Abstract
A large body of research has demonstrated that affective disorders are characterized by attentional biases for emotional stimuli. However, this research relies heavily on manual reaction time (RT) measures that cannot fully delineate the time course and components of attentional bias. Eye tracking technology, which allows relatively direct and continuous measurement of overt visual attention, may provide an important supplement to RT measures. This article reviews eye tracking research on anxiety and depression, evaluating the experimental paradigms and eye movement indicators used to study attentional biases. Also included is a meta-analysis of extant eye tracking research (33 experiments; N = 1579) on both anxiety and depression. Relative to controls, anxious individuals showed increased vigilance for threat during free viewing and visual search, and showed difficulty disengaging from threat in visual search tasks, but not during free viewing. In contrast, depressed individuals were not characterized by vigilance for threat during free viewing, but were characterized by reduced orienting to positive stimuli, as well as reduced maintenance of gaze on positive stimuli and increased maintenance of gaze on dysphoric stimuli. Implications of these findings for theoretical accounts of attentional bias in anxiety and depression are discussed, and avenues for future research using eye-tracking technology are outlined.
Keywords: eye movements, Eye Tracking, Attention, Bias, Anxiety, Depression
Overview
Dating back to the spread of psychology’s “cognitive revolution” to psychotherapy, attentional biases for emotional stimuli have been a key mechanism in theoretical accounts of affective disorders (e.g., Beck, 1976; Williams, Watts, MacLeod, & Mathews, 1988). Several experimental paradigms from cognitive psychology have been adapted to test these theories, resulting in a large literature documenting an attentional bias for threat in anxiety disorders (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoorn, 2007), and a smaller literature suggesting an attentional bias for dysphoric stimuli and possible neglect of positive stimuli in depression (Peckham, McHugh, & Otto, 2010). More recently, cognitive paradigms originally used to demonstrate information-processing biases have been further modified to treat these biases, and some studies have found that attenuating bias toward threatening stimuli leads to lasting symptom relief in anxious individuals (see Hallion & Ruscio, 2011). There is also evidence that training attention away from dysphoric stimuli may alleviate depression (Wells & Beevers, 2010). Thus, in addition to being pervasive in affective disorders, abnormal processing of emotional stimuli appears to be implicated in the etiology and maintenance of some these disorders, in line with early cognitive accounts (Beck, 1976).
Although there is substantial evidence that anxiety and depression are characterized by attentional biases, the nature of these biases is not completely understood. In research on anxiety disorders, it is still unclear to what extent increased processing of threat reflects facilitated orienting toward threat versus difficulty disengaging attention from threat (Weierich, Treat, & Hollingworth, 2008). A smaller number of studies utilizing longer stimulus exposures raise questions about whether anxiety-related biases are always “for” or “toward” threat; at later processing stages, rapid disengagement or sustained attention away from threat have been observed in anxious individuals (e.g., Koster, Verschuere, Crombez, & Van Damme, 2005). In research on depression, it has been hypothesized that an attentional bias for dysphoric stimuli operates at later, more voluntary stages of processing than the attentional bias for threat in anxiety (Mogg & Bradley, 2005); however, there is a paucity of research examining the time course of attentional bias in depression (Peckham et al., 2010), and the components involved in the biases related to dysphoric and positive stimuli remain unclear. As noted in recent reviews, delineating the time course and components of attentional bias is not only essential to advancing theoretical models (Bar-Haim et al., 2007; Cisler, Bacon, & Williams, 2007), it is also crucial for understanding and improving attention modification procedures (Cisler & Koster, 2010). If attention modification procedures indeed work by retraining attention in an antagonistic manner, such that biases are “undone” or “reversed,” then clarifying the time course and components of biases is necessary for specifying treatment targets.
As noted in recent reviews (Bar-Haim et al., 2007; Weierich et al., 2008), further insight into the time course and components of attentional bias may require a broader set of tools for measuring attention. The extensive literature on attentional biases for emotional stimuli in affective disorders is composed mostly of studies using reaction time (RT) measures of attention (emotional Stroop task; Williams, Mathews, & MacLeod, 1996; modified dot probe; MacLeod, Mathews, & Tata, 1986; emotional spatial cueing task; Fox, Russo, Bowles, & Dutton, 2001; see Weierich et al., 2008 for a review). These cost-effective measures have been critical in advancing the study of attentional biases for threat. Further, the application of RT measures to studying attentional biases has been marked by steady innovation, as seen in the refinement of classic paradigms, as well as the integration of novel tasks from cognitive science (Weierich et al., 2008). However, some limitations of RT measures are inherent to using key presses as indices of attention, and thus cannot be surpassed without appealing to additional methodologies. For example, the distal relation between key presses and attention leaves RT measures vulnerable to the confounding effect of emotional information on mediating processes, such as response execution. Likewise, the “snapshot” nature of RT measures (i.e., their restriction to a single time point within a trial) necessary imposes limits on the effectiveness and efficiency with which dynamic attentional processes can be described.
To see beyond the snapshots provided by RT measures of attention, some researchers have turned to eye tracking technology. Eye tracking systems, which sample gaze direction at rates between 60 and 2000 Hz, provide a continuous measure of attentional selection performed via eye movements (EMs; “overt attention”). In between EMs, further selection can be accomplished with “covert” attention, shifts in acuity that occur without reorienting gaze. However, in naturalistic viewing, EMs are the primary means of attentional selection, with covert attention largely relegated to guiding EMs (Hayhoe & Ballard, 2005; Findlay & Gilchrist, 2003). Yet due to the dominance of the passive vision paradigm, EMs have been somewhat disregarded in basic research on visual attention (Pashler, 1998), and consequently, in translational research on attentional biases for emotional stimuli (e.g., Fox et al., 2001). Indeed, the term visual attention has come to denote covert as opposed to overt attention, reflecting the tendency to treat EMs as peripheral, indirect events in visual selection (Findlay & Gilchrist, 2003). Likewise, in the literature on attentional biases in affective disorders, more concerns have been raised regarding how well EMs index covert attention, rather than how well covert attention generalizes to actual viewing behavior. When considering some of the hypothesized proximal effects of attentional bias, such as elevated state anxiety from increased detection of threat (Mogg & Bradley, 1998), or task interference from difficulty disengaging attention from negative stimuli (Cisler & Koster, 2010), it seems very unlikely that either could result from a covert visual bias alone. Given the importance of EMs in selecting stimuli and guiding action in everyday life (Hayhoe & Ballard, 2005), overt attention may be a necessary mediator of the effects of covert visual biases in anxiety and depression.
In light of eye tracking’s potential value for research on attentional biases in anxiety and depression, an integrative methodological and empirical review may guide future research along these lines. Although this growing literature was noted in a narrative review of clinical eye tracking research (Toh, Rossell, & Castle, 2011), a deeper, more rigorous analysis is needed. The present article reviews the application of eye tracking methodology in the study of attentional biases for threat in anxiety and depression, focusing on the EM indicators and experimental paradigms used to test theoretical accounts of attentional bias, as well as the added value of eye tracking relative to RT measures of attention. In addition, a quantitative meta-analysis was conducted to assess the implications of extant eye tracking research for these theories, and to guide future research in this promising area of investigation.
Theoretical accounts of attentional bias in affective disorders
To understand the application of eye tracking to research on affective disorders, it is necessary to consider the theoretical accounts of attentional bias that guide this research. In this section, we review models of attentional bias in anxiety and depression, and the theoretical issues most amenable to eye tracking methodology.
Anxiety
Although there is considerable debate over the specificity of stimuli required to elicit biases (e.g., Becker, Rinck, Margraf, & Roth, 2001), as well as the stages at which evaluative mechanisms influence attention (e.g., Bar-Haim et al., 2007), the attentional components that underlie attentional biases have been the most contested theoretical issue, with starkly contrasting accounts of increased attention towards threat. These competing accounts have been termed the ‘vigilance’ and ‘maintenance’ hypotheses (Weierich et al., 2008), and are best understood in the context of Posner’s model (e.g., Posner & Peterson, 1990) of the components of spatial visual attention. In Posner’s model, a ‘shift’ mechanism orients attention from one location to another, while distinct ‘engage’ and ‘disengage’ mechanisms hold and release attention between shifts.
The vigilance hypothesis posits that individuals with anxiety disorders detect threat more easily, and thus orient (i.e. shift) attention towards threat more often. Inline with this hypothesis, both dichotic listening (Foa & McNally, 1986) and signal detection studies (Wiens, Peira, Golkar, & Öhman, 2008) have found that individuals with anxiety disorders have lower detection thresholds for threatening stimuli, and research on spatial attention suggests that these decreased thresholds lead to increased orienting towards threat (e.g., Mogg & Bradley, 2002). A crucial feature of the vigilance hypothesis is its emphasis on stimulus-driven, ‘exogenous’ shifts of attention. An abundance of behavioral and neuropsychological research suggests a fundamental distinction between a stimulus-driven system and a goal-directed system in the control of attention (Corbetta & Shulman, 2002; Posner & Peterson, 1990). The stimulus-driven system responds to unattended, motivationally-relevant events, enabling their detection through automatic shifts in attention (Corbetta & Shulman, 2002). Accounts of facilitated detection of threat (e.g., LeDoux, 2000; Vuilleumier, 2005) have focused on the role of the amygdala, which receives early sensory input, and returns projections to sensory processing areas. Through this pattern of connectivity, the amygdala can detect motivationally-relevant stimuli early in visual processing and bias competition at later stages of processing, such that threatening stimuli are more likely to capture attention or enter working memory (Sander et al., 2003).
Whereas the vigilance hypothesis has been articulated mostly in relation to the stimulus-driven system, the maintenance hypothesis places more emphasis on the goal-directed system of attention. In contrast to the stimulus-driven system, the goal-directed system selects and maintains focus on stimuli according to ongoing plans, resulting in voluntary, ‘endogenous’ shifts of attention as well as the inhibition of exogenous shifts of attention to distracting stimuli. Whereas the vigilance hypothesis predicts facilitation of exogenous shifts of attention toward threat, the maintenance hypothesis predicts impairment of endogenous shifts of attention away from threat, due to difficulty disengaging attention from threat (Fox et al., 2001). According to this latter account, biases emerge after threat detection, as threatening stimuli hold attention longer in anxious individuals. Attentional control theory (ACT; Eysenck, Derakshan, Santos & Calvo, 2007) provides a useful context for understanding the underlying mechanisms of increased maintenance of attention on threat. ACT specifies three central executive functions involved in endogenous attention control: inhibition (i.e., inhibiting task-irrelevant processing or responding), shifting (i.e., changing mental sets), and updating (i.e., refreshing and monitoring the contents of working memory (Eysenck et al., 2007). Difficulty disengaging attention from threat in anxiety would appear to relate to the inhibition function of the central executive, as the bias involves difficulty inhibiting the initial processing of threat. Although this imbalance in the attentional control systems of anxious individuals could primarily reflect hyperactivity of the exogenous system in response to threat, ACT holds that general deficits in the endogenous system also play a role. This claim is supported by several studies documenting attentional control deficits related to non-threatening stimuli in anxiety (e.g., Bishop, 2009; Ansari & Derakshan, 2011a). Indeed, Derryberry and Reed (2002) found that difficulty disengaging attention from threat in anxiety was contingent on a more generalized deficit in endogenous attentional control.
The vigilance and maintenance hypotheses have somewhat different implications for the possible role of attentional biases in the etiology and maintenance of anxiety disorders (Cisler & Koster, 2010). The vigilance hypothesis suggests that attentional biases may increase state anxiety by causing increased awareness of threats, and over time, may increase estimates of vulnerability to threat. Alternatively, the maintenance hypothesis suggests that attentional biases may increase state anxiety by maintaining cognitive resources on threat (Fox et al., 2001), and may impair daily functioning by increasing the amount of distraction caused by threatening objects or thoughts. AsWeierich et al. (2008) note, the vigilance and maintenance hypotheses need not be mutually exclusive. Both facilitated detection of threat and difficulty disengaging from threat may characterize anxiety disorders and contribute to their etiology and maintenance (Cisler & Koster, 2010).
Although anxious individuals may initially allocate more attention to threat compared to controls, some studies suggest that this pattern may reverse with extended viewing, a phenomenon known as ‘attentional avoidance.’ In addition to operating on a later and more prolonged time scale, attentional avoidance is believed to be voluntary and strategic, whereas vigilance and maintenance biases are considered involuntary and somewhat automatic (Cisler & Koster, 2010). Attentional avoidance is believed to have a functional role similar to behavioral avoidance, in that it prevents reappraisal of threatening stimuli and thereby maintains harm associations (Mogg, Mathews, & Weinman, 1987). For example, by behaviorally avoiding a closet with spiders, a spider phobic is deprived of the opportunity to learn that encountering a spider does not predict a feared outcome (e.g., a venomous bite). If the individual were to enter the shed, but then avoid looking at the regions likely to contain spiders, the outcome for learning and reappraisal could be equivalent to avoiding the situation in the first place.
Depression
Early theoretical accounts (e.g., Beck, 1976) of attentional bias in depression posit a ‘mood-congruent bias,’ in which negative affect causes increased attention to negative stimuli. However, many researchers have had difficulty demonstrating an attentional bias in depression (see Mogg & Bradley, 2005). This difficulty has led some (e.g., MacLeod et al., 1986) to suggest that an attentional bias may be unique to anxiety, whereas a memory bias may be unique to depression (Peckham et al., 2010). However, the experimental parameters typically used to study attentional bias have been largely derived from research on anxiety and may not be ideal for research on depression. Mogg and Bradley (2005) note that attentional bias in depression has been most often observed for dysphoric stimuli at later, more voluntary stages of stimulus processing. Some of the studies failing to observe attentional bias in depression had parameters more typical of research on anxiety disorders, as they employed threatening rather than dysphoric negative stimuli (e.g., MacLeod et al., 1986), or presented stimuli for brief rather than extended durations (e.g., Mogg, Bradley, Williams, & Mathews, 1993).
This pattern of results may suggest that dysphoric stimuli do not exogenously capture attention in depression in a manner resembling the facilitated detection of threat in anxiety (Mogg & Bradley, 2005). Given that exogenous attentional capture is reserved for stimuli that tend to be novel or urgent (Corbetta & Shulman, 2002), stimuli relevant to depression (e.g., a reminder of past loss or of one’s symptoms of depression) would not be expected to trigger exogenous orienting. Attentional bias in depression may instead be a product of a ruminative cognitive style, in which depressed individuals dwell on dysphoric, self-relevant content in an attempt to work through symptoms or learn from failures (Koster, De Lissnyde, Derakshan, & De Raedt, 2011; Nolen-Hoeksema, Wisco, & Lyubomirsky, 2008). Accordingly, attentional bias in depression may involve the goal-directed attentional system, as depressed individuals voluntarily maintain attention on dysphoric content as they engage in elaborative processing. Although the maintenance bias for dysphoric content in depression may begin voluntarily, depressed individuals may eventually attempt to disengage attention from dysphoric content, yet fail to endogenously shift attention elsewhere, in a manner comparable to the maintenance bias predicted in anxiety (Koster et al., 2011). Indeed, attentional control deficits have been observed in depression (see Koster et al., 2011), particularly in individuals high in rumination (De Lissnyder, Derakshan, De Raedt, & Koster, 2011) and in the presence of emotional stimuli (Derakshan, Salt, & Koster, 2009). A tendency to dwell on dysphoric stimuli could play a role in the etiology and maintenance or depression: in the short term, dwelling on dysphoric stimuli could increase negative affect (Peckham et al., 2010); in the long term, this attentional style could lead to distorted beliefs and assumptions about the world.
The mood-congruent attentional bias posited by cognitive theories of depression (e.g., Beck, 1976) may also extend to deficits in positive affect. A small number of studies have observed reduced attention towards positive stimuli in depressed individuals relative to controls (see Peckham et al., 2010). This group difference may reflect the lack of a normative positivity bias in depression (McCabe and Gotlib, 1995), although the underlying attentional components and time course of this bias are unclear. An anhedonic bias in depression could involve insensitivity to reward in the stimulus-driven system, such that rewarding stimuli fail to initially capture attention. Alternatively, insensitivity to reward in depression may have greater implications for the goal-directed attentional system, as depressed individuals lack the motivation to sustain attention on positive stimuli. Reduced attention to positive stimuli could contribute to deficits in positive affect and over time could distort assumptions and beliefs about the world. Given that deficits in positive affect are specific to depression (Clark & Watson, 1991), they are not predicted in anxiety disorders. Indeed, some studies have found increased attention to positive stimuli in anxiety (e.g., Fox et al., 2001, exp. 3; Fox et al., 2002, exp. 1). The “emotionality hypothesis” (see Calvo & Avero, 2005) posits that stimulus-driven system in anxious individuals is hypersensitive to all motivationally-relevant events, leading to biases for positive stimuli in addition to negative stimuli.
Advancing theoretical accounts of attentional bias with eye tracking
Despite decades of research, key issues in theoretical accounts of attentional bias in affective disorders remain unresolved, such as the components of attentional bias in anxiety, and the nature of attentional bias in depression. These issues largely hinge on the empirical delineation of the time course and components of attentional bias in anxiety and depression, which remains a work in progress. RT measures, the main tool for studying attentional biases, have provided important insight into the nature of attentional bias. However, certain limitations of these measures, including the distal relation between key presses and attention and the “snapshot” nature of RT measures, are inherent and cannot be overcome without incorporating other means of measuring attention.
Eye tracking may provide a valuable complement to RT measures, as the methodology is largely unhindered by the inherent limitations of RT measures. Importantly, EMs have a more proximal relation to attention than manual key presses. While eye tracking can only directly measure overt attention, the relation between EMs and covert attention is much closer than that of manual responses and covert attention. As a result, EMs are less susceptible to confounding processes. Critically, saccadic EMs appear to escape the general response slowing or “freezing” (McNaughton, & Corr, 2004) caused by threatening stimuli, which may confound manual RT measures, particularly the emotional Stroop (see Algom, Chajut, & Lev, 2004) and spatial cueing task (see Mogg, Holmes, Garner, & Bradley, 2008). For example, Nummenmaa, Hyönä, and Calvo (2006) presented pairs of images (negative-neutral, pleasant-neutral, neutral-neutral) diagonally, in opposite quadrants of the screen, and found that initial saccade latencies did not differ according to the type of image pair, regardless of whether the initial saccade targeted one of the images or empty space on the screen. Thus, the mere presence of threat does not appear to increase EM latencies in the same manner in which it increases RT latencies.
In addition to providing a relatively proximal measure of attention, EMs can be recorded more or less continuously, with sampling rates ranging from 16.67 ms (60 Hz) to less than one ms (e.g., 2000 Hz). Figure 1a provides a scan path from an eye tracking study (Armstrong, Olatunji, Sarawgi, & Simmons, 2010) that illustrates the continuity of eye tracking data. By allowing virtually continuous recording of attention, eye tracking provides a remarkable increase in the efficiency of measuring attention. Whereas RT measures require multiple conditions to parse components of attention, eye tracking allows precise observation of multiple components within a single trial (Figure 1a), and also provides multiple parameters of each component. For example, both the latency and location of an initial fixation can be recorded, providing both a temporal and a spatial parameter of orienting. In contrast, the emotional spatial cueing task can only provide a measure of the speed of orienting (Fox et al., 2001), whereas the modified dot probe mainly provides an indicator of the direction of orienting.1 As depicted in Figure 1a, the initial orienting of overt attention to a stimulus can be distinguished easily from subsequent dwell time, as orienting is reflected in saccade sequences (where one looks), whereas dwell time is reflected in fixation durations (how long one looks). Thus, the continuous nature of eye tracking provides an important advance in characterizing the time course and components of attentional bias.
Figure 1.
An example of EM data and the eye tracking paradigms considered in the meta-analysis: (a) 3-s scan path in a free viewing task. Circles indicate fixations, diameter of circles indicates duration, and lines indicate saccade sequence. (b–c) Examples of ‘odd-one-out’ visual search task conditions: (b) threat-related target amidst neutral distractors, (c) neutral target amidst threat-related distractors. All facial expressions from the NimStim Face Stimulus Set (Tottenham et al., 2009).
Using EMs as indicators of attentional bias
The body of published eye tracking studies on affective disorders varies considerably in terms of the experimental parameters and operational definitions of attentional bias employed. Some of the methodological variance can be accounted for by the presence of two distinct types of task: free viewing tasks and visual search tasks. In free viewing tasks, which constitute the majority of extant research, attentional biases are not measured in relation to performance on a task, such as making a saccade to a cued location, or searching for a target. In these studies, the only restriction on gaze behavior occurs prior to the onset of a trial, as participants are asked to focus on a central fixation point to ensure that stimuli are presented at equal retinal eccentricity. Participants then examine arrays of stimuli (e.g., faces, Garner, Mogg, & Bradley, 2006; objects, Rinck & Becker, 2006; words, Felmingham, Rennie, Manor, & Bryant, 2012), for periods ranging from 1–60 s. Visual search tasks, in contrast, require participants to search an array of stimuli in order to determine as quickly and accurately as possible if a criterion is met, usually whether or not a stimulus is present. Presence (or absence) is indicated by a speeded key press, which provides an additional source of data that can be integrated with EMs (Derakshan & Koster, 2010). Visual search tasks also differ from free viewing tasks in that they require more items in an array to prevent a floor effect. Whereas arrays in free viewing tasks involve 2 to 4 stimuli, arrays in visual search tasks typically involve between 8 and 16 stimuli in the EM literature on anxiety disorders. Whereas anxiety has been examined with both free viewing and visual search eye tracking paradigms, depression has only been investigated in free viewing eye tracking tasks.
Operational definitions of vigilance and maintenance of attention in studies of anxiety
Free viewing tasks
In free viewing studies of anxiety (Figure 1a), operational definitions of vigilance have focused on the initial orienting of gaze at stimulus onset. Most studies have looked at the direction of orienting, analyzing how frequently threatening images capture initial fixations. Although the speed of orienting has been commonly reported in basic research on EMs and attention (saccade latency: Bannerman, Milders, de Gelder, & Sahraie, 2009; saccade latency and velocity: Nummenmaa et al., 2006) this parameter of orienting has rarely been reported in studies of anxiety. In one of the few studies reporting both variables,Mogg et al. (2000) found that the direction and speed of orienting were moderately correlated, suggesting that these parameters may reflect the same underlying influence of emotion on saccade programming. Overt orienting in free viewing tasks appears to reflect exogenous cueing of attention. Nummenmaa et al. (2006) found that when participants (from an unselected sample) were instructed to look at a neutral image paired with an emotional image (exp. 2), they showed an orienting bias towards emotional images similar to that exhibited when viewing the images freely (exp. 1), suggesting that orienting gaze to motivationally-relevant stimuli is largely involuntary in this viewing context.
Whereas the latency and location of initial fixation are used to test the vigilance hypothesis, the duration of initial fixation is frequently used to test the maintenance hypothesis in anxiety (e.g., Garner et al., 2006). Typically, studies sum the durations of fixations made on the initial viewing of a stimulus (e.g., Garner et al., 2006). Numenmaa et al. (2006) found that initial maintenance of gaze on emotional stimuli likely involves both endogenous and exogenous effects: in an unselected sample, instructions not to look at emotional images (i.e., look at the accompanying neutral image) had more effect on initial maintenance of gaze compared to initial orienting of gaze; however, these instructions were only partially effective, and had a greater effect on a total maintenance of gaze variable that included later stages of processing. Thus, the exogenous effect of emotional stimuli on attention appears to persist beyond initial orienting into the initial maintenance of gaze, where it becomes increasingly subject to the attenuating effects of endogenous attentional control.
Some studies have used time intervals as the sole basis for defining components of attention bias (e.g., Buckner, Maner, & Schmidt, 2010), assuming that fixation duration in an early window reflects facilitated detection of threat, whereas fixation duration in a subsequent window reflects initial maintenance of attention on threat. Rinck and Becker (2006) describe measures such as the latency, direction, and duration of initial fixations as ‘event-related,’ because these variables reference specific EM events. In contrast, they describe measures of fixation duration within time windows as ‘epoch-related,’ because these variables extract fixation duration data from specific gaze events, and re-reference the data to time windows (i.e., epoch) in which fixations occur. Epoch-related analysis has been the primary means of investigating attentional avoidance. Change in fixation duration as a function of time has been assessed on scales ranging from 3 to 60 s. Although attentional avoidance has been conceptualized as a voluntary emotion regulation strategy that emerges later in time (Cisler & Koster, 2010), it has been measured with event-related variables, as well: briefer initial fixation on threat (Garner et al., 2006; Rinck & Becker, 2006), as well as orienting away from threat (Garner et al., 2006), have been construed as indicators of avoidance.
Visual search tasks
In visual search tasks, the vigilance and maintenance hypotheses are tested in separate visual search conditions. To assess facilitated detection of threat, a threatening stimulus is presented as the target in an array of non-threatening distracters (Figure 1b). Latency to fixating the target (often used in conjunction with manual response latency) has been used to provide an overall indicator of facilitated detection of threat (Rinck, Reinecke, Ellwart, Heuer, & Becker, 2005). To further clarify the nature of facilitated detection, the sequence of fixations prior to target detection has been examined. It was previously believed that threatening stimuli could ‘pop-out’ of a search array and capture attention, particularly in anxious individuals, but findings of this effect (Öhman, Flykt, & Esteves, 2001) have been called into question (see Vuilleumier, 2005). When multiple EMs are required to locate a threatening target, facilitated detection can occur in at least two ways. First, a threatening target could be located faster because fewer fixations are required to reach the target. This may reflect exoegenous orienting toward a threat target once gaze is directed to a nearby distracter, bringing the threatening stimulus within a range of eccentricity from which it can attract attention. Alternatively, a threatening target could be located faster due to reduced dwell time on non-threatening distracters (Becker, 2009).
Whereas facilitated detection of threat is assessed with a threatening target in an array of neutral distracters, difficulty disengaging attention from threat is assessed with a neutral target in an array with threatening distracters (Figure 1c). In some studies (Rinck et al., 2005, exp. 1), all of the distracters are threatening, while in others (Miltner, Krieschel, Hecht, Trippe, & Weiss, 2008; Rinck et al., 2005, exp. 2), only a single distracter is threatening, while the remainder are non-threatening. The former design, in which all distracters are threatening, ensures that on most trials, a threatening distracter will be fixated prior to the neutral target. However, when just a single threatening distracter is included in the search array (e.g., Rinck et al., 2005), one can examine whether this distracter delays visual search because it is located more often than other non-threatening distracters (facilitated detection), or because it holds attention longer when it is attended (difficulty disengaging attention).
EM indicators of attentional bias in depression
Eye tracking research on attentional bias in depression has mainly employed the free viewing tasks used in anxiety disorders research, but with longer stimulus presentations that allow insight into the elaborative processing of stimuli. Although the vigilance hypothesis has been less prominent in theoretical accounts of depression, many of these studies have reported the same indicator of vigilance found in studies of anxiety (direction of orienting). In examining the maintenance of attention in depression, studies have placed less emphasis on the initial maintenance of attention, focusing instead on maintenance of attention over the entire trial (e.g., total fixation duration). Fixation time over extended viewing appears to reflect mostly top-down, endogenous control of attention. Whereas emotional stimuli can undermine endogenous control in the initial maintenance of gaze, such stimulus-driven effects appear to wane quickly in the later maintenance of gaze (Nummenmaa, et al., 2006).
Meta-analysis of eye tracking research on attentional biases
Given the potential of eye tracking to address enduring questions in research on attentional biases, a meta-analysis was conducted on the extant literature. The meta-analysis had two primary aims. The first aim was to examine the components of overt attentional bias for threat in anxiety, and to summarize the theoretical implications of these findings. The vigilance hypothesis was tested by examining initial orienting towards threat in free viewing tasks and facilitated detection of threat in visual search tasks. Similarly, the maintenance hypothesis was assessed by examining initial maintenance of gaze on threat in free viewing tasks, and distraction by threat in visual search tasks. By examining initial maintenance of gaze, the present meta-analysis was also able to test for possible attentional avoidance, as this indicator measures a window of stimulus processing in which avoidance has been observed in some RT studies (e.g., Mogg et al., 2004). In exploring the components of attentional bias for threat in anxiety, several population and procedure-related moderators were considered (Bar-Haim et al., 2007).
The second aim was to examine attentional biases for emotional stimuli in depression and to contrast these effects with those observed in anxiety, when possible. Initial orienting towards threatening and positive stimuli was examined in both anxiety and depression in order to determine if vigilance for threat was specific to anxiety and if reduced sensitivity to positive stimuli was specific to depression. In studies of depression, it was also possible to examine orienting towards dysphoric stimuli, as well as biases in extended viewing (10–30 s trials). The hypothesis that depressed individuals show increased elaborative processing of negative stimuli (e.g., Siegle et al., 2001) was tested, and the specificity of this bias to dysphoric stimuli was assessed by examining elaborative processing of threatening stimuli, as well. Lastly, the hypothesis that depressed individuals show reduced elaborative processing of positive stimuli (e.g., Gotlib & McCann, 1984) was tested. Although these latter biases could not be examined in anxiety, these analyses may have important implications for theories of attentional bias in depression.
Methods
Literature base
PsycINFO and PubMed databases were searched for relevant studies using the key words anxiety, phobia, fear, dysphoria, or depression, intersected with eye, tracking, eye-tracking, EMs, gaze, or fixation. The authors of studies revealed by this search were then entered as search terms into the same databases, in order to uncover additional eye tracking studies. In addition, the references of eye tracking studies revealed by these methods were consulted, as were the references of review papers on attentional bias in anxiety or depressive disorders (Bar-Haim et al., 2007; Cisler & Koster, 2010; Mogg & Bradley, 2005; Weierich et al., 2008).
There were three primary criteria for inclusion of studies in the review, in order to ensure commensurability: 1) Studies had to utilize eye tracking, either with video-based infrared systems or electro-oculography; 2) Attentional biases had to be studied in the context of anxiety or depression in adults; and 3) Studies had to present emotional and neutral stimuli simultaneously, in regular arrays, such that these stimuli could compete for attention.
Coding system and coding decisions
Studies were coded in terms of the following population-related variables: 1) sample size (Ns) for high and low symptom group; 2) gender composition (% female) for high and low symptom group and gender difference between groups (high minus low); 3) class of disorder (anxiety versus depression); 4) type of high symptom group (clinical versus analogue); 5) within studies of anxiety, type of anxiety disorder was also coded. For analogue studies, the most relevant anxiety disorder was coded (e.g. studies of contamination fear were coded as OCD; studies of trait anxiety were coded as GAD). Studies were coded in terms of the following procedure-related variables: 1) type of task (free viewing vs. visual search); 2) type of threat stimulus (face versus picture), where picture refers to images of scenes or objects; 3) array size; 4) for free viewing tasks, the retinal eccentricity at which the stimuli were presented, measured from the central fixation point to the centers of stimuli, was also coded; 5) for free viewing tasks, the type of variable (event versus epoch) was coded for both indicators of initial orienting and initial maintenance of attention.
For studies that employed more than one category of threat stimulus, variables related to the most potent (e.g., 100% intensity threat faces; Mogg, Garner, & Bradley, 2007) or the most symptom-relevant threat stimulus (e.g., combat-related threat for PTSD; Kimble, Fleming, Bandy, Kim, & Zambetti, 2010) were selected; if categories of threat stimuli were equally relevant (e.g., neutral face paired with object or angry face paired with neutral face for SAD; Garner et al., 2006), the conditions were averaged together to avoid inflating effect size estimates (see Mitte, 2008). Similar procedures have been used in other reviews of attentional bias for threat in anxiety disorders (e.g., Bar-Haim et al. 2007). Lastly, for visual search tasks, some studies (e.g., Derakshan & Koster, 2010) placed threatening stimuli amongst both neutral and pleasant stimuli. To ensure commensurability with other visual search studies, and to maintain some commensurability with free viewing tasks, variables were selected from conditions with threatening and neutral stimuli.
Operational definition of vigilance and maintenance
Free viewing tasks
For free viewing tasks, the vigilance hypothesis was tested by examining the direction in which gaze was initially oriented (i.e., towards or away from threat). For event-related analyses of vigilance (k = 17), variables indicating how frequently threatening stimuli captured initial fixations were collected. For epoch-related analyses of vigilance (k = 3), variables indicating total fixation time on threatening stimuli within the first 500 ms were collected. Fixation duration in the first 500 ms provides a valid measure of initial orienting, because only the stimulus that captures orienting will be viewed within the first 500 ms on most trials (orienting to a peripheral stimulus is not completed until roughly 225 to 400 ms after stimulus onset, and initial gaze on a stimulus typically lasts at least 300 ms in a free viewing context; e.g., Garner et al., 2006). Also, gaze duration in the initial 500 ms has been found to be highly correlated with the direction of initial orienting (rs = .76 to .84, ps < .001; Armstrong et al., 2010). When both event- and epoch-related orienting indicators were available (e.g., Rinck & Becker, 2006), the event-related variable was selected, because it provides a more direct measure of initial orienting. When a study included two presentation times and one presentation time was potentially too brief to allow EMs (e.g., 175 ms; Stevens, Rist, & Gerlach, 2011), only the longer presentation time was included). Orienting bias was examined for threatening, positive, and dysphoric stimuli.
The initial maintenance of attention on threat was assessed in free viewing tasks of anxiety. Variables indicating the duration of initial fixation on threatening stimuli (event-related) were collected. Also, variables indicating fixation duration on threatening stimuli between 1000 and 2000 ms (epoch-related) were collected for studies that did not report the event-related variable. These indicators have been found to be moderately correlated (rs = .33 to .46, ps < .05; Armstrong et al., 2010). Although the initial fixation on a stimulus begins in the 0–1000 ms epoch, fixation duration within this epoch is still largely determined by the orienting of gaze, because after the initial orienting and maintenance of gaze, there is little time left within the first 1000 ms of the trial for viewing other stimuli. Accordingly, if one type of stimulus disproportionately captures initial fixations, this stimulus type will necessarily be viewed more than other stimulus types during the 0–1000 ms epoch.
For the event-related indicator of maintenance (i.e., initial fixation duration), within-subjects effects (initial maintenance on threatening versus neutral stimuli) were compared between groups. Computing the between groups effect without this within-subjects baseline (i.e., initial maintenance on neutral stimuli) yielded equivalent results. In contrast, epoch-related indicators of maintenance do not offer such a relatively independent neutral baseline, because data points for threatening and neutral variables are drawn from the same trials; in many cases, the epoch-related indicators reported were ratios of time spent viewing threatening relative to neutral stimuli. Thus, in studies reporting epoch-related indicators of maintenance, a within-subjects effect was not computed before comparing groups. Although some studies have examined maintenance of gaze over a larger time scale (e.g., 0–60 s; Rinck & Becker, 2006), there was not a sufficient number of studies to allow meta-analysis of maintenance of gaze beyond 2000 ms in anxiety disorders. However, free viewing eye tracking studies of depression frequently employ longer trials (10 to 30 s). For these studies, measures of total fixation duration on dysphoric, threatening, and pleasant stimuli were examined, in order to shed light on maintenance biases occurring over a larger time scale in depression.
Visual search tasks
In visual search tasks, the vigilance hypothesis was assessed in conditions with threatening targets and non-threatening distractors, whereas the maintenance hypothesis was assessed in conditions with threatening distractors and non-threatening targets. Average fixation time on distractors was the most frequently reported indicator of search efficiency, and was used in both analyses. There was some heterogeneity in how this indicator was reported, as some studies reported average maintenance of gaze on all distractors of a type, whereas some studies reported average maintenance of gaze on individual distractors of a type (i.e., dividing total dwell on distractors by the number of distractors fixated). If neither variable was available, latency to fixating targets was used (k = 1; Miltner et al., 2004). For the analysis of between-groups effects, search conditions with non-threatening stimuli were used as a baseline to control for performance differences between groups unrelated to threat processing. This within-subjects effect was computed, and then used as the basis for the between subjects effect. Computing the between groups effect without this within-subjects baseline yielded equivalent results. The within-groups effects alone were not interpretable, as the search conditions without threatening stimuli were not always matched for difficulty with the conditions containing distractors (e.g., Miltner et al., 2004).
Statistical analysis
Calculation of effects sizes
Individual and combined effect sizes were computed using Hedges’s g (Hedges, 1981) with correction for small samples. This index of effect size can be interpreted similarly to Cohen’s d (0.2 = small, 0.5 = medium, 0.8 = large; Cohen, 1988). The direction of effect size was coded such that increased allocation of attention (in anxious or depressed individuals compared to controls) was reflected in positive values, whereas decreased allocation of attention was reflected in negative values. When means and standard deviations were not available, statistical test values (i.e. F) were used to determine effect size (k = 3). When a result was reported as null without additional information, but the direction of the effect could be inferred from figures (k = 3), a p value of .50 was assumed, in order to ensure that the sample of outcomes was representative (Bar-Haim et al., 2007; Cooper & Hedges, 1994). In studies that did not employ high and low symptom groups (k = 2), correlations between gaze biases and symptom measures were used to compute an effect size. Comprehensive Meta-Analysis, Version 2 (Biostat; Borenstein, Hedges, Higgins, & Rothstein, 2005) was used to construct a database, to compute and weight effect sizes, and to conduct heterogeneity and moderator analyses. Forest plots were generated using the metafor package for R statistical software (Viechtbauer, 2010).
Weighting of effect sizes, tests of heterogeneity, and moderator analysis
Random effects models were used to calculate combined effect sizes, because the studies in each analysis varied somewhat in terms of participants and procedures. For each combined effect size, the extent of heterogeneity was tested using the Q statistic (Hedges & Olkin, 1985) and the I2 statistic (Higgins & Thompson, 2002). A significant Q statistic suggests that variance in effect sizes around the combined effect size cannot be completely accounted for by sampling error; the I2 statistic estimates the percentage of this variance that is due to between-studies variability (25% = low, 50% = medium, and 75% = high heterogeneity; Higgins & Thompson, 2002). The Q statistic is used to determine if moderator analysis is necessary, as significant heterogeneity suggests that there are moderators accounting for between-study variance in effect size. Categorical moderators were tested using a mixed-effect meta-analytic categorical test (meta-analytic analysis of variance); continuous moderators were tested using unrestricted maximum likelihood meta-regression (Hallion & Ruscio, 2011). For the analysis of categorical moderators, levels of the moderator had to have at least two studies to be included in the analysis.
Publication bias
Estimates of combined effect sizes may be inflated by the suppression of null findings. This risk was reduced by searching for dissertation abstracts in addition to published articles, by requesting unpublished studies from authors who frequently publish eye tracking research, and by requesting values in order to compute effect sizes for variables that were not reported in articles. In addition, the number of unreported null findings required to render an effect non-significant, the fail-safe N (FSN), was conducted for each main effect (e.g., Bar-Haim et al., 2007).
Results
The present review integrates a total of 33 eye tracking experiments with 1579 participants (anxious N = 563; non-anxious N = 532; depressed N = 162; non-depressed N = 257; unselected: N = 65). Twenty-eight experiments utilized free viewing tasks (N = 1337). Of the experiments utilizing free viewing tasks, 20 examined anxiety (Table 1) and 9 examined depression (Table 2; one study included both anxious and depressed participants; Mogg et al., 2000).2 Five experiments utilized visual search tasks and examined anxiety (N = 242; Table 3).
Table 1.
Study characteristics: Passiew viewing tasks –– Anxiety
| Study | Anxious n |
Non- anxious n |
N | Type of anxiety disorder |
High symptom group |
Gender difference* |
Type of threat stimulus |
Array size | Stimulus eccentricity |
Type of orienting indicator |
Type of maintenance indicator |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Armstrong et al. (2010) | 23 | 25 | 48 | OCD | Analogue | 18.30 % | Face | 2 | 5.5° | Event | Event |
| Armstrong et al. (2012) | 19 | 20 | 39 | OCD | Analogue | 33.00 % | Picture | 4 | 7° | Event | Event |
| Bradley et al. (2000) | –– | –– | 23 | SAD | Analogue | n/a | Face | 2 | 2.6° | Event | –– |
| Buckner et al. (2009) | 23 | 23 | 46 | SAD | Analogue | −6.20 % | Face | 4 | n/a | Epoch | Epoch |
| Calvo & Avero (2005) | 40 | 40 | 80 | GAD | Analogue | 0 | Picture | 2 | 12.8° | Event | Epoch |
| Felmingham et al. (2011) | 11 | 10 | 21 | PTSD | Clinical | −5.00 % | Word | 4 | 5.5° | Event | Event |
| Gamble & Rapee (2010) | 58 | 29 | 87 | SAD | Clinical | 5.00 % | Face | 2 | 5.15° | Epoch | Epoch |
| Garner et al. (2006) Exp 1 | 16 | 16 | 32 | SAD | Analogue | −0.13 % | Face | 2 | 4.85° | Event | Event |
| Garner et al. (2006) Exp 2 | 16 | 15 | 31 | SAD | Analogue | 0.27 % | Face | 2 | 4.85° | Event | Event |
| Hermans et al. (1999) | 13 | 13 | 26 | SP | Analogue | n/a | Picture | 2 | 3.08° | Epoch | Epoch |
| Kimble et al. (2010) | 9 | 10 | 19 | PTSD | Analogue | −20.00 % | Picture | 2 | 10° | Event | Event |
| Lange et al. (2011) | 22 | 21 | 43 | SAD | Analogue | −0.10 % | Face | 16 | n/a | Event | Event |
| Mogg et al. (2000) | 12 | 10 | 22 | GAD | Clinical | 0.21 % | Face | 2 | 2.5° | Event | –– |
| Mogg et al. (2007) | 21 | 28 | 49 | GAD | Analogue | −0.04 % | Face | 2 | 3.85° | Event | –– |
| Pflugshaupt et al. (2007) | 21 | 21 | 42 | SP | Clinical | 0.14 % | Picture | 2 | 7.25° | Event | Epoch |
| Rinck & Becker (2006) | 22 | 23 | 45 | SP | Analogue | 0.0 9% | Picture | 4 | 6.4° | Event | Event |
| Rohner (2002) | 52 | 48 | 100 | GAD | Analogue | n/a | Face | 2 | 12° | Epoch | Epoch |
| Schofield et al. (2012) | –– | –– | 42 | SAD | Analogue | n/a | Face | 2 | 2.86° | Event | Epoch |
| Stevens et al. (2010) | 16 | 12 | 28 | SAD | Clinical | −0.04 % | Face | 2 | 4° | Event | –– |
| Weiser et al. (2009) | 15 | 15 | 30 | SAD | Analogue | 0.00 % | Face | 2 | 2.5° | Event | Event |
Note:
Positive values for gender difference reflect more females in the anxious group; GAD = generalized anxiety disorder; OCD = obsessive compulsive disorder; SAD = social anxiety disorder; PTSD = post-traumatic stress disorder; SP = specific phobia (spiders); n/a = not available; –– = not applicable (for group n’s, study examined correlations in full sample; for type of maintenance indicator, study presented stimuli too briefly to examine maintenance; i.e. ≤ 600 ms).
Table 2.
Study characteristics: Passiew viewing tasks –– Depression
| Study | Depressed n |
Non- depressed n |
N | High symptom group |
Type of stimuli |
Affective stimuli |
Orienting indicator* |
Trial length ** |
|---|---|---|---|---|---|---|---|---|
| Caseras et al. (2007) | 20 | 23 | 43 | Analogue | picture | D, P | Yes | 3 s |
| Eizenman et al. (2003) | 8 | 9 | 17 | Analogue | picture | D, P, T | No | 10.5 s |
| Ellis et al. (2011) | 23 | 40 | 63 | Analogue | word | D, P, T | No | 10 s |
| Kellough et al. (2008) | 15 | 45 | 60 | Clinical | picture | D, P T | Yes | 30 s |
| Leyman et al. (2011) | 19 | 20 | 39 | Analogue | face | D, P T | No | 10.5 s |
| Mogg et al. (2000) | 10 | 10 | 20 | Clinical | face | D, P T | Yes | 1 s |
| Peña-Esparza (2011) | 23 | 30 | 53 | Analogue | face | D, P | Yes | 2.5 s |
| Sear et al. (2010) | 20 | 52 | 72 | Analogue | picture | D, P T | Yes | 10 s |
| Sear et al. (2011) | 24 | 38 | 62 | Analogue | picture | D, P T | Yes | 10 s |
Note:
All studies reporting orienting indicator reported event-related variable;
studies with trials of 3 s or less not included in analysis of extended viewing; D = dysphoric; P = pleasant; T = threat.
Table 3.
Study characteristics: Visual search tasks –– Anxiety
| Study | Anxious n |
Non- anxious n |
N | High symptom group |
Type of disorder |
Type of stimuli |
Number of items |
Stimulus configuration |
Detection variable | Distraction variable |
|---|---|---|---|---|---|---|---|---|---|---|
| Derakshan & Koster (2010) | 39 | 38 | 77 | Analogue | GAD | Face | 8 | Circle | Fixation time on N distractors for T targets | Fixation time on T distractors for N targets |
| Gerdes et al. (2008) | 21 | 21 | 42 | Clinical | SP | Picture | 7 | Circle | –– | Fixation time on T distractors for N targets |
| Miltner et al. (2004) | 13 | 13 | 26 | Clinical | SP | Picture | 16 | Matrix | Latency to fixating T targets with N distractors | Latency to fixating N target with T distractors |
| Rinck et al. (2005) Exp 1* | 24 | 24 | 48 | Analogue | SP | Picture | 20 | Matrix | Fixation time on N distractors for T targets | Fixation time on T distractors for N targets |
| Rinck et al. (2005) Exp 2 | 24 | 25 | 49 | Analogue | SP | Picture | 20 | Matrix | Fixation time on N distractors for T targets | Fixation time on T distractors for N targets |
Note: GAD = generalized anxiety disorder; SP = specific (spider) phobia; T = threatening; N = non-threatening; –– not examined;
average of ‘odd-one-out’ and ‘target search’ conditions
Vigilance hypothesis: Free viewing tasks
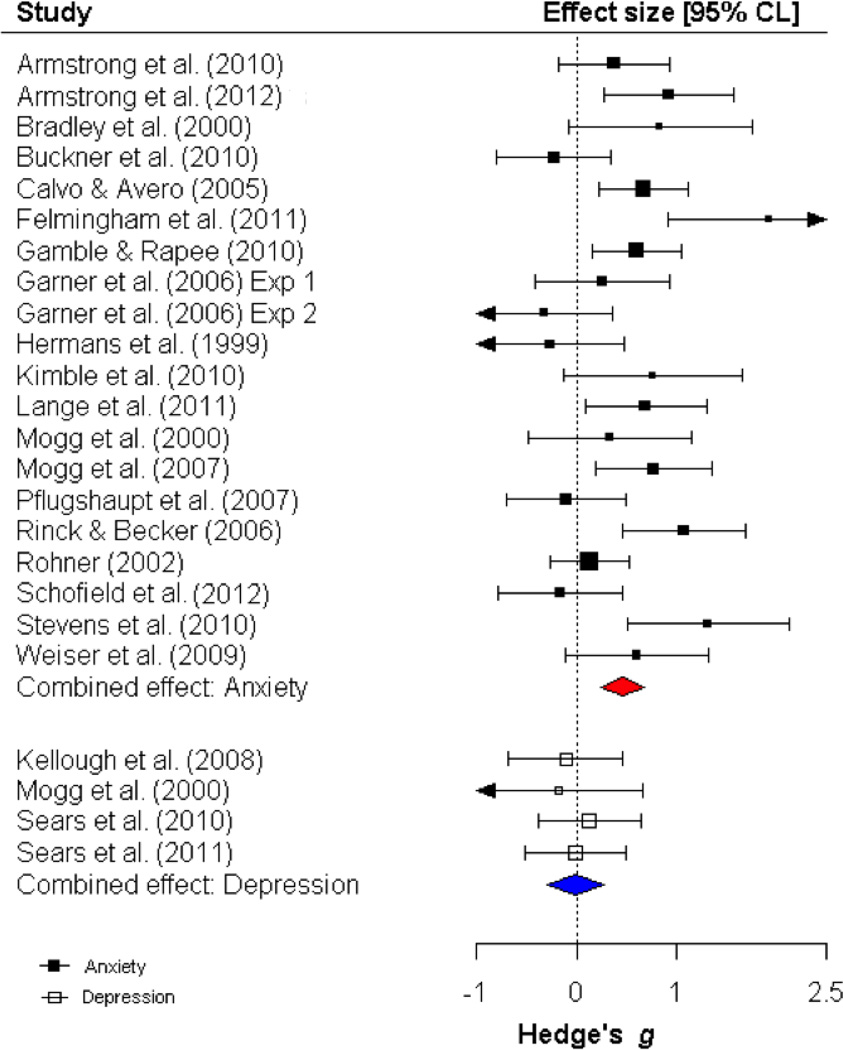
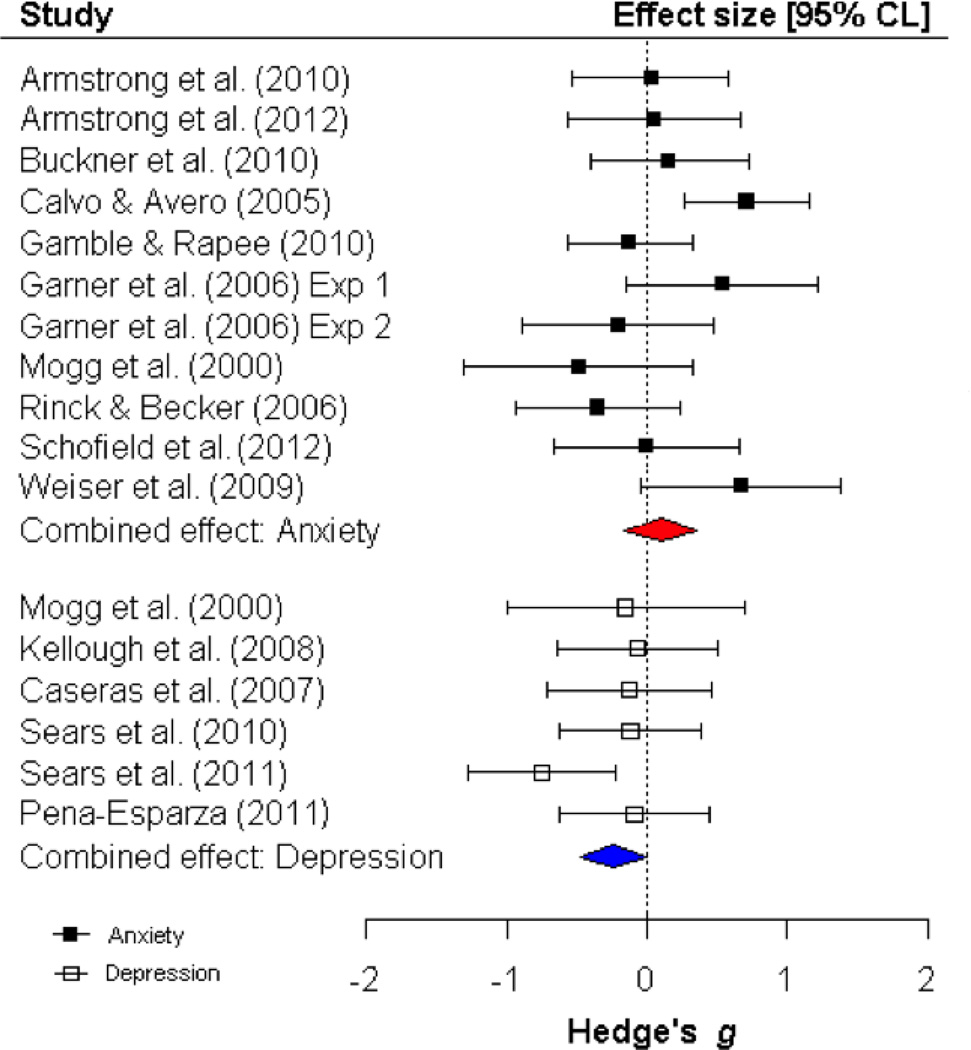
In the analysis of orienting bias in anxious compared to non-anxious individuals, there was significant heterogeneity in the effect sizes for threatening stimuli Q (19) = 47.09, p < .001, I2 = 59.65, and marginal heterogeneity in the effect sizes for pleasant stimuli: Q (10) = 17.66, p = .06, I2 = 43.38. However, in the analysis of orienting bias in depressed compared to non-depressed individuals, there was not significant heterogeneity in the effect sizes for threatening stimuli, Q (3) = .55, p = .95, I2 = 0.0, or for pleasant stimuli, Q (5) = 4.75, p = .55, I2 = 0.0. As depicted in Figure 2, anxious individuals initially oriented gaze towards threat more frequently than non-anxious individuals, as revealed by a significant combined effect size for group differences in orienting bias for threat (k = 20; g = .47, p < .001, CI = .25, .69; FSN: 203). Anxious individuals did not significantly differ from non-anxious individuals in orienting towards pleasant stimuli (k = 11; g = .11, p = .38, CI = −.13, .35; Figure 3).
Figure 2.
Orienting bias for threatening stimuli. In this and all subsequent forest plots, symbol size for point estimates represents study precision. Diamond represents estimate of combined effect size; horizontal edges of diamond represent upper and lower limits of 95% confidence interval.
Figure 3.
Orienting bias for pleasant stimuli.
Depressed individuals did not show an orienting bias towards threat, compared to non-depressed individuals (k = 4; g = −.01, p = .95 CI = −.30, .28). The difference in orienting towards threat in anxious versus non-anxious individuals was significantly different compared to the difference in orienting towards threat in depressed versus non-depressed individuals, Q (1) = 6.72, p = .01 (see Figure 2). Thus, it appears that an overt orienting bias towards threat characterizes anxiety, but not depression. Depressed individuals oriented towards pleasant stimuli less compared to non-depressed individuals (k = 6; g = −.24, p < .05 CI = −.47, −.004; FSN = 6;). This difference in orienting towards pleasant stimuli in depressed versus non-depressed individuals was significantly different compared to the difference in orienting towards pleasant stimuli in anxious versus non-anxious individuals, Q = 4.10, p < .05 (see Figure 3). Thus, it appears that this modest reduction in orienting towards pleasant stimuli is unique to depression. An additional analysis was conducted to explore the possibility of an orienting bias toward dysphoric stimuli in depressed versus non-depressed participants. Although one study found an orienting bias towards dysphoric stimuli in depressed versus never-depressed participants (Sears, Newman, Ference, & Thomas, 2011), the combined effect size was not significant (k = 6; g = .18, p = .27, CI = −.18, .27; heterogeneity: Q (5) = 8.78, p = .12, I2 = 43.02).
Given the finding of significant heterogeneity in studies of anxiety but not depression, possible moderators of orienting towards threat were only examined in studies of anxiety. Table 4 provides full results for the moderator analysis. The only significant moderator was the operational definition of vigilance [event versus epoch-related variable: Q (1) = 3.54, p = .05]. The combined effect size was larger in studies reporting an event-related orienting variable (k = 16, g = .58, CI = .33, .83, p < .001) compared to studies only reporting an epoch-related orienting variable (k = 4, g = .12, CI = −.27, .51, p = .19).
Table 4.
Categorical moderators of orienting bias for threat in anxious versus non-anxious individuals
| Moderator | Effect size |
95% CI | Heterogeneity | Test of moderation | |||
|---|---|---|---|---|---|---|---|
| Type of group | k | g | upper | lower | I2 | Q | p |
| GAD | 4 | 0.47 | 0.14 | 0.79 | 37.68 | 3.05 | 0.55 |
| OCD | 2 | 0.63 | 0.09 | 1.16 | 36.00 | ||
| PTSD | 2 | 1.32 | 0.18 | 2.47 | 65.42 | ||
| SAD | 9 | 0.37 | 0.03 | 0.71 | 59.69 | ||
| SP | 3 | 0.25 | −0.59 | 1.09 | 80.09 | ||
| Type of group | k | g | upper | lower | I2 | Q | p |
| Analogue | 15 | 0.40 | 0.17 | 0.63 | 53.18 | 1.05 | 0.31 |
| Clinical | 5 | 0.74 | 0.13 | 1.36 | 74.30 | ||
| Type of threat stimulus | k | g | upper | lower | I2 | Q | p |
| Face | 13 | 0.37 | 0.13 | 0.61 | 48.10 | 0.35 | 0.55 |
| Picture | 6 | 0.52 | 0.09 | 0.95 | 63.74 | ||
| Array size | k | g | upper | lower | I2 | Q | p |
| 2 stimuli | 15 | 0.37 | 0.15 | 0.59 | 45.38 | 1.30 | 0.25 |
| 4 stimuli | 4 | 0.87 | 0.04 | 1.70 | 83.09 | ||
| Type of variable | k | g | upper | lower | I2 | Q | p |
| Epoch | 4 | 0.12 | −0.27 | 0.51 | 55.54 | 3.84 | 0.05 |
| Event | 16 | 0.58 | 0.33 | 0.83 | 55.44 | ||
Note: GAD = generalized anxiety disorder; OCD = obsessive-compulsive disorder; SAD = social anxiety disorder; PTSD = post-traumatic stress disorder; SP = specific (spider) phobia; CI = confidence interval; significant effects (p < .05) in bold.
Maintenance hypothesis: Free viewing tasks
There was significant heterogeneity in the effect size for initial maintenance of gaze on threat in anxious compared to non-anxious individuals, Q (15) = 58.08, p < .001, I2 = 74.17. Anxious individuals did not differ overall in their initial maintenance of gaze on threat compared to non-anxious individuals (k = 16; g = −.17, p = .26, CI = −.47, .13; Figure 4). To account for the heterogeneity in initial maintenance of gaze on threat in anxiety, possible moderators were examined. Table 5 provides full results of the moderator analysis described below. Type of anxiety disorder significantly moderated group differences in initial maintenance of gaze on threat, Q (4) = 24.13, p < .001. Studies of spider phobia found significantly decreased maintenance of gaze on threat in anxious relative to non-anxious participants (k = 3; g = −1.02, p < .001, CI = −1.41, −.64; Q (2) = .05, p = .79, I2 = 0.0). Studies of generalized/trait anxiety found a similar yet marginally significant effect (k = 2; g = −.25, p < .10, CI = −.54, .04). Studies of contamination-based OCD (k = 2; g = −.24, p = .60, CI = −1.1, .66; Q (4) = 4.54, p < .05, I2 = 77.98) and social anxiety (k = 7; g = .05, p = .83, CI = −.42, .53; Q (4) = 23.45, p < .001, I2 = 74.41) were highly heterogeneous and did not find significant combined effects related to the initial maintenance of gaze. Studies of PTSD found marginally significant increased maintenance of gaze in anxious relative to non-anxious participants (k = 2; g = .60, p = .05, CI = .01, −1.21; Q (1) = .10, p = .76, I2 = 0.0).3
Figure 4.
Initial maintenance bias for threatening stimuli in anxious versus non-anxious individuals.
Table 5.
Categorical moderators of initial maintenance bias for threat in anxious versus non-anxious individuals
| Moderator | Effect size |
95% CI | Heterogeneity | Test of moderation | |||
|---|---|---|---|---|---|---|---|
| Type of group | k | g | upper | lower | I2 | Q | p |
| GAD | 2 | −0.25 | −0.54 | 0.04 | 0.00 | 24.65 | < .001 |
| OCD | 2 | −0.24 | −1.14 | 0.66 | 77.94 | ||
| PTSD | 2 | 0.60 | −0.01 | 1.21 | 0.00 | ||
| SAD | 7 | 0.05 | −0.41 | 0.52 | 74.42 | ||
| SP | 3 | −1.02 | −1.41 | −0.64 | 0.00 | ||
| Type of group | k | g | upper | lower | I2 | Q | p |
| Analogue | 13 | −0.18 | −0.52 | 0.16 | 75.62 | 0.01 | 0.91 |
| Clinical | 3 | −0.13 | −0.88 | 0.62 | 77.23 | ||
| Type of threat stimulus | k | g | upper | lower | I2 | Q | p |
| Face | 9 | 0.02 | −0.33 | 0.37 | 70.30 | 3.70 | 0.06 |
| Picture | 6 | −0.57 | −1.06 | −0.08 | 71.26 | ||
| Array size | k | g | upper | lower | I2 | Q | p |
| 2 stimuli | 11 | −0.12 | −0.46 | 0.21 | 71.49 | 0.01 | 0.94 |
| 4 stimuli | 4 | −0.16 | −1.01 | 0.69 | 85.05 | ||
| Type of variable | k | g | upper | lower | I2 | Q | p |
| Epoch | 8 | −0.11 | −0.53 | 0.32 | 77.87 | 0.18 | 0.67 |
| Event | 8 | −0.24 | −0.71 | 0.22 | 71.78 | ||
Note: GAD = generalized anxiety disorder; OCD = obsessive-compulsive disorder; SAD = social anxiety disorder; PTSD = post-traumatic stress disorder; SP = specific (spider) phobia; CI = confidence interval; significant effects (p < .05) in bold; marginal effects (p < .10) underlined.
Extended maintenance of gaze in depression
There was not significant heterogeneity in the extended maintenance of gaze on threatening, Q (5) = 1.9, p = .86, I2 = 0.0, or pleasant stimuli, Q (5) = .66, p = .97, I2 = 0.0; however, there was marginally significant heterogeneity in the extended maintenance of gaze on dysphoric stimuli, Q (5) = 9.77, p = .08 I2 = 48.82. As depicted in Figure 5, depressed individuals did not maintain gaze on threatening stimuli differently than non-depressed individuals during extended viewing (k = 6; g = .08, p = .50, CI = −.15, .31). However, depressed individuals maintained gaze on dysphoric stimuli longer than non-depressed individuals (k = 6; g = .46, p < .01, CI = .12, .80; FSN = 28). In addition, depressed individuals maintained gaze on positive stimuli less than non-depressed individuals (k = 6; g = −.80, p < .001, CI = −1.04, −.56; FSN = 84). Due to the moderate amount of heterogeneity in the extended maintenance bias for dysphoric stimuli, type of depressed group (clinical or analogue) was examined as a possible moderator. Studies of individuals meeting diagnostic criteria for major depressive disorder found a more robust maintenance bias for dysphoric stimuli in depression (k = 2; g = 1.07, p < .01, CI = .36, 1.80;), compared to studies of individuals meeting a cut-off score for a symptom measure (k = 4; g = .26, p < .06, CI = −.01, .53), Q (1) = 4.40, p < .05.
Figure 5.
Extended viewing of emotional stimuli in depressed versus non-depressed individuals.
Visual search tasks
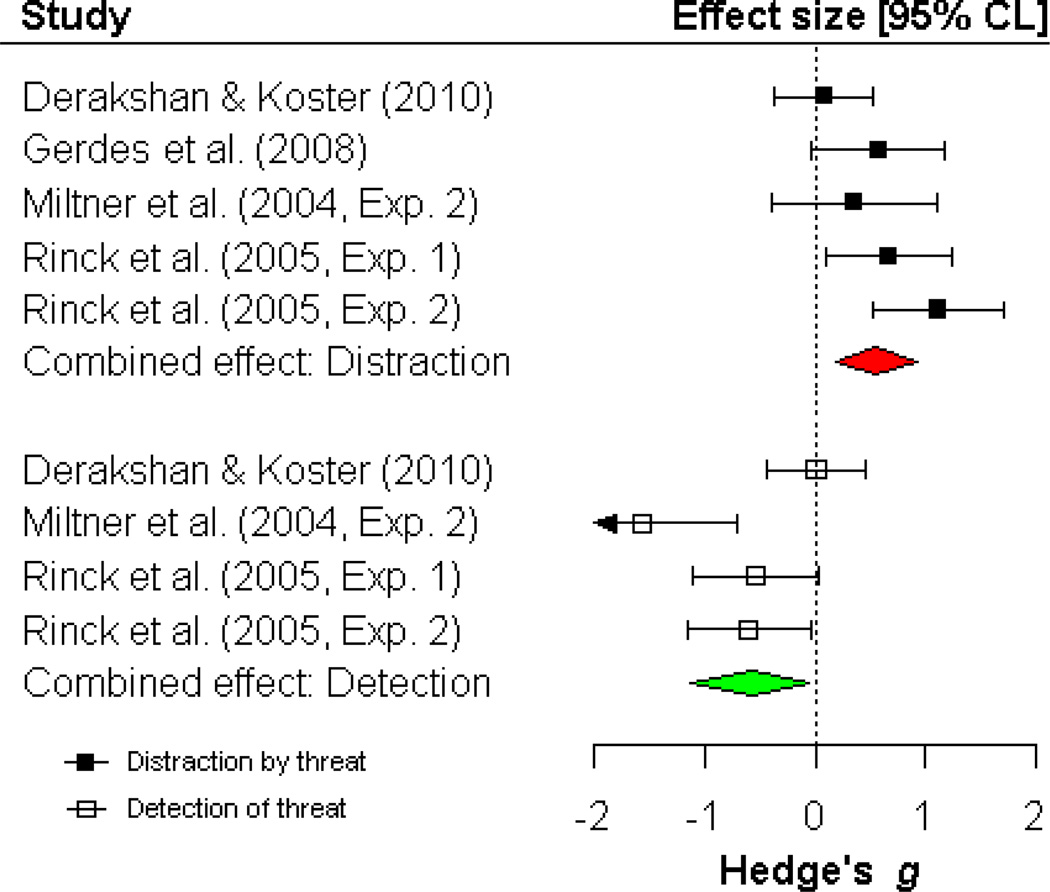
There was significant heterogeneity in the effect size for facilitated detection of threatening targets in anxious compared to non-anxious individuals, Q (3) = 10.89, p < .02, I2 = 72.46. Anxious individuals fixated threatening targets faster, compared to non-anxious individuals (k = 4; g = −.59, p < .04, CI = −1.15, −.04; FSN = 17). There was marginally significant heterogeneity in the effect size for increased maintenance of gaze on threatening distractors in anxious compared to non-anxious individuals, Q (4) = 8.28, p = .08, I2 = 51.66. Anxious individuals maintained attention longer on threatening distractors compared to non-anxious individuals (k = 5; g = .54, p < .01, CI = .17, .92; FSN = 26). Due to the small number of studies, no moderation analyses were conducted for visual search tasks. Meta-analytic findings for visual search tasks are displayed in Figure 6.
Figure 6.
Detection of threat and distraction by threat during visual search in anxious versus non-anxious individuals. For detection effects, negative values reflect facilitated detection in anxious versus non-anxious individuals. For distraction effects, positive values reflect increased distraction in anxious versus non-anxious individuals.
Discussion
The present review provides the first meta-analysis of EM biases for emotional stimuli in affective disorders. In studies of anxiety, the vigilance hypothesis was supported in both free viewing and visual search tasks: anxious individuals were characterized by an orienting bias towards threat during free viewing and facilitated detection of threat during visual search, compared to non-anxious individuals. An orienting bias for threat in free viewing did not characterize depressed individuals compared to non-depressed individuals, suggesting that vigilance for threat is specific to anxiety (Mogg & Bradley, 2005). In contrast, evidence for the maintenance hypothesis was inconsistent. In free viewing tasks, increased initial maintenance of gaze on threat was found only in studies of PTSD. Overall, anxious participants showed a marginal tendency to avoid maintaining gaze on threat compared to non-anxious individuals and this effect was particularly pronounced in spider phobia. However, in visual search tasks, anxious participants showed a strong tendency to maintain gaze longer on threatening distractors compared to non-anxious participants. Interestingly, this effect was based mostly on studies of spider phobic individuals, who showed the strongest tendency to avoid maintaining gaze on threat in free viewing tasks.
The present meta-analysis also has important implications for theoretical accounts of attention bias in depression. Depressed individuals showed a blunted orienting response to pleasant stimuli compared to non-depressed individuals, a bias not seen in anxious individuals compared to non-anxious individuals. An ‘anhedonic bias’ was also observed in the extended maintenance of gaze, where depressed individuals again showed reduced attention to pleasant stimuli, compared to non-depressed individuals. Depressed individuals also showed increased maintenance of gaze on dysphoric stimuli, but not on threatening stimuli, compared to non-depressed individuals.
Implications for the Vigilance hypothesis
The most frequently observed effect in eye tracking studies of anxiety disorders is a spatial orienting bias towards threat in anxious versus non-anxious individuals. The medium effect size of this bias (g = .47) is consistent with the effect size observed for anxious versus non-anxious individuals in Bar-Haim et al.’s (2007) review of RT measures of attentional bias for threat (d = .41). This bias appears to be specific to threatening stimuli, as anxious individuals did not show an orienting bias towards positive stimuli compared to non-anxious individuals, as would be predicted under the emotionality hypothesis (Calvo & Avero, 2005). The orienting bias towards threat in anxiety was heterogeneous in nature, as would be expected given the methodological variance in the free viewing studies reviewed. However, the moderator analysis provided limited insight into this variance. The only factor that appeared to moderate the orienting bias towards threat was its operational definition. Studies reporting only an epoch-related indicator (i.e., fixation duration in initial 500 ms) had difficulty observing the bias, compared to studies using an event-related indicator (i.e., proportion of initial fixations captured). Epoch-related indicators may be too indirect to reliably capture the orienting of gaze, despite two studies finding convergence between event- and epoch-related measures of orienting (Armstrong et al., 2010; Rinck & Becker, 2006).
The robust orienting bias for threat observed in free viewing studies of anxiety is consistent with models of attentional bias emphasizing facilitated detection of threat through exogenous shifts of attention. The studies reviewed herein cannot rule out the possibility that orienting to threat was endogenous in anxious individuals (see Calvo & Lang, 2005); this would require documenting the bias in the presence of voluntary efforts to inhibit orienting towards threat. However, previous research suggests that orienting to an emotional image in this task context is largely unaffected by contrary instructions to look at an accompanying neutral image (Nummenmaa et al., 2006). Further, a study that measured EMs during an emotional spatial cueing task found that anxious individuals involuntarily oriented gaze to threat cues more frequently than non-anxious individuals, despite instructions not to orient gaze to the cues (i.e., instructions to maintain central fixation; Broomfield & Turpin, 2005).
However, this overt orienting bias towards threat may not reflect purely exogenous attentional capture, which would begin pre-attentively, at the earliest stages of sensory processing (Corbetta & Shulman, 2002). The average latency of initial fixations on threat in anxious individuals ranged from roughly 225 to 400 ms (Armstrong et al., 2010; Garner et al., 2006; Mogg et al., 2000). As Nummenmaa and colleagues (2006) note, reflexive saccades typical of purely exogenous capture (e.g., resulting from the sudden onset of a stimulus with a sharply contrasting basic feature) have a latency of 150 to 175 ms (Rayner, 1998). The saccade latencies observed in studies of anxiety suggest a brief window of parafoveal processing in which some semantic content could be derived through covert attention. Such processing has been shown to be capable of guiding EMs on the basis of specific emotional content that goes beyond mere valence (i.e., positive versus negative; Calvo & Nummenmaa, 2007). This is consistent with findings that anxious individuals orient gaze specifically to symptom-related threat as opposed to more generally threatening stimuli (e.g., Armstrong et al., 2011).
The analysis of visual search also suggested that overt orienting to threat in anxiety differs from more prototypical cases of exogenous attentional capture. In the visual search studies reviewed herein, there was no report of facilitated detection of threat targets in the initial fixation of a trial. Thus, the increased perceptual load and/or the greater potential eccentricity of stimuli associated with visual search arrays appeared to eliminate the initial orienting bias that could be observed in smaller arrays associated with free viewing tasks. Although anxious individuals did not immediately orient to threatening visual search targets, they did fixate on these targets sooner than non-anxious individuals. Such facilitated overt threat detection in anxiety may still involve an exogenous orienting bias, albeit one that is capacity-limited, and only effective once gaze is directed to a nearby distracter, bringing the threatening stimulus within a range of eccentricity from which it can attract attention under perceptual load.
However, the present findings leave open the possibility that facilitated threat detection in anxiety also involves more rapid disengagement from neutral stimuli, a process that may rely on endogenous attentional control. Whereas an exogenous orienting bias for threatening targets would result in fewer fixations on neutral distractors, facilitated detection of threatening targets could also be achieved through shorter fixations on neutral distractors (c.f. Rinck & Becker, 2006). One component of hypervigilance for threat in anxiety may be the ability to rapidly disengage from neutral stimuli during the search for threat, a process that would draw on the endogenous, top-down control of attention. Becker (2009), for example, found more rapid disengagement from neutral stimuli following the presentation of a threatening stimulus in an unselected sample. In anxious individuals, this so-called ‘panic search’ may be triggered when individuals anticipate the presence of threat. Unfortunately, the studies reviewed did not report the number and duration of fixations on distractors with enough consistency to fully address this question.
Implications for the maintenance hypothesis
Support for the maintenance hypothesis was far less consistent than support for the vigilance hypothesis. Contrary to the maintenance hypothesis, anxious individuals overall did not show increased initial maintenance of gaze on threat compared to non-anxious individuals in free viewing tasks. However, an initial maintenance bias for threat was found in PTSD during free viewing. This was in sharp contrast to studies of spider phobia, which found a strong tendency to avoid initially maintaining gaze on threat in anxious versus non-anxious individuals. Gaze avoidance may be particularly strong in spider phobia because spider fearful individuals find spiders disgusting (e.g., Mulkens, de Jong, & Merckelbach, 1996), and may be revolted by the perceptual features of spiders (Royzman & Sabini, 2001). Also, differences in the nature of threat encountered in PTSD and spider phobia may help explain this discrepancy. Threats related to traumatic events (combat: Kimble et al., 2012; physical assault: Felmingham et al., 2011) present greater urgency and danger compared to spiders, and thus may be more difficult to ignore. Whereas spider fearful individuals may learn to cope with the presence of spiders by looking elsewhere, individuals with PTSD may perceive the need to maintain attention on trauma cues to avoid immediate harm.
The tendency to avoid maintaining gaze on spiders in spider phobia may reverse when the urgency of threat increases. For example, Lange, Tierney, Reinhardt-Rutland, and Vivekananda-Schmidt (2004) presented a live spider to individuals high and low in spider fear. The spider was presented on either side of a television presenting a program that participants were instructed to watch carefully. Trials lasted 3 minutes, and increased maintenance of gaze on the spider, as well as the room’s exit, was found in high versus low spider fearful individuals. The sustained maintenance of gaze on real spiders found byLange et al. (2004) contrasts with the sustained avoidance of gaze on mere images of spiders found over 3 s (Hermans et al., 1999; Rinck & Becker, 2006), 9 s (Pflugshaupt et al., 2007) and 60 s (Rinck & Becker, 2006) trials. One might conclude that Lange et al.’s findings cast doubt on the clinical significance of attentional avoidance of threatening images, because the phenomenon may be limited to encounters with mere representations of threat. However, an alternative explanation is that anxious individuals decide whether or not to sustain attention on threat according to a cost-benefit analysis. Threatening stimuli, as danger cues, likely occur on a continuum of urgency. Anxious individuals may risk ignoring low urgency danger cues (e.g., an image of a spider) in exchange for the negative reinforcement of anxiety reduction (Mogg & Bradley, 1998). However, these individuals may feel compelled to continue monitoring more urgent danger cues (e.g., a live tarantula in a nearby box) at the cost of increased anxiety. Under this scenario, attentional avoidance could still contribute to anxiety disorders by preventing reappraisal and fear extinction of less urgent danger cues (e.g. an innocuous spider in one’s bedroom), which may be encountered more frequently and thus cause greater impairment.
Although a maintenance bias on threat in anxiety was not consistently observed during free viewing, it was observed for threatening distractors during visual search for non-threatening targets. Compared to non-anxious individuals, anxious individuals were slower locating neutral targets accompanied by a threatening distractor. Although this finding could reflect more frequent shifting of gaze to threatening distractors (Miltner et al., 2008), rather than difficulty disengaging gaze from these distractors (Rinck & Becker, 2006), Gerdes, Alpers, and Pauli (2008) found that spider phobics were slower to detect a neutral target due to longer fixations rather than more frequent fixations on a spider distractor, suggesting difficulty disengaging attention from threat. Interestingly, the spider phobics in Gerdes et al.’s (2008) study showed increased fixations to all distractor types, compared to controls. This finding is consistent with RT research suggesting that when spider phobics anticipate the possibility of a spider distractor, the stimulus-driven system becomes dominant, leading to increased exogenous shifting to all distractors (Devue, Belopolsky, & Theeuwes, 2011). Inhibitory control deficits related to anxiety (Eysenck et al., 2007) may be more threat-specific in the engagement of attention compared to the shifting of attention because more information is available once a stimulus is engaged, reducing ‘false alarms.’
The present meta-analytic findings suggest that difficulty disengaging attention from threat in anxiety may only be observed in certain viewing contexts. The critical contextual factor may be whether or not participants are explicitly required to disengage attention from threat. When threatening stimuli are presented as distracters during visual search, participants must disengage from these stimuli in order to pursue the neutral target. In this context, there is task incentive to disengage attention from threat that applies evenly to all participants. In a free viewing task, there is no task incentive to disengage attention from threat, yet there may be an affective incentive, as threat stimuli arouse anxiety or fear. Given that anxious individuals rate threatening stimuli as more unpleasant (e.g., Rinck & Becker, 2006), and have been observed to voluntarily terminate exposure to threatening images sooner compared to non-anxious individuals (Tolin, Lohr, Lee, & Sawchuk, 1999), it follows that anxious individuals have a greater affective incentive to disengage attention from threat, and may exert more effort towards this end. This additional effort may compensate for, and thus conceal, difficulty disengaging attention from threat in anxious individuals (Ansari & Derakshan, 2011b), reducing the sensitivity of free viewing tasks in detecting this bias. This explanation is inline with the distinction between “effectiveness” and “efficiency” posited by ACT (Eysenck et al., 2006). As Berggren and Derakshan (in press) explain, anxious individuals may be able to disengage attention as effectively as non-anxious individuals in certain contexts, but such disengagement is less efficient, as anxious individuals apply more effort in order to compensate for attentional control deficits. This distinction may be best observed in the anti-saccade task. Derakshan, Ansari, Hansard, Shoker, and Eysenck (2009) found that anxious individuals were able to execute saccades away from a threatening face as effectively as non-anxious individuals (i.e., with similar error rates); however, their correct saccades away from threatening faces had longer latencies, suggesting less efficiency disengaging covert attention from threatening faces in order to execute a saccade to an opposite location.
Gaze biases in anxiety compared to depression
The present meta-analysis has several implications for conceptualizing the differences between attentional biases in anxiety and depression. Whereas a robust orienting bias towards threat was found in anxiety, no orienting bias towards threat was observed in depression. This finding is consistent with a recent meta-analysis of RT studies of attentional bias in depression, which found no bias for threatening stimuli in depressed compared to non-depressed individuals (Peckham et al., 2010; see also Mogg & Bradley, 2005). Also, whereas anxious individuals showed a slight increase in orienting to positive stimuli compared to non-anxious individuals, depressed individuals showed a tendency to orient gaze less to positive stimuli, compared to non-depressed individuals. This finding is also consistent with Peckham et al.’s (2010) recent review, which found reduced initial attention allocation to positive stimuli in depressed individuals (c.f., Derakshan et al., 2009b). This convergent evidence from EM and RT measures suggests that anxiety and depression have distinct effects on the exogenous capture of attention. Anxiety appears to increase the exogenous system’s sensitivity to threatening stimuli, whereas depression appears to decrease the system’s sensitivity to positive stimuli.
In tripartite structural theories of internalizing disorders (e.g., Clark & Watson, 1991), the development of anxiety and depression can be understood in terms of a general factor (e.g., negative affect or neuroticism) that confers risk for both disorders, as well as disorder-specific factors, such as anxious arousal or low positive affect, which specifically confer risk for anxiety or depression, respectively (Oehlberg, Revelle, & Mineka, 2011). The presence of an orienting bias towards threat in anxiety, but not depression, suggests that vigilance for threat is related to an anxiety specific factor, as opposed to a general factor of internalizing. Similarly, the presence of reduced orienting towards positive stimuli in depression, but not anxiety, suggests that an anhedonic bias is related to a depression-specific factor, as opposed to a more general risk factor for internalizing disorders, such as negative affect. The depression-specific factor most commonly cited is low positive affect (e.g., Clark & Watson, 1991), which involves difficulty experiencing pleasure and reduced sensitivity to rewards. Low positive affect may cause the exogenous system to be insensitive to reward cues, leading to a deficit in orienting towards positive stimuli.
The present study could not determine if extended viewing biases related to positive stimuli were unique to depression; however, it seems likely that the strong anhedonic bias in extended viewing in depression is also related to low positive affect. Depressed individuals may voluntarily maintain gaze less on positive stimuli because they are less sensitive to the intrinsic pleasantness of the stimuli, reducing their incentive to maintain gaze. This is consistent with ERP research suggesting happy faces are less salient and receive less attention in depressed individuals, as revealed by the P300, an early component reflecting endogenous processing (Cavanagh & Geisler, 2006). ERP research on working memory in depression may also provide insight into decreased extended maintenance of gaze on positive stimuli. Deldin, Deveney, Kim, Casas, and Best (2001) found that individuals with major depressive disorder show a reduced slow wave (SW) component while maintaining positive adjectives in working memory, compared to controls. The SW component may reflect elaborative processing and other working memory processes between 1 and 5 s after stimulus onset. Behavioral research has also found that depression is characterized by decreased maintenance of positive stimuli in working memory relative to controls (Levens & Gotlib, 2010). Working memory content has been found to guide selective attention (e.g., Downing, 2000), suggesting a possible top-down process in depression in which decreased maintenance of positive stimuli in working memory leads to reduced maintenance of gaze on pleasant stimuli. Reduced attention to positive stimuli could have important clinical implications. Failing to notice or elaborate on positive events could have proximal effects on state affect, depriving depressed individuals of potential pleasure. In addition, these biases could have more distal effects on cognitive schemata. For example, Ellis, Beevers, and Wells (2011) found that reduced gaze towards positive stimuli in depression led to reduced memory for positive stimuli. Over time, failing to attend to and remember positive stimuli could cause depressed individuals to view the world as less abundant with rewards (Gotlib & McCan, 1984; Shane & Peterson, 2007).
Depressed individuals also showed a tendency to maintain gaze on dysphoric stimuli longer than non-depressed individuals during extended viewing (10 to 30 s), and this bias was most pronounced in depressed individuals meeting full diagnostic criteria for major depressive disorder. This finding has generally been interpreted as evidence of increased elaborative processing of dysphoric content in depression (Eizenmann et al., 2003; Kellough et al., 2008; Leyman, De Raedt, Vaeyens, & Philippaerts, 2011). Increased retention of dysphoric content in working memory may drive the extended maintenance of attention on dysphoric stimuli, through the same top down influence of working memory on attention that may underlie the lack of dwell on positive stimuli in depression. Depressed individuals exhibit difficulty removing negative content from working memory (Levens & Gotlib, 2010), and this phenomenon has been observed over a 10 s time window (Joorman & Gotlib, 2008) similar to the time window of extended viewing in many of the studies of depression reviewed herein. If an attentional bias towards dysphoric content in depression indeed requires the maintenance of negative content in working memory, it could explain why an initial orienting bias towards dysphoric content in depression was not found, as such a bias would operate before negative content begins to “stick” in working memory. On this account, the extended maintenance bias for dysphoric stimuli in depression would relate to a deficit in the updating mechanism of attentional control, which manages the content of working memory (Eysenck et al., 2007). This may contrast with the initial maintenance bias in anxiety, which appears to relate to a deficit in the inhibition function, which overrides stimulus-driven effects in attention allocation.
Clinical implications: Eye tracking and attention modification procedures
Recently there have been a number of studies demonstrating that training attention away from threat leads to functional improvement in anxiety (Hallion & Ruscio, 2011), and one study suggests an analogous effect related to dysphoric stimuli in depression (Wells & Beevers, 2010). The present findings may have important implications for attention-based treatment of anxiety. Concerns have been raised about training attention away from threat in anxiety, as it could engender attentional avoidance of threat, which is believed to impair emotional processing and maintain anxiety (Koster, Baert, Bockstaele, & De Raedt, 2010). Indeed, Koster et al., (2010) found that training attention away from threat did not affect vigilance for threat in the modified dot probe (100 ms SOA); however, it did lead to later avoidance of threat (1500 ms SOA), a bias similar to that observed in spider phobia in the present meta-analysis. The congruence between attention training away from threat and attentional avoidance in spider phobia may explain why several studies have failed to observe beneficial effects of attention training in this disorder (Harris & Menzies, 1998; Reese, McNally, Najmi, & Amir, 2010; Van Bockstaele et al., 2011). It is possible that attention training towards threat (Klumpp & Amir, 2010) would be more useful in treating spider phobia and perhaps other specific phobias as well.
The present findings may also have implications for training attention in depression. The finding of an attentional bias in the extended maintenance of gaze supports the use of longer stimulus presentations in the training of attention in depression. Indeed, training attention over longer viewing windows (e.g., 3 s, 4.5 s; Wells & Beevers, 2010) appears to be more effective at modifying attentional bias in depression and reducing symptoms of depression, compared to training over a shorter viewing period (1.5 s; Baert, De Raedt, Schacht, & Koster, 2010). Further, the finding of decreased attention to positive stimuli in depression suggest that researchers should consider training attention toward positive stimuli in depressed individuals (Baert et al., 2010), in addition to training attention away from negative stimuli (Wells & Beevers, 2010). A positivity bias appears to promote resilience (see Wadlinger & Isaacowitz, 2011), and initial evidence suggests that biases for positive stimuli can be induced through training and may have beneficial effects (Wadlinger & Isaacowitz, 2008).
Lastly, eye tracking could help determine the potential role of overt attention in attention training. Currently, it is unclear to what extent individuals make EMs during attention training. In the unweighted version of the dot probe (which is used as a control condition in training studies), EMs to either cue have been found to occur on 30% of trials overall; some individuals make EMs on more than half of trials, whereas others hardly make any EMs (Bradley, Mogg, & Millar, 2000). The efficacy of training may be increased by requiring EMs (through instructions or greater stimulus eccentricity). Given that EMs are the primary means of visual selection in daily life, and that covert attentional shifts do not assure EMs, it is possible that training effects would generalize more to everyday contexts if EMs were required. Also, the additional resources required to make an EM may increase the ‘resistance’ of the exercise, increasing the strength of attentional control gained through training.
Limitations
Eye tracking and covert attention
Although eye tracking addresses many of the shortcomings of RT measures in the study of attentional biases, the measurement of EMs has limitations of its own. Most notably, attentional resources may be allocated covertly, in the absence of saccadic EMs registered by eye tracking. This limitation may have the greatest implications for tests of the maintenance hypothesis. AsGarner et al. (2006) note, eye tracking experiments may fail to observe increased maintenance of attention on threatening stimuli because the phenomenon could occur covertly, prior to initial fixation. These authors cite Fox et al.’s (2001) spatial cueing study, which found greater difficulty disengaging covert attention from incongruent threat cues presented at 100 ms or 250 ms SOAs in anxious individuals compared to controls. Similarly, Fox, Derakshan, and Shoker (2008) found that anxious individuals compared to controls showed an enhanced early N2pc ERP in response to threatening stimuli, a component that reveals covert attention-related activity in the parietal cortex occurring 170–220 ms after stimulus onset. In free viewing tasks, EMs to threatening stimuli were not completed until roughly 225 ms to 400 ms after stimulus onset (Armstrong et al., 2010; Garner et al., 2006; Mogg et al., 2000), suggesting that increased maintenance of visual attention on threat could occur prior to one’s initial fixation on threat. Thus, free viewing studies may fail to observe increased maintenance of attention on threat because the phenomenon occurs early in the covert deployment of attention, and thus eludes EM data. However, if the maintenance bias in anxiety were limited to covert attention in the first 250 ms of stimulus processing, it would call into question the functional significance of the bias.
Limitations of the eye tracking literature
Eye tracking research in affective disorders has been conducted for just over a decade and did not gain momentum until recent years. As a result, some of the analyses in the present study were based on a small number of studies and findings should be interpreted with appropriate caution. Although the analyses of initial orienting towards threat (k = 20) and maintenance of attention on threat (k = 16) in anxiety drew on a moderate number of studies, there were far fewer studies in the analysis of orienting towards threatening (k = 4) and positive stimuli in depression (k = 6), as well as in the analysis of extended maintenance of gaze in depression (k = 6). However, effect sizes were mostly consistent across studies of depression, and the combined effects were largely consistent with RT research on attentional biases in depression (Peckham et al., 2010). There were also a small number of studies in the analysis of visual search tasks (k = 5), and these studies consisted almost entirely of studies of spider phobias (k = 4), raising concern about the generalizability of the present findings to other anxiety disorders.
Another limitation of extant research is that few studies examined attention to threat beyond three seconds of exposure, and many studies employed even shorter trials. Thus, a more thorough investigation of the time course of attentional bias for threat—one of the chief promises of eye tracking methodology— could not be fully realized in the present analysis. However, from the few studies that did employ longer trials, it appears that trends present in the initial maintenance of attention continue over a larger time scale. For example, the present finding of decreased maintenance of gaze on threat in spider phobia was found to hold throughout the course of longer trials (9 s: Pflugshaupte et al., 2007; 60 s: Rinck & Becker, 2006). Also, the present finding of increased initial maintenance of gaze on threat in PTSD was found to continue over 10 s trials (Kimble et al., 2010).
Future Directions
Refining the vigilance hypothesis
The vigilance hypothesis holds that anxious individuals detect threat more readily than non-anxious individuals. The present review firmly established an overt orienting bias as one mechanism of vigilance for threat in anxiety. However, the extant literature leaves much to be learned about the means of facilitated threat detection. For example, the boundary conditions of increased orienting to threat in anxiety have not been clearly outlined. Basic research suggests that the ability of threatening stimuli to capture gaze is affected by how many competing stimuli are present (perceptual load; Yates et al., 2010) and how far into the periphery stimuli are presented (eccentricity; Calvo, Nummenmaa, & Hyönä, 2006). In the analysis of vigilance for threat in visual search tasks, the absence of an initial orienting bias for threat in anxiety suggests that such boundary conditions are present, and likely depend on one of these factors or their interaction (Acunzo & Henderson, 2011). Future eye tracking research that systematically varies these parameters over a wide range of values would help delineate these boundary conditions with more precision. Such findings could inform theoretical models of attentional bias and provide a better sense of the prevalence of this phenomenon in the lives of anxious individuals. Another important question regarding orienting towards threat in anxiety is its relation to endogenous attentional control (Derryberry & Reed, 2002). In free viewing tasks, participants are not required to resist orienting to threat, and thus it is unclear to what extent the bias is moderated by deficits in endogenous attentional control, which have consistently been found in anxiety disorders (e.g., Armstrong, Zald, & Olatunji, 2011). The free viewing paradigms reviewed in the present study could be modified with instructions to make saccades in a specified direction (Nummenmaa, Hyönä, & Calvo, 2009) or towards a specified stimulus (Nummenmaa et al., 2006), requiring participants not to orient gaze towards threat.
Refining the maintenance hypothesis
The present meta-analytic findings suggest that increased maintenance of gaze on threat in anxiety is best observed in a task in which all participants are explicitly required to disengage attention from threat. In addition to visual search tasks, endogenous cueing paradigms provide another means of requiring disengagement from threat (e.g., Sears, Thomas, LeHuquet, & Johnson, 2010). An endogenous cueing test of the maintenance bias could present disorder-relevant stimuli centrally, and then present a tone at random SOAs that required shifting attention to a peripheral location. Saccade latencies following the tone would provide a direct indicator of delayed disengagement. A somewhat similar design was employed by Sears and colleagues (2010) in their study of attentional biases for dysphoric stimuli in depression. In their task, a single stimulus appeared in one of four locations, and on 25% of trials, a central arrow cue directed attention toward a corner of the screen. Sears et al. (2010) found that depressed individuals were slower initiating saccades away from dysphoric images, but not other image types (i.e., threatening, pleasant, neutral) compared to non-depressed individuals, a maintenance bias that was not revealed in a free viewing condition of their study. Testing of the maintenance hypothesis would also benefit from the inclusion of an independent assessment of endogenous attentional control, such that the role of attentional control deficits could be parsed from the role of bottom-up signal enhancement for threatening stimuli. The antisaccade task, which has been shown to reveal general inhibitory deficits related to anxiety (Ansari & Derakshan, 2011a) and depression (Lissnyder et al., 2011), would be ideal for these purposes. The antisaccade task can also be modified to include emotional stimuli, in order to reveal inhibitory deficits specific to affective contents in anxiety (Derakshan et al., 2009a) and depression (Derakshan et al., 2009b).
Refining assessment of attentional avoidance
In the present review, the majority of studies examining attentional avoidance measured fixation duration on stimuli as a function of time. However, attentional avoidance may also be seen in the distance between a fixation point and the nearest threatening stimulus. Pflugshaupte et al. (2005) embedded spiders in images of household scenes and found evidence of avoidance in the distance between a fixation’s coordinates and the nearest spider; further, they examined this relationship as a function of the ordinal number of the fixation, which provided a novel window into the vigilant-avoidant style of attention in anxiety (Mogg & Bradley, 1998). One advantage of embedding threatening stimuli in naturalistic scenes is the variety of locations that can be fixated, which create a spectrum of distance from threat. Embedding threatening stimuli in natural scenes also provides greater insight into how attentional biases operate in “real life” environments, insight which may be unique to eye tracking methodology.
Moving beyond the vigilance and maintenance hypotheses
A large majority of the eye tracking studies reviewed herein were framed as tests of the vigilance and maintenance hypotheses. These purportedly competing hypotheses have been useful for advancing research on attentional biases in anxiety disorders, in that both offer mutually exclusive predictions that are easily testable. Further, the vigilance and maintenance hypothesis are both well-grounded in theory and have been linked to plausible neural substrates (Cisler & Koster, 2010). However, these hypotheses are not mutually exclusive (Weierich et al., 2008). Further, the present analysis revealed that the expression of each bias is context dependent, such that a paradigm will likely favor one hypothesis over the other. The value of pitting these hypotheses against each other may therefore be questionable, particularly when a study employs only one type of task. A more fruitful approach would involve testing the conditions under which either hypothesis holds. Although this approach has not been applied to anxiety disorders,Sears et al. (2010) eye tracking study of depression, which included an effortful disengagement condition in addition to a free viewing condition, provides an illustrative example.
In addition, an over-reliance on broad constructs, such as vigilance, maintenance, or avoidance in the interpretation of EM data has the potential to obscure underlying attentional mechanisms. As noted in the present review, facilitated detection of threat (i.e., vigilance) could derive from an orienting bias for threat or from rapid disengagement from neutral distractors; increased attention on dysphoric stimuli (i.e., maintenance) could occur through voluntary or involuntary attentional engagement; and attentional avoidance could be achieved by rapid disengagement from threat or by maintenance of gaze at a distant location. Theoretical accounts of attentional bias would benefit from a shift in emphasis from attentional outcomes (e.g., facilitated detection of threat) to the processes that determine these outcomes. An over-reliance on broad attentional constructs can also stifle the discovery and exploration of novel gaze biases. For example, there appear to be gaze biases related to the encoding of information that may have clinical significance through their impact on memory. Hyperscanning of threat (greater distance between fixations) has been found in social anxiety disorder for threatening faces (Horley et al., 2002, 2004), and in dysphoria, this pattern of attention for angry faces has been found to increase memory bias for angry faces (Wells, Beevers, Robison, & Ellis, 2010). Likewise, shorter fixations on threat, but not increased overall dwell time, has been found in contamination fear (Armstrong et al., 2012), and this fragmented viewing style was found to predict vulnerability to PTSD in response to combat stress (Beevers, Lee, Wells, Ellis, & Telch, 2011). Whereas hyperscanning may increase retention of threat in memory, fragmented viewing may interfere with habituation to threat. Although these conclusions are highly tentative, such findings highlight the potential for EMs to reveal pathways to abnormal emotional processing that lie beyond the scope of extant constructs.
Highlights.
We conduct the first meta-analysis of eye tracking research on anxiety and depression
Whereas anxiety was characterized by increased orienting to threat, depression was characterized by reduced orienting to positive stimuli
Maintenance of gaze on threat in anxiety was moderated by type of anxiety disorder and type of task
Depression was characterized by increased elaborative processing of dysphoric content and reduced elaborative processing of positive content
Findings provide insight into attentional mechanisms and vulnerability factors related to attentional biases in affective disorders
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Although few studies have examined the concordance between RT and EM measures of attentional bias in the affective disorders, two studies (Bradley, Mogg, & Millar, 2000; Mogg, Garner, & Bradley, 2007)) have found that an attentional bias score (RT) for emotional stimuli in the modified dot probe task is correlated with directional orienting bias when EMs are recorded in the 500 ms version of this task.
Mogg et al.’s (2000) study included a GAD, MDD, and control group. For this study, the same control group was used to determine the between-groups effect size of the orienting bias for GAD versus controls and for MDD versus controls. Excluding this study did not change the outcome of the analyses comparing depression and anxiety. Thus, no attempt to correct for this small violation of independence was made.
Initial maintenance of gaze on threatening stimuli in anxious versus non-anxious participants was marginally moderated by the type of threatening stimulus employed (face versus picture), Q (1) = 3.39, p < .06. However, this finding appeared to be an artifact of the exclusive use of pictures in studies of spider phobia (k = 3), in which a strong bias away from threat was found. When the analysis was repeated with studies of spider phobia removed, the moderation by stimulus type was no longer significant, Q (1) = .34, p = .56, and there was no longer a significant combined effect in the remaining studies that used threatening pictures, k = 3; g = −.12, p = .72, CI = −.78, .54; Q (8) = 6.50, p < .05, I2 = 69.24.
References
*References marked with an asterisk indicate studies included in the qualitative meta-analysis.
- Acunzo DJ, Henderson JM. No emotional "Pop-out" Effect in natural scene viewing. Emotion. 2011;11:1134–1143. doi: 10.1037/a0022586. [DOI] [PubMed] [Google Scholar]
- Algom D, Chajut E, Lev S. A rational look at the emotional Stroop phenomenon: A generic slowdown, not a Stroop effect. Journal of Experimental Psychology: General. 2004;133:323–338. doi: 10.1037/0096-3445.133.3.323. [DOI] [PubMed] [Google Scholar]
- Amir N, Najmi S, Morrison AS. Attenuation of attention bias in obsessive-compulsive disorder. Behaviour Research and Therapy. 2009;43:153–157. doi: 10.1016/j.brat.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari TL, Derakshan N. The neural correlates of impaired inhibitory control in anxiety. Neuropsychologia. 2011a;49:1146–1153. doi: 10.1016/j.neuropsychologia.2011.01.019. [DOI] [PubMed] [Google Scholar]
- Ansari TL, Derakshan N. The neural correlates of cognitive effort in anxiety: Effects on processing efficiency. Biological Psychology. 2011b;86:337–348. doi: 10.1016/j.biopsycho.2010.12.013. [DOI] [PubMed] [Google Scholar]
- *.Armstrong T, Olatunji BO, Sarawgi S, Simmons C. Orienting and maintenance of gaze in contamination fear: Biases for disgust and fear cues. Behaviour Research and Therapy. 2010;48:402–408. doi: 10.1016/j.brat.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Armstrong T, Sarawgi S, Olatunji BO. Attentional bias towards threat in contamination fear: Overt components and behavioral correlates. Journal of Abnormal Psychology. 2012;131:232–237. doi: 10.1037/a0024453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong T, Zald DH, Olatunji BO. Attention control in OCD and GAD: Specificity and associations with core cognitive symptoms. Behaviour Research and Therapy. 2011;49:756–762. doi: 10.1016/j.brat.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baert S, De Raedt R, Schacht R, Koster EHW. Attention bias training in depression: therapeutic effects depend on depression severity. Journal of Behavior Therapy and Experimental Psychiatry. 41:265–274. doi: 10.1016/j.jbtep.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Bannerman RL, Milders MV, Sahraie A. Processing emotional stimuli: Comparison of saccadic and manual choice-reaction times. Cognition and Emotion. 2009;23:930–954. [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and non-anxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Beck AT. Cognitive therapy and the emotional disorders. New York: International Universities Press; 1976. [Google Scholar]
- Becker MW. Panic Search: Fear Produces Efficient Visual Search for Non-threatening Objects. Psychological Science. 2009;20:435–437. doi: 10.1111/j.1467-9280.2009.02303.x. [DOI] [PubMed] [Google Scholar]
- Becker ES, Rinck M, Margraf J, Roth WT. The emotional Stroop effect in anxiety disorders: General emotionality or disorder specificity? Journal of Anxiety Disorders. 2001;15:147–159. doi: 10.1016/s0887-6185(01)00055-x. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Lee H-J, Wells TT, Ellis AJ, Telch MJ. Eye gaze bias for emotion stimuli prospectively predicts PTSD, depression, and anxiety among soldiers deployed in Iraq. American Journal of Psychiatry. 2011;168:735–741. doi: 10.1176/appi.ajp.2011.10091309. [DOI] [PubMed] [Google Scholar]
- Berggren N, Derakshan N. Attentional control deficits in anxiety: Why you see them and why you don't. Biological Psychology. doi: 10.1016/j.biopsycho.2012.03.007. (in press) [DOI] [PubMed] [Google Scholar]
- Bishop SJ. Trait anxiety and impoverished prefrontal control of attention. Nature Neuroscience. 2009;12:92–98. doi: 10.1038/nn.2242. [DOI] [PubMed] [Google Scholar]
- Borenstein MJ, Hedges LV, Higgins JPT, Rothstein HR. Comprehensive Meta-analysis (Version 2) Englewood Cliffs, NJ: Biostat, Inc; 2005. [Google Scholar]
- Bradley BP, Mogg K, Lee SC. Attentional biases for negative stimuli in induced and naturally occurring dysphoria. Behaviour Research and Therapy. 1997;35:911–927. doi: 10.1016/s0005-7967(97)00053-3. [DOI] [PubMed] [Google Scholar]
- *.Bradley BP, Mogg K, Millar NH. Covert and overt orienting of attention to emotion faces in anxiety. Cognition and Emotion. 2000;14:789–808. [Google Scholar]
- Broomfield NM, Turpin G. Covert and overt attention in trait anxiety: A cognitive psychophysiological analysis. Biological Psychology. 2005;68:179–200. doi: 10.1016/j.biopsycho.2004.04.008. [DOI] [PubMed] [Google Scholar]
- *.Buckner JD, Maner JK, Schmidt NB. Difficult Disengaging Attention from Social Threat in Social Anxiety. Cognitive Therapy and Research. 2010;34:99–105. doi: 10.1007/s10608-008-9205-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Calvo MG, Avero P. Time course of attentional bias to emotional scenes in anxiety: Gaze direction and duration. Cognition and Emotion. 2005;19:433–451. doi: 10.1080/02699930441000157. [DOI] [PubMed] [Google Scholar]
- Calvo MG, Lang PJ. Parafoveal semantic processing of emotional visual scenes. Journal of Experimental Psychology: Human Perception and Performance. 2005;31:502–519. doi: 10.1037/0096-1523.31.3.502. [DOI] [PubMed] [Google Scholar]
- Calvo MG, Nummenmaa L, Hyönä J. Emotional scenes in peripheral vision: Selective orienting and gist processing, but not content identification. Emotion. 2008;8:68–80. doi: 10.1037/1528-3542.8.1.68. [DOI] [PubMed] [Google Scholar]
- *.Caseras X, Garner M, Bradley BP, Mogg K. Biases in visual orienting to negative and positive scenes in dysphoria: An eye movement study. Journal of Abnormal Psychology. 2007;116:491–497. doi: 10.1037/0021-843X.116.3.491. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Bacon AK, Williams NL. Phenomenological characteristics of attentional biases towards threat: A critical review. Cognitive Therapy and Research. 2009;33:221–234. doi: 10.1007/s10608-007-9161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Koster EHW. Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clinical Psychology Review. 2010;30:203–216. doi: 10.1016/j.cpr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. Journal of Abnormal Psychology. 1991;100:316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale. NJ: Erlbaum; 1988. [Google Scholar]
- Cooper H, Hedges LV. The handbook of research synthesis. New York: Russell Sage Foundation; 1994. [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- De Lissnyder, Derakshan N, De Raedt R, Koster EHW. Depressive symptoms and attentional control in a mixed antisaccade task: The specific effects of rumination. Cognition & Emotion. 2011;25:886–897. doi: 10.1080/02699931.2010.514711. [DOI] [PubMed] [Google Scholar]
- Derakshan N, Ansari TL, Shoker L, Hansard ME, Eysenck MW. Anxiety, inhibition, efficiency, and effectiveness: An investigation using the antisaccade task. Experimental Psychology. 2009;56:48–55. doi: 10.1027/1618-3169.56.1.48. [DOI] [PubMed] [Google Scholar]
- *.Derakshan N, Koster EHW. Processing efficiency in anxiety: Evidence from eyemovements during visual search. Behaviour Research and Therapy. 2010;46:1180–1185. doi: 10.1016/j.brat.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Derakshan N, Salt M, Koster EH. Attentional control in depression: An investigation using the antisaccade task. Biological Psychology. 2009;80:251–255. doi: 10.1016/j.biopsycho.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Reed MA. Anxiety-related attentional biases and their regulation by attentional control. Journal of Abnormal Psychology. 2002;2:225–223. doi: 10.1037//0021-843x.111.2.225. [DOI] [PubMed] [Google Scholar]
- Downing PE. Interactions between visual working memory and selective attention. Psychological Science. 2000;11:467–473. doi: 10.1111/1467-9280.00290. [DOI] [PubMed] [Google Scholar]
- Devue C, Belopolsky AV, Theeuwes J. The role of fear and expectancies in capture of covert attention by spiders. Emotion. 2011;11:768–775. doi: 10.1037/a0023418. [DOI] [PubMed] [Google Scholar]
- *.Eizenman M, Yu LH, Grupp L, Eizenman E, Ellenbogen M, Gemar M, et al. A naturalistic vision scanning approach to assess selective attention in major depressive disorder. Psychiatry Research. 2003;118:117–128. doi: 10.1016/s0165-1781(03)00068-4. [DOI] [PubMed] [Google Scholar]
- *.Ellis AJ, Beevers CG, Wells TT. Attention allocation and incidental recognition of emotional information in dysphoria. Cognitive Therapy and Research. 2011;35:425–433. [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo M. Anxiety and cognitive performance: The Attentional Control Theory. Emotion. 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- *.Felmingham KL, Rennie C, Manor B, Bryant RA. Eye tracking and physiological reactivity to threatening stimuli in posttraumatic stress disorder. Journal of Anxiety Disorders. 2011;25:668–673. doi: 10.1016/j.janxdis.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Findlay JM, Gilchrist ID. Active vision: The psychology of looking and seeing. Oxford: Oxford University Press; 2003. [Google Scholar]
- Foa EB, McNally RJ. Sensitivity to feared stimuli in obsessive-compulsives: A dichotic listening analysis. Cognitive Therapy and Research. 1986;10:477–485. [Google Scholar]
- Fox E, Derakshan N, Shoker L. Trait anxiety modulates the electrophysiological indices of rapid spatial orienting towards angry faces. Neuroreport. 2008;19:259–263. doi: 10.1097/WNR.0b013e3282f53d2a. [DOI] [PubMed] [Google Scholar]
- Fox E, Russo R, Bowles R, Dutton K. Do threatening stimuli draw or hold visual attention in subclinical anxiety? Journal of Experimental Psychology: General. 2001;130:681–700. [PMC free article] [PubMed] [Google Scholar]
- *.Gamble A, Rapee R. The time-course of attention to emotional faces in social phobia. Journal of Behavior Therapy and Experimental Psychiatry. 2010;41:39–44. doi: 10.1016/j.jbtep.2009.08.008. [DOI] [PubMed] [Google Scholar]
- *.Garner M, Mogg K, Bradley BP. Orienting and maintenance of gaze to facial expressions in social anxiety. Journal of Abnormal Psychology. 2006;115:760–770. doi: 10.1037/0021-843X.115.4.760. [DOI] [PubMed] [Google Scholar]
- *.Gerdes ABM, Alpers GW, Pauli P. When spiders appear suddenly: Spider-phobic patients are distracted by task-irrelevant spiders. Behaviour Research and Therapy. 2008;46:174–187. doi: 10.1016/j.brat.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, McCann CD. Construct accessibility and depression: An examination of cognitive and affective factors. Journal of Personality and Social Psychology. 1984;47:427–439. doi: 10.1037//0022-3514.47.2.427. [DOI] [PubMed] [Google Scholar]
- Graw P, Krauchi K, Knoblauch V, Wirz-Justice A, Cajochen C. Circadian and wakedependent modulation of fastest and slowest reaction times during the psychomotor vigilance task. Physiology and. Behavior. 2004;80:695–701. doi: 10.1016/j.physbeh.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Hallion LS, Ruscio AM. A meta-analysis of the effect of cognitive bias modification on anxiety and depression. Psychological Bulletin. 2011;137:940–958. doi: 10.1037/a0024355. [DOI] [PubMed] [Google Scholar]
- Harris LM, Menzies RG. Changing attentional bias: Can it affect self-reported anxiety? Anxiety, Stress, and Coping. 1998;11:167–179. [Google Scholar]
- Hayhoe M, Ballard D. Eye movements in natural behavior. Trends in Cognitive Sciences. 2005;9:188–193. doi: 10.1016/j.tics.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Hedges LV. Distribution theory for Glass’s estimator of effect size and related estimators. Journal of Educational Statistics. 1981;6:107–128. [Google Scholar]
- Hedges LV, Olkin I. Statistical methods for meta-analysis. Orlando, FL: Academic Press; 1985. [Google Scholar]
- *.Hermans D, Vansteenwegen D, Eelen P. Eye movement registration as a continuous index of attention deployment: data from a group of spider anxious students. Cognition and Emotion. 1999;13:419–434. [Google Scholar]
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Horley K, Williams LM, Gonsalvez C, Gordon E. Social phobics do not see eye to eye: A visual scanpath study of emotional expression processing. Journal of Anxiety Disorders. 2003;17:33–44. doi: 10.1016/s0887-6185(02)00180-9. [DOI] [PubMed] [Google Scholar]
- Horley K, Williams LM, Gonsalvez CJ, Gordon E. Face to face: visual scanpath evidence for abnormal processing of facial expressions in social phobia. Psychiatry Research. 2004;127:43–53. doi: 10.1016/j.psychres.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Updating the contents of working memory in depression: Interference from irrelevant negative material. Journal of Abnormal Psychology. 2008;117:206–213. doi: 10.1037/0021-843X.117.1.182. [DOI] [PubMed] [Google Scholar]
- *.Kellough JL, Beevers CG, Ellis AJ, Wells TT. Time course of selective attention in clinically depressed young adults: An eye tracking study. Behaviour Research and Therapy. 2008;46:1238–1243. doi: 10.1016/j.brat.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Kimble MO, Fleming K, Bandy C, Kim J, Zambetti A. Eye tracking and visual attention to threating stimuli in veterans of the Iraq War. Journal of Anxiety Disorders. 2010;24:293–299. doi: 10.1016/j.janxdis.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, Amir H. Preliminary study of attention training to threat and neutral faces on anxious reactivity to a social stressor in social anxiety. Cognitive Therapy and Research. 2010;34:263–271. [Google Scholar]
- Koster EHW, Baert S, Bockstaele M, De Raedt R. Attentional retraining procedures: Manipulating early or late components of attentional bias? Emotion. 2010;10:230–236. doi: 10.1037/a0018424. [DOI] [PubMed] [Google Scholar]
- Koster EHW, De Lissnyder E, Derakshan N, De Raedt R. Understanding depressive rumination from a cognitive science perspective: The impaired disengagement hypothesis. Clinical Psychology Review. 2011;31:138–145. doi: 10.1016/j.cpr.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Koster EHW, Verschuere B, Crombez G, Van Damme S. Time-course of attention for threatening pictures in high and low trait anxiety. Behaviour Research and Therapy. 2005;43:1087–1098. doi: 10.1016/j.brat.2004.08.004. [DOI] [PubMed] [Google Scholar]
- *.Lange WG, Heuer K, Langner O, Keijsers GPJ, Becker ES, Rinck M. Face value: eye movements and the evaluation of facial crowds in social anxiety. Journal of Behavior Therapy and Experimental Psychiatry. 2011;42:355–363. doi: 10.1016/j.jbtep.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Lange WG, Tierney KJ, Reinhardt-Rutland AH, Vivekananda-Schmidt P. Viewing behaviour of spider phobics and non-phobics in the presence of threat and safety stimuli. British Journal of Clinical Psychology. 2004;43:235–243. doi: 10.1348/0144665031752989. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Reviews Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Levens SM, Gotlib IH. Updating positive and negative stimuli in working memory in depression. Journal of Experimental Psychology: General. 2010;139:654–664. doi: 10.1037/a0020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Leyman L, De Raedt R, Vaeyens R, Phillipaerts R. Attention for emotional facial expressions in dysphoria: An eye-movement registration study. Cognition and Emotion. 2011;25:111–120. doi: 10.1080/02699931003593827. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. Journal of Abnormal Psychology. 1986;95:15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Corr PJ. A two-dimensional neuropsychology of defense: Fear/anxiety and defensive distance. Neuroscience and Biobehavioral Reviews. 2004;28:285–305. doi: 10.1016/j.neubiorev.2004.03.005. [DOI] [PubMed] [Google Scholar]
- *.Miltner WHR, Krieschel S, Hecht H, Trippe R, Weiss T. Eye movements and behavioral responses to threatening and nonthreatening stimuli during visual search in phobic and nonphobic subjects. Emotion. 2004;4:323–339. doi: 10.1037/1528-3542.4.4.323. [DOI] [PubMed] [Google Scholar]
- Mitte K. Memory bias for threatening information in anxiety and anxiety disorders: A metaanalytic review. Psychological Bulletin. 2008;134:886–891. doi: 10.1037/a0013343. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. A cognitive-motivational analysis of anxiety. Behaviour Research and Therapy. 1998;36:809–848. doi: 10.1016/s0005-7967(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. Selective orienting of attention to masked threat faces in social anxiety. Behaviour Research and Therapy. 2002;40:1403–1414. doi: 10.1016/s0005-7967(02)00017-7. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. Attentional bias in generalized anxiety disorder versus depressive disorder. Cognitive Therapy and Research. 2005;29:29–45. [Google Scholar]
- Mogg K, Bradley BP, Williams R, Mathews A. Subliminal processing of emotional information in anxiety and depression. Journal of Abnormal Psychology. 1993;102:304–311. doi: 10.1037//0021-843x.102.2.304. [DOI] [PubMed] [Google Scholar]
- *.Mogg K, Garner M, Bradley BP. Anxiety and orienting of gaze to angry and fearful faces. Biological Psychology. 2007;76:163–169. doi: 10.1016/j.biopsycho.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg K, Holmes A, Garner M, Bradley BP. Effects of threat cues on attentional shifting, disengagement and response slowing in anxious individuals. Behaviour Research and Therapy. 2008;46:656–667. doi: 10.1016/j.brat.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg K, Mathews A, Weinman J. Memory bias in clinical anxiety. Journal of Abnormal Psychology. 1987;96:94–98. doi: 10.1037//0021-843x.96.2.94. [DOI] [PubMed] [Google Scholar]
- *.Mogg K, Millar N, Bradley BP. Biases in eye movements to threatening facial expressions in generalized anxiety disorder and depressive disorder. Journal of Abnormal Psychology. 2000;109:695–704. doi: 10.1037//0021-843x.109.4.695. [DOI] [PubMed] [Google Scholar]
- Müller HJ, Rabbitt PMA. Reflexive and voluntary orienting of visual attention: time course of activation and resistance to interruption. Journal of Experimental Psychology: Human Perception and Performance. 1989;15:315–330. doi: 10.1037//0096-1523.15.2.315. [DOI] [PubMed] [Google Scholar]
- Mulkens SAN, de Jong PJ, Merckelbach H. Disgust and spider phobia. Journal of Abnormal Psychology. 1996;105:464–468. doi: 10.1037//0021-843x.105.3.464. [DOI] [PubMed] [Google Scholar]
- Najmi S, Amir N. The effect of attention training on a behavioral test of contamination fears in individuals with subclinical obsessive-compulsive symptoms. Journal of Abnormal Psychology. 2010;119:136–142. doi: 10.1037/a0017549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Wisco B, Lyubomirsky S. Rethinking rumination. Perspectives on Psychological Science. 2008;3:400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- Nummenmaa L, Hyöna J, Calvo MG. Preferential selective attention to emotional pictures: An eye movement study. Emotion. 2006;6:257–268. doi: 10.1037/1528-3542.6.2.257. [DOI] [PubMed] [Google Scholar]
- Nummenmaa L, Hyönä J, Calvo MG. Emotional scene content drives the saccade generation system reflexively. Journal of Experimental Psychology: Human Perception and Performance. 2009;35:305–323. doi: 10.1037/a0013626. [DOI] [PubMed] [Google Scholar]
- Oehlberg KA, Revelle W, Mineka S. Time-course of Attention to Negative Stimuli: Negative Affectivity, Anxiety, or Dysphoria? Emotion. doi: 10.1037/a0027227. (in press) [DOI] [PubMed] [Google Scholar]
- Öhman A, Flykt A, Esteves F. Emotion drives attention: Detecting the snake in the grass. Journal of Experimental Psychology: General. 2001;130:466–478. doi: 10.1037/0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- Pashler H. The Psychology of Attention. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- Peckham AD, McHugh RK, Otto MW. A meta-analysis of the magnitude of biased attention in depression. Depression and Anxiety. 2010;27:1135–1142. doi: 10.1002/da.20755. [DOI] [PubMed] [Google Scholar]
- *.Peña-Esparza Y. Attentional biases in dysphoric college students. Albuquerque: University of Mexico; 2011. (Unpublished dissertation) [Google Scholar]
- Pflugshaupt T, Mosimann UP, von Wartburg R, Schmitt W, Nyffeler T, Müri RM. Hypervigilance-avoidance pattern in spider phobia. Journal of Anxiety Disorders. 2005;19:105–116. doi: 10.1016/j.janxdis.2003.12.002. [DOI] [PubMed] [Google Scholar]
- *.Pflugshaupt T, Mosimann UP, Schmitt WJ, von Wartburg R, Wurtz P, Lüthi M, et al. To look or not to look at threat? Scanpath differences within a group of spider phobics. Journal of Anxiety Disorders. 2007;21:353–366. doi: 10.1016/j.janxdis.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Posner MI, Peterson SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Reese HE, McNally RJ, Najmi S, Amir N. Attention training for reducing spider fear in spider-fearful individuals. Journal of Anxiety Disorders. 2010;24:657–662. doi: 10.1016/j.janxdis.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Rinck M, Becker ES. Spider fearful individuals attend to threat, then quickly avoid it: evidence from eye movements. Journal of Abnormal Psychology. 2006;115:231–238. doi: 10.1037/0021-843X.115.2.231. [DOI] [PubMed] [Google Scholar]
- *.Rinck M, Reinecke A, Ellwart T, Heuer K, Becker ES. Speeded detection and increased distraction in fear of spiders: evidence from eye movements. Journal of Abnormal Psychology. 2005;114:235–248. doi: 10.1037/0021-843X.114.2.235. [DOI] [PubMed] [Google Scholar]
- *.Rohner JC. The time-course of visual threat processing: high trait anxious individuals eventually avert their gaze from angry faces. Cognition and Emotion. 2002;16:837–844. [Google Scholar]
- Sander D, Grafman J, Zalla T. The human amygdala: An evolved system for relevance detection. Reviews in the Neurosciences. 2003;14:303–316. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- *.Sears CR, Newman KR, Ference JD, Thomas CL. Attention to emotional images in previously depressed individuals: An eye tracking study. Cognitive Therapy and Research. 2011;35:517–528. [Google Scholar]
- *.Sears CR, Thomas CL, LeHuquet JM, Johnson JCS. Attentional biases in dysphoria: An eye-tracking study of the allocation and disengagement of attention. Cognition and Emotion. 2010;24:1349–1368. [Google Scholar]
- *.Schofield CA, Johnson AL, Inhoff AW, Coles ME. Social anxiety and difficulty disengaging threat: Evidence from eye-tracking. Cognition and Emotion. 2012;26:300–311. doi: 10.1080/02699931.2011.602050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane MS, Peterson JB. An evaluation of early and late stage environmental processing of positive and negative information in dysphoria. Cognition and Emotion. 2007;21:789–815. [Google Scholar]
- Siegle GJ, Granholm E, Ingram RE, Matt GE. Pupillary and reaction time measures of sustained processing of negative information in depression. Biological Psychiatry. 2001;49:624–636. doi: 10.1016/s0006-3223(00)01024-6. [DOI] [PubMed] [Google Scholar]
- *.Stevens S, Rist F, Gerlach AL. Eye movement assessment in individuals with social phobia: Differential usefulness for varying presentation times? Journal of Behavior Therapy and Experimental Psychiatry. 42:219–224. doi: 10.1016/j.jbtep.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Toh W, Rossell SL, Castle DJ. Current visual scanpath research: a review of investigations into the psychotic, anxiety, and mood disorders. Comprehensive Psychiatry. 2011;52:567–569. doi: 10.1016/j.comppsych.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Tolin DF, Lohr JM, Lee TC, Sawchuk CN. Visual avoidance in specific phobia. Behaviour Research and Therapy. 1999;37:63–70. doi: 10.1016/s0005-7967(98)00111-9. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, et al. NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele B, Verschuere B, Koster EHW, Tibboel H, De Houwer J, Crombez G. Effects of attention training on self-reported, implicit, physiological and behavioural measures of spider fear. Journal of Behavior Therapy and Experimental Psychiatry. 2011;42:211–218. doi: 10.1016/j.jbtep.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. Journal of Statistical Software. 2010;36:1–48. [Google Scholar]
- Vuilleumier P. How brains beware: Neural mechanisms of emotional attention. Trends in Cognitive Sciences. 2005;9:585–594. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Wadlinger HA, Isaacowitz DM. Looking happy: The experimental manipulation of a positive visual attention bias. Emotion. 2008;8:121–126. doi: 10.1037/1528-3542.8.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadlinger HA, Isaacowitz DM. Fixing our focus: Training attention to regulate emotion. Personality and Social Psychology Review. 2011;15:75–102. doi: 10.1177/1088868310365565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Wieser MJ, Pauli P, Weyers P, Alpers GW, Muhlberger A. Fear of negative evaluation and the hypervigilance-avoidance hypothesis: an eye-tracking study. Journal of Neural Transmission. 2009;116:717–723. doi: 10.1007/s00702-008-0101-0. [DOI] [PubMed] [Google Scholar]
- Weierich MR, Treat TA, Hollingworth A. Theories and measurement of visual attentional processing in anxiety. Cognition and Emotion. 2008;22:985–1018. [Google Scholar]
- Wells TT, Beevers CG, Robison AE, Ellis A. Gaze behavior predicts memory bias for angry faces in stable dysphoria. Emotion. 2010;10:894–902. doi: 10.1037/a0020022. [DOI] [PubMed] [Google Scholar]
- Wiens S, Peira N, Golkar A, Öhman A. Recognizing masked threat: Fear betrays, but disgust you can trust. Emotion. 2008;8:810–819. doi: 10.1037/a0013731. [DOI] [PubMed] [Google Scholar]
- Williams JM, Mathews A, MacLeod C. The emotional Stroop task and psychopathology. Psychological Bulletin. 1996;120:3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]
- Williams JM, Watts FN, MacLeod C, Matthews A. Cognitive psychology and emotional disorders. Chichester, England: Wiley; 1988. [Google Scholar]
- Yates A, Ashwin C, Fox E. Does Emotion Processing Require Attention? The Effects of Fear Conditioning and Perceptual Load. Emotion. 2010;10:822–830. doi: 10.1037/a0020325. [DOI] [PMC free article] [PubMed] [Google Scholar]