Abstract
The development of multicellular organisms is governed in part by temporally and spatially controlled gene expression. DNA methylation, covalent modifications of histones and the use of histone variants are the major epigenetic mechanisms governing gene expression in plant development. In this review, we zoom in onto histone H3 lysine 27 trimethylation (H3K27me3), a repressive mark that plays a crucial role in the dynamic regulation of gene expression in plant development, to discuss recent advances as well as outstanding questions in the deposition, recognition, and removal of the mark and the impacts of these molecular processes on plant development.
Introduction
An angiosperm features a predominant sporophytic form that undergoes several major developmental transitions to culminate in gametogenesis and fertilization to produce zygotes of the next generation. A zygote undergoes embryogenesis, at the end of which the shoot and root apical meristems are established and subsequently produce all the structures of the sporophyte. Mitosis and cell fate specification are key cellular events in the development of the sporophyte and meiosis occurs during gametogenesis to generate gametes. Cell fate specification not only entails temporally and spatially restricted gene expression brought about by transcription factors and posttranscriptional regulators such as microRNAs but also the Polycomb group (PcG) proteins that impart memory of the cell fate decisions through mitosis.
The PcG genes were originally identified from genetic screens as regulators of homeotic (Hox) gene expression in Drosophila [1,2]. In loss-of-function PcG mutants, the patterns of Hox gene expression are correctly established, but the genes become de-repressed in cells in which they are supposed to be repressed, indicating that PcG represses target gene expression and serves as a cellular memory through mitosis. Later studies revealed that PcG introduces histone H3 lysine 27 trimethylation (H3K27me3), a repressive chromatin mark, onto target loci (reviewed in [3]). As the vital roles of PcG are further explored in various organisms including mammals, it has become evident that PcG not only imparts developmental memory of a repressed state of gene expression through mitosis but also participates actively in patterning development as a mechanism of regulating gene expression. For example, PcG is essential for the differentiation of embryonic stem (ES) cells into multiple lineages in mammals [4–6]. Chromatin immunoprecipitation-microarray analyses (ChIP-on-Chip) using anti-H3K27me3 antibodies show that genes acting in all major developmental processes are PcG targets in Drosophila [7,8]. Genome-wide mapping of PcG targets in murine and human cell lines confirms that PcG targets are enriched in developmental regulators [4,9,10].
The plant PcG
The Drosophila PcG is composed of several sub-complexes (reviewed in [3]): Polycomb repressive complex1 (PRC1), Polycomb repressive complex2 (PRC2), pleiohomeotic repressive complex (PhoRC), and a newly identified module named PR-DUB [11]. PRC2 is composed of four subunits, among which Enhancer of zeste (E(Z)) is a SET domain protein that possesses H3K27 methyltransferase activity [12,13]. PRC1 is thought to recognize the H3K37me3 mark to confer stable transcriptional repression. The chromodomain-containing PRC1 subunit, Polycomb (Pc), binds H3K27me3. Among the other three Drosophila PRC1 subunits are two RING finger proteins Posterior sex combs (Psc) and dRING, which are orthologs of mammalian PRC1 subunits BMI1 and RING1A/RING1B, respectively. Mammalian BMI1, RING1A and RING1B form an E3 ubiquitin ligase complex that causes the mono-ubiquitination of histone H2A lysine 119, which confers stable repression of target loci [14,15]. PR-DUB, on the other hand, has ubiquitin hydrolase activity that removes H2A mono-ubiquitination [11]. PhoRC has two subunits, one of which contains a DNA-binding domain.
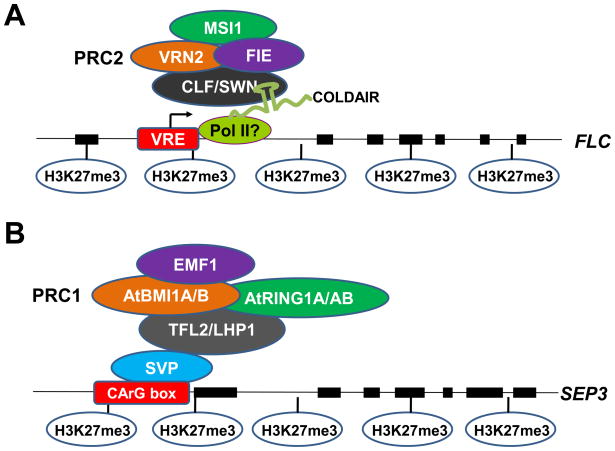
The four Drosphila PRC2 subunits have conserved counterparts in plants (reviewed in [16–19]) (Figure 1A). The three paralogous genes CURLY LEAF (CLF), SWINGER (SWN), and MEDEA are orthologs of Drosophila E(Z). MEDEA appears to function only in gametophyte and seed development. CLF and SWN are broadly expressed and partially redundant in vegetative and reproductive development. The SU(Z)12 subunit of Drosophila PRC2 also has three orthologs in plants, VERNALIZATION2 (VRN2), FERTILIZATION INDEPENDENT SEEDS2 (FIS2), and EMBRYONIC FLOWER2 (EMF2). The ortholog of the Drosophila PRC2 subunit ESC is a single-copy gene FERTILIZATION INDEPENDENT ENDOSPERM (FIE). The Drosophila PRC2 subunit p55 has five orthologs in Arabidopsis, among which MULTICOPY SUPPRESSOR OF IRA1 (MSI1) is essential for multiple aspects of vegetative and reproductive development. Genetic and molecular studies led to the proposal that at least three forms of PRC2 exist in plants: FIS2, VRN2 and EMF2, reflecting different SU(Z)12 paralogs, and each complex acts at a specific developmental phase in Arabidopsis. The FIS2 complex mainly controls seed development; the VRN2 complex acts in vernalization response, a response to prolonged cold treatment that induces flowering; and the EMF2 complex is active in most vegetative and reproductive developmental processes. The FIS2 complex, which contains MEA/SWN, FIS2, FIE, and MSI1, and the VRN2 complex (Figure 1A), which contains CLF/SWN, VRN2, FIE, and MSI1, have been biochemically confirmed [20–22].
Figure 1.
A diagram of the compositions of PcG and mechanisms of its recruitment to targets. (A) Composition of a PRC2 complex and its recruitment to the FLC gene by a noncoding RNA. The VRN2 complex is composed of CLF and/or its paralog SWN, FIE, MSI and VRN2. A vernalization response element (VER) in the first intron of the PcG target FLC serves as a promoter for the transcription of a noncoding RNA (COLDAIR), which binds CLF and recruits PRC2 to FLC. Pol II is probably the polymerase that produces COLDAIR. By analogy to findings from animal systems, CLF may recognize short stem-loop features within COLDAIR. (B) Composition of a PRC1-like complex and its recruitment to the SEPALLATA3 (SEP3) gene. A plant PRC1 complex likely consists of TFL2, AtRING1A/AtRING1B, AtBMI1A/AtBMI1B, and EMF1. The transcription factor SVP binds to its specific recognition site known as a CArG box in SEP3 and recruits TFL2 to SEP3.
Drosophila or mammalian PRC1 subunits have no obvious orthologs in plants, but a functional counterpart of PRC1 has been identified in Arabidopsis. LIKE HETEROCHROMATIN PROTEIN1 (LHP1), also known as TERMINAL FLOWER2 (TFL2), binds H3K27me3 and is co-localized with H3K27me3 marks throughout the genome [23,24]. lhp1/tfl2 mutants show similar phenotypes as other PcG mutants, such as curled leaves, early flowering, and defective vernalization responses [25–27]. Because H3K27me3 marks still remain in lhp1/tfl2 mutants [23], it is thought that LHP1/TFL2 acts downstream of PRC2 as a PRC1-like protein. Molecular genetic evidence also supports a PRC1-like function for two non-sequence-specific DNA binding proteins, EMF1 [28,29] and VRN1 [30–33], but their molecular functions in PcG-mediated silencing remain to be uncovered. Recent bioinformatics and functional studies have identified functional homologs to the Drosophila and mammalian PRC1 RING finger proteins [34–36]. AtRING1A and AtRING1B, homologs of mammalian RING1A and RING1B, interact with LHP1 and repress PcG targets in vivo [36]. AtBMI1A and AtBMI1B, two other Arabidopsis RING finger proteins, are more closely related to mammalian BMI1. They interact with LHP1 and EMF1 and repress PcG targets in vivo [34]. In summary, the Arabidopsis PRC1 complex probably consists of LHP1, AtRING1A/AtRING1B, AtBMI1A/AtBMI1B, and EMF1 (and possibly VRN1) (Figure 1B).
There are no obvious homologs of PhoRC genes in Arabidopsis. Potential Arabidopsis homologs of the newly identified PR-DUB complex will be discussed in a later section.
The developmental functions of the plant PcG are evident from the multitude of developmental defects exhibited by mutants in various PcG subunit genes [37–42]. Mutants in EMF2 skip vegetative development and proceed to flowering soon after germination and display ectopic expression of floral homeotic genes in non-floral tissues [42]. A mutant in CLF shows ecotpic activation of floral homeotic genes in leaves and exhibits pleiotropic phenotypes such as leaf curling, early flowering, and reduced stature [37]. A double mutant in CLF and its paralog SWN is deficient in pattern formation after germination such that the seedling develops into callus-like tissues [43]. Mutations in MEDEA and FIS2 disrupt endosperm development in the seed [38,40]. These mutant phenotypes indicate that PcG, as a repressor of gene expression, plays a crucial role in all major stages of plant development. Consistent with this notion, genome-wide H3K27me3 profiling from 10-day-old seedlings identified ~4000 genes as PcG targets, among which genes involved in transcriptional regulation are enriched [23,24].
Deposition and maintenance of H3K27me3
Although biochemical evidence is lacking, molecular genetic evidence strongly supports the SET domain-containing E(Z) homologs CLF, SWN, and MEA as the methyltransferases that deposit H3K27me3 at target genes. The clf-81 allele with an amino acid substitution at a highly conserved residue in the SET domain resembles null clf mutants in morphology [32,44]. H3K27me3 is completely lost at PcG target genes such as AGAMOUS (AG) or SHOOT MERISTEMLESS (STM) in either a clf single mutant or a clf swn double mutant [32]. Furthermore, CLF is found at the chromatin of PcG target genes [32].
While PRC2 deposits H3K27me3 at PcG targets in vivo, how PRC2 is recruited to specific target genes in a developmentally regulated manner is largely unknown in plants. The recruitment of PcG to targets is best understood in Drosophila. The Hox loci, which are targets of PcG, contain Polycomb responsive elements (PREs), which are necessary and sufficient for PcG recruitment (reviewed in [3]). PREs are sequences of hundreds of basepairs composed of short consensus binding sites for a number of DNA binding proteins. PhoRC is the only PcG subcomplex with DNA binding capability and is one, but not the only, recruiter for the other PcG subcomplexes. A number of transcription factors have also been implicated in the recruitment of PcG to targets in Drosophila. In plants, homologs of PhoRC are not readily recognizable and no PREs have been clearly defined. But persistent efforts to study PRC2 recruitment have begun to implicate DNA sequences, chromatin-related proteins, and non-coding RNAs in the establishment and/or maintenance of H3K27me3 at targets.
Studies with AG and FLOWERING LOCUS C (FLC), two best characterized PcG targets that play crucial roles in reproductive organ development and flowering, respectively, show that DNA sequences in these genes are important for the establishment or maintenance of H3K27 and imply that PRC2 can spread from an initial entry site to methylate histones at adjacent sequences. A portion of the AG genomic region (starting at the 3′ end of the upstream gene and extending into the third exon of AG) confers responsiveness to CLF to the GUS reporter gene in terms of its expression [45]. Strong enrichment of H3K27me3 was found at both the AG and GUS sequences of the transgene in wild type but not in clf mutants [32]. Since GUS is not an endogenous PcG target, these results suggest that the AG sequences recruit PRC2 to the transgene and that PRC2 is able to spread from its site of entry to nearby GUS sequences. Similarly, FLC sequences can confer PRC2-dependent H3K27me3 deposition at nearby NPTII and GUS genes [46,47], which are not endogenous PcG targets. Both the promoter and intron 1 of FLC are required for the initial establishment of H3K27me3 [46]. These studies with AG and FLC have not identified a small (hundreds of bp) region sufficient for PRC2 recruitment, although a small region (289bp; named vernalization response element or VRE) in intron 1 of FLC is required for the maintenance of H3K27me3 after it is induced by vernalization [27]. Although DNA sequences are clearly important in PRC2 recruitment, these studies have not pinpointed elements that may be bound by transcription factors or PhoRC-like proteins analogous to PREs in Drosophila, and leave open alternative mechanisms such as chromatin modification or noncoding RNA production at these sequences playing a role in PRC2 recruitment. In fact, a recent study uncovered the existence of a noncoding RNA (COLDAIR) whose 5′ end lies within the VRE in FLC and revealed a role of this noncoding RNA in the recruitment of PRC2 to FLC [48].
The possibility that other histone marks could play a role in PRC2 recruitment has been suggested by studies on FLC and its paralogs MADS AFFECTING FLOWERING (MAF) 1 to 5. A family of Plant Homeodomain (PHD) Finger proteins consisting of VERNALIZATION INSENSITIVE 3 (VIN3), VERNALIZATION5 (VRN5)/VIN3-LIKE1 (VIL1), and three other VIL/VERNALIZATION LIKE (VEL) proteins is required for the repression of FLC or the MAF genes. In vin3 or vrn5 single mutants, the vernalization-triggered H3K27me3 deposition at FLC is impaired [33,49,50]. In fact, VIN3 was found to associate with the VRN2 PRC2 complex in vivo [22]. The VIN3-like proteins VIL1/VRN5 and VIL2/VEL1 participate in the repression of MAF1 and MAF5 triggered by environmental conditions such as vernalization or photoperiod, and are required for the establishment and/or maintenance of H3K27me3 at MAF1 or MAF5 chromatin [49–51]. VIL2 associates with CLF in vivo and binds dimethylated histone H3 lysine 9 (H3K9me2) in vitro [51]. This implies a crosstalk between H3K9me2 and H3K27me3.
Four recent studies show that a Jumonji C (JmjC) domain protein Jarid2 associates with PRC2, promotes the recruitment of PRC2 to targets, but inhibits the activities of the H3K27 methyltransferase activity of PRC2 in mammalian embryonic stem cells [52–55]. The binding of this protein to PRC2 requires a linear sequence motif GSGFP. The Arabidopsis genome contains at least four Jarid2 homologs (At4g20400, At1g08620, At1g63490, and At2g38950) containing the linear GSGFP motif. It remains to be determined whether these are PRC2-associated proteins and whether they influence PRC2 recruitment to targets.
Noncoding transcripts have been increasingly found to play a role in PcG targeting in animals. For example, the noncoding transcripts HOTAIR and Xist are associated with PRC2 and are required for PcG-mediated silencing of target loci [56,57]. Many long intergenic noncoding transcripts have been found associated with PcG in human cell culture, although the functions of the RNAs still await further investigation [58]. A recent study found short (50 to 200 nucleotides) RNAs transcribed from the 5′ ends of thousands of PcG target genes in human T cells and embryonic stem cells and showed that PRC2 binds these RNAs through their short stem-loop features [59]. Recent studies have identified noncoding RNAs associated with the PcG target FLC in Arabidopsis and demonstrated that PcG recruitment by noncoding RNAs is a conserved mechanism in plants and animals [48,60]. Vernalization induces two groups of noncoding RNAs at the FLC locus, COOLAIR, a group of antisense RNAs originating from the 3′ end of the gene, and COLDAIR, a group of sense RNAs whose transcription initiation sites map within the VRE within the first intron. The induction of COOLAIR is the earliest known molecular response to vernalization, but the role of COOLAIR in FLC repression is currently unknown. COLDAIR, which is induced by cold with a slower kinetics than that of COOLAIR, binds CLF both in vitro and in vivo. RNAi-mediated knockdown of COLDAIR impairs cold-induced CLF occupancy and H3K27me3 enrichment at FLC, stable FLC repression, and early flowering. Therefore, COLDAIR recruits PRC2 to FLC (Figure 1A). Although this is the only example of noncoding RNA-mediated PRC2 recruitment to targets in plants, noncoding RNA-mediated PRC2 recruitment may come to be realized as a general mechanism.
Recognition of H3K27me3
As discussed above, the chromodomain protein LHP1/TFL2 is likely the functional counterpart of Drosophila Pc, a subunit of PRC1 that binds H3K27me3. Although LHP1/TFL2 binds H3K27me3 in vitro [23], its in vivo recognition of H3K27me3 may require additional factors. For example, the MADS-box transcription factor SHORT VEGETATIVE PHASE1 physically interacts with LHP1/TFL2 and helps recruit LHP1/TFL2 to the PcG target SEPALLATA3 [61] (Figure 1B). A recent study showed that the antisense RNA ANRIL from the PcG target INK4b/ARF locus in humans associates with PRC1 and that the ANRIL-PRC1 interaction is crucial for PcG-mediated silencing of INK4b/ARF [62]. It remains to be determined whether noncoding RNAs play a role in the recruitment of LHP1/TFL2 to PcG targets in plants.
How the recognition of H3K27me3 leads to compaction of chromatin and transcriptional gene silencing is little understood in plants. In Drosophila and mammals, PcG-mediated silencing requires histone H2A ubiquitination at lysine 119 by the E3 ubiquitin ligase comprised of RING finger proteins RING and BMI [14,15,63]. Arabidopsis homologs of these proteins have been established as PRC1 subunits [34,36]. The studies by Bratzel et al. demonstrated that the Arabidopsis PRC1 also exhibits H2A monoubiquitination activity [34]. Recombinant AtRING1A, AtRING1B, AtBMI1A, and AtBMI1B proteins were able to monoubiquitinate histone H2A.1 (one of 13 H2A isoforms in Arabidopsis) in vitro. The levels of monoubiquitinated H2A.1 in vivo were drastically reduced in the atbmi1a atbmib double mutant. Levels of H2A.1 monoubiquitination were also reduced in an emf1 mutant. Whether the H2A.1 monoubiquitination activity of PRC1 is essential for gene repression remains to be determined.
Intriguingly, the newly identified PcG subcomplex in Drosophila and humans, PR-DUB, has ubiquitin hydrolase activity that removes H2A mono-ubiquitination and is also required for PcG-mediated silencing, suggesting that the dynamics of H2A ubiquitination somehow influences silencing [11]. The BAP1 protein in PR-DUB contains the ubiquitin C-terminal hydrolase (UCH) domain, which shows approximately 40% sequence identities to that of three Arabidopsis proteins, UCH1, 2, and 3 [64]. It remains to be determined whether these Arabidopsis proteins have PcG-related roles.
Removal of H3K27me3
It is long known that PcG-mediated transcriptional gene silencing can be overcome in response to endogenous or exogenous cues. For example, the floral homeotic MADS-box genes such as AG and APETALA3 that specify floral organ identities are kept silent in leaves by the PcG [37], but are later activated in the proper cells in the floral meristem. Although unknown, the re-activation of PcG target genes later in development probably entails the removal of H3K27me3, which could be through active demethylation or passive dilution upon cell division.
In humans, JmjC domain proteins Hs KDM6A/UTX and Hs KDM6B/JMJD3 demethylate H3K27me3 and H3K27me2 [65]. Although 21 JmjC proteins are found in Arabidopsis, none of them fall in the same clade as the human H3K27me2/3 demethylases [66]. It is possible that a JmjC protein from a different clade has acquired H3K27me3 demethylase activity in plants. In addition to removal of the H3K27me3 mark, PRC2 itself may also need to be displaced from PcG target genes that are to be activated. A recent study in mammalian cells found that phosphorylation of the nearby serine on histone H3 (H3 S28) to generate the H3K27me3S28p double mark accompanies the displacement of PRC2 and the activation of PcG target genes upon developmental or environmental cues [67].
Although the mechanisms of H3K27me3 removal in plants are unknown, a recent study [68] implicated H3K27me3 removal as a means to achieve temporal control in gene activation by transcription factors. The study shows that the PcG target gene KNUCKLES (KNU) is a direct target of the transcription factor AG. While AG expression commences at stage 3 of flower development, the expression of KNU is not activated until stage 6. By controlling the timing of AG activation, it was found that a large reduction in H3K27me3 levels at KNU preceded KNU expression, suggesting that H3K27me3 contributes to the delay in KNU activation. Such a delay is functionally important in flower development, as precocious activation of KNU would lead to the lack of a complete set of floral organs due to its activity in repressing floral stem cells. Interestingly, it was also found that the reduction in H3K27me3 levels at KNU depended on AG activity, suggesting that transcription factors contribute to the removal of H3K27me3 at specific PcG target genes.
The PcG-mediated repression of FLC triggered by vernalization is mitotically stable but is re-set upon meiosis. The mechanisms that re-set H3K27me3 marks at meiosis or fertilization are largely unknown. A recent study that examined the dynamic expression patterns of histone H3 variants in Arabidopsis provides a glimpse into a potential resetting mechanism [69]. This study shows that among the 15 HISTONE THREE RELATED (HTR) genes encoded by the Arabidopsis genome, only a few of the variants are expressed in male and female gametes. Furthermore, a few hours after fertilization, the male and female H3 variants are removed from the zygote chromatin and de novo synthesis of H3 variants restores the somatic H3 composition in the embryo. Therefore, it is conceivable that the somatic H3K27me3 marks are discarded together with their resident histone variants during gametogenesis and early embryogenesis.
Conclusion
PcG-mediated gene silencing is a common mechanism employed by plants and animals to precisely control the expression of a large number of genes in development. With the identification of PcG targets at the genomic scale and the in-depth studies on a few PcG targets in plants, the crucial role of the PcG in plant development is highly appreciated. But the mechanisms of action of the PcG in plants are still poorly understood and this impedes the progress towards a full understanding of plant development. Major questions that await further experimentation include: 1) How is PRC2 recruited to specific target genes and what governs the extent of spreading of PRC2 from its site of entry? 2) How is PRC1 recruited to targets and how does PRC1 lead to transcriptional gene silencing? 3) How is the H3K27me3 mark removed, and if a demethylase exists, how is the demethylase recruited to specific targets in a temporally or spatially controlled manner?
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jurgens G. A group of genes controlling the spatial expression of the bithorax complex in Drosophila. Nature. 1985;316:153–155. [Google Scholar]
- 2.Lewis EB. A gene complex controlling segmentation in Drosophila. Nature. 1978;276:565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz YB, Pirrotta V. Polycomb silencing mechanisms and the management of genomic programmes. Nat Rev Genet. 2007;8:9–22. doi: 10.1038/nrg1981. [DOI] [PubMed] [Google Scholar]
- 4.Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, Levine SS, Wernig M, Tajonar A, Ray MK, et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441:349–353. doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- 5.Pasini D, Bracken AP, Hansen JB, Capillo M, Helin K. The polycomb group protein Suz12 is required for embryonic stem cell differentiation. Mol Cell Biol. 2007;27:3769–3779. doi: 10.1128/MCB.01432-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen X, Liu Y, Hsu YJ, Fujiwara Y, Kim J, Mao X, Yuan GC, Orkin SH. EZH1 mediates methylation on histone H3 lysine 27 and complements EZH2 in maintaining stem cell identity and executing pluripotency. Mol Cell. 2008;32:491–502. doi: 10.1016/j.molcel.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz YB, Kahn TG, Nix DA, Li XY, Bourgon R, Biggin M, Pirrotta V. Genome-wide analysis of Polycomb targets in Drosophila melanogaster. Nat Genet. 2006;38:700–705. doi: 10.1038/ng1817. [DOI] [PubMed] [Google Scholar]
- 8.Tolhuis B, de Wit E, Muijrers I, Teunissen H, Talhout W, van Steensel B, van Lohuizen M. Genome-wide profiling of PRC1 and PRC2 Polycomb chromatin binding in Drosophila melanogaster. Nat Genet. 2006;38:694–699. doi: 10.1038/ng1792. [DOI] [PubMed] [Google Scholar]
- 9.Bracken AP, Dietrich N, Pasini D, Hansen KH, Helin K. Genome-wide mapping of Polycomb target genes unravels their roles in cell fate transitions. Genes Dev. 2006;20:1123–1136. doi: 10.1101/gad.381706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee TI, Jenner RG, Boyer LA, Guenther MG, Levine SS, Kumar RM, Chevalier B, Johnstone SE, Cole MF, Isono K, et al. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell. 2006;125:301–313. doi: 10.1016/j.cell.2006.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheuermann JC, de Ayala Alonso AG, Oktaba K, Ly-Hartig N, McGinty RK, Fraterman S, Wilm M, Muir TW, Muller J. Histone H2A deubiquitinase activity of the Polycomb repressive complex PR-DUB. Nature. 2010;465:243–247. doi: 10.1038/nature08966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czermin B, Melfi R, McCabe D, Seitz V, Imhof A, Pirrotta V. Drosophila enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal Polycomb sites. Cell. 2002;111:185–196. doi: 10.1016/s0092-8674(02)00975-3. [DOI] [PubMed] [Google Scholar]
- 13.Muller J, Hart CM, Francis NJ, Vargas ML, Sengupta A, Wild B, Miller EL, O’Connor MB, Kingston RE, Simon JA. Histone methyltransferase activity of a Drosophila Polycomb group repressor complex. Cell. 2002;111:197–208. doi: 10.1016/s0092-8674(02)00976-5. [DOI] [PubMed] [Google Scholar]
- 14.Cao R, Tsukada Y, Zhang Y. Role of Bmi-1 and Ring1A in H2A ubiquitylation and Hox gene silencing. Mol Cell. 2005;20:845–854. doi: 10.1016/j.molcel.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Wang L, Erdjument-Bromage H, Vidal M, Tempst P, Jones RS, Zhang Y. Role of histone H2A ubiquitination in Polycomb silencing. Nature. 2004;431:873–878. doi: 10.1038/nature02985. [DOI] [PubMed] [Google Scholar]
- 16.Hennig L, Derkacheva M. Diversity of Polycomb group complexes in plants: same rules, different players? Trends Genet. 2009;25:414–423. doi: 10.1016/j.tig.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Kohler C, Villar CB. Programming of gene expression by Polycomb group proteins. Trends Cell Biol. 2008;18:236–243. doi: 10.1016/j.tcb.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Pien S, Grossniklaus U. Polycomb group and trithorax group proteins in Arabidopsis. Biochim Biophys Acta. 2007;1769:375–382. doi: 10.1016/j.bbaexp.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Schatlowski N, Creasey K, Goodrich J, Schubert D. Keeping plants in shape: polycomb-group genes and histone methylation. Semin Cell Dev Biol. 2008;19:547–553. doi: 10.1016/j.semcdb.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 20.De Lucia F, Crevillen P, Jones AM, Greb T, Dean C. A PHD-polycomb repressive complex 2 triggers the epigenetic silencing of FLC during vernalization. Proc Natl Acad Sci U S A. 2008;105:16831–16836. doi: 10.1073/pnas.0808687105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohler C, Hennig L, Bouveret R, Gheyselinck J, Grossniklaus U, Gruissem W. Arabidopsis MSI1 is a component of the MEA/FIE Polycomb group complex and required for seed development. Embo J. 2003;22:4804–4814. doi: 10.1093/emboj/cdg444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wood CC, Robertson M, Tanner G, Peacock WJ, Dennis ES, Helliwell CA. The Arabidopsis thaliana vernalization response requires a polycomb-like protein complex that also includes VERNALIZATION INSENSITIVE 3. Proc Natl Acad Sci U S A. 2006;103:14631–14636. doi: 10.1073/pnas.0606385103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turck F, Roudier F, Farrona S, Martin-Magniette ML, Guillaume E, Buisine N, Gagnot S, Martienssen RA, Coupland G, Colot V. Arabidopsis TFL2/LHP1 specifically associates with genes marked by trimethylation of histone H3 lysine 27. PLoS Genet. 2007;3:e86. doi: 10.1371/journal.pgen.0030086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Germann S, Blus BJ, Khorasanizadeh S, Gaudin V, Jacobsen SE. The Arabidopsis LHP1 protein colocalizes with histone H3 Lys27 trimethylation. Nat Struct Mol Biol. 2007;14:869–871. doi: 10.1038/nsmb1283. [DOI] [PubMed] [Google Scholar]
- 25.Kotake T, Takada S, Nakahigashi K, Ohto M, Goto K. Arabidopsis TERMINAL FLOWER 2 gene encodes a heterochromatin protein 1 homolog and represses both FLOWERING LOCUS T to regulate flowering time and several floral homeotic genes. Plant Cell Physiol. 2003;44:555–564. doi: 10.1093/pcp/pcg091. [DOI] [PubMed] [Google Scholar]
- 26.Mylne JS, Barrett L, Tessadori F, Mesnage S, Johnson L, Bernatavichute YV, Jacobsen SE, Fransz P, Dean C. LHP1, the Arabidopsis homologue of HETEROCHROMATIN PROTEIN1, is required for epigenetic silencing of FLC. Proc Natl Acad Sci U S A. 2006;103:5012–5017. doi: 10.1073/pnas.0507427103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sung S, He Y, Eshoo TW, Tamada Y, Johnson L, Nakahigashi K, Goto K, Jacobsen SE, Amasino RM. Epigenetic maintenance of the vernalized state in Arabidopsis thaliana requires LIKE HETEROCHROMATIN PROTEIN 1. Nat Genet. 2006;38:706–710. doi: 10.1038/ng1795. [DOI] [PubMed] [Google Scholar]
- 28.Aubert D, Chen L, Moon YH, Martin D, Castle LA, Yang CH, Sung ZR. EMF1, a novel protein involved in the control of shoot architecture and flowering in Arabidopsis. Plant Cell. 2001;13:1865–1875. doi: 10.1105/TPC.010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29**.Calonje M, Sanchez R, Chen L, Sung ZR. EMBRYONIC FLOWER1 participates in polycomb group-mediated AG gene silencing in Arabidopsis. Plant Cell. 2008;20:277–291. doi: 10.1105/tpc.106.049957. This study reveals that EMF1 is a PRC1-like protein in that it is recruited to the PcG target gene AG in an EMF2 (PRC2)-dependent manner and that it acts non-redundantly with EMF2 in repressing AG expression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bastow R, Mylne JS, Lister C, Lippman Z, Martienssen RA, Dean C. Vernalization requires epigenetic silencing of FLC by histone methylation. Nature. 2004;427:164–167. doi: 10.1038/nature02269. [DOI] [PubMed] [Google Scholar]
- 31.Levy YY, Mesnage S, Mylne JS, Gendall AR, Dean C. Multiple roles of Arabidopsis VRN1 in vernalization and flowering time control. Science. 2002;297:243–246. doi: 10.1126/science.1072147. [DOI] [PubMed] [Google Scholar]
- 32.Schubert D, Primavesi L, Bishopp A, Roberts G, Doonan J, Jenuwein T, Goodrich J. Silencing by plant Polycomb-group genes requires dispersed trimethylation of histone H3 at lysine 27. Embo J. 2006;25:4638–4649. doi: 10.1038/sj.emboj.7601311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sung S, Amasino RM. Vernalization in Arabidopsis thaliana is mediated by the PHD finger protein VIN3. Nature. 2004;427:159–164. doi: 10.1038/nature02195. [DOI] [PubMed] [Google Scholar]
- 34***.Bratzel F, Lopez-Torrejon G, Koch M, Del Pozo JC, Calonje M. Keeping cell identity in arabidopsis requires PRC1 RING-finger homologs that catalyze H2A monoubiquitination. Curr Biol. 2010;20:1853–1859. doi: 10.1016/j.cub.2010.09.046. This work establishes that the mammalian BMI homologs in Arabidopsis are indeed PRC1 subunits and shows that the AtBMI proteins together with AtRING proteins have E3 ubiquitin ligase activity that monoubiquitinates histone H2A, thus revealing a conserved mechanism in PcG-mediated silencing between plants and animals. [DOI] [PubMed] [Google Scholar]
- 35*.Sanchez-Pulido L, Devos D, Sung ZR, Calonje M. RAWUL: a new ubiquitin-like domain in PRC1 ring finger proteins that unveils putative plant and worm PRC1 orthologs. BMC Genomics. 2008;9:308. doi: 10.1186/1471-2164-9-308. This bioinformatics effort uncovered five Arabidopsis RING finger proteins that are potential homologs of the Drosophila and mammalian PRC1 RING finger proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36**.Xu L, Shen WH. Polycomb silencing of KNOX genes confines shoot stem cell niches in Arabidopsis. Curr Biol. 2008;18:1966–1971. doi: 10.1016/j.cub.2008.11.019. This study establishes AtRING1A and AtRING1B as PRC1-like proteins. [DOI] [PubMed] [Google Scholar]
- 37.Goodrich J, Puangsomlee P, Martin M, Long D, Meyerowitz EM, Coupland G. A Polycomb-group gene regulates homeotic gene expression in Arabidopsis. Nature. 1997;386:44–51. doi: 10.1038/386044a0. [DOI] [PubMed] [Google Scholar]
- 38.Grossniklaus U, Vielle-Calzada JP, Hoeppner MA, Gagliano WB. Maternal control of embryogenesis by MEDEA, a polycomb group gene in Arabidopsis. Science. 1998;280:446–450. doi: 10.1126/science.280.5362.446. [DOI] [PubMed] [Google Scholar]
- 39.Hennig L, Taranto P, Walser M, Schonrock N, Gruissem W. Arabidopsis MSI1 is required for epigenetic maintenance of reproductive development. Development. 2003;130:2555–2565. doi: 10.1242/dev.00470. [DOI] [PubMed] [Google Scholar]
- 40.Luo M, Bilodeau P, Koltunow A, Dennis ES, Peacock WJ, Chaudhury AM. Genes controlling fertilization-independent seed development in Arabidopsis thaliana. Proc Natl Acad Sci U S A. 1999;96:296–301. doi: 10.1073/pnas.96.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohad N, Yadegari R, Margossian L, Hannon M, Michaeli D, Harada JJ, Goldberg RB, Fischer RL. Mutations in FIE, a WD polycomb group gene, allow endosperm development without fertilization. Plant Cell. 1999;11:407–416. doi: 10.1105/tpc.11.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang CH, Chen LJ, Sung ZR. Genetic regulation of shoot development in Arabidopsis: role of the EMF genes. Dev Biol. 1995;169:421–435. doi: 10.1006/dbio.1995.1158. [DOI] [PubMed] [Google Scholar]
- 43.Chanvivattana Y, Bishopp A, Schubert D, Stock C, Moon YH, Sung ZR, Goodrich J. Interaction of Polycomb-group proteins controlling flowering in Arabidopsis. Development. 2004;131:5263–5276. doi: 10.1242/dev.01400. [DOI] [PubMed] [Google Scholar]
- 44.Kim GT, Tsukaya H, Uchimiya H. The CURLY LEAF gene controls both division and elongation of cells during the expansion of the leaf blade in Arabidopsis thaliana. Planta. 1998;206:175–183. doi: 10.1007/s004250050389. [DOI] [PubMed] [Google Scholar]
- 45.Sieburth LE, Meyerowitz EM. Molecular dissection of the AGAMOUS control region shows that cis elements for spatial regulation are located intragenically. Plant Cell. 1997;9:355–365. doi: 10.1105/tpc.9.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheldon CC, Conn AB, Dennis ES, Peacock WJ. Different regulatory regions are required for the vernalization-induced repression of FLOWERING LOCUS C and for the epigenetic maintenance of repression. Plant Cell. 2002;14:2527–2537. doi: 10.1105/tpc.004564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sheldon CC, Finnegan EJ, Peacock WJ, Dennis ES. Mechanisms of gene repression by vernalization in Arabidopsis. Plant J. 2009;59:488–498. doi: 10.1111/j.1365-313X.2009.03883.x. [DOI] [PubMed] [Google Scholar]
- 48***.Heo JB, Sung S. Vernalization-Mediated Epigenetic Silencing by a Long Intronic Noncoding RNA. Science. 2010 doi: 10.1126/science.1197349. This study reveals that a vernalization-induced noncoding RNA from the first intron of FLC binds the PRC2 subunit CURLY LEAF to recruit PRC2 to FLC. This is the first example of noncoding RNA-mediated PRC2 recruitment of targets in plants. Findings from this study in plants and others in animal systems show that noncoding RNA-mediated PRC2 recruitment to targets is a conserved mechanism. [DOI] [PubMed] [Google Scholar]
- 49.Greb T, Mylne JS, Crevillen P, Geraldo N, An H, Gendall AR, Dean C. The PHD finger protein VRN5 functions in the epigenetic silencing of Arabidopsis FLC. Curr Biol. 2007;17:73–78. doi: 10.1016/j.cub.2006.11.052. [DOI] [PubMed] [Google Scholar]
- 50.Sung S, Schmitz RJ, Amasino RM. A PHD finger protein involved in both the vernalization and photoperiod pathways in Arabidopsis. Genes Dev. 2006;20:3244–3248. doi: 10.1101/gad.1493306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim DH, Sung S. The Plant Homeo Domain finger protein, VIN3-LIKE 2, is necessary for photoperiod-mediated epigenetic regulation of the floral repressor, MAF5. Proc Natl Acad Sci U S A. 2010;107:17029–17034. doi: 10.1073/pnas.1010834107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li G, Margueron R, Ku M, Chambon P, Bernstein BE, Reinberg D. Jarid2 and PRC2, partners in regulating gene expression. Genes Dev. 2010;24:368–380. doi: 10.1101/gad.1886410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pasini D, Cloos PA, Walfridsson J, Olsson L, Bukowski JP, Johansen JV, Bak M, Tommerup N, Rappsilber J, Helin K. JARID2 regulates binding of the Polycomb repressive complex 2 to target genes in ES cells. Nature. 2010;464:306–310. doi: 10.1038/nature08788. [DOI] [PubMed] [Google Scholar]
- 54.Peng JC, Valouev A, Swigut T, Zhang J, Zhao Y, Sidow A, Wysocka J. Jarid2/Jumonji coordinates control of PRC2 enzymatic activity and target gene occupancy in pluripotent cells. Cell. 2009;139:1290–1302. doi: 10.1016/j.cell.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shen X, Kim W, Fujiwara Y, Simon MD, Liu Y, Mysliwiec MR, Yuan G-C, Lee Y, Orkin SH. Jumonji modulates Polycomb activity and self-renewal versus differentiation of stem cells. Cell. 2009;139:1303–1314. doi: 10.1016/j.cell.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao J, Sun BK, Erwin JA, Song JJ, Lee JT. Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science. 2008;322:750–756. doi: 10.1126/science.1163045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Rivea Morales D, Thomas K, Presser A, Bernstein BE, van Oudenaarden A, et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A. 2009;106:11667–11672. doi: 10.1073/pnas.0904715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kanhere A, Viiri K, Araujo CC, Rasaiyaah J, Bouwman RD, Whyte WA, Pereira CF, Brookes E, Walker K, Bell GW, et al. Short RNAs are transcribed from repressed polycomb target genes and interact with polycomb repressive complex-2. Mol Cell. 2010;38:675–688. doi: 10.1016/j.molcel.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60**.Swiezewski S, Liu F, Magusin A, Dean C. Cold-induced silencing by long antisense transcripts of an Arabidopsis Polycomb target. Nature. 2009;462:799–802. doi: 10.1038/nature08618. This study first reveals the induction of long noncoding RNAs at the FLC locus by vernalization and the potential role of the antisense RNAs in the repression of FLC expression. [DOI] [PubMed] [Google Scholar]
- 61**.Liu C, Xi W, Shen L, Tan C, Yu H. Regulation of floral patterning by flowering time genes. Dev Cell. 2009;16:711–722. doi: 10.1016/j.devcel.2009.03.011. This study shows that the PRC1 subunit TFL2 interacts with a transcription factor SVP, which aids the recruitment of TFL2 to its target gene SEPALLATA3. [DOI] [PubMed] [Google Scholar]
- 62.Yap KL, Li S, Munoz-Cabello AM, Raguz S, Zeng L, Mujtaba S, Gil J, Walsh MJ, Zhou MM. Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a. Mol Cell. 2010;38:662–674. doi: 10.1016/j.molcel.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Napoles M, Mermoud JE, Wakao R, Tang YA, Endoh M, Appanah R, Nesterova TB, Silva J, Otte AP, Vidal M, et al. Polycomb group proteins Ring1A/B link ubiquitylation of histone H2A to heritable gene silencing and X inactivation. Dev Cell. 2004;7:663–676. doi: 10.1016/j.devcel.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 64.Yang P, Smalle J, Lee S, Yan N, Emborg TJ, Vierstra RD. Ubiquitin C-terminal hydrolases 1 and 2 affect shoot architecture in Arabidopsis. Plant J. 2007;51:441–457. doi: 10.1111/j.1365-313X.2007.03154.x. [DOI] [PubMed] [Google Scholar]
- 65.Swigut T, Wysocka J. H3K27 demethylases, at long last. Cell. 2007;131:29–32. doi: 10.1016/j.cell.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 66.Lu F, Li G, Cui X, Liu C, Wang XJ, Cao X. Comparative analysis of JmjC domain-containing proteins reveals the potential histone demethylases in Arabidopsis and rice. J Integr Plant Biol. 2008;50:886–896. doi: 10.1111/j.1744-7909.2008.00692.x. [DOI] [PubMed] [Google Scholar]
- 67.Gehani SS, Agrawal-Singh S, Dietrich N, Christophersen NS, Helin K, Hansen K. Polycomb group protein displacement and gene activation through MSK-dependent H3K27me3S28 phosphorylation. Mol Cell. 2010;39:886–900. doi: 10.1016/j.molcel.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 68*.Sun B, Xu Y, Ng KH, Ito T. A timing mechanism for stem cell maintenance and differentiation in the Arabidopsis floral meristem. Genes Dev. 2009;23:1791–1804. doi: 10.1101/gad.1800409. This study reveals that H3K27me3 removal precedes the activation of the KNUCKLES gene by the transcription factor AG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69***.Ingouff M, Rademacher S, Holec S, Soljic L, Xin N, Readshaw A, Foo SH, Lahouze B, Sprunck S, Berger F. Zygotic Resetting of the HISTONE 3 Variant Repertoire Participates in Epigenetic Reprogramming in Arabidopsis. Curr Biol. 2010;20:2137–2143. doi: 10.1016/j.cub.2010.11.012. This study examines the dynamic expression of histone H3 variants throughout the life cycle of Arabidopsis and the findings imply that the expression of new histone variants during gemetogenesis and early embryogenesis may underlie the re-setting of histone H3 modifications from one generation to the next. [DOI] [PubMed] [Google Scholar]