INTRODUCTION
Age-related macular degeneration (AMD) is the leading cause of severe low vision and blindness in the United States.1,2 Several large population-based studies have examined differences in the prevalence of AMD between whites and blacks in the United States (Table 1).3–11 These studies have consistently demonstrated that AMD affects whites more than blacks. When examining differences in prevalence of AMD between other racial minorities and whites, there are conflicting findings as to whether Latinos have higher or lower rates of AMD relative to whites (Table 1).4,5,8–10 An analysis of data from the National Health and Nutrition Examination Survey (NHANES) and another from the Multi-ethnic Study of Atherosclerosis (MESA) study found that Latinos had lower rates of early and late nonexudative AMD relative to whites.5,10 In contrast, findings from the Colorado-Wisconsin Study of Age-related Maculopathy4 showed higher rates of nonexudative AMD in Latinos compared with whites and the Proyecto VER identified signs of early AMD in over one quarter of Latinos over 50 years of age.9 While studies have assessed rates of AMD among individuals residing in Asian countries, little is known about the prevalence of AMD among Asian Americans. Given that Latino and Asian Americans constitute the two fastest growing minorities in the United States12, representing nearly 20% of the population, it is becoming increasingly important to have an improved understanding of the epidemiology of AMD for these groups.
Table 1.
Review of Previous Studies Examining Age-related Macular Degeneration and Race and Ethnicity
| Study Name | Primary Author | Year | Population | N | Age |
Early AMD Prevalence % (N) |
Late** AMD Prevalence % (N) |
Exudative AMD Prevalence % (N) |
|---|---|---|---|---|---|---|---|---|
| The Beaver Dam Eye Study3 | Klein R, et al. | 1992 | White (Wisconsin) | 4771 | ≥43 | 15.60% | 1.60% | 1.2%(57) |
| The Colorado-Wisconsin Study of Age-related Maculopathy4 | Cruickshanks KJ, et al. | 1997 | White (Wisconsin) | 3995 | 43–74 | 14.0% (559)§ | 0.60% (24)§ | NA |
| White (Colorado) | 3048 | 43–74 | 9.6% (292)§ | 0.8% (24)§ | NA | |||
| Hispanic$ (Colorado) | 2247 | 43–74 | 14.3% (321)§ | 0.1% (1)§ | NA | |||
| National Health and Nutrition Examination Survey III5 | Klein R, et al. | 1999 | White | 4054 | ≥40 | 9.1% (369) | 0.5% (20) | 0.3%(14) |
| Black | 1996 | ≥40 | 8.3% (165) | 0.13% (3) | 0.1%(2) | |||
| Mexican-Americans$ | 1838 | ≥40 | 7.6% (139) | 0.06% (1) | 0.1%(2) | |||
| The Baltimore Eye Survey6 | Friedman DS, et al. | 1999 | White | 2518 | ≥40 | 20.2% (509)‡ | 1.4% (35) | 0.6% (15) |
| Black | 1843 | ≥40 | 19.8% (365)‡ | 0.2% (4) | 0.1% (2) | |||
| AMD in the Atherosclerosis Risk in Communities Study7 | Klein R, et al. | 1999 | White | 8984 | 48–72 | 5.4% (485) | 0.2% (15) | NA |
| Black | 2548 | 48–72 | 3.7% (94) | 0.0% (0) | NA | |||
| The Los Angeles Latino Eye Study8 | Varma R, et al. | 2004 | Mexican-Americans$ | 5875 | ≥40 | 9.4% (551) | 0.43% (25) | 0.29% (17) |
| Proyecto VER9 | Munoz B, et al. | 2005 | Mexican-Americans$ | 2776 | ≥50 | 27.9% (775)* | 0.59% (15) | 0.14% (4) |
| AMD in the Multi-ethnic Study of Atherosclerosis (MESA)10 | Klein R, et al. | 2006 | White | 2299 | ≥45 | 4.8% (110) | 0.6% (14) | NA |
| Black | 1583 | ≥45 | 2.1% (33) | 0.3% (5) | NA | |||
| Hispanics$ | 1274 | ≥45 | 4% (51) | 0.2% (3) | NA | |||
| Chinese$ | 691 | ≥45 | 3.6% (25) | 1% (7) | NA | |||
| The Salisbury Eye Evaluation Project11 | Bressler SB, et al. | 2008 | White | 1854 | 65–84 | NA | 3.5% (63) | 1.7% (30) |
| Black | 666 | 65–84 | NA | 1.3% (9) | 1.0% (7) | |||
Late AMD includes both study participants with geographic atrophy and exudative AMD
Racial or ethnicity group reported as described in their respective papers
Age-adjusted rate of age-related maculopathy
Adjusted for age and sex
Age-adjusted prevalence 17.8%
AMD = age-related macular degeneration
The purpose of this study is to use healthcare claims data from a large, national managed care network to compare the incidence, prevalence, and hazard of developing nonexudative and exudative AMD among individuals of different races. Such information would be important for clinicians in identifying those most at risk for disease, for developing more appropriate screening protocols of AMD, for researchers designing and recruiting participants for clinical trials, and for health policy-makers when determining resource allocation.
METHODS
Data Source
The i3 InVision Data Mart database (Ingenix, Eden Prairie, MN) contains records of all beneficiaries in a large managed care network in the United States. We analyzed a subset of beneficiaries who had any form of eye care from January 1, 2001 through December 31, 2007, defined as any person who had one or more International Classification of Diseases, Ninth Revision Clinical Modification13 (ICD-9CM) codes for any eye-related diagnosis (360–379.9), or Current Procedural Terminology14 (CPT-4) code for any eye-related visits, diagnostic or therapeutic procedures (65091–68899 or 92002–92499), or any other ICD-9CM or CPT codes assigned by an ophthalmologist or optometrist during their time in the medical plan. For each beneficiary in the sample, we had access to all medical claims (inpatient, outpatient, skilled nursing facility) both for ocular and non-ocular medical conditions. The database also contains detailed records of demographic (age, sex, race, ethnicity) and socioeconomic information (education level, household net worth) for each beneficiary.
Subjects
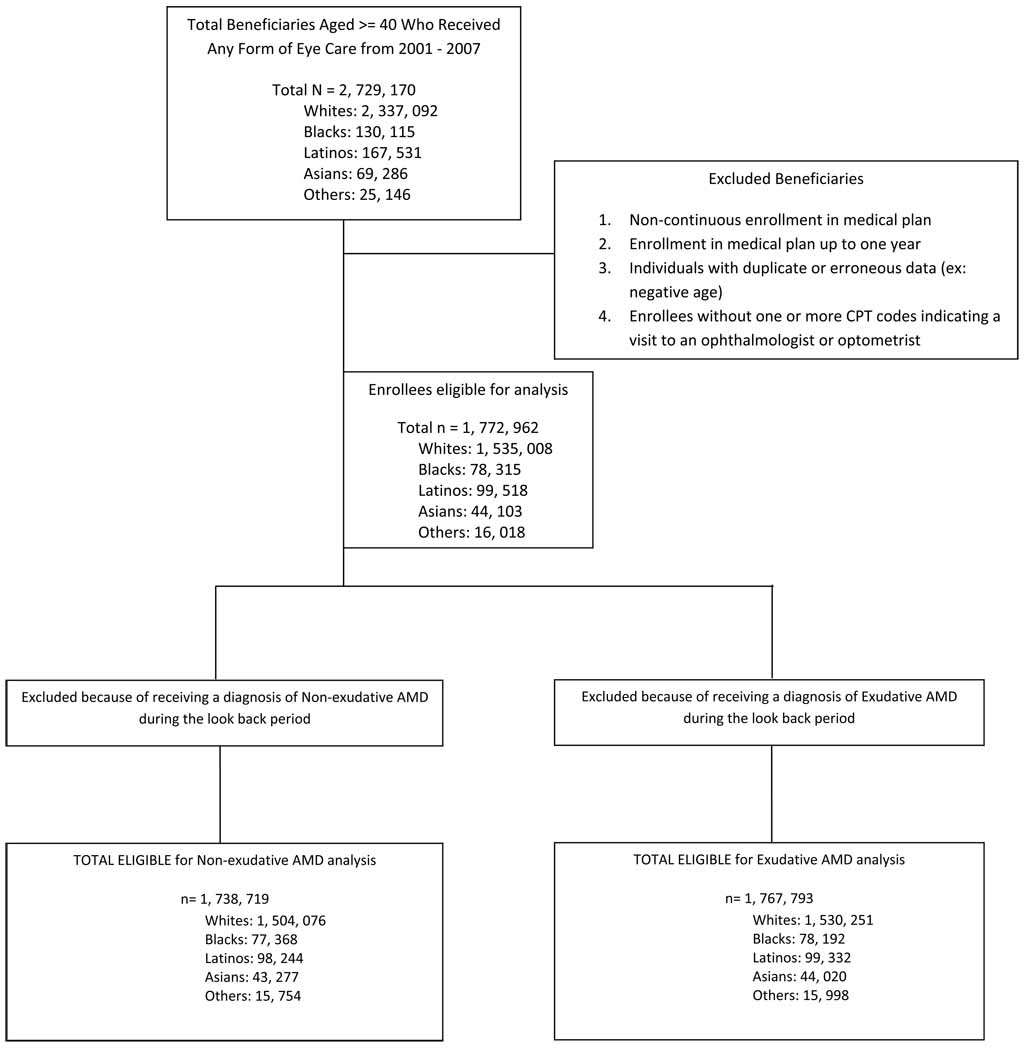
All individuals age 40 or older who were in the i3 InVision Data Mart database for more than one consecutive year and had one or more visits to an eye care provider during their time in the medical plan were identified. Individuals in the medical plan for 365 days or less and those who were not in the medical plan continuously from their beginning to their ending date of enrollment were excluded (Figure 1). The race of each beneficiary was identified by the managed care company using information provided from two sources: public records (drivers license data) and from E-Tech (Ethnic Technologies, LLC., South Hackensack, NJ), a tool that uses information from the name of the beneficiary and the census block he or she lives in to assign race. Although enrollee names and other identifying information is used by Ingenix to assist with the assignment of race, all identifiers were removed before the data is sold to researchers, thus the data we had access to for this analysis was, in essence de-identified. Previous comparisons between assignment of race using E-Tech and information collected from patient self-report demonstrated that E-tech has a positive predictive value of 71%.15 Races were categorized as non-Hispanic white (henceforth referred to as white), black, Latino, and Asian American. All other races were categorized as “Other”. The proportion of enrollees of the different races in this network was found to be relatively similar to that of the 2000 US Census, although blacks were less well represented in the study sample (5.5%) relative to the census data for persons age ≥40 in the U.S. population (9.2%).
Figure 1.
Sample Selection Criteria to Identify Persons with Macular Degeneration for Inclusion in the Study
Incidence and Prevalence of Nonexudative and Exudative AMD
ICD-9CM codes were used to determine whether each beneficiary had one or more diagnoses of AMD during their time in the medical plan. Incidence and prevalence rates were determined for nonexudative AMD (ICD-9CM codes 362.50, 362.51, and 362.57) and exudative AMD (ICD-9CM code 362.52).
Beneficiaries could be diagnosed with more than one form of AMD during their time in the medical plan and were counted in the disease incidence and prevalence estimates for each type of AMD they were documented to have. For example, if a beneficiary was diagnosed with nonexudative AMD in 2002 and diagnosed with exudative AMD in 2005, she was counted as a prevalent case for each of these types of AMD. The database does not contain information to determine whether persons diagnosed with both nonexudative and exudative AMD had these conditions in the same eye over the course of their time in the medical plan. Prevalence and incidence rates of nonexudative and exudative AMD were obtained by identifying the number of individuals diagnosed with each condition divided by the number of beneficiaries in the medical plan during the 7 year time period. When interpreting prevalence estimates, it is important to note that not every beneficiary was in the medical plan for all 7 years. Incidence rates of nonexudative and exudative AMD were calculated by dividing the number of newly diagnosed beneficiaries with each type of AMD by their time in the plan at risk. Diagnoses were considered incident cases if the enrollee did not have any record of the AMD type of interest during their first year in the medical plan. Nonexudative and exudative AMD incidence and prevalence rates for the different races were compared using a test of rate ratios, with white race as the reference group.
Analyses
All analyses were performed by using SAS 9.2 (Cary, NC). Participant characteristics were summarized for the entire sample using means and standard deviations for continuous variables and frequencies and percentages for categorical variables. Incidence and prevalence estimates were generated for nonexudative and exudative AMD and stratified according to race.
Cox regression analysis was performed to determine the hazard of developing nonexudative and exudative AMD.16 We used the first year each beneficiary was enrolled in the medical plan as a look back period. To avoid selection bias, follow-up of all enrollees started at one year after enrollment in the medical plan. The model captures incident cases since individuals diagnosed with nonexudative or exudative AMD during the look back period were excluded from the analysis. Persons were followed one year after enrollment until they either were diagnosed with the condition (nonexudative or exudative AMD) or were censored (either when they left the medical plan or the last day for which we had data, December 31, 2007). For each beneficiary the age to diagnosis or the age to censoring was determined. Using age as the time axis and race as the key predictor of interest, the Cox model was left-truncated at the age of index (one year after entry into the medical plan). Adjustments were made for age (the time axis), sex, region of residence within the US, education level, household net worth, and the following medical and ocular conditions: diabetes mellitus, systemic arterial hypertension, hyperlipidemia, obesity, myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular accident, renal insufficiency, coagulopathy, blood loss anemia, deficiency anemias, systemic hypotension, skin cancer (a surrogate measure of long-term sun exposure), cataract, pseudophakia or aphakia, diabetic retinopathy, and open-angle glaucoma. (Supplemental Table #1 at AJO.com,) For all analyses, a p-value of < 0.05 was considered statistically significant.
RESULTS
Of the 2,259,061 individuals in the medical plan who met the inclusion criteria, 1,772,962 individuals (79%) were able to be classified according to race. There were 1,535,008 whites (87%), 78,315 blacks (4%), 99,518 Latinos (6%), and 44,103 Asian Americans (3%). The median age at entry into the plan was 52 years (range 40–87 years) and the average enrollment time within the plan was 3.75 ± 1.81 years. Table 2 shows the breakdown of individuals with non-exudative and exudative AMD stratified by age, sex, and race. Among all enrollees in the medical plan age, 46% of individuals underwent ≥1 eye examination over the course of their time in the plan. Latinos had the lowest rates of eye examinations (38%), followed by Asian Americans (43%), blacks (45%), and whites (47%). All analyses focus only on individuals who had ≥1 eye examination while in the plan.
Table 2.
Numbers of Beneficiaries with Non-Exudative and Exudative Age-related Macular Degeneration by Age, Race and Sex*
| Persons with Non-exudative AMD |
Persons with Exudative AMD | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Race | Males | Females | Total | Males | Females | Total | Total Eligible |
||||||
| 40–49 | White | 3167 | 0.57% | 4306 | 0.78% | 7473 | 1.36% | 347 | 0.06% | 356 | 0.06% | 703 | 0.13% | 551174 |
| Black | 116 | 0.42% | 240 | 0.87% | 356 | 1.29% | 7 | 0.03% | 20 | 0.07% | 27 | 0.10% | 27696 | |
| Latino | 306 | 0.69% | 379 | 0.85% | 685 | 1.53% | 45 | 0.10% | 51 | 0.11% | 96 | 0.21% | 44667 | |
| Asian | 206 | 1.08% | 196 | 1.03% | 402 | 2.11% | 20 | 0.10% | 12 | 0.06% | 32 | 0.17% | 19082 | |
| Other | 69 | 1.00% | 75 | 1.09% | 144 | 2.09% | 8 | 0.12% | 3 | 0.04% | 11 | 0.16% | 6884 | |
| 50–59 | White | 6996 | 1.32% | 9692 | 1.83% | 16688 | 3.14% | 789 | 0.15% | 941 | 0.18% | 1730 | 0.33% | 530996 |
| Black | 219 | 0.82% | 461 | 1.72% | 680 | 2.53% | 27 | 0.10% | 46 | 0.17% | 73 | 0.27% | 26866 | |
| Latino | 445 | 1.34% | 587 | 1.77% | 1032 | 3.11% | 57 | 0.17% | 61 | 0.18% | 118 | 0.36% | 33232 | |
| Asian | 277 | 1.82% | 404 | 2.66% | 681 | 4.48% | 34 | 0.22% | 37 | 0.24% | 71 | 0.47% | 15199 | |
| Other | 90 | 1.67% | 101 | 1.88% | 191 | 3.55% | 6 | 0.11% | 6 | 0.11% | 12 | 0.22% | 5383 | |
| 60–69 | White | 8745 | 3.17% | 10814 | 3.92% | 19559 | 7.09% | 1154 | 0.42% | 1299 | 0.47% | 2453 | 0.89% | 276023 |
| Black | 240 | 1.64% | 493 | 3.36% | 733 | 5.00% | 38 | 0.26% | 67 | 0.46% | 105 | 0.72% | 14657 | |
| Latino | 451 | 2.98% | 587 | 3.88% | 1038 | 6.85% | 84 | 0.55% | 66 | 0.44% | 150 | 0.99% | 15148 | |
| Asian | 278 | 3.91% | 332 | 4.67% | 610 | 8.58% | 21 | 0.30% | 29 | 0.41% | 50 | 0.70% | 7106 | |
| Other | 103 | 4.11% | 72 | 2.87% | 175 | 6.98% | 8 | 0.32% | 7 | 0.28% | 15 | 0.60% | 2507 | |
| 70–79 | White | 10924 | 8.06% | 14772 | 10.90% | 25696 | 18.96% | 2088 | 1.54% | 2664 | 1.97% | 4752 | 3.51% | 135552 |
| Black | 272 | 3.62% | 509 | 6.78% | 781 | 10.41% | 35 | 0.47% | 72 | 0.96% | 107 | 1.43% | 7504 | |
| Latino | 358 | 6.64% | 435 | 8.06% | 793 | 14.70% | 83 | 1.54% | 82 | 1.52% | 165 | 3.06% | 5393 | |
| Asian | 185 | 8.30% | 209 | 9.37% | 394 | 17.67% | 16 | 0.72% | 22 | 0.99% | 38 | 1.70% | 2230 | |
| Other | 74 | 7.22% | 83 | 8.12% | 157 | 15.33% | 18 | 1.76% | 13 | 1.27% | 31 | 3.03% | 1024 | |
| 80–87 | White | 5222 | 12.70% | 8166 | 19.86% | 13388 | 32.57% | 1228 | 2.99% | 1988 | 4.84% | 3216 | 7.82% | 41110 |
| Black | 88 | 5.55% | 197 | 12.43% | 285 | 17.98% | 17 | 1.07% | 37 | 2.33% | 54 | 3.41% | 1585 | |
| Latino | 102 | 9.55% | 143 | 13.39% | 245 | 22.94% | 25 | 2.34% | 36 | 3.37% | 61 | 5.71% | 1068 | |
| Asian | 58 | 12.01% | 76 | 15.73% | 134 | 27.74% | 9 | 1.86% | 17 | 3.52% | 26 | 5.38% | 483 | |
| Other | 27 | 12.27% | 37 | 16.82% | 64 | 29.09% | 4 | 1.82% | 8 | 3.64% | 12 | 5.45% | 220 | |
Information on sex is missing for 173 persons
Differences in Nonexudative and Exudative AMD Prevalence and Incidence Rates by Race
There were a total of 113,234 persons diagnosed with nonexudative AMD, for an overall prevalence of 5.01%. (Table 3) Whites had the highest prevalence of nonexudative AMD (5.40%), followed by Asian Americans (5.04%), Latinos (3.81%) and blacks (3.62%). The rate ratio test, which compares the prevalence of nonexudative AMD in each race with the prevalence in whites, showed significantly lower prevalence rates of nonexudative AMD in all of the other races relative to whites (p<0.05 for all comparisons).
Table 3.
Prevalence and Incidence Rates for Nonexudative and Exudative Age-Related Macular Degeneration Stratified by Race
| Dry ARMD | Wet ARMD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Prevalence | Incidence | Prevalence | Incidence | |||||||
| Strata | N Total | Weights | N | Rate | N | Rate | N | Rate | N | Rate |
| Overall | 2259061 | 113234 | 5.01% | 69521 | 1.16% | 17181 | 0.76% | 10635 | 0.17% | |
| Race | ||||||||||
| Whites | 1535008 | 82818 | 5.40% | 51886 | 1.18% | 12857 | 0.84% | 8100 | 0.18% | |
| 40–50 | 551213 | 0.33 | 7475 | 1.36% | 5371 | 0.32% | 703 | 0.13% | 484 | 0.03% |
| 50–60 | 531039 | 0.26 | 16689 | 3.14% | 11699 | 0.74% | 1732 | 0.33% | 1255 | 0.08% |
| 60–70 | 276062 | 0.17 | 19562 | 7.09% | 12690 | 1.83% | 2453 | 0.89% | 1606 | 0.22% |
| 70–80 | 135578 | 0.15 | 25702 | 18.96% | 15379 | 4.52% | 4752 | 3.50% | 2955 | 0.76% |
| 80 and over | 41116 | 0.09 | 13390 | 32.57% | 6747 | 8.61% | 3217 | 7.82% | 1800 | 1.81% |
| Age-adjusted rates | 8.08% | 2.02% | 1.47% | 0.34% | ||||||
| Blacks | 78315 | 2836 | 3.62% | 1889 | 0.85% | 366 | 0.47% | 243 | 0.11% | |
| 40–50 | 27696 | 0.42 | 356 | 1.29% | 260 | 0.32% | 27 | 0.10% | 19 | 0.02% |
| 50–60 | 26869 | 0.26 | 680 | 2.53% | 483 | 0.61% | 73 | 0.27% | 55 | 0.07% |
| 60–70 | 14659 | 0.16 | 733 | 5.00% | 472 | 1.25% | 105 | 0.72% | 68 | 0.17% |
| 70–80 | 7504 | 0.11 | 781 | 10.41% | 514 | 2.58% | 107 | 1.43% | 69 | 0.32% |
| 80 and over | 1587 | 0.06 | 286 | 18.02% | 160 | 4.69% | 54 | 3.40% | 32 | 0.83% |
| Age-adjusted rates | 4.11% | 1.03% | 0.57% | 0.14% | ||||||
| Latinos | 99518 | 3793 | 3.81% | 2519 | 0.93% | 591 | 0.59% | 405 | 0.15% | |
| 40–50 | 44673 | 0.46 | 685 | 1.53% | 475 | 0.38% | 97 | 0.22% | 71 | 0.06% |
| 50–60 | 33234 | 0.26 | 1032 | 3.11% | 714 | 0.78% | 118 | 0.36% | 77 | 0.08% |
| 60–70 | 15150 | 0.15 | 1038 | 6.85% | 693 | 1.89% | 150 | 0.99% | 111 | 0.29% |
| 70–80 | 5393 | 0.09 | 793 | 14.70% | 484 | 3.67% | 165 | 3.06% | 109 | 0.75% |
| 80 and over | 1068 | 0.04 | 245 | 22.94% | 153 | 6.58% | 61 | 5.71% | 37 | 1.39% |
| Age-adjusted rates | 4.74% | 1.24% | 0.83% | 0.21% | ||||||
| Asians | 44103 | 2221 | 5.04% | 1395 | 1.17% | 217 | 0.49% | 134 | 0.11% | |
| 40–50 | 19082 | 0.42 | 402 | 2.11% | 270 | 0.49% | 32 | 0.17% | 21 | 0.04% |
| 50–60 | 15200 | 0.28 | 681 | 4.48% | 457 | 1.10% | 71 | 0.47% | 49 | 0.11% |
| 60–70 | 7108 | 0.16 | 610 | 8.58% | 374 | 2.29% | 50 | 0.70% | 30 | 0.17% |
| 70–80 | 2230 | 0.10 | 394 | 17.67% | 225 | 4.23% | 38 | 1.70% | 22 | 0.36% |
| 80 and over | 483 | 0.04 | 134 | 27.74% | 69 | 7.15% | 26 | 5.38% | 12 | 1.00% |
| Age-adjusted rates | 6.38% | 1.59% | 0.70% | 0.15% | ||||||
| Others | 16018 | 731 | 4.56% | 467 | 1.04% | 81 | 0.51% | 61 | 0.13% | |
| 40–50 | 6884 | 0.51 | 144 | 2.09% | 100 | 0.49% | 11 | 0.16% | 11 | 0.05% |
| 50–60 | 5383 | 0.27 | 191 | 3.55% | 126 | 0.82% | 12 | 0.22% | 11 | 0.07% |
| 60–70 | 2507 | 0.13 | 175 | 6.98% | 121 | 1.96% | 15 | 0.60% | 11 | 0.17% |
| 70–80 | 1024 | 0.07 | 157 | 15.33% | 90 | 3.53% | 31 | 3.03% | 22 | 0.78% |
| 80 and over | 220 | 0.03 | 64 | 29.09% | 30 | 7.45% | 12 | 5.45% | 6 | 1.18% |
| Age-adjusted rates | 4.73% | 1.16% | 0.57% | 0.15% | ||||||
The incidence and prevalence rates listed in bold are age-adjusted for each race.
The unbolded rates in the table are unadjusted.
AMD = age-related macular degeneration; C.I. = 95% confidence interval
In the medical plan, there were 69,521 incident cases of nonexudative AMD, for an incidence rate of 1.16%. (Table 3) The incidence of nonexudative AMD was highest in whites (1.18%) and Asian Americans (1.17%) followed by Latinos (0.79%) and blacks (0.72%). When comparing the incident rates of nonexudative AMD using the rate ratio test, there was no difference in the incidence of nonexudative AMD between Asian Americans and whites while blacks (p<0.05) and Latinos (p<0.05) had significantly lower incidence rates of nonexudative AMD compared with whites.
In the overall sample, there were 17,181 cases of exudative AMD, yielding an exudative AMD prevalence of 0.76%. The prevalence of exudative AMD was highest in whites (0.84%) and considerably lower for Latinos (0.59%), Asian Americans (0.49%), and blacks (0.47%). When comparing the prevalence rate ratios, all three of the other races had lower prevalence rates of exudative AMD compared with whites (p<0.05 for all comparisons). Similar findings were observed when comparing the incidence rate ratios for exudative AMD among each of the races (p<0.05 for all comparisons) (Table 3).
Multivariable Analyses
Cox proportional hazard regression analysis was performed to determine the influence of race on the hazard of developing nonexudative and exudative AMD. When performing the analysis, it was noted that the key assumption relating to proportional hazards was violated. According to this assumption, the relative hazard of developing AMD in different races should be constant regardless of age, but what we found was that the relative hazard between races was not consistent over time and varied depending on the age that was being studied. Therefore, to properly run the regression models, it was necessary to include interactions of race with age. Table 4 shows the adjusted hazards of developing nonexudative and exudative AMD stratified by race and age.
Table 4.
The Multivariable Adjusted Hazard of Developing Nonexudative or Exudative AMD Stratified by Race and Age
| Nonexudative AMD |
Exudative AMD |
|||||||
|---|---|---|---|---|---|---|---|---|
| Adjusted HR*$ |
95% Confidence Limits | p-value | Adjusted HR*$ |
95% Confidence Limits | p-value | |||
| Blacks at age 60 | 0.75 | 0.71 | 0.79 | <.0001 | 0.70 | 0.59 | 0.83 | <0.0001 |
| Blacks at age 80 | 0.56 | 0.52 | 0.60 | <.0001 | 0.45 | 0.37 | 0.54 | <0.0001 |
| Latinos at age 60 | 0.99 | 0.94 | 1.04 | 0.65 | 1.28 | 1.13 | 1.45 | <0.0001 |
| Latinos at age 80 | 0.82 | 0.76 | 0.88 | <.0001 | 0.89 | 0.76 | 1.05 | 0.17 |
| Asian Americans at age 60 | 1.28 | 1.20 | 1.36 | <.0001 | 1.08 | 0.89 | 1.31 | 0.44 |
| Asian Americans at age 80 | 0.92 | 0.83 | 1.02 | 0.11 | 0.54 | 0.40 | 0.73 | <0.0001 |
Multivariable analyses adjusted for age, sex, household net worth, education level, geographic region of residence within the US, systemic hypertension, skin cancer, anemia, heart disease, myocardial infarction, stroke, peripheral vascular disease, renal disease, systemic hypotension, obesity, hyperlipidemia, diabetes mellitus, hyperlipidemia, coagulopathies, open-angle glaucoma, cataract, pseudophakia / aphakia, and diabetic retinopathy
Reference group: whites of similar ages
After adjusting for confounding factors, at age 60, blacks (adjusted HR = 0.75 (95% CI: 0.71–0.79)) had a 25% decreased hazard of developing nonexudative AMD and at age 80 (HR = 0.56 (95% CI: 0.52–0.60)) they had a 44% decreased hazard of nonexudative AMD relative to whites. Blacks were also at a decreased hazard for exudative AMD compared to similarly aged whites at both ages 60 and 80, (adjusted HR= 0.70 (95% CI: 0.59–0.83)) and (adjusted HR = 0.45 (95% CI: 0.37–0.54)) respectively.
The hazard for nonexudative AMD among Latinos at age 60 was no different than whites (adjusted HR = 0.98 (95% CI: 0.94–1.04)), but at age 80, there was an 18% decreased hazard (adjusted HR = 0.82 (95% CI: 0.76–0.88)) of nonexudative AMD. However, Latinos had a 28% significantly increased hazard of exudative AMD at age 60 (adjusted HR = 1.28 (95% CI: 1.13–1.45) though there was no significantly different hazard of developing exudative AMD between Latinos and whites at age 80 (adjusted HR = 0.89 (95% CI: 0.76–1.05)).
Asian Americans had a 28% increased hazard (adjusted HR=1.28 (95% CI: 1.21–1.36)) for nonexudative AMD at age 60 relative to whites. However, there was no difference between the groups at age 80 (adjusted HR = 0.82 (95% CI: 0.83–1.02). The hazard of exudative AMD was no different among Asian Americans and whites at age 60 (adjusted HR = 1.08 (95% CI: 0.89–1.31) although by age 80, Asian Americans had a 46% decreased hazard of developing exudative AMD (adjusted HR=0.54 95% CI: 0.40–0.73)).
Due to possible ambiguity in whether enrollees coded exclusively with the ICD-9CM billing code of 362.50 (macular degeneration senile unspecified) had nonexudative or exudative age-related macular degeneration, a sensitivity analysis was conducted. There were no materially significant differences in the results of the Cox regression models for nonexudative or exudative AMD when persons with the billing code 362.50 were omitted (data not shown).
DISCUSSION
In this large national study, we followed a cohort of individuals of each of the four major races longitudinally over time to assess for differences among the races in the hazard of developing nonexudative and exudative AMD. After adjustment for a number of key confounding factors in the multivariable regression analysis, we identified several important differences in the rates of developing AMD among the races. Our analysis confirms the findings of many other studies, demonstrating significantly lower rates of nonexudative and exudative AMD at ages 60 and 80 in blacks relative to whites. No differences were noted in rates of nonexudative AMD between Latinos and whites at age 60, however by age 80 the hazard of developing nonexudative AMD was lower in Latinos relative to whites. In contrast, Latinos exhibited higher rates of exudative AMD relative to whites at age 60, though this difference became insignificant by age 80. The only group found to have and increased hazard of nonexudative AMD relative to whites were 60 year old Asian Americans, though by age 80 no significant differences were noted between these two races. Below are comparisons among the findings from the present analysis with other published studies in the literature.
Blacks
Several large population-based studies, including the Atherosclerosis Risk in Communities Study, the MESA study, and the Salisbury Eye Evaluation Project have reported decreased risk of early (non-exudative) AMD among blacks as compared with whites.7,10,11 Other population-based studies (the Baltimore Eye Survey and NHANES III) also showed less early AMD in blacks relative to whites, though the findings of these studies did not reach statistical significance.5,6 Our analysis confirmed the findings of these population-based studies, demonstrating a 25% decreased hazard of developing nonexudative AMD for blacks at age 60 (p<0.0001) and a 44% decreased hazard of nonexudative AMD at age 80 (p<0.0001) when compared to similar aged whites.
While several studies describe reduced rates of exudative AMD among blacks relative to whites, only two studies have been able to show a statistically significant difference between the two races. 6,17 All of the existing population-based studies which have attempted to compare rates of exudative AMD between blacks and whites have been limited due to small numbers of blacks with exudative AMD (ranging from only 0 to 7 individuals in each study). As many of these studies have acknowledged, they were under-powered to detect a true difference in the rate of exudative AMD between the two races. The present analysis had adequate numbers of blacks (366 persons) and whites (12,857 persons) with exudative AMD to make comparisons among the groups. Our results corroborate the findings of a study by Javitt and colleagues who performed a similar analysis by using Medicare claims data and showed significantly lower rates of exudative AMD in blacks relative to whites.17
Latinos
The prevalence of nonexudative AMD in Latinos (3.81%) in our study was similar to that reported in the MESA study (4.0%), but considerably lower than three other population-based studies (7.0–27.9%).4,5, 8–10 The majority of participants in the latter three studies were of Mexican American ancestry. According to the US Census Bureau, one-third of all Latinos residing in the US originated from Latin American countries other than Mexico.18 Therefore, the rates of AMD generated from these three population-based studies may not be fully reflective of all Latinos residing in the US. For example, Emanuelli and colleagues reported that Puerto Rican Islanders have a nonexudative AMD prevalence rate of only 2.1%.19 Studies which focus predominantly on Mexican-Americans may overestimate the actual prevalence of nonexudative AMD, if generalized to all Latinos. Differences in study design, including the definition used to characterize persons with nonexudative AMD may also contribute to differences in rates noted in the various studies.
In this study we found that Latinos at age 60 had a similar hazard of developing nonexudative AMD relative to whites, but had an 18% decreased hazard by age 80 (p<0.0001). Three previous studies which compared early AMD in whites versus Latinos found no significant differences between these groups, but did not stratify the results by age, making direct comparisons difficult.4,5,10 Our analyses also demonstrated that Latinos had a significantly increased hazard for exudative AMD at age 60 relative to whites (p<0.0001) which is contrary to what has been previously reported, 4,5,10 though at age 80 this hazard was no longer significantly different for Latinos compared to whites (p=0.17). Comparing our findings with others in the existing literature is difficult since these other studies had so few Latinos with exduative AMD.
Asian Americans
While several papers have reported the prevalence of AMD among Asians residing in China, Japan, and other Asian countries, we are aware of only one population-based study that examined rates of AMD among Asian Americans (specifically, Chinese Americans) residing in the US. 10, 20–23 In the present analysis, Asian Americans had a 28% increased hazard for developing nonexudative AMD at age 60, but this difference no longer was significant at age 80 relative to whites. Similar hazards were found when comparing 60 year old Asian Americans to whites for exudative AMD, but the hazard was found to be significantly decreased for 80 year old Asian Americans. Our findings differ from those of the MESA study, which showed a similar rate of early AMD for Chinese Americans compared to whites (OR: 0.74, CI: 0.48–1.15), but a significantly higher risk for exudative AMD (age- and sex-adjusted OR: 4.30, CI:1.30–14.27).10 Reasons for these differences may include study design, particularly the fact that our analysis included Asians of many ethnicities, not just of Chinese ancestry, which was the predominant Asian ethnicity represented in MESA. Another difference between the two studies was the age of the participants, with the mean age of the MESA study being roughly 10 years older than ours. This dissimilarity in study sample may account for why we were able to detect an increased hazard at age 60 and yet have no significant difference at age 80 for nonexudative AMD, similar to the findings of the MESA study. The wide confidence interval for estimating the risk of exudative AMD in MESA also suggests that there is a great deal of variability in their estimates, likely resulting from their small sample size and could explain the difference in risk between the two studies.
Recently, Kawasaki and colleagues (2010) performed a meta-analysis of data from four large population-based studies conducted in Asia along with Chinese Americans from the MESA study. These researchers found that Asians aged 40–79 had a prevalence rate of 6.8% for early AMD and 0.56% for late AMD.24 Despite major differences in study design between these population-based studies included in the meta-analysis and the methods we used, the prevalence estimates for early and late AMD are remarkably similar among the two studies.
One factor that may account for some of the differences in risk of exudative AMD among the races is tobacco use, a known risk factor for exudative AMD.25 A recent study by the Kaiser Family Foundation compared smoking rates among races within the US and showed that both Latinos and Asians were less likely to smoke than whites.26 It is possible that the higher hazards of exudative AMD observed among whites in this study relative to other races may be due, in part, to greater tobacco use in persons of this race. A similar finding has been observed in the Hisayama and Fungata studies. When comparing rates of exudative AMD in Japanese males and females, these studies report higher exudative AMD rates among males, which may be attributable to greater tobacco use among Japanese males .20,21 Unfortunately, our data source does not contain information on smoking so we were not able to explore how tobacco use may affect our study findings.
Study Strengths and Limitations
A major advantage of using health care claims data, relative to other data sources to study the epidemiology of AMD is the large sample size and adequate representation of racial minorities. In this analysis, there were 78,315 blacks, 99,518 Latinos and 44,103 Asian Americans. Moreover, the proportion of individuals of different races in this cohort is similar to the proportion of individuals of each of these races in the most recent US Census estimates.27 In addition, the 17,181 individuals with exudative AMD in this database allowed us to estimate prevalence and incidence rates, by race, for this less-common, though sight-threatening form of AMD. Another strength of this analysis is that our findings may be a better reflection of the prevalence and incidence of AMD in the community, since we have access to eye care provider visit data from throughout the United States, as opposed to findings from other studies which are restricted to a specific academic medical center or city of the country or individuals willing to agree to participate in a population-based study.
There are several limitations that need to be acknowledged. This study only included patients insured through one specific managed care network. Additional studies are necessary to study rates of AMD in other groups including uninsured or underinsured patients, those who receive care through Veterans Affairs Medical Centers, or persons covered through different insurance plans. Also, although all the beneficiaries had insurance coverage, they were not required to obtain regular eye exams. Therefore, selection bias may result in an underestimation of our incidence and prevalence rates, particularly among the oldest of the old, who may have difficulty accessing eye care providers and may have affected persons of different races differently. Second, since the data we had access to was de-identified to us, we had no way of verifying each enrollee’s race. The race of some enrollees may have been misclassified, and if there was differential misclassification of race, this could affect the results. A third limitation is the inability to factor important clinical information into the model that is not included in claims databases such as visual acuity, disease specifics and severity, and the results of ancillary tests (ex. retinal photographic assessment, fluorescein angiography) used to detect the presence or absence of disease. Unlike many population based studies that have retina specialists study retinal photographs of the participants to identify the presence or absence of AMD, when using claims data, we are relying upon the eye care providers to properly diagnose patients with AMD. Given that some providers may be better than others at detecting this condition, there is possibility that some individuals may have been misdiagnosed. While all of the beneficiaries in this study were required to have at least one visit to an eye care provider, there is no way of knowing whether the beneficiary underwent a dilated funduscopic examination during these visits. Since AMD is much easier to identify after dilation, we may be underestimating the true prevalence of these conditions relative to some of the other studies.
Finally, comparing findings among the various studies in the literature with ours can be difficult due to differences in the disease definitions used by different investigators. Several population-based studies report prevalence rates for “early” versus “late” macular degeneration. While many individuals with “early” AMD have nonexudative AMD, those with “late” AMD may include those with geographic atrophy limiting the ability to directly compare those with “late” AMD and those with billing codes for “exudative” AMD. Furthermore, the diagnostic billing code “nonexudative AMD” captures a spectrum of different manifestations of nonexudative AMD from a single druse to geographic atrophy. Without access to clinical data such as visual acuity levels or the results of Amsler grid testing, we cannot distinguish between those who have very early manifestations of this disease and those who are visually impaired by nonexudative AMD.
Implications
The proportion of the U.S. population who are Latino or Asian American is expected to rise to 33% by 2050, and will number over 135 million people. 28 Understanding rates of AMD in these groups is imperative, so that clinicians can have better insight into who is most at risk for developing this disease and health policy-makers can use this information to help guide decisions pertaining to health care resource allocation.
Supplementary Material
Acknowledgments
Funding: Grant support from National Eye Institute K23 Mentored Clinician Scientist Award (JDS; EY019511), Blue Cross Blue Shield of Michigan Foundation (JDS), an unrestricted grant from Research to Prevent Blindness, Research to Prevent Blindness Lew R. Wasserman Merit Award (DCM), Research to Prevent Blindness Sybil B. Harrington Special Scholar Award for Macular Degeneration (DNZ).
Biographies
Joshua D. Stein is an Assistant Professor of Ophthalmology and Visual Sciences at the University of Michigan. He is a health services researcher whose primary research interest involves using large health care claims databases to study utilization patterns and outcomes of eye care throughout the United States.
Brian L. VanderBeek is a Clinical Lecturer and Fellow on the Retina Service at the University of Michigan Department of Ophthalmology and Visual Sciences. He has a background in public health and his research interests involve studying the epidemiology of retinal conditions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
-
B.The authors have no proprietary interest in any material discussed in this manuscript.
- Financial Disclosures: Musch: Pfizer, Inc, Glaukos Corp. Stein: Pfizer, Inc. All other authors: None
-
C.Author Contributions:
-
Preparation of manuscript: BVB, DCM, DNZ, JDSDesign and conduct of study: BN, BVB, DCM, DNZ, JDS, NTCollection and management of study data:JDS, NTAnalysis of data: BN, BVB, DCM, DNZ,JDS, NT
-
-
D.Statement of Conformity: The University of Michigan Institutional Review Board determined this study was exempt from requiring IRB approval since the data are completely de-identified.
-
E.Other acknowledgements: None
REFERENCES
- 1.Sloan FA, Brown DS, Carlisle ES, et al. Estimates of incidence rates with longitudinal claims data. Arch Ophthalmology. 2003;121(10):1462–1468. doi: 10.1001/archopht.121.10.1462. [DOI] [PubMed] [Google Scholar]
- 2.Congdon N, O'Colmain B, Klaver CC, et al. Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmology. 2004;122(4):477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 3.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99(6):933–943. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 4.Cruickshanks KJ, Hamman RF, Klein R, et al. The prevalence of age-related maculopathy by geographic region and ethnicity. The Colorado-Wisconsin Study of Age-Related Maculopathy. Arch Ophthalmol. 1997;115(2):242–250. doi: 10.1001/archopht.1997.01100150244015. [DOI] [PubMed] [Google Scholar]
- 5.Klein R, Klein BE, Jensen SC, et al. Age-related maculopathy in a multiracial United States population: the National Health and Nutrition Examination Survey III. Ophthalmology. 1999;106(6):1056–1065. doi: 10.1016/S0161-6420(99)90255-5. [DOI] [PubMed] [Google Scholar]
- 6.Friedman DS, Katz J, Bressler NM, et al. Racial differences in the prevalence of age-related macular degeneration: the Baltimore Eye Survey. Ophthalmology. 1999;106(6):1049–1055. doi: 10.1016/S0161-6420(99)90267-1. [DOI] [PubMed] [Google Scholar]
- 7.Klein R, Clegg L, Cooper LS, et al. Prevalence of age-related maculopathy in the Atherosclerosis Risk in Communities Study. Arch Ophthalmol. 1999;117(9):1203–1210. doi: 10.1001/archopht.117.9.1203. [DOI] [PubMed] [Google Scholar]
- 8.Los Angeles Latino Eye Study Group. Prevalence of age-related macular degeneration in Latinos: the Los Angeles Latino eye study. Ophthalmology. 2004;111(7):1288–1297. doi: 10.1016/j.ophtha.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 9.Muñoz B, Klein R, Rodriguez J, et al. Prevalence of age-related macular degeneration in a population-based sample of Latino people in Arizona: Proyecto VER. Arch Ophthalmol. 2005;123(11):1575–1580. doi: 10.1001/archopht.123.11.1575. [DOI] [PubMed] [Google Scholar]
- 10.Klein R, Klein BE, Knudtson MD, et al. Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the multi-ethnic study of atherosclerosis. Ophthalmology. 2006;113(3):373–380. doi: 10.1016/j.ophtha.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Bressler SB, Muñoz B, Solomon SD, et al. Racial differences in the prevalence of age-related macular degeneration: the Salisbury Eye Evaluation (SEE) Project. Arch Ophthalmol. 2008;126(2):241–245. doi: 10.1001/archophthalmol.2007.53. [DOI] [PubMed] [Google Scholar]
- 12.US Census Bureau. [Accessed July 8, 2010];US Race/Ethnicity population update press release. 2008 May 1; Available at http://www.census.gov/Press-Release/www/releases/archives/population/011910.html.
- 13.Physician International Classification of Diseases (ICD-9CM); 9th revision, Clinical Modification. Vol 1 and 2. American Medical Association Press; 2006. [Google Scholar]
- 14.Current Procedural Terminology (cpt 2006) Professional Edition. American Medical Association Press; 2006. [Google Scholar]
- 15.DeFrank JT, Bowling JM, Rimer BK, et al. Triangulating differential nonresponse by race in a telephone survey. Prev Chronic Dis. 2007;4(3):A60. [PMC free article] [PubMed] [Google Scholar]
- 16.Cnaan A, Ryan L. Survival analysis in natural history studies of disease. Statistics in Medicine. 1989;Vol. 8(10):1255–1268. doi: 10.1002/sim.4780081009. [DOI] [PubMed] [Google Scholar]
- 17.Javitt JC, Zhou Z, Maguire MG, et al. Incidence of Exudative Age-Related Macular Degeneration among Elderly Americans. Ophthalmology. 2003;110(8):1534–1539. doi: 10.1016/S0161-6420(03)00495-0. [DOI] [PubMed] [Google Scholar]
- 18.US Census Bureau. [Accessed July 8, 2010];Facts for Features: Hispanic Heritage Month 2009. 2009 July 15th; Available at http://www.census.gov/Press-Release/www/releases/archives/facts_for_features_special_editions/013984.html.
- 19.Emanuelli A, Izquierdo NJ, Townsend W. Eye diseases in Puerto Rico. P R Health Sci J. 2005;24(4):287–290. [PubMed] [Google Scholar]
- 20.Yasuda M, Kiyohara Y, Hata Y, et al. Nine-year incidence and risk factors for age-related macular degeneration in a defined Japanese population the Hisayama study. Ophthalmology. 2009;116(11):2135–2140. doi: 10.1016/j.ophtha.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Kawasaki R, Wang JJ, Ji GJ, et al. Prevalence and risk factors for age-related macular degeneration in an adult Japanese population: the Funagata study. Ophthalmology. 2008;115(8):1376–1381. doi: 10.1016/j.ophtha.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Chen SJ, Cheng CY, Peng KL, et al. Prevalence and associated risk factors of age-related macular degeneration in an elderly Chinese population in Taiwan: the Shihpai Eye Study. Invest Ophthalmol Vis Sci. 2008;49(7):3126–3133. doi: 10.1167/iovs.08-1803. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Xu L, Jonas JB, et al. Prevalence of age-related maculopathy in the adult population in China: the Beijing eye study. Am J Ophthalmol. 2006;142(5):788–793. doi: 10.1016/j.ajo.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Kawasaki R, Yasuda M, Song SJ, et al. The prevalence of age-related macular degeneration in Asians: a systematic review and meta-analysis. Ophthalmology. 2010;117(5):921–927. doi: 10.1016/j.ophtha.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Klein R, Knudtson MD, Cruickshanks KJ, Klein BE. Further observations on the association between smoking and the long-term incidence and progression of age-related macular degeneration: the Beaver Dam Eye Study. Arch Ophthalmol. 2008;126(1):115–121. doi: 10.1001/archopht.126.1.115. [DOI] [PubMed] [Google Scholar]
- 26. [Accessed October 2, 2010];United States: Percent of Adults who Smoke by Race/Ethnicity, 2008. 2010 September; Available at http://www.statehealthfacts.org/profileind.jsp?ind=82&cat=2&rgn=1.
- 27.US Census Bureau. [Accessed October 2, 2010];USA Quick Facts from the US Census Bureau. Available at http://quickfacts.census.gov/qfd/states/00000.html.
- 28.US Census Bureau. [Accessed October 2, 2010];Population Projections. 2009 September; Available at http://www.census.gov/population/www/projections/usinterimproj/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.