Abstract
Homing endonucleases are microbial DNA-cleaving enzymes that mobilize their own reading frames by generating double strand breaks at specific genomic invasion sites. These proteins display an economy of size, and yet recognize long DNA sequences (typically 20 to 30 basepairs). They exhibit a wide range of fidelity at individual nucleotide positions, in a manner that is strongly influenced by host constraints on the coding sequence of the targeted gene. The activity of these proteins leads to site-specific recombination events that can result in the insertion, deletion, mutation or correction of DNA sequences. Over the past 15 years the crystal structures of representatives from several homing endonuclease families have been solved, and methods have been described to create variants of these enzymes that cleave novel DNA targets. Engineered homing endonucleases proteins are now being used to generate targeted genomic modifications for a variety of biotech and medical applications.
Endonuclease enzymes are ubiquitous catalysts that are involved in genomic modification, rearrangement, protection and repair. Their specificity spans at least nine orders of magnitude, ranging from nonspecific degradative enzymes up to a variety of gene-specific endonucleases. The most specific of these latter enzymes, termed homing endonucleases, generate double strand breaks at individual loci in their host genomes, and thereby drive site-specific gene conversion events.
Homing is a process in which microbial self-splicing intervening sequences—group I or group II introns or inteins—are specifically duplicated into recipient alleles of their host gene that lack such a sequence (Belfort and Perlman, 1995; Belfort and Roberts, 1997; Chevalier and Stoddard, 2001; Dujon, 1989; Lambowitz and Belfort, 1993). The first observation of homing dates to experiments conducted at the Pasteur Institute in the early 1970s. In these studies, investigators noted the dominant inheritance of a genetic marker, termed 'omega', during yeast mating experiments (mitochondrial genes are passed on biparentally in such studies, and are thus subject to Mendelian laws of inheritance). 'Omega' was determined to be located within the mitochondrial gene that encodes the large ribosomal RNA subunit (LSrRNA) (Bolotin et al., 1971) and was inherited at near 100% frequency in experiments involving homozygous 'omega-plus' and 'omega-minus' yeast strains (Netter et al., 1974). In subsequent experiments, omega was found to correspond to an intervening sequence (later recognized as a self-splicing group I intron) (Bos et al., 1978; Faye et al., 1979).
The dominant inheritance of this intron was eventually determined to be induced by a site-specific endonuclease (now termed I-SceI) that is encoded by an open reading frame harbored within the intron sequence (Jacquier and Dujon, 1985). This enzyme was shown to generate a DNA double-strand break within a long DNA target sequence in the LSrRNA gene that contains the eventual intron insertion site. Repair via homologous recombination, while using the intron-containing allele as a corrective template, leads to duplication of the intron and its endonuclease gene into the target site (Figure 1).
Figure 1. Homing endonucleases and genetic homing.
a: A mobile element consisting of a homing endonuclease gene (red bar) that is embedded within a self-splicing intron or intein (blue bars) resides within a host gene (grey bars). The homing endonuclease (red star) is expressed and cleaves a target site (green bar) that is found in a homologous allele of the host gene that lacks the entire element. The resulting double strand break is repaired by cellular machinery, generally leading either to repair via nonhomologous end-joining (not shown) or to repair via homologous recombination (HR). If HR successfully uses the intron-containing host allele (I+) as a corrective template, then the original uninterrupted allele (intron-minus; I−) is converted to an allele that now contains the intron and homing endonuclease gene (intron-plus; I+). b: A variety of biotechnology and gene therapy applications can potentially make use of the properties of a homing endonuclease, if such an enzyme is introduced into or expressed in a living cell, to drive a gene conversion process. Depending on the presence or absence (as well as the sequence) of a corrective DNA template for break repair, and on the catalytic properties of the endonuclease, such applications can lead to mutation, knockout, modification, or insertion of exogenous coding DNA into the gene target.
A series of studies conducted in several laboratories over the ensuing years led to the discovery of homing endonucleases and mobile introns and inteins within a wide variety of additional microbial genomes (reviewed in (Belfort and Perlman, 1995)). The transfer, duplication and transmission of these sequences was shown to be extremely efficient, leading to unidirectional gene conversion events in diploid genomes (Jacquier and Dujon, 1985) or genetic competition in mixed phage infections (Goodrich-Blair and Shub, 1996). Homing was found to occur even between different subcellular compartments in unrelated organisms (Turmel et al., 1995) and to be capable of driving the rapid spread of mobile introns into related target sites throughout an broad range of biological hosts (Cho et al., 1998).
Although homing endonucleases can also be encoded by free-standing reading frames, their association with self-splicing sequences allows them to invade highly conserved sequences in protein- and RNA-encoding host genes without disrupting their function, and then to persist in microbial genomes that are otherwise subject to strong selective pressure to eliminate extraneous genetic elements (Edgell et al., 2000). The ability of homing endonuclease genes to accumulate within microbial genomes is extraordinary. For example, 15 separate homing endonuclease genes correspond to 11% of the total coding sequence of the T4 phage genome (Edgell et al., 2010).
Intron-encoded proteins: families and structures
Homing endonucleases are extremely widespread and are found in microbes from all biological kingdoms, as well as in those organisms’ corresponding phage and viruses. Despite the close proximity and the frequent symbiotic relationship between multicellular eukaryotes and various microbial species, no examples have been reported of homing endonuclease genes within genomes of those more complex organisms.
At least five different families of homing endonucleases have been identified and characterized; each is primarily associated with a particular biological host range (Stoddard, 2005). Bioinformatic and crystallographic studies of representatives from each of these families (Figure 2) have indicated that they contain unique catalytic cores, and are presumably descended from different ancestral nucleases. Three of these families (corresponding to the GIY-YIG, PD-(D/E)xK, and LAGLIDADG homing endonucleases) are largely constrained to phage, bacterial and archael / eukaryotic hosts, respectively. Two additional structural families of homing endonucleases, that each contain a permutation of an 'HNH' nuclease active site, have also evolved. Enzymes from these latter families display completely different protein scaffolds that are optimized for DNA homing in phage and in protists, respectively.
Figure 2. Homing endonuclease structural families.
In all panels, divalent cations associated with the enzyme active sites are indicated by light green spheres. Bound zinc ions panel d are indicated by smaller dark green spheres. a: The phage-encoded HNH endonuclease I-HmuI (Shen et al., 2004) and GIY-YIG endonuclease I-TevI (VanRoey et al., 2002; VanRoey et al., 2001). These protein displays an extended, monomeric structure consisting of an N-terminal nuclease catalytic domain and a C-terminal DNA binding region that incorporates a C-terminal helix-turn-helix domain. The diagram of I-TevI is a composite of two separate crystal structures of the C-terminal region bount to DNA and the unbound catalytic domain. b: The cyanobacterial PD-(D/E)xK homing endonuclease I-Ssp6803I (a tetrameric intron-associated endonuclease that recognizes a target site in a tRNA host gene) (Zhao et al., 2007). c: The algal LAGLIDADG homing endonuclease I-CreI, which is a homodimer that recognizes a target site in the 23S rRNA encoding gene in the chloroplast genome of Chlamydomonas reinhardtii (Jurica et al., 1998). Similar endonucleases are encoded in fungal mitochondrial genomes and in archaea. These enzymes are found both as homodimers and as tandem repeats of two LAGLIDADG domains that form single chain monomeric proteins. d. The His-Cys box homing endonuclease I-PpoI, which is encoded in the nuclear genome in the slime mold Physarum polycephalum (Flick et al., 1998). This enzyme contains a variant of the HNH nuclease active site, and is therefore distantly related to the phage endonuclease I-HmuI (panel a), but the catalytic cores of these two enzymes have been incorporated into two very different structural scaffolds.
Crystallographic structures of DNA-bound representatives from each of the known homing endonuclease families (Flick et al., 1998; Jurica et al., 1998; Moure et al., 2002; Moure et al., 2003; Shen et al., 2004; VanRoey et al., 2001; Zhao et al., 2007) have provided a clear answer to the question of how these small proteins recognize their long target sites. Two strategies are observed: they either form highly elongated protein folds with minimal hydrophobic cores (Shen et al., 2004; VanRoey et al., 2001)) or they multimerize and thereby double their DNA-contact surface (Flick et al., 1998; Jurica et al., 1998; Zhao et al., 2008). The latter strategy is employed at the cost of being constrained to recognition of DNA sequences with significant palindromic symmetry.
DNA recognition mechanisms vary widely across homing endonucleases. The LAGLIDADG and His-Cys box enzymes (which are the most sequence-specific of these enzymes) rely upon antiparallel β-sheets that dock into the major grooves of their DNA target sites (Flick et al., 1998; Jurica et al., 1998). There they establish a collection of sequence-specific and non-specific contacts that are distributed nonuniformly across multiple consecutive basepairs (Chevalier et al., 2003; Scalley-Kim et al., 2007) (Figure 3). In contrast, the less specific homing endonucleases found primarily in phage and bacteria form a more heterogeneous collection of DNA contacts within both the major and minor groove of their target sites. The phage-derived GIY-YIG and HNH enzymes (typified by I-TevI and I-HmuI, respectively; see Figure 2a) display extended, multi-domain protein structures in which disparate structural elements (including individual α-helices, zinc fingers and/or helix-turn-helix domains) contact a series of DNA regions that can span almost 30 basepairs in length (Shen et al., 2004; VanRoey et al., 2001).
Figure 3. The DNA-binding surface and contacts formed by one subunit of the I-CreI homing endonuclease (from Figure 2c).
a: An antiparallel β-sheet structure presents a group of broadly distributed side chains to the major groove of the DNA target site, where they make a variety of specific and nonspecific contacts. b: A schematic of the contacts made by the structure shown in panel a. Hydrogen-bond acceptor and donor positions on each basepair are shown with concave and convex features on each base; direct contacts are shown with red arrows; water-mediated contacts are shown with blue arrows and blue spheres, which denote the water molecules in the crystal structure). Individual basepairs display a wide variation in the total number of contacts to protein atoms; this variation correlates approximately with the fidelity of recognition that is displayed at each position. Additional specificity is derived by indirect exploitation of sequence-specific conformational preferences of the DNA target, which is often bent as part of endonuclease binding and cleavage.
Finally, examinations of microbial and metagenomic sequence databases indicate that additional homing endonuclease families remain to be discovered and characterized. A recent analysis of the Global Ocean Sampling (GOS) metagenomic sequence database (initially with the goal of finding novel split genes) resulted in the discovery of a previously undescribed homing endonuclease family (Dassa et al., 2009). These proteins display a unique domain organization, with a putative N-terminal DNA binding region fused to a C-terminal catalytic domain that resembles the very short patch repair (Vsr) endonuclease. The Vsr endonuclease is a bacterial mismatch repair enzyme that contains a variation of the canonical PD-(D/E)xK active site. Therefore, two different forms of the PD-(D/E)xK catalytic motif appear to be found in two very different homing endonuclease lineages (similar to the HNH nuclease motif, as described above).
Subsequent to its identification in the metagenomic sequence database, a representative of this new family was found to already have been sequenced within the Bacillus thuriengensis phage 0305ϕ genome. However, this protein was not annotated until its relatives were identified based on their position within metagenomic intervening sequence elements. This protein has now been renamed I-Bth0305I (unpublished data), following the nomenclature convention established for homing endonucleases (Roberts et al., 2003).
Adaptation of homing endonucleases for host-specific functions
In general, homing endonucleases have evolved to act as opportunistic selfish DNA that provides little benefit (but also no significant cost) to their hosts (Stoddard and Belfort, 2010). However, a fraction of homing endonucleases have acquired a more beneficial role for their hosts. In bacteriophage, some homing endonucleases confer an advantage during mixed infections by specifically cleaving the DNA of competing phage (Goodrich-Blair and Shub, 1996). At least one homing endonuclease (I-TevI) acts as a transcriptional regulator, in that case by autorepressing its own transcription (Edgell et al., 2004a). Many homing endonucleases can also participate in the post-transcriptional splicing of their host intron, by assisting the folding of their cognate RNA intron--a function termed 'maturase' activity (Delahodde et al., 1989; Geese et al., 2003; Goguel et al., 1992; Henke et al., 1995; Ho et al., 1997; Longo et al., 2005; Szczepanek and Lazowska, 1996; Wenzlau et al., 1989). Finally, some homing endonucleases have been adopted by the host to act directly as freestanding endonucleases that drive biologically important gene conversion events. For example, the HO endonuclease in yeast, which is responsible for the mating-type genetic switch in that organism, is a LAGLIDADG protein which appears to be derived from an intein-associated homing endonuclease (Jin et al., 1997).
In addition to the direct employment of homing endonucleases for host-specific activities, more distant relatives of these proteins are used for a wide variety of biological functions (Figure 4). The HNH catalytic motif is found in a wide variety of nonspecific bacterial and fungal nucleases, (Friedhoff et al., 1999; Kuhlmann et al., 1999), structure-specific endonucleases (Biertumpfel et al., 2007),restriction endonucleases such as R.Hpy99I and R.PacI (Shen et al., 2010; Sokolowska et al., 2009), and a wide variety of transposases, polymerase editing domains, and DNA packaging factors (Dalgaard et al., 1997; Mehta et al., 2004). Similarly, the GIY-YIG catalytic motif is also found in bacterial restriction enzymes such as R.Eco29kI and R.Hpy88I (Mak et al., 2010; Sokolowska et al., 2010) and in a wide variety of enzymes involved in DNA repair, recombination and fidelity (reviewed in Dunin-Horkawicz et al., 2006).
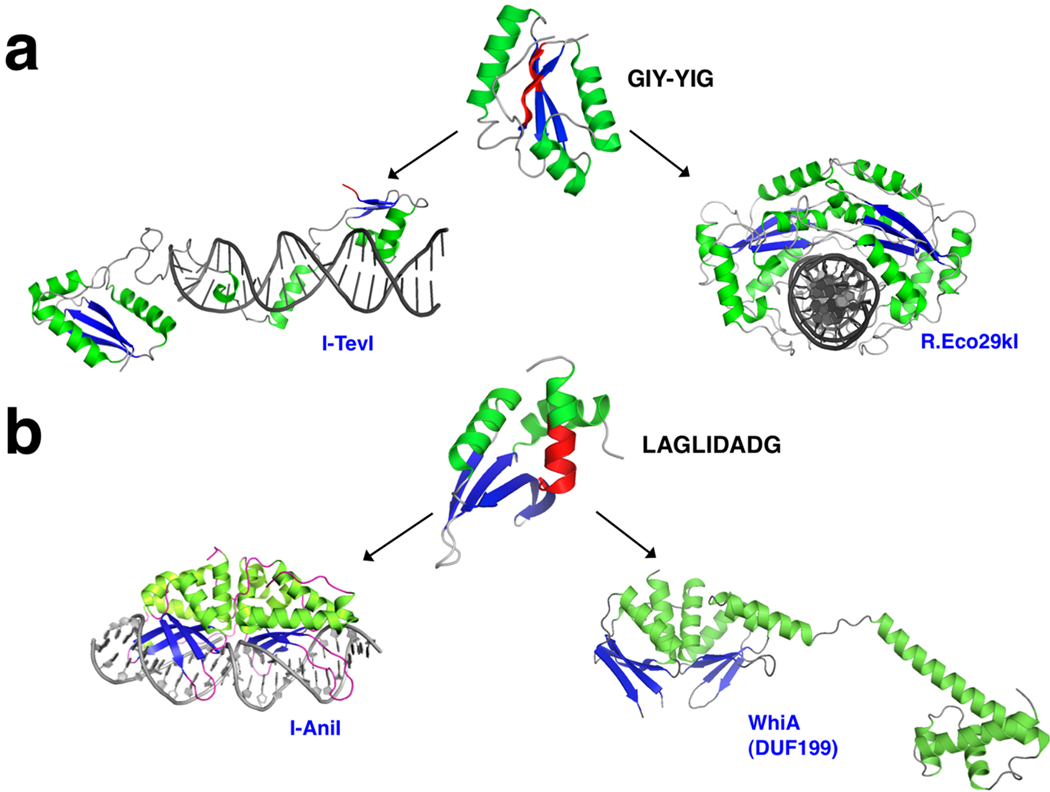
Figure 4. Evolution of homing endonucleases and disparate host gene products and functions from common nuclease ancestors.
a: The GIY-YIG nuclease motif (center) has given rise both to phage-specific, monomeric homing endonucleases such as I-TevI (left) (VanRoey et al., 2002; VanRoey et al., 2001) and multimeric bacterial restriction endonucleases such as R.Eco29k (right) (Mak et al., 2010). The GIY-YIG catalytic motif in the core nuclease fold is shown in red in the upper panel. b: The LAGLIDADG fold (with its namesake catalytic motif shown in red in center panel) has given rise to monomeric and homodimeric homing endonucleases (such as the I-AniI endonuclease from Aspergillus nidulans, left) (Bolduc et al., 2003) and the broadly distributed DUF199 gene family, found in gram positive bacteria (right) (Kaiser et al., 2009; Knizewski and Ginalski, 2007). In this latter gene family, the LAGLIDADG scaffold is fused to a C-terminal helixturn-helix domain; members of this bacterial protein family (such as the WhiA protein from Streptomyces coelicolor) are thought to act as genetic regulators during processes such as sporulation (Ainsa et al., 2000).
In the cases outlined above, the host-specific activities still involve the ability of these enzymes to catalyze phosphotransfer reactions or to promote the rearrangement of nucleic acid substrates. However, at least three instances have been documented where the biological function of a homing endonuclease scaffold has been more dramatically altered--with the protein in each case finding new employment as a transcription factor. In the first example, the DNA-binding domains found in 'Smad' proteins (eukaryotic transcription factors involved in TGF-β signaling) were found to be comprised of an endonuclease fold resembling the I-PpoI endonuclease (Flick et al., 1998)) (Grishin, 2001). Similarly, the DNA binding domain of the AP2/ERF family of plant transcription regulators contain a recognizable HNH endonuclease domain (Magnani et al., 2004).
Finally, two separate bioinformatics reports (Knizewski and Ginalski, 2007; Xie et al., 2007) and a recent crystallographic analysis (Kaiser et al., 2009) have demonstrated that proteins from the bacterial DUF199 gene family contain highly diverged LAGLIDADG domains (that no longer contain recognizable nuclease active site residues) fused to helix-turn-helix domains. The best studied of these factors (known as 'WhiA' from Streptomyces coelicolor) appears to act as an autoregulatory transcription factor for an operon-like cluster of genes that includes its own reading frame (Ainsa et al., 2000). Found in virtually all gram-positive bacteria, the DUF199/WhiA proteins are hypothesized to be involved in the regulation of networks of genes and proteins that are involved in growth transitions during various bacterial life cycles.
Engineering gene targeting reagents with LAGLIDADG proteins
The LAGLIDADG homing endonuclease (LHE) family (Figure 2c), which is the primary source of the enzymes that are used for gene targeting applications, is primarily encoded within archaea and in the chloroplast and mitochondrial genomes of algae and fungi (Chevalier et al., 2005; Dalgaard et al., 1997; Sethuraman et al., 2009). These enzymes are often referred to as 'Meganucleases', a term that reflects the name ‘Omega’ that was coined for the first known homing system, as well as the length of their DNA targets and the exceptional DNA cleavage specificity displayed by most of these enzymes (Paques and Duchateau, 2007). LAGLIDADG homing endonucleases are the scaffold of choice, in addition to zinc finger nucleases, for use in genetic and cell biology applications that require the generation of targeted DNA strand breaks at individual chromosomal loci.
LAGLIDADG endonucleases exist both as homodimers (where the two identical protein subunits are each typically 160 to 200 residues in size) and as monomeric proteins where a tandem repeat of two LAGLIDADG domains is connected by a variable peptide linker. Compared to their homodimeric cousins, the monomeric proteins are rather small: their individual domains are often only 100 to 120 residues in size. Monomeric LAGLIDADG enzymes can recognize fully asymmetric DNA target sites, and they often display disparate rates of cleavage of the two DNA strands in their target sequences, thereby generating transient nicked DNA intermediates en route to the final double strand break (Geese et al., 2003; Moure et al., 2008).
The DNA-binding surfaces of LAGLIDADG endonucleases can accommodate DNA targets of up to 24 base pairs; contacts to the individual halves of these DNA targets are largely segregated to the two corresponding protein domains or subunits. A number of studies have indicated that LAGLIDADG proteins display high fidelity at many of the basepair positions in their target sites (Gimble et al., 2003; Scalley-Kim et al., 2007; Thyme et al., 2009). Those positions that display reduced fidelity often correspond to the basepairs in the reading frame of the host gene that display coding degeneracy or 'wobble' (Edgell et al., 2004b; Gimble et al., 2003; Scalley-Kim et al., 2007). Much of their cleavage specificity appears to be realized at the transition state of the reaction (Jarjour et al., 2009; Thyme et al., 2009). In other words, these proteins may bind many related DNA sequences with comparable affinities, but they actually cleave a much smaller subset of sequences.
The sum of fidelities that is exhibited at individual DNA basepair positions by a typical LAGLIDADG enzyme results in a lower estimate for their overall specificity during in vitro cleavage experiments of at least 1 in 107 to 108. Additional interdependence between adjacent basepair substitutions increases this estimate to at least 1 in 109 (Argast et al., 1998; Arnould et al., 2006; Ashworth et al., 2010; Scalley-Kim et al., 2007). This value (which represents an enzyme’s activity and specificity under optimal in vitro digest conditions in the absence of competing protein factors) is likely to be a conservative estimate relative to the in vivo specificity displayed by a LAGLIDADG enzyme.
Well before their first crystal structures had been determined, the sequence specificity of LAGLIDADG homing endonucleases had been exploited in a series of experiments, using the I-SceI enzyme, that demonstrated the ability of LHEs to induce a targeted genetic modification in a complex eukaryotic genome. In these studies, I-SceI was expressed in transformed murine cells, where its site-specific cleavage activity at a previously integrated I-SceI target site resulted in the generation of a double strand break and the eventual markerless modification of the organism's germ line (Choulika et al., 1995; Rouet et al., 1994).
Based on the results in these and subsequent studies, it became obvious that modification of a homing endonuclease’s cleavage specificity would be required in order to target and modify endogenous target sites in various biological genomes. Consequently, from 2002 to 2005 several academic groups and one biotech company described methods to create mutated variants of LAGLIDADG enzymes that displayed altered DNA recognition specificities, for the purpose of extending DNA targeting applications to endogenous chromosomal recognition sites (reviewed in (Stoddard et al., 2007). These studies were facilitated by previously published descriptions of how protein-DNA recognition is largely accomplished by contacts with chemically compatible amino acid side chains (for example, as summarized in (Pabo and Sauer, 1992).
The earliest experiments to alter LAGLIDADG endonuclease specificity relied upon assays to visually identify mutated endonuclease constructs that displayed altered recognition specificity (Figure 5). These protocols utilized reporters of high affinity DNA binding (for example, through the use of a bacterial two-hybrid screening strategy) (Gimble et al., 2003) or methods that coupled endonuclease activity to the elimination of a reporter gene (Doyon et al., 2006; Gruen et al., 2002; Rosen et al., 2006; Seligman et al., 2002; Sussman et al., 2004). A more direct assay method was described in 2005, in which endonuclease cleavage activity was coupled to the recombination and reconstitution of a reporter gene (Chames et al., 2005).
Figure 5. Selections and direct redesign of homing endonuclease specificity.
a: Several methods have been developed to assay the ability of mutated variants of homing endonucleases to recognize and cleave specific DNA target sites. These assays included the elimination of an integrated β-galactosidase gene in E. coli (top) (Seligman et al., 2002), the elimination of a cell death protein in E. coli (middle) (Doyon et al., 2006; Gruen et al., 2002) or the conversion of two inactive copies of a reporter gene into a single active copy, as a result of endonuclease-induced recombination in yeast (bottom) (Chames et al., 2005). As described in the literature, this latter assay has been incorporated in to a high-throughput screen where activity against a large number of potential target sites is measured in parallel for individual homing endonuclease variants (Arnould et al., 2006; Smith et al., 2006). A change in the position of a positive clone in the array of possible targets (shown by arrows pointing to two adjacent colonies with different sequences) demonstrates a shift in target site specificity. The basis of this latter assay, which directly measures endonuclease-induced recombination in a living cell, has been modified and used in many different eukaryotic cell contexts with a variety of reporters, particularly green fluorescent protein (GFP). b: Direct examination of a homing endonuclease-DNA bound cocrystal structure, often with the use of structure-based computational tools, can also be used to redirect protein-DNA contacts and corresponding specificity (Ashworth et al., 2006; Chevalier et al., 2003). Such methods can be used directly to redirect specificity at individual contact positions, or can be used to focus subsequent mutations to reduced numbers of positions and possible mutated amino acid identities.
Over the same period of time, experiments that relied solely on structure based redesign of the protein-DNA interface, either by direct physical examination or by use of computational algorithms that repack and optimize new protein-DNA contacts, were also discussed and reported (Ashworth et al., 2006; Chevalier et al., 2003). Such methods can be used either to directly redesign homing endonuclease-DNA contacts at individual residues (thus bypassing selection approaches altogether), or to facilitate more efficient mutational screening of enzyme libraries (by reducing the number of protein residues to be randomized).
By 2005 the literature contained multiple examples demonstrating the mutation and alteration of DNA specificity of a LAGLIDADG homing endonuclease (specifically, I-CreI) at individual basepairs (Chames et al., 2005; Gruen et al., 2002; Seligman et al., 2002; Sussman et al., 2004). Soon thereafter, two separate studies described high-throughput experiments to redirect endonuclease specificity across contiguous 'pockets' of sequential basepairs (Arnould et al., 2006; Smith et al., 2006). In those experiments, alterations of specificity were found to display considerable contextdependence. Alteration of individual protein-DNA contacts that caused reduced activity or specificity were sometimes well tolerated in more extensively altered pockets; conversely, some alterations of protein-DNA contacts that behaved well on their own were found to be incompatible with substitutions at adjacent positions. A recent structure-based redesign approach using a closely related enzyme scaffold has recapitulated this result, and provided a high-resolution explanation for the unpredictable behavior of single-point redesigns relative to 'clusters' of altered protein-DNA contacts (Ashworth et al., 2010).
At the same time that the experiments described above were being performed on contact points between amino acid side chains DNA bases, several related studies demonstrated that entire domains or subunits from unrelated LAGLIDADG enzymes could be mixed and fused to create novel chimeric homing endonucleases that recognize corresponding chimeric DNA target sites (Chevalier et al., 2002; Epinat et al., 2003; Steuer et al., 2004). These studies demonstrated that the individual domains and subunits of LAGLIDADG enzymes are largely responsible for the recognition and binding of individual DNA half-sites. Subsequent experiments reinforced this conclusion (Fitzsimons-Hall et al., 2002; Steuer et al., 2004; Silva and Belfort, 2004; Silva et al., 2006). Together, these studies demonstrated that the task of altering a homing endonuclease’s cleavage specificity can be 'broken down' into two separate projects to individually target the left and right half-sites of a DNA target, by systematically altering the DNA-contacting residues of the protein’s N- and C-terminal domains and then combining the final solutions for each domain into a single gene targeting protein.
The present and future: homing endonuclease variants directed towards chromosomal targets
With tools now in hand to redirect the DNA cleavage specificities of homing endonucleases, these enzymes are now being used to study fundamental questions that surround the molecular biology of DNA strand break repair and gene conversion. For example, several laboratories have demonstrated that LAGLIDADG homing endonucleases can be altered to deliver either double strand breaks or single strand breaks (i.e. nicks) in a site-specific manner (McConnell Smith et al., 2009; Niu et al., 2008; Silva and Belfort, 2004). Subsequent studies using one of these altered endonucleases has demonstrated that a homing 'nickase' can stimulate homologous recombination at the enzyme's target site (albeit at a reduced efficiency as compared to a double strand break) but with greatly reduced frequency of end-joining and mutagenesis at that target, and with a corresponding reduction in toxicity (Metzger et al., 2010).
Such use of altered homing endonuclease activities are now being combined with new generations of cell-based reporter systems that can provide information on relative levels of competing strand break repair pathways (particularly homologous recombination versus end-joining) on a per-cell basis. In such experiments, the strand cleavage activity and kinetic behaviors of a homing endonuclease, along with the genomic background of the targeted cell population, can be systematically varied and used in to conduct a wide range of studies of DNA repair and correction (Arnould et al., 2010).
Using the protein design and selection approaches summarized earlier, derivatives of the I-CreI homing endonuclease have been generated that display sequence-specific cleavage and recombination activity against the human RAG1 gene at the site of mutations that give rise to a rare subset of severe combined immunodeficiency disease (or SCID) phenotypes (Grizot et al., 2009). The altered I-CreI enzymes used in this study induced high levels of gene correction via homologous recombination (up to 6% of transformed cells) while exhibiting minimal toxicity. This study represented the first time an engineered homing endonuclease was used in mammalian cells to target and modify a chromosomal target locus.
A number of additional studies reported by the same group have described the production of additional homing endonuclease variants (each using the same wild-type I-CreI protein as the initial scaffold) that are successfully directed at the human genes encoding XPC (Xeroderma Pigmentosum complementation group C) (Arnould et al., 2007) and dystrophin (which is associated with Duchenne muscular dystrophy) (Chapdelaine et al., 2010). Recently, strategies similar to those summarized above have been employed by a separate research group to create a variant of the same well-behaved I-CreI enzyme that could drive a targeted gene disruption event in corn (Gao et al., 2010). This latter study demonstrated that the information and tools developed over the past fifteen years by homing endonuclease investigators may now be employed by a broader community of molecular biologists to create an increasingly wide range of genome engineering tools for biotechnology, agriculture and medicine.
In concert with zinc-finger (Le Provost et al., 2010) and TAL (transcription activator like) proteins (Christian et al., 2010; Li et al., 2010), the discipline of site-specific genome engineering now enjoys a wealth of structural scaffolds for the continued development of gene targeting proteins. Each of these proteins display properties that are particularly well-suited for this field. Zinc finger and TAL proteins contain highly modular architectures that appear broadly engineerable for unique DNA specificities, while LAGLIDADG homing endonuclease tightly couple cognate site recognition to DNA strand cleavage activity and possess small structures that can be coded by short reading frames. Given the wide variety of applications that is envisioned within this field, it seems likely that each of these protein scaffolds will find significant future employment in biotechnology and medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ainsa JA, Ryding NJ, Hartley N, Findlay KC, Bruton CJ, Chater KF. WhiA, a protein of unknown function conserved among gram-positive bacteria, is essential for sporulation in Streptomyces coelicolor A3(2) J Bacteriol. 2000;182:5470–5478. doi: 10.1128/jb.182.19.5470-5478.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argast GM, Stephens KM, Emond MJ, Monnat RJ., Jr I-PpoI and I-CreI homing site sequence degeneracy determined by random mutagenesis and sequential in vitro enrichment. J Mol Biol. 1998;280:345–353. doi: 10.1006/jmbi.1998.1886. [DOI] [PubMed] [Google Scholar]
- Arnould S, Chames P, Perez C, Lacroix E, Duclert A, Epinat JC, Stricher F, Petit AS, Patin A, Guillier S, et al. Engineering of large numbers of highly specific homing endonucleases that induce recombination on novel DNA targets. J Mol Biol. 2006;355:443–458. doi: 10.1016/j.jmb.2005.10.065. [DOI] [PubMed] [Google Scholar]
- Arnould S, Delenda C, Grizot S, Desseaux C, Paques F, Silva GH, Smith J. The I-CreI meganuclease and its engineered derivatives: applications from cell modification to gene therapy. Protein Eng Des Sel. 2010 doi: 10.1093/protein/gzq083. epub ahead of print (doi:10.1093/protein/gzq083). [DOI] [PubMed] [Google Scholar]
- Arnould S, Perez C, Cabaniols J-P, Smith J, Gouble A, Grizot S, Epinat J-C, Duclert A, Duchateau P, Paques F. Engineered I-CreI derivatives cleaving sequences from the human XPC gene can induce highly efficient gene correction in mammalian cells. J Mol Biol. 2007;371:49–65. doi: 10.1016/j.jmb.2007.04.079. [DOI] [PubMed] [Google Scholar]
- Ashworth J, Havranek JJ, Duarte CM, Sussman D, Monnat RJ, Jr, Stoddard BL, Baker D. Computational redesign of endonuclease DNA binding and cleavage specificity. Nature. 2006;441:656–659. doi: 10.1038/nature04818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth J, Taylor GK, Havranek JJ, Quadri SA, Stoddard BL, Baker D. Computational reprogramming of homing endonuclease specificity at multiple adjacent base pairs. Nucleic Acids Res. 2010;38:5601–5608. doi: 10.1093/nar/gkq283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfort M, Perlman PS. Mechanisms of intron mobility. J Biol Chem. 1995;270:30237–30240. doi: 10.1074/jbc.270.51.30237. [DOI] [PubMed] [Google Scholar]
- Belfort M, Roberts RJ. Homing endonucleases - keeping the house in order. Nucleic Acids Res. 1997;25:3379–3388. doi: 10.1093/nar/25.17.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biertumpfel C, Yang W, Suck D. Crystal structure of T4 endonuclease VII resolving a Holliday junction. Nature. 2007;449:616–620. doi: 10.1038/nature06152. [DOI] [PubMed] [Google Scholar]
- Bolduc JM, Spiegel PC, Chatterjee P, Brady KL, Downing ME, Caprara MG, Waring RB, Stoddard BL. Structural and biochemical analyses of DNA and RNA binding by a bifunctional homing endonuclease and group I intron splicing factor. Genes Dev. 2003;17:2875–2888. doi: 10.1101/gad.1109003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolotin M, Coen D, Deutsch J, Dujon B, Netter P, Petrochilo E, Slonimski PP. La recombinaison des mitochondries chez Saccharomyces cerevisiae. Bull Inst Pasteur Paris. 1971;69:215–239. [Google Scholar]
- Bos JL, Heyting C, Borst P, Arnberg AC, Van Bruggen EF. An insert in the single gene for the large ribosomal RNA in yeast mitochondrial DNA. Nature. 1978;275:336–338. doi: 10.1038/275336a0. [DOI] [PubMed] [Google Scholar]
- Chames P, Epinat JC, Guillier S, Patin A, Lacroix E, Paques F. In vivo selection of engineered homing endonucleases using double-strand break induced homologous recombination. Nucleic Acids Res. 2005;33:e178. doi: 10.1093/nar/gni175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapdelaine P, Pichavant C, Rousseau J, Paques F, Tremblay JP. Meganucleases can restore the reading frame of a mutated dystrophin. Gene Therapy. 2010;17:846–858. doi: 10.1038/gt.2010.26. [DOI] [PubMed] [Google Scholar]
- Chevalier B, RJ Monnat J, Stoddard BL. The LAGLIDADG homing endonuclease family. In: Belfort M, Wood D, Derbyshire V, Stoddard B, editors. Homing endonucleases and inteins. Berlin: Springer Verlag; 2005. pp. 34–47. [Google Scholar]
- Chevalier B, Turmel M, Lemieux C, Monnat RJ, Stoddard BL. Flexible DNA target site recognition by divergent homing endonuclease isoschizomers I-CreI and I-MsoI. J Mol Biol. 2003;329:253–269. doi: 10.1016/s0022-2836(03)00447-9. [DOI] [PubMed] [Google Scholar]
- Chevalier BS, Kortemme T, Chadsey MS, Baker D, RJ Monnat J, Stoddard BL. Design, activity and structure of a highly specific artificial endonuclease. Molec Cell. 2002;10:895–905. doi: 10.1016/s1097-2765(02)00690-1. [DOI] [PubMed] [Google Scholar]
- Chevalier BS, Monnat RJ, Jr, Stoddard BL. The homing endonuclease I-CreI uses three metals, one of which is shared between the two active sites. Nature Structural Biology. 2001;8:312–316. doi: 10.1038/86181. [DOI] [PubMed] [Google Scholar]
- Chevalier BS, Stoddard BL. Homing endonucleases: structural and functional insight into the catalysts of intron/intein mobility. Nucleic Acids Research. 2001;29:3757–3774. doi: 10.1093/nar/29.18.3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y, Qiu Y-L, Kuhlman P, Palmer JD. Explosive invasion of plant mitochondria by a group I intron. PNAS USA. 1998;95:14244–14249. doi: 10.1073/pnas.95.24.14244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choulika A, Perrin A, Dujon B, Nicolas JF. Induction of homologous recombination in mammalian chromosomes by using the I-SceI system of Saccharomyces cerevisiae. Mol Cell Biol. 1995;15:1968–1973. doi: 10.1128/mcb.15.4.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian M, Cermak T, Doyle EL, Schmidt C, Zhang F, Hummel A, Bogdanove AJ, Voytas DF. Targeting DNA double-strand breaks with TAL effector nucleases. Genetics. 2010;186:757–761. doi: 10.1534/genetics.110.120717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgaard JZ, Klar AJ, Moser MJ, Holley WR, Chatterjee A, Mian IS. Statistical modeling and analysis of the LAGLIDADG family of site-specific endonucleases and identification of an intein that encodes a site-specific endonuclease of the HNH family. Nucleic Acids Res. 1997;25:4626–4638. doi: 10.1093/nar/25.22.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassa B, London N, Stoddard BL, Schueler-Furman O, Pietrokovski S. Fractured genes: a novel genomic arrangement involving new split inteins and a new homing endonuclease family. Nucleic Acids Res. 2009;37:2560–2573. doi: 10.1093/nar/gkp095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delahodde A, Goguel V, Becam AM, Creusot F, Perea J, Banroques J, Jacq C. Site-specific DNA endonuclease and RNA maturase activities of two homologous intron-encoded proteins from yeast mitochondria. Cell. 1989;56:431–441. doi: 10.1016/0092-8674(89)90246-8. [DOI] [PubMed] [Google Scholar]
- Doyon JB, Pattanayak V, Meyer CB, Liu DR. Directed evolution and substrate specificity profile of homing endonuclease I-SceI. J Am Chem Soc. 2006;128:2477–2484. doi: 10.1021/ja057519l. [DOI] [PubMed] [Google Scholar]
- Dujon B. Group I introns as mobile genetic elements: facts and mechanistic speculations--a review. Gene. 1989;82:91–114. doi: 10.1016/0378-1119(89)90034-6. [DOI] [PubMed] [Google Scholar]
- Dunin-Horkawicz S, Feder M, Bujnicki JM. Phylogenomic analysis of the GIY-YIG nuclease superfamily. BMC Genomics. 2006;7:98. doi: 10.1186/1471-2164-7-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgell DR, Belfort M, Shub DA. Barriers to intron promiscuity in bacteria. J Bacteriology. 2000;182:5281–5289. doi: 10.1128/jb.182.19.5281-5289.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgell DR, Derbyshire V, Van Roey P, LaBonne S, Stanger MJ, Li Z, Boyd TM, Shub DA, Belfort M. Intron-encoded homing endonuclease I-TevI also functions as a transcriptional autorepressor. Nat Struct Mol Biol. 2004a;11:936–944. doi: 10.1038/nsmb823. [DOI] [PubMed] [Google Scholar]
- Edgell DR, Gibb EA, Belfort M. Mobile DNA elements in T4 and related phages: A review in the serieson bacteriophage T4 and its relatives. Virol J. 2010 doi: 10.1186/1743-422X-7-290. Epub ahead of print (doi:10.1186/1743-422X-7-290). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgell DR, Stanger MJ, Belfort M. Coincidence of cleavage sites of intron endonuclease I-TevI and critical sequences of the host thymidylate synthase gene. J Mol Biol. 2004b;343:1231–1241. doi: 10.1016/j.jmb.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Epinat JC, Arnould S, Chames P, rochaix P, Desfontaines D, Puzin C, Patin A, Zanghellini A, Paques F, Lacroix E. A novel engineered meganuclease induces homologous recombination in yeast and mammalian cells. Nucleic Acids Res. 2003;31:2952–2962. doi: 10.1093/nar/gkg375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faye G, Dennebouy N, Kujawa C, Jacq C. Inserted sequence in the mitochondrial 23S ribosomal RNA gene of the yeast Saccharomyces cerevisiae. Mol Gen Genet. 1979;168:101–109. doi: 10.1007/BF00267939. [DOI] [PubMed] [Google Scholar]
- Fitzsimons-Hall M, Noren CJ, Perler FB, Schildkraut I. Creation of an artificial bifunctional intein by grafting a homing endonuclease into a mini-intein. J Mol Biol. 2002;323:173–179. doi: 10.1016/s0022-2836(02)00912-9. [DOI] [PubMed] [Google Scholar]
- Flick KE, Jurica MS, Monnat RJ, Jr, Stoddard BL. DNA binding and cleavage by the nuclear intron-encoded homing endonuclease I-PpoI. Nature. 1998;394:96–101. doi: 10.1038/27952. [DOI] [PubMed] [Google Scholar]
- Friedhoff P, Franke I, Meiss G, Wende W, Krause KL, Pingoud A. A similar active site for non-specific and specific endonucleases. Nat Struct Biol. 1999;6:112–113. doi: 10.1038/5796. [DOI] [PubMed] [Google Scholar]
- Gao H, Smith J, Yang M, Jones S, Djukanovic V, Nicholson MG, West A, Bidney D, Falco SC, Jantz D, Lyznik LA. Heritable targeted mutagenesis in maize using a designed endonuclease. Plant J. 2010;61:176–187. doi: 10.1111/j.1365-313X.2009.04041.x. [DOI] [PubMed] [Google Scholar]
- Geese WJ, Kwon YK, Wen X, Waring RB. In vitro analysis of the relationship between endonuclease and maturase activities in the bi-functional group I intron-encoded protein, I-AniI. Eur J Biochem. 2003;270:1543–1554. doi: 10.1046/j.1432-1033.2003.03518.x. [DOI] [PubMed] [Google Scholar]
- Gimble FS, Moure CM, Posey KL. Assessing the plasticity of DNA target site recognition of the PI-SceI homing endonuclease using a bacterial two-hybrid selection system. J Mol Biol. 2003;334:993–1008. doi: 10.1016/j.jmb.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Goguel V, Delahodde A, Jacq C. Connections between RNA splicing and DNA intron mobility in yeast mitochondria: RNA maturase and DNA endonuclease switching experiments. Molecular & Cellular Biology. 1992;12:696–705. doi: 10.1128/mcb.12.2.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich-Blair H, Shub DA. Beyond homing: competition between intron endonucleases confers a selective advantage on flanking genetic markers. Cell. 1996;84:211–221. doi: 10.1016/s0092-8674(00)80976-9. [DOI] [PubMed] [Google Scholar]
- Grishin NV. Mh1 domain of Smad is a degraded homing endonuclease. J Mol Biol. 2001;307:31–37. doi: 10.1006/jmbi.2000.4486. [DOI] [PubMed] [Google Scholar]
- Grizot S, Smith J, Daboussi F, Prieto J, Redondo P, Merino N, Villate M, Thomas S, Lemaire L, Montoya G, et al. Efficient targeting of a SCID gene by an engineered single-chain homing endonuclease. Nucleic Acids Res. 2009;37:5405–5419. doi: 10.1093/nar/gkp548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruen M, Chang K, Serbanescu I, Liu DR. An in vivo selection system for homing endonuclease activity. Nucleic Acids Res. 2002;30:29–34. doi: 10.1093/nar/30.7.e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke RM, Butow RA, Perlman PS. Maturase and endonuclease functions depend on separate conserved domains of the bifunctional protein encoded by the group I intron aI4 alpha of yeast mitochondrial DNA. EMBO Journal. 1995;14:5094–5099. doi: 10.1002/j.1460-2075.1995.tb00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y, Kim SJ, Waring RB. A protein encoded by a group I intron in Aspergillus nidulans directly assists RNA splicing and is a DNA endonuclease. PNAS USA. 1997;94:8994–8999. doi: 10.1073/pnas.94.17.8994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquier A, Dujon B. An intron-encoded protein is active in a gene conversion process that spreads an intron into a mitochondrial gene. Cell. 1985;41:383–394. doi: 10.1016/s0092-8674(85)80011-8. [DOI] [PubMed] [Google Scholar]
- Jarjour J, West-Foyle H, Certo MT, Hubert CG, Doyle L, Getz MM, Stoddard BL, Scharenberg AM. High-resolution profiling of homing endonuclease binding and catalytic specificity using yeast surface display. Nucleic Acids Res. 2009;37:6871–6880. doi: 10.1093/nar/gkp726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Binkowski G, Simon LD, Norris D. Ho endonuclease cleaves MAT DNA in vitro by an inefficient stoichiometric reaction mechanism. J Biol Chem. 1997;272:7352–7359. doi: 10.1074/jbc.272.11.7352. [DOI] [PubMed] [Google Scholar]
- Jurica MS, Monnat RJ, Jr, Stoddard BL. DNA recognition and cleavage by the LAGLIDADG homing endonuclease I- CreI. Mol Cell. 1998;2:469–476. doi: 10.1016/s1097-2765(00)80146-x. [DOI] [PubMed] [Google Scholar]
- Kaiser BK, Clifton MC, Shen BW, Stoddard BL. The structure of a bacterial DUF199/WhiA protein: domestication of an invasive endonuclease. Structure. 2009;17:1368–1376. doi: 10.1016/j.str.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knizewski L, Ginalski K. Bacterial DUF199/COG1481 proteins including sporulation regulator WhiA are distant homologs of LAGLIDADG homing endonucleases that retained only DNA binding. Cell Cycle. 2007;6:1666–1670. doi: 10.4161/cc.6.13.4471. [DOI] [PubMed] [Google Scholar]
- Kuhlmann UC, Moore GR, James R, Kleanthous C, Hemmings AM. Structural parsimony in endonuclease active sites: should the number of homing endonuclease families be redefined? FEBS Letters. 1999;463:1–2. doi: 10.1016/s0014-5793(99)01499-4. [DOI] [PubMed] [Google Scholar]
- Lambowitz AM, Belfort M. Introns as mobile genetic elements. Annu Rev Biochem. 1993;62:587–622. doi: 10.1146/annurev.bi.62.070193.003103. [DOI] [PubMed] [Google Scholar]
- Le Provost F, Lillico S, Passet B, Young R, Whitelaw B, Vilotte JL. Zinc finger nuclease technology heralds a new era in mammalian transgenesis. Trends Biotechnol. 2010;28:134–141. doi: 10.1016/j.tibtech.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Li T, Huang S, Jiang WZ, Wright D, Spalding MH, Weeks DP, Yang B. TAL nucleases (TALNs): hybrid proteins composed of TAL effectors and FokI DNA-cleavage domain. Nucleic Acids Res. 2010 doi: 10.1093/nar/gkq704. epub ahead of print; DOI 10.1093/nar/gkq704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo A, Leonard CW, Bassi GS, Berndt D, Krahn JM, Tanaka-Hall TM, Weeks KM. Evolution from DNA to RNA recognition by the bI3 LAGLIDADG maturase. Nat Struct Mol Biol. 2005 doi: 10.1038/nsmb976. in press. [DOI] [PubMed] [Google Scholar]
- Magnani E, Sjolander K, Hake S. From endonucleases to transcription factors: evolution of the AP2 DNA binding domain in plants. Plant Cell. 2004;16:2265–2277. doi: 10.1105/tpc.104.023135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak AN-S, Lambert AR, Stoddard BL. Folding, DNA recognition and function of GIY-YIG endonucleases: crystal structures of R.Eco29kI. Structure. 2010;18:1321–1331. doi: 10.1016/j.str.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell Smith A, Takeuchi R, Pellenz S, Davis L, Maizels N, Monnat RJ, Jr, Stoddard BL. Generation of a nicking enzyme that stimulates site-specific gene conversion from the I-AniI LAGLIDADG homing endonuclease. Proc Natl Acad Sci U S A. 2009;106:5099–5104. doi: 10.1073/pnas.0810588106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P, Katta K, Krishnaswamy S. HNH family subclassification leads to identification of commonality in the His-Me endonuclease superfamily. Protein Science. 2004;13:295–300. doi: 10.1110/ps.03115604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger MJ, McConnell-Smith A, Stoddard BL, Miller AD. Single-strand nicks induce homologous recombination with less toxicity than double-strand breaks using an AAV template. Nucleic Acids Res. 2010 doi: 10.1093/nar/gkq826. epub ahead of print (doi: 10.1093/nar/gkq826). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moure C, Gimble F, Quiocho F. Crystal structure of the intein homing endonuclease PI-SceI bound to its recognition sequence. Nature Struct Biol. 2002;9:764–770. doi: 10.1038/nsb840. [DOI] [PubMed] [Google Scholar]
- Moure CM, Gimble FS, Quiocho FA. The crystal structure of the gene targeting homing endonuclease I-SceI reveals the origins of its target site specificity. J Mol Biol. 2003;334:685–696. doi: 10.1016/j.jmb.2003.09.068. [DOI] [PubMed] [Google Scholar]
- Moure CM, Gimble FS, Quiocho FA. Crystal structures of I-SceI complexed to nicked DNA substrates: snapshots of intermediates along the DNA cleavage reaction pathway. Nucleic Acids Res. 2008;36:3287–3296. doi: 10.1093/nar/gkn178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netter P, Petrochilo E, Slonimski PP, Bolotin-Fukuhara M, Coen D, Deutsch J, Dujon B. Mitochondrial genetics. VII. Allelism and mapping studies of ribosomal mutants resistant to chloramphenicol, erythromycin and spiramycin in S. cerevisiae. Genetics. 1974;78:1063–1100. doi: 10.1093/genetics/78.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Y, Tenney K, Li H, Gimble FS. Engineering variants of the I-SceI homing endonuclease with strand-specific and site-specific DNA-nicking activity. J Mol Biol. 2008;382:188–202. doi: 10.1016/j.jmb.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabo CO, Sauer RT. Transcription factors: Structural families and principles of DNA recognition. Ann Rev Biochem. 1992;61:1053–1095. doi: 10.1146/annurev.bi.61.070192.005201. [DOI] [PubMed] [Google Scholar]
- Paques F, Duchateau P. Meganucleases and DNA double-strand break-induced recombination: perspectives for gene therapy. Current Gene Therapy. 2007;7:49–66. doi: 10.2174/156652307779940216. [DOI] [PubMed] [Google Scholar]
- Roberts RJ, Belfort M, Bestor T, Bhagwat AS, Bickle TA, Bitinaite J, Blumenthal RM, Degtyarev S, Dryden DT, Dybvig K, et al. A nomenclature for restriction enzymes, DNA methyltransferases, homing endonucleases and their genes. Nucleic Acids Res. 2003;31:1805–1812. doi: 10.1093/nar/gkg274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen LE, Morrison HA, Masri S, Brown MJ, Springstubb B, Sussman D, Stoddard BL, Seligman LM. Homing endonuclease I-CreI derivatives with novel DNA target specificities. Nucleic Acids Res. 2006;34:4791–4800. doi: 10.1093/nar/gkl645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouet P, Smih F, Jasin M. Introduction of double-strand breaks into the genome of mouse cells by expression of a rare-cutting endonuclease. Mol Cell Biol. 1994;14:8096–8106. doi: 10.1128/mcb.14.12.8096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalley-Kim M, McConnell-Smith A, Stoddard BL. Coevolution of homing endonuclease specificity and its host target sequence. J Mol Biol. 2007;372:1305–1319. doi: 10.1016/j.jmb.2007.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman L, Chisholm KM, Chevalier BS, Chadsey MS, Edwards ST, Savage JH, Veillet AL. Mutations altering the cleavage specificity of a homing endonuclease. Nucleic Acids Res. 2002;30:3870–3879. doi: 10.1093/nar/gkf495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethuraman J, Majer A, Friedrich NC, Edgell DR, Hausner G. Genes within genes: multiple LAGLIDADG homing endonucleases target the ribosomal protein S3 gene encoded within an rnl group I intron of Ophiostoma and related taxa. Mol Biol Evol. 2009;26:2299–2315. doi: 10.1093/molbev/msp145. [DOI] [PubMed] [Google Scholar]
- Shen BW, Heiter DF, Chan S-H, Wang H, Xu S-Y, Morgan RD, Wilson GG, Stoddard BL. Unusual target site disruption by the rare-cutting HNH restriction endonuclease PacI. Structure. 2010;18:734–743. doi: 10.1016/j.str.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen BW, Landthaler M, Shub DA, Stoddard BL. DNA binding and cleavage by the HNH homing endonuclease I-HmuI. J Mol Biol. 2004;342:43–56. doi: 10.1016/j.jmb.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Silva GH, Belfort M. Analysis of the LAGLIDADG interface of the monomeric homing endonuclease I-DmoI. Nucleic Acids Res. 2004;32:3156–3168. doi: 10.1093/nar/gkh618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva GH, Belfort M, Wende W, Pingoud A. From monomeric to homodimeric endonucleases and back: engineering novel specificity of LAGLIDADG enzymes. J Mol Biol. 2006;361:744–754. doi: 10.1016/j.jmb.2006.06.063. [DOI] [PubMed] [Google Scholar]
- Smith J, Grizot S, Arnould S, Duclert A, Epinat JC, Chames P, Prieto J, Redondo P, Blanco FJ, Bravo J, et al. A combinatorial approach to create artificial homing endonucleases cleaving chosen sequences. Nucleic Acids Res. 2006;34:e149. doi: 10.1093/nar/gkl720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowska M, Czapinska H, Bochtler M. Crystal structure of the beta beta alpha-Me type II restriction endonuclease Hpy99I with target DNA. Nucleic Acids Res. 2009;37:3799–3810. doi: 10.1093/nar/gkp228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowska M, Czapinska H, Bochtler M. Hpy188I-DNA pre- and post-cleavage complexes--snapshots of the GIY-YIG nuclease mediated catalysis. Nucleic Acids Res. 2010 doi: 10.1093/nar/gkq821. epub ahead of print (doi: 10.1093/nar/gkq821). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuer S, Pingoud V, Pingoud A, Wende W. Chimeras of the homing endonuclease PI-SceI and the homologous Candida tropicalis intein: a study to explore the possibility of exchanging DNA-binding modules to obtain highly speciric endonucleases with altered specificity. Chembiochem. 2004;5:206–213. doi: 10.1002/cbic.200300718. [DOI] [PubMed] [Google Scholar]
- Stoddard BL. Homing endonuclease structure and function. Quarterly Reviews of Biophysics. 2005;38:49–95. doi: 10.1017/S0033583505004063. [DOI] [PubMed] [Google Scholar]
- Stoddard BL, Belfort M. Social networking between mobile introns and their host genes. Mol Microbiol. 2010;78:1–4. doi: 10.1111/j.1365-2958.2010.07217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard BL, RJ Monnat J, Scharenberg AM. Advances in engineering homing endonucleases for gene targeting: ten years after structures. In: Ozawa RBaK., editor. Progress in Gene Therapy: Autologous and cancer stem cell gene therapy. World Scientific eBooks; 2007. pp. 135–167. [Google Scholar]
- Sussman DJ, Chadsey M, Fauce S, Engel A, Bruett A, RJ Monnat J, Stoddard BL, Seligman LM. Isolation and characterization of new homing endonuclease specificities at individual target site positions. J Mol Biol. 2004;342:31–41. doi: 10.1016/j.jmb.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Szczepanek T, Lazowska J. Replacement of two non-adjacent amino acids in the S.cerevisiae bi2 intron-encoded RNA maturase is sufficient to gain a homing-endonuclease activity. EMBO J. 1996;15:3758–3767. [PMC free article] [PubMed] [Google Scholar]
- Thyme SB, Jarjour J, Takeuchi R, Havranek JJ, Ashworth J, Scharenberg AM, Stoddard BL, Baker D. Exploitation of binding energy for catalysis and design. Nature. 2009;461:1300–1304. doi: 10.1038/nature08508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turmel M, Cote V, Otis C, Mercier JP, Gray MW, Lonergan KM, Lemieux C. Evolutionary transfer of ORF-containing group I introns between different subcellular compartments (chloroplast and mitochondrion) Molecular Biology & Evolution. 1995;12:533–545. doi: 10.1093/oxfordjournals.molbev.a040234. [DOI] [PubMed] [Google Scholar]
- VanRoey P, Meehan L, Kowalski JC, Belfort M, Derbyshire V. Catalytic domain structure and hypothesis for function of GIY-YIG intron endonuclease I-TevI. Nat Struct Biol. 2002;9:806–811. doi: 10.1038/nsb853. [DOI] [PubMed] [Google Scholar]
- VanRoey P, Waddling CA, Fox KM, Belfort M, Derbyshire V. Intertwined structure of the DNA-binding domain of intron endonuclease I-TevI with its substrate. EMBO Journal. 2001;20:3631–3637. doi: 10.1093/emboj/20.14.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzlau JM, Saldanha RJ, Butow RA, Perlman PS. A latent intron-encoded maturase is also an endonuclease needed for intron mobility. Cell. 1989;56:421–430. doi: 10.1016/0092-8674(89)90245-6. [DOI] [PubMed] [Google Scholar]
- Xie Z, Li W, Tian Y, Liu G, Tan H. Identification and characterization of sawC, a whiA-like gene, essential for sporulation in Streptomyces ansochromogenes. Arch Microbiol. 2007;188:575–582. doi: 10.1007/s00203-007-0278-x. [DOI] [PubMed] [Google Scholar]
- Zhao L, Bonocora RP, Shub DA, Stoddard BL. The restriction fold turns to the dark side: a bacterial homing endonuclease with a PD-(D/E)-XK motif. Embo J. 2007;26:2432–2442. doi: 10.1038/sj.emboj.7601672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Pellenz S, Stoddard BL. Activity and Specificity of the Bacterial PD-(D/E)XK Homing Endonuclease I-Ssp6803I. J Mol Biol. 2008;385:1498–1510. doi: 10.1016/j.jmb.2008.10.096. [DOI] [PMC free article] [PubMed] [Google Scholar]