SUMMARY
How information encoded in neuronal spike trains is used to guide sensory decisions is a fundamental question. In olfaction, a single sniff is sufficient for fine odor discrimination but the neural representations on which olfactory decisions are based are unclear. Here, we recorded neural ensemble activity in the anterior piriform cortex (aPC) of rats performing an odor mixture categorization task. We show that odors evoke transient bursts locked to sniff onset and that odor identity can be better decoded using burst spike counts than by spike latencies or temporal patterns. Surprisingly, aPC ensembles also exhibited near-zero noise correlations during odor stimulation. Consequently, fewer than 100 aPC neurons provided sufficient information to account for behavioral speed and accuracy, suggesting that behavioral performance limits arise downstream of aPC. These findings demonstrate profound transformations in the dynamics of odor representations from the olfactory bulb to cortex and reveal likely substrates for odor-guided decisions.
INTRODUCTION
Active sampling is an important component of sensory processing that can result in chunking of information into short, discrete epochs of a fraction of a second, as exemplified by visual fixations. In olfaction, rodents exhibit rapid stereotyped respiration at theta frequency (called sniffing) during active exploration (Wachowiak, 2011; Welker, 1964). Behavioral experiments have shown that a single rapid sniff can support accurate odor discrimination (Uchida and Mainen, 2003; Wesson et al., 2008), suggesting that each sniff generates a relatively complete “snapshot” of an olfactory world, and constitutes a unit of odor coding (Kepecs et al., 2006). Despite these observations, however, how sensory information is represented in this timescale and how it is transformed in the brain to ultimately control behavior remain unclear.
Studies in the olfactory bulb, the first relay in the olfactory neural pathway, have shown that odor stimulation triggers diverse temporal patterns of activity at the level of the olfactory nerve inputs and mitral/tufted cells, the exclusive outputs of the olfactory bulb (Cang and Isaacson, 2003; Friedrich and Laurent, 2001; Hamilton and Kauer, 1989; Junek et al., 2010; Macrides and Chorover, 1972; Margrie and Schaefer, 2003; Meredith, 1986; Spors and Grinvald, 2002; Wehr and Laurent, 1996; Wellis et al., 1989). During sniffing, spiking activity of mitral/tufted cells show diverse and reliable temporal patterns at the resolution of tens of milliseconds (Carey and Wachowiak, 2011; Cury and Uchida, 2010; Shusterman et al., 2011). These dynamic response patterns, in particular, those in the initial portion of the response (~100 ms), convey substantial odor information compared to the total spike counts contained in the entire period of a theta sniff cycle (Cury and Uchida, 2010), suggesting that timing of spikes plays a critical role in rapid and accurate odor coding in the olfactory bulb.
Compared to the olfactory bulb, relatively little is known about how odor information is coded by neurons in the olfactory cortex. Neurons in the olfactory bulb project broadly to the cortex without apparent topography (Ghosh et al., 2011; Miyamichi et al., 2011; Nagayama et al., 2010; Ojima et al., 1984; Sosulski et al., 2011) and odor stimulation activates widely distributed neurons in the cortex again without apparent topography (Illig and Haberly, 2003; Rennaker et al., 2007; Stettler and Axel, 2009), suggesting that the olfactory cortex might use a different mechanism for odor coding than the olfactory bulb. To elucidate coding principles in the olfactory cortex that underlie rapid olfactory decisions, here we examined (1) how active sniffing shapes neural responses, (2) whether spike times or rate carry more information and (3) the nature of odor coding at the ensemble level. We show that odor inhalation triggers a transient burst of spikes time-locked to inhalation onset. In contrast to the olfactory bulb, timing of spikes conveyed little additional information compared to the total spike counts, demonstrating a profound transformation of coding mechanisms between the olfactory bulb and cortex. Furthermore, odor stimulation dramatically reduced correlated noise among neurons, which facilitated the efficiency of population code.
RESULTS
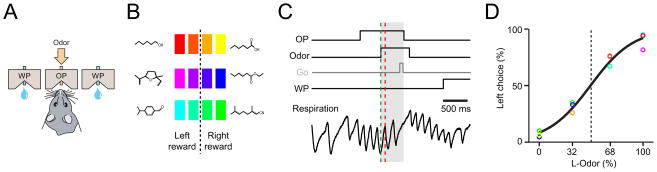
We recorded spiking activity of olfactory cortical neurons in rats while simultaneously monitoring their sniffing and performance in a two-alternative choice odor mixture categorization task (Uchida and Mainen, 2003) (Figure 1A). The stimuli consisted of three or four odor pairs with each delivered either alone (100/0, 0/100) or in mixtures (68/32, 32/68) (Figure 1B). All stimuli were randomly interleaved and one odor of each pair was assigned to the right and the other to the left choice port, with mixtures rewarded according to the dominant component. One set of subjects (n = 5) performed a reaction time version of the task, taking one to two sniffs between odor onset and response initiation (1.71 ± 0.01; Figure S1B) (Uchida and Mainen, 2003). A second set of subjects (n = 3) was trained to wait for a tone (Rinberg et al., 2006) at 700 ms delay from odor valve onset in order to enforce a longer odor sampling period (Figure 1C) and more sniffs (3.84 ± 0.03, P < 0.05 compared to reaction time paradigm; Figure S1B). In both paradigms, rats sniffed at theta frequency during odor sampling (7.18 ± 0.29 and 6.35 ± 0.27 s−1 respectively; Figure 1C). Task performance accuracy was higher for pure than mixture stimuli across all pairs, but was independent of the training paradigm and of the number of sniffs taken within a given paradigm (Figure 1D, Figures S1C, D). Thus, as previously reported (Uchida and Mainen, 2003), a single sniff was sufficient for maximal performance by rats in this odor mixture categorization task.
Figure 1. Behavioral performance and sniff-locked burst responses of aPC neurons.
(A, B) Schematic of odor mixture categorization task. Rats were trained to respond to the left or right reward port depending on the dominant component in a mixture. Task difficulty was varied by changing ratios of two odorants of a given odor pair (A/B: 0/100, 32/68, 68/32, 100/0). Three pairs of odors were used and all the stimuli were randomly interleaved in a session. Odors are indicated by colors; yellow: caproic acid, red: 1-hexanol, blue: ethyl 3-hexenoate, magenta: dehydroxy linalool oxide, green: citralva, cyan: cumin aldehyde. Intermediate colors represent binary mixtures of the pure odors.
(C) Task timing and Respiration patterns. An odor was delivered in the odor port upon entry with a pseudorandom delay of 0.2–0.5 s. In a self-paced version of the task (reaction time paradigm, blank line), rats were allowed to respond as soon as they decided to leave the port. In the go-signal paradigm, rats had to wait until a tone (go-signal, grey line) is played 700 ms after odor onset. Respiration patterns were monitored using a temperature sensor in the nasal cavity (the voltage signal from a nasal thermocouple: V). The gray shading indicates the timing of odor sampling. Scale bar: 500 ms.
(D) Psychometric curve. The behavioral performance for the 12 odors (same color code as a) were fitted by a sigmoid curve as a function of mixture ratio. Task performance accuracy was higher for pure compared to mixture stimuli.
We recorded from ensembles of up to 21 neurons (9.4 ± 4.7, mean ± S.D.) in the anterior piriform cortex (aPC) using chronically-implanted tetrodes during performance of the above tasks (see Experimental Procedures for details). From a total of 460 well-isolated single neurons, 179 neurons recorded using a fixed panel of 6 odorants formed the primary data set for the subsequent analyses. Given the similarity of behavioral performance in reaction-time and go-signal paradigms data from these experiments was pooled (91 neurons from the reaction time paradigm and 88 neurons from the go-tone paradigm).
Sniffing of odors triggers transient spike bursts tightly locked to inhalation onset
Previous studies have noted relatively brief, burst-like responses in PC (McCollum et al., 1991; Wilson, 1998) but these studies did not explicitly compare neural responses with respiration. We found that odor responses in aPC consisted typically of a transient burst of spikes time-locked to the onset of odor inhalation. Aligning spike times relative to the onset of the first sniff after odor onset revealed a much tighter temporal organization than was apparent by aligning on odor valve opening (Figures 2A–C). Indeed some responses were detectable only using sniff-locking (Figures S2A, B). Responses peaked rapidly (Tpeak: 99 ± 45 ms from the first inhalation onset, median ± S.D.; Figure 2D) and returned to baseline rapidly (full-width at half max: 32 ± 24 ms, median ± S.D.; Figure 2E). Thus, odor-evoked transients lasted approximately one sniff cycle (158.1 ± 40.2 ms, mean ± S.D.).
Figure 2. Sniffing of odors triggers transient spike bursts tightly locked to inhalation onset.
(A, B) Activity of an example aPC neuron. Raster plots represent neural activity with each row corresponding to a single trial (N = 37 trials) and each tick mark to a spike. Peri-event histograms are overlaid (green and red, smoothed with a Gaussian filter with the standard deviation of 7.5 ms). Trials are aligned to onset of odor valve opening (A) or first sniff after odor valve opening (B). In (B), periodic spontaneous activity before t=0 that reflects sniffing is evident.
(C) Comparison of peak firing rates between the two alignment conditions (odor valve opening vs. first sniff onset). Instantaneous firing rates were calculated after smoothing the peri-event histogram using a Gaussian filter (S.D.: 7.5 ms). The arrow denotes the example in this figure (A, B). A baseline firing rate (0 to 0.5 sec before odor valve onset) was subtracted for each neuron-odor pair. The peak firing rates are higher when triggered by the first inhalation onset (P<10−10, Wilcoxon signed rank test).
(D) Histogram of temporal half width of peak firing. Data from 243 odor-responsive neurons.
(E) Histogram of peak timing. Same data as in D.
Odor stimulation evokes broadly distributed, moderately sparse ensemble neurons
Single neurons in aPC showed robust and stimulus-specific responses to odor stimuli (Figure 3A). Relatively little selectivity for spatial choices (left vs. right) or reward outcomes was observed (Figure 3B). As a population, 45% of aPC neurons were activated by at least one of the six odors tested while 28% were activated by two or more (Figures 3C, D, S3) (P<0.05, Wilcoxon rank sum test). Conversely, each odor caused significant responses in 16.5 ± 3.1% of aPC neurons (mean ± S.D., n = 6 odors, 10.3% excitatory; 6.2% inhibitory). The probability of response of a piriform neuron to an odor was well-fit by a binomial distribution with an extra allowance for non-responding neurons (Figure 3D). We calculated a population sparseness of 0.41 and a lifetime sparseness of 0.61 (see Experimental Procedures), somewhat lower than previously observed in aPC of anesthetized rats (Poo and Isaacson, 2009). Therefore, aPC responses were observed in broadly distributed, moderately sparse neural populations, largely consistent with previous studies (Poo and Isaacson, 2009; Rennaker et al., 2007; Stettler and Axel, 2009; Zhan and Luo, 2011).
Figure 3. Moderately sparse, distributed population odor responses in aPC.
(A) Odor-evoked responses of an example neuron during first sniff cycle after odor onset. The bottom colors indicate odors tested (same colors as in Figure 1B). The middle plot shows the firing rates in first sniff after odor valve opening (40–160 ms from inhalation onset) as a function of odor stimuli. The dashed line indicates the firing rate at the pre-odor sniff. The top colors indicate the magnitude of odor response to each stimulus. The response magnitudes were calculated as a comparison with blank (no odor) trials using the signal detection analysis (area under the receiver operating characteristics curve, auROC, see Experimental Procedures). Scale is shown in c(red: excitatory response with perfect discriminability, black: no discriminability (no response), blue: inhibitory response with perfect discriminability).
(B) Statistical analysis of neural activity during first sniff (40–160 ms window from sniff onset) (3-way ANOVA performed for each neuron with factors of stimulus identity, choice direction and reward outcome, P < 0.05). Neural responses during this period mostly reflect odor stimuli but not behavioral choice or reward outcomes.
(C) Summary of odor responses (179 neurons). Odor response magnitudes were indicated as in A, top (also see color scale). Non-significant responses (P >0.05, Wilcoxon rank sum test) were black colored. The example neuron in A is indicated by the arrow. Neurons are sorted by pre-odor firing rates in an increasing order.
(D) Histogram of number of pure odorants that activated a given neuron (P < 0.05, Wilcoxon rank sum test). Two lines represent binomial fits with (purple; non-responsive p0=0.50, the other neuron respond with p=0.16/(1−p0)=0.33) or without allowance of extra non-responsive neurons (orange; p=0.16). As a population, 45% of aPC neurons were activated by at least one of the six odors tested while 28% were activated by two or more (<0.05, Wilcoxon rank sum test).
Spike counts carried more reliable and rapid information than temporal patterns
The latency and peak timing of aPC responses varied across neurons and odors, raising the possibility that these parameters may carry odor information (Cury and Uchida, 2010) (Figures 4A, B). However, both of these timing parameters were anti-correlated with spike counts (Figures 4C, D), suggesting that the information conveyed by these variables might be redundant. In order to quantify the amount of information carried by different response variables (i.e. latency, peak timing and spike counts), we performed a decoding analysis to ask how accurately could an ideal observer classify each individual trial as belonging to one of six odor stimuli. By comparing decoding accuracy using vectors consisting of different variables derived from aPC responses, we compared the relative importance of each coding strategy. As decoders (ideal observers), we used linear classifiers including perceptrons and support vector machines with linear kernels. These decoders essentially calculate a weighted sum of inputs followed by a threshold and therefore resemble a biophysical decoding of aPC information that might actually be implemented in downstream areas.
Figure 4. Rapid and accurate readout of odor information based on spike counts in first sniff.
(A) Activity of an example neuron in response to two different odors. This neuron responded to the two odors with different temporal profiles.
(B) Trial-to-trial relationship of peak timing and total spike counts (same neuron and odors as in A). Each dot corresponds to one trial. The peak timing is defined as the timing when the smoothed firing rate profile reaches the maximum firing rate within the first sniff cycle.
(C, D) Correlation coefficients between spike counts and peak timing (C) and latency (D) for 908 neuron-odor pairs. Black bars indicate significant correlations (P < 0.05).
(E) Odor decoding accuracy of a linear decoder based on different firing features. Information contained in an ensemble neural activity (179 neurons) in a sniff (40–160 ms from inhalation onset) was quantified by the accuracy with which a linear classifier (support vector machine with a linear kernel) can correctly identify stimulus out of six odors in a trial-by-trial basis (see Full Experimental Procedures). Decoding accuracy for six pure odors (black) and six mix odors (gray) are plotted separately. Latency: timing of first spike. Peak: timing of peak firing rate. Count: total spike count. L&C: latency and spike count. P&C: peak time and spike count.
(F) Odor decoding accuracy with increasing window lengths. Decoding using peak timing does not result in any faster performance than that using only spike counts. A total of 179 neurons are used. The chance performance level is 16.67% (= 1/6, horizontal thin line). Black horizontal dashed lines indicate the behavioral performance levels for pure odors.
(G) Odor decoding accuracy of a linear classifier, plotted as a function of bin size (10 ms to 160 ms, i.e., temporal resolution). A 160ms time window after the first sniff was first equipartitioned into smaller sized bins (80, 40, 20 or 10ms, respectively) and then the spike counts in all the bins were used for classification. Black: pure, grey: mixture stimuli. Black and gray horizontal dashed lines indicate the behavioral performance levels for pure and mix odors, respectively.
(H) Odor decoding accuracy based on spike counts and phases for pure and mixture odor trials. Spike time: spike counts in 160ms × 1 bin. Phase: spike counts in 8 bins equipartitioning the first sniff cycle. Note that bin widths vary by trials in Phase. For fair comparisons, decoding accuracy was plotted against the mean number of spikes per trial instead of the number of neurons.
Input codes based on the total number or rate of spikes in a sniff cycle provided the most reliable performance in odor classification, whereas codes based on first spike latency or peak timing performed significantly worse (Figure 4E). Furthermore, combining latency or peak timing with rate failed to improve decoding accuracy. Although it has been postulated that spike times may provide a more rapid coding mechanism (Cury and Uchida, 2010; Gollisch and Meister, 2008; Thorpe et al., 2001), we found that decoders using spike count actually performed faster than those based on spike latency or peak timing (Figure 4F), demonstrating that spike counts can convey information in a more reliable manner. Furthermore, decoding based on complete temporal patterns of activity in a sniff cycle did little to improve decoding accuracy (Figure 4G).
Finally, using phase of spike occurrence with respect to sniffing cycle instead of absolute time did not improve the decoding accuracy (Figure 4H). Together, these results suggest that spike rates or counts are the predominant carrier of olfactory information in the aPC, and that the dependence of odor coding on spike timing is greatly reduced compared to the olfactory bulb (Cury and Uchida, 2010).
Information conveyed by the spike counts provided in the burst activity can account for the speed and accuracy of odor discrimination
We next compared the performance of aPC populations decoded using linear classifiers to the performance of the animal. Decoding based on total spike counts in the first sniff using the entire 179 neurons gave nearly perfect performance on pure odors (Figures 5A, B). For both pure and mixture stimuli, the accuracy of the classifier reached a level comparable to that of the animal using only about 70 neurons (Figure 5A). Analysis of the time course of decoding using a short sliding time window showed that the maximum information could be read out from the initial burst of activity within 100 ms after the first inhalation onset and that the rate of information dropped thereafter (Figure 5B, C). Comparing the first and second sniff separately, spikes in the first sniff gave significantly higher accuracy than those in the second sniff or the last sniff before odor port exit (Figure 5D; P<0.05, χ2 test), and using both the first and second sniff cycles resulted in only a small increase in accuracy (Figure 5D). Therefore spike counts in ensembles of aPC neurons appear to be sufficient to explain both the speed and accuracy of decisions in an odor mixture discrimination task.
Figure 5. Information conveyed by the spike counts provide in the burst activity can account for the speed and accuracy of odor discrimination.
(A) Decoding accuracy as a function of the number of neurons. Total spike counts within 40–160 ms after the first sniff onset were used.
(B) Time course of odor decoding accuracy. A vector consisting of instantaneous spike counts of 179 neurons in a sliding window (width: 50 ms, step: 5 ms) was used for the input to a classifier. Training of a classifier and testing were done at every time point. Black: pure, grey: mixture stimuli.
(C) Time course of odor decoding accuracy after the second sniff onset.
(D) Odor decoding accuracy at different sniff cycles. 1: first sniff, 2: second sniff, L: last sniff before odor port exit. +: sum of the spike counts from 1st and 2nd sniffs. &: spike counts from the 1st and 2nd sniffs are treated as independent inputs to a classifier. Note that the last sniffs contain 1st or 2nd sniff depending on how many sniff the animal took in a given trial. The neural response at the first sniff is more informative than the second and the last sniffs. Combining 1st and 2nd sniffs improved decoding accuracy only a little (statistically not significant either for pure or mixture odors, P>0.05, χ2 test).
(E) Comparison of the responses of an example neuron to the same odor on correct trials and error trials.
(F) Choice probabilities: correlations between a trial-to-trial variability in neural activity and a choice toward neuron’s preferred direction. Only mixture odor trials were used to get significant number of error trials. The fraction neurons with significant choice probabilities > 0.5 is significantly larger than the fraction with significant choice probabilities < 0.5 (P < 0.05, χ2 test) although the mean choice probability was not significantly larger than 0.5 (Wilcoxon sign rank test, P > 0.5). A neuron’s preferred choice direction was determined as a direction for a pure odor with significantly higher firing rate than the paired pure odor. Only neuron-mixture odor pairs where two pure odors showed significantly different responses (area under receiver operating characteristic curve > 0.7 or < 0.3) and with numbers of trials for each choice more than five were used for the analysis.
(G–I) Odor decoding accuracy for correct and error trials using simultaneously recorded ensemble neurons (n=19 sessions). Total spike counts within 40–160 ms (F), Peak time (G) and latency (H) from the first sniff onset were used. Only trials with mixture odors, where most of error trials are available, were used. A classifier was first trained using correct trials, and decoding accuracy was obtained using test trials that are composed of correct or error trials. P < 0.05 for spike counts (Wilcoxon test).
Spike counts in ensemble activity correlate with behavioral choices
If firing rates across ensembles of aPC neurons are used by the brain to form behavioral responses, and if sensory uncertainty reduces performance accuracy, as in the mixture trials, then we ought to observe trial-by-trial correlations between decoding based on these neural representations and the animals’ choices. To test this idea, we first compared neuronal firing rates on correct and error choices for a given stimulus, a measure analogous to “choice probability”, a measure that has been used previously to test the role of a neural representation in behavior (Britten et al., 1996; Cury and Uchida, 2010; Parker and Newsome, 1998). We found a low average correlation between the firing rates of individual neurons and subjects’ choices (Avg. choice prob. = 0.51 ± 0.011; Figures 5E, F). This correlation was somewhat smaller than those found in previous observations in visual cortex (0.53–0.7; Britten et al., 1996; Cohen and Newsome, 2009; Dodd et al., 2001; Uka and DeAngelis, 2004). However, if the information for choices is distributed across a large number of uncorrelated aPC neurons such that the contribution of single neurons is diluted (Cohen and Newsome, 2009), then we reasoned that the accuracy of decoding based on simultaneously recorded ensembles may be correlated on a trial-by-trial basis with behavioral choices. Indeed, we found that patterns of spike counts across aPC neurons in correct trials provided significantly higher decoding accuracy than patterns in error trials (Figure 5G, P=0.030, Wilcoxon test). In contrast, decoding using peak timing or latency did not show a significant difference between correct and error trials (Figures 5H, I, P>0.05, Wilcoxon test). Therefore, the spike rates in aPC not only carry substantial stimulus information, they are also correlated at an ensemble level with the behavioral choices of the animal.
Near-zero noise correlations during odor inhalation
The above results indicate that odor information is coded by a large number of neurons in aPC. A critical feature of information coding in neuronal ensembles is the structure and magnitude of correlated fluctuations in firing, which can affect the ability of downstream neurons to decode the information. A simple example of ensemble decoding is population averaging or pooling. By this strategy, neuronal noise can, in principle, be eliminated by averaging the activity of a large number of neurons. However, if noise is not random across neurons, that is, when neural activity co-fluctuates across neurons, the benefit of pooling can be significantly curtailed (Cohen and Kohn, 2011; Zohary et al., 1994). The choice probability analysis suggested that aPC neurons are actually very weakly correlated. To test more directly whether such correlations affect representations of odors in the olfactory cortex, we analyzed the “noise correlations” between pairs of simultaneously recorded aPC neurons (see Experimental Procedures). Noise was defined as the trial-to-trial variability of spike counts in a sniff cycle (40–160 ms after the first sniff onset) around the mean response under a given stimulus condition. Noise correlation was defined as the correlation coefficient between the noise of two neurons to multiple presentations of a given odor stimulus. We found surprisingly low noise correlations amongst aPC neurons (0.0046 ± 0.0988; mean ± S.D.; N=936 pairs, Figures 6A, S5). In fact, both the mean and the standard deviation of noise correlations of the aPC data were similar to trial-shuffled data in which all correlations are removed (0.00011 ± 0.0870; Figures S5C–F) suggesting that deviations from zero were mostly due to the effect of finite sample size (Ecker et al., 2010). Moreover, we observed no dependence of the magnitude of noise correlations on the number of evoked spikes over a range of rates < 5 to > 100 spikes·s−1 (Figure S5A, B). Therefore, near-zero noise correlations in aPC were not a consequence of low firing rates (Cohen and Kohn, 2011; de la Rocha et al., 2007; Kohn and Smith, 2005).
Figure 6. Near zero noise correlations in aPC.
(A) Histogram of noise correlations. Noise correlations were calculated using spike counts in the first sniff cycle after odor onset (40–160 ms). A similar distribution of noise correlations was obtained after trial-shuffling (magenta), indicating that most neuron pairs had zero noise correlations. Black bars indicate correlations significantly different from zero (P < 0.05).
(B) Signal correlations (similarity in odor response tuning for a pair of neurons) compared between neuron pairs from same (S) and different (D) electrodes. Neuron pairs from same electrode showed slightly higher signal correlations (P > 0.05, Wilcoxon rank sum test). The error bars are S.E.M. across neuron-odor pairs.
(C) Noise correlations compared between neuron pairs from same and different electrodes. There was no difference in noise correlations (P > 0.05, Wilcoxon rank sum test). The error bars are SEM across neuron-odor pairs.
(D) No dependency between noise correlations and signal correlations. Neuron pairs recorded from same (S) and different (D) electrodes are indicated by blank and orange dots, respectively. A dot represents a neuron pair. Neither the slope nor intercept of the regression lines were significantly different from 0 (red and black lines, P > 0.05, linear regression), indicating no relationship between noise correlations and signal correlations.
In the neocortex, neighboring neurons with similar stimulus tuning tend to exhibit correlated trial-by-trial fluctuations in firing rate (Bair et al., 2001; Cohen and Kohn, 2011; Zohary et al., 1994), thought to arise from common inputs, and it has been postulated that these “structured” or “limited-range” correlations are particularly detrimental to the efficiency of population coding (Averbeck et al., 2006; Sompolinsky et al., 2001). We therefore examined whether aPC noise correlations are low even when odor tuning is similar. To quantify the similarity of odor tuning between pairs of neurons, we calculated the correlation coefficient of the mean odor responses across all 12 stimuli used (i.e. signal correlation). This analysis showed that signal correlations were low both for aPC neurons recorded on the same tetrode and for those recorded on different tetrodes (P > 0.05, Wilcoxon rank sum test; Figure 6B). Similarly, noise correlations were near-zero regardless of whether neurons were recorded on the same or different tetrodes (P > 0.05, Wilcoxon rank sum test; Figure 6C). Most importantly, the noise correlations of pairs of aPC neurons were independent of their signal correlations (regression slope: 0.0156 ± 0.0090, not significantly different from zero, P > 0.05; Figure 6D). These results suggest that, during odor stimulation, aPC neurons act largely as independent encoders regardless of their distance or the similarity of their odor tuning.
Odor inhalation quenches noise correlations
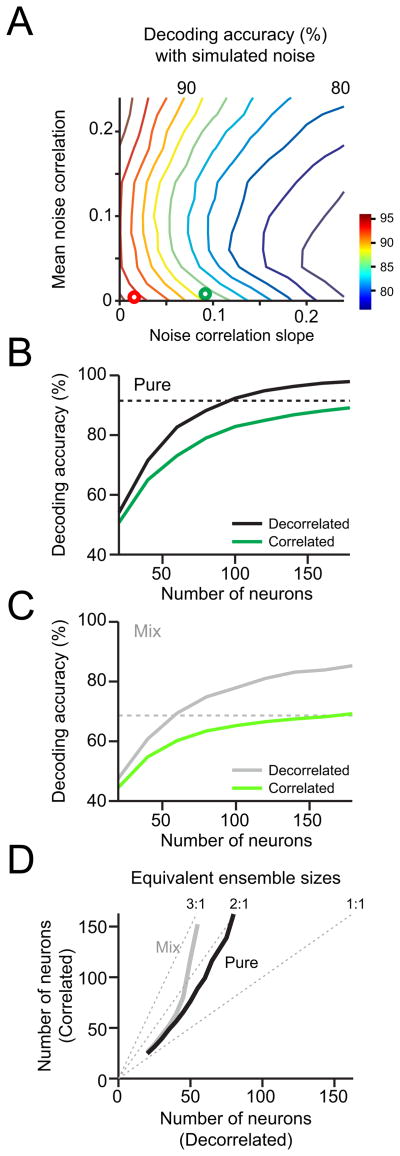
Neuronal variability and noise correlation are not static, but can be modulated by attentional state (Cohen and Maunsell, 2009; Mitchell et al., 2009), perceptual learning (Gu et al., 2011) and stimulus input (Bhandawat et al., 2007; Churchland et al., 2010; de la Rocha et al., 2007; Kazama and Wilson, 2009). Therefore, in order to gain insight into how near zero noise correlations arise in aPC, we tested how trial-to-trial correlations across neurons are modulated during the course of events in a trial. For this analysis, since odor stimuli were not always present, we calculated the correlation coefficients of spike counts without subtracting the mean responses of each stimulus condition (see Experimental Procedures for more details). We found that when rats begin active sampling (sniffing) in anticipation of odor presentation, the aPC population was globally activated, with the mean population firing rate increasing by around 30% (Figure 7A). Surprisingly, during the same period the mean pairwise correlation across the entire population dropped, implying a possible positive impact on population coding (Zohary et al., 1994). However, correlations between similarly tuned pairs increased (Figures 7B–D, S6A–C; regression slope: 0.0916 ± 0.0092, significantly different from zero, P < 0.01), implying a possible negative impact on population coding (Sompolinsky et al., 2001). In order to estimate the net effect, we performed decoding analysis using simulated data in which spike counts obtained during odor stimulation were trial-shuffled to generate noise correlation structures with different means and signal correlations while preserving the mean odor response profile of individual neurons (see Experimental Procedures for details). We found that correlations of the type observed during the pre-odor sampling period, had they persisted into the odor sampling period, would have significantly eroded the efficacy of decoding, reducing classifier performance by more than 5–10% (P < 0.01, t-test)(Figures 8A–C, S7). We calculated that 2–3 times more neurons would have been required to achieve the same level of decoding performance had pre-odor correlation levels been maintained (Figure 8D). The simulation also indicated that the effects would be even larger with larger ensembles. We also found that trial-to-trial variability in spike count, as measured by the Fano factor and the coefficient of variation, was significantly reduced by odor onset (Figures S6D, E). Thus, potentially deleterious population correlations are increased during the period of high sniffing preceding odor onset but these correlations are quenched during the arrival of the stimulus (Churchland et al., 2010).
Figure 7. Odor stimulation quenches structured trial-to-trial correlations that emerge during pre-odor sniffing.
(A) Mean firing rate over the population as a function of task epochs. Spikes in the first sniff after each task event were used. Pre-odor port: 500 ms before odor port in. The bar with a star indicates significance between two epochs and a star without a horizontal bar indicates the significance against all the other epochs (the error bars are across neuron-odor pairs, ANOVA with LSD method).
(B) Mean correlation as a function of task epochs. The error bars are for neuron-odor pairs.
(C) Regression slopes for the signal correlation and trial-to-trial correlation relationship (D) as a function of task epochs. Trial-to-trial correlations were computed at each epoch while signal correlations were computed at the first sniff (generalized linear model with Holm method). The error bars are SEM across neuron-odor pairs.
(D) Trial-to-trial correlations as a function of signal correlations. Two task epochs, pre-odor (green) and 1st sniff (red), are plotted separately (P < 0.05 for slopes, generalized linear model). The error bars are SEM across neuron-odor pairs.
Figure 8. Odor decoding accuracy in the presence of simulated noise correlation structures.
(A) To simulate odor evoked activity that has an arbitorary noise correlation structure, trials were shuffled to artificially induce the mean and slope of the noise and signal correlation relationship. Shuffling was performed multiple times toward minimizing the least square error to achieve the target noise correlation structures defined by its intercept and slope. Odor decoding accuracy was then computed using the trial-shuffled ensemble activity. The green and red circles indicate pre-odor and 1st sniff, respectively.
(B, C) Decoding accuracy using decorrelated (black, gray) and correlated ensembles (green, light green) as a function of number of neurons. Pure (B) and mixture (C) odors were plotted separately. The mean and slope of the correlations observed during the pre-odor period were used to simulate correlated ensembles. The dashed lines denote the animals’ behavioral performance for each condition. The decorrelated ensemble results are the same as in Figure 5A.
(D) Equivalent ensample sizes. The number of neurons required to achieve the same decoding accuracy between decorrelated and correlated ensembles were obtained from B and C. The dotted lines represent ratios (3:1, 2:1, 1:1) of the numbers of neurons between decorrelated vs. correlated ensembles. As the size of a population increases, disproportionately more neurons are required to achieve the same performance in the presence of structured noise correlations.
DISCUSSION
Transformation of odor representation between the olfactory bulb and piriform cortex
Together with recent studies of neural coding in the olfactory bulb (Carey and Wachowiak, 2011; Cury and Uchida, 2010; Shusterman et al., 2011), this study demonstrates that odor representations are profoundly transformed between the bulb and the aPC. While these studies show that odor responses in the olfactory bulb exhibit complex temporal patterns carrying stimulus information, here, we show that those in the aPC consist primarily of a simple burst of firing, locked to respiration. Furthermore, the baseline firing rates are higher in the olfactory bulb compared to the piriform cortex (12.9 ± 6.4 Hz in the olfactory bulb; 6.15 ± 9.01 Hz in the aPC; mean ± S.D.; Cury and Uchida, 2010 and the present study). As the consequence, whereas in the olfactory bulb extracting information from mitral/tufted cells requires decoding of temporal patterns (Cury and Uchida, 2010), in the aPC most odor information can be read out using only spike counts of neurons.
Why might the olfactory bulb and cortex areas use different strategies for odor coding? One important consideration is the substantial anatomical differences between the two areas: while a relatively small number of neurons (20–50 mitral cells) transmit odor information from each of the approximately 1000 input channels (glomeruli) in the olfactory bulb, this information is broadcast to an olfactory cortex that contains an estimated two orders of magnitude more neurons (Shepherd, 2004). Because of this expansion in coding space the necessity to maximize the rate of information transmitted per neuron and per unit time in the olfactory bulb will be much greater than in the aPC. The cortex can therefore better afford to employ a rate-based coding strategy based on a larger number of neurons and a widely-distributed code. One significant advantage of rate-based code over temporal code is that downstream areas can more readily read out such a code or combine it with other kinds of information encoded in rates. This might then facilitate proposed functions of the piriform cortex such as forming associative memories (Franks et al., 2011; Haberly, 2001).
The mechanism of the temporal-to-rate transformation remains to be determined. In insects, temporally dynamic responses in the antennal lobe (AL, considered equivalent to the olfactory bulb) are transformed into sparse responses in the mushroom body (MB, considered equivalent to the PC). Various mechanisms have been proposed to underlie this process, including (1) oscillatory spike synchronization, (2) short membrane time constants of MB neurons, (3) feedforward inhibition and (4) highly convergent connectivity between the AL and MB (Perez-Orive et al., 2004; Perez-Orive et al., 2002). In zebrafish, different mechanisms appear to shape the responsiveness of cortical neurons: neurons in the dorsal telencephalon (Dp) effectively discard information about synchronous firing in the olfactory bulb due to cortical neurons’ slow membrane time constants and relatively weak feedforward inhibition (Blumhagen et al., 2011). It will be important to examine whether PC neurons in mammals are tuned to temporal patterns of activity in the olfactory bulb (Carey and Wachowiak, 2011; Cury and Uchida, 2010; Shusterman et al., 2011), and if so, which aspects of temporal patterns are important.
Neural substrate for rapid olfactory decisions
Our findings bear on the relationship between psychophysical limits and neuronal representations, a central subject in sensory physiology (Parker and Newsome, 1998). We found that, by monitoring spikes from as few as 50–100 aPC neurons, a simple decoder based on firing rates could extract more than enough information in a single sniff cycle to account for the behavioral accuracy of rats in the odor categorization task. We also found that while single neuron activity was not on average different between correct and error trials (low average “choice probability”), population activity-based decoders performed significantly better on correct compared to error trials. Rate information peaked within 100 ms during the first sniff, and aggregating information over longer periods in multiple sniff cycles failed to significantly augment decoding performance, providing an explanation for the rapid speed of olfactory discrimination performance and the lack of speed-accuracy tradeoff over longer periods (Uchida and Mainen, 2003). Therefore, these observations provide substantial evidence linking a rate-based population code to behavioral performance.
Near-zero noise correlations facilitate odor coding
We found that an optimal linear decoder of aPC neurons can reach levels of performance superior to the animal itself using < 100 neurons out of the estimated population of around 106 neurons (Shepherd, 2004). The aPC clearly contains an extremely robust representation of odor identity. What then ultimately limits behavioral accuracy? While similar observations in the visual system have been attributed to the reduced efficiency of pooling in the actual network of neurons due to ensemble correlations (Shadlen et al., 1996; Zohary et al., 1994), this appears not to be the case in the aPC. During odor stimulation, aPC networks have near zero mean noise correlation, more than one order of magnitude lower than that generally reported in the neocortex (0.05–0.2; Cohen and Kohn, 2011; Gawne and Richmond, 1993; Lee et al., 1998; Zohary et al., 1994) (Figure 6A), similar to that reported in the primary auditory cortex of anesthetized rats (Renart et al., 2010) and area V1 of awake monkeys (Ecker et al., 2010). More importantly, aPC neurons also lack the positive relationships between signal and noise correlations that are typically observed (Bair et al., 2001; Gu et al., 2011; Zohary et al., 1994). However, the absence of such correlations is not simply due to the distributed connectivity of the olfactory cortex: Such structured correlated activity can and does emerge prior to odor onset and simulations demonstrated that such correlations would have substantially reduced the efficiency of population coding. However, we found when driven by odor stimulation, these pre-stimulus correlations are quenched. While we cannot rule out the possibility that additional correlations that we were unable to measure with this data set might affect decoding, behavioral performance in the odor mixture categorization task appears to be limited neither by the level of noise of the sensory representation nor by correlated fluctuations amongst the population of neurons. We therefore conclude that the limits of performance must be set either by the ability of downstream circuits to accurately read out of these representations or by other non-sensory sources of variability.
Whether prolonged odor sampling can improve the accuracy of odor discrimination has been controversial. Some studies have suggested that the accuracy of odor discrimination can be improved with longer odor sampling over 500 ms (Rinberg et al., 2006) or more (Friedrich and Laurent, 2001). It has been suggested that the accuracy of discrimination of highly similar odor pairs might depend on the refinement of odor representations through temporal evolution of neural activity (Friedrich and Laurent, 2001) or through temporal integration of sensory evidence. However, the result of the present study suggests that these processes are unnecessary. These findings indicate, instead, that performance accuracy is affected not only by stimulus information but additionally by other task parameters that may affect the ability of the animal to choose accurately based on olfactory stimulus representations (Zariwala et al., 2005). It remains to be seen whether similar conclusions can be drawn in different olfactory tasks such as odor detection, discrimination at low concentrations, or more complex tasks. The present study indicates that neuronal recording in animals performing these behavioral tasks will be a critical step toward addressing these fundamental questions.
EXPERIMENTAL PROCEDURES
All procedures involving animals were carried out in accordance with NIH standards and approved by the Cold Spring Harbor Laboratory and Harvard University Institutional Animal Care and Use Committee (IACUC). All values were represented by mean ± S.E.M unless otherwise noted.
Behavior
Rats were trained and tested on a two-alternative choice odor mixture categorization task where water was used as a reward as described previously (Cury and Uchida, 2010; Uchida and Mainen, 2003). Odor delivery was controlled by a custom made olfactometer (Cury and Uchida, 2010; Uchida and Mainen, 2003). Three rats (two of them trained with go-signals) were tested on a standardized stimulus set of three odor pairs: (1) caproic acid and citralva, (2) ethyl 3-hexenoate and 1-hexanol, and (3) dihydroxy linalool oxide vs. cumin aldehyde (Figure 1B). Each of these odors was diluted 1:10 in mineral oil, and further diluted by filtered air by 1:20 (1:200 total).
Neural recording
After each animal reached an asymptotic performance in behavioral training, each rat was implanted with a custom-made multielectrode drive (Cury and Uchida, 2010) in the left hemisphere in the aPC (3.5 mm anterior to bregma, 2.5 mm lateral to midline) and a bipolar stimulating electrode in the olfactory bulb (Kashiwadani et al., 1999; Schoenbaum and Eichenbaum, 1995) under anesthesia. Extracellular recordings were obtained using six independently adjustable tetrodes. To monitor sniffing, during drive implantation, a temperature sensor (thermocouple) was implanted in one nostril (Cury and Uchida, 2010; Uchida and Mainen, 2003).
Supplementary Material
HIGHLIGHTS.
Firing rate-based coding can account for the speed and accuracy of discrimination
Timing of spikes provides little additional information compared to firing rates
Odor inhalation quenches structured noise correlations
Dramatic transformation of odor coding between the olfactory bulb and cortex
Acknowledgments
We are grateful to Haim Sompolinsky for stimulating discussions on population coding. We thank John Maunsell, Markus Meister, Alex Pouget and Rachel Wilson for their valuable comments on the manuscript. We also thank Kevin Cury, Rafi Haddad, Gabriel Kreiman, Eran Mukamel, Alice Wang and other members of the Uchida lab for discussions. This work was supported by: National Institutes of Health Grant DC006104, Cold Spring Harbor Laboratory and Champlimaud Foundation (Z.F.M.); Swartz Foundation, Smith Family New Investigator Award, Alfred Sloan Foundation, Milton Fund and start-up funding from Harvard University (N.U.).
Footnotes
Supplemental Information includes 8 figures and Supplemental Experimental Procedures.
AUTHOR CONTRIBUTIONS
N.U. and Z.F.M. designed the experiments and wrote the paper. N.U. performed the experiments. K.M. performed the data analysis and helped writing the paper. N.U. and Z.F.M. helped the data analysis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Averbeck BB, Latham PE, Pouget A. Neural correlations, population coding and computation. Nat Rev Neurosci. 2006;7:358–366. doi: 10.1038/nrn1888. [DOI] [PubMed] [Google Scholar]
- Bair W, Zohary E, Newsome WT. Correlated firing in macaque visual area MT: time scales and relationship to behavior. J Neurosci. 2001;21:1676–1697. doi: 10.1523/JNEUROSCI.21-05-01676.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandawat V, Olsen SR, Gouwens NW, Schlief ML, Wilson RI. Sensory processing in the Drosophila antennal lobe increases reliability and separability of ensemble odor representations. Nat Neurosci. 2007;10:1474–1482. doi: 10.1038/nn1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumhagen F, Zhu P, Shum J, Scharer YP, Yaksi E, Deisseroth K, Friedrich RW. Neuronal filtering of multiplexed odour representations. Nature. 2011;479:493–498. doi: 10.1038/nature10633. [DOI] [PubMed] [Google Scholar]
- Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis Neurosci. 1996;13:87–100. doi: 10.1017/s095252380000715x. [DOI] [PubMed] [Google Scholar]
- Cang J, Isaacson JS. In vivo whole-cell recording of odor-evoked synaptic transmission in the rat olfactory bulb. J Neurosci. 2003;23:4108–4116. doi: 10.1523/JNEUROSCI.23-10-04108.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey RM, Wachowiak M. Effect of sniffing on the temporal structure of mitral/tufted cell output from the olfactory bulb. J Neurosci. 2011;31:10615–10626. doi: 10.1523/JNEUROSCI.1805-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Yu BM, Cunningham JP, Sugrue LP, Cohen MR, Corrado GS, Newsome WT, Clark AM, Hosseini P, Scott BB, et al. Stimulus onset quenches neural variability: a widespread cortical phenomenon. Nat Neurosci. 2010;13:369–378. doi: 10.1038/nn.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MR, Kohn A. Measuring and interpreting neuronal correlations. Nat Neurosci. 2011;14:811–819. doi: 10.1038/nn.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MR, Maunsell JH. Attention improves performance primarily by reducing interneuronal correlations. Nat Neurosci. 2009 doi: 10.1038/nn.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MR, Newsome WT. Estimates of the contribution of single neurons to perception depend on timescale and noise correlation. J Neurosci. 2009;29:6635–6648. doi: 10.1523/JNEUROSCI.5179-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cury KM, Uchida N. Robust odor coding via inhalation-coupled transient activity in the mammalian olfactory bulb. Neuron. 2010;68:570–585. doi: 10.1016/j.neuron.2010.09.040. [DOI] [PubMed] [Google Scholar]
- de la Rocha J, Doiron B, Shea-Brown E, Josic K, Reyes A. Correlation between neural spike trains increases with firing rate. Nature. 2007;448:802–806. doi: 10.1038/nature06028. [DOI] [PubMed] [Google Scholar]
- Dodd JV, Krug K, Cumming BG, Parker AJ. Perceptually bistable three-dimensional figures evoke high choice probabilities in cortical area MT. J Neurosci. 2001;21:4809–4821. doi: 10.1523/JNEUROSCI.21-13-04809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker AS, Berens P, Keliris GA, Bethge M, Logothetis NK, Tolias AS. Decorrelated neuronal firing in cortical microcircuits. Science. 2010;327:584–587. doi: 10.1126/science.1179867. [DOI] [PubMed] [Google Scholar]
- Franks KM, Russo MJ, Sosulski DL, Mulligan AA, Siegelbaum SA, Axel R. Recurrent circuitry dynamically shapes the activation of piriform cortex. Neuron. 2011;72:49–56. doi: 10.1016/j.neuron.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich RW, Laurent G. Dynamic optimization of odor representations by slow temporal patterning of mitral cell activity. Science. 2001;291:889–894. doi: 10.1126/science.291.5505.889. [DOI] [PubMed] [Google Scholar]
- Gawne TJ, Richmond BJ. How independent are the messages carried by adjacent inferior temporal cortical neurons? J Neurosci. 1993;13:2758–2771. doi: 10.1523/JNEUROSCI.13-07-02758.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Larson SD, Hefzi H, Marnoy Z, Cutforth T, Dokka K, Baldwin KK. Sensory maps in the olfactory cortex defined by long-range viral tracing of single neurons. Nature. 2011;472:217–220. doi: 10.1038/nature09945. [DOI] [PubMed] [Google Scholar]
- Gollisch T, Meister M. Rapid neural coding in the retina with relative spike latencies. Science. 2008;319:1108–1111. doi: 10.1126/science.1149639. [DOI] [PubMed] [Google Scholar]
- Gu Y, Liu S, Fetsch CR, Yang Y, Fok S, Sunkara A, DeAngelis GC, Angelaki DE. Perceptual learning reduces interneuronal correlations in macaque visual cortex. Neuron. 2011;71:750–761. doi: 10.1016/j.neuron.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberly LB. Parallel-distributed processing in olfactory cortex: new insights from morphological and physiological analysis of neuronal circuitry. Chem Senses. 2001;26:551–576. doi: 10.1093/chemse/26.5.551. [DOI] [PubMed] [Google Scholar]
- Hamilton KA, Kauer JS. Patterns of intracellular potentials in salamander mitral/tufted cells in response to odor stimulation. J Neurophysiol. 1989;62:609–625. doi: 10.1152/jn.1989.62.3.609. [DOI] [PubMed] [Google Scholar]
- Illig KR, Haberly LB. Odor-evoked activity is spatially distributed in piriform cortex. J Comp Neurol. 2003;457:361–373. doi: 10.1002/cne.10557. [DOI] [PubMed] [Google Scholar]
- Junek S, Kludt E, Wolf F, Schild D. Olfactory coding with patterns of response latencies. Neuron. 2010;67:872–884. doi: 10.1016/j.neuron.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Kashiwadani H, Sasaki YF, Uchida N, Mori K. Synchronized oscillatory discharges of mitral/tufted cells with different molecular receptive ranges in the rabbit olfactory bulb. J Neurophysiol. 1999;82:1786–1792. doi: 10.1152/jn.1999.82.4.1786. [DOI] [PubMed] [Google Scholar]
- Kazama H, Wilson RI. Origins of correlated activity in an olfactory circuit. Nat Neurosci. 2009;12:1136–1144. doi: 10.1038/nn.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepecs A, Uchida N, Mainen ZF. The sniff as a unit of olfactory processing. Chem Senses. 2006;31:167–179. doi: 10.1093/chemse/bjj016. [DOI] [PubMed] [Google Scholar]
- Kohn A, Smith MA. Stimulus dependence of neuronal correlation in primary visual cortex of the macaque. J Neurosci. 2005;25:3661–3673. doi: 10.1523/JNEUROSCI.5106-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Port NL, Kruse W, Georgopoulos AP. Variability and correlated noise in the discharge of neurons in motor and parietal areas of the primate cortex. J Neurosci. 1998;18:1161–1170. doi: 10.1523/JNEUROSCI.18-03-01161.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrides F, Chorover SL. Olfactory bulb units: activity correlated with inhalation cycles and odor quality. Science. 1972;175:84–87. doi: 10.1126/science.175.4017.84. [DOI] [PubMed] [Google Scholar]
- Margrie TW, Schaefer AT. Theta oscillation coupled spike latencies yield computational vigour in a mammalian sensory system. J Physiol. 2003;546:363–374. doi: 10.1113/jphysiol.2002.031245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollum J, Larson J, Otto T, Schottler F, Granger R, Lynch G. Short-latency single unit processing in olfatory cortex. J Cogn Neurosci. 1991;3:293–299. doi: 10.1162/jocn.1991.3.3.293. [DOI] [PubMed] [Google Scholar]
- Meredith M. Patterned response to odor in mammalian olfactory bulb: the influence of intensity. J Neurophysiol. 1986;56:572–597. doi: 10.1152/jn.1986.56.3.572. [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Spatial attention decorrelates intrinsic activity fluctuations in macaque area V4. Neuron. 2009;63:879–888. doi: 10.1016/j.neuron.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamichi K, Amat F, Moussavi F, Wang C, Wickersham I, Wall NR, Taniguchi H, Tasic B, Huang ZJ, He Z, et al. Cortical representations of olfactory input by trans-synaptic tracing. Nature. 2011 doi: 10.1038/nature09714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama S, Enerva A, Fletcher ML, Masurkar AV, Igarashi KM, Mori K, Chen WR. Differential axonal projection of mitral and tufted cells in the mouse main olfactory system. Front Neural Circuits. 2010:4. doi: 10.3389/fncir.2010.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojima H, Mori K, Kishi K. The trajectory of mitral cell axons in the rabbit olfactory cortex revealed by intracellular HRP injection. J Comp Neurol. 1984;230:77–87. doi: 10.1002/cne.902300107. [DOI] [PubMed] [Google Scholar]
- Parker AJ, Newsome WT. Sense and the single neuron: probing the physiology of perception. Annu Rev Neurosci. 1998;21:227–277. doi: 10.1146/annurev.neuro.21.1.227. [DOI] [PubMed] [Google Scholar]
- Perez-Orive J, Bazhenov M, Laurent G. Intrinsic and circuit properties favor coincidence detection for decoding oscillatory input. J Neurosci. 2004;24:6037–6047. doi: 10.1523/JNEUROSCI.1084-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Orive J, Mazor O, Turner GC, Cassenaer S, Wilson RI, Laurent G. Oscillations and sparsening of odor representations in the mushroom body. Science. 2002;297:359–365. doi: 10.1126/science.1070502. [DOI] [PubMed] [Google Scholar]
- Poo C, Isaacson JS. Odor representations in olfactory cortex: “sparse” coding, global inhibition, and oscillations. Neuron. 2009;62:850–861. doi: 10.1016/j.neuron.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renart A, de la Rocha J, Bartho P, Hollender L, Parga N, Reyes A, Harris KD. The asynchronous state in cortical circuits. Science. 2010;327:587–590. doi: 10.1126/science.1179850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennaker RL, Chen CF, Ruyle AM, Sloan AM, Wilson DA. Spatial and temporal distribution of odorant-evoked activity in the piriform cortex. J Neurosci. 2007;27:1534–1542. doi: 10.1523/JNEUROSCI.4072-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinberg D, Koulakov A, Gelperin A. Speed-accuracy tradeoff in olfaction. Neuron. 2006;51:351–358. doi: 10.1016/j.neuron.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Eichenbaum H. Information coding in the rodent prefrontal cortex. I. Single-neuron activity in orbitofrontal cortex compared with that in pyriform cortex. J Neurophysiol. 1995;74:733–750. doi: 10.1152/jn.1995.74.2.733. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Britten KH, Newsome WT, Movshon JA. A computational analysis of the relationship between neuronal and behavioral responses to visual motion. J Neurosci. 1996;16:1486–1510. doi: 10.1523/JNEUROSCI.16-04-01486.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM. The synaptic organization of the brain. Oxford: Oxford University Press; 2004. [Google Scholar]
- Shusterman R, Smear MC, Koulakov AA, Rinberg D. Precise olfactory responses tile the sniff cycle. Nat Neurosci. 2011;14:1039–1044. doi: 10.1038/nn.2877. [DOI] [PubMed] [Google Scholar]
- Sompolinsky H, Yoon H, Kang K, Shamir M. Population coding in neuronal systems with correlated noise. Phys Rev E Stat Nonlin Soft Matter Phys. 2001;64:051904. doi: 10.1103/PhysRevE.64.051904. [DOI] [PubMed] [Google Scholar]
- Sosulski DL, Lissitsyna Bloom M, Cutforth T, Axel R, Datta SR. Distinct representations of olfactory information in different cortical centres. Nature. 2011;472:213–216. doi: 10.1038/nature09868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spors H, Grinvald A. Spatio-temporal dynamics of odor representations in the mammalian olfactory bulb. Neuron. 2002;34:301–315. doi: 10.1016/s0896-6273(02)00644-x. [DOI] [PubMed] [Google Scholar]
- Stettler DD, Axel R. Representations of odor in the piriform cortex. Neuron. 2009;63:854–864. doi: 10.1016/j.neuron.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Thorpe S, Delorme A, Van Rullen R. Spike-based strategies for rapid processing. Neural Netw. 2001;14:715–725. doi: 10.1016/s0893-6080(01)00083-1. [DOI] [PubMed] [Google Scholar]
- Uchida N, Mainen ZF. Speed and accuracy of olfactory discrimination in the rat. Nat Neurosci. 2003;6:1224–1229. doi: 10.1038/nn1142. [DOI] [PubMed] [Google Scholar]
- Uka T, DeAngelis GC. Contribution of area MT to stereoscopic depth perception: choice-related response modulations reflect task strategy. Neuron. 2004;42:297–310. doi: 10.1016/s0896-6273(04)00186-2. [DOI] [PubMed] [Google Scholar]
- Wachowiak M. All in a sniff: olfaction as a model for active sensing. Neuron. 2011;71:962–973. doi: 10.1016/j.neuron.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr M, Laurent G. Odour encoding by temporal sequences of firing in oscillating neural assemblies. Nature. 1996;384:162–166. doi: 10.1038/384162a0. [DOI] [PubMed] [Google Scholar]
- Welker WI. Analysis of sniffing of the albino rat. Behavior. 1964;22:223–244. [Google Scholar]
- Wellis DP, Scott JW, Harrison TA. Discrimination among odorants by single neurons of the rat olfactory bulb. J Neurophysiol. 1989;61:1161–1177. doi: 10.1152/jn.1989.61.6.1161. [DOI] [PubMed] [Google Scholar]
- Wesson DW, Carey RM, Verhagen JV, Wachowiak M. Rapid encoding and perception of novel odors in the rat. PLoS biology. 2008;6:e82. doi: 10.1371/journal.pbio.0060082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA. Habituation of odor responses in the rat anterior piriform cortex. J Neurophysiol. 1998;79:1425–1440. doi: 10.1152/jn.1998.79.3.1425. [DOI] [PubMed] [Google Scholar]
- Zariwala H, Uchida A, Mainen ZF. A study of speed and performance accuracy in an olfactory discrimination task. Paper presented at: Society for Neuroscience; Washington. 2005. [Google Scholar]
- Zhan C, Luo M. Diverse patterns of odor representation by neurons in the anterior piriform cortex of awake mice. J Neurosci. 2011;30:16662–16672. doi: 10.1523/JNEUROSCI.4400-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohary E, Shadlen MN, Newsome WT. Correlated neuronal discharge rate and its implications for psychophysical performance. Nature. 1994;370:140–143. doi: 10.1038/370140a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.