Abstract
Foam cells are a pathological feature present at all stages of atherosclerosis. Foam cells develop from monocytes that enter the nascent atheroma and subsequently ingest modified low density lipoproteins (LDL). The regulation of this process has previously been studied in vitro using cultured macrophages fed modified LDL. We used our existing in vitro model of transendothelial migration (TEM) to study this process in a more physiologically relevant setting. In our model, monocytes undergo TEM across a primary endothelial monolayer into an underlying three-dimensional collagen matrix in the presence of 20% human serum. Foam cells were detected by Oil Red O staining for intracellular lipid droplets. We demonstrate that sub-endothelial monocytes can develop into foam cells within 48 hours of TEM across TNF-α activated endothelium, in the absence of additional lipids. Our data indicate a role for both monocyte-endothelial interactions and soluble factors in the regulation of foam cell development, including oxidation of LDL in situ from lipid present in culture medium following TNF-α stimulation of the endothelial cells. Our study provides a simple model for investigating foam cell development in vitro that mimics cell migration in vivo, and demonstrates the critical role of inflammation in regulating early atherogenic events.
Keywords: Foam cell, atherosclerosis, inflammation, modified LDL, cholesterol, macrophage
Introduction
Foam cells are specialized lipid-laden macrophages derived from monocytes, and a key pathological feature of atherosclerotic lesions. Accumulation of macrophages and foam cells within the arterial intima occurs in the earliest atherogenic lesions and continues throughout plaque development (Stary et al., 1994). Foam cells represent the end stage of the response of monocyte/macrophages to accumulation of extracellular modified low-density lipoprotein (LDL), including highly inflammatory oxidized LDL (oxLDL), within the subendothelial atherosclerotic microenvironment (Brown et al., 1979; Schaffner et al., 1980; Henriksen et al., 1983). Internalization of oxLDL by monocyte/macrophages occurs predominantly via the class B scavenger receptor, CD36, and to a lesser extent scavenger receptor-A (SR-A) (Suzuki et al., 1997; Febbraio et al., 2000; Kunjathoor et al., 2002; Kuchibhotla et al., 2008). Internalized cholesterol is converted to cholesteryl esters and stored in large vacuolar lipid droplets that confer the foamy appearance (Brown et al., 1979). In addition to sequestering modified LDL, foam cells secrete inflammatory mediators such as pro-inflammatory cytokines, chemokines and matrix metalloproteinases that indirectly promote plaque development via further leukocyte recruitment and tissue remodeling (see Galkina & Ley, 2009 for review). Conditions of vascular inflammation and oxidative stress are thought to trigger a local inflammatory response that promotes increased entry of blood monocytes and development of early lesions (reviewed in Stocker & Keaney, 2004; Poli et al., 2009). Differentiation of monocyte/macrophages into foam cells reduces their migratory capacity in vivo, and chemotaxis in vitro, in part due to oxLDL-mediated CD36 signaling (Kuchibhotla et al., 2008; Park et al., 2009). However, the atheroma is a complex microenvironment, and in vitro studies that adequately address the regulation of monocyte entry via TEM and subsequent development of foam cells are lacking.
Early studies of foam cell development in vitro focused on the metabolism of LDL and modified LDL in monocultures of endothelial cells (Henriksen et al., 1981; Henriksen et al., 1983; Morel et al., 1984), monocyte/macrophages (Brown et al., 1979; Roma et al., 1990) or smooth muscle cells (SMC) (Morel et al., 1984). These studies demonstrated that extracellular LDL could be oxidatively modified by endothelial cells and SMC, that macrophages had significantly enhanced uptake of oxLDL compared with native LDL, and that oxLDL induced foam cell development. In addition, several studies have assessed the adhesion (Jeng et al., 1993; Maier et al., 1994; Kopprasch et al., 2004) and TEM of monocyte/macrophages across LDL- or oxLDL-treated endothelial cell monolayers (Navab et al., 1991; Mine et al., 2002; Dorweiler et al., 2006). However, weaknesses of these models include reliance on addition of oxLDL for induction of an atheroma-like microenvironment, use of monocyte/macrophage cell lines, and inability to measure foam cell development in situ.
We have previously modeled the process of TEM by monocytes in vitro using a simple co-culture system comprising confluent monolayers of human umbilical vein endothelial cells (HUVEC) grown on Type I collagen matrices in 96-well plates (Muller & Weigl, 1992). In this model, human peripheral blood mononuclear cells (PBMC) are placed on the apical surface of the HUVEC, followed by brief (30 min to 2 h) incubation in which monocytes selectively undergo apical-to-basal (forward) TEM into the collagen beneath, most likely due to constitutive production of chemokines such as CCL2 by the HUVEC (Muller & Weigl, 1992; Randolph & Furie, 1995). After removal of non-migrated PBMC from above the HUVEC, further incubation of the HUVEC/monocyte co-cultures results in the majority of monocytes maturing to macrophages. Approximately half of the subendothelial monocytes differentiate into immature dendritic cells (DC) and undergo reverse TEM across the HUVEC in the basal-to-apical direction (Randolph et al., 1998), mimicking migration of immature DC out of interstitial tissue and into afferent lymphatics in vivo (Randolph et al., 1999). Since the behavior of the reverse migrated mononuclear cells modeled their behavior in vivo, we sought to determine whether this model could be used to study the fate of those monocytes that remain in the subendothelial matrix and become macrophages. Specifically, would they mimic the early events in atherogenesis, in which monocytes migrate across the endothelium, internalize extracellular lipid, and differentiate into foam cells? In the current study, we show that in this co-culture system TNF-α activated endothelial cells promote the generation of foam cells from normal human monocyte-derived macrophages. We further demonstrate that foam cell development from monocytes occurs in response to direct interaction with the endothelium during TEM, as well as via soluble factors including the generation of oxLDL by activated HUVEC. This model is therefore a useful human model to study early events in the formation of atherosclerotic plaques and the factors that influence this process.
Methods
HUVEC isolation and culture
HUVEC were isolated from umbilical cords as previously described (Muller & Weigl, 1992; Westhorpe et al., 2009). Umbilical cords were obtained from consenting women with approval from human research ethics committees at the Royal Women’s Hospital, Melbourne, Australia, and the Institutional Review Board of Weill Cornell Medical Center, and processed immediately. HUVEC were prepared by enzymatic digestion from the umbilical vein of several different cords at once using type II collagenase (Worthington, Lakewood, NJ, USA). Primary HUVEC from each cord were cultured separately on fibronectin-coated 25 cm2 tissue culture flasks in M199 supplemented with 100 U/mL penicillin G, 100 μg/mL streptomycin sulfate and 2 mM glutamine (all from Invitrogen, Carlsbad, CA, USA), plus 20% heat-inactivated human serum (M20), obtained directly from healthy adult donors or from the Australian Red Cross Blood Service (Sydney, Australia). Cells were pooled and passaged once prior to transfer to hydrated type 1 collagen gels in 96-well tissue culture plates (Muller & Weigl, 1992; Westhorpe et al., 2009) (Cultrex type 1 bovine collagen from R&D Systems, Minneapolis, MN, USA). HUVEC were grown to confluence for 3-5 days prior to TEM assays. In some experiments 10 ng/mL TNF-α or 10 U/mL IL-1β was added after TEM to the HUVEC culture media for the final 24-72h. Medium was renewed every 48h for longer experiments.
Transendothelial migration assay
Peripheral blood mononuclear cells (PBMC) were isolated from blood sampled from healthy volunteers who consented to give blood under approved human research ethics applications for this project. PBMC were isolated from blood by centrifugation over Ficoll-Hypaque (Amersham Biosciences, Piscataway, NJ, USA) and interface cells were washed in Hanks’ buffered saline solution (HBSS) containing 0.1% human serum albumin (HSA) and centrifuged at low speed (300 xg) to remove platelets. PBMC were further washed and resuspended to 2×106/ml in supplemented M199 containing 0.1% HSA. HUVEC monolayers were washed twice in warm M199 and 2×105 PBMC were added per well to confluent HUVEC monolayers grown on collagen matrices. PBMC/HUVEC co-cultures were incubated for 1h at 37°C, and non-transmigrated cells were removed by washing gently several times with warm HBSS containing 1 mM ethylene glycol tetraacetic acid (EGTA). Co-cultures were subsequently incubated for up to 5 days (routinely for 48h) in fresh M20 culture medium with or without addition of cytokines (10 ng/mL TNF-α or 10 U/mL IL-1β). In some experiments, co-cultures were incubated with 10 μg/mL of anti-CD36 monoclonal antibody (clone FA6-152 from Immunotech, Beckman Coulter, Marseille, France) or IgG1 isotype control after TEM. At the end of incubation (for one to five days) apical leukocytes that had undergone reverse TEM were removed by repeating the HBSS/EGTA washes, and gels were fixed in 10% formalin overnight at 4°C. Gels were stained using a modified Wright-Giemsa stain, then carefully transferred to microscope slides and placed under a coverslip. Gels were analyzed using Nomarski optics, and multiple high-power fields were scored from each of four to six replicate gels for each experimental condition.
In some experiments, HUVEC were cultured on 3 μm pore size Transwell® inserts (# 3415, Costar, Bremen, Germany). Briefly, Transwell® inserts were coated with fibronectin at 50 μg/ml for 5 min, then HUVEC were added and cultured in M20 as above for a least 3 days. Transwells® were placed in wells containing collagen gel without contact between the Transwell® and the gel. PBMC transmigration proceeded as above, except that cultures were incubated for 3 hours to allow cells to migrate across the filter and into the collagen beneath. Empty Transwells® were used in control wells. Following initial transmigration of monocytes, 10ng/mL TNF-α was added and cultures continued. In some experiments, Transwells® containing HUVEC were removed after TEM to assess the effects of HUVEC on migrated monocytes. In other experiments PBMC were added directly onto the collagen gel, and Transwells® containing HUVEC (or empty Transwells ® as a control) were added above without direct contact with the gel, followed by activation with TNF-α.
Oil Red O staining
Following TEM assays, fixed PBMC/HUVEC co-culture specimens were prepared for Oil Red O staining by incubating for 5 min in 50% (v/v) methanol and then 15 min with 78% (v/v) methanol. Gels were stained with 0.05% Oil Red O (dissolved in 78% methanol, 22% 1N NaOH) for 1h, washed several times with 78% methanol and counterstained with methylene blue before being mounted on microscope slides with a coverslip. Oil Red O positive cells were defined as cells with visible cytoplasmic spherical red-stained vesicles (lipid droplets), and designated as foam cells when more than one-third of the cytoplasm comprised lipid droplets (usually 3-4 visible droplets). Foam cells and non-lipid laden macrophages were counted using the same procedure as for routine TEM assays, and the proportion of foam cells out of total monocyte/macrophages present in the collagen was determined. Some specimens were fixed and examined by electron microscopy using previously published methods (Muller et al., 1989).
Cholesteryl ester analysis
Cholesteryl ester content in monocytes and HUVEC mono- and co-cultures was measured as described (Goldstein et al., 1974). Cells were prepared as above, and following forward TEM incubated with 14C-oleate for a further three days. Cultures were washed and lipids were extracted using chloroform/methanol extraction, and aliquots of extracted lipids were separated by thin layer chromatography. The cholesteryl ester spots were identified by co-migration with known standards, removed from the plates and solubilized, and then counted in a liquid scintillation counter. The data are normalized for total protein content of each sample.
LDL modification
Freshly isolated LDL was oxidized by dialysis with 10 μM CuSO4 in sterile PBS for 6 hours, followed by a wash in 100 μM EDTA/PBS for 2 hours. Aggregated LDL was prepared by incubating 1 mg of LDL protein/mL of PBS with 50 mU/mL sphingomyelinase in the presence of 5 mM MgCl2 at 37°C for 4 hours, then adding 10 mM EDTA to stop the reaction (Sakr et al., 2001). LDL, oxLDL and aggregated LDL (agLDL) were added at a final concentration of 200 μg/mL to the collagen matrices 3 days before the addition of HUVEC.
OxLDL detection
Accumulation of oxLDL in the sub-endothelial collagen gels was measured by immunofluorescence. Cultures were maintained for 48h after TEM, and gels were washed and fixed as above. Gels were permeabilized with 0.1% Triton X-100 and incubated with either anti-oxLDL antibody clone NA59 or MDA2 (a kind gift from Dr. Joseph Witztum) (Palinski et al., 1990). Gels were stained with secondary antibodies conjugated with Alexa-488, mounted on slides and overlaid with a coverslip. Fluorescence intensity was scanned with a Storm scanner (GE Healthcare, Uppsala, Sweden).
Results and Discussion
Induction of foam cell development from monocytes following transendothelial migration in vitro
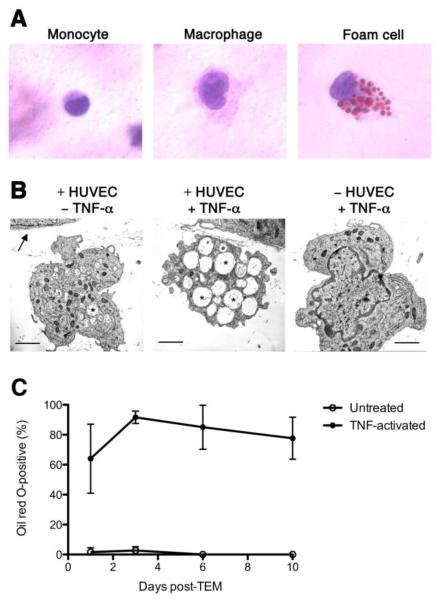
We investigated the effects of inflammation on the capacity of monocytes to develop into foam cells in our model of monocyte transendothelial migration (TEM) (Muller & Weigl, 1992). Monocytes (within PBMC) were allowed to undergo migration across confluent primary HUVEC monolayers into the sub-endothelial collagen for 1 hour, non-migrated cells were removed from the apical surface of the HUVEC, and co-cultures were incubated with TNF-α (Figure 1) or with IL-1β (data not shown) for 48 hours. Subendothelial monocytes rapidly developed a typical macrophage morphology (Figure 1A center panel) and in the presence of pro-inflammatory cytokines, they gradually accumulated intracellular Oil Red O-positive lipid droplets, giving them a foam cell appearance (Fig 1A right panel). By electron microscopy, monocyte-derived cells present beneath unstimulated HUVEC monolayers 48 hours after TEM had a typical macrophage appearance, including an abundance of lysosomes, but few lipid droplets (Fig 1B, left panel). Addition of TNF-α to the co-cultures resulted in macrophages in which the cytoplasm was filled with transparent membrane-bound vesicles that are characteristic of foam cells (Fig 1B, center panel). Significantly, culture of TNF-α treated monocytes in collagen gels in the absence of HUVEC resulted in development of macrophages that lacked lipid droplets (Fig 1B, right panel). Development of foam cells in TNF-α treated monocyte/HUVEC co-cultures peaked at day three post-TEM (Fig 1C).
Figure 1. Sub-endothelial monocyte-derived cells develop a foam cell morphology following TNF-α treatment of HUVEC.
PBMC preparations (2×105 cells) were added to confluent layers of HUVEC which were stimulated with 10 ng/ml TNF-α or left unstimulated and grown on collagen gels for up to 10 days to allow TEM by monocytes. Cells migrating within the collagen were then stained with Oil Red O and counted by light microscopy, or were alternatively fixed in glutaraldehyde, embedded in Epon, and examined by electron microscopy. A) Subendothelial monocytes (left panel) assumed the morphology of macrophages within a few hours (center panel). By 48h incubation of co-cultures with TNF-α, macrophages accumulated Oil Red O-positive lipid droplets, stained red (right panel).
B) Electron micrographs of sub-endothelial macrophages 48 h following TEM across untreated HUVEC (left) or HUVEC treated with TNF-α (centre), and a macrophage cultured in the collagen incubated with TNF-α in the absence of HUVEC (right). Arrow indicates the basal lamina of the HUVEC monolayer; asterisks indicate lipid droplets; scale bar is 2μm.
C) The proportion of cells within the collagen that assumed foam cell morphology was measured over a 10-day period following TEM and co-culture of monocytes/HUVEC in M199 plus 20% human serum containing 10 ng/ml TNF-α (filled circles) or in M199 plus 20% human serum alone (open circles). Data are mean (±SD) of three separate experiments.
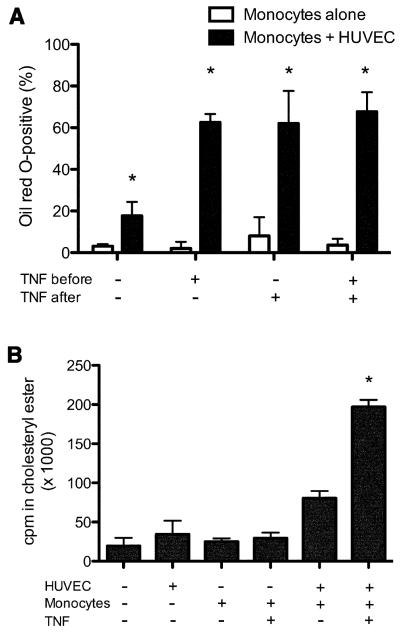
To dissect which components of the HUVEC/monocyte co-cultures were contributing to foam cell development in our model, we measured the numbers of foam cells produced in the sub-endothelial collagen in the presence and absence of HUVEC as well as with and without inflammatory stimulation before and after TEM (Figure 2A). A small proportion of monocytes spontaneously developed into foam cells after several days of culture alone or following migration across unactivated endothelial cells. Exposure of monocytes to TNF-α in the absence of HUVEC did not affect foam cell numbers, whereas TNF-α treatment of HUVEC/monocyte co-cultures produced a dramatic increase in the extent of foam cell development. The same effect was seen in monocytes undergoing TEM across HUVEC treated with IL-1β (data not shown). There was no difference in sub-endothelial development of foam cells whether the TNF-α was applied either prior to or after TEM by monocytes, or both, indicating that endothelial inflammation is the critical step in this process. The absolute percent of macrophages displaying extensive foam cell morphology varied throughout the course of these studies (discussed below). However, these trends were quite reproducible. Since Oil Red O stains all neutral lipids and macrophages can accumulate triglyceride droplets, we biochemically analyzed the composition of lipids accumulating intracellularly. Analysis of lipids extracted from cells embedded in the collagen gel, by thin layer chromatography, showed that the formation of foam cells is associated with an increased content of cholesteryl esters maximal in the TNF-α treated co-cultures, as expected for bona fide foam cells (Fig 2B). We also found no change in intracellular triglyceride levels in sub-endothelial cells in the presence or absence of TNF-α treatment (data not shown). These results confirm the foam cell phenotype of monocyte-derived sub-endothelial macrophages suggested by the morphological data. These data show that in this model inflammatory cytokine-activated HUVEC are sufficient to induce monocyte differentiation into foam cells without the addition of exogenous modified LDL, or HDL depletion.
Figure 2. TNF-α activation of HUVEC is required for optimal induction of monocyte-derived foam cells.
A) Monocytes were allowed to transmigrate across HUVEC monolayers that were unactivated or activated with 10 ng/ml TNF-α before, after, or both before and after transendothelial migration. The proportion of sub-endothelial Oil Red O-positive foam cells was measured as previously. Data indicate mean (±SD) of six replicates per condition; graph is representative of several experiments performed. *Significantly different from cultures without HUVEC (p < 0.05).
B) In vitro-derived foam cells contain cholestryl ester. HUVEC, monocytes, or HUVEC co-cultured with monocytes after transendothelial migration in the presence or absence of TNF-α activation were incubated with 14C-oleate for three days, after which cultures were washed, and cholesteryl ester content determined as described in Methods. Results are mean ± SD of triplicate measurements. *Significantly different from all other samples (p < 0.01).
Foam cell development is stimulated by both contact with and soluble signals from the endothelium
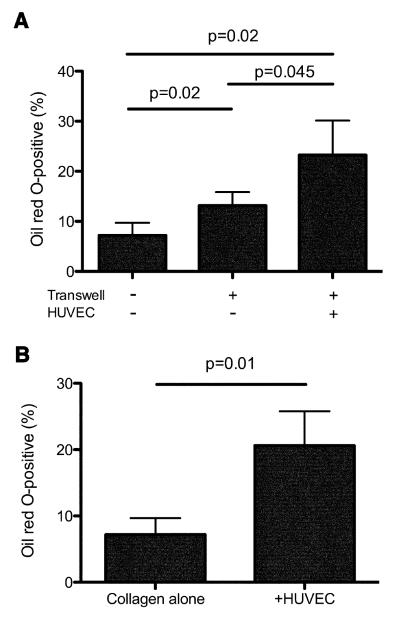
To determine whether monocyte transendothelial migration was required for foam cell development, we used Transwell® tissue culture inserts to provide a cell-permeable membrane separating the HUVEC monolayer from the collagen. We first assessed the role of TEM in foam cell development by allowing monocytes from PBMC to cross a Transwell® insert containing a confluent HUVEC monolayer and move into a collagen matrix beneath, before removing the Transwell® and culturing the cells for 48h in the presence of TNF-α. Migration of monocytes across empty Transwells® produced a modest increase in numbers of foam cells developing in the underlying collagen matrix compared with monocytes placed directly onto the collagen (Figure 3A). Greater numbers of foam cells developed following migration of monocytes across a HUVEC monolayer in the Transwell® insert (Figure 3A) compared with monocytes migrating across empty Transwells®. Thus, TEM across a resting HUVEC monolayer, followed by activation with TNF-α, induces up-regulation of foam cell development from monocytes, although soluble factors from the endothelial monolayer that were present during the first three hours may have contributed (see below).
Figure 3. HUVEC trigger foam cell development from monocytes directly during TEM and by production of soluble factors.
A) PBMC (2×105) were placed directly onto collagen matrices (-Transwell, -HUVEC), onto unseeded Transwells® (+Transwell, -HUVEC) or onto Transwells® containing confluent HUVEC monolayers (+Transwell, +HUVEC) and incubated for 3 hours to allow monocytes to migrate into the underlying collagen. Transwells® were then removed, the collagen was gently washed, and cells were cultured in fresh medium containing 10 ng/ml TNF-α for a further 48 h. Numbers of foam cells present in the collagen were then enumerated as described in Methods and expressed as a proportion of total cells within the collagen. B) PBMC were placed directly onto collagen matrices and cultured for 24 h, then Transwells® containing collagen gels alone or confluent HUVEC monolayers on collagen gels were placed above the collagen and cultures were incubated for a further 48 h in fresh medium containing 10 ng/mL TNF-α. The proportion of Oil Red O-positive foam cells within the collagen matrix was then measured. Data represent mean (±SD) of four determinations.
To determine whether TNF-α activated HUVEC could also up-regulate foam cell development from monocytes via soluble factors or whether cell-cell contact was required, monocytes were placed directly onto collagen matrices and then Transwells® containing confluent HUVEC monolayers placed over the top, preventing direct HUVEC/monocyte contact. Cultures were incubated for two days with TNF-α. Compared with monocytes placed onto the collagen alone, monocytes cultured in the presence of Transwells® containing HUVEC produced approximately three-fold more foam cells (Figure 3B). HUVEC in our model are therefore able to induce foam cell development from monocytes via both direct signals during TEM, and via soluble factor(s).
Sub-endothelial macrophages use CD36 to ingest LDL modified by TNF-α-activated endothelial cells
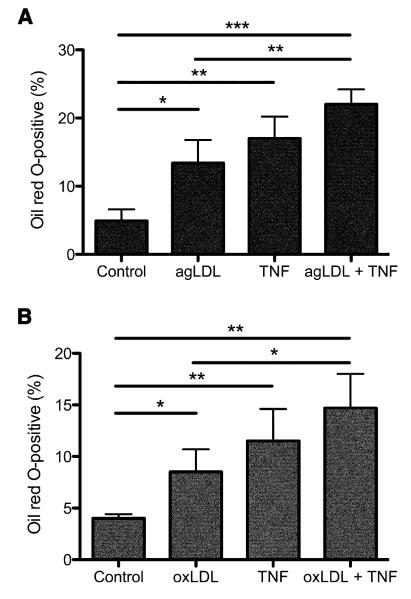
Modified LDL is the primary source of cholesterol in the development of foam cells both in vivo and in vitro (Ghosh et al., 2010). We next determined whether induction of foam cells from sub-endothelial monocytes in the presence of TNF-α activated HUVEC was comparable with foam cell induction by modified LDL. We impregnated the collagen with modified LDL, using either aggregated LDL (agLDL) or oxidized LDL (oxLDL), prior to the addition of HUVEC and performed the TEM assays once the HUVEC reached confluence. In the absence of TNF-α, both agLDL (Fig 4A) and oxLDL (Fig 4B) were able to induce sub-endothelial foam cell formation by monocytes. In both cases, TNF-α treatment of HUVEC/monocyte co-cultures was as effective, if not greater, in the induction of foam cell formation than either agLDL or oxLDL alone. Combination of either agLDL or oxLDL with TNF-α during culture of monocyte/HUVEC co-cultures produced an additive effect. This suggests that the HUVEC may provide signaling to the transmigrating monocytes, in addition to LDL modification, thereby priming them for foam cell formation (as suggested by Fig. 3A), and that either the activated HUVEC signal or levels of modified LDL produced by activated HUVEC is submaximal in our assay.
Figure 4. TNF-α activation of HUVEC is as effective as the presence of modified LDL in promoting foam cell development from monocytes.
Aggregated LDL (agLDL; A) or oxidized LDL (oxLDL; B) were added to the collagen at a concentration of 200 μg/mL three days prior to HUVEC culture. PBMC (2×105) were placed on HUVEC monolayers and monocytes allowed to undergo transmigration. After 1 hour, the proportion of Oil Red O-positive foam cells within the collagen was then enumerated by microscopy, and expressed as a percentage of the total sub-endothelial macrophages present. Results are the mean (±SD) from 3 experiments; *p<0.05; **p<0.01; ***p<0.001.
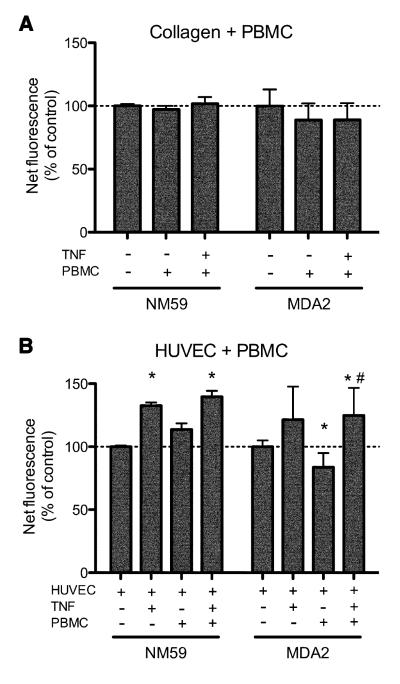
To determine whether TNF-α activation of HUVEC resulted in production of modified LDL, we used two different monoclonal antibodies (NA59 and MDA2) to analyze oxLDL content in our preparations by immunofluorescence (Palinski et al., 1990). Anti-oxLDL immunofluorescence was comparable between collagen preparations without any added cells, and collagen preparations containing either PBMC or PBMC stimulated with TNF-α (Figure 5A) suggesting that mononuclear leukocytes do not appreciably oxidize LDL even when activated. In contrast, activation of HUVEC with TNF-α produced a significant increase in oxLDL content within the collagen matrices, as measured using either monoclonal antibody, which was not further increased by adding PBMC (Figure 5B).
Figure 5. Oxidation of LDL occurs in the presence of TNFα activated HUVEC, but not resting HUVEC, or in the presence of resting or activated macrophages.
(A) Collagen matrices were cultured in the presence of 10 ng/ml TNF-α where indicated with or without PBMC (2×105) for 48h then fixed and stained for oxidized LDL using antibody clones NA59 and MDA2 and an Alexa488-conjugated secondary antibody, and immunofluorescence measured using a Storm fluorescence scanner. Fluorescence readings from blank wells containing medium alone were subtracted from all the samples, and the results were expressed as a percentage relative to the collagen alone control. (B) Collagen matrices seeded with confluent HUVEC monolayers were cultured as above and incubated with 10 ng/ml TNF-a or with PBMC as indicated for 48 h, fixed and stained with anti oxidized LDL monoclonal antibodies. Net fluorescence is expressed relative to that of control wells containing collagen and HUVEC alone. Data are the mean (±SD) of 8-10 replicate wells for each condition. * p<0.05 vs control; # p<0.05 vs HUVEC +PBMC -TNF (Student’s t test).
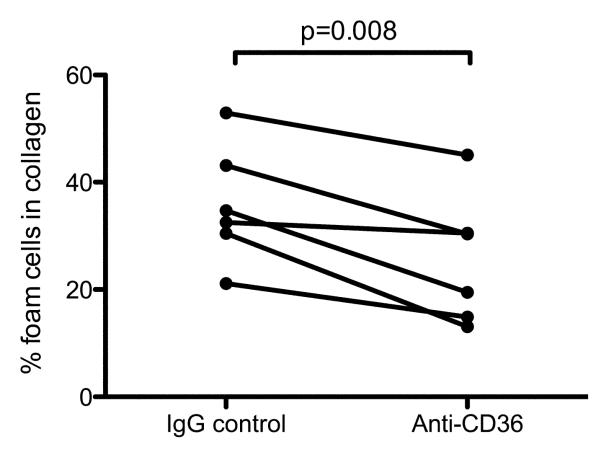
Oxidation of LDL in our model is therefore occurring in the presence of TNF-α activated HUVEC, but not TNF-α activated macrophages. These results indicate that development of sub-endothelial monocyte-derived foam cells is therefore likely to occur mainly via monocyte uptake of extracellular oxidized LDL produced in response to TNF-α activated HUVEC. To further investigate this possibility we undertook a blocking study of CD36, the major receptor for oxLDL on monocyte/macrophages (Kunjathoor et al., 2002). Following TEM across confluent HUVEC monolayers, co-cultures of monocytes and HUVEC were incubated with saturating concentrations of an anti-CD36 antibody that blocks uptake of oxLDL by monocyte/macrophages (Puente Navazo et al., 1996). Compared with the isotype-matched IgG control, anti-CD36 caused a significant decrease in the numbers of sub-endothelial foam cells, which were reduced on average by approximately one-third (Figure 6). Together, these data indicate that HUVEC activation promotes the development of sub-endothelial foam cells from transmigrated monocytes in part via increased abundance of extracellular oxLDL and uptake by macrophages via CD36.
Figure 6. Anti-CD36 antibody inhibits sub-endothelial foam cell development.
Monocytes prepared within 2×105 PBMC from six independent donors were added to confluent monolayers of HUVEC on collagen gels and allowed to undergo TEM across unstimulated HUVEC for 1 hour as above. The monocyte/HUVEC co-cultures were then incubated with 10 ng/ml TNF-α and either 10 ng/ml anti-CD36 clone FA6-152 or isotype-matched control mouse IgG1, and cultured for a further 48h prior to enumeration of sub-endothelial foam cells. Statistical analysis is a paired Student’s t test.
Our novel monocyte/HUVEC co-culture system has enabled analysis of foam cell development in the context of endothelial inflammation in vitro without artificial manipulation of the lipoprotein milieu. Using this system, we have demonstrated that 1) large numbers of human monocytes can differentiate into cholesteryl ester laden foam cells within 48 hours in vitro, 2) the act of TEM itself promotes the differentiation of monocytes into foam cells, 3) soluble factor(s) produced by inflammatory cytokine-activated endothelial cells also contribute, and 4) TNFα in this system works predominantly to activate the endothelial cells for their role in foam cell induction. This model employs human serum as the source of LDL as part of the normal HUVEC culture medium, together with primary human monocytes and endothelial cells, to replicate the microvascular environment in vivo as closely as possible. There was a degree of inter-assay variation in the proportions of sub-endothelial foam cells that developed. This may reflect variation in the LDL content of different batches of human sera, although we found no correlation between LDL levels of donor sera used in the culture medium and foam cell development (data not shown). Nonetheless, the intra-assay data showed a coefficient of variation of 22.7%. Our model is not conducive to removal of LDL from the culture medium, to confirm this as the source of LDL driving foam cell formation, as the HUVEC require serum for growth, and endogenous lipoproteins are likely to become trapped within the sub-endothelial collagen. Regardless, our data suggest that even clinically desirable levels of LDL are sufficient to produce foam cells if inflammatory conditions prevail, similar to what is seen clinically. Large differences in foam cell development from monocytes from different donors has also been reported and is likely to be a factor in our model since we use primary cells. However the overall trends of foam cell development were highly consistent in this assay. We recommend using Oil Red O staining for pilot experiments using this model, which can be validated by more quantitative but technically more difficult methods of intracellular lipid measurement. Our findings highlight the complex cellular interactions that occur during atherogenesis, and provide a view of the earliest vascular events in this process. This model can be adapted to study any of these complex cellular interactions in more detail.
Acknowledgements
This work was funded by the Australian Centre for HIV and Hepatitis Research and the National Institutes of Health of the United States (HL072942). The authors gratefully acknowledge the contribution to this work of the Victorian Operational Infrastructure Support Program. AM is supported by a fellowship within the Postdoctoral Programme of the German Academic Exchange Service (DAAD). SC is a recipient of an NHMRC Principal Research Fellowship. We would like to thank Dr. Joan Muller for initiating these studies and for comments on the manuscript, Professor Joe Witztum (University of California, San Diego) for providing the anti-oxLDL antibodies used in this study, Karen Mendelson for help with some experiments, and Ron Liebman for expert technical assistance.
Abbreviations
- agLDL
aggregated low-density lipoprotein DC dendritic cell
- LDL
low-density lipoprotein
- HUVEC
human umbilical vein endothelial cells
- IL-1β
interleukin-1β
- oxLDL
oxidized low-density lipoprotein
- PBMC
peripheral blood mononuclear cells
- TEM
transendothelial migration
- TNF-α
tumour necrosis factor-α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brown MS, Goldstein JL, Krieger M, Ho YK, Anderson RG. Reversible accumulation of cholesteryl esters in macrophages incubated with acetylated lipoproteins. J. Cell Biol. 1979;82:597–613. doi: 10.1083/jcb.82.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorweiler B, Torzewski M, Dahm M, Ochsenhirt V, Lehr HA, Lackner KJ, Vahl CF. A novel in vitro model for the study of plaque development in atherosclerosis. Thromb. Haemost. 2006;95:182–189. [PubMed] [Google Scholar]
- Febbraio M, Podrez EA, Smith JD, Hajjar DP, Hazen SL, Hoff HF, Sharma K, Silverstein RL. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J. Clin. Invest. 2000;105:1049–1056. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*) Annu. Rev. Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Zhao B, Bie J, Song J. Macrophage cholesteryl ester mobilization and atherosclerosis. Vascul. Pharmacol. 2010;52:1–10. doi: 10.1016/j.vph.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JL, Dana SE, Brown MS. Esterification of low density lipoprotein cholesterol in human fibroblasts and its absence in homozygous familial hypercholesterolemia. Proc. Natl. Acad. Sci. U.S.A. 1974;71:4288–4292. doi: 10.1073/pnas.71.11.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen T, Mahoney EM, Steinberg D. Enhanced macrophage degradation of low density lipoprotein previously incubated with cultured endothelial cells: recognition by receptors for acetylated low density lipoproteins. Proc. Natl. Acad. Sci. U.S.A. 1981;78:6499–6503. doi: 10.1073/pnas.78.10.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen T, Mahoney EM, Steinberg D. Enhanced macrophage degradation of biologically modified low density lipoprotein. Arteriosclerosis. 1983;3:149–159. doi: 10.1161/01.atv.3.2.149. [DOI] [PubMed] [Google Scholar]
- Jeng JR, Chang CH, Shieh SM, Chiu HC. Oxidized low-density lipoprotein enhances monocyte-endothelial cell binding against shear-stress-induced detachment. Biochim. Biophys. Acta. 1993;1178:221–227. doi: 10.1016/0167-4889(93)90013-f. [DOI] [PubMed] [Google Scholar]
- Kopprasch S, Pietzsch J, Westendorf T, Kruse HJ, Grassler J. The pivotal role of scavenger receptor CD36 and phagocyte-derived oxidants in oxidized low density lipoprotein-induced adhesion to endothelial cells. Int. J. Biochem. Cell Biol. 2004;36:460–471. doi: 10.1016/j.biocel.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Kuchibhotla S, Vanegas D, Kennedy DJ, Guy E, Nimako G, Morton RE, Febbraio M. Absence of CD36 protects against atherosclerosis in ApoE knock-out mice with no additional protection provided by absence of scavenger receptor A I/II. Cardiovasc. Res. 2008;78:185–196. doi: 10.1093/cvr/cvm093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunjathoor VV, Febbraio M, Podrez EA, Moore KJ, Andersson L, Koehn S, Rhee JS, Silverstein R, Hoff HF, Freeman MW. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J. Biol. Chem. 2002;277:49982–49988. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- Maier JA, Barenghi L, Pagani F, Bradamante S, Comi P, Ragnotti G. The protective role of high-density lipoprotein on oxidized-low-density-lipoprotein-induced U937/endothelial cell interactions. Eur. J. Biochem. 1994;221:35–41. doi: 10.1111/j.1432-1033.1994.tb18712.x. [DOI] [PubMed] [Google Scholar]
- Mine S, Tabata T, Wada Y, Fujisaki T, Iida T, Noguchi N, Niki E, Kodama T, Tanaka Y. Oxidized low density lipoprotein-induced LFA-1-dependent adhesion and transendothelial migration of monocytes via the protein kinase C pathway. Atherosclerosis. 2002;160:281–288. doi: 10.1016/s0021-9150(01)00582-2. [DOI] [PubMed] [Google Scholar]
- Morel DW, DiCorleto PE, Chisolm GM. Endothelial and smooth muscle cells alter low density lipoprotein in vitro by free radical oxidation. Arteriosclerosis. 1984;4:357–364. doi: 10.1161/01.atv.4.4.357. [DOI] [PubMed] [Google Scholar]
- Muller WA, Ratti CM, McDonnell SL, Cohn ZA. A human endothelial cell-restricted, externally disposed plasmalemmal protein enriched in intercellular junctions. J. Exp. Med. 1989;170:399–414. doi: 10.1084/jem.170.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller WA, Weigl SA. Monocyte-selective transendothelial migration: dissection of the binding and transmigration phases by an in vitro assay. J. Exp. Med. 1992;176:819–828. doi: 10.1084/jem.176.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navab M, Imes SS, Hama SY, Hough GP, Ross LA, Bork RW, Valente AJ, Berliner JA, Drinkwater DC, Laks H, et al. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J. Clin. Invest. 1991;88:2039–2046. doi: 10.1172/JCI115532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palinski W, Yla-Herttuala S, Rosenfeld ME, Butler SW, Socher SA, Parthasarathy S, Curtiss LK, Witztum JL. Antisera and monoclonal antibodies specific for epitopes generated during oxidative modification of low density lipoprotein. Arteriosclerosis. 1990;10:325–335. doi: 10.1161/01.atv.10.3.325. [DOI] [PubMed] [Google Scholar]
- Park YM, Febbraio M, Silverstein RL. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J. Clin. Invest. 2009;119:136–145. doi: 10.1172/JCI35535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli G, Sottero B, Gargiulo S, Leonarduzzi G. Cholesterol oxidation products in the vascular remodeling due to atherosclerosis. Mol. Aspects Med. 2009;30:180–189. doi: 10.1016/j.mam.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Puente Navazo MD, Daviet L, Ninio E, McGregor JL. Identification on human CD36 of a domain (155-183) implicated in binding oxidized low-density lipoproteins (Ox-LDL) Arterioscler. Thromb. Vasc. Biol. 1996;16:1033–1039. doi: 10.1161/01.atv.16.8.1033. [DOI] [PubMed] [Google Scholar]
- Randolph GJ, Beaulieu S, Lebecque S, Steinman RM, Muller WA. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science. 1998;282:480–483. doi: 10.1126/science.282.5388.480. [DOI] [PubMed] [Google Scholar]
- Randolph GJ, Furie MB. A soluble gradient of endogenous monocyte chemoattractant protein-1 promotes the transendothelial migration of monocytes in vitro. J. Immunol. 1995;155:3610–3618. [PubMed] [Google Scholar]
- Randolph GJ, Inaba K, Robbiani DF, Steinman RM, Muller WA. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 1999;11:753–761. doi: 10.1016/s1074-7613(00)80149-1. [DOI] [PubMed] [Google Scholar]
- Roma P, Catapano AL, Bertulli SM, Varesi L, Fumagalli R, Bernini F. Oxidized LDL increase free cholesterol and fail to stimulate cholesterol esterification in murine macrophages. Biochem. Biophys. Res. Commun. 1990;171:123–131. doi: 10.1016/0006-291x(90)91365-y. [DOI] [PubMed] [Google Scholar]
- Sakr SW, Eddy RJ, Barth H, Wang F, Greenberg S, Maxfield FR, Tabas I. The uptake and degradation of matrix-bound lipoproteins by macrophages require an intact actin Cytoskeleton, Rho family GTPases, and myosin ATPase activity. J. Biol. Chem. 2001;276:37649–37658. doi: 10.1074/jbc.M105129200. [DOI] [PubMed] [Google Scholar]
- Schaffner T, Taylor K, Bartucci EJ, Fischer-Dzoga K, Beeson JH, Glagov S, Wissler RW. Arterial foam cells with distinctive immunomorphologic and histochemical features of macrophages. Am. J. Pathol. 1980;100:57–80. [PMC free article] [PubMed] [Google Scholar]
- Stary HC, Chandler AB, Glagov S, Guyton JR, Insull W, Jr., Rosenfeld ME, Schaffer SA, Schwartz CJ, Wagner WD, Wissler RW. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1994;89:2462–2478. doi: 10.1161/01.cir.89.5.2462. [DOI] [PubMed] [Google Scholar]
- Stocker R, Keaney JF., Jr. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, Ueda O, Sakaguchi H, Higashi T, Suzuki T, Takashima Y, Kawabe Y, Cynshi O, Wada Y, Honda M, Kurihara H, Aburatani H, Doi T, Matsumoto A, Azuma S, Noda T, Toyoda Y, Itakura H, Yazaki Y, Kodama T, et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386:292–296. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- Westhorpe CL, Zhou J, Webster NL, Kalionis B, Lewin SR, Jaworowski A, Muller WA, Crowe SM. Effects of HIV-1 infection in vitro on transendothelial migration by monocytes and monocyte-derived macrophages. J. Leukoc. Biol. 2009;85:1027–1035. doi: 10.1189/jlb.0808501. [DOI] [PMC free article] [PubMed] [Google Scholar]