Abstract
Purpose
To analyze the baseline clinicopathologic characteristics of prostate tumors with germline BRCA1 and BRCA2 (BRCA1/2) mutations and the prognostic value of those mutations on prostate cancer (PCa) outcomes.
Patients and Methods
This study analyzed the tumor features and outcomes of 2,019 patients with PCa (18 BRCA1 carriers, 61 BRCA2 carriers, and 1,940 noncarriers). The Kaplan-Meier method and Cox regression analysis were used to evaluate the associations between BRCA1/2 status and other PCa prognostic factors with overall survival (OS), cause-specific OS (CSS), CSS in localized PCa (CSS_M0), metastasis-free survival (MFS), and CSS from metastasis (CSS_M1).
Results
PCa with germline BRCA1/2 mutations were more frequently associated with Gleason ≥ 8 (P = .00003), T3/T4 stage (P = .003), nodal involvement (P = .00005), and metastases at diagnosis (P = .005) than PCa in noncarriers. CSS was significantly longer in noncarriers than in carriers (15.7 v 8.6 years, multivariable analyses [MVA] P = .015; hazard ratio [HR] = 1.8). For localized PCa, 5-year CSS and MFS were significantly higher in noncarriers (96% v 82%; MVA P = .01; HR = 2.6%; and 93% v 77%; MVA P = .009; HR = 2.7, respectively). Subgroup analyses confirmed the poor outcomes in BRCA2 patients, whereas the role of BRCA1 was not well defined due to the limited size and follow-up in this subgroup.
Conclusion
Our results confirm that BRCA1/2 mutations confer a more aggressive PCa phenotype with a higher probability of nodal involvement and distant metastasis. BRCA mutations are associated with poor survival outcomes and this should be considered for tailoring clinical management of these patients.
INTRODUCTION
More than 900,000 new cases of prostate cancer (PCa) are diagnosed worldwide every year.1,2 Although the majority of patients with PCa are cured with radical primary treatment or may only need active surveillance, others will eventually succumb to advanced disease. In fact, PCa accounts for the second commonest cause of male cancer-related deaths in the United States2 and the sixth worldwide, with more than 250,000 deaths a year.1 Thus it is essential to identify up front those patients with a lethal form of PCa.
PCa is rarely diagnosed in men younger than 50 years, but its incidence rises rapidly thereafter. Excluding advanced age, the strongest risk factor for the disease is a family history of PCa,3–5 suggesting the importance of genetic factors in disease development.6 Genome-wide association studies have identified more than 70 susceptibility loci associated with modest relative risks of PCa, which, taken together, explain approximately 30% of the familial PCa risk.7 Rarer genetic variants conferring higher PCa risks have also been identified. Germline BRCA2 mutations are the genetic events that confer the highest risk of PCa known to date (8.6-fold in men ≤ 65 years),8–10 whereas the effect of BRCA1 is more modest (3.4-fold).11 Germline BRCA2 and BRCA1 mutations are present in 1.2% and 0.44% of PCa cases, respectively.10,11
Previous studies have suggested an association of BRCA2 mutations with aggressive tumor phenotype and/or poor overall survival (OS).12–17 The Icelandic BRCA2 999del5 and the Ashkenazi BRCA1 185delAG and BRCA2 6174delT founder mutations have also been associated with poor PCa cause-specific survival (CSS), which is considered a more robust end point than OS.12,15 In general, these series were limited to small number of BRCA1/2 carriers or had little clinical information, and comprehensive multivariable analyses (MVA) were not possible. Thus the real prognostic contribution of BRCA1/2 mutations compared with other classical prognostic factors for PCa outcome remains unresolved.
In the present study, we aimed to analyze the prognostic value of BRCA1 and BRCA2 germline mutations for PCa outcomes in a large series of patients with comprehensive clinicopathologic, therapeutic, and survival data.
PATIENTS AND METHODS
Study Design
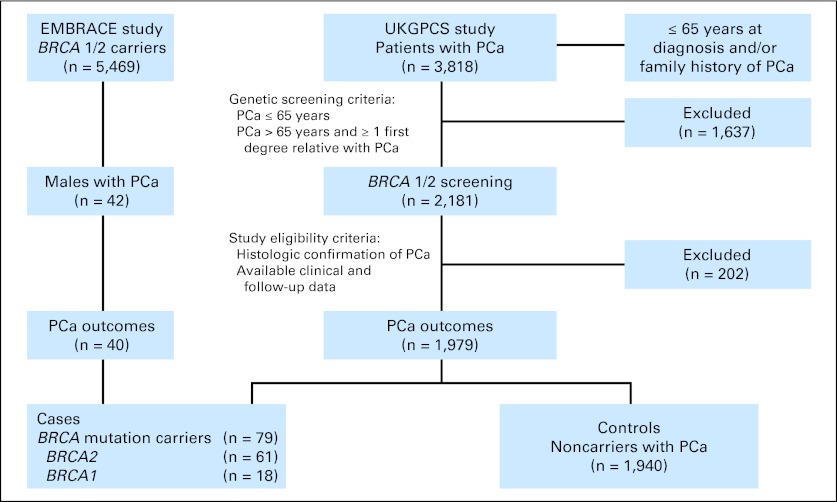
This was a retrospective analysis of PCa outcomes in patients with germline BRCA1 or BRCA2 (BRCA1/2) mutations and noncarriers. Patients with PCa and BRCA1/2 mutations were identified from two ongoing prospective cohort studies: United Kingdom Genetic Prostate Cancer study (UKGPCS; NIHR869)18 and Epidemiological Study of BRCA1/2 Mutation Carriers (EMBRACE; NIHR1358).19 A total of 2,181 patients with PCa, of 3,818 enrolled in UKGPCS, who were ≤ 65 years at diagnosis and/or had a family history of PCa were screened for BRCA1/2 mutations. Those who did not carry BRCA1/2 mutations have been included as noncarriers. The carriers' group was enriched with those carriers participating in EMBRACE who had developed PCa. In addition, all patients included in this analysis met the following criteria: (1) histologic confirmation of PCa, and (2) availability of clinical and follow-up data. Patients without clinical data or who could not be traced were excluded (Fig 1).
Fig 1.
CONSORT diagram. EMBRACE, Epidemiological Study of BRCA1 and BRCA2 Mutation Carriers; PCa, prostate cancer; UKGCPS, United Kingdom Genetic Prostate Cancer Study.
UKGPCS and EMBRACE are observational studies and did not interfere with PCa management. Patients were treated and followed up according to departmental protocols, standardized in 1999 by the National Institute for Clinical Excellence.20
The primary aim of this study was to evaluate the evidence for the independent prognostic value of BRCA1/2 mutation status on PCa cause-specific survival (CSS). Secondary aims included the analysis of the impact of BRCA1/2 mutations as a whole and separately (BRCA1 and BRCA2) on PCa baseline characteristics and outcomes, including CSS, overall survival (OS), CSS in localized PCa (CSS_M0), metastatic disease-free survival (MFS), and CSS from metastatic disease (CSS_M1). This study was approved by the local institutional review boards.
BRCA Mutation Analysis in the UKGPCS Study
Germline DNA was extracted from peripheral-blood samples. The coding region of the BRCA1 and BRCA2 genes were screened using multiplex fluorescent heteroduplex detection, Sanger sequencing,9,10 and multiplex ligation-dependent probe amplification.11
Data Collection
Data from patients enrolled in UKGPCS and EMBRACE were collected at study entry and updated annually. Data sources used in these studies included medical records, pathology reports, and trial standardized questionnaires. Baseline variables collected at diagnosis included date at PCa diagnosis, method of PCa diagnosis, age, TNM stage, Gleason score, and prostate-specific antigen (PSA) level. Other variables related to patients' management and outcomes were PSA doubling-time (PSADT), administered treatments for PCa, date of progression/metastasis, and date/cause of death. Time to biochemical relapse was not analyzed because PSA values were not consistently monitored in a significant proportion of patients.
Statistical Methods
The associations between PCa baseline clinicopathologic data and BRCA carrier status were analyzed using χ2 test, Mantel-Haenszel linear-trend test, or the Mann-Whitney U test, as appropriate. On the basis of previous literature, Gleason scores were categorized into a total score of ≤ 6, 7, and ≥ 8, according to the grade of anaplasia.21 OS time was calculated from the date of PCa diagnosis until date of death from any cause or censored at the last follow-up. CSS was calculated similarly to OS, but if cause of death was different from PCa, CSS time was censored at the time of death. CSS_M0 was only considered for patients without metastatic disease at diagnosis, and CSS_M1 was analyzed in all patients with metastatic disease at presentation, or those who developed metastasis during follow-up. In patients with early disease (M0), MFS was estimated from the date of diagnosis until the date of metastatic disease. Median survival and 5-year survival rates were estimated using the Kaplan-Meier method, and survival curves generated for each group (BRCA1, BRCA2, and noncarriers) were compared using the log-rank test.
To identify the independent prognostic value of BRCA mutations, an MVA model for each survival outcome was created using a Cox regression model to control the effect of other prognostic variables potentially acting as confounding factors. Numeric variables were categorized based on the median distribution value for the pertinent variable or a previously described relevant cutoff. All variables with a P value less than .05 in the univariable analysis (UVA) were included in the MVA.
To analyze the benefit of adding BRCA status to the Kattan nomogram,22 one of the most commonly used predictive tools in patients with local disease, we derived the different logistic regression models and their areas under the receiver operating characteristic curve (AUCs) at 5-, 8-, and 10-year OS, CSS_M0, and MFS.
All P values were two-sided. The SPSS program (version 19.0, SPSS, Chicago, IL) was used for statistical analysis. Data collection cutoff for this analysis was October 31, 2011, when the median follow-up was 50 months (range, 3.5 to 245 months).
RESULTS
Patient Characteristics
A total of 2,019 patients with PCa were eligible, of whom 79 were BRCA carriers (18 BRCA1 and 61 BRCA2) and 1,940 were noncarriers. Mutations in both genes were varied (13 types in BRCA1 and 40 in BRCA2) and not clustered in a single region of either gene (Appendix Table A1, online only). Sixteen percent of patients were ≤ 65 years at diagnosis, and 34% had familial history of PCa.
In our series, PCa was mainly diagnosed as a result of clinical symptoms. However, the proportion of BRCA carriers diagnosed through PSA screening was significantly higher compared with noncarriers (22% v 10%; P = .001), with more BRCA2 than BRCA1 carriers (25% v 11%; P = .021) diagnosed in this way. Median age at diagnosis was similar in both carriers and noncarriers (58 years [range, 42 to 88 years] in carriers v 57 years [range, 32 to 89 years] in noncarriers; P = .14), and no differences were seen in presenting PSA (11.5 v 11.3 ng/mL; P = .93).
Poorly differentiated PCa (Gleason score ≥ 8) was twice as common in BRCA1/2 carriers as in noncarriers (35% v 15%; P = .00003). Advanced stage (T3-T4) was more frequent in BRCA1/2 carriers than in noncarriers (37% v 28%; P = .003) as well as nodal involvement (N1: 15% v 5%; P = .0005) and metastatic spread (M1: 18% v 9%; P = .005). When patients with local disease (N0M0 patients) were stratified according to their risk of relapse,21 no differences were seen between carriers and noncarriers (P = .22; Table 1).
Table 1.
Prostate Cancer Characteristics in BRCA Mutation Carriers and in Noncarriers
| Patient Characteristic |
BRCA Mutation Carriers |
Noncarriers (n = 1,940) |
P (carriers v noncarriers) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n = 79) |
BRCA1 (n = 18) |
BRCA2 (n = 61) |
|||||||
| No. | % | No. | % | No. | % | No. | % | ||
| Age, years | |||||||||
| Median | 58.3 | 60.8 | 57.6 | 57.2 | .142 | ||||
| Range | 41.7-88 | 48.3-73.5 | 41.7-88 | 32.3-88.9 | |||||
| Histologic grade/Gleason score | |||||||||
| Gleason ≤ 6/grade 1 | 20 | 25.3 | 6 | 33.3 | 14 | 23.0 | 733 | 37.8 | < .001 |
| Gleason 7/grade 2 | 19 | 24.1 | 4 | 22.2 | 15 | 24.6 | 511 | 26.3 | |
| Gleason ≥ 8/grade 3 | 28 | 35.4 | 5 | 27.8 | 23 | 37.7 | 299 | 15.4 | |
| Unknown | 12 | 15.2 | 3 | 16.7 | 9 | 14.8 | 397 | 20.5 | |
| Tumor stage, T | |||||||||
| T1, not clinically apparent | 8 | 10.1 | 1 | 5.6 | 7 | 11.5 | 439 | 22.6 | .003 |
| T2, confined to prostate | 25 | 31.6 | 6 | 33.3 | 19 | 31.1 | 550 | 28.4 | |
| T3, palpable, beyond capsule | 22 | 27.8 | 4 | 22.2 | 18 | 29.5 | 474 | 24.4 | |
| T4, fixed or invading locally | 7 | 8.9 | 2 | 11.1 | 5 | 8.2 | 71 | 3.7 | |
| Tx, cannot be assessed | 17 | 21.5 | 5 | 27.8 | 12 | 19.7 | 406 | 20.9 | |
| Nodal stage, N | |||||||||
| N0, no nodal metastasis | 42 | 53.2 | 8 | 44.4 | 34 | 55.7 | 986 | 50.8 | < .001 |
| N1, nodal metastasis | 12 | 15.2 | 2 | 11.1 | 10 | 16.4 | 89 | 4.6 | |
| Nx, cannot be assessed | 25 | 31.6 | 8 | 44.4 | 17 | 27.9 | 865 | 44.6 | |
| Metastasis, M | |||||||||
| M0, no distant metastasis | 65 | 82.3 | 15 | 83.3 | 50 | 82 | 1,774 | 91.4 | .005 |
| M1, distant metastasis | 14 | 17.7 | 3 | 16.7 | 11 | 18 | 166 | 8.6 | |
| PSA at diagnosis, ng/mL | |||||||||
| Median | 11.5 | 8.9 | 15.1 | 11.3 | .926 | ||||
| Range | 0.5-3,000 | 0.7-3,000 | 0.5-761 | 0.2-7,800 | |||||
| Anatomic stage/prognostic group | |||||||||
| Stage I | 8 | 10.1 | 2 | 11.1 | 6 | 9.8 | 373 | 19.2 | .001 |
| Stage IIA | 9 | 11.40 | 1 | 5.6 | 8 | 13.1 | 325 | 16.8 | |
| Stage IIB | 13 | 16.5 | 3 | 16.7 | 10 | 16.4 | 213 | 11.0 | |
| Stage III | 12 | 16.5 | 4 | 22.2 | 11 | 18.1 | 367 | 18.9 | |
| Stage IV | 22 | 27.8 | 3 | 16.7 | 19 | 31.1 | 249 | 12.8 | |
| Cannot be assessed | 14 | 17.7 | 5 | 27.8 | 7 | 11.5 | 413 | 21.3 | |
| Risk stratification for localized PCa | |||||||||
| Low risk | 8 | 17.8 | 2 | 20 | 6 | 17.1 | 373 | 28.6 | .224 |
| Intermediate risk | 22 | 48.9 | 4 | 40 | 18 | 51.4 | 538 | 41.3 | |
| High risk | 15 | 33.3 | 4 | 40 | 11 | 31.4 | 392 | 30.1 | |
Abbreviations: PCa, prostate cancer; PSA, prostate-specific antigen.
Median PSA at diagnosis was higher in BRCA2 than in BRCA1 carriers (15.1 v 8.6 ng/mL; P = .63) who also had a higher frequency of poorly differentiated tumors (38% v 28%), but these differences were not statistically significant. T stage, frequency of nodal involvement, and metastasis at diagnosis were similar in both groups (Table 1).
Treatment
BRCA mutation carriers and noncarriers received similar treatments (Table 2); 79% of noncarriers and 72% of BRCA carriers underwent radical treatment with either surgery or radiotherapy (P = .20), and 36% and 37%, respectively, also received adjuvant androgen-deprivation therapy (P = 0.6). All patients who developed metastasis (n = 330) received palliative hormone treatment; 54% of them had not received any hormone treatment before the diagnosis of metastatic disease. In addition, 17% of noncarriers and 34% of carriers (P = .018) were treated with chemotherapy.
Table 2.
Prostate Cancer Treatments Administered
| Treatment | Noncarriers |
BRCA Carriers |
P | ||||
|---|---|---|---|---|---|---|---|
| No. of Patients | Total No. | % | No. of Patients | Total No. | % | ||
| Primary radical treatment in nonmetastatic disease | |||||||
| External-beam radiotherapy | 794 | 1,774 | 44.8 | 23 | 65 | 35.4 | .135 |
| Radical prostatectomy | 539 | 1,774 | 30.3 | 22 | 65 | 33.8 | .551 |
| Brachytherapy | 67 | 1,774 | 3.8 | 2 | 65 | 3.1 | 1 |
| Any local radical treatment | 1,400 | 1,774 | 78.9 | 47 | 65 | 72.3 | .201 |
| Primary hormone treatment indication | |||||||
| Early disease | .599 | ||||||
| Neoadjuvant-adjuvant | 636 | 1,774 | 35.9 | 24 | 65 | 36.9 | |
| Single therapy | 112 | 1,774 | 6.3 | 6 | 65 | 9.2 | |
| Advanced disease | .210 | ||||||
| Palliative | 165 | 298 | 55.4 | 14 | 32 | 43.8 | |
| Other treatments for metastatic disease | |||||||
| Chemotherapy | 51 | 298 | 17.1 | 11 | 32 | 34.4 | .018 |
CSS From PCa
Two hundred twenty deaths that occurred during follow-up were attributed to PCa (three in BRCA1 carriers, 21 in BRCA2 carriers, and 196 in noncarriers). Median CSS in carriers and noncarriers was 8.6 years and 15.7 years, respectively (P = 7 × 10−8). A trend toward improved CSS was observed for BRCA1 carriers compared with BRCA2 carriers, but this was not significant (median CSS, 10.5 and 8.6 years, P = 0.37; Table 3 and Fig 2C). Cox regression analysis confirmed the independent prognostic value of BRCA1/2 mutations for CSS (P = .015; HR = 1.8; 95% CI, 1.1 to 3.6). Within the carrier group, BRCA2 mutations contributed the most to this risk (P = .007; HR = 1.9; 95% CI, 1.1 to 3.4). Other prognostic factors included age more than 65 years, PSA more than 10 ng/mL, Gleason score, tumor size, and metastasis at diagnosis (Fig 2D).
Table 3.
Outcome of Patients With Prostate Cancer by BRCA Mutation Status
| Group | 5-Year Rate | 95% CI | Median (years) | 95% CI | Log-Rank P |
|---|---|---|---|---|---|
| Overall survival | |||||
| Controls | 86.4 | 84.4 to 88.4 | 12.9 | 11.8 to 14 | < .001* |
| BRCA mutation carriers | 62.0 | 49.1 to 74.9 | 8.1 | 5 to 11.1 | |
| BRCA1 mutation carriers | 82.5 | 60.4 to 100 | 10.5 | 6.7 to 14.5 | .338† |
| BRCA2 mutation carriers | 57.9 | 43.4 to 72.4 | 6.5 | 3.4 to 9.6 | < .001‡ |
| Cause-specific survival | |||||
| Controls | 90.6 | 88.8 to 92.4 | 15.7 | — | < .001* |
| BRCA mutation carriers | 70.1 | 57.2 to 83 | 8.6 | 6.4 to 10.7 | |
| BRCA1 mutation carriers | 80.8 | 56.9 to 100 | 10.5 | — | .200† |
| BRCA2 mutation carriers | 67.9 | 53.4 to 82.4 | 8.6 | 7.7 to 9.5 | < .001‡ |
| Cause-specific survival in M0 patients | |||||
| Controls | 96.2 | 95 to 97.4 | —§ | — | < .001* |
| BRCA mutation carriers | 81.5 | 69 to 94 | 11.3 | 7.1 to 15.4 | |
| BRCA1 mutation carriers | 88.9 | 68.3 to 100 | —§ | — | .576† |
| BRCA2 mutation carriers | 80.2 | 65.9 to 94.5 | 8.8 | 6.2 to 11.5 | < .001‡ |
| Metastasis-free survival in M0 patients | |||||
| Controls | 93.4 | 91.6 to 95.2 | —∥ | — | < .001* |
| BRCA mutation carriers | 77.0 | 62.7 to 91.3 | —∥ | — | |
| BRCA1 mutation carriers | 90.9 | 73.8 to 100 | —∥ | — | .801† |
| BRCA2 mutation carriers | 73.1 | 55.9 to 90.3 | 10.6 | 3.6 to 17.6 | < .001‡ |
| Cause-specific survival from prostate cancer metastasis | |||||
| Controls | 35.2 | 28.5 to 41.9 | 3.4 | 2.8 to 4.0 | .623* |
| BRCA mutation carriers | 22.4 | 5.2 to 39.6 | 2.3 | 2 to 2.5 | |
| BRCA1 mutation carriers | 37.5 | 0 to 93.6 | 2.3 | 0 to 4.9 | .767† |
| BRCA2 mutation carriers | 20.6 | 2.8 to 38.4 | 2.3 | 1.5 to 3.1 | .460‡ |
Univariable P value (log-rank test) for all BRCA carriers versus noncarriers.
Univariable P value (log-rank test) for BRCA1 carriers versus noncarriers.
Univariable P value (log-rank test) for BRCA2 carriers versus noncarriers.
After a median follow-up of 50 months, median CSS_M0 was not reached.
After a median follow-up of 50 months, median MFS was not reached.
Fig 2.
Kaplan-Meier survival curves: (A) overall survival (OS); (C) cause-specific survival (CSS); (E) CSS in early prostate cancer (PCa; CSS_M0); (G) CSS from metastatic disease; and (I) metastasis-free survival (MFS) in early PCa. Survival curves for noncarrier patients are represented in blue; BRCA1 and BRCA2 mutation carriers are illustrated in gold and gray, respectively. Diagrams illustrating the relative strength (hazard ratio [HR]) of each prognostic factor in the multivariate Cox regression: (B) OS; (D) CSS; (F) CSS_M0; (H) CSS from metastatic disease; and (J) MFS. The colored diamonds and the horizontal lines represent the estimated HR and their respective 95% CIs. The vertical discontinuous line represents no effect. If a CI overlaps this line, the effect of this factor did not significantly differ from no effect. PSA, prostate-specific antigen.
OS
After 9,553 person-years of follow-up for the entire cohort, 358 deaths occurred (four in BRCA1 carriers, 29 in BRCA2 carriers, and 325 in noncarriers). Median OS in noncarriers was superior to that in carriers (12.9 v 8.1 years; P = 1 × 10−7). There was also a nonsignificant trend toward improved OS for BRCA1 compared with BRCA2 carriers (10.5 v 6.5 years; P = 0.25; Table 3; Fig 2A). MVA confirmed the independent prognostic value of BRCA1/2 status in PCa for OS (P = .012; HR = 1.9; 95% CI, 1.1 to 3.3); similarly to CSS, BRCA2 mutations contributed the most (P = .004; HR = 1.9; 95% CI, 1.1 to 3.1), but did not confirm any prognostic value for BRCA1 mutations. Other significant risk factors for OS in the Cox regression analysis included age more than 60 years and PSA more than 10 ng/mL at diagnosis, Gleason score, tumor size, and metastasis (Fig 2B).
CSS in Localized PCa
When considering only nonmetastatic patients (M0) at diagnosis, 5-year CSS_M0 in noncarriers was significantly improved compared with carriers (96% v 82%; P = 9 × 10−8), but there was no significant difference between BRCA1 and BRCA2 carriers (89% v 82%; P = 0.29) The independent prognostic value of BRCA1/2 status in CSS_M0 was again confirmed (P = .011; HR = 2.6; 95% CI, 1.2 to 5.3); BRCA2 mutations were once more the main contributor to this risk (Table 3; Fig 1E). Independent prognostic factors for CSS_M0 also included tumor size and Gleason score (Fig 1F).
MFS
During follow-up, 132 noncarriers, 1 BRCA1 carrier, and 17 BRCA2 carriers with localized PCa at diagnosis developed metastasis. Five-year MFS in noncarriers was significantly higher than in BRCA carriers (93% v 77%; P = .0001), but there was no difference between BRCA1 and BRCA2 carriers (91% v 73%; P = .28; Table 3; Fig 2I). MVA confirmed the independent prognostic value of BRCA mutations for MFS (P = .009; HR = 2.7; 95% CI, 1.3 to 5.7; Fig 2J).
CSS From Metastasis
Longer median CSS_M1 was observed in noncarriers compared with carriers (3.4 v 2.3 years) but the difference was not significant (Table 3; Fig 2G). In MVA, patients who presented with metastasis at diagnosis were likely to have longer survival than those who developed metastasis after radical treatment. A Gleason score ≥ 8 was also an independent predictor of poor outcome (Fig 2H).
DISCUSSION
To our knowledge, this is the largest study to date that has investigated the clinical characteristics and outcome of patients with PCa with and without germline BRCA mutations. The study included 1,940 noncarriers and 79 BRCA carriers (61 BRCA2 and 18 BRCA1). The carrier group, with 13 BRCA1 and 40 BRCA2 different mutations, comprises the widest spectrum of mutations in these genes compared with previous studies. Our analyses provide more precise estimates of the prognostic implications of BRCA1/2 mutations in PCa.
We have demonstrated that node involvement and distant metastasis are more common in patients with PCa who have BRCA1/2 mutations than in noncarriers, but also that those carriers with local disease develop metastasis earlier. BRCA1/2 carriers with PCa are currently treated following the same protocols used for noncarriers, as the most appropriate management for this group of patients has not been investigated. Although clinical trials are still needed, radical treatment with either surgery or radiotherapy seems to be preferable to active surveillance for these patients, even for cases classified as low risk.21,23
Our series is the first to report on survival in metastatic patients. Although median CSS_M1 was 2.3 and 3.4 years for carriers and noncarriers, respectively, the difference was not significant, and MVA did not show any statistical trend to shorter CSS_ M1 in the BRCA1/2 patients. Interestingly, BRCA1/2 carriers more frequently had castration-resistant disease at metastatic progression and thus received chemotherapy more often. The lack of difference observed in CSS_M1 could be explained if BRCA1/2 carriers responded to chemotherapy similarly to noncarriers, as has recently been suggested by Gallagher et al,24 although a larger study is needed to confirm this. However, based on the current knowledge of BRCA1/2-related breast and ovarian cancers,25–29 studies evaluating the benefit of platinum-based chemotherapy and poly adenosine diphosphate ribose polymerase inhibitors in these patients are warranted.
The implementation of early diagnosis in these patients may also be crucial, and currently the IMPACT (Identification of Men With a Genetic Predisposition to Prostate Cancer: Targeted Screening in Men at Higher Genetic Risk and Controls) study is evaluating the utility of PSA-based PCa screening in asymptomatic BRCA1/2 carriers.30 In the general population, PSA screening remains controversial,31–35 and a national screening program for PCa has never been implemented in the United Kingdom, where it is estimated that only 6% of men aged 45 to 89 years have routine PSA testing.36 Consequently, PCa in the majority of patients in our series was clinically detected. The aggressive characteristics and worse PCa outcomes observed in BRCA2 carriers could not be attributed to a greater delay in diagnosis compared with the other two groups, as BRCA2 carriers were more frequently diagnosed by PSA screening.
Our study was consistent with others13,15,17,37 that have not observed differences in age at diagnosis between carriers and noncarriers. However, we could not rule out that some specific mutations, such as the Icelandic founder mutation (BRCA2 999del5), could be associated with PCa at a younger age.12
We confirmed that patients with PCa with germline BRCA mutations have poorer OS and CSS compared with noncarriers. Subgroup analyses and MVA suggest that the association with poor outcome was mostly dependent on BRCA2, whereas the contribution of BRCA1 mutations remains unclear, as the number of BRCA1 patients and events were still small to draw any definitive conclusions.
Previously, other series have failed to clarify the clinical value of BRCA1 in PCa due to even smaller number of cases than our series13,15 and the lack of clinicopathologic features14 In our study, we observed that although BRCA1 carriers presented with similar baseline risk characteristics as BRCA2 carriers, their survival parameters (OS, CSS, CSS_M0, and MFS) were more similar to those noncarrier patients. These nonsignficant differences between BRCA1 and BRCA2 carriers should be interpreted cautiously and as hypothesis-generating data rather than conclusive results.
Other studies have reported shorter survival and greater HR associated with BRCA2 mutations.12,15–17 Tryggovadotir et al12 reported a median CSS for BRCA2 of 2.1 years with an MVA HR of 2.4, whereas in our study, median CSS for these patients was 8.6 years with an MVA HR of 1.9. This difference could be mostly attributed to the greater frequency of N1 and/or M1 stage in patients with the Icelandic mutation. Thorne et al17 analyzed a group of 40 patients with 26 different BRCA2 mutations whose baseline characteristics were similar or even less advanced/aggressive than the patients in our series. They reported a remarkably shorter CSS (3.5 years) and a greater HR (4.97) for the BRCA2 carriers compared with our study. Although we cannot explain the differences in survival, the excess of HR in their series could be explained by the small number of variables tested in the MVA.
In a series with 832 patients with localized PCa, including six with the BRCA1 185delAG and 20 with BRCA2 6174delT Ashkenazi mutations, Gallagher et al15 reported a better median CSS in their BRCA2 carriers compared with our series (13.8 v 8.8 years).
The reported HR for BRCA2 status in their study was greater than in ours (5.48 v 3.21), despite the CSS in noncarriers being similar in both series (5-year OS > 95%). MFS in their study was also longer compared with ours. These findings suggest that BRCA2 6174delT may be associated with better outcomes than other BRCA2 mutations.
The addition of BRCA carrier status improved the predictive ability of the commonly used Kattan nomogram22 for MFS and CSS_M0, as shown in Table 4. For some scenarios this was statistically significant; however, this nomogram was originally derived for the prediction of time to treatment failure based on PSA and/or clinical recurrence, an end point that has not been analyzed in our study.
Table 4.
Comparative Kattan Nomogram Versus Kattan Plus BRCA Status
| Model and End Point | 5 Year |
8 Year |
10 Year |
|||
|---|---|---|---|---|---|---|
| AUC (%) | Likelihood Ratio Test P* | AUC (%) | Likelihood Ratio Test P* | AUC (%) | Likelihood Ratio Test P* | |
| All patients with local (N0 and M0) disease | ||||||
| Evaluable patients, n† | 927 | 521 | 267 | |||
| Metastasis-free survival | ||||||
| Kattan model | 81.1 | — | 76.2 | — | 76.6 | — |
| Kattan + BRCA | 82.5 | .048 | 76.6 | .128 | 77.8 | .143 |
| Cause-specific survival | ||||||
| Kattan model | 79.9 | — | 77.6 | — | 76.1 | — |
| Kattan + BRCA | 80.5 | .102 | 78.9 | .083 | 77.0 | .076 |
| Overall survival | ||||||
| Kattan model | 79.6 | — | 78.1 | — | 76.2 | — |
| Kattan + BRCA | 81.5 | .071 | 79.8 | .002 | 77.3 | .096 |
| Patients treated with prostatectomy for N0 and M0 disease | ||||||
| Evaluable patients, n† | 397 | 188 | 79 | |||
| Metastasis-free survival | ||||||
| Kattan model | 82.4 | — | 81.0 | — | 81.4 | — |
| Kattan + BRCA | 91.0 | .122 | 88.7 | .158 | 87.0 | .007 |
| Cause specific survival | ||||||
| Kattan model | 76.7 | — | 75.0 | — | 72.1 | — |
| Kattan + BRCA | 80.9 | .148 | 81.7 | .081 | 82.0 | .002 |
| Overall survival | ||||||
| Kattan model | 88.7 | — | 81.5 | — | 76.0 | — |
| Kattan + BRCA | 91.1 | .277 | 84.5 | .020 | 80.0 | .005 |
Abbreviation: AUC, area under the receiver operating characteristic curve.
Comparison of the logistic regression model for original Kattan nomogram alone (baseline prostate-specific antigen, Gleason score, and clinical tumor [T] stage) score versus the addition of BRCA carrier status using the likelihood ratio test.
All these survival analyses were handled as a simple binary variable for the logistic regression analyses. The number of patients for the different time points differs from the total of patients because those lost to follow-up before the end of the fixed time point were considered not to be evaluable for the analysis.
A potential limitation of our study is the selection of patients with familial PCa; nevertheless numerous reports have shown that a family history of PCa does not affect PCa outcome.38–40 However, our patients were randomly selected with respect to known prognostic factors for PCa. The differences between univariate and multivariate associations in our series suggested that some of these prognostic variables acted as confounding factors.
In conclusion, our results show that a wide spectrum of pathogenic mutations in the BRCA1 and BRCA2 genes confers a more aggressive PCa phenotype with a higher probability of locally advanced and metastatic disease and that the presence of a germline BRCA2 mutation is a prognostic marker associated with poorer survival. Trials analyzing the response of these patients to different treatment modalities and molecular studies to identify the key drivers and therapeutic targets of this PCa subgroup are urgently needed, as this would enable tailored management for these patients.
Acknowledgment
Presented in part at the 47th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 3-7, 2011.
Appendix
Epidemiological Study of BRCA1 & BRCA2 Mutation Carriers (EMBRACE). Douglas F. Easton is the principal investigator of the study. EMBRACE Collaborating Centres are as follows: Coordinating Centre, Cambridge: Susan Peock, Debra Frost, Steve D. Ellis, Elena Fineberg, Radka Platte. North of Scotland Regional Genetics Service, Aberdeen: Zosia Miedzybrodzka, Helen Gregory. Northern Ireland Regional Genetics Service, Belfast: Patrick Morrison, Lisa Jeffers. West Midlands Regional Clinical Genetics Service, Birmingham: Trevor Cole, Kai-ren Ong, Jonathan Hoffman. South West Regional Genetics Service, Bristol: Alan Donaldson, Margaret James. East Anglian Regional Genetics Service, Cambridge: Marc Tischkowitz, Joan Paterson, Sarah Downing, Amy Taylor. Medical Genetics Services for Wales, Cardiff: Alexandra Murray, Mark T. Rogers, Emma McCann. St James's Hospital, Dublin & National Centre for Medical Genetics, Dublin: M. John Kennedy, David Barton. South East of Scotland Regional Genetics Service, Edinburgh: Mary Porteous, Sarah Drummond. Peninsula Clinical Genetics Service, Exeter: Carole Brewer, Emma Kivuva, Anne Searle, Selina Goodman, Kathryn Hill. West of Scotland Regional Genetics Service, Glasgow: Rosemarie Davidson, Victoria Murday, Nicola Bradshaw, Lesley Snadden, Mark Longmuir, Catherine Watt, Sarah Gibson, Eshika Haque, Ed Tobias, Alexis Duncan. South East Thames Regional Genetics Service, Guy's Hospital London: Louise Izatt, Chris Jacobs, Caroline Langman. North West Thames Regional Genetics Service, Harrow: Huw Dorkins. Leicestershire Clinical Genetics Service, Leicester: Julian Barwell. Yorkshire Regional Genetics Service, Leeds: Julian Adlard, Gemma Serra-Feliu. Cheshire & Merseyside Clinical Genetics Service, Liverpool: Ian Ellis, Catherine Houghton. Manchester Regional Genetics Service, Manchester: D Gareth Evans, Fiona Lalloo, Jane Taylor. North East Thames Regional Genetics Service, NE Thames, London: Lucy Side, Alison Male, Cheryl Berlin. Nottingham Centre for Medical Genetics, Nottingham: Jacqueline Eason, Rebecca Collier. Northern Clinical Genetics Service, Newcastle: Fiona Douglas, Oonagh Claber, Irene Jobson. Oxford Regional Genetics Service, Oxford: Lisa Walker, Diane McLeod, Dorothy Halliday, Sarah Durell, Barbara Stayner. The Institute of Cancer Research and Royal Marsden NHS Foundation Trust: Ros Eeles, Susan Shanley, Nazneen Rahman, Richard Houlston, Elizabeth Bancroft, Elizabeth Page, Audrey Ardern-Jones, Kelly Kohut, Jennifer Wiggins Elena Castro, Emma Killick, Sue Martin, Gillian Rea, Anjana Kulkarni. North Trent Clinical Genetics Service, Sheffield: Jackie Cook, Oliver Quarrell, Cathryn Bardsley. South West Thames Regional Genetics Service, London: Shirley Hodgson, Sheila Goff, Glen Brice, Lizzie Winchester, Charlotte Eddy, Vishakha Tripathi, Virginia Attard. Wessex Clinical Genetics Service, Princess Anne Hospital, Southampton: Diana Eccles, Anneke Lucassen, Gillian Crawford, Donna McBride, Sarah Smalley.
United Kingdom Genetic Prostate Cancer study (UKGPCS). Rosalind Eeles is the principal investigator of the UKGPCS study. A complete list with all collaborators can be find at www.icr.ac.uk/ukgpcs.
Table A1.
List of BRCA1 and BRCA2 Mutations Carried by the Patients Included in the Study
| Mutation ID | Mutation Type | Patients |
|---|---|---|
| BRCA1 mutations | ||
| c.1-1447insA | Frameshift | 1 |
| c.68_69delAG (185delAG) | Frameshift | 4 |
| c.212 + 1G> (IVS 5 + 1) | Frameshift | 1 |
| c.1175_1214del40 | Frameshift | 1 |
| c.1961dupA | Frameshift | 1 |
| c.2071delA | Frameshift | 1 |
| c.2073dupA | Frameshift | 1 |
| c.2594delC | Frameshift | 1 |
| c.3756_3759delGTCT | Frameshift | 1 |
| c.4065_4068delTCAA | Frameshift | 3 |
| c.4327C>T | Nonsense | 1 |
| c.4945delA | Frameshift | 1 |
| c.5503C>T | Nonsense | 1 |
| BRCA2 mutations | ||
| c.755_758delACAG | Frameshift | 1 |
| c.1231delA | Frameshift | 1 |
| c.1265delA | Frameshift | 1 |
| c.1787delATGAAACATCTTAA | Frameshift | 1 |
| c.1813insA | Frameshift | 1 |
| c.1929delG | Frameshift | 1 |
| c.2558insA | Frameshift | 1 |
| c.2807delAACA | Frameshift | 1 |
| c.2836delGA | Frameshift | 1 |
| c.3158T>G | Nonsense | 1 |
| c.3405C>A | Nonsense | 1 |
| c.3847delGT | Frameshift | 1 |
| c.4478delAAAG | Frameshift | 2 |
| c.4877delAA | Frameshift | 2 |
| c.4889C>G | Nonsense | 1 |
| c.4965C>G | Nonsense | 1 |
| c.4981delT | Frameshift | 2 |
| c.5303delTT | Frameshift | 1 |
| c.5350_5351delAA | Frameshift | 1 |
| c.5645C>CA | Nonsense | 1 |
| c.5682C>G | Frameshift | 3 |
| c.5946delT (6174delT) | Frameshift | 2 |
| c.6155C>G | Missense | 1 |
| c.6275_6276delTT | Frameshift | 4 |
| c.6405delCTTAA | Frameshift | 2 |
| c.6591_6592delTG | Frameshift | 1 |
| c.6486_6489delACAA | Frameshift | 1 |
| c.7008-?_7805+?del | Large deletion | 1 |
| c.7084delAAAAG | Frameshift | 1 |
| c.7543dupA | Frameshift | 2 |
| c.7757G>A | Nonsense | 1 |
| c.7771insA | Nonsense | 2 |
| c.7966C>T | Nonsense | 1 |
| c.7977-1G>C (IVS 17 G>C) | Splice site | 3 |
| c.8167G>C | Missense | 1 |
| c.8297delC | Frameshift | 2 |
| c.8904delC | Frameshift | 2 |
| c.9097dupA | Frameshift | 1 |
| c.9253insA | Frameshift | 2 |
| c.9294C>G | Nonsense | 1 |
| c.9382C>T | Nonsense | 3 |
| Del exon 14-16 | Large deletion | 1 |
Footnotes
Author affiliations appear at the end of this article.
Written on behalf of the United Kingdom Genetic Prostate Cancer Study and EMBRACE Collaborators.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: Rosalind Eeles, Succinct Healthcare Communications Research Funding: None Expert Testimony: None Other Remuneration: Rosalind Eeles, Vista Clinical Diagnostics, Gen-Probe (formerly Tepnel), Illumina, Janssen
AUTHOR CONTRIBUTIONS
Conception and design: Elena Castro, Chee Goh, David Olmos, Zsofia Kote-Jarai, Rosalind Eeles
Administrative support: Koveela Govindasami, Michelle Guy, Emma Sawyer, Rosemary Wilkinson, Debra Frost, Susan Peock
Provision of study materials or patients: Steve Ellis, M. John Kennedy, Lucy E. Side, Jacqueline Eason, Alex Murray, Douglas F. Easton, Zsofia Kote-Jarai, Rosalind Eeles
Collection and assembly of data: Elena Castro, Chee Goh, David Olmos, Ed Saunders, Daniel Leongamornlert, Malgorzata Tymrakiewicz, Nadiya Mahmud, Tokhir Dadaev, Koveela Govindasami, Michelle Guy, Emma Sawyer, Rosemary Wilkinson, Audrey Ardern-Jones, Steve Ellis, Debra Frost, Susan Peock, D. Gareth Evans, Marc Tischkowitz, Trevor Cole, Rosemarie Davidson, Diana Eccles, Carole Brewer, Fiona Douglas, Mary E. Porteous, Alan Donaldson, Huw Dorkins, Louise Izatt, Jackie Cook, Shirley Hodgson, M. John Kennedy, Lucy E. Side, Jacqueline Eason, Alex Murray, Zsofia Kote-Jarai
Data analysis and interpretation: Elena Castro, David Olmos, Ed Saunders, Daniel Leongamornlert, Malgorzata Tymrakiewicz, Nadiya Mahmud, Tokhir Dadaev, Antonis C. Antoniou, Douglas F. Easton
Manuscript writing: All authors
Final approval of manuscript: All authors
Support
The American Society of Clinical Oncology (ASCO) Cancer Foundation awarded E.C. with a 2011 ASCO Annual Meeting Merit Award. D.O. is supported by CRIS Fundación contra el Cáncer and Fundación científica de la AECC grants. E.C. was also supported by the European Society of Medical Oncology Fellowship program (2010-2011) and the Ronald and Rita McAulay Foundation (2009-2012). C.G. is supported by the GAME-ON (Genetic Associations and Mechanisms in Oncology) initiative (National Institutes of Health ELLIPSE Grant No. U19CA148537). This work was supported by the Ronald and Rita McAulay Foundation and Cancer Research UK Grant No. C5047/A13232. A.C.A. is Cancer Research UK Senior Cancer Research Fellow. Epidemiological Study of BRCA1 and BRCA2 Mutation Carriers (EMBRACE) is supported by Cancer Research UK Grants No. C1287/A10118 and C1287/A11990. D.G.E. is supported by a National Institute for Health Research (NIHR) grant to the Biomedical Research Centre, Manchester. The investigators at the Institute of Cancer Research and the Royal Marsden National Health Service (NHS) Foundation Trust are supported by an NIHR grant to the Biomedical Research Centre at the Institute of Cancer Research and the Royal Marsden NHS Foundation Trust.
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Carter BS, Beaty TH, Steinberg GD, et al. Mendelian inheritance of familial prostate cancer. Proc Natl Acad Sci U S A. 1992;89:3367–3371. doi: 10.1073/pnas.89.8.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eeles RA. Genetic predisposition to prostate cancer. Prostate Cancer Prostatic Dis. 1999;2:9–15. doi: 10.1038/sj.pcan.4500279. [DOI] [PubMed] [Google Scholar]
- 5.Edwards SM, Eeles RA. Unravelling the genetics of prostate cancer. Am J Med Genet C Semin Med Genet. 2004;129C:65–73. doi: 10.1002/ajmg.c.30027. [DOI] [PubMed] [Google Scholar]
- 6.Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer: Analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 7.Eeles RA, Amin Al Olama A, Benlloch S, et al. Identification of 23 new prostate cancer susceptibility loci using the iCOGS custom genotyping array. Nature Genetics. 2013;45:385–391. doi: 10.1038/ng.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cancer risks in BRCA2 mutation carriers. The Breast Cancer Linkage Consortium. J Natl Cancer Inst. 1999;91:1310–1316. doi: 10.1093/jnci/91.15.1310. [DOI] [PubMed] [Google Scholar]
- 9.Edwards SM, Kote-Jarai Z, Meitz J, et al. Two percent of men with early-onset prostate cancer harbor germline mutations in the BRCA2 gene. Am J Hum Genet. 2003;72:1–12. doi: 10.1086/345310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kote-Jarai Z, Leongamornlert D, Saunders E, et al. BRCA2 is a moderate penetrance gene contributing to young-onset prostate cancer: Implications for genetic testing in prostate cancer patients. Br J Cancer. 2011;105:1230–1234. doi: 10.1038/bjc.2011.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leongamornlert D, Mahmud N, Tymrakiewicz M, et al. Germline BRCA1 mutations increase prostate cancer risk. Br J Cancer. 2012;106:1697–1701. doi: 10.1038/bjc.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tryggvadóttir L, Vidarsdóttir L, Thorgeirsson T, et al. Prostate cancer progression and survival in BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99:929–935. doi: 10.1093/jnci/djm005. [DOI] [PubMed] [Google Scholar]
- 13.Mitra A, Fisher C, Foster CS, et al. Prostate cancer in male BRCA1 and BRCA2 mutation carriers has a more aggressive phenotype. Br J Cancer. 2008;98:502–507. doi: 10.1038/sj.bjc.6604132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narod SA, Neuhausen S, Vichodez G, et al. Rapid progression of prostate cancer in men with a BRCA2 mutation. Br J Cancer. 2008;99:371–374. doi: 10.1038/sj.bjc.6604453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallagher DJ, Gaudet MM, Pal P, et al. Germline BRCA mutations denote a clinicopathologic subset of prostate cancer. Clin Cancer Res. 2010;16:2115–2121. doi: 10.1158/1078-0432.CCR-09-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards SM, Evans DG, Hope Q, et al. Prostate cancer in BRCA2 germline mutation carriers is associated with poorer prognosis. Br J Cancer. 2010;103:918–924. doi: 10.1038/sj.bjc.6605822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thorne H, Willems AJ, Niedermayr E, et al. Decreased prostate cancer-specific survival of men with BRCA2 mutations from multiple breast cancer families. Cancer Prev Res (Phila) 2011;4:1002–1010. doi: 10.1158/1940-6207.CAPR-10-0397. [DOI] [PubMed] [Google Scholar]
- 18.Institute of Cancer Research. UK Genetic Prostate Cancer Study. www.icr.ac.uk/ukgpcs.
- 19.University of Cambridge Centre for Cancer Genetic Epidemiology, School of Clinical Medicine. Epidemiological study of BRCA1 and BRCA2 mutation carriers. http://www.srl.cam.ac.uk/genepi/embrace/embrace_home.html.
- 20.National Institute for Health and Clinical Excellence. Prostate cancer: Diagnosis and treatment CG58. 2008. http://publications.nice.org.uk/prostate-cancer-cg58.
- 21.Mohler J, Bahnson RR, Boston B, et al. NCCN clinical practice guidelines in oncology: Prostate cancer. J Natl Compr Canc Netw. 2010;8:162–200. doi: 10.6004/jnccn.2010.0012. [DOI] [PubMed] [Google Scholar]
- 22.Kattan MW, Eastham JA, Stapleton AM, et al. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998;90:766–771. doi: 10.1093/jnci/90.10.766. [DOI] [PubMed] [Google Scholar]
- 23.Graham J, Baker M, Macbeth F, et al. Diagnosis and treatment of prostate cancer: Summary of NICE guidance. BMJ. 2008;336:610–612. doi: 10.1136/bmj.39498.525706.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gallagher DJ, Cronin AM, Milowsky MI, et al. Germline BRCA mutation does not prevent response to taxane-based therapy for the treatment of castration-resistant prostate cancer. BJU Int. 2012;109:713–719. doi: 10.1111/j.1464-410X.2011.10292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 26.Fong PC, Yap TA, Boss DS, et al. Poly(ADP)-ribose polymerase inhibition: Frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol. 2010;28:2512–2519. doi: 10.1200/JCO.2009.26.9589. [DOI] [PubMed] [Google Scholar]
- 27.Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: A proof-of-concept trial. Lancet. 2010;376:235–244. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 28.Byrski T, Gronwald J, Huzarski T, et al. Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J Clin Oncol. 2010;28:375–379. doi: 10.1200/JCO.2008.20.7019. [DOI] [PubMed] [Google Scholar]
- 29.Yang D, Khan S, Sun Y, et al. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA. 2011;306:1557–1565. doi: 10.1001/jama.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitra AV, Bancroft EK, Barbachano Y, et al. Targeted prostate cancer screening in men with mutations in BRCA1 and BRCA2 detects aggressive prostate cancer: Preliminary analysis of the results of the IMPACT study. BJU Int. 2010;107:28–39. doi: 10.1111/j.1464-410X.2010.09648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 32.Andriole GL, Crawford ED, Grubb RL, 3rd, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hugosson J, Carlsson S, Aus G, et al. Mortality results from the Göteborg randomised population-based prostate-cancer screening trial. Lancet Oncol. 2010;11:725–732. doi: 10.1016/S1470-2045(10)70146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chou R, Croswell JM, Dana T, et al. Screening for prostate cancer: A review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:762–771. doi: 10.7326/0003-4819-155-11-201112060-00375. [DOI] [PubMed] [Google Scholar]
- 35.Andriole GL, Crawford ED, Grubb RL, 3rd, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: Mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125–132. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams N, Hughes LJ, Turner EL, et al. Prostate-specific antigen testing rates remain low in UK general practice: A cross-sectional study in six English cities. BJU Int. 2011;108:1402–1408. doi: 10.1111/j.1464-410X.2011.10163.x. [DOI] [PubMed] [Google Scholar]
- 37.Giusti RM, Rutter JL, Duray PH, et al. A twofold increase in BRCA mutation related prostate cancer among Ashkenazi Israelis is not associated with distinctive histopathology. J Med Genet. 2003;40:787–792. doi: 10.1136/jmg.40.10.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grönberg H, Damber L, Tavelin B, et al. No difference in survival between sporadic, familial and hereditary prostate cancer. Br J Urol. 1998;82:564–567. doi: 10.1046/j.1464-410x.1998.00801.x. [DOI] [PubMed] [Google Scholar]
- 39.Roehl KA, Loeb S, Antenor JA, et al. Characteristics of patients with familial versus sporadic prostate cancer. J Urol. 2006;176:2438–2442. doi: 10.1016/j.juro.2006.07.159. [DOI] [PubMed] [Google Scholar]
- 40.Heck MM, Kron M, Gschwend JE, et al. Effect of family history on outcome in German patients treated with radical prostatectomy for clinically localised prostate cancer. Eur J Cancer. 2012;48:1312–1317. doi: 10.1016/j.ejca.2011.10.002. [DOI] [PubMed] [Google Scholar]