Abstract
Purpose
The combination of gemcitabine plus cisplatin (GC) is a standard regimen in patients with locally advanced or metastatic urothelial cancer. A phase I/II study suggested that a three-drug regimen that included paclitaxel had greater antitumor activity and might improve survival.
Patients and Methods
We conducted a randomized phase III study to compare paclitaxel/cisplatin/gemcitabine (PCG) with GC in patients with locally advanced or metastatic urothelial carcinoma. Primary outcome was overall survival (OS). Secondary outcomes were progression-free survival (PFS), overall response rate, and toxicity.
Results
From 2001 to 2004, 626 patients were randomly assigned; 312 patients were assigned to PCG, and 314 patients were assigned to GC. After a median follow-up of 4.6 years, the median OS was 15.8 months on PCG versus 12.7 months on GC (hazard ratio [HR], 0.85; P = .075). OS in the subgroup of all eligible patients was significantly longer on PCG (3.2 months; HR, 0.82; P = .03), as was the case in patients with bladder primary tumors. PFS was not significantly longer on PCG (HR, 0.87; P = .11). Overall response rate was 55.5% on PCG and 43.6% on GC (P = .0031). Both treatments were well tolerated, with more thrombocytopenia and bleeding on GC than PCG (11.4% v 6.8%, respectively; P = .05) and more febrile neutropenia on PCG than GC (13.2% v 4.3%, respectively; P < .001).
Conclusion
The addition of paclitaxel to GC provides a higher response rate and a 3.1-month survival benefit that did not reach statistical significance. Novel approaches will be required to obtain major improvements in survival of incurable urothelial cancer.
INTRODUCTION
Untreated metastatic urothelial carcinoma is associated with a median survival time rarely exceeding 3 to 6 months. It is a chemotherapy-sensitive tumor, and cisplatin-based chemotherapy is the standard treatment.1,2 Historically, the combination of methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) modestly improved survival compared with cisplatin alone3; the combination of cyclophosphamide, doxorubicin, and cisplatin4; and a carboplatin-based regimen.5 However, dose intensification of MVAC did not improve median survival,6–8 and the disappointing long-term outcome with available regimens has led to the search for new active drugs.
Among the agents assessed, the microtubule-stabilizing taxane paclitaxel (Taxol; Bristol-Myers Squibb, Princeton, NJ) and the pyrimidine antimetabolite gemcitabine (Gemzar; Eli Lilly, Indianapolis, IN) have demonstrated high single-agent activity in patients with advanced urothelial cancer. In previously untreated patients, paclitaxel produced a response rate of 42%, with a 27% complete response rate.9 Gemcitabine has single-agent activity against urothelial cancer in previously treated and untreated patients, with overall response rates in the range of 24% to 28%.10–13
The encouraging results with gemcitabine led to a phase III trial comparing a combination of gemcitabine and cisplatin (GC) with MVAC.2 GC provided a similar survival compared with MVAC with a better safety profile and tolerability. This favorable risk-benefit ratio established GC as another standard option for patients with locally advanced and metastatic transitional-cell carcinoma.
Given the different mechanisms of action and the partially nonoverlapping toxicity profiles of cisplatin, gemcitabine, and paclitaxel, the triple combination was assessed by the Spanish Oncology Genitourinary Group.14,15 In 58 patients with advanced urothelial tumors in the combined phase I/II cohort, the overall response rate was 77.6% (95% CI, 60% to 98%). There were 16 complete responses (27.6%), and the median survival time was 15.6 months.14,15 Thus, the three-drug combination was feasible, and the median survival seemed superior to that obtained with the standard MVAC regimen.2 Therefore, the European Organisation for Research and Treatment of Cancer (EORTC) designed a phase III study (EORTC Intergroup Study 30987) to compare the efficacy of GC plus paclitaxel (PCG) with GC alone in patients with locally advanced or metastatic urothelial cancer. Preliminary data from this study, with a median follow-up of 3.4 years, were presented at the 43rd Annual Meeting of the American Society of Clinical Oncology in 200716; this article presents the final mature results after a median follow-up of 4.6 years.
PATIENTS AND METHODS
Design
An open-label randomized, phase III intergroup study was conducted within the framework of the EORTC Genitourinary Group, with the cooperation of the German Association of Urologic Oncology, Groupe d'Etude des Tumeurs Uro-Génitales, National Cancer Institute of Canada Clinical Trials Group, Spanish Oncology Genitourinary Group, Southwest Oncology Group, and the National Cancer Research Institute Bladder Clinical Studies Group.
Eligibility
Eligible patients had histologically confirmed stage IV locally advanced (T4b, any N; or any T, N2-3) or metastatic transitional-cell carcinoma of the urothelium (pure or mixed). Tumor sites included the bladder, urethra, ureter, and renal pelvis. Patients were required to have measurable or nonmeasurable (evaluable) disease according to RECIST,17,18 age ≥ 18 years, WHO performance status of 0 or 1, and a life expectancy of at least 12 weeks. Patients who received prior systemic chemotherapy or investigational agents were not allowed to enter the study. Other inclusion criteria were adequate hematologic (WBC count ≥ 3.0 × 109/L, platelet count ≥ 100 × 109/L, and hemoglobin ≥ 10 g/dL or 6.2 mmol/L), hepatic (serum bilirubin level < 1.25× above the normal range, ALT or AST < 2.5× above the normal range), and renal (creatinine clearance ≥ 60 mL/min) function. Patients with significant cardiac disease, brain metastases, or peripheral neuropathy greater than grade 2 were not eligible. Patients with a secondary primary malignancy, except for in situ carcinoma of the cervix, basal cell carcinoma of the skin, or incidental prostate cancer (T1, Gleason score ≤ 6, prostate-specific antigen < 0.5 ng/mL), were also not eligible.
The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. The study protocol was approved by the institutional review board of each participating center, and relevant patient safeguards were observed. All patients provided written informed consent.
Treatment Schedule
Patients were centrally randomly assigned at the EORTC to receive either PCG (experimental arm) or GC (control arm). Random assignment was stratified by study site, WHO performance status (0 v 1), and the presence or absence of metastatic disease. Treatment schedule and dose adjustments were done according to previously published data.2 In summary, in the GC arm, gemcitabine 1,000 mg/m2 was administered on days 1, 8, and 15, and cisplatin 70 mg/m2 was administered on day 2, every 28 days. The PCG arm consisted of the sequential administration of paclitaxel 80 mg/m2 before the same doses of gemcitabine and paclitaxel as in the GC arm on day 1. Paclitaxel and gemcitabine were administered at the same doses on day 8. Cycles were repeated every 21 days. Patients were treated for a maximum of six cycles or until documentation of progression according to RECIST,17 unacceptable toxicity, or a request for discontinuation by the patient or attending physician.
Study End Points
The primary end point was overall survival (OS), which was defined as the time between random assignment and death from any cause. Secondary end points were progression-free survival, response rate according to RECIST,17 and toxicity using the National Cancer Institute Common Toxicity Criteria (CTC) version 2.0. Patients were assessable for response if they had evaluable disease (measurable and/or non measurable), had received at least one cycle, and had at least one follow-up tumor assessment. Response had to be confirmed after at least 4w. Patients were evaluated every 3m during the first 2y and every 6m thereafter.
Statistical Considerations
The median survival on GC was assumed to be 14 months. The trial was designed to detect an increase in the median survival from 14 months to 18 months on PCG (or equivalently an increase in the 14 month survival rate from 50% to 58.7%), which corresponds to a hazard ratio (HR) of 0.778. It was estimated that a total of 610 patients (305 patients in each arm) was needed to observe the 498 deaths required based on a two-sided log-rank test at error rates of α = .05 and β = .20. Two interim efficacy analyses were carried out in January 2004 and June 2007. To maintain the overall α at 5%, the significance level used for the final analysis was 3.9%.
The primary analysis was carried out in the intent-to-treat (ITT) population of all randomly assigned patients. Time-to-event curves (duration of OS and PFS) were estimated using the Kaplan-Meier method and compared based on a two-sided log-rank test. Response rates were compared using a χ2 test. Survival was also compared in the eligible patients (unplanned, post hoc analysis).
RESULTS
Between May 2001 and June 2004, 626 patients from 137 institutions were randomly assigned, 314 patients to GC and 312 patients to PCG. The 607 patients who started treatment were included in the safety analyses. Forty-seven patients (22 patients on GC and 25 patients on PCG) were ineligible, with an additional four patients with eligibility unverifiable, 41 of whom started protocol treatment (Fig 1).
Fig 1.
Flow chart of the study population. Eligibility before the database lock was assessed by the study coordinator (J.B.) and thereafter reviewed by the statisticians (R.S. and S.C.) and the clinical research physician (S.M.). ITT, intent to treat.
Patient Characteristics
Patient characteristics at random assignment were well balanced between the arms. Baseline data are listed in Table 1.
Table 1.
Patient Demographics and Disease Characteristics at Random Assignment
| Demographic or Characteristic | Paclitaxel/Cisplatin/Gemcitabine (n = 312) |
Gemcitabine/Cisplatin (n = 314) |
Total (N = 626) |
|||
|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Sex | ||||||
| Male | 256 | 82.3 | 252 | 81.0 | 508 | 81.7 |
| Female | 55 | 17.7 | 59 | 19.0 | 114 | 18.3 |
| Age, years | ||||||
| Median | 61 | 61 | 61 | |||
| Range | 27-80 | 32-79 | 27-80 | |||
| WHO performance status | ||||||
| 0 | 171 | 54.8 | 171 | 54.5 | 342 | 54.6 |
| 1 | 141 | 45.2 | 143 | 45.5 | 453 | 45.5 |
| Location of primary tumor | ||||||
| Bladder | 254 | 81.4 | 259 | 82.5 | 513 | 81.9 |
| Renal pelvis | 27 | 8.6 | 25 | 8.0 | 52 | 8.3 |
| Ureter | 13 | 4.2 | 17 | 5.4 | 30 | 4.8 |
| Urethra | 11 | 3.5 | 8 | 2.5 | 19 | 3.0 |
| Other | 6* | 1.9 | 2† | 0.6 | 8 | 1.3 |
| Distant metastases | 275 | 88.1 | 276 | 87.9 | 551 | 88.0 |
| Nonvisceral metastases | 130 | 41.7 | 121 | 38.5 | 251 | 40.1 |
| Visceral metastases‡ | 145 | 46.5 | 155 | 49.4 | 300 | 47.9 |
| Bone | 51 | 16.3 | 57 | 18.2 | 108 | 17.3 |
| Liver | 41 | 13.1 | 51 | 16.2 | 92 | 14.7 |
| Lung | 70 | 22.4 | 84 | 26.8 | 154 | 24.6 |
| Peritoneum | 19 | 6.1 | 12 | 3.8 | 31 | 5.0 |
| No. of metastatic sites | ||||||
| 1 | 108 | 34.6 | 110 | 35.0 | 218 | 34.8 |
| 2 | 86 | 27.6 | 89 | 28.3 | 175 | 28.0 |
| ≥ 3 | 81 | 26.0 | 77 | 24.5 | 158 | 25.2 |
| Prognostic risk group§ | ||||||
| Low | 98 | 31.4 | 93 | 29.6 | 191 | 30.5 |
| Intermediate | 136 | 43.6 | 135 | 43.0 | 271 | 43.3 |
| High | 77 | 24.7 | 83 | 26.4 | 160 | 25.6 |
Abbreviations: GC, gemcitabine + cisplatin; PCG, paclitaxel/cisplatin/gemcitabine.
One patient missing data.
Three patients missing data.
CNS: 1 patient on PCG and 0 on GC; bone marrow: 0 patients on PCG and 2 on GC.
Based on Bajorin et al.19
Survival
After a median follow-up of 4.6 years (maximum, 6.8 years), 504 patients (80.5%) have died, 256 (81.5%) on GC and 248 (79.5%) on PCG. Causes of death were urothelial cancer in 434 patients (226 patients [72%] on GC and 208 patients [66.7%] on PCG), toxicity in nine patients, chronic disease in one patient, other causes in 36 patients, and unknown in 24 patients.
The median OS was 3.1 months longer in the PCG arm; median OS was 15.8 months (95% CI, 13.6 to 17.5 months) on PCG compared with 12.7 months (95% CI, 11.0 to 14.4 months) on GC. However, the difference in median OS did not reach statistical significance (HR, 0.85; 95% CI, 0.72 to 1.02; P = .075; Fig 2). The OS rates at 1 and 4 years were 61.4% (95% CI, 55.7% to 66.6%) and 17.2% (95% CI, 13.0% to 21.8%), respectively, on PCG, compared with 52.8% (95% CI, 47.0% to 58.2%) and 16.4% (95% CI, 12.3% to 20.9%), respectively on CG. Results were similar when adjusted simultaneously by cooperative group, WHO performance status, and presence or absence of metastatic disease.
Fig 2.
Overall duration of survival in the intent-to-treat patient population. O, number of observed events.
All eligibility criteria including laboratory values were checked according to the most recent information available at the time of random assignment. Forty-seven patients (8%) were ineligible, mostly for reasons of disease stage and/or impaired renal function. Ten of these patients did not start the allocated treatment or were not physically fit enough to receive optimal treatment. Hence, we also analyzed OS in the eligible patient population, which showed that patients treated with the triplet had a significantly longer duration of survival (median, 15.9 months; 95% CI, 13.6 to 18.1 months) than patients in the GC arm (median, 12.7 months; 95% CI, 11.4 to 14.4 months; HR, 0.82; 95% CI, 0.68 to 0.98; P = .03; Fig 3).
Fig 3.
Overall duration of survival in the eligible patients. O, number of observed events.
After recent reports that outcome in tumors of the upper urinary tract may differ from outcome in tumors of the lower tract,20 the possible influence of anatomic site on treatment effect was investigated in an analysis that was not preplanned. Among the 81% of patients in whom the bladder was the site of the primary tumor, median OS after PCG was significantly longer than that after GC (15.9 v 11.9 months, respectively; HR, 0.80; 95% CI, 0.66 to 0.97; P = .025).
Prognostic factor analyses in the ITT population, independent of the treatment administered, showed statistically significant differences in survival according to WHO performance status (1 v 0: HR, 1.50; 95% CI, 1.26 to 1.79; P < .001), metastatic disease (presence v absence: HR, 1.38; 95% CI, 1.13 to 1.69; P = .001), visceral metastases (presence v absence; HR, 1.74; 95% CI, 1.46 to 2.08; P < .001), and number of Memorial Sloan-Kettering Cancer Center risk factors (two risk factors v no or one risk factor: HR, 2.17; 95% CI, 1.79 to 2.64; P < .001).
PFS
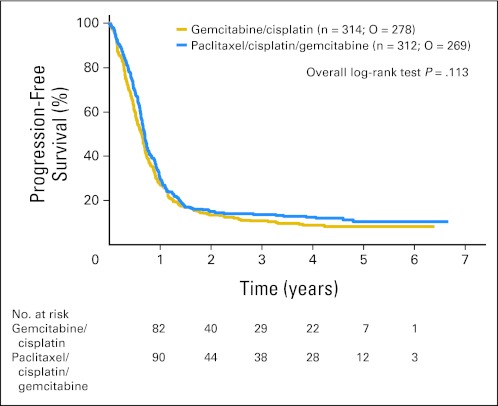
Progression or death was documented in 547 patients, 278 on GC and 269 on PCG. The median PFS was 8.3 months on PCG and 7.6 months on GC (HR, 0.87; 95% CI, 0.74 to 1.03; P = .113; Fig 4).
Fig 4.
Duration of progression-free survival. O, number of observed events.
Response Rate
The overall response rate (complete or partial; blinded review by J.B.) was significantly higher among patients treated with PCG than GC (55.5% v 43.6%, respectively; P = .0031). Response to treatment is shown in Table 2. Overall, 48 patients (21 patients in the PCG arm and 27 patients in the GC arm) underwent postchemotherapy surgical resection.
Table 2.
Overall Response According to RECIST
| Best Overall Response to Treatment | Paclitaxel/Cisplatin/Gemcitabine (n = 312) |
Gemcitabine/Cisplatin (n = 314) |
Total (N = 626) |
|||
|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Complete response | 42 | 13.5 | 35 | 11.1 | 77 | 12.3 |
| Partial response | 131 | 42.0 | 102 | 32.5 | 233 | 37.2 |
| Stable disease | 69 | 22.1 | 97 | 30.9 | 166 | 26.5 |
| Progression of disease | 21 | 6.7 | 47 | 15.0 | 68 | 10.9 |
| Early death | 8 | 2.6 | 7 | 2.2 | 15 | 2.4 |
| Not assessable | 31 | 9.9 | 17 | 5.4 | 48 | 7.7 |
| Treatment never started | 10 | 3.2 | 9 | 2.9 | 19 | 3.0 |
Drug Exposure and Toxicity
Of the 626 randomly assigned patients, 607 started the protocol treatment, 302 on PCG and 305 on GC (three patients refused, three patients had disease progression before start, four patients had other complicating diseases, four patients had other reasons, and information was lacking in five patients). The median duration of treatment was 16.3 weeks (range, 0.1 to 219 weeks). Appendix Table A1 (online only) lists treatment duration, dose reduction, and discontinuation.
Overall, the addition of paclitaxel to the combination of GC had little effect on the frequency or severity of toxic effects. Details of nonhematologic and hematologic adverse events are listed in Table 3.
Table 3.
Nonhematologic and Hematologic Adverse Events
| Adverse Event | Gemcitabine/Cisplatin (n = 305) |
Paclitaxel/Cisplatin/Gemcitabine (n = 302) |
||||||
|---|---|---|---|---|---|---|---|---|
| Grade 3 |
Grade 4 |
Grade 3 |
Grade 4 |
|||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Nonhematologic adverse events | ||||||||
| Vomiting | 19 | 6.2 | 1 | 0.3 | 20 | 6.6 | 1 | 0.3 |
| Pulmonary toxicity | 12 | 3.9 | 3 | 1.0 | 12 | 4.0 | 4 | 1.3 |
| Cardiovascular events* | 36 | 11.8 | 7 | 2.3 | 28 | 9.3 | 5 | 1.6 |
| Allergy | 0 | 0 | 1 | 0.3 | 5 | 1.7 | 4 | 1.3 |
| Fatigue | 34 | 11.1 | 0 | 43 | 14.2 | 4 | 1.3 | |
| Bleeding | 22 | 7.0 | 1 | 0.3 | 9 | 2.9 | 1 | 0.3 |
| Infection | 40 | 13.1 | 4 | 1.3 | 49 | 16.2 | 8 | 2.6 |
| Renal toxicity | 10 | 3.3 | 5 | 1.6 | 11 | 3.6 | 3 | 1.0 |
| Neuropathy/sensory | 1 | 0.3 | 0 | 0 | 0 | 0 | 1 | 0.3 |
| Alopecia | 2 | 0.7 | 0 | 0 | 5 | 1.7 | 0 | 0 |
| Diarrhea | 10 | 3.3 | 1 | 0.3 | 14 | 4.6 | 0 | 0 |
| Hematologic adverse events | ||||||||
| WBC | 102 | 33.4 | 16 | 5.2 | 102 | 33.8 | 53 | 17.5 |
| Neutropenia | 93 | 30.5 | 61 | 20.0 | 86 | 28.5 | 108 | 35.8 |
| Thrombocytopenia | 140 | 45.9 | 19 | 6.2 | 92 | 30.5 | 12 | 4.0 |
| Hemoglobin | 70 | 23.0 | 8 | 2.6 | 60 | 19.9 | 8 | 2.6 |
Includes edema, hypotension, thrombosis/embolism, and other cardiovascular events.
Patients on the PCG arm, compared with patients on the GC arm, experienced more grade 4 neutropenia (35.8% v 20%, respectively; P < .001), more febrile neutropenia (13.2% v 4.3%, respectively; P < .001), and a greater need for granulocyte colony-stimulating factor administration (17% v 11%, respectively; P = .03). However, there was no difference between treatments in the occurrence of neutropenic sepsis. Grade 4 thrombocytopenia was more frequent in the GC arm versus the PCG arm (6.2% v 4.0%, respectively; P = .03). Grade 3 or 4 thrombocytopenia associated with grade 3 bleeding was also more frequent in the GC arm than the PCG arm (11.4% v 6.8%, respectively; P = .05).
Severe acute toxicity (toxic death, grade 3 or 4 thrombocytopenia with grade 3 or 4 hemorrhage, grade 4 thrombocytopenia with hemorrhage, grade 3 asthenia at first cycle, grade 4 asthenia during treatment, grade 3 or 4 renal toxicity, grade 3 or 4 neutropenic fever, or grade 3 or 4 mucositis) was observed in 20.2% of patients on PCG (including six toxic deaths) and in 14.8% of patients on GC (including three toxic deaths).
DISCUSSION
This large, multinational, intergroup, phase III study, to our knowledge the largest study ever conducted in locally advanced or metastatic urothelial carcinoma, enrolling more than 600 patients over 3 years, confirms that cooperative groups on two continents can work together to provide timely answers to important clinical questions in this disease. The study shows that the three-drug combination of PCG provides a better response rate and a 3.1-month prolongation in median survival when compared with standard GC alone. The 15.8-month median OS on the triplet in this trial closely matches the outcome in the phase II study.14,15 The present findings also confirm the tolerability of the PCG regimen.
The trial was designed to detect a difference of 4 months in median survival between GC and PCG. The choice of 4 months was driven by the expected median survival of 18 months initially obtained in the phase I/II dose-finding study.15 The phase III study reported here showed a difference of 3.1 months in the OS in the ITT population, which is a strong trend but did not reach statistical significance. In view of the potential dilution effect of 8% ineligible patients, some of whom either did not receive the allocated treatment or were not physically fit enough to receive optimal treatment, we also carried out an analysis in the 575 eligible patients, which showed a median survival advantage of 3.2 months favoring the triplet compared with GC (15.93 v 12.71 months, respectively) and a reduction of 18% in the risk of death (HR, 0.82), which did reach statistical significance (P = .030). The eligibility was assessed based on measurements taken before random assignment so exclusion of the ineligible patients does not bias the treatment comparison, even though this has the limitation of being an additional unplanned analysis. The planned requisite of a 4-month difference in the median duration of survival based on those data was highly ambitious.15 The fact that the effect sought in the ITT patient population was not attained cannot be attributed to prerandomization differences in prognostic factors between the treatment groups because the two arms were generally well balanced regarding performance status and visceral metastases. This was further demonstrated in the ITT population because the conclusions were not affected after adjusting for these variables.
In addition, the trial has raised an intriguing issue of wide clinical importance. In a post hoc analysis, there was evidence of a greater and statistically significant survival benefit in patients with bladder primaries receiving the triple regimen (median, 15.9 months for PCG v 11.9 months for GC) in contrast to patients with nonbladder primaries, in whom there was no benefit. Pathologic findings in large series of upper tract urothelial cancer reveal that these tumors tend to have higher grade and stage than bladder cancer.20 Despite morphologic similarities, there are genetic and epigenetic differences between transitional-cell carcinoma in the upper and lower urinary tracts. First, embryologically, the urothelium of bladder and ureter arises from different tissues.21 Second, in vitro studies have shown that urothelium from the two sites differs in uroplakin content, keratin expression pattern, growth potential, and propensity to keratinize.22 Extracellular matrix–associated proteins with counter-adhesive properties respond differently in ureteric and bladder urothelial cells.23 Mono- and dinucleotide microsatellite instability, a feature of tumors with deficient mismatch repair, is more common in upper than lower urinary tract cancers,24,25 and these tumors have more extensive methylation than bladder cancers.26 To our knowledge, this study is the first to show a trend in OS advantage in a subgroup of patients with advanced urothelial cancer with bladder being the primary origin. The fact that the benefit by the triplet seems to be obtained particularly in bladder urothelial cancer and that upper tract urothelial cancer may be less responsive to chemotherapy implies that patients with bladder primaries (by far the most common site of urothelial cancer) should perhaps be treated differently from patients with urothelial tumors arising at other sites. Consequently, in the future, trials will need to prospectively analyze this hypothesis in addition to testing the importance of methylating patterns and other molecular factors.
Finally, the present results are consistent with previous findings and confirm that the GC schedule as studied in the randomized phase III study of GC versus MVAC2 may be more toxic in terms of grade 4 thrombocytopenia than most clinicians expect, often resulting in the need for omission of gemcitabine on day 15. Newer regimens with GC using a 21-day schedule are being developed to reduce the need to administer gemcitabine on day 15, which often requires adjustment because of high hematologic toxicity.
The modest survival benefit for the combination of PCG observed in this report has been shown in an exploratory analysis in the eligible patients. The eligible patient population corresponds to the population targeted by the protocol and to whom the results are to be generalized, and therefore, this might be considered to be a more meaningful analysis. In the future, to select patients most likely to benefit from the triple therapy, the development of biomarkers that predict outcome or sensitivity to chemotherapy is an essential first step. Pharmacogenomics and genomics might eventually play a role in the selection of better candidates for treatment and aid in the personalized design of treatment.
In conclusion, this large, multinational, phase III trial in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy shows that the triple combination of PCG provides a higher response rate when compared with GC. The predefined primary end point for OS improvement was not reached in the overall patient population, but the 3.2-month survival difference in the population of all eligible patients reached statistical significance. Moreover, a benefit in patients with a bladder primary was also observed in an analysis that was not preplanned. Finally, the triple combination was not appreciably more toxic than the GC regimen in this population. Ongoing studies may assist to identify patients who will derive the most benefit of taxane-based triple chemotherapy. Novel strategies will be required to have a major impact on survival in this disease.
Acknowledgment
We appreciate the contributions of the study coordinators and data managers of the European Organisation for Research and Treatment of Cancer and intergroup participating centers (Appendix). Anna Orsola, MD, provided nonremunerated editing support.
Appendix
Study Chairs: EORTC (coordinating group), J. Bellmunt; SWOG (North American Coordinator), D. Smith; AUO, A. Boehle; CECOG, C. Zielinski; CTSU, J. Abrams; GETUG, S. Culine; NCIC CTG, M. Moore; RTOG, D. Kaufman; SOGUG, J. Baselga; NCRN, T. Roberts.
With the cooperation of EORTC Genitourinary Tract Cancer Group (GUCG): Kaiser Franz Josef Spital, Vienna, Austria: Dr M. De Santis, Institut Jules Bordet, Brussels, Belgium: Dr T. Gil, Dr R. Van Velthoven, Universitair Ziekenhuis Antwerpen, Antwerp, Belgium: Prof J.B. Vermorken, Universitair Ziekenhuis Brussel, Brussels, Belgium: Prof J. De Greve, Prof F. Keuppens, Onze Lieve Vrouw Ziekenhuis Aalst, Aalst, Belgium, Dr P. Carpentier, Klinikum, Nurnberg, Nurnberg, Germany: Dr G. Kaiser, Rigshospitalet, Copenhagen, Denmark: Dr G. Daugaard, Herlev Hospital–University Copenhagen, Herlev, Denmark: Dr L. Sengelov, Aarhus University Hospital, Aarhus, Denmark: Prof H. Von Der Maase, Hospital General Vall D'Hebron, Barcelona, Spain: Dr J. Bellmunt, Guy's Hospital, London, United Kingdom: Dr P.G. Harper, National Institute of Oncology, Budapest, Hungary: Dr I. Bodrogi, Rabin Medical Center–Tel Aviv University, Petah Tiqva, Israel: Dr Z. Leib, Istituto Scientifico H.S. Raffaele, Milan, Italy: Dr C. Cozzarini, Ospedale Santa Croce, Cuneo, Italy: Dr Bertelli, Dr A. Heouaine, Jeroen Bosch Ziekenhuis, Hertogenbosch, the Netherlands: Dr J.J. Croles, Dr A. Van Der Meijden, The Netherlands Cancer Institute–Antoni Van Leeuwenhoekziekenhuis, Amsterdam, the Netherlands: Dr J.H. Schornagel, Erasmus MC–Daniel Den Hoed Cancer Center, Rotterdam, the Netherlands: Dr R. De Wit, Radboud University Nijmegen Medical Centre, Nijmegen, the Netherlands: Dr P. De Mulder, Dr J.A. Witjes, Universitair Medisch Centrum–Academisch Ziekenhuis, Utrecht, the Netherlands: Dr G. Groenewegen, Leiden University Medical Centre, Leiden, the Netherlands: Dr S. Osanto, Erasmus MC, Rotterdam, the Netherlands: Dr W. Kirkels, Martini Ziekenhuis, Groningen, the Netherlands: Dr R.S. De Jong, Dr. H. Piersma, Maria Sklodowska–Curie Memorial Cancer Centre, Warsaw, Poland: Dr I. Skoneczna, Cancer Research Center, Moscow, Russia: Prof A.M. Garin, Medical Radiological Research Center, Obninsk Kaluga Region, Russia: Dr O. Koriakine, National Cancer Institute, Bratislava, Slovak Republic: Dr J. Mardiak.
German Association of Urologic Oncology (AUO): Diakoniekrankenhaus Rotenburg, Rotenburg–Wuemme, Germany: Dr R. Schoenfelder, Charite–Universitaetsmedizin Berlin–Campus Mitte, Berlin, Germany: Dr A. Wille, Universitaetsklinikum Bonn, Bonn, Germany: Dr R. Siener, Staedtisches Klinikum, Fulda, Germany: Dr C. Greb, Medizinische Universitaet Zu Luebeck, Luebeck, Germany: Dr O. Walden, Heinrich-Heine Universitaetsklinik Dusseldorf, Düsseldorf, Germany: Dr M.-O. Grimm, Martin Luther Universitaet, Halle, Germany: Dr H. Loertzer, Technische Universitaet Muenchen–Klinikum Rechts Der Isar, München, Germany: Dr U. Treiber, Universitaetsklinikum Ulm, Ulm, Germany: Dr J.E. Gschwend, Klinikum Kassel GMBH, Kassel, Germany: Prof P. Albers, Charite–Universitätsmedizin Berlin–Campus Benjamin Franklin, Berlin, Germany: Dr U. Steiner, Universitätsmedizin Mannheim, Mannheim, Germany: Prof M.J. Siegsmund, Klinikum der J.W. Goethe Universität, Frankfurt Am Main, Germany: Dr W.C.D. Beecken, Universitaetsklinikum Muenster, Muenster, Germany: Dr P. Wuelfing, Katharinenhospital, Stuttgart, Germany: Dr J. Schleicher, Helios Klinikum Wuppertal - Klin. Univ Witten/Herdecke, Wuppertal, Germany: Dr L. Fricke, Klinikum St Marien, Amberg, Germany: Dr J. Junginger, Universitaetskliniken Des Saarlandes, Homburg, Germany: Dr J. Lehmann, Klinikum Der Philipps-Universitaet Marburg, Marburg, Germany: Dr O. Carsten-Henning, Universitaetsklinikum Tuebingen, Tuebingen, Germany: Prof M. Kuczyk, Kliniken Der Stadt Ludwigshafen Am Rhein, Ludwigshafen Am Rhein, Germany: Dr M. Koser, Klinikum Neubrandenburg, Neubrandenburg, Germany: Dr J. Rogellin, Kreiskrankenhaus Luedenscheid, Luedenscheid, Germany: Dr M. Gombel, Universitaetsklinikum Jena, Jena, Germany: Dr T. Steiner, Marienkrankenhaus, Hamburg, Germany: Dr C. Ehlert, St Markus Krankenhaus, Frankfurt, Germany: Dr M. Westrich, Staedtische Kliniken Dortmund, Dortmund, Germany: Dr Kwasny, Krankenhaus Hohe Warte, Bayreuth, Germany: Dr S. Reichert, Asklepios Klinik Pasewalk, Pasewalk, Germany: Dr K. Gromoll-Bergmann, Paracelsius Krankenhaus, Ostfildern, Germany: Dr U. Haefele, Johanniter Kankenhaus Stendal, Stendal, Germany: Dr G. Kramer, Helios-Klinik Blankenhain, Blankenhain, Germany: Dr X.A. Krah, St Josefs Hospital Uerdingen, Krefeld, Germany: Dr N. Fuchs.
Groupe d'Etude des Tumeurs Uro-Génitales (GETUG): Centre Hospitalier Universitaire (CHU) Henri Mondor AP-HP, Creteil, France: Dr B. Paule, Centre Regional Francois Baclesse, Caen, France: Dr F. Joly, Centre Henri Becquerel, Rouen, France: Dr M. Debled, Centre Claudius Regaud, Toulouse, France: Dr C. Chevreau, Dr J.P. Delord, Centre Leon Berard, Lyon, France: Dr J.P. Droz, Institut Bergonie, Bordeaux, France: Dr B. Bui-Nguyen, Dr N. Houede-Tchen, Centre Oscar Lambret, Lille, France: Dr A. Caty, Hopital St Andre, Bordeaux, France: Dr A. Ravaud, Centre Alexis Vautrin, Vandoeuvre-Les-Nancy, France: Dr L. Geoffrois, Institut Curie, Paris, France: Dr Beuzeboc, Centre Eugene Marquis, Rennes, France: Prof P. Kerbrat, Assistance Publique–Hôpitaux de Marseille–CHU De La Timone, Marseille, France: Dr M. Baciuchka, Centre Medico-Chirurgical Foch, Suresnes, France: Dr Mignot, CRLC Val D'Aurelle, Montpellier, France: Dr S. Culine, Hopital Lapeyronie, Montpellier, France: Dr E. Legouffe, Institut de Cancerologie de l'Ouest (ICO)–Centre Paul Papin, Angers, France: Dr S. Abadie-Lacourtoisie, Dr R. Delva, Centre Hospitalier Departemental, La Roche Sur Yon, France: Dr F. Priou, Hopital Europeen Georges Pompidou AP-HP, Paris, France: Dr S. Oudard.
Spanish Oncology Genitourinary Group (SOGUG): Corporacio Sanitaria Parc Tauli, Barcelona, Spain: Dr E. Gallardo, Dr Nogue, Hospital Son Dureta, Palma De Mallorca, Spain: Dr A. Gonzalez De Alba, Hospital Juan Canalejo, La Coruna, Spain: Dr A. Aparicio, Instituto Valenciano De Oncologia, Valencia, Spain: Dr V.G. Porta, Hospital Universitario 12 de Octubre, Madrid, Spain: Prof L. Paz-Ares, Hospital Universitario San Carlos, Madrid, Spain: Dr J.L. Gonzalez-Larriba, Hospital Clinic Universitari, Barcelona, Spain: Dr B. Mellado, Hospital Universitario Marques De Valdecilla, Santander, Spain: Dr M. Lopez Brea, ICO Badalona–Hospital Germans Trias i Pujol (Institut Catala, D'Oncologia), Barcelona: Dr A. Font, Hospital Del Mar, Barcelona, Spain: Dr Calceran, Hospital Clinico Universitario Lozano Blesa, Zaragoza, Spain: Dr A. Saenz-Cusi, Hospital Clinico Universitario, Malaga, Spain: Dr L. Alonso Carrion, Hospital Cuidad De Jaen, Jaen, Spain: Dr N. Mohedano Mohedano, Hospital General Universitario De Guadalajara, Guadalajara, Spain: Dr J. Cassinello Espinosa, Hospital Severo Ochoa, Madrid, Spain: Dr R. Garcia-Carbonero.
United Kingdom National Cancer Research Institute (NCRI) Bladder Clinical Studies Group (BCSG): Wales Cancer Trials Unit. NISCHR Cancer Registered Research Group, Cardiff, United Kingdom: G. Griffiths, Princess Royal Hospital, Hull, United Kingdom: Dr J. Hetherington, Christie Hospital, Manchester, United Kingdom: Dr J.P. Wylie, University College Hospital, London, United Kingdom: Dr S. Harland, Addenbrookes Hospital, Cambridge, United Kingdom: Dr H. Patterson, St Mary's Hospital, Portsmouth Hants, United Kingdom: Dr J. Gale, Mount Vernon Hospital, Northwood Middlesex, United Kingdom: Dr P.J. Hoskin, Dr P. Ostler, National Health Service (NHS) Greater Glasgow and Clyde–Western Infirmary, Glasgow, United Kingdom: Dr M. Russell, Newcastle General Hospital, Newcastle, United Kingdom: Dr J.T. Roberts, University Hospitals Bristol NHS Foundation Trust–Bristol Haematology and Oncology Centre, Bristol Avon, United Kingdom: Dr Graham, Cheltenham General Hospital, Cheltenham, United Kingdom: Dr P. Jenkins, Royal United Hospital, Bath, United Kingdom: Dr H. Newman, Nottingham City Hospital, Nottingham, United Kingdom: Dr S. Sundar, Torbay Hospital, Torquay Devon, United Kingdom: Dr A. Lydon, NCRI BCSG + EORTC GUCG: Barts and the London NHS Trust–St Bartholomew's Hospital, London, United Kingdom: Prof R.T.D. Oliver, Royal South Hants Hospital, Southampton, United Kingdom: Dr B. Mead, St James's University Hospital, Leeds, United Kingdom: Dr M. Leahy, Royal Marsden Hospital, Sutton, United Kingdom: Prof A. Horwich, Velindre Hospital, Cardiff, United Kingdom: Dr J. Barber.
National Cancer Institute of Canada Clinical Trials Group (NCIC CTG): Hopital Notre-Dame Du CHUM, Montreal, Quebec, Canada: Dr D. Charpentier, Hôpital Hotel Dieu Du CHUM, Montreal, Quebec, Canada: Dr D. Soulieres, University Health Network-OCI/Princess Margaret Hospital, Toronto, Ontario, Canada: Dr J. Knox, London Health Sciences Centre, London Ontario, Canada: Dr E. Winquist, Cross Cancer Institute, Edmonton, Alberta, Canada: Dr S. North, Southeastern Ontario Regional Cancer Center, Kingston, Ontario, Canada: Dr R. Gregg, Cancercare Manitoba, Winnipeg, Manitoba, Canada: Dr R.Wong, BCCA, Vancouver Cancer Centre, Vancouver, British Columbia, Canada: Dr K. Chi, Odette Cancer Centre-Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada: Dr N. Iscoe, Tom Baker Cancer Centre, Calgary, Alberta, Canada: Dr S. Ernst, Dr D. Ruether, Dr D. Stewart, McGill University, Department of Oncology, Montreal, Quebec, Canada: Dr R. Rajan, Hotel Dieu Hospital, St Catharines, Ontario, Canada: Dr B. Findlay, Hôpital Charles Lemoyne, Greenfield Park, Quebec, Canada: Dr M. Trudeau, William Osler Health Centre, Brampton, Ontario, Canada: Dr D. Roitman.
Southwest Oncology Group (SWOG): University of Southern California/Kenneth Norris Hospital, Los Angeles, CA: Dr D. Quinn, Kansas City CCOP, Kansas City, MO: Dr J. Rigden, Columbus CCOP, Columbus, OH: Dr L. Laufman, Baylor College–UCOP, Houston, TX: Dr S. Lerner, Spartanburg Regional Medical Center, Spartanburg, SC: Dr E. Nelson, Brook Army Medical Center, Ft Sam Houston, TX: Dr M. Osswald, University of Utah Medical Center UCOP, Salt Lake City, UT: Dr A. Beck, Central Illinois CCOP–Decatur Memorial Hospital, Decatur, IL: Dr J. Wade, Montana CCOP, Billings, MT: Dr T. Warr, Good Samaritan Hospital, Kearney, NE: Dr G. Bascom, Loma Linda University, Loma Linda, CA: Dr M. Lilly, City of Hope National Medical Center, Duarte, CA: Dr P. Twardowski, University of Kansas, Kansas City, MO: Dr P. Van Veldhuizen.
Table A1.
Cycles Administered, Dose Reduction, and Discontinuation
| Treatment | Paclitaxel/Cisplatin/Gemcitabine (n = 302) |
Gemcitabine/Cisplatin (n = 305) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| No. of cycles | ||||
| 1 | 27 | 8.9 | 19 | 6.2 |
| 2 | 26 | 8.6 | 34 | 11.1 |
| 3 | 16 | 5.3 | 22 | 7.2 |
| 4 | 36 | 11.9 | 53 | 17.4 |
| 5 | 9 | 3.0 | 25 | 8.2 |
| 6 | 187 | 61.9 | 151 | 49.5 |
| 8 | 1 | 0.3 | 1 | 0.3 |
| ≥ 4 | 233 | 77.1 | 230 | 75.4 |
| Dose reduction* | 202 | 66.9 | 231 | 75.7 |
| Hematologic toxicity | 139 | 46.0 | 177 | 58.0 |
| Renal toxicity | 27 | 8.9 | 21 | 6.9 |
| Treatment delay* | 177 | 58.6 | 177 | 58.0 |
| Reason for treatment discontinuation | ||||
| Treatment completed per protocol criteria | 183 | 60.6 | 141 | 46.2 |
| Disease progression | 23 | 7.6 | 65 | 21.3 |
| Toxicity, adverse effects, or complications | 44 | 14.6 | 48 | 15.7 |
| Other | 52 | 17.2 | 51 | 16.7 |
Gemcitabine/cisplatin arm, n = 305; paclitaxel/cisplatin/gemcitabine arm, n = 302.
Footnotes
Listen to the podcast by Dr Garnick at www.jco.org/podcasts
Written on behalf of the European Organisation for Research and Treatment of Cancer (EORTC), National Cancer Research Institute (NCRI) Bladder Clinical Studies Group, German Association of Urologic Oncology, Groupe d'Etude des Tumeurs Uro-Génitales, Spanish Oncology Genitourinary Group, National Cancer Institute of Canada (NCIC), and Southwest Oncology Group.
Supported by Grants No. 2U10 CA11488-28 through 2U10 CA011488-41 from the National Cancer Institute (NCI; Bethesda, MD), a donation from the EORTC Charitable Trust, and Eli Lilly Study Code B9E-MC-S014. The NCRI involvement was supported by Grant No. C448/A2683-CRUK/02/001 from Cancer Research UK and sponsored by the Medical Research Council. NCIC Clinical Trials Group participation in this trial was supported by funding received from the Canadian Cancer Society Research Institute (Grant No. 10362), the NCI (Grant No. CA077202), a grant from the Associacio per la Recerca Oncologica, Grant No. RD06/0020/0109 from Instituto de Salud Carlos III/FEDER; and Grant No. 2009 SGR 321 from Generalitat de Catalunya. Bristol-Myers Squibb provided support through the free supply of paclitaxel (Taxol), the experimental drug in this study.
Presented in part at the 43rd Annual Meeting of the American Society of Clinical Oncology, June 1-5, 2007, Chicago, IL.
The contents of this article are solely the responsibility of the authors and do not necessarily reflect the official views of the NCI.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00022191.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Joaquim Bellmunt, Eli Lilly (C); Hans von der Maase, Eli Lilly (C); Maria De Santis, Eli Lilly (C), sanofi-aventis (C); Luis Paz-Ares, Eli Lilly (C); Derek Raghavan, Eli Lilly (C), sanofi-aventis (C); Ronald de Wit, Eli Lilly (C) Stock Ownership: None Honoraria: Hans von der Maase, Eli Lilly; Iwona Skoneczna, Eli Lilly Research Funding: Iwona Skoneczna, Eli Lilly; Luis Paz-Ares, Eli Lilly Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Joaquim Bellmunt, Hans von der Maase, Derek Raghavan, Richard Sylvester, Ronald de Wit
Provision of study materials or patients: Joaquim Bellmunt, Hans von der Maase, Graham M. Mead, Iwona Skoneczna, Maria De Santis, Gedske Daugaard, Andreas Boehle, Christine Chevreau, Luis Paz-Ares, Leslie R. Laufman, Eric Winquist, Derek Raghavan, Ronald de Wit
Collection and assembly of data: Joaquim Bellmunt, Hans von der Maase, Derek Raghavan, Sandra Collette, Richard Sylvester,Ronald de Wit
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Sternberg CN, Yagoda A, Scher HI, et al. Methotrexate, vinblastine, doxorubicin, and cisplatin for advanced transitional cell carcinoma of the urothelium: Efficacy and patterns of response and relapse. Cancer. 1989;64:2448–2458. doi: 10.1002/1097-0142(19891215)64:12<2448::aid-cncr2820641209>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 2.von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068–3077. doi: 10.1200/JCO.2000.18.17.3068. [DOI] [PubMed] [Google Scholar]
- 3.Loehrer PJ, Sr, Einhorn LH, Elson PJ, et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: A cooperative group study. J Clin Oncol. 1992;10:1066–1073. doi: 10.1200/JCO.1992.10.7.1066. [DOI] [PubMed] [Google Scholar]
- 4.Logothetis CJ, Dexeus FH, Finn L, et al. A prospective randomized trial comparing MVAC and CISCA chemotherapy for patients with metastatic urothelial tumors. J Clin Oncol. 1990;8:1050–1055. doi: 10.1200/JCO.1990.8.6.1050. [DOI] [PubMed] [Google Scholar]
- 5.Bellmunt J, Ribas A, Eres N, et al. Carboplatin-based versus cisplatin-based chemotherapy in the treatment of surgically incurable advanced bladder carcinoma. Cancer. 1997;80:1966–1972. doi: 10.1002/(sici)1097-0142(19971115)80:10<1966::aid-cncr14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 6.Loehrer PJ, Sr, Elson P, Dreicer R, et al. Escalated dosages of methotrexate, vinblastine, doxorubicin, and cisplatin plus recombinant human granulocyte colony-stimulating factor in advanced urothelial carcinoma: An Eastern Cooperative Oncology Group trial. J Clin Oncol. 1994;12:483–488. doi: 10.1200/JCO.1994.12.3.483. [DOI] [PubMed] [Google Scholar]
- 7.Logothetis CJ, Finn LD, Smith T, et al. Escalated MVAC with or without recombinant human granulocyte-macrophage colony-stimulating factor for the initial treatment of advanced malignant urothelial tumors: Results of a randomized trial. J Clin Oncol. 1995;13:2272–2277. doi: 10.1200/JCO.1995.13.9.2272. [DOI] [PubMed] [Google Scholar]
- 8.Seidman AD, Scher HI, Gabrilove JL, et al. Dose-intensification of MVAC with recombinant granulocyte colony-stimulating factor as initial therapy in advanced urothelial cancer. J Clin Oncol. 1993;11:408–414. doi: 10.1200/JCO.1993.11.3.408. [DOI] [PubMed] [Google Scholar]
- 9.Roth BJ, Dreicer R, Einhorn LH, et al. Significant activity of paclitaxel in advanced transitional-cell carcinoma of the urothelium: A phase II trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 1994;12:2264–2270. doi: 10.1200/JCO.1994.12.11.2264. [DOI] [PubMed] [Google Scholar]
- 10.Lorusso V, Pollera CF, Antimi M, et al. A phase II study of gemcitabine in patients with transitional cell carcinoma of the urinary tract previously treated with platinum: Italian Co-operative Group on Bladder Cancer. Eur J Cancer. 1998;34:1208–1212. doi: 10.1016/s0959-8049(98)00030-6. [DOI] [PubMed] [Google Scholar]
- 11.Moore MJ, Tannock IF, Ernst DS, et al. Gemcitabine: A promising new agent in the treatment of advanced urothelial cancer. J Clin Oncol. 1997;15:3441–3445. doi: 10.1200/JCO.1997.15.12.3441. [DOI] [PubMed] [Google Scholar]
- 12.Pollera CF, Ceribelli A, Crecco M, et al. Weekly gemcitabine in advanced bladder cancer: A preliminary report from a phase I study. Ann Oncol. 1994;5:182–184. doi: 10.1093/oxfordjournals.annonc.a058775. [DOI] [PubMed] [Google Scholar]
- 13.Stadler WM, Kuzel T, Roth B, et al. Phase II study of single-agent gemcitabine in previously untreated patients with metastatic urothelial cancer. J Clin Oncol. 1997;15:3394–3398. doi: 10.1200/JCO.1997.15.11.3394. [DOI] [PubMed] [Google Scholar]
- 14.Bellmunt J, Albanell J, Paz-Ares L, et al. Pretreatment prognostic factors for survival in patients with advanced urothelial tumors treated in a phase I/II trial with paclitaxel, cisplatin, and gemcitabine. Cancer. 2002;95:751–757. doi: 10.1002/cncr.10762. [DOI] [PubMed] [Google Scholar]
- 15.Bellmunt J, Guillem V, Paz-Ares L, et al. Phase I-II study of paclitaxel, cisplatin, and gemcitabine in advanced transitional-cell carcinoma of the urothelium: Spanish Oncology Genitourinary Group. J Clin Oncol. 2000;18:3247–3255. doi: 10.1200/JCO.2000.18.18.3247. [DOI] [PubMed] [Google Scholar]
- 16.Bellmunt J, von der Maase H, Mead GM, et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine (PCG) and gemcitabine/cisplatin (GC) in patients with locally advanced (LA) or metastatic (M) urothelial cancer without prior systemic therapy; EORTC30987/Intergroup Study. J Clin Oncol. 2007;25(suppl):242s. doi: 10.1200/JCO.2011.38.6979. abstr LBA5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 18.Therasse P, Eisenhauer EA, Verweij J. RECIST revisited: A review of validation studies on tumour assessment. Eur J Cancer. 2006;42:1031–1039. doi: 10.1016/j.ejca.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 19.Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol. 1999;17:3173–3181. doi: 10.1200/JCO.1999.17.10.3173. [DOI] [PubMed] [Google Scholar]
- 20.Akdogan B, Dogan HS, Eskicorapci SY, et al. Prognostic significance of bladder tumor history and tumor location in upper tract transitional cell carcinoma. J Urol. 2006;176:48–52. doi: 10.1016/S0022-5347(06)00511-8. [DOI] [PubMed] [Google Scholar]
- 21.Cuckow PM, Nyirady P, Winyard PJ. Normal and abnormal development of the urogenital tract. Prenat Diagn. 2001;21:908–916. doi: 10.1002/pd.214. [DOI] [PubMed] [Google Scholar]
- 22.Riedel I, Liang FX, Deng FM, et al. Urothelial umbrella cells of human ureter are heterogeneous with respect to their uroplakin composition: Different degrees of urothelial maturity in ureter and bladder? Eur J Cell Biol. 2005;84:393–405. doi: 10.1016/j.ejcb.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Hudson AE, Feng WC, Delostrinos CF, et al. Spreading of embryologically distinct urothelial cells is inhibited by SPARC. J Cell Physiol. 2005;202:453–463. doi: 10.1002/jcp.20140. [DOI] [PubMed] [Google Scholar]
- 24.Catto JW, Azzouzi AR, Amira N, et al. Distinct patterns of microsatellite instability are seen in tumours of the urinary tract. Oncogene. 2003;22:8699–8706. doi: 10.1038/sj.onc.1206964. [DOI] [PubMed] [Google Scholar]
- 25.Hartmann A, Zanardo L, Bocker-Edmonston T, et al. Frequent microsatellite instability in sporadic tumors of the upper urinary tract. Cancer Res. 2002;62:6796–6802. [PubMed] [Google Scholar]
- 26.Catto JW, Azzouzi AR, Rehman I, et al. Promoter hypermethylation is associated with tumor location, stage, and subsequent progression in transitional cell carcinoma. J Clin Oncol. 2005;23:2903–2910. doi: 10.1200/JCO.2005.03.163. [DOI] [PubMed] [Google Scholar]