Abstract
We identify here a new class of lung tissue-resident memory CD4 T cells which exhibit tissue tropism and retention independent of antigen or inflammation. Tissue-resident memory CD4 T cells in the lung did not circulate or emigrate from the lung in parabiosis experiments, were protected from in vivo antibody labeling, and expressed elevated levels CD69 and CD11a compared to circulating memory populations. Importantly, influenza-specific lung-resident memory CD4 T cells served as in situ protectors to respiratory viral challenge, mediating enhanced viral clearance and survival to lethal influenza infection. By contrast, memory CD4 T cells isolated from spleen recirculated among multiple tissues without retention, and failed to mediate protection to influenza infection, despite their ability to expand and migrate to the lung. Our results reveal tissue compartmentalization as a major determining factor for immune-mediated protection in a key mucosal site, important for targeting local protective responses in vaccines and immunotherapies.
Keywords: Memory, T cells, Cell trafficking, lung, viral immunity
INTRODUCTION
Migration of activated T cells to peripheral tissue sites during infection promotes local immune responses to clear pathogens. Following clearance, long-lived, pathogen-specific memory T cells can take up residence and recirculate within multiple lymphoid and non-lymphoid compartments, with significant populations in mucosal sites such as lung and intestine (1–2). The presence of memory T cells in tissue sites has been associated with increased protection to virus infections in mice (1–3); however, it has been difficult to establish whether in situ immunity is due to T cells resident in the tissue versus those rapidly recruited from other sites. Defining the protective T cell subsets for specific compartments is critical to the successful design of vaccines which target tissue sites.
Respiratory infection with influenza virus results in the generation and dissemination of memory T cells into lung and lymphoid tissue sites as demonstrated in mouse models (4–5), and in human peripheral blood and lungs (6–7). We recently showed that heterogeneous populations of influenza-specific memory CD4 T cells in mice could direct rapid lung viral clearance independent of CD8 T cells and B cells, although did not protect from morbidity of infection (9–10). As circulating memory CD4 T cells reactive to pandemic influenza strains have been identified in healthy humans (8), further insights into the nature of protective memory CD4 T cells could be beneficial in optimizing this potent response.
In this study, we identify a population of influenza specific memory CD4 T cells in lung which are non-circulating and exhibit lung-tropic migration independent of antigen-specificity. We used parabiosis models to demonstrate irreversible retention of lung-tropic memory CD4 T cells in the lung, and in vivo labeling to identify a non-circulating lung resident polyclonal memory CD4 T cell population persisting after influenza infection--both exhibiting elevated CD69 and CD11a expression. Mice with memory CD4 T cells exclusively in the lung were protected from morbidity and mortality of influenza infection, with spleen-derived memory CD4 T cells affording little protection and even exacerbating mortality despite their diverse tissue residence and migration to the lung. Our results define a new class of memory CD4 T cells resident in lung tissue and reveal tissue compartmentalization as a major determining factor for protection in a key mucosal site, with important implications for targeting site-specific immunity in vaccines and immunotherapies.
MATERIALS AND METHODS
Mice
BALB/c mice (8–16 weeks of age) (NCI Biological Testing Branch), RAG2−/− (BALB/c) mice (Taconic Farms, Germantown, NY), and Influenza Hemagglutinin (HA)-TCR transgenic mice (11) on BALB/c (Thy1.2) or BALB/c(Thy1.1) backgrounds were maintained under specific pathogen-free conditions at the University of Maryland, Columbia University, and the University of Connecticut. Protocols were approved by the Institutional Animal Care and use Committees of all institutions.
Reagents
Fluorescently-conjugated antibodies specific for CD4, CD8, CD44, CD62L, CD69, CD90.1, CD90.2, and CD11a were purchased from BD Pharmingen (San Diego, CA). Influenza HA peptide (110–120, SFERFEIFPKE) was synthesized by the Biopolymer Laboratory (University of Maryland, Baltimore).
Influenza virus infection
Mice were infected intransally with 100–500 TCID50 Influenza virus (A/PR/8/34) for sublethal infection and 5000 TCID50 PR8 Influenza (2LD50) for lethal infection as described (9–10, 12). Morbidity was monitored by daily weighing and examination. Influenza virus titers were determined by Tissue Culture Infectious Dose 50 assay (TCID50) as described (13).
Generation of influenza-specific memory CD4 T-cells
HA-specific memory CD4 T-cells were generated by adoptive transfer of in vitro, antigen-primed HA-TCR CD4 T cells into BALB/c(Thy1.1) or RAG2−/− mice as previously done (4, 14–15). Polyclonal memory CD4 T-cells specific for influenza were recovered 4–8 weeks following intranasal infection of BALB/c mice and quantitated by ELISPOT as described (9, 15).
Homing and Parabiosis Experiments
For in vivo homing assays, HA-specific memory CD4 T cells (Thy1.1+) were isolated from spleens and lungs of RAG2−/− hosts, transferred (2×106/mouse) into BALB/c recipient mice, and recipient tissues were recovered 7 and 21 days later. For parabiosis experiments, BALB/c recipients of 2×106 lung or spleen HA-specific memory CD4 T cells were surgically conjoined seven days post-transfer to BALB/c mouse partners as described (16), and tissues were harvested from mouse pairs 8–21 days later.
In vivo antibody labeling and flow cytometry
For in vivo antibody labeling, naïve or flu-immune BALB/c mice were injected intravenously with 2.5 µg PE-Cy5-conjugated anti-CD4 antibody (clone RM4-5), and after 10 minutes, peripheral blood was obtained and lungs perfused with PBS/500units heparin, and cells isolated. Cells were surface or intracellularly stained with fluorochrome-conjugated antibodies as described (15), and analyzed using an LSRII or FACScanto flow cytometer (BD, San Jose CA) with FACSDiva (BD) or FlowJo software (Tree Star, Inc., Ashland, OR).
Statistics
Results are expressed as the mean value from individual groups +/− SEM unless otherwise designated, indicated by error bars. Significance between experimental groups was determined by two-tailed student T tests, assuming a normal distribution for all groups.
RESULTS AND DISCUSSION
Lung memory CD4 T cells exhibit tissue-specific tropism
Influenza infection resulted in generation of polyclonal influenza-specific memory CD4 T cells in lung and spleen, with lung memory CD4 T cells exhibiting a slightly higher proportion of IFN-γ producers and decreased IL-2 producers compared to spleen memory CD4 T cells (Fig. 1A). To dissect the role of tissue location in the distinct functions of spleen and lung memory CD4 T cells, we generated memory CD4 T cells expressing the same TCR clonotype by adoptive transfer of in vitro-primed influenza HA-specific TCR-transgenic CD4 T cells into lymphocyte-deficient or intact mouse hosts (4, 14, 17). Similar to polyclonal populations, HA-specific memory CD4 T cells in the lung had a lower proportion of IL-2 producers and a similar frequency of IFN-γ producers compared to spleen memory CD4 T cells (Fig. 1B). These results indicate tissue-specific influences on the function of resident memory populations, independent of antigen specificity.
Figure 1. Lung memory CD4 T cells exhibit distinct migration tropism compared to spleen memory CD4 T cells.
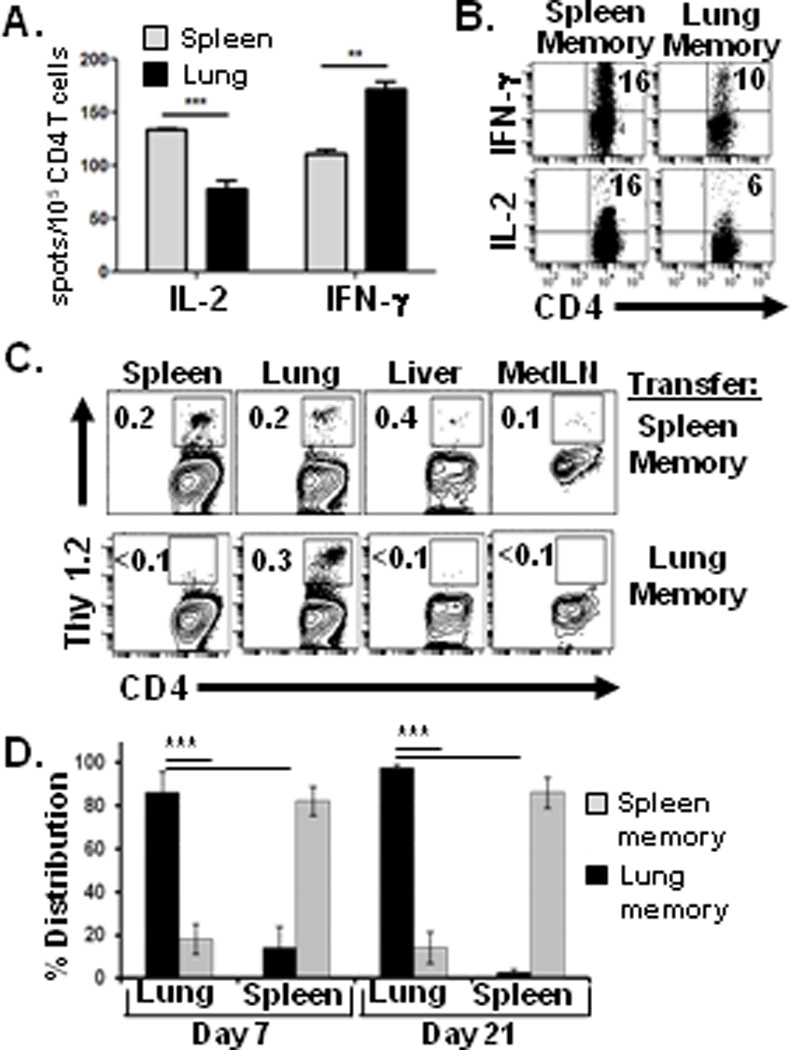
We hypothesized that the tissue location of memory CD4 T cells would influence their intrinsic homing capacities. Lung- and spleen-derived HA-specific memory CD4 T cells isolated as above were transferred into unmanipulated BALB/c congenic hosts, and their resultant distribution into different tissues of recipient mice was assessed 1–3 weeks later. Spleen-derived memory CD4 T cells dispersed into multiple tissues of recipient mice, including spleen, lung, liver and lymph nodes (Fig. 1C), with the majority of spleen-derived cells recovered from the spleen, followed by reduced, yet significant numbers in the lung (Fig. 1D). By contrast, lung-derived memory CD4 T cells distributed almost exclusively in the lungs of recipient mice and not significantly into other sites such as spleen, lymph node or liver (Fig. 1C). Notably, all of the lung-derived memory CD4 T cells were recovered from the lungs of recipient mice three weeks post-transfer (Fig. 1D), demonstrating lung tissue-tropism of this subset. Lung memory CD4 T cells localized predominantly in the tissue parenchyma with much lower numbers in the airways as quantified in bronchiolar lavage (BAL) fluid (Supplemental Fig. 1A). Together, these results demonstrate that lung-resident memory CD4 T cells are distinct in function and migration from spleen-derived counterparts, and possess internal “zip codes” enabling them to home to their tissue of residence.
Lung memory CD4 T cells are specifically retained in the lung
The homing tropism of lung memory CD4 T cells could arise through recirculation and migration back to the lung, and/or specific retention in the lung. We used a parabiosis model (16) to examine the recirculation and/or retention properties of spleen and lung memory CD4 T cells. Host mice of spleen- or lung-derived memory CD4 T cells (Thy1.1+) containing HA-specific memory CD4 T cells in multiple tissues or only in lungs, respectively, were surgically conjoined to a second BALB/c mouse as described (16), to create host and parabiont partner mice with shared circulations. After eight days conjoined, T cell populations in multiple tissues of host and parabiont partner mice were recovered and analyzed. In mouse pairs containing spleen-derived memory CD4 T cells, we detected significant frequencies of HA-specific memory T cells in spleen, lung, and liver, but not lymph nodes (Fig. 2A and data not shown), with the highest overall numbers recovered from spleen and lung (data not shown). In striking contrast, mouse pairs containing lung-derived memory CD4 T cells had memory CD4 T cells exclusively in the lungs of host mice, with no dispersion into additional tissues or lung-draining lymph nodes of host or parabiont partner mice (Fig. 2A, B). Lung-derived memory CD4 T cells further remained in the lungs of the original host mice in long-term parabiosis experiments of three weeks duration (Supplementary Fig. 1B), when spleen-derived memory CD4 T cells had completely dispersed and were not detected in any tissues of host or partner mice (data not shown). These results demonstrate a powerful and specific retention of lung memory CD4 T cells in the lung, independent of antigen and inflammation. These homing and retention properties of lung memory CD4 T cells are distinct from lung memory CD8 T cells, which migrate to multiple lymphoid and non-lymphoid tissue sites (10, 18) and recirculate in vivo in parabiosis studies (16), similar to our findings with spleen-derived memory CD4 T cells. Our results therefore define a new class of tissue-resident memory CD4 T cells retained within a specific compartment.
Figure 2. Irreversible tissue retention of lung memory CD4 T cells.
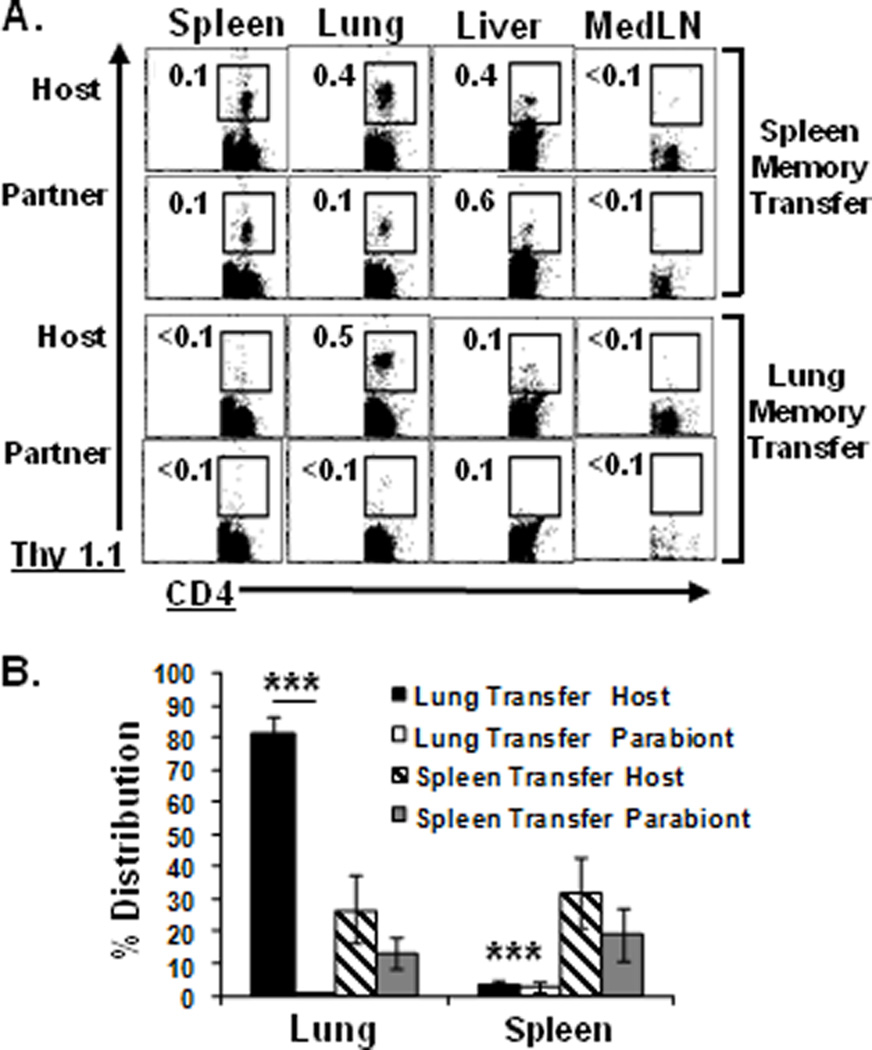
Host mice containing Thy1.1+ lung or spleen-derived HA-specific memory CD4 T cells as in Fig. 1C were surgically conjoined to syngeneic BALB/c partner mice in parabiosis experiments (see methods). (A) Frequency of spleen and lung-derived CD4+Thy1.1+ HA-specific memory CD4 T-cells (of total CD4 T cells) in tissues of host and parabiont partner mice after 8 days conjoined. (B) Percent distribution of HA-specific memory CD4 T cells recovered from host and partner mouse tissues calculated as in Fig. 1D. Results are compiled from eight parabiotic mouse pairs/group and representative of 2 independent experiments. Significant differences between lung and spleen memory migration are indicated by *** (p <0.005).
Distinct phenotypes of circulating and tissue-resident memory CD4 T cell populations
In addition to differences in CD62L expression with lung being predominantly CD62Llo and spleen heterogeneous for CD62L expression (data not shown and (4)), lung-retained HA-specific memory CD4 T cells expressed the activation marker CD69 and high levels of the integrin LFA-1 (CD11a), whereas spleen memory CD4 T cells did not express CD69 and had reduced levels of CD11a (Fig. 3A). We investigated whether subsets with different migratory capacities could be identified within endogenous lung CD4 T cells consisting of naive and memory subsets (4, 19). We adapted an in vivo antibody labeling approach which can distinguish between T cells in the lung interstitium and those accessible to the circulation (20). Administration of fluorescent anti-CD4 antibody to influenza-immune BALB/c mice resulted in >96% of blood T cells labeled (Fig. 3B, left), whereas in the perfused lung, 70% of CD4 T cells were labeled, with 20–30% protected from in vivo labeling (Fig. 3B top). Unlabeled lung T cells exhibited an effector-memory phenotype (CD44hi/CD62Llo) (data not shown) with a high proportion of CD11ahi/CD69+ cells (Fig. 3B, lower), similar to the phenotype of HA-specific lung resident memory CD4 T cells (Fig. 3A). By contrast, in vivo-labeled lung CD4 T cells were predominantly naive (70% CD44lo/CD62Lhi; data not shown), and had reduced levels of CD11a and CD69 compared to the protected population (Fig. 3B, right). These results establish that the lung resident CD69hi/CD11ahi memory CD4 T cell subset is represented among polyclonal populations of lung CD4 T cells. This CD69hi/CD11ahi phenotype is also associated with memory CD4 and CD8 T cells in intestine and skin (21–22), suggesting similar signatures for tissue-resident T cells in other mucosal and non-lymphoid sites.
Figure 3. Distinct phenotype of tissue-resident versus circulating memory CD4 T cells.
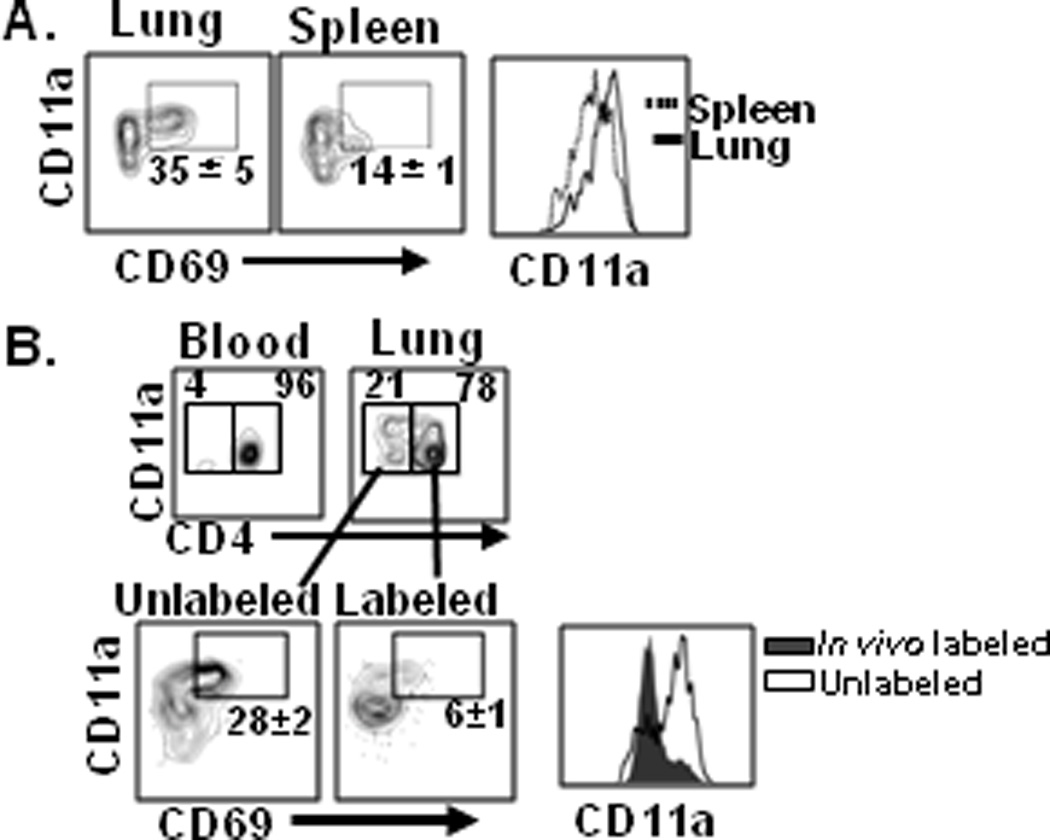
(A) Cell surface CD11a and CD69 expression by lung- and spleen-derived HA-specific memory CD4 T cells gated on live CD4+CD44hiCD62Llo T cells, and are representative of 4 independent experiments. (B) In vivo labeling delineates resident and circulating polyclonal lung memory CD4 T cell subsets. BALB/c mice previously infected with influenza (4–6 weeks post-infection) were injected intravenously with fluorescently labeled anti-CD4 antibody, and blood and lung tissue were harvested. Upper: Proportion of CD3ε+CD8α− γδ− T cells stained by or protected from in vivo administered antibody. Lower: CD11a and CD69 expression by the labeled or protected cell populations, representative of 4 independent experiments.
Enhanced protection from influenza virus challenge by lung-retentive memory CD4 T cells
The distinct migration/retention properties of lung and spleen memory CD4 T cells enabled a novel investigation of protection by tissue-resident versus circulating memory subsets. We challenged mouse recipients of spleen or lung-derived memory CD4 T cells (“spleen-memory” and “lung-memory” recipients, respectively) intranasally with influenza PR8 (H1N1) virus (HA specificity of HA-TCR T cells), and monitored protection by weight loss morbidity and lung viral loads as described (10). Following sublethal infection, naive mice and spleen-memory recipients exhibited profound and progressive weight loss of 25% by 5–6 days post-infection, whereas lung-memory recipients experienced only moderate weight loss (10%) for the entire course of infection (Fig. 4A). Importantly, lung-memory recipients exhibited more rapid and enhanced lung viral clearance compared to infected naive and spleen-memory recipients, with complete clearance by day 8 post-infection (Fig 4B). Lung-memory recipients were also fully protected from lethal influenza challenge compared to naive mice (60% lethality at day 12 post-infection), and spleen-memory recipients which experienced accelerated death at day 7 post-infection (Fig. 4C). Following infection, lung-derived memory CD4 T cells maintained a biased distribution in the lung, whereas spleen-derived memory CD4 T cells were present in high frequencies in both the lung and spleen of recipient mice (Fig. 4D). These findings indicate that lung memory CD4 T cells serve as optimal in situ protectors to influenza challenge, contrasting spleen memory CD4 T cells which exhibit low protective capacities yet extensive expansion.
Figure 4. Lung memory CD4 T cells mediate enhanced protection to influenza challenge compared to spleen memory CD4 T cells.
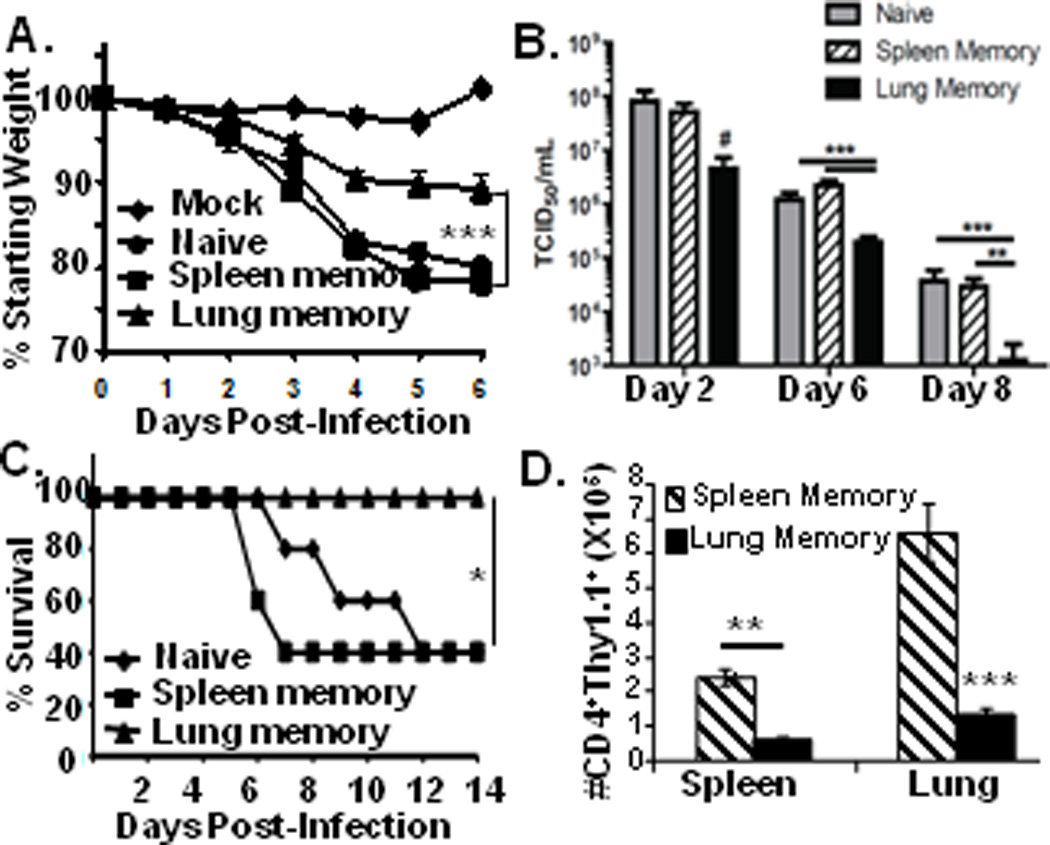
BALB/c mice and recipients of lung- or spleen-derived HA-specific memory CD4 T cells (106/mouse) were challenged with PR8 influenza virus. (A) Daily weight loss following sub-lethal influenza infection, compiled from 6 independent experiments with n=20–25 per group. (B) Kinetic analysis of influenza viral titers in the lungs of naive mice or mice receiving spleen- or lung-derived memory CD4 T cells as in A. Results are expressed as mean TCID50 from 4–9 mice per group, representative of 2–4 experiments at each time-point. (C) Lung memory CD4 T cells protect from lethal challenge. Graph shows survival of naive BALB/c mice or mouse recipients of spleen- or lung-derived memory CD4 T cells infected with 2LD50 of PR8 influenza virus from two independent experiments with 8–10 mice/group. (D) Numbers of lung- and spleen-derived HA-specific memory CD4 T cells recovered from recipient spleen and lungs 6 days post-infection, expressed as the average from 5 mice/group, representative of six experiments. Significance for all sections indicated by *** for p <0.0005, ** for p < 0.005, * for p < 0.05, and #, for p = 0.06.
Our results reveal a novel class of tissue-resident memory CD4 T cells in a key mucosal site that are specifically retained in lung tissue and mediate efficacious protection to respiratory virus infection. These findings indicate that the protective capacity of T cell memory to infections in peripheral sites is intricately linked to their tissue compartmentalization, and quantitative measurements of circulating antigen-specific T cell responses as typically assessed (23) may not reflect the quality of local tissue-specific immune responses. We propose that targeting the generation and maintenance of these tissue-resident memory populations will be critical for vaccines and immunotherapies to promote or regulate compartment-specific immune responses.
Supplementary Material
Abbreviations
- HA
hemagglutinin
- APC
antigen presenting cells
Footnotes
This work was supported by NIH grants AI083022 awarded to D.L.F. and E.J.W., and AI41576 and AI76457 awarded to L.L.
REFERENCES
- 1.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 2.Hogan RJ, Zhong W, Usherwood EJ, Cookenham T, Roberts AD, Woodland DL. Protection from respiratory virus infections can be mediated by antigen-specific CD4(+) T cells that persist in the lungs. J Exp Med. 2001;193:981–986. doi: 10.1084/jem.193.8.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu L, Zhong Q, Tian T, Dubin K, Athale SK, Kupper TS. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell-mediated immunity. Nat Med. 2010;16:224–227. doi: 10.1038/nm.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bingaman AW, Patke DS, Mane VR, Ahmadzadeh M, Ndejembi M, Bartlett ST, Farber DL. Novel phenotypes and migratory properties distinguish memory CD4 T cell subsets in lymphoid and lung tissue. Eur J Immunol. 2005;35:3173–3186. doi: 10.1002/eji.200526004. [DOI] [PubMed] [Google Scholar]
- 5.Marshall DR, Turner SJ, Belz GT, Wingo S, Andreansky S, Sangster MY, Riberdy JM, Liu T, Tan M, Doherty PC. Measuring the diaspora for virus-specific CD8+ T cells. Proc Natl Acad Sci U S A. 2001;98:6313–6318. doi: 10.1073/pnas.101132698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Bree GJ, Daniels H, Schilfgaarde M, Jansen HM, Out TA, van Lier RA, Jonkers RE. Characterization of CD4+ memory T cell responses directed against common respiratory pathogens in peripheral blood and lung. J Infect Dis. 2007;195:1718–1725. doi: 10.1086/517612. [DOI] [PubMed] [Google Scholar]
- 7.Piet B, de Bree GJ, Smids-Dierdorp BS, van der Loos CM, Remmerswaal EB, von der Thusen JH, van Haarst JM, Eerenberg JP, Ten Brinke A, van der Bij W, Timens W, van Lier RA, Jonkers RE. CD8+ T cells with an intraepithelial phenotype upregulate cytotoxic function upon influenza infection in human lung. J Clin Invest. 2011 doi: 10.1172/JCI44675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roti M, Yang J, Berger D, Huston L, James EA, Kwok WW. Healthy Human Subjects Have CD4+ T Cells Directed against H5N1 Influenza Virus. J Immunol. 2008;180:1758–1768. doi: 10.4049/jimmunol.180.3.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teijaro JR, Njau MN, Verhoeven D, Chandran S, Nadler SG, Hasday J, Farber DL. Costimulation modulation uncouples protection from immunopathology in memory T cell responses to influenza virus. J Immunol. 2009;182:6834–6843. doi: 10.4049/jimmunol.0803860. [DOI] [PubMed] [Google Scholar]
- 10.Teijaro JR, Verhoeven D, Page CA, Turner D, Farber DL. Memory CD4 T cells direct protective responses to influenza virus in the lungs through helper-independent mechanisms. J Virol. 2010;84:9217–9226. doi: 10.1128/JVI.01069-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirberg J, Baron A, Jakob S, Rolink A, Karjalainen K, von Boehmer H. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J Exp Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verhoeven D, Teijaro JR, Farber DL. Pulse-oximetry accurately predicts lung pathology and the immune response during influenza infection. Virology. 2009;390:151–156. doi: 10.1016/j.virol.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scherle PA, Palladino G, Gerhard W. Mice can recover from pulmonary influenza virus infection in the absence of class I-restricted cytotoxic T cells. J Immunol. 1992;148:212–217. [PubMed] [Google Scholar]
- 14.Ahmadzadeh M, Farber DL. Functional plasticity of an antigen-specific memory CD4 T cell population. Proc Natl Acad Sci U S A. 2002;99:11802–11807. doi: 10.1073/pnas.192263099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ndejembi MP, Teijaro JR, Patke DS, Bingaman AW, Chandok MR, Azimzadeh A, Nadler SG, Farber DL. Control of Memory CD4 T Cell Recall by the CD28/B7 Costimulatory Pathway. J Immunol. 2006;177:7698–7706. doi: 10.4049/jimmunol.177.11.7698. [DOI] [PubMed] [Google Scholar]
- 16.Klonowski KD, Williams KJ, Marzo AL, Blair DA, Lingenheld EG, Lefrancois L. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 2004;20:551–562. doi: 10.1016/s1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- 17.Moulton VR, Bushar ND, Leeser DB, Patke DS, Farber DL. Divergent generation of heterogeneous memory CD4 T cells. J Immunol. 2006;177:869–876. doi: 10.4049/jimmunol.177.2.869. [DOI] [PubMed] [Google Scholar]
- 18.Masopust D, Vezys V, Usherwood EJ, Cauley LS, Olson S, Marzo AL, Ward RL, Woodland DL, Lefrancois L. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J Immunol. 2004;172:4875–4882. doi: 10.4049/jimmunol.172.8.4875. [DOI] [PubMed] [Google Scholar]
- 19.Cose S, Brammer C, Khanna KM, Masopust D, Lefrancois L. Evidence that a significant number of naive T cells enter non-lymphoid organs as part of a normal migratory pathway. Eur J Immunol. 2006;36:1423–1433. doi: 10.1002/eji.200535539. [DOI] [PubMed] [Google Scholar]
- 20.Galkina E, Thatte J, Dabak V, Williams MB, Ley K, Braciale TJ. Preferential migration of effector CD8+ T cells into the interstitium of the normal lung. J Clin Invest. 2005;115:3473–3483. doi: 10.1172/JCI24482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K, Dowgiert RK, Kupper TS. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006;176:4431–4439. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- 22.Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J Immunol. 2006;176:2079–2083. doi: 10.4049/jimmunol.176.4.2079. [DOI] [PubMed] [Google Scholar]
- 23.Zinkernagel RM. On natural and artificial vaccinations. Annu Rev Immunol. 2003;21:515–546. doi: 10.1146/annurev.immunol.21.120601.141045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


