Abstract
Ciliopathies comprise a group of disorders associated with genetic mutations encoding defective proteins, which result in either abnormal formation or function of cilia. As cilia are a component of almost all vertebrate cells, cilia dysfunction can manifest as a constellation of features that include characteristically, retinal degeneration, renal disease and cerebral anomalies. Additional manifestations include congenital fibrocystic diseases of the liver, diabetes, obesity and skeletal dysplasias. Ciliopathic features have been associated with mutations in over 40 genes to date. However, with over 1,000 polypeptides currently identified within the ciliary proteome, several other disorders associated with this constellation of clinical features will likely be ascribed to mutations in other ciliary genes. The mechanisms underlying many of the disease phenotypes associated with ciliary dysfunction have yet to be fully elucidated. Several elegant studies have crucially demonstrated the dynamic ciliary localisation of components of the Hedgehog and Wnt signalling pathways during signal transduction. Given the critical role of the cilium in transducing “outside-in” signals, it is not surprising therefore, that the disease phenotypes consequent to ciliary dysfunction are a manifestation of aberrant signal transduction. Further investigation is now needed to explore the developmental and physiological roles of aberrant signal transduction in the manifestation of ciliopathy phenotypes. Utilisation of conditional and inducible murine models to delete or overexpress individual ciliary genes in a spatiotemporal and organ/cell-specific manner should help clarify some of the functional roles of ciliary proteins in the manifestation of phenotypic features.
Electronic supplementary material
The online version of this article (doi:10.1007/s00467-010-1731-7) contains supplementary material, which is available to authorised users.
Keywords: Ciliopathy, Renal disease, Retinal disease, Heterogeneous
Introduction
Ciliopathies comprise a group of disorders associated with genetic mutations encoding defective proteins, which result in abnormal formation or function of cilia. As cilia are a component of almost all cells, ciliary dysfunction can manifest as a constellation of features that include primarily retinal degeneration, renal disease and cerebral anomalies. The notion of a “ciliopathic” disorder was first attributed to Bardet–Biedl syndrome (BBS), when Ansley and colleagues identified genetic mutations in BBS8 whereby the encoded protein was noted to have a pilF domain, suggesting a conserved role for BBS8 in prokaryotic pilus formation [1]. Intriguingly, the phenotypic consequences in one family with a homozygous null mutation in BBS8 included situs inversus, a known defect of the embryonic nodal cilium [1]. Subsequent immunohistochemical analysis confirmed the localisation of BBS8 to centrosomes and basal bodies within human embryonic kidney cells (HEK293) in addition to spermatids, the connecting cilium of the retina and the ciliated columnar epithelial cells of the lung [1]. Further supporting evidence for a role in cilia function came from the elegant demonstration that other BBS orthologues in Caenorhabditis elegans, bbs1, bbs2 and bbs7, all localised to the nematode ciliated sensory neurons where osm-5, the orthologue of the mouse polycystic kidney disease gene, polaris, was also previously localised. Thereafter, the innovative utilisation of comparative genomic studies whereby the proteome of the non-flagellated organism, Arabidopsis, was subtracted from the shared proteome of the ciliated/flagellated organisms, Chlamydomonas and human, led to the discovery of mutations in another gene, BBS5, in patients with BBS [2]. Following development of the original ciliary proteome database, subsequent integration of ciliary proteomes from a range of different organisms have contributed to the current ciliary proteome database (http://www.ciliaproteome.org) [3]. The ciliary proteome database was employed by Beales and colleagues to identify mutations in IFT80, which encodes an intraflagellar transport protein in a subset of patients with Jeune asphyxiating thoracic dystrophy (JATD), following the observation that patients with JATD exhibited typical ciliopathy features of retinal degeneration, renal disease and skeletal dysplasia [4].
In the ensuing text, we will provide an overview of the structure and function of cilia, which will provide a basis for the subsequent clinical description of a range of ciliopathic disorders. We will also highlight how ciliopathies can be phenotypically heterogeneous from variation at a single locus while mutations affecting a number of different loci can at the same time result in similar phenotypes. Thereafter, a brief description will follow on the role of ciliary dysfunction in certain phenotypic features that include renal abnormalities, liver disease, retinal degeneration and skeletal dysplasias.
Overview of ciliary biology
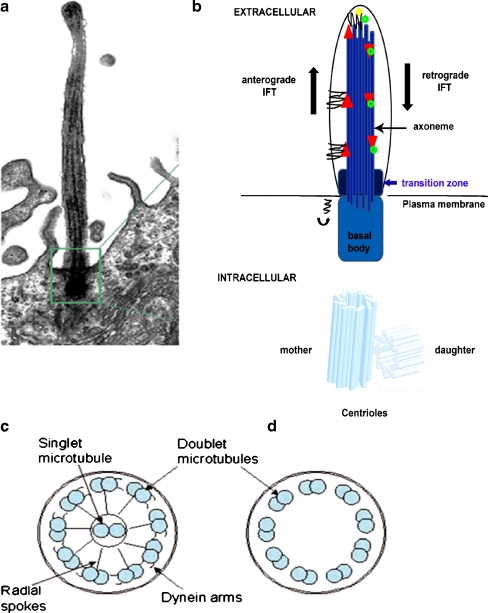
As many as 1,000 different polypeptides are recognised within the current ciliary proteome, highlighting the structural complexity of this highly conserved organelle. Projecting from the cell surface, cilia are microtubule-based, hair-like cytoplasmic extensions with motile and a range of sensory functions, which are critical for developmental and physiological functions [5]. Comprising the microtubular backbone, the ciliary axoneme develops from and is anchored to a specialised centriole called the basal body, which acts as a microtubule organising centre (MTOC) for its ciliary counterpart (Fig. 1). The ciliary axoneme consists of nine doublet microtubules that originate at the triplet microtubules of the basal body centriole and extend the length of the cilium. Cilia fall into two broad categories: motile and immotile. Primary cilia are typically immotile and consist of nine peripheral doublet microtubules, while motile cilia in addition contain a central pair of singlet microtubules (“9 + 2” arrangement) to which they are connected by the radial spoke proteins (Fig. 1c). Immotile cilia are characterised by the absence of the central pair of singlet microtubules (“9 + 0” arrangement; Fig. 1c) [6]. Motile cilia are distinguished from primary cilia by their ability to beat rhythmically, an activity that is powered by adenosine triphosphate (ATP), hydrolysed by dynein proteins, which are anchored to the inner and outer aspects of peripheral doublet microtubules [7]. Motile cilia are utilised in both unicellular and multicellular organisms for locomotion. Defective motile cilia can result in primary ciliary dyskinesias, which comprise a heterogeneous group of disorders characterised by bronchiecstasis, left–right asymmetry and infertility. Primary cilia have chemosensory, osmosensory and phototransduction functions, and will be discussed in more detail later in this review.
Fig. 1.
The primary cilium. a, b Cilia are cytoplasmic extensions projecting from the cell surface and composed of a microtubular-based ciliary axoneme. The transition zone is where the microtubules are reorganised into pairs and anchor the pairs to the membrane. Within this region, proteins involved in trafficking along the axoneme also accumulate. b, c Schematic of a transverse section through c the motile cilium, showing the radial spoke proteins and outer dynein arms, and d the non-motile cilium
Ciliary assembly
Cilia arise from basal bodies, which are formed from centrioles, complex microtubule-based structures located within the cytoplasm (Fig. 1b) [8]. Acting as an intracellular scaffold, the microtubules at the pericentriolar material (PCM) direct the trafficking of vesicles and organelles. Delivery of ciliary cargo occurs in a sequential manner, which involves sorting and packaging into carrier vesicles, docking and fusion of vesicles with the base of the cilium and assembly of cilia from the ciliary base to the tip (Fig. 2). Ciliary targeting and assembly is mediated by several multiprotein complexes that include intraflagellar transport (IFT) proteins and the BBSome, a stable complex of at least eight BBS proteins (BBS 1, 2, 4, 5, 7, 8, 9 and BBIP10), which are modulated by members of the Arf and Rab family of proteins [9]. From the ciliary base, cilia are assembled by IFT, which utilises two microtubule-associated motor proteins called kinesins and dyneins. Structural axonemal components and membrane receptors are transported in an anterograde manner along the ciliary axoneme by kinesin-II. Retrograde transport of recycled proteins down along the axoneme towards the basal body is undertaken by the cytoplasmic dynein motor proteins [10]. Kinesin-II is a heterotrimeric complex comprised of two motor subunits known as KIF3A and KIF3B in addition to a non-motor subunit known as kinesin-associated protein (KAP). IFT particles are composed of two protein complexes, IFTA with 6 protein subunits and IFTB with 13 protein subunits (Fig. 3) [11]. Disruption of either the IFT motors or the basal body proteins essential for their function leads to impaired cilia assembly [12, 13].
Fig. 2.
Intraflagellar transport. Elongation of the axoneme at the distal tip relies on intraflagellar transport (IFT). Anterograde IFT is mediated by kinesin II motors along with axonemal precursors, while retrograde IFT is mediated by a dynein motor. IFT Type A complexes are linked to retrograde transport and IFT Type B complexes are linked to anterograde transport
Fig. 3.
The BBSome and vesicular trafficking to the primary cilium. The BBSome is a multi-protein complex comprising BBS proteins (BBS 1, 2, 4, 5, 7, 8 and 9) that localises predominantly at the ciliary base and mediates vesicular transport to the cilium
Signal transduction at the cilium
Hedgehog signalling
The Hedgehog signalling pathway is a highly conserved and ubiquitous signalling pathway that plays a key role in several developmental contexts regulating a variety of cellular processes that include both cell fate specification and cell proliferation. Hedgehog (Hh) ligands include Sonic hedgehog (Shh) and Indian hedgehog (Ihh), which bind to Patched (Ptch), a transmembrane receptor that represses activation of a G-protein coupled receptor, Smoothened (Smo) [14]. On ligand binding, Smo, moves in a lateral transport pathway from the plasma membrane and accumulates at the ciliary membrane [15]. Activated Smo increases the accumulation of Gli2, a transcription factor at the ciliary tip, which then translocates to the nucleus where it activates Hh target gene expression. In the presence of Hh ligand, Smo interacts within a molecular complex consisting of IFT machinery, Fused (Fu) and Suppressor of Fused (SuFu). Mutations in genes encoding Ift172 and Ift88 were identified in two mouse mutants that showed characteristic defects in Shh signalling following an ethylnitrosourea (ENU) mutagenesis screen undertaken for embryonic patterning mutations. Genetic studies showed that IFT proteins act at the heart of the Shh pathway, downstream of Ptch1 and Smo and upstream of the Gli transcription factors, where they mediate the production of activated to repressor forms of Gli, thereby regulating Hh pathway activity [16].
Wnt signalling
Several studies have implicated ciliary and basal body proteins in the regulation of Wnt signalling [17, 18]. The Wnt family encodes a family of 19 secreted glycoproteins that regulate a variety of biological processes implicated in development and disease. Ligand binding to a complex of the Frizzled (Fz) receptor and the low-density lipoprotein receptors, LRP5 or LRP6, initiates signalling through Dishevelled (Dvl). Inversin (Inv) interacts with Dvl and targets the cytoplasmic fraction of Dvl for degradation [19]. In the absence of Wnt stimuli, β-catenin is constitutively phosphorylated by the β-catenin destruction complex consisting of axin, adenomatous polyposis coli (APC) and glycogen synthase kinase 3β [GSK3β]. Phosphorylated β-catenin is targeted for degradation. In canonical Wnt signalling, the ligand binds to a complex of the Fz receptor and LRP5/6 co-receptor, which then binds to Axin and Dvl, leading to stabilisation of β-catenin in the cytoplasm. β-catenin migrates into the nucleus, replaces TLE, and activates transcription of β-catenin/TCF/LEF1-responsive genes. In non-canonical signalling, activated Dvl is targeted to the membrane and activates downstream targets. Disruption of ciliary or basal body components leads to loss of non-canonical Wnt signalling and stabilisation of both Dvl and β-catenin in the cytoplasm and nucleus, resulting in activation of canonical Wnt signalling.
Emerging evidence suggests that non-canonical Wnt signalling, also known as planar cell polarity signalling (PCP), plays a role in ciliary formation. Mice mutant for the core PCP effector, Fuzzy, have neural tube defects, skeletal dysmorphologies and Hedgehog signalling defects stemming from disrupted ciliogenesis. Further studies demonstrated the key role of Fuz in trafficking ciliary cargo to basal bodies and to the ciliary apex while interaction with a Rab-small GTPase is required for ciliogenesis.
Diseases associated with ciliary dysfunction
As cilia are a component of almost all vertebrate cells, ciliary dysfunction can manifest as a constellation of features that include primarily retinal degeneration, renal disease and cerebral anomalies. Additional manifestations include congenital fibrocystic diseases of the liver and pancreas, diabetes, obesity and skeletal dysplasias. Phenotypically heterogeneous, ciliopathic features can manifest from variation at a single locus while mutations affecting a number of different loci can, at the same time, result in similar phenotypes. Mutations in over 40 genes to date have been associated with ciliopathic features (Tables 1, 2). However, with over 1,000 polypeptides currently identified within the ciliary proteome, several other disorders associated with this constellation of clinical signs will likely be ascribed to mutations in other ciliary genes. A brief description of the characteristic features of some of the ciliopathies encountered by paediatric nephrologists is outlined in the following section.
Table 1.
Phenotypic overlap in the ciliopathies. (Table modified from Gerdes et al. [130], used with permission)
| Phenotype | LCA | SLS | NPHP | MKS | BBS | JBTS |
|---|---|---|---|---|---|---|
| Cerebellar | √ | √ | √ | |||
| hypoplasia | ||||||
| Encephalocele | √ | |||||
| Hepatic disease | √ | √ | √ | √ | √ | |
| Renal disease | √ | √ | √ | √ | √ | |
| Mental retardation | √ | √ | √ | √ | ||
| Obesity | √ | √ | ||||
| Polydactyly | √ | √ | √ | |||
| Retinopathy | √ | √ | √ | √ | √ | |
| Situs inversus | √ | √ | √ | √ | √ | |
| Skeletal dysplasia | √ | |||||
| Cleft palate | √ |
LCA, Leber’s congenital amaurosis; NPHP, nephronophthisis; BBS, Bardet–Biedl syndrome; SLS, Senior–Løken syndrome; JBS, Joubert syndrome; MKS, Meckel–Gruber syndrome
Table 2.
Genotypic overlap in the ciliopathies. (Table modified from Gerdes et al. [130], used with permission)
| Gene | LCA | SLS | NPHP | MKS | BBS | JBTS | OFD |
|---|---|---|---|---|---|---|---|
| CEP290 | √ | √ | √ | √ | √ | √ | |
| NPHP1 | √ | √ | √ | ||||
| INVS | √ | √ | |||||
| NPHP3 | √ | √ | √ | ||||
| NPHP4 | √ | √ | |||||
| NPHP5 | √ | √ | |||||
| GLIS2 | √ | ||||||
| NEK8 | √ | ||||||
| AHI1 | √ | ||||||
| TMEM67 | √ | √ | √ | √ | |||
| RPGRIPL1 | √ | √ | √ | √ | |||
| ARL13B | √ | ||||||
| BBS1 | √ | ||||||
| BBS2 | √ | √ | |||||
| BBS3 | √ | ||||||
| BBS4 | √ | √ | |||||
| BBS5 | √ | ||||||
| BBS6 | √ | √ | |||||
| BBS7 | √ | ||||||
| BBS8 | √ | ||||||
| BBS9 | √ | ||||||
| BBS10 | √ | ||||||
| BBS11 | √ | ||||||
| BBS12 | √ | ||||||
| MGC1203 | √ | ||||||
| MKS1 | √ | √ | |||||
| BBS15 | √ | ||||||
| CC2D2A | √ | √ | |||||
| TMEM216 | √ | √ | √ | ||||
| INPP5E | √ | ||||||
| XNPEP3 | √ | ||||||
| OFD1a | √ | √ |
LCA, Leber’s congenital amaurosis; NPHP, nephronophthisis; BBS, Bardet–Biedl syndrome; SLS, Senior–Løken syndrome; JBS, Joubert syndrome, MKS, Meckel–Gruber syndrome, OMA, oculomotor apraxia, OFD, orofaciodigital syndrome
aIf in males
Joubert syndrome and related disorders
Joubert syndrome (JBTS; MIM ID# 213300) is a rare syndrome that is characterised by hypotonia, ataxia, psychomotor delay, irregular breathing pattern and oculomotor apraxia. Distinctive cerebellar and brain stem malformations associated with JBTS include vermis hypoplasia or agenesis (e.g. abnormalities at the pontomesencephalic junction). The characteristic “molar tooth sign” (MTS) on cranial magnetic resonance imaging (MRI) is demonstrated by elongated but thin superior cerebellar peduncles and mild vermis hypoplasia with the resulting images reminiscent of a section through a molar tooth and is characteristic of JBTS (Fig. 4d). Dandy–Walker malformations may be evident in approximately 10% of cases as a result of abnormal cerebrospinal fluid collections in the posterior fossa. Additional clinical features include retinal degeneration, cystic kidney disease (cystic dysplasia and nephronophthisis [NPHP]), ocular colobomas, occipital encephalocele, hepatic fibrosis, polydactyly, oral hamartomas and endocrine abnormalities.
Fig. 4.
Clinical features of ciliopathies. a Renal ultrasound demonstrating multiple cysts distributed within the renal parenchyma (white arrow). b Renal biopsy demonstrating cystic tubular dilation and interstitial fibrosis characteristic of nephronophthisis (NPHP; black arrow). c Funduscopy of a patient with Bardet–Biedl syndrome demonstrating peripheral pigmentary changes in the retina. d Cranial MRI of a patient with Joubert syndrome demonstrating characteristic “molar tooth” sign (white arrow) as a result of cerebellar vermis hypoplasia
Genetically heterogeneous, JBTS has been associated with mutations in several genes, including INPP5E [20], ARL13B [21], CC2D2A [22], RPGRIP1L [23], TMEM67 [24], NPHP1 [25], AHI1 [26], CEP290 [27], CXORF5 [28] and TMEM216 [29]. Mutations in CEP290, a gene encoding a centrosomal protein with a molecular weight of 290 kDa, are responsible for about 50% of JBTS subgroup of ciliopathies, while they are rarely detected in other related (JSRD) phenotypes that include Leber’s congenital amaurosis (LCA), Senior–Løken syndrome (SLS), nephronophthisis (NPHP), Meckel–Gruber syndrome (MKS), Bardet–Biedl syndrome (BBS) and orofaciodigital (OFD) syndrome [30]. The majority of CEP290 mutations described to date are nonsense, splice-site or frameshift mutations resulting in loss of protein function. Part of the phenotypic heterogeneity associated with CEP290 mutations may be attributed to the number of proteins that CEP290 interacts with [31]. For example, CEP290 exists in a complex with other proteins such as retinitis pigmentosa GTPase regulator, nephrocystin-4 and nephrocystin-8 [32]. Hypomorphic mutations in NPHP6 and NPHP8 (also known as RPGRIP1-L), which are associated with relatively early-onset photoreceptor degeneration, disrupt their association with the retinitis pigmentosa GTPase regulator protein [32–34]. In a similar fashion, other mutated gene products, such as AHI1, which encodes jouberin, has been shown to interact with nephrocystin-1 [35]. For a comprehensive review of the phenotypic and genotypic features associated with CEP290 mutations, the reader is referred to a recent excellent review on this topic [31].
Meckel–Gruber syndrome (MKS, MIM ID #249000) phenotypically overlaps with JBTS. Clinical features include occipital encephalocele and other posterior fossa defects, cystic dysplastic kidneys, hepatic bile duct proliferation and polydactyly. MKS is caused by mutations in several genes including MKS1 [36], MKS3 (TMEM67) [37], CEP290 [24], RPGRIP1L [23], CC2D2A [38] and TMEM216 [29]. Both JSRD and MKS are allelic at several loci (CEP290, TMEM216, TMEM67, RPGRIPL1, CC2D2A) [23, 24, 29, 39, 40]. For example, homozygous missense mutations have recently been described in TMEM216, a tetraspan transmembrane protein required for ciliogenesis in patients with JSRD [29]. Frameshift mutations in TMEM216, resulting in a truncated protein, were found in two Palestinian families with MKS in the same study, emphasising that MKS is thought to represent the severe end of the JSRD clinical spectrum.
Senior–Løken syndrome (SLS, MIM ID #266900) is another rare disorder that shares phenotypic and genotypic overlap with JBTS and other ciliopathies including BBS and NPHP (Table 1). The main clinical features are retinitis pigmentosa (RP) and renal disease. Presentation may occur in infancy or late childhood. RP may present either as congenital retinal blindness caused by retinal hypoplasia or as progressive retinal degeneration later in childhood with a classical fundoscopic appearance of tapetoretinal degeneration. The characteristic renal manifestation is that of nephronophthisis characterised by cystic dilatation of the renal tubules. However, both cystic renal dysplasia and polycystic kidneys have also been observed in SLS. Mutations have been identified in the following genes, which include CEP290 (also known as NPHP6 and MKS4) [27], NPHP1 [41], NPHP3 [42], NPHP4 [43] and NPHP5 (also known as IQCB1) [44]. Significant genetic overlap is evident between SLS and JBTS (Table 2).
Orofaciodigital syndrome
Orofaciodigital syndrome type 1 (OFDI; MIM 311200) is a rare X-linked dominant disorder whereby affected males die in utero. Characteristic features include malformation of the oral cavity, face and digits, in addition to central nervous system (CNS) abnormalities and cystic kidney disease [45]. Mutations in OFD1, which encodes a centrosomal protein localised at the basal bodies at the origin of primary cilia has been described in OFD1 patients [46]. Diminished ciliogenesis has been observed with disease-associated mutations and recent studies suggest that Ofd1 acts at the distal centriole to build distal appendages, recruit IFT proteins and thereafter stabilise centriolar microtubules at a defined length [47]. Ofd1-/- embryos display left–right patterning defects as a result of absent nodal cilia [48]. A recent study has highlighted genetic overlap between OFD and JBTS, whereby OFD1 was found to be mutated in males with Joubert syndrome [28].
Leber’s congenital amaurosis
Leber’s congenital amaurosis (LCA, MIM ID #204000) is a severe retinal dystrophy, which presents within the first year of life. Frequently, visual function is poor and often accompanied by nystagmus, sluggish or near-absent pupillary responses, photophobia, hyperopia and keratoconus. Functionally, visual acuity is rarely better than 20/400 and the electroretinogram (ERG) is characteristically “non-detectable” or severely subnormal. A characteristic finding is Franceschetti’s oculo-digital sign, comprising eye poking, pressing and rubbing. Genes implicated in LCA include GUCY2D [49], RPE65 [50], SPATA7 [51], AIPL1 [52], LCA5 [53], RPGRIPL1 [54], CRX [55], CRB1 [56], IMPD1 [57], RD3 [58], CEP290 [27], NPHP5 [44] and RDH12 [59]. The ophthalmological manifestations of LCA may present as a manifestation of JSRD or SLS. Mutations in many of the same genes are responsible for these three overlapping phenotypes (Table 2).
Bardet–Biedl syndrome
Primary features of Bardet–Biedl syndrome (BBS, MIM# 209900) include rod-cone dystrophy, polydactyly, obesity, learning disabilities, hypogonadism and renal anomalies (Fig. 4). Renal malformations and abnormal renal function leading to end-stage renal disease (ESRD) can be a major cause of morbidity and are present in at least 40% of cases [60]. Renal manifestations include renal dysplasia, cystic tubular disease (e.g. nephronophthisis) and less frequently, focal segmental glomerulosclerosis [61, 62]. Lower urinary tract malformations such as detrusor instability of the bladder occur, but are less common than upper tract malformations [62]. Secondary features include speech delay or disorder, developmental delay, behavioural abnormalities, strabismus/cataracts/astigmatism, brachydactyly/syndactyly, ataxia/poor coordination/imbalance, mild hypertonia, anosmia, diabetes, fibrocystic liver disease, Hirschsprung’s disease, and dental and cardiovascular anomalies [62]. Craniofacial defects such as brachycephaly, macrocephaly, bitemporal narrowing, male frontal balding, large ears, short and narrow palpebral fissures, a long shallow philtrum, nasal anomalies, midfacial hypoplasia and mild retrognathia have been described in BBS [63].
Sixteen genes are known to be associated with BBS (Table 2): BBS1 [64], BBS2 [65], ARL6/BBS3 [66], BBS4 [67], BBS5 [2], MKKS/BBS6 [68], BBS7 [69], TTC8/BBS8 [1, 70], B1/BBS9 [70], BBS10 [71], TRIM32/BBS11 [72], BBS12 [73], MKS1/BBS13 [74], CEP290/BBS14 [74] C2ORF86/FRITZ/BBS15 [75] and SDCCAG8/BBS16 [76]. The recent identification of mutations in MKS1 in BBS has supported the observation that MKS may represent a severe BBS phenotype [74]. Furthermore, previous studies have shown that the BBS phenotype can vary considerably within affected families. Some of this intrafamilial variability can be accounted for by the presence of mutations at more than one BBS locus as well as the presence of additional modifying genes that exert an epistatic effect on known BBS loci. For example, heterozygous mutations in MGC1203, which encodes a pericentriolar protein that interacts with BBS proteins, have been described in BBS patients [77]. As BBS proteins have now been shown to exist in a macromolecular complex, it is likely that mutant proteins within this complex can affect the function of interacting proteins existing within the same complex. Approximately 20% of persons with BBS do not have identifiable mutations in any of the 16 known BBS-related genes; therefore, it is possible that more BBS genes are yet to be identified.
McKusick–Kaufman syndrome (MKKS, MIM#236700) is an autosomal recessive (AR) condition characterised by the triad of hydrometrocolpos (HMC), post-axial polydactyly (PAP) and congenital heart disease (CHD). Many cases of BBS have been misdiagnosed as MKKS in infancy or early childhood prior to the development of other manifestations of BBS. MKKS is caused by mutations in the MKKS gene, which can also cause BBS [68].
Alström syndrome
Alström syndrome (ALS, MIM#203800) is an AR disorder characterised by cone-rod dystrophy, obesity, progressive sensorineural hearing impairment, dilated cardiomyopathy, the insulin resistance syndrome and developmental delay. Over 60% of individuals with Alström syndrome develop cardiac failure as a result of dilated cardiomyopathy at some stage of their lives. Males may have hypogonadotrophic hypogonadism. Renal disease may present as polyuria and polydipsia resulting from a urinary concentrating defect. End-stage renal disease (ESRD) can occur as early as the late teens. In contrast to BBS, Alström syndrome is characterised by relative preservation of cognitive function and the absence of polydactyly. Alström syndrome is caused by mutations in the gene ALMS1 and alms1 localises specifically to the proximal ends of centrioles and basal bodies [78, 79].
Jeune asphyxiating thoracic dystrophy
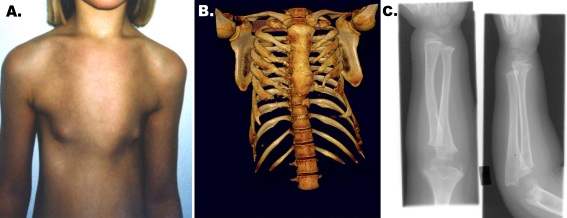
Jeune asphyxiating thoracic dystrophy (JATD; MIM#208500) is a rare AR chondrodysplasia that is frequently associated with infantile death as a result of a severely constricted thoracic cage associated with respiratory insufficiency from pulmonary hypoplasia. Characteristic skeletal findings include a narrow thorax with short ribs, hypoplastic iliac wings, trident acetabular roofs (horizontal acetabular roofs with spur-like projections at the lower margins of the sciatic notches), and rhizomelic limb shortening (Fig. 5). Radiological confirmation of the diagnosis is essential [80]. JATD is characterised by the presence of radiologically irregular metaphyseal ends, histopathologically hyperplastic proliferating chondrocytes and defective endochondral mineralisation. Other skeletal manifestations include post-axial polydactyly, brachydactyly and hydrocephalus [81]. Both RP and retinal aplasia have been noted in JATD [82]. Glomerulosclerosis and cystic renal disease, including NPHP, has been reported in JATD. Fibrocystic disease of both the liver and pancreas has been described [83]. Early death usually occurs in the majority of patients as a consequence of asphyxia with or without pneumonia.
Fig. 5.
Skeletal manifestations of ciliopathies. a A Jeune asphyxiating thoracic dystrophy (JATD) patient with a narrow thorax. b CT of same patient demonstrating short ribs and narrow thoracic cage. c Upper limb radiography of a patient with Mainzer–Saldino syndrome demonstrating acromesomelic shortening with irregular metaphyses and cone-shaped epiphyses
Jeune asphyxiating thoracic dystrophy is a genetically heterogeneous disorder. Beales and colleagues identified two missense mutations and an in-frame deletion in IFT80, the gene encoding the Ift80 protein, thereby linking JATD to ciliary dysfunction [4]. Ift80 was shown to localise to the basal body of cilia in a murine chondrocytic cell line. Aberrant Shh signalling appears to underlie the skeletal manifestations observed in IFT mutants [84]. Zebrafish morphant for ift80 demonstrate downregulation of ptc1, a Shh binding receptor. Phenotypic similarity is observed in Ihh null mice compared with patients with JATD, in that they exhibit extremely short narrow rib cages. Similarly, mice carrying a mutation in Pthrp, a gene regulated by Ihh via Gli3 during chondrocyte differentiation also have short ribs and sternum leading to a narrow rib cage.
Ellis van Creveld syndrome
Ellis van Creveld (EvC, MIM 225500) syndrome is a rare chondroectodermal dysplasia that falls under the differential diagnosis of JATD and is characterised by short limbs, short ribs, post-axial polydactyly and dysplastic nails and teeth [85]. Nail dysplasia and a peculiar upper lip distinguish EvC from Jeune syndrome, while congenital heart disease such as atrial septal defects occur in about 60% of affected individuals and are rare in JATD. Mutations in EVC1 have been described in Amish and Brazilian pedigrees of EvC, but only accounted for a small proportion of affected cases, thereby suggesting that EvC is a heterogeneous disease [86]. More recently, mutations in a second gene, EVC2, have been described in an Ashkenazi child with EvC [87]. The Evc protein was shown to localise to the base of the primary cilium of chondrocytes and defective Ihh signalling was observed in proliferating chondrocytes of Evc-null mice [88].
Sensenbrenner syndrome
Sensenbrenner syndrome (also known as cranioectodermal dysplasia, MIM #218330), a recessive disorder similar to EvC, but with the addition of renal cysts and dolichocephaly (with sagittal suture synostosis) and sparse, slow-growing, fine hair, epicanthal folds, hypodontia and/or microdontia, brachydactyly and a narrow thorax. Mutations in two IFT genes, IFT122 and WDR35, have been implicated in CED with WDR35 recently identified by exome sequencing [89, 90]. As both gene products encode for ciliary proteins, Sensenbrenner syndrome has recently been classified as a ciliopathic disorder.
Jeune asphyxiating thoracic dystrophy has been described in patients with JBTS and intriguingly, no mutation has been identified in any of the known causative genes for either syndrome. Therefore, for such patients it is highly likely that further cases of JATD will be attributed to ciliary dysfunction in the future.
Systemic manifestations of the ciliopathies
While disease manifestation in any organ can occur in the context of ciliopathic dysfunction, the predominant organs affected include the kidney, eye, liver and brain. In the ensuing text, we will outline the range of diseases that can occur as each of these organs in the context of ciliary dysfunction. Within each organ, diseases can be developmental phenotypes presenting at birth or later in childhood. Often this may depend on the severity of the underlying mutation in addition to the number of defective proteins encoded where more than one mutation in a ciliary gene occurs.
Ciliary dysfunction and renal disease
A spectrum of renal diseases has been described as a feature of several ciliopathic syndromes and includes a morphologically heterogeneous group of disorders that have been classified as polycystic, renal medullary cystic disease and cystic renal dysplasia. We will briefly outline the clinical and morphological features that distinguish each of these renal phenotypes. A brief description will follow outlining the underlying genetic aetiology, lessons learned from animal models of cystic kidney disease and the role played by their associated ciliary proteins in the manifestation of disease phenotype.
Polycystic kidney disease
Polycystic kidney disease (PKD) is a group of monogenic disorders that are characterised by the presence of multiple cysts, primarily in the kidney and liver and can present both in the neonatal period as well as in adulthood [91]. PKD is inherited in an autosomal dominant (ADPKD) or recessive (ARPKD) fashion. Typically, ADPKD is diagnosed in the second and third decades of life, while ARPKD presents in utero or in the neonatal period with bilateral enlarged kidneys. ADPKD is one of the most common genetic causes of chronic kidney disease, with an incidence of 1:400–1:1,000, while ARPKD is rare, with an incidence of 1 in 20,000. Macroscopically, ADPKD is characterised by the presence of bilateral grossly enlarged kidneys, which result from the presence of focal cysts occurring at all levels of the nephron. Histologically, ARPKD has a characteristic radial pattern of fusiform cysts present in the dilated collecting ducts. Clinically, patients with enlarged kidney cysts can present with flank pain, haematuria, renal colic, urinary tract infection and hypertension. Cysts may be found in other organs such as the liver, pancreas and seminal vesicles. Rupture of intracranial arterial aneurysms is a significant cause of morbidity and mortality in patients with ADPKD. Childhood-onset end-stage kidney disease is characteristic of ARPKD, with up to 30% requiring renal replacement therapy. ARPKD is suspected when in addition to the renal phenotype, congenital fibrocystic disease of the liver is a manifestation with complications that include portal hypertension, bleeding oesophageal varices and cholangitis.
Autosomal dominant polycystic kidney disease is caused by a mutation in either of two genes, PKD1 or PKD2, encoding polycystin-1 (PC-1) and polycystin-2 (PC-2) respectively. More than 85% of patients have mutations in PKD1, while the remaining 15% have mutations in PKD2 [92, 93]. PC-1 and PC-2, the encoded polycystin protein products of PKD1 and PKD2 respectively form a complex that is located at various cellular sites, which include cell–cell and cell–matrix interactions, the endoplasmic reticulum, in addition to the cilium and/or its basal body [94]. Fibrocystin is the protein encoded by PKHD1 (polycystic kidney and hepatic disease 1), the gene mutated in ARPKD and has been found to be associated with the polycystin complex. Fibrocystin also localises to primary cilia and basal bodies [95].
Homozygous Pkd1 null mice die between 12.5 and 16.5 days postcoitum and phenotypically exhibit gross cystic appearance of the kidneys and pancreas [96]. Conditional inactivation of Pkd1 later than postnatal day 13 results in a much milder course [97]. Although PKD1 and PKD2 mutations are typically autosomal dominant, somatic second hit mutations, where loss of the second PKD1 or PKD2 allele in the tubular epithelium occurs, have been proposed as a mechanism for focal cyst development in mature ADPKD kidneys [98, 99]. Recently, co-inheritance of a truncating mutation in one PKD1 allele in trans with missense mutations in a second PKD1 allele has been shown in cases of PKD with onset in utero, in three pedigrees with otherwise typical ADPKD, suggesting a gene dosage effect in PKD [17]. Rodent Pkhd1 knockout models develop biliary dysgenesis and fibrosis similar to human ARPKD, while kidney disease is generally mild and of later onset [100].
Nephronophthisis
Nephronophthisis (NPHP) is the most common genetic cause of chronic kidney disease within the first three decades of life [101]. The prevalence in a population of childhood end-stage renal failure is estimated at 5%. Patients usually present with symptoms of polyuria and polydipsia, secondary enuresis and anaemia. Presentation may occur during infancy, but more typically in late childhood with progressive renal failure manifesting during early puberty. Ultrasound features demonstrate normal sized kidneys with loss of cortico-medullary differentiation and increased echogenicity. Histologically, NPHP kidneys are characterised by the presence of cortico-medullary cysts, tubular basement membrane disruption and tubulointerstitial nephropathy (Fig. 4b). Extra-renal involvement has been described in over 10% of cases and primarily involves retinal disease, fibrocystic liver disease, cerebellar vermis hypoplasia and skeletal dysplasia.
Inherited in an autosomal recessive mode, NPHP is genetically heterogeneous, with 13 genes currently implicated, (NPHP1–NPHP11, NPHP1L, SDCCAG8), which account for only 30% of cases (Supplementary Tables 1, 2). In the remaining 70% of cases, the causative gene is unknown. Recent studies have employed combined homozygosity mapping with “ciliopathy candidate exome capture” followed by massively parallel sequencing to identify SDCCAG8 as a cause of NPHP [76]. While mutations in a single gene are sufficient to cause NPHP, it has also been shown that more than one gene can be mutated in patients with NPHP [102]. Furthermore, truncating mutations can result in a more severe developmental phenotype such as renal dysplasia in Meckel–Gruber syndrome patients with NPHP3 mutations [103]. A milder phenotype has also been observed for patients with cerebello-oculo-renal syndromes (CORS) syndrome who have missense mutations in RPGRIP1L compared with patients with Meckel–Gruber syndrome who have truncating mutations in the same gene [23, 104].
Similar to the polycystic kidney disease genes, the nephrocystins have all been localised to primary cilia, basal bodies and centrosomes (Supplementary Table 2). Several NPHP gene products have been shown to interact with each other in addition to other ciliary proteins such as BBS proteins and Ofd1 proteins. Furthermore, subcellular localisation other than primary cilia has been described for several NPHP gene products and includes adherens junctions and focal adhesions (nephrocystin-1 and -4), while nephrocystin-2 localises to different subcellular locations in a cell-cycle-dependent manner where it can be found at the mitotic spindle during mitosis, at the mid-body in cytokinesis while in interphase it can be found in cilia at the basal body and centrosome [101]. Genetic inactivation in several murine Nphp genes have yielded a range of phenotypes, which include cystic kidneys in both Inv−/− mice and Nphp3pcy/ko mice [103, 105]. Of interest, Glis2 mutant mice show tubular atrophy and progressive renal fibrosis [106]. For a comprehensive review of nephronophthisis, the reader is referred to a recent excellent review on this topic elsewhere in this series [101].
Renal dysplasia
While cystic renal disease has historically been described as a cardinal feature of a ciliopathic disorder, other renal malformations such as dysplastic kidneys are often an under-recognised feature. Renal dysplasia occurs as a result of defective differentiation of the renal parenchyma during kidney development [107]. Histologically, dysplastic features may include incompletely branched collecting ducts surrounded by undifferentiated mesenchymal stroma. Ultrasonographically, dysplastic kidneys may be small (less than the 50th centile for age), unilateral or bilateral, lack cortico-medullary differentiation and demonstrate increased echogenicity with a variable number of small, subcapsular cysts. The degree of renal impairment will depend on the presence of functional nephron mass in the dysplastic kidney and as a result, an elevated creatinine for age may be observed at birth or later during childhood. Other features have included aplastic and hypoplastic kidneys (reduced nephron number and small size) or multicystic dysplastic kidneys characterised by large cysts and no functioning renal parenchyma. Renal dysplasia has been observed in several ciliopathic disorders, which include BBS and Meckel–Gruber syndrome [108]. As renal dysplasia is essentially a developmental phenotype, its presence in the context of a ciliopathy likely reflects a more severe genotype.
Cystic kidneys and the link to cilia
A role for the primary cilium in cystic disease was suggested following the observation that almost all proteins implicated in cystogenesis are localised to the primary cilium. Evidence that cilia are important in cystic kidney disease comes from the initial observation of renal cysts in the Oak Ridge Polycystic Kidney (orpk) mouse that mimic ARPKD. Orpk mice are hypomorphic for polaris (also known as Tg737), which encodes the mouse orthologue of Chlamydomonas Ift88. Cilia in Tg737orpk animals are structurally defective and shorter than normal cilia while complete Tg737 nulls lack cilia and present with neural tube defects, left–right asymmetry and growth arrest during embryogenesis [109]. Several other mouse models link cilia to cystic kidney disease [110]. For example, kidney-specific inactivation of Kif3a in mice results in a renal epithelium that is devoid of cilia in cystic regions, which appear at 5 days of age [12]. Furthermore, congenital polycystic kidneys (cpk) are observed in the cpk mouse, which carries a mutation in cystin, which localises to the cilium of renal epithelia [110].
Effector pathways implicated in renal cystogenesis
While a role for cilia in cystogenesis has been proposed, the underlying mechanisms are poorly defined. Previous studies have shown a role for cilia in mediating the switch between canonical and non-canonical Wnt signalling [18]. Renal cilia project into the tubular lumen and bend in response to tubular flow. Inversin, the protein product of the gene mutated in NPHP type 2 is localised to the primary cilium and mediates a switch from canonical to non-canonical Wnt signalling, which mediates planar cell polarity (PCP). PCP describes the organisation of cells in the plane of an epithelium. Recent studies have provided further support that loss of PCP signalling can lead to renal cystogenesis. Genetic inactivation of Fat4, a protocadherin and core PCP effector that is localised to the primary cilium leads to renal cyst formation in mice and is evident at embryonic day 16.5 [111]. PCP signalling plays a major role in orientating the mitotic spindle along the longitudinal axis of the developing tubule such that over 95% of cells divide within 34° of the axis of the tubule, a process known as orientated cell division (OCD). Defective OCD has been described in the pck rat, (pck is orthologous to Pkhd1) and in Hnf1ß mice prior to the development of renal cysts [112]. However, recent studies in precystic mouse models of Pkhd1, Pkd1 and Pkd2 failed to identify defective OCD prior to onset of cystogenesis. Loss of OCD was observed, however, during early tubular dilation in both Pkd1 and Pkd2 mouse mutants [113]. Regulation of OCD along the proximo-distal axis during tubular elongation after birth has recently been shown to be regulated by Wnt9b signalling [114]. Furthermore, convergent extension (CE) movements, also regulated by Wnt9b, decrease the number of tubular epithelial cells during tubular morphogenesis until a final tubule diameter is reached. Hypomorphic Wnt9b mutant mice develop renal cysts and exhibit defects in OCD and CE.
Other than PCP, several other signalling pathways have been implicated in renal cystogenesis [115]. In the absence of PCP, canonical Wnt signalling prevails. Over-expression of β-catenin in transgenic mice leads to renal cysts supporting a role for canonical Wnt activation in cystogenesis [116]. Aberrant Shh signalling has also been associated with renal disease. Mutations in NPHP7 (GLIS2), an intracellular Shh effector, have been described in a subset of patients with nephronophthisis [117].
Activation of the mTOR pathway has been demonstrated in polycystic kidney disease and several studies have been undertaken to assess a role for mTOR inhibition in polycystic kidney disease [118]. Altered intracellular calcium homeostasis has been implicated in PKD. Previous studies have shown that calcium influx occurs via the polycystin ion channel complex composed of PC1 and PC2 during tubular flow and is associated with bending of the primary cilium. Furthermore, increased intracellular cAMP has been demonstrated in several PKD animal models. As a result, vasopressin receptor antagonists have been introduced into human clinical trials in ADPKD patients [119]. As several pathways are implicated in cell proliferation and differentiation, it is likely that many other mechanisms are implicated in renal cystogenesis.
Liver disease and cholangiocyte ciliary dysfunction
Congenital fibrocystic diseases (CFD) of the liver are a heterogeneous group of disorders that are characterised by a spectrum of biliary dysgenesis that includes congenital hepatic fibrosis, bile duct dilatation and cyst formation. Hepatic cysts are lined by cholangiocytes, which are specialised biliary epithelial cells. The concept of cholangiociliopathies first evolved with the observation that patients with CFD frequently have other systemic features including renal disease [120]. Ciliary dysfunction has been shown to underlie the pathogenesis of both these cystic disorders following the identification and localisation of fibrocystin and nephrocystins, the genes mutated in ARPKD and in NPHP respectively, to the primary cilium of cholangiocytes and renal tubular epithelial cells [94, 121, 122]. Cholangiocyte cilia regulate bile formation through mechanosensory, osmosensory and chemosensory cues. Defects in cholangiocyte ciliary structure and/or their integrated transducing function lead to a decrease in intracellular calcium and increased cAMP, causing cholangiocyte hyperproliferation, abnormal cell matrix interactions and altered fluid secretion/absorption, which can result in hepatic cystogenesis [123].
Besides the association with cystic kidneys, CFD also occurs as part of the pleiotropic phenotypes of JBTS/COACH syndrome [124], BBS [125], Alström syndrome, Meckel–Gruber syndrome and JATD [120].
Ciliary dysfunction and retinal disease
Degeneration of the retinal photoreceptors is a common feature of ciliopathic disorders and manifests as progressive loss of peripheral vision. Fundoscopic appearances include maculopathy associated with optic disc pallor, pigmentary changes within the peripheral retina and bone spicule formation (Fig. 4c). Several proteins implicated in human ciliopathic diseases have been localised to the photoreceptor cilium. Morphologically, photoreceptors have an outer and an inner segment, which are connected by a modified cilium, called the connecting cilium [126]. Maintenance of photoreceptor integrity relies on continuous IFT. Arrestin, transducin and opsin molecules are synthesised within the inner segment and are then transported via IFT in a light-dependent manner along the ciliary axoneme of the connecting cilium to the outer segment [127]. Here, phototransduction takes place across an extensive array of photosensitive membranes, which are covered in opsin molecules. About 2,000 rhodopsin molecules per minute are transported to the outer segment via the connecting cilium to compensate for lost material each day when at least 10% of the distal ends of the photoreceptor outer segments are shed and phagocytosed by the surrounding retinal pigmentary epithelium.
A range of photoreceptor abnormalities has been described in several murine ciliopathy models and include the absence of outer segments, disorganised outer segments or photoreceptor degeneration without any obvious abnormalities in photoreceptor morphology. Retinal degeneration has been associated with increased cell death in murine models of Bbs [128]. While the underlying mechanisms of photoreceptor degeneration are largely still unknown, defects in vesicular transport, proteosomal-mediated degradation and IFT have been postulated as potential mechanisms [129, 130]. Future studies will need to address the specific molecular pathways that become dysregulated in ciliopathic retinal degeneration.
Conclusion
Since the seminal discovery of BBS8, as a novel ciliary protein, by Ansley and colleagues [1], the primary cilium has been the focus of intense research across a broad range of scientific disciplines over the past few years. With over 1,000 polypeptides identified within the ciliary proteome, it is highly likely that mutations in several more ciliary genes will be identified in patients presenting with a “ciliopathic” phenotype. While identification of new genes and new ciliary proteins are of fundamental biological interest, it is perhaps even more important to understand the mechanisms underlying the functional consequences of ciliary dysfunction in an organ-specifc context. With the recent development of inducible transgenic models, further investigation in both a spatial and temporal manner within individual organ systems should greatly aid our understanding of the functional consequences of ciliary dysfunction.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOCX 18 kb)
(DOCX 26 kb)
(DOCX 28 kb)
Acknowledgements
We graciously acknowledge Professor Neil Sebire (Department of Pathology), Dr Ken Nischal (Department of Opthalmology), Dr Detlef Bockenhauer and Dr Stephen Marks (Department of Nephro-Urology) at Great Ormond Street Hospital for their contribution of the clinical images. Aoife Waters is supported by the Medical Research Council, UK, and Philip Beales is supported by the Wellcome Trust, UK.
Multiple choice questions (answers appear following the references)
- Characteristic clinical features of a “Ciliopathy” DO NOT include:
- Renal disease
- Retinal disease
- Cerebral malformations
- Abdominal distension
- Ciliopathic syndromes DO NOT include:
- Joubert syndrome
- Bardet–Biedl syndrome
- Orofaciodigital syndrome
- Atypical haemolytic uraemic syndrome
- What is the most common genetic cause of NPHP:
- NPHP1
- NPHP4
- SDCCAG8
- NPHP5
- XNPEP3
- A molecular diagnosis can be made in what percentage of cases of NPHP
- 25%
- 40%
- 60%
- 10%
- 70%
Footnotes
Answers
1. d
2. d
3. a
4. a
References
- 1.Ansley SJ, Badano JL, Blacque OE, Hill J, Hoskins BE, Leitch CC, Kim JC, Ross AJ, Eichers ER, Teslovich TM, Mah AK, Johnsen RC, Cavender JC, Lewis RA, Leroux MR, Beales PL, Katsanis N. Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature. 2003;425(6958):628–633. doi: 10.1038/nature02030. [DOI] [PubMed] [Google Scholar]
- 2.Li JB, Gerdes JM, Haycraft CJ, Fan Y, Teslovich TM, May-Simera H, Li H, Blacque OE, Li L, Leitch CC, Lewis RA, Green JS, Parfrey PS, Leroux MR, Davidson WS, Beales PL, Guay-Woodford LM, Yoder BK, Stormo GD, Katsanis N, Dutcher SK. Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell. 2004;117(4):541–552. doi: 10.1016/s0092-8674(04)00450-7. [DOI] [PubMed] [Google Scholar]
- 3.Gherman A, Davis EE, Katsanis N. The ciliary proteome database: an integrated community resource for the genetic and functional dissection of cilia. Nat Genet. 2006;38(9):961–962. doi: 10.1038/ng0906-961. [DOI] [PubMed] [Google Scholar]
- 4.Beales PL, Bland E, Tobin JL, Bacchelli C, Tuysuz B, Hill J, Rix S, Pearson CG, Kai M, Hartley J, Johnson C, Irving M, Elcioglu N, Winey M, Tada M, Scambler PJ. IFT80, which encodes a conserved intraflagellar transport protein, is mutated in Jeune asphyxiating thoracic dystrophy. Nat Genet. 2007;39(6):727–729. doi: 10.1038/ng2038. [DOI] [PubMed] [Google Scholar]
- 5.Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95(6):829–837. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- 6.Satir P, Guerra C, Bell AJ. Evolution and persistence of the cilium. Cell Motil Cytoskeleton. 2007;64(12):906–913. doi: 10.1002/cm.20238. [DOI] [PubMed] [Google Scholar]
- 7.Woolley D. The molecular motors of cilia and eukaryotic flagella. Essays Biochem. 2000;35:103–115. doi: 10.1042/bse0350103. [DOI] [PubMed] [Google Scholar]
- 8.Nigg EA, Raff JW. Centrioles, centrosomes, and cilia in health and disease. Cell. 2009;139(4):663–678. doi: 10.1016/j.cell.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 9.Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, Jackson PK. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129(6):1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol. 2008;85:23–61. doi: 10.1016/S0070-2153(08)00802-8. [DOI] [PubMed] [Google Scholar]
- 11.Cole DG, Snell WJ. SnapShot: intraflagellar transport. Cell. 2009;137(4):784–784. doi: 10.1016/j.cell.2009.04.053. [DOI] [PubMed] [Google Scholar]
- 12.Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, Somlo S, Igarashi P. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci USA. 2003;100(9):5286–5291. doi: 10.1073/pnas.0836980100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moyer JH, Lee-Tischler MJ, Kwon HY, Schrick JJ, Avner ED, Sweeney WE, Godfrey VL, Cacheiro NL, Wilkinson JE, Woychik RP. Candidate gene associated with a mutation causing recessive polycystic kidney disease in mice. Science. 1994;264(5163):1329–1333. doi: 10.1126/science.8191288. [DOI] [PubMed] [Google Scholar]
- 14.Ogden SK, Fei DL, Schilling NS, Ahmed YF, Hwa J, Robbins DJ. G protein Galphai functions immediately downstream of Smoothened in Hedgehog signalling. Nature. 2008;456(7224):967–970. doi: 10.1038/nature07459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milenkovic L, Scott MP, Rohatgi R. Lateral transport of Smoothened from the plasma membrane to the membrane of the cilium. J Cell Biol. 2009;187(3):365–374. doi: 10.1083/jcb.200907126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426(6962):83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- 17.Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, Tan PL, Phillips HM, Leroux MR, Henderson DJ, Murdoch JN, Copp AJ, Eliot MM, Lupski JR, Kemp DT, Dollfus H, Tada M, Katsanis N, Forge A, Beales PL. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37(10):1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- 18.Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, Mlodzik M, Polok B, Driever W, Obara T, Walz G. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37(5):537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gray RS, Abitua PB, Wlodarczyk BJ, Szabo-Rogers HL, Blanchard O, Lee I, Weiss GS, Liu KJ, Marcotte EM, Wallingford JB, Finnell RH. The planar cell polarity effector Fuz is essential for targeted membrane trafficking, ciliogenesis and mouse embryonic development. Nat Cell Biol. 2009;11(10):1225–1232. doi: 10.1038/ncb1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bielas SL, Silhavy JL, Brancati F, Kisseleva MV, Al-Gazali L, Sztriha L, Bayoumi RA, Zaki MS, Abdel-Aleem A, Rosti RO, Kayserili H, Swistun D, Scott LC, Bertini E, Boltshauser E, Fazzi E, Travaglini L, Field SJ, Gayral S, Jacoby M, Schurmans S, Dallapiccola B, Majerus PW, Valente EM, Gleeson JG. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat Genet. 2009;41(9):1032–1036. doi: 10.1038/ng.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cantagrel V, Silhavy JL, Bielas SL, Swistun D, Marsh SE, Bertrand JY, Audollent S, Attie-Bitach T, Holden KR, Dobyns WB, Traver D, Al-Gazali L, Ali BR, Lindner TH, Caspary T, Otto EA, Hildebrandt F, Glass IA, Logan CV, Johnson CA, Bennett C, Brancati F, Valente EM, Woods CG, Gleeson JG. Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. Am J Hum Genet. 2008;83(2):170–179. doi: 10.1016/j.ajhg.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noor A, Windpassinger C, Patel M, Stachowiak B, Mikhailov A, Azam M, Irfan M, Siddiqui ZK, Naeem F, Paterson AD, Lutfullah M, Vincent JB, Ayub M. CC2D2A, encoding a coiled-coil and C2 domain protein, causes autosomal-recessive mental retardation with retinitis pigmentosa. Am J Hum Genet. 2008;82(4):1011–1018. doi: 10.1016/j.ajhg.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delous M, Baala L, Salomon R, Laclef C, Vierkotten J, Tory K, Golzio C, Lacoste T, Besse L, Ozilou C, Moutkine I, Hellman NE, Anselme I, Silbermann F, Vesque C, Gerhardt C, Rattenberry E, Wolf MT, Gubler MC, Martinovic J, Encha-Razavi F, Boddaert N, Gonzales M, Macher MA, Nivet H, Champion G, Bertheleme JP, Niaudet P, McDonald F, Hildebrandt F, Johnson CA, Vekemans M, Antignac C, Ruther U, Schneider-Maunoury S, Attie-Bitach T, Saunier S. The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat Genet. 2007;39(7):875–881. doi: 10.1038/ng2039. [DOI] [PubMed] [Google Scholar]
- 24.Baala L, Romano S, Khaddour R, Saunier S, Smith UM, Audollent S, Ozilou C, Faivre L, Laurent N, Foliguet B, Munnich A, Lyonnet S, Salomon R, Encha-Razavi F, Gubler MC, Boddaert N, Lonlay P, Johnson CA, Vekemans M, Antignac C, Attie-Bitach T. The Meckel-Gruber syndrome gene, MKS3, is mutated in Joubert syndrome. Am J Hum Genet. 2007;80(1):186–194. doi: 10.1086/510499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parisi MA, Bennett CL, Eckert ML, Dobyns WB, Gleeson JG, Shaw DW, McDonald R, Eddy A, Chance PF, Glass IA. The NPHP1 gene deletion associated with juvenile nephronophthisis is present in a subset of individuals with Joubert syndrome. Am J Hum Genet. 2004;75(1):82–91. doi: 10.1086/421846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dixon-Salazar T, Silhavy JL, Marsh SE, Louie CM, Scott LC, Gururaj A, Al-Gazali L, Al-Tawari AA, Kayserili H, Sztriha L, Gleeson JG. Mutations in the AHI1 gene, encoding jouberin, cause Joubert syndrome with cortical polymicrogyria. Am J Hum Genet. 2004;75(6):979–987. doi: 10.1086/425985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sayer JA, Otto EA, O’Toole JF, Nurnberg G, Kennedy MA, Becker C, Hennies HC, Helou J, Attanasio M, Fausett BV, Utsch B, Khanna H, Liu Y, Drummond I, Kawakami I, Kusakabe T, Tsuda M, Ma L, Lee H, Larson RG, Allen SJ, Wilkinson CJ, Nigg EA, Shou C, Lillo C, Williams DS, Hoppe B, Kemper MJ, Neuhaus T, Parisi MA, Glass IA, Petry M, Kispert A, Gloy J, Ganner A, Walz G, Zhu X, Goldman D, Nurnberg P, Swaroop A, Leroux MR, Hildebrandt F. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat Genet. 2006;38(6):674–681. doi: 10.1038/ng1786. [DOI] [PubMed] [Google Scholar]
- 28.Coene KL, Roepman R, Doherty D, Afroze B, Kroes HY, Letteboer SJ, Ngu LH, Budny B, Wijk E, Gorden NT, Azhimi M, Thauvin-Robinet C, Veltman JA, Boink M, Kleefstra T, Cremers FP, Bokhoven H, Brouwer AP. OFD1 is mutated in X-linked Joubert syndrome and interacts with LCA5-encoded lebercilin. Am J Hum Genet. 2009;85(4):465–481. doi: 10.1016/j.ajhg.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valente EM, Silhavy JL, Brancati F, Barrano G, Krishnaswami SR, Castori M, Lancaster MA, Boltshauser E, Boccone L, Al-Gazali L, Fazzi E, Signorini S, Louie CM, Bellacchio E, Bertini E, Dallapiccola B, Gleeson JG. Mutations in CEP290, which encodes a centrosomal protein, cause pleiotropic forms of Joubert syndrome. Nat Genet. 2006;38(6):623–625. doi: 10.1038/ng1805. [DOI] [PubMed] [Google Scholar]
- 30.Brancati F, Barrano G, Silhavy JL, Marsh SE, Travaglini L, Bielas SL, Amorini M, Zablocka D, Kayserili H, Al-Gazali L, Bertini E, Boltshauser E, D’Hooghe M, Fazzi E, Fenerci EY, Hennekam RC, Kiss A, Lees MM, Marco E, Phadke SR, Rigoli L, Romano S, Salpietro CD, Sherr EH, Signorini S, Stromme P, Stuart B, Sztriha L, Viskochil DH, Yuksel A, Dallapiccola B, Valente EM, Gleeson JG. CEP290 mutations are frequently identified in the oculo-renal form of Joubert syndrome-related disorders. Am J Hum Genet. 2007;81(1):104–113. doi: 10.1086/519026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coppieters F, Lefever S, Leroy BP, Baere E. CEP290, a gene with many faces: mutation overview and presentation of CEP290base. Hum Mutat. 2010;31(10):1097–1108. doi: 10.1002/humu.21337. [DOI] [PubMed] [Google Scholar]
- 32.Murga-Zamalloa CA, Desai NJ, Hildebrandt F, Khanna H. Interaction of ciliary disease protein retinitis pigmentosa GTPase regulator with nephronophthisis-associated proteins in mammalian retinas. Mol Vis. 2010;16:1373–1381. [PMC free article] [PubMed] [Google Scholar]
- 33.Chang B, Khanna H, Hawes N, Jimeno D, He S, Lillo C, Parapuram SK, Cheng H, Scott A, Hurd RE, Sayer JA, Otto EA, Attanasio M, O’Toole JF, Jin G, Shou C, Hildebrandt F, Williams DS, Heckenlively JR, Swaroop A. In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Hum Mol Genet. 2006;15(11):1847–1857. doi: 10.1093/hmg/ddl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murga-Zamalloa CA, Swaroop A, Khanna H. RPGR-containing protein complexes in syndromic and non-syndromic retinal degeneration due to ciliary dysfunction. J Genet. 2009;88(4):399–407. doi: 10.1007/s12041-009-0061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eley L, Gabrielides C, Adams M, Johnson CA, Hildebrandt F, Sayer JA. Jouberin localizes to collecting ducts and interacts with nephrocystin-1. Kidney Int. 2008;74(9):1139–1149. doi: 10.1038/ki.2008.377. [DOI] [PubMed] [Google Scholar]
- 36.Kyttala M, Tallila J, Salonen R, Kopra O, Kohlschmidt N, Paavola-Sakki P, Peltonen L, Kestila M. MKS1, encoding a component of the flagellar apparatus basal body proteome, is mutated in Meckel syndrome. Nat Genet. 2006;38(2):155–157. doi: 10.1038/ng1714. [DOI] [PubMed] [Google Scholar]
- 37.Smith UM, Consugar M, Tee LJ, McKee BM, Maina EN, Whelan S, Morgan NV, Goranson E, Gissen P, Lilliquist S, Aligianis IA, Ward CJ, Pasha S, Punyashthiti R, Malik Sharif S, Batman PA, Bennett CP, Woods CG, McKeown C, Bucourt M, Miller CA, Cox P, Algazali L, Trembath RC, Torres VE, Attie-Bitach T, Kelly DA, Maher ER, Gattone VH, 2nd, Harris PC, Johnson CA. The transmembrane protein meckelin (MKS3) is mutated in Meckel-Gruber syndrome and the wpk rat. Nat Genet. 2006;38(2):191–196. doi: 10.1038/ng1713. [DOI] [PubMed] [Google Scholar]
- 38.Tallila J, Jakkula E, Peltonen L, Salonen R, Kestila M. Identification of CC2D2A as a Meckel syndrome gene adds an important piece to the ciliopathy puzzle. Am J Hum Genet. 2008;82(6):1361–1367. doi: 10.1016/j.ajhg.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gorden NT, Arts HH, Parisi MA, Coene KL, Letteboer SJ, Beersum SE, Mans DA, Hikida A, Eckert M, Knutzen D, Alswaid AF, Ozyurek H, Dibooglu S, Otto EA, Liu Y, Davis EE, Hutter CM, Bammler TK, Farin FM, Dorschner M, Topcu M, Zackai EH, Rosenthal P, Owens KN, Katsanis N, Vincent JB, Hildebrandt F, Rubel EW, Raible DW, Knoers NV, Chance PF, Roepman R, Moens CB, Glass IA, Doherty D. CC2D2A is mutated in Joubert syndrome and interacts with the ciliopathy-associated basal body protein CEP290. Am J Hum Genet. 2008;83(5):559–571. doi: 10.1016/j.ajhg.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mougou-Zerelli S, Thomas S, Szenker E, Audollent S, Elkhartoufi N, Babarit C, Romano S, Salomon R, Amiel J, Esculpavit C, Gonzales M, Escudier E, Leheup B, Loget P, Odent S, Roume J, Gerard M, Delezoide AL, Khung S, Patrier S, Cordier MP, Bouvier R, Martinovic J, Gubler MC, Boddaert N, Munnich A, Encha-Razavi F, Valente EM, Saad A, Saunier S, Vekemans M, Attie-Bitach T. CC2D2A mutations in Meckel and Joubert syndromes indicate a genotype-phenotype correlation. Hum Mutat. 2009;30(11):1574–1582. doi: 10.1002/humu.21116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caridi G, Murer L, Bellantuono R, Sorino P, Caringella DA, Gusmano R, Ghiggeri GM. Renal-retinal syndromes: association of retinal anomalies and recessive nephronophthisis in patients with homozygous deletion of the NPH1 locus. Am J Kidney Dis. 1998;32(6):1059–1062. doi: 10.1016/s0272-6386(98)70083-6. [DOI] [PubMed] [Google Scholar]
- 42.Omran H, Sasmaz G, Haffner K, Volz A, Olbrich H, Melkaoui R, Otto E, Wienker TF, Korinthenberg R, Brandis M, Antignac C, Hildebrandt F. Identification of a gene locus for Senior-Loken syndrome in the region of the nephronophthisis type 3 gene. J Am Soc Nephrol. 2002;13(1):75–79. doi: 10.1681/ASN.V13175. [DOI] [PubMed] [Google Scholar]
- 43.Schuermann MJ, Otto E, Becker A, Saar K, Ruschendorf F, Polak BC, Ala-Mello S, Hoefele J, Wiedensohler A, Haller M, Omran H, Nurnberg P, Hildebrandt F. Mapping of gene loci for nephronophthisis type 4 and Senior-Loken syndrome, to chromosome 1p36. Am J Hum Genet. 2002;70(5):1240–1246. doi: 10.1086/340317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Otto E, Hoefele J, Ruf R, Mueller AM, Hiller KS, Wolf MT, Schuermann MJ, Becker A, Birkenhager R, Sudbrak R, Hennies HC, Nurnberg P, Hildebrandt F. A gene mutated in nephronophthisis and retinitis pigmentosa encodes a novel protein, nephroretinin, conserved in evolution. Am J Hum Genet. 2002;71(5):1161–1167. doi: 10.1086/344395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feather SA, Winyard PJ, Dodd S, Woolf AS. Orofaciodigital syndrome type 1 is another dominant polycystic kidney disease: clinical, radiological and histopathological features of a new kindred. Nephrol Dial Transplant. 1997;12(7):1354–1361. doi: 10.1093/ndt/12.7.1354. [DOI] [PubMed] [Google Scholar]
- 46.Ferrante MI, Giorgio G, Feather SA, Bulfone A, Wright V, Ghiani M, Selicorni A, Gammaro L, Scolari F, Woolf AS, Sylvie O, Bernard L, Malcolm S, Winter R, Ballabio A, Franco B. Identification of the gene for orofaciodigital type I syndrome. Am J Hum Genet. 2001;68(3):569–576. doi: 10.1086/318802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singla V, Romaguera-Ros M, Garcia-Verdugo JM, Reiter JF. Ofd1, a human disease gene, regulates the length and distal structure of centrioles. Dev Cell. 2010;18(3):410–424. doi: 10.1016/j.devcel.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferrante MI, Zullo A, Barra A, Bimonte S, Messaddeq N, Studer M, Dolle P, Franco B. Orofaciodigital type I protein is required for primary cilia formation and left-right axis specification. Nat Genet. 2006;38(1):112–117. doi: 10.1038/ng1684. [DOI] [PubMed] [Google Scholar]
- 49.Perrault I, Delphin N, Hanein S, Gerber S, Dufier JL, Roche O, Defoort-Dhellemmes S, Dollfus H, Fazzi E, Munnich A, Kaplan J, Rozet JM. Spectrum of NPHP6/CEP290 mutations in Leber congenital amaurosis and delineation of the associated phenotype. Hum Mutat. 2007;28(4):416. doi: 10.1002/humu.9485. [DOI] [PubMed] [Google Scholar]
- 50.Marlhens F, Bareil C, Griffoin JM, Zrenner E, Amalric P, Eliaou C, Liu SY, Harris E, Redmond TM, Arnaud B, Claustres M, Hamel CP. Mutations in RPE65 cause Leber’s congenital amaurosis. Nat Genet. 1997;17(2):139–141. doi: 10.1038/ng1097-139. [DOI] [PubMed] [Google Scholar]
- 51.Wang H, Hollander AI, Moayedi Y, Abulimiti A, Li Y, Collin RW, Hoyng CB, Lopez I, Abboud EB, Al-Rajhi AA, Bray M, Lewis RA, Lupski JR, Mardon G, Koenekoop RK, Chen R. Mutations in SPATA7 cause Leber congenital amaurosis and juvenile retinitis pigmentosa. Am J Hum Genet. 2009;84(3):380–387. doi: 10.1016/j.ajhg.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sohocki MM, Sullivan LS, Tirpak DL, Daiger SP. Comparative analysis of aryl-hydrocarbon receptor interacting protein-like 1 (Aipl1), a gene associated with inherited retinal disease in humans. Mamm Genome. 2001;12(7):566–568. doi: 10.1007/s003350020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hollander AI, Heckenlively JR, Born LI, Kok YJ, Velde-Visser SD, Kellner U, Jurklies B, Schooneveld MJ, Blankenagel A, Rohrschneider K, Wissinger B, Cruysberg JR, Deutman AF, Brunner HG, Apfelstedt-Sylla E, Hoyng CB, Cremers FP. Leber congenital amaurosis and retinitis pigmentosa with Coats-like exudative vasculopathy are associated with mutations in the crumbs homologue 1 (CRB1) gene. Am J Hum Genet. 2001;69(1):198–203. doi: 10.1086/321263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gerber S, Perrault I, Hanein S, Barbet F, Ducroq D, Ghazi I, Martin-Coignard D, Leowski C, Homfray T, Dufier JL, Munnich A, Kaplan J, Rozet JM. Complete exon-intron structure of the RPGR-interacting protein (RPGRIP1) gene allows the identification of mutations underlying Leber congenital amaurosis. Eur J Hum Genet. 2001;9(8):561–571. doi: 10.1038/sj.ejhg.5200689. [DOI] [PubMed] [Google Scholar]
- 55.Freund CL, Wang QL, Chen S, Muskat BL, Wiles CD, Sheffield VC, Jacobson SG, McInnes RR, Zack DJ, Stone EM. De novo mutations in the CRX homeobox gene associated with Leber congenital amaurosis. Nat Genet. 1998;18(4):311–312. doi: 10.1038/ng0498-311. [DOI] [PubMed] [Google Scholar]
- 56.Hollander AI, Johnson K, Kok YJ, Klebes A, Brunner HG, Knust E, Cremers FP. CRB1 has a cytoplasmic domain that is functionally conserved between human and Drosophila. Hum Mol Genet. 2001;10(24):2767–2773. doi: 10.1093/hmg/10.24.2767. [DOI] [PubMed] [Google Scholar]
- 57.Bowne SJ, Sullivan LS, Mortimer SE, Hedstrom L, Zhu J, Spellicy CJ, Gire AI, Hughbanks-Wheaton D, Birch DG, Lewis RA, Heckenlively JR, Daiger SP. Spectrum and frequency of mutations in IMPDH1 associated with autosomal dominant retinitis pigmentosa and leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2006;47(1):34–42. doi: 10.1167/iovs.05-0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Friedman JS, Chang B, Kannabiran C, Chakarova C, Singh HP, Jalali S, Hawes NL, Branham K, Othman M, Filippova E, Thompson DA, Webster AR, Andreasson S, Jacobson SG, Bhattacharya SS, Heckenlively JR, Swaroop A. Premature truncation of a novel protein, RD3, exhibiting subnuclear localization is associated with retinal degeneration. Am J Hum Genet. 2006;79(6):1059–1070. doi: 10.1086/510021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Janecke AR, Thompson DA, Utermann G, Becker C, Hubner CA, Schmid E, McHenry CL, Nair AR, Ruschendorf F, Heckenlively J, Wissinger B, Nurnberg P, Gal A. Mutations in RDH12 encoding a photoreceptor cell retinol dehydrogenase cause childhood-onset severe retinal dystrophy. Nat Genet. 2004;36(8):850–854. doi: 10.1038/ng1394. [DOI] [PubMed] [Google Scholar]
- 60.O’Dea D, Parfrey PS, Harnett JD, Hefferton D, Cramer BC, Green J. The importance of renal impairment in the natural history of Bardet-Biedl syndrome. Am J Kidney Dis. 1996;27(6):776–783. doi: 10.1016/s0272-6386(96)90513-2. [DOI] [PubMed] [Google Scholar]
- 61.Barakat AJ, Arianas P, Glick AD, Butler MG. Focal sclerosing glomerulonephritis in a child with Laurence-Moon-Biedl syndrome. Child Nephrol Urol. 1990;10(2):109–111. [PMC free article] [PubMed] [Google Scholar]
- 62.Beales PL, Elcioglu N, Woolf AS, Parker D, Flinter FA. New criteria for improved diagnosis of Bardet-Biedl syndrome: results of a population survey. J Med Genet. 1999;36(6):437–446. [PMC free article] [PubMed] [Google Scholar]
- 63.Tobin JL, Franco M, Eichers E, May-Simera H, Garcia M, Yan J, Quinlan R, Justice MJ, Hennekam RC, Briscoe J, Tada M, Mayor R, Burns AJ, Lupski JR, Hammond P, Beales PL. Inhibition of neural crest migration underlies craniofacial dysmorphology and Hirschsprung’s disease in Bardet-Biedl syndrome. Proc Natl Acad Sci USA. 2008;105(18):6714–6719. doi: 10.1073/pnas.0707057105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mykytyn K, Nishimura DY, Searby CC, Shastri M, Yen HJ, Beck JS, Braun T, Streb LM, Cornier AS, Cox GF, Fulton AB, Carmi R, Luleci G, Chandrasekharappa SC, Collins FS, Jacobson SG, Heckenlively JR, Weleber RG, Stone EM, Sheffield VC. Identification of the gene (BBS1) most commonly involved in Bardet-Biedl syndrome, a complex human obesity syndrome. Nat Genet. 2002;31(4):435–438. doi: 10.1038/ng935. [DOI] [PubMed] [Google Scholar]
- 65.Nishimura DY, Searby CC, Carmi R, Elbedour K, Maldergem L, Fulton AB, Lam BL, Powell BR, Swiderski RE, Bugge KE, Haider NB, Kwitek-Black AE, Ying L, Duhl DM, Gorman SW, Heon E, Iannaccone A, Bonneau D, Biesecker LG, Jacobson SG, Stone EM, Sheffield VC. Positional cloning of a novel gene on chromosome 16q causing Bardet-Biedl syndrome (BBS2) Hum Mol Genet. 2001;10(8):865–874. doi: 10.1093/hmg/10.8.865. [DOI] [PubMed] [Google Scholar]
- 66.Chiang AP, Nishimura D, Searby C, Elbedour K, Carmi R, Ferguson AL, Secrist J, Braun T, Casavant T, Stone EM, Sheffield VC. Comparative genomic analysis identifies an ADP-ribosylation factor-like gene as the cause of Bardet-Biedl syndrome (BBS3) Am J Hum Genet. 2004;75(3):475–484. doi: 10.1086/423903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mykytyn K, Braun T, Carmi R, Haider NB, Searby CC, Shastri M, Beck G, Wright AF, Iannaccone A, Elbedour K, Riise R, Baldi A, Raas-Rothschild A, Gorman SW, Duhl DM, Jacobson SG, Casavant T, Stone EM, Sheffield VC. Identification of the gene that, when mutated, causes the human obesity syndrome BBS4. Nat Genet. 2001;28(2):188–191. doi: 10.1038/88925. [DOI] [PubMed] [Google Scholar]
- 68.Katsanis N, Beales PL, Woods MO, Lewis RA, Green JS, Parfrey PS, Ansley SJ, Davidson WS, Lupski JR. Mutations in MKKS cause obesity, retinal dystrophy and renal malformations associated with Bardet-Biedl syndrome. Nat Genet. 2000;26(1):67–70. doi: 10.1038/79201. [DOI] [PubMed] [Google Scholar]
- 69.Badano JL, Ansley SJ, Leitch CC, Lewis RA, Lupski JR, Katsanis N. Identification of a novel Bardet-Biedl syndrome protein, BBS7, that shares structural features with BBS1 and BBS2. Am J Hum Genet. 2003;72(3):650–658. doi: 10.1086/368204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nishimura DY, Swiderski RE, Searby CC, Berg EM, Ferguson AL, Hennekam R, Merin S, Weleber RG, Biesecker LG, Stone EM, Sheffield VC. Comparative genomics and gene expression analysis identifies BBS9, a new Bardet-Biedl syndrome gene. Am J Hum Genet. 2005;77(6):1021–1033. doi: 10.1086/498323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stoetzel C, Laurier V, Davis EE, Muller J, Rix S, Badano JL, Leitch CC, Salem N, Chouery E, Corbani S, Jalk N, Vicaire S, Sarda P, Hamel C, Lacombe D, Holder M, Odent S, Holder S, Brooks AS, Elcioglu NH, Silva ED, Rossillion B, Sigaudy S, Ravel TJ, Lewis RA, Leheup B, Verloes A, Amati-Bonneau P, Megarbane A, Poch O, Bonneau D, Beales PL, Mandel JL, Katsanis N, Dollfus H. BBS10 encodes a vertebrate-specific chaperonin-like protein and is a major BBS locus. Nat Genet. 2006;38(5):521–524. doi: 10.1038/ng1771. [DOI] [PubMed] [Google Scholar]
- 72.Chiang AP, Beck JS, Yen HJ, Tayeh MK, Scheetz TE, Swiderski RE, Nishimura DY, Braun TA, Kim KY, Huang J, Elbedour K, Carmi R, Slusarski DC, Casavant TL, Stone EM, Sheffield VC. Homozygosity mapping with SNP arrays identifies TRIM32, an E3 ubiquitin ligase, as a Bardet-Biedl syndrome gene (BBS11) Proc Natl Acad Sci USA. 2006;103(16):6287–6292. doi: 10.1073/pnas.0600158103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stoetzel C, Muller J, Laurier V, Davis EE, Zaghloul NA, Vicaire S, Jacquelin C, Plewniak F, Leitch CC, Sarda P, Hamel C, Ravel TJ, Lewis RA, Friederich E, Thibault C, Danse JM, Verloes A, Bonneau D, Katsanis N, Poch O, Mandel JL, Dollfus H. Identification of a novel BBS gene (BBS12) highlights the major role of a vertebrate-specific branch of chaperonin-related proteins in Bardet-Biedl syndrome. Am J Hum Genet. 2007;80(1):1–11. doi: 10.1086/510256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leitch CC, Zaghloul NA, Davis EE, Stoetzel C, Diaz-Font A, Rix S, Alfadhel M, Lewis RA, Eyaid W, Banin E, Dollfus H, Beales PL, Badano JL, Katsanis N. Hypomorphic mutations in syndromic encephalocele genes are associated with Bardet-Biedl syndrome. Nat Genet. 2008;40(4):443–448. doi: 10.1038/ng.97. [DOI] [PubMed] [Google Scholar]
- 75.Kim SK, Shindo A, Park TJ, Oh EC, Ghosh S, Gray RS, Lewis RA, Johnson CA, Attie-Bittach T, Katsanis N, Wallingford JB. Planar cell polarity acts through septins to control collective cell movement and ciliogenesis. Science. 2010;329(5997):1337–1340. doi: 10.1126/science.1191184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Otto EA, Hurd TW, Airik R, Chaki M, Zhou W, Stoetzel C, Patil SB, Levy S, Ghosh AK, Murga-Zamalloa CA, Reeuwijk J, Letteboer SJ, Sang L, Giles RH, Liu Q, Coene KL, Estrada-Cuzcano A, Collin RW, McLaughlin HM, Held S, Kasanuki JM, Ramaswami G, Conte J, Lopez I, Washburn J, Macdonald J, Hu J, Yamashita Y, Maher ER, Guay-Woodford LM, Neumann HP, Obermuller N, Koenekoop RK, Bergmann C, Bei X, Lewis RA, Katsanis N, Lopes V, Williams DS, Lyons RH, Dang CV, Brito DA, Dias MB, Zhang X, Cavalcoli JD, Nurnberg G, Nurnberg P, Pierce EA, Jackson PK, Antignac C, Saunier S, Roepman R, Dollfus H, Khanna H, Hildebrandt F. Candidate exome capture identifies mutation of SDCCAG8 as the cause of a retinal-renal ciliopathy. Nat Genet. 2010;42(10):840–850. doi: 10.1038/ng.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Badano JL, Leitch CC, Ansley SJ, May-Simera H, Lawson S, Lewis RA, Beales PL, Dietz HC, Fisher S, Katsanis N. Dissection of epistasis in oligogenic Bardet-Biedl syndrome. Nature. 2006;439(7074):326–330. doi: 10.1038/nature04370. [DOI] [PubMed] [Google Scholar]
- 78.Collin GB, Marshall JD, Ikeda A, So WV, Russell-Eggitt I, Maffei P, Beck S, Boerkoel CF, Sicolo N, Martin M, Nishina PM, Naggert JK. Mutations in ALMS1 cause obesity, type 2 diabetes and neurosensory degeneration in Alstrom syndrome. Nat Genet. 2002;31(1):74–78. doi: 10.1038/ng867. [DOI] [PubMed] [Google Scholar]
- 79.Knorz VJ, Spalluto C, Lessard M, Purvis TL, Adigun FF, Collin GB, Hanley NA, Wilson DI, Hearn T. Centriolar association of ALMS1 and likely centrosomal functions of the ALMS motif-containing proteins C10orf90 and KIAA1731. Mol Biol Cell. 2010;21(21):3617–3629. doi: 10.1091/mbc.E10-03-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pirnar T, Neuhauser EB. Asphyxiating thoracic dystrophy of the newborn. Am J Roentgenol Radium Ther Nucl Med. 1966;98(2):358–364. doi: 10.2214/ajr.98.2.358. [DOI] [PubMed] [Google Scholar]
- 81.Singh M, Ray D, Paul VK, Kumar A. Hydrocephalus in asphyxiating thoracic dystrophy. Am J Med Genet. 1988;29(2):391–395. doi: 10.1002/ajmg.1320290221. [DOI] [PubMed] [Google Scholar]
- 82.Phillips CI, Stokoe NL, Bartholomew RS. Asphyxiating thoracic dystrophy (Jeune’s disease) with retinal aplasia: a sibship of two. J Pediatr Ophthalmol Strabismus. 1979;16(5):279–283. doi: 10.3928/0191-3913-19790901-03. [DOI] [PubMed] [Google Scholar]
- 83.Hudgins L, Rosengren S, Treem W, Hyams J. Early cirrhosis in survivors with Jeune thoracic dystrophy. J Pediatr. 1992;120(5):754–756. doi: 10.1016/s0022-3476(05)80241-0. [DOI] [PubMed] [Google Scholar]
- 84.Haycraft CJ, Zhang Q, Song B, Jackson WS, Detloff PJ, Serra R, Yoder BK. Intraflagellar transport is essential for endochondral bone formation. Development. 2007;134(2):307–316. doi: 10.1242/dev.02732. [DOI] [PubMed] [Google Scholar]
- 85.Brueton LA, Dillon MJ, Winter RM. Ellis-van creveld syndrome, Jeune syndrome, and renal-hepatic-pancreatic dysplasia: separate entities or disease spectrum? J Med Genet. 1990;27(4):252–255. doi: 10.1136/jmg.27.4.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ruiz-Perez VL, Ide SE, Strom TM, Lorenz B, Wilson D, Woods K, King L, Francomano C, Freisinger P, Spranger S, Marino B, Dallapiccola B, Wright M, Meitinger T, Polymeropoulos MH, Goodship J. Mutations in a new gene in Ellis-van Creveld syndrome and Weyers acrodental dysostosis. Nat Genet. 2000;24(3):283–286. doi: 10.1038/73508. [DOI] [PubMed] [Google Scholar]
- 87.Galdzicka M, Patnala S, Hirshman MG, Cai JF, Nitowsky H, Egeland JA, Ginns EI. A new gene, EVC2, is mutated in Ellis-van Creveld syndrome. Mol Genet Metab. 2002;77(4):291–295. doi: 10.1016/s1096-7192(02)00178-6. [DOI] [PubMed] [Google Scholar]
- 88.Ruiz-Perez VL, Blair HJ, Rodriguez-Andres ME, Blanco MJ, Wilson A, Liu YN, Miles C, Peters H, Goodship JA. Evc is a positive mediator of Ihh-regulated bone growth that localises at the base of chondrocyte cilia. Development. 2007;134(16):2903–2912. doi: 10.1242/dev.007542. [DOI] [PubMed] [Google Scholar]
- 89.Walczak-Sztulpa J, Eggenschwiler J, Osborn D, Brown DA, Emma F, Klingenberg C, Hennekam RC, Torre G, Garshasbi M, Tzschach A, Szczepanska M, Krawczynski M, Zachwieja J, Zwolinska D, Beales PL, Ropers HH, Latos-Bielenska A, Kuss AW. Cranioectodermal dysplasia, Sensenbrenner syndrome, is a ciliopathy caused by mutations in the IFT122 gene. Am J Hum Genet. 2010;86(6):949–956. doi: 10.1016/j.ajhg.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gilissen C, Arts HH, Hoischen A, Spruijt L, Mans DA, Arts P, Lier B, Steehouwer M, Reeuwijk J, Kant SG, Roepman R, Knoers NV, Veltman JA, Brunner HG. Exome sequencing identifies WDR35 variants involved in Sensenbrenner syndrome. Am J Hum Genet. 2010;87(3):418–423. doi: 10.1016/j.ajhg.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Harris PC. Homer W. Smith Award: insights into the pathogenesis of polycystic kidney disease from gene discovery. J Am Soc Nephrol. 2009;20(6):1188–1198. doi: 10.1681/ASN.2009010014. [DOI] [PubMed] [Google Scholar]
- 92.Geng L, Segal Y, Peissel B, Deng N, Pei Y, Carone F, Rennke HG, Glucksmann-Kuis AM, Schneider MC, Ericsson M, Reeders ST, Zhou J. Identification and localization of polycystin, the PKD1 gene product. J Clin Invest. 1996;98(12):2674–2682. doi: 10.1172/JCI119090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A, Kimberling WJ, Breuning MH, Deltas CC, Peters DJ, Somlo S. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272(5266):1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- 94.Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol. 2002;13(10):2508–2516. doi: 10.1097/01.asn.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- 95.Ward CJ, Yuan D, Masyuk TV, Wang X, Punyashthiti R, Whelan S, Bacallao R, Torra R, LaRusso NF, Torres VE, Harris PC. Cellular and subcellular localization of the ARPKD protein; fibrocystin is expressed on primary cilia. Hum Mol Genet. 2003;12(20):2703–2710. doi: 10.1093/hmg/ddg274. [DOI] [PubMed] [Google Scholar]
- 96.Lu W, Peissel B, Babakhanlou H, Pavlova A, Geng L, Fan X, Larson C, Brent G, Zhou J. Perinatal lethality with kidney and pancreas defects in mice with a targetted Pkd1 mutation. Nat Genet. 1997;17(2):179–181. doi: 10.1038/ng1097-179. [DOI] [PubMed] [Google Scholar]
- 97.Piontek K, Menezes LF, Garcia-Gonzalez MA, Huso DL, Germino GG. A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat Med. 2007;13(12):1490–1495. doi: 10.1038/nm1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Qian F, Watnick TJ, Onuchic LF, Germino GG. The molecular basis of focal cyst formation in human autosomal dominant polycystic kidney disease type I. Cell. 1996;87(6):979–987. doi: 10.1016/s0092-8674(00)81793-6. [DOI] [PubMed] [Google Scholar]
- 99.Pei Y, Watnick T, He N, Wang K, Liang Y, Parfrey P, Germino G, St George-Hyslop P. Somatic PKD2 mutations in individual kidney and liver cysts support a "two-hit" model of cystogenesis in type 2 autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1999;10(7):1524–1529. doi: 10.1681/ASN.V1071524. [DOI] [PubMed] [Google Scholar]
- 100.Moser M, Matthiesen S, Kirfel J, Schorle H, Bergmann C, Senderek J, Rudnik-Schoneborn S, Zerres K, Buettner R. A mouse model for cystic biliary dysgenesis in autosomal recessive polycystic kidney disease (ARPKD) Hepatology. 2005;41(5):1113–1121. doi: 10.1002/hep.20655. [DOI] [PubMed] [Google Scholar]
- 101.Wolf MT, Hildebrandt F. Nephronophthisis. Pediatr Nephrol. 2010 doi: 10.1007/s00467-010-1585-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tory K, Lacoste T, Burglen L, Moriniere V, Boddaert N, Macher MA, Llanas B, Nivet H, Bensman A, Niaudet P, Antignac C, Salomon R, Saunier S. High NPHP1 and NPHP6 mutation rate in patients with Joubert syndrome and nephronophthisis: potential epistatic effect of NPHP6 and AHI1 mutations in patients with NPHP1 mutations. J Am Soc Nephrol. 2007;18(5):1566–1575. doi: 10.1681/ASN.2006101164. [DOI] [PubMed] [Google Scholar]
- 103.Bergmann C, Fliegauf M, Bruchle NO, Frank V, Olbrich H, Kirschner J, Schermer B, Schmedding I, Kispert A, Kranzlin B, Nurnberg G, Becker C, Grimm T, Girschick G, Lynch SA, Kelehan P, Senderek J, Neuhaus TJ, Stallmach T, Zentgraf H, Nurnberg P, Gretz N, Lo C, Lienkamp S, Schafer T, Walz G, Benzing T, Zerres K, Omran H. Loss of nephrocystin-3 function can cause embryonic lethality, Meckel-Gruber-like syndrome, situs inversus, and renal-hepatic-pancreatic dysplasia. Am J Hum Genet. 2008;82(4):959–970. doi: 10.1016/j.ajhg.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wolf MT, Saunier S, O’Toole JF, Wanner N, Groshong T, Attanasio M, Salomon R, Stallmach T, Sayer JA, Waldherr R, Griebel M, Oh J, Neuhaus TJ, Josefiak U, Antignac C, Otto EA, Hildebrandt F. Mutational analysis of the RPGRIP1L gene in patients with Joubert syndrome and nephronophthisis. Kidney Int. 2007;72(12):1520–1526. doi: 10.1038/sj.ki.5002630. [DOI] [PubMed] [Google Scholar]
- 105.Yokoyama T, Copeland NG, Jenkins NA, Montgomery CA, Elder FF, Overbeek PA. Reversal of left-right asymmetry: a situs inversus mutation. Science. 1993;260(5108):679–682. doi: 10.1126/science.8480178. [DOI] [PubMed] [Google Scholar]
- 106.Kim YS, Kang HS, Herbert R, Beak JY, Collins JB, Grissom SF, Jetten AM. Kruppel-like zinc finger protein Glis2 is essential for the maintenance of normal renal functions. Mol Cell Biol. 2008;28(7):2358–2367. doi: 10.1128/MCB.01722-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Woolf AS, Price KL, Scambler PJ, Winyard PJ. Evolving concepts in human renal dysplasia. J Am Soc Nephrol. 2004;15(4):998–1007. doi: 10.1097/01.asn.0000113778.06598.6f. [DOI] [PubMed] [Google Scholar]
- 108.Sheffield VC, Carmi R, Kwitek-Black A, Rokhlina T, Nishimura D, Duyk GM, Elbedour K, Sunden SL, Stone EM. Identification of a Bardet-Biedl syndrome locus on chromosome 3 and evaluation of an efficient approach to homozygosity mapping. Hum Mol Genet. 1994;3(8):1331–1335. doi: 10.1093/hmg/3.8.1331. [DOI] [PubMed] [Google Scholar]
- 109.Yoder BK, Richards WG, Sweeney WE, Wilkinson JE, Avener ED, Woychik RP. Insertional mutagenesis and molecular analysis of a new gene associated with polycystic kidney disease. Proc Assoc Am Physicians. 1995;107(3):314–323. [PubMed] [Google Scholar]
- 110.Hou X, Mrug M, Yoder BK, Lefkowitz EJ, Kremmidiotis G, D’Eustachio P, Beier DR, Guay-Woodford LM. Cystin, a novel cilia-associated protein, is disrupted in the cpk mouse model of polycystic kidney disease. J Clin Invest. 2002;109(4):533–540. doi: 10.1172/JCI14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Saburi S, Hester I, Fischer E, Pontoglio M, Eremina V, Gessler M, Quaggin SE, Harrison R, Mount R, McNeill H. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet. 2008;40(8):1010–1015. doi: 10.1038/ng.179. [DOI] [PubMed] [Google Scholar]
- 112.Fischer E, Pontoglio M. Planar cell polarity and polycystic kidney disease. Med Sci (Paris) 2006;22(6–7):576–578. doi: 10.1051/medsci/20062267576. [DOI] [PubMed] [Google Scholar]
- 113.Nishio S, Tian X, Gallagher AR, Yu Z, Patel V, Igarashi P, Somlo S. Loss of oriented cell division does not initiate cyst formation. J Am Soc Nephrol. 2010;21(2):295–302. doi: 10.1681/ASN.2009060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Karner CM, Chirumamilla R, Aoki S, Igarashi P, Wallingford JB, Carroll TJ. Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat Genet. 2009;41(7):793–799. doi: 10.1038/ng.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Harris PC, Torres VE. Polycystic kidney disease. Annu Rev Med. 2009;60:321–337. doi: 10.1146/annurev.med.60.101707.125712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Saadi-Kheddouci S, Berrebi D, Romagnolo B, Cluzeaud F, Peuchmaur M, Kahn A, Vandewalle A, Perret C. Early development of polycystic kidney disease in transgenic mice expressing an activated mutant of the beta-catenin gene. Oncogene. 2001;20(42):5972–5981. doi: 10.1038/sj.onc.1204825. [DOI] [PubMed] [Google Scholar]
- 117.Attanasio M, Uhlenhaut NH, Sousa VH, O’Toole JF, Otto E, Anlag K, Klugmann C, Treier AC, Helou J, Sayer JA, Seelow D, Nurnberg G, Becker C, Chudley AE, Nurnberg P, Hildebrandt F, Treier M. Loss of GLIS2 causes nephronophthisis in humans and mice by increased apoptosis and fibrosis. Nat Genet. 2007;39(8):1018–1024. doi: 10.1038/ng2072. [DOI] [PubMed] [Google Scholar]
- 118.Walz G, Budde K, Mannaa M, Nurnberger J, Wanner C, Sommerer C, Kunzendorf U, Banas B, Horl WH, Obermuller N, Arns W, Pavenstadt H, Gaedeke J, Buchert M, May C, Gschaidmeier H, Kramer S, Eckardt KU. Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363(9):830–840. doi: 10.1056/NEJMoa1003491. [DOI] [PubMed] [Google Scholar]
- 119.Hogan MC, Masyuk TV, Page LJ, Kubly VJ, Bergstralh EJ, Li X, Kim B, King BF, Glockner J, Holmes DR, III, Rossetti S, Harris PC, LaRusso NF, Torres VE. Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. J Am Soc Nephrol. 2010;21(6):1052–1061. doi: 10.1681/ASN.2009121291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Johnson CA, Gissen P, Serfi C. Molecular pathology and genetics of congenital hepatorenal fibrocystic syndromes. J Med Genet. 2003;40(5):311–319. doi: 10.1136/jmg.40.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Masyuk AI, Masyuk TV, LaRusso NF. Cholangiocyte primary cilia in liver health and disease. Dev Dyn. 2008;237(8):2007–2012. doi: 10.1002/dvdy.21530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Masyuk TV, Masyuk AI, Torres VE, Harris PC, LaRusso NF. Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3', 5'-cyclic monophosphate. Gastroenterology. 2007;132(3):1104–1116. doi: 10.1053/j.gastro.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 123.Brancati F, Iannicelli M, Travaglini L, Mazzotta A, Bertini E, Boltshauser E, D’Arrigo S, Emma F, Fazzi E, Gallizzi R, Gentile M, Loncarevic D, Mejaski-Bosnjak V, Pantaleoni C, Rigoli L, Salpietro CD, Signorini S, Stringini GR, Verloes A, Zabloka D, Dallapiccola B, Gleeson JG, Valente EM. MKS3/TMEM67 mutations are a major cause of COACH Syndrome, a Joubert Syndrome related disorder with liver involvement. Hum Mutat. 2009;30(2):E432–E442. doi: 10.1002/humu.20924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Meeker WR, Jr, Nighbert EJ. Association of cystic dilatation of intrahepatic and common bile ducts with Laurence-Moon-Biedl-Bardet syndrome. Am J Surg. 1971;122:822–824. doi: 10.1016/0002-9610(71)90454-5. [DOI] [PubMed] [Google Scholar]
- 125.Kennedy B, Malicki J. What drives cell morphogenesis: a look inside the vertebrate photoreceptor. Dev Dyn. 2009;238:2115–2138. doi: 10.1002/dvdy.22010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Young RW. The renewal of photoreceptor cell outer segments. J Cell Biol. 1967;33:61–72. doi: 10.1083/jcb.33.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fath MA, Mullins RF, Searby C, Nishimura DY, Wei J, Rahmouni K, Davis RE, Tayeh MK, Andrews M, Yang B, Sigmund CD, Stone EM, Sheffield VC. Mkks-null mice have a phenotype resembling Bardet-Biedl syndrome. Hum Mol Genet. 2005;14:1109–1118. doi: 10.1093/hmg/ddi123. [DOI] [PubMed] [Google Scholar]
- 128.Pazour GJ, Baker SA, Deane JA, Cole DG, Dickert BL, Rosenbaum JL, Witman GB, Besharse JC. The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J Cell Biol. 2002;157:103–113. doi: 10.1083/jcb.200107108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Moritz OL, Tam BM, Hurd LL, Peranen J, Deretic D, Papermaster DS. Mutant rab8 impairs docking and fusion of rhodopsin-bearing post-Golgi membranes and causes cell death of transgenic Xenopus rods. Mol Biol Cell. 2001;12(8):2341–2351. doi: 10.1091/mbc.12.8.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gerdes JM, Davis EE, Katsanis N. The vertebrate primary cilium in development, homeostasis, and disease. Cell. 2009;137:32–45. doi: 10.1016/j.cell.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
(DOCX 18 kb)
(DOCX 26 kb)
(DOCX 28 kb)