Abstract
Objective: To investigate the clinicopathological characteristics, prognosis, pathology, and differential diagnosis of LPM by analyzing our experience and reviewed relevant literature. We also postulated the necessity of postoperative adjuvant therapy. Methods: 19 patients with LPM underwent surgical treatment from 2007 through 2010 in our department. The clinical charts of the patients, including surgical, histological, and follow-up records, as well as imaging studies, were analyzed retrospectively. Other 43 cases searched from the literature were also included, so that 62 LPM cases were summarized and reviewed together. Results: The summarized 62 patients comprised 30 males and 31 females aged 9 years to 79 years (40.7±18.3 years). The most common locations were convexity, skull base, para-sagittal and cervical canal. Multiple or diffuse lesions were found in 8 cases. There were 13 patients had peripheral blood abnormalities (21%). One-third of the cases had moderate to severe peritumoral brain edema. Thirty-eight patients had total resection, 12 patients not specified while 12 received subtotal resection or only biopsy. MIB-1 was available in 24 cases and a third of them were higher than 3%. Follow-up more than 3 year was only completed in 19/62 cases. Seven cases suffered recurrence and two of them died after 2 years of operation. Conclusion: LPM is a very rare benign variant of intracranial meningioma. Both lesions and hematological abnormalities have a predilection for younger individuals. Preoperative diagnosis of this subtype of meningioma is still difficult. Surgical resection is the primary treatment option, and supportive care for those not totally removed is very important, because the recurrence rate for this subtype is rather low. However, the massive infiltration of lymphocytes and plasma cells in LPMs are still controversial and the long-term follow-ups are needed. Radiotherapy is not recommended, and hormonal or immune-inhibitor therapy might be helpful.
Keywords: Diagnosis, differential diagnosis, edema, lymphoplasmacyte-rich meningioma, prognosis
Introduction
Meningiomas are divided into 3 grades and 15 subtypes according to the 2007 World Health Organization (WHO) classification of tumors of central nervous system (CNS) [1]. Lympho-plasmacyte-rich meningioma (LPM), which was first reported by Banerjee and Blackwood in 1971 [2], is a Grade Isubtype of intracranial meningiomas which has been adapted to the WHO classification in 1993 and ever since. The estimated incidence mentioned by Moradi is less than 1% of meningiomas [3]. LPM features prominent lymphoplasmacytic infiltrates that often over-shadow the inconspicuous meningothelial component, whose atypical clinical course mimics an intracranial inflammatory condition [1]. Nevertheless, its origin, whether neoplastic or inflammatory, is still controversial.
In addition, the number of reported LPM cases in the literature has been insufficient to identify the clinical features and prognosis for differentiation. In this study, we presented our experience on LPM and reviewed relevant literatures in the past three decades to shed light on the clinicopathological characteristics, prognosis, and differential diagnosis of LPM, and to study the necessity of postoperative adjuvant therapy.
Methods
In this retrospective study, all patients (over 14 years old) were diagnosed as LPM by the 2007 WHO classification criteria for tumors of CNS. The patients received surgical treatment at Neurosurgical department, Huashan Hospital, Shanghai Medical College, Fudan University, Shanghai, Chinafrom 2007 to 2010. Written informed consent was obtained in compliance with the Human Subjects Institutional Review Boards at Huashan Hospital. The clinical data and images of each case were reviewed by two neurosurgeons (HD Zhu and Q Xie). All the pathological diagnoses were made by 2 senior neuropathologists at the time of surgery and then those pathological slides were re-checked by Prof. Chen H, a senior neuropathologist, between September and November, 2011. Cases with ambiguous pathological findings were excluded. All patients underwent tumor resection for the first time in our hospital.
The published studies used in this review were searched from PubMed online using the key words, “Lymphoplasmacyte-rich (All Fields) and Meningioma (All Fields) and English (lang)” and “Lymphoplasmacyte-rich Meningioma (Title/Abstract) and English (lang)”. The last search was finished on February 20, 2012. We also searched on Google and Chinese Wanfang database. A total of 35 publications found in full texts, 28 articles in English [2,4-30], 2 online cases [31,32], and 4 articles in Chinese [33-36], which were double-checked by two neurosurgeons (HD Zhu and Q Xie). Six articles in English were exclude [24-29], because of missing detailed clinical information, or merely about pathological differentiation, such as intracranial inflammatory pseudotumor or idiopathic hypertrophic pachymeningitis. Among the remaining relevant publications, 43 reported LPM cases from 1971 to 2011 were presented (Table 1). In this paper, a thorough review of the literatures on LPM plus the 19 cases from Huashan Hospital, a summary of 62 cases, was undertaken to illustrate this very rare entity.
Table 1.
Summary of all previously documentedand present lymphoplasmacyte-rich meningioma cases
| No. | Reference | Sex/Age | Location | Edema | Peripheral blood abnormalities | Treatment | MIB-1 | Outcome (KS) |
|---|---|---|---|---|---|---|---|---|
| 1 | Banerjee, 1971 [2] | M/71 | Right anterior cranial fossa | N/A | Normal | Total Resection | N/A | 8 months, no recurrence |
| 2 | Horten, 1979 [4] | F/15 | Right CPA | N/A | Hypergammaglobulinemia | Subtotal resection | N/A | 2 years, dead |
| 3 | F/53 | Left foramen magnum | N/A | Normal | Total resection | N/A | 7 months, no recurrence | |
| 4 | F/52 | Bilateral frontal parafalco- | N/A | Normal | Total resection | N/A | 5 months, no recurrence | |
| 5 | M/32 | Right frontal Parasagittal | N/A | Normal | Total resection | N/A | 5 years, no recurrence | |
| 6 | M/10 | Bilateral CPA | N/A | N/A | Exploration of right side | N/A | 2 months, no change | |
| 7 | Stam, 1980 [5] | M/59 | Left occipital falcotentorial | N/A | Hyperglobulinemia | Simpson G 2 | N/A | N/A |
| 8 | Mirra, 1983 [6] | F/11 | Multiple (Frontal, temporal, CPA) | +, hyperostotic | Normal | Resection | N/A | 1 year, no recurrence |
| 9 | F/39 | Anterior C3-7 (recurrent) | N/A | N/A | Laminectomy | N/A | 3 years, recurrence | |
| 10 | Gi, 1990 [7] | M/14 | Left CPA (recurrent) | - | Hypergammaglobulinemia | 40 Gy radiation after 1st op. Resection | N/A | Left cerebellar angioblastic meningioma, 6 years recurrent. This time, 1 year recurrent again |
| 11 | Loiseau, 1995 [8] | F/12 | Left parietal parasagittal | +++ | Hypergammaglobulinemia/polyclonal gammopathy | Simpson G 2 | N/A | 11 months, no recurrence |
| 12 | Yamaki, 1997 [9] | M/22 | Diffuse planum sphenoidale, parasellar to C5 | - | Normal | Partial Resection and laminectomy | N/A | 7 years, recurrent at C2-3, but others regressed |
| 13 | F/24 | Multiple bilateral (Clivus to spinal) | - | Normal | Subtotal resection | N/A | 8 years, enlargement, 10 months later spontaneous regression | |
| 14 | Mizushima, 1997 [10] | F/64 | Bilateral parietal parasagittal | +++ | Normal | Simpson G 3 | N/A | 4 years, no recurrence |
| 15 | Katayama, 1997 [11] | F/47 | Left parietal | +++ | N/A | Simpson G 2 | N/A | 1 month, well |
| 16 | Yoneyama, 1999 [12] | M/36 | Left parietal | - | One patient with anemia | Resection | 5 years, recurrence | |
| 17 | F/41 | Left frontal | ++ | Resection | N/A | 5 years, no recurrence | ||
| 18 | M/22 | Left parafalco- | ++ | Resection | N/A | 8 months, no recurrence | ||
| 19 | Miklossy, 2000 [31] | M/43 | Left parietal | - | N/A | Total resection | N/A | N/A |
| 20 | Pittella, 2001 [13] | M/47 | Multiple, C1-6 (recurrent) | - | N/A | Supportive | N/A | 2 years after 1st operation, dead |
| 21 | Bruno, 2004 [14] | F/45 | Left frontal | - | Hypergammaglobulinemia/anemia | Simpson G 3 | N/A | 1 year, no recurrence |
| 22 | Guo, 2005 [33] | M/45 | Left mastoid | N/A | N/A | Resection | N/A | N/A |
| 23 | Zheng, 2006 [35] | M/41 | Left temporal | + | Hypergammaglobulinemia/anemia | Resection | N/A | N/A |
| 24 | M/28 | Right frontal | N/A | N/A | Resection | N/A | N/A | |
| 25 | Loh, 2006 [15] | F/22 | Left sphenoid ridge | ++ | Normal | Total Resection | N/A | 24 months, no recurrence |
| 26 | Hirunwiwatkul, 2007 [16] | M/24 | Bilateral diffuse lesion from sphenoid planum to foramen magnum | + | Normal | Biopsy, then prednisone & azathioprine | 7% | 6 months, very slight reduction |
| 27 | Nohara, 2007 [17] | M/12 | Right CPA | - | Normal | Simpson G 3 | 5% | Atypical? 2 years, no recurrence |
| 28 | Ghosal, 2007 [18] | M/25 | Right frontal | +++ | N/A | Simpson G 1 | N/A | Atypical? 1 year, no recurrence |
| 29 | Wang, 2008 [34] | F/70 | Left cerebellar | N/A | Hypergammaglobulinemia/anemia | Total resection | N/A | 5 months, no recurrence |
| 30 | F/54 | Multiple right parietal | N/A | Normal | Total resection | N/A | 7 months, no recurrence | |
| 31 | Pandey, 2008 [32] | ?/55 | Multiple | N/A | N/A | Resection | N/A | 1 year, no recurrence |
| 32 | Avninder, 2008 [19] | F/22 | Foramen magnum | - | N/A | Total resection | N/A | 1 year, no recurrence |
| 33 | Bodi, 2008 [20] | F/72 | Right frontal lobe | +++ | Normal | Resection | 3% | 6 months, no recurrence |
| 34 | Zhu, 2009 [36] | M/38 | Frontal-parietal | N/A | N/A | Total resection | <3% | N/A |
| 35 | Liu, 2011 [21] | M(4), F(3), average 38 yrs, 9-63 yrs | 2 Frontotemporal, 1 Parafalx, 1 Sphenoid ridge, 1 Occipital parasagittal, 1 CPA, 1 Lateral ventricle | All seven cases displayed apparent heavy peritumoral brain edema | Three patients showed polyclonal immunoglobulinopathy, whose hematopoietic systems were completely backe to normal after surgery. | Resection | N/A | Over 1-4 years, one case recurred in the first years after operation, no recurrence was found in the twoyears following the second surgery in this case |
| 36 | ||||||||
| 37 | ||||||||
| 38 | ||||||||
| 39 | ||||||||
| 40 | ||||||||
| 41 | ||||||||
| 42 | Kanno, 2011 [22] | F/55 | Right jugular foramen | - | Normal | Total resection | N/A | 9 months, no recurrence |
| 43 | Nakayama, 2011 [23] | F/37 | Right frontal | +++ | Normal | Simpson G 1 | <1% | 1 year, no recurrence |
| 44 | Present case 1 | F/20 | Left occipital | + | Normal | Simpson G 2 | 5% | Lost follow-up |
| 45 | Present case 2 | F/28 | Right frontoparietal | +++ | Hyperglobulinemia/anemia | Simpson G 2 | 1% | 63 months, no recurrence, KS100 |
| 46 | Present case 3 | M/42 | Left temporal | - | Normal | Simpson G 3 | <1% | 59 months, no recurrence, KS100 |
| 47 | Present case 4 | F/48 | Left frontotemporal | N/A | Normal | Simpson G 1 | 2% | Lost follow-up |
| 48 | Present case 5 | M/77 | Right frontal falx | - | Normal | Simpson G 1 | 2% | 50 months, no recurrence, KS70 |
| 49 | Present case 6 | M/46 | Right frontal | + | Normal | Simpson G 1 | <1% | 50 months, no recurrence, KS100 |
| 50 | Present case 7 | M/46 | Left occipital | - | Normal | Simpson G 2 | <1% | 50 months, no recurrence, KS100 |
| 51 | Present case 8 | M/57 | Right frontoparietal | + | Normal | Simpson G 1 | <1% | 49 months, no recurrence, KS100 |
| 52 | Present case 9 | F/58 | Left sphenoid ridge | ++ | Normal | Simpson G 4 | 3% | 46 months, no recurrence, KS90 |
| 53 | Present case 10 | M/79 | Right frontal falx | - | Normal | Simpson G 1 | 2% | 45 months, no recurrence, KS100 |
| 54 | Present case 11 | F/63 | Right frontal falx | + | Normal | Simpson G 1 | 5% | 41 months, no recurrence, KS100 |
| 55 | Present case 12 | M/71 | Right frontal falx | ++ | Normal | Simpson G 2 | 2% | 39 months, no recurrence, KS80 |
| 56 | Present case 13 | F/46 | Right frontal | ++ | Normal | Simpson G 2 | 5% | 39 months, no recurrence, KS100 |
| 57 | Present case 14 | M/25 | C1-2 | - | Anemia | Simpson G 1 | <1% | 37 months, no recurrence, KS100 |
| 58 | Present case 15 | F/57 | Foramen magnum to C1-4 | - | Normal | Simpson G 4 | 3% | 33 months, no recurrence, KS40 |
| 59 | Present case 16 | F/21 | Left cerebellum & tentorium | - | Anemia | Simpson G 1 | 4% | 32 months, no recurrence, KS100 |
| 60 | Present case 17 | F/40 | Right frontoparietal | + | Normal | Simpson G 2 | 10% | 31 months, no recurrence, KS60 |
| 61 | Present case 18 | M/51 | C3-4 | - | Normal | Simpson G 2 | 5% | 28 months, no recurrence, KS90 |
| 62 | Present case 19 | F/24 | Left frontoparietal | +++ | Normal | Simpson G 2 | 3% | 24 months, no recurrence, KS100 |
CPA: Cerebellopontine angle; N/A: Not available; C: Cervical. Simpson G: Simpson Grade; KS: Karnofsky score. Peritumoral brain edema was easily observed in this study and several criteria were applied. “N/A” was information unavailable. No edema was marked as “-”. Slight edema was marked as “+”, which represented that the volume of tumor was bigger than edema. Moderate edema was as “++”, which represented similar volume of tumor and edema. Severe edema was marked as “+++”, which represented the volume of edema was bigger than the tumor.
Results
Clinical presentation
Among our own 19 patients (From case #44-62, Table 1), the age of diagnosis ranged from 20 to 79 years old (47.3±18.2 years old) and the male to female ration was approximately 1 (9:10). While among the summed 62 LPM cases, the age of diagnosis ranged from 9 to 79 years old (40.7±18.3 years old) and the male to female ratio was approximately 1 (30:31, except one not specified), mainly from 30 to 50 years old (Figure 1).
Figure 1.

Age and sex distribution of lymphoplasmacyte-rich meningioma. Among these 62 LPM cases, the age of diagnosis ranged from 9 years to 79 years (40.7±18.3 years) and the male to female ratio was approximately 1 (30:31, except one not specified), mainly from 30 to 50 years old.
The most common initial symptom was headache, and others were hemiparesis, seizure, vomiting, dizziness, visual disturbance, and slurred speech. Only 3 recurrent cases with diagnosis of other subtype meningiomas had been reported, but lacking detailed information [6,7,13]. The most common locations were convexity (25/62, frontal 12, parietal 7, temporal 2, occipital 4), cranial base (23/62, anterior 1, middle 6, posterior 16), para-sagittal or para-falco (12/62), and cervical canal (7/62). Occasionally, the lesions located at the lateral ventricle (1/62), or mastoid cavity (1/62). Multiple or diffuse lesions were found in 8 cases (Case #8, #12, #13, #20, #26, #30, #31, and #58), which were more severe and inclined to recur.
Preoperative laboratory tests disclosed peripheral blood abnormalities in 14 cases (14/62), including hyperglobulinemia (11 cases) and microcytic hypochromic anemia (3 cases) (Table 1), which may gradually return to normal after surgery. There were only 3 cases of our 19 patients had peripheral blood abnormalities before operation, including hyperglobulinemia (1 case) and microcytic hypochromic anemia (2 cases).
Radiological findings
In our 19 patients study, magnetic resonance imaging (MRI) data and CT scan image were available in 14 and 11 cases respectively. Most of lesions exhibited low to iso-intense signal on T1-weighted images and iso- to high intense signal on T2-weighted images, usually with homogenous enhancement after gadolinium administration; dural tail signs were observed in some cases. These cases had all been diagnosed as meningiomas but not LPM before operation. In addition, 50% (7/14) of the lesions exhibited peritumoral edema, and 21.4% (3/14) showed irregular or heterogeneous enhancement. Peritumoral or intra-tumoral cystic changes were observed in 21.4% (3/14) of the patients (Figures 2, 3). For those tumors with this atypical pattern, only one (1/6) had been correctly diagnosed. The other four (4/6) lesions had been diagnosed as gliomas or metastatic tumors. One special lesion that showed lobular and irregular enhancement with a relatively clear boundary had been diagnosed as a teratoma (Case #46) (Figure 2).
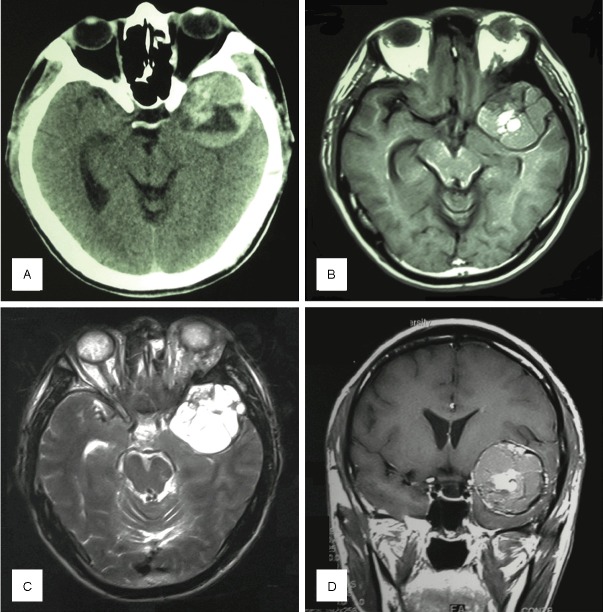
Figure 2.
Case #3: A 42-year-old male with left temporal lobe lesion. A: CT showed a high-density mass at the left temporal lobe and middle cranial fossa. B: The tumor’s signal was iso- to hyper-intense on T2WI image and the tumor was lobulated. C: The tumor’s signal was much higher on T1WI images. D: Contrast-enhanced T1WI coronal image showed a lobular and slightly irregular enhanced mass with relatively clear boundary.
Figure 3.

Case #19: A 24-year-old female with left fronto-parietal para-sagittal LPM. A: The tumor’s signal was iso- to hypo-intense on T1WI image. B: The tumor’s signal was much higher on T2WI images with severe peritumoral brain edema. C: Contrast-enhanced T1WI coronal image showed a lobular significantly enhanced mass with rather clear boundary. The midline was shifted due to the lesion and edema. D: The tumor was totally resected and contrast-enhanced T1WI coronal image showed the tumor was totally resected and the midline was restored.
Among the summed 62 LPM cases, peritumoral brain edema was easily observed and several criteria were applied. “N/A” was information unavailable. No edema was marked as “-”. Slight edema was marked as “+”, which represented that the volume of tumor was bigger than edema. Moderate edema was as “++”, which represented similar volume of tumor and edema. Severe edema was marked as “+++”, which represented the volume of edema was bigger than the tumor. Description of peritumoral brain edema was recorded in 75.8% (47/62) of the cases, in which 38.3% (18/47) were no edema, 17.0% (8/47) were slight edema, 44.7% (21/47) were moderate to severe edema. Nonetheless, the preoperative diagnosis of LPM was still hard to establish because peritumoral brain edema might be seen in other types of meningioma, such as secretory, microcystic, angiomatous and chordoid meningiomas.
Nohara described that 18F-2-fluoro-2-deoxy-D-glucose positron emission tomography ([18F]FDG PET) revealed relatively high uptake ratio (tumor/contralateral cortex) of 1.38 [17], which suggested a possible way for differentiation.
Surgical results
Based on the Simpson Grading Criteria, 16 of our 19 patients had total tumor resection (Simpson Grade I or II), while 3 patients had subtotal resection or biopsy (Simpson Grade III or IV). Meanwhile, 38 of the summed 62 patients received total tumor resection (Simpson Grade I or II). The extent of surgery was not specified in about 12 cases. And for other 12 cases, a complete resection was not possible because of the tumor tightly adhering to the dura of skull base, wrapping around ICA or on ventral medulla, so they received subtotal resection or biopsy (Simpson Grade III, IV or V). After operation, patients were treated with supportive care. One patient received prednisone and azathioprine after tumor biopsy, and the tumor slightly reduced on 6 months follow-up [16].
Pathological findings
Microscopic examination of the surgically resected tumor revealed that polygonal or spindle tumor cells, some of which were bundled in whirlpool arrangements, psammoma bodies, and vascular tissues were always observed in the inflammatory background with diffused infiltration of plasma cells and lymphocytes (Figure 4A). Immunohistochemical assays revealed that tumors were positive for epithelial membrane antigen (EMA) (Figure 4B) and vimentin (Figure 4C) in all 19 cases, and lymphocytes andplasma cells were positive forleukocyte common antigen (LCA) (Figure 4D). Glial fibrillary acidic protein (GFAP) was negative in most cases.
Figure 4.

Histology of LPM (Case #15). A: Hematoxylin & Eosin staining (H&E, ×400): H&E showed the chronic inflammatory cells infiltrated in the lobules of meningothelial cells. B: Epithelial Membrane Antigen staining (EMA, ×400): Both the tumor cells and the reactive plasma cells were positive for EMA. C: Vimentin staining (Vim, ×400): The meningothelial components were Vim positive. D: Leukocyte common antigen staining (LCA, ×400): Lymphocytes andplasma cells were positive for LCA. E: MIB-1 index: 3%. F: Progesterone receptor staining (PR, ×400): The meningothelial components were PR positive. Scale bar = 200 μm.
MIB-1 was studied in all specimens of our 19 patients, ranging from <1% to 10%, in which 9 cases higher than 3%. For the 62 patients, MIB-1 was available in 24 cases, ranging from <1% to 10%, while a third of them were higher than 3% (Table 1).
Follow-up
In our 19 cases, the prognoses and postoperative neurological condition of the patients were determined by outpatient services and telephone contact. The latest follow-ups were made on August 20, 2012. There were 2 patients lost contact although both recovered well after operations. The mean follow-up period for 17 patients was 42.1±10.8 months (ranging from 24-63 months). No patient suffered recurrence or died during the follow-up period, all patients had a good outcome, except one patient (Case #15) with the tumor adhering to the medulla was subjected to tracheotomy after the operation, and the Karnofsky score was 40%.
In summed 62 patients, follow-up more than 1 year was achieved in 34/62 cases. Follow-up over 3 years were achieved in 19/62 cases. Three patients received postoperative radiotherapy, and only one case was reported had a very slight reduction in the size of the mass [16]. Among these 62 patients, seven patients suffered recurrence and two of them died after 2 years of operation.
Discussion
Lymphoplasmacyte-rich meningioma is a rare WHO Grade I subtype of meningiomas. LPM usually occurred in young and middle age patients without sex predominance, which was different from other common types of meningiomas. The most common locations were convexity, skull base, para-sagittal and cervical canal, while some of them were multiple or diffuse lesions. More than 20% patients had peripheral blood abnormalities, andone-third of the cases had moderate to severe peritumoral brain edema. Incidence of tumor related recurrence or death was rather low although total resection was achieved only in about 60% cases, and MIB-1 in a third of cases was higher than 3%.
The clinical and biological features of patients with LPM vary in terms of the tumor location and biological behavior of the tumor cells. Most patients had nonspecific clinical manifestations. Headache, hemiparesis, seizure, vomiting, dizziness, visual disturbance or loss, dyscalculia, dysgraphia and slurred speech might occur according to the location of the tumor. Horten et al. reported a patient that presented recurrent subarachnoid hemorrhage as the first clinical manifestation [4]. The natural history of LPM is often over one year, while a few might take place suddenly, due to the location of lesions or inflammatory cell infiltration and edema [21].
Systemic hematological abnormalities such as hyperglobulinemia and iron refractory anemia had been documented in some patients with LPM [4-8,14,21]. This special phenomenon had only been reported in 2 subtypes of meningiomas, LPM (WHO grade I) and chordoid meningioma (WHO grade II) [2]. Among the reviewed 62 cases, 13 patients (21%) showed hematological abnormalities, i.e., polyclonal immunoglobulinopathy and/or iron refractory anemia. Among them, eight patients had detailed information, except for the report of Liu and Yoneyama, and 37.5% (3/8) of them were adolescents, showing a marked predilection for a younger age group. Gi reported a 14-year-old boy with a recurrent meningioma and a high serum immunoglobulin level that immediately diminished after surgery and increased again with tumor recurrence [7]. It is believed that the plasmacyte infiltrates led to blood abnormalities and that the inflammatory cell reaction in this type of meningioma is secondary, presumably reflecting an unusual immunological response of the host individual [8]. The significance of hematological abnormalities, however, in this type of meningioma is still unclear, and more cases and long-term follow-ups are needed. Kepes reported that chordoid meningioma is more likely to be accompanied with LPM and causes Castleman’s syndrome in children and young adults (delayed somatic and sexual development, hepatosplenomegaly, iron refractory hypochromic microcysticanemia, and bone marrow plasmocytosis with dysgammaglobulinemia) [30]. Thus we speculate that the particular histological manifestation and high proportion of dysgammaglobulinemia and/or iron refractory hypochromic microcysticanemia in LPM patients may imply similar pathogenesis of these two variants.
The typical characters of LPM on MRI were mainly lower-isointense on T1-weighted images andhyperintense on T2-weighted images, with strongly homogenousenhancement after the administration of gadolinium; obvious peritumoral brain edema and dural tail signswere usually present. Sometimes, however, cystic components [11], and heterogeneous enhancement can also be encountered, rendering preoperative diagnosis difficult.
The correlation between histological type and peritumoral brain edema had been reported by several authors. Osawa and coworkers classified meningothelial, transitional, and fibrous meningiomas as ‘‘WHO grade I common type’’ and the other subtypes of grade I as ‘‘WHO grade I uncommon type’’ [37]. They reported that the uncommon type had obviously higher edema indices than the common type (69% vs 34%). Recently, Park et al. found that IL-6 protein localizedin the cytoplasm of the tumor cells, and was detected in 75% of edematous meningiomas, and indicated that IL-6 expression may contribute to the formation of brain edema in meningiomas [38]. We speculate that the massive infiltration of lymphocytes and plasma cells may play a central role in the development of brain edema associated with LPM.
Recent studies showed that most meningiomas with slow growth rate, especially for WHO grade I types, might exhibit a moderately increased glucose metabolism and thus may not reliably be detected with 18F-FDG PET [39]. Usually, 18F-FDG uptake for meningiomas was similar to normal gray matter or lower [40]. However, Nohara [17] demonstrated a case of LPM with a relative higher 18F-FDG uptake ratio (tumor/contralateral cortex) of 1.38 by PET scan. It has been reported that 18F-FDG uptake may also be increased by inflammation, infection, or granulomas. This characteristic is surprisingly consistent with the inflammatory feature of LPM, but its clinical potential has to be further evaluated.
Nakayama et al. reported a LPM wherein inflammatory cells were extensively distributed in the subarachnoid and Virchow-Robin spaces [23]. The atypical MRI features, including linear enhancement spreading in the brain parenchyma can be attributed to the diffused infiltration of the inflammatory cells. Therefore, they suggested that the cells did not solely target the meningiomatous component, and that certain soluble factors may have induced the diffuse infiltration. Overall, the typical pattern of meningioma addition to peritumoral brain edema may be the MRI features of LPM, but preoperative diagnosis is still very difficult because of its rare and variable nature. Furthermore, the proliferating plasma cells and lymphocytes in LPM are not neoplastic but probablyan immune reaction of hosts [8,10,11]. This theory is further strengthened by the disappearance of hematological abnormalities after complete removal of the tumor and their reappearance with relapse [7].
The use of staining for EMA and vimentin is reportedly useful in indicating the meningothelial origin of the tumor, and differentiates LPM from other intracranial lesions such as IHP, chordoid meningioma inflammatory pseudotumors, and inflammatory fibrous histiocytomas [4-7,11,13,15]. In our study, polygonal or spindle tumor cells that exhibited a strong immunoreactivity for EMA and vimentin in the inflammatory background with diffused infiltration of plasma cells and lymphocytes were typical of LPM. However, Yamaki reported two cases with skull base meningeal masses mainly consisting of the inflammatory component with small foci of meningiomatous tissue that showed spontaneous regression and recurred at multiple sites during follow-up periods of 7 and 12 years, respectively [9]. Given that the peculiar biological behavior is more typical of intracranial granulomas than of meningiomas, another hypothesis is put forward: the meningothelial nests embedded within the inflammatory infiltrate may be hyperplastic rather than neoplastic in nature.
Given that many intracranial masses may resemble LPM, differential diagnosis including IHP, chordoid meningioma, inflammatory pseudotumor, and meningeal sinus histiocytosis mimicking lymphoplasmacyte-rich meningioma should be considered.
The pathological findings of IHP usually include thickened fibrotic dura with marked infiltration of lymphocytes and plasma cells, occasionally accompanied with small islands of meningothelial proliferation [27,41,42], mimicking those of LPM. Localized nodular lesion can sometimes rule out this diagnosis in that IHP usually shows diffused lamellar thickenings or plaque-like features [22].
Chordoid meningiomas often contain regions that are histologically similar to chordoma, with cords or trabeculae of eosinophilic, vacuolated cells inan abundant mucoid matrix background [2]. Detailed histological studies can aid the differential diagnosis.
Inflammatory pseudotumor (plasma cell granuloma) should also be considered in the differential diagnosis, because it shows clinical, radiological, and surgical similarities with LPM [29]. Inflammatory pseudotumor is a distinct clinical entity often observed in the lung or visceral organs [43-45], and featured by varying numbers of myofibroblastic spindle cells admixed with a lymphoplasmacytic infiltrate. Among reported primary intracranial inflammatory pseudotumor, meningeal lesions are the most frequently encountered although they are rare [29,46-50]. When plasma cell granuloma occurs as a meningeal mass, definitive histological differentiation from lymphoplasmacyte-rich meningioma ispossible only if the meningothelial component is absent in the former [46].
Therefore, comprehensive evaluation with radiologic and pathological findings is necessary to establish an accurate diagnosis of LPM. And we postulated if LPM is suspected or diagnosed, total resection should be achieved only under safe conditions, especially for skull base, multiple or diffuse tumors, and radiotherapy is not recommended. Hormonal or immune-inhibitor therapy might be helpful due to the mechanism of this disease, but this still needs further observation.
Conclusions
Lymphoplasmacyte-rich meningioma is a very rare benign variant of intracranial meningioma, featured by massive inflammatory cell infiltration and often a less proportion of meningothelial tumorous elements. Surgical resection is still the primary treatment, and most of the patients have relatively favorable clinical outcomes. However, the mechanisms underlying the formation of lesions and the massive infiltration of lymphocytes and plasma cells in LPM are still unclear, and longer follow-up time was needed. Radiotherapy is not recommended, and hormonal or immune-inhibitor therapy might be helpful.
Acknowledgement
We appreciate Prof. Hong Chen and Prof. Yin Wang for providing pathological pictures and re-checking all the pathological slides in this article. We also appreciate Prof. Kang Zheng, Prof. Yongfei Wang, Prof. Weimin Bao, MD, and Prof. Bojie Yang for their operations on some patients in this study and allowance to access them in follow-ups.
This work was supported by Grant No.0841-1965100 and 12JC1401800 from Science and Technology Commission of Shanghai Munici-pality, and both Grant No.30872675 and No.30901549 from National Natural Science Foundation of China.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerjee AK, Blackwood W. A subfrontal tumour with the features of plasmocytoma and meningioma. Acta Neuropathol. 1971;18:84–88. doi: 10.1007/BF00684477. [DOI] [PubMed] [Google Scholar]
- 3.Moradi A, Semnani V, Djam H, Tajodini A, Zali AR, Ghaemi K, Nikzad N, Madani-Civi M. Pathodiagnostic parameters for meningioma grading. J Clin Neurosci. 2008;15:1370–1375. doi: 10.1016/j.jocn.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Horten BC, Urich H, Stefoski D. Meningiomas with conspicuous plasma cell-lymphocytic components: a report of five cases. Cancer. 1979;43:258–264. doi: 10.1002/1097-0142(197901)43:1<258::aid-cncr2820430137>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 5.Stam FC, van Alphen HA, Boorsma DM. Meningioma with conspicuous plasma cell components. A histopathological and immunohistochemical study. Acta Neuropathol. 1980;49:241–243. doi: 10.1007/BF00707113. [DOI] [PubMed] [Google Scholar]
- 6.Mirra SS, Tindall SC, Check IJ, Brynes RK, Moore WW. Inflammatory meningeal masses of unexplained origin. An ultrastructural and immunological study. J Neuropathol Exp Neurol. 1983;42:453–468. doi: 10.1097/00005072-198307000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Gi H, Nagao S, Yoshizumi H, Nishioka T, Uno J, Shingu T, Fujita Y. Meningioma with hypergammaglobulinemia. Case report. J Neurosurg. 1990;73:628–629. doi: 10.3171/jns.1990.73.4.0628. [DOI] [PubMed] [Google Scholar]
- 8.Loiseau H, Pedespan JM, Vital A, Marchal C, Vital C, Cohadon F. Lymphoplasmacyte-rich meningioma in a child. Case report. J Neurosurg. 1995;83:1075–1079. doi: 10.3171/jns.1995.83.6.1075. [DOI] [PubMed] [Google Scholar]
- 9.Yamaki T, Ikeda T, Sakamoto Y, Ohtaki M, Hashi K. Lymphoplasmacyte-rich meningioma with clinical resemblance to inflammatory pseudotumor. Report of two cases. J Neurosurg. 1997;86:898–904. doi: 10.3171/jns.1997.86.5.0898. [DOI] [PubMed] [Google Scholar]
- 10.Mizushima M, Tanaka Y, Kawakami S, Hori T, Ohama E. Lymphoplasmacyte-rich meningioma: a case report with histological and immunohistochemical studies. Brain Tumor Pathol. 1997;14:59–62. doi: 10.1007/BF02478870. [DOI] [PubMed] [Google Scholar]
- 11.Katayama S, Fukuhara T, Wani T, Namba S, Yamadori I. Cystic lymphoplasmacyte-rich meningioma--case report. Neurol Med Chir (Tokyo) 1997;37:275–278. doi: 10.2176/nmc.37.275. [DOI] [PubMed] [Google Scholar]
- 12.Yoneyama T, Kasuya H, Kubo O, Hori T. [Lymphoplasmacyte-rich meningioma: a report of three cases and a review of the literature] . No Shinkei Geka. 1999;27:383–389. [PubMed] [Google Scholar]
- 13.Pittella JE, da Costa CC, Giannetti AV, Perpetuo FO. October 2000: a 47 year old man with long-standing progressive tetraparesis. Brain Pathol. 2001;11:261–262. [PubMed] [Google Scholar]
- 14.Bruno MC, Ginguene C, Santangelo M, Panagiotopoulos K, Piscopo GA, Tortora F, Elefante A, De Caro ML, Cerillo A. Lymphoplasmacyte rich meningioma. A case report and review of the literature. J Neurosurg Sci. 2004;48:117–124. discussion 124. [PubMed] [Google Scholar]
- 15.Loh JK, Hwang SL, Tsai KB, Kwan AL, Howng SL. Sphenoid ridge lymphoplasmacyte-rich meningioma. J Formos Med Assoc. 2006;105:594–598. doi: 10.1016/S0929-6646(09)60156-X. [DOI] [PubMed] [Google Scholar]
- 16.Hirunwiwatkul P, Trobe JD, Blaivas M. Lymphoplasmacyte-rich meningioma mimicking idiopathic hypertrophic pachymeningitis. J Neuroophthalmol. 2007;27:91–94. doi: 10.1097/WNO.0b013e31806773a5. [DOI] [PubMed] [Google Scholar]
- 17.Nohara H, Furuya K, Kawahara N, Iijima A, Yako K, Shibahara J, Kirino T. Lymphoplasmacyte-rich meningioma with atypical invasive nature. Neurol Med Chir (Tokyo) 2007;47:32–35. doi: 10.2176/nmc.47.32. [DOI] [PubMed] [Google Scholar]
- 18.Ghosal N, Furtado SV, Santosh V, Sridhar M, Hegde AS. Co-existing fibrous dysplasia and atypical lymphoplasmacyte-rich meningioma. Neuropathology. 2007;27:269–272. doi: 10.1111/j.1440-1789.2007.00753.x. [DOI] [PubMed] [Google Scholar]
- 19.Avninder S, Gupta V, Sharma KC. Lymphoplasmacyte-rich meningioma at the foramen magnum. Br J Neurosurg. 2008;22:702–704. doi: 10.1080/02688690802040645. [DOI] [PubMed] [Google Scholar]
- 20.Bodi I, Hortobagyi T, Buk S. A 72-year-old woman with right frontal extra-axial mass. Brain Pathol. 2008;18:279–282. doi: 10.1111/j.1750-3639.2008.00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu JL, Zhou JL, Ma YH, Dong C. An analysis of the magnetic resonance imaging and pathology of intracal lymphoplasmacyte-rich meningioma. Eur J Radiol. 2012;81:968–973. doi: 10.1016/j.ejrad.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 22.Kanno H, Nishihara H, Hara K, Ozaki Y, Itoh T, Kimura T, Tanino M, Tanaka S. A case of lymphoplasmacyte-rich meningioma of the jugular foramen. Brain Tumor Pathol. 2011;28:341–345. doi: 10.1007/s10014-011-0048-y. [DOI] [PubMed] [Google Scholar]
- 23.Nakayama Y, Watanabe M, Suzuki K, Usuda H, Emura I, Toyoshima Y, Takahashi H, Kawaguchi T, Kakita A. Lymphoplasmacyte-rich meningioma: a convexity mass with regional enhancement in the adjacent brain parenchyma. Neuropathology. 2012;32:174–179. doi: 10.1111/j.1440-1789.2011.01236.x. [DOI] [PubMed] [Google Scholar]
- 24.Aizawa T, Sato T, Tanaka Y, Kishimoto K, Watanabe M, Kokubun S. Intramedullary plasma cell granuloma in the cervicothoracic spine. Case report. J Neurosurg. 2002;97:235–238. doi: 10.3171/spi.2002.97.2.0235. [DOI] [PubMed] [Google Scholar]
- 25.Bertalanffy A, Roessler K, Koperek O, Gelpi E, Prayer D, Neuner M, Knosp E. Intraventricular meningiomas: a report of 16 cases. Neurosurg Rev. 2006;29:30–35. doi: 10.1007/s10143-005-0414-5. [DOI] [PubMed] [Google Scholar]
- 26.Hartmann C, Sieberns J, Gehlhaar C, Simon M, Paulus W, von Deimling A. NF2 mutations in secretory and other rare variants of meningiomas. Brain Pathol. 2006;16:15–19. doi: 10.1111/j.1750-3639.2006.tb00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hosler MR, Turbin RE, Cho ES, Wolansky LJ, Frohman LP. Idiopathic hypertrophic pachymeningitis mimicking lymphoplasmacyte-rich meningioma. J Neuroophthalmol. 2007;27:95–98. doi: 10.1097/WNO.0b013e318064c53a. [DOI] [PubMed] [Google Scholar]
- 28.Kitai R, Sato K, Kubota T, Kabuto M, Kawano H, Kobayashi H, Tsuji T. Meningeal sinus histiocytosis mimicking lymphoplasmacyte-rich meningioma. Case report. J Neurosurg. 1996;84:1051–1054. doi: 10.3171/jns.1996.84.6.1051. [DOI] [PubMed] [Google Scholar]
- 29.Suri V, Shukla B, Garg A, Singh M, Rishi A, Sharma MC, Sarkar C. Intracranial inflammatory pseudotumor: report of a rare case. Neuropathology. 2008;28:444–447. doi: 10.1111/j.1440-1789.2008.00870.x. [DOI] [PubMed] [Google Scholar]
- 30.Kepes JJ, Chen WY, Connors MH, Vogel FS. “Chordoid” meningeal tumors in young individuals with peritumoral lymphoplasmacellular infiltrates causing systemic manifestations of the Castleman syndrome. A report of seven cases. Cancer. 1988;62:391–406. doi: 10.1002/1097-0142(19880715)62:2<391::aid-cncr2820620226>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 31.Miklossy J, Kopniczky Z, Uske A, Delacraz F, Chaubert P, Porchet F. Case 225--Epileptic seizures. Neuropathology. 2000 [PubMed] [Google Scholar]
- 32.Pandey R, Khurana P, Sethi S, Suneja S, Shankar J. Lymphoplasmacyte-rich Meningioma Mimicking Tubercular Meningitis: A Case Report. The Internet Journal of Radiology. 2008;9 [Google Scholar]
- 33.Guo SP, Yang L. Lymphoplasmacyte-rich meningioma in the mastoid: a case report and review of the literature. Chinese Journal of Otology. 2005;3:301–303. [Google Scholar]
- 34.Wang XM, Li FC, Lu MS, Hou QY, Zhang MY, Chen F. Lymphoplasmoacyte-rich meningioma: A report of two cases and review of literature. Central China Medical Journal. 2008;32:254–265. [Google Scholar]
- 35.Zheng HG, Sun BC, Yang YQ, Jiang YH. Lymphoplasmacyte-rich meningioma: a study of clinicopathological and Immunohistochemical results [Chinese] . J Diag Pathol. 2006;13:72–73. [Google Scholar]
- 36.Zhu XM, Ji LL, Xu K. Lymphoplasmoacyte-rich meningioma: A Case Report [Chinese] . Journal of Qiqihar Medical College. 2009;30 [Google Scholar]
- 37.Osawa T, Tosaka M, Nagaishi M, Yoshimoto Y. Factors affecting peritumoral brain edema in meningioma: special histological subtypes with prominently extensive edema. J Neurooncol. 2013;111:49–57. doi: 10.1007/s11060-012-0989-y. [DOI] [PubMed] [Google Scholar]
- 38.Park KJ, Kang SH, Chae YS, Yu MO, Cho TH, Suh JK, Lee HK, Chung YG. Influence of interleukin-6 on the development of peritumoral brain edema in meningiomas. J Neurosurg. 2010;112:73–80. doi: 10.3171/2009.4.JNS09158. [DOI] [PubMed] [Google Scholar]
- 39.Cornelius JF, Langen KJ, Stoffels G, Hanggi D, Sabel M, Jakob Steiger H. Positron emission tomography imaging of meningioma in clinical practice: review of literature and future directions. Neurosurgery. 2012;70:1033–1041. doi: 10.1227/NEU.0b013e31823bcd87. discussion 1042. [DOI] [PubMed] [Google Scholar]
- 40.Liu RS, Chang CP, Guo WY, Pan DH, Ho DM, Chang CW, Yang BH, Wu LC, Yeh SH. 1-11C-acetate versus 18F-FDG PET in detection of meningioma and monitoring the effect of gamma-knife radiosurgery. J Nucl Med. 2010;51:883–891. doi: 10.2967/jnumed.109.070565. [DOI] [PubMed] [Google Scholar]
- 41.Kleiter I, Hans VH, Schuierer G, Marienhagen J, Hau P, Schutz H, Bogdahn U, Steinbrecher A. Intraventricular cytarabine in a case of idiopathic hypertrophic pachymeningitis. J Neurol Neurosurg Psychiatry. 2004;75:1346–1348. doi: 10.1136/jnnp.2003.024653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reichenthal E, Rubinstein AB, Shevach I, Cohen ML. Meningioma presenting at a site of a previously aspirated brain abscess. Acta Neurochir (Wien) 1991;109:142–144. doi: 10.1007/BF01403010. [DOI] [PubMed] [Google Scholar]
- 43.Bahadori M, Liebow AA. Plasma cell granulomas of the lung. Cancer. 1973;31:191–208. doi: 10.1002/1097-0142(197301)31:1<191::aid-cncr2820310127>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 44.Fisch AE, Brodey PA. Plasma cell granuloma of kidney. Urology. 1976;8:89–91. doi: 10.1016/0090-4295(76)90066-2. [DOI] [PubMed] [Google Scholar]
- 45.Pettinato G, Manivel JC, Insabato L, De Chiara A, Petrella G. Plasma cell granuloma (inflammatory pseudotumor) of the breast. Am J Clin Pathol. 1988;90:627–632. doi: 10.1093/ajcp/90.5.627. [DOI] [PubMed] [Google Scholar]
- 46.Hsiang J, Moorhouse D, Barba D. Multiple plasma cell granulomas of the central nervous system: case report. Neurosurgery. 1994;35:744–747. doi: 10.1227/00006123-199410000-00024. [DOI] [PubMed] [Google Scholar]
- 47.Hausler M, Schaade L, Ramaekers VT, Doenges M, Heimann G, Sellhaus B. Inflammatory pseudotumors of the central nervous system: report of 3 cases and a literature review. Hum Pathol. 2003;34:253–262. doi: 10.1053/hupa.2003.35. [DOI] [PubMed] [Google Scholar]
- 48.Jeon YK, Chang KH, Suh YL, Jung HW, Park SH. Inflammatory myofibroblastic tumor of the central nervous system: clinicopathologic analysis of 10 cases. J Neuropathol Exp Neurol. 2005;64:254–259. doi: 10.1093/jnen/64.3.254. [DOI] [PubMed] [Google Scholar]
- 49.Lui PC, Fan YS, Wong SS, Chan AN, Wong G, Chau TK, Tse GM, Cheng Y, Poon WS, Ng HK. Inflammatory pseudotumors of the central nervous system. Hum Pathol. 2009;40:1611–1617. doi: 10.1016/j.humpath.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 50.Yavuzer D, Dalbayrak S, Oz B, Yilmaz M, Akansel G. Intracranial inflammatory pseudotumor: case report and review of the literature. Clin Neuropathol. 2010;29:151–155. doi: 10.5414/npp29151. [DOI] [PubMed] [Google Scholar]