Abstract
Progression of fibrosis involves interstitial hypercellularity, matrix accumulation and atrophy of epithelial structures, resulting in the loss of normal function and ultimately organ failure. There is common agreement that the fibroblast/myofibroblast is the cell type most responsible for interstitial matrix accumulation and consequent structural deformations associated with fibrosis. During wound healing and progressive fibrotic events fibroblasts transform into myofibroblasts acquiring smooth muscle features, most notably the expression of alpha-smooth muscle actin (α-SMA) and synthesis of mesenchymal cell related matrix proteins. In renal disease, glomerular mesangial cells also acquire a myofibroblast phenotype and synthesize the same matrix proteins. The origin of interstitial myofibroblasts during fibrosis is a matter of debate where the cells are proposed to derive from resident fibroblasts, pericytes, perivascular adventitial, epithelial, and/or endothelial sources. Regardless of the origin of the cells, TGF-β1 is the principal growth factor responsible for myofibroblast differentiation to a profibrotic phenotype and exerts its effects via Smad signaling pathways involving MAPK and Akt/PKB. Additionally, reactive oxygen species (ROS) play important roles in progression of fibrosis. ROS are derived from a variety of enzyme sources of which the NAD(P)H oxidase family has been identified as a major source of superoxide and hydrogen peroxide generation in the cardiovasculature and the kidney during health and disease. Recent evidence indicates that the NAD(P)H oxidase homolog Nox4 is most accountable for ROS-induced fibroblast and mesangial cell activation where it has an essential role in TGF-β1 signaling of fibroblast activation and differentiation into a profibrotic myofibroblast phenotype and matrix production. Information on the role of ROS in mesangial cell and fibroblast signaling is incomplete and further research on myofibroblast differentiation during fibrosis is warranted.
Keywords: fibrosis, smooth muscle actin, extracellular matrix, reactive oxygen species, Nox4
Introduction
Progression of fibrosis is remarkably similar in most organs involving pathogenic processes of interstitial hypercellularity, matrix accumulation and atrophy of epithelial structures that lead to loss of normal function and organ failure. Three phases of fibrogenesis have been described in the kidney [1] that predictably apply to other organ systems as well. There is an induction phase characterized by infiltration of inflammatory cells, principally mononuclear cells or macrophages, that release profibrogenic cytokines and growth factors. This initial event is followed by the activation of fibroblasts to transition into myofibroblasts that also secrete biological active products and matrix proteins. The third phase is ongoing synthesis and accumulation of matrix despite resolution of the primary stimulus. Central to the activation of fibroblast to a profibrotic myofibrobroblast is transforming growth factor beta (TGF-β1) that can be released through paracrine and autocrine pathways. The roles inflammatory, tubular and vascular cells play in fibrosis is complex and multifactorial, a topic adroitly covered in several recent reviews [2–4]. In the kidney, interstitial fibrosis is a common pathway of progressive renal diseases leading to end stage renal disease regardless of the eitiology [5]. Also, interstitial fibrosis is the strongest morphologic predictor of clinical outcome and is most tightly linked to progression of disease [6]. Despite primary glomerular origin of most renal diseases, interstitial involvement indicates a more ominous outcome [7]. With increasing morbidity associated with fibrosis related to obesity, diabetes, and heart disease there is imminent importance to understand the pathophysiology of the myofibroblast for the design of future therapeutics. This review will discuss biological factors that lead to myofibroblast differentiation and the origin of these cells in the interstitium. Attention is placed on TGF-β and reactive oxygen species (ROS) as principal mediators of fibrosis with special emphasis on the NAD(P)H oxidase of the Nox family (particularly Nox4) as requisite elements in signaling of TGF-β-induced myofibroblast differentiation.
Myofibroblasts in Fibrosis
Activation and transformation of fibroblasts into myofibroblasts was first described by Gabbiani showing that after injury, during wound healing, or progressive fibrotic events, activated cells acquired smooth muscle features including expression of desmin, caldesmon, and SM-myosin heavy chains and an actin isoform (alpha-smooth muscle actin, α-SMA) [8;9]. Consequently, α-SMA positive myofibroblasts were identified as the primary cell type responsible for interstitial matrix accumulation in fibrotic diseases including the kidney [8–13]. Moreover, an α-SMA phenotype is considered a useful marker for myofibroblast differentiation in a number of disease settings [8–27]. Similarly, an α-SMA + phenotype in intrinsic mesenchymal cells from a variety of organs can be induced by prolonged culture, exposure to cytokines such as interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF-α) and growth factors including platelet-derived growth factor (PDGF), angiotensin II, and TGF–β. These include liver fat storing cells (Ito cells), periportal fibroblasts, pancreatic stellate cells, breast stromal cells, lung fibroblasts, and renal mesangial cells and kidney fibroblasts [28–35]. These cells express little to no α-SMA in their resting state, however, upon activation in vitro or during disease, they highly express this protein along with extracellular mesenchymal matrix proteins collagen I, collagen III, and cellular fibronectin. More recently, epithelial cells have been reported to transition into matrix producing fibroblasts and α-SMA + fibroblasts (myofibroblasts), see section on EMT below. Collectively, these cells are referred to as “myofibroblasts” despite their origin.
Control of the transition of fibroblasts to myofibroblasts is complex and involves a variety of chemical factors, extracellular matrix proteins, and mechanical microenvironment [8;13], see below. Hinz [13] categorized the differentiation of fibroblasts into myofibroblasts functionally, by a two-step process where fibroblasts are activated to form proto-myofibroblasts with contractile stress fibers in response to locally released cytokines or changes in the composition, organization and mechanical property of the extracellular matrix (ECM). Proto-myofibroblasts further differentiate into myofibroblasts by neo-expressing α-SMA [13]. These steps are believed to be responsible for migration and re-population of damaged tissues followed by matrix synthesis and contraction of connective tissue during remodeling. Prolonged exposure to an insult results in inappropriate matrix expansion by myofibroblasts leading fibrosis and loss of tissue function [36].
Activation of Mesangial Cells and Interstitial Fibroblasts in Renal Disease
The bulk of excess extracellular matrix synthesis during the progression of chronic renal disease can be attributed to two similar mesenchymal cell types, the glomerular mesangial cell and the interstitial fibroblast. Mesangial cells have been suggested to be specialized pericytes [37] juxtaposed to the capillaries within the renal glomerulus and fibroblast occupy the peritubular interstitial space. Other than their location, the two cell types are quite similar in their response to profibrotic stimuli during disease by acquiring an α-SMA positive myofibroblast phenotype and the subsequent synthesis of matrix proteins including fibronectin, laminins and type IV collagen. When activated, both cell types also acquire the expression of type I and type III collagens [10–12;16;21;23;38–40] and activation of both cell types upregulates an alternatively spliced isoform of fibronectin containing extra domain EIIIA (equivalent to ED-A in human tissues) that plays an essential role in myofibroblast differentiation (see below). Also, α-SMA deficient mice, show enhanced cell proliferation and type-1 procollagen expression in the interstitium after UUO and in mesangioproliferative glomerulonephritis, suggesting that the function of α-SMA in these two mesenchymal cell types is similar (41). The interpretation of these seemingly paradoxical results was that the presence or absence of an α-SMA phenotype have opposing functions of maintaining a cell in a contractile or productive state, respectively. Thus, the relative presence of α-SMA may provide suppressing or accentuating roles in fibrosis progression (41).
The Origin(s) of Myofibroblasts
The origin(s) of interstitial myofibroblasts in fibrosis is currently under extensive investigation and scientific reports in kidney, liver and lung indicate the cells may be derived from one or more sources (Figure 1). Originally, myofibroblasts were thought to be derived from intrinsic fibroblasts that reside within the interstitium, but this simple concept has evolved into a much more complex and controversial area of investigation. The origins of interstitial myofibroblasts now include activation of resident fibroblasts or pericytes [19;20;22–26;42], expansion of perivascular fibroblasts [19;20;22;43–48], infiltration of circulating bone marrow-derived fibrocytes [49–51], epithelial to mesenchymal transition (EMT, see below) and/or endothelial-mesenchymal (EndoMT) transition [52;53]. Each mode of interstitial encroachment results by myofibroblast migration, proliferation, and ultimately matrix expansion within the interstitial space. EMT has become the preeminent hypothesis of renal fibroblasts and myofibroblast encroachment into the peritubular interstitium during kidney fibrosis (reviewed in [54–58]. EMT is based on the reversal of an essential developmental process, known as mesenchymal to epithelial transition (MET) whereby embryonic mesenchymal cells differentiate into the tubular epithelium [59]. During the process of EMT, mechanisms are believed to exist to reverse the MET and epithelial cells lose specialized differentiation markers such as E-cadherin and cytokeratins, and acquire mesenchymal proteins including α-SMA, vimentin, and fibroblast specific protein-1 (FSP-1) that has been associated with cell migration and invasiveness. According to this hypothesis, the transformed epithelial cells synthesize matrix proteinases that digest basement membrane proteins, thereby allowing their migration into the interstitial space where they proliferate and synthesize extracellular matrix proteins commonly associated with mesenchymal cells [54–58]. Such local conversion of epithelium was reported by Strutz et al after the discovery the expression of FSP-1 by tubular epithelial cells during tubulointerstitial nephritis [60]. Since this original description, expression of FSP-1 in injured proximal tubules, with or without co-localization of mesenchymal markers, has been taken as evidence of EMT as the major contributor to fibrosis in numerous models of renal fibrosis including ureteral obstruction, renal ablation, diabetic nephropathy, nephrotoxic serum nephritis, and polycystic kidney disease [54–58, see listings in 56;61;62]. A role for EMT in liver and lung fibrosis has subsequently been described [63;64].
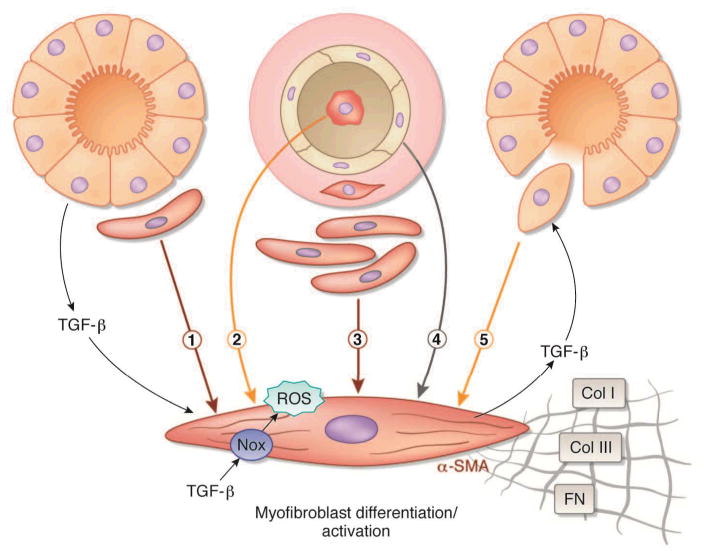
Figure 1. Proposed origin(s) of interstitial myofibroblasts during fibrosis.
Proposed origin(s) of interstitial myofibroblasts during fibrosis. Myofibroblasts responsible for interstitial expansion and structural damage during progressive organ injury have been proposed to be derived from one or more sources: (1) activation of resident fibroblasts or pericytes, (2) infiltration of circulating bone marrow-derived fibrocytes, (3) expansion of perivascular adventitial fibroblasts, (4) endothelial–mesenchymal (EndoMT) transition, and/or (5) epithelial-to-mesenchymal transition (EMT). Transforming growth factor-beta (TGF-β) released via paracrine or autocrine pathways induces myofibroblast differentiation expressed by the acquisition of an alpha-smooth muscle actin (α-SMA) phenotype and consequent synthesis of mesenchymal matrix proteins collagen type I (Col I), collagen type III (Col III), and fibronectin EIIIA (FN). Nox NAD(P)H oxidase (Nox4)-derived reactive oxygen species (ROS) have a central role in TGF-β signaling of myofibroblast differentiation.
The existence of EMT as a source of interstitial fibroblast (myofibroblast) encroachment has recently become a matter of debate (61;62;65). A large number of studies postdating the inception of the EMT hypothesis reported that myofibroblasts and their secreted matrix proteins are located exclusively in the interstitial space during experimental fibrosis without evidence of epithelial locations [17–26;66–74]. Moreover, recent studies specifically designed to determine the origin of myofibroblasts by utilizing state-of-the-art protein marker, gene reporter or epithelial tracking strategies observe myofibroblasts exclusively within the peritubular interstitium in the absence of evidence for EMT in a variety of models of renal fibrosis [19;20;22–26;42;66;72–75]. The above studies are in contrast to a number of reporter studies illustrating a tubular origin of myofibroblasts or α-SMA negative collagen producing fibroblasts that migrate into the interstitial space (76, see the following reviews for a comprehensive discussion 56;61;62). Also, elimination of cells expressing FSP-1 was reported to reduce fibrosis (77).
Much of the controversy over the origin of interstitial cells rests on selection and specificity of markers for cells destined to become interstitial matrix producing (myo)fibroblasts. Arguments have been made that FSP-1, also known as S100A4 is a superior and more consistent marker of tissue fibroblasts because α-SMA does not define the “universe of fibroblasts” in that not all fibroblasts express α-SMA” [60;76] and α-SMA is not expressed in all FSP-1 positive fibroblasts [54;78;79]. Indeed, the presence of FSP-1 in epithelial cells is now used to define EMT [61]. On the other hand, FSP-1, also known as S100A4 and a variety of other names, is not only observed in fibroblasts as the name implies but can be expressed by a wide variety of cell types [61,80–82], including resident fibroblasts, endothelial cells, monocytes, or macrophages residing in or near the renal interstitial space during fibrosis [19;20;25;26;66;83]. Conversely, others show no cross-reactivity of FSP-1 with macrophages in renal interstitial fibrosis (76;84;85). An ongoing debate exists over the selection of antibodies and materials used to detect S100A4, macrophages, and other cell types and whether “preferred” reagents are being used (25;66;84). S100A4 is also associated with cellular behaviors other than migration or invasiveness including cell stress, proliferation and apoptosis [80;81;86] that are present during tubular injury [42;69;87]. Thus, expression of FSP-1 may also be viewed as a marker for cell activation and reflect a generalized response to cell injury as well as cell migration and invasiveness. Varying viewpoints exist for and against the use of either α-SMA or S100A4 as suitable markers of interstitial matrix producing cells. Answers to this debate will require additional experimentation or the discovery of specific markers to determine the contributions of the various aforementioned cell types in renal interstitial fibrosis.
We are of the opinion that interstitial myofibroblasts are derived from local fibroblasts, pericytes, or perivascular cells [19;20;22;72,73]. A cellular location of myofibroblasts in the perivascular space also supports the histological descriptions that mesenchymal cell-derived collagen type I or type III, and/or fibronectin are first expressed in the perivascular spaces of arteries or arterioles in fibrosis related to cardiovascular [88], liver [89], lung [90] and kidney [19;22;43–48] disease. A perivascular source of myofibroblast encroachment becomes noteworthy because myofibroblasts in the adventitium is a major site of reactive oxygen species (ROS) in the aorta during vascular disease. The adventitia layer is less structured in smaller arteries and arterioles, but like the aortic adventitia, it may be the source of perivascular myofibroblasts during renal disease.
Myofibroblast Differentiation: Roles of TGF-β and Alternatively Spliced Fibronectin EIIIA
A number of growth factors are associated with myofibroblast differentiation including PDGF, angiotensin II, CTGF, and TGF-β1 [13]. TGF-β1 is frequently associated with a myofibroblast α-SMA phenotype in liver, lung, and kidney disease [8;91–93] and has been determined to be the preeminent growth factor responsible for fibroblast activation and matrix synthesis in vitro and during vascular disease and fibrosis [93–95]. Also, TGF-β1 plays a pivotal role in fibrogenesis in which a number of growth factors, including PDGF and angiotensin II, exert their effects by directly stimulating TGF-β1 production [92–98]. Additionally, TGF-β1 directly promotes myofibroblast development by inducing expression of α-SMA phenotype [34;35;95;99;100]. Similarly, TGF-β1 is considered the foremost growth factor for induction of EMT in culture and in vivo [101].
TGF-β1 contributes to fibrosis by the direct activation of myofibroblast synthesis of fibronectin, laminins collagen types I, III, IV and VI. Of these matrix proteins, collagen type VI and fibronectin (EIIIA) are closely associated with myofibroblast differentiation in vitro and in disease [13]. Fibronectin EIIIA is abundantly expressed during embryogenesis [21;102] and at the margins of healing wounds [103]; whereas, this domain is spliced out of plasma fibronectin (hepatocyte-derived) and many tissue-specific cells in adult organs [104], suggesting that this matrix protein has important and specialized functions in tissue remodeling [105]. α-SMA myofibroblasts and fibronectin EIIIA frequently co-localize in fibrotic disease as well as in glomerular and interstitial lesions in kidney diseases [21;22;38;39;106;107].
TGF-β1 differentially regulates the expression of fibronectin EIIIA [108] and induces expression of α-SMA in a variety of mesenchymal cells in culture including renal fibroblasts and mesangial cells [34;100;109]. Moreover, fibronectin EIIIA is mandatory for TGF-β1 induction of myofibroblast differentiation and α-SMA expression [109;110]. The close proximity of the EIIIA splice site to the Arg-Gly-Asp-Ser (RGDS) cell binding domain suggests that this variant has specific functional roles [105]. Certainly, fibronectin EIIIA is closely associated with activated cells undergoing high rates of migration, proliferation, and differentiation [21;38;39;102;103;105] and fibronectin EIIIA is considered the most reliable marker of myofibroblast-derived extracellular matrix [13].
Reactive Oxygen Species (ROS) in Fibroblast and Vascular Pericyte Activation
Accumulating evidence also indicates that oxidative stress resulting in generation of ROS, mainly in the form of superoxide, and hydrogen peroxide play a significant role in the initiation and progression of cardiovascular and renal disease [111–113]. Superoxide and its dismuted derivative hydrogen peroxide are formed by the univalent reduction of oxygen, generally mediated by several enzyme systems such as xanthine oxidase, uncoupled nitric oxide synthase, myeloperoxidase, mitochondrial respiratory oxidases, lipoxygenases, and the NAD(P)H oxidases of the Nox family. Exacerbated production of ROS may directly cause macromolecular damage or function as signaling molecules that induce cellular damage or fibrogenic responses through stress-sensitive pathways. It is important to mention that there is growing evidence indicating that ROS are not always damaging, especially at low levels, and in fact may act as physiological signaling molecules.
Examples of ROS-induced fibrogenic responses include distinct cell functions such as hypertrophy, migration, proliferation, apoptosis and regulation of extracellular matrix [34;106;111;112;113]. Perivascular adventitial fibroblasts in the rabbit and rat aorta [112;114–116], pericytes from the microvasculature [117], and renal myofibroblasts [34] are a major source of superoxide. These observations become highly relevant to fibrogenesis because not only are fibroblast responsible for ROS generation, but ROS are directly linked to transmodulation of fibroblasts to α-SMA expressing myofibroblasts [34;35;115;118;119]. Moreover, the perivascular space is noticeably reactive and is the location where myofibroblasts have been shown to first appear during the course of renal fibrosis (see above).
A role for ROS in the pathogenesis of chronic renal disease has been observed in a number of diverse models including diabetic nephropathy, proliferative glomerulonephritis; IgA nephropathy, hypertensive renal disease, and fibrosis due to transient ischemic injury, renal ablation, and ureteral obstruction (thoroughly referenced in [113;120]). Importantly, the NAD(P)H oxidases of the Nox family have recently gained heightened attention as principal mediators of injury associated with vascular and renal disease [111;112;120–123]. Moreover, NAD(P)H oxidase has been identified as the enzyme system most responsible for superoxide generation by adventitial fibroblasts after vascular injury of the aorta [112;116;124], and is now recognized as a key mediator of cell proliferation and matrix accumulation in renal disease [106;120;125–127]; a topic that will be reviewed in depth below.
NAD(P)H Oxidases of the Nox Family as Major Sources of ROS in the Cardiovasculature and the Kidney
Early studies on NAD(P)H oxidases were performed in neutrophils and phagocytic cells, investigating the respiratory burst NADPH oxidase system [128]. The phagocyte respiratory burst oxidase or phagocyte NADPH oxidase catalyzes the NADPH-dependent reduction of molecular oxygen to generate superoxide anion which is dismuted to hydrogen peroxide [128;129]. The phagocyte oxidase consists of two plasma membrane-associated proteins, gp91phox (the catalytic subunit) and p22phox which comprise flavocytochrome b558, as well as cytosolic factors, p47phox, p67phox, p40phox and the small GTPase Rac. Homologues of gp91phox, termed Nox (for NAD(P)H oxidase) proteins, have been found in all vascular and renal cells [111;1112;120;121;130–134] (Figure 2). To date, the Nox family comprises seven members: Nox1 to -5, of which Nox2 is gp91phox, and the dual oxidases Duox1 and -2 [122;130;135;136] (Figure 2). Nox1, gp91phox/Nox2 and Nox4 are the NAD(P)H oxidase isoforms that are predominantly expressed in the cardiovascular and cardiorenal systems. Note that the calcium-dependent isoform Nox5 is also found in the human vasculature but the fact that the enzyme is not present in rodents has hampered the investigation of its role in cardiovascular pathologies [121;134]. Moreover, no data related to Nox5 expression in the human kidney are yet available.
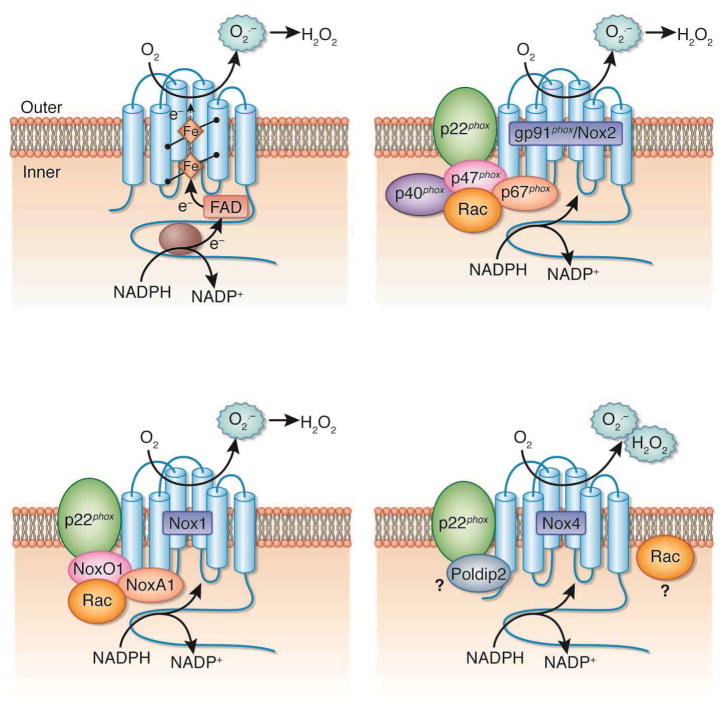
Figure 2. Structure and molecular organization of the cardiorenal nicotinamide adenine dinucleotide phosphate (NAD(P)H) oxidases of the Nox family.
The top right panel illustrates the topology and the enzymatic reaction catalyzed by the Nox enzymes. The other panels represent the molecular structure of the different isoforms of Nox oxidases predominantly expressed in the cardiorenal system, gp91phox/Nox2, Nox1, and Nox4. All cardiorenal Nox proteins form a complex with p22phox, but the cytosolic subunits differ from the Nox oxidase isoforms. FAD, flavin adenine dinucleotide; H2O2, hydrogen peroxide; O2•−, superoxide.
While the mechanism by which the activity of the Nox enzymes is regulated in cardiovascular or renal cells and how they generate reactive oxygen species (ROS) is not fully understood, a number of regulatory subunits have been identified. Importantly, the isoforms Nox1, gp91phox/Nox2 and Nox4 (but not Nox5) appear to require p22phox as a docking subunit [130;135;136]. Activation mechanisms for Nox1 are similar to that of gp91phox/Nox2, and involve complex formation with regulatory cytosolic subunits upon agonist stimulation. Although Nox1 seems to primarily interact with the p47phox homologue NoxO1 (Nox organizer 1), the p67phox homologue NoxA1 (Nox activator 1) and Rac upon activation, it was reported that p47phox and p67phox can partially supplant NoxO1 and NoxA1, respectively [121;122;130;1132;134–136] (Figure 2).
The most abundant Nox isoform in the cardiorenal system is Nox4 [120;130;131;135]. The isoform Nox4/Renox was cloned from the kidney and is highly expressed in renal tubules, fibroblasts, glomerular mesangial cells and podocytes [34;106;137–141]. Nox4 is a 578 amino acid protein that exhibits 39 % identity to the phagocyte gp91phox/Nox2 with special conservation in the six membrane-spanning regions and binding sites for NADPH, FAD and heme, the electron transfer centers that are required to pass electrons from NAD(P)H to oxygen to form superoxide and hydrogen peroxide [121;130;133;135–138]. Evidence to date suggest that Nox4 heterodimerization with p22phox is sufficient to enhance the enzyme activity and it does not require cytosolic subunits that are essential for other Nox isoforms [130;135;136;142;143]. Moreover, it was shown that the Nox4 dehydrogenase domain exists in a conformation that allows the spontaneous transfer of electrons from NADPH to FAD, a property explaining the constitutive activity of the enzyme [144]. Hence, Nox4 has been referred to as a “constitutively active” enzyme that is regulated primarily at the level of its expression in response to various stimuli [130;133;136]. As a corollary, the overall ROS output of Nox4 may be directly Nox4 governed by its expression level. Interestingly, it seems that transcriptional events are involved in the chronic control of Nox4 and protein expression [118;141;145–153] whereas Nox4 is acutely regulated through translational mechanisms without change in its mRNA levels [34;154–157]. It was suggested that stimulation with certain agonists cause early Nox4 protein accumulation independent of mRNA transcription by promoting translation of existing mRNA copies [154;156;157]. These types of translational-mediated regulation mechanisms were also described for other proteins in the kidney and implicated in the pathogenesis of renal disease [154;158–160]. Importantly, the fact that release of ROS by Nox4 does not appear to be triggered acutely after recruitment of cytosolic subunits upon agonist stimulation as for gp91phox/Nox2 and Nox1 does not mean that oxidant production by Nox4 is not controlled by activation of receptors by their ligands (i.e. agonist stimulation). Indeed, it has been reported that Nox4 expression is rapidly upregulated by binding of agonists such as TGF-β [34;118], angiotensin II [154], insulin or insulin-like growth factor [156;161] to their membrane receptors. Since the enzyme is constitutively active, receptor activation by these ligands results in increased Nox4-dependent ROS production. Finally, it should be noted that even if Nox4 regulation may occur primarily at the expression level, Nox4 regulatory proteins that enhance its activity named Poldip2 and NoxR1 have been recently identified [162;163]. A potential implication of Rac in the control of Nox4 function was suggested in endothelial cells and mesangial cells [139;152;164] but it remains a contentious topic.
Nox4 complexity is further illustrated by the difficulty to identify the type of ROS (superoxide or hydrogen peroxide) produced by the enzyme. While it is clear that gp91phox/Nox2 and Nox1 release primarily superoxide, the nature of the ROS produced by Nox4 in cells or tissues is controversial. It was documented that Nox4 generates mostly hydrogen peroxide in vascular smooth muscle cells or heterologous overexpression systems [130;133;145;165;166] while other studies in vascular smooth cells as well as in cardiac or renal cells and tissue were able to detect Nox4-dependent superoxide and hydrogen peroxide production [34;106;118;141;155;167–169]. It was proposed that Nox4 differs from other Nox enzymes because the superoxide produced by Nox4 is rapidly converted to hydrogen peroxide, thereby rendering superoxide release from the enzyme practically undetectable [133;165]. It is of interest to note that Nox4 has been shown to be associated to intracellular compartments or organelles such as endoplasmic reticulum, mitochondria or nucleus [121;130;133;143;147;155;170]. Although effective probes designed to measure intracellular superoxide exists, the particular subcellular localization of Nox4 may render more difficult the detection of the superoxide generated by the oxidase. In addition, negatively charged superoxide anion does not permeate biological membranes whereas hydrogen peroxide obtained after superoxide dismutation is readily permeable [130;145].
Nox-Derived ROS as Mediators of Cardiovascular and Kidney Disease. Role of Nox4 in Myofibroblast and Mesangial Cell Activation
A growing array of evidence suggests that Nox enzymes contribute to the pathogenesis of cardiovascular and renal disease, including hypertension, atherosclerosis, renal or cardiac fibrosis as well as diabetic nephropathy or cardiomyopathy [106;111;120;121;130–134;136;163;171–173]. This is due to the fact that multiple stimuli and agonists implicated in these pathologies like TGF-β, PDGF, angiotensin II, hyperglycemia, thrombin, urotensin, oxidized LDL, IGF-I, vascular endothelial growth factor (VEGF), and aldosterone have been shown to alter the activity or expression of the Nox proteins and subunits, and ultimately the amount of ROS produced [34;106;114;118;120;121;130;141;147;148;151;152;154–156;174–180]. For instance, upregulation of gp91phox/Nox2, Nox1, Nox4 and p22phox (mRNA and protein) together with increased superoxide or hydrogen peroxide generation has been reported in response to angiotensin II in the vasculature and the kidney in vitro as well as in vivo [111;112;120;121;148;150;154;165;174;181–183]. Similar to angiotensin II, enzyme activity or gp91phox/Nox2, Nox1, Nox4 and p22phox expression are upregulated in response to hyperglycemia in vascular and renal cells as well as in experimental models of diabetes [106;112;141;148;151;152;155;171;181–192]. Although most of the vascular and renal Nox oxidases are regulated by pro-fibrotic stimuli such as TGF-β, angiotensin II, or hyperglycemia, it is important to point out that Nox4 appears to play a predominant role in the activation of fibroblasts into the myofibroblast phenotype and the subsequent fibrotic processes taking place in vitro as well as in vivo. Interestingly, it is established that TGF-β, the most potent pro-fibrotic factor, specifically increases the expression of Nox4 and ROS production in a myriad of cell types including smooth muscle cells, endothelial cells, hepatocytes and fibroblasts [34;35;118;169;193–196]. Moreover, we have documented that increased Nox4 expression accounts for TGF-β-, angiotensin II- and high glucose-mediated oxidative stress and renal cell activation that is accompanied by increased extracellular matrix protein and α-SMA expression [34;106;154;155]. More specifically, Nox4 was clearly identified as a critical mediator of high glucose- or angiotensin II-induced mesangial cell activation [106;154;155] as well as of the effects of TGF-β on kidney fibroblast differentiation [34]. However, it should be noted that it has not been directly demonstrated that the oxidase is implicated in TGF-β signaling in mesangial cells. Similar observations were made in cardiac fibroblasts where it was shown that the induction of Nox4-dependent ROS production by TGF-β is required for the conversion of fibroblasts into myofibroblasts, which in turn produce a large amount of extracellular matrix [118]. Nox4 is also closely linked to TGF-β1-induced cytoskeletal changes including filipodia formation and F-actin assembly in human vascular endothelial cells [196] and with stress fibers in differentiated vascular smooth muscle cells that constitutively express α-SMA [167], indicating that Nox-derived ROS are essential to the maintenance of cell shape and differentiation.
The fact that these factors are known as critical mediators of vascular, cardiac or renal fibrosis further supports the concept that Nox4 is the principal source of ROS that promote oxidants-mediated tissue injury and fibrotic processes associated to cardiovascular diseases. A causative relationship between Nox4-derived ROS and fibrogenic responses to renal injury was demonstrated in vivo by showing that treatment of type 1 diabetic rats with Nox4 antisense oligonucleotides reduced ROS production and prevented fibronectin EIIIA accumulation in the kidney [106]. Similarly, genetic silencing of Nox4 with small interfering RNA abrogates myofibroblast differentiation and fibrogenesis in a murine model of lung fibrosis [35]. Given that TGF-β-dependent myofibroblast or modified pericyte activation is a critical component of the fibrogenic processes leading to tissue injury in these diseases, it is reasonable to speculate that the deleterious effects of TGF-β may be also mediated by Nox4-derived ROS in vivo. However, despite that it is established that Nox4 is a target of TGF-β in cultured renal cells, direct in vivo evidence of Nox4 recruitment as an intermediate of the pathway linking TGF-β to glomerular and tubulointerstitial fibrosis is not yet available.
TGF-β1 and Redox Signaling in Fibroblast and Vascular Pericyte Activation
Smad/ERK Transcriptional Pathway in Mesangial Cell and Fibroblast Signaling
TGFβ-1 exerts its effects on the cell surface by binding TGFβ receptor type II TGFR-II and subsequent phosphorylation of a Type 1 receptor subunit forming a heterodimeric complex. Both TGFR-I and TGFR-II possess tyrosine kinase activity that subsequently signal through a canonical pathway involving the Smad family of transcriptional activators [197–200]. In fibroblasts, TGFβ-1 regulation of α-SMA transcription and myofibroblast differentiation is mediated via TGFR1 phosphorylation of Smad2/3 that subsequently complexes with Smad4 and translocates to the nucleus where the dimer binds to the promoter region of the α-SMA gene [13]. Transcriptional control is regulated by a variety of transcription factors and the upregulation of an inhibitory Smad7 [13]. Phenotypic and functional changes associated with TGF-β1-induced fibroblast terminal differentiation are differentially regulated by Smad proteins [200]. In some cell types, ERK is required for activation of the Smad pathway [201]. In mesangial cells and fibroblasts, TGF-β/Smad signaling (Smad 2/3) is tightly controlled by MAP kinase (Ras/MEK/ERK) signaling cascades [202;203]. Also, ERK and Akt/PKB act as alternative pathways in TGFβ-1 signaling of matrix proteins [203–208], making these three signaling proteins the predominant transduction pathways by this growth factor. Additionally, TGF-β signaling of tubular epithelial cells in EMT may involve both Smad dependent through integrin-linked kinase and Smad independent signal transduction pathways such as p38 mitogen-activated protein kinase (MAPK) [199;209;210].
Role of ROS and Nox4 in Mesangial Cell and Fibroblast Signaling (Figure 3)
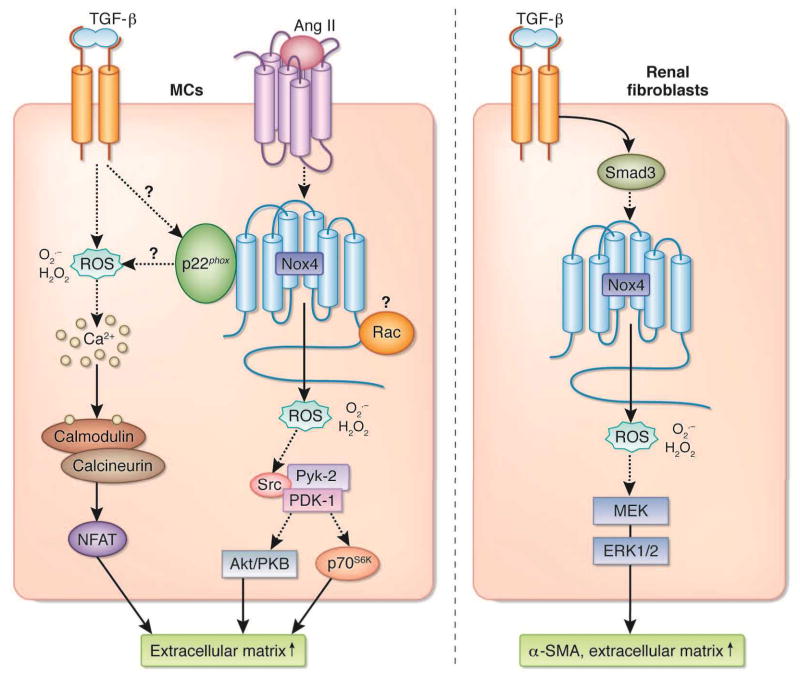
Figure 3.
Pathways of Nox-dependent signal transductions implicated in mesangial cells (MCs) (left panel) and renal fibroblasts (right panel) activation. See text for detail. Ang II, angiotensin II; ERK, extracellular receptor kinase; H2O2, hydrogen peroxide; MEK, MAP kinase kinase; NFAT, nuclear factor of activated T cell; O2•−, superoxide; PKB, protein kinase B; ROS, reactive oxygen species; α-SMA, alpha-smooth muscle actin; TGF-β, transforming growth factor-beta.
The observations that both TGF-β1 and ROS activate fibroblasts transition to an α-SMA myofibroblast and enhanced matrix synthesis [34;35] indicate that these two molecules are interrelated and may share signaling pathways in myofibroblast differentiation during fibrosis.
ROS are known to activate many tyrosine and serine threonine kinases [133;211;212], indicating that products of oxidation play a central role in cell signaling. Indeed, a number of hormones, growth factors, and cytokines induce the production of hydrogen peroxide in a variety of target cells [211;212]. A regulatory role for ROS in PDGF- and angiotensin II-induced signal transduction is well documented [213;214]. Also, TGF-β1-induced fibronectin synthesis by mesangial cells occurs via calcium mobilization and calmodulin/calcineurin-dependent activation of transcription factor NFAT in a ROS-dependent manner [215]. Similar results were observed for TGF-β1 induced tubule epithelial cells and renal fibroblasts [215].
NAD(P)H oxidases of the Nox family have received heightened interest as source of ROS signaling in a number of cell types. [120;121;133;173;178;216]. Receptor binding of PDGF, angiotensin II, TGF-β and/or TNF-α, rapidly activates NAD(P)H oxidase followed by intracellular ROS generation and activation of protein tyrosine kinases, serine/threonine kinases, phospholipases (e.g. phospholipases C and A2), and calcium-dependent pathways [120;121;133;173;178;216]. NAD(P)H oxidase signaling pathways can be complex and diverse often with conflicting data among cell type, signaling pathway and cell behavior examined [120;121;133;173;178;216]. Accumulating evidence points toward the NAD(P)H oxidase of the Nox family and particularly Nox4 as the predominant enzyme source for ROS generation in fibrotic disease, placing this enzyme family in a pivotal position in cell signaling (see above). Studies by our group indicate that angiotensin II-induced ROS generation in mesangial cells acts principally through Nox4 as an upstream activator of ERK1/ERK2 [140], Pyk-2/Src/PDK-1 [154], Akt/PKB and/or p70S6K [139;154;217;218]; pathways that lead to cell hypertrophy and increased protein synthesis and/or fibronectin expression. (Figure 3). More specifically, Pyk-2 appears to act as a molecular scaffold, binding to both PDK-1 and Src, thereby allowing Src to tyrosine-phosphorylate and activate PDK-1, that in turn activate its downstream effectors, Akt/PKB and p70S6K [154]. Interestingly, Nox4 and p22phox both contribute to angiotensin II-dependent oxidative stress and fibronectin accumulation in mesangial cells [139;154;218]. Because it is known that p22phox interacts with Nox4 and enhance its activity (see above), it is reasonable to think that in these cells, Nox4 and p22phox may form a complex that accounts for angiotensin II-induced ROS generation and the subsequent fibrotic response. However, given that gp91phox/Nox2 and Nox1 also interact with p22phox, their role cannot be rule out in conditions where these subunits are also expressed in the cells. These in vitro studies were extrapolated in vivo to type 1 diabetic nephropathy showing co-localization of Nox4 with α-SMA positive mesangial cells and that antisense oligonucleotides to Nox4 reduced ERK1/2 and Akt/PKB phosphorylation and inhibited glomerular hypertrophy and fibronectin EIIIA expression [106]. Interestingly, other studies with mesangial cells exposed to advanced oxidation protein products place a NAD(P)H oxidase likely to be Nox4 distal to PKC activation, resulting in ECM overproduction and upregulation of TGF-β1 [219], raising the possibility that Nox enzymes may also function as upstream modulators of TGF-β1 synthesis. As mentioned earlier, we would like to point out that the role of Nox4 in TGF-β1-mediated ROS generation and matrix protein accumulation has not been directly established in mesangial cells. However, it was documented that p22phox is required for TGF-β1-induced ROS production [220] and that Src, PDK-1 or Akt/PKB, targets of Nox4-derived ROS, play a role in TGF-β1-dependent matrix accumulation [205;221] in mesangial cells. Thus, given that TGF-β1 is a major regulator of Nox4 in most of the cell type examined (see above), it is tempting to speculate that as for angiotensin II the p22phox/Nox4 complex may also mediates TGF-β1 fibrotic effects in mesangial cells. It is appealing to consider that a Nox4- and p22phox-containing oxidase may be a pivotal signal transducer commonly shared by both fibrotic stimuli in mesangial cells. This is highly relevant for the design of therapeutic strategies to prevent the initiation or progression of fibrotic renal diseases. Whether TGF-β1-induced mesangial cell activation share portions of these ROS signaling cascades in Smad-related cellular events remains to be elucidated.
Studies with fibroblasts derived from heart, lung, and kidney also indicate that Nox4 is central to TGF-β1-induced ROS generation and myofibroblast differentiation to a profibrotic phenotype. For example, TGF-β1-induced transition of cardiac or pulmonary fibroblasts to α-SMA positive myofibroblasts is dependent on Nox4 regulation of Smad2/3 [35;118]. Nox4 small interfering RNA or dominant negative Smad3 significantly reduced these effects in vitro [35;195] as well as myofibroblast expansion and fibronectin synthesis in bleomycin induced fibrosis in vivo [35]. We also showed that Nox4 is the predominant Nox oxidase homolog in kidney myofibroblast differentiation and expression of fibronectin (EIIIA) [34]. However, unlike cardiac and pulmonary myofibroblasts, our results place Nox4 downstream of Smad3 and proximal to ERK (Figure 3). Chemical inhibitors of NAD(P)H oxidase and Nox4 siRNA, specifically, had no effect on Smad3 phosphorylation, but blocked downstream ERK phosphorylation and subsequent α-SMA expression and fibronectin EIIIA synthesis (Figure 3). These results follow a similar course described in pulmonary vascular smooth muscle cells where TGF-β-induced proliferation occurs through a Nox4-dependent pathway downstream of Smad3 [195]. Moreover, recent studies by Haurani et al indicate that Nox4 causes feedback inhibition of its own expression and regulates migration in adventitial fibroblast [114], although the signaling pathway of this loop remains to be elucidated. TGF-β1 also, induces PAI-1 expression in human lung fibroblasts where nuclear MAPK phosphatase MKP-1 is a molecular target of Nox4-generated ROS leading to a sustained activation of JNK and p38 MAPKs [169]. The location of these signaling molecules with respect to Smad has not been determined. Taken together, the data summarized above indicate that Nox4 is essential in TGF-β-induced myofibroblast differentiation and profibrotic cell behaviors; however, the position of Nox4 derived ROS in the various signaling pathways varies among fibroblast organ type.
Concluding remarks
The role of the myofibroblast in progression of fibrosis cannot be overstated. Despite the controversy over the origin of the interstitial myofibroblast during fibrosis, there is common agreement that it is responsible for much of the accumulated matrix and ultimate organ damage. Similarly, the mesangial cell, when activated, acquires myofibroblast features and is most responsible for matrix synthesis in the glomerulus during disease. Angiotensin II and TGF-β are the growth factors with the greatest impact on myofibroblast differentiation and synthesis of mesenchymal matrix proteins. The signaling pathways used by mesangial cells and fibroblasts are diverse depending on the cell type and agonist they are exposed to; however recent studies indicate that NAD(P)H oxidase-generated ROS are common to cell activation. It seems also that Nox4 may be the isoform that that is most responsible for growth factor-induced ROS generation and occupies a central role in mesangial cell and myofibroblast differentiation and matrix synthesis. Information on the role of ROS in angiotensin II- and TGF-β-induced mesangial cell and fibroblast signaling is incomplete, however recent evidence indicates they most likely share similar pathways. Further research in this area will shed important information on myofibroblast differentiation and aid in development of therapeutic approaches to fibrosis.
Footnotes
Disclosure
The authors declared no competing interests.
References
- 1.Strutz F, Muller GA. Mechanisms of renal fibrogenesis. In: Neilson EG, Couser WG, editors. Immunologic renal diseases. 2. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 73–101. [Google Scholar]
- 2.Hewitson TD. Renal tubulointerstitial fibrosis: common but never simple. Am J Physiol - Renal Physiol. 2009;296:F1239–F1244. doi: 10.1152/ajprenal.90521.2008. [DOI] [PubMed] [Google Scholar]
- 3.Hardie WD, Glasser SW, Hagood JS. Emerging concepts in the pathogenesis of lung fibrosis. Am J Pathol. 2009;175:3–16. doi: 10.2353/ajpath.2009.081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiao J, Friedman SL, Aloman C. Hepatic fibrosis. Cur Opinion Gastroenterol. 2009;25:223–229. doi: 10.1097/mog.0b013e3283279668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughson MD. End-stage renal disease. In: Jennette JC, Olson JL, Schwartz MM, Silva FG, editors. Heptinstall’s Pathology of the Kidney. 6. Philadelphia: Lippincott-Raven Publishers; 2007. pp. 1307–1346. [Google Scholar]
- 6.Schwartz MM, Korbet SM, Rydell J, et al. Primary focal segmental glomerular sclerosis in adults: prognostic value of histologic variants. Am J Kidney Dis. 1995;25:845–852. doi: 10.1016/0272-6386(95)90566-9. [DOI] [PubMed] [Google Scholar]
- 7.Risdon RA, Sloper JC, De Wardener HE. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet. 1968;2(7564):363–366. doi: 10.1016/s0140-6736(68)90589-8. [DOI] [PubMed] [Google Scholar]
- 8.Desmouliere A, Darby IA, Gabbiani G. Normal and pathologic soft tissue remodeling: role of the myofibroblast, with special emphasis on liver and kidney fibrosis. Lab Invest. 2003;83:1689–1707. doi: 10.1097/01.lab.0000101911.53973.90. [DOI] [PubMed] [Google Scholar]
- 9.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 10.Alpers CE, Pichler R, Johnson RJ. Phenotypic features of cortical interstitial cells potentially important in fibrosis. Kidney Int. 1996;49:S-28–S-31. [PubMed] [Google Scholar]
- 11.Alpers CE, Hudkins KL, Gown AM, et al. Enhanced expression of “muscle-specific” actin in glomerulonephritis. Kidney Int. 1992;41:1134–1142. doi: 10.1038/ki.1992.173. [DOI] [PubMed] [Google Scholar]
- 12.Johnson RJ, Floege J, Yoshimura A, et al. The activated mesangial cell: a glomerular “myofibroblast”? J Am Soc Nephrol. 1992;2:S190–S197. doi: 10.1681/ASN.V210s190. [DOI] [PubMed] [Google Scholar]
- 13.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 14.Johnson RJ, Alpers CE, Yoshimura A, et al. Renal injury from angiotensin II-mediated hypertension. Hypertension. 1992;19:464–474. doi: 10.1161/01.hyp.19.5.464. [DOI] [PubMed] [Google Scholar]
- 15.Diamond JR, van GH, Ding G, Engelmyer E. Myofibroblasts in experimental hydronephrosis. Am J Pathol. 1995;146:121–129. [PMC free article] [PubMed] [Google Scholar]
- 16.Kliem V, Johnson RJ, Alpers CE, et al. Mechanisms involved in the pathogenesis of tubulointerstitial fibrosis in 5/6-nephrectomized rats. Kidney Int. 1996;49:666–678. doi: 10.1038/ki.1996.95. [DOI] [PubMed] [Google Scholar]
- 17.Taneda S, Hudkins KL, Topouzis S, et al. Obstructive uropathy in mice and humans: potential role for PDGF-D in the progression of tubulointerstitial injury. J Am Soc Nephrol. 2003;14:2544–2555. doi: 10.1097/01.asn.0000089828.73014.c8. [DOI] [PubMed] [Google Scholar]
- 18.Kimura M, Asano M, Abe K, et al. Role of atrophic changes in proximal tubular cells in the peritubular deposition of type IV collagen in a rat renal ablation model. Nephrol Dialysis Transplant. 2005;20:1559–1565. doi: 10.1093/ndt/gfh872. [DOI] [PubMed] [Google Scholar]
- 19.Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humphreys BD, Lin SL, Kobayashi A, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnes VL, Musa J, Mitchell RJ, et al. Expression of embryonic fibronectin isoform EIIIA parallels alpha-smooth muscle actin in maturing and diseased kidney. J Histochem Cytochem. 1999;47:787–798. doi: 10.1177/002215549904700608. [DOI] [PubMed] [Google Scholar]
- 22.Faulkner JL, Szcykalski LM, Springer F, et al. Origin of interstitial fibroblasts in an accelerated model of angiotensin II-induced renal fibrosis. Am J Pathol. 2005;167:1193–1205. doi: 10.1016/S0002-9440(10)61208-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chai Q, Krag S, Chai S, et al. Localisation and phenotypical characterisation of collagen-producing cells in TGF-beta 1-induced renal interstitial fibrosis. Histochem Cell Biol. 2003;119:267–280. doi: 10.1007/s00418-003-0513-8. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki T, Kimura M, Asano M, et al. Role of atrophic tubules in development of interstitial fibrosis in microembolism-induced renal failure in rat. Am J Pathol. 2001;158:75–85. doi: 10.1016/S0002-9440(10)63946-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Hir M, Hegyi I, Cueni-Loffing D, et al. Characterization of renal interstitial fibroblast-specific protein 1/S100A4-positive cells in healthy and inflamed rodent kidneys. Histochem Cell Biol. 2005;123:335–346. doi: 10.1007/s00418-005-0788-z. [DOI] [PubMed] [Google Scholar]
- 26.Picard N, Baum O, Vogetseder A, et al. Origin of renal myofibroblasts in the model of unilateral ureter obstruction in the rat. Histochem Cell Biol. 2008;130:141–155. doi: 10.1007/s00418-008-0433-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson RJ, Iida H, Alpers CE, et al. Expression of smooth muscle cell phenotype by rat mesangial cells in immune complex nephritis. Alpha-smooth muscle actin is a marker of mesangial cell proliferation. J Clin Invest. 1991;87:847–858. doi: 10.1172/JCI115089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elger M, Drenckhahn D, Nobiling R, et al. Cultured rat mesangial cells contain smooth muscle alpha-actin not found in vivo. Am J Pathol. 1993;142:497–509. [PMC free article] [PubMed] [Google Scholar]
- 29.Jarnagin WR, Rockey DC, Koteliansky VE, et al. Expression of variant fibronectins in wound healing: cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis. J Cell Biol. 1994;127:2037–2048. doi: 10.1083/jcb.127.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sappino A-P, Schurch W, Gabbiani G. Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as marker of phenotypic modulations. Lab Invest. 1990;63:144–161. [PubMed] [Google Scholar]
- 31.Schmitt-Graff A, Desmouliere A, Gabbiani G. Heterogeneity of myofibroblast phenotypic features: an example of fibroblastic cell plasticity. Virchows Archiv. 1994;425:3–24. doi: 10.1007/BF00193944. [DOI] [PubMed] [Google Scholar]
- 32.Masamune A, Watanabe T, Kikuta K, et al. Roles of pancreatic stellate cells in pancreatic inflammation and fibrosis. Clin Gastroenterol Hepatol. 2009;7(11 Suppl):S48–54. doi: 10.1016/j.cgh.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 33.Schnaper HW, Hayashida T, Hubchak SC, et al. TGF-beta signal transduction and mesangial cell fibrogenesis. Am J of Physiol - Renal Physiol. 2003;284:F243–F252. doi: 10.1152/ajprenal.00300.2002. [DOI] [PubMed] [Google Scholar]
- 34.Bondi CD, Manickam N, Lee DY, et al. NAD(P)H oxidase mediates TGF-beta1-induced activation of kidney myofibroblasts. J Am Soc Nephrol. 2010;21:93–102. doi: 10.1681/ASN.2009020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hecker L, Vittal R, Jones T, et al. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nature Medicine. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Border WA, Ruoslahti E. Transforming growth factor-beta in disease: the dark side of tissue repair. J Clin Invest. 1992;90:1–7. doi: 10.1172/JCI115821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlondorff D. The glomerular mesangial cell: an expanding role for a specialized pericyte. FASEB Journal. 1987;1:272–281. doi: 10.1096/fasebj.1.4.3308611. [DOI] [PubMed] [Google Scholar]
- 38.Barnes JL, Hastings RR, De la Garza MA. Sequential expression of cellular fibronectin by platelets, macrophages, and mesangial cells in proliferative glomerulonephritis. Am J Pathol. 1994;145:585–597. [PMC free article] [PubMed] [Google Scholar]
- 39.Barnes JL, Torres ES, Mitchell RJ, Peters JH. Expression of alternatively spliced fibronectin variants during remodeling in proliferative glomerulonephritis. Am J Pathol. 1995;147:1361–1371. [PMC free article] [PubMed] [Google Scholar]
- 40.Tang WW, Van GY, Qi M. Myofibroblast and α1(III) collagen expression in experimental tubulointerstitial nephritis. Kidney Int. 1997;51:926–931. doi: 10.1038/ki.1997.131. [DOI] [PubMed] [Google Scholar]
- 41.Takeji M, Moriyama T, Oseto S, et al. Smooth muscle α-actin deficiency in myofibroblasts leads to enhanced renal tissue fibrosis. J Biol Chem. 2006;281:40193–40200. doi: 10.1074/jbc.M602182200. [DOI] [PubMed] [Google Scholar]
- 42.Pozdzik AA, Salmon IJ, Debelle FD, et al. Aristolochic acid induces proximal tubule apoptosis and epithelial to mesenchymal transformation. Kidney Int. 2008;73:595–607. doi: 10.1038/sj.ki.5002714. [DOI] [PubMed] [Google Scholar]
- 43.Wiggins R, Goyal M, Merritt S, et al. Vascular adventitial cell expression of collagen I messenger ribonucleic acid in anti-glomerular basement membrane antibody-induced crescentic nephritis in the rabbit. A cellular source for interstitial collagen synthesis in inflammatory renal disease. Lab Invest. 1993;68:557–565. [PubMed] [Google Scholar]
- 44.Lloyd CM, Dorf ME, Proudfoot A, et al. Role of MCP-1 and RANTES in inflammation and progression to fibrosis during murine crescentic nephritis. J Leukocyte Biol. 1997;62:676–680. doi: 10.1002/jlb.62.5.676. [DOI] [PubMed] [Google Scholar]
- 45.Lloyd CM, Minto AW, Dorf ME, et al. RANTES and monocyte chemoattractant protein-1 (MCP-1) play an important role in the inflammatory phase of crescentic nephritis, but only MCP-1 is involved in crescent formation and interstitial fibrosis. J Exp Med. 1997;185:1371–1380. doi: 10.1084/jem.185.7.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu Y, Appay MD, Heudes D, et al. Colocalization of collagen overexpression and inflammatory cell infiltration in the two-kidney one-clip rat model from the early days of hypertension onward. Virchows Archiv. 1998;432:267–277. doi: 10.1007/s004280050165. [DOI] [PubMed] [Google Scholar]
- 47.Hewitson TD, Darby IA, Bisucci T, et al. Evolution of tubulointerstitial fibrosis in experimental renal infection and scarring. J Am Soc Nephrol. 1998;9:632–642. doi: 10.1681/ASN.V94632. [DOI] [PubMed] [Google Scholar]
- 48.Muchaneta-Kubara EC, el Nahas AM. Myofibroblast phenotypes expression in experimental renal scarring. Nephrol Dial Transplant. 1997;12:904–915. doi: 10.1093/ndt/12.5.904. [DOI] [PubMed] [Google Scholar]
- 49.Wada T, Sakai N, Matsushima K, et al. Fibrocytes: a new insight into kidney fibrosis. Kidney Int. 2007;72:269–273. doi: 10.1038/sj.ki.5002325. [DOI] [PubMed] [Google Scholar]
- 50.Broekema M, Harmsen MC, van Luyn MJ, et al. Bone marrow-derived myofibroblasts contribute to the renal interstitial myofibroblast population and produce procollagen I after ischemia/reperfusion in rats. J Am Soc Nephrol. 2007;18:165–175. doi: 10.1681/ASN.2005070730. [DOI] [PubMed] [Google Scholar]
- 51.Li J, Deane JA, Campanale NV, et al. The contribution of bone marrow-derived cells to the development of renal interstitial fibrosis. Stem Cells. 2007;25:697–706. doi: 10.1634/stemcells.2006-0133. [DOI] [PubMed] [Google Scholar]
- 52.Li J, Qu X, Bertram JF. Endothelial-myofibroblast transition contributes to the early development of diabetic renal interstitial fibrosis in streptozotocin-induced diabetic mice. Am J Pathol. 2009;175:1380–1388. doi: 10.2353/ajpath.2009.090096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeisberg EM, Potenta SE, Sugimoto H, et al. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol. 2008;19:2282–2287. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strutz F, Neilson EG. New insights into mechanisms of fibrosis in immune renal injury. Springer Sem Immunopathol. 2003;24:459–476. doi: 10.1007/s00281-003-0123-5. [DOI] [PubMed] [Google Scholar]
- 55.Iwano M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. Cur Opin Nephrol Hypertension. 2004;13:279–284. doi: 10.1097/00041552-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 56.Zeisberg M, Kalluri R. Fibroblasts emerge via epithelial-mesenchymal transition in chronic kidney fibrosis. Front Biosci. 2008;13:6991–6998. doi: 10.2741/3204. [DOI] [PubMed] [Google Scholar]
- 57.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69:213–217. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- 59.Hay ED, Zuk A. Transformations between epithelium and mesenchyme: normal, pathological, and experimentally induced. Am J Kidney Dis. 1995;26:678–690. doi: 10.1016/0272-6386(95)90610-x. [DOI] [PubMed] [Google Scholar]
- 60.Strutz F, Okada H, Lo CW, et al. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol. 1995;130:393–405. doi: 10.1083/jcb.130.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barnes JL, Glass W. Renal interstitial fibrosis: A critical evaluation of the origin of myofibroblasts. In: Herrera GAS, Karger AG, editors. Experimental Models of Renal Diseases: Impact on Understanding Pathogenesis and Improving Diagnosis. Medical and Scientific Publishers; 2010. In Press. [Google Scholar]
- 62.Zeisberg M, Duffield JS. Resolved: EMT produces fibroblasts in the kidney. Debates. J Am Soc Nephrol. 2010;21:1247–1253. doi: 10.1681/ASN.2010060616. [DOI] [PubMed] [Google Scholar]
- 63.Zeisberg M, Yang C, Martino M, et al. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem. 2007;282:23337–23347. doi: 10.1074/jbc.M700194200. [DOI] [PubMed] [Google Scholar]
- 64.Willis BC, duBois RM, Borok Z. Epithelial origin of myofibroblasts during fibrosis in the lung. Proc Am Thoracic Soc. 2006;3:377–382. doi: 10.1513/pats.200601-004TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cook HT. The origin of renal fibroblasts and progression of kidney disease. Am J Pathol. 2010;176:22–24. doi: 10.2353/ajpath.2010.090898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Le Hir M, Kaissling B. Antibodies against macrophages that overlap in specificity with fibroblasts. Kidney Int. 2005;68:2400. doi: 10.1111/j.1523-1755.2005.00704_1.x. [DOI] [PubMed] [Google Scholar]
- 67.Fujigaki Y, Muranaka Y, Sun D, et al. Transient myofibroblast differentiation of interstitial fibroblastic cells relevant to tubular dilatation in uranyl acetate-induced acute renal failure in rats. Virchows Archiv. 2005;446:164–176. doi: 10.1007/s00428-004-1155-5. [DOI] [PubMed] [Google Scholar]
- 68.Pat B, Yang T, Kong C, et al. Activation of ERK in renal fibrosis after unilateral ureteral obstruction: Modulation by antioxidants. Kidney Int. 2005;67:931–943. doi: 10.1111/j.1523-1755.2005.00157.x. [DOI] [PubMed] [Google Scholar]
- 69.Zhang G, Oldroyd SD, Huang LH, et al. Role of apoptosis and Bcl-2/Bax in the development of tubulointerstitial fibrosis during experimental obstructive nephropathy. Exp Nephrol. 2001;9:71–80. doi: 10.1159/000052597. [DOI] [PubMed] [Google Scholar]
- 70.Hewitson TD, Mookerjee I, Masterson R, et al. Endogenous relaxin is a naturally occurring modulator of experimental renal tubulointerstitial fibrosis. Endocrinol. 2007;148:660–669. doi: 10.1210/en.2006-0814. [DOI] [PubMed] [Google Scholar]
- 71.Ma FY, Liu J, Kitching AR, et al. Targeting renal macrophage accumulation via c-fms kinase reduces tubular apoptosis but fails to modify progressive fibrosis in the obstructed rat kidney. Am J Physiol - Renal Physiol. 2009;296:F177–F185. doi: 10.1152/ajprenal.90498.2008. [DOI] [PubMed] [Google Scholar]
- 72.Li L, Zepeda-Orozoco D, Black R, et al. Autophagy is a component of epithelial cell fate in obstructive uropathy. Am J Pathol. 2010;176:1767–1778. doi: 10.2353/ajpath.2010.090345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koesters R, Kaissling B, LeHir M, et al. Tubular overexpression of transforming growth factor-β1 induces autophagy and fibrosis but not mesenchymal transition of renal epithelial cells. Am J Pathol. 2010;177:632–643. doi: 10.2353/ajpath.2010.091012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taura K, Miura K, Iwaisako K, et al. Hepatocytes do not undergo epithelial-mesenchymal transition in liver fibrosis in mice. Hepatol. 2010;51:1027–1036. doi: 10.1002/hep.23368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hickling KC, Hitchcock JM, Chipman JK, et al. Induction and progression of cholangiofibrosis in rat liver injured by oral administration of furan. Toxicologic Pathol. 2010;38:213–229. doi: 10.1177/0192623309357945. [DOI] [PubMed] [Google Scholar]
- 76.Iwano M, Plieth D, Danoff TM, et al. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iwano M, Fischer A, Okada H, Plieth D, Xue C, Danoff TM, Neilson EG. Conditional abatement of tissue fibrosis using nucleoside analogs to selectively corrupt DNA replication in transgenic fibroblasts. Molecular Therapy. 2001;3:149–59. doi: 10.1006/mthe.2000.0251. [DOI] [PubMed] [Google Scholar]
- 78.Okada H, Ban S, Nagao S, et al. Progressive renal fibrosis in murine polycystic kidney disease: an immunohistochemical observation. Kidney Int. 2000;58:587–597. doi: 10.1046/j.1523-1755.2000.00205.x. [DOI] [PubMed] [Google Scholar]
- 79.Nishitani Y, Iwano M, Yamaguchi Y, et al. Fibroblast-specific protein 1 is a specific prognostic marker for renal survival in patients with IgAN. Kidney Int. 2005;68:1078–1085. doi: 10.1111/j.1523-1755.2005.00500.x. [DOI] [PubMed] [Google Scholar]
- 80.Mazzucchelli L. Protein S100A4: too long overlooked by pathologists? Am J Pathol. 2002;160:7–13. doi: 10.1016/S0002-9440(10)64342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garrett SC, Varney KM, Weber DJ, Bresnick AR. S100A4, a mediator of metastasis. J Biol Chem. 2006;281:677–680. doi: 10.1074/jbc.R500017200. [DOI] [PubMed] [Google Scholar]
- 82.Gibbs FE, Barraclough R, Platt-Higgins A, et al. Immunocytochemical distribution of the calcium-binding protein p9Ka in normal rat tissues: variation in the cellular location in different tissues. J Histochem Cytochem. 1995;43:169–180. doi: 10.1177/43.2.7822773. [DOI] [PubMed] [Google Scholar]
- 83.Ito K, Chen J, El CM, et al. Renal damage progresses despite improvement of renal function after relief of unilateral ureteral obstruction in adult rats. Am J Physiol - Renal Physiol. 2004;287:F1283–F1293. doi: 10.1152/ajprenal.00441.2003. [DOI] [PubMed] [Google Scholar]
- 84.Inoue T, Plieth D, Venkov CD, et al. Antibodies against macrophages that overlap in specificity with fibroblasts. Kidney Int. 2005;67:2488–2493. doi: 10.1111/j.1523-1755.2005.00358.x. [DOI] [PubMed] [Google Scholar]
- 85.Rossini M, Cheunsuchon B, Donnert E, Ma LJ, Thomas JW, Neilson EG, Fogo AB. Immunolocalization of fibroblast growth factor-1 (FGF-1), its receptor (FGFR-1), and fibroblast-specific protein-1 (FSP-1) in inflammatory renal disease. Kidney Int. 2005;68:2621–2628. doi: 10.1111/j.1523-1755.2005.00734.x. [DOI] [PubMed] [Google Scholar]
- 86.Rivard CJ, Brown LM, Almeida NE, et al. Expression of the calcium-binding protein S100A4 is markedly up-regulated by osmotic stress and is involved in the renal osmoadaptive response. J Biol Chem. 2007;282:6644–6652. doi: 10.1074/jbc.M609432200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kimura M, Suzuki T, Hishida A. A rat model of progressive chronic renal failure produced by microembolism. Am J Pathol. 1999;155:1371–1380. doi: 10.1016/S0002-9440(10)65239-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shi Y, O’Brien JE, Jr, la-Kokko L, et al. Origin of extracellular matrix synthesis during coronary repair. Circulation. 1997;95:997–100. doi: 10.1161/01.cir.95.4.997. [DOI] [PubMed] [Google Scholar]
- 89.Nakatsukasa H, Nagy P, Evarts RP, et al. Cellular distribution of transforming growth factor-beta 1 and procollagen types I, III, and IV transcripts in carbon tetrachloride-induced rat liver fibrosis. J Clin Invest. 1990;85:1833–1843. doi: 10.1172/JCI114643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hardie WD, Le Cras TD, Jiang K, et al. Conditional expression of transforming growth factor-alpha in adult mouse lung causes pulmonary fibrosis. Am J Physiol - Lung Cell Mol Physiol. 2004;286:L741–L749. doi: 10.1152/ajplung.00208.2003. [DOI] [PubMed] [Google Scholar]
- 91.Milani S, Herbst H, Schuppan D, et al. Transforming growth factors β1 and β2 are differentially expressed in fibrotic liver disease. Am J Pathol. 1991;139:1221–1229. [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang H-Y, Gharaee-Kermani M, Zhang K, et al. Lung fibroblast alpha-smooth muscle actin expression and contractile phenotype in bleomycin-induced pulmonary fibrosis. Am J Pathol. 1996;148:527–537. [PMC free article] [PubMed] [Google Scholar]
- 93.Abbate M, Zoja C, Rottoli D, et al. Proximal tubular cells promote fibrogenesis by TGF-beta1-mediated induction of peritubular myofibroblasts. Kidney Int. 2002;61:2066–2077. doi: 10.1046/j.1523-1755.2002.00380.x. [DOI] [PubMed] [Google Scholar]
- 94.Zalewski A, Shi Y. Vascular myofibroblasts. Lessons from coronary repair and remodeling. Arterioscler Thromb Vasc Biol. 1997;17:417–422. doi: 10.1161/01.atv.17.3.417. [DOI] [PubMed] [Google Scholar]
- 95.Shi Y, O’Brien JE, Jr, Fard A, et al. Transforming growth factor-beta 1 expression and myofibroblast formation during arterial repair. Arterioscler Thromb Vasc Biol. 1996;16:1298–1305. doi: 10.1161/01.atv.16.10.1298. [DOI] [PubMed] [Google Scholar]
- 96.Wolf G. Renal injury due to renin-angiotensin-aldosterone system activation of the transforming growth factor-beta pathway. Kidney Int. 2006;70:1914–1919. doi: 10.1038/sj.ki.5001846. [DOI] [PubMed] [Google Scholar]
- 97.Kagami S, Border WA, Miller DE, et al. Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-beta expression in rat glomerular mesangial cells. J Clin Invest. 1994;93:2431–2437. doi: 10.1172/JCI117251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gaedeke J, Peters H, Noble NA, et al. Angiotensin II, TGF-beta, and renal fibrosis. Contrib Nephrol. 2001;135:153–160. doi: 10.1159/000060162. [DOI] [PubMed] [Google Scholar]
- 99.Ronnov-Jessen L, Petersen OW. Induction of alpha-smooth muscle actin by transforming growth factor-β1 in quiescent human breast gland fibroblasts-implications for myofibroblast generation in breast neoplasia. Lab Invest. 1993;68:696–707. [PubMed] [Google Scholar]
- 100.Desmouliere A, Geinoz A, Gabbiani F, et al. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bottinger EP. TGF-beta in renal injury and disease. Sem Nephrol. 2007;27:309–320. doi: 10.1016/j.semnephrol.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 102.Peters JH, Hynes RO. Fibronectin isoform distribution in the mouse. I. The alternatively spliced EIIIB, EIIIA, and V segments show widespread codistribution in developing mouse embryo. Cell Adhesion Comm. 1996;4:103–125. doi: 10.3109/15419069609010766. [DOI] [PubMed] [Google Scholar]
- 103.ffrench-Constant C, Van De Water L, Dvorak HF, et al. Reappearance of an embryonic pattern of fibronectin splicing during wound healing in the adult rat. J Cell Biol. 1989;109:903–914. doi: 10.1083/jcb.109.2.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Peters JH, Chen G, Hynes RO. Fibronectin isoform distribution in the mouse. II. Differential distribution of the alternatively spliced EIIIB, EIIIA, and V segments in the adult mouse. Cell Adhesion Comm. 1996;4:127–148. doi: 10.3109/15419069609010767. [DOI] [PubMed] [Google Scholar]
- 105.ffrench-Constant C. Alternative splicing of fibronectin--many different proteins but few different functions. Exp Cell Res. 1995;221:261–271. doi: 10.1006/excr.1995.1374. [DOI] [PubMed] [Google Scholar]
- 106.Gorin Y, Block K, Hernandez J, et al. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem. 2005;280:39616–39626. doi: 10.1074/jbc.M502412200. [DOI] [PubMed] [Google Scholar]
- 107.Zuk A, Bonventre JV, Matlin KS. Expression of fibronectin splice variants in the postischemic rat kidney. Am J Physiol- Renal Physiol. 2001;280:F1037–F1053. doi: 10.1152/ajprenal.2001.280.6.F1037. [DOI] [PubMed] [Google Scholar]
- 108.Borsi L, Castellani P, Risso AM, et al. Transforming growth factor-β regulates the splicing pattern of fibronectin messenger RNA precursor. FEBS Letters. 1990;261:175–178. doi: 10.1016/0014-5793(90)80664-5. [DOI] [PubMed] [Google Scholar]
- 109.Serini G, Bochaton-Piallat ML, Ropraz P, et al. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol. 1998;142:873–881. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tomasek JJ, Gabbiani G, Hinz B, et al. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nature Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 111.Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol - Reg Integrative Compar Physiol. 2003;285:R277–R297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 112.Haurani MJ, Pagano PJ. Adventitial fibroblast reactive oxygen species as autacrine and paracrine mediators of remodeling: bellwether for vascular disease? Cardiovasc Res. 2007;75:679–89. doi: 10.1016/j.cardiores.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 113.Sachse A, Wolf G. Angiotensin II-induced reactive oxygen species and the kidney. J Am Soc Nephrol. 2007;18:2439–2446. doi: 10.1681/ASN.2007020149. [DOI] [PubMed] [Google Scholar]
- 114.Haurani MJ, Cifuentes ME, Shepard AD, et al. Nox4 oxidase overexpression specifically decreases endogenous Nox4 mRNA and inhibits angiotensin II-induced adventitial myofibroblast migration. Hypertension. 2008;52:143–149. doi: 10.1161/HYPERTENSIONAHA.107.101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shi Y, Niculescu R, Wang D, et al. Increased NAD(P)H oxidase and reactive oxygen species in coronary arteries after balloon injury. Arterioscler Thromb Vasc Biol. 2001;21:739–745. doi: 10.1161/01.atv.21.5.739. [DOI] [PubMed] [Google Scholar]
- 116.Pagano PJ, Clark JK, Cifuentes-Pagano ME, et al. Localization of a constitutively active, phagocyte-like NADPH oxidase in rabbit aortic adventitia: enhancement by angiotensin II. Proc Natl Acad Sci USA. 1997;94:14483–14488. doi: 10.1073/pnas.94.26.14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Manea A, Raicu M, Simionescu M. Expression of functionally phagocyte-type NAD(P)H oxidase in pericytes: effect of angiotensin II and high glucose. Biol Cell. 2005;97:723–734. doi: 10.1042/BC20040107. [DOI] [PubMed] [Google Scholar]
- 118.Cucoranu I, Clempus R, Dikalova A, et al. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res. 2005;97:900–907. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 119.Shi Y, O’Brien JE, Fard A, et al. Adventitial myofibroblasts contribute to neointimal formation in injured porcine coronary arteries. Circulation. 1996;94:1655–1664. doi: 10.1161/01.cir.94.7.1655. [DOI] [PubMed] [Google Scholar]
- 120.Gill PS, Wilcox CS. NADPH oxidases in the kidney. Antioxidants Redox Signal. 2006;8:1597–1607. doi: 10.1089/ars.2006.8.1597. [DOI] [PubMed] [Google Scholar]
- 121.Lassegue B, Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol. 2010;30:653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lambeth J, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radical Biol Med. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ha H, Hwang IA, Park JH, Lee HB. Role of reactive oxygen species in the pathogenesis of diabetic nephropathy. Diabetes Res Clin Prac. 2008;82(Suppl 1):S42–S45. doi: 10.1016/j.diabres.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 124.Pagano PJ, Chanock SJ, Siwik DA, et al. Angiotensin II induces p67phox mRNA expression and NADPH oxidase superoxide generation in rabbit aortic adventitial fibroblasts. Hypertension. 1998;32:331–337. doi: 10.1161/01.hyp.32.2.331. [DOI] [PubMed] [Google Scholar]
- 125.Gaertner SA, Janssen U, Ostendorf T, et al. Glomerular oxidative and antioxidative systems in experimental mesangioproliferative glomerulonephritis. J Am Soc Nephrol. 2002;13:2930–2937. doi: 10.1097/01.asn.0000034908.43113.5d. [DOI] [PubMed] [Google Scholar]
- 126.Kitada M, Koya D, Sugimoto T, et al. Translocation of glomerular p47phox and p67phox by protein kinase C-beta activation is required for oxidative stress in diabetic nephropathy. Diabetes. 2003;52:2603–2614. doi: 10.2337/diabetes.52.10.2603. [DOI] [PubMed] [Google Scholar]
- 127.Fujii M, Inoguchi T, Maeda Y, et al. Pitavastatin ameliorates albuminuria and renal mesangial expansion by downregulating NOX4 in db/db mice. Kidney Int. 2007;72:473–480. doi: 10.1038/sj.ki.5002366. [DOI] [PubMed] [Google Scholar]
- 128.Clark RA. Activation of the neutrophil respiratory burst oxidase. J Infectious Dis. 1999;179(Suppl 2):S309–S317. doi: 10.1086/513849. [DOI] [PubMed] [Google Scholar]
- 129.Leusen JH, Verhoeven AJ, Roos D. Interactions between the components of the human NADPH oxidase: a review about the intrigues in the phox family. Frontiers Biosci. 1996;1:d72–90. doi: 10.2741/a117. [DOI] [PubMed] [Google Scholar]
- 130.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiological Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 131.Nistala R, Whaley-Connell A, Sowers JR. Redox control of renal function and hypertension. Antioxidants Redox Signal. 2008;10:2047–2089. doi: 10.1089/ars.2008.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Brandes RP, Schroder K. Composition and functions of vascular nicotinamide adenine dinucleotide phosphate oxidases. Trends Cardiovasc Med. 2008;18:15–19. doi: 10.1016/j.tcm.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 133.Brown DI, Griendling KK. Nox proteins in signal transduction. Free Rad Biol Med. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brandes RP, Weissmann N, Schroder K. NADPH oxidases in cardiovascular disease. Free Radical Biol Med. 2010 doi: 10.1016/j.freeradbiomed.2010.04.030. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 135.Geiszt M. NADPH oxidases: new kids on the block. Cardiovasc Res. 2006;71:289–299. doi: 10.1016/j.cardiores.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 136.Selemidis S, Sobey CG, Wingler K, et al. NADPH oxidases in the vasculature: molecular features, roles in disease and pharmacological inhibition. Pharmacol Therapeutics. 2008;120:254–291. doi: 10.1016/j.pharmthera.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 137.Geiszt M, Kopp JB, Varnai P, Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Nat’l Acad Sci USA. 2000;97:8010–8014. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shiose A, Kuroda J, Tsuruya K, et al. A novel superoxide-producing NAD(P)H oxidase in kidney. J Biol Chem. 2001;276:1417–1423. doi: 10.1074/jbc.M007597200. [DOI] [PubMed] [Google Scholar]
- 139.Gorin Y, Ricono JM, Kim NH, et al. Nox4 mediates angiotensin II-induced activation of Akt/protein kinase B in mesangial cells. Am J Physiol - Renal Physiol. 2003;285:F219–F229. doi: 10.1152/ajprenal.00414.2002. [DOI] [PubMed] [Google Scholar]
- 140.Gorin Y, Ricono JM, Wagner B, et al. Angiotensin II-induced ERK1/ERK2 activation and protein synthesis are redox-dependent in glomerular mesangial cells. Biochem J. 2004;381:231–239. doi: 10.1042/BJ20031614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Eid AA, Gorin Y, Fagg BM, et al. Mechanisms of podocyte injury in diabetes: role of cytochrome P450 and NADPH oxidases. Diabetes. 2009;58:1201–1211. doi: 10.2337/db08-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ambasta RK, Kumar P, Griendling KK, et al. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem. 2004;279:45935–45941. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- 143.Martyn KD, Frederick LM, von LK, et al. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 144.Nisimoto Y, Jackson HM, Ogawa H, et al. Constitutive NADPH-dependent electron transferase activity of the Nox4 dehydrogenase domain. Biochem. 2010;49:2433–2442. doi: 10.1021/bi9022285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Serrander L, Cartier L, Bedard K, et al. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jiang F. NADPH oxidase in the kidney: a Janus in determining cell fate. Kidney Int. 2009;75:135–137. doi: 10.1038/ki.2008.478. [DOI] [PubMed] [Google Scholar]
- 147.Pedruzzi E, Guichard C, Ollivier V, et al. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol Cell Biol. 2004;24:10703–10717. doi: 10.1128/MCB.24.24.10703-10717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wingler K, Wunsch S, Kreutz R, et al. Upregulation of the vascular NAD(P)H-oxidase isoforms Nox1 and Nox4 by the renin-angiotensin system in vitro and in vivo. Free Rad Biol Med. 2001;31:1456–1464. doi: 10.1016/s0891-5849(01)00727-4. [DOI] [PubMed] [Google Scholar]
- 149.Moe KT, Aulia S, Jiang F, et al. Differential upregulation of Nox homologues of NADPH oxidase by tumor necrosis factor-alpha in human aortic smooth muscle and embryonic kidney cells. J Cell Mol Med. 2006;10:231–239. doi: 10.1111/j.1582-4934.2006.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yamagishi S, Nakamura K, Ueda S, et al. Pigment epithelium-derived factor (PEDF) blocks angiotensin II signaling in endothelial cells via suppression of NADPH oxidase: a novel anti-oxidative mechanism of PEDF. Cell Tissue Res. 2005;320:437–445. doi: 10.1007/s00441-005-1094-8. [DOI] [PubMed] [Google Scholar]
- 151.Tong X, Schroder K. NADPH oxidases are responsible for the failure of nitric oxide to inhibit migration of smooth muscle cells exposed to high glucose. Free Rad Biol Medicine. 2009;47:1578–1583. doi: 10.1016/j.freeradbiomed.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chai D, Wang B, Shen L, et al. RXR agonists inhibit high-glucose-induced oxidative stress by repressing PKC activity in human endothelial cells. Free Rad Biol Med. 2008;44:1334–1347. doi: 10.1016/j.freeradbiomed.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 153.Mittal M, Roth M, Konig P, et al. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res. 2007;101:258–267. doi: 10.1161/CIRCRESAHA.107.148015. [DOI] [PubMed] [Google Scholar]
- 154.Block K, Eid A, Griendling KK, et al. Nox4 NAD(P)H oxidase mediates Src-dependent tyrosine phosphorylation of PDK-1 in response to angiotensin II: role in mesangial cell hypertrophy and fibronectin expression. J Bio Chem. 2008;283:24061–24076. doi: 10.1074/jbc.M803964200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Block K, Gorin Y, Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proc Nat’l Acad Sci U S A. 2009;106:14385–14390. doi: 10.1073/pnas.0906805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Meng D, Lv DD, Fang J. Insulin-like growth factor-I induces reactive oxygen species productioncell migration through Nox4Rac1 in vascular smooth muscle cells. Cardiovasc Res. 2008;80:299–308. doi: 10.1093/cvr/cvn173. [DOI] [PubMed] [Google Scholar]
- 157.Peshavariya H, Jiang F, Taylor CJ, et al. Translation-linked mRNA destabilization accompanying serum-induced Nox4 expression in human endothelial cells. Antioxidants Redox Signal. 2009;11:2399–2408. doi: 10.1089/ars.2009.2579. [DOI] [PubMed] [Google Scholar]
- 158.Kasinath BS, Mariappan MM, Sataranatarajan K, et al. mRNA translation: unexplored territory in renal science. J Am Soc Nephrol. 2006;17:3281–3292. doi: 10.1681/ASN.2006050488. [DOI] [PubMed] [Google Scholar]
- 159.Mariappan MM, Feliers D, Mummidi S, et al. High glucose, high insulin, and their combination rapidly induce laminin-beta1 synthesis by regulation of mRNA translation in renal epithelial cells. Diabetes. 2007;56:476–485. doi: 10.2337/db05-1334. [DOI] [PubMed] [Google Scholar]
- 160.Kasinath BS, Feliers D, Sataranatarajan K, et al. Regulation of mRNA translation in renal physiology and disease. Am J Physiol - Renal Physiol. 2009;297:F1153–F1165. doi: 10.1152/ajprenal.90748.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mahadev K, Motoshima H, Wu X, et al. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol. 2004;24:1844–1854. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lyle AN, Deshpande NN, Taniyama Y, et al. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res. 2009;105:249–259. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sedeek M, Hebert RL, Kennedy CR, et al. Molecular mechanisms of hypertension: role of Nox family NADPH oxidases. Curt Opin Nephrol Hyperten. 2009;18:122–127. doi: 10.1097/MNH.0b013e32832923c3. [DOI] [PubMed] [Google Scholar]
- 164.Wu RF, Ma Z, Myers DP, Terada LS. HIV-1 Tat activates dual Nox pathways leading to independent activation of ERK and JNK MAP kinases. J Biol Chem. 2007;282:37412–37419. doi: 10.1074/jbc.M704481200. [DOI] [PubMed] [Google Scholar]
- 165.Dikalov SI, Dikalova AE, Bikineyeva AT, et al. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Rad Biol Med. 2008;45:1340–1351. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.von LK, Noack D, Jesaitis AJ, et al. Mutational analysis reveals distinct features of the Nox4-p22 phox complex. J Biol Chem. 2008;283:35273–35282. doi: 10.1074/jbc.M804200200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Clempus RE, Sorescu D, Dikalova AE, et al. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2007;27:42–48. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ago T, Kuroda J, Pain J, et al. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circulation Research. 2010;106:1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Liu RM, Choi J, Wu JH, et al. Oxidative modification of nuclear mitogen-activated protein kinase phosphatase 1 is involved in transforming growth factor beta1-induced expression of plasminogen activator inhibitor 1 in fibroblasts. J Biol Chem. 2010;285:16239–16247. doi: 10.1074/jbc.M110.111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hilenski LL, Clempus RE, Quinn MT, et al. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 171.Lambeth JD. Nox enzymes ROS and chronic disease: an example of antagonistic pleiotropy. Free Rad Biol & Med. 2007;43:332–347. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cave AC, Brewer AC, Narayanapanicker A, et al. NADPH oxidases in cardiovascular health and disease. Antioxidants Redox Signal. 2006;8:691–728. doi: 10.1089/ars.2006.8.691. [DOI] [PubMed] [Google Scholar]
- 173.Akki A, Zhang M, Murdoch C, et al. NADPH oxidase signaling and cardiac myocyte function. J Mol & Cell Cardiol. 2009;47:15–22. doi: 10.1016/j.yjmcc.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 174.Lyle AN, Griendling KK. Modulation of vascular smooth muscle signaling by reactive oxygen species. Physiol. 2006;21:269–280. doi: 10.1152/physiol.00004.2006. [DOI] [PubMed] [Google Scholar]
- 175.Diebold I, Djordjevic T, Petry A, et al. Phosphodiesterase 2 mediates redox-sensitive endothelial cell proliferation and angiogenesis by thrombin via Rac1 and NADPH oxidase 2. Circ Res. 2009;104:1169–1177. doi: 10.1161/CIRCRESAHA.109.196592. [DOI] [PubMed] [Google Scholar]
- 176.Djordjevic T, BelAiba RS, Bonello S, et al. Human urotensin II is a novel activator of NADPH oxidase in human pulmonary artery smooth muscle cells. Arterioscler Thromb Vasc Biol. 2005;25:519–525. doi: 10.1161/01.ATV.0000154279.98244.eb. [DOI] [PubMed] [Google Scholar]
- 177.Wagner B, Ricono JM, Gorin Y, et al. Mitogenic signaling via platelet-derived growth factor beta in metanephric mesenchymal cells. Journal of the American Society of Nephrology. 2007;18:2903–2911. doi: 10.1681/ASN.2006111229. [DOI] [PubMed] [Google Scholar]
- 178.Ushio-Fukai M. VEGF signaling through NADPH oxidase-derived ROS. Antioxidants Redox Signaling. 2007;9:731–739. doi: 10.1089/ars.2007.1556. [DOI] [PubMed] [Google Scholar]
- 179.Lee CF, Qiao M, Schroder K, et al. Nox4 is a novel inducible source of reactive oxygen species in monocytes and macrophages and mediates oxidized low density lipoprotein-induced macrophage death. Circ Res. 2010;106:1489–1497. doi: 10.1161/CIRCRESAHA.109.215392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Miyata K, Rahman M, Shokoji T, et al. Aldosterone stimulates reactive oxygen species production through activation of NADPH oxidase in rat mesangial cells. J Am Soc Nephrol. 2005;16:2906–2912. doi: 10.1681/ASN.2005040390. [DOI] [PubMed] [Google Scholar]
- 181.Mollnau H, Wendt M, Szocs K, et al. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res. 2002;90:E58–E65. doi: 10.1161/01.res.0000012569.55432.02. [DOI] [PubMed] [Google Scholar]
- 182.Li Y, Lappas G, nand-Srivastava MB. Role of oxidative stress in angiotensin II-induced enhanced expression of Gi(alpha) proteins and adenylyl cyclase signaling in A10 vascular smooth muscle cells. Am J Physiol - Heart Circ Physiol. 2007;292:H1922–H1930. doi: 10.1152/ajpheart.01166.2006. [DOI] [PubMed] [Google Scholar]
- 183.Dikalova A, Clempus R, Lassegue B, et al. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005;11:2668–2676. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- 184.Coughlan MT, Cooper ME, Forbes JM. Renal microvascular injury in diabetes: RAGE and redox signaling. Antioxidants & Redox Signal. 2007;9:331–342. doi: 10.1089/ars.2006.1469. [DOI] [PubMed] [Google Scholar]
- 185.Inoguchi T, Sonta T, Tsubouchi H, et al. Protein kinase C-dependent increase in reactive oxygen species (ROS) production in vascular tissues of diabetes: role of vascular NAD(P)H oxidase. J Am Soc Nephrol. 2003;14(8 Suppl 3):S227–S232. doi: 10.1097/01.asn.0000077407.90309.65. [DOI] [PubMed] [Google Scholar]
- 186.Etoh T, Inoguchi T, Kakimoto M, et al. Increased experssion of NAD(P)H oxidase subunits, NOX4 and p22phox, in the kidney of streptozotocin-induced diabetic rats and its reversibility by interventive insulin treatment. Diabetologia. 2003;46:1428–1437. doi: 10.1007/s00125-003-1205-6. [DOI] [PubMed] [Google Scholar]
- 187.Proctor G, Jiang T, Iwahashi M, et al. Regulation of renal fatty acid and cholesterol metabolism, inflammation, and fibrosis in Akita and OVE26 mice with type 1 diabetes. Diabetes. 2006;55:2502–2509. doi: 10.2337/db05-0603. [DOI] [PubMed] [Google Scholar]
- 188.Guzik TJ, Mussa S, Gastaldi D, et al. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002;105:1656–1662. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- 189.Hink U, Li H, Mollnau H, et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88:E14–E22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]
- 190.Xia L, Wang H, Goldberg HJ, et al. Mesangial cell NADPH oxidase upregulation in high glucose is protein kinase C dependent and required for collagen IV expression. Am J Physiol - Renal Physiol. 2006;290:F345–F356. doi: 10.1152/ajprenal.00119.2005. [DOI] [PubMed] [Google Scholar]
- 191.San Martin A, Du P, Dikalova A, et al. Reactive oxygen species-selective regulation of aortic inflammatory gene expression in Type 2 diabetes. Am J Physiol - Heart Circ Physiol. 2007;292:H2073–H2082. doi: 10.1152/ajpheart.00943.2006. [DOI] [PubMed] [Google Scholar]
- 192.Onozato ML, Tojo A, Goto A, et al. Oxidative stress and nitric oxide synthase in rat diabetic nephropathy: effects of ACEI and ARB. Kidney Int. 2002;61:186–94. doi: 10.1046/j.1523-1755.2002.00123.x. [DOI] [PubMed] [Google Scholar]
- 193.Carmona-Cuenca I, Roncero C, Sancho P, et al. Upregulation of the NADPH oxidase NOX4 by TGF-beta in hepatocytes is required for its pro-apoptotic activity. J Hepatol. 2008;49:965–976. doi: 10.1016/j.jhep.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 194.Sturrock A, Huecksteadt TP, Norman K, et al. Nox4 mediates TGF-beta1-induced retinoblastoma protein phosphorylation, proliferation, and hypertrophy in human airway smooth muscle cells. Am J Physiol - Lung Cell Mol Physiol. 2007;292:L1543–L1555. doi: 10.1152/ajplung.00430.2006. [DOI] [PubMed] [Google Scholar]
- 195.Sturrock A, Cahill B, Norman K, et al. Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol - Lung Cell Mol Physiol. 2006;290:L661–L673. doi: 10.1152/ajplung.00269.2005. [DOI] [PubMed] [Google Scholar]
- 196.Hu T, Ramachandrarao SP, Siva S, et al. Reactive oxygen species production via NADPH oxidase mediates TGF-beta-induced cytoskeletal alterations in endothelial cells. Am J Physiol - Renal Physiol. 2005;289:F816–825. doi: 10.1152/ajprenal.00024.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Develop. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 198.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 199.Bottinger EP, Bitzer M. TGF-beta signaling in renal disease. J Am Soc Nephrol. 2002;13:2600–2610. doi: 10.1097/01.asn.0000033611.79556.ae. [DOI] [PubMed] [Google Scholar]
- 200.Evans RA, Tian YC, Steadman R, Phillips AO. TGF-beta1-mediated fibroblast-myofibroblast terminal differentiation-the role of Smad proteins. Exp Cell Res. 2003;282:90–100. doi: 10.1016/s0014-4827(02)00015-0. [DOI] [PubMed] [Google Scholar]
- 201.Kim YK, Bae GU, Kang JK, et al. Cooperation of H2O2-mediated ERK activation with Smad pathway in TGF-beta1 induction of p21WAF1/Cip1. Cell Signal. 2006;18:236–243. doi: 10.1016/j.cellsig.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 202.Yue J, Mulder KM. Requirement of Ras/MAPK pathway activation by transforming growth factor beta for transforming growth factor beta 1 production in a Smad-dependent pathway. J Biol Chem. 2000;275:30765–30773. doi: 10.1074/jbc.M000039200. [DOI] [PubMed] [Google Scholar]
- 203.Hayashida T, Decaestecker M, Schnaper HW. Cross-talk between ERK MAP kinase and Smad signaling pathways enhances TGF-beta-dependent responses in human mesangial cells. FASEB Journal. 2003;17:1576–1578. doi: 10.1096/fj.03-0037fje. [DOI] [PubMed] [Google Scholar]
- 204.Massague J, Gomis RR. The logic of TGFbeta signaling. FEBS Letters. 2006;580:2811–2820. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 205.Ghosh Choudhury G, Abboud HE. Tyrosine phosphorylation-dependent PI 3 kinase/Akt signal transduction regulates TGFbeta-induced fibronectin expression in mesangial cells. Cell Signal. 2004;16:31–41. doi: 10.1016/s0898-6568(03)00094-9. [DOI] [PubMed] [Google Scholar]
- 206.Hubchak SC, Sparks EE, Hayashida T, Schnaper HW. Rac1 promotes TGF-beta-stimulated mesangial cell type I collagen expression through a PI3K/Akt-dependent mechanism. Am J Physiol - Renal Physiol. 2009;297:F1316–F1323. doi: 10.1152/ajprenal.00345.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hayashida T, Wu MH, Pierce A, et al. MAP-kinase activity necessary for TGFbeta1-stimulated mesangial cell type I collagen expression requires adhesion-dependent phosphorylation of FAK tyrosine 397. J Cell Sci. 2007;120:4230–4240. doi: 10.1242/jcs.03492. [DOI] [PubMed] [Google Scholar]
- 208.Venkatesan B, Mahimainathan L, Das F, et al. Downregulation of catalase by reactive oxygen species via PI 3 kinase/Akt signaling in mesangial cells. J Cell Physiol. 2007;211:457–467. doi: 10.1002/jcp.20953. [DOI] [PubMed] [Google Scholar]
- 209.Li Y, Yang J, Dai C, et al. Role for integrin-linked kinase in mediating tubular epithelial to mesenchymal transition and renal interstitial fibrogenesis. J Clin Invest. 2003;112:503–516. doi: 10.1172/JCI17913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15:1–12. doi: 10.1097/01.asn.0000106015.29070.e7. [DOI] [PubMed] [Google Scholar]
- 211.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol - Lung Cell Mol Physiol. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 212.Rhee SG. Cell signaling H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 213.Xie Z, Singh M, Singh K. ERK1/2 and JNKs, but not p38 kinase, are involved in reactive oxygen species-mediated induction of osteopontin gene expression by angiotensin II and interleukin-1beta in adult rat cardiac fibroblasts. J Cell Physiol. 2004;198:399–407. doi: 10.1002/jcp.10419. [DOI] [PubMed] [Google Scholar]
- 214.Suzuki H, Frank GD, Utsunomiya H, et al. Current understanding of the mechanism and role of ROS in angiotensin II signal transduction. Curr Pharmaceutical Biotechnol. 2006;7:81–86. doi: 10.2174/138920106776597667. [DOI] [PubMed] [Google Scholar]
- 215.Gooch JL, Gorin Y, Zhang BX, Abboud HE. Involvement of calcineurin in transforming growth factor-beta-mediated regulation of extracellular matrix accumulation. J Biol Chem. 2004;279:15561–15570. doi: 10.1074/jbc.M308759200. [DOI] [PubMed] [Google Scholar]
- 216.Garrido AM, Griendling KK. NADPH oxidases and angiotensin II receptor signaling. Mol Cell Endocrinol. 2009;302:148–158. doi: 10.1016/j.mce.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Gorin Y, Kim NH, Feliers D, et al. Angiotensin II activates Akt/protein kinase B by an arachidonic acid/redox-dependent pathway and independent of phosphoinositide 3-kinase. FASEB J. 2001;15:1909–1920. doi: 10.1096/fj..01-0165com. [DOI] [PubMed] [Google Scholar]
- 218.Block K, Ricono JM, Lee DY, et al. Arachidonic acid-dependent activation of a p22(phox)-based NAD(P)H oxidase mediates angiotensin II-induced mesangial cell protein synthesis and fibronectin expression via Akt/PKB. Antioxidants Redox Signal. 2006;8:1497–1508. doi: 10.1089/ars.2006.8.1497. [DOI] [PubMed] [Google Scholar]
- 219.Wei XF, Zhou QG, Hou FF, et al. Advanced oxidation protein products induce mesangial cell perturbation through PKC-dependent activation of NADPH oxidase. Am J Physiol - Renal Physiol. 2009;296:F427–F437. doi: 10.1152/ajprenal.90536.2008. [DOI] [PubMed] [Google Scholar]
- 220.Xia L, Wang H, Munk S, et al. High glucose activates PKC-zeta and NADPH oxidase through autocrine TGF-beta1 signaling in mesangial cells. Am J Physiol - Renal Physiol. 2008;295:F1705–F1714. doi: 10.1152/ajprenal.00043.2008. [DOI] [PubMed] [Google Scholar]
- 221.Runyan CE, Schnaper HW, Poncelet AC. The phosphatidylinositol 3-kinase/Akt pathway enhances Smad3-stimulated mesangial cell collagen I expression in response to transforming growth factor-beta1. J Biol Chem. 2004;279:2632–2639. doi: 10.1074/jbc.M310412200. [DOI] [PubMed] [Google Scholar]