Abstract
Diarrhea is one of the main drawbacks for cancer patients. Possible etiologies could be radiotherapy, chemotherapeutic agents, decreased physical performance, graft versus host disease and infections. Chemotherapy-induced diarrhea (CID) is a common problem, especially in patients with advanced cancer. The incidence of CID has been reported to be as high as 50–80% of treated patients (≥30% CTC grade 3–5), especially with 5-fluorouracil bolus or some combination therapies of irinotecan and fluoropyrimidines (IFL, XELIRI). Regardless of the molecular targeted approach of tyrosine kinase inhibitors and antibodies, diarrhea is a common side effect in up to 60% of patients with up to 10% having severe diarrhea. Furthermore, the underlying pathophysiology is still under investigation. Despite the number of clinical trials evaluating therapeutic or prophylactic measures in CID, there are just three drugs recommended in current guidelines: loperamide, deodorized tincture of opium and octreotide. Newer strategies and more effective agents are being developed to reduce the morbidity and mortality associated with CID. Recent research focusing on the prophylactic use of antibiotics, budesonide, probiotics or activated charcoal still have to define the role of these drugs in the routine clinical setting. Whereas therapeutic management and clinical work-up of patients presenting with diarrhea after chemotherapy are rather well defined, prediction and prevention of CID is an evolving field. Current research focuses on establishing predictive factors for CID like uridine diphosphate glucuronosyltransferase-1A1 polymorphisms for irinotecan or dihydropyrimidine-dehydrogenase insufficiency for fluoropyrimidines.
Keywords: chemotherapy-induced diarrhea, frequency, irinotecan, loperamide, octreotide, pathophysiology, prevention management
Introduction
In oncological patients, diarrhea can occur in several different situations. Possible etiologies could be radiotherapy, chemotherapeutic agents, decreased physical performance, graft versus host disease and infections. Careful analysis of the causative agent can lead to a more accurate management and early intervention possibly helps to prevent severe complications that may be irreversible [Davila and Bresalier, 2008; Vincenzi et al. 2008]. In particular, chemotherapy-induced diarrhea (CID) is a common problem in patients with advanced cancer and has to be carefully differentiated from other causes of diarrhea [Gibson and Stringer, 2009].
Chemotherapy-induced diarrhea
CID can occur in 50–80% of patients depending on the chemotherapy regimen [Benson et al. 2004; Gibson and Stringer, 2009]. A review of early toxic deaths occurring in two National Cancer Institute-sponsored cooperative group trials of irinotecan plus high-dose fluorouracil and leucovorin for advanced colorectal cancer has led to the recognition of a life-threatening gastrointestinal syndrome and highlighted the need for vigilant monitoring and aggressive therapy for this serious complication [Conti et al. 1996; Arbuckle et al. 2000; Saltz et al. 2000]. CID can cause depletion of fluids and electrolytes, malnutrition, dehydration and hospitalization, all of which can lead to cardiovascular compromise and death. In addition, diarrhea can interfere with and detract from cancer treatment by causing dosing delays or reductions which may have an impact on survival [Engelking et al. 1998; Ippoliti, 1998]. Therapeutic agents commonly causing diarrhea include 5-fluorouracil (5-FU), capecitabine and irinotecan (CPT-11) [Benson et al. 2004; Keefe et al. 2004]. Usually it is a dose-related adverse effect and may be associated with other features of toxicity. CID appears to be a multifactorial process whereby acute damage to the intestinal mucosa (including loss of intestinal epithelium, superficial necrosis and inflammation of the bowel wall) causes an imbalance between absorption and secretion in the small bowel [Keefe et al. 2000; Keefe, 2007; Gibson and Stringer, 2009].
Frequency
The frequency of CID depends on the drug and schedule, with the highest rate of diarrhea occurring with weekly irinotecan and bolus 5-FU (Table 1). Late diarrhea from irinotecan occurs at all dose levels, whereas early-onset diarrhea (≤24 hours after administration) is dose dependent, developing in up to 10% of patients (grade 3/4). The median time to onset of late diarrhea is about 6 days with the 350 mg/m2 every 3 weeks schedule and 11 days with the weekly schedule (125 mg/m2).
Table 1.
Rates of grade 3/4 diarrhea (CTC grades) for different therapeutic agents and combinations.
| Agent | Grade 3/4 diarrhea | |
|---|---|---|
| Chemotherapy | Single agent | Combination therapy |
| 5-FU (bolus) | 32% (G3) | 26% XELIRI |
| 5-FU (CI) | 6–13% | 25–28% IFL (bolus) |
| irinotecan (late diarrhea) | 16–22% | 11–14% FOLFIRI (bolus/CI) |
| capecitabine | 11% | |
| docetaxel/paclitaxel | 4% | 14% docetaxel + capecitabine 19% DCF |
| Targeted agents | ||
| anti-EGFR-antibodies | 1–2% | 15% cetuximab + FOLFIRI |
| anti-EGFR-TKI | 6–9% | 13% lapatinib + capecitabine 15% lapatinib + paclitaxel 6% erlotinib + gemcitabine |
| sorafenib/sunitinib | 2–8% (G3) | |
| m-TOR inhibitors | 1–4% (G3) | |
5-FU, 5-fluorouracil; DCF, docetaxel + cisplatin + 5-FU; EGFR, epidermal growth factor receptor; FOLFIRI, irinotecan + leucovorin + 5-FU; IFL, irinotecan + leucovorin + 5-FU; m-TOR, mammalian target of rapamycin; TKI, tyrosine kinase inhibitor; XELIRI, irinotecan + capecitabine.
Fluoropyrimidines have also been associated with severe diarrhea. Both the therapeutic efficacy and frequency of diarrhea associated with 5-FU are increased when given with leucovorin (LV).
Clinical manifestations and evaluation
CID can be debilitating and, in some cases, life threatening. Findings in such patients include volume depletion, renal failure, and electrolyte disorders such as metabolic acidosis and depending upon water intake, hyponatremia (increased water intake that cannot be excreted because of the hypovolemic stimulus to the release of antidiuretic hormone) or hypernatremia (insufficient water intake to replace losses) [Benson et al. 2004; Maroun et al. 2007].
Diagnosis of CID begins with a history to determine the severity according to the NCI CTC grades (recently updated National Cancer Institute Common Toxicity Criteria, Table 2). The volume and duration of diarrhea should also be determined, and the history should include questions concerning foods or drugs that might play a contributory role.
Table 2.
Common Toxicity Criteria (version 3.0 and 4.02) for diarrhea, adapted from the National Cancer Institute.
| Grade |
|||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Diarrhea (version 3.0) | Increase of <4 stools per day over baseline; mild increase in ostomy output compared to baseline | Increase of 4–6 stools per day over baseline; iv fluids indicated <24 hrs; moderate increase in ostomy output compared to baseline; not interfering with ADL | Increase of ≥7 stools per day over baseline; incontinence; iv fluids ≥24 hrs; hospitalization; severe increase in ostomy output compared to baseline; inter-fering with ADL | Life-threatening consequences (eg hemodynamic collapse) | Death |
| Diarrhea (version 4.02) | Increase of <4 stools per day over baseline; mild increase in ostomy output compared to baseline | Increase of 4–6 stools per day over baseline; moderate increase in ostomy output compared to baseline | Increase of ≥7 stools per day over baseline; incontinence; hospitalization indicated; severe increase in ostomy output compared to baseline; limiting self care ADL | Life-threatening consequences; urgent intervention indicated | Death |
ADL, activities of daily living; iv, intravenous.
It should also be considered that other factors can contribute to diarrhea in cancer patients treated with 5-FU or irinotecan. These include intestinal infection (e.g. Clostridium difficile), radiation, and a history of prior intestinal resection [Davila and Bresalier, 2008; Vincenzi et al. 2008].
Irinotecan-induced diarrhea
Irinotecan is frequently used in first- and second-line treatment of metastatic colorectal cancer [Saltz et al. 2000, 2007; Hurwitz et al. 2004; Jordan et al. 2004; Van Cutsem et al. 2009]. Regardless of its schedule of administration, myelosuppression and delayed-type diarrhea are the most common side effects [Davila and Bresalier, 2008].
Irinotecan can cause acute diarrhea (immediately after drug administration) or delayed diarrhea. Immediate-onset diarrhea is caused by acute cholinergic properties and is often accompanied by other symptoms of cholinergic excess, including abdominal cramping, rhinitis, lacrimation, and salivation. The mean duration of symptoms is 30 minutes and they usually respond rapidly to atropine. Delayed-type diarrhea is defined as diarrhea occurring more than 24 hours after administration of irinotecan and is noncumulative and occurs at all dose levels.
Main clinical predictive factors for irinotecan-related diarrhea are weekly administration, poor performance status, high serum creatinine levels, prior abdominopelvic irradiation, low leukocyte counts, age over 70 years, Gilbert syndrome and Crigler-Najjar syndrome type 1 [Vincenzi et al. 2008].
Pathophysiology of irinotecan-induced diarrhea
Irinotecan is converted by hepatic and peripheral carboxylesterase to its active metabolite 7-ethyl-10-hydroxycamptothecin (SN38), which is subsequently glucuronidated by hepatic uridine diphosphate glucuronosyltransferase-1A1 (UDP-GT 1A1) to SN38-glucuronide (SN38G) as depicted in Figure 1 [Voigt et al. 1998; Gibson and Stringer, 2009].
Figure 1.
Metabolism of irinotecan. UGT, UDP glucuronosyltransferase; SN-38, 7-ethyl-10-hydroxycamptothecin;, CYP, cytochrome P450;, CES carboxylesterases; APC, 7-ethyl-10-[4-N-(5-aminopentanoic acid)-1-piperidino]carbonyloxycamptothecin; NPC, 7-ethyl-10-(4-amino-1-piperidino)carbonyloxy-camptothecin; M, oxidized metabolite; ABCB/C, ATP-binding cassette, sub-family B/C.
Both SN-38 and SN-38G are excreted via urine and bile. Mass balance studies using 14carbon–labelled irinotecan have demonstrated that the fecal route of excretion is the major route eliminating 63.7% of the administered drug. SN38G, once in the intestinal lumen, is deconjugated by bacterial ß-glucuronidase to SN38. In feces, SN38 was found to be in excess relative to SN38G, which is suggestive of substantial ß-glucuronidase activity in the human intestinal contents [Saliba et al. 1998; Stringer et al. 2008].
The free intestinal luminal SN38, either from bile or SN38G deconjugation, is responsible for irinotecan-induced diarrhea. The different mechanisms in detail by which the free SN38 induces diarrhea are still a matter of debate [Stringer et al. 2007].
In summary the different mechanisms are discussed as follows:
Free intestinal luminal SN38 induces direct mucosal damage with water and electrolyte malabsorption and mucous hypersecretion in rats [Takasuna et al. 1995].
The luminal environment is altered by irinotecan and as a result may favor different genera of bacteria, allowing them to proliferate. The bacterial ß-glucuronidase then deconjugates SN38G to the active form SN38 at an increased rate causing significant damage and diarrhea. [Takasuna et al. 1998; Stringer et al. 2007, 2008].
The distribution and severity of histological damage within the rat intestine after administration of irinotecan has been correlated to the luminal ß-glucuronidase activity in rodents [Takasuna et al. 1998; Fittkau et al. 2004].
Irinotecan causes severe colonic damage (increased apoptosis, crypt hypoplasia and dilation) with accompanying excessive mucous secretion, as well as the usual chemotherapy-induced small intestinal damage, like villous atrophy and crypt hypoplasia. Increased levels of cell apoptosis combined with the histopathological changes in both the jejunum and colon and the changes in goblet cell numbers may cause changes in absorption rates, possibly leading to diarrhea [Gibson et al. 2003].
Irinotecan causes an increase in mucin secretion, accompanied by a significant decrease of mucin expression in the jejunum and colon of rats shown by immunohistochemistry for Muc2 and Muc4. Therefore the increase of mucin secretion is likely to be related to altered mucine gene expression, and may contribute to diarrhea induced by irinotecan [Stringer et al. 2009].
Molecular factors predictive of irinotecan-induced toxicities
Considering the complex metabolism of irinotecan (Figure 1) there are a couple of molecular factors that are potentially predictive for toxicities. There is no established factor for the prediction of irinotecan-induced diarrhea yet. However, pharmacogenomic research revealed a predictive factor for hematologic toxicities, the UGT isoform, UDP-glucuronosyltransferase, or UGT1A1. UGT1A1 was one of the first factors to be investigated, due to the observation of severe toxicities in patients with inherited disorders characterized by decreased bilirubin glucuronidation like Gilbert’s syndrome (i.e. mild unconjugated hyperbilirubinemia) [Wasserman et al. 1997a]. Patients with homozygosity for UGT1A1*28 allele have lower UGT1A1 expression, a decreased SN-38 glucuronidation and therefore a higher risk for developing severe irinotecan toxicities. [Iyer et al. 2002; Innocenti et al. 2004]. Regarding a frequency of around 9% for the homozygous allele, every tenth patient has an enhanced risk for hematological toxicities, leading to the approval of a genotyping method by the US Food and Drug Administration in 2005. [Hoskins et al. 2007; Kim et al. 2007]. A recommendation for an upfront dose reduction in this group of patients was added to the irinotecan package insert. However, recent studies reveal varying results regarding the need for dose reductions during irinotecan treatment in case of UGT1A1*28 genotype. In a retrospective analysis of the Dutch CAIRO trial, a reduced performance status and not the UGT1A1*28 genotype was a predictor for febrile neutropenia in a bivariate analysis [Kweekel et al. 2008; Liu et al. 2008].
Recently, the role of other UGT1A1 polymorphisms and genetic variations of SLCO1B1 and ABC-transporters were investigated, with the latter playing a pivotal role in the excretion of SN-38 in the active form into the blood and in the glucuronidated form into the bile (Figure 1) [De Jong et al. 2007; Han et al. 2009; Innocenti et al. 2009]. The variability of the ABCC2 gene seems to be a determinant for irinotecan induced diarrhea. However, the clinical relevance of these factors still has to be determined. Further research to establish predictive factors for daily practice is absolutely essential.
Fluoropyrimidines (5-FU, capecitabine, tegafur/uracil)
The severity and prevalence of diarrhea caused by 5-FU treatment is increased by the addition of leucovorin (LV) to the treatment regimen. Diarrhea is reported in up to 50% of patients receiving weekly 5-FU/LV combined treatment. Moreover, the severity of the diarrhea can increase when 5-FU is administered by bolus injection as opposed to intravenous infusion [Vincenzi et al. 2008]. Clinical factors predictive for fluoropyrimidine-induced diarrhea are female sex, caucasian race and presence of diabetes [Zalcberg et al. 1998; McCollum et al. 2002; Meyerhardt et al. 2004]. The gender- and race-related differences are possibly influenced by the variable activity of dihydropyrimidine-dehydrogenase (DPD) [Mattison et al. 2006a]. The leading polymorphism, which accounts for nearly 50% of nonfunctional alleles, is the DPYD*2A, resulting in a decreased drug clearance and prolonged exposure with severe toxicities. Complete DPD deficiency is extremely rare, but a partial deficiency is present in 3–5% of all cancer patients. DPD activity can be evaluated by peripheral blood mononuclear cell radioassay, DPD radioassaygenotyping of DPYD gene by denaturing high performance liquid chromatography (DHPLC), or 2-13C uracil breath test (UraBT). The current genotyping strategies are not yet available for routine use [Yen and McLeod, 2007]. Potentially, the simple breath test (UraBT) could be used as a screening tool [Mattison et al. 2006b].
Of further predictive value are polymorphisms of the thymidilate synthase (TS) and methylenetetrahydrofolate reductase (MTHFR) genes. However, taking into account the multifactorial nature of fluoropyrimidine induced diarrhea, in daily practice genotyping for DPD will be initiated after occurrence of unusual toxicity.
Pathophysiology of fluoropyrimidine-induced diarrhea
Although 5-FU is routinely used in the treatment of cancer and is known to cause diarrhea, very few basic research papers have attempted to elucidate the mechanisms underlying the pathophysiology. Early investigations revealed 5-FU being the causative agent for mitotic arrest of intestinal crypt cells, decrease of the relative fraction of villous enterocytes and the surface area for resorption [Siber et al. 1980]. Further research focused on different dose schedules of this cytotoxic agent using 5-FU in animal models [Cao et al. 1998].
Incidence of diarrhea with molecularly targeted agents
Epidermal growth factor receptor-targeted therapies
The rate of severe diarrhea (grade 3/4) with epidermal growth factor receptor (EGFR) targeting therapies is less than 10%. For monoclonal antibodies (mAb), such as the chimeric IgG1 mAb cetuximab or the fully human IgG2 mAb panitumumab, rates of grade 2 diarrhea are up to 21% and for grade 3 (ie greater than 7 stools per day or requiring intravenous fluids) between 1 and 2% [Van Cutsem et al. 2007; Davila and Bresalier, 2008; Vincenzi et al. 2008]. Diarrhea is more common in patients receiving small molecule EGFR tyrosine kinase inhibitors (TKIs), such as erlotinib, gefitinib or lapatinib. Occurrence of diarrhea is up to 60% for all grades. Grade 3 diarrhea develops in about 6–9%. However, dose reduction due to EGFR-targeting therapy induced diarrhea is seldom necessary. In combination with radiotherapy diarrhea could be a more serious problem for EGFR-targeting drugs.
Multitargeting tyrosine kinase inhibitors
Sorafenib and sunitinib cause diarrhea in 30–50% of patients (all grades) with a rate of less than 10% of grade 3 diarrhea [Llovet et al. 2008; Gore et al. 2009; Motzer et al. 2009]. Imatinib, an inhibitor of the Bcr-Abl protein tyrosine kinase, causes diarrhea in about 30% of the patients, but severe diarrhea is also rare.
m-TOR inhibitors
Everolimus and temsirolimus (inhibitors of the mammalian target of rapamycin [m-TOR]) were both recently approved for treatment of renal cell cancer, causing diarrhea in up to 40% with a rate of severe diarrhea in less than 5% of patients [Hudes et al. 2007; Motzer et al. 2008; Hess et al. 2009].
Pathophysiology of molecularly targeted agent-induced diarrhea
The mechanisms of targeted agent-induced diarrhea are not adequately investigated yet. The antitumor activity is based on apoptosis induction, antiangiogenesis and tyrosine kinase inhibition by targeting receptors or signaling pathways that are present in normal cells as well, including the mucosa. Increased levels of EGFR are found in inflamed mucosa, particulary in goblet cells, which seem to play a role in CID [Threadgill et al. 1995]. However, there was no increase in toxicity of head and neck radiation by addition of cetuximab in a phase III trial despite a possible correlation between EGFR targeting and maturation of squamous eptithelium of the tongue and nasal cavity [Bonner et al. 2006; Keefe and Gibson, 2007]. The high expression of Kit in the interstitial cells of Cajal, which function as pacemaker cells of the intestinal motility, might be a potential mechanism for diarrhea induced by imatinib or sunitinib [Deininger et al. 2003].
Regarding the increasing utilization of targeted therapies further research to gain the ability to prevent diarrhea is urgently warranted [Keefe and Anthony, 2008].
Therapeutic approaches
The treatment of CID includes nonpharmacologic and pharmacologic interventions to control diarrhea and careful serial evaluation to rule out significant volume depletion or comorbidities that would require specific intervention or hospitalization [Benson et al. 2004; Maroun et al. 2007]. Initial nonpharmacologic measures include avoidance of foods that would aggravate the diarrhea and aggressive oral rehydration with fluids that contain water, salt, and sugar [Dupont, 1997]. These principles are similar to those used for infectious diarrhea.
Given the lack of predictability for CID, significant effort has been made to evaluate prophylactic and therapeutic measures to reduce its severity. A broad variety of drugs have been tested for those measures.
Prophylactic measures
Antibiotics
Based on the assumption that bacterial ß-glucuronidase in the intestine is essential for activating SN-38G which plays a crucial role in the development of irinotecan-induced mucosal damage, the eradication of bacteria with marginally absorbable antibiotics, like neomycin, seems to be an interesting approach [Kehrer et al. 2001]. Despite promising results in a small series for secondary prophylaxis [Schmittel et al. 2004], a recent randomized phase II study displayed only a nonsignificant reduction of grade 3 diarrhea from 32.4 to 17.9% [De Jong et al. 2006]. In contrast, in a further nonrandomized study with 51 patients using levofloxacin just one patient experienced grade 3 diarrhea with no grade 4 at all [Flieger et al. 2007]. Regarding these controversial results, the role of antibiotics in prevention of irinotecan-induced diarrhea has to be further investigated.
Budesonide
Pathophysiologically, the reduction of inflammation in the bowel could possibly reduce the occurrence of diarrhea. The published data however, only reveal a trend towards a reduction of CID from 4.2 to 1.8 days in a randomized phase II study when concomitantly loperamide was used [Karthaus et al. 2005]. In contrast, in loperamide-refractory patients the CID grade could be reduced in more than half of the patients treated with either irinotecan or 5-FU in a small case series [Lenfers et al. 1999]. Larger studies are necessary to determine the actual role of budesonide.
Glutamine
Preclinical data suggests that glutamine stimulates intestinal mucosa growth, displaying less gastrointestinal toxictiy in rodents treated with chemotherapy [Fox et al. 1988; Xue et al. 2008]. Results from randomized studies showed a nonsignificant reduction in the CID rate [Daniele et al. 2001], and no effect in the prevention of radiation-induced diarrhea for the oral application form of glutamine was revealed [Kozelsky et al. 2003]. Importantly, a trial in patients receiving high-dose chemotherapy and glutamine-containing intravenous solutions showed significantly more relapses and deaths in the glutamine group [Pytlik et al. 2002]. Recently, a series with 44 patients showed a significant reduction in diarrhea with prophylactic use of intravenous glutamine — any influence on survival was not reported [Li et al. 2009]. Considering these results, a further development of glutamine for CID seems to be questionable.
Celecoxib
In animal models, celecoxib enhanced the antitumor activity of irinotecan and reduced the rate of diarrhea [Trifan et al. 2002]. The rate of grade 3 diarrhea was only 8% in one trial of 43 patients suffering from malignant gliomas treated with irinotecan and celecoxib [Reardon et al. 2005], whereas in another study with the same sample size using a combination of celecoxib and glutamine with the IFL-regimen the rate was 45% for grade 3 diarrhea, which is even higher than the expected margin [Pan et al. 2005]. In a recent review, no improvement with the usage of celecoxib in reducing CID was observed in the analyzed studies [Fakih and Rustum, 2009].
Long-acting formulation of octreotide
The efficacy of long-acting octreotide in the therapeutic setting has been demonstrated, as has its use in secondary prophylaxis in a small case series with doses ranging from 20 mg up to 40 mg every 4 weeks [Rosenoff, 2004a,b]. A large randomized study resulted in a nonsignificant reduction of severe diarrhea (61.7 versus 48.4%) favoring a dose of 40 mg over 30 mg every 4 weeks as secondary prophylaxis [Rosenoff et al. 2006]. However, preliminary results of a study presented by Zacchariah and colleagues at ASCO 2007 in the primary prophylaxis of diarrhea in 215 rectal cancer patients receiving 5-FU based chemoradiation was negative, revealing no difference between placebo and 30 mg of long-acting octreotide [Zachariah et al. 2007]. In patients receiving pelvic radiation the prophylactic treatment with 20 mg of long-acting octreotide versus placebo showed even worse tolerability regarding gastrointestinal symptoms and no change in diarrhea [Martenson et al. 2008].
Probiotics
Probiotics have been shown to prevent diarrhea in inflammatory bowel disease. Preclinical data yielded a similar efficacy in CID [Von Bultzingslowen et al. 2003; Bowen et al. 2007]. In the clinical setting, a combination of Lactobacillus rhamnosus and fiber resulted in a significant reduction of grade 3/4 diarrhea (37 versus 22%) in a randomized study of patients treated with either bolus (Mayo) or bolus and infusional (simplified de Gramont) 5-FU with leucovorin for adjuvant treatment of colorectal cancer [Osterlund et al. 2007].
Activated charcoal
The prophylactic use of activated charcoal in irinotecan-induced diarrhea seems to have interesting potential. Two small studies, one conducted in children, displayed a reduction in grade 3/4 diarrhea (7.1 versus 25% and 4.4 versus 52.3%) with excellent compliance and tolerability. The discontinuation rate of irinotecan was much lower and less loperamide was used [Michael et al. 2004; Sergio et al. 2008]. This approach should be further investigated in a phase III trial.
A further possible approach is the modulation of irinotecan pharmacokinetics, by the addition of phenobarbital, phenytoin and cyclosporine, to downsize SN-38 biliary excretion and induce glucuronidation, limited by the small therapeutic range of the used drugs and the possible decremental impact on efficacy, due to reduced concentration of active metabolites. Attempts have also been made to pharmacologically upregulate intestinal mucosal UDP-GT 1A1 with the plant flavonoid, chrysin. Other therapeutic measures assessed include an encephalinase inhibitor (acetorphan), which seems to be equally effective as loperamide in the treatment of non-CID diarrhea.
Guideline-based drug recommendations
So far, only loperamide, octreotide and tincture of opium are recommended in the updated treatment guidelines by the consensus conference on the management of CID from Benson and colleagues due to a lack of efficacy or insufficient evidence level of the other mentioned therapeutic approaches [Benson et al. 2004].
Opioids
Loperamide is an opioid which functions by decreasing intestinal motility by directly affecting the smooth muscle of the intestine and has no systemic effects due to a minimal absorption. The recommendation in current treatment guidelines [Benson et al. 2004] is based on an effective reduction in fecal incontinence, frequency of bowel movements and stool weight. The dosage of loperamide is an initial 4 mg dose followed by 2 mg every 2–4 hours or after every unformed stool. In case of CID, especially irinotecan-containing therapies, the more aggressive regimen should be chosen.
Deodorized tincture of opium (DTO) is another widely used antidiarrheal agent, despite the absence of literature to support its use in CID treatment. DTO contains the equivalent of 10 mg/ml morphine. The recommended dose is 10–15 drops in water every 3–4 hours [Benson et al. 2004]. The camphorated (alcohol-based) tincture is a less concentrated preparation containing the equivalent of 0.4 mg/ml morphine, leading to a dose of 5 ml (one teaspoon) every 3–4 hours.
Octreotide
Octreotide, a synthetic somatostatin analog, acts via several mechanisms: decreased secretion of a number of hormones, such as vasoactive intestinal peptide (VIP); prolongation of intestinal transit time and reduced secretion and increased absorption of fluid and electrolytes. It is approved by the US Food and Drug Administration for the treatment of diarrhea related to VIP-secreting tumors and symptoms due to carcinoid syndrome. Octreotide is beneficial in patients with CID from fluoropyrimidines, irinotecan, and 5-FU-based chemoradiotherapy [Gebbia et al. 1993; Goumas et al. 1998; Barbounis et al. 2001]. Although one randomized trial in 41 5-FU-treated patients showed that octreotide was more effective than standard-dose loperamide (90 versus 15% resolution of diarrhea by day 3) [Cascinu et al. 1993], octreotide is generally reserved as a second-line treatment for patients who are refractory after 48 hours, despite a loperamide escalation, because of its high cost [Zidan et al. 2001]. Patients developing a gastrointestinal syndrome including severe diarrhea, nausea, vomiting, anorexia, and abdominal cramping should receive an aggressive management with intravenous fluids and upfront octreotide. These recommendations by the consensus conference mentioned above reflect the risk of life-threatening complications and the reduced activity of loperamide in cases of severe diarrhea [Cascinu et al. 2000].
The optimal dosage of octreotide is not well defined. Current treatment guidelines recommend a starting dose of 100–150 µg subcutaneously (sc) or intravenously (iv) three times a day. Doses could be escalated to 500 µg sc/iv three times a day or by continuous iv infusion 25–50 µg/hr showing a dose-response relationship without significant toxicities [Wadler et al. 1995; Wasserman et al. 1997b].
Summary of the consensus recommendations
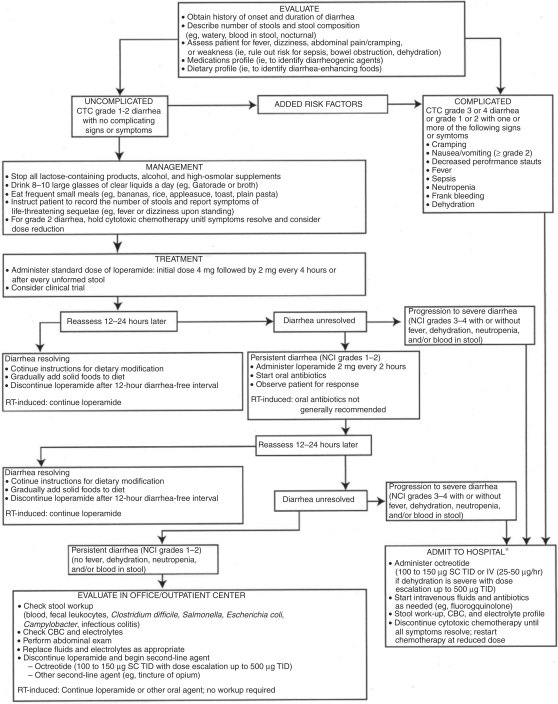
The recommendations of a consensus conference on the management of CID were published in 1998 and updated in 2004. Guidelines for evaluation and management of patients with CID are presented in Figure 2 [Wadler et al. 1998, Benson et al. 2004]. The tempo and specific nature of treatment is guided by the classification of the symptom constellation as complicated or uncomplicated. Uncomplicated patients may be managed conservatively in the outpatient setting (at least initially), while those with severe diarrhea or a potentially exacerbating condition (eg abdominal cramping, nausea, vomiting, fever, sepsis, neutropenia or bleeding) should be admitted to the hospital and treated aggressively with octreotide, intravenous fluids, antibiotics and a diagnostic workup.
Figure 2.
Consensus guideline for the treatment of chemotherapy induced diarrhea [Benson et al. 2004]. Reprinted with permission © 2008 American Society of Clinical Oncology. All rights reserved.
Conclusion
CID is caused by changes in intestinal absorption and might be accompanied by excessive electrolyte and fluid secretion. Furthermore, this type of diarrhea may be a consequence of biochemical changes caused by chemotherapy. Depending on the chemotherapeutic regimen, rates of severe or life-threatening CID can be up to 30% (grade 3–5 diarrhea), especially with 5-FU bolus or combination therapies of irinotecan and fluoropyrimidines (IFL, XELIRI). Regarding the tremendous effects on patients’ safety and quality of life, the possible occurrence of CID has to be carefully considered. Current research focuses on establishing predictive factors for toxicities caused by therapeutic agents like UGT1A1-polymorphisms for irinotecan or DPD-insufficiency for fluoropyrimidines. Despite the amount of clinical trials evaluating therapeutic or prophylactic measures in CID, there are just three drugs recommended in current guidelines: loperamide, deodorized tincture of opium and octreotide. Further evaluation of treatment options is absolutely essential for the management of this debilitating toxicity.
Conflict of interest statement
None declared.
References
- Arbuckle R.B., Huber S.L., Zacker C. (2000) The consequences of diarrhea occurring during chemotherapy for colorectal cancer: a retrospective study. Oncologist 5: 250–259 [DOI] [PubMed] [Google Scholar]
- Barbounis V., Koumakis G., Vassilomanolakis M., Demiri M., Efremidis A.P. (2001) Control of irinotecan-induced diarrhea by octreotide after loperamide failure. Support Care Cancer 9: 258–260 [DOI] [PubMed] [Google Scholar]
- Benson A.B., 3rd,, Ajani J.A., Catalano R.B., Engelking C., Kornblau S.M., Martenson J.A., Jr,, et al. (2004) Recommended guidelines for the treatment of cancer treatment-induced diarrhea. J Clin Oncol 22: 2918–2926 [DOI] [PubMed] [Google Scholar]
- Bonner J.A., Harari P.M., Giralt J., Azarnia N., Shin D.M., Cohen R.B., et al. (2006) Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354: 567–578 [DOI] [PubMed] [Google Scholar]
- Bowen J.M., Stringer A.M., Gibson R.J., Yeoh A.S., Hannam S., Keefe D.M. (2007) Vsl#3 probiotic treatment reduces chemotherapy-induced diarrhea and weight loss. Cancer Biol Ther 6: 1449–1454 [DOI] [PubMed] [Google Scholar]
- Cao S., Troutt A.B., Rustum Y.M. (1998) Interleukin 15 protects against toxicity and potentiates antitumor activity of 5-fluorouracil alone and in combination with leucovorin in rats bearing colorectal cancer. Cancer Res 58: 1695–1699 [PubMed] [Google Scholar]
- Cascinu S., Fedeli A., Fedeli S.L., Catalano G. (1993) Octreotide versus loperamide in the treatment of fluorouracil-induced diarrhea: a randomized trial. J Clin Oncol 11: 148–151 [DOI] [PubMed] [Google Scholar]
- Cascinu S., Bichisao E., Amadori D., Silingardi V., Giordani P., Sansoni E., et al. (2000) High-dose loperamide in the treatment of 5-fluorouracil-induced diarrhea in colorectal cancer patients. Support Care Cancer 8: 65–67 [DOI] [PubMed] [Google Scholar]
- Conti J.A., Kemeny N.E., Saltz L.B., Huang Y., Tong W.P., Chou T.C., et al. (1996) Irinotecan is an active agent in untreated patients with metastatic colorectal cancer. J Clin Oncol 14: 709–715 [DOI] [PubMed] [Google Scholar]
- Daniele B., Perrone F., Gallo C., Pignata S., De Martino S., De Vivo R., et al. (2001) Oral Glutamine in the prevention of fluorouracil induced intestinal toxicity: a double blind, placebo controlled, randomised trial. Gut 48: 28–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila M., Bresalier R.S. (2008) Gastrointestinal complications of oncologic therapy. Nat Clin Pract Gastroenterol Hepatol 5: 682–696 [DOI] [PubMed] [Google Scholar]
- De Jong F.A., Kehrer D.F., Mathijssen R.H., Creemers G.J., De Bruijn P., Van Schaik R.H., et al. (2006) Prophylaxis of irinotecan-induced diarrhea with neomycin and potential role for Ugt1a1*28 genotype screening: a double-blind, randomized, placebo-controlled study. Oncologist 11: 944–954 [DOI] [PubMed] [Google Scholar]
- De Jong F.A., Scott-Horton T.J., Kroetz D.L., McLeod H.L., Friberg L.E., Mathijssen R.H., et al. (2007) Irinotecan-induced diarrhea: functional significance of the polymorphic abcc2 transporter protein. Clin Pharmacol Ther 81: 42–49 [DOI] [PubMed] [Google Scholar]
- Deininger M.W., O'Brien S.G., Ford J.M., Druker B.J. (2003) Practical management of patients with chronic myeloid leukemia receiving imatinib. J Clin Oncol 21: 1637–1647 [DOI] [PubMed] [Google Scholar]
- Dupont H.L. (1997) Guidelines on acute infectious diarrhea in adults. The Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol 92: 1962–1975 [PubMed] [Google Scholar]
- Engelking C., Rutledge D.N., Ippoliti C., Neumann J., Hogan C.M. (1998) Cancer-related diarrhea: a neglected cause of cancer-related symptom distress. Oncol Nurs Forum 25: 859–860 [PubMed] [Google Scholar]
- Fakih M.G., Rustum Y.M. (2009) Does celecoxib have a role in the treatment of patients with colorectal cancer? Clin Colorectal Cancer 8: 11–14 [DOI] [PubMed] [Google Scholar]
- Fittkau M., Voigt W., Holzhausen H.J., Schmoll H.J. (2004) Saccharic acid 1.4-lactone protects against Cpt-11-induced mucosa damage in rats. J Cancer Res Clin Oncol 130: 388–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flieger D., Klassert C., Hainke S., Keller R., Kleinschmidt R., Fischbach W. (2007) Phase II clinical trial for prevention of delayed diarrhea with cholestyramine/levofloxacin in the second-line treatment with irinotecan biweekly in patients with metastatic colorectal carcinoma. Oncology 72: 10–16 [DOI] [PubMed] [Google Scholar]
- Fox A.D., Kripke S.A., De Paula J., Berman J.M., Settle R.G., Rombeau J.L. (1988) Effect of a glutamine-supplemented enteral diet on methotrexate-induced enterocolitis. JPEN J Parenter Enteral Nutr 12: 325–331 [DOI] [PubMed] [Google Scholar]
- Gebbia V., Carreca I., Testa A., Valenza R., Curto G., Cannata G., et al. (1993) Subcutaneous octreotide versus oral loperamide in the treatment of diarrhea following chemotherapy. Anticancer Drugs 4: 443–445 [DOI] [PubMed] [Google Scholar]
- Gibson R.J., Stringer A.M. (2009) Chemotherapy-induced diarrhoea. Curr Opin Support Palliat Care 3: 31–35 [DOI] [PubMed] [Google Scholar]
- Gibson R.J., Bowen J.M., Inglis M.R., Cummins A.G., Keefe D.M. (2003) Irinotecan causes severe small intestinal damage, as well as colonic damage, in the rat with implanted breast cancer. J Gastroenterol Hepatol 18: 1095–1100 [DOI] [PubMed] [Google Scholar]
- Gore M.E., Szczylik C., Porta C., Bracarda S., Bjarnason G.A., Oudard S., et al. (2009) Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: an expanded-access trial. Lancet Oncol 10: 757–763 [DOI] [PubMed] [Google Scholar]
- Goumas P., Naxakis S., Christopoulou A., Chrysanthopoulos C., Nikolopoulou V.V., Kalofonos H.P. (1998) Octreotide acetate in the treatment of fluorouracil-induced diarrhea. Oncologist 3: 50–53 [PubMed] [Google Scholar]
- Han J.Y., Lim H.S., Park Y.H., Lee S.Y., Lee J.S. (2009) Integrated pharmacogenetic prediction of irinotecan pharmacokinetics and toxicity in patients with advanced non-small cell lung cancer. Lung Cancer 63: 115–120 [DOI] [PubMed] [Google Scholar]
- Hess G., Herbrecht R., Romaguera J., Verhoef G., Crump M., Gisselbrecht C., et al. (2009) Phase III study to evaluate temsirolimus compared with investigator's choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol 27: 3822–3829 [DOI] [PubMed] [Google Scholar]
- Hoskins J.M., Goldberg R.M., Qu P., Ibrahim J.G., McLeod H.L. (2007) Ugt1a1*28 genotype and irinotecan-induced neutropenia: dose matters. J Natl Cancer Inst 99: 1290–1295 [DOI] [PubMed] [Google Scholar]
- Hudes G., Carducci M., Tomczak P., Dutcher J., Figlin R., Kapoor A., et al. (2007) Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 356: 2271–2281 [DOI] [PubMed] [Google Scholar]
- Hurwitz H., Fehrenbacher L., Novotny W., Cartwright T., Hainsworth J., Heim W., et al. (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350: 2335–2342 [DOI] [PubMed] [Google Scholar]
- Innocenti F., Undevia S.D., Iyer L., Chen P.X., Das S., Kocherginsky M., et al. (2004) Genetic variants in the Udp-glucuronosyltransferase 1a1 gene predict the risk of severe neutropenia of irinotecan. J Clin Oncol 22: 1382–1388 [DOI] [PubMed] [Google Scholar]
- Innocenti F., Kroetz D.L., Schuetz E., Dolan M.E., Ramirez J., Relling M., et al. (2009) Comprehensive pharmacogenetic analysis of irinotecan neutropenia and pharmacokinetics. J Clin Oncol 27: 2604–2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ippoliti C. (1998) Antidiarrheal agents for the management of treatment-related diarrhea in cancer patients. Am J Health Syst Pharm 55: 1573–1580 [DOI] [PubMed] [Google Scholar]
- Iyer L., Das S., Janisch L., Wen M., Ramirez J., Karrison T., et al. (2002) Ugt1a1*28 Polymorphism as a determinant of irinotecan disposition and toxicity. Pharmacogenomics J 2: 43–47 [DOI] [PubMed] [Google Scholar]
- Jordan K., Kellner O., Kegel T., Schmoll H.J., Grothey A. (2004) Phase II trial of capecitabine/irinotecan and capecitabine/oxaliplatin in advanced gastrointestinal cancers. Clin Colorectal Cancer 4: 46–50 [DOI] [PubMed] [Google Scholar]
- Karthaus M., Ballo H., Abenhardt W., Steinmetz T., Geer T., Schimke J., et al. (2005) Prospective, double-blind, placebo-controlled, multicenter, randomized phase III study with orally administered budesonide for prevention of irinotecan (Cpt-11)-induced diarrhea in patients with advanced colorectal cancer. Oncology 68: 326–332 [DOI] [PubMed] [Google Scholar]
- Keefe D.M. (2007) Intestinal mucositis: mechanisms and management. Curr Opin Oncol 19: 323–327 [DOI] [PubMed] [Google Scholar]
- Keefe D., Anthony L. (2008) Tyrosine kinase inhibitors and gut toxicity: a new era in supportive care. Curr Opin Support Palliat Care 2: 19–21 [DOI] [PubMed] [Google Scholar]
- Keefe D.M., Gibson R.J. (2007) Mucosal injury from targeted anti-cancer therapy. Support Care Cancer 15: 483–490 [DOI] [PubMed] [Google Scholar]
- Keefe D.M., Brealey J., Goland G.J., Cummins A.G. (2000) Chemotherapy for cancer causes apoptosis that precedes hypoplasia in crypts of the small intestine in humans. Gut 47: 632–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe D.M., Gibson R.J., Hauer-Jensen M. (2004) Gastrointestinal mucositis. Semin Oncol Nurs 20: 38–47 [DOI] [PubMed] [Google Scholar]
- Kehrer D.F., Sparreboom A., Verweij J., De Bruijn P., Nierop C.A., Van De Schraaf J., et al. (2001) Modulation of irinotecan-induced diarrhea by cotreatment with neomycin in cancer patients. Clin Cancer Res 7: 1136–1141 [PubMed] [Google Scholar]
- Kim T.W., Innocenti F. (2007) Insights, challenges, and future directions in irinogenetics. Ther Drug Monit 29: 265–270 [DOI] [PubMed] [Google Scholar]
- Kozelsky T.F., Meyers G.E., Sloan J.A., Shanahan T.G., Dick S.J., Moore R.L., et al. (2003) Phase III double-blind study of glutamine versus placebo for the prevention of acute diarrhea in patients receiving pelvic radiation therapy. J Clin Oncol 21: 1669–1674 [DOI] [PubMed] [Google Scholar]
- Kweekel D.M., Gelderblom H., Van Der Straaten T., Antonini N.F., Punt C.J., Guchelaar H.J. (2008) Ugt1a1*28 genotype and irinotecan dosage in patients with metastatic colorectal cancer: a dutch colorectal cancer group study. Br J Cancer 99: 275–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenfers B.H., Loeffler T.M., Droege C.M., Hausamen T.U. (1999) Substantial activity of budesonide in patients with irinotecan (cpt-11) and 5-fluorouracil induced diarrhea and failure of loperamide treatment. Ann Oncol 10: 1251–1253 [DOI] [PubMed] [Google Scholar]
- Li Y., Ping X., Yu B., Liu F., Ni X., Li J. (2009) Clinical trial: prophylactic intravenous alanyl-glutamine reduces the severity of gastrointestinal toxicity induced by chemotherapy: a randomised crossover trial. Aliment Pharmacol Ther 30(5): 452–458 [DOI] [PubMed] [Google Scholar]
- Liu C.Y., Chen P.M., Chiou T.J., Liu J.H., Lin J.K., Lin T.C., et al. (2008) Ugt1a1*28 polymorphism predicts irinotecan-induced severe toxicities without affecting treatment outcome and survival in patients with metastatic colorectal carcinoma. Cancer 112: 1932–1940 [DOI] [PubMed] [Google Scholar]
- Llovet J.M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J.F., et al. (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359: 378–390 [DOI] [PubMed] [Google Scholar]
- Maroun J.A., Anthony L.B., Blais N., Burkes R., Dowden S.D., Dranitsaris G., et al. (2007) Prevention and management of chemotherapy-induced diarrhea in patients with colorectal cancer: a consensus statement by the canadian working group on chemotherapy-induced diarrhea. Curr Oncol 14: 13–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martenson J.A., Halyard M.Y., Sloan J.A., Proulx G.M., Miller R.C., Deming R.L., et al. (2008) Phase III, double-blind study of depot octreotide versus placebo in the prevention of acute diarrhea in patients receiving pelvic radiation therapy: results of North Central Cancer Treatment Group N00ca. J Clin Oncol 26: 5248–5253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison L.K., Fourie J., Desmond R.A., Modak A., Saif M.W., Diasio R.B. (2006a) Increased prevalence of dihydropyrimidine dehydrogenase deficiency in African-Americans compared with Caucasians. Clin Cancer Res 12: 5491–5495 [DOI] [PubMed] [Google Scholar]
- Mattison L.K., Fourie J., Hirao Y., Koga T., Desmond R.A., King J.R., et al. (2006b) The uracil breath test in the assessment of dihydropyrimidine dehydrogenase activity: pharmacokinetic relationship between expired 13CO2 and plasma [2-13c]dihydrouracil. Clin Cancer Res 12: 549–555 [DOI] [PubMed] [Google Scholar]
- McCollum A.D., Catalano P.J., Haller D.G., Mayer R.J., Macdonald J.S., Benson A.B., 3rd,, et al. (2002) Outcomes and toxicity in African-American and Caucasian patients in a randomized adjuvant chemotherapy trial for colon cancer. J Natl Cancer Inst 94: 1160–1167 [DOI] [PubMed] [Google Scholar]
- Meyerhardt J.A., Tepper J.E., Niedzwiecki D., Hollis D.R., McCollum A.D., Brady D., et al. (2004) Impact of body mass index on outcomes and treatment-related toxicity in patients with stage II and III rectal cancer: findings from Intergroup Trial 0114. J Clin Oncol 22: 648–657 [DOI] [PubMed] [Google Scholar]
- Michael M., Brittain M., Nagai J., Feld R., Hedley D., Oza A., et al. (2004) Phase II study of activated charcoal to prevent irinotecan-induced diarrhea. J Clin Oncol 22: 4410–4417 [DOI] [PubMed] [Google Scholar]
- Motzer R.J., Escudier B., Oudard S., Hutson T.E., Porta C., Bracarda S., et al. (2008) Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 372: 449–456 [DOI] [PubMed] [Google Scholar]
- Motzer R.J., Hutson T.E., Tomczak P., Michaelson M.D., Bukowski R.M., Oudard S., et al. (2009) Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 27: 3584–3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund P., Ruotsalainen T., Korpela R., Saxelin M., Ollus A., Valta P., et al. (2007) Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: a randomised study. Br J Cancer 97: 1028–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan C.X., Loehrer P., Seitz D., Helft P., Juliar B., Ansari R., et al. (2005) A phase II trial of irinotecan, 5-fluorouracil and leucovorin combined with celecoxib and glutamine as first-line therapy for advanced colorectal cancer. Oncology 69: 63–70 [DOI] [PubMed] [Google Scholar]
- Pytlik R., Benes P., Patorkova M., Chocenska E., Gregora E., Prochazka B., et al. (2002) Standardized parenteral alanyl-glutamine dipeptide supplementation is not beneficial in autologous transplant patients: a randomized, double-blind, placebo controlled study. Bone Marrow Transplant 30: 953–961 [DOI] [PubMed] [Google Scholar]
- Reardon D.A., Quinn J.A., Vredenburgh J., Rich J.N., Gururangan S., Badruddoja M., et al. (2005) Phase II trial of irinotecan plus celecoxib in adults with recurrent malignant glioma. Cancer 103: 329–338 [DOI] [PubMed] [Google Scholar]
- Rosenoff S. (2004a) Resolution of refractory chemotherapy-induced diarrhea (CID) with octreotide long-acting formulation in cancer patients: 11 case studies. Support Care Cancer 12: 561–570 [DOI] [PubMed] [Google Scholar]
- Rosenoff S.H. (2004b) Octreotide Lar resolves severe chemotherapy-induced diarrhoea (CID) and allows continuation of full-dose therapy. Eur J Cancer Care (Engl) 13: 380–383 [DOI] [PubMed] [Google Scholar]
- Rosenoff S.H., Gabrail N.Y., Conklin R., Hohneker J.A., Berg W.J., Warsi G., et al. (2006) A multicenter, randomized trial of long-acting octreotide for the optimum prevention of chemotherapy-induced diarrhea: results of the stop trial. J Support Oncol 4: 289–294 [PubMed] [Google Scholar]
- Saliba F., Hagipantelli R., Misset J.L., Bastian G., Vassal G., Bonnay M., et al. (1998) Pathophysiology and therapy of irinotecan-induced delayed-onset diarrhea in patients with advanced colorectal cancer: a prospective assessment. J Clin Oncol 16: 2745–2751 [DOI] [PubMed] [Google Scholar]
- Saltz L.B., Cox J.V., Blanke C., Rosen L.S., Fehrenbacher L., Moore M.J., et al. (2000) Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 343: 905–914 [DOI] [PubMed] [Google Scholar]
- Saltz L.B., Niedzwiecki D., Hollis D., Goldberg R.M., Hantel A., Thomas J.P., et al. (2007) Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: results of Calgb 89803. J Clin Oncol 25: 3456–3461 [DOI] [PubMed] [Google Scholar]
- Schmittel A., Jahnke K., Thiel E., Keilholz U. (2004) Neomycin as secondary prophylaxis for irinotecan-induced diarrhea. Ann Oncol 15: 1296–1296 [DOI] [PubMed] [Google Scholar]
- Sergio G.C., Felix G.M., Luis J.V. (2008) Activated charcoal to prevent irinotecan-induced diarrhea in children. Pediatr Blood Cancer 51: 49–52 [DOI] [PubMed] [Google Scholar]
- Siber G.R., Mayer R.J., Levin M.J. (1980) Increased gastrointestinal absorption of large molecules in patients after 5-fluorouracil therapy for metastatic colon carcinoma. Cancer Res 40: 3430–3436 [PubMed] [Google Scholar]
- Stringer A.M., Gibson R.J., Logan R.M., Bowen J.M., Yeoh A.S.J., Burns J., et al. (2007) Chemotherapy-induced diarrhea is associated with changes in the luminal environment in the da rat. Experimental Biology and Medicine 232: 96–106 [PubMed] [Google Scholar]
- Stringer A.M., Gibson R.J., Logan R.M., Bowen J.M., Yeoh A.S., Keefe D.M. (2008) Faecal microflora and beta-glucuronidase expression are altered in an irinotecan-induced diarrhea model in rats. Cancer Biol Ther 7: 1919–1925 [DOI] [PubMed] [Google Scholar]
- Stringer A.M., Gibson R.J., Logan R.M., Bowen J.M., Yeoh A.S., Laurence J., et al. (2009) Irinotecan-induced mucositis is associated with changes in intestinal mucins. Cancer Chemother Pharmacol 64: 123–132 [DOI] [PubMed] [Google Scholar]
- Takasuna K., Kasai Y., Kitano Y., Mori K., Kakihata K., Hirohashi M., et al. (1995) [Study on the mechanisms of diarrhea induced by a new anticancer camptothecin derivative, irinotecan hydrochloride (Cpt-11), in Rats]. Nippon Yakurigaku Zasshi 105: 447–460 [DOI] [PubMed] [Google Scholar]
- Takasuna K., Hagiwara T., Hirohashi M., Kato M., Nomura M., Nagai E., et al. (1998) Inhibition of intestinal microflora beta-glucuronidase modifies the distribution of the active metabolite of the antitumor agent, irinotecan hydrochloride (Cpt-11) in rats. Cancer Chemother Pharmacol 42: 280–286 [DOI] [PubMed] [Google Scholar]
- Threadgill D.W., Dlugosz A.A., Hansen L.A., Tennenbaum T., Lichti U., Yee D., et al. (1995) Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science 269: 230–234 [DOI] [PubMed] [Google Scholar]
- Trifan O.C., Durham W.F., Salazar V.S., Horton J., Levine B.D., Zweifel B.S., et al. (2002) Cyclooxygenase-2 inhibition with celecoxib enhances antitumor efficacy and reduces diarrhea side effect of Cpt-11. Cancer Res 62: 5778–5784 [PubMed] [Google Scholar]
- Van Cutsem E., Peeters M., Siena S., Humblet Y., Hendlisz A., Neyns B., et al. (2007) Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 25: 1658–1664 [DOI] [PubMed] [Google Scholar]
- Van Cutsem E., Kohne C.H., Hitre E., Zaluski J., Chang Chien C.R., Makhson A., et al. (2009) Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 360: 1408–1417 [DOI] [PubMed] [Google Scholar]
- Vincenzi B., Schiavon G., Pantano F., Santini D., Tonini G. (2008) Predictive factors for chemotherapy-related toxic effects in patients with colorectal cancer. Nat Clin Pract Oncol 5: 455–465 [DOI] [PubMed] [Google Scholar]
- Voigt W., Matsui S., Yin M.B., Burhans W.C., Minderman H., Rustum Y.M. (1998) Topoisomerase-I inhibitor Sn-38 can induce DNA damage and chromosomal aberrations independent from DNA synthesis. Anticancer Res 18: 3499–3505 [PubMed] [Google Scholar]
- Von Bultzingslowen I., Adlerberth I., Wold A.E., Dahlen G., Jontell M. (2003) Oral and intestinal microflora in 5-fluorouracil treated rats, translocation to cervical and mesenteric lymph nodes and effects of probiotic bacteria. Oral Microbiol Immunol 18: 278–284 [DOI] [PubMed] [Google Scholar]
- Wadler S., Haynes H., Wiernik P.H. (1995) Phase I trial of the somatostatin analog octreotide acetate in the treatment of fluoropyrimidine-induced diarrhea. J Clin Oncol 13: 222–226 [DOI] [PubMed] [Google Scholar]
- Wadler S., Benson A.B., 3rd,, Engelking C., Catalano R., Field M., Kornblau S.M., et al. (1998) Recommended guidelines for the treatment of chemotherapy-induced diarrhea. J Clin Oncol 16: 3169–3178 [DOI] [PubMed] [Google Scholar]
- Wasserman E., Hidalgo M., Hornedo J., Cortes-Funes H. (1997b) Octreotide (Sms 201-995) for hematopoietic support-dependent high-dose chemotherapy (Hsd-Hdc)-related diarrhoea: dose finding study and evaluation of efficacy. Bone Marrow Transplant 20: 711–714 [DOI] [PubMed] [Google Scholar]
- Wasserman E., Myara A., Lokiec F., Goldwasser F., Trivin F., Mahjoubi M., et al. (1997a) Severe Cpt-11 toxicity in patients with Gilbert's syndrome: two case reports. Ann Oncol 8: 1049–1051 [DOI] [PubMed] [Google Scholar]
- Xue H., Sawyer M.B., Field C.J., Dieleman L.A., Murray D., Baracos V.E. (2008) Bolus oral glutamine protects rats against Cpt-11-induced diarrhea and differentially activates cytoprotective mechanisms in host intestine but not tumor. J Nutr 138: 740–746 [DOI] [PubMed] [Google Scholar]
- Yen J.L., McLeod H.L. (2007) Should DPD analysis be required prior to prescribing fluoropyrimidines? Eur J Cancer 43: 1011–1016 [DOI] [PubMed] [Google Scholar]
- Zachariah B., James J., Gwede C.X., Ajani J., Chin L., Donath D., et al. (2007) RTOG 0315: a randomized, double-blind, placebo-controlled phase III study to determine the efficacy of octreotide acetate in preventing or reducing the severity of chemoradiation-induced diarrhea in patients with anal or rectal cancer. J Clin Oncol 2007 ASCO Annual Meeting Proceedings Part I 25: 4032–4032 [Google Scholar]
- Zalcberg J., Kerr D., Seymour L., Palmer M. (1998) Haematological and non-haematological toxicity after 5-fluorouracil and leucovorin in patients with advanced colorectal cancer is significantly associated with gender, increasing age and cycle number. Tomudex International Study Group. Eur J Cancer 34: 1871–1875 [DOI] [PubMed] [Google Scholar]
- Zidan J., Haim N., Beny A., Stein M., Gez E., Kuten A. (2001) Octreotide in the treatment of severe chemotherapy-induced diarrhea. Ann Oncol 12: 227–229 [DOI] [PubMed] [Google Scholar]