Summary
The anterior choroidal artery has the cortical branches to the temporal, parietal, and occipital lobes in the early embryological stage, which later become the posterior cerebral artery distal to the posterior communicating artery (P2-4). Acute embolic stroke occurred in a 57-year-old man with an anterior choroidal artery having such a persistent embryonic branch to the temporal lobe. Recognition of this embryological form of the anterior choroidal artery is clinically important in acute cerebral ischaemia because the cerebral region between the territories supplied by the middle cerebral artery and the anterior choroidal artery is shown on carotid angiography as an avascular area, which could be misunderstood as a region of the acute ischaemia.
Key words: anterior choroidal artery, acute cerebral ischaemia, intervention, posterior cerebral artery
Introduction
Embryologically, the primitive internal carotid artery can be divided into the rostral (cranial) and caudal divisions. The former consists of the anterior cerebral artery, middle cerebral artery, and anterior choroidal artery (AchA). The latter consists of the posterior communicating artery, P1 portion of the posterior cerebral artery (PCA) and the basilar artery distal to the primitive trigeminal artery1-3. AchA is embryologically an old artery and its telencephalic branches of the temporal, parietal, and occipital lobes are annexed to the diencephalicmesencephalic arteries or primitive posterior choroidal artery of the caudal division of the primitive internal carotid artery, of whose process is called “distal annexation” 2,3. Annexed cortical branches of the AchA become the PCA distal to the posterior communicating artery (P2-4).
When the distal annexation does not occur or is incomplete, AchA keeps the cortical branches of the temporo-parieto-occipital lobes in varying degree, which are normally supplied by the P2-4 segments of the PCA. We present a patient having such an embryological form of the AchA, who suffered from acute cardio-embolic stroke. Knowledge of such an AchA variant is important for a proper understanding of cerebral angiography, and we discuss its clinical implications.
Case Report
This left-handed 57-year-old man developed temporary numbness in the left hand lasting three minutes, followed by speech disturbance. The patient was transferred to us within an hour of ictus by ambulance.
At admission, the patient was alert without apparent motor weakness. The eyes were deviated rightward and motor aphasia was observed. The speech center seemed be located in the right cerebral hemisphere. Six years prior to this admission, this patient had undergone cardiac surgery for stenosis and regurgitation of the aortic valve and for regurgitation of the mitral valve. Warfarin and aspirin had been given thereafter, but thrombo test value on admission was 33.1, which was ineffective for anticoagulation.
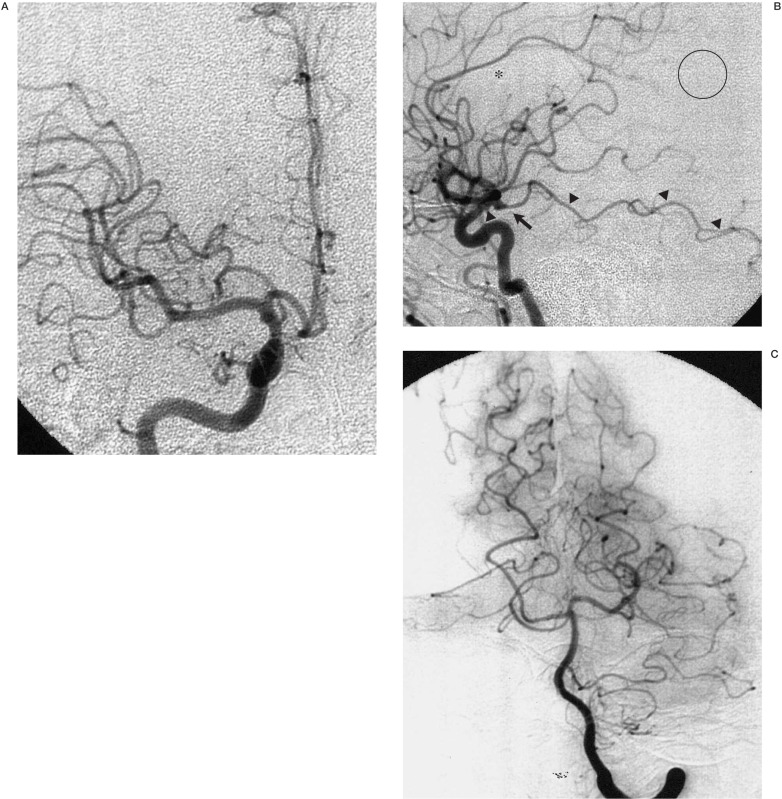
Computed tomography of the brain at admission was normal without an apparent low-density area. Emergency cerebral angiography showed occlusion of the right precentral artery of the middle cerebral artery and a small AchA aneurysm at the origin of the internal carotid artery. AchA had the large cortical branches to the temporal lobe. (figure 1). Right carotid angiography showed a non-opacified cortical region between the territories supplied by the middle cerebral artery and the AchA, which mimicked an avascular region caused by embolus. Careful interpretation of the vertebral angiography showed that this “avascular” region was supplied by the parieto-occipital branches of the right PCA. Selective local fibrinolysis using 180 k units of urokinase to the prefrontal artery resulted in partial recanalisation. Careful manipulation of the microcatheter was required to avoid an injury to the AchA aneurysm.The aneurysm was not treated at this time. This patient developed mild left hemiparesis postoperatively, which subsided in several days. Motor aphasia improved gradually by speech therapy. Intense warfarin administration was give to the patient controlling the international normalised ratio of the prothrombin time around 2.0-2.5.
Figure 1.
Right carotid angiograms: (A frontal and B lateral views) show a small saccular aneurysm (arrow) at the origin of the anterior choroidal artery, which has embryological branches to the temporal lobe (closed arrowheads). The ring indicates an avascular area caused by embolic occlusion of the prefrontal artery. The cortical region between the territories of the distal middle cerebral artery and the anterior choroidal artery (open circle) mimics the avascular area suggestive of acute cerebral ischaemia. The open arrowhead indicates the posterior communicating artery. Left vertebral angiogram (C frontal view) shows the right posterior cerebral artery without temporal branches.
The AchA aneurysm was electively clipped three months later without any sequelae. This patient had minimal motor aphasia at the last follow-up of 1.5 years from the ischaemic attack.
Discussion
Normally, AchA supplies the critical brain structures including the optic tract, the posterior limb of the internal capsule, globus pallidus, cerebral peduncle, uncus, lateral geniculate body, and optic radiation as well as choroid plexus1,4,5. AchA has a connection with the lateral and medial branches of the posterior choroidal artery as well as a choroidal branch of the anterior cerebral artery. AchA may have several branches supplying the temporal lobe, and possibly the medial parietal and occipital lobes. The latter two are telencephalic branches normally supplied by the PCA.
AchA originates normally from the internal carotid artery and less frequently from the posterior communicating artery or from the middle cerebral artery1,5-9. Transposition of the origins of the AchA and the posterior communicating artery10,11 and an absence of the AchA6 are extremely rare. Large AchA supplying the temporal lobe and/or parieto-occipital lobes could be misunderstood as a duplicated posterior communicating artery or as a hyperplastic AchA 9, both of which do not express the embryological backgrounds.
AchA with persistent embryological cortical branches has been reported in patients with arteriovenous malformations 12 and cerebral aneurysms9,12,13, but no patients had been reported in association with acute cerebral ischaemia. In acute cerebral ischaemia, prompt interpretation of cerebral angiograms is mandatory because local fibrinolysis is indicated in the selected situation. In the patient with embryological form of the AchA, the cerebral region between the frontal and temporal regions, which is not opacified on carotid angiograms, should not be misunderstood as a territory of acute ischaemia. Knowledge of such a primitive form of the AchA is of clinical importance especially to avoid misunderstanding of the territory of the acute ischaemia. Concomitant vertebral angiography may help to understand the AchA variations.
In conclusion, an AchA variant may supply the temporal and/or parieto-occipital lobes, which are normally supplied by the PCA. In patients suffering from0 acute cerebral ischaemia with such an AchA variant, carotid angiograms could be misunderstood as showing an avascular region in the parieto-occipital lobe. Knowledge of the AchA variants is important to avoid such a misinterpretation.
References
- 1.Abbie AA. The clinical significance of the anterior choroidal artery. Brain. 1933;56:234–246. [Google Scholar]
- 2.Moffat DB. The development of the posterior cerebral artery. J Anat. 1961;95:485–497. [PMC free article] [PubMed] [Google Scholar]
- 3.Lasjaunias P, Berenstein A, Ter Brugge KG. Surgical Neuroangiography. Vol. 1. Berlin: Springer-Verlag; 2001. Intradural arteries, Clinical vascular anatomy and variations; pp. 479–629. [Google Scholar]
- 4.Herman LH, Fernando OU, Gurdjian ES. The anterior choroidal artery: An anatomical study of its area of distribution. Anat Rec. 1966;154:95–102. doi: 10.1002/ar.1091540109. [DOI] [PubMed] [Google Scholar]
- 5.Rhoton AL, Fujii K, Fradd B. Microsurgical anatomy of the anterior choroidal artery. Surg Neurol. 1979;12:171–187. [PubMed] [Google Scholar]
- 6.Carpenter MB, Noback CR, Moss ML. The anterior choroidal artery. Its origins, course, distribution, and variations. AMA Arch Neurol Psychiatry. 1954;71:714–722. doi: 10.1001/archneurpsyc.1954.02320420042005. [DOI] [PubMed] [Google Scholar]
- 7.Sjogren WE. The anterior choroidal artery. Acta Radiol. 1956;46:143–157. doi: 10.3109/00016925609170823. [DOI] [PubMed] [Google Scholar]
- 8.Otomo E. The anterior choroidal artery. Arch Neurol. 1965;13:656–658. doi: 10.1001/archneur.1965.00470060092009. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi S, Suga T, et al. Anterior choroidal artery: Angiographic analysis of variations and anomalies. Am J Neuroradiol. 1990;11:719–729. [PMC free article] [PubMed] [Google Scholar]
- 10.Hara N, Koike T, et al. Anomalous origin of anterior choroidal artery. Neuroradiol. 1989;31:88. doi: 10.1007/BF00342038. [DOI] [PubMed] [Google Scholar]
- 11.Moyer DJ, Flamm ES. Anomalous arrangement of the origins of the anterior choroidal and posterior communicating arteries. J Neurosurg. 1992;76:1017–1018. doi: 10.3171/jns.1992.76.6.1017. [DOI] [PubMed] [Google Scholar]
- 12.Abrahams JM, Hurst RW, et al. Anterior choroidal artery supply to the posterior cerebral artery distribution: Embryological basis and clinical implications. Neurosurgery. 1999;44:1308–1314. [PubMed] [Google Scholar]
- 13.Koyama T, Gibo H, Kobayashi S. A large anomalous anterior choroidal artery associated with internal carotid artery-posterior communicating artery aneurysm. Case report. Neurosurg Rev. 1998;21:299–301. doi: 10.1007/BF01105790. [DOI] [PubMed] [Google Scholar]