Abstract
Stressful experiences during early-life can modulate the genetic programming of specific brain circuits underlying emotional and cognitive aspects of behavioral adaptation to stressful experiences later in life. Although this programming effect exerted by experience-related factors is an important determinant of mental health, its outcome depends on cognitive inputs and hence the valence an individual assigns to a given environmental context. From this perspective we will highlight, with studies in rodents, non-human primates and humans, the three-hit concept of vulnerability and resilience to stress-related mental disorders, which is based on gene-environment interactions during critical phases of perinatal and juvenile brain development. The three-hit (i.e., hit-1: genetic predisposition, hit-2: early-life environment, and hit-3: later-life environment) concept accommodates the cumulative stress hypothesis stating that in a given context vulnerability is enhanced when failure to cope with adversity accumulates. Alternatively, the concept also points to the individual’s predictive adaptive capacity, which underlies the stress inoculation and match/mismatch hypotheses. The latter hypotheses propose that the experience of relatively mild early-life adversity prepares for the future and promotes resilience to similar challenges in later-life; when a mismatch occurs between early and later-life experience, coping is compromised and vulnerability is enhanced. The three-hit concept is fundamental for understanding how individuals can either be prepared for coping with life to come and remain resilient or are unable to do so and succumb to a stress-related mental disorder, under seemingly identical circumstances.
Keywords: vulnerability, resilience, stress, adaptation, three hit, cumulative stress, mismatch, stress inoculation, Cortisol, corticosterone, HPA axis, developmental programming, epigenetics, genetic predisposition, early life environment, later life environment, rats, primates, humans
1. Introduction
It is well-documented that during critical periods of brain development stressful experiences can modulate the functioning of specific circuits that underlie adult emotional and cognitive functioning, and behavior (Taylor, 2010). These effects exerted by stress are mediated by the autonomic nervous system and the hypothalamus – pituitary – adrenal (HPA) -axis. Hence, decades of research have been devoted to understand how the mediators of these systems such as adrenaline and other biogenic amines, neuropeptides and hormones can modulate brain function and behavior for life (Maras and Baram, 2012). These programming effects induced by the stress mediators suggest enduring changes in the transcriptome underlying DNA methylation and chromatin modifications. In fact, recent research has revealed that the mediators and their receptors of the HPA-axis themselves are prime targets of epigenetic modification. This includes corticotrophin releasing hormones (CRH), vasopressin and their receptors, and also the receptors for circulating adrenal corticoids in the limbic-cortical circuitry (Murgatroyd and Spengler, 2012). Although rapid progress has been made in unraveling this epigenetic mechanism induced by early experience, it is still unresolved how this modulation of programming by the environment precisely occurs (Franklin et al., 2012).
Here we focus on the HPA-axis and its glucocorticoid endproducts, i.e. Cortisol and corticosterone in human and non-human primates and only corticosterone in rodents, collectively called CORT. These hormones coordinate and synchronize daytime and sleep-related events, regulate the organism’s response to stress and facilitate adaptation (de Kloet et al., 2005). We ask the question how CORT action during stress can change from a protective into a harmful signal by focusing on the environmental programming effects powered by the hormone.
To address this question this review reflects the content of the Presidential Symposium held under the title “Resilience and vulnerability: adaptations to early-life adversity outcome” at the 42nd ISPNE Conference in the New York Academy of Sciences. Thus we first briefly discuss, for rodents, monkeys and humans, the development of the pup’s /infant’s HPA-axis at perinatal life when they are particularly susceptible to environmental influences. We briefly review rodent (Daskalakis et al., 2011a) and non-human primate (Parker et al., 2006) animal models, that are appropriate to exploit gene-environment interactions at these critical periods in the perspective of later-life outcome. We focus on a mechanism involving excitatory neurotransmission and stress mediators (Champagne et al., 2008; Bagot et al., 2012a). Next this analysis is projected to the human situation. We conclude with the presentation of the three-hit concept of vulnerability and resilience. This concept unifies the currently dominant viewpoints that have precipitated as the cumulative stress hypothesis, and the stress inoculation and match/mismatch hypotheses.
2. Early-life stress in animal models
Mother-pup interactions during the first postnatal period have been studied the last 60 years in rodents and in non-human primates to evaluate the significance of early-life experiences for individual differences in adult neuroendocrine activity, emotional responses, cognitive performance and behavior. Some researchers studied the impact of experimental early-life manipulations such as neonatal handling and maternal separation (Levine, 2005), and others examined the outcome of naturally occurring variations of maternal care (Meaney, 2001). Also significant progress has been made with studies using monkeys exposed to moderate postnatal stressors (Parker et al., 2006).
2.1 Stress hypo-responsive period (SHRP) and the short-term effects of maternal absence
The SHRP is a developmental period from postnatal days (pnd) 1–10 in mice and pnd 3–14 in rats, in which the elevation of CORT is attenuated after exposure to mild stressors, that otherwise trigger a profound response in the adult animals (Schapiro et al., 1962; Sapolsky and Meaney, 1986; Levine, 2001; Schmidt et al., 2003a). The human HPA-axis development is in concordance with that of rats (even though rats are prematurely born) since the axis is not yet fully developed at birth and CORT secretion manifests a comparable SHRP during postnatal months 6 to 12. During this period human babies are dependent on their caregivers for normal development, and adverse experiences in this period can have a long-lasting impact (Gunnar and Quevedo, 2007).
There are phylogenic differences, however, in HPA-axis development between rodents and primates. Detailed studies with the New World monkeys, the marmosets, have shown basal hyperactivity of the HPA-axis in neonates, but without an apparent circadian rhythmicity. From infancy to adulthood the pattern of stress responsiveness remains similar. Hence in view of its neonatal hypercorticism the marmoset is an interesting animal model to study the outcome of early manipulations of the stress system under a high steroid titer as compared to rodents, humans and other non-human primates (Pryce et al., 2002).
For the neonate’s hypo-responsiveness to stressors a lot of factors have been implicated like: adrenal inhibition/insensitivity (Stanton et al., 1988; Chatelain et al., 1989; Walker, 1995; Okimoto et al., 2002), enhanced glucocorticoid receptor (GR) mediated negative feedback (Walker et al., 1986; van Oers et al., 1998a; Schmidt et al., 2005), inhibition of the brain renin-angiotensin system (Muret et al., 1992; Liebl et al., 2009), CRHR1 and CRHR2 receptors functions (Eghbal-Ahmadi et al., 1998; Schmidt et al., 2003b; Fenoglio et al., 2005; Schmidt et al., 2006a), central α2 adrenoreceptor control of pituitary adrenocorticotropic hormone (ACTH) release (Grino et al., 1994) and central action of metabolic factors (Proulx et al., 2001; Salzmann et al., 2004; Schmidt et al., 2006b; Schmidt et al., 2008) as well as immaturity of the hypothalamus-pituitary connection (Suchecki et al., 1993) Yet, the most proximal cause for the transient hypo-responsiveness to stress is a strongly reduced responsiveness of the adrenals to ACTH (Rosenfeld et al., 1991; Okimoto et al., 2002). In fact, during the SHRP, the central stress response after a challenge does not translate into adrenal corticosterone secretion.
Prolonged (≥8h) maternal absence (i.e., maternal separation; MS), implemented within the SHRP, causes neonatal rodents to emerge from the SHRP and to display elevated basal and stress-induced levels of CORT (Stanton et al., 1988). These observations showed that a central mechanism required to elicit an endocrine response following stress can be effective already early in development (Walker et al., 1986; Walker et al., 1990; Walker et al., 1991; van Oers et al., 1998a). Various components of the dam’s behavior (mostly feeding and tactile stimulation) seem capable of inhibiting or dampening the MS-induced responsiveness of the HPA-axis, but do so at different levels. Feeding during maternal absence acts as an inhibitory factor to the neonate’s basal and stress-induced adrenal activity (Stanton et al., 1988; Stanton and Levine, 1990; Suchecki et al., 1993; van Oers et al., 1998b; Schmidt et al., 2002; Schmidt et al., 2006b), and stroking (e.g., 45sec every 8h) can inhibit the activation of pituitary ACTH release as well as of excitability of the limbic and hypothalamic brain areas over the 24h period (Suchecki et al., 1993; van Oers et al., 1998b; Zhang et al., 2002).
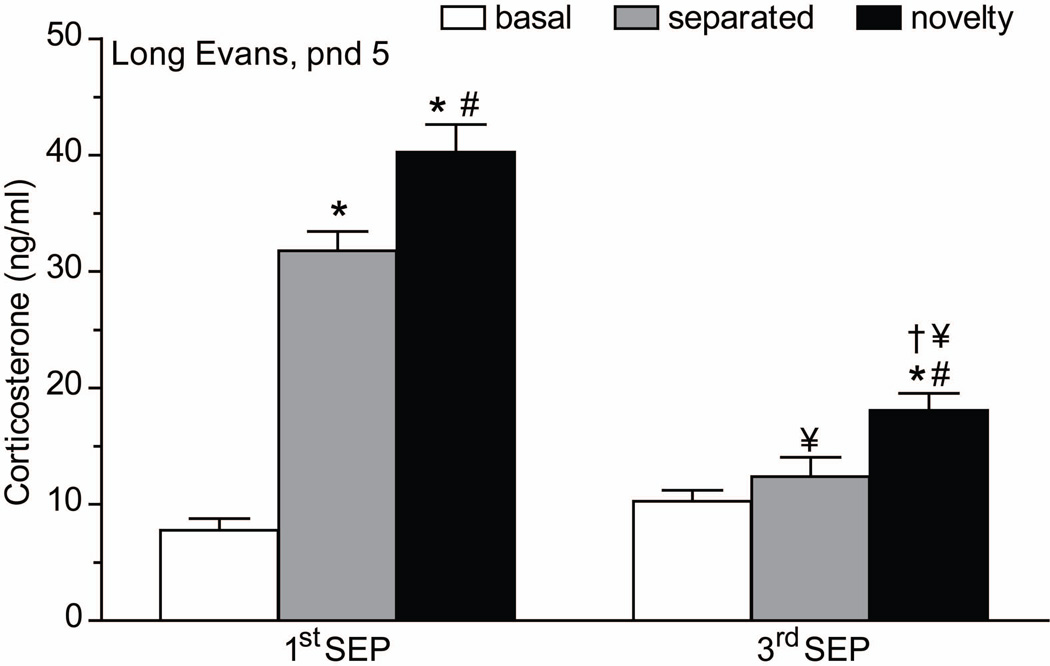
Short MS for periods of 3min to 3h are insufficient to increase basal CORT-secretion. If repeated daily, this MS procedure is capable to induce sensitization of the CORT stress-response in parallel with adrenal growth (D’Amato et al., 1992; Huot et al., 2002; Knuth and Etgen, 2005; Levine et al., 1991; McCormick et al., 1998; Schmidt et al., 2004; Vazquez and Akil, 1992). Extension of MS to periods beyond 3h causes increased basal and stress-induced CORT levels. If the 8h-MS procedure was repeated the next day this rise in basal CORT is abolished, however. The explanation of this habituation or adaptation of the pup’s HPA-axis to the experience of repeated MS is probably psychological. Apparently, already after one episode of maternal absence the pup has learned to predict the return of the mother if it is exposed to MS the next day, a phenomenon that occurs irrespective of genotype or species. Single MS causes, however, also increased adrenal sensitivity to heterotypic stressors and exogenous ACTH (Levine et al., 1991; Rosenfeld et al., 1992; Okimoto et al., 2002). Furthermore, we show that even though basal levels of ACTH and CORT remain low if separations are repeated, the pups stay on alert and retain the ability to respond to stressors with increases in ACTH and CORT levels (Enthoven et al., 2008; Daskalakis et al., 2011a) (Figure 1).
Figure 1. The newborn rat’s stress system readily habituates to repeated and prolonged maternal separation, while continuing to respond to stressors.
Corticosterone (ng/ml) blood plasma levels measured at basal conditions (“basal”; white bars), after 8h of maternal separation (“separated”; gray bars) or 8h of maternal separation with an additional 30min of exposure to novelty (“novelty”; black bars). First separation rat pups (“1st SEP”) had no previous history of treatments. Third separation rat pups (“3rd SEP”) were exposed to 8h of maternal separation on postnatal day (pnd) 3 and 4. For the experiment, Long Evans litters were used. Data represented mean ± SEM. Significance level was set at p < 0.05. * vs. basal, # vs. separated levels of the same treatment group, † vs. basal of 1st HOME SEP, ¥ vs. correspondent value of 1st HOME SEP. n = 14–16 per time point of each treatment group. Adapted from Daskalakis et al., 2011a
We studied post-MS stress sensitivity using two different MS-conditions, in which the rat pups were either with their siblings together in the home cage (HOME-SEP) or isolated and housed individually for 8h in a novel cage (NOVEL-SEP). Our studies showed that habituation of basal CORT secretion to repeated-MS occurs irrespective of the home or novel context (Daskalakis et al., 2011a). However, the two separation procedures demonstrated a different response to a subsequent 30min novelty stressor. Rat pups separated in a novel environment adapt and do therefore not respond to the novelty stressor, while the repeated “home separation” animals do. The adrenal ACTH receptor (MCR2) varies in parallel with these changes in adrenal responsiveness; the home separation group displayed increased MCR2 expression (Daskalakis et al., 2011a).
It is well established in squirrel monkeys that brief maternal separations elicit increases in plasma cortisol levels in infants (Mendoza et al., 1978; Levine et al., 1989). These separations evoke also species-typical distress peep-calls and locomotor agitation. Recovery of baseline measures is achieved after each social reunion (Coe et al., 1983; Stanton and Levine, 1985; Hennessy, 1986). The neuroendocrine responses are fairly resistant to habituation, as they have been shown to persist after as many as 80 intermittent maternal separations (Hennessy, 1986). The social environment has been shown to be an important mediator of the magnitude of this adrenocortical activation (Levine, 2000). Infants removed from both their mother and home cage and then housed in a novel environment exhibit larger cortisol increases as compared to infants whose mothers were removed, but which remained in the familiar home cage surrounded by known cage-mates. The increase in cortisol was larger when the infants were housed in isolation rather than with familiar conspecifics, and a further increase was noted when housed with non-familiar conspecific (Levine and Wiener, 1988).
The above findings suggest that in the newborn familiar signals can buffer against stress-induced HPA-axis activation and that rodents and monkeys respond comparably in this respect. The question is whether this type of buffering occurs in human neonates or infants. The studies by Gunnar and Cheatam (2003) suggest that the quality of care of children and the degree of attachment are capable to attenuate stress-induced Cortisol secretion suggesting the existence of a SHRP (Gunnar and Cheatham, 2003). Childers’s attachment security and cortisol-reactivity depend on both genetic factors and the early environment (Bakermans-Kranenburg and van Ijzendoorn, 2007; Ouellet-Morin et al., 2008). Interestingly, infants in transition to day-care, which is an unfamiliar setting, show higher cortisol response to the daily MS in comparison with infants that stay at home and show the first signs of habituation after more than a week (Ahnert et al., 2004).
2.2 Long-term effects of early-life manipulations
Adversity of the maternal environment, either experimentally-induced or naturally-occurring, can predict alterations in social behavior, susceptibility to drugs of abuse, learning & memory processes and sensorimotor gating of the adult offspring (Liu et al., 1997; Ellenbroek et al., 1998; Ellenbroek and Cools, 2000; Liu et al., 2000; Plotsky et al., 2005; Zhang et al., 2005; Veenema et al., 2006; Parent and Meaney, 2008; van der Veen et al., 2008; Veenema and Neumann, 2009; Oomen et al., 2010; Lukas et al., 2011). Originally, maternal behavior per se was proposed to mediate the effect of early-life experience (Meaney, 2001). However, the exclusivity of the role of maternal behavior was questioned, since the pup’s environmental input appears to be equally important (Macri et al., 2004; Macri and Wurbel, 2006; Tang et al., 2006).
Early handling(EH; i.e., brief MS of 3min–15min the first two or three weeks of life) results in increased maternal care in the reunion periods and across the day (Liu et al., 1997; Pryce et al., 2001; Macri et al., 2004; Claessens et al., 2012) and can lead to better coping with stressful situations later in life (Levine, 1957; DeNelsky and Denenberg, 1967; Meaney and Aitken, 1985). Handled offspring exhibiting good performance in cognitive tasks, show reduced anxiety and display a lower endocrine response to a mild stressor in comparison with non-handled rats (Levine, 1967; Meaney and Aitken, 1985; Meerlo et al., 1999; Kosten et al., 2012). Interestingly, apart from maternal care, the individual components of the early-handling procedure (i.e., maternal absence, novelty exposure, tactile stimulation) also contribute to the observed phenotype (Tang, 2001; Daskalakis et al., 2009b). Taken together, the early handing experiments demonstrated that exposure to a mild predictable stressor early in life can be beneficial (Macri and Wurbel, 2006; Claessens et al., 2011). This finding was replicated in other species (non-human primates; see below) and served as the basis for the stress-inoculation theory (Parker and Maestripieri, 2011).
MS(i.e., prolonged MS of typically 3h to 8h daily during the first two or three weeks of life): Maternally separated pups, as compared to handled individuals or controls (animal facility reared), display as adults increased stress-responsiveness, enhanced emotional reactivity and impaired cognitive performance. MS also results in enhanced acoustic startle, and lower prepulse or latent inhibition. The MS outcome is a function of strain and gender of the animals, the frequency and duration of the separation as well as the pup’s age, time point within the light cycle and ambient temperature at the moment the separation occurs (Lehmann and Feldon, 2000; Claessens et al., 2011; Kosten et al., 2012).
The environmental context such as the housing in groups or in isolation, inside the home cage or in a novel environment, is important (Lehmann and Feldon, 2000; Ruedi-Bettschen et al., 2004). We found that HOME-SEP animals maintain a remarkably enhanced stress-induced CORT response throughout life. The enhancement of their CORT response occurs in the face of attenuated stress-induced ACTH-release indicating a profound adrenal hyper-responsiveness. The endocrine phenotype of the NOVEL-SEP offspring also persisted into adulthood as judged from its enhanced stress-induced amygdala c-Fos activation and ACTH-release (Daskalakis et al., 2009a; Daskalakis, 2011).
In Table 1, the long-term effects of the NOVEL and HOME-SEP MS paradigms on a wide spectrum of behavioral endophenotypes are summarized. Our data suggest that the social and environmental context in which the adverse early-life experience took place is an important determinant of the long-term outcome of early-life adversity. HOME-SEP rats have behavioral deficits mainly in the cognitive domain (↓ working and emotional memory vs. non-separated controls); NOVEL-SEP rats display behavioral alterations in all domains (↑stereotypy, ↑gating, ↑emotional memory, and negative ↓ social interaction vs. non-separated controls) (Daskalakis et al., 2009a; Daskalakis, 2011; Daskalakis et al., 2012). Apart from its scientific relevance, the difference in the long-term outcome of the two paradigms asks for standardization of methodology in animal studies of early-life adversity. In human studies, early-life history is also increasingly implicated. Very often these studies classify the participants according to the presence or absence of early-life adversity based on self-report, but only to a limited extent the desired contextual information is provided.
Table 1.
Long-term behavioral outcome of maternal separation depends on context.
| Behavioral endophenotypes |
HOME SEP |
NOVEL SEP |
|---|---|---|
| Stereotypy | No change | ↑ |
| Sensorimotor gating | No change | ↓ |
| Working Memory | ↓ | No change |
| Emotional Memory | ↓ | ↑ |
| Social Interaction | No change | ↓ |
Note. Repeatedly separated pups were exposed to 8h–MS on postnatal day 3, 4 & 5 in a home (HOME-SEP; home separated) or novel context (NOVEL-SEP; novel separated) and tested in the behavioral tasks post-weaning. Rats, non-separated as pups (no history of postnatal treatments) served as controls. Based on data from Daskalakis et al., 2009a; Daskalakis, 2011; Daskalakis et al., 2012.
Naturally occurring variations in maternal care
In the absence of any experimental manipulations, there is a considerable variation in maternal care in the rat (Champagne et al., 2003). The frequency with which a dam licks/grooms her pups (LG) is a stable behavioral trait and is normally distributed across dams (Champagne et al., 2003). Pup LG is a major source of tactile stimulation for the neonatal rat that regulates several physiological systems of the neonate (Bagot and Meaney, 2010). There is considerable evidence, mainly from studies using Long Evans rats, for a sustained effect of maternal care on the behavioral and endocrine responses to stress in the offspring. Maternal LG frequency correlates with hippocampus-dependent learning in adulthood. Offspring of High LG mothers more rapidly acquires the location of a hidden platform in the Morris water maze (Liu et al., 2000) and also shows enhanced memory in an object recognition task (Bredy et al., 2003). Low LG offspring displays potentiated acoustic startle responses and deficits in prepulse inhibition (Zhang et al., 2005).
Consistent with enhanced hippocampal-dependent learning and memory, long-term potentiation (LTP) in the hippocampal DG and CA1 areas of High LG offspring is increased compared to Low LG offspring (Bredy et al., 2003; Champagne et al., 2008; Bagot et al., 2009). The high LG offspring, moreover, displays increased spine densities and enhanced dendritic arborization (Champagne et al., 2008; Bagot et al., 2009). Although under low stress conditions the High LG offspring shows enhanced hippocampal-dependent learning and corresponding hippocampal plasticity compared to Low LG offspring, when the High LG animals are exposed to stress or high corticosterone, both measures become compromised (Champagne et al., 2008) (see also section 2.6).
The large variation in maternal care used by this paradigm can be interpreted as variation in maternal strategies and maternal investment, evoked by environmental adversity. In other words, maternal care would be a response to environmental demands and pups’ distress, and its evolutionary goal would be to preserve the fitness of the offspring (Beery and Francis, 2011). Whatever the evolutionary significance might be, the combined exposure to care, moderate environmental challenges and corticosteroids seem to prepare the pup for life to come.
Nesting conditions
Standard nesting conditions may represent an impoverished environment (Levine, 2005; Macri and Wurbel, 2006). Enrichment of the nesting conditions is a way to manipulate maternal care behavior and the pup’s development in a naturalistic way. Two environmental enrichment paradigms studied are the communal nesting (CN) (Branchi, 2009) and the double mothering paradigm (D’Amato et al., 2011). In the former, three females breed and keep pups together, and share care-giving behavior in a single nest from birth to weaning. In the latter, pups are raised by two dams (one or two are lactating; i.e., Lactating/Non-Lactating or Lactating/Lactating).
In both paradigms pups are provided with high levels of maternal care compared to mice reared in standard laboratory conditions, but yet the phenotype is diverging (Branchi, 2009; D’Amato et al., 2011). Paradoxically, CN mice display increased anxiety-related behavior and normal cognitive performance, but enhanced social competences and resilience to social stress (D’Andrea et al., 2007; Branchi, 2009; Branchi et al., 2010). Moreover, this effect depends on interaction with both the peers and the mother (Branchi et al., 2013a). Also, in later-life the vulnerability to psychosocial stressors rather than to physical stressors is attenuated in response to this type of early social enrichment (Branchi et al., 2013b). Mice experiencing double mothering do not differ in their emotional or stress-hormone profile from mice reared by their mothers alone, but do exhibit enhanced cognitive performance (D’Amato et al., 2011).
Opposite effects on maternal care levels (i.e., reduced levels) are achieved by the limited nesting material model where mothers are provided with reduced nesting and bedding material from pnd 2 to 9 of their litter. This manipulation results in inconsistent and fragmented maternal care (Ivy et al., 2008), producing a continuously stressful environment for the offspring that is reflected in high basal CORT levels of the pups and long-lasting HPA-axis hyperactivity and cognitive impairment in hippocampal-dependent cognitive tasks (Rice et al., 2008).
Beyond maternal mediation
The maternal mediation hypothesis might not be the sole mechanism explaining the effects of early-life stress. Instead it is proposed that the combination of the extent of environmental adversity and the maternal repertoire determines the lasting alterations of the offspring’s HPA-axis response (Macri and Wurbel, 2006; Tang et al., 2011). The pup’s stress reactivity is proposed to be a function of the additive effects of the environmental stress and maternal care responsivity to environmental stress, acting as two independent factors (Macri and Wurbel, 2006; Tang et al., 2011). Thus, as noted above enhanced maternal care occurs in response to either maternal perception of environmental stress and demands (Beery and Francis, 2011) or the stress signals produced by the neonate after periods away from the mother (Hofer, 1996; Macri and Wurbel, 2006).
2.3. Long-term effects in non-human primates
Although there is considerable evidence for the enduring effects of stressful early experiences on HPA-axis physiology in adult rodents, surprisingly few studies have examined the outcome of similar early-life stress in adult monkeys. The vast majority of early stress paradigms in monkeys have focused on effects that persist only into late infancy. Nevertheless, data are available from several primate paradigms which have examined the persistent effects of early-life stress on HPA-axis physiology into adolescence and early adulthood. Some of these models are reviewed briefly below.
A primate model of early-life adversity similar to the rodent maternal separation paradigm is the parental deprivation model in common marmosets. This model is unique, because it involves both parents. Common marmoset social ecology is characterized by monogamous breeding pairs and intensive biparental care of young (Pryce, 1996). Infants are in constant (i.e., 24h a day) contact with their parents throughout the first 2–3 weeks of life (Ingram, 1977; Pryce, 1993). To examine the consequences of early stressful experience, Pryce and colleagues exposed marmoset infants to daily parental separations on pnds 2–28 (Dettling et al., 2002). Each infant is exposed to a total of 9h of social deprivation each week, with each session being variable in duration (30–120min per session) and time of day, a feature implemented to increase stressor unpredictability. Control infants are briefly handled on their parents’ backs daily during pnds 2–28. No baseline or stress-induced HPA-axis physiology data are available from this model, but repeated parental separations in monkeys have been shown to induce mild reductions in mineralocorticoid receptor (MR) and GR gene expression in the hippocampus (in CA 1–2 subfield) in late adolescence. These effects are specific to the hippocampus, as no differences in MR or GR gene expression were observed in the prefrontal cortex, other cortical areas, or the hypothalamus (Arabadzisz et al., 2010).
A different developmental outcome occurred in another New World monkey, the squirrel monkey, by using a brief intermittent maternal separation paradigm. This paradigm has been termed the “stress inoculation” protocol. Various separation protocols have been used. We summarize here the results of rearing experiments that occurred between 17–27 weeks of age for the offspring, a developmental period during which young monkeys are becoming independent from their mothers (Boinski and Fragaszy, 1989). In 1h sessions of maternal separation which occurred every week for 10 weeks, the monkeys were maintained in a room different from their colony room, while the control monkeys remained in their home cages (Parker et al., 2004). The stress-inoculated monkeys were less anxious than the non-inoculated controls as appeared from a number of behavioral tests including increased food intake, decreased clinging to the mother and increased exploratory behavior. The stress-inoculated monkeys also displayed reduced stress-induced ACTH and cortisol responses as compared to controls. The findings are robust and replicated in other independent cohorts using slightly different stress inoculation protocols (Lyons et al., 1999; Lyons et al., 2000; Levine and Mody, 2003).
Accordingly, inappropriate stress-induced activation of the infant primate HPA-axis does not necessarily lead to stress hyper-reactivity in adulthood, which is often considered a hallmark for psychopathology. Moreover, in view of the profound glucocorticoid resistance characteristic, the New World monkey’s programming likely involves a mechanism distinct from that in the rodent (Parker et al., 2006).
2.4 Biological basis of early-life programming
Genetic variation in combination with external non-genetic factors has an effect on the regulation and expression of genes influencing protein functions. The biological basis of this is the epigenome. Epigenetics refers to the functionally relevant modifications of the genome that do not involve a change in nucleotide sequence. Such modifications include chemical marks that regulate the transcription of the genome (e.g., DNA-methylation, histone modifications and chromatin remodeling). There is now evidence that environmental events can directly modify the epigenetic state of the genome during sensitive developmental periods, but also - possibly to a lesser extent - in adulthood leading to changes in gene expression and neural function (Fagiolini et al., 2009). These studies define a biological interplay between environmental signals and the genome in the regulation of individual differences in behavior, cognition, and physiology. Some recent findings suggest the intriguing possibility that the epigenetic signature of the environment induced early in life or in adulthood may be transferred through generations (Franklin et al., 2010; Dietz et al., 2011; Rodgers et al., 2013), although the mechanism by which this may occur is unclear. Possible paths of transmission include environmental influences on gametes, gestational development, postnatal care and social context during development (Curley et al., 2009; Drake et al., 2011). Factors influencing environmentally-induced transgenerational transmission of phenotypes can be the gender of the parent, the age of the parent at exposure, parental pathology or psychopathology induced by the exposure (Pembrey et al., 2006; Yehuda, 2011; Champagne, 2013).
The first example of epigenetic programming by early-life experience in the rodent was demonstrated in Long Evans rats by researchers in McGill University using the naturally-occurring variations in the maternal care model. They proved that these variations in maternal care permanently affect the extent of hippocampal GR expression, through epigenetic changes occurring the first week of life. These changes involved DNA methylation changes in a GR promoter region (17 ortholog of human 1F), where NGFI-A binds as transcription factor (Weaver et al., 2004). This lasting modulation of the GR has in turn consequences for HPA-axis activity later in life and reportedly appears linked to hyperactivity in case of the Low LG offspring and hypo-activity of the axis of the High LG offspring. This phenotype can be transmitted in a non-genomic way to the next generation (Liu et al., 1997; Francis et al., 1999). After this seminal example of epigenetic programming, more studies appeared using the various models of early-life adversity to study epigenetic programming of the HPA-axis including its afferent projections areas in hippocampus and prefrontal cortex. In Table 2, we summarize recent rodent literature on the topic. The last years the first monkey and human data on the topic appeared leading to more convergent conclusions on the interaction of early-life adversity with the glucocorticoid-related genes leading to epigenetic modifications observed in brain post-mortem and blood samples (Oberlander et al., 2008; McGowan et al., 2009; Perroud et al., 2011; Radtke et al., 2011; Provencal et al., 2012; Tyrka et al., 2012; Klengel et al., 2013).
Table 2.
Evidence of epigenetic alterations of the HPA-axis in rodent models of early-life adversity
| Early-life stress paradigm |
Species/Strain | DNA methylation | Refs |
|---|---|---|---|
| Early Handling | Rat, Sprague Dawley | Hypothalamus: ↑Crh1 gene promoter | (Korosi et al., 2010; McClelland et al., 2011) |
| (vs. Non Handling) | |||
| Maternal separation | Rat, Sprague Dawley | Hippocampus: Not changed Nr3cl Exon 17 promoter |
(Daniels et al., 2009) |
| (vs. Non Handling or Animal Facility Rearing) |
Mouse, C57BL/6N Mouse, C57BL/6J |
Parvocellular PVN: ↓Avp enhancer Hippocampus: ↑ Avp enhancer, Not changed Nr3cl promoter, ↓Nr4al promoter |
(Murgatroyd et al., 2009) (Kember et al., 2012) |
| Mouse, DBA/2J | Hippocampus: ↑ Avp enhancer, ↑ Nr3cl promoter, Not changed Nr4al promoter |
(Kember et al., 2012) | |
| Natural occurring variations of maternal care |
Rat, Long Evans | Hippocampus: ↑ Nr3cl Exon 17 promoter, ↑Pcdh α, β, and γ gene families regulatory regions, ↑Grml |
(Weaver et al., 2004; Champagne et al., 2006; McGowan et al., 2011; Bagot et al., 2012b; Suderman et al., 2012) |
| (Low LG vs. High LG) | MPOA: ↑ERα Exon lb promoter | ||
| Communal nesting | Mice, CD1 | Hippocampus: Not changed Bdnf promoters 1, 4, 6 and 7. (but ↑ levels of the acetylated form of histone H3) |
(Branchi et al., 2011) |
| (vs. Standard nesting | |||
| Limited access to nesting material |
Rat, Long Evans | PFC: ↑Bdnf (Exon 4 & 9 promoters) | (Roth et al., 2009) |
| (vs. Non Handling) |
Abbreviations: LG (Licking & Grooming), Crh (corticotropin-releasing hormone), Nr3cl (nuclear receptor subfamily 3, group C, member 1; i.e., glucocorticoid receptor), Nr3cl (nuclear receptor subfamily 3, group C, member 1), Avp (arginine vasopressin), Nr4al (nuclear receptor subfamily 4, group A, member 1; i.e., nerve Growth factor IB), Pcdh (protocadherins), Grml (glutamate receptor, metabotropic 1), ERα (estrogen receptor α), Bdnf (brain-derived neurotrophic factor).
Another recent development in the field was the evidence that these epigenetic marks of developmental programming can be modified in adulthood by stressful experiences. Chronic CORT exposure was able to decrease methylation in the intronic regions of the gene encoding FK506 binding protein 5 (FKBP5) in the brain and periphery. FKBP5 operates in an ultrashort negative feedback loop regulating of the GR responsiveness (Lee et al., 2010; Lee et al., 2011). Similarly, chronic, but not acute, restraint stress increased methylation of GR 17 promoter in the adrenal and pituitary (Witzmann et al., 2012).
2.5 Early-life stress and the development of fear aversion-learning
Besides the epigenetic modifications in the HPA-axis by early-life stress, recent findings suggest the involvement of amygdala-dependent fear-learning in early-life programming and ask for experiments on the epigenetic alterations within the amygdala subnuclei (Davidson and McEwen, 2012). Rat pups are dependent on maternal care for survival and, undergo under the age of pnd 10, a sensitive period of facilitated preference-learning to maternal odor. During this period, pups show an attenuated learning of fear. The absence of fear aversion-learning is crucial for forming dam-pup attachments (Sullivan and Holman, 2010). After pnd 10 (post-sensitive period), just a short-term maternal absence allows odor aversion-learning (Moriceau and Sullivan, 2006). Elevation of CORT levels is crucial for transition to aversion. CORT needs to activate the amygdala to prematurely trigger aversion to noxious stimuli (Moriceau et al., 2006).
In our studies the MS paradigm was combined with the ontogeny of the amygdala-dependent fear aversion-learning. Briefly, pups were at an age (pnd 3–5 during the SHRP) that permitted formation of memories only during long-term absence of the dam. After being separated from their mothers for the first time, pups experienced high amounts of CORT that permits priming of the amygdala. Yet, the outcome is profoundly diverging as a function of the MS-context. At pnd 5, HOME-SEP pups responded to a mild novelty stressor with an enhanced CORT response, since in this condition their adrenal cortex was primed for hyper-responsiveness. In contrast, NOVEL-SEP pups displayed enhanced amygdala c-Fos expression and increased ACTH-release in response to the same stressor, while they did not show a peripheral adrenocortical stress-response (Daskalakis et al., 2009a; Daskalakis, 2011; Daskalakis et al., 2011a). In the NOVEL-SEP offspring, the relatively enhanced amygdala c-Fos activation and ACTH-release persisted into adulthood and were paralleled by higher retention of the freezing response (Daskalakis et al., 2009a; Daskalakis, 2011; Daskalakis et al., 2011a), in agreement with studies linking early-life stressful experience with the adult fear-response through long-lasting changes in amygdala processing (Sevelinges et al., 2007; Sevelinges et al., 2008).
Taken together, these findings suggest a modulation in programming of amygdala function through early-life stress, as previously reported in other stress-regulated brain regions. Amygdala programming seems to be context-specific, since the interaction of maternal absence with early-life context predicts the adult outcome rather than the maternal absence per se.
2.6 Maternal care and glucocorticoid regulation of hippocampal plasticity
In addition to effects on learning and memory, maternal care influences stress reactivity and HPA-axis. High levels of LG early in life associate with decreased stress responsivity in adulthood. Compared to the adult offspring of Low LG mothers, offspring of High LG mothers have lower plasma levels of ACTH and CORT both during and following the termination of acute restraint stress (Liu et al., 1997). Up-regulation of hippocampal GR expression in offspring of High LG mothers, in part, appears associated with enhanced negative feedback control (Liu et al., 1997; Francis et al., 1999; Weaver et al., 2004). Hippocampal control of stress-induced HPA-axis activity is mediated by stimulation of GR by CORT during stress-induced CORT elevations, although the outcome is disinhibitory rather than inhibitory raising the possibility of implication of other feedback sites as well (De Kloet et al., 1998; Furay et al., 2008). The central role of the hippocampus as target and regulator of the HPA-axis suggests that alterations of HPA-axis activity should have wide ranging consequences for hippocampal learning and plasticity. Indeed, brief CORT treatment suppresses LTP in the hippocampal CA1 (Champagne et al., 2008) and DG (Bagot et al., 2009) of High LG offspring. However, CORT facilitates LTP in Low LG offspring. Stress also enhances hippocampus-dependent learning in Low LG offspring in contextual fear-conditioning (Bagot et al., 2009). Thus the maternal effect on stress responsivity influences hippocampus-dependent learning and synaptic plasticity. The interaction between maternal care and CORT effects on hippocampal function may be mediated through effects on NMDA receptor function.
Although maternal care might be expected to differentially affect CORT-regulation of NMDAR function, the direction of such an effect is difficult to predict based on previous findings. Since High LG offspring are less stress responsive than Low LG offspring, one might expect CORT to exert a stronger impact on NMDAR in Low LG offspring. Alternatively, since High LG offspring express higher levels of GR in the hippocampus, and GR activation is necessary for CORT-induced enhancement of NMDAR function (Tse et al., 2011), CORT may more potently regulate NMDAR function in High LG offspring. In fact, the stress-level CORT (100 nM) significantly enhances NMDAR function in High LG offspring and increases the normalized NMDAR-fEPSP. In contrast, CORT treatment does not alter NMDAR-fEPSPs in Low LG offspring.
The mechanism underlying the loss of CORT-regulation of NMDAR in Low LG offspring is unclear. Since NMDAR function is maintained at a high and possibly saturated level in Low LG offspring in basal conditions, the capacity for further enhancement of NMDAR function after CORT treatment could be limited. Interestingly, the time-course of CORT-induced enhancement of NMDAR function (within 20 min) suggests that a classical genomic action requiring cytoplasmic corticosteroid receptors is not involved. Indeed a BSA-CORT conjugate replicates the effect of CORT, implicating the involvement of a membrane-bound corticosteroid receptor. Thus, similar to non-genomic effects of CORT in facilitating AMPAR (Karst et al., 2005) and LTP formation (Wiegert et al., 2006), CORT-induced facilitation of synaptic NMDAR in the adult hippocampus of High LG offspring is likely mediated by non-genomic mechanisms.
Functional Implications
Offspring of Low LG rats is largely insensitive to the acute effects of CORT on NMDAR function. In contrast, in offspring of High LG mothers, CORT increases postsynaptic NMDAR current (Bagot et al., 2012a). This differential sensitivity is consistent with the maternal effect on HPA-axis negative feedback sensitivity (Liu et al., 1997). Thus, as a consequence of reduced glucocorticoid sensitivity, Low LG offspring is exposed to more sustained elevations of CORT; yet the acute effects of CORT exposure, at least in terms of NMDAR function, are attenuated. Altered HPA-negative feedback sensitivity is in part a consequence of epigenetic effects of maternal care on hippocampal GR expression. Maternal effects on sensitivity to rapid effects of CORT are likely independent of classical GR expression. Membrane-bound corticosteroid receptors might also be differentially regulated in High and Low LG offspring.
Glutamate receptors are gaining increased attention as therapeutic targets in depression research. The glutamatergic hypothesis of depression implicates NMDAR hyperfunction in the pathophysiology of the disorder (Pittenger et al., 2007; Skolnick et al., 2009). Expression of NMDARs is bidirectionally regulated by antidepressants and chronic-stress. Clinically, the NMDAR-antagonist ketamine alleviates symptoms of treatment-resistant depression after a single acute treatment (Berman et al., 2000; Zarate et al., 2006). NMDAR-antagonists also produce rapid antidepressant-like responses and reverse some of the behavioral and physiological consequences of chronic stress in rodent models of depression (Autry et al., 2011; Li et al., 2011). Moreover, chronic stress induces certain depression-like behaviors and sustained increases in NMDAR/AMPAR ratio in hippocampal neurons (Kole et al., 2002). This is similar to the basal profile observed in Low LG offspring. Low LG offspring exhibits phenotypes associated with vulnerability for depression, such as reduced glucocorticoid negative-feedback sensitivity and increased HPA-axis responses to stress as well as increased fearfulness (Liu et al., 1997; Caldji et al., 1998; Weaver et al., 2004) and increased immobility in the forced swim test (Weaver et al., 2005).
In conclusion, studies of Low LG offspring may have utility in uncovering endophenotypes that predict an increased risk for depression in humans. We suggest that variations in early-life environments might, in part, influence the risk for depression through effects on NMDAR function.
3. Gene-environment interaction studies in the human: Examples from the GR/MR genetic variation
There is convincing evidence that (traumatic) stress, especially during early-life, is a major risk factor for the development of almost all psychiatric disorders, including posttraumatic stress disorder (Bremner et al., 1993), major depressive disorder (Heim et al., 2008), and schizophrenia (van Os et al., 2010). However, despite decades of research, it is currently unknown which combination of stressful life events are the most etiologically relevant to predict the development of psychopathology and how stressful events interact at different periods of life.
In the literature, several hypotheses exist to explain the effect of variable outcomes after (repeated) exposure to traumatic events. Methodological limitations in the current literature preclude firm conclusions on the validity of these models for human psychopathology. For example, human studies generally have a retrospective methodological design. Also, the simultaneous retrospective assessment of adversity is subject to recall bias and cannot determine any causality or disentangle the temporal course of stressful experiences across the life span. Importantly, across studies, diverse phenomena have been defined to represent ‘stress’, ranging from prenatal adverse events, maternal psychopathology, infections during pregnancy, childhood abuse and neglect, a specific life event, and chronic perceived stress due to daily hassles. These events represent different aspects of stress but do not necessarily measure the same construct. A good example of the methodological issues surrounding stress assessment methods is the moderating effect of the serotonin transporter gene polymorphism (5-HTTLPR) on the relationship between stressful life events and depression (Caspi et al., 2010).
Another important factor in the assessment of adversity is the possibility that certain time windows of vulnerability exist in which traumatic and/or stressful experiences disproportionately affect at-risk individuals. Obviously, it is also of great importance to take personality characteristics, prior psychiatric symptoms, and coping mechanisms into account. Especially the role of personality traits is of importance in light of the existence of dependent stressful life events, i.e. events that are directly related to an individual’s own behavior (Kendler et al., 1999).
Considerable inter-individual differences exist in outcomes after (repeated) adversity, and many individuals do not develop psychopathology (Southwick and Charney, 2012). The leading hypothesis is that stress exposure only affects individuals with a susceptible genetic background. Therefore, an individual’s history of adversity should be interpreted in light of genetic predisposition. While multiple genetic factors are thought to be involved, the HPA-axis is pivotal in shaping dynamic stress reactivity. Indeed, exposure to adverse events across the life span has been commonly associated with abnormal basal and stress-induced HPA-axis activity, even though the developmental course of such changes has not yet been elucidated.
Several clinical gene-environment studies have shown that genetic variation in the HPA-axis moderates the long-term effects of stress, conferring either an adaptive advantage or a risk for psychiatric disorders. Corticosteroids bind to both GR and MR, regulating gene transcription and triggering a cascade of cellular and network changes in the brain underlying physiological and behavioral adaptations. Several gene-environment studies have been published on the GR (van Rossum et al., 2006; Bet et al., 2009), including a pivotal role for the GR chaperone FKBP5 (Binder et al., 2008; Klengel et al., 2013). In contrast, the role of the MR has received less attention. MR play a critical role in stress appraisal (Oitzl and de Kloet, 1992), not only via the (classical) slow genomic pathway but also – as recently revealed - through rapid non-genomic effects which require elevated hormone levels (Karst et al., 2010). Since MR are closely involved in stress appraisal, several studies have put the MR rs5522/rs2070951 haplotype forward as a prominent and plausible player in stress research (Klok et al., 2011a; Klok et al., 2011b). Moreover, several seminal studies have shown that genetic variation in the CRHR1 receptor is involved in the subsequent development of depression (Bradley et al., 2008; Polanczyk et al., 2009) (see (Binder and Nemeroff, 2010) for review).
To our knowledge, no studies have been published on the interaction between adversity across the entire life span and genetic variation in the HPA-axis. However, investigating biologically plausible genetic variation in HPA-axis activity may enhance our understanding of the large variation in outcomes after accumulating acute and chronic adversity. An important advantage of a comprehensive and integrated assessment of adversity across the life span is that the causal relation between proximal and more distal adverse events as well as their interaction may be studied. Also, multiple assessments across various stress domains may increase the validity and reliability of the impact of stress exposure compared to a single assessment.
Thus, we believe that assessment of an individual’s life time adversity history should receive special consideration in future clinical studies. Such studies may eventually elucidate to what extent the accumulation of stress is context-dependent or whether cumulative stress exposure has negative long-term consequences in the context of both psychiatric and somatic disorders. Instead of focusing on a single stress domain, a comprehensive measure of stressful experiences in conjunction with the genetic background may lead to a fundamentally new way of understanding the variable outcomes after (repeated) traumatic stress. The study of individual developmental trajectories which are uniquely shaped by the environment may ultimately lead to the identification who is and who is not at risk for psychiatric disorders. In conclusion, individuals with a particular genetic variation in the HPA-axis may be associated with certain specific developmental trajectories in terms of psychopathology in response to accumulating environmental stress.
4. The three-hit concept of resilience and vulnerability
The previous sections did highlight that adversity is often found associated with psychopathology. Accordingly, in studies on the cumulative stress model (the “classic” diathesis-stress model) it is postulated that if the accumulation of stressors across the life span exceeds a certain threshold, the development of psychopathology is enhanced in at-risk individuals (McEwen, 1998). Alternatively, in an evolutionary perspective, early-life experience could induce (epigenetic) changes underlying predictive adaptive responses (Gluckman et al., 2009). In this view plasticity evolved to match an organism to its predicted environment. Hence, a mismatch between the phenotypic outcome of adaptive plasticity and the ability to cope with the current environmental experience is thought to increase the risk of disease (i.e., developmental match/mismatch hypothesis).
A major differentiating factor between the cumulative stress model and the match/mismatch model is the possible adaptive value of experienced early-life adversity. Whereas the cumulative model asserts that the extent of cumulative adversity does generally not have any advantageous effect but progressively increases disease risk, the mismatch model explicitly assumes that adverse events during development may constitute a possible source of adaptation for certain individuals. In this context, exposure to a challenging, but still moderate stressful environment which promotes active coping (“stress inoculation”) should be distinguished from severe physical and sexual abuse. Early exposure to severe stress in childhood increases the incidence of mood and anxiety disorders in adulthood. Moderate early stress exposure results in subsequent resilience rather than enhanced vulnerability.
Consistent with this view is also the differential susceptibility to environmental influence (“for-better-and-for-worse”) model, which assumes that genetic susceptibility should be contextually interpreted: vulnerability in one environment may actually constitute an adaptive benefit in another environment (Belsky and Beaver, 2011). Individuals with reactive (to the environment) alleles are expected to display heightened susceptibility to environmental influences for better or worse. Hence in a stressful environment these reactive alleles will increase on the one hand the risk for disease in an adverse context, but also increase sensitivity to beneficial effects of the environment on the other hand. Individuals with less reactive alleles will be less susceptible to environmental influence (Belsky, 1997; Boyce and Ellis, 2005; Bakermans-Kranenburg and van Ijzendoorn, 2007; Belsky et al., 2007; Belsky et al., 2009; Ellis et al., 2011).
Attempts to reconcile the different models were proposed from the perspective of individual differences in programming sensitivity (Nederhof and Schmidt, 2012), which is most likely defined by the interaction of genetic predisposition and early-life environment. For gathering experimental evidence supporting this concept’s face, predictive and construct validity, the three-hit concept of vulnerability and resilience offers a testable framework. This concept is depicted in Figure 2 and can be formulated as follows: the interaction of genetic factors (hit-1) with early-life environmental factors (hit-2), as reflected in altered endocrine regulations and epigenetic modifications, programs during brain development gene expression patterns relevant for an evolving phenotype. A certain programmed phenotype when exposed to a later-life environment (hit-3) mental functions may become compromised and a higher risk of psychiatric symptoms may arise (vulnerability), but when exposed to another type of environment the same individual is expected to be resistant to mental dysfunction (resilience).
Figure 2. The three-hit concept of vulnerability and resilience.
Multi-genic inputs (hit-1) interact with early-life environmental inputs and experience-related factors (hit-2), programming phenotypes with differential susceptibility to later-life challenges (hit-3). Depending on the interaction of “programmed phenotypes” with later-life environment vulnerability or resilience may precipitate.
Abbreviations: 5-HTTLPR (serotonin-transporter-linked polymorphic region), Aktl(RAC-alpha serine/threonine-protein kinase), BDNF (brain-derived neurotrophic factor), COMT (Catechol-O-methyltransferase), CRHR1 (Corticotropin releasing hormone receptor 1), DAOA (D-amino acid oxidase activator), DRD2 (Dopamine receptor D2), DRD4 (Dopamine receptor D4), FKBP5 (FK506 binding protein 5), GR (glucocorticoid receptor), MAOA (Monoamine oxidase A), MR (mineralocorticoid receptor), NPY (neuropeptide Y).
This contribution on adaptation to early-life adversity outcome documents progress at the verge of a new era, where complex interactions of genes with the environment will be tested in animals carrying inducible, brain site-specific or even brain cell-specific, gene modifications. Natural variation in genetic make-up can be also be used for this purpose, as is the case for the apomorphine-susceptible (APO-SUS) rat line (Ellenbroek et al., 2000; Ellenbroek and Cools, 2002; Daskalakis, 2011; Daskalakis et al., 2011b). These studies suggest that in the APO-SUS rat line, selected for enhanced dopamine responsiveness, a severe behavioral disruption precipitates upon a combined exposure to early-life adversity and peri-pubertal social isolation that allows discriminating between gene-environment conditions programming resilience vs. vulnerability. CORT, and the HPA-axis, are in the driver’s seat playing a key role in early programming effects, and are important determinants of the functional and structural plasticity underlying the adaptations to early-life adversity. These findings underscore the amazing plasticity of the brain; after all as the late Seymour Levine used to say “nothing is written in stone”.
Acknowledgments
We would like to thank the organizers of 42nd ISPNE Conference for choosing our proposal for a Symposium. We would like to thank Dr. Esther Nederhof for participating as a speaker in the Symposium. ND and EdK were supported by the Dutch Top-Institute Pharma T5-209, and KP by the NIH-R01HD67175. EdK was also supported by the Royal Netherlands Academy for Sciences.
Role of funding source
All finding resources had no role in the writing of the manuscript and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have no conflict of interest to report.
Contributors
NPD and EdK proposed the Symposium with the same topic to ISPNE, conceptualized the review article and prepared the first draft of the manuscript. RB, KP and CV edited the manuscript. All authors contributed to and have approved the final manuscript.
References
- Ahnert L, Gunnar MR, Lamb ME, Barthel M. Transition to child care: associations with infant-mother attachment, infant negative emotion, and Cortisol elevations. Child Dev. 2004;75:639–650. doi: 10.1111/j.1467-8624.2004.00698.x. [DOI] [PubMed] [Google Scholar]
- Arabadzisz D, Diaz-Heijtz R, Knuesel I, Weber E, Pilloud S, Dettling AC, Feldon J, Law AJ, Harrison PJ, Pryce CR. Primate early life stress leads to long-term mild hippocampal decreases in corticosteroid receptor expression. Biol. Psychiatry. 2010;67:1106–1109. doi: 10.1016/j.biopsych.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot RC, Meaney MJ. Epigenetics and the biological basis of gene x environment interactions. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49:752–771. doi: 10.1016/j.jaac.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Bagot RC, Tse YC, Nguyen HB, Wong AS, Meaney MJ, Wong TP. Maternal Care Influences Hippocampal N-Methyl-D-Aspartate Receptor Function and Dynamic Regulation by Corticosterone in Adulthood. Biol. Psychiatry. 2012a doi: 10.1016/j.biopsych.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Bagot RC, van Hasselt FN, Champagne DL, Meaney MJ, Krugers HJ, Joels M. Maternal care determines rapid effects of stress mediators on synaptic plasticity in adult rat hippocampal dentate gyrus. Neurobiol. Learn. Mem. 2009;92:292–300. doi: 10.1016/j.nlm.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Bagot RC, Zhang TY, Wen X, Nguyen TT, Nguyen HB, Diorio J, Wong TP, Meaney MJ. Variations in postnatal maternal care and the epigenetic regulation of metabotropic glutamate receptor 1 expression and hippocampal function in the rat. Proc. Natl. Acad. Sci. U. S. A. 2012b;109(Suppl 2):17200–17207. doi: 10.1073/pnas.1204599109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van Ijzendoorn MH. Research Review: genetic vulnerability or differential susceptibility in child development: the case of attachment. J. Child Psychol. Psychiatry. 2007;48:1160–1173. doi: 10.1111/j.1469-7610.2007.01801.x. [DOI] [PubMed] [Google Scholar]
- Beery AK, Francis DD. Adaptive significance of natural variations in maternal care in rats: a translational perspective. Neurosci. Biobehav. Rev. 2011;35:1552–1561. doi: 10.1016/j.neubiorev.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J. Variation in Susceptibility to Environmental Influence: An Evolutionary Argument. Psychological inquiry. 1997;8:182–186. [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, van Uzendoorn MH. For Better and For Worse. Current Directions in Psychological Science. 2007;16:300–304. [Google Scholar]
- Belsky J, Beaver KM. Cumulative-genetic plasticity, parenting and adolescent self-regulation. Journal of child psychology and psychiatry, and allied disciplines. 2011;52:619–626. doi: 10.1111/j.1469-7610.2010.02327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes? Mol. Psychiatry. 2009;14:746–754. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Bet PM, Penninx BW, Bochdanovits Z, Uitterlinden AG, Beekman AT, van Schoor NM, Deeg DJ, Hoogendijk WJ. Glucocorticoid receptor gene polymorphisms and childhood adversity are associated with depression: New evidence for a gene-environment interaction. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:660–669. doi: 10.1002/ajmg.b.30886. [DOI] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Nemeroff CB. The CRF system, stress, depression and anxiety-insights from human genetic studies. Mol Psychiatry. 2010;15:574–588. doi: 10.1038/mp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boinski S, Fragaszy DM. The ontogeny of foraging in squirrel monkeys, Saimiri oerstedi. Anim. Behav. 1989;37:415–428. [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev. Psychopathol. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, Gillespie CF, Berg T, Evces M, Newport DJ, Stowe ZN, Heim CM, Nemeroff CB, Schwartz A, Cubells JF, Ressler KJ. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch. Gen. Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchi I. The mouse communal nest: investigating the epigenetic influences of the early social environment on brain and behavior development. Neurosci. Biobehav. Rev. 2009;33:551–559. doi: 10.1016/j.neubiorev.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Branchi I, Curley JP, D’Andrea I, Cirulli F, Champagne FA, Alleva E. Early interactions with mother and peers independently build adult social skills and shape BDNF and oxytocin receptor brain levels. Psychoneuroendocrinology. 2013a;38:522–532. doi: 10.1016/j.psyneuen.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchi I, D’Andrea I, Cirulli F, Lipp HP, Alleva E. Shaping brain development: mouse communal nesting blunts adult neuroendocrine and behavioral response to social stress and modifies chronic antidepressant treatment outcome. Psychoneuroendocrinology. 2010;35:743–751. doi: 10.1016/j.psyneuen.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Branchi I, Karpova NN, D’Andrea I, Castren E, Alleva E. Epigenetic modifications induced by early enrichment are associated with changes in timing of induction of BDNF expression. Neurosci. Lett. 2011;495:168–172. doi: 10.1016/j.neulet.2011.03.038. [DOI] [PubMed] [Google Scholar]
- Branchi I, Santarelli S, D’Andrea I, Alleva E. Not all stressors are equal: early social enrichment favors resilience to social but not physical stress in male mice. Horm. Behav. 2013b;63:503–509. doi: 10.1016/j.yhbeh.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Humpartzoomian RA, Cain DP, Meaney MJ. Partial reversal of the effect of maternal care on cognitive function through environmental enrichment. Neuroscience. 2003;118:571–576. doi: 10.1016/s0306-4522(02)00918-1. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Southwick SM, Johnson DR, Yehuda R, Charney DS. Childhood physical abuse and combat-related posttraumatic stress disorder in Vietnam veterans. Am. J. Psychiatry. 1993;150:235–239. doi: 10.1176/ajp.150.2.235. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc. Natl. Acad. Sci. U. S. A. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am. J. Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne DL, Bagot RC, van Hasselt F, Ramakers G, Meaney MJ, de Kloet ER, Joels M, Krugers H. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J. Neurosci. 2008;28:6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA. Effects of stress across generations: why sex matters. Biol. Psychiatry. 2013;73:2–4. doi: 10.1016/j.biopsych.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol. Behav. 2003;79:359–371. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Weaver IC, Diorio J, Dymov S, Szyf M, Meaney MJ. Maternal care associated with methylation of the estrogen receptor-alphalb promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology. 2006;147:2909–2915. doi: 10.1210/en.2005-1119. [DOI] [PubMed] [Google Scholar]
- Chatelain A, Durand P, Naaman E, Dupouy JP. Ontogeny of ACTH(1–24) receptors in rat adrenal glands during the perinatal period. J. Endocrinol. 1989;123:421–428. doi: 10.1677/joe.0.1230421. [DOI] [PubMed] [Google Scholar]
- Claessens SE, Daskalakis NP, Oitzl MS, de Kloet ER. Early handling modulates outcome of neonatal dexamethasone exposure. Horm. Behav. 2012;62:433–441. doi: 10.1016/j.yhbeh.2012.07.011. [DOI] [PubMed] [Google Scholar]
- Claessens SE, Daskalakis NP, van der Veen R, Oitzl MS, de Kloet ER, Champagne DL. Development of individual differences in stress responsiveness: an overview of factors mediating the outcome of early life experiences. Psychopharmacology (Berl.) 2011;214:141–154. doi: 10.1007/s00213-010-2118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe CL, Glass JC, Wiener SG, Levine S. Behavioral, but not physiological, adaptation to repeated separation in mother and infant primates. Psychoneuroendocrinology. 1983;8:401–409. doi: 10.1016/0306-4530(83)90019-7. [DOI] [PubMed] [Google Scholar]
- Curley JP, Davidson S, Bateson P, Champagne FA. Social enrichment during postnatal development induces transgenerational effects on emotional and reproductive behavior in mice. Front. Behav. Neurosci. 2009;3:25. doi: 10.3389/neuro.08.025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amato FR, Zanettini C, Sgobio C, Sarli C, Carone V, Moles A, Ammassari-Teule M. Intensification of maternal care by double-mothering boosts cognitive function and hippocampal morphology in the adult offspring. Hippocampus. 2011;21:298–308. doi: 10.1002/hipo.20750. [DOI] [PubMed] [Google Scholar]
- D’Andrea I, Alleva E, Branchi I. Communal nesting, an early social enrichment, affects social competences but not learning and memory abilities at adulthood. Behav. Brain Res. 2007;183:60–66. doi: 10.1016/j.bbr.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Daniels WM, Fairbairn LR, van Tilburg G, McEvoy CR, Zigmond MJ, Russell VA, Stein DJ. Maternal separation alters nerve growth factor and corticosterone levels but not the DNA methylation status of the exon 1(7) glucocorticoid receptor promoter region. Metab. Brain Dis. 2009;24:615–627. doi: 10.1007/s11011-009-9163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis N. Division of Medical Pharmacology of the Leiden/Amsterdam Center for Drug Research (LACDR) Faculty of Medicine/Leiden University Medical Center (LUMC), Leiden University; 2011. Nurturing nature: testing the three-hit hypothesis of schizophrenia. [Google Scholar]
- Daskalakis N, Diamantopoulou A, Tjälve M, Meelis W, Oitzl MS, de Kloet ER. Early-life adversity load predicts psychosis-like behaviour in the rat. Vol. 2009. Chicago, IL: Society for Neuroscience; 2009a. Online, Program No. 341.349/0318. [Google Scholar]
- Daskalakis NP, Claessens SE, Laboyrie JJ, Enthoven L, Oitzl MS, Champagne DL, de Kloet ER. The newborn rat’s stress system readily habituates to repeated and prolonged maternal separation, while continuing to respond to stressors in context dependent fashion. Horm. Behav. 2011a;60:165–176. doi: 10.1016/j.yhbeh.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Daskalakis NP, Kaperoni M, Koros C, de Kloet ER, Kitraki E. Environmental and tactile stimulation modulates the neonatal handling effect on adult rat spatial memory. Int. J. Dev. Neurosci. 2009b;27:747–755. doi: 10.1016/j.ijdevneu.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Daskalakis NP, Martens GJ, Cools AR, de Kloet ER. Schizophrenia endophenotypes and stress hyper-reactivity co-precipitate following adverse life events in genetically susceptible rats. Eur. Neuropsychopharmacol. 2011b;21:S85–S86. [Google Scholar]
- Daskalakis NP, Oitzl MS, Schachinger H, Champagne DL, de Kloet ER. Testing the cumulative stress and mismatch hypotheses of psychopathology in a rat model of early-life adversity. Physiol. Behav. 2012;106:707–721. doi: 10.1016/j.physbeh.2012.01.015. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, McEwen BS. Social influences on neuroplasticity: stress and interventions to promote well-being. Nat. Neurosci. 2012;15:689–695. doi: 10.1038/nn.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Sibug RM, Helmerhorst FM, Schmidt MV. Stress, genes and the mechanism of programming the brain for later life. Neurosci. Biobehav. Rev. 2005;29:271–281. doi: 10.1016/j.neubiorev.2004.10.008. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- DeNelsky GY, Denenberg VH. Infantile stimulation and adult exploratory behaviour in the rat: effects of handling upon visual variation-seeking. Anim. Behav. 1967;15:568–573. doi: 10.1016/0003-3472(67)90060-7. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Feldon J, Pryce CR. Repeated parental deprivation in the infant common marmoset (Callithrix jacchus, primates) and analysis of its effects on early development. Biol. Psychiatry. 2002;52:1037–1046. doi: 10.1016/s0006-3223(02)01460-9. [DOI] [PubMed] [Google Scholar]
- Dietz DM, Laplant Q, Watts EL, Hodes GE, Russo SJ, Feng J, Oosting RS, Vialou V, Nestler EJ. Paternal transmission of stress-induced pathologies. Biol. Psychiatry. 2011;70:408–414. doi: 10.1016/j.biopsych.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake AJ, Liu L, Kerrigan D, Meehan RR, Seckl JR. Multigenerational programming in the glucocorticoid programmed rat is associated with generation-specific and parent of origin effects. Epigenetics. 2011;6 doi: 10.4161/epi.6.11.17942. [DOI] [PubMed] [Google Scholar]
- Eghbal-Ahmadi M, Hatalski CG, Lovenberg TW, Avishai-Eliner S, Chalmers DT, Baram TZ. The developmental profile of the corticotropin releasing factor receptor (CRF2) in rat brain predicts distinct age-specific functions. Brain Res. Dev. Brain Res. 1998;107:81–90. doi: 10.1016/s0165-3806(98)00002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbroek BA, Cools AR. The long-term effects of maternal deprivation depend on the genetic background. Neuropsychopharmacology. 2000;23:99–106. doi: 10.1016/S0893-133X(00)00088-9. [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA, Cools AR. Apomorphine susceptibility and animal models for psychopathology: genes and environment. Behav. Genet. 2002;32:349–361. doi: 10.1023/a:1020214322065. [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA, Sluyter F, Cools AR. The role of genetic and early environmental factors in determining apomorphine susceptibility. Psychopharmacology (Berl.) 2000;148:124–131. doi: 10.1007/s002130050033. [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA, van den Kroonenberg PT, Cools AR. The effects of an early stressful life event on sensorimotor gating in adult rats. Schizophr. Res. 1998;30:251–260. doi: 10.1016/s0920-9964(97)00149-7. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Differential susceptibility to the environment: an evolutionary-neurodevelopmental theory. Dev. Psychopathol. 2011;23:7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Enthoven L, Oitzl MS, Koning N, van der Mark M, de Kloet ER. Hypothalamic-pituitary-adrenal axis activity of newborn mice rapidly desensitizes to repeated maternal absence but becomes highly responsive to novelty. Endocrinology. 2008;149:6366–6377. doi: 10.1210/en.2008-0238. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Jensen CL, Champagne FA. Epigenetic influences on brain development and plasticity. Curr. Opin. Neurobiol. 2009;19:207–212. doi: 10.1016/j.conb.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio KA, Brunson KL, Avishai-Eliner S, Stone BA, Kapadia BJ, Baram TZ. Enduring, handling-evoked enhancement of hippocampal memory function and glucocorticoid receptor expression involves activation of the corticotropin-releasing factor type 1 receptor. Endocrinology. 2005;146:4090–4096. doi: 10.1210/en.2004-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Franklin TB, Russig H, Weiss IC, Graff J, Under N, Michalon A, Vizi S, Mansuy IM. Epigenetic transmission of the impact of early stress across generations. Biol. Psychiatry. 2010;68:408–415. doi: 10.1016/j.biopsych.2010.05.036. [DOI] [PubMed] [Google Scholar]
- Franklin TB, Saab BJ, Mansuy IM. Neural mechanisms of stress resilience and vulnerability. Neuron. 2012;75:747–761. doi: 10.1016/j.neuron.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Furay AR, Bruestle AE, Herman JP. The role of the forebrain glucocorticoid receptor in acute and chronic stress. Endocrinology. 2008;149:5482–5490. doi: 10.1210/en.2008-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Buklijas T, Low FM, Beedle AS. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nat. Rev. Endocrinol. 2009;5:401–408. doi: 10.1038/nrendo.2009.102. [DOI] [PubMed] [Google Scholar]
- Grino M, Paulmyer-Lacroix O, Faudon M, Renard M, Anglade G. Blockade of alpha 2-adrenoceptors stimulates basal and stress-induced adrenocorticotropin secretion in the developing rat through a central mechanism independent from corticotropin-releasing factor and arginine vasopressin. Endocrinology. 1994;135:2549–2557. doi: 10.1210/endo.135.6.7988443. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annu. Rev. Psychol. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Cheatham CL. Brain and behavior interface: Stress and the developing brain. Infant mental healthjournal. 2003;24:195–211. [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Hennessy MB. Multiple, brief maternal separations in the squirrel monkey: Changes in hormonal and behavioral responsiveness. Physiol. Behav. 1986;36:245–250. doi: 10.1016/0031-9384(86)90011-9. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Multiple regulators of ultrasonic vocalization in the infant rat. Psychoneuroendocrinology. 1996;21:203–217. doi: 10.1016/0306-4530(95)00042-9. [DOI] [PubMed] [Google Scholar]
- Ingram JC. Interactions between parents and infants, and the development of independence in the common marmoset (Callithrixjacchus) Anim. Behav. 1977;25:811–827. [Google Scholar]
- Ivy AS, Brunson KL, Sandman C, Baram TZ. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience. 2008;154:1132–1142. doi: 10.1016/j.neuroscience.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst H, Berger S, Erdmann G, Schutz G, Joels M. Metaplasticity of amygdalar responses to the stress hormone corticosterone. Proc. Natl. Acad. Sci. U. S. A. 2010;107:14449–14454. doi: 10.1073/pnas.0914381107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst H, Berger S, Turiault M, Tronche F, Schutz G, Joels M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc. Natl. Acad. Sci. U. S. A. 2005;102:19204–19207. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kember RL, Dempster EL, Lee TH, Schalkwyk LC, Mill J, Fernandes C. Maternal separation is associated with strain-specific responses to stress and epigenetic alterations to Nr3c1, Avp, and Nr4a1 in mouse. Brain Behav. 2012;2:455–467. doi: 10.1002/brb3.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, Pace TW, Mercer KB, Mayberg HS, Bradley B, Nemeroff CB, Holsboer F, Heim CM, Ressler KJ, Rein T, Binder EB. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat. Neurosci. 2013;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok MD, Giltay EJ, Van der Does AJ, Geleijnse JM, Antypa N, Penninx BW, de Geus EJ, Willemsen G, Boomsma DI, van Leeuwen N, Zitman FG, de Kloet ER, DeRijk RH. A common and functional mineralocorticoid receptor haplotype enhances optimism and protects against depression in females. Transl Psychiatry. 2011a;1:e62. doi: 10.1038/tp.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok MD, Vreeburg SA, Penninx BW, Zitman FG, de Kloet ER, DeRijk RH. Common functional mineralocorticoid receptor polymorphisms modulate the Cortisol awakening response: Interaction with SSRIs. Psychoneuroendocrinology. 2011b;36:484–494. doi: 10.1016/j.psyneuen.2010.07.024. [DOI] [PubMed] [Google Scholar]
- Kole MH, Swan L, Fuchs E. The antidepressant tianeptine persistently modulates glutamate receptor currents of the hippocampal CA3 commissural associational synapse in chronically stressed rats. Eur. J. Neurosci. 2002;16:807–816. doi: 10.1046/j.1460-9568.2002.02136.x. [DOI] [PubMed] [Google Scholar]
- Korosi A, Shanabrough M, McClelland S, Liu ZW, Borok E, Gao XB, Horvath TL, Baram TZ. Early-life experience reduces excitation to stress-responsive hypothalamic neurons and reprogramsthe expression of corticotropin-releasing hormone. J. Neurosci. 2010;30:703–713. doi: 10.1523/JNEUROSCI.4214-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Kim JJ, Lee HJ. Early life manipulations alter learning and memory in rats. Neurosci. Biobehav. Rev. 2012 doi: 10.1016/j.neubiorev.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RS, Tamashiro KL, Yang X, Purcell RH, Harvey A, Willour VL, Huo Y, Rongione M, Wand GS, Potash JB. Chronic corticosterone exposure increases expression and decreases deoxyribonucleic acid methylation of Fkbp5 in mice. Endocrinology. 2010;151:4332–4343. doi: 10.1210/en.2010-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RS, Tamashiro KL, Yang X, Purcell RH, Huo Y, Rongione M, Potash JB, Wand GS. A measure of glucocorticoid load provided by DNA methylation of Fkbp5 in mice. Psychopharmacology (Berl.) 2011;218:303–312. doi: 10.1007/s00213-011-2307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J, Feldon J. Long-term biobehavioral effects of maternal separation in the rat: consistent or confusing? Rev. Neurosci. 2000;11:383–408. doi: 10.1515/revneuro.2000.11.4.383. [DOI] [PubMed] [Google Scholar]
- Levine S. Infantile experience and resistance to physiological stress. Science. 1957;126:405. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- Levine S. Maternal and environmental influences on the adrenocortical response to stress in weanling rats. Science. 1967;156:258–260. doi: 10.1126/science.156.3772.258. [DOI] [PubMed] [Google Scholar]
- Levine S. Influence of psychological variables on the activity of the hypothalamic-pituitary-adrenal axis. Eur. J. Pharmacol. 2000;405:149–160. doi: 10.1016/s0014-2999(00)00548-3. [DOI] [PubMed] [Google Scholar]
- Levine S. Primary social relationships influence the development of the hypothalamic-pituitary-adrenal axis in the rat. Physiol. Behav. 2001;73:255–260. doi: 10.1016/s0031-9384(01)00496-6. [DOI] [PubMed] [Google Scholar]
- Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005;30:939–946. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Levine S, Huchton DM, Wiener SG, Rosenfeld P. Time course of the effect of maternal deprivation on the hypothalamic-pituitary-adrenal axis in the infant rat. Dev. Psychobiol. 1991;24:547–558. doi: 10.1002/dev.420240803. [DOI] [PubMed] [Google Scholar]
- Levine S, Mody T. The long-term psychobiological consequences of intermittent postnatal separation in the squirrel monkey. Neurosci. Biobehav. Rev. 2003;27:83–89. doi: 10.1016/s0149-7634(03)00011-3. [DOI] [PubMed] [Google Scholar]
- Levine S, Weiner S, Coe C. The psychoneuroendocrinology of stress: A psychobiological perspective. In: Brush FR, Levine S, editors. Psychoneuroendocrinology. New York: Academic Press; 1989. pp. 341–377. [Google Scholar]
- Levine S, Wiener SG. Psychoendocrine aspects of mother-infant relationships in nonhuman primates. Psychoneuroendocrinology. 1988;13:143–154. doi: 10.1016/0306-4530(88)90011-x. [DOI] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol. Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebl C, Panhuysen M, Putz B, Trumbach D, Wurst W, Deussing JM, Muller MB, Schmidt MV. Gene expression profiling following maternal deprivation: involvement of the brain Renin-Angiotensin system. Front. Mol. Neurosci. 2009;2:1. doi: 10.3389/neuro.02.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat. Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Lukas M, Bredewold R, Landgraf R, Neumann ID, Veenema AH. Early life stress impairs social recognition due to a blunted response of vasopressin release within the septum of adult male rats. Psychoneuroendocrinology. 2011;36:843–853. doi: 10.1016/j.psyneuen.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Martel FL, Levine S, Risch NJ, Schatzberg AF. Postnatal experiences and genetic effects on squirrel monkey social affinities and emotional distress. Horm. Behav. 1999;36:266–275. doi: 10.1006/hbeh.1999.1547. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Yang C, Mobley BW, Nickerson JT, Schatzberg AF. Early environmental regulation of glucocorticoid feedback sensitivity in young adult monkeys. J. Neuroendocrinol. 2000;12:723–728. doi: 10.1046/j.1365-2826.2000.00505.x. [DOI] [PubMed] [Google Scholar]
- Macri S, Mason GJ, Wurbel H. Dissociation in the effects of neonatal maternal separations on maternal care and the offspring’s HPAand fear responses in rats. Eur. J. Neurosci. 2004;20:1017–1024. doi: 10.1111/j.1460-9568.2004.03541.x. [DOI] [PubMed] [Google Scholar]
- Macri S, Wurbel H. Developmental plasticity of HPA and fear responses in rats: a critical review of the maternal mediation hypothesis. Horm. Behav. 2006;50:667–680. doi: 10.1016/j.yhbeh.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Maras PM, Baram TZ. Sculpting the hippocampus from within: stress, spines, and CRH. Trends Neurosci. 2012;35:315–324. doi: 10.1016/j.tins.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland S, Korosi A, Cope J, Ivy A, Baram TZ. Emerging roles of epigenetic mechanisms in the enduring effects of early-life stress and experience on learning and memory. Neurobiol. Learn. Mem. 2011;96:79–88. doi: 10.1016/j.nlm.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Allostasis and allostatic load. Vol. 840. Annals of the New York Academy of Sciences; 1998. Stress, adaptation, and disease; pp. 33–44. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Suderman M, Sasaki A, Huang TC, Hallett M, Meaney MJ, Szyf M. Broad epigenetic signature of maternal care in the brain of adult rats. PLoS ONE. 2011;6:e14739. doi: 10.1371/journal.pone.0014739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH. The effects of early postnatal handling on hippocampal glucocorticoid receptor concentrations: temporal parameters. Brain Res. 1985;354:301–304. doi: 10.1016/0165-3806(85)90183-x. [DOI] [PubMed] [Google Scholar]
- Meerlo P, Horvath KM, Nagy GM, Bohus B, Koolhaas JM. The influence of postnatal handling on adult neuroendocrine and behavioural stress reactivity. J. Neuroendocrinol. 1999;11:925–933. doi: 10.1046/j.1365-2826.1999.00409.x. [DOI] [PubMed] [Google Scholar]
- Mendoza SP, Smotherman WP, Miner MT, Kaplan J, Levine S. Pituitary-adrenal response to separation in mother and infant squirrel monkeys. Dev. Psychobiol. 1978;11:169–175. doi: 10.1002/dev.420110209. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nat. Neurosci. 2006;9:1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Wilson DA, Levine S, Sullivan RM. Dual circuitry for odor-shock conditioning during infancy: corticosterone switches between fear and attraction via amygdala. J. Neurosci. 2006;26:6737–6748. doi: 10.1523/JNEUROSCI.0499-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muret L, Priou A, Oliver C, Grino M. Stimulation of adrenocorticotropin secretion by insulin-induced hypoglycemia in the developing rat involves arginine vasopressin but not corticotropin-releasing factor. Endocrinology. 1992;130:2725–2732. doi: 10.1210/endo.130.5.1315256. [DOI] [PubMed] [Google Scholar]
- Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmuhl Y, Fischer D, Holsboer F, Wotjak CT, Almeida OF, Spengler D. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat. Neurosci. 2009;12:1559–1566. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- Murgatroyd C, Spengler D. Genetic variation in the epigenetic machinery and mental health. Curr. Psychiatry Rep. 2012;14:138–149. doi: 10.1007/s11920-012-0255-1. [DOI] [PubMed] [Google Scholar]
- Nederhof E, Schmidt MV. Mismatch or cumulative stress: toward an integrated hypothesis of programming effects. Physiol. Behav. 2012;106:691–700. doi: 10.1016/j.physbeh.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant Cortisol stress responses. Epigenetics. 2008;3:97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- Oitzl MS, de Kloet ER. Selective corticosteroid antagonists modulate specific aspects of spatial orientation learning. Behav. Neurosci. 1992;106:62–71. doi: 10.1037//0735-7044.106.1.62. [DOI] [PubMed] [Google Scholar]
- Okimoto DK, Blaus A, Schmidt MV, Gordon MK, Dent GW, Levine S. Differential expression of c-fos and tyrosine hydroxylase mRNA in the adrenal gland of the infant rat: evidence for an adrenal hyporesponsive period. Endocrinology. 2002;143:1717–1725. doi: 10.1210/endo.143.5.8819. [DOI] [PubMed] [Google Scholar]
- Oomen CA, Soeters H, Audureau N, Vermunt L, van Hasselt FN, Manders EM, Joels M, Lucassen PJ, Krugers H. Severe early life stress hampers spatial learning and neurogenesis, but improves hippocampal synaptic plasticity and emotional learning under high-stress conditions in adulthood. J. Neurosci. 2010;30:6635–6645. doi: 10.1523/JNEUROSCI.0247-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet-Morin I, Boivin M, Dionne G, Lupien SJ, Arseneault L, Barr RG, Perusse D, Tremblay RE. Variations in heritability of Cortisol reactivity to stress as a function of early familial adversity among 19-month-old twins. Arch. Gen. Psychiatry. 2008;65:211–218. doi: 10.1001/archgenpsychiatry.2007.27. [DOI] [PubMed] [Google Scholar]
- Parent CI, Meaney MJ. The influence of natural variations in maternal care on play fighting in the rat. Dev. Psychobiol. 2008;50:767–776. doi: 10.1002/dev.20342. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. Prospective investigation of stress inoculation in young monkeys. Arch. Gen. Psychiatry. 2004;61:933–941. doi: 10.1001/archpsyc.61.9.933. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Sundlass K, Schatzberg AF, Lyons DM. Maternal mediation, stress inoculation, and the development of neuroendocrine stress resistance in primates. Proc. Natl. Acad. Sci. U. S. A. 2006;103:3000–3005. doi: 10.1073/pnas.0506571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Maestripieri D. Identifying key features of early stressful experiences that produce stress vulnerability and resilience in primates. Neurosci. Biobehav. Rev. 2011;35:1466–1483. doi: 10.1016/j.neubiorev.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pembrey ME, Bygren LO, Kaati G, Edvinsson S, Northstone K, Sjostrom M, Golding J. Sex-specific, male-line transgenerational responses in humans. Eur. J. Hum. Genet. 2006;14:159–166. doi: 10.1038/sj.ejhg.5201538. [DOI] [PubMed] [Google Scholar]
- Perroud N, Paoloni-Giacobino A, Prada P, Olie E, Salzmann A, Nicastro R, Guillaume S, Mouthon D, Stouder C, Dieben K, Huguelet P, Courtet P, Malafosse A. Increased methylation of glucocorticoid receptor gene (NR3C1) in adults with a history of childhood maltreatment: a link with the severity and type of trauma. Transl Psychiatry. 2011;1:e59. doi: 10.1038/tp.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger C, Sanacora G, Krystal JH. The NMDA receptor as a therapeutic target in major depressive disorder. CNS Neurol. Disord. Drug Targets. 2007;6:101–115. doi: 10.2174/187152707780363267. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30:2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, Caspi A, Williams B, Price TS, Danese A, Sugden K, Uher R, Poulton R, Moffitt TE. Protective effect of CRHR1 gene variants on the development of adult depression following childhood maltreatment: replication and extension. Arch Gen Psychiatry. 2009;66:978–985. doi: 10.1001/archgenpsychiatry.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proulx K, Clavel S, Nault G, Richard D, Walker CD. High neonatal leptin exposure enhances brain GR expression and feedback efficacy on the adrenocortical axis of developing rats. Endocrinology. 2001;142:4607–4616. doi: 10.1210/endo.142.11.8512. [DOI] [PubMed] [Google Scholar]
- Provencal N, Suderman MJ, Guillemin C, Massart R, Ruggiero A, Wang D, Bennett AJ, Pierre PJ, Friedman DP, Cote SM, Hallett M, Tremblay RE, Suomi SJ, Szyf M. The signature of maternal rearing in the methylome in rhesus macaque prefrontal cortex and T cells. J. Neurosci. 2012;32:15626–15642. doi: 10.1523/JNEUROSCI.1470-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce CR. The regulation of maternal behaviour in marmosets and tamarins. Behav Proc. 1993;30:201–224. doi: 10.1016/0376-6357(93)90133-C. [DOI] [PubMed] [Google Scholar]
- Pryce CR. Socialization, hormones, and the regulation of maternal behavior in nonhuman simian primates. Adv Stud Anim Behav. 1996;25:423–473. [Google Scholar]
- Pryce CR, Bettschen D, Feldon J. Comparison of the effects of early handling and early deprivation on maternal care in the rat. Dev. Psychobiol. 2001;38:239–251. doi: 10.1002/dev.1018. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Palme R, Feldon J. Development of pituitary-adrenal endocrine function in the marmoset monkey: infant hypercortisolism is the norm. J. Clin. Endocrinol. Metab. 2002;87:691–699. doi: 10.1210/jcem.87.2.8244. [DOI] [PubMed] [Google Scholar]
- Radtke KM, Ruf M, Gunter HM, Dohrmann K, Schauer M, Meyer A, Elbert T. Transgenerational impact of intimate partner violence on methylation in the promoter of the glucocorticoid receptor. Transl Psychiatry. 2011;1:e21. doi: 10.1038/tp.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice CJ, Sandman CA, Lenjavi MR, Baram TZ. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology. 2008;149:4892–4900. doi: 10.1210/en.2008-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal Stress Exposure Alters Sperm MicroRNA Content and Reprograms Offspring HPA Stress Axis Regulation. J. Neurosci. 2013;33:9003–9012. doi: 10.1523/JNEUROSCI.0914-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld P, Gutierrez YA, Martin AM, Mallett HA, Alleva E, Levine S. Maternal regulation of the adrenocortical response in preweanling rats. Physiol. Behav. 1991;50:661–671. doi: 10.1016/0031-9384(91)90001-5. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P, Wetmore JB, Levine S. Effects of repeated maternal separations on the adrenocortical response to stress of preweanling rats. Physiol. Behav. 1992;52:787–791. doi: 10.1016/0031-9384(92)90415-x. [DOI] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol. Psychiatry. 2009;65:760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruedi-Bettschen D, Feldon J, Pryce CR. Circadian- and temperature-specific effects of early deprivation on rat maternal care and pup development: short-term markers for long-term effects? Dev. Psychobiol. 2004;45:59–71. doi: 10.1002/dev.20014. [DOI] [PubMed] [Google Scholar]
- Salzmann C, Otis M, Long H, Roberge C, Gallo-Payet N, Walker CD. Inhibition of steroidogenic response to adrenocorticotropin by leptin: implications for the adrenal response to maternal separation in neonatal rats. Endocrinology. 2004;145:1810–1822. doi: 10.1210/en.2003-1514. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 1986;396:64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Schapiro S, Geller E, Eiduson S. Neonatal adrenal cortical response to stress and vasopressin. Proc. Soc. Exp. Biol. Med. 1962;109:937–941. doi: 10.3181/00379727-109-27384. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Enthoven L, van der Mark M, Levine S, de Kloet ER, Oitzl MS. The postnatal development of the hypothalamic-pituitary-adrenal axis in the mouse. Int J Dev Neurosci. 2003a;21:125–132. doi: 10.1016/s0736-5748(03)00030-3. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Okimoto DK, Dent GW, Gordon MK, Levine S. Maternal regulation of the hypothalamic-pituitary-adrenal axis in the 20-day-old rat: consequences of laboratory weaning. J. Neuroendocrinol. 2002;14:450–457. doi: 10.1046/j.1365-2826.2002.00797.x. [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Deussing JM, Oitzl MS, Ohl F, Levine S, Wurst W, Holsboer F, Muller MB, de Kloet ER. Differential disinhibition of the neonatal hypothalamic- pituitary-adrenal axis in brain-specific CRH receptor 1-knockout mice. Eur. J. Neurosci. 2006a;24:2291–2298. doi: 10.1111/j.1460-9568.2006.05121.x. [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Levine S, Alam S, Harbich D, Sterlemann V, Ganea K, de Kloet ER, Holsboer F, Muller MB. Metabolic signals modulate hypothalamic-pituitary-adrenal axis activation during maternal separation of the neonatal mouse. J. Neuroendocrinol. 2006b;18:865–874. doi: 10.1111/j.1365-2826.2006.01482.x. [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Levine S, Oitzl MS, van der Mark M, Muller MB, Holsboer F, de Kloet ER. Glucocorticoid receptor blockade disinhibits pituitary-adrenal activity during the stress hyporesponsive period of the mouse. Endocrinology. 2005;146:1458–1464. doi: 10.1210/en.2004-1042. [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Liebl C, Sterlemann V, Ganea K, Hartmann J, Harbich D, Alam S, Muller MB. Neuropeptide Y mediates the initial hypothalamic-pituitary-adrenal response to maternal separation in the neonatal mouse. J. Endocrinol. 2008;197:421–427. doi: 10.1677/JOE-07-0634. [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Oitzl MS, Muller MB, Ohl F, Wurst W, Holsboer F, Levine S, De Kloet ER. Regulation of the developing hypothalamic-pituitary-adrenal axis in corticotropin releasing hormone receptor 1-deficient mice. Neuroscience. 2003b;119:589–595. doi: 10.1016/s0306-4522(03)00097-6. [DOI] [PubMed] [Google Scholar]
- Sevelinges Y, Moriceau S, Holman P, Miner C, Muzny K, Gervais R, Mouly AM, Sullivan RM. Enduring effects of infant memories: infant odor-shock conditioning attenuates amygdala activity and adult fear conditioning. Biol. Psychiatry. 2007;62:1070–1079. doi: 10.1016/j.biopsych.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Sevelinges Y, Sullivan RM, Messaoudi B, Mouly AM. Neonatal odor-shock conditioning alters the neural network involved in odor fear learning at adulthood. Learn. Mem. 2008;15:649–656. doi: 10.1101/lm.998508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnick P, Popik P, Trullas R. Glutamate-based antidepressants: 20 years on. Trends Pharmacol. Sci. 2009;30:563–569. doi: 10.1016/j.tips.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Charney DS. The science of resilience: implications for the prevention and treatment of depression. Science. 2012;338:79–82. doi: 10.1126/science.1222942. [DOI] [PubMed] [Google Scholar]
- Stanton M, Levine S. Brief separation elevates Cortisol in mother and infant squirrel monkeys. Physiol. Behav. 1985;34:1007–1008. doi: 10.1016/0031-9384(85)90029-0. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Gutierrez YR, Levine S. Maternal deprivation potentiates pituitary-adrenal stress responses in infant rats. Behav. Neurosci. 1988;102:692–700. doi: 10.1037//0735-7044.102.5.692. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Levine S. Inhibition of infant glucocorticoid stress response: specific role of maternal cues. Dev. Psychobiol. 1990;23:411–426. doi: 10.1002/dev.420230504. [DOI] [PubMed] [Google Scholar]
- Suchecki D, Rosenfeld P, Levine S. Maternal regulation of the hypothalamic-pituitary-adrenal axis in the infant rat: the roles of feeding and stroking. Brain Res. Dev. Brain Res. 1993;75:185–192. doi: 10.1016/0165-3806(93)90022-3. [DOI] [PubMed] [Google Scholar]
- Suderman M, McGowan PO, Sasaki A, Huang TC, Hallett MT, Meaney MJ, Turecki G, Szyf M. Conserved epigenetic sensitivity to early life experience in the rat and human hippocampus. Proc. Natl. Acad. Sci. U. S. A. 2012;109(Suppl 2):17266–17272. doi: 10.1073/pnas.1121260109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Holman PJ. Transitions in sensitive period attachment learning in infancy: the role of corticosterone. Neurosci. Biobehav. Rev. 2010;34:835–844. doi: 10.1016/j.neubiorev.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang AC. Neonatal exposure to novel environment enhances hippocampal-dependent memory function during infancy and adulthood. Learn. Mem. 2001;8:257–264. doi: 10.1101/lm.43101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang AC, Akers KG, Reeb BC, Romeo RD, McEwen BS. Programming social, cognitive, and neuroendocrine development by early exposure to novelty. Proc. Natl. Acad. Sci. U. S. A. 2006;103:15716–15721. doi: 10.1073/pnas.0607374103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang AC, Reeb-Sutherland BC, Yang Z, Romeo RD, McEwen BS. Neonatal novelty-induced persistent enhancement in offspring spatial memory and the modulatory role of maternal self-stress regulation. J. Neurosci. 2011;31:5348–5352. doi: 10.1523/JNEUROSCI.6808-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE. Mechanisms linking early life stress to adult health outcomes. Proc. Natl. Acad. Sci. U.S. A. 2010;107:8507–8512. doi: 10.1073/pnas.1003890107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse YC, Bagot RC, Hutter JA, Wong AS, Wong TP. Modulation of synaptic plasticity by stress hormone associates with plastic alteration of synaptic NMDA receptor in the adult hippocampus. PLoS ONE. 2011;6:e27215. doi: 10.1371/journal.pone.0027215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Marsit C, Walters OC, Carpenter LL. Childhood adversity and epigenetic modulation of the leukocyte glucocorticoid receptor: preliminary findings in healthy adults. PLoS ONE. 2012;7:e30148. doi: 10.1371/journal.pone.0030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen R, Koehl M, Abrous DN, de Kloet ER, Piazza PV, Deroche-Gamonet V. Maternal environment influences cocaine intake in adulthood in a genotype-dependent manner. PLoS ONE. 2008;3:e2245. doi: 10.1371/journal.pone.0002245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oers HJ, de Kloet ER, Li C, Levine S. The ontogeny of glucocorticoid negative feedback: influence of maternal deprivation. Endocrinology. 1998a;139:2838–2846. doi: 10.1210/endo.139.6.6037. [DOI] [PubMed] [Google Scholar]
- van Oers HJ, de Kloet ER, Whelan T, Levine S. Maternal deprivation effect on the infant’s neural stress markers is reversed by tactile stimulation and feeding but not by suppressing corticosterone. J. Neurosci. 1998b;18:10171–10179. doi: 10.1523/JNEUROSCI.18-23-10171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010;468:203–212. doi: 10.1038/nature09563. [DOI] [PubMed] [Google Scholar]
- van Rossum EF, Binder EB, Majer M, Koper JW, Ising M, Modell S, Salyakina D, Lamberts SW, Holsboer F. Polymorphisms of the glucocorticoid receptor gene and major depression. Biol Psychiatry. 2006;59:681–688. doi: 10.1016/j.biopsych.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Blume A, Niederle D, Buwalda B, Neumann ID. Effects of early life stress on adult male aggression and hypothalamic vasopressin and serotonin. Eur. J. Neurosci. 2006;24:1711–1720. doi: 10.1111/j.1460-9568.2006.05045.x. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Neumann ID. Maternal separation enhances offensive play-fighting, basal corticosterone and hypothalamic vasopressin mRNA expression in juvenile male rats. Psychoneuroendocrinology. 2009;34:463–467. doi: 10.1016/j.psyneuen.2008.10.017. [DOI] [PubMed] [Google Scholar]
- Walker CD. Chemical sympathectomy and maternal separation affect neonatal stress responses and adrenal sensitivity to ACTH. Am. J. Physiol. 1995;268:R1281–1288. doi: 10.1152/ajpregu.1995.268.5.R1281. [DOI] [PubMed] [Google Scholar]
- Walker CD, Akana SF, Cascio CS, Dallman MF. Adrenalectomy in the neonate: adult-like adrenocortical system responses to both removal and replacement of corticosterone. Endocrinology. 1990;127:832–842. doi: 10.1210/endo-127-2-832. [DOI] [PubMed] [Google Scholar]
- Walker CD, Sapolsky RM, Meaney MJ, Vale WW, Rivier CL. Increased pituitary sensitivity to glucocorticoid feedback during the stress nonresponsive period in the neonatal rat. Endocrinology. 1986;119:1816–1821. doi: 10.1210/endo-119-4-1816. [DOI] [PubMed] [Google Scholar]
- Walker CD, Scribner KA, Cascio CS, Dallman MF. The pituitary-adrenocortical system of neonatal rats is responsive to stress throughout development in a time-dependent and stressor-specific fashion. Endocrinology. 1991;128:1385–1395. doi: 10.1210/endo-128-3-1385. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Champagne FA, Brown SE, Dymov S, Sharma S, Meaney MJ, Szyf M. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J. Neurosci. 2005;25:11045–11054. doi: 10.1523/JNEUROSCI.3652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegert O, Joels M, Krugers H. Timing is essential for rapid effects of corticosterone on synaptic potentiation in the mouse hippocampus. Learn. Mem. 2006;13:110–113. doi: 10.1101/lm.87706. [DOI] [PubMed] [Google Scholar]
- Witzmann SR, Turner JD, Meriaux SB, Meijer OC, Muller CP. Epigenetic regulation of the glucocorticoid receptor promoter 1(7) in adult rats. Epigenetics. 2012;7:1290–1301. doi: 10.4161/epi.22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R. Are different biological mechanisms involved in the transmission of maternal versus paternal stress-induced vulnerability to offspring? Biol. Psychiatry. 2011;70:402–403. doi: 10.1016/j.biopsych.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zhang LX, Levine S, Dent G, Zhan Y, Xing G, Okimoto D, Kathleen Gordon M, Post RM, Smith MA. Maternal deprivation increases cell death in the infant rat brain. Brain Res. Dev. Brain Res. 2002;133:1–11. doi: 10.1016/s0926-6410(01)00118-5. [DOI] [PubMed] [Google Scholar]
- Zhang TY, Chretien P, Meaney MJ, Gratton A. Influence of naturally occurring variations in maternal care on prepulse inhibition of acoustic startle and the medial prefrontal cortical dopamine response to stress in adult rats. J. Neurosci. 2005;25:1493–1502. doi: 10.1523/JNEUROSCI.3293-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]