molecule E-selectin. Although increased local and systemic expression of IL6 were found, this was not necessary for neutrophil recruitment. A subsequent report83 compared the effect of a low and a high fluence (total light energy), each delivered at a low and high fluence rate, against Colo 26 murine tumours treated with HPPH. It has previously been proposed that PDT is less efficient when light is delivered at a high fluence rate because the tissue oxygen is completely used up and cannot be supplied fast enough by the microvasculature to keep up with photochemical consumption84. Oxygen-conserving low fluence rate PDT at a high fluence resulted in 70–80% tumour cures, whereas the same fluence at the oxygen-depleting high fluence rate resulted in 10–15% tumour cures. High fluence at a low fluence rate led to the ablation of blood vessels. The highest levels of inflammatory cytokines and neutrophilic infiltrates were observed when low fluence was delivered at a low fluence rate (10–20% cures). The optimally curative PDT regimen (high fluence at a low fluence rate) produced minimal inflammation. The depletion of neutrophils did not significantly change the high cure rates of that regimen, but abolished curability in the maximally inflammatory regimen. These data indicate that tumour cure can be mediated by maximizing the photochemical action of PDT, but the importance of causing inflammation and neutrophil infiltration is less clear.
Sluiter et al.85 first observed that neutrophils adhere to the microvascular wall after PDT in vivo, but PDT did not stimulate the expression of P-selectin (one of the principal adhesion molecules that bind leukocytes) by endothelial cells (ECs). The ECs retracted after PDT, which enabled neutrophils to adhere to the subendothelial matrix by their β2-integrin adhesion receptors, and this could be blocked by anti-β2-integrin antibodies86. This finding was supported by a report which showed that expression levels of the adhesion molecules intercellular adhesion molecule 1 (ICAM1) and vascular cell adhesion molecule 1 (VCAM1) were downregulated in ECs after PDT87. The administration of anti-rat neutrophil serum with PDT in rhabdomyosarcoma-bearing rats completely abrogated the normal PDT-induced retardation of tumour growth88, which shows that an influx of neutrophils is required for an effective anti-tumour response in this model. An increase in the number of peripheral-blood neutrophils was found 4 hours after PDT treatment, and lasted for 24 hours. The increase in neutrophils was preceded by an increase in serum levels of IL1β. Anti-GCSF (granulocyte colony-stimulating factor) antibodies decreased neutrophil numbers and decreased the efficacy of PDT. The reasons why neutrophils are so important in producing an effective response to PDT in some (but not all) tumour models are still uncertain.
Krosl and co-workers89 measured cellular populations in the murine squamous cell carcinoma VII (SCCVII) model treated with Photofrin-mediated PDT. They found a 200-fold increase in the number of neutrophils within 5 minutes of PDT, followed immediately by an increase in the levels of mast cells. Another type of myeloid cell, most likely monocytes, invaded the tumour 2 hours after PDT. Cecic et al.90 found that pronounced neutrophilia developed rapidly after Photofrin or mTHPC-mediated PDT of mice with SCCVII or EMT6 mammary carcinomas. Neutrophilia was also observed after PDT treatment of normal dorsal skin, but not in the footpad of tumour-free mice. Complement inhibition completely prevented the development of PDT-induced neutrophilia. Complement fragments from C3 and C5a proteins can induce neutrophilia either by mobilizing bone-marrow pools or as a response to transient neutropaenia caused by the adhesion of neutrophils to the endothelium91. Korbelik et al. went on to show that complement activation occurred after Lewis lung carcinomas (LLC) were treated with Photofrin-mediated PDT’s and observed increased levels of C3 in the tumour and serum92. Increased alternative complement pathway activity in the serum was evident 1–3 days after PDT. Blocking C3a or C5a receptors in the host mice decreased the efficacy of PDT in producing LLC tumour cures. Korbelik and colleagues also showed that blocking ICAM1 with monoclonal antibodies reduced the number of tumour cures93. A marked upregulation of the ICAM1 ligands CD11b and CD11c, which are found on neutrophils, was also associated with PDT-treated tumours. IL1-neutralizing antibodies diminished the number of cures of PDT-treated tumours. Neutrophils express MHC class II molecules, which suggests that they are engaged as antigen-presenting cells and involved in the development of the anti-tumour immune response. Korbelik et al. also found that IL1 and TNFα both function as potent promoters of the early phase of PDT-induced neutrophilia, but do not seem to have a significant role in the advanced phase94. The data attained by blocking two other cytokines, GCSF and IL10, showed that they are important contributors to advanced-phase neutrophilia, with no apparent influence in the early phase.
The reports described above show that the acute inflammation, which is produced by PDT, and both a systemic and tumour-localized increase in neutrophils is important in obtaining tumour cures. It is highly probable (although difficult to show) that these phenomena will also be important in the development of a memory T-cell anti-tumour immune response after PDT.
PDT and anti-tumour immunity
The introduction of transplantable tumours grown in inbred mouse or rat strains that share the same MHC haplotype (syngeneic animals) and have intact immune systems, has enabled researchers to study anti-tumour immunity after PDT. Canti and colleagues95 examined the effects of PDT with the PS aluminium disulphonated phthalocyanine on the anti-tumour immune response in both immunosuppressed and normal mice bearing MS-2 fibrosarcomas. All mice were cured and survived indefinitely, but resistance to MS-2 rechallenge was evident only in normal surviving animals cured by PDT, whereas immunosuppressed surviving animals and animals cured by surgery died after tumour rechallenge. Different syngeneic murine leukaemias were not rejected.
Korbelik et al.96 reported that Photofrin-based PDT cured 100% of EMT6 mammary sarcomas in syngeneic BALB/c mice, but no long-term cures were observed in non-obese diabetic (NOD), severe combined immunodeficient (SCID) or nude mice. The adoptive transfer of splenic T-lymphocytes from naive BALB/c mice into SCID mice before PDT postponed the recurrence of treated tumours, whereas adoptive transfer carried out immediately or 7 days after PDT had no benefit. Adoptive transfer of non-adherent splenocytes (a mixture of CD4+ and CD8+ T cells with some B cells, NK cells and monocytes) from normal mice cured of EMT6 tumours by PDT 5 weeks previously, fully restored the curative effect of PDT on EMT6 tumours that were growing in SCID mice. Splenocytes obtained from donors that were cured by X-rays were much less effective. The depletion of specific T-cell populations from donor splenocytes indicated that CD8+ cytotoxic T-lymphocytes had the most curative effect, whereas CD4+ helper T cells played a supportive role97. Analogous studies were performed by a different group98 using PDT with the PS 2-iodo-5-ethylamino-9-diethylaminobenzo[a]-phenothiazinium chloride.
A recent report99 showed that BPD-mediated PDT of RIF1 tumours (a poorly immunogenic murine sarcoma) in wild-type C3H/HeN mice leads to initial tumour disappearance but not to permanent cures because of local recurrence. By contrast, when the tumours were genetically engineered to express GFP from jellyfish, 100% cures and long-term resistance to rechallenge was obtained after PDT. PDT (but not surgical removal) induced immune recognition of the foreign GFP as a model tumour antigen. As additional tumour-rejection antigens are identified in mouse tumour cell lines100, a more rational approach can be taken to studying the factors that govern the relative strength of anti-tumour immune responses stimulated by PDT for different tumours and PDT regimens.
PDT-produced cancer vaccines
A related approach that takes advantage of the immunostimulatory effects of PDT is the preparation of cancer vaccines using in vitro PDT of cell cultures. Gollnick et al.101 compared the cancer-vaccine potential of PDT-generated cell lysates (EMT6 and P815 tumour cells) with lysates generated by UV or ionizing radiation. PDT-generated vaccines were tumour-specific, induced a cytotoxic T-cell response and, unlike the other methods, did not require the co-administration of an adjuvant to be effective. PDT-generated lysates were able to induce phenotypic DC maturation and IL12 expression. Korbelik and Sun102 produced a vaccine by treating SCCVII cells with BPD-mediated PDT and later with a lethal X-ray dose, and showed that these cells, when injected peritumorally into mice with established SCCVII tumours, produced a significant therapeutic effect, including growth retardation, tumour regression and cures. Importantly, vaccine cells that were retrieved from the treatment site at 1 hour after injection were intermixed with dendritic cells (DCs), HSP70 was expressed on their surface and they were opsonized by complement C3. This observation verifies some of the earlier findings in mouse models and in vitro.
PDT combined with other therapies
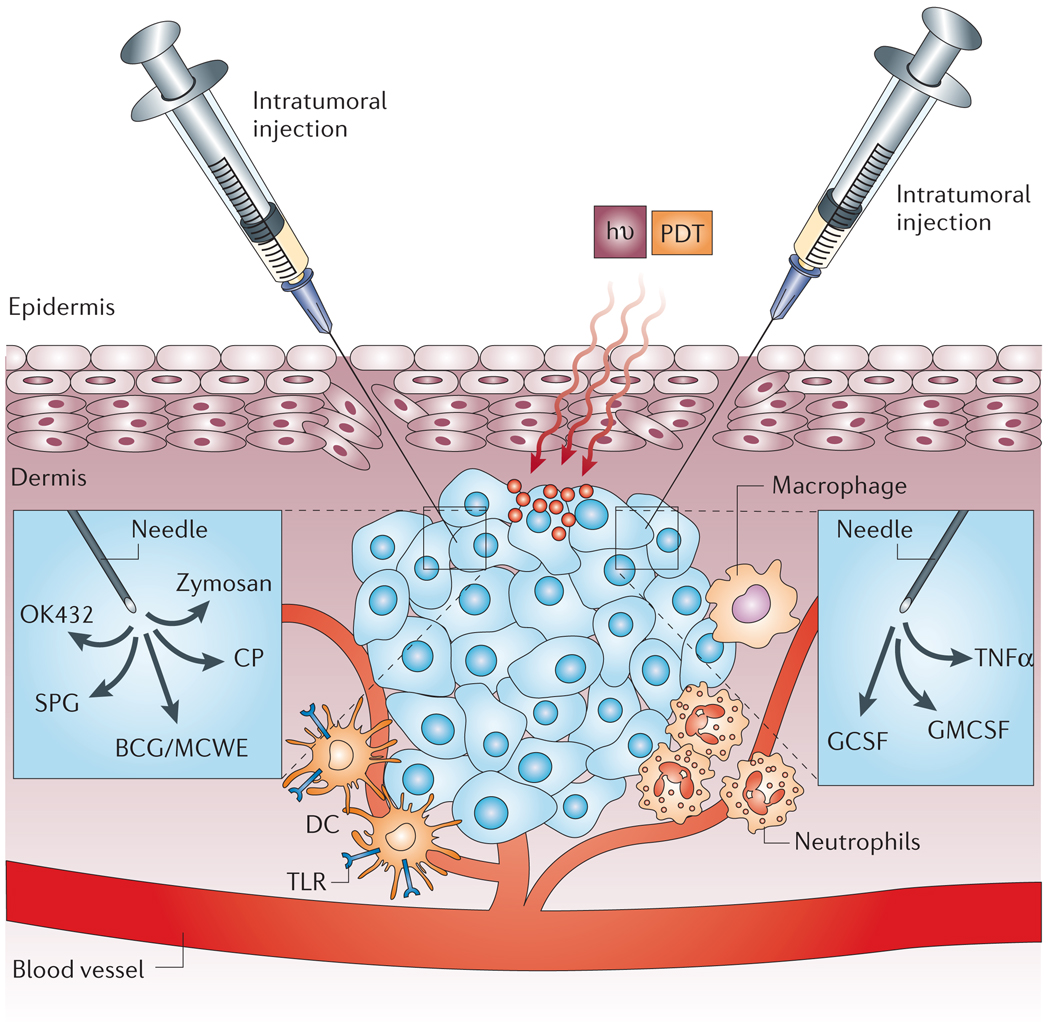
Reports of PDT combined with other immunostimulatory agents or strategies can be divided into three broad classes (FIG. 4).
Figure 4. Combination of photodynamic therapy with immunostimulants.
The intratumoral injection of various Toll-like receptor (TLR) ligands: bacillus Calmette–Guerin (BCG), Mycobacterial cell-wall extract (MCWE), OK432, zymosan, schizophyllan (SPG) or Corynebacterium parvum (CP), effectively activates dendritic cells (DCs) and increases antigen presentation and local inflammation. The injection of various cytokines, such as granulocyte-macrophage colony-stimulating factor (GMCSF), granulocyte colony-stimulating factor (GCSF) and tumour-necrosis factor-α (TNFα), results in increased infiltration by macrophages, activation of neutrophils, and direct destruction of tumour vessels, respectively. hν, light; PDT, photodynamic therapy.
PDT and microbial adjuvants
First, agents that are derived from microbial stimulators of innate immunity can be injected into the tumour or surrounding area before, during or after PDT (FIG. 4). Their role is to activate Toll-like receptors (TLRs) or similar pattern-recognition molecules that are present on macrophages and dendritic cells103. So far, 13 TLR family members have been identified on monocytes and macrophages, dendritic cells, mast cells and some epithelial cells104–106. Their principal function is thought to be as detectors of danger signals; early warning systems of imminent infection. The activation of TLR pathways can induce NFκB and, consequently, the expression of several genes involved in the activation of the immune sytem that are also important for the anti-tumour immune response107. These findings gave rise to the hypothesis that combination therapy that involves the administration of immunoadjuvants (often potential TLR ligands) and different PDT regimens might prove effective.
Myers et al.108 combined haematoporphyrin derivative (HPD)-mediated PDT with a killed preparation of Corynebacterium parvum (CP, now Propionibacterium acnes) in a mouse model of subcutaneous bladder cancer. Giving a high dose of CP after PDT was shown to have a significantly greater effect than CP treatment before PDT. Subcutaneous mouse EMT6 tumours were treated with a single dose of BCG in combination with PDT using six PSs109. Regardless of the PS used, BCG significantly increased the number of cured tumours and the number of memory T cells in tumour-draining lymph nodes compared with PDT alone. PDT was combined with a single dose of Mycobacterium cell-wall extract immediately after light exposure110, and produced significantly more long-term cures of EMT6 tumours in BALB/c mice and more tumour-infiltrating leukocytes at 22 hours after PDT. OK432 is a preparation derived from killed streptococcal bacteria, and increased the tumour-free time in mice with NRS1 squamous cell carcinomas when it was injected intratumorally 3 hours before HPD-mediated PDT. OK432 injected immediately after PDT, or OK432 alone had little effect111. The intratumoral injection of OK432 also potentiates PDT-induced anti-tumour immunity against EMT6 tumours (A.P.C., P.M. and M.R.H., unpublished observations). Schizophyllan (SPG) is an example of a β-D-glucan fungal polysaccharide, which are thought to be potent inducers of humoral and cell-mediated immunity by the macrophage dectin-1 receptor112, as well as TLRs113. The tumour cure rate increased threefold when SPG was given intramuscularly before the Photofrin-mediated PDT of mice with SCCVII, whereas SPG given after PDT had little effect114. A report from Chen and colleagues115 showed that a preparation of glycated chitosan derived from shrimp shells injected intratumorally increased the curative effects of Photofrin-mediated PDT on EMT6 tumours and Line1 lung tumours. The receptors that are responsible for mediating the effects of glycated chitosan are unknown.
Korbelik’s group has observed the activation of the complement system during PDT92, and has proposed this as an additional mechanism of anti-tumour response. Tumour-localized treatment with zymosan, an alternative complement pathway activator, and TLR2 and TLR6 ligands, reduced the number of recurrent tumours after PDT116. However, a similar treatment with heat-aggregated γ-globulin (a classical complement pathway activator) had no significant effect as a PDT adjuvant. Systemic complement activation with streptokinase treatment had no detectable effect on complement deposition at the tumour site without PDT, but it augmented the complement activity in PDT-treated tumours. Photofrin-mediated PDT was tested against SSCVII tumours in combination with serum vitamin D3 binding protein-derived macrophage-activating factor (DBPMAF)117. DBPMAF markedly improved the outcome of PDT, but as a single agent had no significant effect on the growth of SCCVII tumours.
PDT and cytokine therapy
Another class of combination therapies concerns the administration of cytokines such as TNFα, which was shown by Bellnier118 to potentiate Photofrin-mediated PDT of murine SMT-F adenocarcinoma after a single dose of intravenously administered recombinant human material. Localized tumour treatment with GCSF in combination with Photofrin-mediated PDT resulted in a significant reduction of tumour growth and an increase in the length of survival of BALB/c mice bearing two types of tumour: colo 26 tumours and Lewis lung carcinomas119 (FIG. 4). Moreover, 33% of colo 26 tumour-bearing mice were completely cured after combined therapy, and developed a specific and long-lasting immunity. Krosl et al.120 repeatedly injected lethally irradiated SCCVII cells that were genetically engineered to produce GMCSF and showed augmented anti-tumour effectiveness of Photofrin- and BPD-based PDT in mice with SCCVII. The treatment with GMCSF resulted in higher cytotoxic activity of tumour-associated macrophages against SCCVII cells.
Regulatory T cells and adoptive cellular therapies
A third group of PDT combination therapies includes interventions that are designed to alter or augment the cellular arm of the anti-tumour immune response. There is a growing realization that CD4+CD25+ T-regulatory cells have an important function in suppressing the immune response against multiple targets, and these cells are depleted by a low dose of cyclophosphamide (CY), therefore potentiating immunity121, whereas a high dose of CY is immunosuppressive122. Low-dose CY combined with BPD-mediated PDT, using a short drug to light interval that predominantly targeted the tumour blood vessels, led to a significant number of long-term J774 reticulum cell sarcoma cures and resistance to tumour rechallenge, whereas each treatment alone led to 100% death from progressive tumours or metastasis123. The examination of splenocytes recovered from tumour-bearing mice after low-dose CY showed that CD4+CD25+ T cells were reduced in number, and the splenocytes secreted significantly less transforming growth factor-β (TGFβ). TGFβ is an important immunosuppressive cytokine that is secreted by T-regulatory cells, and also stimulates T-regulatory cells124,125. Golab and colleagues126 showed that the injection of immature dendritic cells into tumours that were treated with Photofrin-mediated PDT resulted in effective homing to regional and peripheral lymph nodes and stimulation of CTLs and NK cells. The combination treatment produced the best tumour response and some resistance to a tumour rechallenge. A recent paper127 studied the combination of intratumoral dendritric cells and PDT mediated by the chlorin-type PS ATX-S10 Na(II) against CT26 tumours in BALB/c mice. The combination therapy produced tumour cures that were not seen with either treatment alone. Furthermore, when mice bearing two tumours had only one treated with the combination of PDT and dendritic cells, the contralateral untreated tumour underwent regression. The presence of tumour-specific lymphocytes was shown by chromium-release CTL assay and by IFNγ production. Korbelik and Sun128 used adoptive transfer of a human NK cell line that was genetically altered to produce IL2 combined with mTHPC-mediated PDT of subcutaneous human squamous cell carcinoma growing in SCID mice. Peritumoral or intravenous injection of cells immediately after PDT produced an improvement in the outcome of PDT, which was not seen with a cell line that did not produce IL2.
Immunosuppressive effects of PDT
Paradoxically, considering the discussions above, there are also several reports that PDT can induce various forms of immmunosuppression129. These have nearly all been concerned with the suppression of the contact hypersensitivity (CHS) reaction in mice130. This involves application of a hapten such as dinitrofluorobenzene to skin, followed by a rechallenge at a distant site, and can be suppressed for up to 28 days after PDT (FIG. 5). It seems that this suppression involves systemic IL10 release in cases where the PDT illumination penetrates the skin (red light)131, but is independent of IL10 when the PDT is confined to the skin layers (blue light)132. In contrast to UVB irradiation that suppresses both CHS and delayed type hypersensitivity (DTH) responses, PDT does not suppress DTH133. One difference between CHS and DTH is that CHS is thought to be an MHC-class-I-mediated process, whereas DTH is mediated by MHC class II, (REF. 134). As dendritic cells present antigens derived from destroyed tumour cells by MHC class II it could be argued that this difference explains why PDT-induced immmunosuppression does not abrogate anti-tumour immunity. MHC class I molecules usually present endogenous molecules to CD8+ T cells, whereas MHC class II molecules present exogenous molecules to CD4+ T cells. It is likely that the efficient induction of immune response against tumours requires the priming of CD4+ T cells by MHC class II molecules and CD8+ T cells by MHC class I molecules around the time of treatment, followed by the recognition of antigens presented on MHC class I molecules at the effector stage.
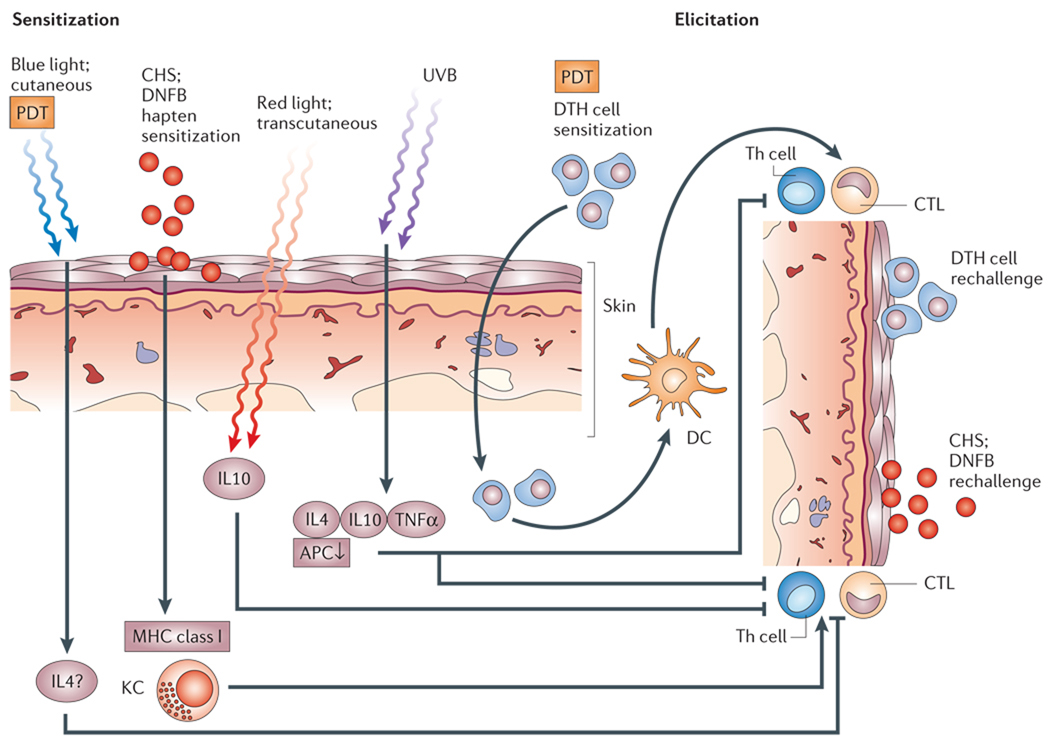
Figure 5. Mechanism of photodynamic therapy-induced immune suppression.
Contact hypersensitivity (CHS) is induced by the application of a hapten, such as dinitrofluorobenzene (DNFB), to the skin, and is mediated by the expression of the major histocompatibility complex (MHC) class I on keratinocytes (KC). A subsequent rechallenge with DNFB elicits an inflammatory response caused by the cytotoxic T cells. Delayed type hypersensitivity (DTH) is induced by the injection of cellular antigens such as foreign proteins, and is mediated by MHC class II expressed by dendritic cells (DCs) that are recognized by T-helper (CD4+) lymphocytes. CHS is suppressed by photodynamic therapy (PDT) using blue light that does not penetrate the skin and red light that does penetrate. Only by using red light does the suppression of CHS depend on the secretion of interleukin 10 (IL10). DTH is not suppressed by PDT, whereas ultraviolet light (UVB) suppresses both CHS and DTH. These differences might explain the paradoxical observation that PDT can both simultaneoulsy stimulate and suppress parts of the immune system, whereas UVB is only found to be immunosuppressive. APC, antigen-presenting cells; CTL, cytotoxic T cells; Th, T-helper cells.
Clinical studies and future outlook
Considering the number of patients (several thousand) that have been treated with PDT for various cancers over the previous three decades, there have been remarkably few studies that have even attempted to determine the effects of PDT on the human immune system, or to detect anti-tumour immunity after patients were treated. Scattered reports exist about the measurement of PDT-induced cytokine expression in patients64, and there are anecdotal reports about the unexpectedly long survival of patients who were treated with PDT for recurrent cancer135. There have been two reports about affects on the immune system in PDT of human papilloma virus lesions in patients136,137. A systematic study designed to detect the possible immune recognition of tumour cells after PDT for patients with cancer is long overdue. A recent meeting abstract (S.O. Gollnick, personal communication) reported that patients who were treated with PDT for basal cell carcinoma (BCC) demonstrated a significant increase (50–130%) in the numbers of peripheral-blood T cells that produced IFNγ when they recognized the sonic hedgehog ligand, hedgehog interacting protein (HIP1). HIP1 is not mutated in BCC, and has been shown to function as a tumour-associated antigen. For other cancers, measuring PDT-induced immune response might involve tumour-reactive serum antibodies or tumour-specific CD8+ or CD4+ T cells after PDT, but would involve taking tumour biopsy samples before PDT.
As we learn more it should be possible to understand how PDT can influence the precise cellular aspects of anti-tumour immunity. For instance, T-regulatory cells might be specifically inactivated by IL6 (REF. 138), a cytokine that is abundantly produced after PDT82. One point to be considered in the design of future clinical trails is suggested by the report from Henderson et al.83, which is referred to above. It is entirely possible that the optimal PDT regimen for producing local tumour cures will be different from the optimal PDT regimen for producing inflammation and stimulating immune response. Time will determine whether PDT-induced anti-tumour immunity is a clinically useful phenomenon that could benefit patients and potentially save lives, or whether it is a curiosity only applicable to mice and rats in the laboratory.
Acknowledgements
A.P.C. was supported by a US Department of Defense CDMRP Breast Cancer Research Grant. M.R.H. was supported by the US National Institutes of Health. We thank T. N. Demidova for help and advice.
Glossary
- Neutropaenia
A reduction in numbers of circulating neutrophils that predisposes to infection.
- Erysipelas
A skin disease caused by Streptococcus pyogenes.
- Antigen
A macromolecule (usually a protein or polysaccharide) that is perceived as foreign and stimulates an immune response.
- Major histocompatibility complex
Cell membrane proteins that bind short peptides and are recognized by T-cell receptors.
- Innate immune response
The immediately available nonspecific defence against invading pathogens, which consists of cellular (neutrophils, macrophages and natural killer cells) and non-cellular (complement and antibacterial peptides) arms.
- Adaptive immune response
An antigen-specific defence that develops with time, which consists of cellular (cytotoxic and helper T-lymphocytes) and humoral (B-lymphocytes and antibody) arms.
- Fluence
The light energy delivered per unit area (J cm−2).
- Fluence rate
The rate at which light energy is delivered per unit area (W cm−2).
- Antigen-presenting cells
Phagocytic cells such as dendritic cells, macrophages and B cells, which take up foreign antigens, and present then through major histocompatibility complex class II and express co-stimulatory molecules to ensure an effective T-cell response.
- Cross-presentation
The process by which exogenous antigens that would normally be presented by dendritic cells in the context major histocompatibility complex (MHC) class II to CD4+ T cells are also presented in the context of MHC class I to CD8+ T cells.
- Complement
A group of proteins in serum that function with antibodies (classical pathway) or in response to microbial stimuli (alternative pathway) to achieve the destruction of foreign blood cells or bacteria.
- Toll-like receptors
First discovered in Drosophila, these represent a conserved set of pattern-recognition molecules that are triggered by motifs present on bacteria, viruses and fungi to initiate signalling which attracts and activates immune cells.
- Hapten
A small reactive molecule that can bind to host proteins and stimulate an immune response.
- Delayed type hypersensitivity
A delayed-onset, cytokine-induced localized inflammatory reaction characterized by a large influx of macrophages.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
DATABASES
The following terms in this article are linked online to:
Entrez Gene: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene
COX2 | FOS | GCSF | GRP78 | GRP94 | HSP47 | HSP60 | ICAM1 | IL1β | IL6 | IL8 | IL10 | JUN | PI3K | PKCα | TGFβ | TLR2 | TLR6 | TNFα | VCAM1 | vascular endothelial growth factor
National Cancer Institute: http://www.cancer.gov hepatocellular carcinoma | Hodgkin Disease
FURTHER INFORMATION
Michael Hamblin’s homepage: http://www.massgeneral.org/wellman/people/mhamblin.asp
Access to this links box is available online.
References
- 1.Moan J, Peng Q. An outline of the hundred-year history of PDT. Anticancer Res. 2003;23:3591–3600. [PubMed] [Google Scholar]
- 2. Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nature Rev. Cancer. 2003;3:380–387. doi: 10.1038/nrc1071. A recent review on the theory and applications of PDT in cancer treatment.
- 3.Detty MR, Gibson SL, Wagner SJ. Current clinical and preclinical photosensitizers for use in photodynamic therapy. J. Med. Chem. 2004;47:3897–3915. doi: 10.1021/jm040074b. [DOI] [PubMed] [Google Scholar]
- 4.Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: part two — cellular signalling, cell metabolism and modes of cell death. Photodiagn. Photodyn. Ther. 2005;2:1–23. doi: 10.1016/S1572-1000(05)00030-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: part three-photosensitizer pharmacokinetics, biodistribution, tumor localization and modes of tumor destruction. Photodiagn. Photodyn. Ther. 2005;2:91–106. doi: 10.1016/S1572-1000(05)00060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oleinick NL, Morris RL, Belichenko I. The role of apoptosis in response to photodynamic therapy: what, where, why, and how. Photochem. Photobiol. Sci. 2002;1:1–21. doi: 10.1039/b108586g. [DOI] [PubMed] [Google Scholar]
- 7.Krammer B. Vascular effects of photodynamic therapy. Anticancer Res. 2001;21:4271–4277. [PubMed] [Google Scholar]
- 8.Dolmans DE, et al. Vascular accumulation of a novel photosensitizer, MV6401, causes selective thrombosis in tumor vessels after photodynamic therapy. Cancer Res. 2002;62:2151–2156. [PubMed] [Google Scholar]
- 9.Korbelik M. Induction of tumor immunity by photodynamic therapy. J. Clin. Laser Med. Surg. 1996;14:329–334. doi: 10.1089/clm.1996.14.329. [DOI] [PubMed] [Google Scholar]
- 10.van Duijnhoven FH, et al. The immunological consequences of photodynamic treatment of cancer, a literature review. Immunobiology. 2003;207:105–113. doi: 10.1078/0171-2985-00221. [DOI] [PubMed] [Google Scholar]
- 11.Canti G, De Simone A, Korbelik M. Photodynamic therapy and the immune system in experimental oncology. Photochem. Photobiol. Sci. 2002;1:79–80. doi: 10.1039/b109007k. [DOI] [PubMed] [Google Scholar]
- 12.Lugade AA, et al. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J. Immunol. 2005;174:7516–7523. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 13.Friedman EJ. Immune modulation by ionizing radiation and its implications for cancer immunotherapy. Curr. Pharm. Des. 2002;8:1765–1780. doi: 10.2174/1381612023394089. [DOI] [PubMed] [Google Scholar]
- 14.Crum ED. Effect of cisplatin upon expression of in vivo immune tumor resistance. Cancer Immunol. Immunother. 1993;36:18–24. doi: 10.1007/BF01789126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sierra-Rivera E, Voorhees GJ, Freeman ML. γ irradiation increases hsp-70 in Chinese hamster ovary cells. Radiat. Res. 1993;135:40–45. doi: 10.2307/3578394. [DOI] [PubMed] [Google Scholar]
- 16.Ng CS, et al. Thoracotomy is associated with significantly more profound suppression in lymphocytes and natural killer cells than video-assisted thoracic surgery following major lung resections for cancer. J. Invest. Surg. 2005;18:81–88. doi: 10.1080/08941930590926320. [DOI] [PubMed] [Google Scholar]
- 17.Fontana G, et al. Immunological response to prostatic cancer cryotherapy: certainties, controversies, hypotheses. Prog. Clin. Biol. Res. 1989;303:299–300. [PubMed] [Google Scholar]
- 18.Ivarsson K, et al. Resistance to tumour challenge after tumour laser thermotherapy is associated with a cellular immune response. Br. J. Cancer. 2005;93:435–440. doi: 10.1038/sj.bjc.6602718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Challis GB, Stam HJ. The spontaneous regression of cancer. A review of cases from 1900 to 1987. Acta Oncol. 1990;29:545–550. doi: 10.3109/02841869009090048. A collection of 744 cases of spontaneous regressions of cancer in humans, showing the theoretical justification for immunotherapy.
- 20.Hoption Cann SA, van Netten JP, van Netten C. Dr William Coley and tumour regression: a place in history or in the future. Postgrad. Med. J. 2003;79:672–680. [PMC free article] [PubMed] [Google Scholar]
- 21.Starnes CO. Coley’s toxins in perspective. Nature. 1992;357:11–12. doi: 10.1038/357011a0. [DOI] [PubMed] [Google Scholar]
- 22.Coley WB. Treatment of inoperable malignant tumors with toxins of erysipelas and the bacillus prodigiosus. Trans. Am. Surg. Assn. 1894;12:183–212. [Google Scholar]
- 23.Bassi P. BCG (Bacillus of Calmette Guerin) therapy of high-risk superficial bladder cancer. Surg. Oncol. 2002;11:77–83. doi: 10.1016/s0960-7404(02)00008-7. [DOI] [PubMed] [Google Scholar]
- 24.Zinkernagel RM, Doherty PC. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv. Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]
- 25.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 26.Belardelli F, Ferrantini M. Cytokines as a link between innate and adaptive antitumor immunity. Trends Immunol. 2002;23:201–208. doi: 10.1016/s1471-4906(02)02195-6. [DOI] [PubMed] [Google Scholar]
- 27.Kern DE, Klarnet JP, Jensen MC, Greenberg PD. Requirement for recognition of class II molecules and processed tumor antigen for optimal generation of syngeneic tumor-specific class I-restricted CTL. J. Immunol. 1986;136:4303–4310. [PubMed] [Google Scholar]
- 28.Ahmad M, Rees RC, Ali SA. Escape from immunotherapy: possible mechanisms that influence tumor regression/progression. Cancer Immunol. Immunother. 2004;53:844–854. doi: 10.1007/s00262-004-0540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nature Rev. Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 30.Agostinis P, Buytaert E, Breyssens H, Hendrickx N. Regulatory pathways in photodynamic therapy induced apoptosis. Photochem. Photobiol. Sci. 2004;3:721–729. doi: 10.1039/b315237e. [DOI] [PubMed] [Google Scholar]
- 31.Moor AC. Signaling pathways in cell death and survival after photodynamic therapy. J. Photochem. Photobiol. B. 2000;57:1–13. doi: 10.1016/s1011-1344(00)00065-8. [DOI] [PubMed] [Google Scholar]
- 32.Plaetzer K, Kiesslich T, Oberdanner CB, Krammer B. Apoptosis following photodynamic tumor therapy: induction, mechanisms and detection. Curr. Pharm. Des. 2005;11:1151–1165. doi: 10.2174/1381612053507648. [DOI] [PubMed] [Google Scholar]
- 33.Chen B, et al. Photodynamic therapy with hypericin induces vascular damage and apoptosis in the RIF-1 mouse tumor model. Int. J. Cancer. 2002;98:284–290. doi: 10.1002/ijc.10175. [DOI] [PubMed] [Google Scholar]
- 34.Kaneko T, Chiba H, Yasuda T, Kusama K. Detection of photodynamic therapy-induced early apoptosis in human salivary gland tumor cells in vitro and in a mouse tumor model. Oral Oncol. 2004;40:787–792. doi: 10.1016/j.oraloncology.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Lilge L, Portnoy M, Wilson BC. Apoptosis induced in vivo by photodynamic therapy in normal brain and intracranial tumour tissue. Br. J. Cancer. 2000;83:1110–1117. doi: 10.1054/bjoc.2000.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magner WJ, Tomasi TB. Apoptotic and necrotic cells induced by different agents vary in their expression of MHC and costimulatory genes. Mol. Immunol. 2005;42:1033–1042. doi: 10.1016/j.molimm.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 37.Bartholomae WC, et al. T cell immunity induced by live, necrotic, and apoptotic tumor cells. J. Immunol. 2004;173:1012–1022. doi: 10.4049/jimmunol.173.2.1012. [DOI] [PubMed] [Google Scholar]
- 38.Scheffer SR, et al. Apoptotic, but not necrotic, tumor cell vaccines induce a potent immune response in vivo. Int. J. Cancer. 2003;103:205–211. doi: 10.1002/ijc.10777. [DOI] [PubMed] [Google Scholar]
- 39.Shaif-Muthana M, et al. Dead or alive: immunogenicity of human melanoma cells when presented by dendritic cells. Cancer Res. 2000;60:6441–6447. [PubMed] [Google Scholar]
- 40.Zitvogel L, et al. Immune response against dying tumor cells. Adv. Immunol. 2004;84:131–179. doi: 10.1016/S0065-2776(04)84004-5. [DOI] [PubMed] [Google Scholar]
- 41.Melcher A, Gough M, Todryk S, Vile R. Apoptosis or necrosis for tumor immunotherapy: what’s in a name? J. Mol. Med. 1999;77:824–833. doi: 10.1007/s001099900066. [DOI] [PubMed] [Google Scholar]
- 42.Yenari MA, et al. Antiapoptotic and anti-inflammatory mechanisms of heat-shock protein protection. Ann. NY Acad. Sci. 2005;1053:74–83. doi: 10.1196/annals.1344.007. [DOI] [PubMed] [Google Scholar]
- 43.Korbelik M, Sun J, Cecic I. Photodynamic therapy-induced cell surface expression and release of heat shock proteins: relevance for tumor response. Cancer Res. 2005;65:1018–1026. [PubMed] [Google Scholar]
- 44.Todryk S, et al. Heat shock protein 70 induced during tumor cell killing induces Th1 cytokines and targets immature dendritic cell precursors to enhance antigen uptake. J. Immunol. 1999;163:1398–1408. [PubMed] [Google Scholar]
- 45. Gomer CJ, et al. Photodynamic therapy-mediated oxidative stress can induce expression of heat shock proteins. Cancer Res. 1996;56:2355–2360. Demonstrates that PDT induces HSP expression both in vitro and in vivo.
- 46.Mitra S, Goren EM, Frelinger JG, Foster TH. Activation of heat shock protein 70 promoter with meso-tetrahydroxyphenyl chlorin photodynamic therapy reported by green fluorescent protein in vitro and in vivo. Photochem. Photobiol. 2003;78:615–622. doi: 10.1562/0031-8655(2003)078<0615:aohspp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 47.Verwanger T, et al. Gene expression pattern following photodynamic treatment of the carcinoma cell line A-431 analysed by cDNA arrays. Int. J. Oncol. 2002;21:1353–1359. [PubMed] [Google Scholar]
- 48.Verrico AK, Haylett AK, Moore JV. In vivo expression of the collagen-related heat shock protein HSP47, following hyperthermia or photodynamic therapy. Lasers Med. Sci. 2001;16:192–198. doi: 10.1007/pl00011354. [DOI] [PubMed] [Google Scholar]
- 49.Hanlon JG, et al. Induction of Hsp60 by Photofrin-mediated photodynamic therapy. J. Photochem. Photobiol. B. 2001;64:55–61. doi: 10.1016/s1011-1344(01)00189-0. [DOI] [PubMed] [Google Scholar]
- 50.Gomer CJ, et al. Glucose regulated protein induction and cellular resistance to oxidative stress mediated by porphyrin photosensitization. Cancer Res. 1991;51:6574–6579. [PubMed] [Google Scholar]
- 51.Nowis D, et al. Heme oxygenase-1 protects tumor cells against photodynamic therapy-mediated cytotoxicity. Oncogene. 2006 Feb 6; doi: 10.1038/sj.onc.1209378. (doi 10. 1038/sj.onc1209378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang H, et al. Selective depletion of a thymocyte subset in vitro with an immunomodulatory photosensitizer. Clin. Immunol. 1999;91:178–187. doi: 10.1006/clim.1999.4695. [DOI] [PubMed] [Google Scholar]
- 53.Jiang H, et al. Selective action of the photosensitizer QLT0074 on activated human T lymphocytes. Photochem. Photobiol. 2002;76:224–231. doi: 10.1562/0031-8655(2002)076<0224:saotpq>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 54.Boumedine RS, Roy DC. Elimination of alloreactive T cells using photodynamic therapy. Cytotherapy. 2005;7:134–143. doi: 10.1080/14653240510027109. [DOI] [PubMed] [Google Scholar]
- 55.Hunt DW, Levy JG. Immunomodulatory aspects of photodynamic therapy. Expert Opin. Investig. Drugs. 1998;7:57–64. doi: 10.1517/13543784.7.1.57. [DOI] [PubMed] [Google Scholar]
- 56.Edstrom DW, Porwit A, Ros AM. Photodynamic therapy with topical 5-aminolevulinic acid for mycosis fungoides: clinical and histological response. Acta Derm. Venereol. 2001;81:184–188. doi: 10.1080/000155501750376276. [DOI] [PubMed] [Google Scholar]
- 57.Steubing RW, et al. Activation of macrophages by Photofrin II during photodynamic therapy. J. Photochem. Photobiol. B. 1991;10:133–145. doi: 10.1016/1011-1344(91)80218-7. [DOI] [PubMed] [Google Scholar]
- 58. Evans S, et al. Effect of photodynamic therapy on tumor necrosis factor production by murine macrophages. J. Natl Cancer Inst. 1990;82:34–39. doi: 10.1093/jnci/82.1.34. Shows that PDT produces TNFα from macrophages.
- 59.Yamamoto N, et al. Photodynamic immunopotentiation: in vitro activation of macrophages by treatment of mouse peritoneal cells with haematoporphyrin derivative and light. Eur. J. Cancer. 1991;27:467–471. doi: 10.1016/0277-5379(91)90388-t. [DOI] [PubMed] [Google Scholar]
- 60.Yamamoto N, Naraparaju VR. Immunotherapy of BALB/c mice bearing Ehrlich ascites tumor with vitamin D-binding protein-derived macrophage activating factor. Cancer Res. 1997;57:2187–2192. [PubMed] [Google Scholar]
- 61.Korbelik M, Krosl G. Enhanced macrophage cytotoxicity against tumor cells treated with photodynamic therapy. Photochem. Photobiol. 1994;60:497–502. doi: 10.1111/j.1751-1097.1994.tb05140.x. [DOI] [PubMed] [Google Scholar]
- 62.Marshall JF, Chan WS, Hart IR. Effect of photodynamic therapy on anti-tumor immune defenses: comparison of the photosensitizers hematoporphyrin derivative and chloro-aluminum sulfonated phthalocyanine. Photochem. Photobiol. 1989;49:627–632. doi: 10.1111/j.1751-1097.1989.tb08434.x. [DOI] [PubMed] [Google Scholar]
- 63. Gollnick SO, et al. Role of cytokines in photodynamic therapy-induced local and systemic inflammation. Br. J. Cancer. 2003;88:1772–1779. doi: 10.1038/sj.bjc.6600864. Describes the production of pro-inflammatory cytokines after PDT in vivo.
- 64.Yom SS, et al. Elevated serum cytokine levels in mesothelioma patients who have undergone pleurectomy or extrapleural pneumonectomy and adjuvant intraoperative photodynamic therapy. Photochem. Photobiol. 2003;78:75–81. doi: 10.1562/0031-8655(2003)078<0075:esclim>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 65.Henderson BW, Donovan JM. Release of prostaglandin E2 from cells by photodynamic treatment in vitro. Cancer Res. 1989;49:6896–6900. [PubMed] [Google Scholar]
- 66.Henderson BW, Owczarczak B, Sweeney J, Gessner T. Effects of photodynamic treatment of platelets or endothelial cells in vitro on platelet aggregation. Photochem. Photobiol. 1992;56:513–521. doi: 10.1111/j.1751-1097.1992.tb02195.x. [DOI] [PubMed] [Google Scholar]
- 67.Fingar VH, Wieman TJ, Doak KW. Role of thromboxane and prostacyclin release on photodynamic therapy-induced tumor destruction. Cancer Res. 1990;50:2599–2603. [PubMed] [Google Scholar]
- 68.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 69.Sun SC, Xiao G. Deregulation of NF-κB and its upstream kinases in cancer. Cancer Metastasis Rev. 2003;22:405–422. doi: 10.1023/a:1023733231406. [DOI] [PubMed] [Google Scholar]
- 70.Baeuerle PA, Henkel T. Function and activation of NF-κB in the immune system. Annu. Rev. Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 71.Haefner B. NF-κB: arresting a major culprit in cancer. Drug Discov. Today. 2002;7:653–663. doi: 10.1016/s1359-6446(02)02309-7. [DOI] [PubMed] [Google Scholar]
- 72. Ryter SW, Gomer CJ. Nuclear factor κB binding activity in mouse L1210 cells following photofrin II-mediated photosensitization. Photochem. Photobiol. 1993;58:753–756. doi: 10.1111/j.1751-1097.1993.tb04964.x. First report of PDT triggering NFκB signalling.
- 73.Kick G, et al. Photodynamic therapy induces expression of interleukin 6 by activation of AP-1 but not NF-κB DNA binding. Cancer Res. 1995;55:2373–2379. [PubMed] [Google Scholar]
- 74.Matroule JY, et al. Pyropheophorbide-a methyl ester-mediated photosensitization activates transcription factor NF-κB through the interleukin-1 receptor-dependent signaling pathway. J. Biol. Chem. 1999;274:2988–3000. doi: 10.1074/jbc.274.5.2988. [DOI] [PubMed] [Google Scholar]
- 75.Matroule JY, et al. Role of nuclear factor-κB in colon cancer cell apoptosis mediated by aminopyropheophorbide photosensitization. Photochem. Photobiol. 1999;70:540–548. [PubMed] [Google Scholar]
- 76.Granville DJ, et al. Nuclear factor-κB activation by the photochemotherapeutic agent verteporfin. Blood. 2000;95:256–262. [PubMed] [Google Scholar]
- 77.Ferrario A, et al. Cyclooxygenase-2 inhibitor treatment enhances photodynamic therapy- mediated tumor response. Cancer Res. 2002;62:3956–3961. [PubMed] [Google Scholar]
- 78.Mitsuhashi M, et al. Regulation of interleukin-12 gene expression and its anti-tumor activities by prostaglandin E2 derived from mammary carcinomas. J. Leukoc. Biol. 2004;76:322–332. doi: 10.1189/jlb.1203641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Makowski M, et al. Inhibition of cyclooxygenase-2 indirectly potentiates antitumor effects of photodynamic therapy in mice. Clin. Cancer Res. 2003;9:5417–5422. [PubMed] [Google Scholar]
- 80.Volanti C, et al. Distinct transduction mechanisms of cyclooxygenase 2 gene activation in tumour cells after photodynamic therapy. Oncogene. 2005;24:2981–2991. doi: 10.1038/sj.onc.1208481. [DOI] [PubMed] [Google Scholar]
- 81.Hendrickx N, et al. Up-regulation of cyclooxygenase-2 and apoptosis resistance by p38 MAPK in hypericin-mediated photodynamic therapy of human cancer cells. J. Biol. Chem. 2003;278:52231–52239. doi: 10.1074/jbc.M307591200. [DOI] [PubMed] [Google Scholar]
- 82.Gollnick SO, et al. Altered expression of interleukin 6 and interleukin 10 as a result of photodynamic therapy in vivo. Cancer Res. 1997;57:3904–3909. [PubMed] [Google Scholar]
- 83.Henderson BW, et al. Choice of oxygen-conserving treatment regimen determines the inflammatory response and outcome of photodynamic therapy of tumors. Cancer Res. 2004;64:2120–2126. doi: 10.1158/0008-5472.can-03-3513. [DOI] [PubMed] [Google Scholar]
- 84.Coutier S, et al. Effect of irradiation fluence rate on the efficacy of photodynamic therapy and tumor oxygenation in meta-tetra (hydroxyphenyl) chlorin (mTHPC)-sensitized HT29 xenografts in nude mice. Radiat. Res. 2002;158:339–345. doi: 10.1667/0033-7587(2002)158[0339:eoifro]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 85.Sluiter W, de Vree WJ, Pietersma A, Koster JF. Prevention of late lumen loss after coronary angioplasty by photodynamic therapy: role of activated neutrophils. Mol. Cell. Biochem. 1996;157:233–238. doi: 10.1007/BF00227904. [DOI] [PubMed] [Google Scholar]
- 86.de Vree WJ, Fontijne-Dorsman AN, Koster JF, Sluiter W. Photodynamic treatment of human endothelial cells promotes the adherence of neutrophils in vitro. Br. J. Cancer. 1996;73:1335–1340. doi: 10.1038/bjc.1996.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Volanti C, et al. Downregulation of ICAM-1 and VCAM-1 expression in endothelial cells treated by photodynamic therapy. Oncogene. 2004;23:8649–8658. doi: 10.1038/sj.onc.1207871. [DOI] [PubMed] [Google Scholar]
- 88. de Vree WJ, et al. Evidence for an important role of neutrophils in the efficacy of photodynamic therapy in vivo. Cancer Res. 1996;56:2908–2911. Shows that neutrophils have a crucial role in the PDT response in vivo.
- 89.Krosl G, Korbelik M, Dougherty GJ. Induction of immune cell infiltration into murine SCCVII tumour by photofrin-based photodynamic therapy. Br. J. Cancer. 1995;71:549–555. doi: 10.1038/bjc.1995.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cecic I, Parkins CS, Korbelik M. Induction of systemic neutrophil response in mice by photodynamic therapy of solid tumors. Photochem. Photobiol. 2001;74:712–720. doi: 10.1562/0031-8655(2001)074<0712:iosnri>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 91.Kajita T, Hugli TE. C5a-induced neutrophilia. A primary humoral mechanism for recruitment of neutrophils. Am. J. Pathol. 1990;137:467–477. [PMC free article] [PubMed] [Google Scholar]
- 92.Cecic I, Serrano K, Gyongyossy-Issa M, Korbelik M. Characteristics of complement activation in mice bearing Lewis lung carcinomas treated by photodynamic therapy. Cancer Lett. 2005;225:215–223. doi: 10.1016/j.canlet.2004.11.059. [DOI] [PubMed] [Google Scholar]
- 93.Sun J, Cecic I, Parkins CS, Korbelik M. Neutrophils as inflammatory and immune effectors in photodynamic therapy-treated mouse SCCVII tumours. Photochem. Photobiol. Sci. 2002;1:690–695. doi: 10.1039/b204254a. [DOI] [PubMed] [Google Scholar]
- 94.Cecic I, Korbelik M. Mediators of peripheral blood neutrophilia induced by photodynamic therapy of solid tumors. Cancer Lett. 2002;183:43–51. doi: 10.1016/s0304-3835(02)00092-7. [DOI] [PubMed] [Google Scholar]
- 95.Canti G, et al. Antitumor immunity induced by photodynamic therapy with aluminum disulfonated phthalocyanines and laser light. Anti-Cancer Drugs. 1994;5:443–447. doi: 10.1097/00001813-199408000-00009. [DOI] [PubMed] [Google Scholar]
- 96. Korbelik M, Krosl G, Krosl J, Dougherty GJ. The role of host lymphoid populations in the response of mouse EMT6 tumor to photodynamic therapy. Cancer Res. 1996;56:5647–5652. One of the first reports to show T-cell mediated immune response against cancer after PDT.
- 97.Korbelik M, Dougherty GJ. Photodynamic therapy-mediated immune response against subcutaneous mouse tumors. Cancer Res. 1999;59:1941–1946. [PubMed] [Google Scholar]
- 98.Hendrzak-Henion JA, et al. Role of the immune system in mediating the antitumor effect of benzophenothiazine photodynamic therapy. Photochem. Photobiol. 1999;69:575–581. [PubMed] [Google Scholar]
- 99.Castano AP, Liu Q, Hamblin MR. A green fluorescent protein-expressing murine tumour but not its wild-type counterpart is cured by photodynamic therapy. Br. J. Cancer. 2006;94:391–397. doi: 10.1038/sj.bjc.6602953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Uenaka A, Nakayama E. Murine leukemia RL male 1 and sarcoma Meth A antigens recognized by cytotoxic T lymphocytes (CTL) Cancer Sci. 2003;94:931–936. doi: 10.1111/j.1349-7006.2003.tb01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Gollnick SO, Vaughan L, Henderson BW. Generation of effective antitumor vaccines using photodynamic therapy. Cancer Res. 2002;62:1604–1608. Shows that PDT is especially effective in preparing vaccines from tumour cell lysates.
- 102.Korbelik M, Sun J. Photodynamic therapy-generated vaccine for cancer therapy. Cancer Immunol. Immunother. 2005;55:900–909. doi: 10.1007/s00262-005-0088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu. Rev. Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 104.Kadowaki N, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Krug A, et al. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur. J. Immunol. 2001;31:3026–3037. doi: 10.1002/1521-4141(2001010)31:10<3026::aid-immu3026>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 106.Supajatura V, et al. Protective roles of mast cells against enterobacterial infection are mediated by Toll-like receptor 4. J. Immunol. 2001;167:2250–2256. doi: 10.4049/jimmunol.167.4.2250. [DOI] [PubMed] [Google Scholar]
- 107.Seya T, et al. Role of toll-like receptors and their adaptors in adjuvant immunotherapy for cancer. Anticancer Res. 2003;23:4369–4376. [PubMed] [Google Scholar]
- 108.Myers RC, et al. Modulation of hematoporphyrin derivative-sensitized phototherapy with corynebacterium parvum in murine transitional cell carcinoma. Urology. 1989;33:230–235. doi: 10.1016/0090-4295(89)90399-3. [DOI] [PubMed] [Google Scholar]
- 109.Korbelik M, Sun J, Posakony JJ. Interaction between photodynamic therapy and BCG immunotherapy responsible for the reduced recurrence of treated mouse tumors. Photochem. Photobiol. 2001;73:403–409. doi: 10.1562/0031-8655(2001)073<0403:ibptab>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 110.Korbelik M, Cecic I. Enhancement of tumour response to photodynamic therapy by adjuvant mycobacterium cell-wall treatment. J. Photochem. Photobiol. B. 1998;44:151–158. doi: 10.1016/S1011-1344(98)00138-9. [DOI] [PubMed] [Google Scholar]
- 111.Uehara M, et al. Enhancement of the photodynamic antitumor effect by streptococcal preparation OK-432 in the mouse carcinoma. Cancer Immunol. Immunother. 2000;49:401–409. doi: 10.1007/s002620000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Taylor PR, et al. The β-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J. Immunol. 2002;169:3876–3882. doi: 10.4049/jimmunol.169.7.3876. [DOI] [PubMed] [Google Scholar]
- 113.Roeder A, et al. Toll-like receptors as key mediators in innate antifungal immunity. Med. Mycol. 2004;42:485–498. doi: 10.1080/13693780400011112. [DOI] [PubMed] [Google Scholar]
- 114.Krosl G, Korbelik M. Potentiation of photodynamic therapy by immunotherapy: the effect of schizophyllan (SPG) Cancer Lett. 1994;84:43–49. doi: 10.1016/0304-3835(94)90356-5. [DOI] [PubMed] [Google Scholar]
- 115.Chen WR, et al. Enhancement of laser cancer treatment by a chitosan-derived immunoadjuvant. Photochem. Photobiol. 2005;81:190–195. doi: 10.1562/2004-07-20-RA-236. [DOI] [PubMed] [Google Scholar]
- 116.Korbelik M, Sun J, Cecic I, Serrano K. Adjuvant treatment for complement activation increases the effectiveness of photodynamic therapy of solid tumors. Photochem. Photobiol. Sci. 2004;3:812–816. doi: 10.1039/b315663J. [DOI] [PubMed] [Google Scholar]
- 117.Korbelik M, Naraparaju VR, Yamamoto N. Macrophage-directed immunotherapy as adjuvant to photodynamic therapy of cancer. Br. J. Cancer. 1997;75:202–207. doi: 10.1038/bjc.1997.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bellnier DA. Potentiation of photodynamic therapy in mice with recombinant human tumor necrosis factor-α. J. Photochem. Photobiol. B. 1991;8:203–210. doi: 10.1016/1011-1344(91)80060-u. [DOI] [PubMed] [Google Scholar]
- 119.Golab J, et al. Potentiation of the anti-tumour effects of Photofrin-based photodynamic therapy by localized treatment with G-CSF. Br. J. Cancer. 2000;82:1485–1491. doi: 10.1054/bjoc.1999.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Krosl G, Korbelik M, Krosl J, Dougherty GJ. Potentiation of photodynamic therapy-elicited antitumor response by localized treatment with granulocyte-macrophage colony-stimulating factor. Cancer Res. 1996;56:3281–3286. [PubMed] [Google Scholar]
- 121.North RJ. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J. Exp. Med. 1982;155:1063–1074. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zagozdzon R, Golab J. Immunomodulation by anticancer chemotherapy: more is not always better (review) Int. J. Oncol. 2001;18:417–424. doi: 10.3892/ijo.18.2.417. [DOI] [PubMed] [Google Scholar]
- 123.Castano AP, Hamblin MR. Anti-tumor immunity generated by photodynamic therapy in a metastatic murine tumor model. Proc. SPIE. 2005;5695:7–16. [Google Scholar]
- 124.Fu S, et al. TGF-β induces Foxp3 + T-regulatory cells from CD4 + CD25- precursors. Am. J. Transplant. 2004;4:1614–1627. doi: 10.1111/j.1600-6143.2004.00566.x. [DOI] [PubMed] [Google Scholar]
- 125.Wahl SM, Swisher J, McCartney-Francis N, Chen W. TGF-β: the perpetrator of immune suppression by regulatory T cells and suicidal T cells. J. Leukoc. Biol. 2004;76:15–24. doi: 10.1189/jlb.1103539. [DOI] [PubMed] [Google Scholar]
- 126.Jalili A, et al. Effective photoimmunotherapy of murine colon carcinoma induced by the combination of photodynamic therapy and dendritic cells. Clin. Cancer Res. 2004;10:4498–4508. doi: 10.1158/1078-0432.CCR-04-0367. [DOI] [PubMed] [Google Scholar]
- 127. Saji H, et al. Systemic antitumor effect of intratumoral injection of dendritic cells in combination with local photodynamic therapy. Clin. Cancer Res. 2006;12:2568–2574. doi: 10.1158/1078-0432.CCR-05-1986. The immune response produced by PDT and dendritic cells can regress a distant untreated tumour.
- 128.Korbelik M, Sun J. Cancer treatment by photodynamic therapy combined with adoptive immunotherapy using genetically altered natural killer cell line. Int. J. Cancer. 2001;93:269–274. doi: 10.1002/ijc.1326. [DOI] [PubMed] [Google Scholar]
- 129.Hunt DW, Levy JG. Immunomodulatory aspects of photodynamic therapy. Expert Opin. Investig. Drugs. 1998;7:57–64. doi: 10.1517/13543784.7.1.57. [DOI] [PubMed] [Google Scholar]
- 130.Musser DA, Camacho SH, Manderscheid PA, Oseroff AR. The anatomic site of photodynamic therapy is a determinant for immunosuppression in a murine model. Photochem. Photobiol. 1999;69:222–225. [PubMed] [Google Scholar]
- 131. Simkin GO, Tao JS, Levy JG, Hunt DW. IL-10 contributes to the inhibition of contact hypersensitivity in mice treated with photodynamic therapy. J. Immunol. 2000;164:2457–2462. doi: 10.4049/jimmunol.164.5.2457. Describes PDT-induced immune suppression as evidenced by reduction of CHS.
- 132.Gollnick SO, et al. IL-10 does not play a role in cutaneous Photofrin photodynamic therapy- induced suppression of the contact hypersensitivity response. Photochem. Photobiol. 2001;74:811–816. doi: 10.1562/0031-8655(2001)074<0811:idnpar>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 133.Musser DA, Oseroff AR. Characteristics of the immunosuppression induced by cutaneous photodynamic therapy: persistence, antigen specificity and cell type involved. Photochem. Photobiol. 2001;73:518–524. doi: 10.1562/0031-8655(2001)073<0518:cotiib>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 134.Ohtani M, Kobayashi Y, Watanabe N. Gene expression in the elicitation phase of guinea pig DTH and CHS reactions. Cytokine. 2004;25:246–253. doi: 10.1016/j.cyto.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 135.Lou PJ, et al. Interstitial photodynamic therapy as salvage treatment for recurrent head and neck cancer. Br. J. Cancer. 2004;91:441–446. doi: 10.1038/sj.bjc.6601993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Abdel-Hady ES, et al. Immunological and viral factors associated with the response of vulval intraepithelial neoplasia to photodynamic therapy. Cancer Res. 2001;61:192–196. One of the few papers describing immune response after PDT in humans.
- 137.Shikowitz MJ, et al. Clinical trial of photodynamic therapy with meso-tetra (hydroxyphenyl) chlorin for respiratory papillomatosis. Arch. Otolaryngol. Head Neck Surg. 2005;131:99–105. doi: 10.1001/archotol.131.2.99. [DOI] [PubMed] [Google Scholar]
- 138.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]