Abstract
Photodynamic therapy (PDT) is a rapidly developing cancer treatment that utilizes the combination of nontoxic dyes and harmless visible light to destroy tumors by generating reactive oxygen species. PDT produces tumor-cell destruction in the context of acute inflammation that acts as a ‘danger signal’ to the innate immune system. Activation of the innate immune system increases the priming of tumor-specific T lymphocytes that have the ability to recognize and destroy distant tumor cells and, in addition, lead to the development of an immune memory that can combat recurrence of the cancer at a later point in time. PDT may be also successfully combined with immunomodulating strategies that are capable of overcoming or bypassing the escape mechanisms employed by the progressing tumor to evade immune attack. This article will cover the role of the immune response in PDT anti-tumor effectiveness. It will highlight the milestones in the development of PDT-mediated anti-tumor immunity and emphasize the combination strategies that may improve this therapy.
Keywords: anti-tumor immunity, cancer vaccines, cytotoxic T-lymphocytes, damage-associated molecular patterns, dendritic cells, photodynamic therapy, Toll-like receptor agonists, tumor-associated antigens
Since Richard Nixon’s declaration to make the ‘conquest of cancer a national crusade’, our understanding of the development and propagation of cancer has considerably improved. As a result of major investments in cancer research and cancer prevention, treatment and survival has significantly improved over the last 40 years [1]. Consequently, the increasing knowledge created by basic scientific research becomes gradually translated into more (and sometimes more effective) treatment options [2]. Despite the increasing emergence of drugs produced by biotechnological techniques, in 2008 half a million individuals diagnosed with cancer died from their disease in the USA [1].
Some of these drugs directed against tumor-associated factors such as ligands, receptors and transduction signaling factors are expensive, intrinsically cannot be used in a broad population of cancer patients and often fail to demonstrate their superiority over conventional chemotherapeutic drugs [3–6]. Furthermore, treating tumors with such ‘one-target’ drugs poses other problems to physicians. Some tumors remain persistently resistant to treatment and others are only detectable in advanced stages [7–9]. In addition, some tumors seem to adapt to these specialized medicines. Each time a portion of their biological pathways is blocked, they circumvent these obstacles by developing alternative routes for survival. Despite their known drawbacks, conventional intervention including surgery, radiation therapy and chemotherapy remain the first option in the oncologist’s toolbox for the treatment of patients.
Photodynamic therapy (PDT) has been proven to be an interesting alternative to the three described treatment modalities in several indications [10,11]. This technique is based on the administration of a photosensitizing agent to a patient via topical or parenteral routes. Depending on its pharmacokinetic and pharmacodynamic properties, the intrinsically nontoxic photosensitizer (PS) selectively accumulates in the tumor cells and in the associated (morphologically modified) vasculature (Figure 1). Activation with light of an appropriate wavelength results in the activation of the PS from its electronic ground state (S0) into one of its excited states (Sn). In biological media, the excited PS then returns to its lowest excited state (S1) through internal conversion. From there, it can return to its ground state through heat dissipation, emission of fluorescence or convertion through spin-forbidden intersystem crossing (ISC) into its lowest triplet state. This long-lived triplet state can lose its energy through the emission of phosphorescence or by exchange of energy with its direct environment via collisional energy transfer. In the case of the presence of molecular oxygen, 3O2 is converted into highly reactive 1O2, which in turn gives rise to reactive oxygen species, ultimately destroying vital cellular targets in the close proximity [12]. Importantly, only the concomitant presence of all three constituents in PDT will result in a notable photodynamic action. Therefore, even in the case of a less selective accumulation of the PS in the target tissue, the selectivity of PDT can be managed by the presence/absence of one of the other two components.
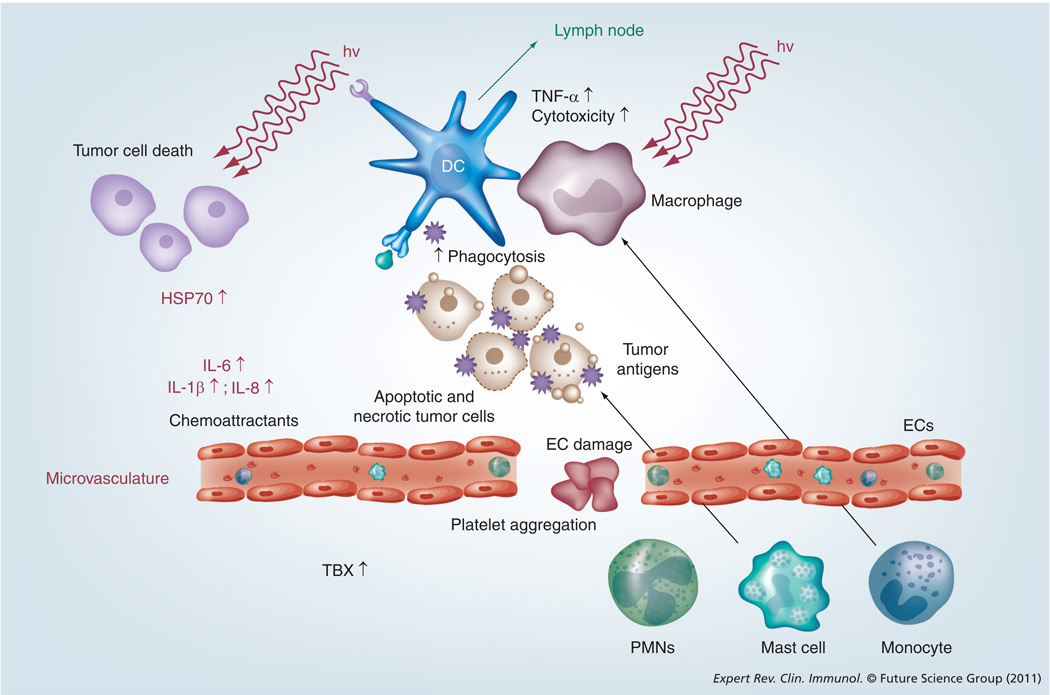
Figure 1. Anti-tumor mechanisms of photodynamic therapy.
Jablonski diagram illustrates the absorption of light by photosensitizer ground state to form a short-lived excited singlet state that can lose energy by fluorescence, internal conversion to heat, or can undergo intersystem crossing to long-lived PS triplet state that can carry out photochemistry. Subsequently this photochemistry leads to the local production of reactive oxygen species that are cytotoxic to tumor and endothelial cells.
HSP: Heat-shock protein; hv: Light; ISC: Intersystem crossing; PDT: Photodynamic therapy; S0: Ground state; S1: First excited singlet state; S2: Second excited singlet state; T1: First excited triplet state.
Photodynamic therapy has several advantages over conventional treatment modalities. Along with its aforementioned selectivity, it can be repeated several times, side effects are rare, it is relatively inexpensive compared with biotechnological products, and resistance to this treatment has only been reported in rare cases [13,14]. To date, several PSs are commercially available and some of them are approved for the treatment for oncologic indications. Most of these marketed PSs belong to chemical class of porphyrins, chlorins or precursors thereof (Figure 2) [15]. Since its early beginnings, PDT has made substantial progress. Prolonged skin photosensitization, a problem that essentially hampered the broad implementation of PDT, has been nearly eliminated by the development of second-generation PSs [16]. Furthermore, these compounds absorb in regions of the visible spectrum, optimal for deep-tissue penetration, and problems encountered with respect to the formulation of mostly lipophilic PSs have been resolved by pharmaceutical sciences [17]. However, PDT is not yet a front-line therapy for most indications, presumably owing to the lack of large randomized clinical trials. Furthermore, severe side effects can be observed with suboptimal PDT parameters, such as PDT schedules, PS and light doses, especially in hollow organs.
Figure 2. Clinically approved photosensitizers for photodynamic therapy.
5-ALA: 5-aminolevulinic acid; BPD-MA: Benzoporphyrin derivative monoacid ring A; HP: Hematoporphyrin; mTHPC: m-tetrahydroxyphenylchlorin.
In vivo, the curative or palliative effect from a photodynamic insult can be attributed to several, sometimes interconnected, biological and physiological effects (Figure 1). Depending on the localization of the PS within the organism and target cell, its concentration and also the light dose administered, PDT can exert its effect either through direct cell killing, occlusion of the tumor-associated vasculature or modulation of the immune system. At a cellular level, both necrosis and apoptosis have been observed as a primary reaction to the photodynamic insult [11,18–21]. Recently, it has also been reported that autophagy seems to play a role in PDT [22,23].
Previously, it was assumed that PDT-induced damage is confined to the irradiated area. However, in 1941, Blum hypothesized the presence of so-called dilator substances leading to the increased permeability of the minute vasculature due to the release of a ‘histamine-like’ or H-substance [24]. Indeed, it is now accepted that the direct damage of tumor cells initiates the initiation of several cell-signaling cascades. Furthermore, damage to, and damage of, endothelial cells (ECs) will ultimately lead to the formation of thromboses and eventually to vascular occlusion. In both cases, the release of cell fragments, cytokines and inflammatory mediators is triggered. This reaction, in turn, activates a multifaceted spectrum of the host’s response elements, including inflammation and innate or adaptive immunity. Both events are of essential importance for a pronounced systemic effect and the therapeutic outcome after PDT. In this article we will summarize how PDT stimulates the immune system. Early and late effects of PDT on the key players of the immune system and the consequences for the tumor on a local and systemic level will also be described.
PDT & anti-tumor immune response
The ideal cancer treatment modality should cause local tumor regression and eradication, as well as inducing a systemic anti-tumor immunity that could effectively eradicate distant metastases without toxicity to normal tissues. PDT may meet these expectations, since it produces acute inflammation and attracts immune cells to treat distant tumors. PDT-treated dying cells produce danger signals, which both increase the antigen presentation by dendric cells (DCs) and the recruitment of antigen-specific cytotoxic T lymphocytes (CTLs). This favorable response to the treatment can be impaired, however, by the mechanisms of immune escape employed by the tumor, in particular by intratumoral accumulation T regulatory cells (Tregs) [25]. It is therefore of the utmost importance that we understand the mechanisms that govern the PDT-induced anti-tumor immunity to be able to successfully exploit this phenomenon for the benefit of cancer patients.
The last 20 years of dedicated research have cast some light on PDT-mediated anti-tumor immune responses and our understanding of these processes has never been greater. In the following section, we review the significant body of work in this area.
Damage-associated molecular patterns
Researchers have recently discovered a second array of molecular motifs that may play a similar role to that of pathogen-associated molecular patterns (PAMPs). Instead of being associated with pathogenic microbes, these molecules are associated with host tissue damage. By analogy with the terminology used for PAMPs, this second class of early warning signals to the immune system was named damage-associated molecular patterns (DAMPs) [26–28]. DAMPs are intracellular molecules normally ‘hidden’ within live cells, which acquire various different properties such as immunostimulation upon exposure or secretion by sudden and uncontrolled induction of damaged and/or dying cells. DAMPs are thought to mediate the possible immunogenicity of dying cells. It has been proposed that hydrophobicity is probably the most ‘ancient’ danger signal capable of activating the innate immune system and hence most known DAMPs tend to contain many hydrophobic regions such as the chaperones belonging to the heat-shock protein (HSP) family [29]. A list of reported DAMPs is presented in Table 1.
Table 1.
Damage-associated molecular pattern molecules that may be released after photodynamic therapy of tumors.
| DAMP | Name | Function | Responder cells | Ref. |
|---|---|---|---|---|
| CRT | Calreticulin | ER-located calcium-binding protein | [145] | |
| HSP70, HSP90, gp96 | Heat-shock proteins | Inducible molecular chaperones on cell surface |
Monocytes, neutrophils | [146] |
| HMGB1 | High mobility group box-1 | Nuclear chromatin-binding protein | Monocytes, neutrophils | [147] |
| ATP | Adenosine triphosphate | High-energy molecule, normally intracellular |
Dendritic cells, microglia | [148] |
| S100S | Calgranulin family members | Calcium-binding proteins | Monocytes, neutrophils | [149] |
| SAP130 | Spliceasome-associated protein 130 |
Histone deacetylase complex subunit | Macrophages | [150] |
| dRP S19 | Covalent dimer of ribosomal protein S19 |
Constituent of small ribosomal subunit | Monocytes, neutrophils | [151] |
| Uric acid | Mono sodium urate | Derived from degradation of RNA and DNA |
Dendritic cells, neutrophils, CD4+ and CD8+ T cells |
[152] |
DAMP: Damage-associated molecular patterns; ER: Endoplasmic reticulum; HSP: Heat-shock protein; PDT: Photodynamic therapy.
The discovery of DAMPs may explain some contradictory reports concerning whether or not tumor cells that have been killed by apoptotic or by necrotic cell death pathways are immunogenic [30]. While it is generally accepted that necrotic tumor cells are proinflammatory and therefore likely to be immunogenic, the importance of necrotic tumor cell death for generating an immune response has not been specifically demonstrated in the case of PDT of cancer. It was considered that apoptotic cells in general and tumor cells that have been killed by apoptosis in particular were silently disposed of by macrophages and other phagocytic cells in a non-inflammatory fashion and were therefore unlikely to stimulate anti-tumor immunity [31,32]. However, other reports suggested that under certain circumstances, apoptotic tumor cells could be effective in generating an immune response [33,34]. Recently, researchers have begun to refer to immunogenic and non-immunogenic apoptosis [32,35,36]. It is likely that the original concept of programmed cell death that is known to be a major aspect of embryogenesis is non-inflammatory and non-immunogenic, but some modalities of cancer therapy that cause tumor damage (by certain chemotherapy agents or by PDT regimens) produce an inflammatory and therefore an immunogenic form of apoptosis, largely characterized by the release of DAMPS.
Although there have not as yet been many studies that have looked extensively at the release of DAMPs after PDT, the subject has begun to be studied [27,28]. The most frequently reported example of DAMP expression after PDT is the upregulation and translocation of HSPs to the cell membrane [37,38].
Involvement of innate immune response in anti-tumor PDT
The innate immune system consists of all the immune defenses that lack immunologic memory. Thus, a characteristic of innate responses is that they remain unchanged regardless of how often the antigen is encountered. The innate immune system consists of several immune cells that all participate is the first line of defense [39] and PDT has been shown to effectively engage them in the host’s inflammatory responses to cancer [40,41]. PDT alters the tumor microenvironment by stimulating the release or expression of various proinflammatory and acute-phase response mediators from the PDT-treated site (Figure 3). They include complement proteins, HSPs, arachidonic acid derivatives, chemokines and cytokines such as TNF-α, IL-6 and IL-1 [42]. It is thought that PDT causes this inflammatory response in treated solid tumors by causing a significant rise in oxidative stress, thereby severely damaging cellular membranes and cytoplasmic structures. The body recognizes the presence of local trauma threatening the integrity of the affected site, and releases proinflammatory mediators to maintain homeostasis [43]. PDT thereby prompts a powerful acute inflammatory response, causing activation of complement and the accumulation of neutrophils and other inflammatory cells in large numbers at the treated site and to attack tumor cells [44,45]. In particular, the complement system has emerged as a powerful mediator of the effects of PDT on tumor cells and in vitro studies have indicated that PDT induces fixation of complement C3 protein to tumor cells [46]. Complement fixation, in turn, marks cells as targets for destruction by the innate immune system [47–49]. Complement not only acts as a direct mediator of inflammation but also stimulates cells to release secondary inflammatory mediators, including the cytokines IL-1β, TNF-α, IL-6, IL-10, granulocyte colony-stimulating factor, thromboxane, prostaglandins, leukotrienes, histamine and coagulation factors [50].
Figure 3. Photodynamic therapy of tumors leads to the development of local inflammation mediated by the localized release of danger signals, cytokines and derivatives of arachidonic acid.
The infiltration of the treated area by various cells of the immune system follows.
EC: Endothelial cell; HSP: Heat-shock protein; hv: Light; PMN: Polymorphonuclear neutrophil; TBX: Thromboxane.
Adapted with permission from [25].
In addition to stimulating local inflammation, PDT acts systemically to induce a potent acute phase response [45]. Using animal tumor models subjected to PDT, researchers observed a dramatic rise in serum levels of established acute-phase reactants, including serum amyloid P component and mannose-binding lectin A. Upregulation of genes encoding C-reactive protein was also noted [51]. Furthermore, the acute-phase response causes marked neutrophilia by accelerating maturation of neutrophils in the bone marrow as well as increasing neutrophil recruitment from storage pools [50]. In the following section, we discuss in detail the involvement of several classes of immune cells in the PDT anti-tumor response.
PDT & macrophages
Macrophages are phagocytic cells derived from blood-borne monocytes that are known to express a wide range of membrane cellular receptors that can recognize numerous endogenous and exogenous ligands [52]. In addition, macrophages have receptors for antibodies and complement, so that the coating of microorganisms with antibodies, complement or both enhances phagocytosis. The subsequent response is central to their functions in homeostasis as well as to host defense and they can be directly cytotoxic to tumor cells as well as engage in the activation of adaptive immunity through presentation of tumor antigens (TAs).
There are reports based on in vitro data that PDT can have an effect on monocyte/macrophage cell lineages. Macrophages can be activated by low sublethal doses of PDT [53] and secrete TNF-α [54] by a PDT-related increase in macrophage -activating factor [55,56]. Evidence also indicates that macrophages can show preferential cytotoxicity towards tumor cells treated with a sub-lethal dose of PDT [57] and that this effect may be due to potential interaction between macrophages and natural killer cells (NK) [58]. Macrophage functions can also be enhanced by several cytokines and when Krosl et al. repeatedly injected lethally irradiated squamous cell carcinoma (SCC) VII cells genetically engineered to produce granulocyte/macrophage colony stimulating factor (GM-CSF), they observed higher cytotoxic activity of tumor-associated macrophages against PDT-treated tumors [59].
PDT & neutrophils
The granulocytes are another group of cells that form the innate immune system. This group of cells is formed by neutrophils, basophils and eosinophils [60]. Contrary to macrophages, they are only weakly phagocytic and their main function is to secrete leukotrienes, prostaglandins and other cytokines to facilitate the development of the inflammatory response. The neutrophils and their responses are probably the most studied subject in the ‘PDT and immunology’ field.
Gollnick et al. demonstrated that 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-α (HPPH)-mediated-PDT caused neutrophil migration into the treated tumor area due to a transient and local increase in the expression of the chemokine macrophage inflammatory protein-2 (the murine equivalent of IL-8), together with increased expression of the adhesion molecule E-selectin [42]. Interestingly, they also found that the local and systemic increase in expression of IL-6 was not necessary for neutrophil recruitment. A subsequent report compared a low and a high fluence (total light energy) each delivered at a low and high fluence rate against Colo 26 murine tumor treated with HPPH [61]. The oxygen-conserving low fluence rate PDT at a high fluence yielded 70–80% tumor cures, whereas the same fluence at the oxygen-depleting high fluence rate yielded 10–15% tumor cures. The highest levels of inflammatory cytokines and neutrophilic infiltrates were measured with low fluence delivered at low fluence rate (10–20% cures) while the optimally curative PDT regimen (high fluence at low fluence rate) produced minimal inflammation. Interestingly, the depletion of neutrophils did not significantly change the high cure rates of that regimen but abolished curability in the maximally inflammatory regimen. This group went on to subsequently demonstrate that mice defective in neutrophil homing to peripheral tissues (CXCR2(−/−) mice) or mice depleted of neutrophils were unable to mount strong anti-tumor CD8+ T-cell responses following PDT [62]. The lack of neutrophils seemed to be disturbing T-cell proliferation and/or survival and these results further support the notion that tumor-infiltrating neutrophils play an essential role in the establishment of anti-tumor immunity following PDT. Sluiter et al. first observed that neutrophils adhere to the microvascular wall after PDT in vivo [63] and that EC retracted after PDT allowing the adherence of neutrophils by their β2-integrin adhesion receptors to the subendothelial matrix [64]. In agreement with this finding was a report describing that expression levels of the adhesion molecules ICAM-1 and VCAM-1 were downregulated on ECs after PDT [65]. The administration of anti-neutrophil serum together with PDT in rhabdomyosarcoma-bearing rats completely abrogated the expected anti-tumor PDT effects, providing additional information that neutrophil infiltration of the PDT-treated area is essential for an effective anti-tumor response [66]. Blocking ICAM-1 with monoclonal antibodies also reduced the number of tumor cures and a noticeable upregulation of ICAM1 ligands CD11b/c expressed by neutrophils was also associated with PDT-treated tumors [67]. An increase in the number of peripheral blood neutrophils was also found 4 h after PDT treatment and lasted for 24 h. It was preceded by an increase in serum levels of IL-1β. Anti-G-CSF antibodies decreased neutrophil numbers and decreased the efficacy of PDT. Krosl and colleagues investigated cellular infiltrate in the murine SCCVII model treated with Photofrin® PDT (Axcan Pharma, AL, USA) [44]. They reported a 200-fold rise in neutrophils. Cecic et al. established prominent and rapid neutrophilia after PDT (Photofrin or m-tetrahydroxyphenylchlorin) of mice with SCCVII or EMT6 mammary carcinomas and found that complement inhibition completely prevented this [68]. de Bruijn et al. published a study in which a treatment of rat rhabdomyosarcoma tumors with 5-aminolevulinic acid-PDT lead to a significant increase in blood neutrophils during the first few days after illumination, with the highest observed levels at 16 and 24 h post-treatment [69].
PDT & NK-cell recruitment
In a recent report, Kabingu et al. used NK cells depletion in severe combined immunodeficient (SCID) mice to assess the involvement of this cell lineage in PDT-induced immunity [70]. Mice were injected subcutaneously and intravenously at the same time with EMT/6 tumor cells to establish both lung and subcutaneous tumors; the subcutaneous tumors alone received PDT treatment. They observed that the number of lung tumors per mouse 10 days after PDT was significantly higher in NK-depleted animals, suggesting that NK cells participate in PDT-induced immune control of tumors. Moreover, in the absence of NK cells, SCID mice replenished with CD8+ T cells exhibited a significant increase in lung tumor number. The authors concluded that that NK cells may play an important role in post-PDT activity of CD8+ T cells and control of distant nontreated metastases.
PDT & DCs
Dendritic cells are the most potent APCs and a key element in the development of an anti-tumor immune response. There are two well-established maturation states for DCs that include the ‘immature’ and ‘mature’ states [71]. The decision ‘to mature’ is hugely influenced by the signals coming from the environment. In many instances, the tumor microenvironment not only fails to provide the proinflammatory signals needed for efficient DC activation, but also provides additional immunosuppressive mechanisms that actively inhibit it. These factors affect the differentiation of DCs, leading to decreased levels of functionally competent, mature APCs, and accumulation of immature dendritic cells [72]. The immature DCs are those that constantly sample their environment, capture antigens and migrate in small numbers to draining lymph nodes. They display a phenotype reflecting their specialized function as antigen-capturing cells. They are highly endocytic, able to acquire fluid-phase antigens by macropinocytosis, take up protein or antigen-antibody immune complexes by receptor-mediated endocytosis, and ingest entire cells by phagocytosis. They express relatively low levels of surface MHCI and MHCII gene products and costimulatory molecules such as CD80 and CD86.
In the absence of inflammation, the DCs remain in an immature state, and antigens are presented to T cells in the lymph node without costimulation, leading to either the deletion of T cells or the generation of inducible Tregs. Tissue inflammation induces the maturation of DCs and the migration of large numbers of mature DCs to draining lymph nodes. The mature DCs express peptide–MHC complexes at the cell surface, as well as appropriate costimulatory molecules. This allows the priming of CD4+ T helper cells and CD8+ CTLs, the activation of B cells and the initiation of an adaptive immune response.
It has been rapidly realized that DC may play an important role in PDT-mediated anti-tumor immunity. One of the major cellular factors induced by PDT and released from tumor cells is extracellular HSP70. HSP70 expression is prompted by cellular stress and, when HSP remains intracellular, it chaperones unfolded proteins and inhibits cell death by preventing the aggregation of cellular proteins [73]. This forms stable complexes with cytoplasmic TAs that can then either be displayed at the surface of cellular membrane or escape intact from dying necrotic cells to interact with APCs such as DCs and stimulate an anti-tumor immune response [37]. DCs as well as other APCs have high-affinity receptors on their surface and binding of HSP–antigen complexes leads to the effective activation and maturation of DCs and the subsequent presentation of the peptide antigen to CD8+ cytotoxic T cells [74]. PDT mediated by three different PSs has been shown to increase HSP70 mRNA, but only mono-l-aspartyl chlorin-e6 and tin etiopurpurin, and not Photofrin, increased HSP70 protein levels in mouse tumor cells in vitro and in tumors in vivo [38]. The release of HSP-bound TAs that can easily be taken up by DCs from PDT-induced necrotic tumor cells may therefore explain the particular efficiency of PDT in stimulating an immune response against tumors. It has also been observed that PDT-generated lysates were able to induce phenotypic DC maturation and IL-12 expression. Korbelik and Sun produced a vaccine by treating SCCVII cells with benzoporphyrin derivative (BPD)-PDT and later with a lethal x-ray dose, and showed that these cells, when injected peritumorally in mice with established SCCVII tumors, produced a significant therapeutic effect, including growth retardation, tumor regression and cure [75]. Importantly, vaccine cells retrieved from the treatment site at 1 h postinjection were intermixed with DCs, exhibited HSP70 on their surface, and were opsonized by complement C3.
PDT & adaptive immunity
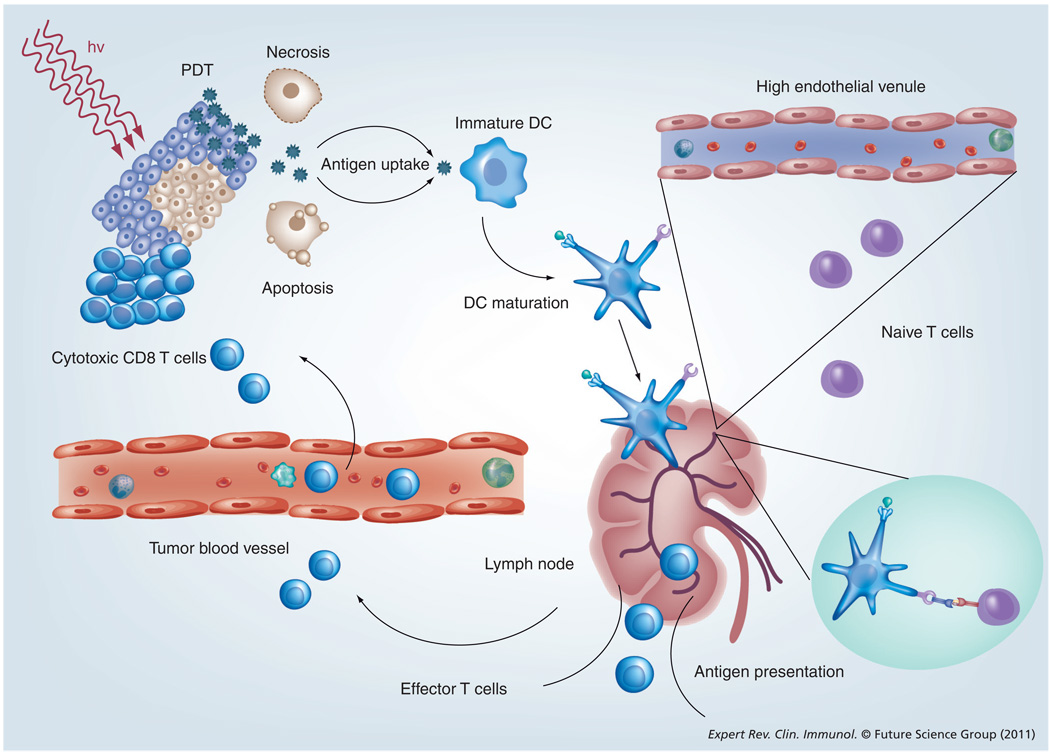
Adaptive immunity is provided by antigen-specific B and T cells. B cells produce immunoglobulins, the antigen-specific antibodies responsible for eliminating extracellular microorganisms. T cells are divided into several populations and they can be involved in helping B cells to make antibody or eradication of intracellular pathogens, activation of macrophages, killing of virally infected or cancer cells and finally immunosuppression. The involvement of the adaptive immune system in PDT is depicted in Figure 4.
Figure 4. Photodynamic therapy-induced local inflammation leads to the development of systemic immunity.
Antigens released from PDT-treated tumor cells are phagocytosed by DCs and presented to naive T cells in regional lymph nodes. Activated T cells return to the circulation and then track down and destroy tumors. BCG: Bacillus Calmette–Guérin; C.P.: Corynebacterium parvum; DC: Dendritic cell; G-CSF: Granulocyte colony-stimulating factor; GM-CSF: Granulocyte-macrophage colony-stimulating factor; hv: Light; MCWE: Mycobacterium cell wall extract; PDT: Photodynamic therapy; SPG: Schizophylan.
Adapted with permission from [25].
PDT activation of CD8+ T cells
The first evidence on the involvement of cytotoxic T cells in the PDT anti-tumor effects comes from Canti and colleagues [76] who examined the effects of PDT in both immunosuppressed and normal mice bearing MS-2 fibrosarcomas. All mice were cured and survived indefinitely, but resistance to MS-2 rechallenge was evident only in normal surviving animals cured by PDT, while immunosuppressed surviving animals and animals cured by surgery died after tumor rechallenge. Different syngeneic murine leukemias were not rejected. The subsequent studies with the adoptive transfer of splenic T lymphocytes from naive BALB/c mice into SCID mice performed before PDT provided additional evidence. It postponed the recurrence of treated tumors, while adoptive transfer performed immediately or 7 days after PDT had no benefit [77]. Adoptive transfer of non adherent splenocytes (a mixture of CD4+ and CD8+ T cells together with some B cells, NK cells and monocytes) from normal mice cured of EMT6 by PDT 5 weeks previously fully restored the curative effect of PDT on EMT6 tumors growing in SCID mice. Splenocytes obtained from donors cured by x-rays were much less effective. Additional studies used depletion to prove the role of cytotoxic T cells. The depletion of specific T-cell populations from donor splenocytes indicated that CD8+ CTLs had the most effect, while CD4+ helper T cells played a supportive role [78]. Analogous studies were performed by a different group using PDT with the PS 2-iodo-5-ethylamino-9-diethylaminobenzo-phenothiazinium chloride [79].
PDT & CD4+ T cells
Gollnick et al. demonstrated that CD4+ T cell depletion had no effect on the ability of PDT to control tumors present outside the treated area [70]. Splenocytes transferred to SCID mice after depletion of CD4+ T cells successfully controlled the growth of tumors and these observations were also confirmed in mice lacking CD40, a CD4+ T cell costimulatory molecule necessary for interaction with DCs. The tumor growth control after PDT was not significantly different compared with wild-type mice. To further elucidate the necessity of CD4+ T cells for PDT-induced immunity, they reconstituted SCID mice with only CD8+ cells and next inoculated them with EMT/6 tumors. The following PDT treatment resulted in 40 days of tumor-free survival and the development of memory immunity demonstrated by rejection of rechallenge with intravenous administration of the same tumor.
PDT & TAs
The mechanism by which the immune system can tackle tumors is by recognition of TAs presented by MHC class I molecules on the tumor cell surface and the subsequent destruction of these tumor cells by CTLs. The molecular identity of a number of these TAs has been well defined, both in mouse and human tumors [80]. The TAs defined to date broadly belong to three major groups:
Antigens encoded by cancer-testis genes of the melanoma antigen (MAGE)-type expressed in various tumors but not in normal tissues such as the mouse gene P1A and human genes of the MAGE, B melanoma antigen (BAGE) and G antigen (GAGE) families [81–86]. These antigens are tumor specific and absent on most normal adult cells. If they are present in normal tissues they are usually expressed in the immune-privileged sites like the testes and therefore are not exposed to the host immune system. They are also shared because they are present on many tumors of several histological types;
Differentiation antigens of the melanocytic lineage, which are present on most melanomas but also on normal melanocytes [86–88];
Antigens that result from tumor-specific mutations in genes, which are expressed in all tissues [89–93]. In most cases, the mutation is unique to a single tumor and these antigens are therefore individually specific.
Efforts have been made to introduce defined TAs into mouse tumor cell lines in order to have reproducible models to study. These are usually ‘foreign’ proteins such as β-galactosidase from bacteria [94], ovalbumin from chicken eggs [95] and viral influenza hemagglutinin [96], among others. For many of these TAs, the peptide sequence displayed in MHC-I molecules is known, and specific CTL lines that kill the tumor cells expressing these TAs have been produced. However successful this approach may be in the laboratory, the artifcial TAs are not clinically applicable and it would be preferable to study naturally occurring TAs. Therefore, we propose to study the antigen-specific PDT-induced anti-tumor immune response in a more clinically relevant setting by employing two naturally occurring cancer antigens: the mouse MAGE-type P1A antigen, the best described unmutated mouse cancer/testis tumor antigen that has been identified [81,97,98], and the E7 antigen associated with human papilloma virus-induced cervical cancer [99].
Our laboratory was the first to recognize the role of TAs in the anti-tumor immune response after PDT. We showed that a vascular PDT regimen was able to produce 100% long-term cures and rejection of rechallenge when tumors were formed in C3H mice by RIF1 cells that had been transduced to stably express enhanced green fluorescent protein by a retroviral vector [100]. The recently completed study goes even further and showed that PDT can induce a highly potent antigen-specific systemic immune response capable of causing regression in distant established tumors that received no light [101].
Additional information on the role of TAs in the PDT immune response comes from other laboratories. The report by Abdel-Hady et al. observed a short-term response in a third of patients with high-grade vulval intraepithelial neoplasia (VIN 2–3) treated with 5-aminolevulinic acid PDT [102]. In this study, Abdel-Hady investigated the level of human papilloma virus infection, the levels of HLA expression as well as the infiltration of the lesions by the cells of the immune system in biopsies from responders and nonresponders. Interestingly the nonresponders were more likely to show HLA class I loss while the responders showed increased infiltration by CD4+ and CD8+ CTLs. These data indirectly point to the role of tumor antigen presentation in the anti-tumor PDT immunity.
Recently, the role of tumor antigen in PDT anti-tumor immunity has been studied in the clinical setting. In the study by Kabingu et al., basal cell carcinoma lesions were either treated with PDT or surgically removed and the results showed that the immune recognition of hedgehog-interacting protein 1, a TA that is increased in some patients with basal cell carcinoma lesions, was significantly better in patients treated with PDT [103]. These findings showed that local tumor PDT can enhance systemic immune responses to tumor antigen in patients, and helped to validate previous preclinical findings.
PDT & Tregs
A special population among CD4+ T cells is Tregs. Tregs can be defined as a T-cell population that functionally suppresses an immune response. Tregs were initially described by Gershon et al. in the early 1970s and were called suppressor T cells [104]. There has recently been an explosion of interest in the role of Tregs in both mice and humans that led to many studies describing their involvement in both autoimmune disease [105] and cancer [106]. Tregs were initially characterized by coexpression of CD4 and high levels of CD25 (the high-affinity component of the IL-2R complex [IL-2R α-chain]) [107]. It was subsequently determined that the most specific marker for Tregs is the transcription factor Foxp3 [108], as other Treg markers (CD25, CTLA-4 and glucocorticoid-induced tumor necrosis factor receptor [GITR]) can be found on other T-cell subsets, especially on activated CD4+ T cells [109,110]. The transcription factor Foxp3 is specifically expressed in Tregs and is required for their development [111]. CD25+ Tregs comprise 5–10% of CD4+ T cells and have been divided into two main classes: naturally occurring Tregs found in the thymus and inducible Tregs found in the periphery [112]. Naturally occurring Tregs are thought to have T-cell receptors (TCRs) that recognize self-antigens and to play a major role in the prevention of autoimmune disease. Inducible Tregs can be induced and differentiate in the periphery, such as in the tumor microenvironment. Thymus-derived Tregs in the tumor microenvironment might clonally expand following stimulation by tumor-associated DCs that frequently have an immature phenotype. Tregs can also be present in a tumor as a result of conversion from CD4+CD25− T cells [113] under the influence of TGF-β, which is present at high levels in the tumor microenvironment [114]. Therefore the tumor (or tumor draining lymph nodes) might contain thymus-derived natural Tregs, expanded and converted natural Tregs, and locally differentiated and expanded T inducible regulatory type 1 cells.
It is thought that Tregs mediate their immunosuppressive effects by multiple pathways [115]. Tregs express CTLA-4, which binds to B7-1 and B7-2 costimulatory molecules on APCs but with affinities much higher than CD28, and produces negative signaling, rather than the positive signaling produced by the equivalent molecule, CD28 expressed on normal T cells [116]. T-cell activation is a dynamic process that is determined by the strength of the TCR signal; the strength of costimulation provided by CD28; and the magnitude of inhibitory signals generated by CTLA-4. Tregs also express GITR, however, this appears to reduce suppressor function on binding its ligand [117]. Treg can express TGF-β, which is an immunosuppressive cytokine [118] that can induce further proliferation of Tregs [119]. Recent evidence also suggests that Tregs control T-cell activation by suppression of DC activation [120]. In addition, imaging studies have suggested that Tregs diminish the ability of DCs to form stable contacts with self-reactive T cells and thereby diminish their activation [121].
Tumor-induced expansion of Tregs is a major obstacle to successful cancer immunotherapy. It has been shown that Tregs inhibit the generation of immune responses against tumors [112]. Many studies report that increased tumor Tregs predict reduced survival or treatment response. Tregs mediate their immunosuppressive effects by multiple pathways [115,116,118–120], and the targeting of these cells using antibodies[112] or cyclophosphamide (CY) [122] promotes rejection of several transplantable murine tumors [123,124], leading to complete and permanent regressions of established tumors [125] or an increase in the fraction of both CD4+ and CD8+ T cells with a memory phenotype [126].
Our laboratory was the first to realize that Tregs may play an important and negative role in PDT anti-tumor immunity. We observed that Tregs can be efficiently depleted by a low dose of CY and that low-dose CY combined with BPD-PDT led to a significant number of long-term J774 reticulum cell sarcoma cures and resistance to tumor rechallenge, while either treatment alone led to 100% death from progressive tumors or metastasis [127]. Cured mice had tumor-specific T cells in spleens and low-dose CY was shown to significantly reduce the numbers of Tregs. Our recent preliminary data revealed that Treg depletion by CY can unravel PDT-mediated immune response to mouse autoantigen gp70 in the colon adenocarcinoma CT26WT model [Mroz P et al., Unpublished Data]. Moreover, this combination treatment leads to the development of long-lasting immune memory that could only be uncovered by CY administration prior to rechallenge.
PDT & immunostimulants
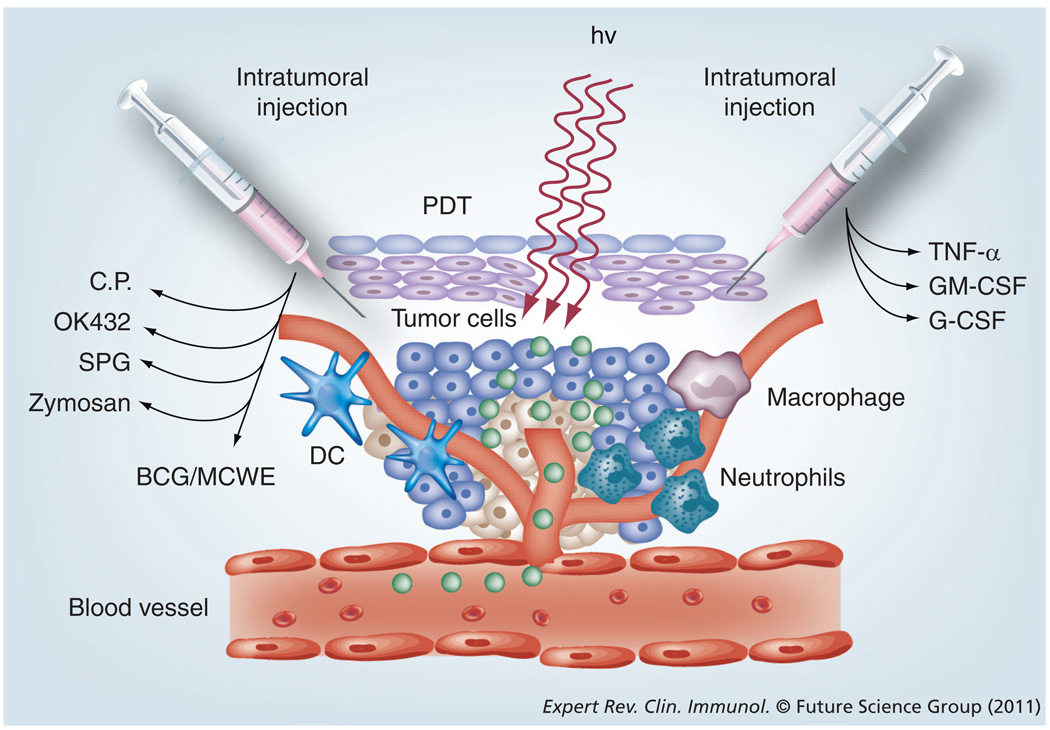
Because of the infrequency and generally unsatisfactory nature of the observable immune response after PDT, a considerable amount of work has gone into testing strategies designed to administer some sort of immunostimulant or adjuvant in combination with PDT to increase the frequency or strength of the anti-tumor immune response. Many of these combination adjuvants fall into the classification of Toll-like receptor (TLR) agonists. TLRs were discovered in 1994 and named after the Toll gene in the fruit fly Drosophila melanogaster responsible for immunity to fungal infection, which it achieved by activating the synthesis of antimicrobial peptides [128]. It is now known that most mammalian species have between ten and 15 types of TLR and their role is to act as early warning systems for microbial invasion [129]. Various molecular motifs (PAMPs) derived from Gram-positive bacteria (TLR-1, −2 and −9), from Gram-negative bacteria (TLR-4, −5 and −9) and from viruses (TLR-3, −7 and −8) bind to TLR and thereby activate a cell-signaling pathway that results in secretion of cytokines, activation of the inflammatory cascade and the recruitment of innate immune cells that will destroy the microbial invaders [130]. Because of this ability to activate the immune system, several groups have studied whether or not it is beneficial to combine these substances with PDT. Owing to the nature of most TLR agonists, they cannot be administered systemically because they would cause unacceptable toxicity due to indiscriminate activation of innate immune cells all over the body and uncontrolled secretion of cytokines such as TNF-α. For these reasons, the combination of TLR agonists and other related immunostimulatory preparations is usually carried out by local administration by means of an intratumoral or peritumoral injection of small amounts of the substance. This is schematically illustrated in Figure 5. Table 2 gives a listing of the studies in which microbial preparations have been successfully combined with PDT.
Figure 5. Local activation of the innate immune system can be strongly potentiated by strategies that facilitate better activation of dendritic cells, macrophages or neutrophils.
Intratumoral injection greatly enhances the effectiveness of combination strategies.
DC: Dendritic cell; hv: Light; PDT: Photodynamic therapy.
Adapted with permission from [25].
Table 2.
Studies combining local application of microbial products with photodynamic therapy of tumors.
| Adjuvant | Source | PDT agent | Tumor model | Ref. |
|---|---|---|---|---|
| OK432 | Penicillin-killed streptococci | Hematoporphyrin derivative | Murine NR-S1 squamous cell carcinoma | [153] |
| CpG | Oligodeoxynucleotide | BPD (verteporfin) | Murine 4T1 mammary carcinoma | [154] |
| MCWE | Mycobacterium cell wall extract |
Photofrin, BPD, mTHPC, ZnPc | Murine EMT6 sarcoma | [155] |
| BCG | Live mycobacterial vaccine | Photofrin, BPD, mTHPC, ZnPc | Murine EMT6 sarcoma | [156] |
|
Cryptosporidium parvum |
Killed bacterial vaccine | Hematoporphyrin derivative | Murine MBT2 transitional cell carcinoma | [157] |
| Glycated chitosan | Polysaccharide preparation | Photofrin, mTHPC | Murine EMT6 sarcoma, line 1 lung cancer |
[133] |
| Schizophyllan | Fungal β-glucan | Photofrin | Murine SCCVII squamous carcinoma | [134] |
| Zymosan | Yeast cell wall extract | Photofrin, mTHPC | Murine SCCVII squamous and LLC lung carcinomas |
[135] |
| Imiquimod topical |
Small molecule TLR8 agonist | ALA–PPIX | Human HPV-associated vulval intraepithelial neoplasia |
[158] |
ALA–PPIX: 5-aminolevulinic acid–protoporphyrin IX; BCG: Bacillus Calmette–Guérin; BPD: Benzoporphyrin derivative; HPV: Human papilloma virus; LLC: Lewis lung carcinoma; MCWE: Mycobacterium cell wall extract; mTHPC: M-tetrahydroxyphenylchlorin; PDT: Photodynamic therapy; SCC: Squamous cell carcinoma; ZnPc: Zinc phtalocyanine.
In addition to TLR agonists, other microbial substances (especially those derived from fungi) activate other aspects of the innate immune system such as complement and C-type lectins [131,132]. The adjuvants known as glycated chitosan [133] and schizophyllan [134] that have been combined with PDT are both probably recognized by C-type lectins. On the other hand, zymosan (also a fungal constituent) was proposed to act as a classical activator of complement when combined with PDT [135].
PDT & combination approaches
There have been studies that have used combination therapies to increase response of tumors to PDT by affecting various elements of the immune system (see Table 3 for a summary). These combination approaches include cytokines such as TNF-α, which was shown by Bellnier to potentiate Photofrin-mediated PDT of murine SMT-F adenocarcinoma after a single dose of intravenously administered recombinant human material [136]. Photofrin-mediated PDT was tested against SCCVII tumors in combination with serum vitamin D3-binding protein-derived macrophage-activating factor [137]. This markedly improved the outcome of PDT, but as a single agent had no significant effect on the growth of SCCVII tumors. Localized tumor treatment with G-CSF in combination with Photofrin-mediated PDT resulted in a significant reduction of tumor growth and an increase in the length of survival of BALB/c mice bearing two types of tumor: colo 26 tumors and Lewis lung carcinomas [138]. Moreover, 33% of colo 26 tumor-bearing mice were completely cured after combined therapy and developed a specific and long-lasting immunity. Korbelik and Cooper used intralesional γ-inulin (a potent classical complement activator) in delaying the recurrence of B16BL6 melanomas after PDT [139]. This effect of γ-inulin was further enhanced by IFN-γ pretreatment. Tumor C3 protein levels, already elevated after individual PDT or γ-inulin treatments, increased much more after their combination. With fibrosarcomas MCA205 and FsaR, adjuvant γ-inulin proved to be highly effective in reducing recurrence rates following PDT using four different PSs (BPD, ce6, Photofrin and m-tetrahydroxyphenylchlorin). At 3 days after PDT plus γ-inulin treatment, over 50% of cells found at the tumor site were CTLs engaged in killing specific targets via the perforin–granzyme pathway.
Table 3.
Studies of other immunostimulants that have been combined with photodynamic therapy of tumors.
| Agent | Mechanism | PDT agent | Tumor model | Ref. |
|---|---|---|---|---|
| DBPMAF (ip. and peritumoral) |
Serum vitamin D3-binding protein- derived macrophage-activating factor |
Photofrin® | Murine SCCVII squamous carcinoma | [137] |
| TNF-α (single dose iv.) | Cytokine activation Antivascular effect |
Photofrin | Murine SMT-F adenocarcinoma |
[136] |
| DMXAA | TNF-α induction Antivascular effect | HPPH (Photochlor) | Murine CT-26 colon carcinoma | [159] |
| GM-CSF (intratumor) | Killed cells expressing GM-CSF Stimulates macrophages |
BPD (Verteporfin) | Murine SCCVII squamous carcinoma | [160] |
| G-CSF | Stimulates neutrophils | Photofrin | Murine CT26 colon and LLC lung carcinomas |
[138] |
| γ-inulin (intratumor) |
Classical complement activator | BPD, ce6, Photofrin, mTHPC |
Murine B16 melanoma MCA205 and FsaR fibrosarcomas |
[139] |
| Low-dose CY (systemic ip.) |
Regulatory T-cell depletion | BPD (15 min drug-light interval) |
Murine J774 reticulum cell sarcoma | [161] |
BPD: Benzoporphyrin derivative; CT: Colon tumor; CY: Cyclophosphamide; DBPMAF: Vitamin D-binding protein macrophage-activating factor; G-CSF: Granulocyte colony-stimulating factor; GM-CSF: Granulocyte-macrophage colony-stimulating factor; HPPH: 2- (1-hexyloxyethyl)-2-devinyl pyropheophorbide-a; ip.: Intraperitoneal; iv.: Intravenous; LLC: Lewis lung carcinoma; mTHPC: M-tetrahydroxyphenylchlorin; PDT: Photodynamic therapy; SCC: Squamous cell carcinoma.
PDT-induced Immunity & cancer vaccines
If the antimicrobial vaccines can be considered as one of the seminal accomplishments of the 21st Century, the cancer vaccines that may reduce or eliminate morbidity and mortality from debilitating and fatal malignancies show considerable promise to be the next generation’s greatest exploit [140]. Recent research indicates that PDT may be used to produce such cancer vaccines. PDT-derived anticancer vaccines have clinical potential to become beneficial adjuvant or primary therapy in the treatment of various cancers.
The concept behind conventional vaccination is the introduction of attenuated or killed forms of the microbe (or its toxins), which the body recognizes as foreign and produces protective antibodies against. Researchers have produced cancer vaccines with a similar mechanism in mind, exposing tumor cells to lethal doses of radiation and then introducing these killed tumor cells to animal subjects with the hope that the host’s immune system will recognize the killed tumor cells and develop immunity. However, many tumor cells are poorly immunogenic and therefore are not the best target for vaccine-based therapies. It has been postulated, however, that PDT may be used to augment the immunogenicity of the tumor cells and increase the effectiveness of anticancer vaccines. Korbelik and Sun incubated SCC cells with the photosensitizing agent BPD and exposed them to light (690 nm, 1 J/cm2) before killing them via radiation exposure [75]. These PDT-treated tumor cells, which constitute a cancer vaccine, were injected peritumorally in mice bearing subcutaneous SCC. The PDT vaccine resulted in a significant therapeutic effect, including tumor growth retardation, regression and cures. Interestingly, increasing the inoculum of cancer cells in the PDT vaccine further improved the outcome, reaching the maximum benefit with 2 × 107 cells/vaccination. A further increase in cells per inoculum proved counterproductive and decreased vaccine efficacy, presumably due to a self-regulatory downregulation of host immune response due to excessive antigen load [141]. Remarkably, no significant difference was found between two concentrations of the PS agent BPD and both doses were equally effective. In an attempt to dissect the mechanism responsible for the observed phenomenon, Korbelik et al. excised lymph nodes from the mice 4 days post-vaccination, and noted a dramatic rise in lymph node cells in PDT-vaccinated mice as compared with controls [75]. A five-to-sixfold increase in T cells, an over 17-fold increase in B cells and a greater than tenfold increase in DCs has been noted. Furthermore, a greater percentage of T cells bore the CD44+CD45RB− memory phenotype. Retrieved PDT vaccine tumor cells were found to be coated by C3 surface proteins, as were lymph node cells. The relevance of complement involvement was demonstrated by the fact that the PDT vaccine was deemed ineffective in C3 complement-deficient mice. Retrieved tumor cells were also found to express HSP70 on their cell surface. Gollnick et al. also explore the effectiveness of PDT vaccines in cancer therapy [142]. They compared the PDT vaccine potential with vaccines generated by ultraviolet radiation or ionizing irradiation. PDT-generated vaccines were tumor specific, induced a cytotoxic T-cell response and, unlike the other methods of producing vaccines, did not require coadministration of an adjuvant to be effective. Moreover, PDT-generated lysates were able to induce phenotypic DC maturation and IL-12 expression.
Expert commentary
It is now widely accepted that there is a pronounced activation of the immune system after PDT for cancer in both animal models and also in patients. However, there is no agreement on the molecular and cellular determinants of the effect and more importantly on how to improve it. T-cell responses (CD4 and CD8) have been observed and these have been shown to be tumor specific and to lead to memory immunity. Other reports have demonstrated the activation of neutrophils, NK cells and macrophages after PDT. It has been argued that PDT regimens that have a high degree of acute inflammation are better at immune activation than those in which the acute inflammation is lower. However, it is conceivable that PDT-induced inflammation may instead actually increase the rate of recurrence or regrowth of remaining tumor cells. It is known that an increase in inflammatory mediators can promote tumor cell growth in many situations [143]. Acute-phase response and complement activation have been proposed to be important pathways in immune responses after PDT. The realization that tumors have evolved particular pathways and mechanisms for evading the host immune response has only recently been appreciated by the PDT community. Large and diverse arrays of therapies that combine PDT and an immunostimulant have been tested but there is no agreement on which is best and which could be clinically applied.
Five-year view
We believe that the next 5 years will see great advances in the field of anti-tumor immune response after PDT. These advances will be largely driven by three considerations. First, there will be increased understanding from the tumor immunology community of how tumors interact with the immune system of their hosts. The different pathways that tumors use to evade the host immune response and suppress the immune system will be better understood and more effective ways of overcoming these pathways will be discovered. Second, there will be advances from the PDT community. These will include better PSs and improved light sources and light delivery technology that will make obtaining a good local response in PDT-treated tumors more predictable and achievable. The PDT community will also increasingly study immunological aspects, especially in small animal tumor models. Combination studies will continue to increase. It is important that more collaboration and discussion between PDT specialists and tumor immunologists take place. Third, there will be more involvement by clinicians who treat cancer patients with PDT. We are only aware of one clinical paper that demonstrated effective induction of adaptive anti-tumor immunity after PDT [144]. Clinical PDT trials will include one or more measures of immune function and immune response in patients, and these metrics will be correlated with outcome. We must realize that in all probability it will take considerably longer than 5 years for the use of PDT to induce a sufficiently powerful anti-tumor immune response that results in improved survival in advanced cancer patients to become clinically accepted practice.
Acknowledgements
Norbert Lange would like to dedicate his participation to this review to his beloved father.
The authors are grateful to Mladen Korbelik, Jakub Golab and Abhishek Garg for helpful discussions.
Pawel Mroz was partly supported by Partners Genzyme Translational Grant. Norbert Lange was supported by the Swiss Science Foundation (Grants #205320-122144, #IZLSZ2_123011, #310030-119938 and #K-32K1-116460). Michael R Hamblin was supported by NIH grant R01AI050875, Center for Integration of Medicine and Innovative Technology (DAMD17-02-2-0006), CDMRP Program in TBI (W81XWH-09-1-0514) and Air Force Office of Scientific Research (FA9950-04-1-0079).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J. Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Sheridan C. Fresh from the biologic pipeline – 2009. Nat. Biotechnol. 2010;28(4):307–310. doi: 10.1038/nbt0410-307. [DOI] [PubMed] [Google Scholar]
- 3.Simon R. Lost in translation: problems and pitfalls in translating laboratory observations to clinical utility. Eur. J. Cancer. 2008;44(18):2707–2713. doi: 10.1016/j.ejca.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu X, Lee VC, Chevalier E, Hwang ST. Chemokine receptors as targets for cancer therapy. Curr. Pharm. Des. 2009;15(7):742–757. doi: 10.2174/138161209787582165. [DOI] [PubMed] [Google Scholar]
- 5.Klastersky J. Adverse effects of the humanized antibodies used as cancer therapeutics. Curr. Opin Oncol. 2006;18(4):316–320. doi: 10.1097/01.cco.0000228734.32261.62. [DOI] [PubMed] [Google Scholar]
- 6.Barrett A, Roques T, Small M, Smith RD. How much will Herceptin really cost? Br. Med. J. 2006;333(7578):1118–1120. doi: 10.1136/bmj.39008.624051.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.La Porta CA. Mechanism of drug sensitivity and resistance in melanoma. Curr. Cancer Drug Targets. 2009;9(3):391–397. doi: 10.2174/156800909788166574. [DOI] [PubMed] [Google Scholar]
- 8.Laconi E, Pani P, Farber E. The resistance phenotype in the development and treatment of cancer. Lancet Oncol. 2000;1:235–241. doi: 10.1016/s1470-2045(00)00154-6. [DOI] [PubMed] [Google Scholar]
- 9.Bianco R, Damiano V, Gelardi T, Daniele G, Ciardiello F, Tortora G. Rational combination of targeted therapies as a strategy to overcome the mechanisms of resistance to inhibitors of EGFR signaling. Curr. Pharm. Des. 2007;13(33):3358–3367. doi: 10.2174/138161207782360564. [DOI] [PubMed] [Google Scholar]
- 10.Robertson CA, Evans DH, Abrahamse H. Photodynamic therapy (PDT): a short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B. Biol. 2009;96(1):1–8. doi: 10.1016/j.jphotobiol.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Moan J, Peng Q. An outline of the hundred-year history of PDT. Anticancer Res. 2003;23(5A):3591–3600. [PubMed] [Google Scholar]
- 12.Ogilby PR. Singlet oxygen: there is indeed something new under the sun. Chem. Soc. Rev. 2010;39(8):3181–3209. doi: 10.1039/b926014p. [DOI] [PubMed] [Google Scholar]
- 13.Robey RW, Steadman K, Polgar O, Bates SE. ABCG2-mediated transport of photosensitizers: potential impact on photodynamic therapy. Cancer Biol. Ther. 2005;4(2):187–194. [PubMed] [Google Scholar]
- 14.Xue LY, Chiu SM, Oleinick NL. Atg7 deficiency increases resistance of MCF-7 human breast cancer cells to photodynamic therapy. Autophagy. 2010;6(2):248–255. doi: 10.4161/auto.6.2.11077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Detty MR, Gibson SL, Wagner SJ. Current clinical and preclinical photosensitizers for use in photodynamic therapy. J. Med. Chem. 2004;47(16):3897–3915. doi: 10.1021/jm040074b. [DOI] [PubMed] [Google Scholar]
- 16.Boyle RW, Dolphin D. Structure and biodistribution relationships of photodynamic sensitizers. Photochem. Photobiol. 1996;64(3):469–485. doi: 10.1111/j.1751-1097.1996.tb03093.x. [DOI] [PubMed] [Google Scholar]
- 17.Konan YN, Gurny R, Allemann E. State of the art in the delivery of photosensitizers for photodynamic therapy. J. Photochem. Photobiol. B. Biol. 2002;66(2):89–106. doi: 10.1016/s1011-1344(01)00267-6. [DOI] [PubMed] [Google Scholar]
- 18.Oleinick NL, Morris RL, Belichenko I. The role of apoptosis in response to photodynamic therapy: what, where, why, and how. Photochem. Photobiol. Sci. 2002;1(1):1–21. doi: 10.1039/b108586g. [DOI] [PubMed] [Google Scholar]
- 19.Almeida RD, Manadas BJ, Carvalho AP, Duarte CB. Intracellular signaling mechanisms in photodynamic therapy. Biochim. Biophys. Acta. 2004;1704(2):59–86. doi: 10.1016/j.bbcan.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Granville DJ, McManus BM, Hunt DW. Photodynamic therapy: shedding light on the biochemical pathways regulating porphyrin-mediated cell death. Histol. Histopathol. 2001;16(1):309–317. doi: 10.14670/HH-16.309. [DOI] [PubMed] [Google Scholar]
- 21.Girotti AW. Photosensitized oxidation of membrane lipids: reaction pathways, cytotoxic effects, and cytoprotective mechanisms. J. Photochem. Photobiol. B. Biol. 2001;63(1–3):103–113. doi: 10.1016/s1011-1344(01)00207-x. [DOI] [PubMed] [Google Scholar]
- 22.Kessel D, Reiners JJ., Jr Initiation of apoptosis and autophagy by the Bcl-2 antagonist HA14-1. Cancer Lett. 2007;249(2):294–299. doi: 10.1016/j.canlet.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xue LY, Chiu SM, Azizuddin K, Joseph S, Oleinick NL. The death of human cancer cells following photodynamic therapy: apoptosis competence is necessary for Bcl-2 protection but not for induction of autophagy. Photochem. Photobiol. 2007;83(5):1016–1023. doi: 10.1111/j.1751-1097.2007.00159.x. [DOI] [PubMed] [Google Scholar]
- 24.Blum HF. Photodynamic Action and Diseases Caused by Light (No. 85) Reinhold Publishing Corporation, NY, USA; 1941. pp. 239–250. [Google Scholar]
- 25. Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer. 2006;6(7):535–545. doi: 10.1038/nrc1894. • Authoritative review of anti-tumor immunity after photodynamic therapy (PDT).
- 26.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 2007;81(1):1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 27. Garg AD, Nowis D, Golab J, Agostinis P. Photodynamic therapy: illuminating the road from cell death towards anti-tumour immunity. Apoptosis. 2010;15(9):1050–1071. doi: 10.1007/s10495-010-0479-7. • Describes damage-associated molecular patterns released by PDT.
- 28.Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P. Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Biochim. Biophys. Acta. 2010;1805(1):53–71. doi: 10.1016/j.bbcan.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat. Rev. Immunol. 2004;4(6):469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 30.Melcher A, Gough M, Todryk S, Vile R. Apoptosis or necrosis for tumor immunotherapy: what’s in a name? J. Mol. Med. 1999;77(12):824–833. doi: 10.1007/s001099900066. [DOI] [PubMed] [Google Scholar]
- 31.Melcher A, Todryk S, Hardwick N, Ford M, Jacobson M, Vile RG. Tumor immunogenicity is determined by the mechanism of cell death via induction of heat shock protein expression. Nat. Med. 1998;4(5):581–587. doi: 10.1038/nm0598-581. [DOI] [PubMed] [Google Scholar]
- 32.Tesniere A, Panaretakis T, Kepp O, et al. Molecular characteristics of immunogenic cancer cell death. Cell Death Differ. 2008;15(1):3–12. doi: 10.1038/sj.cdd.4402269. [DOI] [PubMed] [Google Scholar]
- 33.Scheffer SR, Nave H, Korangy F, et al. Apoptotic, but not necrotic, tumor cell vaccines induce a potent immune response in vivo. Int. J. Cancer. 2003;103(2):205–211. doi: 10.1002/ijc.10777. [DOI] [PubMed] [Google Scholar]
- 34.Goldszmid RS, Idoyaga J, Bravo AI, Steinman R, Mordoh J, Wainstok R. Dendritic cells charged with apoptotic tumor cells induce long-lived protective CD4+ and CD8+ T cell immunity against B16 melanoma. J. Immunol. 2003;171(11):5940–5947. doi: 10.4049/jimmunol.171.11.5940. [DOI] [PubMed] [Google Scholar]
- 35.Kepp O, Tesniere A, Schlemmer F, et al. Immunogenic cell death modalities and their impact on cancer treatment. Apoptosis. 2009;14(4):364–375. doi: 10.1007/s10495-008-0303-9. [DOI] [PubMed] [Google Scholar]
- 36.Zitvogel L, Kroemer G. The immune response against dying tumor cells: avoid disaster, achieve cure. Cell Death Differ. 2008;15(1):1–2. doi: 10.1038/sj.cdd.4402267. [DOI] [PubMed] [Google Scholar]
- 37.Korbelik M, Sun J, Cecic I. Photodynamic therapy-induced cell surface expression and release of heat shock proteins: relevance for tumor response. Cancer Res. 2005;65(3):1018–1026. [PubMed] [Google Scholar]
- 38.Gomer CJ, Ryter SW, Ferrario A, Rucker N, Wong S, Fisher AM. Photodynamic therapy-mediated oxidative stress can induce expression of heat shock proteins. Cancer Res. 1996;56(10):2355–2360. [PubMed] [Google Scholar]
- 39.Delves PJ, Roitt IM. The immune system. First of two parts. N. Engl. J. Med. 2000;343(1):37–49. doi: 10.1056/NEJM200007063430107. [DOI] [PubMed] [Google Scholar]
- 40.Dougherty TJ, Gomer CJ, Henderson BW, et al. Photodynamic therapy. J. Natl Cancer Inst. 1998;90(12):889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korbelik M. Induction of tumor immunity by photodynamic therapy. J. Clin. Laser Med. Surg. 1996;14(5):329–334. doi: 10.1089/clm.1996.14.329. [DOI] [PubMed] [Google Scholar]
- 42.Gollnick SO, Evans SS, Baumann H, et al. Role of cytokines in photodynamic therapy-induced local and systemic inflammation. Br. J. Cancer. 2003;88(11):1772–1779. doi: 10.1038/sj.bjc.6600864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Korbelik M. PDT-associated host response and its role in the therapy outcome. Lasers Surg. Med. 2006;38(5):500–508. doi: 10.1002/lsm.20337. [DOI] [PubMed] [Google Scholar]
- 44.Krosl G, Korbelik M, Dougherty GJ. Induction of immune cell infiltration into murine SCCVII tumour by photofrin-based photodynamic therapy. Br. J. Cancer. 1995;71(3):549–555. doi: 10.1038/bjc.1995.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cecic I, Stott B, Korbelik M. Acute phase response-associated systemic neutrophil mobilization in mice bearing tumors treated by photodynamic therapy. Int. Immunopharmacol. 2006;6(8):1259–1266. doi: 10.1016/j.intimp.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 46.Korbelik M, Cecic I. Complement activation cascade and its regulation: relevance for the response of solid tumors to photodynamic therapy. J. Photochem. Photobiol. B. Biol. 2008;93(1):53–59. doi: 10.1016/j.jphotobiol.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 47.Cecic I, Korbelik M. Deposition of complement proteins on cells treated by photodynamic therapy in vitro. J. Environ. Pathol. Toxicol. Oncol. 2006;25(1–2):189–203. doi: 10.1615/jenvironpatholtoxicoloncol.v25.i1-2.110. [DOI] [PubMed] [Google Scholar]
- 48.Cecic I, Sun J, Korbelik M. Role of complement anaphylatoxin C3a in photodynamic therapy-elicited engagement of host neutrophils and other immune cells. Photochem. Photobiol. 2006;82(2):558–562. doi: 10.1562/2005-09-09-RA-681. [DOI] [PubMed] [Google Scholar]
- 49.Stott B, Korbelik M. Activation of complement C3, C5, and C9 genes in tumors treated by photodynamic therapy. Cancer Immunol. Immunother. 2007;56(5):649–658. doi: 10.1007/s00262-006-0221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cecic I, Korbelik M. Mediators of peripheral blood neutrophilia induced by photodynamic therapy of solid tumors. Cancer Lett. 2002;183(1):43–51. doi: 10.1016/s0304-3835(02)00092-7. [DOI] [PubMed] [Google Scholar]
- 51.Korbelik M, Cecic I, Merchant S, Sun J. Acute phase response induction by cancer treatment with photodynamic therapy. Int. J. Cancer. 2008;122(6):1411–1417. doi: 10.1002/ijc.23248. [DOI] [PubMed] [Google Scholar]
- 52.Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu. Rev. Immunol. 2005;23:901–944. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- 53.Steubing RW, Yeturu S, Tuccillo A, Sun CH, Berns MW. Activation of macrophages by Photofrin II during photodynamic therapy. J. Photochem. Photobiol. B. Biol. 1991;10(1–2):133–145. doi: 10.1016/1011-1344(91)80218-7. [DOI] [PubMed] [Google Scholar]
- 54. Evans S, Matthews W, Perry R, Fraker D, Norton J, Pass HI. Effect of photodynamic therapy on tumor necrosis factor production by murine macrophages. J. Natl Cancer Inst. 1990;82(1):34–39. doi: 10.1093/jnci/82.1.34. • One of the first papers to propose the importance of the immune response in PDT effects.
- 55. Yamamoto N, Homma S, Sery TW, Donoso LA, Hoober JK. Photodynamic immunopotentiation: in vitro activation of macrophages by treatment of mouse peritoneal cells with haematoporphyrin derivative and light. Eur. J. Cancer. 1991;27(4):467–471. doi: 10.1016/0277-5379(91)90388-t. • Emphasizes the role of macrophages activation after PDT.
- 56.Yamamoto N, Naraparaju VR. Immunotherapy of BALB/c mice bearing Ehrlich ascites tumor with vitamin D-binding protein-derived macrophage activating factor. Cancer Res. 1997;57(11):2187–2192. [PubMed] [Google Scholar]
- 57.Korbelik M, Krosl G. Enhanced macrophage cytotoxicity against tumor cells treated with photodynamic therapy. Photochem. Photobiol. 1994;60(5):497–502. doi: 10.1111/j.1751-1097.1994.tb05140.x. [DOI] [PubMed] [Google Scholar]
- 58.Marshall JF, Chan WS, Hart IR. Effect of photodynamic therapy on anti-tumor immune defenses: comparison of the photosensitizers hematoporphyrin derivative and chloro-aluminum sulfonated phthalocyanine. Photochem. Photobiol. 1989;49(5):627–632. doi: 10.1111/j.1751-1097.1989.tb08434.x. [DOI] [PubMed] [Google Scholar]
- 59.Krosl G, Korbelik M, Krosl J, Dougherty GJ. Potentiation of photodynamic therapy-elicited antitumor response by localized treatment with granulocyte-macrophage colony-stimulating factor. Cancer Res. 1996;56(14):3281–3286. [PubMed] [Google Scholar]
- 60.Delves PJ, Roitt IM. The immune system. N. Engl. J. Med. 2000;343:37–49. doi: 10.1056/NEJM200007063430107. [DOI] [PubMed] [Google Scholar]
- 61.Henderson BW, Gollnick SO, Snyder JW, et al. Choice of oxygen-conserving treatment regimen determines the inflammatory response and outcome of photodynamic therapy of tumors. Cancer Res. 2004;64(6):2120–2126. doi: 10.1158/0008-5472.can-03-3513. [DOI] [PubMed] [Google Scholar]
- 62.Kousis PC, Henderson BW, Maier PG, Gollnick SO. Photodynamic therapy enhancement of antitumor immunity is regulated by neutrophils. Cancer Res. 2007;67(21):10501–10510. doi: 10.1158/0008-5472.CAN-07-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sluiter W, de Vree WJ, Pietersma A, Koster JF. Prevention of late lumen loss after coronary angioplasty by photodynamic therapy: role of activated neutrophils. Mol. Cell. Biochem. 1996;157(1–2):233–238. doi: 10.1007/BF00227904. [DOI] [PubMed] [Google Scholar]
- 64.de Vree WJ, Fontijne-Dorsman AN, Koster JF, Sluiter W. Photodynamic treatment of human endothelial cells promotes the adherence of neutrophils in vitro. Br. J. Cancer. 1996;73(11):1335–1340. doi: 10.1038/bjc.1996.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Volanti C, Gloire G, Vanderplasschen A, Jacobs N, Habraken Y, Piette J. Downregulation of ICAM-1 and VCAM-1 expression in endothelial cells treated by photodynamic therapy. Oncogene. 2004;23(53):8649–8658. doi: 10.1038/sj.onc.1207871. [DOI] [PubMed] [Google Scholar]
- 66.de Vree WJ, Essers MC, de Bruijn HS, Star WM, Koster JF, Sluiter W. Evidence for an important role of neutrophils in the efficacy of photodynamic therapy in vivo. Cancer Res. 1996;56(13):2908–2911. [PubMed] [Google Scholar]
- 67.Sun J, Cecic I, Parkins CS, Korbelik M. Neutrophils as inflammatory and immune effectors in photodynamic therapy-treated mouse SCCVII tumours. Photochem. Photobiol. Sci. 2002;1(9):690–695. doi: 10.1039/b204254a. [DOI] [PubMed] [Google Scholar]
- 68.Cecic I, Parkins CS, Korbelik M. Induction of systemic neutrophil response in mice by photodynamic therapy of solid tumors. Photochem. Photobiol. 2001;74(5):712–720. doi: 10.1562/0031-8655(2001)074<0712:iosnri>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 69.de Bruijn HS, Sluiter W, van der Ploeg-van den Heuvel A, Sterenborg HJ, Robinson DJ. Evidence for a bystander role of neutrophils in the response to systemic 5-aminolevulinic acid-based photodynamic therapy. Photodermatol. Photoimmunol. Photomed. 2006;22(5):238–246. doi: 10.1111/j.1600-0781.2006.00240.x. [DOI] [PubMed] [Google Scholar]
- 70. Kabingu E, Vaughan L, Owczarczak B, Ramsey KD, Gollnick SO. CD8+ T cell-mediated control of distant tumours following local photodynamic therapy is independent of CD4+ T cells and dependent on natural killer cells. Br. J. Cancer. 2007;96(12):1839–1848. doi: 10.1038/sj.bjc.6603792. • Provides evidence for the role of natural killer cells in immune response after PDT.
- 71.Adams S, O’Neill DW, Bhardwaj N. Recent advances in dendritic cell biology. J. Clin. Immunol. 2005;25(3):177–188. doi: 10.1007/s10875-005-4086-2. [DOI] [PubMed] [Google Scholar]
- 72.Gabrilovich DI, Chen HL, Girgis KR, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat. Med. 1996;2(10):1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 73.Yenari MA, Liu J, Zheng Z, Vexler ZS, Lee JE, Giffard RG. Antiapoptotic and anti-inflammatory mechanisms of heat-shock protein protection. Ann. NY Acad. Sci. 2005;1053:74–83. doi: 10.1196/annals.1344.007. [DOI] [PubMed] [Google Scholar]
- 74.Todryk S, Melcher AA, Hardwick N, et al. Heat shock protein 70 induced during tumor cell killing induces Th1 cytokines and targets immature dendritic cell precursors to enhance antigen uptake. J. Immunol. 1999;163(3):1398–1408. [PubMed] [Google Scholar]
- 75.Korbelik M, Sun J. Photodynamic therapy-generated vaccine for cancer therapy. Cancer Immunol. Immunother. 2006;55(8):900–909. doi: 10.1007/s00262-005-0088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Canti G, Lattuada D, Nicolin A, Taroni P, Valentini G, Cubeddu R. Antitumor immunity induced by photodynamic therapy with aluminum disulfonated phthalocyanines and laser light. Anticancer Drugs. 1994;5(4):443–447. doi: 10.1097/00001813-199408000-00009. • First paper to describe memory immunity after PDT.
- 77.Korbelik M, Krosl G, Krosl J, Dougherty GJ. The role of host lymphoid populations in the response of mouse EMT6 tumor to photodynamic therapy. Cancer Res. 1996;56(24):5647–5652. [PubMed] [Google Scholar]
- 78. Korbelik M, Dougherty GJ. Photodynamic therapy-mediated immune response against subcutaneous mouse tumors. Cancer Res. 1999;59(8):1941–1946. • Emphasizes role of T lymphocytes in immune response after PDT.
- 79.Hendrzak-Henion JA, Knisely TL, Cincotta L, Cincotta E, Cincotta AH. Role of the immune system in mediating the antitumor effect of benzophenothiazine photodynamic therapy. Photochem. Photobiol. 1999;69(5):575–581. [PubMed] [Google Scholar]
- 80.Van den Eynde BJ, van der Bruggen P. T cell defined tumor antigens. Curr. Opin. Immunol. 1997;9(5):684–693. doi: 10.1016/s0952-7915(97)80050-7. [DOI] [PubMed] [Google Scholar]
- 81.Van den Eynde B, Lethe B, Van Pel A, De Plaen E, Boon T. The gene coding for a major tumor rejection antigen of tumor P815 is identical to the normal gene of syngeneic DBA/2 mice. J. Exp. Med. 1991;173(6):1373–1384. doi: 10.1084/jem.173.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Van den Eynde B, Peeters O, De Backer O, Gaugler B, Lucas S, Boon T. A new family of genes coding for an antigen recognized by autologous cytolytic T lymphocytes on a human melanoma. J. Exp. Med. 1995;182(3):689–698. doi: 10.1084/jem.182.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van der Bruggen P, Traversari C, Chomez P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254(5038):1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 84.Gaugler B, Van den Eynde B, van der Bruggen P, et al. Human gene MAGE-3 codes for an antigen recognized on a melanoma by autologous cytolytic T lymphocytes. J. Exp. Med. 1994;179(3):921–930. doi: 10.1084/jem.179.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Traversari C, van der Bruggen P, Luescher IF, et al. A nonapeptide encoded by human gene MAGE-1 is recognized on HLA-A1 by cytolytic T lymphocytes directed against tumor antigen MZ2-E. J. Exp. Med. 1992;176(5):1453–1457. doi: 10.1084/jem.176.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Coulie PG, Brichard V, Van Pel A, et al. A new gene coding for a differentiation antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J. Exp. Med. 1994;180(1):35–42. doi: 10.1084/jem.180.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brichard V, Van Pel A, Wolfel T, et al. The tyrosinase gene codes for an antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J. Exp. Med. 1993;178(2):489–495. doi: 10.1084/jem.178.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bakker AB, Schreurs MW, de Boer AJ, et al. Melanocyte lineage-specific antigen gp100 is recognized by melanoma-derived tumor-infiltrating lymphocytes. J. Exp. Med. 1994;179(3):1005–1009. doi: 10.1084/jem.179.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Coulie PG, Lehmann F, Lethe B, et al. A mutated intron sequence codes for an antigenic peptide recognized by cytolytic T lymphocytes on a human melanoma. Proc. Natl Acad. Sci. USA. 1995;92(17):7976–7980. doi: 10.1073/pnas.92.17.7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mandelboim O, Berke G, Fridkin M, Feldman M, Eisenstein M, Eisenbach L. CTL induction by a tumour-associated antigen octapeptide derived from a murine lung carcinoma. Nature. 1994;369(6475):67–71. doi: 10.1038/369067a0. [DOI] [PubMed] [Google Scholar]
- 91.Monach PA, Meredith SC, Siegel CT, Schreiber H. A unique tumor antigen produced by a single amino acid substitution. Immunity. 1995;2(1):45–59. doi: 10.1016/1074-7613(95)90078-0. [DOI] [PubMed] [Google Scholar]
- 92.Robbins PF, El-Gamil M, Li YF, et al. A mutated β-catenin gene encodes a melanoma-specific antigen recognized by tumor infiltrating lymphocytes. J. Exp. Med. 1996;183(3):1185–1192. doi: 10.1084/jem.183.3.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dubey P, Hendrickson RC, Meredith SC, et al. The immunodominant antigen of an ultraviolet-induced regressor tumor is generated by a somatic point mutation in the DEAD box helicase p68. J. Exp. Med. 1997;185(4):695–705. doi: 10.1084/jem.185.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang M, Bronte V, Chen PW, et al. Active immunotherapy of cancer with a nonreplicating recombinant fowlpox virus encoding a model tumor-associated antigen. J. Immunol. 1995;154(9):4685–4692. [PMC free article] [PubMed] [Google Scholar]
- 95.McCabe BJ, Irvine KR, Nishimura MI, et al. Minimal determinant expressed by a recombinant vaccinia virus elicits therapeutic antitumor cytolytic T lymphocyte responses. Cancer Res. 1995;55(8):1741–1747. [PMC free article] [PubMed] [Google Scholar]
- 96.Marzo AL, Lake RA, Robinson BW, Scott B. T-cell receptor transgenic analysis of tumor-specific CD8 and CD4 responses in the eradication of solid tumors. Cancer Res. 1999;59(5):1071–1079. [PubMed] [Google Scholar]
- 97.Ramarathinam L, Sarma S, Maric M, et al. Multiple lineages of tumors express a common tumor antigen, P1A, but they are not cross-protected. J. Immunol. 1995;155(11):5323–5329. [PubMed] [Google Scholar]
- 98.Uyttenhove C, Godfraind C, Lethe B, et al. The expression of mouse gene P1A in testis does not prevent safe induction of cytolytic T cells against a P1A-encoded tumor antigen. Int. J. Cancer. 1997;70(3):349–356. doi: 10.1002/(sici)1097-0215(19970127)70:3<349::aid-ijc17>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 99.Woodman CB, Collins SI, Young LS. The natural history of cervical HPV infection: unresolved issues. Nat. Rev. Cancer. 2007;7(1):11–22. doi: 10.1038/nrc2050. [DOI] [PubMed] [Google Scholar]
- 100.Castano AP, Liu Q, Hamblin MR. A green fluorescent protein-expressing murine tumour but not its wild-type counterpart is cured by photodynamic therapy. Br. J. Cancer. 2006;94(3):391–397. doi: 10.1038/sj.bjc.6602953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mroz P, Szokalska A, Wu MX, Hamblin M. Photodynamic therapy of tumors can lead to development of systemic antigen-specific immune response. PLoS ONE. 2011 doi: 10.1371/journal.pone.0015194. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Abdel-Hady ES, Martin-Hirsch P, Duggan-Keen M, et al. Immunological and viral factors associated with the response of vulval intraepithelial neoplasia to photodynamic therapy. Cancer Res. 2001;61(1):192–196. [PubMed] [Google Scholar]
- 103. Kabingu E, Oseroff AR, Wilding GE, Gollnick SO. Enhanced systemic immune reactivity to a Basal cell carcinoma associated antigen following photodynamic therapy. Clin. Cancer Res. 2009;15(13):4460–4466. doi: 10.1158/1078-0432.CCR-09-0400. • First demonstration that an antigen-specific immune response can be observed after PDT in patients.
- 104.Gershon RK, Cohen P, Hencin R, Leibhaber SA. Suppressor T cells. J. Immunol. 1972;108:586–590. [PubMed] [Google Scholar]
- 105.Dejaco C, Duftner C, Grubeck-Loebenstein B, Schirmer M. Imbalance of regulatory T cells in human autoimmune diseases. Immunology. 2006;117(3):289–300. doi: 10.1111/j.1365-2567.2005.02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yamaguchi T, Sakaguchi S. Regulatory T cells in immune surveillance and treatment of cancer. Semin. Cancer Biol. 2006;16(2):115–123. doi: 10.1016/j.semcancer.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 107.Antony PA, Restifo NP. CD4+CD25+ T regulatory cells, immunotherapy of cancer, and interleukin-2. J. Immunother. 2005;28(2):120–128. doi: 10.1097/01.cji.0000155049.26787.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat. Immunol. 2005;6(4):331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 109.Scotto L, Naiyer AJ, Galluzzo S, et al. Overlap between molecular markers expressed by naturally occurring CD4+CD25+ regulatory T cells and antigen specific CD4+CD25+ and CD8+CD28-T suppressor cells. Hum. Immunol. 2004;65(11):1297–1306. doi: 10.1016/j.humimm.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 110.Albers AE, Ferris RL, Kim GG, Chikamatsu K, DeLeo AB, Whiteside TL. Immune responses to p53 in patients with cancer: enrichment in tetramer+ p53 peptide-specific T cells and regulatory T cells at tumor sites. Cancer Immunol. Immunother. 2005;54(11):1072–1081. doi: 10.1007/s00262-005-0670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ziegler SF. FOXP3: of mice and men. Annu. Rev. Immunol. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 112.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat. Rev. Immunol. 2006;6(4):295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 113.Curotto de Lafaille MA, Lino AC, Kutchukhidze N, Lafaille JJ. CD25− T cells generate CD25+Foxp3+ regulatory T cells by peripheral expansion. J. Immunol. 2004;173(12):7259–7268. doi: 10.4049/jimmunol.173.12.7259. [DOI] [PubMed] [Google Scholar]
- 114.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25−naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 2003;198(12):1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bluestone JA, Tang Q. How do CD4+CD25+ regulatory T cells control autoimmunity? Curr. Opin. Immunol. 2005;17(6):638–642. doi: 10.1016/j.coi.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 116.Tang Q, Boden EK, Henriksen KJ, Bour-Jordan H, Bi M, Bluestone JA. Distinct roles of CTLA-4 and TGF-β in CD4+CD25+ regulatory T cell function. Eur. J. Immunol. 2004;34(11):2996–3005. doi: 10.1002/eji.200425143. [DOI] [PubMed] [Google Scholar]
- 117.Stephens GL, McHugh RS, Whitters MJ, et al. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J. Immunol. 2004;173(8):5008–5020. doi: 10.4049/jimmunol.173.8.5008. [DOI] [PubMed] [Google Scholar]
- 118.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-β1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J. Exp. Med. 2005;201(7):1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ghiringhelli F, Puig PE, Roux S, et al. Tumor cells convert immature myeloid dendritic cells into TGF-β-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J. Exp. Med. 2005;202(7):919–929. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Veldhoen M, Moncrieffe H, Hocking RJ, Atkins CJ, Stockinger B. Modulation of dendritic cell function by naive and regulatory CD4+ T cells. J. Immunol. 2006;176(10):6202–6210. doi: 10.4049/jimmunol.176.10.6202. [DOI] [PubMed] [Google Scholar]
- 121.Rudensky AY, Campbell DJ. In vivo sites and cellular mechanisms of T reg cell-mediated suppression. J. Exp. Med. 2006;203(3):489–492. doi: 10.1084/jem.20060214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ghiringhelli F, Larmonier N, Schmitt E, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur. J. Immunol. 2004;34(2):336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 123.Rollinghoff M, Starzinski-Powitz A, Pfizenmaier K, Wagner H. Cyclophosphamide-sensitive T lymphocytes suppress the in vivo generation of antigen-specific cytotoxic T lymphocytes. J. Exp. Med. 1977;145(2):455–459. doi: 10.1084/jem.145.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Glaser M. Regulation of specific cell-mediated cytotoxic response against SV40-induced tumor associated antigens by depletion of suppressor T cells with cyclophosphamide in mice. J. Exp. Med. 1979;149(3):774–779. doi: 10.1084/jem.149.3.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Berendt MJ, North RJ. T-cell-mediated suppression of anti-tumor immunity. An explanation for progressive growth of an immunogenic tumor. J. Exp. Med. 1980;151(1):69–80. doi: 10.1084/jem.151.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Loeffler M, Kruger JA, Reisfield RA. Immunostimulatory effects of low-dose cyclophosphamide are controlled by inducible nitric oxide synthase. Cancer Res. 2005;65(12):5027–5030. doi: 10.1158/0008-5472.CAN-05-0646. [DOI] [PubMed] [Google Scholar]
- 127.Castano AP, Hamblin MR. Anti-tumor immunity generated by photodynamic therapy in a metastatic murine tumor model. Proc. SPIE. 2005;5695:7–16. [Google Scholar]
- 128.Muzio M, Mantovani A. Toll-like receptors. Microbes Infect. 2000;2(3):251–255. doi: 10.1016/s1286-4579(00)00303-8. [DOI] [PubMed] [Google Scholar]
- 129.Oshiumi H, Matsuo A, Matsumoto M, Seya T. Pan-vertebrate Toll-like receptors during evolution. Curr. Genomics. 2008;9(7):488–493. doi: 10.2174/138920208786241234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Armant MA, Fenton MJ. Toll-like receptors: a family of pattern-recognition receptors in mammals. Genome Biol. 2002;3(8) doi: 10.1186/gb-2002-3-8-reviews3011. reviews3011.1–reviews3011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.van Kooyk Y. C-type lectins on dendritic cells: key modulators for the induction of immune responses. Biochem. Soc. Trans. 2008;36(Pt 6):1478–1481. doi: 10.1042/BST0361478. [DOI] [PubMed] [Google Scholar]
- 132.Cambi A, Koopman M, Figdor CG. How C-type lectins detect pathogens. Cell Microbiol. 2005;7(4):481–488. doi: 10.1111/j.1462-5822.2005.00506.x. [DOI] [PubMed] [Google Scholar]
- 133.Chen WR, Korbelik M, Bartels KE, Liu H, Sun J, Nordquist RE. Enhancement of laser cancer treatment by a chitosan-derived immunoadjuvant. Photochem. Photobiol. 2005;81(1):190–195. doi: 10.1562/2004-07-20-RA-236. [DOI] [PubMed] [Google Scholar]
- 134.Krosl G, Korbelik M. Potentiation of photodynamic therapy by immunotherapy: the effect of schizophyllan (SPG) Cancer Lett. 1994;84(1):43–49. doi: 10.1016/0304-3835(94)90356-5. [DOI] [PubMed] [Google Scholar]
- 135.Korbelik M, Sun J, Cecic I, Serrano K. Adjuvant treatment for complement activation increases the effectiveness of photodynamic therapy of solid tumors. Photochem. Photobiol. Sci. 2004;3(8):812–816. doi: 10.1039/b315663J. [DOI] [PubMed] [Google Scholar]
- 136.Bellnier DA. Potentiation of photodynamic therapy in mice with recombinant human tumor necrosis factor-α. J. Photochem. Photobiol. B. Biol. 1991;8(2):203–210. doi: 10.1016/1011-1344(91)80060-u. [DOI] [PubMed] [Google Scholar]
- 137.Korbelik M, Naraparaju VR, Yamamoto N. Macrophage-directed immunotherapy as adjuvant to photodynamic therapy of cancer. Br. J. Cancer. 1997;75(2):202–207. doi: 10.1038/bjc.1997.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Golab J, Wilczynski G, Zagozdzon R, et al. Potentiation of the anti-tumour effects of Photofrin-based photodynamic therapy by localized treatment with G-CSF. Br. J. Cancer. 2000;82(8):1485–1491. doi: 10.1054/bjoc.1999.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Korbelik M, Cooper PD. Potentiation of photodynamic therapy of cancer by complement: the effect of γ-inulin. Br. J. Cancer. 2007;96(1):67–72. doi: 10.1038/sj.bjc.6603508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Weiner LM, Surana R, Murray J. Vaccine prevention of cancer: can endogenous antigens be targeted? Cancer Prev. Res. 2010;3(4):410–415. doi: 10.1158/1940-6207.CAPR-10-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Critchfield JM, Racke MK, Zuniga-Pflucker JC, et al. T cell deletion in high antigen dose therapy of autoimmune encephalomyelitis. Science. 1994;263(5150):1139–1143. doi: 10.1126/science.7509084. [DOI] [PubMed] [Google Scholar]
- 142. Gollnick SO, Vaughan L, Henderson BW. Generation of effective antitumor vaccines using photodynamic therapy. Cancer Res. 2002;62(6):1604–1608. • Shows that killing tumor cells in vitro by PDT makes them good vaccines.
- 143.Sgambato A, Cittadini A. inflammation and cancer: a multifaceted link. Eur. Rev. Med. Pharmacol. Sci. 2010;14(4):263–268. [PubMed] [Google Scholar]
- 144.Thong PS, Ong KW, Goh NS, et al. Photodynamic-therapy-activated immune response against distant untreated tumours in recurrent angiosarcoma. Lancet Oncol. 2007;8(10):950–952. doi: 10.1016/S1470-2045(07)70318-2. [DOI] [PubMed] [Google Scholar]
- 145.Zitvogel L, Kepp O, Senovilla L, Menger L, Chaput N, Kroemer G. Immunogenic tumor cell death for optimal anticancer therapy: the calreticulin exposure pathway. Clin. Cancer Res. 2010;16(12):3100–3104. doi: 10.1158/1078-0432.CCR-09-2891. [DOI] [PubMed] [Google Scholar]
- 146.Spisek R, Dhodapkar MV. Towards a better way to die with chemotherapy: role of heat shock protein exposure on dying tumor cells. Cell Cycle. 2007;6(16):1962–1965. doi: 10.4161/cc.6.16.4601. [DOI] [PubMed] [Google Scholar]
- 147.Tang D, Kang R, Zeh HJ, 3rd, Lotze MT. High-mobility group box 1 and cancer. Biochim. Biophys. Acta. 2010;1799(1–2):131–140. doi: 10.1016/j.bbagrm.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Martins I, Tesniere A, Kepp O, et al. Chemotherapy induces ATP release from tumor cells. Cell Cycle. 2009;8(22):3723–3728. doi: 10.4161/cc.8.22.10026. [DOI] [PubMed] [Google Scholar]
- 149.Donato R. RAGE: a single receptor for several ligands and different cellular responses: the case of certain S100 proteins. Curr. Mol. Med. 2007;7(8):711–724. doi: 10.2174/156652407783220688. [DOI] [PubMed] [Google Scholar]
- 150.Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K, Saito T. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat. Immunol. 2008;9(10):1179–1188. doi: 10.1038/ni.1651. [DOI] [PubMed] [Google Scholar]
- 151.Horino K, Nishiura H, Ohsako T, et al. A monocyte chemotactic factor, S19 ribosomal protein dimer, in phagocytic clearance of apoptotic cells. Lab. Invest. 1998;78(5):603–617. [PubMed] [Google Scholar]
- 152.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425(6957):516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 153.Uehara M, Sano K, Wang ZL, Sekine J, Ikeda H, Inokuchi T. Enhancement of the photodynamic antitumor effect by streptococcal preparation OK-432 in the mouse carcinoma. Cancer Immunol. Immunother. 2000;49(8):401–409. doi: 10.1007/s002620000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Castano AP, Hamblin MR. Biophotonics and Immune Responses. San Jose: The International Society for Optical Engineering, WA, USA; 2006. Enhancing photodynamic therapy of a metastatic mouse breast cancer by immune stimulation. [Google Scholar]
- 155.Korbelik M, Cecic I. Enhancement of tumour response to photodynamic therapy by adjuvant mycobacterium cell-wall treatment. J. Photochem. Photobiol. B. Biol. 1998;44(2):151–158. doi: 10.1016/S1011-1344(98)00138-9. [DOI] [PubMed] [Google Scholar]
- 156.Korbelik M, Sun J, Posakony JJ. Interaction between photodynamic therapy and BCG immunotherapy responsible for the reduced recurrence of treated mouse tumors. Photochem. Photobiol. 2001;73(4):403–409. doi: 10.1562/0031-8655(2001)073<0403:ibptab>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 157.Myers RC, Lau BH, Kunihira DY, Torrey RR, Woolley JL, Tosk J. Modulation of hematoporphyrin derivative-sensitized phototherapy with corynebacterium parvum in murine transitional cell carcinoma. Urology. 1989;33(3):230–235. doi: 10.1016/0090-4295(89)90399-3. [DOI] [PubMed] [Google Scholar]
- 158.Winters U, Daayana S, Lear JT, et al. Clinical and immunologic results of a Phase II trial of sequential imiquimod and photodynamic therapy for vulval intraepithelial neoplasia. Clin. Cancer Res. 2008;14(16):5292–5299. doi: 10.1158/1078-0432.CCR-07-4760. [DOI] [PubMed] [Google Scholar]
- 159.Seshadri M, Bellnier DA. The vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid improves the antitumor efficacy and shortens treatment time associated with Photochlor-sensitized photodynamic therapy in vivo. Photochem. Photobiol. 2009;85(1):50–56. doi: 10.1111/j.1751-1097.2008.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Krosl G, Korbelik M, Krosl J, Dougherty GJ. Potentiation of photodynamic therapy-elicited antitumor response by localized treatment with granulocyte-macrophage colony-stimulating factor. Cancer Res. 1996;56(14):3281–3286. [PubMed] [Google Scholar]
- 161. Castano AP, Mroz P, Wu MX, Hamblin MR. Photodynamic therapy plus low-dose cyclophosphamide generates antitumor immunity in a mouse model. Proc. Natl Acad. Sci. USA. 2008;105(14):5495–5500. doi: 10.1073/pnas.0709256105. • Provides evidence for the role of regulatory T cells in inhibiting the immune response after PDT.