Abstract
Discrete fetal androgen excess during early gestation in rhesus monkeys (Macaca mulatta) promotes endocrine antecedents of adult polycystic ovary syndrome (PCOS)-like traits in female offspring. Because developmental changes promoting such PCOS-like metabolic dysfunction remain unclear, the present study examined time-mated, gravid rhesus monkeys with female fetuses, of which nine gravid females received 15 mg of testosterone propionate (TP) subcutaneously daily from 40 to 80 days (first to second trimesters) of gestation [term, mean (range): 165 (155–175) days], whereas an additional six such females received oil vehicle injections over the same time interval. During gestation, ultrasonography quantified fetal growth measures and was used as an adjunct for fetal blood collections. At term, all fetuses were delivered by cesarean section for postnatal studies. Blood samples were collected from dams and infants for glucose, insulin, and total free fatty acid (FFA) determinations. TP injections transiently accelerated maternal weight gain in dams, very modestly increased head diameter of prenatally androgenized (PA) fetuses, and modestly increased weight gain in infancy compared with concurrent controls. Mild to moderate glucose intolerance, with increased area-under-the-curve circulating insulin values, occurred in TP-injected dams during an intravenous glucose tolerance test in the early second trimester. Moreover, reduced circulating FFA levels occurred in PA fetuses during a third trimester intravenous glucagon-tolbutamide challenge (140 days gestation), whereas excessive insulin sensitivity and increased insulin secretion relative to insulin sensitivity occurred in PA infants during an intravenous glucose-tolbutamide test at ∼1.5 mo postnatal age. Data from these studies suggest that experimentally induced fetal androgen excess may result in transient hyperglycemic episodes in the intrauterine environment that are sufficient to induce relative increases in pancreatic function in PA infants, suggesting in this nonhuman primate model that differential programming of insulin action and secretion may precede adult metabolic dysfunction.
Keywords: fetal programming, polycystic ovary syndrome, hyperglycemic pregnancy, metabolic syndrome
reproductive and metabolic traits reminiscent of polycystic ovary syndrome (PCOS) are prevalent in a wide variety of female mammals exposed to testosterone excess during gestation (5, 28). Reproductive PCOS-like traits found in prenatally androgenized (PA) nonhuman females include combinations of hyperandrogenism, intermittent or absent ovulatory cycles, and polycystic ovaries, traits that comprise the diagnostic criteria required for PCOS in women (4). Metabolic PCOS-like traits in animal models of fetal androgen excess, moreover, are diverse and can include hyperinsulinemia, insulin resistance, pancreatic β-cell defects, increased incidence of type 2 diabetes, enhanced adiposity, and hyperlipidemia [monkeys (1), sheep (58), and rats (25)]. Such heterogeneity of PCOS-like trait expression in PA females resembles that found in women with PCOS (4, 5), providing a novel perspective into potential developmental origins of PCOS, particularly with regard to metabolic phenotype (33).
Experimentally induced PCOS-like traits in PA monkeys and sheep allow investigation of fetal origins (3, 32), providing insight into how altered developmental trajectories and mechanistic derangements can precede adult PCOS-like traits in these animal models. Understanding prepubertal expression of PCOS-like traits is important, since daughters of women with PCOS manifest PCOS-like antecedents, such as ovarian hormone hypersecretion, as early as 2 mo of age (63) and increased ovarian volume and hyperinsulinemia before or during pubertal progression (24, 45, 64). In this regard, endocrine and ovarian antecedents of adult PCOS-like traits exist in PA female offspring in both monkeys and sheep beginning in late fetal/early neonatal development (2, 72), presumably because female fetuses exposed to fetal male levels of testosterone (or its androgenic or estrogenic metabolites) develop permanent epigenetic and pathophysiological changes in endocrine and reproductive target tissues. Although little attention has focused on the physiological responses of pregnant dams to exogenous testosterone administration and their effects on female fetal development, maternal metabolic dysfunction, particularly from hyperglycemia (18–19, 35–37), may reprogram fetal and subsequent postnatal metabolic traits of offspring from PA females.
Insulin resistance can be induced in humans by administrating testosterone to regularly menstruating women for 10–12 days, (27), to reproductive-aged female and male transsexuals for 4 mo (56), or to postmenopausal women for 3 mo (74). In the latter treated group of women, androgen rather than estrogen receptor-mediated action has been implicated in insulin resistance, since estrogen administration does not impair insulin action (74). Such androgen specificity also occurs in a fetal androgen excess sheep model for PCOS in which gestational treatment of dams with the nonaromatizable androgen, dihydrotestosterone, induces comparable insulin resistance in exposed female offspring to that found in females exposed to testosterone excess during gestation (51). In vitro androgen specificity also exists as androgen receptor blockade prevents testosterone-induced insulin resistance in human subcutaneous adipocytes (21).
Testosterone treatment of gravid rhesus monkeys, therefore, may induce maternal insulin resistance in addition to elevating circulating androgen levels in both maternal and fetal compartments (2). Because pregnancy-induced insulin resistance in primates [female rhesus monkeys (43) and women (66)] may be exacerbated by androgen excess, as evident by hyperandrogenic PCOS women being at risk for gestational diabetes (13), testosterone treatment of rhesus monkey dams may expose their female offspring to hyperglycemia (4, 25, 51). Understanding the development of metabolic PCOS-like traits in PA female monkeys, therefore, is crucial since maternal hyperglycemia is readily conveyed to the fetus via transplacental transport, causing phenotypic changes in both the exposed human fetus (hyperinsulinemia and macrosomia) and subsequent neonate (hypoglycemia and increased adiposity) (35, 36).
The present study, therefore, examines whether discrete, early gestation testosterone treatment of rhesus monkey dams causes predictable metabolic antecedents of PCOS-like traits in PA female offspring, such as insulin resistance [as found in young PA female sheep and rats (25, 58)] and failure of pancreatic β-cells to appropriately compensate for insulin resistance (β-cell defect). This study also examines whether gestational testosterone treatment disrupts maternal glucoregulation and whether preconception maternal body weight exaggerates any glucoregulatory perturbation. Evidence for testosterone inducing any form of hyperglycemia during pregnancy, with maternal metabolic dysfunction linked to a postnatal metabolic phenotype in PA female offspring, would support the proposed “second hit” on the female fetus (3, 4), in which relative hyperinsulinemia induced by fetal exposure programs postnatal metabolic dysfunction (26). This metabolic second hit, coexisting with the direct effects of testosterone (or its metabolites) on fetal and postnatal development (i.e., “first hit”), could cause insulin-amplified reproductive dysfunction, as found in PA adult female monkeys (76) and sheep (67). Such implications for fetal origins of metabolic (and reproductive) phenotypes in adult female PA monkeys also may apply to the pathogenesis of PCOS in women (26).
MATERIALS AND METHODS
Animals and Fetal Programming
Animal procedures conformed to the requirements of the Animal Welfare Act, and animal protocols were approved before implementation by the Institutional Animal Care and Use Committee at the University of California, Davis. Fifteen adult, nonprimiparous, pregnant female rhesus monkeys, housed at the California National Primate Research Center, were selected for study, based upon the absence of Y-chromosomal DNA in their peripheral circulation (39) at 30 days of gestation (range: 28–32 days), confirming a female fetus. Between 40 and 80 days of gestation (late first to midsecond trimester), gravid animals received daily subcutaneous injections of either 15 mg testosterone propionate (TP, n = 9) or vehicle (100–200 μl sesame oil; control, n = 7; Fig. 1). Such maternal TP treatment has been shown to induce fetal androgen excess in PA females equivalent to that found in fetal males and programs for a PCOS phenotype in adulthood (1, 2, 60).
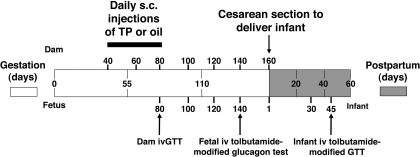
Fig. 1.
Diagrammatic representation of the experimental design, illustrating days of maternal subcutaneous injections [15 mg testosterone propionate (TP): n = 9; oil: n = 6] during 40–80 days gestation; days of blood sample collection in dams, fetuses, and infants; timing of cesarean section delivery; and dam, fetal, and infant tests of glucoregulation [dam: iv glucose tolerance test (ivGTT); fetus: iv tolbutamide-modified glucagon test; infant: iv tolbutamide-modified glucose test].
As previously reported (2), before conception, adult females in TP and control groups had similar ages [TP: 11.2 ± 1.5 (SE) yr; control: 8.4 ± 2.0 (SE) yr] and body weights (TP: 7.0 ± 0.4 kg; control: 6.8 ± 0.5 kg). At term, newborns were delivered by cesarean section from dams of comparable term body weights (TP: 8.5 ± 0.4 kg; control: 8.1 ± 0.5 kg). Routine newborn assessments, including body weights and crown-rump lengths (see Fig. 3), and gross appearance of the placenta, were unremarkable and similar between the groups. Infants were nursery reared under standard operating procedures for housing conditions and diet for animals in this age group (71). One control dam and the associated offspring were omitted from the data analyses because they were identified as outliers [by extreme studentized deviate test] for elevated maternal/fetal (P < 0.05/P < 0.01) basal serum glucose levels in the third trimester and elevated (138 mg/dl) infant (P < 0.05) basal serum glucose levels at 2 mo postnatal age. The birth weight of this latter infant (546 g) was ∼23% greater than the mean of remaining controls (445 ± 19 g), although within the normative weight range for newborn rhesus monkeys.
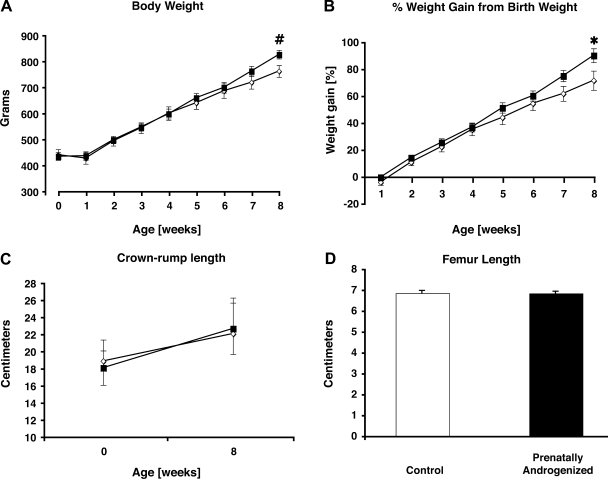
Fig. 3.
Mean ± SE body weight (A), %birth weight (B), crown-rump length (C), and femur length (D) in 5 concurrent control (◊) and 9 prenatally androgenized (PA) (■) female infants. #P < 0.04 and *P < 0.05 vs. control infants at 8 wk postnatal age.
Morphometrics
Crown-rump and femur lengths were assessed at birth and at the time of tissue harvest (∼2 mo postnatal age) using standardized methods, and placental weights were assessed at delivery (71). Infants were weighed daily or weekly during the study period until the endpoint of ∼2 mo postnatal age. Organ weights were assessed at tissue harvest (71).
Glucoregulatory Assessment
Dams.
After an overnight fast, maternal blood was collected from a peripheral vessel under telazol (5–8 mg/kg im) or ketamine (10 mg/kg im) approximately every 3 wk from 40 days of gestation (first trimester) through term. A modified intravenous glucose tolerance test (ivGTT) was also performed at 80 days of gestation. Glucose (300 mg/kg at 0 min) was administered intravenously, and blood samples were collected at select time points via a peripheral vessel. Insulin sensitivity (SI) was determined using the modified minimal model method (9). Further measures obtained from the ivGTT were basal (−5 min relative to glucose infusion) circulating levels of insulin (basal insulin), glucose (basal glucose), and total free (nonesterified) fatty acids (basal FFA); acute insulin responses to glucose (elevation of posthepatic plasma insulin concentration above baseline at 5 min); glucose disappearance rate (slope of the log linear regression of plasma glucose concentration between 10 and 20 min); and disposition index (β-cell compensation index; product of SI and acute insulin responses to glucose) (42). Area under the curve for glucose (AUCglucose), insulin (AUCinsulin), and FFA (AUCFFA) were determined using the trapezoidal rule. The validity of using such limited numbers of time points to determine dam (and infant monkey, see below) SI, and the derived measure of disposition index, were validated by correlating SI and disposition index values calculated from a 180-min ivGTT performed on 12 normal adult female rhesus monkeys with SI and disposition index values calculated from the same adult ivGTTs, but only including the time points used in the current study. SI values derived from the reduced number of time points, −5 to 60 min (dam) or 45 min (infant), were positively correlated with those SI values obtained using all time points across the 180-min ivGTT (r2 values, 0.47–0.55; P values, 0.01–0.006, respectively; slopes and y-axis intercepts did not differ from 1 and 0, respectively, P values >0.10). Disposition index comparisons were similarly correlated (r2 values, 0.52–0.71; P values, 0.005–0.001, respectively; slopes and y-axis intercepts did not differ from 1 and 0, respectively, P values >0.40).
Fetuses.
Fetal blood samples for basal glucose and insulin determinations were collected when the dams were sedated for ultrasound-guided fetal cardiocentesis at 80, 100, 120, and 140 days of gestation using established techniques (69). Fetal blood samples were also obtained at −5, 5, 15, 25, 35, and 45 min after a direct injection of 20 mg glucagon in the fetal circulation at 0 min at 140 days gestation (third trimester). Tolbutamide (20 mg/kg) was also injected directly in the fetal circulation 20 min following the glucagon injection (portal vein). The following measures were derived from this glucagon-tolbutamide test: basal insulin, glucose, and FFA; acute fetal insulin responses to glucagon (fetal insulin concentration above baseline at 5 min) and to tolbutamide (fetal insulin concentration at 25 min above 15 min value); as well as AUCglucose, AUCinsulin, and AUCFFA.
Infants.
A modified ivGTT was performed in overnight-fasted infants on postnatal day 45 (0.5 g glucose/kg iv at 0 min, tolbutamide 20 mg/kg iv at 20 min; blood samples taken at −5, 5, 15, 25, 35, and 45 min). SI was determined using the modified minimal model method (9). Further measures derived from the ivGTT were basal insulin, glucose, and FFA; acute insulin responses to both glucose and tolbutamide; disposition index; AUCglucose; AUCinsulin; and AUCFFA.
Statistical Analyses
Glucoregulatory parameters not normally distributed were log transformed to achieve homogeneity of variance and to increase linearity. Treatment group comparisons were conducted using Student's t-test or repeated-measures-design ANOVA (version 7.0; Systat, Evanston, IL). Log-transformed glucoregulatory or morphometric parameters not normally distributed were analyzed using nonparametric statistics. A P value of <0.05 was considered significant. All log-transformed parameters are expressed as the antilog of the transformed means (95% confidence limit), nonnormal data are expressed as the median (interquartile range), and all other data are shown as means ± SE.
RESULTS
Morphometrics
Dams.
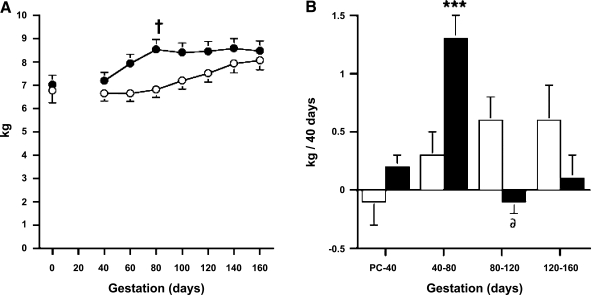
While pregnancy induced the expected increase in weight gain, dams receiving TP injections exhibited accelerated body weight gain when compared with concurrent controls (Fig. 2). The body weights of TP-injected dams exceeded those of control dams by ∼25% at 80 days of gestation (P < 0.029). TP-injected dams maintained this body weight until term delivery and did not differ from gestational controls in terms of body weight in the second half of gestation. This difference in the temporal progression of body weight represented an over fourfold weight gain in TP-injected dams vs. controls during TP treatment (P < 0.001, Fig. 2), although similar rates of weight gain occurred in both groups before and after TP treatment, except for a transiently diminished (P < 0.015) weight gain for 40 days immediately after TP injections (80–120 days of gestation; Fig. 2). During this latter time interval, TP-injected dams lost an average of 0.08 kg/40 days, whereas control dam body weight increased by an average of 0.60 kg/40 days.
Fig. 2.
Mean ± SE body weight (A) and rate of weight gain (B) during pregnancy in 5 oil (○)- and 9 TP-injected (●) dams. †P < 0.03 vs. control dams at 80 days gestation. ***P < 0.001 vs. control dams at 40–80 days gestation. δP < 0.015 vs. control dams at 80–120 days gestation.
Fetuses.
Normal trends in fetal growth were observed across gestation in both groups. Ultrasound biometrics included biparietal diameter and femur length and were within the normative range at all time points assessed compared with historical controls (n ∼ 150) (70), although PA females exhibited an overall increase in fetal head size compared with concurrent control female fetuses (P < 0.043, Table 1).
Table 1.
Fetal biparietal diameter and femur length in five concurrent control and nine PA female rhesus monkeys determined from digitized caliper measurement during transabdominal ultrasonography of oil- or TP-injected gravid dams, respectively
| Biparietal Diameter, mm |
Femur Length, mm |
|||
|---|---|---|---|---|
| Gestational Age, days | Control | PA* | Control | PA |
| 60 | 17.0 ± 0.4 | 17.9 ± 0.3 | 5.2 ± 0.3 | 6.0 ± 0.2 |
| 80 | 27.0 ± 0.5 | 27.9 ± 0.4 | 15.4 ± 0.6 | 15.3 ± 0.4 |
| 100 | 34.0 ± 0.6 | 35.9 ± 0.4 | 23.8 ± 0.7 | 23.4 ± 0.5 |
| 120 | 40.6 ± 0.6 | 41.6 ± 0.5 | 31.2 ± 1.0 | 33.1 ± 0.7 |
| 140 | 45.6 ± 0.7 | 46.0 ± 0.5 | 39.0 ± 0.9 | 39.2 ± 0.6 |
Data are means ± SE. PA, prenatally androgenized.
P < 0.043 vs. control females, overall type of female effect across all days of gestation.
Infants.
As previously reported, PA female monkeys had birth weights within the normative range when compared with historical controls (n ∼ 130) (70) at delivery by cesarean section (Fig. 3). PA female infants, however, demonstrated approximately a 10% increase in body weight compared with concurrent controls by 8 wk postnatal age, both by absolute weight and percent birth weight (type of female × age interactions: P < 0.005 and P < 0.016, respectively). Crown-rump lengths at delivery by cesarean section and at 9 wk of postnatal age were similar between control and PA infants (Fig. 3), as were femur lengths at 9 wk postnatal age (Fig. 3). The body mass index of infants, defined as the body weight relative to crown-rump length, did not differ significantly between female infant groups (cesarean section: control, 11.9 ± 0.4 kg/m2 and PA, 12.2 ± 0.3 kg/m2, P = 0.55; 8 wk postnatal age: control, 15.2 ± 0.4 kg/m2 and PA 15.8 ± 0.3 kg/m2, P = 0.24). Organ weights at tissue harvest, corrected for body weight, also were similar between female infant groups at 8 wk postnatal age (Table 2).
Table 2.
Infant organ weights corrected for infant body weight in five concurrent control and nine PA female rhesus monkeys determined at tissue harvest at 9 wk postnatal age
| Organ Wt/Infant Body Wt, mg/kg |
||
|---|---|---|
| Organ | Control | PA |
| Liver | 21.8 ± 1.3 | 24.2 ± 1.0 |
| Spleen | 1.7 ± 1.0 | 1.7 ± 1.0 |
| Thymus | 4.5 ± 0.5 | 4.7 ± 0.3 |
| Right adrenal | 0.2 ± 0.1 | 0.2 ± 0.1 |
| Left adrenal | 0.3 ± 0.1 | 0.2 ± 0.1 |
| Right kidney | 2.9 ± 0.1 | 2.7 ± 0.1 |
| Left kidney | 2.9 ± 0.1 | 2.6 ± 0.1 |
| Right ovary (×10−1) | 0.6 ± 0.1 | 0.5 ± 0.1 |
| Left ovary (×10−1) | 0.6 ± 0.1 | 0.6 ± 0.1 |
Data are means ± SE.
Glucoregulation
Dams.
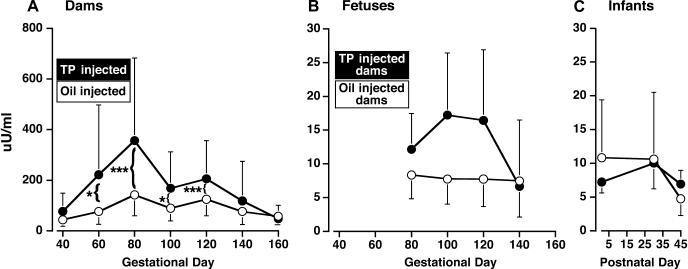
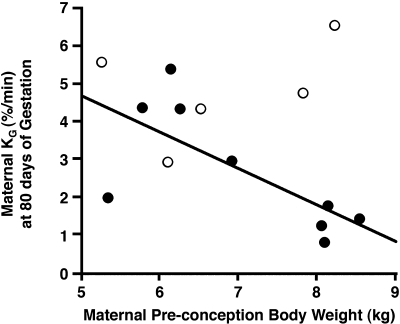
Basal (overnight-fasted) circulating glucose and insulin levels did not differ between oil- and TP-injected dams between 40 days gestation and term (Figs. 4 and 5). In both groups combined, basal glucose levels did not vary across this time period (time effect, P < 0.70), whereas between 60 and 120 days gestation (P < 0.018–0.001) basal insulin levels increased above the values at study initiation (40 days gestation; time effect, P < 0.001). Gestational peak values in maternal basal insulin levels occurred at a similar median of 80 days of gestation in both groups of dams, with basal insulin levels in TP-injected dams tending (P < 0.067) to be ∼130% higher [379.3 (190.2, 756.6) μU/ml; backtransformed mean (95% confidence limits)] than those in controls [164.8 (108.8, 279.6) μU/ml]. Maternal basal insulin levels only returned to early gestation values at 140 days gestation (third trimester). During the ivGTT (80 days of gestation), glucose disappearance rate diminished by ∼60% (P ≤ 0.03) and AUCglucose tended (P ≤ 0.057) to increase by ∼20% in TP-injected dams (Table 3), indicating impaired glucose tolerance in the latter dams despite elevated AUCinsulin values (P ≤ 0.019). There were no significant differences between oil- and TP-injected dams with respect to SI, disposition index, and acute insulin response to glucose (Table 3). Glucose disappearance rate was negatively correlated (r2 = 0.50, P < 0.033) with dam preconception body weight in TP- but not oil-injected dams (Fig. 6), whereas none of the other glucose or insulin parameters was correlated with glucose disappearance in either group of dams. There were no between-group differences in maternal FFA parameters during the ivGTT (Table 3) or basal FFA levels during gestation (data not shown).
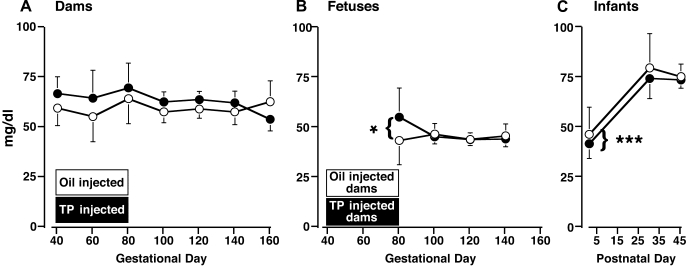
Fig. 4.
Mean (95% confidence limits) of basal circulating glucose levels in 5 concurrent control (○) and 9 TP-exposed (●) dams between 40 and 160 days gestation (A), fetuses between 40 and 140 days gestation (B), and infants between postnatal days 1 and 45 (C). *P < 0.025 vs. 100, 120, and 140 days gestation, both female fetal groups combined. The duration of gestational treatment is indicated by the open (control) and filled (TP-treated) boxes designating the female groups. ***P < 0.001 vs. postnatal days 30 and 45, both female infant groups combined.
Fig. 5.
Mean (95% confidence limits) of basal circulating insulin levels in 5 concurrent control (○) and 9 TP-exposed (●) dams between 40 and 160 days gestation (A), fetuses between 40 and 140 days gestation (B), and infants between postnatal days 1 and 45 (C). The duration of gestational treatment is indicated by the open (control) and filled (TP-treated) boxes designating the female groups. *P < 0.018–0.01 and ***P < 0.001 vs. 40 days of gestation, both groups of dams combined.
Table 3.
Circulating insulin and glucose parameters during an ivGTT performed on oil- or TP-injected dams at 80 days of gestation, an iv glucagon-tolbutamide test performed on control or PA female fetuses at 140 days of gestation, and an iv glucose-tolbutamide test performed on control and PA female infants at postnatal day 45
| TP-Injected Dam (n = 9) | |||
|---|---|---|---|
| Female and Parameter | Control (n = 5) | PA Fetus or Infant (n = 9) | P Value |
| Dam at 80 days of gestation | |||
| Basal glucose,* mg/dl | 63.1 (53.6, 66.5) | 64.6 (56.7, 106.2) | 0.3 |
| Basal insulin,†μU/ml | 89.7 (39.1, 205.9) | 231.2 (120.2, 444.8) | 0.1 |
| SI† | 4.2 (0.7, 23.9) | 1.3 (0.4, 4.8) | 0.3 |
| DI, min−1 | 10.3 ± 3.3 | 8.2 ± 2.4 | 0.6 |
| KG, %/min | 4.8 ± 0.7 | 2.7 ± 0.5 | 0.03 |
| AIRg5min, μU/ml | 265 ± 83 | 180 ± 62 | 0.5 |
| AUCglucose,* mg·dl−1·60 min−1 | 4,110 (3,190, 4,478) | 4,831 (3,953, 8,418) | 0.057 |
| AUCinsulin,*μU·ml−1·60 min−1 | 5,479 (4,977, 6,970) | 15,076 (6,440, 36,383) | 0.019 |
| Basal free fatty acids,†μM·l−1·60 min−1 | 325 (118, 897) | 452 (212, 964) | 0.6 |
| AUC free fatty acids,†μM·l−1·60 min−1 | 25,704 (11,053, 59,774) | 28,840 (15,402, 54,005) | 0.8 |
| Fetus at 140 days of gestation | |||
| Basal glucose,† mg/dl | 44.4 (39.6, 49.7) | 43.2 (39.7, 47.1) | 0.7 |
| Basal insulin,†μU/ml | 9.7 (5.5, 17.0) | 7.3 (4.7, 11.4) | 0.5 |
| AIRglucagon5min* | 6.0 (−11.5, 18.0) | 15.8 (5.7, 68.2) | 0.2 |
| AIRtol5min* | −3.0 (−8.3, 9.2) | −3.9 (−29.7, 2.4) | 0.5 |
| AUCglucose,† mg·dl−1·45 min−1 | 3,467 (3,168, 3,795) | 3,419 (3,196, 3,659) | 0.8 |
| AUCinsulin,†μU·ml−1·45 min−1 | 657 (361, 1,199) | 1,208 (752, 1,940) | 0.2 |
| Basal free fatty acids,†μM/l | 269 (121, 598) | 77 (42, 140) | 0.03 |
| AUC free fatty acids,†μM·l−1·45 min−1 | 14,060 (7,081, 27,920) | 5,012 (3,010, 8,346) | 0.036 |
| Infant at postnatal day 45 | |||
| Basal glucose,† mg/dl | 75 ± 3.2 | 74.1 ± 2.6 | 0.8 |
| Basal insulin,†μU/ml | 4.8 (2.2, 10.2) | 7.3 (4.0, 13.3) | 0.4 |
| SI | 12.2 ± 4.8 | 28.4 ± 3.6 | 0.02 |
| DI, min−1 | 2.3 ± 2.1 | 6.9 ± 4.9 | 0.03 |
| AIRg5min, μU/ml | 24.8 ± 7.2 | 26.0 ± 5.3 | 0.9 |
| AIRtol5min, μU/ml | 12.0 ± 6.3 | −10.6 ± 4.7 | 0.014 |
| AUCglucose, mg·dl−1·45 min−1 | 5,751 ± 290 | 4,605 ± 245 | 0.013 |
| AUCinsulin, μU·ml−1·45 min−1 | 1,199 ± 120 | 991 ± 95 | 0.2 |
| Basal free fatty acids, μM/l | 902 ± 163 | 591 ± 122 | 0.2 |
| AUC free fatty acids, μM·l−1·45 min−1 | 32,038 ± 5,843 | 19,286 ± 4,355 | 0.2 |
Data are means ± SE unless indicated otherwise. TP, testosterone proprionate; SI, insulin sensitivity; DI, disposition index; KG, glucose disappearance rate; AIRg5min, acute insulin response to glucose at 5 min postglucose infusion; ivGTT, iv glucose tolerance test; AUCglucose, area under the curve (AUC) for glucose; AUCinsulin, area under the curve for insulin; AIRglucagon5min, acute insulin response to glucagon at 5 min postglucagon infusion; AIRto15min, acute insulin response to tolbutamide at 5 min posttolbutamide infusion.
Median (interquartile range).
Backtransformed logarithmic mean (95% confidence limits).
Fig. 6.
Negative relationship between ivGTT-determined glucose clearance (KG) in dams at 80 days of gestation and dam preconception body weight (○, oil-injected control dams; ●, TP-injected dams).
Fetuses.
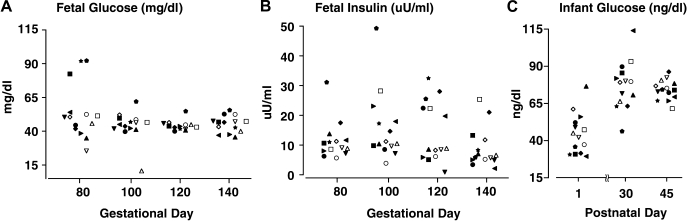
Overall, circulating glucose and insulin levels did not differ between control and PA female fetuses between 80 and 140 days gestation, nor were there gestational changes in circulating insulin (time effect, P > 0.17) levels (Figs. 4 and 5). Circulating glucose levels, however, did change across gestation (time effect, P < 0.021), with values at 80 days gestation exceeding all later gestational values (Fig. 4). Unique to prenatal TP exposure, preconception dam body weight, and circulating glucose levels and glucose clearance rates in TP-injected dams at 80 days gestation, significantly influenced PA fetal glucose levels. PA fetal glucose levels at 80 days gestation showed a strong, positive correlation with the preconception body weight of their dams (r2 = 0.72, P < 0.004), implicating dam adiposity before conception as a key contributor in the glucoregulatory associations found between PA fetuses and their TP-injected dams. PA fetal glucose levels showed a similarly strong negative correlation with glucose disappearance rates (r2 = 0.64, P < 0.01) and a positive correlation with simultaneous basal glucose values (r2 = 0.98, P < 0.001) in their TP-injected dams, with four of the nine (44%) PA fetuses exhibiting serum glucose levels (54.6–92.3 mg/dl) above concurrent control maximal values (52.4 mg/dl; Fig. 7). The positive correlation between fetal and maternal glucose levels persisted at 100 days gestation (r2 = 0.62, P < 0.021) for PA fetuses and TP-injected dams only. There were no significant associations between fetal insulin levels and maternal glucose or insulin concentrations despite 44% of PA fetuses having serum insulin levels (11.7–31.3 and 22.5–32.5 μU/ml) above respective control maximal values (11.3 and 22.4 μU/ml) on 100 and 120 days gestation, respectively (Fig. 7).
Fig. 7.
Individual values for fetal circulating levels of glucose (mg/dl) (A) and fetal circulating levels of insulin (μU/ml) (B) between 80 and 140 days of gestation and infant circulating levels of glucose (mg/dl) between postnatal days 1 and 45 (C) in 5 concurrent control (open symbols) and 9 PA (filled symbols) infants showing the PA values that exceed control ranges. The same individuals designated by the same symbol appear in all three panels, as either fetus or infant.
During the intravenous glucagon-tolbutamide test at 140 days of gestation, there were no significant female-type differences for any glucose or insulin parameters (Table 3). In contrast, during ivGTT at 140 days gestation, both basal FFA and AUCFFA values in PA fetuses were ∼29–36% (P ≤ 0.03–0.036), respectively, of controls (Table 3).
Infants.
In both female groups combined and typical of newborns (Fig. 4), basal circulating glucose levels reached a nadir (time effect, P < 0.001) on the first postnatal day of life vs. values on both postnatal days 30 and 45. Basal glucose levels in five out of nine (55%) PA infants, however, ranged from 29 to 36 mg/dl, below the minimum level (38 mg/dl) found in control infants on the first postnatal day (Fig. 7). Consistent with this finding, PA but not control infants demonstrated a negative correlation (r2 = 0.45, P < 0.048) between glucose levels on postnatal day 1 and their fetal glucose levels on 80 days of gestation, significantly associating elevated fetal glucose levels with initially low newborn infant glucose values in the androgen-exposed group. In contrast, basal circulating insulin levels did not vary between infant groups or across postnatal days (time effect, P < 0.70; Fig. 5). During the intravenous glucose-tolbutamide test at postnatal day 45, SI and the disposition index in PA infants, however, both increased to ∼230% (P ≤ 0.02) and 300% (P ≤ 0.03), respectively, above control values (Table 3). Increased disposition index values were consistent with ∼20% lower (P ≤ 0.013) AUCglucose values in PA than in control infants. The acute insulin responses to tolbutamide in PA infants were diminished (P ≤ 0.014) compared with controls. There were no other significant differences between PA and control infants during the intravenous glucose-tolbutamide test, including FFA parameters (Table 3).
DISCUSSION
In women and ewes, preconception adiposity and enhanced maternal weight gain can impair glucoregulation during pregnancy, causing increased fetal growth and altered glycemic control in the offspring [humans (19, 37, 43) and sheep (31)]. In this study, despite similar preconception body weights of all dams, body weight before conception [as a measure of female rhesus monkey adiposity (30)] was negatively correlated with glucose clearance in TP-injected monkey dams following accelerated maternal weight gain during TP treatment, suggesting that preconception adiposity interacts with TP-induced maternal weight gain to disrupt maternal glucoregulation. Correspondingly, PA female monkey offspring exposed to fetal androgen excess demonstrate a subtle increase in fetal head growth compared with concurrent controls, along with indications of transient fetal hyperglycemia and relative hyperinsulinemia in neonates. PA female infants demonstrate increased SI and relative increases in insulin release following glucose challenge, unlike the diminished SI and reduced insulin release following glucose challenge found in adult PA female monkeys (1, 29) and diminished SI observed in young female PA sheep (51) as well as adult PA female rats (25). Consequently, PA fetal and neonatal monkeys may exhibit these metabolic abnormalities more from TP-induced maternal glucose intolerance than from direct actions of hyperandrogenism on fetal target tissues.
Such metabolic mechanisms underlying the ability of fetal androgen excess to induce PCOS-like metabolic phenotypes in female rhesus monkeys may explain differences in PCOS-like traits between various fetal androgen excess models (1, 33). In our study, early to midgestation treatment of rhesus monkey dams with TP [intentionally exceeding placental/hepatic steroid deactivation and aromatization to produce fetal male levels of testosterone in the female fetuses (2)] modestly accelerates maternal weight gain to elevate circulating glucose and insulin levels following glucose challenge and to diminish glucose clearance relative to preconception adiposity, likely adding a metabolic perturbation of postprandial hyperglycemia to in utero androgen exposure. Postprandial hyperglycemia is now recognized as an important pathophysiological component in cardiovascular disease (15, 20, 54), glucose intolerant and diabetic pregnancies (12, 48), and type 2 diabetes (8, 46, 59, 66). In addition, gestational maternal postprandial hyperglycemia has recently been shown to be a sensitive marker for maternal glucoregulatory impairment and postpartum development of metabolic syndrome (61). Such TP-induced maternal metabolic disruption may explain why both adult female (4, 29) and male (16) offspring of TP-injected monkey dams exhibit insulin resistance and pancreatic β-cell defects, as two sequelae of a transient hyperglycemic gestational environment (5, 35, 36), rather than fetal androgen excess per se (2, 58). As in human pregnancies, relatively mild changes in gestational glucoregulation, as manifest in the present TP-injected dams and PA fetuses and infants, may have consequences for adult metabolic dysfunction (7, 18, 19, 62, 35, 36, 53).
Increased Maternal Weight Gain During Gestation
The modestly accelerated maternal weight gain found in this study emulates that found by Kemnitz and colleagues (44) when they administered TP at 2 mg·kg−1·day−1 to gonadectomized adult PA and control female rhesus monkeys and induced increased fat-free mass. The TP dose in the latter study closely mimics the ∼1.8–2.3 mg TP·kg−1·day−1 range employed in the present study (15 mg·dam−1·day−1; Fig. 1). Accelerated weight gain in rhesus monkey dams has potential adverse consequences on the fetus, as evident in human studies in which women in the highest quartile of weight gain during pregnancy are more likely to manifest maternal hyperglycemia and impaired glucose tolerance (37), and offspring born to overweight women are more likely to manifest metabolic syndrome in adulthood (19). Although body weight is positively correlated with body mass index (68) and total body fat (30) in female rhesus monkeys, the TP-accelerated weight gain in dams in the present study may well represent increased fat-free mass (44).
In addition, we observed impaired glucoregulation in TP-injected monkey dams, via diminished glucose clearance during midgestational ivGTT, equivalent to mild to moderate glucose intolerance found in obese pregnant rhesus monkeys (43). The degree of glucose intolerance shown by TP-injected dams resembles that of obese, untreated rhesus dams with preconception body weights ∼2–5 kg (∼30–70%) greater than those of the dams included in this study, implying that androgen excess may exaggerate the adverse metabolic consequence of adiposity accumulated before and during pregnancy (43), even though such adiposity may be within the range of normal body weight.
Impaired Maternal Glucoregulation
As expected, testosterone treatment of rhesus monkey dams induced subtle hyperinsulinemia presumably from testosterone-induced insulin resistance (21, 27, 56, 74), with increased pancreatic insulin responsiveness to glucose (elevated AUCinsulin) and a tendency for increased basal insulin levels, but neither prevented glucose intolerance (diminished glucose clearance) in TP-injected dams at 80 days of gestation. Because no additional glucose challenges were performed on the dams, it is not known whether maternal glucoregulatory impairments persist beyond the period of TP treatment. In this regard, insignificantly diminished SI in TP- vs. oil-injected dams likely represents heterogeneity of SI in controls from variability of pregnancy-related insulin resistance in rhesus monkeys (43, 55). Because preconception maternal body weight negatively correlates with maternal glucose disappearance in TP-injected dams alone, prepregnancy adiposity may additionally impair glucoregulatory function (19, 31, 37, 43), rendering TP-injected dams particularly susceptible to testosterone-induced insulin resistance. As previously shown in female rats (25), monkeys (47), and sheep (51), hypercaloric, diet-induced obesity mimics the diminished glucoregulatory effects of testosterone treatment so that the combination of increased adiposity and testosterone treatment may exaggerate impaired glucoregulation induced by testosterone treatment alone. Consistent with this hypothesis, increased lipolysis from daily TP injections of adult female rhesus monkeys (44) may contribute to diminished maternal glucose clearance (6, 38, 73).
Fetal Glucose, Insulin, and Growth
In hyperglycemia, excess glucose crosses the placenta by facilitated diffusion and induces compensatory hyperinsulinemia in the fetus (31, 34, 52), whereas circulating maternal insulin does not cross the placenta in any significant amount (41). Transient gestational maternal postprandial hyperglycemia, as found in TP-injected dams in this study, likely has an analogous effect on exposed female fetuses. Such a transient increase in transplacental glucose transport in TP-injected dams can explain how 1) circulating glucose levels in 44% of PA female fetuses exceed those of all fetal controls at 80 days gestation (first fetal sample day, last day of dam treatment) and 2) PA female fetal glucose levels positively correlate with those in their respective dams (at both 80 and 100 days gestation), while negatively correlating with dam glucose clearance (at 80 days gestation). As a consequence of such fetal hyperglycemia, providing principal energy substrate, and likely subtle increases in fetal insulin, as a growth promoter (31, 34, 52), PA monkey fetuses exhibited modestly enhanced biparietal diameter compared with concurrent controls during and after androgen excess treatment of their dams.
In such a fetal environment, circulating FFA levels in PA female fetal monkeys are only ∼30% of those found in controls by late gestation. Their respective dams, however, exhibit circulating FFA levels similar to those found in control dams. Although increasing glucose load on human placental tissue in vitro does not alter placental transport of fatty acids (50), gestational diabetes alters placental processing of fatty acids and lipids that may diminish (11) or enhance (57) their release to the fetus. It is thus unclear whether diminished circulating FFA levels in PA female fetuses reflect reduced placental delivery or enhanced fetal extraction. Either alteration in FFA availability to PA female fetuses may perturb early acquisition of fat stores without fetal growth restriction, as observed in this study. In humans, early acquisition of body fat in fetuses and infants leads to increased prevalence of adult adiposity and metabolic syndrome (17, 19).
Neonatal Glucose, Insulin, and Growth
Increases in PA fetal dimensions, combined with increased dam weight gain during pregnancy, would be expected to increase the postnatal risk of metabolic derangements in mammalian offspring (7, 17, 19, 31, 35). A degree of neonatal hypoglycemia was found in PA neonates on postnatal day 1 when 55% exhibited serum glucose levels of 29–36 mg/dl, all of which were lower than the minimum level of 38 mg/dl found in concurrent controls. With this modest glucose difference, PA neonates also demonstrated a negative correlation between their first postnatal day circulating glucose levels and those obtained from fetal blood samples at 80 days gestation, significantly associating low postnatal glucose levels with high fetal glucose levels in PA females, alone. Analogous associations between newborn hypoglycemia and maternal hyperglycemia have also been found in human pregnancies, including modest maternal hyperglycemia (17, 35).
Insulin hypersecretion following glucose challenge in PA 45-day-old infant monkeys, as shown by excessive acute insulin responses to glucose relative to SI (i.e., increased disposition index), are biologically relevant, as confirmed by increased glucose clearance (diminished AUCglucose), and may represent persistent pancreatic insulin hypersecretion entrained by transient maternal postprandial hyperglycemia (36, 53). The mechanism of exaggerated SI in PA compared with control infant monkeys is unknown but may reflect increased skeletal muscle insulin metabolic signaling, as manifest in neonatal lambs with fetal growth restriction (49), or increased adipose SI, as observed in children with increased fetal growth and early postnatal weight gain (14). Relatively excessive insulin release (increased disposition index) in an insulin-sensitive environment (elevated SI) likely explains why a modest increase in weight gain occurs in PA infants at ∼8 wk of age, particularly because insulin's anabolic action (7, 10) may synergize with PA infant hyperandrogenism (2) to increase lipogenesis and muscle protein synthesis (7, 22, 75), thereby enhancing insulin-sensitive tissue mass in PA monkey neonates.
Clinical Relevance
The present study shows that fetal pancreatic β-cells entrained by transient maternal postprandial hyperglycemia cause relative insulin hypersecretion in neonatal life, linking pregnancy-related metabolic antecedents with metabolic dysfunction in PA female rhesus monkeys (4) and possibly in PCOS women. Cresswell and colleagues (23) have associated the development of polycystic ovaries with increased birth weight and mother adiposity during pregnancy, indicating an increased likelihood for postnatal hyperinsulinemia from insulin resistance. Studies of two PCOS populations of Spanish descent (26, 65) have proposed that hyperinsulinemia accompanying postnatal catch-up growth amplifies the development of PCOS traits. In support of this, preadolescent and adolescent daughters of PCOS women have subtle hyperinsulinemia from insulin resistance without apparent changes in glucose parameters preceding an obvious PCOS phenotype (45, 64), whereas preadolescent daughters of glucose-intolerant PCOS women have greater insulin resistance and inferior insulin secretory compensation (D. H. Geller, personal communication). Postnatal insulin defects may thus provide an important developmental component in the expression of PCOS phenotype.
That such developmental origins for PCOS may be strongly influenced by clinically unrecognizable events in the postnatal environment, yet go virtually unrecognized until adulthood, raises profound health care concerns regarding the effect of maternal obesity and/or increased pregnancy weight gain on the transgenerational development of obesity as a susceptibility factor to later adult chronic diseases. With a progressive epidemic of obesity likely to induce metabolic abnormalities amplifying fetal androgen excess programming, the need for primate models to understand the developmental origins of PCOS-like reproductive and metabolic dysfunction is crucial to develop new clinical strategies that treat abnormalities during maternal-fetal development and postnatal life development to reduce the risk of adult disease.
GRANTS
This work was supported by National Institutes of Health Grants P50 HD-044405, P51 RR-000167 (WNPRC base operating grant), and RR-00169 (CNPRC base operating grant) and was partly conducted at a facility (WNPRC) constructed with support from Research Facilities Improvement Program Grant nos. RR-15459-01 and RR-020141-01.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank J. M. Turk, S. J. Muller, J. R. Lange, and Assay Services of the Wisconsin National Primate Research Center (WNPRC) for assistance with endocrine determinations; members of the animal care staff at the California National Primate Research Center (CNPRC) for expert technical assistance; Dr. R. N. Bergmann for advice on Minimal Model derivations; and R. A. Becker and J. L. Peterson at WNPRC for assistance with preparation of the figures and manuscript, respectively.
REFERENCES
- 1.Abbott DH, Barnett DK, Bruns CM, Dumesic DA. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update 11: 357–374, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Abbott DH, Barnett DK, Levine JE, Padmanabhan V, Dumesic DA, Jacoris S, Tarantal AF. Endocrine antecedents of polycystic ovary syndrome in fetal and infant prenatally androgenized female rhesus monkeys. Biol Reprod 79: 154–163, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abbott DH, Bruns CM, Barnett DK, Dumesic DA. Fetal programming of polycystic ovary syndrome ( 2nd ed.). In: Polycystic Ovary Syndrome, edited by Kovacs WG, Norman RL. Cambridge, UK: Cambridge Univ Press, 2007, p. 262–287 [Google Scholar]
- 4.Abbott DH, Dumesic DA. Fetal androgen excess provides a developmental origin for polycystic ovary syndrome. Expert Rev Obstet Gynecol 4: 1–7, 2009 [Google Scholar]
- 5.Abbott DH, Tarantal AF, Dumesic DA. Fetal, infant, adolescent and adult phenotypes of polycystic ovary syndrome in prenatally androgenized female rhesus monkeys. Am J Primatol 71: 776–784, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdul-Ghani MA, Muller FL, Liu Y, Chavez AO, Balas B, Zuo P, Chang Z, Tripathy D, Jani R, Molina-Carrion M, Monroy A, Folli F, Van Remmen H, DeFronzo RA. Deleterious action of FA metabolites on ATP synthesis: possible link between lipotoxicity, mitochondrial dysfunction, and insulin resistance. Am J Physiol Endocrinol Metab 295: E678–E685, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Aerts L, Van Assche FA. Animal evidence for the transgenerational development of diabetes mellitus. Int J Biochem Cell Biol 38: 894–903, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Bell DS. Importance of postprandial glucose control. South Med J 94: 804–809, 2001 [PubMed] [Google Scholar]
- 9.Bergman RN. Toward physiological understanding of glucose tolerance. Minimal-model approach. Diabetes 38: 1512–1527, 1989 [DOI] [PubMed] [Google Scholar]
- 10.Biolo G, Declan Fleming RY, Wolfe RR. Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J Clin Invest 95: 811–819, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bitsanis D, Ghebremeskel K, Moodley T, Crawford MA, Djahanbakhch O. Gestational diabetes mellitus enhances arachidonic and docosahexaenoic acids in placental phospholipids. Lipids 41: 341–346, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Bonomo M, Corica D, Mion E, Gonçalves D, Motta G, Merati R, Ragusa A, Morabito A. Evaluating the therapeutic approach in pregnancies complicated by borderline glucose intolerance: a randomized clinical trial. Diabet Med 22: 1536–1541, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Boomsma CM, Eijkemans MJ, Hughes EG, Visser GH, Fauser BC, Macklon NS. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update 12: 673–683, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Bouhours-Nouet N, Dufresne S, de Casson FB, Mathieu E, Douay O, Gatelais F, Rouleau S, Coutant R. High birth weight and early postnatal weight gain protect obese children and adolescents from truncal adiposity and insulin resistance: metabolically healthy but obese subjects? Diabetes Care 31: 1031–1036, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Brand-Miller J, Dickinson S, Barclay A, Allman-Farinelli M. Glycemic index, glycemic load, thrombogenesis. Semin Thromb Hemost 35: 111–118, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Bruns CM, Baum ST, Colman RJ, Eisner JR, Kemnitz JW, Weindruch R, Abbott DH. Insulin resistance and impaired insulin secretion in prenatally androgenized male rhesus monkeys. J Clin Endocrinol Metab 89: 6218–6223, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Catalano PM, Thomas A, Huston-Presley L, Amini SB. Increased fetal adiposity: a very sensitive marker of abnormal in utero development. Am J Obstet Gynecol 189: 1698–1704, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, Damm P. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care 31: 340–346, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, Schmidt L, Damm P. Overweight and the metabolic syndrome in adult offspring of women with diet-treated gestational diabetes mellitus or type 1 diabetes. J Clin Endocrinol Metab 94: 2464–2470, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Clement S. What are the best options for controlling prandial glycemia? Curr Diab Rep 9: 355–359, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Corbould A. Chronic testosterone treatment induces selective insulin resistance in subcutaneous adipocytes of women. J Endocrinol 192: 585–594, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Corbould A. Effects of androgens on insulin action in women: is androgen excess a component of female metabolic syndrome? Diabetes Metab Res Rev 24: 520–532, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Cresswell JL, Barker DJ, Osmond C, Egger P, Phillips DI, Fraser RB. Fetal growth, length of gestation, and polycystic ovaries in adult life. Lancet 350: 1131–1135, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Crisosto N, Codner E, Maliqueo M, Echiburú B, Sánchez F, Cassorla F, Sir-Petermann T. Anti-Müllerian hormone levels in peripubertal daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab 92: 2739–2743, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Demissie M, Lazic M, Foecking EM, Aird F, Dunaif A, Levine JE. Transient prenatal androgen exposure produces metabolic syndrome in adult female rats. Am J Physiol Endocrinol Metab 295: E262–E268, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Zegher F, Ibáñez L. Prenatal growth restraint followed by catch-up of weight: a hyperinsulinemic pathway to polycystic ovary syndrome. Fertil Steril 86, Suppl 1: S4–S5, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Diamond MP, Grainger D, Diamond MC, Sherwin RS, Defronzo RA. Effects of methyltestosterone on insulin secretion and sensitivity in women. J Clin Endocrinol Metab 83: 4420–4425, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Dumesic DA, Abbott DH, Padmanabhan V. Polycystic ovary syndrome and its developmental origins. Rev Endocr Metab Disord 8: 127–141, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eisner JR, Dumesic DA, Kemnitz JW, Abbott DH. Timing of prenatal androgen excess determines differential impairment in insulin secretion and action in adult female rhesus monkeys. J Clin Endocrinol Metab 85: 1206–1210, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Eisner JR, Dumesic DA, Kemnitz JW, Colman RJ, Abbott DH. Increased adiposity in female rhesus monkeys exposed to androgen excess during early gestation. Obes Res 11: 279–286, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Ford SP, Zhang L, Zhu M, Miller MM, Smith DT, Hess BW, Moss GE, Nathanielsz PW, Nijland MJ. Maternal obesity accelerates fetal pancreatic β-cell but not alpha-cell development in sheep: prenatal consequences. Am J Physiol Regul Integr Comp Physiol 297: R835–R843, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foster DL, Jackson LM, Padmanabhan V. Novel concepts about normal sexual differentiation of reproductive neuroendocrine function and the developmental origins of female reproductive dysfunction: the sheep model. Soc Reprod Fertil Suppl 64: 83–107, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Franks S. Do animal models of polycystic ovary syndrome help to understand its pathogenesis and management? Yes, but their limitations should be recognized. Endocrinology 150: 3983–3985, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Freinkel N. Of pregnancy and progeny. Diabetes 29: 1023–1035, 1980 [DOI] [PubMed] [Google Scholar]
- 35.HAPO Study Cooperative Research Group, Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, Oats JJ, Persson B, Rogers MS, Sacks DA. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 358: 1991–2002, 2008 [DOI] [PubMed] [Google Scholar]
- 36.HAPO Study Cooperative Research Group Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: associations with neonatal anthropometrics. Diabetes 58: 453–459, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herring SJ, Oken E, Rifas-Shiman SL, Rich-Edwards JW, Stuebe AM, Kleinman KP, Gillman MW. Weight gain in pregnancy and risk of maternal hyperglycemia. Am J Obstet Gynecol 201: e1–e7, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jensen MD. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab 93, Suppl 1: S57–S63, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jimenez DF, Tarantal AF. Fetal gender determination in early first trimester pregnancies of rhesus monkeys (Macaca mulatta) by fluorescent PCR analysis of maternal serum. J Med Primatol 32: 315–319, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Jones RH, Ozanne SE. Fetal programming of glucose-insulin metabolism. Mol Cell Endocrinol 297: 4–9, 2009 [DOI] [PubMed] [Google Scholar]
- 41.Kalhan SC, Schwartz R, Adam PA. Placental barrier to human insulin-I125 in insulin-dependent diabetic mothers. J Clin Endocrinol Metab 40: 139–142, 1975 [DOI] [PubMed] [Google Scholar]
- 42.Kemnitz JW, Elson DF, Roecker EB, Baum ST, Bergman RN, Meglasson MD. Pioglitazone increases insulin sensitivity, reduces blood glucose, insulin, and lipid levels, and lowers blood pressure, in obese, insulin-resistant rhesus monkeys. Diabetes 43: 204–211, 1994. [DOI] [PubMed] [Google Scholar]
- 43.Kemnitz JW, Engle MJ, Flitsch TJ, Perelman RH, Farrell PM. Obesity in pregnancy: consequences for maternal glucoregulation and fetal growth. In: Nonhuman Primate Studies on Diabetes, Carbohydrate Intolerance and Obesity. Monographs in Primatology, edited by Howard CF., Jr New York, NY: Liss, 1988, vol. 12, p. 29–41 [Google Scholar]
- 44.Kemnitz JW, Sladky KK, Flitsch TJ, Pomerantz SM, Goy RW. Androgenic influences on body size and composition of adult rhesus monkeys. Am J Physiol Endocrinol Metab 255: E857–E864, 1988 [DOI] [PubMed] [Google Scholar]
- 45.Kent SC, Gnatuk CL, Kunselman AR, Demers LM, Lee PA, Legro RS. Hyperandrogenism and hyperinsulinism in children of women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab 93: 1662–1669, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krssak M, Brehm A, Bernroider E, Anderwald C, Nowotny P, Dalla Man C, Cobelli C, Cline GW, Shulman GI, Waldhäusl W, Roden M. Alterations in postprandial hepatic glycogen metabolism in type 2 diabetes. Diabetes 53: 3048–3056, 2004 [DOI] [PubMed] [Google Scholar]
- 47.McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE, Grove KL. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest 119: 323–335, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McLachlan K, Jenkins A, O'Neal D. The role of continuous glucose monitoring in clinical decision-making in diabetes in pregnancy. Aust N Z J Obstet Gynaecol 47: 186–190, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Muhlhausler BS, Duffield JA, Ozanne SE, Pilgrim C, Turner N, Morrison JL, McMillen IC. The transition from fetal growth restriction to accelerated postnatal growth: a potential role for insulin signalling in skeletal muscle. J Physiol 587: 4199–4211, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nandakumaran M, al-Rayyes S, al-Yatama M, Sugathan TN. Effect of glucose load on the transport kinetics of palmitic acid in the human placenta: an in vitro study. Clin Exp Pharmacol Physiol 26: 669–673, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Padmanabhan V, Veiga-Lopez A, Abbott DH, Recabarren SE, Herkimer C. Developmental programming: impact of prenatal testosterone excess and postnatal weight gain on insulin sensitivity index and transfer of traits to offspring of overweight females. Endocrinology 151: 595–605, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pedersen J. Weight and length at birth of infants of diabetic mothers. Acta Endocrinol (Copenh) 16: 330–342, 1954 [DOI] [PubMed] [Google Scholar]
- 53.Persson B. Neonatal glucose metabolism in offspring of mothers with varying degrees of hyperglycemia during pregnancy. Semin Fetal Neonatal Med 14: 106–110, 2009 [DOI] [PubMed] [Google Scholar]
- 54.Peter R, Okosieme OE, Evans LM. Insulin therapy in type 2 diabetes: insulin analogue mix 50, a potential role in reducing postprandial hyperglycaemia and cardiovascular disease. Expert Opin Pharmacother 11: 33–39, 2010 [DOI] [PubMed] [Google Scholar]
- 55.Pitkin RM, Van Orden DE, Reynolds WA. Plasma insulin response and glucose tolerance in pregnant rhesus monkeys. Endocrinology 86: 435–437, 1970 [DOI] [PubMed] [Google Scholar]
- 56.Polderman KH, Gooren LJ, Asscheman H, Bakker A, Heine RJ. Induction of insulin resistance by androgens and estrogens. J Clin Endocrinol Metab 79: 265–271, 1994 [DOI] [PubMed] [Google Scholar]
- 57.Radaelli T, Lepercq J, Varastehpour A, Basu S, Catalano PM, Hauguel-De Mouzon S. Differential regulation of genes for fetoplacental lipid pathways in pregnancy with gestational and type 1 diabetes mellitus. Am J Obstet Gynecol 201: e1–e10, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Recabarren SE, Padmanabhan V, Codner E, Lobos A, Durán C, Vidal M, Foster DL, Sir-Petermann T. Postnatal developmental consequences of altered insulin sensitivity in female sheep treated prenatally with testosterone. Am J Physiol Endocrinol Metab 289: E801–E806, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Rendell MS, Jovanovic L. Targeting postprandial hyperglycemia. Metabolism 55: 1263–1281, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Resko JA, Buhl AE, Phoenix CH. Treatment of pregnant rhesus macaques with testosterone propionate: observations on its fate in the fetus. Biol Reprod 37: 1185–1191, 1987 [DOI] [PubMed] [Google Scholar]
- 61.Retnakaran R, Qi Y, Sermer M, Connelly PW, Zinman B, Hanley AJ. Isolated hyperglycemia at 1 hour on oral glucose tolerance test in pregnancy resembles gestational diabetes mellitus in predicting postpartum metabolic dysfunction. Diabetes Care 31: 1275–1281, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salihu HM, Mbah AK, Alio AP, Kornosky JL, Bruder K, Belogolovkin V. Success of programming fetal growth phenotypes among obese women. Obstet Gynecol 114: 333–339, 2009 [DOI] [PubMed] [Google Scholar]
- 63.Sir-Petermann T, Codner E, Maliqueo M, Echiburú B, Hitschfeld C, Crisosto N, Pérez-Bravo F, Recabarren SE, Cassorla F. Increased anti-Müllerian hormone serum concentrations in prepubertal daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab 91: 3105–3109, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Sir-Petermann T, Codner E, Pérez V, Echiburú B, Maliqueo M, Ladrón de Guevara A, Preisler J, Crisosto N, Sánchez F, Cassorla F, Bhasin S. Metabolic and reproductive features before and during puberty in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab 94: 1923–1930, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sir-Petermann T, Hitchsfeld C, Maliqueo M, Codner E, Echiburú B, Gazitúa R, Recabarren S, Cassorla F. Birth weight in offspring of mothers with polycystic ovarian syndrome. Hum Reprod 20: 2122–2126, 2005 [DOI] [PubMed] [Google Scholar]
- 66.Sivan E, Boden G. Free fatty acids, insulin resistance, and pregnancy. Curr Diab Rep 3: 319–322, 2003 [DOI] [PubMed] [Google Scholar]
- 67.Steckler T, Wang J, Bartol FF, Roy SK, Padmanabhan V. Fetal programming: prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment. Endocrinology 146: 3185–3193, 2005 [DOI] [PubMed] [Google Scholar]
- 68.Sullivan EL, Daniels AJ, Koegler FH, Cameron JL. Evidence in female rhesus monkeys (Macaca mulatta) that nighttime caloric intake is not associated with weight gain. Obes Res 13: 2072–2080, 2005 [DOI] [PubMed] [Google Scholar]
- 69.Tarantal AF. Interventional ultrasound in pregnant macaques: embryonic/fetal applications. J Med Primatol 19: 47–58, 1990 [PubMed] [Google Scholar]
- 70.Tarantal AF. Ultrasound imaging in rhesus and long-tailed macaques: Reproductive and research applications. In: The Laboratory Primate. New York, NY: Elsevier, 2005, chapt. 20, p. 317–351 [Google Scholar]
- 71.Tarantal AF, McDonald RJ, Jimenez DF, Lee CI, Plopper CG, Kohn DB. Intrapulmonary and intramyocardial gene transfer in rhesus monkeys: Safety and efficiency of lentiviral vectors for fetal gene delivery. Mol Ther 12: 87–98, 2005 [DOI] [PubMed] [Google Scholar]
- 72.West C, Foster DL, Evans NP, Robinson J, Padmanabhan V. Intra-follicular activin availability is altered in prenatally-androgenized lambs. Mol Cell Endocrinol 185: 51–59, 2001 [DOI] [PubMed] [Google Scholar]
- 73.Wilding JP. The importance of free fatty acids in the development of Type 2 diabetes. Diabet Med 24: 934–945, 2007 [DOI] [PubMed] [Google Scholar]
- 74.Zang H, Carlström K, Arner P, Hirschberg AL. Effects of treatment with testosterone alone or in combination with estrogen on insulin sensitivity in postmenopausal women. Fertil Steril 86: 136–144, 2006 [DOI] [PubMed] [Google Scholar]
- 75.Zhang HH, Huang J, Düvel K, Boback B, Wu S, Squillace RM, Wu CL, Manning BD. Insulin stimulates adipogenesis through the Akt-TSC2-mTORC1 pathway. PLoS One 4: e6189, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou R, Bruns CM, Bird IM, Kemnitz JW, Goodfriend TL, Dumesic DA, Abbott DH. Pioglitazone improves insulin action and normalizes menstrual cycles in a majority of prenatally androgenized female rhesus monkeys. Reprod Toxicol 23: 438–448, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]