Abstract
Acute graft-versus-host disease (aGVHD) following allogeneic hematopoietic cell transplant (HCT) is the major reason for non-relapse mortality and thus is a major determinant of long term survival. Clinical trials of new aGVHD treatments are needed in order to identify approaches that will ultimately improve upon HCT survival. At present it is not clear how quickly response to GVHD treatment needs to be established in order to reliably categorize patients at high risk for death or to promptly identify those who might benefit from alternate treatment. Therefore, we analyzed time to response from onset of aGVHD treatment in 180 patients who were enrolled on a national, randomized, phase II aGVHD treatment clinical trial whose initial treatment of GVHD consisted of high dose steroids plus a second immunosuppressive agent. The aim of this analysis was to determine whether time to aGVHD treatment response predicts patient outcomes, especially survival. We used response at 14, 28 and 56 days from initiation of aGVHD treatment to categorize patients for non-relapse mortality and survival. Multivariate analyses and specificity/sensitivity analyses identified that day 28 response (complete or partial response) best categorized patients by non-relapse mortality and survival at 9 months from start of aGVHD treatment. If verified as a reliable predictor of late outcomes following other aGVHD treatment approaches, day 28 response should serve as a standard early endpoint for future trials of aGVHD therapy.
INTRODUCTION
Allogeneic hematopoietic cell transplant (HCT) is used as a curative therapy for a large number of malignant and non-malignant hematologic diseases. Graft-versus-host disease (GVHD) is the major reason for non-relapse mortality and is staged and graded according to severity of symptoms in three target organs (skin, liver, and gastrointestinal tract)1. Effective GVHD prevention remains an area of active clinical research, but at present most patients are administered prophylactic agents that interfere with T-lymphocyte numbers or function 2–4. Nevertheless, large numbers of patients develop acute (aGVHD) requiring treatment. Patients whose GVHD does not respond to treatment, generally high doses of corticosteroids, experience particularly high rates of mortality5,6 and new treatments for steroid-refractory patients are needed7. However, it is not clear at present how quickly response to GVHD treatment needs to be established in order to reliably categorize patients at high-risk for death or to promptly identify those who might benefit from alternate treatment. Therefore, we analyzed time to response from onset of aGVHD treatment in 180 patients who were enrolled on a national, randomized, phase II aGVHD treatment clinical trial whose initial treatment of GVHD consisted of high dose steroids plus a second immunosuppressive agent8. The aim of this secondary analysis was to determine whether time to GVHD treatment response predicts patient outcomes, especially survival.
METHODS
The patients, their GVHD characteristics and GVHD treatment for this study have all been previously described8. Briefly, patients 6 years of age or older who had undergone an allogeneic HCT in which bone marrow (BM), peripheral blood (PB), or umbilical cord blood (UCB) grafts were used and had newly diagnosed acute GVHD requiring systemic therapy were eligible for inclusion. Biopsy confirmation of GVHD was encouraged, but not required. Patients could not have received previous systemic immunosuppressive therapy for the treatment of GVHD except for a maximum 48 hours of previous steroid therapy (≥ 1 mg/kg per day methylprednisolone). Patients with uncontrolled infections, absolute neutrophil counts (ANCs) less than 500 µL, or creatinine clearances of less than 30 mL/min/1.73 m2 were excluded. Also ineligible were patients who received a donor lymphocyte infusion, unless as part of their originally planned transplant therapy (and not for persistent/recurrent disease), or patients whose clinical condition made deviation from the protocol-mandated therapy likely, including the suggested steroid taper schedule. The protocol and informed consentswere approved by the Protocol Review Committee and Data and Safety Monitoring Board of the NHLBI and the review boards of all participating institutions. All patients or their parents signed institutionally approved informed consents.
Study design
The study was a multicenter, randomized, four-arm phase II trial conducted by the Blood and Marrow Transplant Clinical Trials Network (BMT CTN). The trial was designed to evaluate the safety and efficacy of four agents, each in combination with corticosteroids. Eligible patients who had not received mycophenolate mofetil (MMF, Cellcept®) for GVHD prophylaxis within 7 days of enrollment were randomized to 1 of the 4 treatment arms consisting of MMF, etanercept, denileukin diftitox, or pentostatin, each in combination with methylprednisolone 2 mg/kg per day intravenously (or prednisone 2.5 mg/kg per day orally) in a 2:1:1:1 ratio, respectively. Patients who had receivedMMF for GVHD prophylaxis within 7 days of onset of GVHD were not randomized to MMF, but were randomized to the 3 non-MMF arms in equal proportions. This randomization scheme balanced all four arms such that approximately equal numbers of patients received each of the four study drugs. The primary endpoint on the study was the proportion of patients in complete response (all GVHD target organs staged as zero) on day 28 from start of study treatment.
Treatment of GVHD
Study treatment began within 48 hours of randomization and included corticosteroids plus one of the four study drugs. Patients received methylprednisolone 2 mg/kg per day intravenously (or prednisone 2.5 mg/kg per day orally) divided in 2 to 3 daily doses for no less than seven days. Steroids could then be tapered as tolerated to no less than methylprednisolone 0.6 mg/kg per day (prednisone 0.75 mg/kg per day) at day 28. A suggested steroid taper schedule was provided; however, adherence was optional apart from days 7 and 28 stipulations. Patients whose GVHD was progressing after 7 days, had no response by 14 days, or were not in a CR at day 28 could receive secondary therapy, but were still followed for study end points. Patients who achieved a day 28 CR and had a subsequent flare of aGVHD could receive the same or alternative agents at the treating physician's discretion or, alternatively, receive a temporary increase in the dose of steroids. Patientswere maintained on therapeutic levels of calcineurin inhibitors and received standard supportive care, including transfusions and anti-infective prophylaxis per institutional practices.
GVHD scoring and response assessment
GVHD was scored by the consensus criteria1 and reported weekly to the BMT CTN Data and Coordinating Center along with biopsy results, differential diagnosis, and GVHD therapy through week 8 and at 3, 4, 6, and 9 months after study enrollment. Complete response (CR) was defined as resolution of all signs and symptoms of GVHD in all organs without intervening salvage therapies. A partial response (PR) was defined as an improvement of one stage in one or more organs without progression in any organ. Mixed response was considered an improvement in at least one organ with progression or newly developed GVHD in another organ(s). No response was defined as absence of improvement or deterioration within 14 days of therapy initiation. Progression was defined as worsening by one or more stages without improvement in any involved organ. In patients who received secondary therapy, subsequent GVHD evaluations were classified as non-responding. Toxicity, infections, and GVHD flares (increase in symptoms or therapy for GVHD after an initial response) were recorded through day 90. GVHD-free survival was calculated by the proportion of patients in each study arm who were alive at day 56, achieved a CR and who had not experienced a GVHD-flare, chronic GVHD, or need for additional therapy. A study endpoint committee, blinded to study drug assignment, reviewed all response and endpoint data prior to analysis.
Statistical analysis
Baseline pre-transplant factors and GVHD characteristics at study entry were described and their association with response outcomes were assessed using chi-square tests or Fisher’s exact test as appropriate. Survival probabilities at 180 days and 270 days after randomization were estimated according to response status at day 14, 28, or 56 using the Kaplan-Meier estimate restricted to patients alive at each response assessment time. Probabilities of non-relapse mortality were estimated using the cumulative incidence technique. Log transformations were used to construct confidence intervals for the survival probabilities and cumulative incidence probabilities. Cox proportional hazards regression models with backwards selection were used to build prognostic factor models for mortality and non-relapse mortality. A p-value of <=0.05 was considered significant for remaining in the model. The proportionality assumption for Cox regression was tested by adding a time-dependent covariate for each risk factor and each outcome. Tests indicated that all variables met the proportional-hazards assumption. Results were expressed as relative risks (RR) of occurrence of the event. The following variables were considered in multivariate analyses: age at transplant, donor type, conditioning intensity, source of stem cells, time to onset of GVHD, grade of GVHD at study entry, and multi-organ GVHD involvement. In addition, response assessment at day 14, 28, or 56 as well as the occurrence of flare after CR were incorporated into the proportional hazards model using time-dependent covariates. Sensitivity and specificity calculations were used to assess the accuracy of GVHD response at each timepoint as a surrogate outcome for 180 day mortality.
RESULTS
Effect of baseline characteristics on response to treatment
Response rates by day 28 based on baseline characteristics (pre-transplant and at study enrollment) are shown in Table 1. Donor type, conditioning intensity, stem cell source and age did not influence the likelihood of response to initial GVHD treatment at day 28 or at the other time points analyzed (days 14 and 56). A higher proportion of patients whose GVHD developed more than 30 days from the transplant achieved a complete response within 28 days of initiation of GVHD treatment (69% vs. 55%), but this difference was not statistically significant (p=0.09).
Table 1.
Baseline characteristics (pre-transplant and at GVHD onset) by day 28 response
| Baseline characteristic | Patient number | CR, % (n=81) | PR, % (n=30) | ORR, % (n=111) | p-value |
|---|---|---|---|---|---|
| Donor type | 0.85 | ||||
| Related | 84 | 48% | 15% | 63% | |
| Unrelated | 94 | 44% | 18% | 62% | |
| Conditioning regimen | 0.66 | ||||
| Myeloablative | 116 | 45% | 16% | 61% | |
| Reduced intensity | 62 | 47% | 18% | 65% | |
| Stem cell source | 0.2 | ||||
| Peripheral blood | 109 | 44% | 14% | 58% | |
| Bone marrow | 44 | 48% | 18% | 66% | |
| Cord blood | 25 | 48% | 28% | 76% | |
| Age group | 0.84 | ||||
| <35y | 46 | 48% | 15% | 63% | |
| 35–54y | 73 | 38% | 21% | 59% | |
| ≥55y | 61 | 51% | 13% | 64% | |
| Time to onset of aGVHD | 0.09 | ||||
| <30 days | 89 | 39% | 16% | 55% | |
| >=30 | 91 | 51% | 18% | 69% | |
| GVHD grade at treatment onset | 0.05 | ||||
| 0–I | 22 | 55% | 4% | 59% | |
| II | 102 | 46% | 23% | 69% | |
| III–IV | 56 | 39% | 11% | 50% | |
| Multi-organ involvement | 0.53 | ||||
| No | 114 | 46% | 14% | 60% | |
| Yes | 66 | 44% | 21% | 65% |
Note: ORR (Object Response Rate) is CR + PR. P-value is for comparison of CR, PR, NR with risk factor. Two subjects are missing baseline information of donor type, conditioning regimen and stem cell source.
Effect of acute GVHD characteristics at study entry on outcomes
Not surprisingly, GVHD grade at initiation of treatment correlated with response to treatment. Specifically, patients with grade III/IV acute GVHD at study entry were less likely to have responded to treatment by day 28 compared to patients with less severe acute GVHD (p=0.05). Because GVHD grade represents a composite score that includes staging of each target organ, we investigated whether target organ involvement and severity (for example, GI vs. skin GVHD) predicted response to treatment. Patients with grade I GVHD have only skin involvement, while visceral involvement is a requirement for grade III GVHD. For grade II GVHD we tested whether patients with skin GVHD alone were likely to have different response rates than patients with multiorgan GVHD. As shown in Table 2, the day 28 overall response rate (ORR) for skin GVHD alone (70%) was not different than the ORR for multiorgan GVHD (67%, p=0.94). There were too few patients with grade III/IV disease to permit valid statistical comparisons based upon organ staging, but the frequencies of response are as shown.
Table 2.
Day 28 response by GVHD grade and organ at treatment onset
| GVHD grade/organ | Number | CR, % (n=81) | PR, % (n=30) | ORR, % (n=111) | p value |
|---|---|---|---|---|---|
| Grade 0/I | 22 | 55% | 4% | 59% | NA |
| Grade II | 102 | 46% | 23% | 69% | 0.94 |
| Skin alone | 59 | 46% | 24% | 70% | |
| Gut/Liver +/− skin | 43 | 47% | 21% | 67% | |
| Grade III/IV | 56 | 39% | 11% | 50% | NA |
Given the GVHD is the major cause of non-relapse mortality (NRM), we then performed a multivariate analysis using a Cox-regression model to determine which characteristics present at the time of study entry significantly impacted on NRM and overall survival (OS) over the 9 month follow-up period. Only GVHD grade at study entry had a significant impact on NRM. The relative risk of NRM was 1.72 (95% confidence interval 1.13–2.59) for patients with grade III–IV GVHD at study entry, compared to patients with GVHD grade II or lower. As shown in Table 3, using a backwards elimination regression method to identify independent factors affecting OS, GVHD grade at study entry, donor type, and stem cell source were found to significantly affect OS. After accounting for GVHD grade, organ involvement had no statistically significant impact either as a main effect or as an interaction. As noted above, this is likely because GVHD organ involvement is a major factor in determining GVHD grade.
Table3.
Cox regression model for baseline characteristics on OS
| Variable | N | OS RR |
p value |
|---|---|---|---|
| GVHD grade at onset | |||
| 0–II | 123 | 1.00 | |
| III–IV | 55 | 1.92 (1.20–3.06) | 0.006 |
| Donor Type | |||
| Related | 84 | 1.00 | |
| Unrelated | 94 | 1.80 (1.11–2.90) | 0.02 |
| Stem Cell Source | |||
| Peripheral Blood | 109 | 1.00 | |
| Bone Marrow | 44 | 0.63 (0.37–1.07) | 0.09 |
| Cord Blood | 25 | 0.38 (0.18–0.81) | 0.01 |
Effect of time to response to treatment on outcomes
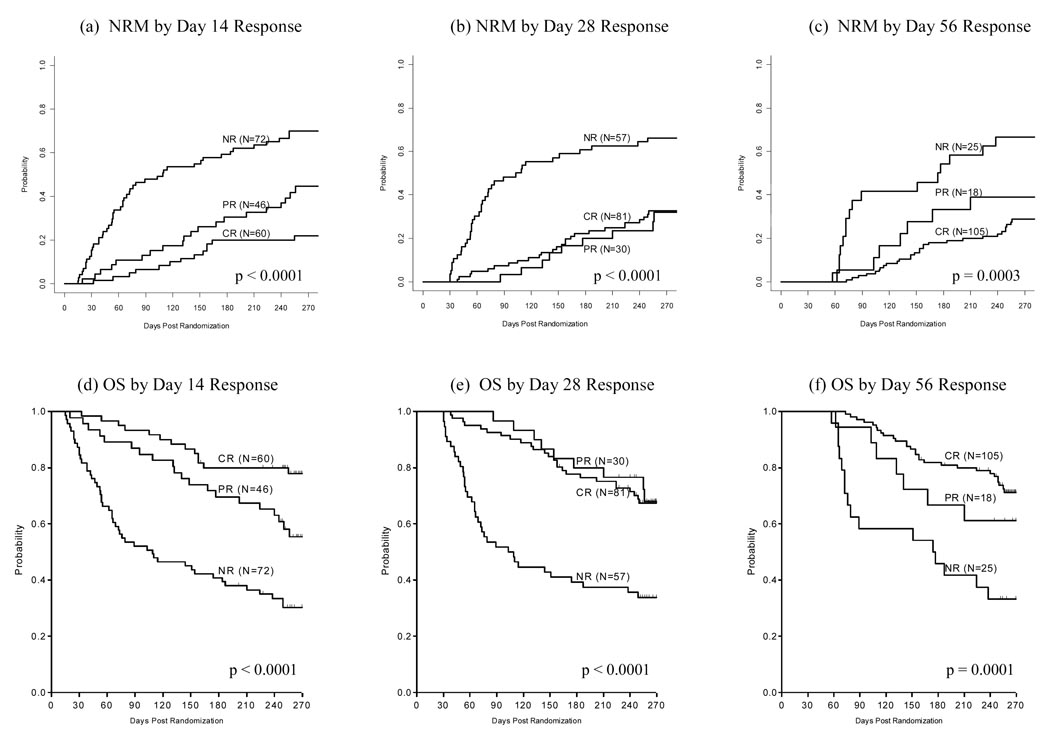
GVHD responses for survivors at each timepoint were categorized according to whether patients were in complete or partial response at day 14, day 28, and day 56. We then determined which of these categories correlated best with survival at 6 and 9 months following initiation of GVHD treatment. Survival significantly varied according to CR or PR status at day 14. However, survival was similar for both CR and PR at day 28 and day 56. The poorest 6-month survival was seen in those patients with no response (NR) at day 28 (Figure 1d–f and Table 4). Non-relapse mortality followed a similar pattern. NRM was significantly improved based on CR vs. PR at day 14, but at both day 28 and day 56, patients who achieved either CR or PR experienced similar NRM (Figure 1a–c).
Figure 1. Cumulative Incidence of Non-Relapse Mortality (NRM*) and Kaplan-Meier Curves of Overall Survival (OS) by Response Status.
FIGURE DESCRIPTION:
* overall p-value computed using Gray’s test
(a) NRM by Day 14 Response
(b) NRM by Day 28 Response
(c) NRM by Day 56 Response
(d) OS by Day 14 Response
(e) OS by Day 28 Response
(f) OS by Day 56 Response
Table 4.
Overall survival among patients alive and evaluable for response at day 14, 28, and 56
| Time Points |
Response Status |
Number alive & evaluable for response |
6 month survival (95% CI) |
9 month survival (95% CI) |
p-value by 9 month |
|---|---|---|---|---|---|
| Day 14 | 177 | ||||
| CR | 60 | 80.0% (67.4%, 88.1%) | 77.9% (65.0%, 86.6%) | <0.0001 | |
| PR | 46 | 69.6% (54.1%, 80.7%) | 55.4% (39.6%, 68.6%) | ||
| NR | 71 | 40.8% (29.4%, 51.9%) | 30.2% (19.9%, 41.2%) | ||
| Day 28 | 167 | ||||
| CR | 81 | 77.7% (67.0%, 85.3%) | 67.4% (55.9%, 76.5%) | <0.0001 | |
| PR | 30 | 80.0% (60.8%, 90.5%) | 68.0% (47.2%, 82.1%) | ||
| NR | 56 | 39.3% (26.6%, 51.7%) | 33.8% (21.8%, 46.1%) | ||
| Day 56 | 147 | ||||
| CR | 105 | 81.9% (73.1%, 88.0%) | 71.2% (61.2%, 79.1%) | 0.0001 | |
| PR | 18 | 66.7% (40.4%, 83.4%) | 61.1% (35.3%, 79.2%) | ||
| NR | 24 | 45.8% (25.6%, 64.0%) | 33.3% (15.9%, 51.9%) |
p-value computed using log-rank test
We also determined the sensitivity and specificity for responses at day 14, day 28, and day 56 to predict survival. Sensitivity was defined as the proportion of patients who did not respond to treatment among those who died by 6 months. As shown in Table 5, patients who did not achieve CR by day 14 accounted for the highest proportion of deaths (83%) within 6 months of initiating GVHD treatment. Specificity was defined as the proportion of patients who responded to treatment among those who were alive at 6 months. Complete plus partial (CR+PR) response at every time point was a better predictor for 6 month survival than complete response alone, particularly showing greater specificity for predicting survival. Not surprisingly, specificity improves when likelihood of survival at 6 months is analyzed at time points closer to 6 months.
Table 5.
Sensitivity and Specificity statistics for various response outcomes at day 14, 28, and 56 with survival status at 6 months
| Time Points | Response Status |
Dead at 6 months |
Alive at 6 months |
Sensitivity | Specificity |
|---|---|---|---|---|---|
| Day 14 | Not CR | 58 | 61 | 83% | 44% |
| CR | 12 | 47 | |||
| Day 28 | Not CR | 52 | 46 | 74% | 57% |
| CR | 18 | 62 | |||
| Day 56 | Not CR | 51 | 23 | 73% | 79% |
| CR | 19 | 85 | |||
| Day 14 | Not CR+PR | 44 | 29 | 63% | 73% |
| CR+PR | 26 | 79 | |||
| Day 28 | Not CR+PR | 46 | 22 | 66% | 80% |
| CR+PR | 24 | 86 | |||
| Day 56 | Not CR+PR | 45 | 11 | 64% | 90% |
| CR+PR | 25 | 97 |
In order to further interpret these early response findings as predictors of later outcome, we analyzed the effect of response to treatment on NRM and OS after adjusting for baseline characteristics. As shown in Table 6, patients who did not achieve PR or CR at each time point were at significantly greater risk for NRM and death by 9 months. Patients with either CR or PR at day 28 had almost identical long term outcomes. Altogether, these analyses indicate that either CR or PR (versus no response) to GVHD treatment at day 28 is the best surrogate for later outcomes, such as NRM and survival.
Table 6.
Cox Regression Model on NRM and OS including effect of response assessment at day 14, 28, 56, adjusted for baseline characteristics from Table 3.
| NRM | OS | |||||
|---|---|---|---|---|---|---|
| Time Points | Response Status |
n | RR | p-value | RR | p-value |
| Day 14 | CR | 60 | 1.00 | 1.00 | ||
| PR | 46 | 2.09 (1.12–3.88) | 0.02 | 2.00 (1.03–3.90) | 0.04 | |
| NR | 71 | 4.04 (2.32–7.02) | <0.001 | 4.36 (2.42–7.83) | <0.001 | |
| Day 28 | CR | 81 | 1.00 | |||
| PR | 30 | 0.99 (0.52–1.89) | 0.98 | 0.96 (0.48–1.94) | 0.91 | |
| NR | 56 | 2.32 (1.44–3.73) | <0.001 | 2.79(1.71–4.55) | <0.001 | |
| Day 56 | CR | 105 | 1.00 | 1.00 | ||
| PR | 18 | 1.16 (0.54–2.51) | 0.698 | 1.31 (0.60–2.85) | 0.50 | |
| NR | 24 | 1.65 (1.09–2.50) | 0.004 | 4.27 (2.28–7.98) | <0.001 | |
We also considered whether a GVHD flare (return of GVHD symptoms in patients who had previously achieved a CR) influenced these findings. In a multivariate analysis, a flare after an earlier CR did not increase the risk of NRM (RR=1.18 [95% CI 0.66–2.13], p=0.58). Finally, we performed a multivariate analysis to determine whether response assessed at day 14, 28, or 56 correlated with risk of developing chronic GVHD. There was no significant effect of response status on cGVHD, using the Cox proportional hazards model.
DISCUSSION
One challenge in GVHD treatment is determining the key predictive factors for outcomes; particularly early findings which predict later outcomes. In this study of 180 patients newly diagnosed with GVHD who were prospectively treated with systemic steroids plus a second agent, treatment response at multiple time points were highly predictive of NRM and survival, even after adjustment for pre-transplant characteristics. As previously reported, the study was not designed to test for statistically significant differences in response rates or long-term outcomes between the four different agents added to high dose systemic steroid therapy8, therefore, the distinction between the second agents was not further pursued as a variable in this analysis.
The relationship between GVHD grade and long-term outcomes is complex and imperfect9. It is well established that as GVHD severity increases, mortality increases, even though the correlation is not strictly linear6. Other factors that predict outcome have been less well characterized. Time to GVHD response might be an important predictor of outcome, but the incorporation of this variable in clinical trial design and interpretation requires validation10. The three response timepoints tested all showed utility as surrogate markers for later outcomes. In this analysis, we identified overall response rate (CR + PR) at day 28 being most predictive of both 9 month non-relapse mortality and survival. In addition, patients who failed to achieve any response by day 14 experienced high mortality rates. Given the steep decrease in survival for patients with no response by day 14, the day 14 response endpoint may have value for determining which patients are unlikely to realize benefit from agents under study and may require additional intervention.
Interestingly, pre-transplant characteristics that categorize patients for risk of developing GVHD, such as donor type (related vs. unrelated), age, and conditioning intensity did not predict for response to GVHD treatment. Identification of predictors for GVHD responsiveness (or lack thereof) will be important for future clinical trials. Recent GVHD biomarker studies provide hope that advances along these lines may be forthcoming11–13.
These survival analyses, together with the sensitivity and specificity calculations, highlight that the probability of long-term survival depends only in part on response to GVHD treatment. Many patients die despite achieving GVHD response, often due to opportunistic infections14,15 and/or chronic GVHD16,17. Nonetheless, the overall complete plus partial response rate at day 28 to GVHD treatment appears to function well as a surrogate endpoint for clinical benefit, namely, long-term survival. Importantly, patients with active, but responding GVHD (PR) by day 28 contribute to long-term survival rates. Many of these patients eventually achieve CR, highlighting the importance of responding, rather than complete response, by day 28.
A similar analysis of a large cohort of patients (n=864) was recently reported from the University of Minnesota. The Minnesota study population differs from the BMT CTN study population in several relevant ways. These differences include a younger population all treated at a single center over a 17-year time period with a disproportionate representation of unrelated cord blood as a stem cell source. Therefore, it is reassuring that the Minnesota analysis also found that the day 28 response to GVHD therapy correlated best with long-term outcomes such as 2 year NRM18.
This analysis, which is derived from a prospective multicenter trial of initial aGVHD treatment, is strengthened by the fact that GVHD grading was done prospectively and GVHD scores and responses were confirmed by an independent blinded GVHD grading panel. However, its conclusions may be limited by the modest sample size and the smaller numbers of patients with severe aGVHD. It should be noted that the response rates on this study were higher than those historically reported with steroids alone5. Although these results are concordant with the large single center results recently reported18, applicability of findings in this analysis to patients treated with steroids alone, second line therapy, or to cohorts with more severe aGVHD, will need further testing.
ACKNOWLEDGMENTS
This work was supported in part by the National Heart, Lung, and Blood Institute and the National Cancer Institute. The authors gratefully acknowledge the participating centers and co-investigators for the BMT CTN 0302 study: MD Anderson Cancer Research Center (Amin M. Alousi, M.D.), Johns Hopkins University (Javier Bolaños-Meade, M.D. and Georgia Vogelsang, M.D.), University of Minnesota (Daniel Weisdorf, M.D.), Dana Farber Brigham Women’s Partners (Vincent Ho, M.D., Robert Soiffer, M.D., and Joseph Antin, M.D.), University of Michigan (John Levine, M.D., M.S. and James L. Ferrara, M.D.), Oregon Health Sciences University (Eneida Nemecek, M.D.), University of Florida College of Medicine (John Wingard, M.D.), University of Pennsylvania Hospital Center (Steven Goldstein, M.D., and Edward Stadtmauer, M.D.), University of Nebraska Medical Center (Marcel Devetten, M.D.), Washington University of St. Louis (John DiPersio, M.D., and Peter Westervelt, M.D.), Stanford Hospital and Clinics (Laura Johnston, M.D.), University of California at San Diego (Edward Ball, M.D.), Duke University Medical Center (Nelson Chao, M.D., and Joanne Kurtzberg, M.D.), University Hospitals of Cleveland (CWRU) (Hillard Lazarus, M.D.), Memorial Sloan-Kettering Cancer Center (Nancy Kernan, M.D., and Miguel-Angel Perales, M.D.), Texas Transplant Institute (Carlos Bachier, M.D., Michael Grimley, M.D., and Paul Shaughnessy, M.D.), City of Hope National Medical Center (Pablo Parker, M.D.), Fred Hutchinson Cancer Research Center (Richard Nash, M.D.), and Hackensack University Medical Center (Joel Brochstein, M.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors report no relevant financial disclosures.
REFERENCES
- 1.Cahn JY, Klein JP, Lee SJ, et al. Prospective evaluation of 2 acute graft-versus-host (GVHD) grading systems: A joint Societe Francaise de Greffe de Moelle et Therapie Cellulaire (SFGM-TC), Dana Farber Cancer Institute (DFCI), and International Bone Marrow Transplant Registry (IBMTR) prospective study. Blood. 2005;106:1495–1500. doi: 10.1182/blood-2004-11-4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ram R, Gafter-Gvili A, Yeshurun M, Paul M, Raanani P, Shpilberg O. Prophylaxis regimens for GVHD: systematic review and meta-analysis. Bone Marrow Transplant. 2009;43:643–653. doi: 10.1038/bmt.2008.373. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chao NJ, Chen BJ. Prophylaxis and treatment of acute graft-versus-host disease. Semin Hematol. 2006;43:32–41. doi: 10.1053/j.seminhematol.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 5.MacMillan ML, Weisdorf DJ, Wagner JE, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transpl. 2002;8:387–394. doi: 10.1053/bbmt.2002.v8.pm12171485. [DOI] [PubMed] [Google Scholar]
- 6.Pasquini MC. Impact of graft-versus-host disease on survival. Best Pract Res Clin Haematol. 2008;21:193–204. doi: 10.1016/j.beha.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Antin JH, Chen AR, Couriel DR, Ho VT, Nash RA, Weisdorf D. Novel approaches to the therapy of steroid-resistant acute graft-versus-host disease. Biol Blood Marrow Transplant. 2004;10:655–668. doi: 10.1016/j.bbmt.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Alousi AM, Weisdorf DJ, Logan BR, et al. Etanercept, mycophenolate, denileukin or pentostatin plus corticosteroids for acute graft vs. host disease: a randomized phase II trial from the BMT CTN. Blood. 2009;114:511–517. doi: 10.1182/blood-2009-03-212290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leisenring WM, Martin PJ, Petersdorf EW, et al. An acute graft-versus-host disease activity index to predict survival after hematopoietic cell transplantation with myeloablative conditioning regimens. Blood. 2006;108:749–755. doi: 10.1182/blood-2006-01-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin PJ. Study design and endpoints in graft-versus-host disease. Best Pract Res Clin Haematol. 2008;21:357–372. doi: 10.1016/j.beha.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paczesny S, Krijanovski OI, Braun TM, et al. A biomarker panel for acute graft-versus-host disease. Blood. 2009;113:273–278. doi: 10.1182/blood-2008-07-167098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paczesny S, Braun T, Levine JE, et al. Elafin is a biomarker of graft-versus-host disease of the skin. Science Translational Medicine. 2010;2:50–57. doi: 10.1126/scitranslmed.3000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fondi C, Nozzoli C, Benemei S, et al. Increase in FOXP3+ regulatory T cells in GVHD skin biopsies is associated with lower disease severity and treatment response. Biol Blood Marrow Transplant. 2009;15:938–947. doi: 10.1016/j.bbmt.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Wingard JR. Fungal infections after bone marrow transplant. Biol Blood Marrow Transplant. 1999;5:55–68. doi: 10.1053/bbmt.1999.v5.pm10371357. [DOI] [PubMed] [Google Scholar]
- 15.Ljungman P, Perez-Bercoff L, Jonsson J, et al. Risk factors for the development of cytomegalovirus disease after allogeneic stem cell transplantation. Haematologica. 2006;91:78–83. [PubMed] [Google Scholar]
- 16.Martin PJ, Carpenter PA, Sanders JE, Flowers ME. Diagnosis and clinical management of chronic graft-versus-host disease. Int J Hematol. 2004;79:221–228. doi: 10.1532/ijh97.03176. [DOI] [PubMed] [Google Scholar]
- 17.Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9:215–233. doi: 10.1053/bbmt.2003.50026. [DOI] [PubMed] [Google Scholar]
- 18.Macmillan ML, Defor TE, Weisdorf DJ. The best endpoint for acute GVHD treatment trials. Blood. doi: 10.1182/blood-2009-12-258442. prepublished online April 13, 2010; DOI 101182/blood-2009-12-258442. [DOI] [PubMed] [Google Scholar]