Abstract
The salivary glands are important effector sites for IgA-mediated humoral immunity to protect oral surfaces. Within murine submandibular glands (SMG), we identified a memory CD8 T-cell population that exhibited a unique cell-surface phenotype distinct from memory CD8 T cells in spleen but similar to memory T cells resident in the intraepithelial lymphocyte compartment of the intestinal mucosa. In mice immune to lymphocytic choriomeningitis virus (LCMV) or vesicular stomatitis virus(VSV), virus-specific memory CD8 T cells with this unusual phenotype were present in SMG at remarkably high frequencies. LCMV-specific memory CD8 T cells in SMG showed potent functional activities in vivo, including cytokine-induced bystander proliferation, antigen-triggered IFNγ production, and viral clearance. Adoptive transfer experiments further revealed that the capacity to accumulate in SMG decreased during CD8 T-cell differentiation and that SMG CD8 T cells were poorly replenished from the circulation, indicating that they were tissue-resident. Moreover, they preferentially relocalized within their tissue of origin after adoptive transfer and antigen rechallenge, thus revealing an imprinted differentiation status. Accumulation of memory CD8 T cells within SMG did not require local antigen presentation but was promoted by the epithelial differentiation molecule E-cadherin intrinsically expressed by these CD8 T cells. This finding extends the epithelial-restricted function of E-cadherin to an impact on lymphocyte accumulation within epithelial tissues.
Keywords: epithelial tissues, viral infections
The establishment of a multilayered memory T-cell system provides high flexibility to combat reinfections with different pathogens. Central memory T cells (TCM) build up a long-lasting pool of rapidly replicating cells in secondary lymphoid organs whereas effector memory T cells (TEM) patrolling blood, spleen, and nonlymphoid tissues are crucial to fighting incoming infections by immediate effector functions (1, 2). Parabiosis experiments, however, revealed that entry of blood-borne memory T cells into brain and gut lamina propria is restricted (3). Moreover, recent studies have suggested a prominent role of tissue-resident memory T cells (TRM) in providing local protection (4). Virus-specific CD8 TRM cells have been described in sensory ganglia, skin, brain, and intestinal mucosa (4–11). Common features of differently localized TRM cells are the expressions of the αEβ7 integrin CD103 and of CD69 (4, 5, 7, 9).
Submandibular glands (SMG) are accessory organs of the oral mucosa and well-characterized effector sites of the mucosal IgA response (12, 13). In addition to B cells, SMG also accommodate αβ and γδ T cells, NK cells, and other innate immune cell populations (13, 14). The glandular tissue further represents an important target organ for cytomegalovirus infections and for autoimmune reactions (15, 16). As SMG are exocrine epithelial tissues, the epithelial differentiation molecule E-cadherin is highly expressed in these organs. E-cadherin is a well-known homotypic cell–cell adhesion molecule for epithelial cells, and heterotypic binding of E-cadherin on epithelial tissues to CD103 on lymphocytes is well documented (17–19). In contrast, the functional role of E-cadherin on the surface of lymphocytes has not yet been addressed in vivo (20–24).
Examining the tissue distribution of virus-specific memory CD8 T cells after systemic lymphocytic choriomeningitis virus (LCMV) infection, we detected an enrichment of LCMV-specific memory T cells in SMG that resembled TRM cells. In the present report, we characterize the unique phenotype and the functional features of these T cells.
Results
Accumulation of CD8 T Cells in SMG After LCMV Infection.
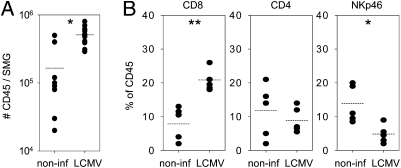
Infection of C57BL/6 (B6) mice with LCMV-WE (200 pfu) induces a robust virus-specific CD8 T-cell response followed by rapid virus clearance and stable memory formation. We observed that absolute numbers of CD45 leukocytes within SMG of LCMV-immune mice [4–16 wk post infection (p.i.)] increased approximately threefold compared with noninfected controls (Fig. 1A). Flow cytometric analysis further revealed a proportional increase of CD8 (8 ± 2% vs. 21 ± 1%) and a decrease of NKp46 (14 ± 2% vs. 5 ± 1%) cells in noninfected vs. infected mice (Fig. 1B). Nearly all CD8 cells isolated from SMG expressed αβ TCR and CD8 α- and β-chains, indicating that these cells represented CD8 T lymphocytes (Fig. S1A). Percentages of the other main leukocyte subsets—including CD4 T cells (12 ± 4%/9 ± 2%), CD19 B cells (26 ± 6%/29 ± 3%), CD11c/CD11b macrophages/DC (40 ± 11%/28 ± 5%), and Gr-1 granulocytes (3 ± 1%)—were comparable between infected and noninfected mice (Fig. 1B and Fig. S1B). Immunohistological examination of SMG sections from infected mice further showed a scattered distribution of CD45 and CD8 cells within the glandular tissue and no evidence of the formation of leukocyte clusters (Fig. S1C).
Fig. 1.
Accumulation of CD8 T cells in SMG of LCMV-immune mice. (A) Absolute numbers of CD45 leukocytes per SMG in noninfected vs. LCMV-immune B6 mice (4–16 wk p.i.). *P < 0.05. (B) Percentage of corresponding cell populations within CD45 cells of SMG in noninfected vs. LCMV-immune B6 mice. Dots represent values of SMG pools from three to five mice. *P < 0.05; **P < 0.01.
High Frequencies of Antigen-Specific Memory CD8 T Cells in SMG.
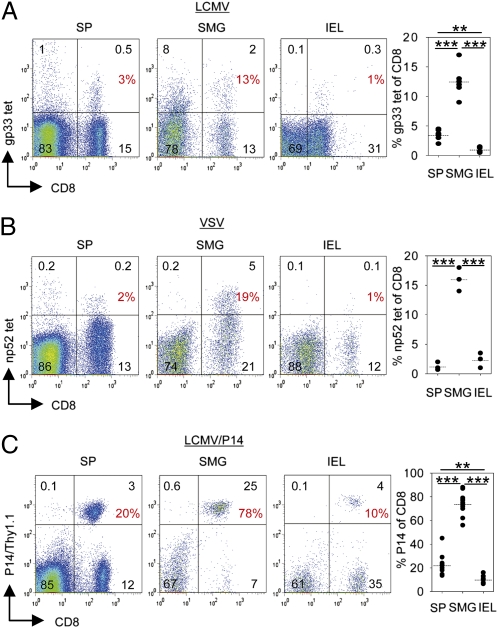
The frequencies of LCMV-specific CD8 T cells in SMG and spleen of LCMV-immune B6 mice were determined by MHC class I tetramers (gp33/H-2Db). Because SMG are part of the digestive tract and the intestine is known to harbor antigen-experienced CD8 T cells, intraepithelial lymphocytes (IEL) from gut epithelium of the small bowel were included in the analysis. As shown in Fig. 2A, percentages of gp33-specific memory CD8 T cells were considerably higher in SMG (13 ± 1%) than in IEL (1 ± 0.2%). In spleen, intermediate percentages (4 ± 0.4%) of LCMV-specific memory CD8 T cells were found. To determine whether the high frequencies of virus-specific CD8 T cells in SMG represented a phenomenon restricted to the LCMV infection, virus-specific memory CD8 T cells were analyzed in VSV-immune B6 mice (28 wk p.i.) by MHC class I tetramers (np52/H-2Kb). As depicted in Fig. 2B, 16 ± 1% of SMG CD8 memory T cells were specific for VSV np52 whereas the corresponding frequencies were considerably lower in IEL and spleen (2 ± 0.7% and 1 ± 0.4%). Similar results were obtained when VSV-immune mice were analyzed 4 wk p.i. (Fig. S2). To extend the analyses to a monoclonal antigen-specific system, LCMV gp33-specific CD8 T cells from P14 TCR transgenic mice were used. A tracer population of naive P14 CD8 T cells (Thy1.1+) was transferred into B6 recipient mice followed by LCMV infection. At the stationary phase of infection (4–16 wk p.i.), the frequencies of P14 memory T cells within the CD8 T-cell population isolated from SMG and spleen were determined. In LCMV-immune P14 chimeric mice, the tissue distribution of P14 memory T cells (spleen: 22 ± 3%; SMG: 74 ± 3%; IEL: 10 ± 1%) was comparable to non–TCR-transgenic memory CD8 T cells in LCMV-immune B6 mice. P14 memory T cells were around seven times more frequent among SMG CD8 T cells compared with CD8αβ IEL (Fig. 2C). Taken together, these data revealed a long-term persistence of virus-specific memory CD8 T cells within SMG after systemic viral infection with considerably higher frequencies than in the IEL compartment and in spleen.
Fig. 2.
High frequencies of antigen-specific memory CD8 T cells in SMG. (A) Percentages of CD8 memory T cells specific for LCMV gp33 peptide in spleen (SP), SMG, and IEL of LCMV-immune (4–16 wk p.i.) B6 mice determined by MHC class I tetramer (gp33/H-2Db) staining. (B) Percentages of CD8 memory T cells specific for VSV np52 in spleen (SP), SMG, and IEL of VSV-immune B6 mice determined by MHC class I tetramer (np52/H-2Kb) staining 28 wk p.i. (C) Percentages of CD8 memory T cells carrying the P14 TCR (Thy1.1) in spleen (SP), SMG, and IEL determined in LCMV-immune (4–16 wk p.i.) P14 chimeric mice. Representative dot plots and statistical graphs are shown. All samples were gated on CD45 cells. Percentages of CD8 T cells specific for the indicated markers are depicted. Dots in statistical graphs represent values from individual mice. **P < 0.01; ***P < 0.001.
CD8 T Cells in SMG Express a Unique Cell-Surface Phenotype.
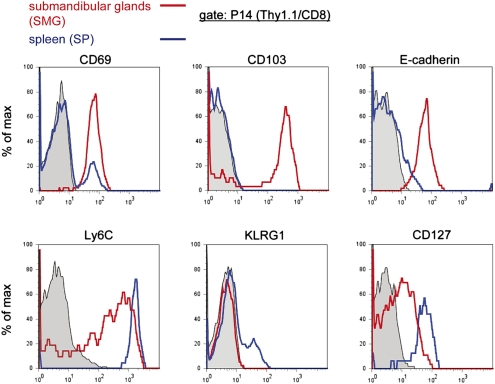
In striking contrast to splenic P14 memory T cells, P14 T cells isolated from SMG of LCMV-immune P14 chimeric mice expressed high levels of CD69, CD103, and E-cadherin (Fig. 3). Ly6C and CD127 expression levels were reduced, and P14 T cells positive for KLRG1 were lacking. CD44, CD62L, and CD27 could not be used for analyses because these markers were sensitive to collagenase treatment, required for efficient lymphocyte isolation from SMG. Importantly, non–TCR-transgenic CD8 T cells in SMG of LCMV-immune B6 mice showed the same cell-surface phenotype (Fig. S3A). The high levels of CD69 and CD103 in SMG P14 memory T cells were reminiscent of the previously described phenotype of P14 memory T cells isolated from the IEL compartment (7). In contrast to P14 memory T cells in SMG, P14 IEL were negative for Ly6C but could also be stained with E-cadherin–specific mAb (Fig. S3B).
Fig. 3.
Unique cell-surface phenotype of SMG CD8 memory T cells. P14 memory T cells from SMG (red line) and spleen (SP, blue line) of LCMV-immune (week 14 p.i.) P14 chimeric mice were analyzed with the markers indicated. Representative flow cytometry profiles are depicted. All samples were pregated on CD45 cells. Histograms were further gated on P14 T cells (Thy1.1/CD8). Isotype controls are shown in gray.
Functional Activities of SMG CD8 Memory T Cells.
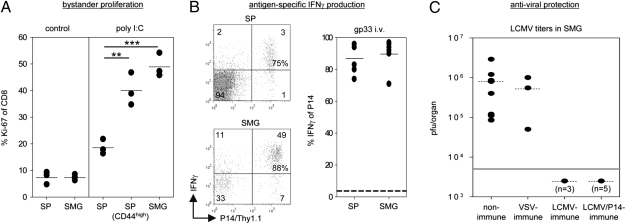
Poly I:C treatment is known to induce bystander proliferation of memory CD8 T cells via induction of type I IFN and IL-15 (25). To determine whether SMG memory CD8 T cells were capable of responding to this cytokine stimulation in vivo, LCMV-immune B6 mice were treated with poly I:C, and T-cell proliferation was determined by Ki-67 expression. As shown in Fig. 4A, poly I:C treatment induced Ki-67 expression in SMG CD8 T cells (49 ± 3%) at levels comparable to those in CD44high memory-phenotype CD8 T cells in the spleen (40 ± 4%). To evaluate the antigen-specific response of SMG CD8 T cells, we systemically administered the cognate LCMV gp33 peptide into LCMV-immune P14 chimeric mice. Soon (6 h) after gp33 peptide treatment, P14 T cells from SMG and spleen were isolated and directly stained for intracellular IFNγ. Similar percentages of SMG and splenic P14 memory T cells produced IFNγ (spleen: 87 ± 4%; SMG: 90 ± 4%) in response to gp33 antigen stimulation in vivo (Fig. 4B). P14 memory T cells from SMG restimulated with gp33 peptide in vitro were also able to produce IFNγ (19 ± 4%) and to degranulate (CD107a: 39 ± 6%), but the proportions of responding cells in these assays were about two- to threefold lower compared with gp33-restimulated splenic P14 memory T cells (CD107a: 78 ± 3%; IFNγ: 60± 8%) (Fig. S4A). To determine whether SMG CD8 T cells can provide immunity to local infection, LCMV titers in SMG were determined in different groups of mice 3 d after intraglandular (i.g.) infection of the salivary glands with the fast-replicating LCMV strain Docile. In nonimmune mice, this infection route led to significantly higher viral titers (∼10-fold) in SMG compared with spleen whereas systemic infection (i.v.) resulted in high titers in spleen but not in SMG (Fig. S4B). To prevent lymphocyte egress from nearby lymphoid tissues, mice were treated with FTY720 during viral rechallenge. The results from these experiments revealed that LCMV was completely cleared in SMG of LCMV-immune B6 and P14 chimeric mice whereas high LCMV titers were found in SMG of the nonimmune control (8 × 105 ± 3 × 105 pfu/SMG) and of VSV-immune mice (5 × 105 ± 3 × 105 pfu/SMG), confirming antigen specificity (Fig.4C). Neutralizing antibodies could not be detected in the sera of these LCMV-immune mice (Fig. S4C), rendering humoral immunity as the responsible effector mechanism in this experimental setting very unlikely.
Fig. 4.
Functional activities of SMG CD8 memory T cells. (A) Poly I:C (250 μg) was administered i.p. into LCMV-immune B6 mice, and intranuclear Ki-67 expression was evaluated 72 h later. Controls are derived from untreated LCMV-immune B6 mice. Percentages of splenic total CD8 T cells or CD44high CD8 T cells and total SMG CD8 T cells positive for Ki-67 are depicted. Dots represent values from individual mice. **P < 0.01; ***P < 0.001. (B) LCMV-immune P14 chimeric mice were injected (i.v.) with gp33 peptide (200 μg). Six hours later, P14 memory T cells from spleen (SP) and SMG were isolated and immediately stained for intracellular IFNγ. Shown are representative dot plots and a statistical graph depicting percentages of P14 memory T cells positive for IFNγ. Dots represent values from individual mice. Horizontal dashed black line represents mean percentage of P14 memory T cells positive for IFNγ from untreated LCMV-immune P14 chimeric mice. (C) The indicated groups of mice were infected i.g. with LCMV-Docile, and viral titers in SMG were determined on day 3 p.i. Dots represent titers in plaque-forming units per organ from individual mice. Horizontal gray line marks the detection limit (5 × 103 pfu/organ).
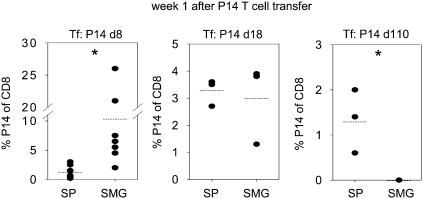
P14 Effector but Not Resting P14 Memory Cells Accumulate in SMG.
To test whether effector or memory CD8 T cells from spleen were able to accumulate in SMG in the absence of a LCMV infection, we adoptively transferred P14 effector (day 8 p.i.), late effector (day 18 p.i.), or resting memory (day 110 p.i.) cells from LCMV-immune P14 chimeric mice into B6 recipient mice. The recipient mice were analyzed 1 wk after cell transfer (Fig. 5). P14 effector (day 8) T cells were found at clearly higher frequencies in the CD8 T-cell population of SMG (10.4 ± 3.5%) compared with the spleen (1.3 ± 0.4%) whereas the relative tissue distribution of P14 late effector (day 18) T cells was similar between spleen (3.3 ± 0.3%) and SMG (3.0 ± 0.9%). In striking contrast, after transfer of resting P14 memory (day 110) T cells, we failed to recover any P14 T cells from SMG whereas 1.3 ± 0.4% of CD8 T cells in spleen carried the P14 TCR in the same mice. Taken together, these data indicate that the capacity to accumulate in SMG decreases during T-cell differentiation and further suggest that SMG CD8 T cells were not rapidly replenished from the circulation in the resting state.
Fig. 5.
Only P14 effector but not resting P14 memory T cells accumulate in SMG in the absence of a LCMV infection. P14 effector T cells (day 8 p.i., 5 × 106), P14 late effector T cells (day 18 p.i., 4 × 106), or resting P14 memory T cells (day 110 p.i., 2 × 106) from spleen of P14 chimeric mice were transferred (i.v.) into naive B6 mice. Percentages of CD8 T cells carrying the P14 TCR (Thy1.1) recovered from spleen (SP) and SMG of the recipient mice were determined 1 wk after adoptive transfer. Dots in statistical graphs represent values from individual mice. *P < 0.05.
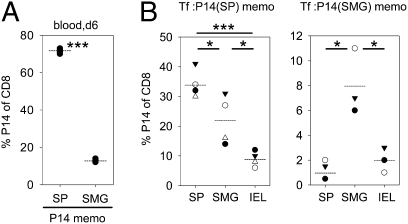
Tissue-Specific Accumulation of SMG P14 Memory T Cells After LCMV Rechallenge.
To assess the impact of the SMG environment during the differentiation of CD8 memory T cells, P14 memory T cells from SMG and spleen were transferred into B6 mice followed by LCMV infection. Six days later, the recall response in blood of the recipient mice was monitored. As shown in Fig. 6A, SMG P14 memory T cells were able to mount a secondary proliferative response in blood, but the magnitude of the response was considerably lower compared with splenic P14 memory T cells (13 ± 1% vs. 72 ± 1% of CD8 T cells carrying P14 TCR). To determine whether the origin of P14 memory T cells influenced their tissue distribution after recall, we examined these mice 3 wk p.i. (Fig. 6B). As expected from the marked difference in clonal burst after rechallenge, overall frequencies of secondary P14 memory T cells were considerably higher in mice that had been repopulated with splenic P14 memory T cells. In these mice, percentages of secondary P14 memory T cells among CD8 T cells were highest in spleen (34 ± 2%), followed by SMG (22 ± 4%) and IEL (9 ± 1%). Strikingly, mice repopulated with SMG P14 memory T cells showed higher frequencies in SMG (8 ± 2%), followed by IEL (2 ± 1%) and spleen (1 ± 0.4%). Thus, P14 memory T cells from SMG preferentially reaccumulate in SMG after LCMV rechallenge.
Fig. 6.
Tissue-specific accumulation of SMG P14 memory T cells after LCMV rechallenge. (A) B6 mice were seeded with 105 P14 memory T cells from spleen (SP) or SMG, and proliferative capacity was assessed in blood of the recipient mice 6 d after LCMV rechallenge. (B) Tissue distribution of P14 memory T cells derived from spleen (SP) or SMG determined 3 wk after retransfer and rechallenge. Dots in statistical graphs represent values from individual mice. *P < 0.05; ***P < 0.001.
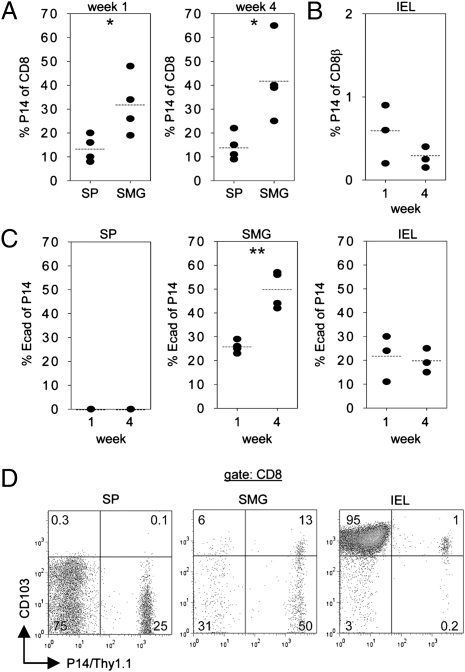
Accumulation of P14 Memory T Cells in SMG Is Independent of Local Antigen.
In the experimental conditions used here to generate LCMV-immune mice, LCMV was not detectable in SMG by virus plaque assay either at the acute or at the stationary phase of the infection (Fig. S5), suggesting that accumulation of LCMV-specific CD8 memory T cells in SMG was antigen independent. To provide further evidence for this notion, short-term (day 3) gp33 peptide-activated P14 T cells were transferred into B6 mice. One and four weeks after adoptive cell transfer, P14 T cells were traced in spleen, SMG, and IEL. As shown in Fig. 7 A and B, P14 T cells could be detected in all of these tissues, but their frequencies varied considerably. At both time points, frequencies of P14 T cells within the CD8 T-cell population were highest in SMG (week 1: 32 ± 6%; week 4: 42 ± 8%), lower in spleen (weeks 1 and 4: 14 ± 3%), and only marginal in IEL (week 1: 0.6 ± 0.2%; week 4: 0.3 ± 0.1%). Similar to the LCMV infection model, a significant proportion of P14 T cells (26 ± 1%) recovered from SMG expressed E-cadherin 1 wk after adoptive cell transfer. This proportion was further increased (50 ± 4%) 4 wk after transfer. E-cadherin was also found on a fraction (week 1: 22 ± 6%; week 4: 20 ± 3%) of the few P14 IEL but not on splenic P14 T cells (Fig. 7C). Next, CD103 expression on P14 T cells isolated from spleen, SMG, and small intestine epithelium was examined (Fig. 7D). CD103 was expressed at high levels by almost all (79 ± 7%) P14 T cells in the IEL compartment but not at all by P14 T cells from spleen. Among SMG P14 T cells, CD103high expression was found on a clearly smaller fraction (32 ± 7%) than in P14 IEL. Altogether, these data indicate that accumulation of antigen-specific memory CD8 T cells in SMG was independent of local antigen and also occurred in the absence of inflammatory conditions. In vitro-activated P14 T cells used for adoptive transfer completely lacked E-cadherin and CD103 surface expression (Fig. S6A), indicating that these adhesion molecules were induced in P14 T cells within glandular and small intestinal tissues. This was also confirmed by the observation that P14 T cells present in SMG at an early time point (day 3) after transfer did not express E-cadherin (Fig. S6B).
Fig. 7.
Antigen-independent accumulation of P14 T cells in SMG and tissue-specific induction of E-cadherin and CD103 expression. P14 T cells (5 × 106) activated in vitro with LCMV gp33 peptide for 3 d were transferred into naive B6 mice. Percentage of CD8 T cells carrying the P14 TCR (Thy1.1) recovered from spleen (SP) and SMG (A) and from IEL (B) were determined 1 and 4 wk after adoptive cell transfer. (C) E-cadherin expression by in vitro-activated and transferred P14 T cells (Thy1.1) recovered from spleen (SP), SMG, and IEL. (D) Representative dot plots depicting CD103 expression of P14 T cells (Thy1.1) collected from spleen (SP), SMG, and IEL 3 wk after adoptive transfer of in vitro-activated P14 T cells. All samples were pregated on CD45. Dots in statistical graphs represent values from individual mice. *P < 0.05; **P < 0.01.
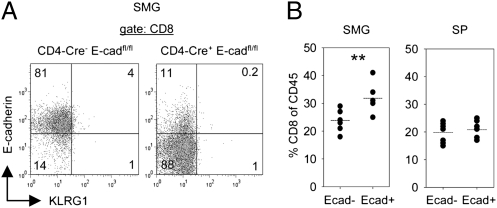
E-Cadherin Expressed by SMG CD8 T Cells Promotes Their Tissue Accumulation.
SMG CD8 T cells showed an intriguing E-cadherin expression. To examine whether E-cadherin was synthesized directly by CD8 T cells or acquired from the surrounding SMG epithelium that expresses high levels of E-cadherin, mice carrying a T-cell–specific deletion of the E-cadherin gene were analyzed. These mice were generated by breeding CD4-Cre transgenic mice with mice carrying a floxed allele of the E-cadherin gene. For the analysis, littermates carrying CD4-Cre+E-cadfl/fl and CD4-Cre−E-cadfl/fl genotypes were used. As depicted in Fig. 8A, E-cadherin staining of SMG CD8 T cells from LCMV-immune (4–16 wk p.i.) CD4-Cre+E-cadfl/fl mice decreased strongly compared with the respective cells derived from CD4-Cre−E-cadfl/fl mice. This result indicated that E-cadherin was synthesized by SMG CD8 T cells. Notably, E-cadherin ablation in SMG CD8 T cells did not restore KLRG1 expression in these cells (Fig. 8A). The role of E-cadherin in cell adhesion mediated by homotypic interactions is well known. Therefore, E-cadherin expressed by SMG CD8 T cells might be important for accumulation of these cells within the submandibular gland tissue. Indeed, percentages of CD8 T cells among CD45 leukocytes from SMG were significantly lower in LCMV-immune CD4-Cre+E-cadfl/fl (24 ± 1%) mice compared with CD4-Cre−E-cadfl/fl (32 ± 2%) mice (Fig. 8B). Importantly, the corresponding percentages did not differ in splenocytes (20 ± 1% vs. 21 ± 1%) of the same animals (Fig. 8B). Thus, these data indicate that E-cadherin expressed by SMG CD8 T cells promotes accumulation of these cells in this epithelial tissue.
Fig. 8.
E-cadherin expressed by SMG CD8 T cells promotes their tissue accumulation. (A) Expression of E-cadherin and KLRG1 by SMG CD8 T cells of LCMV-immune CD4-Cre+E-cadfl/fl and CD4-Cre−E-cadfl/fl mice. Representative dot plots of CD45 pregated samples are shown. (B) Percentage of CD8 T cells within CD45 cells in spleen (SP) and SMG of LCMV-immune CD4-Cre+E-cadfl/fl (E-cad−) and CD4-Cre−E-cadfl/fl (E-cad+) mice. Dots in statistical graphs represent values from individual mice. **P < 0.01.
Discussion
The phenotype of SMG memory CD8 T cells was clearly different from splenic memory CD8 T cells but were reminiscent of αβTCR CD8αβ cells in the IEL compartment (7). In LCMV-immune P14 chimeric mice, P14 T cells in both SMG and IEL were positive for CD69, CD103, and E-cadherin and lacked KLRG1 expression. However, Ly6C expression was found only on P14 T cells in SMG, indicating that antigen-specific CD8 T cells in SMG and IEL exhibit a similar but not an identical phenotype. Moreover, percentages of virus-specific CD8 T cells in the CD8αβ T-cell com-partment were strikingly higher in SMG than in IEL. This finding indicates that the mechanisms that govern entry, retention, and/or survival of antigen-specific CD8 T cells in SMG and IEL are different. This notion is further supported by the finding that short-term (day 3) in vitro-activated P14 T cells accumulated efficiently in SMG but not in IEL after adoptive transfer.
P14 memory T cells isolated from SMG exhibited decreased functional activity compared with their splenic counterparts when tested in restimulation assays in vitro. However, when the responsiveness of these cells was examined after antigen stimulation in vivo, P14 memory T cells from SMG and spleen showed comparable levels of IFNγ production. These different results fit well with the observation by Wakim et al. (5) that tissue-resident memory T cells from brain show impaired functional activity after dissociation from the tissue in which they reside. The decreased expansion rate of isolated and adoptively transferred P14 memory T cells from SMG upon recall may also be, at least partially, due to this effect. Nonetheless, these recall experiments demonstrated that P14 memory T cells from spleen preferentially resided in spleen after rechallenge whereas P14 memory T cells from SMG accumulated mainly within SMG. Thus, the tissue origin of “primary” P14 memory T cells influenced tissue localization of the corresponding “secondary” P14 memory T cells. Adoptive transfer experiments with effector and memory P14 T cells from spleen further revealed that the capacity to accumulate in SMG decreases during the course of T-cell differentiation. Short-term (day 3) in vitro-stimulated P14 T cells and P14 effector T cells (day 8 p.i.) exhibited a high competence to enrich in SMG followed by “late” P14 effector T cells (day 18) whereas resting P14 memory T cells (day 110 p.i.) failed to accumulate in SMG. Hence, not only the tissue of origin but also the differentiation stage determines the extent of accumulation in SMG. The failure of peripheral resting P14 memory T cells to accumulate in SMG further implies that SMG memory CD8 T cells are poorly replenished from the circulation, and thus they can be considered as tissue resident.
After skin scarification with HSV or after intranasal VSV infection, there is good evidence that local antigen presentation is required for the induction of TRM cells (4, 5). Nonetheless, long-term residence of these cells may be independent of persisting antigen (5). VSV-specific TRM cells in the brain have been shown to cluster around former hot spots of local antigen (5). Our data reveal a widely scattered distribution of CD8 T cells within the glandular tissue of LCMV-immune mice and the lack of leukocyte clusters, suggesting an accumulation process independent of local antigen. This conclusion is further based on the finding that gp33 peptide-activated P14 T cells accumulated in SMG after adoptive transfer. As the gp33 peptide has a very short half-life in vivo (26), residual traces of gp33 peptides, possibly present in the transfer inoculum, would be degraded within minutes in the recipient mice. In addition, LCMV could not be detected in SMG by standard virus plaque assay either at the acute or at the stationary phase after low-dose systemic LCMV infection used here for generation of LCMV-immune mice. In this respect, accumulation of antigen-specific CD8 T cells in SMG differs from the local viral infection models (4, 5); it resembles more the entry of “early” LCMV-specific effector CD8 T cells into the gut epithelium after systemic infection, a process that is also independent of local priming events (6).
The interaction of CD103 expressed by CD8 T cells with E-cadherin on epithelial tissues has been thoroughly investigated (17–19). On the contrary, expression of E-cadherin on lymphocytes has been analyzed in only a few studies. E-cadherin has been found on fetal thymocytes, IEL, and dendritic epidermal T cells (DETC) in the skin (21–23), and it has also recently been postulated to function as an inhibitory receptor on DETC (24). Interestingly, leukocytes infiltrating SMG of autoimmunity-prone NOD mice have further been reported to express E-cadherin (20). The possibility that the positive E-cadherin staining of these cells was due to adsorption from the surrounding epithelial tissue, however, was not addressed. The adoptive cell transfer experiments with short-term (day 3) in vitro-stimulated P14 T cells indicate that E-cadherin expression by CD8 T cells within SMG was driven by the glandular environment. Our data in combination with the reported infiltration of E-cadherin–positive leukocytes during autoimmunity affecting SMG imply that E-cadherin–assisted accumulation of lymphocytes in SMG not only may be relevant for foreign-specific but also for self-specific T cells. The diminished enrichment of E-cadherin–deficient compared with wild-type CD8 T cells in SMG of LCMV-immune mice can readily be rationalized by a decreased adhesiveness of the cells to E-cadherin–expressing epithelial cells. Because E-cadherin is also involved in signaling pathways (27), E-cadherin ligation on T cells may alternatively impact their function or survival. This issue, as well as the role of CD103 and VLA-1 that have been shown to be important for retention and survival of memory T cells in other epithelial tissues, need to be addressed for SMG memory CD8 T cells (5, 28).
The salivary glands are important effector sites of IgA-mediated humoral immunity to protect oral surfaces (12, 13). We now demonstrate that these glands also harbor antigen-specific CD8 memory T cells with features of TRM cells at surprisingly high frequencies and that these cells can provide local immunity to reinfection. It is further intriguing to speculate that these cells may also play a role in the autoimmune processes of Sjögren's syndrome affecting this gland.
Materials and Methods
Mice.
C57BL/6J (B6) mice were obtained from Janvier. Thy1.1+ P14 TCR transgenic mice [B6; D2-Tg (TcrLCMV) 318Sdz/JDvsJ] specific for aa 33–41 (gp33 epitope) of the LCMV glycoprotein (29) were maintained in our colony. CD4-Cre transgenic mice (30) were kindly provided by Ulrich Kalinke (Twincore, Hannover, Germany), and mice carrying the floxed E-cadherin gene (31) were a kind gift from Rolf Kemler (Max Planck Institute of Immunobiology and Epigenetics, Freiburg, Germany). Mice were kept under specific pathogen-free conditions. Animal care and use was approved by the Regierungspräsidium Freiburg. Mice infected i.v. with 200 pfu LCMV-WE or 2 × 106 pfu VSV-Indiana were considered to be virus-immune after 4 wk p.i.
Isolation of Lymphocytes.
Both splenic and SMG leukocytes were isolated by mincing, followed by incubation with collagenase type II (2,000 U/mL; GIBCO) at 37 °C for 30 min. All lymph nodes and connective tissue in SMG were removed before leukocyte isolation. Small-intestine IEL were prepared by shaking with low agitation of the cleaned small intestine in cell culture medium containing 10% FCS and antibiotics for 45 min at 37 °C.
Adoptive Cell Transfer.
P14 chimeric mice were generated by adoptive transfer (i.v.) of 105 naive splenic P14 T cells into B6 recipients 1 d before infection with 200 pfu LCMV-WE (i.v.). For secondary transfers, 105 P14 T cells isolated from spleen or SMG of LCMV-immune P14 chimeric mice were transferred (i.v.) into B6 followed by LCMV-WE (200 pfu i.v.) infection 1 d later. For generation of in vitro-activated P14 T cells, P14 splenocytes (2 × 106/mL) were stimulated with 10−7 M LCMV gp33 peptide (KAVYNFATM) for 3 d. Peptide-stimulated P14 T cells (5 × 106) were transferred i.v. into B6 recipient mice.
Flow Cytometry.
Antibodies and MHC tetramers are listed in SI Materials and Methods. Samples were analyzed using a FACSCalibur flow cytometer (BD Biosciences) and FlowJo software (Tree Star).
Antiviral Protection Assay.
Mice were infected i.g. (32) with 5 × 103 pfu LCMV-Docile (in 50 μL), and viral titers were determined 3 d p.i. by standard viral plaque assay (33). During viral rechallenge, mice were treated i.v. with FTY720 (US Biological; dose: 1 mg/kg body weight) on days 0 and 2.
Statistical Analyses.
Statistical significance was evaluated with Student's t test using SigmaPlot software. SEM was used to determine the mean.
Supplementary Material
Acknowledgments
The authors thank Peter Aichele, Selina Keppler, and Tobias Straub for critical comments on the manuscript; Ulrich Kalinke and Rolf Kemler for providing mice; and Mechthild Brundiers, Oliver Schweier, and Smiljka Vucikuja for technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft (Grant SFB 620, B2 to H.P.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107200108/-/DCSupplemental.
References
- 1.Masopust D, Vezys V, Marzo AL, Lefrançois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 2.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 3.Klonowski KD, et al. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 2004;20:551–562. doi: 10.1016/s1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- 4.Gebhardt T, et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 5.Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci USA. 2010;107:17872–17879. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masopust D, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med. 2010;207:553–564. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R. Cutting edge: Gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J Immunol. 2006;176:2079–2083. doi: 10.4049/jimmunol.176.4.2079. [DOI] [PubMed] [Google Scholar]
- 8.Hawke S, Stevenson PG, Freeman S, Bangham CR. Long-term persistence of activated cytotoxic T lymphocytes after viral infection of the central nervous system. J Exp Med. 1998;187:1575–1582. doi: 10.1084/jem.187.10.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity. 2003;18:593–603. doi: 10.1016/s1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark RA, et al. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006;176:4431–4439. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- 11.Verjans GM, et al. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc Natl Acad Sci USA. 2007;104:3496–3501. doi: 10.1073/pnas.0610847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mega J, McGhee JR, Kiyono H. Cytokine- and Ig-producing T cells in mucosal effector tissues: Analysis of IL-5- and IFN-gamma-producing T cells, T cell receptor expression, and IgA plasma cells from mouse salivary gland-associated tissues. J Immunol. 1992;148:2030–2039. [PubMed] [Google Scholar]
- 13.Oudghiri M, Seguin J, Deslauriers N. The cellular basis of salivary immunity in the mouse: Incidence and distribution of B cells, T cells and macrophages in single-cell suspensions of the major salivary glands. Eur J Immunol. 1986;16:281–285. doi: 10.1002/eji.1830160313. [DOI] [PubMed] [Google Scholar]
- 14.O'Sullivan NL, Skandera CA, Montgomery PC. Lymphocyte lineages at mucosal effector sites: Rat salivary glands. J Immunol. 2001;166:5522–5529. doi: 10.4049/jimmunol.166.9.5522. [DOI] [PubMed] [Google Scholar]
- 15.Fox RI, Robinson CA, Curd JG, Kozin F, Howell FV. Sjögren's syndrome: Proposed criteria for classification. Arthritis Rheum. 1986;29:577–585. doi: 10.1002/art.1780290501. [DOI] [PubMed] [Google Scholar]
- 16.Jordan MC, Shanley JD, Stevens JG. Immunosuppression reactivates and disseminates latent murine cytomegalovirus. J Gen Virol. 1977;37:419–423. doi: 10.1099/0022-1317-37-2-419. [DOI] [PubMed] [Google Scholar]
- 17.Cepek KL, et al. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature. 1994;372:190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- 18.Feng Y, et al. CD103 expression is required for destruction of pancreatic islet allografts by CD8(+) T cells. J Exp Med. 2002;196:877–886. doi: 10.1084/jem.20020178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schön MP, et al. Mucosal T lymphocyte numbers are selectively reduced in integrin alpha E (CD103)-deficient mice. J Immunol. 1999;162:6641–6649. [PubMed] [Google Scholar]
- 20.Esch TR, Jonsson MV, Levanos VA, Poveromo JD, Sorkin BC. Leukocytes infiltrating the submandibular glands of NOD mice express E-cadherin. J Autoimmun. 2000;15:387–393. doi: 10.1006/jaut.2000.0451. [DOI] [PubMed] [Google Scholar]
- 21.Inagaki-Ohara K, Sawaguchi A, Suganuma T, Matsuzaki G, Nawa Y. Intraepithelial lymphocytes express junctional molecules in murine small intestine. Biochem Biophys Res Commun. 2005;331:977–983. doi: 10.1016/j.bbrc.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 22.Lee MG, Sharrow SO, Farr AG, Singer A, Udey MC. Expression of the homotypic adhesion molecule E-cadherin by immature murine thymocytes and thymic epithelial cells. J Immunol. 1994;152:5653–5659. [PubMed] [Google Scholar]
- 23.Lee MG, Tang A, Sharrow SO, Udey MC. Murine dendritic epidermal T cells (DETC) express the homophilic adhesion molecule E-cadherin. Epithelial Cell Biol. 1994;3:149–155. [PubMed] [Google Scholar]
- 24.Uchida Y, Kawai K, Ibusuki A, Kanekura T. Role for E-cadherin as an inhibitory receptor on epidermal gammadelta T cells. J Immunol. 2011;186:6945–6954. doi: 10.4049/jimmunol.1003853. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 26.Stemmer C, et al. Protection against lymphocytic choriomeningitis virus infection induced by a reduced peptide bond analogue of the H-2Db-restricted CD8(+) T cell epitope GP33. J Biol Chem. 1999;274:5550–5556. doi: 10.1074/jbc.274.9.5550. [DOI] [PubMed] [Google Scholar]
- 27.Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4:118–132. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- 28.Ray SJ, et al. The collagen binding alpha1beta1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity. 2004;20:167–179. doi: 10.1016/s1074-7613(04)00021-4. [DOI] [PubMed] [Google Scholar]
- 29.Pircher H, Bürki K, Lang R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342:559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 30.Wolfer A, et al. Inactivation of Notch 1 in immature thymocytes does not perturb CD4 or CD8T cell development. Nat Immunol. 2001;2:235–241. doi: 10.1038/85294. [DOI] [PubMed] [Google Scholar]
- 31.Boussadia O, Kutsch S, Hierholzer A, Delmas V, Kemler R. E-cadherin is a survival factor for the lactating mouse mammary gland. Mech Dev. 2002;115:53–62. doi: 10.1016/s0925-4773(02)00090-4. [DOI] [PubMed] [Google Scholar]
- 32.Grewal JS, et al. Salivary glands act as mucosal inductive sites via the formation of ectopic germinal centers after site-restricted MCMV infection. FASEB J. 2011;25:1680–1696. doi: 10.1096/fj.10-174656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Battegay M, et al. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24- or 96-well plates. J Virol Methods. 1991;33:191–198. doi: 10.1016/0166-0934(91)90018-u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.