Introduction
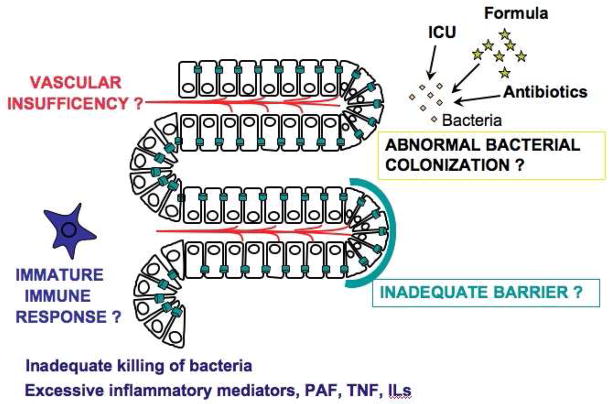
The pathogenesis of necrotizing enterocolitis (NEC) remains poorly understood. Many factors potentially predispose the premature intestine to injury: 1) A lack of adequate substrate and O2 delivery to the intestinal epithelial cells, due to an incomplete microvasculature development or to an immature regulation of the intestinal vascular tone; 2) an inadequate intestinal barrier; 3) an inflammatory response triggered by abnormal bacterial colonization; and 4) an immature immune response, leading to inefficient killing of microbes which then translocate through the epithelium (Fig. 1). At the same time, an excessive production of inflammatory mediators leads to the recruitment of neutrophils and subsequent tissue injury and necrosis.
Fig. 1.
Schematic representation of the risk factors of the premature intestine to injury.
The pathogenesis of NEC is complex and its speed of progression is quite variable. In an attempt to gain understanding of the disease, researchers have examined tissues resected from patients with NEC. However, as these are obtained at late stages of the disease, they do not yield clues about the early pathogenic events leading to NEC. Therefore animal models have been used and have helped to identify a role for several mediators of the inflammatory network in NEC. In this chapter, we discuss the evidence for the role of these inflammatory mediators and conclude with a current unifying hypothesis regarding NEC pathogenesis.
Bacterial – Lipopolysaccharide
During the birth process, the newborn intestine is exposed to the many microbes from the environment and colonization occurs. In term infants, the intestine is colonized with microbes derived from the maternal birth canal and the environment. Exposure to breast milk induces the development of a rich balanced microflora and the growth of bifidobacteria, which have many protective properties for the neonatal gut. Compared with full term infants, the colonization process in preterm infants is altered as the growth of fewer but more aggressive bacteria1 is facilitated by the lack of breast milk, the use of antibiotics and anti-acids2 and the NICU environment. This flora contains more “pro-inflammatory” bacteria and less commensals, which are known to induce genes that promote the epithelial barrier, digesting enzymes and angiogenesis3. Bacterial overgrowth is further facilitated by the immaturities of intestinal motility and digestion, the intestinal barrier and innate immunity. There is evidence that bacteria play a role in acute bowel injury in animal models. For example, germ-free rats were protected against injury in a model of acute bowel injury induced by PAF4. In humans, an abnormal intestinal microflora skewed toward gram-negative bacteria has been found to be associated with the early stage of NEC5. Gram-negative bacteria contain lipopolysaccharides (LPS or endotoxin) on their outer membranes. LPS is a potent activator of the host immune response and may play a central role in NEC. LPS, when injected to rats and mice, produces intestinal injury and shock6. Increased serum LPS has been found in patients with NEC7. LPS is a potent activator of the transcription factor nuclear factor-κB (NF-κB)8 and stimulates the production of many cytokines, including IL1, IL6, chemokines, TNF9 and PAF10, which all amplify the inflammatory response.
Cytokines – Inflammation
The intestinal epithelial cells (IECs) are in contact with bacteria and their products. The bacterial-IEC interaction may be facilitated in the premature intestine by bacterial overgrowth and by an immature mucous layer. Components of the bacteria (called microbial-associated molecular patterns (MAMPs) interact with Toll-like receptors (TLRs) on the IEC and on the submucosal inflammatory cells. Once activated, TLRs elicit the activation of several signal transduction pathways including the inhibitor of NF-κB (IκB) kinase (IKK)11, a critical upstream kinase that activates NF-κB, a major regulator of inflammation. This leads to the production of many inflammatory mediators, including cytokines and chemokines, which lead to immune cell recruitment, especially neutrophils, and to intestinal inflammation.
There are several pieces of evidence which suggest a role for excessive inflammation in NEC: 1) In patients with the disease, several cytokines have been found to be increased in the plasma and intestinal tissue12, 13; 2) The incidence of NEC is prevented by prenatal use of glucocorticoids14, which are known potent inhibitors of the inflammatory response; 3) The production of IL-8 by enterocytes is developmentally regulated15; and 4) Blocking the activation of NF-κB in a neonatal rat model protects against NEC16.
NF-κB
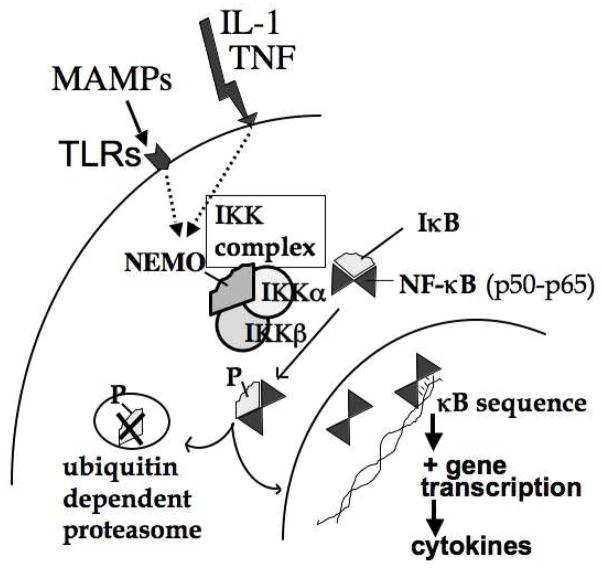
NF-κB is a transcription factor that regulates the expression of many pro-inflammatory cytokines, chemokines and leukocyte adhesion molecules17, 18. It consists of five subunits (p50, p65, p52, cRel and RelB) that homo- or heterodimerize to form active NF-κB17, 18. The dimers of NF-κB mostly found in intestinal tissues are p50-p50 and p50-p6519, 20. NF-κB is constitutively present in the cytoplasm of most cells, bound to inhibitory proteins IκBs (Fig. 2). Following stimulation, IκB is phosphorylated by the upstream IKK complex17. This complex consists of two catalytic subunits, IKKα and IKKβ and a regulatory component, NEMO (NF-κB essential modulator)21. Phosphorylation of IκB by the IKK complex leads to its ubiquitination and subsequent degradation by the 26S proteasome, leaving NF-κB free to translocate to the nucleus and regulate the gene expression of many inflammatory mediators17, 21. Our laboratory has shown that NF-κB is constitutively present at low levels in adult rat intestine, and is activated in PAF-induced acute bowel injury19. We also found that, in fetal rat intestines, the constitutive NF-κB activation appears at 20 days of gestation (length of gestation: 21 days)16.
Fig. 2.
Signaling events leading to NF-κB activation
NF-κB has multiple functions in the intestine, some protective, other potentially detrimental. Several interventions known to decrease NEC such as breastfeeding and use of probiotics attenuate IEC NF-κB activation22, 23: Breast milk induces the production of IκBα in IECs22 and probiotics inhibit NF-κB activation through the proteasome23. Mice with deletion of IKKβ in IECs are protected against systemic inflammation and multiple organ dysfunction syndromes following intestinal ischemia-reperfusion24. However, in several in vivo models, the activation of IKKβ and NF-κB in IECs has been shown to be protective and to limit intestinal mucosal damage24–26. Therefore, NF-κB in IECs might have both protective and detrimental properties in the intestine.
Previous work done in animal models by our laboratory and others suggests a role for NF-κB in NEC. In a neonatal rat NEC model, increased NEC severity (by histology) correlated with increased IEC staining for TLR-2 and activated NF-κB as well as increased IEC apoptosis and impaired IEC proliferation27. We have shown that NF-κB is persistently activated in the intestine in a neonatal rat model of NEC16 and neonatal rats treated with a specific NF-κB inhibitory peptide but not a control peptide had decreased mortality and bowel injury16, suggesting a central role for NF-κB in NEC. This same NF-κB inhibitory peptide inhibits LPS-induced chemokine CXCL2 (or MIP-2) gene expression in IECs28 and inhibits LPS-induced interleukin-1 β (IL-1β), IL-6 and TNF-alpha gene expression in macrophages in vitro (J774.1)29.
There is evidence that NF-κB is developmentally regulated with higher activation and cytokine production in immature IECs15 and inflammatory cells30, 31. Intestinal NF-κB is strongly activated at birth, and is downregulated within a day in dam fed newborn rats16. In contrast, NF-κB remains strongly activated at both day 1 (D1) and D2 in stressed animals and this is accompanied by a significant decrease in the levels of the endogenous NF-κB inhibitory proteins IκBα and IκBβ at D216. Immature enterocytes expressed lower levels of specific IkappaB genes compared with mature enterocytes15 and had a higher IL-8 response to bacterial infection15.
While activation of NF-κB is an essential component of host immunity against pathogens32, in premature infants, a marked and prolonged NF-κB activation may contribute to intestinal tissue injury16.
TLR
TLRs are receptors for bacterial products (also called microbial-associated molecular patterns (MAMPs)), and are found on many cells, including IECs and inflammatory cells. 10 different TLRs have been identified to date in humans. In the intestine, when MAMPs, generally from commensal bacteria, interact with TLRs located on the intestinal epithelium, epithelial cell proliferation, IgA production, integrity of tight junctions and antimicrobial peptide production are promoted, all of which help maintaining a healthy intestinal barrier33. However, the interaction of MAMPs with TLRs on underlying lamina propria immune cells33 can trigger a pro-inflammatory response. The location of these interactions may influence the response: Apical exposure of IECs with CpG-DNA results in inhibition of NF-κB activation, while basolateral exposure leads to activation of NF-κB34. This suggests that invasive bacteria that can penetrate the epithelial barrier elicit a pro-inflammatory response at the basolateral site, while bacteria that cannot cross the barrier, generally non-pathogenic bacteria, remaining on the apical site, elicit a homeostatic, anti-inflammatory response33.
Murine and human NEC have been associated with increased intestinal TLR2 and TLR435 and decreased TLR9 expression36.
TLR4 is the receptor for endotoxin. While a study found that TLR4 was protective in ischemia/reperfusion injury in neonatal mice37, there are many pieces of evidence that suggest that TLR4 has an injurious role in NEC 1) Intestinal TLR4 gene expression is increased in animals exposed to formula feeding and cold asphyxia stress during experimental NEC, while it is normally downregulated in dam fed animals during the first 72 hours of life38; and 2) TLR4-deficient mice are protected against NEC38,39. TLR4 activation in IECs has been found to delay mucosal repair by inducing HMGB1 signaling which increases stress fibers and focal adhesions40 and reduce enterocyte proliferation by inhibiting beta-catenin signaling41.
While the activation of TLR4 contributes to NEC, TLR9, a cell receptor for unmethylated CpG dinucleotides originated from bacterial DNA, has been shown to be protective. Indeed, TLR9-deficient mice exhibited increased NEC severity36 and the activation of TLR9 by its ligand CpG-DNA inhibits LPS-mediated TLR4 signaling in enterocytes and reduces NEC severity36.
IFN gamma
Interferon gamma (or type II interferon)(IFNγ) is a cytokine produced mainly by T cells and natural killer cells, but also by B cells, NK-T cells, dendritic cells and macrophages42. IFNγ is produced by macrophages early during infection. Its synthesis is induced in response to IL 12 and IL 18 and is inhibited by IL-4, IL-10, TGFβ and glucocorticoids42. IFNγ activates several signaling pathways, including STAT1, PI-3kinase/Akt and MAPKs which regulates the transcription of over 500 genes in the cell that modulate several cellular functions, such as apoptosis, proliferation, leukocyte migration and epithelial permeability42.
A role for IFNγ in NEC has been suggested by the following evidence: A trend toward an increase in IFNγ in ileal tissues has been shown in experimental NEC43. IFNγ knock-out mice are protected against NEC and show increased epithelial cell restitution compared to wild-type controls when exposed to the NEC model44. IFNγ has been shown to inhibit enterocyte migration by reversibly displacing connexin43 from lipid rafts45.
IL-6
IL-6 is a cytokine produced by macrophages, T cells and endothelial cells under the control of NF-κB. IL-6 triggers the production of acute phase proteins in the liver, B cell proliferation and antibody production. IL-6 levels have been found to be elevated in the plasma and the stools of patients with NEC46 and correlated with the severity of disease12.
IL-8
IL-8 is a chemokine produced by macrophages, endothelial cells and epithelial cells. IL-8 is a potent chemoattractant for neutrophils and an angiogenic factor. IL-8 has been shown to be elevated in the plasma of infants with NEC47 and to correlate with disease severity47. Fetal enterocytes have upregulated IL-8 gene expression compared to mature enterocytes15 which might contribute to the susceptibility of the premature intestine to inflammation.
IL-10
IL-10 is a cytokine secreted by Th2-cells that inhibits cytokine production in macrophages and other antigen presenting cells (APCs)48. IL-10 modulates both innate and adaptive immune responses48 and is a major anti-inflammatory cytokine in the intestine. In humans, IL-10 has been found to be increased in infants with severe NEC47, which could be a compensatory mechanism to attenuate the intestinal inflammatory response. A role for IL10 in NEC is suggested by the following work: IL10-deficient mice are predisposed to inflammatory colitis49. The production of IL-10 by stimulated blood mononuclear leukocytes is diminished in the premature infant compared to term infant50 and may increase the susceptibility of the premature infant to inflammation. IL-10 levels were more frequently not detectable in the breast milk of premature infants that developed NEC compared to these that did not51. In a mouse model of NEC, IL-10 has been shown to be protective against NEC by attenuating the degree of intestinal inflammation, epithelial apoptosis, decreased junctional adhesion molecule-1 localization, and increased intestinal inducible nitric oxide synthase expression52.
IL-12
IL-12 is a cytokine released by macrophages, neutrophils, B cells and dendritic cells in response to bacteria, viruses and their products. It induces IFNγ and activates Th1 cells and macrophages. While one study found that IL-12 is downregulated in NEC43, another has shown that IL-12 is upregulated in the ileum of neonatal rats with NEC which correlates with the progression of the tissue damage53.
IL-18
IL-18, a pro-inflammatory cytokine released by macrophages, dendritic cells and IECs, induces the production of IL-1β, IL-8, TNF-alpha and IFNγ by inflammatory cells. Several pieces of evidence suggest an involvement of IL-18 in NEC: 1) A polymorphism of IL18 has been associated with NEC54; 2) IL18 is upregulated in the ileum of neonatal rats with NEC53, 55; 3) IL-18 knock-out mice have been shown to have decreased incidence of NEC56. They were also found to have higher levels of IkappaB-alpha and IkappaB-beta, suggesting less NF-κB activation56. They were also shown to have fewer ileal macrophages56.
TNF-alpha
TNF-alpha has been shown to mediate inflammatory bowel disease in adults57. While TNF-alpha levels have not been found to be consistently increased in the plasma of infants with NEC12, 46, 58, TNF-alpha protein has been found to be increased in resected NEC intestinal tissues59. When examined by in situ hybridization, tissue obtained from patients with NEC had a marked increase in TNF-alpha mRNA in Paneth cells, as well as in infiltrating eosinophils and macrophages60. TNF-alpha has been found to be increased in a model of bowel injury in neonatal rats induced by hypoxia/reoxygenation61 but not in a neonatal rat NEC model43. Our laboratory also did not find any increase in intestinal TNF-alpha in the neonatal rat NEC model (unpublished data). Two independent studies found that anti-TNF-alpha antibodies improved the intestinal injury in a neonatal rat NEC model62, 63. Also, pentoxifylline, a drug with many effects including TNF-alpha inhibition has been shown to decrease the incidence of NEC in neonatal rats64. However, our lab did not find any protective effect of anti-TNF in both a model of acute bowel injury induced by PAF and in a neonatal rat NEC model where we compared rats treated with anti-TNF antibodies versus. control immunoglobulin (unpublished data). TNF-alpha has been shown to cause a marked loss of mucous-containing goblet cells in immature mice65. TNF-alpha causes apoptosis in IECs via production of mitochondrial ROS (reactive oxygen species) and activation of the JNK/p38 signaling pathway59.
PAF
Platelet-activating factor (PAF) is an endogenous phospholipid mediator released by many cells, including neutrophils, mast cells, eosinophils, macrophages, platelets, endothelial cells and bacteria, including E. coli66–68. PAF binds to a G protein-coupled receptor, PAF-R, preferentially expressed in the ileum, but also abundant in the jejunum and the spleen69. PAF-R is present in many cells, e.g. neutrophils, macrophages and epithelial cells69, 70. Activation of PAF-R causes a prolonged effect in vivo by the activation of a downstream cascade (e.g. activation of NF-κB19 and of PI3kinase/Akt71), and the production of endogenous PAF (by phospholipase A2 (PLA2))72, oxygen radicals by xanthine oxidase (a major oxidant producing enzyme in the small intestine)73 and TNF-alpha74. In vivo, free PAF is rapidly degraded by its degrading enzyme, PAF-acetylhydrolase (PAF-AH). PAF has been shown to play an important role in NEC: 1) PAF-receptor antagonists prevent experimental NEC in a neonatal rat model75 and in a piglet model76, and PAF-AH prevents experimental NEC in rats77; 2) Infants with NEC have elevated circulating PAF78 and decreased amounts of plasma PAF-acetylhydrolase79, compared to age-matched controls; 3) PAF-AH is present in human milk80; 4) intravenous infusion of recombinant PAF-AH prevents NEC in rats81, and PAF-AH knock-out mice are more susceptible to NEC, although their survival is higher82; and 5) the ileum, the site of predilection of NEC, has the highest amounts of PAF-R69. PAF also mediates the intestinal injury induced by hypoxia/reperfusion83, TNF-alpha84 and LPS85. Exogenous administration of PAF induces systemic hypotension, increased vascular permeability, hemoconcentration, and a dose-dependent isolated bowel necrosis86 which predominates in the small intestine (frequently the ileum) as seen in NEC. Therefore, PAF administration has been used as a model of acute bowel necrosis and NEC86. LPS administration potentiates PAF-induced bowel injury6 and PAF mediates LPS87 and hypoxia-induced bowel necrosis87. When injected intravenously, even at a dose below that which causes bowel necrosis, PAF activates NF-κB very rapidly (within 5 minutes, peaking at 30 minutes)19 and induces the release of leukotrienes C488, the gene expression of PLA272, TNF-alpha74, NF-κB p50 precursor p10589, PAF-R69 and TLR4 in IECs90. PAF is an important mediator of the allergic and inflammatory response and causes systemic and mesenteric vasodilation, increased permeability, platelet aggregation and neutrophil aggregation91.
NO
Nitric oxide (NO) is a free radical that regulates numerous physiological as well as pathological processes in the gastrointestinal tract. Small amounts of endogenous NO are constitutively produced in the intestine, mainly via the 2 constitutive isoforms of the enzyme NO synthase: endothelial NOS (eNOS) and neuronal NOS (nNOS). Several studies have found that eNOS is protective against intestinal inflammation in a DSS colitis model92, 93. However, using the same model, other investigators showed that eNOS and iNOS were detrimental while nNOS was beneficial94. In normal rat small intestine, nNOS was found to suppress the gene expression of iNOS through NF-κB down-regulation95. nNOS suppression led to IκB alpha degradation, NF-κB activation, and iNOS expression95. In neonatal rats, formula feeding downregulates eNOS and nNOS, while it upregulates iNOS96 and in piglets, it downregulates eNOS97. iNOS is upregulated in a neonatal rat NEC model43. Intravenous infusion of L-arginine, a NO synthase substrate, attenuates intestinal injury in this neonatal piglet model of NEC while L-NAME, a NO synthase inhibitor, worsened the injury98. NO released by activated macrophages has been found to inhibit enterocyte migration99. The localized production of nitric oxide by villus enterocytes results in an increase in enterocyte apoptosis100 and impaired proliferation101. NO mediates dendritic cell apoptosis102, while protecting against apoptosis in other cell types103.
ROS
Reactive oxygen species (ROS) are likely the final effector of PAF and many other cytokines. In the intestine, one of the main producers of ROS is the xanthine oxidase/dehydrogenase system. This enzyme is first synthetized as xanthine dehydrogenase, which catalyzes the transformation of xanthine into uric acid (Xanthine+H2O+NAD→ Uric acid+NADH+H+). Xanthine dehydrogenase is constitutively and abundantly expressed in the intestinal villous epithelium104. However, during ischemia, xanthine dehydrogenase is converted into xanthine oxidase, which not only catalyzes the transformation of xanthine into uric acid, but also leads to the production of superoxide (Xanthine+H2O+O2→ Uric acid+2O2−.+2H+). During oxidative stress, ROS leads to activation of the intestinal mitochondrial apoptotic signaling pathway105 and IEC apoptosis via the activation of intestinal p38 MAPK106. In experimental NEC, intestinal ischemia has been shown to be associated with a shift form NO* to O2* production in a NOS-dependent manner107. Xanthine oxidase and superoxide have been shown to play a central role in intestinal reperfusion injury108. A central role for XO and ROS in causing intestinal injury is further supported by the protective role of allopurinol, a xanthine oxidase inhibitor, on PAF-induced bowel necrosis73.
Neutrophils/Macrophages
Neutrophils are thought to play an important role in NEC. Neutrophils have been shown to mediate PAF-induced bowel injury, hypotension, hemoconcentration109 and NF-κB activation19. Mice deficient in P-selectin110 and mice treated with antibody against beta-2 integrin111 (adhesion molecules necessary for neutrophils to roll and adhere to the endothelium) are protected against PAF-induced bowel injury. Our laboratory has found that treatment of mice with antibodies against CXCL2, a major chemokine for neutrophils, attenuated the systemic inflammation, the hypotension and the acute intestinal injury induced by PAF112. However, in studies using live bacteria to induce NEC, e.g., NEC induced by Cronobacter sakazakii infection, depletion of PMN and macrophages from the lamina propria impeded bacterial killing, decreased C. sakazaki clearance and exacerbated cytokine production and bowel injury113. In this same model, C. sakazakii infection was shown to result in epithelial damage by recruiting dendritic cells (DCs) into the gut114. The interaction of DC with IECs led to increased TGF-β production, iNOS production, apoptosis and epithelial cell damage114. The role of macrophages has been recently explored by other investigators who have found that prematurity is associated with a hyper-inflammatory intestinal macrophage phenotype that leads to increased bowel injury115. They showed that, during pregnancy, intestinal macrophages progressively acquire a non-inflammatory profile. They found that TGF-β(2) isoform, suppresses macrophage inflammatory responses in the developing intestine and protects against inflammatory mucosal injury115. Activated macrophages have been shown to block IEC restitution by inhibiting enterocyte gap junction via the release of nitric oxide99.
Systemic inflammation
In NEC, the inflammatory response is not limited to the intestine, and there is evidence that the liver might play an important role in amplifying the inflammation116. Hepatic IL-18 and TNF-alpha and the number of Kupffer cells (KC) were found to be increased in experimental NEC and to correlate with the progression of the intestinal damage116. TNF-alpha levels found in the intestinal lumen of rats with NEC were significantly decreased when KC were inhibited with gadolinium chloride116.
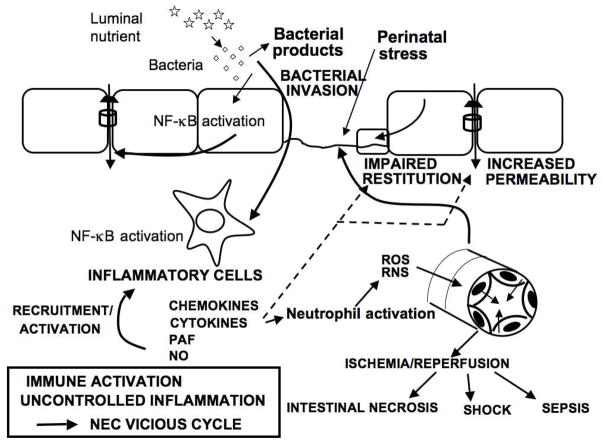
SUMMARY - Current Hypothesis (Fig. 3)
Fig. 3.
Pathogenesis of NEC-Current hypothesis.
The pathogenesis of NEC remains poorly understood. The current but limited understanding of NEC pathogenesis is that it results from a local intestinal inflammation initiated by perinatal stress. Following the introduction of feedings, there is proliferation of intestinal bacteria, favored by the immaturity of the neonatal mucosal immune system. Intestinal bacteria and their products adhere to the epithelium, breach the immature and fragile intestinal mucosal barrier and activate NF-κB in lamina propria immunocytes, causing them to secrete pro-inflammatory mediators, chemokines (CXCL2), cytokines (TNF, IL), prostanoids, platelet-activating factor, and nitric oxide. (We hypothesized that the bowel injury in NEC results from inappropriately elevated and prolonged NF-κB activity in inflammatory cells). These inflammatory agents attract further inflammatory cells, in particular neutrophils, induce the production of reactive oxygen species, and inflict further damage to the intestinal barrier resulting in increased bacterial translocation, intestinal epithelial damage, impaired epithelial cell restitution, apoptosis and mucosal necrosis. Thus, a vicious cycle characteristic of severe NEC is created by bacterial invasion, immune activation, uncontrolled inflammation with production of reactive oxygen and nitrogen species, vasoconstriction followed by ischemia-reperfusion injury, gut barrier failure, intestinal necrosis, sepsis and shock.
Key points.
The pathogenesis of NEC remains poorly defined and likely due to a complex mechanism.
The production of many inflammatory mediators is developmentally regulated in the intestine.
Immaturities of several pathways that regulate inflammation may predispose the premature infants to inflammation.
Excessive inflammation may play an important role in NEC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang Y, Hoenig JD, Malin KJ, et al. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. The ISME journal. 2009;3:944–954. doi: 10.1038/ismej.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dinsmore JE, Jackson RJ, Smith SD. The protective role of gastric acidity in neonatal bacterial translocation. J Pediatr Surg. 1997;32:1014–1016. doi: 10.1016/s0022-3468(97)90389-4. [DOI] [PubMed] [Google Scholar]
- 3.Hooper LV, Wong MH, Thelin A, et al. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 4.Rozenfeld RA, Liu X, DePlaen I, et al. Role of gut flora on intestinal group II phospholipase A2 activity and intestinal injury in shock. Am J Physiol Gastrointest Liver Physiol. 2001;281:G957–G963. doi: 10.1152/ajpgi.2001.281.4.G957. [DOI] [PubMed] [Google Scholar]
- 5.Carlisle EM, Poroyko V, Caplan MS, et al. Gram negative bacteria are associated with the early stages of necrotizing enterocolitis. PloS one. 2011;6:e18084. doi: 10.1371/journal.pone.0018084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez-Crussi F, Hsueh W. Experimental model of ischemic bowel necrosis. The role of platelet-activating factor and endotoxin. Am J Pathol. 1983;112:127–135. [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma R, Tepas JJ, III, Hudak ML, et al. Neonatal gut barrier and multiple organ failure: role of endotoxin and proinflammatory cytokines in sepsis and necrotizing enterocolitis. J Pediatr Surg. 2007;42:454–461. doi: 10.1016/j.jpedsurg.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 8.Essani NA, McGuire GM, Manning AM, et al. Endotoxin-induced activation of the nuclear transcription factor kappa B and expression of E-selectin messenger RNA in hepatocytes, Kupffer cells, and endothelial cells in vivo. J Immunol. 1996;156:2956–2963. [PubMed] [Google Scholar]
- 9.Hawiger J. Innate immunity and inflammation: a transcriptional paradigm. Immunologic research. 2001;23:99–109. doi: 10.1385/IR:23:2-3:099. [DOI] [PubMed] [Google Scholar]
- 10.Ferraris L, Karmeli F, Eliakim R, et al. Intestinal epithelial cells contribute to the enhanced generation of platelet activating factor in ulcerative colitis. Gut. 1993;34:665–668. doi: 10.1136/gut.34.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carmody RJ, Chen YH. Nuclear factor-kappaB: activation and regulation during toll-like receptor signaling. Cell Mol Immunol. 2007;4:31–41. [PubMed] [Google Scholar]
- 12.Harris MC, Costarino AT, Jr, Sullivan JS, et al. Cytokine elevations in critically ill infants with sepsis and necrotizing enterocolitis. J Pediatr. 1994;124:105–111. doi: 10.1016/s0022-3476(94)70264-0. [DOI] [PubMed] [Google Scholar]
- 13.Viscardi RM, Lyon NH, Sun CC, et al. Inflammatory cytokine mRNAs in surgical specimens of necrotizing enterocolitis and normal newborn intestine. Pediatr Pathol Lab Med. 1997;17:547–559. [PubMed] [Google Scholar]
- 14.Israel EJ, Schiffrin EJ, Carter EA, et al. Cortisone strengthens the intestinal mucosal barrier in a rodent necrotizing enterocolitis model. Adv Exp Med Biol. 1991;310:375–380. doi: 10.1007/978-1-4615-3838-7_48. [DOI] [PubMed] [Google Scholar]
- 15.Claud EC, Lu L, Anton PM, et al. Developmentally regulated IkappaB expression in intestinal epithelium and susceptibility to flagellin-induced inflammation. Proc Natl Acad Sci USA. 2004;101:7404–7408. doi: 10.1073/pnas.0401710101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Plaen IG, Liu SX, Tian R, et al. Inhibition of nuclear factor-kappaB ameliorates bowel injury and prolongs survival in a neonatal rat model of necrotizing enterocolitis. Pediatr Res. 2007;61:716–721. doi: 10.1203/pdr.0b013e3180534219. [DOI] [PubMed] [Google Scholar]
- 17.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 18.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 19.De Plaen IG, Tan XD, Chang H, et al. Intestinal NF-kappaB is activated, mainly as p50 homodimers, by platelet-activating factor. Biochim Biophys Acta. 1998;1392:185–192. doi: 10.1016/s0005-2760(98)00024-1. [DOI] [PubMed] [Google Scholar]
- 20.De Plaen IG, Tan XD, Chang H, et al. Lipopolysaccharide activates nuclear factor kappaB in rat intestine: role of endogenous platelet-activating factor and tumour necrosis factor. Br J Pharmacol. 2000;129:307–314. doi: 10.1038/sj.bjp.0703055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DiDonato JA, Hayakawa M, Rothwarf DM, et al. A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 22.Minekawa R, Takeda T, Sakata M, et al. Human breast milk suppresses the transcriptional regulation of IL-1beta-induced NF-kappaB signaling in human intestinal cells. Am J Physiol Cell Physiol. 2004;287:C1404–C1411. doi: 10.1152/ajpcell.00471.2003. [DOI] [PubMed] [Google Scholar]
- 23.Petrof EO, Kojima K, Ropeleski MJ, et al. Probiotics inhibit nuclear factor-kappaB and induce heat shock proteins in colonic epithelial cells through proteasome inhibition. Gastroenterology. 2004;127:1474–1487. doi: 10.1053/j.gastro.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Chen LW, Egan L, Li ZW, et al. The two faces of IKK and NF-kappaB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nat Med. 2003;9:575–581. doi: 10.1038/nm849. [DOI] [PubMed] [Google Scholar]
- 25.Egan LJ, Eckmann L, Greten FR, et al. IkappaB-kinasebeta-dependent NF-kappaB activation provides radioprotection to the intestinal epithelium. Proc Natl Acad Sci USA. 2004;101:2452–2457. doi: 10.1073/pnas.0306734101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chae S, Eckmann L, Miyamoto Y, et al. Epithelial cell I kappa B-kinase beta has an important protective role in Clostridium difficile toxin A-induced mucosal injury. J Immunol. 2006;177:1214–1220. doi: 10.4049/jimmunol.177.2.1214. [DOI] [PubMed] [Google Scholar]
- 27.Le Mandat Schultz A, Bonnard A, Barreau F, et al. Expression of TLR-2, TLR-4, NOD2 and pNF-kappaB in a neonatal rat model of necrotizing enterocolitis. PloS one. 2007;2:e1102. doi: 10.1371/journal.pone.0001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Plaen IG, Han XB, Liu X, et al. Lipopolysaccharide induces CXCL2/macrophage inflammatory protein-2 gene expression in enterocytes via NF-kappaB activation: independence from endogenous TNF-alpha and platelet-activating factor. Immunology. 2006;118:153–163. doi: 10.1111/j.1365-2567.2006.02344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shibata W, Maeda S, Hikiba Y, et al. Cutting edge: The IkappaB kinase (IKK) inhibitor, NEMO-binding domain peptide, blocks inflammatory injury in murine colitis. J Immunol. 2007;179:2681–2685. doi: 10.4049/jimmunol.179.5.2681. [DOI] [PubMed] [Google Scholar]
- 30.Vancurova I, Bellani P, Davidson D. Activation of nuclear factor-kappaB and its suppression by dexamethasone in polymorphonuclear leukocytes: newborn versus adult. Pediatr Res. 2001;49:257–262. doi: 10.1203/00006450-200102000-00021. [DOI] [PubMed] [Google Scholar]
- 31.Kilpinen S, Henttinen T, Lahdenpohja N, et al. Signals leading to the activation of NF-kappa B transcription factor are stronger in neonatal than adult T lymphocytes. Scand J Immunol. 1996;44:85–88. doi: 10.1046/j.1365-3083.1996.d01-277.x. [DOI] [PubMed] [Google Scholar]
- 32.Kelly D, Conway S. Bacterial modulation of mucosal innate immunity. Mol Immunol. 2005;42:895–901. doi: 10.1016/j.molimm.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nature reviews Immunology. 2010;10:131–144. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 34.Lee J, Mo JH, Katakura K, et al. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nature cell biology. 2006;8:1327–1336. doi: 10.1038/ncb1500. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Zhu L, Fatheree NY, et al. Changes in intestinal Toll-like receptors and cytokines precede histological injury in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2009;297:G442–G450. doi: 10.1152/ajpgi.00182.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gribar SC, Sodhi CP, Richardson WM, et al. Reciprocal expression and signaling of TLR4 and TLR9 in the pathogenesis and treatment of necrotizing enterocolitis. Journal of immunology (Baltimore, Md: 1950) 2009;182:636–646. doi: 10.4049/jimmunol.182.1.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tatum PM, Jr, Harmon CM, Lorenz RG, et al. Toll-like receptor 4 is protective against neonatal murine ischemia-reperfusion intestinal injury. Journal of pediatric surgery. 2010;45:1246–1255. doi: 10.1016/j.jpedsurg.2010.02.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jilling T, Simon D, Lu J, et al. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol. 2006;177:3273–3282. doi: 10.4049/jimmunol.177.5.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leaphart CL, Cavallo J, Gribar SC, et al. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. Journal of immunology (Baltimore, Md: 1950) 2007;179:4808–4820. doi: 10.4049/jimmunol.179.7.4808. [DOI] [PubMed] [Google Scholar]
- 40.Dai S, Sodhi C, Cetin S, et al. Extracellular high mobility group box-1 (HMGB1) inhibits enterocyte migration via activation of Toll-like receptor-4 and increased cell-matrix adhesiveness. The Journal of biological chemistry. 2010;285:4995–5002. doi: 10.1074/jbc.M109.067454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sodhi CP, Shi XH, Richardson WM, et al. Toll-like receptor-4 inhibits enterocyte proliferation via impaired beta-catenin signaling in necrotizing enterocolitis. Gastroenterology. 2010;138:185–196. doi: 10.1053/j.gastro.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beaurepaire C, Smyth D, McKay DM. Interferon-gamma regulation of intestinal epithelial permeability. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research. 2009;29:133–144. doi: 10.1089/jir.2008.0057. [DOI] [PubMed] [Google Scholar]
- 43.Nadler EP, Dickinson E, Knisely A, et al. Expression of inducible nitric oxide synthase and interleukin-12 in experimental necrotizing enterocolitis. J Surg Res. 2000;92:71–77. doi: 10.1006/jsre.2000.5877. [DOI] [PubMed] [Google Scholar]
- 44.Leaphart CL, Qureshi F, Cetin S, et al. Interferon-gamma inhibits intestinal restitution by preventing gap junction communication between enterocytes. Gastroenterology. 2007;132:2395–2411. doi: 10.1053/j.gastro.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 45.Leaphart CL, Dai S, Gribar SC, et al. Interferon-gamma inhibits enterocyte migration by reversibly displacing connexin43 from lipid rafts. American journal of physiology Gastrointestinal and liver physiology. 2008;295:G559–569. doi: 10.1152/ajpgi.90320.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morecroft JA, Spitz L, Hamilton PA, et al. Plasma cytokine levels in necrotizing enterocolitis. Acta Paediatr Suppl. 1994;396:18–20. doi: 10.1111/j.1651-2227.1994.tb13235.x. [DOI] [PubMed] [Google Scholar]
- 47.Edelson MB, Bagwell CE, Rozycki HJ. Circulating pro- and counterinflammatory cytokine levels and severity in necrotizing enterocolitis. Pediatrics. 1999;103:766–771. doi: 10.1542/peds.103.4.766. [DOI] [PubMed] [Google Scholar]
- 48.Paul G, Khare V, Gasche C. Inflamed gut mucosa: downstream of interleukin-10. European journal of clinical investigation. 2012;42:95–109. doi: 10.1111/j.1365-2362.2011.02552.x. [DOI] [PubMed] [Google Scholar]
- 49.Kuhn R, Lohler J, Rennick D, et al. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 50.Chheda S, Palkowetz KH, Garofalo R, et al. Decreased interleukin-10 production by neonatal monocytes and T cells: relationship to decreased production and expression of tumor necrosis factor-alpha and its receptors. Pediatric research. 1996;40:475–483. doi: 10.1203/00006450-199609000-00018. [DOI] [PubMed] [Google Scholar]
- 51.Fituch CC, Palkowetz KH, Goldman AS, et al. Concentrations of IL-10 in preterm human milk and in milk from mothers of infants with necrotizing enterocolitis. Acta paediatrica (Oslo, Norway: 1992) 2004;93:1496–1500. doi: 10.1080/08035250410022314. [DOI] [PubMed] [Google Scholar]
- 52.Emami CN, Chokshi N, Wang J, et al. Role of interleukin-10 in the pathogenesis of necrotizing enterocolitis. American journal of surgery. 2012;203:428–435. doi: 10.1016/j.amjsurg.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Halpern MD, Holubec H, Dominguez JA, et al. Up-regulation of IL-18 and IL-12 in the ileum of neonatal rats with necrotizing enterocolitis. Pediatric research. 2002;51:733–739. doi: 10.1203/00006450-200206000-00012. [DOI] [PubMed] [Google Scholar]
- 54.Heninger E, Treszl A, Kocsis I, et al. Genetic variants of the interleukin-18 promoter region (-607) influence the course of necrotising enterocolitis in very low birth weight neonates. European journal of pediatrics. 2002;161:410–411. doi: 10.1007/s00431-002-0968-y. [DOI] [PubMed] [Google Scholar]
- 55.Xu J, Treem WR, Roman C, et al. Ileal immune dysregulation in necrotizing enterocolitis: role of CD40/CD40L in the pathogenesis of disease. Journal of pediatric gastroenterology and nutrition. 2011;52:140–146. doi: 10.1097/MPG.0b013e3182039bad. [DOI] [PubMed] [Google Scholar]
- 56.Halpern MD, Khailova L, Molla-Hosseini D, et al. Decreased development of necrotizing enterocolitis in IL-18-deficient mice. American journal of physiology Gastrointestinal and liver physiology. 2008;294:G20–26. doi: 10.1152/ajpgi.00168.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Dullemen HM, van Deventer SJ, Hommes DW, et al. Treatment of Crohn’s disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2) Gastroenterology. 1995;109:129–135. doi: 10.1016/0016-5085(95)90277-5. [DOI] [PubMed] [Google Scholar]
- 58.Morecroft JA, Spitz L, Hamilton PA, et al. Plasma interleukin-6 and tumour necrosis factor levels as predictors of disease severity and outcome in necrotizing enterocolitis. J Pediatr Surg. 1994;29:798–800. doi: 10.1016/0022-3468(94)90374-3. [DOI] [PubMed] [Google Scholar]
- 59.Baregamian N, Song J, Bailey CE, et al. Tumor necrosis factor-alpha and apoptosis signal-regulating kinase 1 control reactive oxygen species release, mitochondrial autophagy, and c-Jun N-terminal kinase/p38 phosphorylation during necrotizing enterocolitis. Oxid Med Cell Longev. 2009;2:297–306. doi: 10.4161/oxim.2.5.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tan X, Hsueh W, Gonzalez-Crussi F. Cellular localization of tumor necrosis factor (TNF)-alpha transcripts in normal bowel and in necrotizing enterocolitis. TNF gene expression by Paneth cells, intestinal eosinophils, and macrophages. Am J Pathol. 1993;142:1858–1865. [PMC free article] [PubMed] [Google Scholar]
- 61.Akisu M, Baka M, Yalaz M, et al. Supplementation with Saccharomyces boulardii ameliorates hypoxia/reoxygenation-induced necrotizing enterocolitis in young mice. European journal of pediatric surgery: official journal of Austrian Association of Pediatric Surgery = Zeitschrift fur Kinderchirurgie. 2003;13:319–323. doi: 10.1055/s-2003-43580. [DOI] [PubMed] [Google Scholar]
- 62.Halpern MD, Clark JA, Saunders TA, et al. Reduction of experimental necrotizing enterocolitis with anti-TNF-alpha. Am J Physiol Gastrointest Liver Physiol. 2006;290:G757–G764. doi: 10.1152/ajpgi.00408.2005. [DOI] [PubMed] [Google Scholar]
- 63.Seitz G, Warmann SW, Guglielmetti A, et al. Protective effect of tumor necrosis factor alpha antibody on experimental necrotizing enterocolitis in the rat. J Pediatr Surg. 2005;40:1440–1445. doi: 10.1016/j.jpedsurg.2005.05.043. [DOI] [PubMed] [Google Scholar]
- 64.Travadi J, Patole S, Charles A, et al. Pentoxifylline reduces the incidence and severity of necrotizing enterocolitis in a neonatal rat model. Pediatr Res. 2006;60:185–189. doi: 10.1203/01.pdr.0000228325.24945.ac. [DOI] [PubMed] [Google Scholar]
- 65.McElroy SJ, Prince LS, Weitkamp JH, et al. Tumor necrosis factor receptor 1-dependent depletion of mucus in immature small intestine: a potential role in neonatal necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2011;301:G656–G666. doi: 10.1152/ajpgi.00550.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Denizot Y, Dassa E, Kim HY, et al. Synthesis of paf-acether from exogenous precursors by the prokaryote Escherichia coli. FEBS Lett. 1989;243:13–16. doi: 10.1016/0014-5793(89)81207-4. [DOI] [PubMed] [Google Scholar]
- 67.Denizot Y, Dassa E, Thomas Y, et al. Production of paf-acether by various bacterial strains. Gastroenterol Clin Biol. 1990;14:681–682. [PubMed] [Google Scholar]
- 68.Benveniste J, Chignard M, Le Couedic JP, et al. Biosynthesis of platelet-activating factor (PAF-ACETHER). II. Involvement of phospholipase A2 in the formation of PAF-ACETHER and lyso-PAF-ACETHER from rabbit platelets. Thromb Res. 1982;25:375–385. doi: 10.1016/0049-3848(82)90128-1. [DOI] [PubMed] [Google Scholar]
- 69.Wang H, Tan X, Chang H, et al. Regulation of platelet-activating factor receptor gene expression in vivo by endotoxin, platelet-activating factor and endogenous tumour necrosis factor. Biochem J. 1997;322 (Pt 2):603–608. doi: 10.1042/bj3220603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang H, Tan X, Chang H, et al. Platelet-activating factor receptor mRNA is localized in eosinophils and epithelial cells in rat small intestine: regulation by dexamethasone and gut flora. Immunology. 1999;97:447–454. doi: 10.1046/j.1365-2567.1999.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu J, Caplan MS, Li D, et al. Polyunsaturated fatty acids block platelet-activating factor-induced phosphatidylinositol 3 kinase/Akt-mediated apoptosis in intestinal epithelial cells. American journal of physiology Gastrointestinal and liver physiology. 2008;294:G1181–1190. doi: 10.1152/ajpgi.00343.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tan XD, Wang H, Gonzalez-Crussi FX, et al. Platelet activating factor and endotoxin increase the enzyme activity and gene expression of type II phospholipase A2 in the rat intestine. Role of polymorphonuclear leukocytes. J Immunol. 1996;156:2985–2990. [PubMed] [Google Scholar]
- 73.Qu XW, Rozenfeld RA, Huang W, et al. The role of xanthine oxidase in platelet activating factor induced intestinal injury in the rat. Gut. 1999;44:203–211. doi: 10.1136/gut.44.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang L, Tan X, Crawford SE, et al. Platelet-activating factor and endotoxin induce tumour necrosis factor gene expression in rat intestine and liver. Immunology. 1994;83:65–69. [PMC free article] [PubMed] [Google Scholar]
- 75.Caplan MS, Hedlund E, Adler L, et al. The platelet-activating factor receptor antagonist WEB 2170 prevents neonatal necrotizing enterocolitis in rats. J Pediatr Gastroenterol Nutr. 1997;24:296–301. doi: 10.1097/00005176-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 76.Ewer AK, Al-Salti W, Coney AM, et al. The role of platelet activating factor in a neonatal piglet model of necrotising enterocolitis. Gut. 2004;53:207–213. doi: 10.1136/gut.2003.024521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Caplan MS, Lickerman M, Adler L, et al. The role of recombinant platelet-activating factor acetylhydrolase in a neonatal rat model of necrotizing enterocolitis. Pediatric research. 1997;42:779–783. doi: 10.1203/00006450-199712000-00010. [DOI] [PubMed] [Google Scholar]
- 78.MacKendrick W, Hill N, Hsueh W, et al. Increase in plasma platelet-activating factor levels in enterally fed preterm infants. Biol Neonate. 1993;64:89–95. doi: 10.1159/000243976. [DOI] [PubMed] [Google Scholar]
- 79.Caplan M, Hsueh W, Kelly A, et al. Serum PAF acetylhydrolase increases during neonatal maturation. Prostaglandins. 1990;39:705–71. doi: 10.1016/0090-6980(90)90030-y. [DOI] [PubMed] [Google Scholar]
- 80.Furukawa M, Narahara H, Yasuda K, et al. Presence of platelet-activating factor-acetylhydrolase in milk. Journal of lipid research. 1993;34:1603–1609. [PubMed] [Google Scholar]
- 81.Muguruma K, Gray PW, Tjoelker LW, et al. The central role of PAF in necrotizing enterocolitis development. Adv Exp Med Biol. 1997;407:379–382. doi: 10.1007/978-1-4899-1813-0_56. [DOI] [PubMed] [Google Scholar]
- 82.Lu J, Pierce M, Franklin A, et al. Dual roles of endogenous platelet-activating factor acetylhydrolase in a murine model of necrotizing enterocolitis. Pediatric research. 2010;68:225–230. doi: 10.1203/PDR.0b013e3181eb2efe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Caplan MS, Sun XM, Hsueh W. Hypoxia causes ischemic bowel necrosis in rats: the role of platelet- activating factor (PAF-acether) Gastroenterology. 1990;99:979–986. doi: 10.1016/0016-5085(90)90616-9. [DOI] [PubMed] [Google Scholar]
- 84.Sun XM, Hsueh W. Bowel necrosis induced by tumor necrosis factor in rats is mediated by platelet-activating factor. J Clin Invest. 1988;81:1328–1331. doi: 10.1172/JCI113459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hsueh W, Gonzalez-Crussi F, Arroyave JL. Platelet-activating factor: an endogenous mediator for bowel necrosis in endotoxemia. FASEB J. 1987;1:403–405. doi: 10.1096/fasebj.1.5.3678700. [DOI] [PubMed] [Google Scholar]
- 86.Hsueh W, Gonzalez-Crussi F, Arroyave JL. Platelet-activating factor-induced ischemic bowel necrosis. An investigation of secondary mediators in its pathogenesis. Am J Pathol. 1986;122:231–239. [PMC free article] [PubMed] [Google Scholar]
- 87.Caplan MS, Kelly A, Hsueh W. Endotoxin and hypoxia-induced intestinal necrosis in rats: the role of platelet activating factor. Pediatr Res. 1992;31:428–434. doi: 10.1203/00006450-199205000-00002. [DOI] [PubMed] [Google Scholar]
- 88.Hsueh W, Gonzalez-Crussi F, Arroyave JL. Release of leukotriene C4 by isolated, perfused rat small intestine in response to platelet-activating factor. J Clin Invest. 1986;78:108–114. doi: 10.1172/JCI112538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tan X, Sun X, Gonzalez-Crussi FX, et al. PAF and TNF increase the precursor of NF-kappa B p50 mRNA in mouse intestine: quantitative analysis by competitive PCR. Biochim Biophys Acta. 1994;1215:157–162. doi: 10.1016/0005-2760(94)90105-8. [DOI] [PubMed] [Google Scholar]
- 90.Soliman A, Michelsen KS, Karahashi H, et al. Platelet-activating factor induces TLR4 expression in intestinal epithelial cells: implication for the pathogenesis of necrotizing enterocolitis. PloS one. 2010;5:e15044. doi: 10.1371/journal.pone.0015044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ford-Hutchinson AW. Neutrophil aggregating properties of PAF-acether and leukotriene B4. International journal of immunopharmacology. 1983;5:17–21. doi: 10.1016/0192-0561(83)90067-x. [DOI] [PubMed] [Google Scholar]
- 92.Sasaki M, Bharwani S, Jordan P, et al. Increased disease activity in eNOS-deficient mice in experimental colitis. Free radical biology & medicine. 2003;35:1679–1687. doi: 10.1016/j.freeradbiomed.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 93.Vallance BA, Dijkstra G, Qiu B, et al. Relative contributions of NOS isoforms during experimental colitis: endothelial-derived NOS maintains mucosal integrity. American journal of physiology Gastrointestinal and liver physiology. 2004;287:G865–874. doi: 10.1152/ajpgi.00187.2004. [DOI] [PubMed] [Google Scholar]
- 94.Beck PL, Xavier R, Wong J, et al. Paradoxical roles of different nitric oxide synthase isoforms in colonic injury. American journal of physiology Gastrointestinal and liver physiology. 2004;286:G137–147. doi: 10.1152/ajpgi.00309.2003. [DOI] [PubMed] [Google Scholar]
- 95.Qu XW, Wang H, De Plaen IG, et al. Neuronal nitric oxide synthase (NOS) regulates the expression of inducible NOS in rat small intestine via modulation of nuclear factor kappa B. FASEB journal. 2001;15:439–446. doi: 10.1096/fj.99-0343com. [DOI] [PubMed] [Google Scholar]
- 96.D’Souza A, Fordjour L, Ahmad A, et al. Effects of probiotics, prebiotics, and synbiotics on messenger RNA expression of caveolin-1, NOS, and genes regulating oxidative stress in the terminal ileum of formula-fed neonatal rats. Pediatric research. 2010;67:526–531. doi: 10.1203/PDR.0b013e3181d4ff2b. [DOI] [PubMed] [Google Scholar]
- 97.van Haver ER, Oste M, Thymann T, et al. Enteral feeding reduces endothelial nitric oxide synthase in the caudal intestinal microvasculature of preterm piglets. Pediatric research. 2008;63:137–142. doi: 10.1203/PDR.0b013e31815f00f9. [DOI] [PubMed] [Google Scholar]
- 98.Di Lorenzo M, Bass J, Krantis A. Use of L-arginine in the treatment of experimental necrotizing enterocolitis. J Pediatr Surg. 1995;30:235–240. doi: 10.1016/0022-3468(95)90567-7. [DOI] [PubMed] [Google Scholar]
- 99.Anand RJ, Dai S, Rippel C, et al. Activated macrophages inhibit enterocyte gap junctions via the release of nitric oxide. American journal of physiology Gastrointestinal and liver physiology. 2008;294:G109–119. doi: 10.1152/ajpgi.00331.2007. [DOI] [PubMed] [Google Scholar]
- 100.Potoka DA, Upperman JS, Nadler EP, et al. NF-kappaB inhibition enhances peroxynitrite-induced enterocyte apoptosis. The Journal of surgical research. 2002;106:7–14. doi: 10.1006/jsre.2002.6423. [DOI] [PubMed] [Google Scholar]
- 101.Potoka DA, Upperman JS, Zhang XR, et al. Peroxynitrite inhibits enterocyte proliferation and modulates Src kinase activity in vitro. American journal of physiology Gastrointestinal and liver physiology. 2003;285:G861–869. doi: 10.1152/ajpgi.00412.2002. [DOI] [PubMed] [Google Scholar]
- 102.Stanford A, Chen Y, Zhang XR, et al. Nitric oxide mediates dendritic cell apoptosis by downregulating inhibitors of apoptosis proteins and upregulating effector caspase activity. Surgery. 2001;130:326–332. doi: 10.1067/msy.2001.116411. [DOI] [PubMed] [Google Scholar]
- 103.Chen Y, Stanford A, Simmons RL, et al. Nitric oxide protects thymocytes from gamma-irradiation-induced apoptosis in correlation with inhibition of p53 upregulation and mitochondrial damage. Cellular immunology. 2001;214:72–80. doi: 10.1006/cimm.2001.1880. [DOI] [PubMed] [Google Scholar]
- 104.Hsueh W, Caplan MS, Qu XW, et al. Neonatal necrotizing enterocolitis: clinical considerations and pathogenetic concepts. Pediatr Dev Pathol. 2003;6:6–23. doi: 10.1007/s10024-002-0602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Baregamian N, Song J, Papaconstantinou J, et al. Intestinal mitochondrial apoptotic signaling is activated during oxidative stress. Pediatric surgery international. 2011;27:871–877. doi: 10.1007/s00383-011-2880-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhou Y, Wang Q, Evers MB, et al. Oxidative stress-induced intestinal epithelial cell apoptosis is mediated by p38 MAPK. Biochemical and biophysical research communications. 2006;350:860–865. doi: 10.1016/j.bbrc.2006.09.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Whitehouse JS, Xu H, Shi Y, et al. Mesenteric nitric oxide and superoxide production in experimental necrotizing enterocolitis. The Journal of surgical research. 2010;161:1–8. doi: 10.1016/j.jss.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Parks DA, Bulkley GB, Granger DN, et al. Ischemic injury in the cat small intestine: role of superoxide radicals. Gastroenterology. 1982;82:9–15. [PubMed] [Google Scholar]
- 109.Musemeche C, Caplan M, Hsueh W, et al. Experimental necrotizing enterocolitis: the role of polymorphonuclear neutrophils. J Pediatr Surg. 1991;26:1047–1049. doi: 10.1016/0022-3468(91)90671-f. [DOI] [PubMed] [Google Scholar]
- 110.Sun X, Rozenfeld RA, Qu X, et al. P-selectin-deficient mice are protected from PAF-induced shock, intestinal injury, and lethality. Am J Physiol. 1997;273:G56–G61. doi: 10.1152/ajpgi.1997.273.1.G56. [DOI] [PubMed] [Google Scholar]
- 111.Sun XM, Qu XW, Huang W, et al. Role of leukocyte beta 2-integrin in PAF-induced shock and intestinal injury. Am J Physiol. 1996;270:G184–G190. doi: 10.1152/ajpgi.1996.270.1.G184. [DOI] [PubMed] [Google Scholar]
- 112.Han XB, Liu X, Hsueh W, et al. Macrophage inflammatory protein-2 mediates the bowel injury induced by platelet-activating factor. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1220–G1226. doi: 10.1152/ajpgi.00231.2004. [DOI] [PubMed] [Google Scholar]
- 113.Emami CN, Mittal R, Wang L, et al. Role of neutrophils and macrophages in the pathogenesis of necrotizing enterocolitis caused by Cronobacter sakazakii. The Journal of surgical research. 2012;172:18–28. doi: 10.1016/j.jss.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Emami CN, Mittal R, Wang L, et al. Recruitment of dendritic cells is responsible for intestinal epithelial damage in the pathogenesis of necrotizing enterocolitis by Cronobacter sakazakii. Journal of immunology (Baltimore, Md: 1950) 2011;186:7067–7079. doi: 10.4049/jimmunol.1100108. [DOI] [PubMed] [Google Scholar]
- 115.Maheshwari A, Kelly DR, Nicola T, et al. TGF-beta2 suppresses macrophage cytokine production and mucosal inflammatory responses in the developing intestine. Gastroenterology. 2011;140:242–253. doi: 10.1053/j.gastro.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Halpern MD, Holubec H, Dominguez JA, et al. Hepatic inflammatory mediators contribute to intestinal damage in necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2003;284:G695–G702. doi: 10.1152/ajpgi.00353.2002. [DOI] [PubMed] [Google Scholar]