Abstract
The current study investigated the hypothesis that the duration of the pro-inflammatory environment plays a critical role in the brain’s response that result in negative consequences upon cognition, biochemistry and pathology. Lipopolysaccharide (LPS) or aCSF was slowly (250 ηg/hr) infused into the IVth ventricle of young (3 mo), adult (9 mo) or aged (23 mo) male F-344 rats for 21 or 56 days. The rats were then tested in the water pool task and endogenous hippocampal levels of pro- and anti-inflammatory proteins and genes as well as indicators of glutamatergic function were determined. The duration of the LPS infusion, as compared to the age of the rat, had the greatest impact upon 1) spatial working memory, 2) the density and distribution of activated microglia within the hippocampus, and 3) the cytokine protein and gene expression profiles within the hippocampus. The duration- and age-dependent consequences of neuroinflammation may explain why adult humans respond positively to anti-inflammatory therapies while aged humans do not.
1. Introduction
Advanced age and the duration of neuroinflammation may equally contribute to the frequent co-occurrence of Alzheimer’s disease (AD) and Parkinson’s disease (PD, Aarsland et al., 2001; Akiyama et al., 2000; Hobson and Meara, 2007; Hughes et al., 2000). Recent reports have shown that the co-occurrence of these disorders increases in a duration-dependent manner from 28% six years after diagnosis to around 83% twenty years after diagnosis (Hely et al., 2008; Perez et al., 2012). We and others have speculated that the consequences of neuroinflammation associated with microglial activation, operating across a time scale of decades, are carefully regulated until, due to normal aging, there is a gradual shift to a non-equilibrium state that is permissive for neurodegenerative processes (Colton and Wilcock, 2010; Smith et al., 2012; Wenk and Hauss-Wegrzyniak, 2001). Consistent with this hypothesis are epidemiological evidence that very long duration inflammatory diseases in humans, such as atherosclerosis, obesity, diabetes, depression and periodontitis increase the risk of AD (Andersen et al., 2005; Balakrishnan et al., 2005; Biessels and Kappelle, 2005; Casserly and Topol, 2004; Dowlati et al., 2010; Kamer et al., 2009; Ownby et al., 2006).
Microglia activation is detectable many years prior to the onset of neuropathological changes associated with AD and PD and is predictive of later symptom severity, as demonstrated by positron emission tomography studies (Cagnin et al., 2007; Gerhard et al., 2006; Imamura et al., 2003). Microglia can assume various activation states that are associated either with elevations of pro-inflammatory cytokines and the release of potentially destructive oxidative enzymes or the expression of a cytokine activation profile that sustains repair, recovery and growth (Colton and Wilcock, 2010; Sudduth et al., 2013). This spectrum of activation states have been categorized as M1or M2 in macrophages (Mantovani et al., 2004). Vulnerable brain regions, particularly the hippocampus, are likely exposed for many decades to a complex and varying blend of microglia in alternative activation states (Bilbo, 2010; Eikelenboom et al., 2010; Heneka et al., 2010; Herrup, 2010; Sudduth et al., 2013). The current study investigated the differential influence of brain age and the duration of the pro-inflammatory stimulus upon the profile of hippocampal pro- and anti-inflammatory genes and proteins, the number of histologically identified activated microglia and performance in a spatial memory task.
2. Methods
2.1. Experimental Design
Young, adult and aged male F-344 rats received chronic infusion of LPS, or its vehicle, into the IVth ventricle for 21 or 56 days and were then evaluated by behavioral, histological, biochemical and genetic analyses. Multiple counter-balanced iterations of this study were performed so that at the end of the investigation each group contained eleven rats; thus, 132 rats completed all aspects of the investigation.
2.2. Subjects
Young (3 mo), adult (9 mo) and aged (23 mo) male F-344 rats (Harlan Sprague–Dawley) were maintained on a 12/12-h light–dark cycle in a temperature-controlled room (22° C) with free access to food and water and with lights off at 09:00. All rats were sacrificed during the dark phase of the diurnal cycle. Body weights and general health were closely monitored throughout the study. All rats were given health checks, handled upon arrival and allowed at least one week to adapt to their new environment prior to surgery. We certify that the experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23) revised 1996. We also certify that the formal approval to conduct the experiments has been obtained from the animal subjects review board from Ohio State University.
2.3. Surgery
Artificial cerebrospinal fluid (aCSF; 140 mM NaCl, 3.0 mM KCl, 2.5 mM CaCl2, 1.0 mM MgCl2, and 1.2 mM Na2HPO4 adjusted to pH 7.4) or LPS (0.25μg/hr, 1.66 mg/ml prepared in aCSF; E. coli, serotype 055:B5, TCA extraction, Sigma) were chronically infused via a cannula that was implanted into the IVth ventricle (−2.5 mm AP and −7.0 mm DV relative to lambda) and attached (via Tygon tubing, 0.06 O.D.) to an osmotic minipump (Alzet model #2006, to deliver 0.15μl/hr; Durect Corp., Cupertino, CA) as previously described (Hauss-Wegrzyniak et al., 1998; Marchalant et al., 2007; Rosi et al., 2005). Calculations using the average fill volume for this pump allows for release of LPS or aCSF for up to 56 days. Post-operative care included lidocaine 1% solution applied to the exposed skin upon closure and 2 ml of isotonic saline by subcutaneous injection to prevent dehydration during recovery.
2.4. Behavioral Testing
Water Pool Task: The rats were handled daily for five days before behavioral testing began. Spatial learning ability was assessed for all rats using a 170 cm diameter water pool with grey walls. The water was maintained at room temperature (RT, 21–22°C). The pool was in the center of a room with multiple visual stimuli as distal and proximal cues. The circular escape platform was 10 cm in diameter. For the spatial (hidden-platform) version of the water task, a circular escape platform was present in a constant location, submerged 2.5 cm below the water surface. The rats were tracked using Noldus Ethovision 3.1 tracking and analysis system (Noldus, Leesburg, VA).
Each rat performed six trials per day for four consecutive days (24 trials total), with a 60-min inter-trial interval. The rat was released into the water on each trial from one of seven locations spaced evenly at the side of the pool, which varied so that the rats did not start from any location twice in one day. After the rat found the escape platform or swam for a maximum of 60 sec, it was allowed to remain on the platform for 30 sec. At the end of the fourth day, the platform was removed and a standard probe trial was conducted. After the probe test, all rats were also tested using with the platform raised 2 cm above the surface of the water in order to control for possible aging or LPS-induced deficits in visual acuity. The effects of aging and/or inflammation were assessed through the comparison of the latency to find the platform.
2.5. Histology
Tissue collection
All the rats were deeply anesthetized prior to sacrifice. Rats used for histology (five rats/group) were prepared for a transcardiac perfusion with cold saline containing 1 U/ml heparin, followed by 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. The brains were then post-fixed overnight in the same fixative and then stored (4°C) in phosphate buffer saline (PBS), pH 7.4. Rats used for biochemistry (six rats/group) from each group were briefly anesthetized and then rapidly decapitated; their hippocampi were quickly dissected on ice. The left and right hippocampi were randomly chosen for either protein or gene expression analyses. The hippocampi were stored at −80°C until processed. Blood was collected during the rapid decapitation procedure. After centrifugation at 4°C for 15min at 2500g, serum was collected and assayed.
Immunocytochemistry
Free-floating coronal sections (40 μm) were obtained using a vibratome from perfused tissues for staining with standard avidin/biotin peroxidase labeling methods. The monoclonal antibody OX-6 (final dilution 1:200, Pharmigen, San Diego, CA) was used to visualize activated microglial cells only. This antibody is directed against Class II major histocompatibility complex (MHC II) antigen,3an indicator of activation (Akiyama et al., 2000; Streit and Xue, 2009). After quenching endogenous peroxidase/activity and blocking nonspecific binding, the sections were incubated (4°C) overnight with the primary antibody. Thereafter, the sections were incubated for 1h30 at RT with the secondary polyclonal antibody, rat adsorbed biotinylated horse anti-mouse immunoglobulin G (final dilution 1:200, Vector, Burlingame, CA). Sections were then incubated for 1h at RT with avidin-biotinylated horseradish peroxidase (Vectastain, ABC kit, Vector). After washing again in PBS, the sections were incubated with 0.05% 3, 3′-diaminobenzidine tetrahydrochloride (Vector) as chromogen. The reaction was stopped by washing the section with PBS. No staining was detected in the absence of the primary or secondary antibodies. Sections were mounted on slides, air-dried and cover-slipped with cytoseal (Allan Scientific, Kalamazoo, MI) mounting medium. The location of immunohistochemically-defined cells was examined by light microscopy. Quantification of cell density in the reconstructed hippocampal coronal sections (at least five sections from each rat) was assessed with Nikon 80i documentation system with DS-5M-L1 digital camera using Elements 3.1 (Nikon Instruments, Melville, NY) and quantified using MetaMorph imaging software (Universal Image Corporation, West Chester, PA).
2.6. Biochemistry
Protein analysis
Frozen (−80°C) hippocampi were placed in a BioPlex Cell Lysis solution (BioRad, Richmond, CA) and total proteins were extracted according to the manufacturer’s instructions. Hippocampal levels of tumor necrosis factor (TNF)-α, interleukin-1-alpha (IL-1α), IL-1β, IL-2,3IL-4, IL-6, IL-10, IL-12 IL-13, IL-18 (interferon-γ inducing factor), interferon (IFN)-γ, and granulocyte-macrophage-stimulating factor (GM-CSF) were quantified simultaneously with a bead-based flow cytometric immunoassay (Bio-Rad, BioPlex Pro Rat Standard, 171-K1002M). Briefly, a mixture of distinct capture beads (fluorescently dyed microspheres) each with a specific spectral address and conjugated to an antibody against one of the cytokines listed above were dispensed across a 96-well plate and protected from light. Samples and antigen standards were added in duplicate and incubated for 1 h at 700 RPM at room temperature; unbound materials were washed away (3x). Then biotinylated detection antibodies directed against each of the proteins were added for 30 min at 700 RPM at RT; unbound materials were washed away (3x). Each well was then incubated for 10 min at 700 RPM at room temperature with a reporter dye, streptavidin-phycoerythrin conjugate (SA-PE), which binds to the detection antibody; unbound materials were washed away (3x). Each well was then suspended in assay buffer and shaken at 1100 RPM for 30 sec. Finally, the contents of each well was passed through a dual detection flow cytometer with a classification laser that distinguishes each of the proteins by color of its bound antigen-specific bead and a reporter laser that quantifies each molecule based upon the fluorescence of bound antigen-specific SA-PE reporter dye. Values were compared to standard curves; all of the biomarkers were well above the background detection level of the assay. Calculated values were standardized to the total protein content of the homogenate. GM-CSF was examined because it can activate microglia and may play a role in the pathophysiology of AD (Parajuli et al., 2012; Tarkowski et al., 2001), multiple sclerosis (Carrieri et al., 1998) and HIV-associated dementia (Perrella et al., 1992); expression of the TLR4 gene was examined because its protein is the principle target of LPS (Akashi et al., 2003) and can be up regulated by exposure to GM-CSF (Parajuli et al., 2012). Total protein was quantified in brain homogenates using the Bio-Rad protein assay (Bio-Rad). The results are reported as picograms/mg protein.
rtPCR mRNA analysis
Total RNAs were extracted from hippocampi by using Trizol reagent (Life Technologies, Carlsbad, CA) followed by NucleoSpin RNA II kit (Macherey-Nagel, Düren, Germany) according to the manufacturers’ instructions. Total RNAs (1 μg) form each sample were reverse-transcribed using the iScript reverse transcription Supermix for RT-qPCR (Bio-Rad). Primers were designed for each gene using the PrimerQuest software (Integrated DNA Technologies, Coralville, IA; Table 1). Primer alignments were performed with the BLAST database to ensure specificity of primers. Primers and Sso Advanced SYBR Green Supermix (BioRad) were prepared with RNase-free water. For PCR amplification, mix (19μl) was added to RT reaction (1 μl) previously diluted (1:20). Assays were run in triplicate on the CFX96, C1000 Thermal Cycler (Bio-Rad). Amplification conditions were as follows: 95°C for 30 sec; 40 cycles of PCR (denaturation: 95°C for 5sec, annealing/extension: 60°C for 30sec) and melting curves. Two negative controls were performed during each quantitative PCR experiment: reaction without the reverse transcription to confirm the absence of genomic DNA contamination, and samples with no added cDNA template to prove the absence of primer dimers. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene. The cycle (Ct) at which expression levels crossed threshold was normalized to the Ct of the housekeeper GAPDH, producing ΔCt with arbitrary units of total gene expression. All plates were evaluated with respect to –RT and H2O controls.
Table 1.
Primer sequences and RT-PCR conditions
| Gene | Accession # | Primer Sequence | Annealing Temp (°C) | Product Length |
|---|---|---|---|---|
| TNFα | X66539.1 | CTGGCCAATGGCATGGATCTCAAA | 59.7 | 97 bp |
| IL1β | NM_031512 | ACCTGCTAGTGTGTGATGTTCCCA | 59.9 | 109 bp |
| TGFβ | NM_021578 | TGATACGCCTGAGTGGCTGTCTTT | 60 | 115 bp |
| TLR4 | NM_019178 | TCTGCCCTGCCACCATTTACAGTT | 60.7 | 135 bp |
| CX3CL1 | NM_134455.1 | ACTTCTGTGCTGACCCAAAGGAGA | 59.9 | 105 bp |
| CX3CR1 | NM_133534.1 | GTGCAAGCTCACGACTGCTTTCTT | 59.8 | 133 bp |
| GLT1 | AY_069978 | TCTTGCCAGCTTCCTGTTGTCTCA | 60.2 | 116 bp |
| GADPH | NM_017008 | TGACTCTACCCACGGCAAGTTCAA | 59.9 | 141 bp |
| XcT | NM_001107673 | TGTATGACTGGGAAACCACAGCGA | 60 | 175 bp |
| TH | NM_012740 | AGCCTGTGTACTTTGTGTCCGAGA | 59.9 | 91 bp |
2.7. Statistical analysis
Results are expressed as means ± SEM. Statistical analyses were conducted using SigmaStat 12.0 (Systat, San Jose, CA). ANOVA were performed followed by Fisher’s PLSD for post hoc comparisons.3Graphs are shown with SEMs represented by error bars. Control aCSF is shown in some graphs as one collapsed group, but aCSF groups were not collapsed for statistical analysis. A p<0.05 was considered statistically significant. * Indicates significant difference between aCSF and LPS within the same age group. # Indicates significant difference between LPS 21 days and LPS 56 days within the same age group. † Indicates significant difference between age groups (3, 9 or 23 mo) within treatment (aCSF, LPS 21days or LPS 56 days).
3. Results
3.1 Behavior
The latency to find the submerged platform (Figure 1) was significantly impaired by the age of the rat (F3,2495 = 140.5, p < 0.001) and LPS treatment (F1,2495 = 7.2, p = 0.007); furthermore, the response of the rat to LPS was influenced by the age of the rat (p=0.029) and the duration of the exposure to LPS (p=0.014). Performance in the probe trial (Figure 2) was also impaired by the rat’s age (F2,103 = 29.6, p < 0.001) and the LPS treatment (F1,103 = 4.8, p = 0.03). Overall, the age-dependent decline in performance was potentiated by LPS-infusion in a duration-dependent manner in young and middle-aged rats. In contrast, the baseline performance of the aged rats was so poor that the LPS exposure did not worsen performance.
Figure 1.
Water maze performance. The latency to find the submerged platform was significantly influenced by an interaction between the rats’ age and the LPS infusion; furthermore, the effects of the rats’ age and treatment upon performance depended upon the duration of the LPS infusion. All young and adult rats had significantly (p<0.05) shorter latencies to find the submerged platform across testing days. *p<0.01 vs. aCSF-infused rats within the same age group; †p<0.01, comparing across age groups; #p<0.01, 3 mo rats LPS 56 days vs. 21 days.
Figure 2.
Probe Trial. The age of the rat and the LPS infusion reduced the time spent searching in the target quadrant once the platform was removed. LPS reduced the time spent in the vicinity of the missing platform in young and middle-aged rats, *p<0.05, but not aged rats. Search time was reduced with increasing age, †p<0.05
3.2 Histology
The increased density of MHC II-immunoreactive (MHC II-IR) microglia (Figure 3) in the dentate gyrus (DG), CA3 and CA1 regions of the hippocampus was significantly (all F >25, p<0.001) influenced by the LPS infusion, the age of the rat and the duration of the exposure. Furthermore, the interaction between age and LPS treatment significantly (p<0.001) depended upon the duration of the LPS infusion.
Figure 3.
Photomicrographs and density quantification of MHC II-IR microglial cells in hippocampus. Young rats – aCSF (A), LPS 21 days (B), LPS 56 days (C); Aged rats – aCSF (D), LPS 21 days (E), LPS 56 days (F). Scale bar: 200 μm. Throughout all three regions of interest, the density of MHC II-IR microglia was influenced by aging, LPS treatment and the duration of the LPS infusion. Furthermore, there were significant interactions between the effects of aging and the LPS treatment, aging and the duration of the LPS infusion and the LPS treatment and its duration. Finally, the interaction between age and LPS treatment depended significantly upon the duration of the LPS infusion. *p<0.01 vs. aCSF; #p<0.01 vs. 21 days LPS; †p<0.01.
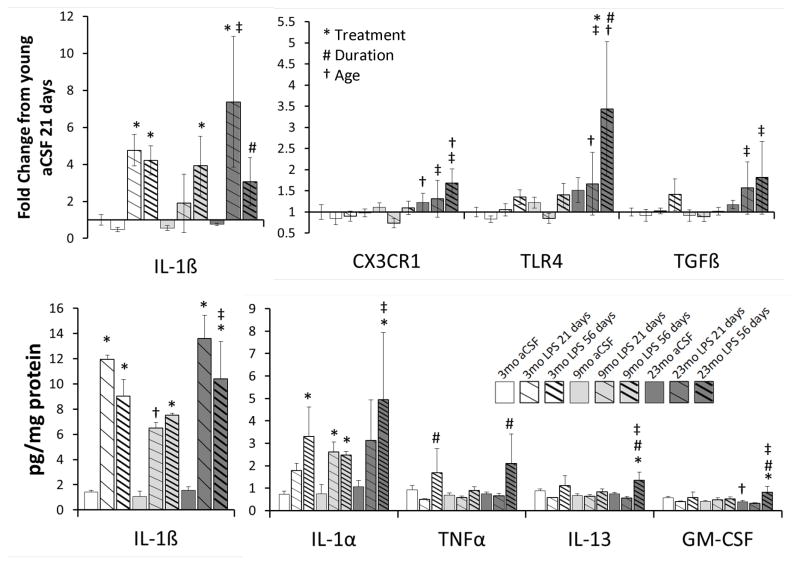
3.3 Biochemistry
Hippocampal Proteins
LPS treatment significantly (p<0.001) increased IL-1α and IL-1β levels (Figure 4). In contrast, TNFα, GM-CSF and IL-13 levels were significantly (p<0.01) influence by the duration of the LPS infusion. GM-CSF also demonstrated a duration-dependent increase (p=0.05) in which it was elevated only in aged rats, and only after 56 days infusion, regardless if LPS or aCSF was infused. The anti-inflammatory cytokine IL-13 was also elevated after 56 days LPS infusion in young and aged rats only (See Figure 4). Endogenous hippocampal levels of the other biomarkers, i.e. IL-2, IL-4, IL-5, IL-6, IL-10, IL-12 and INFγ, were unchanged across all age groups, treatments and duration of infusion (data not shown).
Figure 4.
Hippocampal3cytokines levels (bottom panel) and gene expression (top panel). The infusion of LPS into the IVth ventricle significantly increased the protein levels of IL-1α and IL-1β (notice the scale difference for IL-1β). The increased levels of TNFα and IL-13 depended upon the duration of the LPS infusion. The increased levels of GM-CSF depended upon an interaction between the influences of the LPS treatment and duration and the age of the rat. Hippocampal levels of IL-2, IL-4, IL-5, IL-6, IL-10, IL-12 and INFγ were unchanged. The infusion of LPS into the IVth ventricle significantly increased the gene expression of IL-1β, TGFβ and the TLR4 receptor (notice the scale difference for IL-1β). The increased expression of the TLR4 and fractalkine genes depended upon the duration of the LPS infusion. The age of the rat was responsible for increased gene expression for CX3CR1, TGFβ and the TLR4 receptor. Expression of GLT1, TNFα, XcT, fractalkine ligand and BDNF genes were unchanged. All post-hoc comparisons p<0.05: * aCSF vs LPS within respective age group; # LPS 21 days vs. 56 days; † vs. 3 mo; ± vs. 9 mo; †± vs. 3 and 9 mo.
Serum Proteins
Serum levels of IL-1α., IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, IL-13, GM-CSF, INFγ and TNFα were unchanged across all age and treatment groups (data not shown).
Hippocampal Gene Expression
LPS exposure significantly (p<0.03) increased the gene expression of IL-1β, TGFβ and the TLR4 receptor (Figure 4). The duration of the LPS infusion had its greatest impact (F> 5, p<0.02) on fractalkine receptor and TLR4 receptor gene expression whereas the age of the rat had the greatest impact (F >6, p<0.01) on fractalkine receptor, TGFβ and the TLR4 receptor gene expression.3The changes in TGFβ levels are similar to those seen in AD (Rota et al., 2006). 3The duration- and age-dependent profile of TLR4 gene expression mirrored that of GM-CSF, increasing in aged rats after 56 days of either aCSF or LPS infusion. Pro-inflammatory TNFα gene expression was significantly increased after 56 days LPS infusion in young and aged rats, following the same profile of anti-inflammatory IL-13 protein expression (data not shown). Gene expression of the anti-inflammatory cytokine TGFβ and the fractalkine receptor were both elevated (p<0.04) in a duration-dependent manner following LPS infusion of either 21 or 56 days in aged rats. Changes in the expression of GLT1, the major glutamate transporter, XcT, a sodium-independent cystine-glutamate transporter, TNFα, fractalkine ligand and BDNF genes were not statistically significant; their data are not shown.
4. Discussion
In the current study, we produced a pro-inflammatory environment in brains of young, adult and aged male rats and then systematically documented an evolving series of behavioral, biochemical and gene expression changes induced by the chronic infusion of picomolar levels of LPS3into the IVth ventricle. These changes represent a complex interplay of glia (both astrocytes and microglia) and neurons leading to a series of changes that transpire in the presence of continued stimulation of TLR4 receptors on microglia. In the current study, LPS was used as a chemical tool to selectively stimulate the TLR4/CD14 complex expressed by microglia; administered in this fashion, the overall dose of LPS is not sufficient to produce any peripheral manifestations of infectious disease processes (such as elevated serum levels of inflammatory cytokines) or down-regulation in TLR4 gene expression in the brain. We have previously documented an array of duration-dependent changes following infusion of LPS in this manner (Wenk et al., 2000) particularly within the hippocampus (Hauss-Wegrzyniak et al., 1998, 2000, 2002; Marchalant et al., 2008, 2009; Rosi et al., 2004, 2008, 2009; Wenk et al., 2000).
Hippocampal-dependent spatial learning measured by performance in the water maze showed a duration-dependent decline in young and middle-aged rats; in contrast, performance of aged rats was not further impaired by the LPS infusion, as compared to the performance of age-matched aCSF-infused rats. Throughout the hippocampus an increase in the density of MHC II-IR microglia depended upon the3duration of the LPS infusion and, inversely, the age of the rat. 3Generally, the number of activated microglia increased in parallel with the infusion duration across all age groups; however, the magnitude of the increase in the number of MHC II-IR microglia was more dynamic in younger rats and diminished in aged rats. Surprisingly, the blunted response to LPS by the aged rat hippocampus occurred in the presence of increased expression of the TLR4 gene suggesting that endotoxin tolerance does not develop in the aged brain. Furthermore, increased TLR4 gene expression in response to the LPS infusion was only observed in the aged rats and these changes were duration dependent. Surprisingly, the increased TLR4 gene expression in response to the LPS infusion in old rats occurred in the presence of significantly fewer indicators of microglial activation. The diminished response might be due to an age-related decline in the number of microglia available for activation; however, significant changes in microglia density do not occur in normal aged rats (Long et al., 1998). The blunted response of aged rats to LPS may also be due to the aged microglia’s impaired ability to regulate its response to inflammogens such as LPS (Cerbai et al., 2012) or mutant proteins such as beta-amyloid (Cameron et al., 2012; Cribbs et al., 2012; Flanary et al., 2007; Streit et al., 2009). An age-related decline in the normal microglia response may also underlie the fact that adult humans and young rodents respond positively to anti-inflammatory therapies (Breitner et al., 2011; Hauss-Wegrzyniak et al., 1999; Leoutsakos et al., 2012) while aged humans and rodents do not (Bachstetter et al., 2012; Breitner et al., 2011; Drye and Zandi, 2012; Hauss-Wegrzyniak et al., 1999).
Ordinarily, GM-CSF levels are barely detectable in the adult brain (Dame et al., 1999). However, in the current study, GM-CSF levels increased within the hippocampus in response to prolonged LPS exposure, but only in the aged rats. GM-CSF is a pro-inflammatory factor released by astrocytes in response to IL-1β or LPS that binds to a specific receptor on microglia leading to up-regulation of MHC II expression (Pierson et al., 2012) and TLR4 gene expression (Parajuli et al., 2012) in young adult rats. GM-CSF can also enhance the LPS-induced NF-κB nuclear translocation and production of IL-1β and TNF-α (Parajuli et al., 2012). Our results demonstrated that the parallel changes in TLR4 gene expression and GM-CSF production was significantly influenced by the duration of the neuroinflammatory environment as well as the age of the rat. Overall, young rats responded with a robust M1 type response similar to that seen during the early stages of AD (Sudduth et al., 2013), while the aged rats responded with a more complex profile of M1 (IL-1β, IL-1α, CX3CR1, TLR4, TNFα, GM-CSF), M2a (IL-13, MHC II), M2b (MHC II) and M2c (TGFβ) biomarkers (Mantovani et al., 2004). The results of the current study further demonstrated that aged microglia display a blunted up-regulation of MHC II expression in response to elevated levels of both IL-1β and GM-CSF in response to LPS exposure. The profile of cytokine biomarkers in the aged rats showed a greater response for the markers of alternative activation states, such as TGF-β, IL-13 and CX3CR1; all of which might be expected to mute the microglial response to LPS. Thus, the lack of MHC II expression, typically associated with an M1 or M2b response, may be explained by a greater chronic M2 activation state in response to LPS in the aged rat brain. Our results suggest that chronically elevated levels of GM-CSF in the aged brain may function to accelerate the progression of AD by increasing TLR4 expression on microglia in the absence of any observable changes in MHC II expression by resident microglia. These results suggest that in the aged brain MHC II expression is not a useful indicator of microglia activation state.
The LPS infusion increased IL-1α in aged rats and IL-1β in all rats. IL-1β gene and protein levels correlated significantly with each other (p<0.001) and were elevated in aged rats in spite of the blunted MHC II expression in response to LPS. These robust changes in IL-1α and IL-1β gene expression and protein levels in the brain further highlight the important role played by the interleukin-1 cytokines in age-associated cognitive decline (Mrak and Griffin, 2001) and as the driving force in the phosphorylation of tau (Ghosh et al., 2013). In contrast to the correlation seen for the interleukins, LPS-induced changes in TNFα gene expression did not correlate with TNFα protein levels in the hippocampus. The current study demonstrated a duration-dependent, but not age-dependent, increase in hippocampal TNFα protein levels. The level of TNFα and its receptor are increased in the AD brain; the TNFα receptor may be necessary for beta-amyloid-induced cell death (Cheng et al., 2010). TNFα also plays a critical role in neuroplasticity via its interaction with glutamatergic neurotransmission and the role of glutamate in cytotoxicity (Santello and Volterra, 2012), thus it is not surprising that in the current study TNFα protein levels correlated significantly (p=0.02) with GLT1, the major glutamate transporter, gene expression. GLT1 is responsible for glutamate clearance in hippocampal tissue and is almost exclusively expressed by astrocytes, particularly perisynaptic astrocytes in regions receiving dense glutamatergic innervation, such as the molecular layer of the DG (Lehre et al., 1995). The absence of a significant increase in GLT1 gene expression was likely due to the elevated levels of IL-1β (Prow and Irani, 2008). Microglia and neurons do not generally express GLT1 (Milton et al. 1997), however, GLT1 is present on the surface of microglia exposed to LPS (Persson et al., 2005). Consistent with this finding, our flow cytometry investigation found a significant increase in the number of CD11b/c-immunopositive microglia expressing both GLT1 protein and TLR4 following chronic exposure to LPS (H.M. Brothers, unpublished observation).
There are two competing perspectives on the activation state of aged microglia that are crucial to determine how to reduce the consequences of neuroinflammation: 1) that microglia become primed and more reactive with age (Barrientos et al., 2010; Henry et al., 2009) and 2) microglia become senescent and less reactive with age (Flanary et al., 2007; Streit et al., 2009). The first suggests that immunotherapy should be used to suppress microglia activation in the diseased brain, and the latter proposes that we attempt to augment microglia reactivity and promote their function. Ordinarily the number of MHC II-IR microglia in the hippocampus increases with normal aging, however, in our current and previous studies (Bardou et al., 2012; Hauss-Wegrzyniak et al., 1999), the number of MHC II-IR microglia was not enhanced by chronic LPS exposure in aged rats. In the current study we found no significant age by treatment interactions that were independent of the influence of duration; thus, overall, our results are consistent with the hypothesis that microglia become senescent and less responsive to both pro- and anti-inflammatory molecules.
Our behavioral and biochemical results in aged rats are consistent with those reported previously by Cribbs et al. (2012) that inflammation-related genes for IL-1β, IL-6 and IL-10, but not TNFα, undergo a progressive activation in the aging human hippocampus; the authors concluded that these changes prime the brain for neurodegenerative cascades, cognitive decline, and progression to AD. In the current study, LPS produced a duration-dependent increase in IL-13 protein levels; IL-13 induces a class of protein-degrading enzymes, known as matrix metalloproteinases that may also contribute to the development of disorders that involve neuroinflammation such as amyotrophic lateral sclerosis (Shi et al., 2007), PD and multiple sclerosis (Kim and Joh, 2012) and the degeneration of hippocampal neurons in the presence of beta-amyloid (Nam et al., 2012). It remains unanswered whether similar inflammatory events cause, or result from, the neurodegeneration that leads to these degenerative disorders in aging humans.
Overall, the current results emphasize the critical role of the duration of the pro-inflammatory challenge initiated by mutant proteins, disease or injury. We speculate that an uncontrolled pro-inflammatory environment could drive a self-propagating cycle in which abnormal cellular responses promulgate the release of both M1 and M2 mediators, such as seen in the current study, which in turn may exacerbate local neuronal injury within discrete brain regions leading to impaired cognition (Mantovani et al., 2004; Sudduth et al., 2013).
Acknowledgments
Supported by U.S. Public Health Service, RO1 AG030331, RO1 AG037320 and The Ohio State University Women and Philanthropy Program to GLW.
Footnotes
All authors have contributed to the work, agree with the presented findings, and that the work has not been published before nor is being considered for publication in another journal.
No authors report any conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarsland D, Andersen K, Larsen JP, Lolk A, Nielsen H, Kragh-Sorensen P. Risk of dementia in Parkinson’s disease: a community based, prospective study. Neurol. 2001;56:730–736. doi: 10.1212/wnl.56.6.730. [DOI] [PubMed] [Google Scholar]
- Akashi S, Saitoh S, Wakabayashi Y, Kikuchi T, Takamura N, Nagai Y, Kusumoto Y, Fukase K, Kusumoto S, Adachi Y, Kosugi A, Miyake K. Lipopolysaccharide interaction with cell surface Toll-like receptor 4-MD-2: higher affinity than that with MD-2 or CD14. J Exp Med. 2003;198:1035–1042. doi: 10.1084/jem.20031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cooper NR, Eikelenboom P, Emmerling M, Fiebich B, Finch CE, Frautschy S, Griffin WST, Hampel H, Landreth G, McGeer PL, Mrak R, MacKenzie I, O’Banion K, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray A. Inflammation in Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen K, Lolk A, Kragh-Sorensen P, Petersen NE, Green A. Depression and the risk of Alzheimer disease. Epidemiol. 2005;16:233–238. doi: 10.1097/01.ede.0000152116.32580.24. [DOI] [PubMed] [Google Scholar]
- Bachstetter AD, Norris CM, Sompol P, Wilcock DM, Goulding D, Neltner JH, Clair DS, Watterson DM, Van Eldik LJ. Early stage drug treatment that normalizes proinflammatory cytokine production attenuates synaptic dysfunction in a mouse model that exhibits age-dependent progression of Alzheimer’s disease-related pathology. J Neurosci. 2012;32:10201–10210. doi: 10.1523/JNEUROSCI.1496-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan K, Verdile G, Mehta PD, Beilby J, Nolan D, Galvao DA, Newton R, Gandy SE, Martins RN. Plasma Aβ42 correlates positively with increased body fat in healthy individuals. J Alzheim Dis. 2005;8:269–282. doi: 10.3233/jad-2005-8305. [DOI] [PubMed] [Google Scholar]
- Bardou I, DiPatrizio N, Brothers HM, Kaercher RM, Baranger K, Mitchem M, Hopp SC, Wenk GL, Marchalant Y. Pharmacological manipulation of cannabinoid neurotransmission reduces neuroinflammation associated with normal aging. Health. 2012;4:679–684. [Google Scholar]
- Barrientos RM, Frank MG, Watkins LR, Maier SF. Memory impairments in healthy aging: Role of aging-induced microglial sensitization. Aging Dis. 2010;1:212–231. [PMC free article] [PubMed] [Google Scholar]
- Bartels AL, Leenders KL. Neuroinflammation in the pathophysiology of Parkinson’s disease: evidence from animal models to human in vivo studies with [11C] PK11195 PET. Mov Disord. 2005;22:1852–1856. doi: 10.1002/mds.21552. [DOI] [PubMed] [Google Scholar]
- Biessels G, Kappelle LJ. Increased risk of Alzheimer’s disease in Type II diabetes: insulin resistance of the brain or inslin-induced amyloid pathology? Biochem Soc Trans. 2005;33:1041–1044. doi: 10.1042/BST0331041. [DOI] [PubMed] [Google Scholar]
- Bilbo SD. Early-life infection is a vulnerability factor for aging-related glial alterations and cognitive decline. Neurobiol Learn Mem. 2010;94:57–64. doi: 10.1016/j.nlm.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitner JC, Baker LD, Montine TJ, Meinert CL, Lyketsos CG, Ashe KH, Brandt J, Craft S, Evans DE, Green RC, Ismail MS, Martin BK, Mullan MJ, Sabbagh M, Tariot PN ADAPT Research Group. Extended results of the Alzheimer’s disease anti-inflammatory prevention trial. Alzheimers Dement. 2011;7:402–411. doi: 10.1016/j.jalz.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagnin A, Kassiou M, Meikle SR, Banati RB. In vivo evidence for microglial activation in neuro-degenerative dementia. Acta Neurolog Scand. 2006;114:107–114. doi: 10.1111/j.1600-0404.2006.00694.x. [DOI] [PubMed] [Google Scholar]
- Cameron B, Tse W, Lamb R, Li XX, Lamb BT, Landreth GE. Loss of interleukin receptor-associated kinase 4 signaling suppresses amyloid pathology and alters microglial phenotype in a mouse model of Alzheimer’s disease. J Neurosci. 2012;32:15112–15123. doi: 10.1523/JNEUROSCI.1729-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrieri PB, Provitera V, De Rosa T, Tartaglia G, Gorga F, Perrella O. Profile of cerebrospinal fluid and serum cytokines in patients with relapsing–remitting multiple sclerosis: a correlation with clinical activity. Immunopharmacol Immunotoxicol. 1998;20:373–382. doi: 10.3109/08923979809034820. [DOI] [PubMed] [Google Scholar]
- Casserly I, Topol E. Convergence of atherosclerosis and Alzheimer’s disease: inflammation, cholesterol, and misfolded proteins. Lancet. 2004;363:1139–1146. doi: 10.1016/S0140-6736(04)15900-X. [DOI] [PubMed] [Google Scholar]
- Cheng X, Yang L, He P, Li R, Shen Y. Differential activation of tumor necrosis factor receptors distinguishes between brains from Alzheimer’s disease and non-demented patients. J Alz Dis. 2010;19:621–630. doi: 10.3233/JAD-2010-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton CA, Wilcock DM. Assessing Activation States in Microglia. CNS and Neurol Disord Drug Targets. 2010;9:174–191. doi: 10.2174/187152710791012053. [DOI] [PubMed] [Google Scholar]
- Cribbs DH, Berchtold NC, Perreau V, Coleman PD, Rogers J, Tenner AJ, Cotman CW. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study. J Neuroinflamm. 2012;9:179. doi: 10.1186/1742-2094-9-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dame JB, Christensen RD, Juul SE. The distribution of granulocyte-macrophage colony-stimulating factor and its receptor in the developing human fetus. Pediatr Res. 1999;46:358–366. doi: 10.1203/00006450-199910000-00002. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sahm L, Reim EK, Lanctot KL. A meta-analysis of cytokines in major depression. Biol Psychiat. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Drye LT, Zandi PP. Role of APOE and age at enrollment in the Alzheimer’s disease anti-inflammatory prevention trial (ADAPT) Dement Geriatr Cogn Dis Extra. 2012;2:304–311. doi: 10.1159/000341783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikelenboom P, van Exel E, Hoozemans JJM, Veerhuis R, Rosemuller AJM, van Gool WA. Neuroinflammation – An early event in both the history and pathogenesis of Alzheimer’s disease. Neurodegen Dis. 2010;7:38–41. doi: 10.1159/000283480. [DOI] [PubMed] [Google Scholar]
- Flanary BE, Sammons NW, Nguyen C, Walker D, Streit WJ. Evidence that aging and amyloid promote microglial cell senescence. Rejuvenation research. 2007;10:61–74. doi: 10.1089/rej.2006.9096. [DOI] [PubMed] [Google Scholar]
- Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, Eggert K, Oertel W, Banati RB, Brooks DJ. In vivo imaging of microglial activation with [C-11](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol Dis. 2006;21:404–412. doi: 10.1016/j.nbd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Wu MD, Shaftel SS, Kyrkanides S, La Ferla FM, Olschowka JA, O’Banion MK. Sustained Interleukin-1β overexpression exacerbates tau pathology despite reduced amyloid burden in an Alzheimer’s mouse model. J Neurosci. 2013;33:5053–5064. doi: 10.1523/JNEUROSCI.4361-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Dobrzanski P, Stoehr JD, Wenk GL. Chronic neuroinflammation in rats reproduces components of the neurobiology of Alzheimer’s disease. Brain Res. 1998;780:294–303. doi: 10.1016/s0006-8993(97)01215-8. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Galons JP, Wenk GL. Quantitative volumetric analysis of brain magnetic resonance imaging from rat with chronic neuroinflammation and correlation with histology. Exp Neurol. 2000;165:347–354. doi: 10.1006/exnr.2000.7469. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Lynch MA, Vraniak PD, Wenk GL. Chronic brain inflammation results in cell loss in the entorhinal cortex and impaired LTP in perforant path-granule cell synapses. Exp Neurol. 2002;341:336–341. doi: 10.1006/exnr.2002.7966. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Vraniak P, Wenk GL. The effects of a novel NSAID upon chronic neuroinflammation are age dependent. Neurobiol Aging. 1999;20:305–313. doi: 10.1016/s0197-4580(99)00028-7. [DOI] [PubMed] [Google Scholar]
- Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23:837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- Heneka MT, O’Banion MK, Terwel D, Kummer MP. Neuroinflammatory processes in Alzheimer’s disease. J Neural Trans. 2010;117:919–947. doi: 10.1007/s00702-010-0438-z. [DOI] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrup K. Reimagining Alzheimer’s disease- an age-based hypothesis. J Neurosci. 2010;30:16755–16762. doi: 10.1523/JNEUROSCI.4521-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson P, Meara J. Risk and incidence of dementia in a cohort of older subjects with Parkinson’s disease in the United Kingdom. Mov Disord. 2004;19:1043–1049. doi: 10.1002/mds.20216. [DOI] [PubMed] [Google Scholar]
- Hughes TA, Ross HF, Musa S, Bhattacherjee S, Nathan RN, Mindham RH, Spokes EG. A 10-year study of the incidence of and factors predicting dementia in Parkinson’s disease. Neurol. 2000;54:1596–1602. doi: 10.1212/wnl.54.8.1596. [DOI] [PubMed] [Google Scholar]
- Imamura K, Hishikawa N, Sawada M, Nagatsu T, Yoshida M, Hashizume Y. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropath. 2003;106:518. doi: 10.1007/s00401-003-0766-2. [DOI] [PubMed] [Google Scholar]
- Kamer AR, Craig RG, Pirraglia E, Dassanayake AP, Norman RG, Boylan RJ, Nehorayoff A, Glodzik L, Brys M, de Leon MJ. TNF-α and antibodies to periodontal bacteria discriminate between Alzheimer’s disease patients and normal subjects. J Neuroimmunol. 2009;216:92–97. doi: 10.1016/j.jneuroim.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Joh TH. Matrix metalloproteinases, new insights into the understanding of neurodegenerative disorders. Biomolec Therapeut. 2012;20:133–143. doi: 10.4062/biomolther.2012.20.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC. Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. J Neurosci. 1995;15:1835–1853. doi: 10.1523/JNEUROSCI.15-03-01835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoutsakos JMS, Muthen BO, Breitner JC, Lyketsos CG ADAPT Res Team. Effects of non-steroidal anti-inflammatory drug treatments on cognitive decline vary by phase of pre-clinical Alzheimer disease: findings from the randomized controlled Alzheimer’s Disease Anti-inflammatory Prevention Trial. Int J Geriat Psychiat. 2012;27:364–374. doi: 10.1002/gps.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JM, Kalehua AN, Muth NJ, Calhoun ME, Jucker M, Hengemihle JM, Ingram DK, Mouton PR. Stereological analysis of astrocyte and microglia in aging mouse hippocampus. Neurobiol Aging. 1998;19:497–503. doi: 10.1016/s0197-4580(98)00088-8. [DOI] [PubMed] [Google Scholar]
- Madhavan L, Daley BF, Paumier KL, Collier TJ. Transplantation of subventricular zone neural precursors induces an endogenous precursor cell response in a rat model of Parkinson’s disease. J Comp Neurol. 2009;515:102–115. doi: 10.1002/cne.22033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677e686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Marchalant Y, Brothers HM, Norman GJ, Karolina K, DeVries C, Wenk GL. Cannabinoids attenuate the effects of aging upon neuroinflammation and neurogenesis. Neurobiol Dis. 2009;34:300–307. doi: 10.1016/j.nbd.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Marchalant Y, Cerbai F, Brothers H, Wenk GL. Cannabinoid receptor stimulation is anti-inflammatory and improves memory in old rats. Neurobiol Aging. 2008;29:1894–1901. doi: 10.1016/j.neurobiolaging.2007.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalant Y, Rosi S, Wenk GL. Anti-inflammatory property of the cannabinoid agonist WIN-55212–2 in a rodent model of chronic brain inflammation. Neurosci. 2007;144:1516–1522. doi: 10.1016/j.neuroscience.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton ID, Banner SJ, Ince PG, Piggott NH, Fray AE, Thatcher N, Home CH, Shaw PJ. Expression of the glial glutamate transporter EAAT2 in the human CNS: an immunohistochemical study. Brain Res Molec Brain Res. 1997;52:17–31. doi: 10.1016/s0169-328x(97)00233-7. [DOI] [PubMed] [Google Scholar]
- Mrak RE, Griffin WS. Interleukin-1, neuroinflammation and Alzheimer’s disease. Neurobiol Aging. 2001;22:903–908. doi: 10.1016/s0197-4580(01)00287-1. [DOI] [PubMed] [Google Scholar]
- Nam JH, Park KW, Park ES, Lee YB, Lee HG, Baik HH, Kim YS, Maeng S, Park J, Jin BK. Interleukin-13/−4-induced oxidative stress contributes to death of hippocampal neurons in A beta(1–42)-treated hippocampus in vivo. Antiox Redox Signal. 2012;16:1369–1383. doi: 10.1089/ars.2011.4175. [DOI] [PubMed] [Google Scholar]
- Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiat. 2006;63:530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parajuli B, Sonobe Y, Kawanokuchi J, Doi Y, Noda M, Takeuchi H, Mizuno T, Suzumura A. GM-CSF increases LPS-induced production of proinflammatory mediators via upregulation of TLR4 and CD14 microglia. J Neuroinflam. 2012;9:268. doi: 10.1186/1742-2094-9-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez F, Helmer C, Foubert-Samier A, Auriacombe S, Dartigues JF, Tison F. Risk of dementia in an elderly population of Parkinson’s disease patients: a 15-year population-based study. Alz Dement. 2012;8:463–469. doi: 10.1016/j.jalz.2011.09.230. [DOI] [PubMed] [Google Scholar]
- Perrella O, Guerriero M, Izzo E, Soscia M, Carrieri PB. Interleukin-6 and granulocyte macrophage-CSF in the cerebrospinal fluid from HIV infected subjects with involvement of the central nervous system. Arq Neuro-Psiquiatr. 1992;50:180–182. doi: 10.1590/s0004-282x1992000200008. [DOI] [PubMed] [Google Scholar]
- Persson M, Brantefjord M, Hansson E, Ronnback L. Lipopolysaccharide increases microglial GLT-1 expression and glutamate uptake capacity in vitro by a mechanism dependent on TNF-alpha. Glia. 2005;51:111–120. doi: 10.1002/glia.20191. [DOI] [PubMed] [Google Scholar]
- Pierson E, Simmons SB, Castelli L, Goverman JM. Mechanisms regulating regional localization of inflammation during CNS autoimmunity. Immunol Rev. 2012;248:205–215. doi: 10.1111/j.1600-065X.2012.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prow NA, Irani DN. The inflammatory cytokine, interleukin-1 beta, mediates loss of astroglial glutamate transport and drives excitotoxic motor neuron injury in the spinal cord during acute viral encephalomyelitis. J Neurochem. 2008;105:1276–1286. doi: 10.1111/j.1471-4159.2008.05230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi S, Ramirez-Amaya V, Hauss-Wegrzyniak B, Wenk GL. Chronic brain inflammation leads to a decline in hippocampal NMDA R1 receptors. J Neuroinflamm. 2004;1:12–18. doi: 10.1186/1742-2094-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi S, Ramirez-Amaya V, Vazdarjanova A, Esperanza E, Larkin P, Fike JR, Wenk GL, Barnes CA. Accuracy of hippocampal network activity is disrupted by neuroinflammation: rescue by memantine. Brain. 2009;132:2464–2486. doi: 10.1093/brain/awp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi S, Ramirez-Amaya V, Vazdarjanova A, Worley PF, Barnes CA, Wenk GL. Neuroinflammation alters the hippocampal pattern of behaviorally induced Arc expression. J Neurosci. 2005;25:723–731. doi: 10.1523/JNEUROSCI.4469-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rota E, Bellone G, Rocca P, Bergamasco B, Emanuelli G, Ferrero P. Increased intrathecal TGF-beta1, but not IL-12, IFN-gamma and IL-10 levels in Alzheimer’s disease patients. Neurol Sci. 2006;27:33–39. doi: 10.1007/s10072-006-0562-6. [DOI] [PubMed] [Google Scholar]
- Santello M, Volterra A. TNFα in synaptic function: switching gears. Trends Neurosci. 2012;35:638–647. doi: 10.1016/j.tins.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Shi N, Kawano Y, Tateishi T, Kikuchi H, Osoegawa M, Ohyagi Y, Kira J. Increased IL-13-producing T cells in ALS: Positive correlations with disease severity and progression rate. J Neuroimmunol. 2007;182:232–235. doi: 10.1016/j.jneuroim.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Smith JA, Das A, Ray SK, Banik NL. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull. 2012;87:10–20. doi: 10.1016/j.brainresbull.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman L. A brief history of TH17, the first major revision in the TH1/TH2 hypothesis of T cell-mediated tissue damage. Nature Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Xue QS. Life and death of microglia. J Neuroimm Pharmacol. 2009;4:371–379. doi: 10.1007/s11481-009-9163-5. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Braak H, Xue QS, Bechmann I. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer’s disease. Acta Neuropathol. 2009;118:475–85. doi: 10.1007/s00401-009-0556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudduth TL, Schmitt FA, Nelson PT, Wilcock DM. Neuroinflammatory phenotype in early Alzheimer’s disease. Neurobiol Aging. 2013;34:1051–1059. doi: 10.1016/j.neurobiolaging.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkowski E, Wallin A, Regland B, Blennow K, Tarkowski A. Local and systemic GM-CSF increase in Alzheimer’s disease and vascular dementia. Acta Neurol Scand. 2001;103:166–174. doi: 10.1034/j.1600-0404.2001.103003166.x. [DOI] [PubMed] [Google Scholar]
- Wenk GL, Hauss-Wegrzyniak B. Animal models of chronic neuroinflammation as a model of Alzheimer’s Disease. In: Bondy S, Campbell A, editors. Inflammatory Events in Neurodegeneration. Scottsdale, AZ: Prominent Press; 2001. pp. 83–87. [Google Scholar]
- Wenk GL, Hauss-Wegrzyniak B, Willard LB. Pathological and biochemical studies of chronic neuroinflammation may lead to therapies for Alzheimer’s disease. In: Patterson P, Kordon C, Christen Y, editors. Research and Perspectives in Neurosciences: Neuro-Immune Neurodegenerative and Psychiatric Disorders and Neural Injury. Heidelberg: Springer-Verlag; 2000. pp. 73–77. [Google Scholar]