Abstract
Although flowering time is often associated with plant size, little is known about how flowering time genes affect plant architecture. We grew four rice lines having different flowering time genotypes (hd1 ehd1, hd1 Ehd1, Hd1 ehd1 and Hd1 Ehd1) under distinct photoperiod conditions. By using genotype–treatment combinations that resulted in similar flowering times, we were able to compare the effects of flowering time genes on traits related to plant architecture. The results revealed that the combination of Heading-date 1 (Hd1) and Early heading date 1 (Ehd1) can reduce the number of primary branches in a panicle, resulting in smaller spikelet numbers per panicle; this occurs independently of the control of flowering time. In addition, expression of the Hd3a and Rice Flowering-locus T 1 (RFT1) florigen genes was up-regulated in leaves of the Hd1 Ehd1 line at the time of the floral transition. We further revealed that Hd1 and/or Ehd1 caused up-regulation of Terminal Flower 1-like genes and precocious expression of panicle formation-related genes at shoot apical meristems during panicle development. Therefore, two key flowering time genes, Hd1 and Ehd1, can control panicle development in rice; this may affect crop yields in the field through florigen expression in leaf.
Keywords: Florigen, Flowering time gene, Panicle development, Rice
Introduction
Recent molecular genetics studies have revealed a complicated gene network controlling flowering time in rice. An evolutionarily conserved key flowering time controller gene, Heading-date 1 (Hd1), which is an ortholog of Arabidopsis CONSTANS (CO), promotes floral transition (the transition from vegetative to reproductive growth) under short-day (SD) conditions and represses it strongly under long-day (LD) conditions (Yano et al. 2000, Izawa et al. 2003). In addition, a type-B response regulator unique to rice (or monocots), termed Early heading date 1 (Ehd1), promotes floral transition more strongly under SD than under LD conditions (Doi et al. 2004). Natural variation in another unique strong floral repressor gene, Grain number, plant height, and heading date 7 (Ghd7), which encodes a CCT-motif protein, contributes to adaptation of rice cultivars to cold climate regions (Xue et al. 2008). We have recently revealed that Ghd7 transcription is mediated through phytochrome signaling and is gated in a photoperiod-dependent manner. Ehd1 transcriptional induction by blue light is also gated; in this case, the gate for Ehd1 opens mainly in the morning, regardless of the photoperiod. The Ghd7 induction under LD conditions subsequently represses transcription of Ehd1 in the morning; this partly explains the critical daylength recognition of Heading date 3a (Hd3a; a rice florigen) transcriptional control (Itoh et al. 2010).
In studies of crop productivity, flowering time control is often associated with yield-related traits, because people believe that longer vegetative periods result in greater biomass. In fact, it has been reported that Ghd7 affects grain number in field tests (Xue et al. 2008). Pleiotropic effects of core flowering time genes have also been reported in Arabidopsis. CO is involved in determination of the timing of xylem expansion during the vegetative phases of Arabidopsis development (Sibout et al. 2008). Critical evaluations, however, have not been done yet on the effects of rice flowering time genes on the developmental mechanisms of plant architecture including panicle formation (morphogenesis) at the molecular level.
In this work, we developed four rice lines having distinct alleles for Hd1 and Ehd1, and we examined flowering time under various photoperiod conditions. With comparisons of panicle traits where the corresponding plants flowered with similar timing, we were able to evaluate the direct effects of Hd1 and Ehd1 on panicle formation (i.e. their effects independent of changes in flowering time). These results indicated that Hd1 and Ehd1 together control the number of primary branches in the panicle. Further analysis suggested that this control of panicle size by flowering time genes may be mediated by the levels of expression of the Hd3a and Rice Flowering-locus T 1 (RFT1) florigen genes (Kojima et al. 2002, Tamaki et al. 2007) in rice leaves at the time of floral transition.
Results
Flowering time phenotypes under various photoperiodic induction conditions
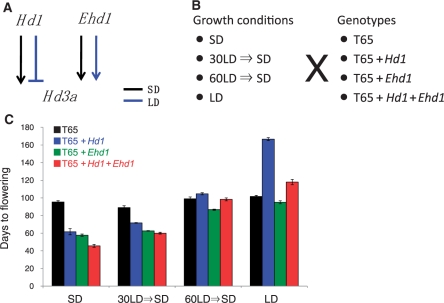
In a previous study, we transformed functional alleles of Hd1 and Ehd1 into the cultivar Taichung 65 (T65), which contains defective alleles of Hd1 and Ehd1 (Doi et al. 2004), and examined flowering time phenotypes. Both lines exhibited good complementation phenotypes. Here, we crossed the Hd1 and Ehd1 lines to produce a rice line having functional copies of both Hd1 and Ehd1. This resulted in four T65-related rice lines with distinct combinations of flowering time genes: hd1 ehd1 (T65), Hd1 ehd1 (T65 + Hd1), hd1 Ehd1 (T65 + Ehd1) and Hd1 Ehd1 (T65 + Hd1 + Ehd1) (Fig. 1). We grew these four lines to maturity under four distinct floral induction conditions: (i) SD conditions; (ii) SD conditions after 1 month of LD growth (30LD→SD); (iii) SD conditions after 2 months of LD growth (60LD→SD); and (iv) LD conditions (Fig. 1), and examined their flowering times and yield-related traits (Supplementary Table S1). We independently performed the same experiment four times to evaluate the reproducibility.
Fig. 1.
(A) Schematic summary of rice flowering time genes tested in this work. Hd1 promotes Hd3a (and RFT1) expression and flowering only under SD conditions, whereas Ehd1 promotes Hd3a (and RFT1) expression and flowering under both SD and LD conditions. (B) Experimental design. SD, short-day conditions (10 h light, 14 h dark); LD, long-day conditions (14.5 h light, 9.5 h dark); 30LD, 30 d under LD conditions; 60LD, 60 d under LD conditions. Each of the three plant materials was subjected to each of the four growth conditions. The experiments were repeated independently four times. (C) Days to heading of T65, T65 + Hd1, T65 + Ehd1 and T65 + Hd1 + Ehd1 under different daylength conditions (mean ± SE, n = 3). Data represent a single experiment. Similar results were obtained in three additional independent experiments.
Under SD conditions, either Hd1 or Ehd1 alone reduced days to flowering by >30 d compared with T65, and both Hd1 and Ehd1 together reduced days to flowering by >40 d compared with T65 (Fig. 1, Supplementary Table S1). This indicated the presence of additive effects between Hd1 and Ehd1 under SD conditions. Because it often takes about 30 d from the start of floral transition at the apex to heading, T65 + Hd1 + Ehd1 is presumed to undergo floral transition at about 20 d after germination.
Under 30LD→SD conditions, Hd1 and Ehd1 decreased days to flowering by approximately 15 and 20 d, respectively, compared with T65. The combination of Hd1 and Ehd1 caused slightly early flowering by <5 d compared with Ehd1 alone. Considering the timing of floral transitions, the floral transitions for T65 + Ehd1 and T65 + Hd1 + Ehd1 might occur just after the shift to SD conditions. Under 60LD→SD conditions, T65, T65 + Hd1 and T65 + Hd1 + Ehd1 flowered at similar times, whereas T65 + Ehd1 flowered about 15 d earlier than the others.
Under LD conditions, Hd1 alone delayed flowering by >70 d, and Ehd1 alone reduced flowering time slightly, compared with T65. When combined with Hd1 under LD, Ehd1 dramatically antagonized the floral repression by Hd1: T65 + Hd1 + Ehd1 flowered >40 d earlier than T65 + Hd1. These results were basically consistent with results reported previously (Doi et al. 2004). Across our experiments, flowering time varied from just after 40 d to >170 d after sowing. The flowering time of T65 did not vary much among the four growth conditions. In the first experiment of the four experiments, we moved T65 + Hd1 plants from LD to SD conditions at 150 d after sowing for floral induction (Fig. 1).
Effects of Hd1 and Ehd1 on tiller number
We also examined the maximum tiller number (stem number) and panicle number per plant of the same plants used for the flowering time experiments (Supplementary Fig. S1). In rice development, the tiller normally increases early in rice development after breaks of dormancy. However, some of the tillers are later retarded and do not undergo heading. Other tillers do not grow when dormancy is not broken. Therefore, panicle number is usually less than the maximum tiller number. Dynamic changes of rice tiller development were monitored (Supplementary Fig. S1A). The results clearly indicated that more tillers were produced during the early stages of rice development under the three LD conditions than under the SD conditions (Supplementary Fig. S1). Some reduction in the rate of new tiller production was observed after the shift to SD conditions in the 30LD→SD treatment (Supplementary Fig. S1A). Tiller numbers peaked at about 50–60 d after sowing, then started to decrease. Panicle numbers and tiller numbers exhibited similar changes across the four growth conditions (Supplementary Fig. S1B). Some flowering time gene effects were observed, but not so drastic (Supplementary Table S1). One of exceptions was the panicle and stem number of T65 + Hd1 plants grown under LD conditions. Under LD conditions, most T65 + Hd1 plants did not exhibit heading even 160 d after sowing, resulting in reduced panicle numbers.
Effects of Hd1 and Ehd1 on panicle development
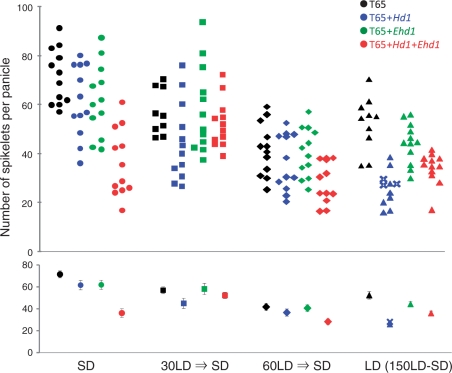
We next examined spikelet number per panicle of the same plants used for the flowering time experiments (Fig. 2). There were some clear differences observed among the four growth conditions. In general, we observed higher spikelet numbers under SD conditions than under the conditions involving an LD step (Fig. 2). The growth condition had some effect on panicle size: some reduction of spikelet number per panicle was observed in T65, T65 + Hd1 and T65 + Ehd1 under 30LD→SD, 60LD→SD, and LD conditions, compared with the same genotypes under SD conditions, though not all of the reductions were significant. Furthermore, among plants grown with the same growth conditions, we observed some significant differences among the four lines (Supplementary Table S1), indicating some effects of Hd1 and Ehd1 on the number of spikelets per panicle. As the control of flowering time by Hd1 and Ehd1 apparently affects accumulation of biomass and primary metabolism in rice plants, it would be of great interest to evaluate the effects of Hd1 and/or Ehd1 on panicle formation. However, such a simple comparison would not allow us to understand these effects fully because of lack of consideration of the variation in flowering time.
Fig. 2.
Number of spikelets per panicle. The upper panel in each part shows all data from four independent experiments (n = 9–12). The lower panel shows average values and standard errors (n = 9–12, except for T65 + Hd1 under 150LD→SD conditions, shown as ‘x’, for which n = 3). In T65 + Hd1 under LD, some plants did not flower. The statistical significance of these data is summarized in Supplementary Table S1.
We therefore decided to control the effects of variation of vegetative phase length by grouping the genotype–treatment combinations on the basis of flowering time (Fig. 3). Examples of possible combinations with similar flowering time in the series of experiments are: T65 (SD), T65 + Ehd1 (LD) and T65 (LD), which all flowered around 95 d after sowing (Fig. 3A, B, Supplementary Table S1); T65 + Hd1 (SD), T65 + Ehd1 (SD) and T65 + Hd1 + Ehd1 (SD), which all flowered about 55 d after sowing (Fig. 3C, D, Supplementary Table S1); and T65 (60LD→SD), T65 + Hd1 (60LD→SD) and T65 + Hd1 + Ehd1 (60LD→SD), which all flowered about 90 d after sowing (Fig. 3E, Supplementary Table S1).
Fig. 3.
Comparisons of spikelet number per panicle of plants that flowered with similar timing. Comparisons between (A) T65 and T65 + Hd1 (SD and LD); (B) T65 and T65 + Ehd1; (C) T65 + Hd1 and T65 + Hd1 + Ehd1; (D) T65 + Ehd1 and T65 + Hd1 + Ehd1; and (E) All four lines. The left-hand panels show all data from four independent experiments; the right-hand panels show mean values ± SE (n = 9–12). Circles are data from SD conditions. Triangles are data from LD conditions. Diamonds are data from 60LD→SD. Statisical significance (P < 0.01 t-test): T65 (SD) vs. T65 + Hd1 (LD), T65 + Hd1 (SD) vs. T65 + Hd1 + Ehd1 (SD), T65 + Ehd1 (SD) vs. T65 + Hd1 + Ehd1 (SD) For (E), refer to Supplementary Table S1.
Two lines among these combinations seemed to be appropriate for evaluation of the direct effects of Hd1 and/or Ehd1 on panicle formation of rice. As shown in re-summarized data for spikelet number per panicle (Fig. 3), spikelet number per panicle in T65 + Hd1 + Ehd1 (SD) was smaller than in either T65 + Hd1 (SD) or T65 + Ehd1 (SD) (significant by t-test at P < 0.01) (Fig. 3C, D), whereas spikelet number per panicle in T65 (SD) was comparable with that in both T65 + Hd1 (SD) and T65 + Ehd1 (SD) (Fig. 3A, B). The results suggested that both Hd1 and Ehd1 together can control panicle formation, especially under SD conditions, although neither Hd1 nor Ehd1 alone had a clear effect on spikelet number per panicle under SD conditions. In addition, spikelet number per panicle in T65 + Ehd1 (LD) was slightly less than that in T65 (LD) (although not significant by t-test) (Fig. 3B), suggesting that Ehd1 may control panicle formation under LD conditions. Similarly, spikelet number per panicle in T65 + Hd1 + Ehd1 (60LD→SD) was less than that in either T65 + Hd1 (60LD→SD) or T65 (60LD→SD) (Fig. 3E), even though all three had comparable flowering times. Conversely, the spikelet number per panicle in T65 + Ehd1 (60LD→SD) is similar to that of T65 + Hd1 and T65, even though T65 + Ehd1 (60LD→SD) flowered earlier than the other two (Figs. 1, 3E).
Regarding the relationship between panicle sizes and duration of vegetative growth, spikelet number per panicle in T65 + Hd1 (SD) and T65 + Ehd1 (SD) was greater than that in T65 + Hd1 (LD) and T65 + Ehd1 (LD), respectively, though they flowered later under LD than SD conditions (Figs. 1, 3A, C, D). These results clearly indicated that longer periods of vegetative growth did not always mean bigger panicle sizes. In addition, spikelet number per panicle in T65 (LD) was slightly less than that in T65 (SD) (significant by t-test at P < 0.01) (Fig. 3A). Since the amounts of primary acclimation (per day) by photosynthesis were very likely to be higher under LD than under SD conditions, the larger panicle sizes observed in T65 grown under SD conditions than in those grown under LD conditions may reflect some unknown photoperiodic response for panicle formation in rice.
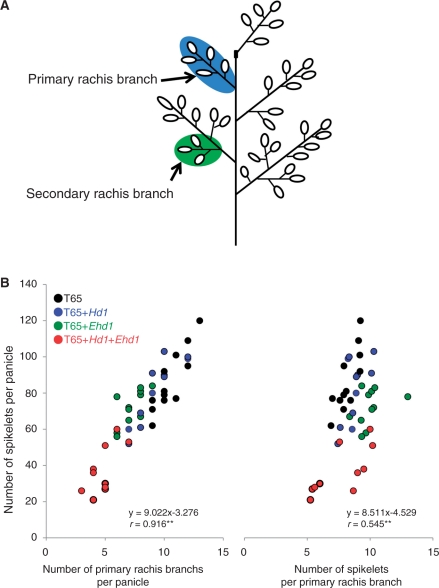
To evaluate further the effects of Hd1 and Ehd1 on panicle development, we next examined the panicle architecture of all four genotypes under SD conditions. The results clearly indicated that spikelet number per panicle was correlated with the number of primary rachis branches per panicle, whereas spikelet number per primary rachis branch was not significantly affected by the presence of Hd1 and/or Ehd1 (Fig. 4).
Fig. 4.
Changes of panicle development. (A) A scheme of the rice panicle. (B) Correlation between the number of spikelets per panicle and the number of primary rachis branches per panicle (left) and number of spikelets per primary rachis branch (right) under SD conditions. Data were collected from the main stem. **Correlation is significant at the 1% level.
Florigen gene expression in leaves at the time of floral transition
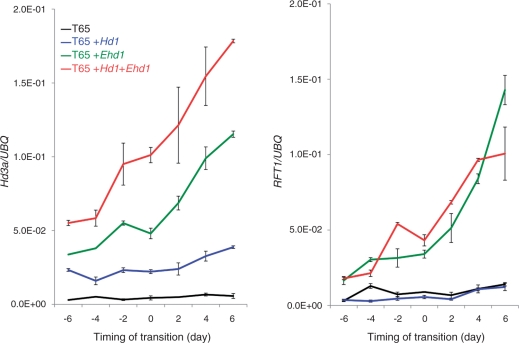
Both Hd1 and Ehd1 can control the expression of both Hd3a and RFT1 florigen genes in leaves (Izawa et al. 2002, Itoh et al. 2010). We therefore examined Hd3a and RFT1 mRNA expression in leaves of rice plants undergoing floral transitions under SD conditions. The timing of the floral transitions of the four lines was monitored by microscopic observation of shoot apical meristem (SAM) development of plants grown under the same growth conditions as the tested plants, and then the developmental stages of tested plants were estimated (see the Materials and Methods). Up-regulation of Hd3a in leaves was clearly observed in T65 + Hd1 + Ehd1, T65 + Hd1 and T65 + Ehd1 6 d before the floral transition compared with T65 (Fig. 5). The expression level of Hd3a gradually increased in all genotypes except T65 until 6 d after the floral transition. The expression levels of Hd3a were highest for T65 + Hd1 + Ehd, and expression levels of Hd3a in T65 + Ehd1 were higher than those in T65 + Hd1. The expression levels of Hd3a in T65 were the lowest, and the expression was not clearly induced upon floral transition. For RFT1, up-regulation was clearly observed in T65 + Hd1 + Ehd1 and T65 + Ehd1, whereas T65 + Hd1 and T65 showed little to no induction (Fig. 5). These changes in florigen mRNA amounts in leaves might reflect the levels of florigen protein at meristem regions undergoing floral transitions since the florigen protein is a mobile signal for the leaf to the apex (Tamaki et al. 2007).
Fig. 5.
Temporal expression patterns of Hd3a and RFT1 in mature leaves. Quantitative RT–PCR analyses of Hd3a (left panel) and RFT1 (right panel) in T65, T65 + Hd1, T65 + Ehd1 and T65 + Hd1 + Ehd1 under SD conditions. Timing of transition was determined by the microscopic observation of SAM parts of test samples for all sampling dates. Day 2 indicates the timing of the start of primary rachis differentiation. mRNAs were prepared from the corresponding leaf samples grown under SD conditions. The results are the mean ± SE (n = 3 individual plants). Three RT–PCRs were done for each cDNA sample from one plant. Leaf samples were collected 3 h after dawn from leaf blades. The graph shows the result of a single experiment (means ± SE, n = 3). Another experiment also gave similar results.
Related gene expression in SAMs at the time of floral transition
Temporal gene expression patterns of genes related to panicle development at the shoot apex were also examined in these rice lines. Total RNA was prepared from the shoot apex regions of the plants grown under SD conditions. In addition to Gn1a, LOG, APO1, RFL and OsSPL14 genes (Kyozuka et al, 1998, Ashikari et al. 2005, Ikeda et al. 2007, Kurakawa et al. 2007, Jiao et al. 2010, Miura et al. 2010), we examined the expression of OsMADS1/14/15, PAP2 (OsMADS34), RCN1 and RCN2 genes (Jeon et al. 2000, Kyozuka et al. 2000, Nakagawa et al. 2002, Kobayashi et al. 2010). These genes are reported to be involved in panicle formation in rice. In addition, it has been reported that OsMADS1/14/15 genes function genetically downstream of the Ehd1 gene (Doi et al. 2004). After the start of floral transitions in SAM regions, there were some trends of changes among the four tested lines in the expression of OsMADS1, 14, 15, PAP2, RCN1 and RCN2 genes (Fig. 6), but no such difference was detected in APO1, RFL Gn1a, LOG and OsSPL14 genes (data not shown). Here we observed some precocious gene expression of OsMADS1, 14, 15 and 34 in SAMs of the lines with lower grain number such as T65 + Hd1 + Ehd1, T65 + Hd1 and T65 + Ehd1. In addition, up-regulation of RCN1 and RCN2 in those lines with lower grain number by Hd1 and/or Ehd1 may be involved in the changes of panicle development since ectopic expression of RCN genes can affect the panicle size in rice (Nakagawa et al. 2002). Together with the panicle size effects (Fig. 3) and florigen expression in leaves (Fig. 4), these data have clearly indicated that Hd1 and/or Ehd1can control panicle formation independently of determination of timing of floral transition (or heading date). Note that we also confirmed the temporal development stages for mRNA samples based on microscopic observation using the corresponding test samples, like the expression analysis for Hd3a and RFT1 in rice leaves (Fig. 5).
Fig. 6.
Temporal gene expression patterns of rice panicle development-related genes in SAM regions during early stages of panicle development. Levels of mRNA accumulation were examined by quantitative RT–PCR. Timing of transition was determined by the microscopic observation of SAM parts of test samples for all sampling dates. Day 2 indicates the timing of the start of primary rachis differentiation. The results are the mean ± SE (n = 3 individual plants). Three RT–PCRs were done for each cDNA sample from one plant. mRNAs were prepared from the corresponding SAM samples grown under SD conditions. Similar data were obtained when normalized by number of SAMs for mRNA preparation.
Effects of ectopic expression of Hd3a on panicle size in rice
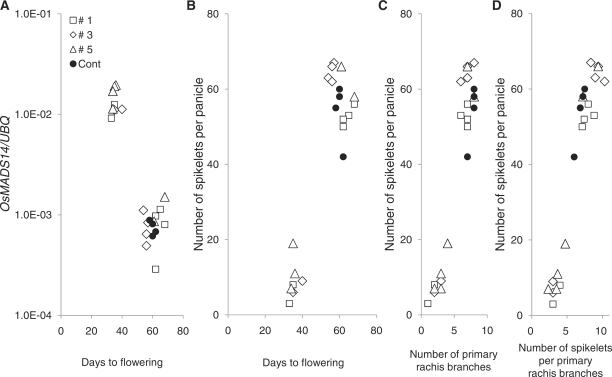
Because we found an association between reduced panicle size (i.e. reduced number of spikelets per panicle) and the increased expression of Hd3a and RFT1 among the four genotypes (Figs. 4, 5), we next examined the effect of ectopic Hd3a expression on panicle size (Fig. 7). For that purpose, we made transgenic Nipponbare (an Ehd1 Hd1 cultivar) rice lines containing Hd3a driven by the Oshsp16.9C heat-shock gene promoter (hereafter referred to as HSP) to promote flowering time. Unexpectedly, background expression from the HSP promoter was sufficient to induce early-flowering phenotypes even in the absence of heat shock, so we examined the panicle sizes of those early-flowering T1 plants. Note that we tried to increase Hd3a expression by heat-shock treatment upon floral transition, but we were not able to control it as planned. We instead examined the levels of OsMADS14 expression in leaves as a possible gene downstream of ectopic Hd3a expression (Fig. 7A). As expected, several plants exhibiting precocious OsMADS14 expression among T1 plants resulted in extremely early-flowering phenotypes (Fig. 7A). This result indicates that ectopic Hd3a expression induced the precocious OsMADS14 expression in rice leaves. Panicle size (i.e. spikelet number per panicle), primary rachis branch number per panicle and spikelet number per primary branch were drastically reduced in the presence of ectopic Hd3a expression (Fig. 7). There was rare secondary branch formation in these early-flowering plants (data not shown). This suggests a direct role for Hd3a in controlling panicle architecture, although the small meristem size of the early-flowering plants at the time of floral transition may affect the results.
Fig. 7.
Effects of ectopic Hd3a expression on days to flowering and panicle size traits under SD conditions. Segregating T1 plants from three independent lines were grown. Open symbols indicate the spikelet numbers on the main stem for transgenic plants [n = 2–4 main panicles (plants) per line]; filled circles indicate spikelet numbers on the main stem for the non-promoter control (in which only Hd3a cDNA without promoter was transformed) plants (n = 4 samples). (A) Correlation between ‘days to flowering’ and OsMADS14 expression in leaves. The ectopic Hd3a expression was able to induce endogenous OsMADS14 in rice leaves. (B) Correlation between ‘days to flowering’ and number of spikelets per panicle. (C) Correlation between number of spikelets per panicle and number of primary rachis branches. (D) Correlation between number of spikelets per panicle and number of spikelets per primary rachis branch. Data were collected only from main stems. Heat-shock treatment was not given. **Correlation is significant at the 1% level.
Relationship among biomass, grain number and flowering time
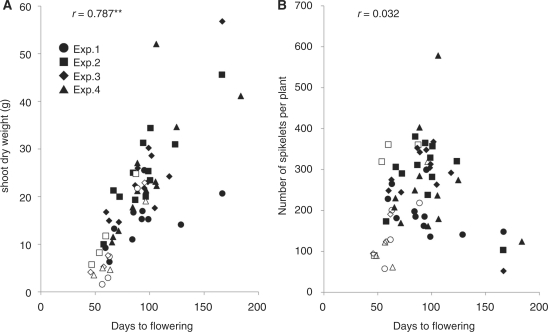
Control of biomass and grain (spikelet) numbers per plant is an important research target related to grain yield in rice. Therefore, we next examined the shoot dry weight and spikelet number for each plant in our experiments after harvesting, and plotted them to study the relationships between these characteristics and flowering time (Fig. 8). The results clearly showed a strong positive correlation between shoot dry weight and flowering time (Fig. 8A). This association was likely to reflect the carbon accumulation rate when the plants grew, though we were not able to detect clear differences in a related ratio, dry shoot weight/day to flowering (final amount of growth/duration of vegetative growth), between the SD treatment and the three other LD-related growth treatments (Fig. 8A). This finding may imply that plants grown under LD conditions spend more energy for housekeeping activities than those grown under SD conditions.
Fig. 8.
Relationship among biomass, grain number and flowering time. (A) Correlation between ‘days to flowering’ and dry weight of shoot. SD conditions (open symbols), 30LD→SD, 60LD→SD, 150LD→SD and LD conditions (filled symbols). (B) Correlation between days to flowering and number of spikelets per plant. Shoot dry weight excludes the weight of panicles. The panels in each part show all data from four independent experiments. **Correlation is significant at the 1% level.
In contrast to the results observed for shoot dry weight, there was no clear correlation between spikelet number per plant and flowering time (Fig. 8B). The main reason for these results is the relatively small panicles obtained from very late-flowering plants. In plants grown under these artificial conditions, very late-flowering phenotypes were often associated with ectopic stem elongation and relatively small meristem sizes before floral transition. In the normal development of rice, stem elongation starts just before floral transition at the SAM (Matsuo et al. 1990).
Discussion
Rice flowering time genes Hd1 and Ehd1 may control sink size through the control of florigen expression in leaves
We demonstrated that some key flowering time genes of rice, Hd1 and Ehd1, can affect spikelet number (grain number) per panicle, a trait that is deeply involved in crop yield potential, in some cases independently of the control of flowering time. Several rice genes have been reported to control grain number per panicle. For example, reduced activity of Gn1a genes, which encode a cytokinin oxidase, increases grain number per panicle (Ashikari et al. 2005). The gene Wealthy Farmer's Panicle (also called Ideal Panicle Architecture), which encodes an SPL (SQUAMOSA promoter-binding protein-like) transcription factor protein (OsSPL14) that is similar to Arabidopsis SPL genes, can also affect grain number per panicle (Jiao et al. 2010, Miura et al. 2010). Aberrant Panicle Organization 1, a rice F-box gene orthologous to Arabidopsis UFO, can control grain number of rice (Ikeda et al. 2007). The genetic network that controls grain number in rice panicles has not yet been elucidated, although it is possible to speculate which genetic networks are involved based on some evolutionary relationships in the control of inflorescence architecture between Arabidopsis and rice. Therefore, how the plant architecture of rice including panicle formation would be affected by changes in ambient environmental conditions is completely unknown. This means that we are not able to explain how environmental changes affect yield of plants at the molecular level. Most yield-related traits such as panicle size are often believed to be just developmental events controlled by complex genetic programs and somehow affected by the conditions of primary metabolites through photosynthesis. On the other hand, flowering time genes control the duration of vegetative growth of plants, resulting in a strong association with biomass and grain yield. However, how directly flowering time genes affect biomass, yield and plant architecture is still an open question. Since the expression of flowering time genes is controlled by both circadian clock and ambient light signals (Itoh et al. 2010), in addition to ambient temperature conditions, it is possible that ambient environmental conditions may control the inflorescence architecture of plants through the action of some flowering time genes. Indeed, the flowering time locus is often considered as a sort of yield-related quantitative trait locus (QTL) in crop breeding. For example, the rice Ghd7 gene was reported to be a yield-related gene that can control grain number per panicle in addition to its critical control of flowering time (Xue et al. 2008). This hypothesis has not been critically tested, however, because changes in flowering time are likely to affect plant sizes through changes (or accumulation) in primary metabolites. Therefore, it is difficult to evaluate the relationship between crop yield and flowering time in rice. In general, it is simply believed that a longer vegetative period increases the size of rice plants. Leaf primordia are produced periodically in the regions adjacent to the SAM until floral transition has occurred (Matsuo et al. 1990). An axial bud is formed next to each leaf primordium and often becomes dormant. Prolonged vegetative growth leads to more breaks in dormancy and produces more tillers. This increase in tiller number is often associated with greater panicle number, resulting in higher grain yields. Experienced rice breeders believe that the increase in tiller number is associated with a decrease in the number of grains in each panicle owing to a type of homeostasis. It is also likely that SAM size is affected by environmental conditions such as ambient temperature, light quantity, photoperiod and soil nutrient contents. Thus, variation in the timing of the floral transition itself may affect inflorescence architecture indirectly owing to the above effects. In order to evaluate the direct effects of flowering time genes on inflorescence formation, inflorescence-related traits should be compared among only those plants that exhibit similar flowering time phenotypes.
To satisfy these criteria as much as possible, we modified the growth conditions of rice plants by changing the photoperiod to produce variation in timing of floral induction (Fig. 1B). Under these conditions, and by using rice lines with different combinations of two flowering time genes, Hd1 and Ehd1, we succeeded in obtaining a range of flowering times from around 40 d to >170 d after sowing (Fig. 1, Supplementary Table S1). Using these materials, we examined the rates of tiller formation, panicle number, branch number per panicle and grain number (or spikelet number) per panicle (Figs. 2, 3; Supplementary Fig. S1). By grouping the data to allow comparisons among plants with similar flowering time phenotypes, we demonstrated that functional Hd1 and Ehd1 genes can decrease grain number per panicle, especially when combined, independently of the control of flowering time (Fig. 3). We further revealed that this decrease in grain number was due to a decrease in primary branch number per panicle (Fig. 4), suggesting developmental control rather than indirect metabolic effects. Interestingly, either Ehd1 or Hd1 can reduce flowering time significantly under SD conditions without an apparent decrease in grain number (Fig. 3A, B). The results imply that a certain combination of specific environmental conditions and some genetic backgrounds of flowering time genes may be required for rice plants to have a decrease in grain number per panicle. Therefore, our results imply that ambient environmental conditions can control panicle formation in rice through the action of Hd1 and Ehd1 flowering time genes. Therefore, this work can be one example to show that a plant can critically respond to changes in ambient environments through the flowering time genetic networks in order to control such yield-related traits as panicle formation.
Possible downstream processes by which Hd1 and Ehd1 affect panicle formation in rice
We also demonstrated that the increase in primary branch number per panicle induced by Hd1 and Ehd1 coincided with the level of Hd3a (and/or RFT1) expression in leaves at the time of floral transition (Fig. 5). This makes sense since we have demonstrated how Hd1 and Ehd1 control Hd3a and RFT1 genes in rice leaves (Izawa et al. 2002, Doi et al. 2004, Itoh et al. 2010). This is also consistent with previous results showing that ectopic FT-like expressions resulted in early-flowering phenotypes and small panicle sizes in transgenic rice plants (Izawa et al. 2002). In this study, we further confirmed that ectopic Hd3a expression resulted in early-flowering phenotypes and small panicles, although both primary branch number and floret (spikelet) number per primary branch were also decreased (Fig. 7). The high expression levels of Hd3a after (and/or before) floral transition in the transgenic plants might have led to extremely small panicle sizes, although the small SAMs of the early-flowering plants upon floral transition in this experiment may have had a partial effect. Our analysis of the related gene expression at the SAMs during panicle formation of the four lines (Fig. 6) implies that the induction of Hd3a/RFT1 by Hd1 and Ehd1 under SD conditions is a trigger for floral transitions, but may not be enough to start the floral transitions at the apex since Hd3a and RFT1 expression levels in leaves varied at the same developmental stage in panicle development of rice in our experiments (Fig. 5). This may generate some differences in protein levels of florigen (possibly at the apex) after the start of floral transition between the four lines and may cause precocious expression of some panicle formation-related genes such as OsMADS1/14/15/34 in T65 + Hd1 + Ehd1 and other lines (Fig. 7). This precocious expression of the MADS box genes was likely to be involved in the smaller size of panicles. For example, overexpression of OsMADS14 often resulted in flower-like organs when regenerated in culture plates (data not shown). We also observed an ectopic expression of RCN1 and RCN2 in SAM regions at the start of floral transition (Fig. 6). It has been reported that the Terminal Flower 1 gene, an Arabidopsis RCN1 and RCN2 ortholog, is a mobile signal to control plant architecture (Conti and Bradley 2007). Therefore, we have considered that the differential expression of RCN1 and 2 by Hd1/Ehd1 may also be involved in panicle development of rice. However, the numbers of primary branches in plants overexpressing RCN1 and 2 were not affected, although some increase in spikelet number per panicle was reported in rice (Nakagawa et al. 2002). Therefore, it is quite possible that we were not able to detect other target genes of Hd1/Ehd1 (otherwise, Hd3a/RFT1) in rice panicle development. It still remains to be determined whether this ectopic expression of RCN1 and RCN2 caused the changes of panicle sizes in our case since it is still unknown whether RCN1 and RCN2 genetically affect flowering time and panicle size in rice.
Crop yield, sink size and flowering time in rice
In rice breeding, both sink size (e.g. panicle size) and flowering time are important agronomic traits. Until now, it has been believed that these traits are independently controlled, although some secondary effects due to the duration of vegetative growth have been considered. We revealed here that genetic control of these two traits; flowering time and panicle size, may have more direct links. Our data further imply that ambient environmental conditions may partly control sink size through the action of the flowering time genes. The total accumulation of photosynthates is also an important factor controlling yields. The combinations of photosynthate accumulation and this flowering time control may interact in complex ways to determine the total sink size of a plant. In this work, we also demonstrated that, as expected, longer flowering times exhibited a strong correlation with greater dry weights, suggesting a possible increase in the total photosynthate accumulation during the vegetative period, resulting in more tillers and taller shoots (Fig. 8). This increase in biomass was, however, weakly correlated with an increase in spikelet (grain) number. Indeed, in the case of long flowering time, the total grain number per plant decreased although the biomass still increased (Fig. 8). Therefore, decoding of genetic programs for plant architecture formation and elucidation of control of plant architecture formation by ambient environmental changes will be a big challenge in plant biology in addition to understanding how to produce biomass efficiently by use of photosynthesis.
Materials and Methods
Plant materials
T65 + Hd1 and T65 + Ehd1 rice lines were described by Doi et al. (2004). T65 + Hd1 + Ehd1 was selected from the progeny of a cross between the T65 + Hd1 and the T65 + Ehd1 for this study.
Plant growth conditions
Seeds were imbibed in darkness (2 d at 30°C) and then sown in soil (5 d at 30°C). Seedlings were then transplanted into pots under SD (10 h of light/14 h of dark) or LD (14.5 h of light/9.5 h of dark) conditions. Some parts of plants were shifted from LD to SD conditions after 30, 60 or 150 d of LD treatment. Additional fertilizer (N : P : K = 14 : 14 : 14) was applied every 2 weeks (0.3 g was used for the first experiment, and 1.0 g for the second, third and fourth experiments). Metal halide lamps were used as the light source in the growth chambers. Photosynthetic photon flux density ranged from 450 to 500 μmol m−2 s−1. Flowering time was defined as the time when the first panicle emerged.
Plasmid construction
The 5′-flanking region of Oshsp16.9C (Guan et al. 2004), a rice ortholog of the heat-shock protein gene Gmhsp17.3-B, and Hd3a cDNA were amplified by PCR using the primers (restriction sites underlined) Hd3a-F-XbaI (5′-tctagaatggccggaagtgg-3′), Hd3a-R-KpnI (5′-ggtaccctagttgtagaccc-3′), Oshsp16.9C-F (5′-ggaagcttcagtgtaaagcagtgaattg-3′) and Oshsp16.C-R (5′-ggggatccagctcgatcaaatgcttcagt-3′). The resulting PCR products were cloned into the pCR 8/GW/TOPO vector (Invitrogen). These clones were sequenced to confirm that there was no nucleotide substitution. The Hd3a fragment was digested with XbaI and KpnI and then inserted into the pRiceFOX-based (Nakamura et al. 2007) binary vector pRiceFOX/GATE to generate pRiceFOX/GATE/Hd3a. Finally, the HSP promoter fragment was used in the LR reaction with the destination vector pRiceFOX/GATE/Hd3a to create a binary plasmid containing HSP::Hd3a. The LR reaction was performed as described in the manufacturer's manual (Invitrogen).
Plant transformation
The binary vector containing HSP:Hd3a was introduced into Agrobacterium tumefaciens strain EHA105 by electroporation. Plasmids were transformed into wild-type Nipponbare. Transgenic plants were selected on medium containing 50 mg l−1 hygromycin. Hygromycin-resistant plants were transplanted into soil and grown as described above.
mRNA sampling and quantitative RT–PCR analysis of gene expression
A series of plants were grown under SD conditions. A few shoot apices of plants for test sampling were observed for each plant line using microscopy to estimate the developmental stages every 2 d when leaves and SAMs are collected for RNA preparation. Total RNA was extracted by using an RNeasy Plant Mini Kit (Qiagen) in accordance with the manufacturer's instructions. The reverse transcription reaction was performed with SuperScript II reverse transcriptase (Invitrogen), oligo(dT)12–18 (Invitrogen) and 3 μg of total RNA in accordance with the manufacturer's instructions. Real-time quantitative reverse transcription–PCR (RT–PCR) analysis was performed as described previously. Briefly, quantitative RT–PCR was performed by the Taq-Man or MESA-Green PCR method using an ABI PRISM 7900 Sequence Detection System in accordance with the manufacturer's instructions. Primer and probe sequence information for the quantitative RT–PCR is listed in Supplementary Table S2.
Supplementary data
Supplementary data are available at PCP online.
Funding
This work was supported by the Ministry of Economy, Trade and Industry (METI) of Japan [a grant termed ‘Development of Fundamental Technologies for Production of High-value Materials using Transgenic Plants (2006–2010) project’]; the Ministry of Agriculture, Forestry, and Fisheries of Japan [Genomics of Agricultural Innovation grant GPN-0001].
Supplementary Material
Acknowledgments
We thank Sungshin Lee, Reiko Matsuzaki and Mieko Tomiyama for helping to grow rice plants and measure traits; and Hiroaki Ichikawa for providing pRice FOX binary vector.
Glossary
Abbreviations
- CO
CONSTANS
- Ehd1
Early heading date 1
- Ghd7
Grain number, plant height, and heading date 7
- LD
long day
- Hd1
Heading-date 1
- RFT1
Rice Flowering-locus T1
- RT–PCR
reverse transcription–PCR
- SAM
shoot apical meristem
- SD
short day
- T65
Taichung 65.
References
- Ashikari M, Sakakibara H, Lin S, Yamamoto T, Takashi T, Nishimura A, et al. Cytokinin oxidase regulates rice grain production. Science. 2005;309:741–745. doi: 10.1126/science.1113373. [DOI] [PubMed] [Google Scholar]
- Conti L, Bradley D. TERMINAL FLOWER1 is a mobile signal controlling Arabidopsis architecture. Plant Cell. 2007;19:767–778. doi: 10.1105/tpc.106.049767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi K, Izawa T, Fuse T, Yamanouchi U, Kubo T, Shimatani Z, et al. Ehd1, a B-type response regulator in rice, confers short-day promotion of flowering and controls FT-like gene expression independently of Hd1. Genes Dev. 2004;18:926–936. doi: 10.1101/gad.1189604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan JC, Jinn TL, Yeh CH, Feng SP, Chen YM, Lin CY. Characterization of the genomic structures and selective expression profiles of nine class I small heat shock protein genes clustered on two chromosomes in rice (Oryza sativa L.) Plant Mol. Biol. 2004;56:795–809. doi: 10.1007/s11103-004-5182-z. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Ito M, Nagasawa N, Kyozuka J, Nagato Y. Rice ABERRANT PANICLE ORGANIZATION 1, encoding an F-box protein, regulates meristem fate. Plant J. 2007;51:1030–1040. doi: 10.1111/j.1365-313X.2007.03200.x. [DOI] [PubMed] [Google Scholar]
- Itoh H, Nonoue Y, Yano M, Izawa T. A pair of floral regulators sets critical day length for Hd3a florigen expression in rice. Nat. Genet. 2010;42:635–638. doi: 10.1038/ng.606. [DOI] [PubMed] [Google Scholar]
- Izawa T, Oikawa T, Sugiyama N, Tanisaka T, Yano M, Shimamoto K. Phytochrome mediates the external light signal to repress FT orthologs in photoperiodic flowering of rice. Genes Dev. 2002;16:2006–2020. doi: 10.1101/gad.999202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa T, Takahashi Y, Yano M. Comparative biology comes into bloom: genomic and genetic comparison of flowering pathways in rice and Arabidopsis. Curr. Opin. Plant Biol. 2003;6:113–120. doi: 10.1016/s1369-5266(03)00014-1. [DOI] [PubMed] [Google Scholar]
- Jeon JS, Jang S, Lee S, Nam J, Kim C, Lee SH, et al. leafy hull sterile1 is a homeotic mutation in a rice MADS box gene affecting rice flower development. Plant Cell. 2000;12:871–884. doi: 10.1105/tpc.12.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Wang Y, Xue D, Wang J, Yan M, Liu G, et al. Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nat. Genet. 2010;42:541–544. doi: 10.1038/ng.591. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Maekawa M, Miyao A, Hirochika H, Kyozuka J. PANICLE PHYTOMER2 (PAP2), encoding a SEPALLATA subfamily MADS-box protein, positively controls spikelet meristem identity in rice. Plant Cell Physiol. 2010;51:47–57. doi: 10.1093/pcp/pcp166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Takahashi Y, Kobayashi Y, Monna L, Sasaki T, Araki T, et al. Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short-day conditions. Plant Cell Physiol. 2002;43:1096–1105. doi: 10.1093/pcp/pcf156. [DOI] [PubMed] [Google Scholar]
- Kurakawa T, Ueda N, Maekawa M, Kobayashi K, Kojima M, Nagato Y, et al. Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature. 2007;445:652–655. doi: 10.1038/nature05504. [DOI] [PubMed] [Google Scholar]
- Kyozuka J, Konishi S, Nemoto K, Izawa T, Shimamoto K. Down-regulation of RFL, the FLO/LFY homolog of rice, accompanied with panicle branch initiation. Proc. Natl Acad. Sci. USA. 1998;95:1979–1982. doi: 10.1073/pnas.95.5.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyozuka J, Kobayashi T, Morita M, Shimamoto K. Spatially and temporally regulated expression of rice MADS box genes with similarity to Arabidopsis class A, B and C genes. Plant Cell Physiol. 2000;41:710–718. doi: 10.1093/pcp/41.6.710. [DOI] [PubMed] [Google Scholar]
- Matsuo T, Ishii R, Hirata H, Kumazawa K, Ishihara K. Science of the Rice Plant. Tokyo: Rural Culture Association; 1990. [Google Scholar]
- Miura K, Ikeda M, Matsubara A, Song XJ, Ito M, Asano K, et al. OsSPL14 promotes panicle branching and higher grain productivity in rice. Nat. Genet. 2010;42:545–549. doi: 10.1038/ng.592. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Shimamoto K, Kyozuka J. Overexpression of RCN1 and RCN2, rice TERMINAL FLOWER 1/CENTRORADIALIS homologs, confers delay of phase transition and altered panicle morphology in rice. Plant J. 2002;29:743–750. doi: 10.1046/j.1365-313x.2002.01255.x. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Hakata M, Amano K, Miyao A, Toki N, Kajikawa M, et al. A genome-wide gain-of-function analysis of rice genes using the FOX-hunting system. Plant Mol. Biol. 2007;65:357–371. doi: 10.1007/s11103-007-9243-y. [DOI] [PubMed] [Google Scholar]
- Sibout R, Plantegenet S, Hardtke CS. Flowering as a condition for xylem expansion in Arabidopsis hypocotyl and root. Curr. Biol. 2008;18:458–463. doi: 10.1016/j.cub.2008.02.070. [DOI] [PubMed] [Google Scholar]
- Tamaki S, Matsuo S, Wong HL, Yokoi S, Shimamoto K. Hd3a protein is a mobile flowering signal in rice. Science. 2007;316:1033–1036. doi: 10.1126/science.1141753. [DOI] [PubMed] [Google Scholar]
- Xue W, Xing Y, Weng X, Zhao Y, Tang W, Wang L, et al. Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat. Genet. 2008;40:761–767. doi: 10.1038/ng.143. [DOI] [PubMed] [Google Scholar]
- Yano M, Katayose Y, Ashikari M, Yamanouchi U, Monna L, Fuse T, et al. Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell. 2000;12:2473–2484. doi: 10.1105/tpc.12.12.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.