Abstract
Long-term survivors of glioblastoma (GB) are rare. Several variables besides tumor size and location determine a patient's survival chances: age at diagnosis, where younger patients often receive more aggressive treatment that is multimodal; functional status, which has a significant negative correlation with age; and histologic and genetic markers.
Introduction
Of the estimated 17,000 primary brain tumors diagnosed in the US each year, approximately 60% are gliomas.1,2 Glioblastoma (GB), or grade IV astrocytoma, is the most aggressive of primary tumors of the brain for which no cure is available.1,3 Management remains palliative and includes surgery, radiotherapy, and chemotherapy. With optimal treatment, patients with GBs have a median survival of less than one year.1 About 2% of patients survive three years.4 Previously reported long-term survivors (LTSs) of GB may have been patients who actually harbored other low-grade gliomas.5 The overall prognosis for GB has changed little since the 1980s, despite major improvements in neuroimaging, neurosurgery, radiotherapy, and chemotherapy techniques.
Methods
LTSs are defined as those who survive longer than two years.1 Despite extensive clinical trials, prediction of clinical outcome for individual patients has remained an elusive goal. In search of factors or predictors of long-term survival, we queried the literature using PubMed and Google and the keywords glioblastoma prognostic factor long survival, and then reviewed the articles, comparing their results for common findings.
Results
We found that patient survival depends on the following clinical and biologic parameters: tumor size and location, treatment, age at presentation, Karnofsky performance score (KPS) at presentation, histologic findings, and molecular genetic factors.
Tumor Size and Location
GB is a highly infiltrating tumor and most of the time cannot be resected completely; hence, surgery often consists of incomplete debulking. The feasibility and extent of surgical resection depends on tumor size and eloquence of the brain areas (location). Supratentorial and cerebellar tumors are more amenable to surgical treatment and thus carry better prospects than tumors in the brainstem or diencephalon. Stereotactic biopsy, followed by radiotherapy, may be a more appropriate treatment for these patients.6 Case management with best supportive care for patients with unresectable, primary, biopsy-proven GB results in a median survival time of three months.6,7
Treatment
Clinical evidence suggests that an aggressive and multimodal treatment results in longer survival.8–14 Total or subtotal resection, combined with radiotherapy and chemotherapy, is the mainstay of treatment. New therapies that are still under investigation have shown some promising results. For example, in a 2007 report of a study by Dehdashti et al, brachytherapy was used as a boost to radiotherapy: three patients lived 11, 16, and 18 years, respectively, in the basic group, but unfortunately, statistics did not reveal any significant association with brachytherapy.15 In another example, temozolomide has recently proved to significantly prolong survival when used as an adjuvant chemotherapy to radiotherapy.16 Regarding intra-arterial chemotherapy, a survival benefit in comparison with intravenous administration was not established.17
Age at Presentation
Nearly all studies showed a significant negative relationship between advancing age and duration of postoperative survival.8–18 In a 2005 report of a study by Korshunov et al,18 the percentage of patients younger than age 40 years who survived more than five years was 34%, compared with 6% for patients age 40 years old and older. The researchers suggested age 40 years as the most appropriate cutoff for dividing patients with GB into groups according to prognosis.
Karnofsky Performance Score at Presentation
Many studies' findings show that higher KPS at presentation correlates with improved outcome.4,15,19–21 This is most probably linked to the factor of younger age at diagnosis.
Tumor size and location, treatment, age at presentation, and KPS at presentation allow stratification of patients into risk groups. Using recursive partitioning analysis, Lamborn et al22 identified four risk groups. The two lower-risk groups included patients younger than age 40 years, the lowest risk group being young patients with tumor in the frontal lobe only. An intermediate-risk group included patients with a KPS >70, subtotal or total resection, and between ages 40 and 65 years. The highest-risk group included all patients older than age 65 years and patients between ages 40 and 65 years with either KPS <80 or biopsy only. Subgroup analyses indicated that inclusion of adjuvant chemotherapy provides an increase in survival, although that improvement tends to be minimal for patients older than age 65 years, for patients older than age 40 years with KPS <80, and for those treated with brachytherapy.
… aggressive and multimodal treatment results in longer survival.8–14
Histologic Findings
The higher the grade of tumor, the more malignant the tumor is and the worse the prognosis is. Tumors are graded mainly on the basis of their proliferation index, which is an important prognostic factor in GB. The Ki-67 protein is expressed in all phases of the cell cycle except G0 and serves as a good marker for proliferation. Studies that have evaluated proliferation index by Ki-67 immunohistochemistry in GB have shown a significant correlation between high proliferation rates and shorter disease-free and overall survival.5,12,13
The cytologic and histologic composition of glioblastoma has an impact on survival. Microcystic change, the presence of cells with obvious astrocytic differentiation (fibrillary astrocytes), and the subjective impression that areas of better differentiation are present has been associated with a better outcome.23 Another histologic factor, calcification, was in one study associated with a better prognosis.24 A significant relationship also exists between the presence of necrosis and poor outcome.23 Korshunov et al25 found that some histologic and genetic markers that were significant for outcome appeared to be closely related to biology of single cytologic subsets (see “Molecular Genetic Factors”), so they divided GB into three cytologic subsets: small-cell GB (SGB), pleomorphic-cell GB (PGB), and gemistocytic GB (GGB).
Molecular Genetic Factors
Cytogenetic and molecular genetic studies of GB have shown that the most frequent alterations encountered in these tumors are loss of heterozygosity on chromosome arm 10q (60%–90%), mutations in p53 (25%–40%), PTEN mutations (30%), overexpression of MDM2 (10%–15%), and epidermal growth factor receptor (EGFR) gene amplification.1 More p53 expression was reported in LTSs (>3 years) and overexpression of MDM2 in short-term survivors (<3 years).26 Korshunov et al25 found that the number of p53-positive tumors prevailed among the PGB, whereas the number of tumors with EGFR and MDM2 positivity was significantly greater in SGB. GGB contained the significantly lowest mean proliferating cell nuclear antigen (PCNA) labeling index (LI), greater number of p21ras-positive cases, and higher mean apoptotic index (AI). Thus, there is a relationship between histologic and genetic markers. Survival time in patients with SGB, EGFR, and MDM2 positivity and PCNA LI >40% was found to be significantly shorter, whereas presence of p21ras and AI >0.5% were associated with prolonged survival. In another study, Korshunov et al18 found that being younger than age 40 years is strongly associated with a favorable prognosis. EGFR amplification, loss of 9p21, and gain of chromosome 9 had prognostic significance for all patients, whereas gain of chromosome 7 and loss of 10q23/PTEN showed clinical importance only for patients age 40 years and older. Krex et al19 studied 55 patients with GB who lived more than three years. They found significantly more frequent O6-methylguanine–DNA methyltransferase (MGMT) hypermethylation in LTSs.19 Interestingly, the protein product of MGMT gene, 06 alkylguanine–DNA alkyltransferase, was shown to be involved in tumor resistance to alkylating agents. Silencing of the MGMT gene by promoter methylation compromises DNA repair and has been associated with longer survival in patients with glioblastoma who receive alkylating agents.27–30 Clinical trials for malignant gliomas now often include determination of MGMT expression status.
Recently, Marko et al31 identified a set of 1478 genes with significant differential expression (p < 0.01) between long-term and short-term survivors and, with additional mathematic filtering, isolated a 43-gene “fingerprint” that distinguished survival phenotypes. Gene ontology analysis of the fingerprint demonstrated pathophysiologic functions for the gene products that are consistent with current models of tumor biology, suggesting that differential expression of these genes may contribute etiologically to the observed differences in survival.
Conclusion
GBS are highly malignant tumors that are difficult (but not impossible) to eradicate and that carry a dismal prognosis. LTSs are rare. Several factors besides tumor size and location determine patient's survival chances after diagnosis of GB. Age and functional status are two important prognostic aspects that seem to be correlated. Proliferation index and genetic markers have also been related to age.32,33 Moreover, younger patients often receive aggressive and multimodal treatment. Thus, age at diagnosis plays a pivotal role in the prognosis for GB patients (Figure 1).
Figure 1.
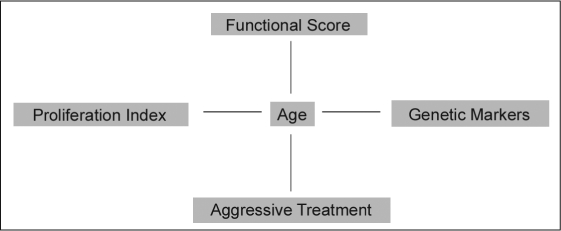
Interaction of prognostic factors for patients with glioblastoma.
Disclosure Statement
The author(s) have no conflicts of interest to disclose.

Acknowledgments
Katharine O'Moore-Klopf of KOK Edit provided editorial assistance.
References
- Bruce JN, Cronk K, Waziri A, et al. Glioblastoma multiforme [monograph on the Internet] Nebraska: eMedicine from WebMD; last updated 2006 Aug 4 [cited 2008 Jul 18]. Available from: www.emedicine.com/med/topic2692.htm. [Google Scholar]
- Salah Uddin ABM, Jarmi T.Glioblastoma multiforme [monograph on the Internet] Nebraska: eMedicine from WebMD; 2007 Jan 10. last updated 2008 May 21 [cited 2008 Jul 18]. Available from: www.emedicine.com/NEURO/topic147.htm. [Google Scholar]
- Burger PC, Vogel FS, Green SB, Strike TA. Glioblastoma multiforme and anaplastic astrocytoma. Pathologic criteria and prognostic implications. Cancer. 1985 Sep 1;56(5):1106–11. doi: 10.1002/1097-0142(19850901)56:5<1106::aid-cncr2820560525>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Scott JN, Rewcastle NB, Brasher PM, et al. Which glioblastoma multiforme patient will become a long-term survivor? A population-based study. Ann Neurol. 1999 Aug;46(2):183–8. [PubMed] [Google Scholar]
- McLendon RE, Halperin EC. Is the long-term survival of patients with intracranial glioblastoma multiforme overstated? Cancer. 2003 Oct 15;98(8):1745–8. doi: 10.1002/cncr.11666. [DOI] [PubMed] [Google Scholar]
- Coffey RJ, Lunsford LD, Taylor FH. Survival after stereotactic biopsy of malignant gliomas. Neurosurgery. 1988 Mar;22(3):465–73. doi: 10.1227/00006123-198803000-00003. [DOI] [PubMed] [Google Scholar]
- Nieder C, Grosu AL, Astner S, Molls M. Treatment of unresectable glioblastoma multiforme. Anticancer Res. 2005 Nov–Dec;25(6C):4605–10. [PubMed] [Google Scholar]
- Kleinschmidt-De Masters B, et al. The burden of radiation-induced central nervous system tumors: a single institution's experience. J Neuropathol Exp Neurol. 2006 Mar;65(3):204–16. doi: 10.1097/01.jnen.0000205146.62081.29. [DOI] [PubMed] [Google Scholar]
- Deb P, Sharma MC, Mahapatra AK, Agarwal D, Sarkar C. Glioblastoma multiforme with long term survival. Neurol India. 2005 Sep;53(3):329–32. [PubMed] [Google Scholar]
- Cervoni L, Celli P, Salvati M. Long-term survival in a patient with supratentorial glioblastoma: clinical considerations. Ital J Neurol Sci. 1998 Aug;19(4):221–4. doi: 10.1007/BF02427606. [DOI] [PubMed] [Google Scholar]
- Yamada S, Endo Y, Hirose T, et al. Autopsy findings in a long-term survivor with glioblastoma multiforme—case report. Neurol Med Chir (Tokyo) 1998 Feb;38(2):95–9. doi: 10.2176/nmc.38.95. [DOI] [PubMed] [Google Scholar]
- Chandler KL, Prados MD, Malec M, Wilson CB. Long-term survival in patients with glioblastoma multiforme. Neurosurgery. 1993 May;32(5):716–20. doi: 10.1227/00006123-199305000-00003. discussion 720. [DOI] [PubMed] [Google Scholar]
- Rutz HP, de Tribolet N, Calmes JM, Chapuis G. Long-time survival of a patient with glioblastoma and Turcot's syndrome. Case report. J Neurosurg. 1991 May;74(5):813–5. doi: 10.3171/jns.1991.74.5.0813. [DOI] [PubMed] [Google Scholar]
- Salford LG, Brun A, Nirfalk S. Ten-year survival among patients with supratentorial astrocytomas grade III and IV. J Neurosurg. 1988 Oct;69(4):5069. doi: 10.3171/jns.1988.69.4.0506. [DOI] [PubMed] [Google Scholar]
- Dehdashti AR, Sharma S, Laperriere N, Bernstein M. Coincidence vs cause: cure in three glioblastoma patients treated with brachytherapy. Can J Neurol Sci. 2007 Aug;34(3):339–42. doi: 10.1017/s031716710000679x. [DOI] [PubMed] [Google Scholar]
- Minniti G, De Sanctis V, Muni R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma in elderly patients. J Neurooncol. 2008 May;88(1):97–103. doi: 10.1007/s11060-008-9538-0. [DOI] [PubMed] [Google Scholar]
- Imbesi F, Marchioni E, Benericetti E, et al. A randomized phase III study: comparison between intravenous and intraarterial ACNU administration in newly diagnosed primary glioblastomas. Anticancer Res. 2006 Jan–Feb;26(1B):553–8. [PubMed] [Google Scholar]
- Korshunov A, Sycheva R, Golanov A. The prognostic relevance of molecular alterations in glioblastomas for patients age < 50 years. Cancer. 2005 Aug 15;104(4):825–32. doi: 10.1002/cncr.21221. [DOI] [PubMed] [Google Scholar]
- Krex D, Klink B, Hartmann C, et al. German Glioma Network Long-term survival with glioblastoma multiforme. Brain. 2007 Oct;130(Pt 10):2596–606. doi: 10.1093/brain/awm204. [DOI] [PubMed] [Google Scholar]
- Ulutin C, Fayda M, Aksu G, et al. Primary glioblastoma multiforme in younger patients: a single-institution experience. Tumori. 2006 Sep–Oct;92(5):407–11. doi: 10.1177/030089160609200507. [DOI] [PubMed] [Google Scholar]
- Hung KS, Howng SL. Prognostic significance of annexin VII expression in glioblastomas multiforme in humans. J Neurosurg. 2003 Nov;99(5):886–92. doi: 10.3171/jns.2003.99.5.0886. [DOI] [PubMed] [Google Scholar]
- Lamborn KR, Chang SM, Prados MD. Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis. Neuro Oncol. 2004 Jul;6(3):227–35. doi: 10.1215/S1152851703000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger PC, Green SB. Patient age, histologic features, and length of survival in patients with glioblastoma multiforme. Cancer. 1987 May 1;59(9):1617–25. doi: 10.1002/1097-0142(19870501)59:9<1617::aid-cncr2820590916>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Hoshino K. Statistical analysis of factors affecting survival after glioblastoma multiforme. Acta Neurochir (Wien) 1977;37(1–2):57–73. doi: 10.1007/BF01401926. [DOI] [PubMed] [Google Scholar]
- Korshunov A, Golanov A, Sycheva R. Immunohistochemical markers for prognosis of cerebral glioblastomas. J Neurooncol. 2002 Jul;58(3):217–36. doi: 10.1023/a:1016218117251. [DOI] [PubMed] [Google Scholar]
- Burton EC, Lamborn KR, Forsyth P, et al. Aberrant p53, mdm2, and proliferation differ in glioblastomas from long-term compared with typical survivors. Clin Cancer Res. 2002 Jan;8(1):180–7. [PubMed] [Google Scholar]
- Crinière E, Kaloshi G, Laigle-Donadey F, et al. MGMT prognostic impact on glioblastoma is dependent on therapeutic modalities. J Neurooncol. 2007 Jun;83(2):173–9. doi: 10.1007/s11060-006-9320-0. [DOI] [PubMed] [Google Scholar]
- Donson AM, Addo-Yobo SO, Handler MH, Gore L, Foreman NK. MGMT promoter methylation correlates with survival benefit and sensitivity to temozolomide in pediatric glioblastoma. Pediatr Blood Cancer. 2007 Apr;48(4):403–7. doi: 10.1002/pbc.20803. [DOI] [PubMed] [Google Scholar]
- Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005 Mar 10;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- Idbaih A, Omuro A, Ducray F, Hoang-Xuan K. Molecular genetic markers as predictors of response to chemotherapy in gliomas. Curr Opin Oncol. 2007 Nov;19(6):606–11. doi: 10.1097/CCO.0b013e3282f075f3. [DOI] [PubMed] [Google Scholar]
- Marko NF, Toms SA, Barnett GH, Weil R. Genomic expression patterns distinguish long-term from short-term glioblastoma survivors: a preliminary feasibility study. Genomics. 2008 May;91(5):395–406. doi: 10.1016/j.ygeno.2008.01.002. [DOI] [PubMed] [Google Scholar]
- McKeever PE, Junck L, Strawderman MS, et al. Proliferation index is related to patient age in glioblastoma. Neurology. 2001 May 8;56(9):1216–8. doi: 10.1212/wnl.56.9.1216. [DOI] [PubMed] [Google Scholar]
- Stark AM, Hugo HH, Witzel P, Mihajlovic Z, Mehdorn HM. Age-related expression of p53, Mdm2, EGFR and Msh2 in glioblastoma multiforme. Zentralbl Neurochir. 2003;64(1):30–6. doi: 10.1055/s-2003-37149. [DOI] [PubMed] [Google Scholar]


