Abstract
Background
Impaired vasodilator function is an early manifestation of coronary artery disease and may precede angiographic stenosis. It is unknown whether non-invasive assessment of coronary vasodilator function in patients with suspected or known coronary artery disease (CAD) carries incremental prognostic significance.
Methods and Results
2783 consecutive patients referred for rest/stress PET were followed for a median of 1.4 years (inter-quartile range: 0.7–3.2 years). The extent and severity of perfusion abnormalities were quantified by visual evaluation of myocardial perfusion images (MPI). Rest and stress myocardial blood flow (MBF) were calculated using factor analysis and a 2-compartment kinetic model, and were used to compute coronary flow reserve (CFR=stress/rest MBF). The primary endpoint was cardiac death. Overall 3-year cardiac mortality was 8.0%. The lowest tertile of CFR (<1.5) was associated with a 5.6-fold increase in the risk of cardiac death (95%CI 2.5–12.4, p<0.0001) compared to the highest tertile. Incorporation of CFR into cardiac death risk assessment models resulted in an increase in the c-index from 0.82 (95%CI 0.78–0.86) to 0.84 (95%CI 0.80–0.87, p=0.02) and in a net reclassification improvement (NRI) of 0.098 (95%CI 0.025–0.180). Addition of CFR resulted in correct reclassification of 34.8% of intermediate risk patients (NRI=0.487, 95%CI 0.262–0.731). Corresponding improvements in risk assessment for mortality from any cause were also demonstrated.
Conclusions
Non-invasive quantitative assessment of coronary vasodilator function using PET is a powerful, independent predictor of cardiac mortality in patients with known or suspected CAD and provides meaningful incremental risk stratification over clinical and gated MPI variables.
Keywords: coronary disease, blood flow, imaging, atherosclerosis, ischemia
Introduction
Traditional approaches for cardiac risk assessment in coronary artery disease (CAD) include information about left ventricular (LV) function, the angiographic extent and severity of epicardial coronary stenoses, and the magnitude of inducible myocardial ischemia by non-invasive testing (1). Although very powerful, these traditional risk markers do not together fully account for the risk of cardiac death, especially among high risk subgroups (2,3). This suggests that other mechanisms may contribute to clinical risk. One such mechanism may involve the adverse effects of atherosclerosis and its risk factors on coronary epicardial and microcirculatory function, thereby increasing the potential for ischemic and atherothrombotic complications.
Coronary circulatory dysfunction is present in the earliest stages of atherogenesis and precedes significant angiographic stenosis (4,5), identifiable perfusion deficits and wall motion abnormalities with traditional imaging methods (6–8). Furthermore, abnormalities in vascular function identified by specialized invasive methods have been shown to identify patients at increased risk for adverse cardiovascular events (9–11), and to improve selection of patients for revascularization (12). Coronary vasodilator function can also be assessed non-invasively using Positron Emission Tomography (PET).
This study was designed to test the hypothesis that among patients referred for cardiac testing, abnormalities of coronary vasodilator function add incremental value for the identification of those at risk of cardiac death beyond clinical risk factors and traditional semi-quantitative assessment of myocardial ischemia and left ventricular function.
Methods
Study Population
All patients referred for rest/stress cardiac PET at the Brigham & Women’s Hospital (Boston, MA) between January 1, 2006 and June 30, 2010 were included in this study, excluding those whose images were missing or uninterpretable due to poor image quality. In cases of repeat evaluations during the study period, only the earliest evaluable study was included. The study was approved by the Partners Healthcare Institutional Review Board and conducted in accordance with institutional guidelines.
Risk Factor Assessment
Demographic factors and key elements of the patients’ history including risk factors and medication use were ascertained at the time of the study by patient interview and review of medical records.
Positron Emission Tomographic Imaging
Patients were studied using a whole body PET-CT scanner (Discovery RX or STE LightSpeed 64, GE Healthcare, Milwaukee, WI) after an overnight fast. Patients refrained from caffeine and methylxanthine containing substances and drugs for 24 hours prior to their scans. Myocardial blood flow (MBF) was measured during rest and peak stress using 82Rubidium as a perfusion tracer, as described previously (13). Briefly, after transmission imaging and beginning with the intravenous bolus administration of 82Rubidium (1,480–2,200 MBq), list mode images were acquired for seven minutes. Then, a standard intravenous infusion of dipyridamole, adenosine, regadenoson or dobutamine was given. At peak stress, a second dose of 82Rubidium was injected and images were recorded in the same manner. The average radiation exposure per study was 4.6 mSv (14). Heart rate, blood pressure, and 12-lead electrocardiogram were recorded at baseline and every minute during and after pharmacological stress.
Image Analysis
Semiquantitative Analysis of Myocardial Perfusion
Semi-quantitative 17-segment visual interpretation of the gated myocardial perfusion images was performed by experienced observers using a standard 5-point scoring system (15). Summed rest (SRS) and stress scores (SSS) were calculated as the sum of individual segmental scores on the respective images, and their difference was recorded as summed difference score (SDS). For each of these variables, higher scores reflect larger areas of myocardial ischemia and/or scar. Summed rest, stress and difference scores were converted into percentages of total myocardium by division the maximum possible score.
Left Ventricular Systolic Function
Rest and stress LV ejection fraction (LVEF) were calculated from gated myocardial perfusion images using commercially available software. Left ventricular ejection fraction reserve was considered present when LVEF increased from rest to stress.
Quantitative Myocardial Blood Flow and Flow Reserve
Absolute MBF (in ml/g/min) was computed from the dynamic rest and stress imaging series using commercially available software (Corridor4DM; Ann Arbor, Michigan) and previously validated methods (13,16). Automated factor analysis was used to generate blood pool (arterial input function) and tissue time-activity curves (17). Regional and global rest and peak stress MBF were calculated by fitting the 82Rubidium time-activity curves to a two-compartment tracer kinetic model as described previously (16). Per-patient global coronary flow reserve (CFR) was calculated as the ratio of absolute MBF at stress over rest for the entire left ventricle. Quantitation of MBF was performed by four operators. The intra-class correlation coefficient for CFR among these four readers was 0.94 (95%CI 0.88–0.98), indicating excellent reproducibility.
Assessment of Outcomes
The primary outcome was death from any cardiac cause. Patients who died from non-cardiac causes were censored. Vital status of all patients was ascertained by integrating data from the Social Security Death Index, the National Death Index and the Partners Healthcare Research Patient Data Registry. Cause of death was determined by blinded adjudication of hospital records and death certificates. Early revascularization (within 90 days) was ascertained from the Partners Healthcare Research Patient Data Registry and hospital records. Mortality from any cause was used as a secondary endpoint.
Statistical Analysis
Statistical significance was assessed using Wilcoxon tests, Fisher exact and chi-square tests for continuous, dichotomous and categorical variables, respectively. Two sided p-values < 0.05 were considered significant. All statistical analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, NC).
Multivariable Modeling
The Cox proportional hazards model was used to assess the impact of CFR on cardiac mortality after controlling for the effects of critical covariates. A series of models were developed starting with one containing only clinical covariates (i.e. age, gender, hypertension, dyslipidemia, diabetes mellitus, family history of CAD, tobacco use, history of CAD (including recent or prior myocardial infarction and revascularization), body mass index, chest pain, dyspnea and early revascularization). Rest LVEF, the combined extent of myocardial scar and ischemia, stress-induced LVEF augmentation and CFR (in tertiles) were then sequentially incorporated into the model. In order to investigate the effects of absolute peak stress MBF we generated an additional model containing absolute stress MBF instead of CFR. The models were examined for the validity of the proportional hazards assumption and additive value, taking care to avoid over-fitting. Survival was plotted using direct adjusted survival probabilities (18) from the Cox survival model.
To assess for biases introduced by early revascularization, analyses were repeated censoring all patients who underwent early revascularization (19). In an exploratory analysis, we considered the effect of any revascularization, including those >90 days after the PET scan, as a time-dependent covariate.
Assessment of Incremental Value
Incremental prognostic value of CFR was assessed with the likelihood ratio test to determine the improvement in prediction power of each sequential Cox model. Model calibration was evaluated with the Nam-D’Agostino χ2 statistic (20). The c-index was calculated for each model (21) with comparisons using the method of Antolini and colleagues (22). The potential impact of CFR on risk stratification was assessed by net reclassification improvement (NRI) (23)at 1.5-years using threshold annual rates of cardiac mortality of 1% and 3% based on ACC/AHA guidelines for management of chronic stable angina (1).
Exploratory Analyses
The effect size of CFR on cardiac mortality was evaluated in a series of subgroups by constructing Cox proportional hazard models for each subgroup containing only CFR tertiles as predictor variables (i.e. unadjusted models). Effect modification was explored using Cox models incorporating interaction terms between key covariates and CFR.
To explore the relative contribution of epicardial stenoses and microvascular dysfunction, findings on coronary angiography within 90 days of PET were correlated to CFR tertile among patients with no evidence of scar or ischemia by visual PET evaluation and without history of coronary artery bypass graft surgery or intervening MI. Lesions greater than 70% stenosis were considered significant. Angiographic disease was categorized as non-obstructive or normal (<50% stenosis), 1-, 2- or 3-vessel disease.
Results
Patient Characteristics
A total of 2783 of 3404 (81.8%) consecutive patients met inclusion criteria during the study period and followed for a median of 1.4 years (IQR: 0.7–3.2 years). Baseline characteristics are given in table 1. PET scans represented 19.8% of all stress imaging scans performed at our institution during the time period of this study. The remainder of stress imaging tests were performed with SPECT (54.6%) or echocardiographic imaging (25.7%). The risk profiles of patients referred for these tests were comparable using the Duke clinical score: 49.2 ± 32.3 (mean ± SD) for PET, 47.8 ± 31.6 for SPECT and 37.9 ± 30.9 for echocardiography (24).
Table 1.
Patient Characteristics
| Variable | No Cardiac Death (n=2646) | Cardiac Death (n=137) | All Patients (n=2783) | p-Value |
|---|---|---|---|---|
| Demographics | ||||
| Age (y) | 64.3 [55.8–74.6] | 74.6 [63.7–81.4] | 64.8 [56.1–75.2] | <0.0001 |
| Male gender | 1242 (46.9) | 91 (66.4) | 1333 (47.9) | <0.0001 |
| Hispanic | 300 (11.3) | 3 (2.2) | 303 (10.9) | 0.0002 |
| Race | 0.06 | |||
| White | 1662 (62.8) | 100 (73.0) | 1762 (63.3) | |
| Black | 436 (16.5) | 17 (12.4) | 453 (16.3) | |
| Other/Unknown | 548 (20.7) | 20 (14.6) | 568 (20.4) | |
| COPD | 283 (10.7) | 33 (24.1) | 316 (11.4) | <0.0001 |
| Risk Factors | ||||
| BMI (kg/m2) | 29 [25.1–34.7] | 26.4 [23–30.4] | 28.8 [25.1–34.4] | <0.0001 |
| BMI ≥ 30 kg/m2 | 1167 (44.1) | 37 (27.0) | 1204 (43.3) | <0.0001 |
| Hypertension | 2154 (81.4) | 117 (85.4) | 2271 (81.6) | 0.26 |
| Dyslipidemia | 1756 (66.4) | 97 (70.8) | 1853 (66.6) | 0.31 |
| Diabetes | 940 (35.5) | 68 (49.6) | 1008 (36.2) | 0.001 |
| Family history of CAD | 723 (27.3) | 33 (24.1) | 756 (27.2) | 0.43 |
| Tobacco Use | 276 (10.4) | 18 (13.1) | 294 (10.6) | 0.06 |
| Dialysis | 118 (4.5) | 15 (11.0) | 133 (4.8) | 0.003 |
| Medications | ||||
| Aspirin | 1613 (61.0) | 92 (67.2) | 1705 (61.3) | 0.15 |
| B-adrenergic blockers | 1655 (62.6) | 107 (78.1) | 1762 (63.3) | 0.0002 |
| Cholesterol-lowering agents | 1624 (61.4) | 99 (72.3) | 1723 (61.9) | 0.01 |
| Insulin | 407 (15.4) | 36 (26.3) | 443 (15.9) | 0.002 |
| Oral hypoglycemic agents | 274 (10.4) | 10 (7.3) | 284 (10.2) | 0.31 |
| Ca-channel blockers | 594 (22.5) | 27 (19.7) | 621 (22.3) | 0.53 |
| ACE inhibitors | 1040 (39.3) | 64 (46.7) | 1104 (39.7) | 0.09 |
| Nitrates | 324 (12.2) | 32 (23.4) | 356 (12.8) | 0.0005 |
| Diuretics | 948 (35.8) | 77 (56.2) | 1025 (36.8) | <0.0001 |
| Indications | ||||
| Chest Pain | 1268 (47.9) | 38 (27.7) | 1306 (46.9) | <0.0001 |
| Dyspnea | 791 (29.9) | 61 (44.5) | 852 (30.6) | 0.0006 |
| Post-MI | 223 (8.4) | 28 (20.4) | 251 (9.0) | <0.0001 |
| Pre-operative | 380 (14.4) | 28 (20.4) | 408 (14.7) | 0.06 |
| Cardiovascular History | ||||
| Any prior CAD | 1070 (40.4) | 105 (76.6) | 1175 (42.2) | <0.0001 |
| Recent MI (≤30 days) | 279 (10.5) | 37 (27.0) | 316 (11.4) | <0.0001 |
| Remote MI (>30 days) | 462 (17.5) | 49 (35.8) | 511 (18.4) | <0.0001 |
| Prior PCI | 565 (21.4) | 47 (34.3) | 612 (22.0) | 0.0007 |
| Prior CABG | 317 (12.0) | 52 (38.0) | 369 (13.3) | 0.0007 |
| Congestive Heart Failure | 115 (4.4) | 28 (20.4) | 143 (5.1) | <0.0001 |
| Cerebrovascular Disease | 158 (6.0) | 12 (8.8) | 170 (6.1) | 0.2 |
| Peripheral Vascular Disease | 157 (5.9) | 24 (17.5) | 181 (6.5) | <0.0001 |
| Early Revascularization (≤90 days post-PET) | 217 (8.2) | 18 (13.1) | 235 (8.4) | 0.0564 |
| Stress Protocol | <0.0001 | |||
| Adenosine | 205 (7.8) | 11 (8.0) | 216 (7.8) | |
| Dipyridamole | 1280 (48.4) | 96 (70.1) | 1376 (49.4) | |
| Dobutamine | 115 (4.4) | 9 (6.6) | 124 (4.5) | |
| Regadenoson | 1046 (39.5) | 21 (15.3) | 1067 (38.3) | |
| Imaging Parameters | ||||
| Rest LVEF(%) | 59 [49–66] | 39 [27–54] | 58 [48–66] | <0.0001 |
| Stress-induced ↑LVEF | 2060 (77.9) | 87 (63.5) | 2147 (77.2) | 0.0002 |
| Scar + Ischemic Myocardium (%) | 0 [0–10.3] | 16.2 [4.4–35.3] | 0 [0–10.3] | <0.0001 |
| Ischemic Myocardium(%) | 0 [0–4.4] | 4.4 [0–10.3] | 0 [0–5.9] | <0.0001 |
| Global CFR | 1.76 [1.37–2.25] | 1.31 [1.12–1.56] | 1.73 [1.34–2.22] | <0.0001 |
| Stress Global MBF (ml/g/min) | 1.82 [1.25–2.54] | 1.26 [0.92–1.82] | 1.80[1.23–2.52] | <0.0001 |
| Rest Global MBF (ml/g/min) | 1.01 [0.79–1.33] | 1.00[0.75–1.34] | 1.01 [0.78–1.33] | 0.41 |
Continuous variables are presented as median (inter-quartile range). Dichotomous variables are presented as number (%). Patients whose LVEF at stress was greater than that at rest were considered to have positive stress-induced increase in LVEF. BMI = body mass index. ACE = angiotensin converting enzyme. MI = myocardial infarction. CAD = coronary artery disease. PCI = percutaneous coronary intervention. CABG = coronary artery bypass graft. LVEF = left ventricular ejection fraction. CFR = coronary flow reserve. MBF = myocardial blood flow.
The most common indications for testing were evaluation for chest pain, dyspnea, or their combination. Approximately half of all studies were normal by semi-quantitative visual analysis. Scar and ischemia were more common in patients who experienced cardiac mortality. However, scar rather than ischemia accounted for a larger portion of defects among patients who died.
At rest, myocardial blood flow was comparable across tertiles of CFR (1.17, 1.14 and 1.01 mL/min/g for patients in high, intermediate, and low tertiles of CFR, respectively, p<0.0001; Figure 1). During peak stress, there was a significant stepwise decline in myocardial blood flow with decreasing tertiles of CFR (2.61, 1.98, and 1.35 mL/min/g for patients in high, intermediate, and low tertiles of CFR, respectively, p<0.0001), indicating that reduced CFR reflected primarily a reduction in coronary vasodilator function.
Figure 1. Myocardial Blood Flow by CFRTertile.
Box plots of rest and stress blood flow distributions of the population divided into tertiles by coronary flow reserve (CFR). Overall median blood flow for rest and stress are indicated by the dashed horizontal lines. Although the blood flows significantly vary across tertiles at both rest and stress, the differences are more pronounced at stress and are small in magnitude at rest.
Patient Outcomes
Mortality from any cause occurred in 279 (10.3%) patients, of which 137 (49.1%) were due to cardiac causes (Table 2). Three-year cardiac mortality was 8.0%. Compared to patients without cardiac death, those who experienced cardiac death were older, more likely to be male with diabetes, had a lower body mass index, more likely to be referred for dyspnea evaluation, have prior CAD, lower rest LVEF, and larger abnormalities on PET scans (Table 1).
Table 2.
Causes of Death
| Cause of Death | All Patients (n=2783) | Upper CFR Tertile | Middle CFR Tertile | Lower CFR Tertile | p-Value |
|---|---|---|---|---|---|
| Cardiac | 137 (4.9%) | 7 (0.8%) | 37 (4.0%) | 93 (10.0%) | <0.0001 |
| Vascular | 2 (0.1%) | 0 | 0 | 2 (0.2%) | 0.11 |
| Non-hemorrhagic stroke | 9 (0.3%) | 0 | 4 (0.4%) | 5 (0.5%) | 0.06 |
| Other | 115 (4.1%) | 24(2.6%) | 31(3.3%) | 60 (6.5%) | <0.0001 |
| Unknown | 16 (0.6%) | 1 (0.1%) | 8 (0.9%) | 7 (0.8%) | 0.04 |
|
| |||||
| Any Cause | 279 (10.3%) | 32 (3.5%) | 80 (8.0%) | 167 (18.0%) | <0.0001 |
Patients were divided into tertiles of CFR corresponding to <1.5, 1.5–2.0 and >2.0. CFR = coronary flow reserve.
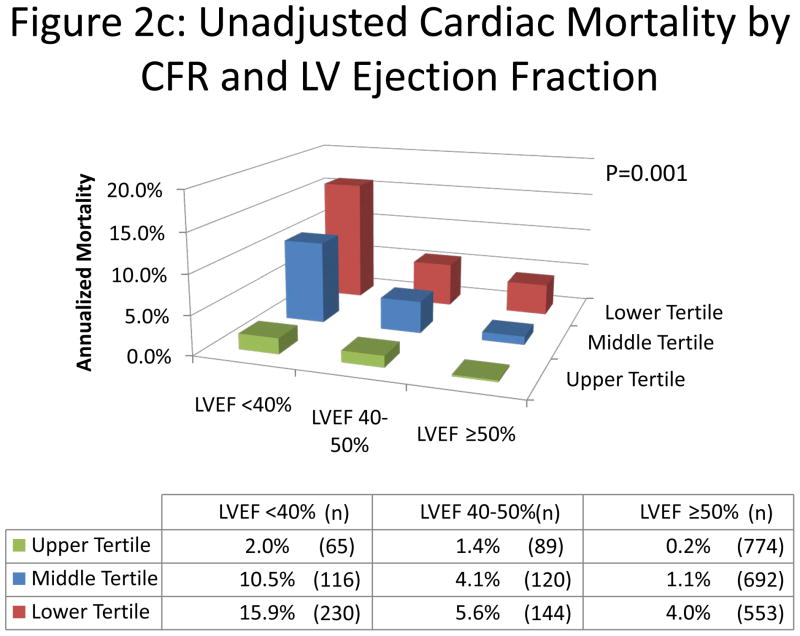
The annual rate of cardiac death increased with increasing myocardial scar and ischemia and decreasing CFR (Figure 2a,b). Importantly, lower CFR consistently identified higher risk patients at every level of myocardial scar/ischemia, including among those with visually normal PET scans. Likewise, in each category of LVEF, increasing tertiles of CFR were associated with a significant decrease in the risk of cardiac mortality (Figure 2c).
Figure 2. Unadjusted Cardiac Mortality.
Unadjusted annualized cardiac mortality by tertiles of CFR and categories of myocardial ischemia and scar (panel A); by categories of myocardial ischemia (panel B); and by tertiles of CFR and categories of left ventricular ejection fraction (panel C). The annual rate of cardiac death increased with increasing summed stress score, decreasing LVEF and CFR. Importantly, lower CFR consistently identified higher risk patients at every level of myocardial scar/ischemia and LVEF, including among those with visually normal PET scans and normal LV function.
Univariate Predictors of Mortality
Compared to the highest tertile of CFR (values >2), the lowest tertile (values <1.5) was associated with a 16-fold increased risk whereas the intermediate tertile was associated a 5.7-fold increased risk for cardiac death. Other significant predictors of increased risk included age, male gender, renal failure requiring dialysis and prior CAD (Figure 3). Chest pain as a reason for testing and obesity were unexpectedly associated with a decreased risk, possibly reflecting confounding due to more aggressive testing and treatment in these groups, although alternative mechanisms have been suggested for similar observations in other cohorts (25). In addition, dyspnea, a decrease in rest LVEF, the absence of stress-induced LVEF augmentation, as well as increasing burden of myocardial scar, ischemia or their combination on semi-quantitative visual analysis were all significantly associated with increased risk.
Figure 3. Univariate Predictors of Cardiac Death.
Univariate predictors of cardiac mortality are shown. Hazard ratios are presented for a one unit increase except for age (increase of 10 years), left ventricular ejection fraction (LVEF; decrease of 10%), extent of myocardial ischemia and scar combined and each separately (increase of 10%). CAD indicates patient reported coronary artery disease, known angiographic coronary stenosis, prior myocardial infarction or history of coronary revascularization. Hx = history of. ASA = aspirin. BMI = body mass index.
Multivariable Survival Analysis and Incremental Prognostic Value
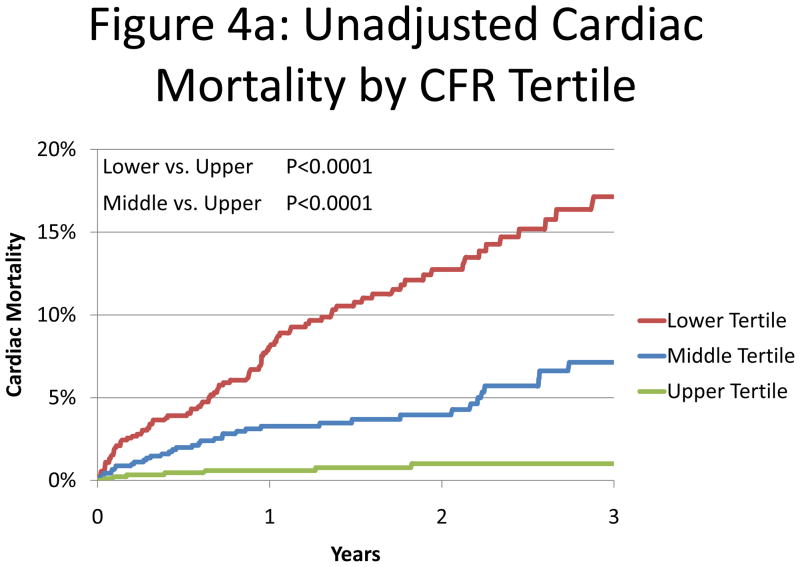
A series of multivariable models were constructed to assess the incremental value of CFR after adjustment for critical covariates known to be associated with increased risk of cardiac mortality (Table 3). Addition of CFR was associated with a significant increase in global χ2 and decrease in Akaike information criterion, indicating improved model fit, and a significant increase in the c-index from 0.82 to 0.84 (p=0.02). Compared to the highest tertile of CFR, the adjusted hazard ratio for cardiac death was 5.6 (95%CI 2.5–12.4, p<0.0001) for the lowest tertile and 3.4 (95%CI 1.5–7.7, p=0.003) for the intermediate tertile (Figure 4).
Table 3.
Multivariable Survival Analysis
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fit Statistic | p-value | Fit Statistic | p-value | Fit Statistic | p-value | Fit Statistic | p-value | Fit Statistic | p-value | |
| Global χ2* | 154.74 | ref | 209.78 | <.0001 | 227.06 | <.0001 | 227.27 | n/s | 253.57 | <.0001 |
| AIC* | 1919.08 | ref | 1866.03 | <.0001 | 1850.76 | <.0001 | 1852.54 | n/s | 1830.24 | 0 |
| Calibration χ2 | 12.71 | 0.18 | 6.2 | 0.72 | 10.33 | 0.32 | 9.18 | 0.42 | 10.97 | 0.28 |
| c-index* | 0.78 (0.74–0.82) | ref | 0.81 (0.77–0.85) | 0.01 | 0.82 (0.78–0.86) | 0.17 | 0.82 (0.78–0.86) | 0.87 | 0.84 (0.8–0.87) | 0.02 |
| Covariate | HR | p-value | HR | p-value | HR | p-value | HR | p-value | HR | p-value |
|
| ||||||||||
| Age (y) | 1.03 (1.01–1.05) | 0.0002 | 1.03 (1.02–1.05) | <.0001 | 1.03 (1.02–1.05) | <.0001 | 1.03 (1.02–1.05) | <.0001 | 1.03 (1.01–1.05) | 0.001 |
| Male Gender | 1.52 (1.05–2.21) | 0.03 | 0.97 (0.66–1.43) | 0.89 | 0.94 (0.64–1.39) | 0.75 | 0.94 (0.64–1.38) | 0.75 | 1.00 (0.68–1.48) | 1.00 |
| Hypertension | 1.02 (0.62–1.68) | 0.94 | 1.06 (0.64–1.74) | 0.83 | 1.15 (0.70–1.89) | 0.58 | 1.15 (0.70–1.88) | 0.59 | 1.04 (0.63–1.71) | 0.88 |
| Dyslipidemia | 0.88 (0.60–1.30) | 0.53 | 0.90 (0.61–1.33) | 0.59 | 0.80 (0.54–1.19) | 0.28 | 0.80 (0.54–1.19) | 0.28 | 0.84 (0.57–1.25) | 0.40 |
| Diabetes | 1.73 (1.22–2.45) | 0.002 | 1.51 (1.06–2.15) | 0.02 | 1.53 (1.07–2.17) | 0.02 | 1.52 (1.07–2.17) | 0.02 | 1.39 (0.97–1.98) | 0.07 |
| Family Hx CAD | 0.84 (0.56–1.25) | 0.39 | 0.85 (0.57–1.26) | 0.41 | 0.86 (0.58–1.29) | 0.47 | 0.87 (0.58–1.30) | 0.49 | 0.86 (0.58–1.29) | 0.47 |
| Tobacco Use | 1.43 (0.85–2.41) | 0.17 | 1.44 (0.86–2.43) | 0.17 | 1.48 (0.88–2.49) | 0.14 | 1.47 (0.88–2.48) | 0.14 | 1.56 (0.92–2.64) | 0.10 |
| CAD | 3.02 (1.95–4.67) | <.0001 | 2.26 (1.45–3.55) | 0.0004 | 1.81 (1.13–2.88) | 0.01 | 1.80 (1.13–2.87) | 0.01 | 1.60 (1.01–2.55) | 0.05 |
| BMI (kg/m2) | 0.94 (0.91–0.97) | 0.0003 | 0.96 (0.94–1) | 0.03 | 0.96 (0.93–0.99) | 0.02 | 0.96 (0.93–0.99) | 0.02 | 0.96 (0.93–0.99) | 0.02 |
| Chest Pain | 0.55 (0.38–0.81) | 0.003 | 0.7 (0.47–1.03) | 0.07 | 0.71 (0.48–1.05) | 0.08 | 0.71 (0.48–1.05) | 0.08 | 0.79 (0.54–1.17) | 0.24 |
| Dyspnea Early | 1.69 (1.2–2.37) | 0.0025 | 1.37 (0.97–1.93) | 0.07 | 1.46 (1.03–2.06) | 0.03 | 1.45 (1.03–2.05) | 0.03 | 1.45 (1.03–2.05) | 0.03 |
| Revascularization | 0.97 (0.58–1.62) | 0.91 | 0.88 (0.53–1.46) | 0.62 | 0.62 (0.37–1.07) | 0.08 | 0.62 (0.36–1.06) | 0.08 | 0.57 (0.33–0.97) | 0.04 |
| Rest LVEF (%) | 0.96 (0.95–0.97) | <.0001 | 0.97 (0.96–0.98) | <.0001 | 0.97 (0.95–0.98) | <.0001 | 0.97 (0.96–0.98) | <.0001 | ||
| Ischemia+Scar (%) | 1.03 (1.01–1.04) | <.0001 | 1.03 (1.01–1.04) | <.0001 | 1.02 (1.01–1.03) | 0.0005 | ||||
| LVEF Reserve | 0.92 (0.64–1.32) | 0.64 | 1.03 (0.72–1.48) | 0.86 | ||||||
| CFR Lower Tertile | 5.55 (2.49–12.4) | <.0001 | ||||||||
| CFR Middle Tertile | 3.40 (1.50–7.72) | 0.003 | ||||||||
Summary of characteristics of five nested models.
P-values for fit statistics are for comparison of each model to the next simpler model (e.g. model 5 s model 4). C-indices and Hosmer-Lemeshow statistics are calculated for 1.5-year event data. Global χ2 = likelihood ratio chi-squared statistic for the entire model. AIC = Akaike Information Criterion. Calibration χ2 = Nam-D’Agostino goodness of fit statistic. Hx = history of. CAD = coronary artery disease. BMI = body mass index. LVEF = left ventricular ejection fraction. CFR = coronary flow reserve.
Figure 4. Cardiac Mortality.
Cumulative incidence of cardiac mortality for tertiles of coronary flow reserve presented in Kaplan-Meier format (panel A) and after adjustment (18) for age, sex, body mass index, hypertension, dyslipidemia, diabetes mellitus, family history of coronary artery disease (CAD), tobacco use, prior CAD, chest pain, dyspnea, early revascularization, rest left ventricular ejection fraction (LVEF), summed stress score and LVEF reserve (panel B) showing a significant association between CFR and cardiac mortality. HR = hazard ratio.
In contrast, replacement of CFR by peak stress myocardial blood flow in a model containing clinical covariates, rest LVEF, the combined extent of myocardial scar and ischemia, and stress-induced LVEF augmentation resulted in a more modest increase in the global χ2 from 227.3 to 230.6 (p=0.07). The adjusted hazard ratio for cardiac death was 1.1 (95%CI 1.0–1.3, p=0.07).
Risk Reclassification
Addition of CFR estimates to the model resulted in the reclassification of 15%, 48%, and 12% of patients at low, intermediate, and high cardiac risk, respectively, based on the pre-CFR model (Table 3, Model 4) (Figure 5). The net reclassification improvement (NRI) was 0.098 (95%CI 0.025–0.180) across clinically meaningful risk categories of <1, 1–3, and ≥3% annual rate of cardiac death (Table 4). The continuous NRI was 43.5% (95%CI 23.9–62.2). The benefit of CFR on risk reclassification was greatest among patients with an intermediate pre-CFR risk, in whom addition of CFR to risk estimation downgraded risk in 32% (0% observed 3-year cardiac mortality) and upgraded it in 16% (13.6% observed 3-year cardiac mortality). Overall 34.9% of these intermediate pre-CFR risk patients were correctly reclassified. In these patients, the NRI across risk categories of <1%, 1–3 and ≥3% annual rate of cardiac death was 0.484 (95%CI 0.262–0.731). Among patients considered low and high risk without CFR data the NRI was 0.478 (95%CI −0.157–0.933) and 0.112 (95%CI 0.061–0.162), respectively.
Figure 5. Risk Reclassification.
Illustration of risk reclassification by addition of coronary flow reserve (CFR) to a model containing clinical risk factors, left ventricular ejection fraction (LVEF), LVEF reserve and combined extent of myocardial scar and ischemia. The upper horizontal bar graph represents the distribution of risk across categories of <1 (green), 1–3 (blue) and >3% (red) per year risk of cardiac death as estimated by a model containing clinical risk factors, rest LVEF, LVEF reserve and the combination of myocardial scar and ischemia (Model 4, Table 3). The pie graphs represent the proportions of patients in each pre-CFR category reassigned to each risk category after the addition of CFR to the risk model(Model 5, Table 3). The vertical bar charts at the bottom represent the annualized rates of cardiac mortality in each of the post-CFR risk categories.
Table 4.
Risk Reclassification
| Model without CFR | Model with CFR | |||
|---|---|---|---|---|
|
| ||||
| <1% Annual Risk | 1–3% Annual Risk | >3% Annual Risk | Total | |
| Patients with Cardiac Death | ||||
| <1% Annual Risk | 2.2 (38.4) | 3.6 (61.6) | 0 (0) | 5.8 |
| 1–3% Annual Risk | 1 (3.4) | 19.6 (63.9) | 10 (32.8) | 30.7 |
| >3% Annual Risk | 0 (0) | 4.7 (4.4) | 101 (95.6) | 105.6 |
| Total | 3.3 | 27.8 | 27.8 | 142.1 |
| Patients without Cardiac Death | ||||
| <1% Annual Risk | 924.8 (84.5) | 169.4 (15.5) | 0 (0) | 1094.2 |
| 1–3% Annual Risk | 303 (34.9) | 425.4 (49) | 139 (16) | 867.3 |
| >3% Annual Risk | 22 (3.2) | 84.3 (12.4) | 573 (84.3) | 679.4 |
| Total | 1249.7 | 679.2 | 712 | 2640.9 |
Reclassification table for censored data using method of Steyerberg and Pencina (37) from 1.5 year event data. Parentheses indicate percentages of each pre-test category reclassified to each post-stress category.
Subgroup Analysis & Effect Modification
The impact of CFR on the risk of cardiac death was consistent across 29 of 33 subgroups evaluated (Figure 6), suggesting the broad potential impact of this measure in clinical practice. Interaction terms between CFR and age, gender and diabetes were non-significant, underscoring the consistent effect of CFR on cardiac mortality.
Figure 6. Subgroup Analysis.
Analysis of the hazard ratio for cardiac death across patient subgroups between the upper and lower tertiles of coronary flow reserve (CFR). The graph demonstrates the consistent effect of impaired CFR on cardiac mortality. Hazard ratios were computed without adjustment for other covariates. The left vertical dashed line indicates unity. The right vertical dashed line corresponds to the point estimate for the hazard ratio in the entire population with the grey bar indicating the 95% confidence interval for this estimate. In three subgroups, each containing 20% or fewer of patients in the study (i.e. normotensive individuals, those without stress-induced left ventricular ejection fraction (LVEF) augmentation and those who underwent early revascularization), the Cox model did not generate meaningful estimates for the hazard ratios (indicated by ★). Hx = history of. CAD = coronary artery disease. LVEF = left ventricular ejection fraction.
Transient ischemic dilation (TID) has been associated with poor prognosis and severe disease on coronary angiography. Among 1883 patients (67.7%) for whom TID was available, incorporation of TID into a model containing clinical risk factors, LVEF, extent of ischemia and scar and stress-induced LVEF augmentation was not associated with an improved model fit, increase in c-index or positive NRI. However, addition of CFR to the comprehensive model containing TID continued to improve model fit (p<0.0001), the c-index (0.84 vs. 0.86, p=0.05) and was associated with favorable risk reclassification (NRI=0.068).
In order to assess the relative impact of epicardial stenosis and microvascular dysfunction on impaired CFR, we correlated CFR findings to angiographic findings in the 41 of 1444 patients (2.8%) with normal PET scans on semi-quantitative visual assessment and no history of coronary artery bypass graft surgery who underwent coronary angiography within 90 days of their PET scan and did not have intervening myocardial infarction or revascularization. Nineteen of the 41 patients (46%) had non-obstructive disease by coronary angiography (<70% stenosis). Likewise, 12 of 25 patients with abnormal CFR (low or intermediate CFR tertiles) had no obstructive epicardial stenosis by angiography.
All-Cause Mortality
Analyses were repeated using mortality from any cause as a secondary outcome and the results remained significant, although somewhat attenuated. After correction for a wide array of risk factors, CFR remained a significant predictor of mortality with the lowest tertile of CFR associated with a hazard ratio of 2.82 (1.87–4.26, p<0.0001) compared to the highest tertile. Similarly, the middle tertile of CFR carried a hazard ratio of 1.79 (1.17–2.72, p=0.007) compared to the highest tertile. The continuous NRI for all-cause mortality was 0.408 (95%CI 0.272–0.537). Using thresholds of 2 and 6% annually for mortality from any cause, the NRI was 0.058 (95%CI 0.006–0.111).
Discussion
These data from a large patient cohort demonstrate the incremental prognostic value of non-invasive measures of coronary flow reserve over the combination of comprehensive clinical assessment, LV systolic function and semi-quantitative measures of myocardial ischemia and scar for identification of patients at risk of cardiac mortality. Indeed, patients with the highest tertile of CFR, indicating preserved vasodilator function, had an extremely low rate of cardiac mortality (<0.5%/year). Conversely, intermediate and severely reduced CFR were associated with an adjusted HR for cardiac mortality of 3.4 and 5.6, respectively. The addition of CFR to clinical variables, rest LVEF, LVEF reserve and the extent of myocardial scar and ischemia resulted in the correct reclassification of approximately one third of all intermediate risk patients.
Prior Studies
Prior studies have evaluated the diagnostic value of CFR compared to coronary angiography and fractional flow reserve, especially with respect to detection of multi-vessel CAD (26,27). More recent studies have examined the prognostic value of non-invasive estimates of CFR in less than 700 patients with limited numbers of outcome events (28–31). Although these studies suggested an association between impaired CFR and adverse cardiovascular events (including hospitalization for cardiac causes and late revascularization), they were underpowered to demonstrate an association between impaired CFR and cardiac mortality. The demonstration of incremental prognostic value was also limited by incomplete adjustment for important markers of clinical risk (28,30) and measures of LV systolic function (29,30).
Our study included a large number of patients with known or suspected CAD encompassing a wide spectrum of clinical risk, and was sufficiently powered to adjust for a broad array of clinical and imaging risk markers, and demonstrate an association between decreased CFR and risk of cardiac mortality. More importantly, the inclusion of CFR in risk prediction models resulted in the correct reclassification of risk in a substantial proportion of patients, including 35% of intermediate risk patients (NRI=60.3%). Improved risk estimates obtained with CFR may impact patient management by triggering more targeted management of risk factors and/or referral for revascularization. These quantitative measures of CFR can be obtained at no additional cost, imaging time or radiation exposure.
Mechanistic Link Between Abnormal Coronary Circulatory Function and Cardiac Mortality
The exact mechanism relating non-invasive measures of coronary flow reserve to increased cardiac mortality cannot be determined from this study. However, several potential mechanisms could explain our findings. The observation that the reduction of CFR was primarily due to progressive reductions in peak stress myocardial blood flow suggests a primary abnormality in coronary vasodilator function.
As our study cohort included patients with both known and suspected CAD, it is likely that the observed abnormalities in coronary function integrate the fluid dynamic effects of epicardial stenosis with those of coronary risk factors and atherosclerosis on microcirculatory function. However, the fact that CFR improved risk stratification not only in the setting of demonstrable ischemia and/or scar as identified by conventional methods, but also among those patients with visually normal PET scans suggests that diffuse atherosclerosis and vascular remodeling, microvascular dysfunction, or both may also partially account for the excess cardiac mortality among those with impaired CFR. Indeed, the fact that approximately half the patients with visually normal PET scans who underwent coronary angiography had non-obstructive CAD, supports the notion that the observed excess coronary risk may be related in part to microvascular dysfunction. Such abnormalities have been described in early stages of atherogenesis in patients without overt CAD or angiographically normal coronary arteries (32), and have been linked to disease progression (10) and adverse cardiovascular events including sudden cardiac death, myocardial infarction, congestive heart failure, and coronary revascularization (9,11,33,34).
Limitations of our analyses merit consideration. The current study is a single-center, non-randomized, observational study and carries all of the inherent limitations of that study design. As such, it is likely that some amount of residual confounding remains, despite adjustment for a wide array of clinically relevant covariates. On the other hand, compared to data derived from patients selectively enrolled in a randomized trial, these data, with very limited exclusion criteria, may be more representative of patients seen in routine clinical practice.
Another side-effect of our broad inclusion criteria is that we may understate the value of parameters which have been previously shown to be markers of poor prognosis in more restricted subgroups (35). The breadth of the inclusion criteria of this study underscores the generalizability of the findings.
The demonstrated improvement in the c-index is only moderate in size, especially when compared to the more substantial impact on risk reclassification. However, this reflects the relative insensitivity of the c-index (36) rather than limited clinical value of CFR measures.
Peak stress blood flow on its own was a less powerful predictor of outcomes than CFR, possibly because CFR taken as the ratio of peak stress and rest blood flows may better isolate vasodilator capacity and reduce systematic errors in measurement.
Finally, we are not able to evaluate the downstream impact of CFR on patient management decisions as referring clinicians were not informed of CFR results in clinical reports. However, this reduces bias in estimates of CFR effect size introduced by subsequent treatment decisions based on this result.
Conclusion
Non-invasive assessment of coronary vasodilator function provides incremental risk stratification beyond routine measures of clinical risk, including estimates of LV systolic function and the extent and severity of myocardial ischemia and scar, and results in a meaningful incremental risk reclassification of patients with known or suspected CAD.
Qyantitative estimates of myocardial blood flow and coronary flow reserve (CFR) integrate the fluid dynamic effects of epicardial atherosclerosis and microvascular function. The present study demonstrates that CFR improves stratification of risk of cardiac and all-cause mortality beyond clinical risk factors, left ventricular ejection fraction (LVEF), extent of myocardial ischemia and scar, and stress-induced LVEF augmentation. Patients with CFR <1.5 were at 5.6-fold increased risk of cardiac mortality compared to those with CFR >2.0. Among patients whose clinical risk factors, LVEF and stress imaging findings placed them at intermediate risk of cardiac death (1–3% per year), 35% were reclassified as either high risk (>3% cardiac mortality per year) or low risk (<1% cardiac mortality per year). These findings demonstrate that incorporation of coronary vasodilator function assessment into stress testing by quantification of CFR improves risk stratification in patients with known or suspected coronary artery disease.
Acknowledgments
Funding Sources
The study was funded in part by grants from the National Institutes of Health (RC1 HL101060-01, T32 HL094301-01A1, and K23HL092299-03).
Footnotes
DISCLOSURES
Dr. Di Carli receives research grant support from Toshiba. Dr. Dorbala receives research grant support from Astellas.
References
- 1.Gibbons RJ, Chatterjee K, Daley J, Douglas JS, Fihn SD, Gardin JM, Grunwald MA, Levy D, Lytle BW, O’Rourke RA, Schafer WP, Williams SV, Ritchie JL, Cheitlin MD, Eagle KA, Gardner TJ, Garson A, Jr, Russell RO, Ryan TJ, Smith SC., Jr ACC/AHA/ACP-ASIM guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients With Chronic Stable Angina) J Am Coll Cardiol. 1999;33:2092–2197. doi: 10.1016/s0735-1097(99)00150-3. [DOI] [PubMed] [Google Scholar]
- 2.Giri S, Shaw LJ, Murthy DR, Travin MI, Miller DD, Hachamovitch R, Borges-Neto S, Berman DS, Waters DD, Heller GV. Impact of Diabetes on the Risk Stratification Using Stress Single-Photon Emission Computed Tomography Myocardial Perfusion Imaging in Patients With Symptoms Suggestive of Coronary Artery Disease. Circulation. 2002;105:32–40. doi: 10.1161/hc5001.100528. [DOI] [PubMed] [Google Scholar]
- 3.Al-Mallah MH, Hachamovitch R, Dorbala S, Di Carli MF. Incremental Prognostic Value of Myocardial Perfusion Imaging in Patients Referred to Stress Single-Photon Emission Computed Tomography With Renal Dysfunction. Circulation: Cardiovascular Imaging. 2009;2:429–436. doi: 10.1161/CIRCIMAGING.108.831164. [DOI] [PubMed] [Google Scholar]
- 4.Quyyumi AA, Dakak N, Andrews NP, Husain S, Arora S, Gilligan DM, Panza JA, Cannon RO. Nitric oxide activity in the human coronary circulation. Impact of risk factors for coronary atherosclerosis. J Clin Invest. 1995;95:1747–1755. doi: 10.1172/JCI117852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox DA, Vita JA, Treasure CB, Fish RD, Alexander RW, Ganz P, Selwyn AP. Atherosclerosis impairs flow-mediated dilation of coronary arteries in humans. Circulation. 1989;80:458–465. doi: 10.1161/01.cir.80.3.458. [DOI] [PubMed] [Google Scholar]
- 6.Laine H, Raitakari OT, Niinikoski H, Pitkänen O-P, Iida H, Viikari J, Nuutila P, Knuuti J. Early impairment of coronary flow reserve in young men with borderline hypertension. Journal of the American College of Cardiology. 1998;32:147–153. doi: 10.1016/s0735-1097(98)00222-8. [DOI] [PubMed] [Google Scholar]
- 7.Yokoyama I, Momomura S-ichi, Ohtake T, Yonekura K, Nishikawa J, Sasaki Y, Omata M. Reduced Myocardial Flow Reserve in Non-Insulin-Dependent Diabetes Mellitus. Journal of the American College of Cardiology. 1997;30:1472–1477. doi: 10.1016/s0735-1097(97)00327-6. [DOI] [PubMed] [Google Scholar]
- 8.Iwado Y, Yoshinaga K, Furuyama H, Ito Y, Noriyasu K, Katoh C, Kuge Y, Tsukamoto E, Tamaki N. Decreased endothelium-dependent coronary vasomotion in healthy young smokers. Eur J Nucl Med Mol Imaging. 2002;29:984–990. doi: 10.1007/s00259-002-0818-1. [DOI] [PubMed] [Google Scholar]
- 9.Halcox JPJ, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KRA, Quyyumi AA. Prognostic Value of Coronary Vascular Endothelial Dysfunction. Circulation. 2002;106:653–658. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 10.Schachinger V, Britten MB, Zeiher AM. Prognostic Impact of Coronary Vasodilator Dysfunction on Adverse Long-Term Outcome of Coronary Heart Disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 11.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Lerman A. Long-Term Follow-Up of Patients With Mild Coronary Artery Disease and Endothelial Dysfunction. Circulation. 2000;101:948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 12.Tonino PAL, De Bruyne B, Pijls NHJ, Siebert U, Ikeno F, van ’t Veer M, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF. Fractional Flow Reserve versus Angiography for Guiding Percutaneous Coronary Intervention. New England Journal of Medicine. 2009;360:213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 13.El Fakhri G, Sitek A, Guerin B, Kijewski MF, Di Carli MF, Moore SC. Quantitative Dynamic Cardiac 82Rb PET Using Generalized Factor and Compartment Analyses. J Nucl Med. 2005;46:1264–1271. [PubMed] [Google Scholar]
- 14.Senthamizhchelvan S, Bravo PE, Lodge MA, Merrill J, Bengel FM, Sgouros G. Radiation Dosimetry of 82Rb in Humans Under Pharmacologic Stress. J Nucl Med. 2011;52:485–491. doi: 10.2967/jnumed.110.083477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. Standardized Myocardial Segmentation and Nomenclature for Tomographic Imaging of the Heart: A Statement for Healthcare Professionals From the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 16.El Fakhri G, Kardan A, Sitek A, Dorbala S, Abi-Hatem N, Lahoud Y, Fischman A, Coughlan M, Yasuda T, Di Carli MF. Reproducibility and Accuracy of Quantitative Myocardial Blood Flow Assessment with 82Rb PET: Comparison with 13N-Ammonia PET. J Nucl Med. 2009;50:1062–1071. doi: 10.2967/jnumed.104.007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sitek A, Gullberg GT, Huesman RH. Correction for ambiguous solutions in factor analysis using a penalized least squares objective. Medical Imaging, IEEE Transactions on. 2002;21:216–225. doi: 10.1109/42.996340. [DOI] [PubMed] [Google Scholar]
- 18.Nieto FJ, Coresh J. Adjusting Survival Curves for Confounders: A Review and a New Method. American Journal of Epidemiology. 1996;143:1059–1068. doi: 10.1093/oxfordjournals.aje.a008670. [DOI] [PubMed] [Google Scholar]
- 19.Hachamovitch R, Di Carli MF. Methods and Limitations of Assessing New Noninvasive Tests: Part II: Outcomes-Based Validation and Reliability Assessment of Noninvasive Testing. Circulation. 2008;117:2793–2801. doi: 10.1161/CIRCULATIONAHA.107.714006. [DOI] [PubMed] [Google Scholar]
- 20.Hosmer DW, Hosmer T, Le Cessie S, Lemeshow S. A comparison of goodness-of-fit tests for the logistic regression model. Stat Med. 1997;16:965–980. doi: 10.1002/(sici)1097-0258(19970515)16:9<965::aid-sim509>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 21.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Statist Med. 2004;23:2109–2123. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 22.Antolini L, Nam B-H, D’Agostino RB. Inference on Correlated Discrimination Measures in Survival Analysis: A Nonparametric Approach. Communications in Statistics: Theory & Methods. 2004;33:2117–2135. [Google Scholar]
- 23.Pencina MJ, D’ Agostino RB, Sr, D’ Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Statistics in Medicine. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 24.Pryor DB, Shaw L, McCants CB, Lee KL, Mark DB, Harrell FE, Muhlbaier LH, Califf RM. Value of the History and Physical in Identifying Patients at Increased Risk for Coronary Artery Disease. Annals of Internal Medicine. 1993;118:81–90. doi: 10.7326/0003-4819-118-2-199301150-00001. [DOI] [PubMed] [Google Scholar]
- 25.Romero-Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Allison TG, Mookadam F, Lopez-Jimenez F. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. The Lancet. 2006;368:666–678. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 26.Kajander S, Joutsiniemi E, Saraste M, Pietila M, Ukkonen H, Saraste A, Sipila HT, Teras M, Maki M, Airaksinen J, Hartiala J, Knuuti J. Cardiac Positron Emission Tomography/Computed Tomography Imaging Accurately Detects Anatomically and Functionally Significant Coronary Artery Disease. Circulation. 2010;122:603–613. doi: 10.1161/CIRCULATIONAHA.109.915009. [DOI] [PubMed] [Google Scholar]
- 27.Parkash R, deKemp RA, Ruddy TD, Kitsikis A, Hart R, Beauchesne L, Beauschene L, Williams K, Davies RA, Labinaz M, Beanlands RSB. Potential utility of rubidium 82 PET quantification in patients with 3-vessel coronary artery disease. J Nucl Cardiol. 2004;11:440–449. doi: 10.1016/j.nuclcard.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Tio RA, Dabeshlim A, Siebelink H-MJ, de Sutter J, Hillege HL, Zeebregts CJ, Dierckx RAJO, van Veldhuisen DJ, Zijlstra F, Slart RHJA. Comparison Between the Prognostic Value of Left Ventricular Function and Myocardial Perfusion Reserve in Patients with Ischemic Heart Disease. J Nucl Med. 2009;50:214–219. doi: 10.2967/jnumed.108.054395. [DOI] [PubMed] [Google Scholar]
- 29.Herzog BA, Husmann L, Valenta I, Gaemperli O, Siegrist PT, Tay FM, Burkhard N, Wyss CA, Kaufmann PA. Long-Term Prognostic Value of 13N-Ammonia Myocardial Perfusion Positron Emission Tomography: Added Value of Coronary Flow Reserve. Journal of the American College of Cardiology. 2009;54:150–156. doi: 10.1016/j.jacc.2009.02.069. [DOI] [PubMed] [Google Scholar]
- 30.Fukushima K, Javadi MS, Higuchi T, Lautamaki R, Merrill J, Nekolla SG, Bengel FM. Prediction of Short-Term Cardiovascular Events Using Quantification of Global Myocardial Flow Reserve in Patients Referred for Clinical 82Rb PET Perfusion Imaging. J Nucl Med. 2011;52:726–732. doi: 10.2967/jnumed.110.081828. [DOI] [PubMed] [Google Scholar]
- 31.Ziadi MC, deKemp RA, Williams KA, Guo A, Chow BJW, Renaud JM, Ruddy TD, Sarveswaran N, Tee RE, Beanlands RSB. Impaired Myocardial Flow Reserve on Rubidium-82 Positron Emission Tomography Imaging Predicts Adverse Outcomes in Patients Assessed for Myocardial Ischemia. J Am Coll Cardiol. 2011;58:740–748. doi: 10.1016/j.jacc.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 32.Zeiher A, Drexler H, Wollschlager H, Just H. Endothelial dysfunction of the coronary microvasculature is associated with coronary blood flow regulation in patients with early atherosclerosis. Circulation. 1991;84:1984–1992. doi: 10.1161/01.cir.84.5.1984. [DOI] [PubMed] [Google Scholar]
- 33.von Mering GO, Arant CB, Wessel TR, McGorray SP, Bairey Merz CN, Sharaf BL, Smith KM, Olson MB, Johnson BD, Sopko G, Handberg E, Pepine CJ, Kerensky RA. Abnormal Coronary Vasomotion as a Prognostic Indicator of Cardiovascular Events in Women: Results From the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109:722–725. doi: 10.1161/01.CIR.0000115525.92645.16. [DOI] [PubMed] [Google Scholar]
- 34.Cecchi F, Olivotto I, Gistri R, Lorenzoni R, Chiriatti G, Camici PG. Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N Engl J Med. 2003;349:1027–1035. doi: 10.1056/NEJMoa025050. [DOI] [PubMed] [Google Scholar]
- 35.Dorbala S, Hachamovitch R, Curillova Z, Thomas D, Vangala D, Kwong RY, Di Carli MF. Incremental Prognostic Value of Gated Rb-82 Positron Emission Tomography Myocardial Perfusion Imaging Over Clinical Variables and Rest LVEF. JACC: Cardiovascular Imaging. 2009;2:846–854. doi: 10.1016/j.jcmg.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cook NR. Use and Misuse of the Receiver Operating Characteristic Curve in Risk Prediction. Circulation. 2007;115:928–935. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
- 37.Steyerberg EW, Pencina MJ. Reclassification Calculations for Persons With Incomplete Follow-up. Annals of Internal Medicine. 2010;152:195–196. doi: 10.7326/0003-4819-152-3-201002020-00019. [DOI] [PubMed] [Google Scholar]