Abstract
This review aims to disentangle cause and effect in the relationship between anisometropia and amblyopia. Specifically, we examine the literature for evidence to support different possible developmental sequences that could ultimately lead to the presentation of both conditions. The prevalence of anisometropia is around 20% for an inter-ocular difference of 0.5D or greater in spherical equivalent refraction, falling to 2-3%, for an inter-ocular difference of 3D or above. Anisometropia prevalence is relatively high in the weeks following birth, in the teenage years coinciding with the onset of myopia and, most notably, in older adults starting after the onset of presbyopia. It has about one-third the prevalence of bilateral refractive errors of the same magnitude. Importantly, the prevalence of anisometropia is higher in highly ametropic groups, suggesting that emmetropization failures underlying ametropia and anisometropia may be similar.
Amblyopia is present in 1-3% of humans and around one-half to two-thirds of amblyopes have anisometropia either alone or in combination with strabismus. The frequent co-existence of amblyopia and anisometropia at a child’s first clinical examination promotes the belief that the anisometropia has caused the amblyopia, as has been demonstrated in animal models of the condition. In reviewing the human and monkey literature however it is clear that there are additional paths beyond this classic hypothesis to the co-occurrence of anisometropia and amblyopia. For example, after amblyopia secondary to either deprivation or strabismus has emerged, anisometropia often follows. In cases of anisometropia with no apparent deprivation or strabismus, questions remain about the failure of the emmetropization mechanism that routinely eliminates infantile anisometropia. Also, the chronology of amblyopia development is poorly documented in cases of ‘pure’ anisometropic amblyopia. Although indirect, the therapeutic impact of refractive correction on anisometropic amblyopia provides strong support for the hypothesis that the anisometropia caused the amblyopia. Direct evidence for the aetiology of anisometropic amblyopia will require longitudinal tracking of at-risk infants, which poses numerous methodological challenges. However, if we are to prevent this condition, we must understand the factors that cause it to develop.
1. Introduction and Rationale
The majority of human ametropes can be characterised as isoametropic, in that the refractive status of their two eyes is very similar. For example, in the large-scale study by Qin et al. (2005, their Fig. 3A), at least 85% of those aged up to 70 years had right and left eye ocular refractions which were matched to within 1 dioptre. In a minority of humans, however, there are significant interocular differences in refractive error (anisometropia), which can be accompanied by an interocular difference in visual acuity that is optically uncorrectable, at least initially (amblyopia). The co-occurrence of these two anomalies, with no additional abnormality, is labelled ‘anisometropic amblyopia’. Anisometropia, therefore, is a special case of an emmetropization failure that is commonly accompanied by a serious neurological deficit. Le Cat (1713, reviewed in Ciuffreda et al., 1991) is credited with providing the first accurate description of amblyopia, and anisometropic amblyopia has been identified clinically since 1743 when George Louis Leclerc, Count de Buffon, proposed a treatment for this condition which is as relevant now as it was when it was first proposed: refractive correction and occlusion of the better eye. Anisometropic amblyopia continues to be treated by refractive correction alone or in combination with patching or other therapies that differentially stimulate the two eyes (Ciuffreda et al., 1991; Simons, 2005; Shotton et al., 2008; Taylor, 2012).
Although anisometropia and amblyopia are often discovered at the same time, for example during a school vision screening, it is widely held that the anisometropia is a precursor to, and indeed the cause of the amblyopia. However, definitive evidence that anisometropia universally precedes development of the amblyopia is lacking, and the simplicity of this cause and effect relationship continues to be challenged (Almeder et al., 1990; Barrett et al., 2005; Lempert, 2000, 2003, 2004, 2008a, 2008b; Lempert and Porter, 1998, Smith and Hung, 1999). In light of the general uncertainty about the aetiology of anisometropia and anisometropic amblyopia, an examination of the literature is timely as part of the continuing effort to refine approaches to vision screening and clinical care. This review examines the human and non-human primate literature concerning the co-occurrence of these two conditions in an attempt to gain insight into their origins and the underlying relationships between them. It is timely because it coincides with a recent surge in the number of published articles on the topic, from around 1000 per decade between 1960 and 2000 to around 1900 during the last decade (a PubMed search conducted on July 1st 2012 using the term ‘anisometropic amblyopia’ yielded 7046 citations, Figure 1). Anisometropic amblyopia is also of major significance from a clinical perspective. In 1980 it was estimated that each year in the USA 1.2 million office visits for medical eye care were related to amblyopia and its associated conditions (National Society to Prevent Blindness, 1980). Given that anisometropia is present in half to two-thirds of amblyopes (Flynn and Cassady, 1978; Flom and Bedell, 1985; Attebo et al., 1998; Robaei et al., 2006; Friedman et al., 2009; MEPEDS, 2009), it is clear that anisometropic amblyopia represents an important public health concern. Also, understanding the aetiology of anisometropic amblyopia could provide general insight into failures of emmetropization that are so apparent in the general global epidemic of myopia (Saw, 2003).
Figure 1.

Number of publications about ‘anisometropic amblyopia’ per decade, identified using PubMed searches. [Search conducted, July 1st, 2012].
A thoughtful discussion of the origins of anisometropia amblyopia by Abrahamsson and Sjostrand (1996) raised a series of central questions about its aetiology, providing context and motivation for this review: “…is the marked anisometropia at 1 year of age already present at birth and the result of a [prenatal] growth delay in the more hyperopic eye? Is transitory anisometropia during childhood a part of natural development? Does it cause amblyopia? Does amblyopia cause the emmetropization to stop and thereby make the anisometropia persistent? Does amblyopia create anisometropia?” (p.862). The discussion below surveys the extensive experimental and clinical literature about anisometropia and amblyopia to explore these and related questions.
2. Definitions
Anisometropia
The term anisometropia refers to a difference in sphero-cylindrical refractive error between the right and left eyes. Strictly speaking any interocular difference in refractive error could be termed anisometropia. However, since test-retest studies of refractive error measurement indicate a substantial degree of variability, measurement precision needs to be taken into account when defining and diagnosing anisometropia. Successive refractions of the same eye by different clinicians or repeated refractions by the same clinician can differ by up to 0.75 dioptres (Goss and Grosvenor, 1996; Bullimore et al., 1998; MacKenzie, 2008; Shah et al., 2009). For this reason, the term ‘anisometropia’ is usually reserved for clinically significant differences in refractive error that exceed some criterion amount, e.g. ≥±0.75D. It is typically considered a fundamental axial length anomaly in which the size of the right and left eyes differ (Sorsby et al., 1962b; Bradley et al., 1983; Rabin et al., 1983; Smith and Hung, 1999; Smith et al., 1999; Hung et al., 1995; Tong et al., 2004; Cass and Tromans, 2008; O’Donoghue et al., 2013), but in some instances it can be refractive when the optical power of the eyes differ. Anisometropia is not purely an issue of right- versus left-eye axial length difference in that individuals with anisometropia also tend to exhibit high levels of astigmatism (Ingram, 1979; Qin et al., 2005; Dobson et al., 2008a) and individuals with aniso-astigmatism (defined as the absolute difference in refractive astigmatism, e.g. Huynh et al., 2006; O’Donoghue et al., 2013) exhibit differences in corneal toricity. When considering higher order monochromatic aberrations, it appears that there are no significant inter-ocular differences between the two eyes of anisometropic adults (Vincent et al., 2011; Tian et al., 2011).
Amblyopia
Amblyopia is a neurological disorder of vision that is believed to follow abnormal binocular interaction or visual deprivation during early life. von Noorden (1977) defines amblyopia as “a decrease in visual acuity in one eye when caused by abnormal binocular interaction, or in one or both eyes as a result of pattern vision deprivation during visual immaturity, for which no cause can be detected during the physical examination of the eye(s) and which in appropriate cases is reversible by therapeutic measures”. Most definitions of amblyopia, like the one just quoted, are clinically-based, exclusionary definitions that do not incorporate the true neurological nature of this condition, but rather define amblyopia based upon criterion levels of a very specific visual capability (visual acuity) in the absence of other causal factors. Some more recent definitions (e.g. Ciuffreda et al., 1991) make explicit the need for ‘amblyogenic’ factors to be present to confirm the diagnosis. The underlying neurological anomaly forming the basis of amblyopia appears to be located in the primary and secondary visual cortex (for reviews see: Hess, 2001; Barrett et al., 2004; Anderson and Swettenham, 2006; Hess et al., 2009; Hess et al., 2010; although see Brown et al., 2013 in which multi-focal electro-retinogram responses were reduced in a sample (n=5) of amblyopic individuals who also exhibited reduced function of the lateral geniculate nucleus). This neurological anomaly in the visual pathway generates a wide range of visual deficits beyond the clinical acuity-based definition (for review see Levi and Carkeet, 1993) including, for example, reduced contrast sensitivity at high and medium spatial frequencies (Hess and Howell, 1977; Hess, 1979; Levi and Harwerth, 1977; Bradley and Freeman, 1981; Levi, 1988), reduced stereoacuity (Reinecke, 1979; McKee et al., 2003; O’Connor et al., 2010), impaired sensitivity to shape changes and abnormalities in contour processing (Hess et al., 1999; Kovacs et al., 2000; Chandna et al., 2001; Levi et al., 2007) plus abnormal patterns of lateral interaction (Bonneh, 2004; Polat et al., 2005). Amblyopes also exhibit poor performance on spatial localisation tasks (Bedell and Flom, 1981; Bedell and Flom, 1983; Levi et al., 1987; Fronius et al., 2004) such as vernier acuity tasks (Levi and Klein, 1982a, 1982b; Levi and Klein, 1983, 1985; Levi et al., 1985) and often exhibit dramatic misperception of gratings (Hess et al., 1978; Barrett et al., 2003) and other targets (Pugh, 1958; Lagreze and Sireteanu, 1991; Sireteanu et al., 2008).
Anisometropic Amblyopia
From the outset, the term ‘anisometropic amblyopia’ must be defined carefully. For the purposes of this review the term is used to describe the co-occurrence of anisometropia and amblyopia without invoking any specific causal relationship. It is worth noting that there is no indication that the anisometropia which accompanies amblyopia is qualitatively any different from anisometropia in the absence of amblyopia. The same, however, cannot be said for the amblyopia that accompanies anisometropia. There are many claims in the literature that the amblyopia associated with anisometropia differs from that associated with strabismus (for reviews see Ciuffreda et al., 1991 and Levi and Carkeet, 1993; see also Levi and Klein, 1982a, 1982b, 1983, 1985; Levi et al., 1985; Hess and Pointer, 1985; Thiel and Sireteanu, 2009) although more recent studies appear to question the strength of the distinction between anisometropic and strabismic amblyopia (McKee et al., 2003). It is possible, for example, that the apparent distinction between these amblyopias may in fact reflect differences in the severity of the amblyopia (Birch and Swanson, 2000) or in the extent to which there is any residual binocularity (McKee et al., 2003).
3. Prevalence
Whereas amblyopia has been the subject of many literature reviews (Awaya and Watanabe, 1995; Barrett et al., 2004; Hess, 2001; Hess et al., 2011; Hoyt, 2005; Kanonidou, 2011; Kiorpes and McKee, 1999; Levi, 2006; Simons, 2005; von Noorden, 1978) over the past forty years, the same cannot be said for anisometropia (Weale, 2002 & 2003). Thus, we begin with a comprehensive review of anisometropia prevalence. A contemporary summary of amblyopia prevalence is then provided (section 3.2) before we examine the co-occurrence of anisometropia and amblyopia (section 3.3).
In trying to establish prevalence figures for anisometropia, amblyopia and anisometropic amblyopia it is worth noting some influential factors. Although there is a vast literature describing the prevalence of refractive errors and amblyopia, a number of factors make comparisons between studies problematic. The issue of whether the data are drawn from population-based or clinical samples is key. Individuals with amblyopia and refractive error are more likely to present for eye examinations than those without these conditions. Hence amblyopia and anisometropia prevalence estimates from clinical samples are likely to over-estimate the population-based values, even when identical examination methods and criteria for diagnosis are employed. Aside from the study sample, the issue of whether prevalence data (for example, as a function of age) are drawn longitudinally or from a study with a cross-sectional design is also important, as will be discussed below. Other relevant factors include the criterion used to diagnose amblyopia and anisometropia, whether the study was prospective or retrospective in design, and the methodology used. Anisometropia diagnosis and measurement, for example, can be made using photorefraction without a cycloplegic agent whereas other studies may need to use cycloplegia if the two eyes are being examined sequentially. Also, some studies of very young children have used motor fixation preference to make a diagnosis of amblyopia, rather than a direct estimate of sensory visual acuity. It is also possible that subtle pathology may have been missed, leading to inappropriate diagnosis of amblyopia. Issues also surround the assessment of fixation status (as central or eccentric) in individuals without manifest strabismus. This assessment will drive a diagnosis of either “pure anisometropic amblyopia” or “microstrabismus”. All of these factors must be borne in mind when interpreting studies of prevalence. Some of these factors (e.g. influence of criterion) are considered explicitly in sections below while others permeate the discussion of prevalence that now follows.
3.1 Prevalence of Anisometropia
Comparison with Isoametropia
Interocular differences in spherical and astigmatic refractive error seem to be considerably less common than approximately matched refractive errors in the two eyes or isoametropia. For example, Ingram (1979) reported bilateral hyperopia of ≥+2D in ~11.8% of a sample that was recruited in the community and which consisted of 1648 UK infants aged from 11 to 13 months; however, only 6.5% of the same sample exhibited anisometropia (difference of 1D or more in sphere and/or cylinder). Ingram’s findings are listed in Table 1 along with the results of other studies sampling different ages and geographical locations that have compared anisometropia prevalence with the prevalence of bilateral spherical or astigmatic ametropia. Unfortunately many large-sample, population-based studies of refractive error do not report anisometropia prevalence and so are not listed here (e.g. Refractive Error Study in Children (RESC), Zhao et al., 2000; Maul et al., 2000; Kempen et al., 2004).
Table 1.
A comparison of the prevalence of different forms of refractive error. Studies of younger age groups are listed closer to the top.
| Authors (year) |
n (country, age range, methodology) |
Myopia | Hyperopia | Astigmatism | Anisometropia |
|---|---|---|---|---|---|
| Ingram (1979) | 1648 (UK, 11-13 month olds, retinoscopy following atropine cycloplegia). Clinic- based study. Parents of children who attended for hearing test at age 9 months were invited to have their child’s eyes examined. |
Not Reported |
11.8% (≥+2D) |
13.3% (≥1.5D) |
6.5% (≥1D difference in sphere or cylinder) |
| Dirani et al. (2010) | 3009 (Singapore, 6 to 72 month olds, cycloplegic autorefraction). Population- based study in southwest Singapore featuring door-to- door recruitment of participants. |
11% (SE ≤−0.5D) |
7.8% (SE ≥+3D) |
8.6% (≥0.75DC) |
0.6% (difference in SE ≥2D) |
| Borchert et al (2010) | 6024 (USA, 6 to 72 month olds, cycloplegic autorefraction) 2994 (African American, AA) 3030 (Hispanic, H). Population- based survey of 6-72 month-old subjects identified by door-to- door screening of families. |
6.6% (AA) 3.7% (H) (SE ≤−1D) |
20.8% (AA) 26.9% (H) (SE ≥+2D) |
Not stated Not stated |
4.2% (AA) 4.3% (H)(difference in SE ≥1D) |
| Giordano et al. (2009) | 2298 (USA, 6 to 71 month olds, cycloplegic autorefraction) 1268 (African American, AA) 1030 (White, W). Population- based evaluation of the prevalence of ocular disorders in children aged 6 to 71 months in Baltimore, Maryland, USA. (The Baltimore Pediatric Eye Disease Study, BPEDS). |
5.5% (AA) 0.7% (W) (SE ≤−1D in eye with lesser refractive error) |
4.4% (AA) 8.9% (W) (SE ≥+3D in eye with lesser refractive error) |
13.1% (AA) 11.4% (W) (≥1.5DC) |
1% (AA) 1.5% (W) (difference in SE ≥2D) |
| Huynh et al. (2006) | 1765 (Australia, 6 year olds, cycloplegic autorefraction). Part of the “Sydney Myopia Study” is a population based study of eye health in Australian schoolchildren. Six year old children were recruited during 2003–4 from 34 primary schools using random cluster sampling. Informed consent obtained from parents prior to participation. |
1.5% (SE ≤−0.5D) |
13.2% (SE ≥+2D) |
Not stated | 1.6% (difference in SE ≥1D) |
|
O’Donoghue et al. (2010); O’Donoghue et al. (2011); O’Donoghue et al. (2013); |
661 (Northern Ireland, 12-13 year olds) and 392 (Northern Ireland, 6-7 year olds. Cycloplegic autorefraction. Cross-sectional, population- based study (Northern Ireland Childhood Errors of Refraction Study). |
2.8% in 6-7 year-olds, 17.7% in 12- 13 year olds (SE ≤−0.5D) |
26% in 6-7 year-olds, 14.7% in 12- 13 year olds (SE ≥+2D) |
24% in 6-7 year-olds, 20% in 12-13 year olds (≥1DC) |
8.5% in 6-7 year-olds, 9.4% in 12-13 year olds (difference in SE ≥1D) |
| Yekta et al. (2010) | 1872 (Iran, 7-15 year olds, cycloplegic autorefraction). Cross-sectional, population- based study. Sampling was carried out using a random cluster approach by taking schools as clusters. |
4.35% (SE ≤−0.5D) |
5.0% (SE ≥+2D) |
11.3% (≥0.75DC) |
2.6% (difference in SE ≥1D) |
| Tong et al. (2004) | 1979 (Singapore, 7-to 9-year olds, cycloplegic autorefraction). Population- based, cross-sectional study of children attending 3 Singapore schools. Informed, written consent was obtained from the parents of the children. |
36.4% (SE ≤−0.5D) |
34.4% (SE ≥+0.5D) |
Not stated | 1.6%(differenc e in SE ≥1.5D) |
| Ohlsson et al. (2003) | 1035 (Mexico, 12- &13-year olds, retinoscopy following installation of 0.5% tropicamide). Population-based study of 12-13 year old children in Monterrey, Mexico. Parental consent was required prior to participation. |
40% (SE ≤−0.5D) |
9.3% (SE ≥+1D) |
9.5% (≥1.5DC) |
5.3%(≥1.5D difference in sphere or cylinder) |
| Ohlsson et al. (2001) | 1046 (Sweden, 12- &13-year olds, retinoscopy following installation of 0.5% tropicamide). Population-based study of 12-13 year old children in Gothenburg, Sweden. Parental consent was required prior to participation. |
45% (SE ≤−0.5D) |
8.4% (SE ≥+1D) |
5.2% (≥1.5DC) |
3.3% (≥1.5D difference in sphere or cylinder) |
| Sorsby et al. (1960); Sorsby et al. (1962) | 1033 (UK, 17 to 27 year old male army recruits, cycloplegic refraction) |
5.6% (SE ≤-1D) |
13.6% (SE ≥+2D) |
10.0% (≥1DC) |
6.6% (≥1D difference in sphere or cylinder) |
| Katz et al. (1997). | 613 (USA, African-Americans aged 40-49 year olds, non- cycloplegic refraction). Population-based survey of ocular disorders among non- institutionalized subjects 40 years of age and older living in east Baltimore, USA. |
28.9% (F) 34.0% (M) (SE ≤−0.5D) |
16.7% (F) 11.8% (M) (SE ≥+0.5D) |
22.9% (F) 21.2% (M) (≥0.5DC) |
4.5% (F) 2.8% (M) (difference in SE ≥1D) |
| Wong et al. (2000). | 1232 (Chinese Singaporeans, 40 to 79 year olds, non- cycloplegic refraction). Population-based survey of ocular disorders among adults living in Singapore. The 1996 Singapore electoral register was used for sampling. |
38.7% (SE ≤−0.5D) |
28.4%(SE ≥+0.5D) |
37.8% (≥0.5DC) |
15.9% (difference in SE ≥1D) |
| Attebo et al. (1999) | 3654 (Australia, 49 to 97 year- olds, non-cycloplegic refraction). Population-based, cross-sectional study of eye disease in elderly people living in the community (‘Blue Mountains Eye Study’). All identified, eligible residents were invited to attend a clinic appointment. |
15% (SE ≤−0.5D) |
57% (SE ≥+0.5D |
37.0% (≥0.75DC) |
14.1% (difference in SE ≥1D) |
| Anton et. al. (2009) | 569 (Spain, 49 to 79 years, non-cycloplegic refraction). Subjects were randomly selected in a stratified manner according to gender and age in a cross-sectional, population-based epidemiologic study, |
25.4% (SE≤−0.5D) |
43.6% (SE≥+0.5D) |
53.5% (≥−0.5DC) |
12.3% (SE ≥1D) |
| Krishnaiah et al. (2009) | 10293 (South India, adults 40 years and above, subjective refraction). Population-based, cross-sectional epidemiologic study. |
34.6% (SE≤−0.5D) |
18.4% (SE ≥+0.5D) |
37.6% (≥−0.5DC) |
13.0% (SE ≥0.5D) |
| Liang et al. (2009) | 6491 (Rural China, phakic adults aged 30 years and above, subjective refraction). Population-based, cross- sectional study that employed a clustered, random sampling procedure to select participants. |
26.7% (SE≤−0.5D) |
15.9% (SE ≥+0.5D) |
24.5% (≥0.5DC) |
7.7% (SE ≥1D) |
| Tan et al. (2011) | 1835 (Singapore, 55-85 year olds, autorefraction). Population-based, cross- sectional study in Singapore(‘Singapore Longitudinal Aging Study, SLAS) of adults living in a geographically defined area in district of Singapore. Subjects were identified using door-to-door census, and all respondents signed written informed consent. |
30.0% (SE≤−0.5D) |
41.5% (SE ≥+1D) |
43.5% (≥1DC) |
22.1% (SE ≥1D) |
F: female; M: Male; AA: African-American; W: White; H: Hispanic; SE: Spherical Equivalent. Only studies with >500 participants were considered for inclusion in this table of comparison of refractive error prevalence. Note that whereas some studies cited above report raw prevalence (e.g. Ingram, 1979), others (e.g. Liang et al., 2009) report prevalence figures that have been adjusted so as to match, for example, the age and gender characteristics of their sample to full population (i.e. census) data.
The between-study differences in anisometropia prevalence shown in Table 1 most likely reflect some combination of difference in criteria and the significant co-variates of age, refractive error and racial profile of the samples. Despite these differences in prevalence, the studies summarised in the table show very clearly that anisometropia is less prevalent than isoametropic refractive error. For example, anisometropia prevalence is approximately 50% (SD±40%) of that observed for myopia, and 34% (±21%) and 36% (±19%) of the hyperopia and astigmatism rates, respectively (Table 1). One could argue from these data that it is more important for the visual system to achieve correspondence between the refractive status of the two eyes than to achieve emmetropia, or that ametropia of a similar magnitude in the two eyes may be in some sense preferable to anisometropia. An alternative, simpler explanation, may be that just like limb length (Tanner, 1978), ocular dimensions, and thus refraction, exhibit approximate bilateral symmetry because the genetic, and many of the environmental factors, that determine the refractive error of the right eye also exist for the left eye (for review see Wallman and Winawer, 2004).
Whatever the underlying explanation, there is strong empirical evidence from numerous species including the chicken (Schmid and Wildsoet, 1997), guinea pig (Jiang et al., 2009), marmoset (Graham and Judge, 1999; Troilo et al., 2009) and macaque (Smith et al., 1999; Kiorpes et al., 1998; Zhong et al., 2004) that an interocular difference in refractive error is typically actively compensated for by differential eye growth during early postnatal development (see section 4.1). There appears, therefore, to be strong pressure for the visual system to attain matching refractive status in the two eyes and thus to limit the magnitude of anisometropia.
The following sections now consider additional factors that relate to prevalence:
Dependence on Criterion
The prevalence of a condition depends critically upon the criterion used to diagnose it, and numerous different criteria have been employed to define anisometropia. For example, anisometropia has been defined as a difference in the spherical-equivalent refraction (i.e. sphere + cylinder/2) of the two eyes of ≥1D (e.g. Katz et al., 1997; Wong et al., 2000), ≥1.5D (Phelps and Muir, 1977) or ≥2D (Voo et al., 1998). Other studies have defined anisometropia as a difference in refractive power of either sphere or cylinder that reaches some minimum level (e.g. 1.5D, Ohlsson et al., 2011), while others define it as a difference between the two eyes in corresponding meridia that reaches some threshold level (e.g. 1.5D, Kuo et al., 2003) or as a difference of ≥2D in the meridian with the greatest refractive error (Stigmar et al., 1978). More recent studies of anisometropia (e.g. Qin et al., 2005) have reported both vector-based (mean spherical equivalent, J0 and J45; Thibos et al., 1997) and non-vector-based (spherical and cylindrical powers) analyses of interocular differences in refraction.
Figure 2 shows how anisometropia prevalence is linked to the criterion used to diagnose it. Prevalence estimates can be high (e.g. over 40% in Cheng et al.’s (2003) study of individuals aged 65 years and above) but fall rapidly when the criterion for diagnosis is increased. For example, in Tarczy-Hornoch et al.’s (2011) study of 5710 children aged between 30 and 72 months from the Multi-Ethnic Pediatric Eye Disease (MEPEDS) and Baltimore Pediatric Eye Disease studies, 20%, 3.8% and 0.7% of the sample had spherical equivalent anisometropia of ≥0.50D, ≥1D and ≥2D, respectively.
Figure 2.
Dependence of prevalence on the criterion used to diagnose anisometropia. Anisometropia is given as the inter-ocular difference in spherical-equivalent refractive error. Results are shown from studies that quoted prevalence figures using different criteria in a single sample. (Cheng et al., 2003; Chia, 2010; Deng and Gwiazda, 2012; Giordano et al., 2009; Gupta et al., 2008; Guzowski et al., 2003; Huynh et al., 2006; Ohlsson et al., 2003; Qin et al., 2005; Quek et al., 2004; Saw et al., 2002; Tarczy-Hornoch et al., 2011; Tong et al., 2004).
The impact of the criterion used to define anisometropia is nicely demonstrated in the MEPEDS population study of 6- to 72-month-old children in the USA by Borchert et al. (2010). The authors found that the prevalence of cylindrical anisometropia (defined as cylinder amplitude, regardless of axis) or spherical equivalent anisometropia did not diminish beyond 1 year of age whereas the prevalence of vector-based cylindrical anisometropia (calculated using Power Vectors; Thibos et al., 1997) decreased steadily beyond the age of 1. Ingram (1979) commented on this issue when he stated: “It is also a timely reminder of how arbitrarily the criteria for ‘anisometropia’ were selected and that in reality isoametropia gradually merges into anisometropia” (p.346). Adopting a consistent criterion for diagnosis would facilitate comparisons across studies. Figure 2 suggests a prevalence of anisometropia of around 20% for a criterion of ≥0.5D inter-ocular difference in spherical equivalent, falling to 15%, 3 to 4%, 2 to 3%, and around 1% when the criterion changes to ≥1D, ≥2D, ≥3D and ≥4D, respectively.
Relationship between Anisometropia and other Components of Refractive Error
Many studies have reported a positive association between the prevalence and severity of anisometropia, and the level of spherical ametropia (Guzowski et al., 2003; Parssinen, 1990; Goldschmidt et al. 2004; Tong et al., 2004; Fledelius, 1984; Attebo et al., 1999) and astigmatism (Ingram et al., 1979; Dobson et al., 2008b; Guzowski et al., 2003; Qin et al., 2005). Fledelius (1984) noted that anisometropia is more commonly found in cases of high ametropia, in particular amongst individuals with large amounts of myopia, and many others have corroborated these findings (e.g. Attebo et al., 1999; Qin et al., 2005). For example, in their population study of over 3400 adults aged 49 years and above, Guzowski et al. (2003) reported that both the prevalence and the severity of anisometropia increased with increasing levels of ametropia in myopes and hyperopes, but the rise was more dramatic in myopic individuals. A large-scale study of 6-year-old children (Huynh et al., 2006) also noted a much greater prevalence of anisometropia (≥1.0D difference in spherical equivalent refractive error) in children with moderate hyperopia (≥+2D spherical equivalent, anisometropia prevalence 10.1%) compared to those with mild hyperopia (>0.51 to <+2D spherical equivalent, anisometropia prevalence 0.1%). Qin et al.’s (2005) study of around 91,000 individuals employed a multiple regression model to examine whether spherical-equivalent ametropia and astigmatism are independently associated with anisometropia (factoring out potentially significant co-variates such as age). They showed that anisometropia is independently associated with both spherical ametropia and astigmatism. Anisometropia prevalence increased from 10% to almost 20% as the level of ametropia in the least ametropic eye increased from myopia of −1D to myopia of −3 to −4D. They found a roughly linear increase in anisometropia prevalence and severity with increasing levels of myopia. In hyperopes the trend was similar but less linear. It should be pointed out however, that although Qin et al. (2005) found levels of spherical ametropia to be significantly associated with both the prevalence and severity of anisometropia, it was cylindrical refractive error that was the parameter most strongly associated with anisometropia. The co-occurrence between anisometropia, astigmatism and amblyopia is briefly discussed in section 4.4.
Studies of anisometropia that are based on clinic records generally find anisomyopes to be about two to five times more prevalent than anisohyperopes (Sorsby et al., 1962b: 63% vs. 27%, United Kingdom, criterion ≥2D difference; Tanlamai and Goss, 1979: 71% vs. 22%, Thailand, criterion ≥2D difference; Tanlamai and Goss, 1979: 76% vs. 16%, USA, criterion ≥1D difference). Antimetropia (where one eye is myopic, but the other eye is hyperopic) was reported in about 8% by Tanlamai and Goss in both of their samples. One exception to this pattern was observed by de Vries (1985) in a sample of anisometropic (≥2D difference in spherical or cylindrical power) children attending a hospital eye clinic. The proportions of anisomyopes, anisohyperopes and antimetopes in that sample were 20%, 70% and 10%, respectively. The difference between de Vries (1985) and other clinical populations (Sorsby et al., 1962a; Tanlamai and Goss, 1979) is probably accounted for by the fact that the de Vries sample contained only children (aged up to 10 years, who are less likely to exhibit myopia), and because a high proportion (42%) of them also had strabismus (see section 4.3).
The studies summarized above compared anisomyopia and anisohyperopia in clinical samples. As a result they are likely to show substantial bias, because for example, young hyperopes may be asymptomatic and thus not present for an eye examination. Nevertheless, a number of population-based studies in adults (Attebo et al., 1999; Guzowski et al., 2003) and children (Huynh et al., 2006) have confirmed the qualitative findings from clinical populations, namely that while a greater prevalence and severity of anisometropia is associated with higher levels of ametropia, the link is particularly evident in myopia. The relative proportion of anisomyopes to anisohyperopes in any given sample, clinical or population-based, is therefore associated with underlying myopia and hyperopia prevalence in that group. In samples with a similar or greater proportion of myopia compared to hyperopia, anisomyopia presents up to six times more commonly than anisohyperopia (Sorsby et al., 1962a; Tanlamai and Goss, 1979; Tong et al., 2004) whereas in populations with greater a proportion of hyperopes, the numbers of anisomyopes and anisohyperopes are more balanced (Tong et al., 2004) or anisohyperopes are more commonly encountered (de Vries, 1985; Huynh et al., 2006).
The link between the level of ametropia and the prevalence and severity of anisometropia indicates that an increasing failure of emmetropization is also associated with an increasing failure of coordinated eye growth across the eyes. Thus, understanding the origins of anisometropia could have important implications for our understanding of the origins of ametropia in general.
Effect of Age
Many studies have provided prevalence estimates for anisometropia derived from samples containing a broad age range (e.g. Giles, 1950; Woodruff and Samek, 1977; Phelps and Muir, 1977; Aine, 1984; Fledelius, 1984). For example, Woodruff and Samek (1977) studied the Amerind population in Ontario and found that 7.25% of their population (n=3722) aged from birth to 90+ years had anisometropia of ≥1D, which is similar to the 7.4% that Giles quoted from a study (n=2500) conducted in Great Britain (Giles, 1950) and Aine’s figure of 7% from a Finnish population (n=611) aged 6-85 years (≥1.25D in spherical equivalent) (Aine, 1984). Fledelius (1984) estimated anisometropia (≥1D in spherical equivalent) to be 9.3% in a Danish population (n=1200) aged 16-85 years. These figures for samples spanning a wide age range mask large differences in anisometropia prevalence at different times during life, however.
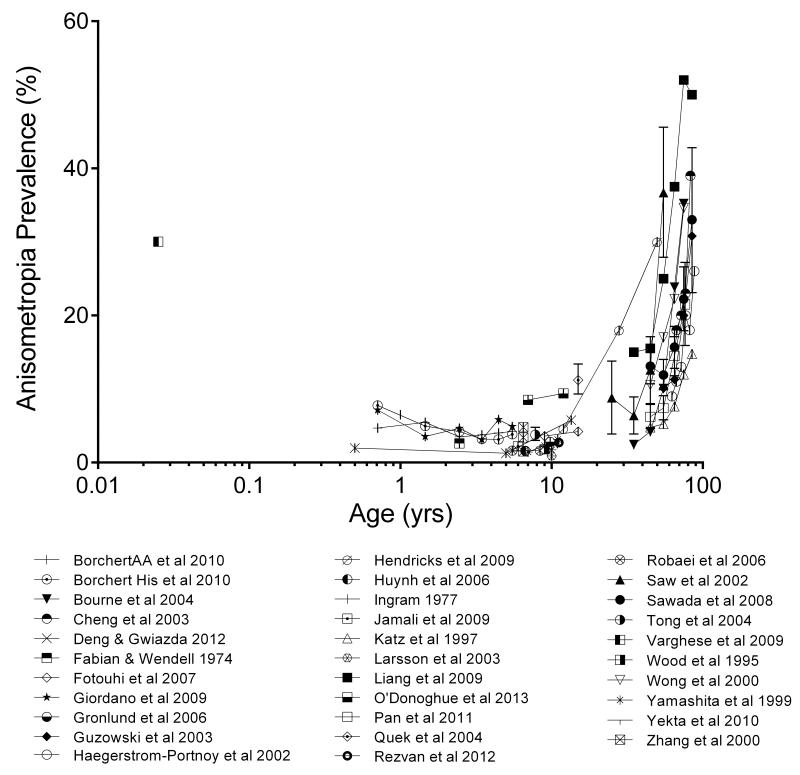
Despite difficulties in comparing anisometropia across studies (due to criterion differences, for example, see Figure 2), a picture emerges of anisometropia prevalence over the lifespan (Figure 3). Weale (2002) described the pattern as being ‘u’-shaped. Prevalence is high in the first few weeks of postnatal life (Zonis and Miller, 1974; Fulton et al., 1980; Varghese et al., 2009), but then decreases in early childhood (e.g. Atkinson et al., 1996), only to increase again in the teenage years as myopia emerges (e.g., Parssinen, 1990; Laird, 1991; Lin et al., 1999). Prevalence is then stable in early adulthood (between 20 and 40 years, e.g. Qin et al., 2005) but progressively increases after the onset of presbyopia, at first modestly but then rapidly in later life (Weale, 2002; Figure 3).
Figure 3.
Prevalence of anisometropia as a function of age. To account for differences in criterion used to diagnose anisometropia, only studies employing the most commonly used criterion are included, a difference of 1D or greater in spherical equivalent refraction between the right and left eyes.
Few studies have examined anisometropia in infancy and in those that have, participant numbers are typically small. At or soon after birth, anisometropia prevalence has been reported to be 17.3% (n=300, >1D difference between vertical or horizontal meridia, Zonis and Miller, 1974) to 30% (n= 256, >1D difference in SE, Varghese et al., 2009). Larger scale, more population-based studies have been conducted on children from the age of 6 months (e.g., Dirani et al., 2010; Borchert et al., 2010) and, if the high anisometropia prevalence figures in the perinatal period generalise to a larger population, prevalence appears to drop considerably by the age of one year. For example, Borchert et al. (2010) report a (≥1D difference in SE) prevalence in children aged 6-11 months of 7.8% (Hispanics) or 4.8% (African-Americans). Ingram (1979) reported a prevalence of 6.5% (n=1648 one-year old children, ≥1D difference in sphere or cylinder), while Ingram and Barr (1979a; n=148, ≥1D difference in sphere or cylinder) reported a prevalence of 8.1-8.8% in their sample of 1-year olds. Other studies, using different clinical assessment methods (Atkinson et al., 1996; n=8000, 7 to 9 month olds, prevalence 1%; Wood et al. 1995; 2 of 113 (<2%) infants of whom 43% had a family history of strabismus or amblyopia) or different criteria have found much lower prevalences towards the end of the first year of life. In relation to criterion, for example, Dirani et al. (2010) found that none of their 165 children aged 6 to 11.9 months exhibited an interocular difference of ≥2D spherical equivalent refractive error.
Cross-sectional, population-based studies of anisometropia prevalence should provide more representative data than studies of clinical populations. A number of cross-sectional, population-based studies of typically-developing children up to six years of age indicate a prevalence range of 0.6% to ~4% (Dirani et al., 2010, n=2600, 0.6%; Fabian and Wendell, 1974, n=1200, 2.6%; Huynh et al., 2006, n=1765, 1.6%; Borchert et al., 2010, n=6024, 4.2%). Differences between studies may simply reflect criterion differences (Figure 2); in fact cross-sectional, population-based studies that span a range of ages up to the age of six have reported stable anisometropia prevalence after 1 year (Borchert et al., 2010; Dirani et al., 2010). In older children, anisometropia prevalence is in the region of 2.7% (7-year olds) to 5.8% (9-year olds) (Tong, et al., 2006, n=~2000, ≥1D difference in SE refraction). These studies lay out a range that is consistent with other groups (Flom and Bedell, 1985, 3.4% of 2700, 5 to 12 year olds; de Vries, 1985, 3.9% of 1336 children up to the age of 10), although higher anisometropia prevalence (≥1D difference in SE refraction) figures were recently reported by O’Donoghue et al. (2013) amongst 6 to 7 (8.5%; n=389) and 12 to 13 (9.4%; n=661) year-olds. Several studies have found an increased prevalence as a function of age in the teenage years apparently mirroring the increase in myopia prevalence (e.g. Hirsch, 1967; Hendricks et al., 2009; Lin et al., 1999; but see Czepita et al., 2005).
The large-scale study by Qin et al. (2005) provides strong evidence that the prevalence of anisometropia is stable between the ages of 20 and 40 years with around 11 to 13% of subjects exhibiting anisometropia of ≥1D difference in spherical equivalent. Katz et al.’s (1997) sample of ~4,500 US adults aged 40 years and above found that the prevalence of anisometropia (≥1D difference in SE refraction) increased with age in the population, from ~4.8% in the 40-49 age group to ~14.8% in those aged 80 years and above. Broadly similar increases with age have been reported in a large number of other cross-sectional, population-based studies (e.g. Anton et al., 2009; Saw et al., 2008; Wong et al., 2000; Lavery et al., 1988) (Figure 3) with those including older adults being careful to exclude subjects who have undergone cataract extraction..
Beyond prevalence, the magnitude of anisometropia also appears to grow with age. A cross-sectional, population-based study (Attebo et al., 1999) of around 3650 Australian adults aged 49-97 years found anisometropia of >1D in spherical equivalent between the eyes in 14.1% of the sample as a whole, with the mean increasing from ~0.4D in those aged 49-59 years to ~0.9D in persons aged 80 years and above. Similarly, in the study by Anton et al. (2009), anisometropia increased with increasing age from a mean of 0.22D in persons aged 40 to 49 years to an average of 0.57D in those 70 years or older.
The vast majority of studies that have examined how anisometropia prevalence changes with age are cross-sectional rather than longitudinal in design. However, a small pool of longitudinal studies has investigated changes in anisometropia over restricted age ranges (e.g. Hirsch, 1967; Ingram and Barr, 1979b; Almeder et al., 1990; Abrahamsson et al., 1990b; Wood et al., 1995). Longitudinal studies offer a clear advantage in that they allow the persistence of anisometropia in individual subjects to be examined. The longitudinal studies conducted by Ingram and Barr (1979b, n=148 between 1 and 3.5 years) and Almeder et al. (1990, n= 686, 3 months to 9 years) showed that, in spite of a stable overall prevalence of anisometropia in early childhood, the majority of the children who were anisometropic at one examination were not anisometropic on subsequent examination, and children who did not exhibit anisometropia at earlier visits had replaced them. The same result was obtained by Abrahamsson et al (1990b) who found that although the overall prevalence of anisometropia was stable in their sample of longitudinally-studied children aged between 1 and 4 years, less than half of children who exhibited anisometropia at any stage during the period of follow-up remained anisometropic throughout the whole test period. By contrast, anisometropia in older children appears to be more permanent (Hirsch, 1967; Parssinen, 1990; Weale, 2002). The issue of the persistence of anisometropia in early life is clearly of relevance to the association between anisometropia and amblyopia (e.g. Almeder et al., 1990; Abrahamsson and Sjostrand, 1996; Fielder and Moseley, 1996) and we return to this topic in section 4.4.
To summarise, the association between age and anisometropia prevalence has been described as a u-shaped function (Weale, 2002) although there is uncertainty about the early limb of the function in peri-natal humans; while very high prevalence of anisometropia has been reported at or shortly after birth (Zonis and Miller, 1974; Varghese et al., 2009), evidence to support this is somewhat limited (small participant numbers). At older ages, prevalence is low and stable from the age of one to the pre-teenage years before showing an increase linked to the onset of myopia. Anisometropia in early adulthood is very stable before increasing, first modestly after the onset of presbyopia and then steeply in later life (Figure 3).
Influence of Race/Ethnicity and Gender
Anisometropia prevalence has been estimated in a wide range of racial/ethnic groups (Ohlsson et al., 2001; Ohlsson et al., 2003; Katz et al., 1999; Macias et al., 1999; Borchert et al., 2010; Wong et al., 2000; Saw et al., 2008; Dobson et al., 2008b; Pai et al., 2011). Studies that have adopted the same criterion when comparing anisometropia amongst groups are listed in Table 2.
Table 2. A comparison of the prevalence of anisometropia in different racial and ethnic groups. Only studies comparing prevalence in two or more groups are included.
|
Authors (year) (n)
(country) |
Anisometropia
Criterion/ Examination Methods |
Anisometropia Prevalence (%) for
different Ethnic Groups (age) |
Conclusion/Notes |
|---|---|---|---|
|
| |||
|
*Katz et al. (1997) (n=5028) (Population-based survey of ocular disorders among non- institutionalized subjects 40 years of age and older living in east Baltimore, USA) |
>1D difference in SE. Subjects underwent Subjective refraction with AO Reichert SR- TV Programmed Subjective Refractor. Refractions were refined as needed for possible overcorrection to eliminate the effect of instrument accommodation. Those who could not respond adequately to automated refraction were refracted using retinoscopy or with manual techniques. Subjects did not undergo cycloplegia. |
Caucasian (40-49 yrs.) 5.9% African-American (40-49 yrs.) 3.6% Caucasian (50-59 yrs.) 5.8% African-American (50-59 yrs.) 4.6% Caucasian (60-69 yrs.) 8.4% African-American (60-69 yrs.) 6.8% Caucasian (70-79 yrs.) 14.6% African-American (70-79 yrs.) 9.3% Caucasian (80+ yrs.) 15.5% African-American (80+ yrs.) 14.0% |
Caucasians had significantly greater anisometropia than African-Americans. African-Americans had lower rates of all refractive errors, except for hyperopia prevalence in women which was similar in the two groups |
|
| |||
|
Borchert et al. (2010) (n=6024) (Population-based survey. 6-72 month-old subjects identified by door- to-door screening of families within 44 census tracts in/around the city of Inglewood, Los Angeles County, California USA) |
≥1D difference in SE. Retinomax autorefraction was performed on all participants after cycloplegia |
Hispanic (6 to 72 months) 4.3% African-American (6 to 72 months) 4.2% |
Cylinder vector anisometropia more prevalent in African- American children (11.9% versus 10.4%) but no difference between groups for any other anisometropia index. |
|
| |||
|
Saw et al. (2008) (n=2974) (Singapore) |
>1D difference in SE. | Singapore Malay population (40 to 80 yrs) 9.9% |
Figures quoted for these 3 studies represent the crude/raw figures, not those age-adjusted to Singapore census data. |
|
Wong et al. (2000) (n=1076) (Singapore) |
>1D difference in SE. | Singapore Chinese population (40 to 79 yrs) 20.0% |
|
|
Pai et al. (2011) (n=2762) (Singapore) These are population-based surveys of ocular disorders among adults living in Singapore. The 1996 Singapore electoral register was used for sampling in these studies |
>1D difference in SE. Objective refraction result was recorded using an autorefractor (Retinomax K-plus; Nikon, Tokyo, Japan). Manual subjec- tive refraction was then attempted to refine vision, using the results of the objective refraction,. No cycloplegia was used. Those who did not attend the clinic visit were offered an examination in their homes. These refraction were conducted using a handheld autorefractor (Retinomax K-plus; Nikon, Toyko, Japan). No manual subjective refraction was conducted on these subjects. |
Singapore Indian population (40+ yrs) 9.9% |
|
|
| |||
|
Ohlsson et al. (2001) (n=1046) (Sweden) Ohlsson et al. (2003) (n=1035) (Mexico) Population-based studies of 12-13 year old children born in Sweden (conducted in schools in Gothenburg, Sweden) or Mexico (conducted in Monterrey). Parental consent was required prior to participation. |
≥1.5D difference in sphere or cylinder ≥1.5D difference in sphere or cylinder. Retinoscopy performed after installation of one or two drops of 0.5% Tropicamide. |
Caucasian (12- to 13-yr. olds) 3.3% Central America (12- to 13-yr. olds) 5.3% |
|
|
| |||
|
Macias et al. (1999) (n=5226). Not population- based. Retrospective study of self-selected adults (aged 25 to 74 year) who received vision screenings/eye examinations in a mobile eye clinic in Los Angeles, USA between 1987 and 1997. |
>1D difference in SE. Retinoscopy/ subjective refraction. |
Hispanic (18 to 93 years, n=2970): 2.2%* African-American (18 to 94 years, n=1028): 22.3%* Caucasian (18 to 97 years, n=1228): 26.8%* |
Caucasians had higher rates of anisometropia, astigmatism and hyperopia compared to the other racial groups. Myopia was more common among African-Americans than the other racial groups. |
|
| |||
|
Giordano et al. (2009) (n=2298, White, n=1030, African- American, n=1268), (Baltimore, USA) Population-based evaluation of the prevalence of ocular disorders in children aged 6 to 71. |
≥2D difference in SE. ≥3D difference in SE. |
White: 1.5% African-American: 1% White: 0.7% African-American: 0.2% |
As well as greater anisometropia prevalence amongst whites, the prevalence of hyperopia of 3D or more in the eye with the lesser refractive error was 8.9% in white children and 4.4% in African- American children. Also, the prevalence of emmetropia (refractive correction from −1D to +1D) was 35.6% in white children and 58.0% in African- American children. |
Prevalence figures for males and females have been averaged.
In their population-based study of Hispanic and African-American children aged from 6 to 72 months, Borchert et al. (2010) found a prevalence (≥1D difference in SE refraction) amongst 6 to11 month-olds of 7.8% in Hispanic children but only 4.8% in African-American children of the same age. The prevalence was found to fall by the age of one in the Hispanic but not in the African American children, however. Borchert et al found no effect of age on prevalence beyond 1 year of age and a similar overall prevalence in the two samples (4.3%, Hispanic; 4.2% African-American). Using an identical anisometropia criterion, Giordano et al. (2009) found an overall prevalence of 4.3% and 5% in samples of African-American and white children, respectively, aged from 6 to 72 months. Interestingly, larger amounts of anisometropia may be considerably more prevalent in white compared to African-American children; for anisometropia of ≥3D difference in SE refraction, the prevalence in white children was 0.5% compared to only 0.2% in the African-American children. Dirani et al.’s (2010) anisometropia criterion was ≥2D difference in SE refraction in their study of 6-72 month children in Singapore. It yielded a prevalence of 0.6% compared to 0.6% and 1.2% for the African-American and white children, respectively, in Giordano et al. (2009) when an identical criterion was applied. In Huynh et al.’s (2006) sample of 6-year old Australians (63.6% of whom had an ethnic origin defined as ‘White European’), the prevalence of anisometropia (again for ≥2D difference in SE refraction) was 0.5%.
In adults, statistically significant but generally small differences in anisometropia prevalence have been reported between adult Caucasian Americans and other racial and ethnic groups (Table 2). In their population-based study of adults aged 40 years and older, Katz et al. (1997) reported that prevalence may be slightly higher in Caucasian Americans compared to African-Americans. Relatively few studies of Asian populations have been conducted, although a series of population-based studies comparing Singaporean populations of adults of 40 years and above of Malay (Saw et al., 2008), Chinese (Wong et al., 2000) and Indian (Pan et al., 2011) origin suggest that anisometropia prevalence in Singapore Chinese may be twice that of Singapore Malays and Indians, and also considerably greater than in Caucasian Americans and African-Americans adults (Katz et al., 1997) (Table 2). Whether such racial/ethnic differences in anisometropia arise secondary to differences between populations in the level or type of ametropia (see previous section on the influence of other components of refractive error) is far from clear. Interestingly, one study (Macias et al., 1999) found much lower anisometropia prevalence amongst their self-selected sample of Hispanic adults compared to their self-selected samples of Caucasian and African-American adults (Table 2). However, the interpretation of this result needs to take account of the fact that Macias et al. (1999) was not a population-based study.
Overall therefore, there is limited evidence that racial or ethnic differences exert a major influence on anisometropia prevalence. This is supported by a number of population studies (e.g., Tong et al., 2004; Huynh et al., 2006) that have included race/ethnicity as a factor in the statistical analyses of their results. They have generally concluded that race/ethnicity does not influence anisometropia prevalence. Similarly, while gender differences have occasionally been reported in population studies of refractive error in adults (e.g. Katz et al., 1997; Wong et al., 2000) and children (e.g. Maul et al., 2000; Zhao et al., 2000; Ip et al., 2008), gender does not appear to be a significant factor in anisometropia prevalence (Guzowski et al., 2003; Tong et al., 2004; Qin et al., 2005; Saw et al., 2008; Dobson et al., 2008b; Wu et al., 2008; Anton et al., 2009; Krishnaiah et al., 2009; Borchert et al., 2010).
Influence of Family History
A small number of studies have reported familial anisometropia. For example, Blatt (1924) found anisometropia of more than 20D (unilateral high myopia) in a mother, her sister and her daughter, and lower amounts of anisometropia (ranging from 3D to 11.5D) in a mother, and four of her offspring (two daughters and two sons). De Jong et al. (1993) also reported high (more than 20D) anisometropia (again one eye highly myopic) in a pair of monozygotic twins. Interestingly, the anisometropia was symmetric (left eye was highly myopic in both cases) in the De Jong study whereas other twin studies have found mirror-symmetric anisometropia where there was a right eye myopic anisometropia in one twin, and a left eye myopic anisometropia in the other (Okamoto et al., 2001; Stankovid-Babid et al., 2011). Stankovid-Babid et al. (2011) reported mirror symmetric anisometropia in two pairs of monozygotic twins (one pair was a case of myopic astigmatism, while the second pair had esotropia and hyperopia). While there is evidence for familial anisometropia, there is also evidence that isometropia has a genetic basis. Sorsby et al. (1962) reported that 78.5% of 78 pairs of monozygoyic twins had anisometropia of less than 0.5D but only 30% of 40 pairs of dizygoyic twin exhibited this similarity. Thus, there is evidence for at least some genetic predisposition to both the presence and absence of anisometropia.
Influence of Other Factors
Borchert et al.’s (2010) study of around 6000 children aged 6 to 72 months found that maternal age, gestational age, prematurity, birth weight, prenatal exposure to alcohol or tobacco, cerebral palsy, and family history of strabismus or spectacle use were not significantly associated with anisometropia. Similarly, Huynh et al.’s (2006) study of 1765 6-year old children did not find a statistically significant association between anisometropia and low birth weight, prematurity, smoking or breast-feeding. The failure to find an association with low birth weight or prematurity is consistent with the results from studies which show that in the absence of retinopathy of prematurity, anisometropia tends to disappear within the first year of life. Huynh et al. (2006) did, however, find a statistically greater chance of anisometropia in cases where there was a history of having been admitted to a neo-natal intensive care unit, and in cases of exotropia or amblyopia. There is a clear link between anisometropia and disruption to visual experience in early life and this is described in detail in 4.2.
Summary of Anisometropia Prevalence and the Factors that affect it
Although complicated by differences in the criteria used to diagnose it (Figure 2), advanced age (Figure 3), higher refractive error (especially myopia), and the presence of ocular disease are all associated with significant increases in anisometropia prevalence. Gender does not influence prevalence but modest racial differences may exist. Regardless of the criterion used to diagnose it or the characteristics of the population sample, anisometropia is much less common than isoametropia (Table 1). If a criterion of ≥1D difference in spherical equivalent refractive error is employed, anisometropia prevalence is proposed to be relatively high in newborns, but stable at around 5% from the age of 1 year until the teenage years when it increases with myopia onset. Prevalence is stable at around 10% in early adulthood but grows significantly (e.g., to 30-40%) in later life (Figure 3).
3.2 Prevalence of Amblyopia
When overall amblyopia prevalence is reported, the figure quoted includes both unilateral and bilateral cases. However, overall prevalence figures mask the fact that unilateral amblyopia is much more common than the bilateral form (Table 3).
Table 3.
A comparison of the prevalence of amblyopia reported in population studies.
| Authors (year) | Country, Age of Sample, (n) | Amblyopia Criterion |
Equipment to
determine VA |
Amblyopia
Prevalence |
Unilateral
versus Bilateral Ratio |
Refractive Error
among Amblyopes |
Amblyopia
Severity |
Presumed
Amblyopia Aetiology ☖ |
|---|---|---|---|---|---|---|---|---|
| Oliver and Nawratzki (1971). | Israel, 1.5-6 year olds, (n=5232). Population-based study. Random sample of children attending Mother & Child Care Clinics or kindergartens. |
VA difference of ≥2 lines. When VA test could not be performed (young children) diagnosis was established from unilateral strabismus or an obvious difference in the behavior of the child when one eye was covered as compared with the other. |
Equipment included small toys, matching tests, picture charts and illiterate E charts. Different tests used according to age. |
1.2% | --- | Hyperopia of ≥+3D: 46.3% Astigmatism of ≥2D: 13.4% Myopia (any amount): 6% |
--- | Strabismus: 68.7% (Esotropia 89%/Exotropia 11%) Anisometropia (≥2D): 29.9% |
| Lim et al. (2004) | Korea, 3-5 year olds (n=7116). Population-based study (‘Seoul Metropolitan Preschool Vision Screening Programme’). A home vision test kit was sent to the parents of kindergarten children. Children were identified from the Ministry of Health and Welfare database, The enrolled children represented 10.1% of the total same-age group of children who lived in Seoul. |
VA of 20/40 or worse (age ≤3 years) or 20/32 (>3 years), or any case with ≥2 line difference between eyes. |
Picture tests or single optotype tests. |
0.4% | --- | --- | --- | Ametropia 48.3%; Anisometropia 34.2%; Strabismus 12.8%; Unclassified 4.7%. |
| Pai et al. (2012) | Australia, 30 to 72 months, (n=1422). Population-based, cross-sectional study (‘The Sydney Paediatric Eye Disease Study’). |
Unilateral: ≥2-line difference in VA between 2 eyes with VA of 20/32 or below in the worse-seeing eye with 1 or more amblyogenic factor(s) Bilateral: VA of less than 20/50 (children aged <48 moths) or less than 20/40 in children aged ≥48months ,with either past history of bilateral visual axis obstruction, or bilateral significant ametropia |
Most with ‘Electronic Visual Acuity’ system, some using LogMAR chart |
1.9% | 63%/37% | Hyperopia ≥+4D SE: 51.9% Astigmatism of ≥1D: 48.1% Myopia of ≤− 0.50D SE: 7.4% Mean SE refractive error of amblyopic eyes +3.6D, compared to +1.25D in non- amblyopic eyes |
Mean VA of amblyopic eyes 20/50 compared to 20/25 in non- amblyopic eyes |
Amongst the unilateral cases: Anisometropia only, 41.2%; Strabismus only, 29.4%; Strabismus and Anisometropia, 29.4% |
|
Robaei et al. (2005)
Robaei et al. (2006) |
Australia, 6 year-olds (n=1741). Population-based, cross- sectional study (‘The Sydney Myopia Study’). The study area was stratified by socioeconomic status using Australian Bureau of Statistics 2001 National Census data. These data were used to select 34 primary schools across Sydney. |
Unilateral: Corrected visual acuity <0.3 logMAR units (poorer than 20/40) in the affected eye not attributable to any underlying structural abnormality of the eye or visual pathway, together with a 0.2− logMAR difference between the eyes and presence of an amblyogenic risk factor. Bilateral: Visual acuity worse than 0.3 in presence of bilateral hyperopia >+4D, bilateral myopia of −6 or greater or bilateral astigmatism of 2.5D or greater. |
LogMAR) visual acuity measured in both eyes before and after pinhole correction and with spectacles if worn. |
0.7% rising to 1.8% if children with successfully treated amblyopia are included. |
93.7%/6.3% | Most amblyopic eyes (58.7%) were significantly hyperopic (spherical equivalent > or = +3.00 D); 8.7% were myopic. |
Mean corrected VA in amblyopic eyes was <20/40 compared to 20/25 for overall sample of 6 year olds. |
Strabismus or strabismus surgery history : 37.5%; Anisometropia: 34.4%, both Anisometropia and strabismus: 18.8%; Isoametropia: 6.3% |
| Friedman et al. (2009) | USA. 30-71 month olds (n= 2546). Population-based, cross- sectional study (‘The Baltimore Pediatric Eye Disease Study’) that enrolled subjects from 54 contiguous census tracts in northeastern and eastern Baltimore City and adjacent portions of eastern Baltimore County. |
As in MEPEDS (2008) (see below). |
As in MEPEDS (2008) (see below). |
Caucasian: 1.8% African- American: 0.8% |
94.7%/5.3% | --- | --- | Unilateral cases: Anisometropia only, 31.6%; Strabismus only, 31.6%; Strabismus and Anisometropia, 10.5%. |
| MEPEDS (2008) | USA. 30-72month olds (n= 3817). Population-based, cross- sectional study (‘the multi- ethnic pediatric eye disease study, MEPEDS’) of children in 44 census tracts in Los Angeles County. |
Unilateral: a 2-line inter-ocular difference in best-corrected VA, VA of 20/32 or worse in the worse eye, and ≥1 amblyopia risk factor(s). Bilateral: Best- corrected VA of 20/50 or worse in children aged 30 to 47 months or 20/40 or worse in children ≥48 months) with either bilateral evidence of visual axis obstruction or bilateral ametropia |
Monocular, single- surrounded HOTV VA tested using the Electronic Visual Acuity system and the Amblyopia Treatment Study VA protocol (Holmes et al., 2001) |
Hispanic/ Latin: 2.6% African American: 1.5% |
76.8%/23.2% | --- | --- | Unilateral cases: Anisometropia. only, 73.6%; Strabismus only, 18.9%; Strabismus and Anisometropia, 5.7%. |
|
Preslan and Novak (1996)
Preslan and Novak (1998) |
USA (n=680) (3-6 year old children) USA (n=285) (4-6 year old children) Study of school children from an inner-city elementary school in Baltimore, USA (‘Baltimore Vision Screening Project’). Screening and treatment study. |
Criterion not explicitly stated but children examined if VA at screening was 10/15 or lower or if the motility examination was failed. |
Isolated Snellen-E optotypes presented at 3m. |
3.9% 5.3% |
88%/12% (1996 sample) 100%/0% (1998) |
Refractive error of anisometropic amblyopic eyes evenly split between hyperopia, myopia and astigmatism. (1996 study) |
VA in amblyopic eye ranged from 20/40 to 20/200 (1996) 60% had VA of 20/50 or better; 40% worse than 20/50 (1998). |
Strabismus: 44% (1996) (all but one was esotropic) 33% (1998); Anisometropia: 44% (1996) Anisometropia 33%, Astigmatism 33%. (1998) |
| Flom and Neumaier (1966) | USA, 10 to 50 year olds (n=7017). Not population- based. Records of patients who had presented for free eye examinations at an Optometry clinic. |
Best-corrected VA of 20/40-or worse and more than one line difference between the eyes,. |
---- | 1.7% | ---- | ---- | ---- | ---- |
| Lithander (1998) | Oman, 6/7 and 11/12 year- olds (n=6292). Cross-sectional study of school-children. |
Best-corrected VA of 20/40 or worse |
Snellen E- chart for 12 year olds, Kolt-test for 6 year olds. |
0.92% | 100%/0% | --- | 86.2% of amblyopic eyes had VA of 20/200 or better. |
Anisometropia only was present in 47.8% of amblyopes. Strabismus only was present in 32.2% of amblyopes. Strabismus and anisometropia were found in 20% of children. |
| Faghihi et al. (2011) | Iran, 6-21 years (mean 13.2 years) (n=2150). Cross-sectional study with cluster sampling from schools of district 1 in Mashhad. |
Reduction of BCVA to 20/30 or less in one eye or 2-line interocular optotype acuity differences in the absence of pathological causes. First to 8th grade students underwent auto-refraction following cycloplegia. Older children had subjective refraction. |
Snellen E- chart. |
1.9% | 73%/27% | Among myopic, hyperopic, and astigmatic students, 3.7%, 27.8%, and 6.5% had amblyopia, respectively. . |
--- | Anisometropia:65.9 %; Strabismus in 24.4%; Isoametropia: 9.8%. |
| Williams et al. (2008) | United Kingdom, 7 year olds, (n=7825). Participants consistent of children who participated in a birth –cohort study. |
History of patching treatment and/or with an interocular difference in best acuity for each eye of >0.2 logMAR where the worse-seeing eye had a best acuity of worse than 0.3 logMAR, and the eye looked normal on dilated funduscopy. |
VA measured monocularly, using the “2000” series ETDRS charts. |
3.6% | 83.7%/16.6% | 43.3% of those with hyperopia (≥+2D in either eye) had past or present amblyopia. |
--- | --- |
| Yassur et al. (1972) | Rwanda, 10-18 year olds (n=1552). Random sample consisting from six schools in the two main cities of Rwanda. |
VA of 20/40 or worse in at least one eye. |
Snellen chart. | 1.2% | --- | Hyperopia of ≥+2D:23% Astigmatism of ≥2D: 12% Myopia of ≤-2: 33% |
VA worse than 6/60: 44% VA 6/18 to 6/60: 44% 6/12: 12% |
Strabismus: 72% Anisometropia (>2D difference): 28% |
| Chang et al. (2007) | Taiwan, 3 to 6 year olds (n=5232). Population-based vision screening tests conducted in children in eastern Taiwan. |
Diagnosis by senior ophthalmologist. Best- corrected VA of less than decimal 1.0. |
--- | 2.2% | --- | --- | --- | Strabismus: 2.6%; Refractive errors: 62.6%; Anisometropia: 24.3%; Organic 10.4%. |
|
He et al. (2004)
He et al. (2007) |
China, 5 to 15 year olds (n=4364) China, 13 to 17 year olds (n=2400) Population-based study. Cluster sampling was used to select the study sample. Eye examinations were conducted in schools. |
Best-corrected VA of 20/40 or worse, no apparent organic lesion and with one or more amblyogenic factor(s) present. |
Retro- illuminated (LogMAR) chart with tumbling-E optotypes. |
0.87%/1.97 %* 0.50%/1.05 %^ |
--- | --- | --- | Strabismus: 25%; Anisometropia (≥2D SE difference): 66.6%; Anisometropia & Strabismus: 8.3%. |
| Goh et al. (2005) | Malaysia, 7 to 15 year olds (n=4634). Population-based, cross-sectional survey. Random selection of geographically defined clusters was used to identify the study sample. Children in 34 clusters were enumerated through a door-to- door survey and examined in 140 schools. |
Amblyopia was diagnosed when there was no apparent organic lesion but ≥1 from: 1) esotropia, exotropia, or vertical tropia at 4m, or esotropia or vertical tropia at 0.5m (‘strabismic amblyopia’); 2) anisometropia: ≥ 2D difference in SE (‘anisometropic amblyopia’); or 3) bilateral ametropia of ≥+6D SE. |
LogMAR (tumbling-E optotypes). |
0.65%/3.53 %◆ |
80%/20% | --- | --- | Strabismus: 23.3%; Anisometropia (≥2D SE difference): 63.3%; Anisometropia & Strabismus: 10%. |
|
Ohlsson et al. (2001). Ohlsson et al. (2003). |
Sweden, 12 to 13 years olds (n=1046). Population-based study of 12-13 year old children in Gothenburg, Sweden. Mexico, 12 to 13 years olds (n=1035). Population-based study of 12-13 year old children in Monterrey, Mexico. |
VA of 20/40 or worse or ≥2-line VA difference between the eyes and amblyogenic factors. VA of 20/40 or worse, no organic cause. |
Landolt C LogMAR Landolt C LogMAR |
1.1%■ 2.5% |
82.9%/17.1% |
--- --- |
--- 62.1%: logMAR 0.30 - 0.60; 24.1% logMAR 0.7- 0.9; 13.8%: logMAR<0.3 |
Unilateral cases: Anisometropia (56.5%), strabismus (30.4%), mixed strabismus and anisometropia (13.1%), --- |
| Helveston (1965) | USA, 17 to 25 year old males (n=9000). Army recruits??.CHECK |
VA of worse than 20/40 in one eye with ‘normal vision’ in the other eye with no detectable organic disease and no history of trauma or disease |
--- | 1% | --- | --- | --- | 52% of cases had ‘no detectable strabismus (i.e. anisometropia or history of strabismus)’®; 40% had esotropia; 8% had exotropia. |
| Chia et al. (2010) | Singapore Chinese, 30 to 72 months, (n=1682). Population- based study. Chinese children were recruited from Housing Development Board townships through a door-to-door recruitment exercise. The study area included a large part of the South-Western region of Singapore. |
Unilateral: ≥2-line difference in VA between 2 eyes with VA of 20/30 or below in the worse-seeing eye with at least one amblyogenic factor Bilateral: VA of less than 20/50 (children aged <48 moths) or less than 20/40 in children aged 48-72 months with either past or present visual axis obstruction, or bilateral significant ametropia |
LogMAR chart. Sheridan- Gardner when logMAR not possible |
1.2% ⊖ | 69.7%/ 30.3% |
Not stated but astigmatism identified as most frequent amblyogenic risk factor. |
--- | Unilateral cases: Anisometropia only, 78.3% Strabismus only, 21.7%, Strabismus and Anisometropia, 0% |
| Wang et al. (2011). | China, Adults aged 30 to 80 years, (n=6830). Population- based, cross-sectional study. Thirteen villages in the Yongnian County of Handan were selected randomly, and residents of these selected villages 30 years of age or older were invited to participate in the Handan Eye Study. |
Unilateral: best- corrected VA of 20/32 or worse, not attributable to any underlying structural abnormality of the eye or visual pathway. Bilateral: best- corrected VA of 20/32 or less in both eyes and a history of form deprivation during the sensitive period of visual development. |
LogMAR chart | Crude: 3% Adjusted: 2.8%◇ |
60.7%/39.3% | Of the amblyopia cases, 47.6% were hyperopic. |
--- | Anisometropia (67.3%), strabismus (5.4%), mixed strabismus and anisometropia (4.4%), visual deprivation (9.8%), astigmatism association (9.8%), and other (3.4%). |
| Rosman et al. (2005). | Chinese, Indian, and Malay men, aged 18 to 19 in Singapore (n=122,596). Population-based, cross- sectional study of all Singaporean men born in the years 1978 to 1983 were measured before enlistment into military service. |
Best corrected visual acuity of 20/40 or worse, not attributable directly to any underlying structural abnormality of the eye or visual pathways. |
--- | 0.35% | --- | --- | --- | Anisometropia: 37.1%; Strabismus 5.7%; Meridional: 14.3% |
| Brown et al. (2000). | Australia, 40-92 year olds (n=4721). The Visual Impairment Project is a population-based study of age- related eye disease in the state of Victoria, Australia. |
Unilateral: Best- corrected visual acuity of 20/30 or worse in one eye, with at least one line difference in VA between the eyes without attributable pathological cause. Bilateral: Best- corrected visual acuity of 20/30 or worse in both eyes with history of form deprivation or high uncorrected ametropia. |
LogMAR chart, using current glasses. Refraction performed if VA found to be less than 20/20. |
Unilateral: 3.1% |
>99% unilateral |
Spherical equivalent hyperopia (>+0.50D) was present in 52%; myopia (more than −0.5D) was present in 38.6% of amblyopic eyes.. |
54% of amblyopic eyes had visual acuity of worse than 20/40; 27.9% had VA of worse than 20/60; 10.9% had VA worse than 20/80. |
53.9% of amblyopes had anisometropia (≥1D difference in spherical equivalent refraction). 46% of amblyopic eyes had astigmatism of 1D or more. Cover test not performed so no strabismus data reported. |
| Nowak et al. (2009). | Poland, Military 18-34 year olds (n=969).Retro spectively study of males of European Caucasian origin, most of whom live and have lived in Poland, and who were selected from the original database comprising 105017 subjects examined in the period 1993-2004. |
Distance visual acuity of worse than 20/40 in one or both eyes. |
Retro- illuminated Snellen chart. |
0.8% | 87.5%/12.5% | ---- | ---- | ---- |
| Quah et al. (1991). | Singapore, Males 18-19 year olds, (n=6556). Vision screening of National Service pre- enlistees. |
VA of 20/40 or less in one or both eyes in the absence of ocular pathology. |
Snellen chart. | 0.73% | 98%/2% | 75% of anisometropic amblyopes were myopic, 25% wer e hyperopic. |
Anisometropia: 50%, strabismus: 18.7%; high astigmatism: 14.5%; Other causes/ combination of factors: 16.7%. |
|
| Attebo et al. (1998). | Australia, 49 years and older, (n=3647, of whom 2068 were female). Population-based, cross-sectional study of eye disease in elderly people living in the community (‘Blue Mountains Eye Study’). All identified, eligible residents were invited to attend a clinic appointment. |
Reduced best- corrected visual acuity in the absence of any other cause |
LogMAR chart, using current glasses and pinhole disc. Refraction performed if VA found to be less than 20/20. |
VA of 20/40 or worse: 2.9%. Prevalence was 3.2% for criterion of 20/30 or worse. |
99%/1% | Spherical equivalent hyperopia up to +5D was present in 50% of amblyopic eyes, ~20% had hyperopia >+5D, and 25% were myopic |
19% of amblyopic eyes had VA of 20/200 or worse, 19% had VA of 20/80 to 20/160, 52% had 20/40 to 20/63, and 11% had VA of 20/30. Poorest VA in visual deprivation amblyopes. VA in strabismic amblyopic eyes was poorer than in eyes with strabismus &anisometro pia and poorer than VA in eyes with anisometropi c amblyopia |
Anisometropia (50%), strabismus (19%), mixed strabismus and anisometropia (27%), and visual deprivation (4%). Amongst strabismic amblyopes, 59% were esotropes, 28% were exotropes and 11% were microtropes |
Adj: prevalence figure adjusted so that the sample population matches overall population according to census in that country. ‘Crude’ refers to actual prevalence in the sample under test.
Aetiology is presumed since the studies reported here are cross-sectional in nature not longitudinal.
In He et al. (2004) amblyopia was diagnosed in 0.87% of the population but in a further 1.1% of the population amblyopia was considered the principal cause of the “unexplained reduction” in best-corrected visual acuity, even though none of the explicit criteria for amblyopiadiagnosis were met.
In He et al. (2007) amblyopia was diagnosed in 0.50% of the population but in a further 0.54% of the population amblyopia was considered the principal cause of the “unexplained reduction” in best-corrected visual acuity, even though none of the explicit criteria for amblyopia diagnosis were met.
In Goh et al. (2005) amblyopia was diagnosed in 0.65% of the population but in a further 2.88% of the population amblyopia was considered the principal cause of the “unexplained reduction” in best-corrected visual acuity, even though none of the explicit criteria for amblyopia diagnosis were met.
Ohlsson et al. (2001) make it clear that this is the prevalence of residual amblyopia since they sampled a population that had previously been screened
Some of Helveston’s (1965) amblyopia cases without ‘detectable strabismus’ may have been microtropes.
-—-- means this information was not provided in the paper.
In Chia et al. (2010), 2.8% of the sample met the visual acuity criterion for amblyopia but 58% of these cases were not diagnosed as have amblyopia because insufficient amblyopic risk factors were identified.
A relatively recent large-scale review of the amblyopia literature estimated that the prevalence of amblyopia is between 1.6% and 3.6% (Simons, 2005). A number of large-scale, population-based studies of amblyopia prevalence have been conducted and several (e.g. Robaei et al., 2006; Chia et al., 2010) have also included summary tables reporting the prevalence found in individual studies.
In a recent population-based study of Australian children aged 30 to 72 months, Pai et al. (2012) report an overall prevalence of amblyopia of 1.9% (see Table 3 for criterion). Some population-based studies have reported a lower amblyopia prevalence (e.g. 0.4% in Lim et al.’s 2004 study of 3- to 5 year old Korean children; 0.8% amongst African-American children in the USA, Friedman et al., 2009). However Pai et al.’s (2012) prevalence figure accords well with studies of 6 year-old Australian children by Robaei et al (2005, 2006) in which amblyopia prevalence was 1.8% and with Friedman et al.’s (2009) figure of 1.8% in Caucasian children aged 30 to 71 months (Table 3). However, a number of recent studies report a higher amblyopia prevalence figure. For example, Williams et al. (2008) reported amblyopia prevalence of 3.6% in 7 year old children in the United Kingdom and Attebo et al.’s (1998) study of Australian adults reported a prevalence of 2.9%, similar to Brown et al.’s (2000) figure of 3.1% that was also gathered from Australian adults (Table 3). While there are some recent examples of very low prevalence (e.g., Chia et al., 2010; Friedman et al., 2009) the majority of recent population-based studies find that amblyopia prevalence falls in the 1.6% to 3.6% range noted in the review by Simons (2005) (Table 3).
Amblyopia has been found to be the first, second, or third leading cause of visual impairment in adults of various age distributions (Sachsenweger, 1968; National Eye Institute. Office of & Epidemiology, 1984; Klein et al., 1995; Attebo et al., 1996; Robaei et al., 2005; Robaei et al., 2006; Gilbert et al., 2008). Clearly, criterion differences account for at least of some of the variation in prevalence. In the paragraphs that follow we also examine the impact of laterality, age, race, refractive error, pathology and gender, and whether or not individuals with successfully treated amblyopia are included.
Dependence on Criterion
As with anisometropia, the estimate of prevalence of amblyopia is critically dependent upon the criterion used for diagnosis. There are many examples in the literature of authors indicating how the prevalence varies in their sample when different criteria are applied (e.g. Robaei et al., 2006). Flom and Neumaier’s (1966) sample of over 7000 people examined in a university eye clinic revealed an amblyopia prevalence of 0.4% based upon a VA criterion of 20/200 or worse. The figure rose to 2%, 3.4% and 9% when the criterion was altered to 20/40 or worse, 20/30 or worse and 20/25 or worse, respectively [see their figure 2]. Individuals presenting to an eye clinic are not representative of the population in general, and therefore the absolute values are not informative about overall amblyopia prevalence but Flom and Neumaier’s figures do highlight the importance of criterion used for diagnosis. Similarly, Flom and Bedell’s (1985) school-based study of 2762 eleven- and twelve-year old children reported an amblyopia prevalence of 0.1% for 20/200 or worse and 1.4% for 20/40 or worse. Chia et al.’s (2010) population-based study of Singaporean children aged 6 to 72 months yielded a prevalence figure of 1.19% (Table 3), but the authors indicated that this figure would rise 2.7 fold to 3.27% if a criterion more similar to that used by the American Association of Pediatric Ophthalmology & Strabismus (AAPOS, for 3-5 year olds: VA of 20/40 or worse, or two or more lines of VA difference between the eyes, even when both eyes achieve better than 20/40) had been used (Committee on Practice and Ambulatory Medicine (CPAM), 1996). In their population-based study of Australian adults aged 49 and older, Attebo et al. (1998) reported amblyopia in 3.2% of the population for 20/30 or worse and 2.9% for 20/40 or worse. When they added the need for a minimum two-line visual acuity difference between the eyes, the figures dropped to 2.6% and 2.5%, respectively.
The critical dependence of amblyopia prevalence upon criterion is also highlighted in Goh et al.’s (2005) population-based study of Malaysian children aged between 7 and 15 years of age. Based upon their adopted criterion (see Table 3), the prevalence of amblyopia was just 0.65%. However, a sizeable proportion of children had reduced visual acuity and although amblyopia was the presumed cause, this loss was listed as ‘unexplained’ because the specific criterion for amblyopia diagnosis was not met (Table 3). Had this unexplained loss been considered to be amblyopia, prevalence would have risen from 0.65% to 3.3%. Similarly, in Chia et al.’s (2010) population-based study of 6 to 72 month-old Singaporean children, 2.8% of the sample met the visual acuity criterion for amblyopia but only 1.2% were actually diagnosed with amblyopia because insufficient amblyogenic risk factors were identified in the other 1.6% (Table 3). Other recent population-based studies of amblyopia have also required the presence at least one amblyogenic factor in order to diagnose cases of reduced visual acuity as being due to amblyopia (MEPEDS, 2009; Friedman et al. (BPEDS), 2009). In addition, current treatment studies of amblyopia typically use inclusion criteria of a one-, two- or three-line difference in acuity and the presence of an amblyogenic factor (Agervi et al., 2009; Cotter et al., 2006 & 2012; Hwang et al., 2010; Scheiman et al., 2008; Stewart et al., 2004, 2005 & 2007; Wallace et al., 2006).
Bilateral versus Unilateral Amblyopia
While amblyopia can exist unilaterally or bilaterally, all of the studies that distinguish between cases of unilateral and bilateral amblyopia find that the unilateral form of the condition is much more common (Table 3). Bilateral amblyopia has been defined as reduced best-corrected visual acuity in both eyes together with a history of form deprivation during the sensitive period of visual development (e.g. Wang et al., 2011). This form deprivation could consist of bilateral visual axis obstruction or, more usually, significant bilateral ametropia (e.g., defined by Robaei et al. (2006) as 4D or more of hyperopia, 6D or more of myopia or 2.5D or greater astigmatism). While some population-based studies find that bilateral cases account for less than 10% of all cases (e.g., Brown et al., 2000; Attebo et al., 1998; Quah et al., 1991; Friedman et al., 2009; Table 3) others indicate that a considerably higher proportion (e.g., 17%-30%, Table 3) of amblyopia cases are of the bilateral form (e.g. Ohlsson et al., 2001; Ohlsson et al., 2003; MEPEDS, 2008; Chia et al., 2010; Table 3). Differences in unilateral versus bilateral proportions between studies are not solely due to criterion differences (Tarczy-Hornoch et al., 2011). For example, sixteen of 69 cases of amblyopia (23%) were bilateral in the MEPEDS (2008) study whereas, using identical criteria, Friedman et al (2009, Baltimore Pediatric Eye Disease Study (BPEDS)) found only one bilateral case amongst 19 individuals with amblyopia (5.3%). Instead, differences between studies in bilateral versus unilateral proportions of amblyopia cases probably reflect differences between samples; ethnicity, esotropia and anisometropia were all associated with interocular differences in visual acuity in Tarcy-Hornoch et al.’s (2011) examination of data from both MEPEDS and BPEDS. Bilateral reductions in visual acuity were, on the other hand, found by Tarcy-Hornoch et al. (2011) to be particularly associated with the presence of astigmatism and high hyperopia (≥+4D) (Tarczy-Hornoch et al., 2011).
Prevalence in the Right- versus the Left-Eye
Most studies that report amblyopia prevalence figures for right and left eyes separately found no difference between eyes (Oliver and Nawratzki, 1971; Goh et al., 2005; Robaei et al., 2006). Interestingly, however, a recent study by Repka et al. (2010) found that among subjects with anisometropic amblyopia (with or without strabismus), amblyopia was present more often in left (59%) than in right (41%) eyes. The same was not true in the case of amblyopes with strabismus only (i.e. without anisometropia). The cause of this interesting finding is yet to be determined but it accords with Woodruff et al. (1994) who also reported greater prevalence of left- (109 cases) than right-sided (59 cases) amblyopia among their ‘pure’ anisometropic amblyopes. Woodruff et al.’s (1994) amblyopes with both anisometropia and strabismus showed a less marked, but still statistically significant preponderance of left-sided amblyopia (115 right versus 147 left) with no statistically significant asymmetry in strabismic amblyopes without anisometropia (252-right, 283-left).
Influence of Treatment History and Age
Unlike anisometropia, which is currently correctable with appropriate refractive correction but not curable, amblyopia is generally considered to be a curable condition (Cotter et al., 2012; Holmes et al., 2011; Stewart et al., 2005; Stewart et al., 2007). This suggests amblyopia prevalence rates should be lower when those who have been successfully treated are identified as non-amblyopic. Prevalence would also be expected to be lower in populations with access to treatment (e.g. through screenings) and possibly to fall during the typical treatment ages of 3 to 10 years if the treatment rate is higher than the incidence of new cases. The first two predictions are borne out in the data but the third is not. Firstly, Robaei et al. (2006) diagnosed amblyopia in only 0.7% of their six-year-old Australian children, but this figure rose to 1.8% with inclusion of children who previously had amblyopia; thus 1.1% of the sample had been successfully treated. Secondly, there is convincing evidence that amblyopia prevalence rates are lower in populations of older children who have gone through screening/treatment when compared to those who have not. For example, Eibschitz-Tsimhoni et al. (2000) reported a prevalence of amblyopia (corrected visual acuity of < 5/10 (20/40), or >1 line difference in corrected visual acuity between the two eyes) of 1.0% in an 8-year-old population screened in infancy, compared with 2.6% in an 8-year-old population that had not had the screening. The benefit of early detection and treatment also receives strong support from Williams et al. (2002) who reported that at 7.5 years, amblyopia was significantly less prevalent in the intensively screened group than in the control group (0.6% v 1.8%), and from Kvarnstrom et al. (1998) and Kvarnstrom et al. (2001) who reported better visual outcomes when multiple early screenings take place. These results provide solid evidence for the view expressed by Simons (2005) and others (Williamson et al., 1995; Williams et al., 1998; Preslan and Novak, 1996, 1998) that amblyopia is more prevalent in socially disadvantaged and medically-underserved communities (see ‘Other Factors’ section below). However, it is noteworthy that even in populations that have access to screenings and therapy (e.g. Sweden, UK) amblyopia prevalance rates are never zero. That is, either therapy effectiveness is low, or a significant proportion of amblyopes do not receive or comply with treatment.
Interestingly, the prediction that amblyopia prevalence would fall during the typical treatment ages of 3 to 10 years is not borne out by the data. A number of studies of children have examined whether amblyopia prevalence varies across the age of the sample under test. Oliver and Nawratzki (1971) found amblyopia to be more prevalent in older children (5 and 6 year olds) than younger ones (1.5 years), suggesting that the incidence of new cases was equal to or higher than the treatment rate. One important consideration, however, is the quality of visual acuity estimates from younger children and thus the accuracy with which amblyopia can be diagnosed; visual acuity can be difficult to measure in very young children and they are unable to complete crowded recognition acuity tests. Greater variability in visual acuity estimates from younger children has been offered as the explanation in Tarczy-Hornoch et al.’s (2011) analysis of the combined MEPEDS and BPEDS data for the finding that unilateral and bilateral reduction in visual acuity was more common in their younger children compared to their oldest group of children. A positive response to treatment cannot explain these findings because the amblyopia in MEPEDS and BPEDS was largely untreated (Tarczy-Hornoch et al., 2011).
Chia et al. (2010), found no age trend in amblyopia prevalence in their study of 30 to 72 month-old children. Similarly, in the MEPEDS (2008) study, also of 30 to 72 month-old children, amblyopia prevalence was stable after 3 years of age. Lithander (1998) reported statistically significantly more amblyopia in their 11-12 year olds when compared with their 6-7 year olds but this difference was only found among strabismic children. Huynh et al. (2006) found that 6- and 12-year-olds had a similar prevalence of amblyopia and Pai et al. (2012) also failed to find an effect of age on prevalence in their sample of children, as did Flom and Neumaier (1966) in kindergarten children and children in grades 1 through 6. The reasons why amblyopia prevalence is not seen to fall across the typical treatment age-range are not clear but they are likely to include incidence rates, age of onset, access to screening and treatment, treatment efficacy, and/or compliance with treatment. It is also possible that there is a reduction in overall amblyopia prevalence due to successful treatment but that this is countered by an increase in the numbers of children who develop amblyopia as they age.
Since amblyopia is a developmental disorder of vision one would not expect to see a change in prevalence across the later lifespan in individuals who are not undergoing amblyopia treatment. Consistent with this in the Brown et al. (2000) study of 40-92 year olds, amblyopia prevalence was not found to differ with age. Indeed, as Flom and Neumaier point out “Since amblyopia seems to develop only rarely after children reach school age, a similar prevalence is expected in [untreated] children and adults” (1966, p.340). Thus, beyond the age at which amblyopia is typically treated, the prevalence of this condition remains stable across the lifespan. Evidence from a variety of sources indicates that amblyopia is treatable and in populations who are offered and successfully comply with amblyopia treatment, the prevalence can fall to less than 1%.
Influence of Race/Ethnicity and Gender
Racial and ethnic differences in amblyopia prevalence have been observed in population-based studies (Friedman et al., 2009; MEPEDS, 2009). In US children aged 30 to 72 months, amblyopia prevalence was higher in Hispanic/Latino children (2.6%) than in African-American children (1.5%) (Table 3). Using an identical criterion, Friedman et al. (2009) found a lower amblyopia prevalence in African-American children aged 30 to 71 months (0.8%) compared to similarly-aged Caucasian children (1.8%) (Table 3). In Rosman et al.’s (2005) study of adult Asian males, however, amblyopia prevalence was similar among Chinese (0.34%), Malays (0.37%), and Indians (0.41%). Thus the evidence for inter-racial differences in amblyopia prevalence is mixed and the basis for any difference that may exist remains unknown. For example, the reasons may have to do with differences in the prevalence of amblyogenic factors or to differences in vulnerability to these factors. As is the case for anisometropia, there does not appear to be any gender bias in the amblyopia prevalence data (Brown et al., 2000; MEPEDS, 2008; Chia et al., 2010; Faghihi et al., 2011; Pai et al., 2012).
Influence of Other Factors
Pai et al. (2012) found no significant associations between amblyopia and low birth-weight (<2500 g), preterm birth (<37 weeks), maternal smoking, age, gender, ethnicity, or measures of socioeconomic status. In the study of 6-year-old Australian school children by Robaei et al. (2006), amblyopia was not statistically associated with any measures of socioeconomic status, including parental education, parental employment status, or home ownership. It was also not associated with maternal age at conception, history of breastfeeding, or the duration of breastfeeding. Unlike Pai et al. (2012), however, highly significant associations were found with neonatal factors, including gestational age, birth weight, and parent-reported admission to a neonatal intensive care unit. Children born at less than 37 weeks gestation had a 5-fold greater risk of having amblyopia and those with birth weights less than 2500g were almost 5 times more likely to have amblyopia at the time of examination. There was also a borderline association with maternal smoking during pregnancy. Tarczy-Hornoch et al.’s (2011) analysis of demographic factors from BPEDS/MEPEDS found significant effects but only for the risk for bilateral decreased visual acuity. Specifically the risk for bilateral decreased visual acuity was higher in those without health insurance and in children whose primary care-giver lacked high school education. Active maternal smoking during pregnancy and lack of breastfeeding were also associated with bilaterally reduced visual acuity, but only amongst the MEPEDS subgroup. Similarly, Williams et al. (2008) found an association between amblyopia and maternal smoking during pregnancy. A family history of amblyopia and/or strabismus was also a predictive factor as was lower social class. This differs from Brown et al. (2000) who, like Robaei et al. (2006) and Pai et al. (2012), found no effect of place of birth on prevalence although it accords with the review by Simons (2005) who concluded that amblyopia is more prevalent amongst lower social groups. Thus, while a number of factors, in particular maternal smoking and lower socioeconomic status have shown an association with amblyopia in more than one study, there is as yet little consensus on precisely which factors are predictive of future amblyopia occurrence.
Summary of Amblyopia Prevalence and the Factors that affect it
Amblyopia prevalence, like anisometropia prevalence, is critically dependent on the criterion used for diagnosis, but typically, population-based studies indicate that it exists in 1.6% to 3.6% of the population (Table 3). Prevalence does not vary with age once attempts to treat it have ceased. Amblyopia prevalence does not depend on gender but modest differences may exist between races. In populations that screen for amblyopia, and where necessary treat, prevalence falls. This is consistent with suggestions that amblyopia prevalence is higher amongst socially disadvantaged and medically-underserved communities.
3.3. Co-occurrence of Anisometropia and Amblyopia
Co-occurrence Rates
When one considers the proportion of amblyopes with anisometropia or conversely the proportion of anisometropes with amblyopia, it quickly becomes clear that the two conditions frequently co-occur. Anisometropia appears to be the only associated abnormality in around one-third of cases of human amblyopia (Flom and Neumaier, 1966; Flom and Bedell, 1985; Attebo et al., 1998; Robaei et al., 2006; Friedman et al., 2009; Pai et al., 2012), and is present together with strabismus in approximately a further 10% (Friedman et al., 2009), 19% (Robaei et al., 2006) or 28% of cases (Flom and Neumaier, 1966; Flom and Bedell, 1985). Thus, most studies find that anisometropia is present in around half of all cases of human amblyopia (Robaei et al., 2006; Friedman et al., 2009; Chia et al., 2010; Pai et al., 2012). Some have found that anisometropia is present in an even higher proportion (61%, MEPEDS, 2009; 66%, Flom and Bedell, 1985; 75%, Attebo et al., 1998) (also see Table 3). Thus, the prevalence of anisometropia in amblyopes vastly exceeds the proportion of the general population that exhibit anisometropia (Figure 3). Conversely, several studies have examined the proportion of anisometropes who exhibit amblyopia. For example, Flom and Bedell, (1985) found that 14% of non-strabismic anisometropes had amblyopia, and this figure rose to 20% when individuals with both strabismus and anisometropia were considered. Robaei et al. (2006) found amblyopia in around 57% of their six-year-old anisometropes but only 0.8% of children in the whole study sample (n=1741) had amblyopia without anisometropia. Very similar figures have been reported by other authors; in population-based studies, the proportion of individuals with amblyopia without anisometropia was 0.5% (Chia et al., 2010), 0.7% (Friedman et al. 2009, BPEDS) or 0.8% (Pai et al., 2012). In Tarczy-Hornoch et al.’s (2011) analysis of MEPEDS and BPEDS population-based data, 59.5% of children with ≥2D of spherical-equivalent anisometropia had a ≥2 line difference in interocular acuity and an acuity of 20/32 or worse in the worse eye. Similarly, although their data were not from a population-based study, Tanlamai and Goss (1979) reported an amblyopia prevalence (VA of 20/30 or less in the weaker eye, with 20/25 or better in the fellow eye) of 35% amongst their hyperopes with 1D or more of spherical-equivalent anisometropia. Again, this figure vastly exceed the proportion of amblyopes in the general population (see amblyopia prevalence section).
Tarczy-Hornoch et al. (2011) examined the ‘risk’ of reduced visual acuity with anisometropia in two population-based samples of pre-school children using a multivariate analysis approach that allowed them to control for the presence of strabismus. The chances of finding a ≥2 line inter-ocular difference in visual acuity were substantially greater (odds-ratio of 4.5) in those with 1D to <2D of spherical equivalent anisometropia compared to children with 0 to <1D anisometropia. Furthermore, they examined the relationship between a vector-based measure of anisometropia and the prevalence of an interocular difference in visual acuity. They found a strong linear relationship between anisometropia magnitude (range 1 to 5D) and prevalence of reduced visual acuity with the latter reaching almost 100% at 5D of anisometropia.
All of these data show that while it is possible to have one condition without the other, anisometropia and amblyopia routinely co-occur establishing that these two conditions, one refractive and the other sensory/neural, are not independent.
Co-occurrence of Anisometropia and Amblyopia with Significant Hyperopia
Examination of refractive error data from amblyopes reveals a much stronger association between amblyopia and hyperopia than between amblyopia and myopia. For example, Robaei et al. (2006) found that 58.6% of amblyopic eyes had significant hyperopia (≥+3D SE) whereas only 8.7% had myopia. Several other studies have reported similar findings (Attebo et al., 1998; Wang et al., 2011; Pai et al., 2012). Similarly, many studies report that a greater proportion of anisometropic amblyopes are anisohyperopes than anisomyopes (e.g. Copps, 1944; Rutstein and Corliss, 1999; Tanlamai and Goss, 1979) and that the severity of the associated amblyopia is also generally greater in anisohyperopes than in anisomyopes (e.g. Tanlamai and Goss, 1979; Leon et al., 2008; Levi et al., 2011). These studies point to the fact that the prevalence and severity of amblyopia associated with anisometropia varies with both the type (i.e. the sign) and the magnitude of the anisometropia, as summarised in Figures 4 and 5. Given that the prevalence of anisomyopia is higher than anisohyperopia (section 3.1), the fact that so many more amblyopes have anisohyperopia than anisomyopia is even more striking.
Figure 4.

Prevalence of amblyopia (%) as a function of the magnitude of anisometropia (D). Positive values on the abscissa indicate anisohyperopia, negative values represent anisomyopia. Studies were included only if they reported on individuals without manifest strabismus and if they distinguished between cases anisomyopia and anisohyperopia. Studies were not included if the whole sample was amblyopic (e.g. Townshend et al., 1993; Kutschke et al., 1991). The criteria for diagnosis of amblyopia are those that appeared in the respective studies.
Figure 5.
Severity of amblyopia (logMAR VA) as a function of magnitude of anisometropia (D). Positive values on the abscissa indicate anisohyperopia, negative values represent anisomyopia. Studies were included only if they reported on individuals without manifest strabismus and if they distinguished between cases anisomyopia and anisohyperopia.
Relationship between Anisometropia Type and Amblyopia Prevalence
Generally, amblyopia prevalence rates increase with the amount of anisometropia, but are higher in populations of anisohyperopes than anisomyopes (Figure 4). Interestingly, there are reports of patients with very high levels of anisomyopia (e.g. −10D) who do not have amblyopia whereas most studies report that all individuals with high anisohyperopia have amblyopia. For example, in Tanlamai and Goss (1979) amblyopia prevalence (acuity of 20/30 or worse in poorer eye with 20/25 or better in the fellow eye) was 100% of anisohyperopes with ≥4.5D SE of anisometropia and 50% in anisohyperopes with ≥2.5D SE of anisometropia. In contrast, 100% amblyopia prevalence was reached only when there was at least 6.5D of anisomyopia and only 50% of individuals with anisomyopia of 4.5D were amblyopic. Tanlamai and Goss (1979) noted that, irrespective of how anisometropia was calculated (e.g., as inter-ocular difference in spherical equivalent refractive error or inter-ocular difference between meridians with least refractive error), the prevalence of amblyopia amongst anisohyperopes consistently matched that among anisomyopes with ~2D more of anisometropia. Similar findings have been observed in more recent studies (e.g. Dobson et al., 2008a; Levi et al., 2011; Weakley, 1999, 2001). In the study by Levi et al. (2011), it was estimated that the risk of amblyopia was around twice as high in the hyperopic anisometropes than the myopic anisometropes with the same level of anisometropia.
Relationship between Anisometropia Type and Amblyopia Severity
The link between amblyopia severity and anisometropia magnitude has proven particularly difficult to establish. While some early studies reported that the severity of amblyopia does increase with the amount of anisometropia (e.g. Copps, 1944; Jampolsky et al., 1955; Phillips, 1959; Kivlin and Flynn, 1981; de Vries, 1985) others found little or no association (e.g. Horwich, 1964; Helveston, 1966; Malik et al., 1968). The issue remains contentious (Kutschke et al., 1994; Townshend et al., 1994; Lempert, 2006; Donahue, 2006). One possible reason for the discrepant findings relates to the fact that many did not differentiate between anisomyopes and anisohyperopes. A second reason is that fixation status was not always reported and so some individuals diagnosed as anisometropic amblyopes may in fact have been microstrabismic (e.g. Helveston, 1966). Also, some studies examining the relationship between anisometropia magnitude and visual acuity have included non-amblyopic anisometropes (e.g. Weakley, 1999, 2001; Levi et al., 2011) while others have included only amblyopic anisometropes (e.g. Kutschke et al., 1991; Townshend et al., 1993). Another factor that could account for differences between studies of adult anisometropia magnitude and amblyopia severity is the fact that the anisometropia or even spherical refractive error may not remain stable from childhood to adulthood (Abrahamsson et al., 1990b; Almeder et al., 1990). In other words, the anisometropia present in an adult amblyope may not reflect the anisometropia that existed (if indeed any did exist) when amblyopia was developing in early life. In addition, the acuity of the adult amblyope may also reflect any response to treatment.
Figure 5 shows how severity of amblyopia is linked to the sign and magnitude of anisometropia. The picture that emerges is similar to the pattern evident in Figure 4 in that the severity of amblyopia associated with a given level of anisohyperopia is greater than for similar levels of anisomyopia. Thus, although acuity worsens with increasing magnitude of both anisomyopia and anisohyperopia, the fall-off is less steep in anisomyopes than in anisohyperopes. Interestingly, reduced binocularity also appears to be more prevalent with higher levels of anisometropia, and is more often associated with anisohyperopia than anisomyopia (Rutstein and Corliss, 1999; Weakley, 2001; Levi et al., 2011), although this issue has received comparatively little attention.
Implications for Understanding Aetiology of Anisometropic Amblyopia
Differences in amblyopia prevalence and severity among anisohyperopes and anisomyopes have both been included in discussions of the mechanisms by which amblyopia develops. Notably, it has been argued that anisohyperopia will likely lead to habitual defocus in the more hyperopic eye whereas anisomyopes can alternately use the more myopic eye for near work and less myopic eye for distance work, avoiding habitual defocus of one eye (von Noorden, 1977, 2002). Alternatively it can argued that amblyopia may be more prevalent and more severe in anisohyperopes because anisohyperopia typically develops much earlier in life than anisomyopia (Schapero, 1971), since myopia arises later in life than hyperopia (Hirsch, 1967; Parssinen, 1990; Pointer, 2004). Also, anisomyopia has a much lower prevalence than anisohyperopia in infants (e.g. Abrahamsson and Sjostrand, 1996; Zonis and Miller, 1974) and young children (e.g. Donnelly et al., 2005; Leon et al., 2008). Both of these hypotheses and the evidence to support or refute them are elaborated upon in the next section.
As summarized in Figures 4 and 5, as the levels of anisometropia increase, both the prevalence and severity of amblyopia increase. These studies confirm the tight relationship that exists between anisometropia and amblyopia, and in the next section we examine the nature of this relationship.
4. Cause and effect: a classic chicken and egg story
Strabismus and anisometropia are considered to be the two primary causes of amblyopia. In both the clinical and basic science literatures authors regularly assert that anisometropia is a leading cause of amblyopia (Flom and Bedell, 1985; Smith et al. 1985; Kiorpes et al., 1987; Ciuffreda et al., 1991; von Noorden, 2002; Simons, 2005; MEPEDS, 2009). For example, “anisometropia is frequently considered to be the most common cause of amblyopia” (Ciuffreda et al., 1991), “strabismus and anisometropia are the two major causes of functional amblyopia” (Flom and Bedell, 1985), anisometropia is “one of the most frequent causes of amblyopia in the human population” (Smith et al., 1985), “in humans, amblyopia frequently occurs as result of anisometropia” (Kiorpes et al., 1987), and “amblyopia is usually caused by abnormal refractive error”, typically “purely anisometropic unilateral amblyopia without strabismus” (MEPEDS, 2008).
Although most studies state the single cause and effect relationship noted above, several laboratory and clinical studies have made additional observations beyond the standard explanation (Almeder et al., 1990; Barrett et al., 2005; Lempert, 2000, 2003, 2004, 2008b; Lempert and Porter, 1998). In one instance the question has been cast as an interesting case of “which came first, the chicken or egg?” (Fielder and Moseley, 1996). Abrahamsson and Sjostrand (1996) captured the potential complexity of this topic in their 1996 paper (see section 1).
Because prospective studies of animal models clearly show that anisometropia can precede amblyopia (Smith et al., 1985), that amblyopia can precede anisometropia (Kiorpes and Wallman, 1995), and that monocular deprivation can affect both ocular growth (Raviola and Wiesel, 1978; Wiesel and Raviola, 1977, 1979) and cortical synaptic circuitry (Hubel et al., 1977; Wiesel and Hubel, 1963, 1965) resulting in both anisometropia and amblyopia, three hypotheses emerge as viable explanations for why anisometropia and amblyopia are so often discovered together (Figure 6): (1) Hypothesis 1: anisometropia causes amblyopia (e.g. due to chronic monocular blur and thus a form of monocular deprivation, which leads to abnormal binocular interaction because of interocular difference in retinal image clarity, Ciuffreda et al. 1991), (2) Hypothesis 2: amblyopia causes anisometropia (due to interference in the emmetropization process), and (3) Hypothesis 3: a third factor causes a disruption of both emmetropization of the eye and cortical function. In fact, all three of these hypotheses require some initial anomaly to trigger anisometropia and/or amblyopia to develop. In most cases, clinicians are unable to identify this apparent trigger unless it consists of clinically observable signs, such as ptosis or strabismus.
Figure 6.

Hypothesis 1 is the standard clinical explanation: something causes emmetropiziation to fail, and chronic, unilateral blurring from anisometropia causes the amblyopia. Hypothesis 2: the anisometropia is caused by the amblyopia. Hypothesis 3: the amblyopia and the anisometropia are not linked causally but are both triggered by a third factor. Hypotheses 2 and 3 are theoretically distinct but they are difficult to distinguish because amblyopia tends to arise quickly following deprivation whereas anisometropia onset and progression is much slower (see section 4.1). Thus, even if Hypothesis 3 is correct, the earlier appearance of the amblyopia could falsely be interpreted as evidence that the amblyopia has caused the anisometropia (Hypothesis 2). Cases of “pure ” anisometropic amblyopia (where there is no apparent triggering factor) are believed to represent instances where the anisometropia has caused the amblyopia (Hypothesis 1) but Hypotheses 2 or 3 may be correct if a sub-clinical anomaly exists now or if one existed in the past.
Because both anisometropia and amblyopia can exist without overt signs and symptoms, young patients with amblyopia and/or anisometropia are commonly not found until they have entered the school system (Ingram, 1977; Shaw et al., 1988; Woodruff et al., 1994), at which point the initial event that triggered either the amblyopia or anisometropia may be in the distant past. In contrast, patients with strabismus typically present at a clinic at much younger ages because of the overt nature of esotropia and exotropia. Children with strabismus present at a mean age of around 3.5 years, whereas those with anisometropia and amblyopia (and no obvious strabismus) are on average 3 years older (Ingram, 1977; Shaw et al., 1988; Woodruff et al., 1994). Also, adult patients with strabismus and amblyopia reported being diagnosed at a mean age of 7.7 years, while those with anisometropic amblyopia reported diagnosis at 12.7 years (Attebo et al., 1998). This discrepancy may be further exaggerated in more socially and economically disadvantaged groups (Smith et al., 1994). This unfortunate reality means that the diagnosis of anisometropia is routinely made at the same time as the diagnosis of amblyopia (Shaw et al., 1988; Woodruff et al., 1994), often long after some estimates of the human critical period have ended (Banks et al, 1975; Daw, 1998; Vaegan and Taylor, 1979). Thus, this standard clinical presentation provides little insight into the aetiology of either condition.
Hypothesis 1 (anisometropia causes amblyopia, Figure 6) requires chronic blur of one eye’s retinal image to serve as an amblyogenic monocular deprivation early in life (Maguire et al., 1982; Smith et al., 1985). For anisometropia to generate chronic unilateral blur, two conditions must co-exist. First, accommodation must remain yoked in the two eyes as unequal or uncoupled accommodation responses could theoretically bring both retinal images into focus. Most studies (Ball, 1952; Flitcroft et al., 1992; Koh and Charman, 1998; Bharadwaj and Candy, 2011; but see Horwood and Riddell, 2010) find that accommodation is consensual in the presence of anisometropia but small amounts of aniso-accommodation have been reported by Marran and Schor (1998, 1999), which may reflect the need for small amounts of aniso-accommodation with eccentric gaze (Charman, 2011). Second, for anisometropia to generate chronic defocus in one eye, anisometropes must habitually focus the other eye. It has been argued that anisometropes, like isometropes (e.g. Heath, 1956), will employ the least amount of accommodation required to focus, and thus anisomyopes can employ the more myopic eye at near (McMullen, 1939; Freeman, 1975; Ciuffreda et al., 1991; Horwood and Riddell, 2010; Koh and Charman, 1998; von Noorden, 2002), while anisohyperopes will habitually focus the less hyperopic eye for all target distances (McMullen, 1939; von Noorden, 2002). Linke et al. (2012) have recently reported that greater hyperopia exists in the non-dominant eye compared to the dominant eye in a large sample of human hyperopes, which also suggests that the less hyperopic eye in bilateral hyperopes is habitually focused. This difference in the pattern of accommodation between anisomyopes and anisohyperopes has been proposed to explain the higher prevalence and severity of amblyopia in anisohyperopes (see previous section). However, there is some recent evidence to suggest that unilaterally myopic humans may focus with the distance-corrected eye at both distance and near, consistent with their vergence demand (Phillips, 2005). Phillips studied eighteen, 11-year-old myopic children who wore a monovision spectacle correction over a 30 month period. Although participant numbers are small and though it contained only myopes, this study tentatively suggests that anisometropes may habitually focus the less ametropic eye, generating chronic defocus in the more ametropic eye (also see Bharadwaj and Candy, 2011). Consistent with this observation, amblyopia is generally found in the more ametropic eye of both anisohyperopes and anisomyopes (Rutstein and Corliss, 2004). The lower prevalence of amblyopia in anisomyopes may, therefore, not be due to alternating focus with changing target distance, but instead reflect the earlier development of hyperopia and anisohyperopia in young children when myopia is rare (Abrahamsson and Sjostrand, 1996). Thus, chronic unilateral blur in many anisomyopes may appear late in the sensitive period (Vaegan and Taylor, 1979; Harwerth et al., 1986; Daw, 1998;) and consequently have less impact on the maturation of visual cortex (Hubel and Wiesel, 1970), and thus generate less amblyopia.
These arguments assume that anisometropic amblyopia arises due to monocular deprivation caused by chronic unilateral defocus of the more ametropic eye. This hypothesis (Hypothesis 1) predicts that (1) amblyopia should be more severe as anisometropia increases (more unilateral blur deprivation), and (2) anisometropias that emerge earlier in the sensitive period will be associated with more prevalent and more serious amblyopia. There is strong evidence to support the first of these predictions (Figure 5, section 3.3) but very little is known about how the timing of the onset of anisometropia interacts with amblyopia onset or severity.
Although Hypothesis 2 (amblyopia causes anisometropia, Figure 6) and Hypothesis 3 (a third factor causes both amblyopia and anisometropia, Figure 6) are theoretically distinct, they are difficult to distinguish in practice. Amblyopia can develop extremely quickly (e.g. in hours in kittens, Freeman and Olson, 1982; Olson and Freeman, 1975; days or weeks in non-human primates, e.g. Hubel and Wiesel, 1970; Harwerth et al., 1981; Kiorpes and Wallman, 1995; months in humans, Hardesty, 1959 reporting a single case-study), while anisometropia results from slower differential growth of the two eyes that can take months or years (non-human primates: Raviola and Wiesel, 1985; Troilo and Judge, 1993; Kiorpes and Wallman, 1995; humans: Lepard, 1975; Nastri et al., 1984). Therefore, although a third factor could trigger the two quite separate processes, the amblyopia would likely appear to precede the anisometropia, and thus the progression described by Hypothesis 3 could appear consistent with Hypothesis 2 (Figure 6).
One examination of a series of monkeys raised with either artificial strabismus or unilateral defocus revealed a close relationship between the magnitude of the emergent amblyopia and the magnitude of the anisometropia recorded at the time when the inter-ocular visual acuity differences were noted (Kiorpes and Wallman, 1995). Kiorpes and Wallman (1995) noted that the amblyopia appeared (typically at around 10 weeks) significantly before the anisometropia (at ~30 weeks). They argue that these data support the hypothesis that it is the amblyopia (and not the strabismus or defocus) that is responsible for “altered eye growth”. Also, Troilo and Judge (1993) observed anisometropia developing in non-human primates after short term monocular deprivation was completed, presumably due to the continued presence of the neural amblyopia even though the deprivation source had been removed, and Smith et al. (1999) also came to this conclusion. Contrary to this interpretation, however, Raviola and Wiesel (1985, 1990) found that monocular deprivation-induced eye growth occurs in monkeys with both optic nerves sectioned or with the visual cortex removed. They argue that these data reject the hypothesis that cortical amblyopia causes the anomalous eye growth and instead, disruption of some local (e.g. in the eye) growth mechanism is responsible. This may be consistent with Huang at el. (2009) who found that in addition to producing central refractive errors, abnormal visual experience can alter the shape of the posterior globe and the pattern of peripheral refractive errors in infant primates. Perhaps the abnormal ocular shape that results from the deprivation could also lead to ocular growth after the deprivation is eliminated.
The animal literature, therefore, presents a rather complex set of results. Those studies in which anisometropia was introduced experimentally and amblyopia resulted (Maguire et al., 1982; Smith et al., 1985; Kiorpes et al., 1993), of course, are consistent with Hypothesis 1 (Figure 6). Several studies, however, point to amblyopia preceding anisometropia and thus potentially causing the anisometropia (Hypothesis 2, Figure 6), which is consistent with the notion that a cortical deficit can influence eye growth, and there are studies that point to a third factor (e.g. deprivation) triggering two quite independent processes, one in the cortex and one in the eye, to generate the amblyopia and the anisometropia (Hypothesis 3, Figure 6).
In the following sections we provide a comprehensive review of the experimental evidence for and against these three different hypotheses. Prospective experimental studies of primates provide insight into the potential chronology of emerging amblyopia and anisometropia, and thus provide an opportunity to examine the aetiology of anisometropic amblyopia (section 4.1). Section 4.2 describes studies of neural, visual and refractive development in children who have had early onset, clinically-demonstrable pathology and/or disruption of visual experience (the triggering event) and who go on to develop anisometropia and/or amblyopia. The special case of children who develop strabismus and amblyopia and then go on to develop anisometropia is examined in section 4.3, and then the more challenging analysis of children who appear at the clinic with both amblyopia and anisometropia but without any apparent pathology or strabismus is provided in section 4.4. Finally studies of children undergoing amblyopia therapy are discussed in section 4.5.
4.1. Experimental studies of non-human primates
Experimental studies of non-human primates provide the most detailed chronology of the emergence of amblyopia and anisometropia. Early examples of these studies in which the visual input to one eye was degraded generally had one of two quite separate goals, either to examine the impact of unilateral interruption of visual input on the sensory system (neural and/or behavioural responses, e.g. Blakemore, 1978; Harwerth et al., 1981), or to examine the impact of visual disruption on eye growth and refraction (Raviola and Wiesel, 1985). Later studies (e.g. Kiorpes and Wallman, 1995; Smith et al., 1999) have examined both the visual/neural as well as refractive anomalies associated with abnormal visual experience during early life. Several general results emerge from this literature, but there are some intriguing inconsistencies.
First, as shown initially by Hubel and Wiesel (Wiesel and Hubel, 1963, 1965; Hubel and Wiesel, 1970; Hubel et al., 1977), interruption of the visual input to one eye early in life has the potential to completely eliminate that eye’s ability to activate neurons in the primary visual cortex (monocular deprivation paradigm), which can result in almost complete loss of spatial vision (Harwerth et al., 1981). These effects can be quite rapid, e.g. very significant imbalance in cortical ocular dominance can occur after 8 hours of monocular deprivation in kittens (e.g. Freeman and Olson, 1982; Olson and Freeman, 1975) and after days (Hubel et al., 1977) or weeks in young monkeys (e.g. Hubel et al., 1977; Kiorpes and Wallman, 1995). Two weeks of monocular deprivation are sufficient to generate almost complete loss of spatial vision in young monkeys (Harwerth et al., 1981). Neurological and visual deficits in monkeys also emerge after 2-10 weeks of artificial strabismus (Kiorpes, 1992; Kiorpes and Movshon, 1989) or two months of monocular defocus (Smith et al., 1985).
In a series of studies of visually deprived monkeys, Raviola and Wiesel (1985, 1990) revealed that monocular deprivation of the types that produce neurological amblyopia also produce significant anomalies in eye growth. Notably, the same monocular deprivation that generates a rapid neurological and visual deficit in the deprived eye’s pathway produced long and thus myopic eyes. This result has been replicated by a number of laboratories (e.g. Bradley et al., 1996; Greene and Guyton, 1986; Smith and Hung, 2000), with varying forms of deprivation (opaque and translucent contact lenses, high power defocusing lenses, ocular lens removal, and scattering in the cornea). When introduced monocularly, these manipulations generally lead to anisometropia, but not always myopic eye growth (see below).
The generally rapid neurological and visual consequences of interruption of visual input to one eye have been contrasted with the slow emergence of refractive error changes following deprivation. For example, Raviola and Wiesel (1985) showed that anisometropia continued to develop over one year during which monocular deprivation persisted. Their data reveal anisometropia first appearing at 2 months after initiation of deprivation but the anomalous eye growth continues for a far longer period than the amblyopia development, because the neuro-sensory effects of monocular deprivation may be complete after about 2 months or less (Hubel and Wiesel, 1970; Hubel et al., 1977; Harwerth et al., 1981). These studies clearly show that unilateral interruption of visual input early in life can generate both amblyopia and anisometropia by interrupting normal refractive and sensory development and are thus consistent with Hypothesis 3 (Figure 6). However, because of the more rapid development of the neuro-sensory deficit in visual cortex, these results could be interpreted as evidence in support of Hypothesis 2 (amblyopia precedes and then causes anisometropia, Figure 6).
The Effect of the Type of Disrupted Visual Experience on Refraction and Neural Function
Interestingly, some methods of degrading the visual input to one eye trigger faster eye growth resulting in a larger and more myopic eye, while other forms of deprivation seem to trigger a slowing of eye growth relative to the fellow, undeprived eye. For example, with lid suture, all eyes became myopic in the Raviola and Wiesel studies (Raviola and Wiesel, 1985, 1990), a result that was repeated by Greene and Guyton (1986). However, only two of the four monocularly deprived monkeys of Harwerth et al. (1983) developed myopia in the lid-sutured eye, while two developed hyperopia. The larger myopic eyes had the standard characteristics associated with myopia: long axial lengths, and in some cases the associated fundus signs of a “stretched” retina (temporal crescent at the disk, visible choroidal vessels). Unilateral myopia was also observed by Tigges et al. (1990) and Bradley et al. (1996) who created monocular deprivation using opaque contact lenses instead of lid suture, whereas less severe disruption of the visual input created with optical diffusers (e.g. diffusing contact lenses, Bradley et al., 1996) resulted in aniso-hyperopia. When the cornea was used as a diffuser (Raviola and Wiesel, 1985) or diffusing goggles were used (Smith and Hung, 2000), anisomyopia developed. Also, less severe monocular deprivation has been created with high-powered defocusing contact lenses. In cases where, for example, a negative lens is introduced before one eye, a case of refractive anisometropia is generated that may be corrected by eye growth. When the lens is then removed, the animal now has axial myopic anisometropia which is also potentially correctable by modulating eye growth. The important feature is that emmetropization (controlled growth of the vitreous chamber) can effectively correct for either refractive or axial anisometropia.
Crewther et al. (1988) used +6D, −6D, or −9D lens powers starting between 7 and 46 weeks and lasting for up to 15 months. They found no anisometropia in 5 of their 9 monkeys and anisohyperopia in the remaining four. Smith et al. (1994) employed −9D lenses, and observed anisohyperopia in five of eight monkeys, anisomyopia in two and isometropia in one. Smith et al.’s treatment started at between 12 and 30 days, and lasted between 2 and 4 months, after which many of the treated eyes began to emmetropize. Kiorpes and Wallman (1995) reared five monkeys with a −10D contact lens in one eye, starting at between 10 and 25 days and lasting for 7-10 months. Three of the five monkeys developed unusually high hyperopia in the treated eye. It is important to note that if the −6D, −9D, and −10 D were driving an active emmetropization process, these eyes should have developed myopia. The image plane was also moved far behind the retina by removing the eye’s lens in monkeys (Wilson et al., 1987; Tigges et al., 1990). These animals did not emmetropize, and rather than increase the growth rate (to place the retina at the image plane), growth slowed resulting in extra hyperopia. The interpretation of these studies is complicated by the obvious interactions between eye growth and deprivation. For example, it appears that when lower levels of anisometropia are introduced experimentally, compensatory eye growth (emmetropization, e.g. Smith et al., 1999) will eliminate the monocular blur produced by the experimental anisometropia, and thus, as expected, the animals that re-emmetropize do not develop amblyopia. These experiments emphasize that the visual system is capable of correcting for anomalous eye growth (anisometropia) and in doing so can eliminate the amblyogenic monocular blur deprivation and thus prevent amblyopia from developing. Evidence of a similar capability in humans is reviewed in section 4.4.
The study of marmosets by Troilo and Judge (1993) is very interesting because it shows that short-term monocular lid suture initially generates some slight hyperopia, which is then followed by emmetropization and then finally myopia. An initial hyperopic shift followed by a myopic shift was also seen in some of the monkeys reared with monocular diffusers (Smith and Hung, 2000).
Interestingly, interruption of vision during the normal eye growth period in monkeys does not guarantee that amblyopia or anisometropia will develop. For example, only four of the nine contact-lens reared monkeys of Crewther et al. (1988) became amblyopic, and only five of eight of Smith et al.’s (1994) −9D contact-lens reared animals became anisometropic. It appears therefore, that when vision is interrupted slightly (e.g. blur, diffuser) about half of the eyes continue to emmetropize normally and do not exhibit amblyopia while half do not emmetropize and exhibit slowed eye growth and amblyopia. Why milder deprivation generates anomalous eye growth and amblyopia in some animals but not in others is unknown, but a similar pattern is seen in young humans (section 4.4).
Visual interruption that is started too late will not result in amblyopia or anisometropia (Raviola and Wiesel, 1985). Also, after visual disruption started early in life that is then removed early enough, recovery via emmetropization can occur if the initial visual interruption was milder (Qiao-Grider et al., 2004; Smith and Hung, 2000). Such recovery is absent if the initial monocular deprivation was complete as in lid suture (Qiao-Grider et al., 2004). Perhaps most significantly, lower levels of induced anisometropia (typically 3 or 6 diopters) fail to generate amblyopia and instead typically result in a corrective emmetropization response (Smith et al., 1999).
The results of the studies summarised above clearly indicate that interruption of vision during the period of normal eye growth in monkeys can lead to rapid development of amblyopia and can prevent normal emmetropization due to anomalous growth rates of the deprived/amblyopic eye. Rather surprisingly, if the interruption in vision is almost complete (lid suture, black contact lens), most eyes develop severe amblyopia (almost total blindness) and grow too much, becoming highly myopic (Raviola and Wiesel, 1985; Greene and Guyton, 1986). If the deprivation is less severe, most investigators find a consistent slowing of ocular growth and resulting anisohyperopia with less amblyopia (Wilson et al., 1987; Crewther et al., 1988; Kiorpes and Wallman, 1995; Smith et al., 1994; Bradley et al., 1996), although Smith and Hung (2000) and Raviola and Wiesel (1985) found myopia in such cases. The Troilo and Judge (1993) and Smith and Hung (2000) results indicate that during the period in which anisometropia is emerging, the type (i.e. sign) of the anisometropia may change, but the general relationship between amblyopia and anisometropia is clear, in that higher levels of anisometropia are associated with deeper amblyopia (Kiorpes and Wallman, 1995).
Nathan et al.’s (1985) study of humans with naturally occurring visual deprivation led them to propose a hypothesis to explain these differences in the type of anisometropia precipitated by mild and severe forms of visual disruption. Nathan et al. proposed that visual deprivation restricted to the fovea (or equivalently to higher spatial frequencies, Kiorpes and Wallman, 1995) will generate hyperopic anisometropia, whereas more complete deprivation that affects the whole retina will lead to myopic anisometropia. We now ask whether data from studies of non-human primates support this hypothesis. Recent studies by Smith and colleagues (Smith et al., 2007) reveal the emergence of deprivation myopia in monkeys reared with unilateral diffusers after foveal ablation. Interestingly, although this study emphasizes that contrast deprivation in the peripheral retina can precipitate abnormal myopic eye growth, more than half of the eyes treated this way did not become myopic. When introducing a −3D blurring lens after foveal ablation, however, all monkeys emmetropized in the same way as control monkeys with an intact fovea. Also, when the anisometropia was introduced into one hemifield (Smith et al., 2009), the eyes grew toward emmetropia only in one hemiretina. This recent work from Smith’s group emphasizes the critical role of the peripheral retina in emmetropization, but it does not explain why or how high spatial frequency deprivation at the fovea leads to hyperopic anisometropia (Nathan et al’s hypothesis).
Although the animal studies clearly show that anisometropia and amblyopia can both follow unilateral interruption of vision early in life, they do not distinguish clearly between the three causal hypotheses outlined above. Yes, anisometropia-induced blur can lead to amblyopia (Hypothesis 1, Figure 6). Yes, eyes made amblyopic by monocular deprivation can grow abnormally resulting in anisometropia even after the deprivation has been removed (Hypothesis 2 or 3, Figure 6), and, consistent with Hypothesis 3 (Figure 6), experimental monocular deprivation that precipitates amblyopia can also lead to anomalous eye growth. There is weak evidence that it is the induced amblyopia that causes the emmetropization failures (Troilo and Judge, 1993; Kiorpes and Wallman, 1995) (Hypothesis 2), but somewhat more compelling data suggest that the emmetropization failures are triggered locally and are independent of any neural deficits in the cortex (Raviola and Wiesel, 1985, 1990) (Hypothesis 3) (Figure 6).
The monkey literature, therefore, supports the idea that anisometropia and amblyopia development can both be triggered by the same types of monocular disruption in visual input, but these processes may have quite different time-courses. Anisometropia may follow after amblyopia has emerged, or proceed without a cortex. Also, these monkey studies reveal that more severe monocular deprivation generally (but not always) leads to myopic eye growth, whereas milder forms of monocular deprivation have a bias to slow eye growth and generate unilateral hyperopia. Anisometropia in some animals leads to amblyopia, whereas in others it triggers active emmetropization to correct the anisometropia and then amblyopia does not emerge. These monkey studies, therefore fail to reject any of the three hypotheses.
4.2. Humans with Disrupted Visual Experience Early in Life
As discussed above, the greatest challenge to uncovering the causal relationships in human anisometropic amblyopia is the fact that patients tend to present clinically with both conditions, potentially long after they have developed (e.g. >6 years of age; Ingram, 1977; Shaw et al., 1988 Woodruff et al., 1994). There are, however, retrospective studies of patients who had well documented events that disrupted visual experience early in life. In this section we examine refraction and visual function in cases of early visual disruption. These studies have direct parallels to the studies of monkeys described in the previous section.
Amblyopia and anisometropia routinely develop in humans who have experienced serious early monocular image degradation caused by unilateral lid closure (Salleras and JC, 1950; Robb, 1977; Hoyt et al., 1981; Rabin et al., 1981; Rabin et al, 1981; Stone et al., 1981; von Noorden and Lewis, 1987; Inatomi et al., 2004; Oral et al., 2010) even when the lid closure is incomplete (O’Leary and Millodot, 1979; Gusek-Schneider and Martus, 2000), or by unilateral congenital cataracts (Frey et al., 1973; Rabin et al., 1981; von Noorden and Lewis, 1987; Shih et al., 1989), unilateral traumatic cataracts (Rabin et al., 1981; Shih et al., 1989; Vanathi et al., 2002), unilateral vitreous hemorrhage or debris (Rabin et al., 1981; Miller-Meeks et al., 1990; Mohney, 2002), unilateral corneal opacification (Shih et al., 1989), and numerous forms of unilateral retinal disease (Rabin et al., 1981; Nathan et al., 1985; Shih et al., 1989). These studies all demonstrate an often dramatic failure of emmetropization (e.g. spherical equivalent refractive error of −15D or −17D, even −25D) and a high incidence of significant astigmatism (Shih et al., 1989) with a sometimes catastrophic loss of vision (Frey et al., 1973; Vaegan and Taylor, 1979; Birch and Stager, 1988). Most significantly, these studies emphasize that emmetropization failures and amblyopia can originate from a wide variety of ocular problems manifest in all parts of the eye (from cornea to retina). For example, Stark and Walther (1984) reported anisometropia (≥1.25D interocular difference in sphere or ≥0.75D difference in cylinder) in 56% of their 54 patients with congenital ptosis. Common to all of these conditions, however, is the reduction in retinal activity in the affected eye, either due to reduced image contrast, or reduced neural function. In all these cases, as with the animal studies, the presumed initiating causal agent is well documented, but the retrospective study design provides no insights into the developmental sequence of refractive and visual anomalies. For example, both the eye growth and the amblyopia could be triggered by the pathology (though with different time scales) (Hypothesis 3) or the pathology could trigger only one (the eye growth or the amblyopia), which in turn leads to the other (Hypotheses 1 & 2) (Figure 6).
In relation to the impact upon eye growth, Nathan et al. (1985) observed a large range of refractive errors in a heterogeneous population of low vision patients indicating that emmetropization failures can occur across a wide range of causes of reduced visual function. The leptokurtosis seen in the refractive error distribution of normal eyes was also absent in a population with childhood visual anomalies and emmetropization failures were not restricted to cases of monocular visual disturbance (see also Rabin et al., 1981).
As with the animal studies of failed emmetropization during and subsequent to deprivation, post-deprivation ametropia in humans results from anomalous axial growth of the eye (Hoyt et al., 1981; Shih et al., 1989; Mohney, 2002; Vanathi et al., 2002). Following unilateral childhood cataracts, Inatomi et al. (2004) report some cases of exaggerated eye growth that continues over the ten years following cataract removal and lens implantation (see also Vanathi et al., 2002). These results are consistent with significant disruption of normal eye growth after childhood cataract removal, although it must be remembered that these patients can no longer exert accommodation and therefore they are likely to experience significant retinal defocus at some viewing distances. These studies are somewhat related to studies of monkeys after removal of monocular deprivation (section 4.1), some of which report anisometropia associated with anomalous eye growth after the deprivation source was removed (Troilo and Judge, 1993; Raviola and Wiesel, 1985). Nathan et al. (1985) also noted that disruption of vision during the period of rapid postnatal eye growth (up to 3 years) results in larger refractive errors, and those that had disease limited to the fovea tended to become hyperopic. The latter finding may be related to findings from animal models (reviewed in Section 4.1) in which the effects on eye growth appear to depend upon the severity of the deprivation.
Because amblyopia can emerge quickly in humans if monocular deprivation is present early in life (e.g. a somewhat extreme, single case-study where 1 month of full-time patching at age 2 years produced a visually unresponsive eye Hardesty, 1959), and abnormal eye growth may take years to materialize (Lepard, 1975; Nastri et al., 1984), these studies of disruption of visual experience in early in human life provide complex evidence regarding the relationship between anisometropia and amblyopia. The literature reviewed in this section shows that anisometropia and amblyopia can both follow monocular deprivation resulting from a wide variety of ocular pathologies and is therefore consistent with Hypotheses 2 or 3. It is important to acknowledge that once significant anisometropia developed in these cases, it could have precipitated amblyopia, but it is also reasonable to assume the initial visual disruption likely caused the amblyopia.
In addition to these studies reporting the consequences of postnatal visual deprivation, problems related to prematurity can also lead to anisometropia. When retinopathy of prematurity (ROP) develops there is evidence that both the prevalence of anisometropia and its magnitude increase and it is less likely to resolve than in typically developing visual systems (Schaffer et al., 1984; Snir et al., 1988; Holmstrom et al., 1998). For example, Schaffer et al. (1984) reported an anisometropia prevalence (≥1D difference in SE) of 15.4% (4 of 26 patients, examined at ~1 year) in individuals with fully resolved retinopathy of prematurity (ROP) compared to a 0% prevalence amongst 38 premature infants examined at the same age who had never had ROP. Holmstrom et al.’s (1998) study of 248 premature infants also found that anisometropia was more common in infants with ROP at 6 and 12 months of age (12.4% and 13.8%, respectively) than in those without ROP (2.7% and 4.5%, respectively) and Snir et al.’s (1988) study of 187 premature babies first examined at the age of 6 months and then longitudinally followed for between 4 and 11 years (mean follow-up 6.5 years) found a much higher prevalence of anisometropia (≥2.5D difference in spherical equivalent refractive error) in premature individuals with ROP (n=48; 10%) compared to premature infants without ROP (n=139; 0%).
Further evidence of a strong link between anisometropia in premature infants and the presence of retinal pathology has come from a large-scale, longitudinal study (initial n=2916) of low birth-weight infants by Quinn et al. (1992) who noted that the prevalence of anisometropia (defined as ≥2D difference in spherical equivalent) increased with the severity of ROP and with decreasing birth-weight. The presence of anisometropia was highly correlated with myopia. For example, the prevalence of high myopia (more than −5D spherical equivalent) amongst those with anisometropia at 3, 12 and 24 months of age was 18%, 28.6% and 34.2%, respectively, compared with only 0.7% to 1.8% of those who did not have anisometropia. Commenting on their results, Quinn et al. (1992) stated the following: “although there are many infants with anisometropia who do not develop high myopia and many infants with high myopia who do not develop anisometropia, the factors that predispose infants to develop high myopia or anisometropia are similar and are related to severity of ROP and to the prematurity of infants”. The link between severity of ROP and high myopia and anisometropia was further demonstrated in Quinn et al. (1998). Finally, there is evidence that anisometropia arises more commonly in cases where pathology is more advanced in one eye than its fellow or where treatment has been more effective in one eye (Quinn, personal communication, 2010).
In summary, there is a clear association between anisometropia and the presence in early life of retinal disease or pre-retinal pathology that interferes with the formation of a retinal image. This anisometropia can exist with or without amblyopia (Schaffer et al., 1984; Piotrowski et al., 2010). In this section we have summarized studies of humans in which anisometropia and amblyopia coexist following a history of ocular pathology. These studies generally provide no direct evidence of relative chronology of the amblyopia and anisometropia development. However, it is certainly feasible that the visual deprivation caused the amblyopia, and also that either the amblyopia (Hypothesis 2) or the deprivation (Hypothesis 3) was responsible for the failure of emmetropization (Figure 6).
4.3 Amblyopia and Anisometropia associated with Strabismus
Experimentally induced strabismus leads to amblyopia in some monkeys (Harwerth et al., 1983; Kiorpes and Wallman, 1995) and Kiorpes and Wallman’s (1995) strabismic monkeys who ended up amblyopic also developed anisometropia. Therefore, just as we examined the impact of monocular deprivation or disease on the development of amblyopia and anisometropia, in this section we examine evidence that strabismus also acts as a trigger for both amblyopia and anisometropia.
In humans, population-based studies indicate that amblyopia is strongly associated with the presence of anisometropia and/or strabismus in childhood and young adulthood, and that around half of the amblyopes with anisometropia also have strabismus (Flom and Neumaier, 1966; Ohlsson et al., 2001; Ohlsson et al., 2003; He et al., 2004; He et al., 2007; Goh et al., 2005; Robaei et al., 2006; Faghihi et al., 2011; Table 3). It is widely accepted that strabismus, like monocular deprivation, can cause amblyopia. Also, as observed by Flom and colleagues (Flom and Neumaier, 1966; Flom and Bedell, 1985), amblyopia is most prevalent in those children with both strabismus and anisometropia (prevalence is almost four times higher than in children with only strabismus or anisometropia) generally supporting the idea that both strabismus and anisometropia can contribute as causal agents. That is, when they co-exist, there are two causal agents present, and thus amblyopia is more likely to develop. Alternatively, if strabismus were the single causal agent for the amblyopia and the anisometropia, this would represent an example of hypothesis 3 (Figure 6). If strabismus causes amblyopia, which in turn disrupts emmetropization, we may be seeing an example of hypothesis 2 (Figure 6).
Three general approaches have been employed to investigate the development of anisometropia and amblyopia in patients who are strabismic. First, prospective longitudinal studies examined refractive, visual acuity and oculomotor status of children who were believed to be at risk for amblyopia or strabismus because of their family history (Aurell and Norrsell, 1990) or other clinical characteristics (e.g. significant hyperopia or anisometropia) (Abrahamsson et al., 1990a; Abrahamsson et al., 1990b). A second group of studies employed a retrospective analysis of prior refraction, visual acuity and oculomotor data collected from very large samples of young children (Ingram et al., 1994; Ingram et al., 2003; Ingram et al., 2009; Abrahamsson et al., 1992). These large data sets were later mined to identify early characteristics of children who eventually developed strabismus or amblyopia (Ingram et al., 1994; Ingram et al., 2003; Ingram et al., 2009). The studies by Weakley and colleagues (Weakley and Birch, 2000; Weakley et al., 2001) and Colburn and colleagues (2010) took a similar approach, though with smaller numbers. A third study design examines the longitudinal changes in refraction that take place over many years (in some cases decades) following the onset of strabismus in childhood (Lepard, 1975; Nastri et al., 1984; Birch et al, 2010).
Interestingly, in the studies of strabismic patients reported in this section (Ingram et al., 2009; Abrahamsson et al., 1992; Lepard, 1975; Birch et al., 2010), we find little direct evidence that anisometropia causes amblyopia in these patients (Hypothesis 1, Figure 6). There is, however, a consistent pattern in which, early esotropia and amblyopia precede anisometropia. These studies, therefore, examine the complex dynamic interplay between refractive, oculomotor and visual performance anomalies in the developing visual system of young humans, and they generally support the hypothesis that the presence of strabismus and amblyopia can precede anisometropia (Hypotheses 2 & 3, Figure 6). The complexity and significance of these studies and their interpretation warrants careful scrutiny.
When strabismus is present in an anisometropic individual, it is almost always of the convergent type and is generally found in anisohyperopes but not anisomyopes. For example, in the de Vries (1985) study of 64 children with anisometropia examined in an eye hospital, 40% of the anisometropes had strabismus and 89% of these had esotropia. There was no case of myopia amongst the strabismic anisometropes. Similarly, Phillips (1959) sampled 93 anisometropes with bilateral hyperopia and found 53 (57%) cases of strabismus, whereas only one of the 19 cases of anisometropia with bilateral myopia had a strabismus and this was of the divergent type. The association between anisometropia, hyperopia and esotropia has also been noted in a number of other studies (Aurell and Norrsell, 1990; Otsuka and Sato, 1984; Abrahamsson et al., 1992).
Longitudinal Study of ‘At Risk’ Children during Early Life
In the prospective longitudinal study by Aurell and Norrsell (1990), the refractive, oculomotor, and visual status of thirty-four children with a family history of strabismus were evaluated from the age of three months up to at least four years. Spectacles were only prescribed if strabismus developed. Thirteen of the 34 (38%) had 4 or more dioptres of hyperopia at the age of six months. Of these 13, seven showed a decrease in their hyperopia from the age of six months, with the reduction being most pronounced before the child reached their second birthday. None of these seven emmetropizing children became strabismic. In the remaining six who were highly hyperopic as infants, the hyperopia remained almost unchanged over the period of study and five of the six developed esotropia between the ages of 18 and 30 months; the sixth child exhibited esotropia around the age of four years. None of the seven children who showed signs of emmetropization were amblyopic at age four but three of the six who failed to show signs of emmetropization and who became esotropic exhibited amblyopia at this age. Although anisometropia (>1D difference in spherical equivalent refraction) was present at six months in three of the seven who emmetropized, none of these children were anisometropic at four years because emmetropization had lowered the levels of both hyperopia and anisometropia, and these children remained non-strabismic. At six months, anisometropia of ~1D was observed in 2 of the 6 children with high hyperopia who went on to develop esotropia, and by four years, three of these six children (one of the original two plus two others who were not anisometropic at 6 months) had anisometropia. Aurell and Norrsell’s (1990) study thus suggests a key role for high levels of persistent hyperopia in the first few months in developing esotropia, amblyopia and in some cases anisometropia.
Abrahamsson et al. (1992) examined the refractive errors of 41 esotropic children at the time when the strabismus was diagnosed; the age at diagnosis was evenly distributed between the age of 1 and 4 years. More than 60% had hyperopia of 2D or more in the deviating eye when the strabismus was detected and no child with esotropia was myopic. In contrast, in 21 exotropic children, significant refractive errors were much less common. Abrahamsson et al. (1992) were able to mine earlier refractions of 9,000 children to gather the longitudinal refraction changes in fourteen of the children who became esotropic, most of whom had hyperopia >+2D at one year of age. Ten of these fourteen children showed an increase in hyperopia of 0.5D or more in the deviating eye between 1 and 4 years of age, while the non-deviating eye emmetropized normally. Therefore, although only one of the 14 cases who ultimately became esotropic had anisometropia (defined as ≥1D interocular difference) at the onset of the strabismus, almost all (13 of the 14 cases) had anisometropia about 1 year after the esotropia developed. That is, when anisometropia and esotropia co-existed the esotropia preceded the anisometropia in all but one case. In stark contrast, amongst the children who became exotropic, there were no cases of anisometropia developing after the onset of the strabismus. Abrahamsson et al.’s results in esotropes are also in accord with a study by Birch and Stager (1985). Birch and Stager studied 88 infants longitudinally from the diagnosis of esotropia between 3 and 7 months of age up to a maximum of 14 months of age. None of them exhibited anisometropia of >1D at the time esotropia was identified. Unfortunately, data about later development of refractive errors and anisometropia are not available from this cohort but the results of this study do show that significant anisometropia was not typically present in the early period.
The Aurell and Norrsell (1990) and Abrahamsson et al. (1992) studies support a rather complex, four-component model of the emergence of anisometropia in strabismic amblyopes: First, a significant proportion of hyperopic infants fail to emmetropize and the greater the hyperopia the greater the chances that emmetropization will fail (Mutti, 2007). The children who do emmetropize, do not develop refractive accommodative esotropia, anisometropia or amblyopia. Second, many of those who do not emmetropize go on to develop esotropia (e.g. all 6 cases in Aurrell and Norrsell, 1990; approximately 20% in Anker et al., 2004; Atkinson et al., 1996; Ingram et al., 1990), presumably due to neural coupling between the accommodative and vergence systems producing a chronic pressure to overconverge if the children accommodate to correct for their hyperopia (Bharadwaj and Candy, 2009; Candy, 2012). Third, once unilateral esotropia has developed, amblyopia follows (Birch and Stager, 1985; Aurell and Norrsell, 1990). Finally, although the fixing eye may emmetropize, the non-fixing eye can fail to do so and can become even more hyperopic (Lepard, 1975; Nastri et al., 1984; Aurell and Norrsell, 1990; Abrahamsson et al., 1992). Thus the final result is a child with esotropia, amblyopia, and hyperopic anisometropia. Since most emmetropization of nonstrabismics occurs during the first year of life (Saunders, 1995; Saunders et al., 1995; Wood et al., 1995; Pennie et al., 2001; Mutti et al., 2005; Mutti, 2007), the emmetropization of the fixing eye of strabismics might be termed ‘abnormal’ late emmetropization in many cases.
Retrospective Analysis of Clinical Data from Children who Eventually Developed Strabismus or Amblyopia
Ingram et al. (2003) retrospectively examined the results of 2920 cycloplegic refractions conducted between the ages of age 5 and 7 months and again at around 42 months of age. Specifically, they compared data from children who developed typically (n=2710) with data from children who developed a microtropia or larger strabismus (n=210). Esotropia was diagnosed in more than 95% of cases of manifest strabismus. In the strabismic children there were higher levels of hyperopia at age 3-4 years than in non-strabismic children, and this was true for strabismic children who started with high (>+2.75D) or lower (<+2.75 D) levels of hyperopia at 5-7 months. They also found that the emmetropization failures were more pronounced in the non-fixing eyes of the strabismics. In the children who ended up without strabismus, the mean anisometropia increased slightly from 0.02D in infancy to 0.09D by the age of ~42 months. Also, in the group who ended up nonstrabismic, 92% of those children with infantile anisometropia >0.75D ended up with <0.75D of anisometropia, and only 1.3% (36/2710) without anisometropia in infancy exhibited it later. The anisometropia results were very different in the strabismic children where the mean ‘early’ anisometropia was also low (0.15D), but grew to 0.44D at the later age. Also, 53% of those who had strabismus at 3-4 years showed increasing anisometropia. Although more of the strabismic group had abnormal anisometropia (>0.75D) in infancy (31/210, 15%) compared to normals (123/2710, 4.5%), the majority (72%) of children with abnormal early anisometropia did not develop strabismus.
In a subsequent study that included 20,176 infants (Ingram et al., 2009) who underwent a cycloplegic refraction at six months, 879 went on to develop strabismus. In 853 strabismic children who did not wear glasses from the age of 6 months, the level of anisometropia present at six months or when strabismus was detected was generally not related to the final visual acuity. The only exceptions to this observation were the 26 infants with high (>1.5D) anisometropia and hyperopia of +5D or more in the non-fixing eye at age 6 months. These children ultimately exhibited poorer visual acuity by comparison with similarly hyperopic children who had between 0.75 and 1.5D of early anisometropia. One potential explanation for this finding is that the early anisometropia was (in addition to the strabismus) responsible for the emerging amblyopia. Alternatively, one could perhaps interpret the data from early anisometropes as evidence that amblyopia was present early and this eye was already failing to emmetropize by the age of 6 months.
The studies by Ingram’s group monitored refraction at two ages (around 6 months and then later in childhood, 3-7 years), and thus the sequence of any changes that occurred in between these two measurements is largely unknown. However, these studies suggest that it is esotropia and not early hyperopia or anisometropia that is associated with the emergence of amblyopia. Also, it is a failure of emmetropization (and the resultant high levels of hyperopia) that is associated with the emergence of esotropia. The Ingram et al. (2003 and 2009) studies are thus generally consistent with the observations of Aurell and Norrsell (1990) and Abrahamsson et al. (1992) in that high hyperopia that is not lowered by emmetropization is associated with esotropia and amblyopia. The deviating, amblyopic eye fails to emmetropize; indeed it can become even more hyperopic. Therefore, anisometropia develops after strabismus in the esotropic group because the fixing eye emmetropizes, and the deviating eye does not (Abrahamsson et al., 1992; Ingram et al., 1994; Ingram et al., 2003; Ingram et al., 2009).
The link between hyperopia, esotropia and amblyopia is also demonstrated in the studies by Colburn et al. (2010) and Weakley et al. (2001). Colburn et al. (2010) conducted a retrospective observational evaluation of the refraction and oculomotor status of 149 children (mean age at presentation 40 months, range 5 to 108) identified with high hyperopia (>+3.74D) but without anisometropia. Colburn et al. (2010) found amblyopia in 19% of cases at presentation. In the 108 (72%) cases for which follow-up data were available (mean follow-up period 40 months), amblyopia developed in a further 24% of the remaining children. Thirty-two percent of children had accommodative esotropia at presentation and esotropia developed in a further 33% during follow-up.
Weakley et al.’s (2001) study of children presenting at an ophthalmology clinic (mean age at presentation 49 months) found that a high proportion (60%) of children with hyperopia >+2.00 D had esotropia, and that esotropia was present in >90% of the children with amblyopia. However, Weakley et al. (2001) concluded that it is the anisometropia that is a risk factor for the development of accommodative esotropia rather than vice-versa. This is clearly at odds with the studies described above in which anisometropia was found to emerge after the development of strabismus.
Longitudinal Studies of Changes in Refraction following the onset of Strabismus in Childhood
Typically-developing children show a steady decline in hyperopia during the first 9-12 months of life. This is evident in the study by Mutti et al. (2009) in which 262 normal birth-weight children were evaluated longitudinally at 3, 9 and 19 months postnatally. Similarly, Wood et al.’s (1995) longitudinal study of 113 infants examined at three monthly intervals revealed a significant emmetropic shift during the first year of life. Pennie et al.’s (2001) study showed a steady reduction in spherical equivalent hyperopia during the first year of life, although the sample size in the latter study was modest (n=20). Saunders et al. (1995) gathered refraction data (using a near retinoscopy technique) in 22 children during the first six months of life, and again between 12 and 17 months of age. As well as showing a decline in hyperopia across the period of study, they found evidence that the rate of emmetropization is related to the initial refractive error; the rate of emmetropization was more rapid in the presence of high refractive errors. Cross-sectional studies reveal a similar pattern of refractive development in typically-developing children. Mayer et al.’s (2001) study of over 500 healthy, full-term children aged from 1 to 48 months, revealed a significant reduction in both hyperopia and the variability of the spherical-equivalent refractive error across this age range. Refractive development in strabismic infants shows a different pattern, however. For example, in a recent longitudinal study of 143 children who presented initially (age < 6 months) with infantile esotropia, Birch et al. (2010) carefully tracked the refractive development of their right eyes (note, however, that there was no indication how many were fixating and how many were the deviating eyes) and found that strabismic infants show either little change in hyperopia in infancy or they became more hyperopic. Birch et al. (2010) showed a drift toward emmetropia at about age 7 years.
The co-existence of failed emmetropization with amblyopia also seems to be present during later childhood. Lepard monitored spherical equivalent refractive error in strabismic amblyopes (n=55) from age one to four years through to the teenage years (Lepard, 1975). During this time, there is no evidence that the deviated amblyopic eyes shift from their high hyperopic starting points (average +2.75D), but after age four there is a gradual shift in the fixing eye toward emmetropia and myopia. These data seem to show that well after normal eye growth is complete (Mutti, 2007), the fixing eyes continue to emmetropize (or drift toward myopia). This study of mean refractive error is consistent with Nastri et al.’s (1984) study of 61 strabismic amblyopic children (aged 3-5 years) who were studied longitudinally for at least 10 years. The mean refractive error for the non-amblyopic eye was found to drift to emmetropia while the deviating, amblyopic eye did not show this pattern. However, careful inspection of the refractive error distributions reveals that most non-amblyopic eyes remained slightly hyperopic throughout this period (modal refractive error remains +1.00D), while in some eyes there is a progressive drift into myopia. Thus, although most of the eyes remain hyperopic, the mean drifts toward emmetropia during this period after the classic emmetropization period is complete. Both the Lepard (1975) and Nastri et al. (1984) papers imply that some combination of esotropia and amblyopia may not only prevent emmetropization in younger eyes, but they also prevent the drift to myopia in older children’s eyes, which may suggest that the drift away from hyperopia in young children may require normal alignment and visual function as might the drift away from emmetropia in emerging myopia in older children. This seems contrary to the reports of myopia developing in eyes that have amblyopia and early deprivation (e.g. cataract or ptosis) (see section 4.2).
This section reports on some very challenging issues that have been investigated in a small number of creative studies, which although imperfect in design (e.g., clinical-rather than population-based, and cross-sectional rather than longitudinal) provide powerful insights into the developmental sequence that leads to the common co-existence of anisometropia, strabismus and amblyopia. Although early hyperopia generally reduces during the first year and a half of life, this emmetropization fails in some. A significant proportion of these children develop esotropia, and experience less emmetropization in their deviating eye than in their dominant eye. This results in young children with esotropia, amblyopia and anisometropia. Early (and transitory) anisometropia seems to be a minor factor in determining the final outcome for these children with strabismus. The data that have been interpreted as evidence that anisometropia is a risk factor for accommodative esotropia and amblyopia (Hypothesis 1) have come from cross-sectional studies in which anisometropia, esotropia and amblyopia co-exist at diagnosis and thus are unable to provide an insight into the developmental sequence. The literature thus provides little evidence to support Hypothesis 1 (anisometropia causes amblyopia) in these strabismic cases. The available data are more consistent with Hypothesis 3 (amblyopia and anisometropia are both effects of failed emmetropization and the associated accommodative esotropia in young hyperopes) or Hypothesis 2 (amblyopia is the cause of the anisometropia in that strabismus may generate amblyopia first, and then anisometropia follows) (Figure 6).
4.4 Anisometropia Development in the Absence of Known Early Disruption of Visual Experience
The literature discussed in the immediately preceding sections demonstrates examples of timing consistent with hypotheses 2 and 3 laid out above. In other words, that clinically significant anisometropia can become evident after amblyopia has developed. Given that amblyopia is an experience-dependent phenomenon, a third factor must be required to either disrupt experience and cause the amblyopia, which then causes anisometropia (Hypothesis 2) or causes both the amblyopia and the anisometropia (Hypothesis 3) (Figure 6). The weight of current opinion (Raviola and Wiesel, 1990; Smith et al., 2010; Wallman et al., 1987; Wildsoet, 2003) might favor local factors in the eye impacting axial length, rather than cortical influences of amblyopia, and therefore potentially favors Hypothesis 3. The central and key topic remaining to be discussed, however, is Hypothesis 1, the potential for anisometropia to cause amblyopia. There are certainly a number of studies in the literature that have recruited and studied young anisometropes with no clinically detectable amblyopia, strabismus or pathology (e.g. Abrahamsson and Sjostrand, 1996; Almeder et al., 1990). Some of these patients have gone on to exhibit visual acuity deficits and are therefore consistent with the traditional concept of anisometropic amblyopia and Hypothesis 1. This topic is addressed in the following sections.
Anisometropic Amblyopia: A Primary Failure of Emmetropisation?
In the simplest form of Hypothesis 1, an infant is born with stable anisometropia. He/she accommodates to keep the image in one eye focused enough to develop good visual function while the other, chronically defocused eye becomes amblyopic through form deprivation because accommodation is consensual. While there is evidence from animal models that induced anisometropia (and aniseikonia) can precipitate cortical deficits (section 4.1), there are numerous challenges to this story. First, as discussed above, studies of animal models suggest that the primate visual system is able to differentially adjust eye growth to eliminate anisometropia (section 4.1), and in agreement, human anisometropia is often transitory during infancy and early childhood (Abrahamsson et al., 1990b; Abrahamsson and Sjostrand, 1996; Almeder et al., 1990; Ingram et al., 2003). Second, there are individuals with anisometropia during infancy and early childhood who apparently do not develop amblyopia; for example, seven of Abrahamsson and Sjostrand’s (1996) sample of twenty children (35%) with marked anisometropia (≥3D difference in spherical equivalent refraction) at age 1 did not develop amblyopia when studied longitudinally between the ages of 3 and 10 years. So, in developing a theory of anisometropic amblyopia in the absence of strabismus or other pathology in which the anisometropia precedes and ultimately causes the amblyopia, the task is actually to explain why the visual system does not eliminate the anisometropia and why only some anisometropic individuals exhibit amblyopia. Therefore, in cases of Hypothesis 1, what would cause an individual to develop anisometropia without amblyopia, so that the anisometropia could then precipitate amblyopia?
In thinking about anisometropia in the context of Hypothesis 1, we need to understand why emmetropization does not remove infantile anisometropia in some humans as it does in animal models and most humans, and, if an infant starts life without anisometropia, why they go on to develop it? Infants and young children are rarely truly emmetropic (Cook and Glasscock, 1951; Mayer et al., 2001; Mutti et al., 2005; Saunders et al., 1995), and therefore any postnatal change from isometropia to anisometropia implies that emmetropization is unequal in the two eyes. This asymmetry is consistent with the idea that eye growth is controlled at least to some degree by factors local to the eye (Abrahamsson et al., 1990b; Raviola and Wiesel, 1990; Smith et al., 2010; Wallman et al., 1987; Wildsoet, 2003) rather than purely by central mechanism(s) acting entirely symmetrically in the two eyes. There may be two different mechanistic paths to an anisometropia that could cause chronic retinal image defocus leading to cortical deficit and amblyopia: i) Retention of a neonatal anisometropia through at least moderately coordinated eye growth in the two eyes, or ii) Poor coordination of postnatal growth in the two eyes resulting in development of a new anisometropia. The evidence from animal models suggests local control of eye growth and thus supports the second rather than the first path.
Very little is known about why anisometropia would be retained or develop in the absence of demonstrable pathology or strabismus, and how it is related to family history (Abrahamsson and Sjostrand, 1996; Aurell and Norrsell, 1990, Wood et al., 1995). Interestingly, most of the animal studies employ anisometropia as a starting point, from which they monitor the process of unequal eye growth that reduces or compensates for the refractive anisometropia. In these animal models, it is key to remember that the unequal eye growth is a sign of successful iso-emmetropization, perhaps revealing a normal process present in many young humans. Conceptually this could be viewed either as the two eyes working in combination to achieve matched refractive error, or as two eyes undergoing emmetropization independently (Tanner, 1978; Wallman and Winawer, 2004). In the second scenario, it is interesting to contemplate why in those individuals with anisometropia, the two eyes both stabilize at an equal, non-emmetropic endpoint when they are acting entirely independently. In either case, the animal models do not clarify issues surrounding the development and retention of anisometropia in the absence of amblyopia, strabismus or pathology.
The emergence and subsequent elimination of anisometropia during the period of postnatal eye growth (e.g. Almeder et al., 1990) may reflect normal variability between the eyes in the growth process (e.g. as suggested by Abrahamsson et al., 1990b) followed by an active and protective emmetropization process. During this period of transitory anisometropia, it is possible that amblyopia may develop, but then resolve with the elimination of the anisometropia (Smith et al., 1999). Alternatively, one must always ask how much of this apparent appearance and then disappearance of anisometropia is related to the limits of agreement of the measurement technique (i.e. how much of it is really there, and not due to measurement variability?). The intra-examiner and intra-subject repeatability of typical techniques used to measure refractive error in infants and young children, retinoscopy and autorefraction, have not been reported extensively. Hodi and Wood (1993) noted a standard deviation of 0.16D in repeated intra-examiner retinoscopy estimates of spherical equivalent from cyclopleged one year-old subjects. Harvey et al. (1997) studied 47 children under 8 years of age and found up to 0.75D difference in cycloplegic Retinomax readings on repeated testing, while Adams et al. (2002) tested 74, 2 to 12 month-olds with the Suresight autorefractor without cycloplegia and found 86% of infants to have a difference in spherical equivalent of less than 1D on repeat testing. It is important to remember when measuring anisometropia that the difference between the eyes also includes the measurement error in the estimate from each eye. The combined impact of this variance on the anisometropia estimate is calculated as the square root of the sum of the two variances of the repeated measurements from each eye alone. For example, if the standard deviation of repeated measurements of one eye by the same observer is 0.16D (Hodi and Wood, 1993), the standard deviation of the combined estimate of anisometropia would be 0.23D () and therefore a crude estimate of the 95% limit of agreement for estimates taken at the same visit would be 1.96*0.23 = 0.45D, a value which is somewhat less than the 1D limit of agreement from Saunders and Westall (1992) (also Saunders, 2012, personal communication). Even so, infants and young children are routinely found to lose larger amounts of anisometropia than this (Abrahamsson et al., 1990b; Almeder et al., 1990; Ingram et al.,2003).
Interestingly, while there is strong evidence that spherical anisometropia induced in animal models by ophthalmic lenses is linked in a causal fashion to amblyopia (section 4.1), astigmatism based in the optical components of the eye can also be present. Astigmatism is corneal or lenticular in nature (e.g. Shankar and Bobier, 2004). In this case elimination of a difference in spherical equivalent through vision-dependent growth would be dependent on an active process detecting and correcting astigmatism. Studies of animal models have shown inconsistent and incomplete compensation for this type of blur and therefore astigmatism could theoretically contribute to one form of sustained anisometropia (Schmid and Wildsoet, 1997; Thomas and Schaeffel, 2000; Kee et al., 2004). If the astigmatism were to remain into adulthood, this could explain the association with anisometropia noted in section 3.1 and with amblyopia noted in Table 3.
Anisometropic Amblyopia: Questions of Magnitude, Timing and Duration?
Why do some young anisometropes develop amblyopia, while others do not (e.g. de Vries, 1985)? For Hypothesis 1 to be true, does the presence of amblyopia at a vision screening require anisometropia to be present at a critical age within the cortical critical period? Equivalently, for amblyopia to develop must chronic blur exist when the visual system is particularly vulnerable to the effects of abnormal visual experience? Or, is a particular duration of anisometropia required to result in sustained amblyopia? Donahue (2006), for example, noted that in children screened for refractive error with photo-retinoscopy, approximately 40% of individuals with more than 1D of anisometropia in any meridian at two years of age were diagnosed with amblyopia, and that the figure rose to approximately 70% by four years of age. He also noted that amblyopia increased in severity but not frequency after age 4 years. Leon and colleagues (2008) determined that the likelihood of amblyopia goes up with both an earlier age at clinical presentation and with greater amounts of anisometropia during early childhood. Although these studies indicate that anisometropia that is more severe and in existence from an earlier age and for a longer duration is more likely to lead not only to amblyopia but also to more severe amblyopia, the picture is not as clear as these data might suggest. The studies by Donahue (2006) and Leon et al. (2008) suffer from a number of methodological limitations that were acknowledged by the authors, who also stated that their study “probably overestimates the prevalence of amblyopia in anisometropic children” (p.138, Donahue, 2006). First, these were screenings in which, only 75% of those referred were examined and also the children were examined in a non-standardized way by different eye-care providers. Also, cross-sectional studies fail to capture the emergence and development of amblyopia and anisometropia in individuals.
The question of how anisometropia severity, age at anisometropia onset and duration interact is particularly difficult to address, in that it involves both prevalence of a condition that is not outwardly easy to observe (anisometropia), and the use of longitudinal studies to determine the point at which amblyopia develops, raising ethical questions of spectacle treatment being withheld under current prescribing guidelines (American Academy of Ophthalmology, 1997). Also, because amblyopia is typically defined in terms of visual acuity (section 3.2), amblyopia in young children may go unnoticed because of difficulties measuring visual acuity in the very young.
It is feasible that with additional data it will be possible to develop the concept of a ‘dosage’ of anisometropia required to result in amblyopia, in terms of age, amplitude and duration. A parallel concept has been discussed in the context of a dosage of visual experience during amblyopia treatment (Stewart et al., 2007 & Loudon et al., 2003), but could also presumably apply to abnormal visual experience as the condition develops. As little as 1D of anisometropia during early childhood substantially increased the risk of amblyopia (Tarczy-Hornoch et al., 2011; discussed in section 3.3.) and these data provide evidence to support the consensus-based guidelines of the American Academy of Ophthalmology (1997) which refer to the amblyogenic potential of 1D or greater of hyperopic anisometropia. Also, based on the data from children with known disruptions (strabismus or fulltime patching, for example) just weeks of abnormal experience are sufficient to lead to reduced acuity. If 1D or more of hyperopic anisometropia for weeks were all it took to cause amblyopia, it would seem that amblyopia could develop on a faster timescale than the iso-emmetropization process could function, and therefore that it would be difficult for patients with these refractive errors to avoid amblyopia. In fact, based on these amplitude and duration criteria one could ask whether there is a subset of individuals who develop anisometropia and amblyopia and then lose them both without intervention?
Anisometropic Amblyopia: More Than a Failure of Emmetropisation?
As described above, pure anisometropic amblyopes are defined as having no strabismus or other pathology. These are typically considered to be the patients who have developed according to Hypothesis 1 (Figure 6). They have, for an unknown reason, apparently retained their anisometropia and subsequently developed cortical deficit. Here we discuss the hypothesis that some sub-clinical undetected pathology is the root cause of this failure to emmetropize. This topic has been discussed in the literature for some time.
The evidence that subtle pathology or structural anomalies exist unilaterally in eyes diagnosed with anisometropic amblyopia is both equivocal and controversial (Lempert, 2000; 2003; 2004; Archer, 2000; Bacal, 2004; Yen et al., 2004; Colen et al., 2000; Bozkurt et al., 2003), although there is recent evidence that bilateral structural anomalies of the globe (Pineles and Demer, 2009) or foveal pit (Bruce et al., 2012) may exist in amblyopes. The extent to which they could ultimately be responsible for a diagnosis of anisometropic amblyopia is uncertain. If they represent the primary anomaly causing both conditions, they would be consistent with Hypothesis 3. Theoretically, subtle structural anomalies may also cause anisometropia or amblyopia only, which could then lead to the other condition (Hypothesis 1 or 2). Alternatively, these structural anomalies could arise after the onset of anisometropia and amblyopia. Thus the significance and prevalence of subtle structural anomalies prior to or during the development of anisometropia and/or amblyopia is far from clear.
Microstrabismus (Helveston and Von Noorden 1967; de Vries 1985) is another factor that may be ‘sub-clinical’ during early development, as it is currently very difficult to detect in infants and young children (but see Loudon et al., 2011). It could serve as a trigger for both amblyopia and anisometropia (section 4.3). To complicate things further, associated eccentric fixation rather than an amblyopic cortical deficit may result in a reduced monocular acuity in these cases. This acuity reduction could mimic and be diagnosed as amblyopia.
4.5 Implications from Studies of Amblyopia Therapy
Currently, the initial treatment for young patients found to have anisometropia and amblyopia starts with optical correction and then adds additional treatment (e.g. occlusion) as necessary (e.g. Holmes et al., 2006). As noted above, if the human visual system exhibits an iso-emmetropization process, it is feasible to leave these patients untreated to allow the anisometropia to disappear and therefore retinal image quality to improve in the amblyopic eye. However, consistent with the literature discussed in sections 4.1 through 4.3, an amblyopic eye may not undergo normal emmetropization. For example, Smith and Hung (1999) found that a greater emmetropization response could be observed if measures were taken to prevent amblyopia from developing in monkeys reared with high powered unilateral defocusing lenses. A study of children who were not compliant with treatment (Simons and Preslan, 1999) and another of a group of children who did not receive treatment for a year (Clarke et al., 2003) both demonstrated that anisometropia and amblyopia were unlikely to resolve without treatment.
Strictly speaking the diagnosis of pure anisometropic amblyopia can only really be confirmed when vision improves with manipulation of visual experience alone (glasses, patching or atropine). If vision does not improve, one could question whether the vision loss was due to some associated ocular anomaly (Lempert, 2000; 2004; 2008b) such as subtle pathology or non-foveal fixation. Lack of compliance with prescribed treatment also remains as an alternative explanation to be discussed below, however. If patients reach an age when the critical synapses are no longer plastic, the diagnosis of anisometropic amblyopia can only be inferred based on the presence of anisometropia and thorough testing of a range of spatial tasks associated with an amblyopia diagnosis in adults (e.g. McKee et al., 2003; Levi et al., 2011).
Treatment of anisometropic amblyopia is now discussed in the context of insights into its origins:
Glasses alone
Prescribing glasses for anisometropia manipulates visual experience to effectively eliminate differences in retinal defocus between the eyes. This spectacle-based elimination of the relative retinal defocus should eliminate the imbalance in neural activity arriving in visual cortex, and therefore it could affect the amblyopia if the relevant cortical synapses are still plastic. Significantly, successful amblyopia treatment with glasses alone indicates a role for visual-experience in the amblyopia consistent with Hypothesis 1 (in the treatment phase at least). However, optical correction of any anisometropia should prevent any iso-emmetropization growth process that requires asymmetric retinal defocus, and therefore it is unlikely to ‘treat’ the anisometropia, and indeed, may interfere with any natural treatment effected by emmetropization.
If wearing glasses is sufficient to treat amblyopia it also suggests that treatment biasing activity to the amblyopic eye (e.g. patching) is not actually required, merely the restoration of more equal activity driven by the two eyes (Mitchell and Gingras, 1998; Kind, 1999). A number of studies in the human literature have looked at the impact of treatment with optical correction alone. Abrahamsson and Sjostrand (1996), looked at spectacle correction in a preventative sense. They studied 20 subjects, with no signs of ocular disease or structural defects of the eye, who had 3-5.5D anisometropia at one year of age. They provided full optical correction for the anisometropia at 2-3 years of age and followed the subjects until they reached 10 years of age, with typical clinical treatment of any amblyopia they discovered. Ninety percent of the patients were still anisometropic at 5 years of age and 14 of 20 were anisometropic at 10 years of age (with a 1D criterion). In six patients the ametropia and anisometropia increased and they developed amblyopia, another seven lost most of their anisometropia and developed no amblyopia or strabismus, and of the remaining seven who retained most of their anisometropia, six developed amblyopia and one developed only a strabismus. In all cases the most dramatic changes in anisometropia occurred between one and four years of age, and in the group who lost their anisometropia, astigmatism also decreased. Two puzzles emerge from this study. 1. How can a patient who has essentially been made isometropic and emmetropic by spectacles possibly emmetropize to overcome their anisometropia? 2. If it was the monocular blur from anisometropia that caused the amblyopia, none of these patients should have developed amblyopia if they were wearing their optical correction. The authors state that compliance with spectacle wear did not differ between the different outcomes, although compliance was judged only on the basis of reports from parents. If compliance was indeed similar for the different outcomes, the fact that some of the patients continued on to develop amblyopia and strabismus with increased anisometropia suggests a role for additional factors (specifically Hypothesis 3).
The Pediatric Eye Disease Investigator Group (Cotter et al., 2006) provided optical correction to 84 previously untreated subjects aged 3-7 years in whom anisometropia and amblyopia had both been diagnosed. These subjects had no clinical evidence of strabismus and had greater than or equal to 3 lines of acuity difference between the eyes and >=0.5D SE (mean of 3.21D) anisometropia or >=1.50 D aniso-astigmatism. The average improvement in 5 weeks from baseline was 1.8±1.3 lines of acuity with 59% having an improvement of ≥2lines. Improvement continued for 48% of those tested at 5 weeks and the longest period of improvement was 30 weeks before acuity stabilized in the amblyopic eye. Ultimately 77% had improvement of ≥2 lines and 27% had resolution of their amblyopia to an interocular acuity difference of one line or less. There was no effect of age in the 3-7 year window and the likelihood of resolution was higher for patients with better baseline acuities in the amblyopic eye and lower amounts of anisometropia. Although there was no group of untreated patients in this study, to control for the effects of repeated testing and increasing age, the reduction in interocular difference in acuity is consistent with a true treatment effect.
Broadly consistent results have been achieved by a number of other groups, some of whom included amblyopes with small angle strabismus in their studies (Kivlin and Flynn 1981; Moseley et al., 2002; Clarke et al., 2003; Stewart et al., 2004; Steele et al., 2006; Chen et al., 2007). This body of work suggests that anisometropic amblyopes are a heterogeneous group in that at least some patients respond to the optical treatment in a manner consistent with Hypothesis 1, and their amblyopia could be fully treated by removing the anisometropic retinal blur. In thinking about synaptic plasticity in cortex, a simple Hebbian mechanism (Hebb, 1949) responding to interocular competition would not predict improvement in acuity with balancing of neural activity alone (consistent with this, see Mitchell and Gingras, 1998; Kind, 1999; Mitchell et al., 2001, for evidence of activity-dependent recovery in animal models with both eyes open). However, in a monkey study that closely parallels the glasses only treatment scenario (Smith et al., 1985), it was found that amblyopia present at the end of a period of induced anisometropia recovers as long as it has only been present for a short duration.
Treatment beyond optical correction
From the optical correction studies it is clear that some “pure anisometropic amblyopia” is responsive to the presence or absence of anisometropia (consistent with Hypothesis 1), but it is routine in the clinic to employ treatment strategies that bias input to visual cortex to the amblyopic eye (patching or atropine). What can be learned about the origin of anisometropic amblyopia from these studies? Classical amblyopia therapy with patching or atropine acts to bias cortical activity to input from the amblyopic eye, and therefore provides synapses driven by that eye with a competitive advantage in the Hebbian sense (reviewed by Barrett et al., 2004). There have been numerous recent trials, discussed below, of the effectiveness of these treatments for amblyopes with strabismus and/or anisometropia, looking at dosage and patient age in particular. In the context of the origins of anisometropic amblyopia, two aspects of these studies are particularly interesting. First the issue of poor responders to treatment, and second, the question of the ages at which the visual cortex is plastic and responding to treatment.
With regard to treatment success, there have been two categories of study design. In the first, success is assessed as a function of prescribed treatment and in the second, success is assessed as a function of monitored treatment dosage completed. For example, the first Pediatric Eye Disease Investigator Group (PEDIG) Amblyopia Treatment Study (2002) looked at response to prescribed patching or atropine treatment in 419 moderate amblyopes (20/40 to 20/100) aged less than 7 years. In that study approximately 75% of the participants achieved 20/30 or better or a three-line improvement in acuity over a six-month period. There was no significant difference between the two forms of treatment or between types of amblyopia (anisometropic, strabismic or mixed). Thus in this study design, it is clear that there are individuals left with residual amblyopia either through poor response to treatment or poor compliance with treatment (Archer, 2012; Repka and Holmes, 2012). After a further 18 months of standard clinical care the full set of patients still had on average a two line difference in acuity between the eyes (Repka et al., 2005), so while their amblyopia did respond to manipulation of visual experience (average improvement was 3-4 lines), the difference in acuity between the eyes was not routinely fully eliminated, and when 169 of these patients were tested at 10 years of age the results had been maintained in that the average amblyopic eye visual acuity was 20/32 with only 46% of amblyopic eyes having an acuity of 20/25 or better (Repka et al., 2008). Interestingly this group has recently noted that the amblyopic eye of non-strabismic patients is likely to undergo a small amount of emmetropization over this period up to 10 years of age (Kulp et al., 2012).
Other studies have addressed the question of treatment compliance with patching by incorporating a device designed to monitor occlusion compliance (Fielder et al., 1995). Two groups have noted that acuity outcome is correlated with amount of occlusion undertaken in young amblyopes (Stewart et al., 2007 & Loudon et al., 2003), with Stewart et al. noting that most of their subjects achieved their best visual acuity with 150 to 250 hours of cumulative dose. In both cases the authors recruited anisometropic, strabismic and mixed factor amblyopes, and then did not note any difference between the different classifications. There are still individuals however, who do not appear to respond fully, or in some cases at all, to treatment and thus their vision loss is not entirely dependent on or responsive to retinal image blur or manipulations of visual experience in treatment (also, PEDIG, 2002; Clarke et al., 2003). These are the patients who have been suggested to have sub-clinical pathology (Lempert and Porter, 1998; Lempert, 2003, 2004, 2008; Barrett et al., 2005), although poor treatment compliance certainly represents an alternative explanation when the patching is not monitored. If, however, there is sub-clinical pathology, these individuals may have developed with the chronology of Hypothesis 2 or 3 (Figure 6).
With regard to the age at which treatment is successful, a number of studies (Hardman Lea et al., 1989; Cobb et al. 2002; PEDIG 2002) have noted minimal effect of age on treatment success between 3 and 7 years of age, and that treatment success tends to reduce after that point (Kivlin and Flynn 1981; Sen, 1982; de Vries 1985; Hussein et al., 2004; Fronius et al., 2009). This treatment typically takes on the order of three to five months to reach the endpoint when amblyopia is considered treated or stable (Oliver et al., 1986; Lithander and Sjostrand, 1991; Stewart et al., 2007). While there are likely to be a number of critical periods for different visual functions in the primate visual system (e.g. von Noorden and Crawford, 1979) and more evidence is being acquired showing some treatment effects at later ages and into adulthood (e.g., Scheiman et al., 2005, Scheiman et al., 2008; Astle et al., 2011; for recent reviews see Levi and Li (2009) and Levi, (2012)), it is clear that visual experience has its most dramatic impact on cortical neural circuitry during the pre-school years. Therefore, as suggested above, the magnitude and timing of anisometropia may be key to understanding how some individuals escape amblyopia during this period, presenting with anisometropia alone at a school age vision screening (Weakley, 2001).
To summarise, while there is persuasive evidence from animal models demonstrating that induced unilateral blur can lead to cortical deficits, support for Hypothesis 1 in humans hinges on the visual system’s ability to develop anisometropia in the absence of any factor that could also cause amblyopia (a third factor causing both conditions would be consistent with Hypothesis 3). There is potential for subtle pathology and/or microstrabismus to cause both conditions but the likelihood of this is not well established. Our current clinical ability to detect these additional factors is an area that clearly needs further insight and understanding. Interestingly, Loudon et al. (2011) describe a new device called a Pediatric Vision Screener (PVS) which simultaneously assesses ocular fixation in the two eyes. On the basis of the results they obtained in their examination of twenty-two patients with anisometropic amblyopia, they claim that all anisometropic amblyopes may have a microstrabismus or fixation instability that is too small to be measured by the clinical gold standard (the prism-and-cover test) and which is impossible to measure using photorefractive methods, but which is detected by the PVS.
Given the timelines in cases of observable pathology (cataract or ptosis for example), it would be atypical for a third factor to produce clinical anisometropia before amblyopia. Thus, the longitudinal studies finding anisometropia and then amblyopia appear to provide support for Hypothesis 1, although the significant variability of normative clinical acuity results in the first years after birth raises the concern that amblyopia may be missed in the younger patients even though their anisometropia is detected. Technically- and ethically-challenging, longitudinal studies in which visual acuity and refractive error can be precisely determined in infants from birth are required to address the relative timelines of the two conditions, and to provide definitive evidence about their order of appearance.
Perhaps the strongest support for Hypothesis 1 currently comes from studies of treatment of anisometropic amblyopia. Although the lack of a control group for repeat testing is a widespread shortcoming of these studies, the average improvement in acuity substantially exceeds that which might be expected based upon a learning effect or an age effect. These studies demonstrate the response of the cortex and visual function to the presence and absence of anisometropic retinal blur, and they clearly indicate that the two conditions are not independent. In moving forward, in particular, it is important to note that the prevalence of hyperopic anisometropia of >1D is greater than the prevalence of anisometropic amblyopia in older children and therefore, if we are to attempt efficient prevention and treatment, we need to understand more about why young individuals with anisometropia are unable to emmetropize and at what age and in what circumstances an anisometropic child is destined for amblyopia.
5. Overall Summary & Conclusion
Although the text-book explanation that anisometropia causes amblyopia is comfortingly simple, Abrahamsson and Sjostrand in 1996, and others (Almeder et al., 1990; Fielder and Moseley, 1996; Lempert, 2000, 2003, 2004, 2008; Lempert and Porter, 1998; Barrett et al., 2005; Smith et al., 1999) have challenged the broad application of this simple cause and effect hypothesis. Clinical experience and cross-sectional studies document the common association between anisometropia and amblyopia (Section 2), and also the quantitative relationship between magnitude and sign of anisometropia and prevalence and depth of amblyopia (Section 3). However, insights into the broader cause and effect relationship come from two quite different sources: (1) experiments on infant monkeys in which a known factor is introduced and its refractive or sensory consequences monitored later in time (Section 4.1), and (2) the few studies of human children in which refractive, oculomotor and/or sensory data are collected longitudinally (Sections 4.2-4.4). This review has examined both sets of literature to develop a comprehensive examination of the different cause and effect relationships that result in presentation of both amblyopia and anisometropia.
Although the data from studies of infant monkeys employing experimentally-induced monocular deprivation, strabismus, or anisometropia (section 4.1) have provided insight into the development of amblyopia and anisometropia, they have not been able to solve the mystery of which came first in many cases of human anisometropic amblyopia. Indeed, anisometropia can lead to amblyopia in some monkeys, but also experimentally-induced amblyopia (or the conditions that generate the amblyopia) can precipitate the development of anisometropia. That is, within the animal literature there are studies to support all three hypotheses for the origins of anisometropic amblyopia: Hypothesis 1, anisometropia causes amblyopia; Hypothesis 2, amblyopia causes anisometropia; and Hypothesis 3, some other event (e.g. monocular deprivation) causes both amblyopia and anisometropia (Figure 6).
The animal studies that show anisometropia secondary to monocular deprivation and amblyopia have direct parallels in the human literature in those rare cases of monocular deprivation during infancy due, for example, to unilateral ptosis or monocular cataract (section 4.2). Deep amblyopia and often very large amounts of anisometropia are found in these cases, as was found in monkeys. Although there is no support in this literature for Hypothesis 1, insufficient data prevent rejection of either of the competing hypotheses (Hypothesis 2 and Hypothesis 3). That is, the anisometropia may be triggered locally (in the eye) by the deprivation (Hypothesis 3), or may follow due to the emergence of amblyopia in the visual cortex (Hypothesis 2) (Figure 6).
Early monocular deprivation is relatively rare. By contrast, anisometropic amblyopia and amblyopia are commonly associated with strabismus; about half of all patients with anisometropia and amblyopia also have strabismus. Studies of these individuals (section 4.3) do not provide support for the hypothesis that anisometropia develops and then causes amblyopia (Hypothesis 1). The available evidence suggests that young hyperopic children who develop esotropia are rarely anisometropic, but tend to become so after the strabismus and amblyopia have emerged. In these children, significant infantile hyperopia that does not emmetropize can precede esotropia, and in many of these cases amblyopia will also develop. Finally, the ensuing failure of emmetropization in the deviating amblyopic eye is contrasted with the somewhat successful late emmetropization of the fixating eye, leading to hyperopic anisometropia. Again, anisometropia appears to be the last anomaly to develop in these children, and since there is no obvious change within the deviating eye, these data support the hypotheses (Hypothesis 2 & Hypothesis 3) that it is the amblyopia and/or strabismus that trigger the unilateral emmetropization failure (Figure 6).
Around half of the amblyopes with anisometropia do not have strabismus or monocular deprivation, and thus there is no obvious other factor that can explain the amblyopia (section 4.4). Significantly, these children are generally asymptomatic, and thus are routinely detected at an older age, perhaps many years after any triggering event might have happened (Section 2). In the absence of any other observable amblyogenic factors, it is reasonable to assume that the anisometropia caused the amblyopia (Hypothesis 1). The role of this hypothesis across the population, however, is complicated by the compelling monkey and human data indicating that, in the absence of amblyopia, even large amounts of anisometropia are routinely eliminated by active emmetropization and thus are only transitory and do not lead to amblyopia. Also, there are suggestions but little hard evidence that these cases may also represent instances of Hypotheses 2 or 3 by virtue of the possible presence of an undetected anomaly in the eye, or an undetected microstrabismus. Interestingly, some of the most compelling evidence in support of Hypothesis 1 comes from the amblyopia therapy literature. Full optical correction of an anisometropic amblyope (equivalent to removal of the anisometropia) is often an effective treatment, which is consistent with Hypothesis 1 (because amblyopia is treated by removing the cause). The cross-sectional, population-based studies that find increased prevalence and severity of amblyopia in older anisometropes seem to support the idea that anisometropia of longer duration causes more amblyopia (Hypothesis 1). However, since the timeline of anisometropia and amblyopia development remains unknown in cross-sectional studies, Hypothesis 2 (the amblyopia caused the anismometropia) is just as plausible. Also, there are two related ways that prevalence and severity of amblyopia will appear to increase in anisometropes even when anisometropia is not causing the amblyopia. Firstly, because amblyopia is difficult to detect in young children, especially in a screening environment, more detectable cases of amblyopia would be expected in older children even if amblyopia prevalence was stable with age. Secondly, if amblyopia is in reality limited resolution, yet we define it as an inter-ocular difference in visual acuity, as the better eye visual acuity improves with age, the amblyopia may appear to deepen. These points serve to highlight how examination of even a relatively simple question such as how anisometropia magnitude and amblyopia severity are linked quickly becomes complex. In the end, it is not currently possible to definitively reject any of the three hypotheses listed above to find a single cause of “pure” anisometropic amblyopia.
To conclude we return to the thought provoking questions posed in Abrahamsson and Sjostrand (1996) and ask whether the literature now provides us with any answers.
Question: “…is the marked anisometropia at 1 year of age already present at birth and the result of a [prenatal] growth delay in the more hyperopic eye?”
Answer: Probably not, in that studies of infantile anisometropia all show that, in the absence of amblyopia, it will most likely be transitory. However, no infant who ends up amblyopic and anisometropic has had their refraction tracked from birth.
Question: “Is transitory anisometropia during childhood a part of natural development?”
Answer: Yes, in most cases, anisometropia present during the first year of life is gone by 2 years of age (Almeder et al., 1990; Abrahamsson et al., 1990b), but there is evidence from animal models (e.g. Smith et al., 1999) that the chances that anisometropia will disappear during visual development appear to be linked to the magnitude of the anisometropia. Larger amounts were less likely to disappear and were more likely to be associated with amblyopia. Details concerning the magnitude of anisometropia that can be overcome by emmetropization in humans, and the reasons why some children overcome their anisometropia but others do not, are far from clear.
Question: “Does it [anisometropia] cause amblyopia?”
Answer: While there is evidence from animal models to show that it can (section 4.1), there is relatively little compelling and definitive evidence in humans to show that it does. The best evidence currently available to support this hypothesis comes from cross-sectional studies (e.g. Donahue, 2006) which find a higher prevalence of amblyopia and more severe amblyopia in older anisometropic children. Although this is indirectly consistent with longer duration of anisometropia causing more amblyopia, conclusive data in support of the hypothesis that anisometropia causes amblyopia is lacking and will require tracking visual performance longitudinally in anisometropic individuals during their early life.
Question: “Does amblyopia cause the emmetropization to stop and thereby make the anisometropia persistent?”
Answer: Abrahamsson and Sjostrand’s (1996) longitudinal study of twenty individuals with marked anisometropia at 1 year of age suggests that the presence of amblyopia is associated with poor emmetropization. However, the volume of evidence supporting this assertion remains very low and the underlying mechanism is yet to be determined.
Question: “Does amblyopia create anisometropia?”
Answer: There is evidence (sections 4.1, 4.2, 4.3) that amblyopia develops over a much shorter period (e.g. weeks/months) than anisometropia (months/years), although it is true to say that the majority of this evidence is from studies of animal models. Based solely on chronology it is feasible that anisometropia is created by amblyopia (Hypothesis 2) in cases of monocular deprivation (4.2) and strabismus (4.3), but it is impossible to reject Hypothesis 3, that the deprivation or strabismus caused a local change in the eye that precipitated emmetropization failure independent of amblyopia, just more slowly.
The questions posed by Abrahamsson and Sjostrand (1996) are as relevant today as they were when they were first posed, showing that we are still struggling to understand the relationships between anisometropia and amblyopia.
Future Directions
Answers to the questions posed by Abrahamsson and Sjostrand (1996) could become available if we were able to follow children longitudinally from birth, simultaneously examining their visual acuity, binocularity, and refractive development. Also, given the appealing logic to the hypothesis that a sub-clinical condition is disrupting emmetropization to cause anisometropia in at least some young subjects, ocular health/structure would also need to be studied longitudinally. Testing this hypothesis clearly requires equipment and methodology capable of reliably detecting ocular pathology, visual acuity and refractive error in infants. There are many challenges for such a study. First, methodologically these are difficult tests to perform. Second, to avoid having to enroll many thousands of newborns, an effective strategy for identifying those most likely to develop anisometropia is required. Also, as anisometropia and amblyopia emerge, significant ethical concerns arise pressing for intervention. The magnitude of these challenges may prevent a rapid advance in our understanding of the cause and effect relationships between human anisometropia and amblyopia.
Acknowledgements
We are grateful to the reviewers who made many excellent suggestions for improvement. Author RC is supported by NIH R01 grant EY014460.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahamsson M, Fabian G, Andersson AK, Sjostrand J. A longitudinal study of a population based sample of astigmatic children. I. Refraction and amblyopia. Acta Ophthalmologica. 1990a;68(4):428–434. doi: 10.1111/j.1755-3768.1990.tb01671.x. [DOI] [PubMed] [Google Scholar]
- Abrahamsson M, Fabian G, Sjostrand J. A longitudinal study of a population based sample of astigmatic children. II. The changeability of anisometropia. Acta Ophthalmologica. 1990b;68(4):435–440. doi: 10.1111/j.1755-3768.1990.tb01672.x. [DOI] [PubMed] [Google Scholar]
- Abrahamsson M, Fabian G, Sjostrand J. Refraction changes in children developing convergent or divergent strabismus. British Journal of Ophthalmology. 1992;76(12):723–727. doi: 10.1136/bjo.76.12.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamsson M, Sjostrand J. Natural history of infantile anisometropia. British Journal of Ophthalmology. 1996;80(10):860–863. doi: 10.1136/bjo.80.10.860. see comment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RJ, Dalton SM, Murphy AM, Hall HL, Courage ML. Testing young infants with the Welch Allyn suresight non-cycloplegic autorefractor. Ophthalmic & Physiological Optics. 2002;22(6):546–551. doi: 10.1046/j.1475-1313.2002.00073.x. [DOI] [PubMed] [Google Scholar]
- Agervi P, Kugelberg U, Kugelberg M, Simonsson G, Fornander M, Zetterstrom C. Treatment of anisometropic amblyopia with spectacles or in combination with translucent Bangerter filters. Ophthalmology. 2009;116(8):1475–1480. doi: 10.1016/j.ophtha.2009.02.023. [DOI] [PubMed] [Google Scholar]
- Aine E. Refractive errors in a Finnish rural population. Acta Ophthalmologica (Copenh) 1984;62:944–954. doi: 10.1111/j.1755-3768.1984.tb08447.x. [DOI] [PubMed] [Google Scholar]
- Almeder LM, Peck LB, Howland HC. Prevalence of anisometropia in volunteer laboratory and school screening populations. Investigative Ophthalmology & Visual Science. 1990;31(11):2448–2455. [PubMed] [Google Scholar]
- Anderson SJ, Swettenham JB. Neuroimaging in human amblyopia. Strabismus. 2006;14:21–35. doi: 10.1080/09273970500538082. [DOI] [PubMed] [Google Scholar]
- Anker S, Atkinson J, Braddick O, Nardini M, Ehrlich D. Non-cycloplegic refractive screening can identify infants whose visual outcome at 4 years is improved by spectacle correction. Strabismus. 2004;12(4):227–245. doi: 10.1080/09273970490517935. doi: 10.1080/09273970490517935. [DOI] [PubMed] [Google Scholar]
- Anton A, Andrada MT, Mayo A, Portela J, Mareya J. Epidemiology of refractive errors in an adult population: the Segovia study. Ophthamic Epidemiology. 2009;16:231–237. doi: 10.3109/09286580903000476. [DOI] [PubMed] [Google Scholar]
- Archer SM. Amblyopia? Journal of AAPOS. 2000;4(5):257. doi: 10.1067/mpa.2000.110552. [DOI] [PubMed] [Google Scholar]
- Astle AT, McGraw PV, Webb BS. Can human amblyopia be treated in adulthood? Strabismus. 2011;19(3):99–109. doi: 10.3109/09273972.2011.600420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J, Braddick O, Robier B, Anker S, Ehrlich D, King J, Moore A. Two infant vision screening programmes: prediction and prevention of strabismus and amblyopia from photo- and videorefractive screening. Eye. 1996;10(Pt 2):189–198. doi: 10.1038/eye.1996.46. [DOI] [PubMed] [Google Scholar]
- Attebo K, Ivers RQ, Mitchell P. Refractive errors in an older population: the Blue Mountains Eye Study. Ophthalmology. 1999;106(6):1066–1072. doi: 10.1016/S0161-6420(99)90251-8. [DOI] [PubMed] [Google Scholar]
- Attebo K, Mitchell P, Cumming R, Smith W, Jolly N, Sparkes R. Prevalence and causes of amblyopia in an adult population. Ophthalmology. 1998;105(1):154–159. doi: 10.1016/s0161-6420(98)91862-0. [DOI] [PubMed] [Google Scholar]
- Attebo K, Mitchell P, Smith W. Visual acuity and the causes of visual loss in Australia. The Blue Mountains Eye Study. Ophthalmology. 1996;103(3):357–364. doi: 10.1016/s0161-6420(96)30684-2. [DOI] [PubMed] [Google Scholar]
- Aurell E, Norrsell K. A longitudinal study of children with a family history of strabismus: factors determining the incidence of strabismus. British Journal of Ophthalmology. 1990;74:589–594. doi: 10.1136/bjo.74.10.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awaya S, Watanabe Y. Amblyopia. Current Opinion in Ophthalmology. 1995;6(5):9–14. doi: 10.1097/00055735-199510000-00003. [DOI] [PubMed] [Google Scholar]
- Bacal DA. An argument against axial length-disc area ratio as the cause of esotropic amblyopia. Archives of Ophthalmology. 2004;122(11):1732. doi: 10.1001/archopht.122.11.1732-a. author reply 1732-3. [DOI] [PubMed] [Google Scholar]
- Ball EA. A study of consensual accommodation. American Journal of Optometry & Archives of American Academy of Optometry. 1952;29:561–574. [PubMed] [Google Scholar]
- Banks MS, Aslin RN, Letson RD. Sensitive period for the development of human binocular vision. Science. 1975;190:675. doi: 10.1126/science.1188363. [DOI] [PubMed] [Google Scholar]
- Barrett BT, Bradley A, McGraw PV. Understanding the neural basis of amblyopia. Neuroscientist. 2004;10(2):106–117. doi: 10.1177/1073858403262153. [DOI] [PubMed] [Google Scholar]
- Barrett BT, Candy TR, McGraw PV, Bradley A. Probing the causes of visual acuity loss in patients diagnosed with functional amblyopia. Ophthalmic & Physiological Optics. 2005;25(3):175–178. doi: 10.1111/j.1475-1313.2005.00280.x. [DOI] [PubMed] [Google Scholar]
- Barrett BT, Pacey IE, Bradley A, Thibos LN, Morrill P. Nonveridical visual perception in human amblyopia. Investigative Ophthalmology & Visual Science. 2003;44(4):1555–1567. doi: 10.1167/iovs.02-0515. [DOI] [PubMed] [Google Scholar]
- Bedell HD, Flom MC. Monocular spatial distortion in strabismic amblyopia. Investigative Ophthalmology & Visual Science. 1981;20:263–268. [PubMed] [Google Scholar]
- Bedell HE, Flom MC. Normal and abnormal space perception. American Journal of Optometry & Physiological. Optics. 1983;60:426–435. doi: 10.1097/00006324-198306000-00003. [DOI] [PubMed] [Google Scholar]
- Bharadwaj SR, Candy TR. Accommodative and vergence responses to conflicting blur and disparity stimuli during development. Journal of Vision. 2009;9(11):4, 1–18. doi: 10.1167/9.11.4. doi: 10.1167/9.11.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj SR, Candy TR. The effect of lens-induced anisometropia on accommodation and vergence during human visual development. Investigative Ophthalmology & Visual Science. 2011;52(6):3595–3603. doi: 10.1167/iovs.10-6214. doi: 10.1167/iovs.10-6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE, Stager DR. Monocular acuity and stereopsis in infantile esotropia. Investigative Ophthalmology & Visual Science. 1985;26(11):1624–1630. [PubMed] [Google Scholar]
- Birch EE, Stager DR. Prevalence of good visual acuity following surgery for congenital unilateral cataract. Archives of Ophthalmology. 1988;106(1):40–43. doi: 10.1001/archopht.1988.01060130046025. [DOI] [PubMed] [Google Scholar]
- Birch EE, Stager DR, Sr., Wang J, O’Connor A. Longitudinal changes in refractive error of children with infantile esotropia. Eye (Lond) 2010;24:1814–1821. doi: 10.1038/eye.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE, Swanson WH. Hyperacuity deficits in anisometropic and strabismic amblyopes with known ages of onset. Vision Research. 2000;40(9):1035–1040. doi: 10.1016/s0042-6989(00)00011-0. [DOI] [PubMed] [Google Scholar]
- Blakemore C, Garey LJ, Vital-Durand F. The physiological effects of monocular deprivation and their reversal in the monkey’s visual cortex. Journal of Physiology. 1978;283:223–262. doi: 10.1113/jphysiol.1978.sp012498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt N. Die Vererbung von Anisometropie. von Graefes Archive for Clinical and Experimental Ophthalmology. 1924;114:604. [Google Scholar]
- Bonneh YS, Sagi D, Polat U. Local and non-local deficits in amblyopia: acutiy and spatial interactions. Vision Research. 2004;44:3099–3110. doi: 10.1016/j.visres.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Borchert M, Tarczy-Hornoch K, Cotter SA, Liu N, Azen SP, Varma R, MEPEDS Group Anisometropia in Hispanic and African-American infants and young children: the multi-ethnic pediatric eye disease study. Ophthalmology. 2010;117:148–153. doi: 10.1016/j.ophtha.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne RR, Dineen BP, Ali SM, Noorul Huq DM, Johnson GJ. Prevalence of refractive error in Bangladeshi adults: results of the National Blindness and Low Vision Survey of Bangladesh. Ophthalmology. 2004;111(6):1150–1160. doi: 10.1016/j.ophtha.2003.09.046. [DOI] [PubMed] [Google Scholar]
- Bozkurt B, Irkeç M, Orhan M, Karaağaoğlu E. Thickness of the retinal nerve fiber layer in patients with anisometropic and strabismic amblyopia. Strabismus. 2003;11(1):1–7. doi: 10.1076/stra.11.1.1.14091. [DOI] [PubMed] [Google Scholar]
- Bradley A, Freeman RD. Contrast sensitivity in anisometropic amblyopia. Investigative Ophthalmology & Visual Science. 1981;21(3):467–476. [PubMed] [Google Scholar]
- Bradley A, Rabin J, Freeman RD. Non-optical determinants of aniseikonia. Investigative Ophthalmology & Visual Science. 1983;24:507–512. [PubMed] [Google Scholar]
- Bradley DV, Fernandes A, Tigges M, Boothe RG. Diffuser contact lenses retard azial elongation in infant rhesus monkeys. Vision Research. 1996;36:509–514. doi: 10.1016/0042-6989(95)00279-0. [DOI] [PubMed] [Google Scholar]
- Brown B, Feigl B, Gole GA, Mullen K, Hess RF. Assessment of neuroretinal function in a group of functional Amblyopes with documented LGN deficits. Ophthalmic and Physiological Optics. 2013;33:138–149. doi: 10.1111/opo.12024. [DOI] [PubMed] [Google Scholar]
- Brown SA, Weih LM, Fu CL, Dimitrov P, Taylor HR, McCarty CA. Prevalence of amblyopia and associated refractive errors in an adult population in Victoria, Australia. Ophthalmic Epidemiology. 2000;7:249–258. [PubMed] [Google Scholar]
- Bruce A, Pacey IE, Bradbury JA, Scally AJ, Barrett BT. Bilateral Changes in Foveal Structure in Individuals with Amblyopia. Ophthalmology. 2012 Sep 29; doi: 10.1016/j.ophtha.2012.07.088. [DOI] [PubMed] [Google Scholar]
- Bullimore MA, Fusaro RE, Adams CW. The repeatability of automated and clinician refraction. Optometry & Vision Science. 1998;75(8):617–622. doi: 10.1097/00006324-199808000-00028. [DOI] [PubMed] [Google Scholar]
- Candy TR. Which hyperopic patients are destined for trouble? Journal of AAPOS. 2012;16(2):107–109. doi: 10.1016/j.jaapos.2012.02.004. doi: 10.1016/j.jaapos.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass K, Tromans C. A biometric investigation of ocular components in amblyopia. Ophthalmic & Physiological Optics. 2008;28:429–440. doi: 10.1111/j.1475-1313.2008.00585.x. [DOI] [PubMed] [Google Scholar]
- Chandna A, Pennefather PM, Kovács I, Norcia AM. Contour integration deficits in anisometropic amblyopia. Investigative Ophthalmology & Visual Science. 2001;42:875–878. [PubMed] [Google Scholar]
- Chang CH, Tsai RK, Sheu MM. Screening amblyopia of preschool children with uncorrected vision and stereopsis tests in Eastern Taiwan. Eye. 2007;21:1482–1488. doi: 10.1038/sj.eye.6702568. [DOI] [PubMed] [Google Scholar]
- Charman WN. Keeping the world in focus: how might this be achieved? Optometry & Vision Science. 2011;88(3):373–376. doi: 10.1097/OPX.0b013e31820b052b. doi: 10.1097/OPX.0b013e31820b052b. [DOI] [PubMed] [Google Scholar]
- Cheng C-Y, Hsu W-M, Liu J-H, Tsai S-Y, Chou P. Refractive Errors in an Elderly Chinese Population in Taiwan: The Shihpai Eye Study. Investigative Ophthalmology & Visual Science. 2003;44(11):4630–4638. doi: 10.1167/iovs.03-0169. doi: 10.1167/iovs.03-0169. [DOI] [PubMed] [Google Scholar]
- Chia A, Dirani M, Chan YH, Gazzard G, Au Eong KG, Selvaraj P, Ling Y, Quah BL, Young TL, Mitchell P, Varma R, Wong TY, Saw SM. Prevalence of amblyopia and strabismus in young Singaporean Chinese children. Investigative Ophthalmology & Visual Science. 2010;51:3411–3417. doi: 10.1167/iovs.09-4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuffreda KJ, Levi DM, Selenow A. Amblyopia: Basic and Clinical Aspects. Butterworth-Heineman; Boston; London: 1991. [Google Scholar]
- Clarke MP, Wright CM, Hrisos S, Anderson JD, Henderson J, Richardson SR. Randomised controlled trial of treatment of unilateral visual impairment detected at preschool vision screening. British Medical Journal. 2003;327:1251. doi: 10.1136/bmj.327.7426.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colburn JD, Morrison DG, Estes RL, Li C, Lu P, Donahue SP. Longitudinal follow-up of hypermetropic children identified during preschool vision screening. Journal of AAPOS. 2010;14:211–215. doi: 10.1016/j.jaapos.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Colen TP, de Faber JT, Lemij HG. Retinal nerve fiber layer thickness in human strabismic amblyopia. Binocul Vis Strabismus Q. 2000;15(2):141–6. [PubMed] [Google Scholar]
- Committee on Practice and Ambulatory Medicine (CPAM), S. o. O. Eye examination and vision screening in infants, children and young adults. Pediatrics. 1996;98:153–157. [PubMed] [Google Scholar]
- Cook RC, Glasscock RE. Refractive and ocular findings in the newborn. Amercian Journal of Ophthalmology. 1951;34(10):1407–1413. doi: 10.1016/0002-9394(51)90481-3. [DOI] [PubMed] [Google Scholar]
- Copps LA. Vision in anisometropia. American Journal of Ophthalmology. 1944;27:641–644. [Google Scholar]
- Cotter SA, Pediatric Eye Disease Investigator Group. Edwards AR, Wallace DK, Beck RW, Arnold RW, Weise KK. Treatment of anisometropic amblyopia in children with refractive correction. Ophthalmology. 2006;113(6):895–903. doi: 10.1016/j.ophtha.2006.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter SA, Foster NC, Holmes JM, Melia BM, Wallace DK, Repka MX, Suh DW. Optical treatment of strabismic and combined strabismic-anisometropic amblyopia. Ophthalmology. 2012;119(1):150–158. doi: 10.1016/j.ophtha.2011.06.043. doi: 10.1016/j.ophtha.2011.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crewther SG, Nathan J, Kiely PM, Brennan NA, Crewther DP. The Effect of Defocusing Contact-Lenses on Refraction in Cynomolgus Monkeys. Clinical Vision Sciences. 1988;3(3):221–228. [Google Scholar]
- Czepita D, Goslawski W, Mojsa A. Occurrence of anisometropia among students ranging from 6 to 18 years of age. Klinika Oczna. 2005;107(4-6):297–299. [PubMed] [Google Scholar]
- Daw NW. Critical periods and amblyopia. Archives of Ophthalmology. 1998;116:502–505. doi: 10.1001/archopht.116.4.502. [DOI] [PubMed] [Google Scholar]
- de Vries J. Anisometropia in children: analysis of a hospital population. British Journal of Ophthalmology. 1985;69(7):504–507. doi: 10.1136/bjo.69.7.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Gwiazda JE. Anisometropia in children from infancy to 15 years. Investigative Ophthalmology & Visual Science. 2012;53(7):3782–3787. doi: 10.1167/iovs.11-8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De-Tong PTVM, Oostra BA, De Faber JTHN. High Symmetric Anisometropia in Monozygotic Twins. Ophthalmic Paediatrics. 1993;14(1):29–32. doi: 10.3109/13816819309087620. [DOI] [PubMed] [Google Scholar]
- Dirani M, Chan YH, Gazzard G, Hornbeak DM, Leo SW, Selvaraj P, Zhou B, Young TL, MItchell P, Varma R, Wong TY, Saw SM. Prevalence of refractive error in Singaporean Chinese children: the strabismus, amblyopia, and refractive error in young Singaporean Children (STARS) study. Investigative Ophthalmology & Visual Science. 2010;51:1348–1355. doi: 10.1167/iovs.09-3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson V, Harvey EM, Miller JM, Clifford-Donaldson CE. Anisometropia prevalence in a highly astigmatic school-aged population. Optometry & Vision Science. 2008b;85:512–529. doi: 10.1097/OPX.0b013e31817c930b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson V, Miller JM, CLifford-Donaldson CE, Harvey EM. Associations between anisometropia, amblyopia, and reduced stereoacuity in a school-aged population with a gigh prevalence of astigmatism. Investigative Ophthalmology & Visual Science. 2008a;49:4427–4436. doi: 10.1167/iovs.08-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue SP. Relationship between anisometropia, patient age, and the development of amblyopia. American Journal of Ophthalmology. 2006;142(1):132–140. doi: 10.1016/j.ajo.2006.02.040. see comment. [DOI] [PubMed] [Google Scholar]
- Donnelly UM, Stewart NM, Hollinger M. Prevalence and outcomes of childhood visual disorders. Ophthalmic Epidemiology. 2005;12(4):243–250. doi: 10.1080/09286580590967772. [DOI] [PubMed] [Google Scholar]
- Eibschitz-Tsimhoni M, Friedman T, Naor J, Eibschitz N, Friedman Z. Early screening for amblyogenic risk factors lowers the prevalence and severity of amblyopia. Journal of AAPOS. 2000;4(4):194–199. doi: 10.1067/mpa.2000.105274. [DOI] [PubMed] [Google Scholar]
- Fabian G, Wendell ME. Ophthalmologic and orthoptic examination of 1,200 children up to age 2. American Orthoptic Journal. 1974;24:86–90. [PubMed] [Google Scholar]
- Faghihi M, Ostadimoghaddam H, Yekta AA. Amblyopia and strabismus in Iranian schoolchildren, Mashhad. Strabismus. 2011;19(4):147–152. doi: 10.3109/09273972.2011.622341. [DOI] [PubMed] [Google Scholar]
- Fielder AR, Moseley MJ. Anisometropia and amblyopia--chicken or egg? British Journal of Ophthalmology. 1996;80(10):857–858. doi: 10.1136/bjo.80.10.857. comment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fledelius HC. Prevalences of astigmatism and anisometropia in adult danes. With reference to presbyopes’ possible use of supermarket standard glasses. Acta Ophthalmologica. 1984;62(3):391–400. doi: 10.1111/j.1755-3768.1984.tb08419.x. [DOI] [PubMed] [Google Scholar]
- Flitcroft DI, Judge SJ, Morley JW. Binocular interactions in accommodation control: effects of anisometropic stimuli. Journal of Neuroscience. 1992;12(1):188–203. doi: 10.1523/JNEUROSCI.12-01-00188.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flom MC, Bedell HE. Identifying amblyopia using associated conditions, acuity, and nonacuity features. American Journal of Optometry & Physiological Optics. 1985;62(3):153–160. doi: 10.1097/00006324-198503000-00001. [DOI] [PubMed] [Google Scholar]
- Flom MC, Neumaier RW. Prevalence of amblyopia. Public Health Reports. 1966;81(4):329–341. [PMC free article] [PubMed] [Google Scholar]
- Flynn JT, Cassady JC. Current trends in amblyopia therapy. Ophthalmology. 1978;85(5):428–450. doi: 10.1016/s0161-6420(78)35651-7. [DOI] [PubMed] [Google Scholar]
- Fotouhi A, Hashemi H, Khabazkhoob M, Mohammad K. The prevalence of refractive errors among schoolchildren in Dezful, Iran. British Journal of Ophthalmology. 2007;91(3):287–292. doi: 10.1136/bjo.2006.099937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman RD. Asymmetries in human accomodation and visual experience. Vision Research. 1975;15(4):483–492. doi: 10.1016/0042-6989(75)90025-5. [DOI] [PubMed] [Google Scholar]
- Freeman RD, Olson C. Brief periods of monocular deprivation in kittens: effects of delay prior to physiological study. Journal of Neurophysiology. 1982;47(2):139–150. doi: 10.1152/jn.1982.47.2.139. [DOI] [PubMed] [Google Scholar]
- Frey T, Friendly D, Wyatt D. Re-evaluation of monocular cataracts in children. American Journal of Ophthalmology. 1973;76(3):381–388. doi: 10.1016/0002-9394(73)90495-9. [DOI] [PubMed] [Google Scholar]
- Friedman DS, Repka MX, Katz J, Giordano L, Ibironke J, Hawse P, Tielsch JM. Prevalence of amblyopia and strabismus in white and African American children aged 6 through 71 months the Baltimore Pediatric Eye Disease Study. Ophthalmology. 2009;116(11):2128–2134. e2121–2122. doi: 10.1016/j.ophtha.2009.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fronius M, Sireteanu R, Zubcov A. Deficits of spatial localization in children with strabismic amblyopia. Graefes Archive for Clinical and Experimental Ophthalmology. 2004;242(10):827–839. doi: 10.1007/s00417-004-0936-5. [DOI] [PubMed] [Google Scholar]
- Fulton AB, Dobson V, Salem D, Mar C, Petersen RA, Hansen RM. Cycloplegic refractions in infants and young children. American Journal of Ophthalmology. 1980;90(2):239–247. doi: 10.1016/s0002-9394(14)74861-5. [DOI] [PubMed] [Google Scholar]
- Gilbert CE, Ellwein LB, Refractive Error Study in Children Study, G. Prevalence and causes of functional low vision in school-age children: results from standardized population surveys in Asia, Africa, and Latin America. Investigative Ophthalmology & Visual Science. 2008;49(3):877–881. doi: 10.1167/iovs.07-0973. [DOI] [PubMed] [Google Scholar]
- Giles GH. The distribution of visual defects. British Journal of Physiological Optics. 1950;7(4):179–208. [PubMed] [Google Scholar]
- Giordano L, Friedman DS, Repka MX, Katz J, Ibironke J, Hawes P, Tielsch JM. Prevalence of refractive error among preschool children in an urban population: the Baltimore Pediatric Eye Disease Study. Ophthalmology. 2009;116(4):739–746. doi: 10.1016/j.ophtha.2008.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh PP, Abqariyah Y, Pokharel GP, Ellwein LB. Refractive error and visual impairment in school-age children in Gombak District, Malaysia. Ophthalmology. 2005;112(4):678–685. doi: 10.1016/j.ophtha.2004.10.048. [DOI] [PubMed] [Google Scholar]
- Goldschmidt E, Lyhne N, Lam CS. Ocular anisometropia and laterality. Acta Ophthalmologica Scandinavica. 2004;82(2):175–178. doi: 10.1111/j.1600-0420.2004.00230.x. [DOI] [PubMed] [Google Scholar]
- Goss DA, Grosvenor T. Reliability of refraction--a literature review. Journal of the American Optometric Association. 1996;67(10):619–630. [PubMed] [Google Scholar]
- Graham B, Judge SJ. The effects of spectacle wear in infancy on eye growth and refractive error in the marmoset (Callithrix jacchus) Vision Research. 1999;39(2):189–206. doi: 10.1016/s0042-6989(98)00189-8. [DOI] [PubMed] [Google Scholar]
- Granet DB, Christian W, Gomi CE, Banuelos L, Castro E. Treatment options for anisohyperopia. J Pediatr Ophthalmol Strabismus. 2006;43(4):207–211. doi: 10.3928/01913913-20060701-01. quiz 231-202. [DOI] [PubMed] [Google Scholar]
- Greene PR, Guyton DL. Time course of rhesus lid-suture myopia. Experimental Eye Research. 1986;42(6):529–534. doi: 10.1016/0014-4835(86)90043-6. [DOI] [PubMed] [Google Scholar]
- Grönlund MA, Andersson S, Aring E, Hård A-L, Hellström A. Ophthalmological findings in a sample of Swedish children aged 4-15 years. Acta Ophthalmologica Scandinavica. 2006;84(2):169–176. doi: 10.1111/j.1600-0420.2005.00615.x. doi: 10.1111/j.1600-0420.2005.00615.x. [DOI] [PubMed] [Google Scholar]
- Gupta A, Casson RJ, Newland HS, Muecke J, Landers J, Selva D, Aung T. Prevalence of Refractive Error in Rural Myanmar: The Meiktila Eye Study. Ophthalmology. 2008;115(1):26–32. e22. doi: 10.1016/j.ophtha.2007.02.025. [DOI] [PubMed] [Google Scholar]
- Gusek-Schneider GC, Martus P. Stimulus deprivation amblyopia in human congenital ptosis: a study of 100 patients. Strabismus. 2000;8(4):261–270. doi: 10.1076/stra.8.4.261.687. [DOI] [PubMed] [Google Scholar]
- Guzowski M, Fraser-Bell S, Rochtchina E, Wang JJ, Mitchell P. Asymmetric refraction in an older population: the Blue Mountains Eye Study. American Journal of Ophthalmology. 2003;136(3):551–553. doi: 10.1016/s0002-9394(03)00246-0. [DOI] [PubMed] [Google Scholar]
- Haegerstrom-Portnoy G, Schneck ME, Brabyn JA, Lott LA. Development of refractive errors into old age. Optometry & Vision Science. 2002;79(10):643–649. doi: 10.1097/00006324-200210000-00010. [DOI] [PubMed] [Google Scholar]
- Hardesty HH. Occlusion amblyopia; report of a case. AMA Arch Ophthalmol. 1959;62(2):314–316. doi: 10.1001/archopht.1959.04220020140020. [DOI] [PubMed] [Google Scholar]
- Hardman Lea SJ, Loades J, Rubinstein MP. The sensitive period for anisometropic amblyopia. Eye. 1989;3(Pt 6):783–790. doi: 10.1038/eye.1989.122. [DOI] [PubMed] [Google Scholar]
- Harvey EM, Miller JM, Wagner LK, Dobson V. Reproducibility and accuracy of measurements with a hand held autorefractor in children. British Journal of Ophthalmology. 1997;81(11):941–8. doi: 10.1136/bjo.81.11.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwerth RS, Crawford ML, Smith EL, 3rd, Boltz RL. Behavioral studies of stimulus deprivation amblyopia in monkeys. Vision Research. 1981;21(6):779–789. doi: 10.1016/0042-6989(81)90175-9. [DOI] [PubMed] [Google Scholar]
- Harwerth RS, Smith EL, 3rd, Boltz RL, Crawford ML, von Noorden GK. Behavioral studies on the effect of abnormal early visual experience in monkeys: spatial modulation sensitivity. Vision Research. 1983;23(12):1501–1510. doi: 10.1016/0042-6989(83)90162-1. [DOI] [PubMed] [Google Scholar]
- Harwerth RS, Smith EL, 3rd, Duncan GC, Crawford ML, von Noorden GK. Multiple sensitive periods in the development of the primate visual system. Science. 1986;232(4747):235–238. doi: 10.1126/science.3952507. [DOI] [PubMed] [Google Scholar]
- Heath GG. The influence of visual acuity on accommodative responses of the eye. Am J Optom Arch Am Acad Optom. 1956;33(10):513–24. doi: 10.1097/00006324-195610000-00001. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The organization of behavior; a neuropsychological theory. Wiley; New York: 1949. [Google Scholar]
- He M, Huang W, Zheng Y, Huang L, Ellwein LB. Refractive error and visual impairment in school children in rural southern China. Ophthalmology. 2007;114(2):374–382. doi: 10.1016/j.ophtha.2006.08.020. [DOI] [PubMed] [Google Scholar]
- He M, Zeng J, Liu Y, Xu J, Pokharel GP, Ellwein LB. Refractive error and visual impairment in urban children in southern china. Investigative Ophthalmology & Visual Science. 2004;45(3):793–799. doi: 10.1167/iovs.03-1051. [DOI] [PubMed] [Google Scholar]
- Helveston EM. The Incidence of Amblyopia Ex Anopsia in Young Adult Males in Minnesota in 1962-63. American Journal of Ophthalmology. 1965;60:75–77. doi: 10.1016/0002-9394(65)92394-9. [DOI] [PubMed] [Google Scholar]
- Helveston EM. Relationship between degree of anisometropia and depth of amblyopia. American Journal of Ophthalmology. 1966;62(4):757–759. doi: 10.1016/0002-9394(66)92207-0. [DOI] [PubMed] [Google Scholar]
- Helveston EM, Von Noorden GK. Microtropia. A newly defined entity. Archives of Ophthalmology. 1967;78(3):272–281. doi: 10.1001/archopht.1967.00980030274003. [DOI] [PubMed] [Google Scholar]
- Hendricks TJ, de Brabander J, Vankan-Hendricks MH, van der Horst FG, Hendrikse F, Knottnerus JA. Prevalence of habitual refractive errors and anisometropia among Dutch schoolchildren and hospital employees. Acta Opthalmologica. 2009;87(5):538–543. doi: 10.1111/j.1755-3768.2008.01251.x. [DOI] [PubMed] [Google Scholar]
- Hess RF. Contrast sensitivity assessment of functional amblyopia in humans. Transactions of the Ophthalmological Societies of the United Kingdom. 1979;99(3):391–397. [PubMed] [Google Scholar]
- Hess RF. Amblyopia: site unseen. Clinical & Experimental Optometry. 2001;84(6):321–336. doi: 10.1111/j.1444-0938.2001.tb06604.x. [DOI] [PubMed] [Google Scholar]
- Hess RF, Campbell FW, Greenhalgh T. On the nature of the neural abnormality in human amblyopia; neural aberrations and neural sensitivity loss. Pflugers Archiv - European Journal of Physiology. 1978;377(3):201–207. doi: 10.1007/BF00584273. [DOI] [PubMed] [Google Scholar]
- Hess RF, Howell ER. The threshold contrast sensitivity function in strabismic amblyopia: evidence for a two type classification. Vision Research. 1977;17(9):1049–1055. doi: 10.1016/0042-6989(77)90009-8. [DOI] [PubMed] [Google Scholar]
- Hess RF, Li X, Lu G, Thompson B, Hansen BC. The contrast dependence of the cortical fMRI deficit in amblyopia; a selective loss at higher contrasts. Human Brain Mapping. 2010;31(8):1233–1248. doi: 10.1002/hbm.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RF, Pointer JS. Differences in the neural basis of human amblyopia: the distribution of the anomaly across the visual field. Vision Research. 1985;25(11):1577–1594. doi: 10.1016/0042-6989(85)90128-2. [DOI] [PubMed] [Google Scholar]
- Hess RF, Thompson B, Gole G, Mullen KT. Deficient responses from the lateral geniculate nucleus in humans with amblyopia. European Journal of Neuroscience. 2009;29(5):1064–1070. doi: 10.1111/j.1460-9568.2009.06650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RF, Thompson B, Gole GA, Mullen KT. The amblyopic deficit and its relationship to geniculo-cortical processing streams. Journal of Neurophysiology. 2010;104(1):475–483. doi: 10.1152/jn.01060.2009. [DOI] [PubMed] [Google Scholar]
- Hess RF, Wang YZ, Demanins R, Wilkinson F, Wilson HR. A deficit in strabismic amblyopia for global shape detection. Vision Research. 1999;39(5):901–914. doi: 10.1016/s0042-6989(98)00157-6. [DOI] [PubMed] [Google Scholar]
- Hess RF, Mansouri B, Thompson B. Restoration of binocular vision in amblyopia. Strabismus. 2011;19(3):110–118. doi: 10.3109/09273972.2011.600418. [DOI] [PubMed] [Google Scholar]
- Hirsch MJ. Anisometropia: a preliminary report of the Ojai Longitudinal Study. American Journal of Optometry & Archives of American Academy of Optometry. 1967;44(9):581–585. [PubMed] [Google Scholar]
- Holmes JM, Beck RW, Repka MX, Leske DA, Kraker RT, Blair RC, Moke PS, Birch EE, Saunders RA, Hertle RW, Quinn GE, Simons KA, Miller JM, Pediatric Eye Disease Investigator Group The amblyopia treatment study visual acuity testing protocol. Archives of Ophthalmology. 2001;119(9):1345–53. doi: 10.1001/archopht.119.9.1345. [DOI] [PubMed] [Google Scholar]
- Holmes JM, Lazar EL, Melia BM, Astle WF, Dagi LR, Donahue SP, Weise KK. Effect of age on response to amblyopia treatment in children. Archives of Ophthalmology. 2011;129(11):1451–1457. doi: 10.1001/archophthalmol.2011.179. doi: 10.1001/archophthalmol.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes JM, Repka MX, Kraker RT, Clarke MP. The treatment of amblyopia. Strabismus. 2006;14(1):37–42. doi: 10.1080/09273970500536227. [DOI] [PubMed] [Google Scholar]
- Holmstrom M, el Azazi M, Kugelberg U. Ophthalmological long-term follow up of preterm infants: a population based, prospective study of the refraction and its development. British Journal of Ophthalmology. 1998;82(11):1265–1271. doi: 10.1136/bjo.82.11.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwich H. Anisometropia Amblyopia. American Orthoptic Journal. 1964;14:99–104. [PubMed] [Google Scholar]
- Horwood AM, Riddell PM. Independent and reciprocal accommodation in anisometropic amblyopia. Journal of AAPOS. 2010;14(5):447–449. doi: 10.1016/j.jaapos.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt CS, Stone RD, Fromer C, Billson FA. Monocular axial myopia associated with neonatal eyelid closure in human infants. American Journal of Ophthalmology. 1981;91(2):197–200. doi: 10.1016/0002-9394(81)90173-2. [DOI] [PubMed] [Google Scholar]
- Hoyt CS. Amblyopia: a neuro-ophthalmic view. Journal of Neuroophthalmology. 2005;25(3):227–31. doi: 10.1097/01.wno.0000177304.67715.ba. [DOI] [PubMed] [Google Scholar]
- Huang J, Hung LF, Ramamirtham R, Blasdel TL, Humbird TL, Bockhorst KH, Smith EL., 3rd Effects of form deprivation on peripheral refractions and ocular shape in infant rhesus monkeys (Macaca mulatta) Investigative Ophthalmology & Visual Science. 2009;50(9):4033–4044. doi: 10.1167/iovs.08-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. Journal of Physiology. 1970;206(2):419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN, LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philosophical Transactions of the Royal Society of London - Series B: Biological Sciences. 1977;278(961):377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- Hung LF, Crawford ML, Smith EL. Spectacle lenses alter eye growth and the refractive status of young monkeys. Nature Medicine. 1995;1(8):761–765. doi: 10.1038/nm0895-761. [DOI] [PubMed] [Google Scholar]
- Huynh SC, Wang XY, Ip J, Robaei D, Kifley A, Rose KA, Mitchell P. Prevalence and associations of anisometropia and aniso-astigmatism in a population based sample of 6 year old children. British Journal of Ophthalmology. 2006;90(5):597–601. doi: 10.1136/bjo.2005.083154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang DJ, Kim YJ, Lee JY. Effect and sustainability of part-time occlusion therapy for patients with anisometropic amblyopia aged > or = 8 years. British Journal of Ophthalmology. 2010;94(9):1160–1164. doi: 10.1136/bjo.2009.167817. [DOI] [PubMed] [Google Scholar]
- Inatomi M, Kora Y, Kinohira Y, Yaguchi S. Long-term follow-up of eye growth in pediatric patients after unilateral cataract surgery with intraocular lens implantation. Journal of AAPOS. 2004;8(1):50–55. doi: 10.1016/j.jaapos.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Ingram RM. Refraction as a basis for screening children for squint and amblyopia. British Journal of Ophthalmology. 1977;61(1):8–15. doi: 10.1136/bjo.61.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram RM. Refraction of 1-year-old children after atropine cycloplegia. British Journal of Ophthalmology. 1979;63(5):343–347. doi: 10.1136/bjo.63.5.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram RM, Arnold PE, Dally S, Lucas J. Results of a randomised trial of treating abnormal hypermetropia from the age of 6 months. British Journal of Ophthalmology. 1990;74(3):158–159. doi: 10.1136/bjo.74.3.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram RM, Barr A. Refraction of 1-year-old children after cycloplegia with 1% cyclopentolate: comparison with findings after atropinisation. British Journal of Ophthalmology. 1979a;63(5):348–352. doi: 10.1136/bjo.63.5.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram RM, Barr A. Changes in refraction between the ages of 1 and 3.5 years. British Journal of Ophthalmology. 1979b;63(5):339–342. doi: 10.1136/bjo.63.5.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram RM, Gill LE, Goldacre MJ. Emmetropisation and accommodation in hypermetropic children before they show signs of squint--a preliminary analysis. Bulletin de la Societe Belge d Ophtalmologie. 1994;253:41–56. [PubMed] [Google Scholar]
- Ingram RM, Gill LE, Lambert TW. Emmetropisation in normal and strabismic children and the associated changes of anisometropia. Strabismus. 2003;11(2):71–84. doi: 10.1076/stra.11.2.71.15104. [DOI] [PubMed] [Google Scholar]
- Ingram RM, Lambert TW, Gill LE. Visual outcome in 879 children treated for strabismus: insufficient accommodation and vision deprivation, deficient emmetropisation and anisometropia. Strabismus. 2009;17(4):148–157. doi: 10.3109/09273970903376010. [DOI] [PubMed] [Google Scholar]
- Ingram RM, Traynar MJ, Walker C, Wilson JM. Screening for refractive errors at age 1 year: a pilot study. British Journal of Ophthalmology. 1979;63(4):243–250. doi: 10.1136/bjo.63.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip JM, Robaei D, Kifley A, Wang JJ, Rose KA, Mitchell P. Prevalence of hyperopia and associations with eye findings in 6- and 12-year-olds. Ophthalmology. 2008;115(4):678–685. e671. doi: 10.1016/j.ophtha.2007.04.061. [DOI] [PubMed] [Google Scholar]
- Jamali P, Fotouhi A, Hashemi H, Younesian M, Jafari A. Refractive errors and amblyopia in children entering school: Shahrood, Iran. Optometry & Vision Science. 2009;86(4):364–369. doi: 10.1097/OPX.0b013e3181993f42. [DOI] [PubMed] [Google Scholar]
- Jampolsky A, Flom BC, Weymouth FW, Moses LE. Unequal corrected visual acuity as related to anisometropia. Archives of Ophthalmology. 1955;54(6):893–905. doi: 10.1001/archopht.1955.00930020899013. [DOI] [PubMed] [Google Scholar]
- Jiang L, Schaeffel F, Zhou X, Zhang S, Jin X, Pan M, Qu J. Spontaneous axial myopia and emmetropization in a strain of wild-type guinea pig (Cavia porcellus) Investigative Ophthalmology & Visual Science. 2009;50(3):1013–1019. doi: 10.1167/iovs.08-2463. [DOI] [PubMed] [Google Scholar]
- Kanonidou E. Amblyopia: a mini review of the literature. International Ophthalmology. 2011;31(3):249–256. doi: 10.1007/s10792-011-9434-z. [DOI] [PubMed] [Google Scholar]
- Katz J, Tielsch JM, Sommer A. Prevalence and risk factors for refractive errors in an adult inner city population. Investigative Ophthalmology & Visual Science. 1997;38(2):334–340. [PubMed] [Google Scholar]
- Kee CS, Hung LF, Qiao-Grider Y, Roorda A, Smith EL., 3rd Effects of optically imposed astigmatism on emmetropization in infant monkeys. Investigative Ophthalmology & Visual Science. 2004;45(6):1647–1659. doi: 10.1167/iovs.03-0841. [DOI] [PubMed] [Google Scholar]
- Kempen JH, Mitchell P, Lee KE, Tielsch JM, Broman AT, Taylor HR, Ikram MK, Congdon NG, O’Colmain BJ. The prevalence of refractive errors among adults in the United States, Western Europe, and Australia. Archives of Ophthalmology. 2004;122:495–505. doi: 10.1001/archopht.122.4.495. [DOI] [PubMed] [Google Scholar]
- Kind PC. Cortical plasticity: is it time for a change? Current Biology. 1999;9(17):640–643. doi: 10.1016/s0960-9822(99)80412-6. [DOI] [PubMed] [Google Scholar]
- Kiorpes L. Effect of strabismus on the development of vernier acuity and grating acuity in monkeys. Visual Neuroscience. 1992;9(3-4):253–259. doi: 10.1017/s095252380001066x. [DOI] [PubMed] [Google Scholar]
- Kiorpes L, Boothe RG, Hendrickson AE, Movshon JA, Eggers HM, Gizzi MS. Effects of early unilateral blur on the macaque’s visual system. I. Behavioral observations. Journal of Neuroscience. 1987;7(5):1318–1326. doi: 10.1523/JNEUROSCI.07-05-01318.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiorpes L, Kiper DC, Movshon JA. Contrast sensitivity and vernier acuity in amblyopic monkeys. Vision Research. 1993;33(16):2301–2311. doi: 10.1016/0042-6989(93)90107-8. [DOI] [PubMed] [Google Scholar]
- Kiorpes L, Kiper DC, O’Keefe LP, Cavanaugh JR, Movshon JA. Neuronal correlates of amblyopia in the visual cortex of macaque monkeys with experimental strabismus and anisometropia. Journal of Neuroscience. 1998;18(16):6411–6424. doi: 10.1523/JNEUROSCI.18-16-06411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiorpes L, McKee SP. Neural mechanisms underlying amblyopia. Current Opinion in Neurobiology. 1999;9(4):480–486. doi: 10.1016/s0959-4388(99)80072-5. [DOI] [PubMed] [Google Scholar]
- Kiorpes L, Movshon JA. Differential development of two visual functions in primates. Proc Natl Acad Sci U S A. 1989;86(22):8998–9001. doi: 10.1073/pnas.86.22.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiorpes L, Wallman J. Does experimentally-induced amblyopia cause hyperopia in monkeys? Vision Research. 1995;35(9):1289–1297. doi: 10.1016/0042-6989(94)00239-i. [DOI] [PubMed] [Google Scholar]
- Kivlin JD, Flynn JT. Therapy of anisometropic amblyopia. Journal of Pediatric Ophthalmology & Strabismus. 1981;18(5):47–56. doi: 10.3928/0191-3913-19810901-12. [DOI] [PubMed] [Google Scholar]
- Klein R, Wang Q, Klein BE, Moss SE, Meuer SM. The relationship of age-related maculopathy, cataract, and glaucoma to visual acuity. Investigative Ophthalmology & Visual Science. 1995;36(1):182–191. [PubMed] [Google Scholar]
- Knox-Laird I. Anisometropia. In: Grosvenor T, Flom MC, editors. Refractive Anomalies: Research and Clinical Applications. Butterworth-Heinemann; Stoneham, MA: 1991. pp. 174–198. [Google Scholar]
- Koh LH, Charman WN. Accommodative responses to anisoaccommodative targets. Ophthalmic & Physiological Optics. 1998;18(3):254–262. [PubMed] [Google Scholar]
- Kovacs I, Polat U, Pennefather PM, Chandna A, Norcia AM. A new test of contour integration deficits in patients with a history of disrupted binocular experience during visual development. Vision Research. 2000;40(13):1775–1783. doi: 10.1016/s0042-6989(00)00008-0. [DOI] [PubMed] [Google Scholar]
- Krishnaiah S, Srinivas M, Khanna RC, Rao GN. Prevalence and risk factors for refractive errors in the South Indian adult population: The Andhra Pradesh Eye disease study. Clinical Ophthalmology. 2009;3:17–27. [PMC free article] [PubMed] [Google Scholar]
- Kulp MT, Foster NC, Holmes JM, Kraker RT, Melia BM, Repka MX, Tien DR, Pediatric Eye Disease Investigator Group Effect of ocular alignment on emmetropization in children <10 years with amblyopia. American Journal of Ophthalmology. 2012;154(2):297–302. doi: 10.1016/j.ajo.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo A, Sinatra RB, Donahue SP. Distribution of refractive error in healthy infants. Journal of Aapos: American Association for Pediatric Ophthalmology & Strabismus. 2003;7(3):174–177. doi: 10.1016/s1091-8531(03)00017-x. [DOI] [PubMed] [Google Scholar]
- Kutschke PJ, Scott WE, Keech RV. Anisometropic amblyopia. Ophthalmology. 1991;98(2):258–263. doi: 10.1016/s0161-6420(91)32307-8. [DOI] [PubMed] [Google Scholar]
- Kutschke PJ, Scott WE, Keech RV. Depth of anisometropic amblyopia and difference in refraction. American Journal of Ophthalmology. 1994;117(5):681–682. doi: 10.1016/s0002-9394(14)70087-x. comment. [DOI] [PubMed] [Google Scholar]
- Kvarnstrom G, Jakobsson P, Lennerstrand G. Screening for visual and ocular disorders in children, evaluation of the system in Sweden. Acta Paediatrica. 1998;87(11):1173–1179. doi: 10.1080/080352598750031176. [DOI] [PubMed] [Google Scholar]
- Kvarnstrom G, Jakobsson P, Lennerstrand G. Visual screening of Swedish children: an ophthalmological evaluation. Acta Ophthalmol Scand. 2001;79(3):240–244. doi: 10.1034/j.1600-0420.2001.790306.x. [DOI] [PubMed] [Google Scholar]
- Lagreze WD, Sireteanu R. Two-dimensional spatial distortions in human strabismic amblyopia. Vision Research. 1991;31(7-8):1271–1288. doi: 10.1016/0042-6989(91)90051-6. [DOI] [PubMed] [Google Scholar]
- Larsson EK, Rydberg AC, Holmstrom GE. A population-based study of the refractive outcome in 10-year-old preterm and full-term children. Archives of Ophthalmology. 2003;121(10):1430–1436. doi: 10.1001/archopht.121.10.1430. [DOI] [PubMed] [Google Scholar]
- Lavery JR, Gibson JM, Shaw DE, Rosenthal AR. Refraction and refractive errors in an elderly population. Ophthalmic & Physiological Optics. 1988;8(4):394–396. doi: 10.1111/j.1475-1313.1988.tb01175.x. [DOI] [PubMed] [Google Scholar]
- Lempert P. Journal of AAPOS. 2000;4(5):258–266. doi: 10.1067/mpa.2000.106963. Optic nerve hypoplasia and small eyes in presumed amblyopia. see comment. [DOI] [PubMed] [Google Scholar]
- Lempert P. Axial length-disc area ratio in esotropic amblyopia. Archives of Ophthalmology. 2003;121(6):821–824. doi: 10.1001/archopht.121.6.821. see comment. [DOI] [PubMed] [Google Scholar]
- Lempert P. The axial length/disc area ratio in anisometropic hyperopic amblyopia: a hypothesis for decreased unilateral vision associated with hyperopic anisometropia. Ophthalmology. 2004;111(2):304–308. doi: 10.1016/j.ophtha.2003.05.020. [DOI] [PubMed] [Google Scholar]
- Lempert P. Relationship between anisometropia, patient age, and the development of amblyopia. American Journal of Ophthalmology. 2006;142(5):891. doi: 10.1016/j.ajo.2006.07.059. author reply 891-892. [DOI] [PubMed] [Google Scholar]
- Lempert P. Optic disc area and retinal area in amblyopia. Seminars in Ophthalmology. 2008a;23(5):302–306. doi: 10.1080/08820530802505997. [DOI] [PubMed] [Google Scholar]
- Lempert P. Retinal area and optic disc rim area in amblyopic, fellow, and normal hyperopic eyes: a hypothesis for decreased acuity in amblyopia. Ophthalmology. 2008b;115(12):2259–2261. doi: 10.1016/j.ophtha.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Lempert P, Porter L. Dysversion of the optic disc and axial length measurements in a presumed amblyopic population. Journal of Aapos: American Association for Pediatric Ophthalmology & Strabismus. 1998;2(4):207–213. doi: 10.1016/s1091-8531(98)90054-4. [DOI] [PubMed] [Google Scholar]
- Leon A, Donahue SP, Morrison DG, Estes RL, Li C. The age-dependent effect of anisometropia magnitude on anisometropic amblyopia severity. Journal of AAPOS. 2008;12(2):150–156. doi: 10.1016/j.jaapos.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Lepard CW. Comparative changes in the error of refraction between fixing and amblyopic eyes during growth and development. American Journal of Ophthalmology. 1975;80(3 Pt 2):485, 490. doi: 10.1016/0002-9394(75)90212-3. [DOI] [PubMed] [Google Scholar]
- Levi DM. The Glenn A. Fry award lecture: the “spatial grain” of the amblyopic visual system. American Journal of Optometry & Physiological Optics. 1988;65(10):767–786. [PubMed] [Google Scholar]
- Levi DM. Visual processing in amblyopia: human studies. Strabismus. 2006;14(1):11–19. doi: 10.1080/09273970500536243. [DOI] [PubMed] [Google Scholar]
- Levi DM. Prentice award lecture: removing the brakes on plasticity in the amblyopic brain. Optometry & Vision Science. 2011;89(6):827–38. doi: 10.1097/OPX.0b013e318257a187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM, Carkeet AD. Amblyopia: a consequence of abnormal visual development. In: Simons K, editor. Early Visual Develeopment, Normal and Abnormal. Oxford University Press; New York: 1993. pp. 391–408. [Google Scholar]
- Levi DM, Harwerth RS. Spatio-temporal interactions in anisometropic and strabismic amblyopia. Investigative Ophthalmology & Visual Science. 1977;16(1):90–95. [PubMed] [Google Scholar]
- Levi DM, Klein S. Differences in vernier discrimination for grating between strabismic and anisometropic amblyopes. Investigative Ophthalmology & Visual Science. 1982a;23(3):398–407. [PubMed] [Google Scholar]
- Levi DM, Klein S. Hyperacuity and amblyopia. Nature. 1982b;298(5871):268–270. doi: 10.1038/298268a0. [DOI] [PubMed] [Google Scholar]
- Levi DM, Klein SA. Spatial localization in normal and amblyopic vision. Vision Research. 1983;23(10):1005–1017. doi: 10.1016/0042-6989(83)90011-1. [DOI] [PubMed] [Google Scholar]
- Levi DM, Klein SA. Vernier acuity, crowding and amblyopia. Vision Research. 1985;25(7):979–991. doi: 10.1016/0042-6989(85)90208-1. [DOI] [PubMed] [Google Scholar]
- Levi DM, Klein SA, Aitsebaomo AP. Vernier acuity, crowding and cortical magnification. Vision Research. 1985;25(7):963–977. doi: 10.1016/0042-6989(85)90207-x. [DOI] [PubMed] [Google Scholar]
- Levi DM, Li RW. Perceptual learning as a potential treatment for amblyopia: a mini-review. Vision Research. 2009;49(21):2535–49. doi: 10.1016/j.visres.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM, McKee SP, Movshon JA. Visual deficits in anisometropia. Vision Research. 2011;51(1):48–57. doi: 10.1016/j.visres.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM, Yu C, Kuai SG, Rislove E. Global contour processing in amblyopia. Vision Research. 2007;47(4):512–524. doi: 10.1016/j.visres.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang YB, Wong TY, Sun LP, Tao QS, Wang JJ, Yang XH, Friedman DS. Refractive errors in a rural Chinese adult population the Handan eye study. Ophthalmology. 2009;116(11):2119–2127. doi: 10.1016/j.ophtha.2009.04.040. [DOI] [PubMed] [Google Scholar]
- Lim HT, Yu YS, Park SH, Ahn H, Kim S, Lee M, Koo BS. The Seoul Metropolitan Preschool Vision Screening Programme: results from South Korea. British Journal of Ophthalmology. 2004;88(7):929–933. doi: 10.1136/bjo.2003.029066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LL, Shih YF, Tsai CB, Chen CJ, Lee LA, Hung PT, Hou PK. Epidemiologic study of ocular refraction among schoolchildren in Taiwan in 1995. Optometry & Vision Science. 1999;76(5):275–281. doi: 10.1097/00006324-199905000-00013. [DOI] [PubMed] [Google Scholar]
- Linke SJ, Baviera J, Richard G, Katz T. Association between ocular dominance and spherical/astigmatic anisometropia, age and sex: analysis of 1274 hyperopic individuals. Investigative Ophthalmology & Visual Science. 2012;53(9):5362–5369. doi: 10.1167/iovs.11-8781. [DOI] [PubMed] [Google Scholar]
- Lithander J. Prevalence of amblyopia with anisometropia or strabismus among schoolchildren in the Sultanate of Oman. Acta Ophthalmologica Scandinavica. 1998;76(6):658–662. doi: 10.1034/j.1600-0420.1998.760604.x. [DOI] [PubMed] [Google Scholar]
- Loudon SE, Polling JR, Simonsz HJ. Electronically measured compliance with occlusion therapy for amblyopia is related to visual acuity increase. Graefes Arch Clin Exp Ophthalmol. 2003;241(3):176–80. doi: 10.1007/s00417-002-0570-z. [DOI] [PubMed] [Google Scholar]
- Loudon SE, Rook CA, Nassif DS, Piskun NV, Hunter DG. Rapid, high-accuracy detection of strabismus and amblyopia using the pediatric vision scanner. Investigative Ophthalmology & Visual Science. 2011;52(8):5043–5048. doi: 10.1167/iovs.11-7503. [DOI] [PubMed] [Google Scholar]
- Macias EP, Lee DA, Oelrich FO. Refractive errors and visual acuity impairment among self-selected Hispanic, white, and black adults examined by the UCLA Mobile Eye Clinic. Journal of the American Optometric Association. 1999;70(11):724–734. [PubMed] [Google Scholar]
- MacKenzie GE. Reproducibility of sphero-cylindrical prescriptions. Ophthalmic & Physiological Optics. 2008;28(2):143–150. doi: 10.1111/j.1475-1313.2008.00549.x. [DOI] [PubMed] [Google Scholar]
- Maguire GW, Smith EL, 3rd, Harwerth RS, Crawford ML. Optically induced anisometropia in kittens. Investigative Ophthalmology & Visual Science. 1982;23(2):253–264. [PubMed] [Google Scholar]
- Malik SR, Gupta AK, Choudhry S. Anisometropia--its relation to amblyopia and eccentric fixation. British Journal of Ophthalmology. 1968;52(10):773–776. doi: 10.1136/bjo.52.10.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marran L, Schor CM. Lens induced aniso-accommodation. Vision Research. 1998;38(22):3601–3619. doi: 10.1016/s0042-6989(98)00064-9. [DOI] [PubMed] [Google Scholar]
- Marran L, Schor CM. The effect of target proximity on the aniso-accommodative response. Ophthalmic & Physiological Optics. 1999;19(5):376–392. doi: 10.1046/j.1475-1313.1999.00452.x. [DOI] [PubMed] [Google Scholar]
- Maul E, Barroso S, Munoz SR, Sperduto RD, Ellwein LB. Refractive Error Study in Children: results from La Florida, Chile. American Journal of Ophthalmology. 2000;129(4):445–454. doi: 10.1016/s0002-9394(99)00454-7. [DOI] [PubMed] [Google Scholar]
- Mayer DL, Hansen RM, Moore BD, Kim S, Fulton AB. Cycloplegic refractions in healthy children aged 1 through 48 months. Archives of Ophthalmology. 2001;119(11):1625–1628. doi: 10.1001/archopht.119.11.1625. [DOI] [PubMed] [Google Scholar]
- McKee SP, Levi DM, Movshon JA. The pattern of visual deficits in amblyopia. Journal of Vision. 2003;3(5):380–405. doi: 10.1167/3.5.5. [DOI] [PubMed] [Google Scholar]
- McMullen WH. Some points in anisometropia in discussion on problem of refraction. Trans Ophthalmol Soc. UK. 1939;59:119. [Google Scholar]
- Miller-Meeks MJ, Bennett SR, Keech RV, Blodi CF. Myopia induced by vitreous hemorrhage. American Journal of Ophthalmology. 1990;109(2):199–203. doi: 10.1016/s0002-9394(14)75987-2. [DOI] [PubMed] [Google Scholar]
- Mitchell DE, Gingras G. Visual recovery after monocular deprivation is driven by absolute, rather than relative, visually evoked activity levels. Current Biology. 1998;8(21):1179–1182. doi: 10.1016/s0960-9822(07)00489-7. [DOI] [PubMed] [Google Scholar]
- Mitchell DE, Gingras G, Kind PC. Initial recovery of vision after early monocular deprivation in kittens is faster when both eyes are open. Proc Natl Acad Sci. 2001;98(20):11662–11667. doi: 10.1073/pnas.201392698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohney BG. Axial myopia associated with dense vitreous hemorrhage of the neonate. Journal of AAPOS. 2002;6(6):348–353. doi: 10.1067/mpa.2002.129044. [DOI] [PubMed] [Google Scholar]
- Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet. 2012;379(9827):1739–1748. doi: 10.1016/S0140-6736(12)60272-4. [DOI] [PubMed] [Google Scholar]
- Multi-Ethnic Pediatric Eye Disease Study Prevalence and causes of visual impairment in African-American and Hispanic preschool children: the multi-ethnic pediatric eye disease study. Ophthalmology. 2009;116(10):1990–2000. e1991. doi: 10.1016/j.ophtha.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multi-ethnic Pediatric Eye Disease Study, G. Prevalence of amblyopia and strabismus in African American and Hispanic children ages 6 to 72 months the multi-ethnic pediatric eye disease study. Ophthalmology. 2008;115(7):1229–1236. e1221. doi: 10.1016/j.ophtha.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutti DO. To emmetropize or not to emmetropize? The question for hyperopic development. Optometry & Vision Science. 2007;84(2):97–102. doi: 10.1097/OPX.0b013e318031b079. [DOI] [PubMed] [Google Scholar]
- Mutti DO, Mitchell GL, Jones LA, Friedman NE, Frane SL, Lin WK, Zadnik K. Axial growth and changes in lenticular and corneal power during emmetropization in infants. Investigative Ophthalmology & Visual Science. 2005;46(9):3074–3080. doi: 10.1167/iovs.04-1040. [DOI] [PubMed] [Google Scholar]
- Mutti DO, Mitchell GL, Jones LA, Friedman NE, Frane SL, Lin WK, Zadnik K. Accommodation, acuity, and their relationship to emmetropization in infants. Optometry & Vision Science. 2009;86(6):666–676. doi: 10.1097/OPX.0b013e3181a6174f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastri G, Perugini GC, Savastano S, Polzella A, Sbordone G. The evolution of refraction in the fixing and the amblyopic eye. Documenta Ophthalmologica. 1984;56(3):265–274. doi: 10.1007/BF00159077. [DOI] [PubMed] [Google Scholar]
- Nathan J, Kiely PM, Crewther SG, Crewther DP. Disease-associated visual image degradation and spherical refractive errors in children. American Journal of Optometry & Physiological Optics. 1985;62(10):680–688. doi: 10.1097/00006324-198510000-00003. [DOI] [PubMed] [Google Scholar]
- National Eye Institute. Office of, B., & Epidemiology . Report on the National Eye Institute’s Visual Acuity Impairment Survey Pilot Study. Office of Biometry and Epidemiology, National Eye Institute, National Institutes of Health, Public Health Service, Dept. of Health and Human Services; Washington, D.C.: 1984. [Google Scholar]
- National Society to Prevent Blindness . Vision problems in the U.S.: data analysis. 1980. [Google Scholar]
- O’Connor AR, Birch EE, Anderson S, Draper H, Group FR. The functional significance of stereopsis. Investigative Ophthalmology & Visual Science. 2010;51(4):2019–2023. doi: 10.1167/iovs.09-4434. [DOI] [PubMed] [Google Scholar]
- O’Donoghue L, McClelland JF, Logan NS, Rudnicka AR, Owen CG, Saunders KJ. Refractive error and visual impairment in school children in Northern Ireland. British Journal of Ophthalmology. 2010;94(9):1155–9. doi: 10.1136/bjo.2009.176040. [DOI] [PubMed] [Google Scholar]
- O’Donoghue L, McClelland JF, Logan NS, Rudnicka AR, Owen CG, Saunders KJ. Profile of Anisometropia and aniso-astigmatism in children: prevalence and association with age, ocular biometric measures and refractive status. Investigative Ophthalmology & Visual Science. 2013;21(1):602–8. doi: 10.1167/iovs.12-11066. [DOI] [PubMed] [Google Scholar]
- O’Donoghue L, Rudnicka AR, McClelland JF, Logan NS, Owen CG, Saunders KJ. Refractive and corneal astigmatism in white school children in Northern Ireland. Investigative Ophthalmology & Visual Science. 2011;52(7):4048–53. doi: 10.1167/iovs.10-6100. [DOI] [PubMed] [Google Scholar]
- O’Leary DJ, Millodot M. Eyelid closure causes myopia in humans. Experientia. 1979;35(11):1478–1479. doi: 10.1007/BF01962795. [DOI] [PubMed] [Google Scholar]
- Ohlsson J, Villarreal G, Sjostrom A, Abrahamsson M, Sjostrand J. Visual acuity, residual amblyopia and ocular pathology in a screened population of 12-13-year-old children in Sweden. Acta Ophthalmologica Scandinavica. 2001;79(6) doi: 10.1034/j.1600-0420.2001.790609.x. [DOI] [PubMed] [Google Scholar]
- Ohlsson J, Villarreal G, Sjöström A, Abrahamsson M, Sjöstrand J. Visual acuity, residual amblyopia and ocular pathology in a screened population of 12-13-year-old children in Sweden. Acta Ophthalmologica Scandinavica. 2001;79(6):589–595. doi: 10.1034/j.1600-0420.2001.790609.x. doi: 10.1034/j.1600-0420.2001.790609.x. [DOI] [PubMed] [Google Scholar]
- Ohlsson J, Villarreal G, Sjostrom A, Cavazos H, Abrahamsson M, Sjostrand J. Visual acuity, amblyopia, and ocular pathology in 12- to 13-year-old children in Northern Mexico. Journal of AAPOS. 2003;7(1):47–53. doi: 10.1067/mpa.2003.S1091853102420113. [DOI] [PubMed] [Google Scholar]
- Ohlsson J, Villarreal G, Sjöström A, Cavazos H, Abrahamsson M, Sjöstrand J. Visual acuity, amblyopia, and ocular pathology in 12- to 13-year-old children in Northern Mexico. Journal of AAPOS. 2003;7(1):47–53. doi: 10.1067/mpa.2003.S1091853102420113. [DOI] [PubMed] [Google Scholar]
- Oliver M, Nawratzki I. Screening of pre-school children for ocular anomalies. II. Amblyopia. Prevalence and therapeutic results at different ages. British Journal of Ophthalmology. 1971;55(7):467–471. doi: 10.1136/bjo.55.7.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson CR, Freeman RD. Progressive changes in kitten striate cortex during monocular vision. Journal of Neurophysiology. 1975;38(1):26–32. doi: 10.1152/jn.1975.38.1.26. [DOI] [PubMed] [Google Scholar]
- Okamoto F, Nonoyama T, Hommura S. Mirror image myopic anisometropia in two pairs of monozygotic twins. Ophthalmologica. 2001;215(6):435–8. doi: 10.1159/000050904. [DOI] [PubMed] [Google Scholar]
- Oral Y, Ozgur OR, Akcay L, Ozbas M, Dogan OK. Congenital ptosis and amblyopia. Journal of Pediatric Ophthalmology & Strabismus. 2010;47(2) doi: 10.3928/01913913-20100308-08. [DOI] [PubMed] [Google Scholar]
- Otsuka J, Sato Y. Comparison of refraction in orthophoric hyperopic eyes and accommodative esotropic eyes. Nippon Ganka Gakkai Zasshi - Acta Societatis Ophthalmologicae Japonicae. 1984;88(1):58, 64. [PubMed] [Google Scholar]
- Ostadimoghaddam H, Fotouhi A, Hashemi H, Yekta AA, Heravian J, Hemmati B, Jafarzadehpur E, Rezvan F, Khabazkhoob M. The prevalence of anisometropia in population base study. Strabismus. 2012;20(4):152–7. doi: 10.3109/09273972.2012.680229. [DOI] [PubMed] [Google Scholar]
- Pai AS, Rose KA, Leone JF, Sharbini S, Burlutsky G, Varma R, Mitchell P. Amblyopia prevalence and risk factors in Australian preschool children. Ophthalmology. 2012;119(1):138–144. doi: 10.1016/j.ophtha.2011.06.024. [DOI] [PubMed] [Google Scholar]
- Pai AS, Wang JJ, Samarawickrama C, Burlutsky G, Rose KA, Varma R, Mitchell P. Prevalence and risk factors for visual impairment in preschool children the sydney paediatric eye disease study. Ophthalmology. 2011;118(8) doi: 10.1016/j.ophtha.2011.01.027. [DOI] [PubMed] [Google Scholar]
- Pan CW, Wong TY, Lavanya R, Wu RY, Zheng YF, Lin XY, Saw SM. Prevalence and risk factors for refractive errors in Indians: the Singapore Indian Eye Study (SINDI) Investigative Ophthalmology & Visual Science. 2011;52(6):3166–3173. doi: 10.1167/iovs.10-6210. [DOI] [PubMed] [Google Scholar]
- Parssinen O. Anisometropia and changes in anisometropia in school myopia. Optometry & Vision Science. 1990;67(4):256–259. doi: 10.1097/00006324-199004000-00005. [DOI] [PubMed] [Google Scholar]
- Pennie FC, Wood IC, Olsen C, White S, Charman WN. A longitudinal study of the biometric and refractive changes in full-term infants during the first year of life. Vision Research. 2001;41(21):2799–2810. doi: 10.1016/s0042-6989(01)00169-9. [DOI] [PubMed] [Google Scholar]
- Phelps WL, Muir J. Anisometropia and strabismus. American Orthoptic Journal. 1977;27:131–133. [PubMed] [Google Scholar]
- Phillips CI. Strabismus, anisometropia, and amblyopia. British Journal of Ophthalmology. 1959;43:449–460. doi: 10.1136/bjo.43.8.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JR. Monovision slows juvenile myopia progression unilaterally. British Journal of Ophthalmology. 2005;89(9):1196–1200. doi: 10.1136/bjo.2004.064212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineles SL, Demer JL. Bilateral abnormalities of optic nerve size and eye shape in unilateral amblyopia. American Journal of Ophthalmology. 2009;148(4):551–557. doi: 10.1016/j.ajo.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowski JT, Diehl NN, Mohney BG. Neonatal dacryostenosis as a risk factor for anisometropia. Archives of Ophthalmology. 2010;128(9):1166–9. doi: 10.1001/archophthalmol.2010.184. doi: 10.1001/archophthalmol.2010.184. [DOI] [PubMed] [Google Scholar]
- Pointer JS, Gilmartin B. Clinical characteristics of unilateral myopic anisometropia in a juvenile optometric practice population. Ophthalmic & Physiological Optics. 2004;24:458–463. doi: 10.1111/j.1475-1313.2004.00226.x. [DOI] [PubMed] [Google Scholar]
- Polat U, Bonneh Y, Ma-Naim T, Belkin M, Sagi D. Spatial interactions in amblyopia: effects of stimulus parameters and amblyopia type. Vision Research. 2005;45(11):1471–1479. doi: 10.1016/j.visres.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Preslan MW, Novak A. Baltimore Vision Screening Project. Ophthalmology. 1996;103(1):105–109. doi: 10.1016/s0161-6420(96)30753-7. [DOI] [PubMed] [Google Scholar]
- Preslan MW, Novak A. Baltimore Vision Screening Project. Phase 2. Ophthalmology. 1998;105(1):150–153. doi: 10.1016/s0161-6420(98)91813-9. [DOI] [PubMed] [Google Scholar]
- Pugh M. Visual distortion in amblyopia. British Journal of Ophthalmology. 1958;42(8):449–460. doi: 10.1136/bjo.42.8.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao-Grider Y, Hung LF, Kee CS, Ramamirtham R, Smith EL., 3rd Recovery from form-deprivation myopia in rhesus monkeys. Investigative Ophthalmology & Visual Science. 2004;45(10):3361, 3372. doi: 10.1167/iovs.04-0080. [DOI] [PubMed] [Google Scholar]
- Qin XJ, Margrain TH, To CH, Bromham N, Guggenheim JA. Anisometropia is independently associated with both spherical and cylindrical ametropia. Investigative Ophthalmology & Visual Science. 2005;46(11):4024–4031. doi: 10.1167/iovs.05-0120. [DOI] [PubMed] [Google Scholar]
- Quah BL, Tay MT, Chew SJ, Lee LK. A study of amblyopia in 18-19 year old males. Singapore Medical Journal. 1991;32(3):126–129. [PubMed] [Google Scholar]
- Quek TPL, Chua CG, Chong CS, Chong JH, Hey HW, Lee J, Saw S-M. Prevalence of refractive errors in teenage high school students in Singapore. Ophthalmic and Physiological Optics. 2004;24(1):47–55. doi: 10.1046/j.1475-1313.2003.00166.x. doi: 10.1046/j.1475-1313.2003.00166.x. [DOI] [PubMed] [Google Scholar]
- Quinn GE, Dobson V, Kivlin J, Kaufman LM, Repka MX, Reynolds JD, Stone RA, Cryotherapy for Retinopathy of Prematurity Cooperative Group Prevalence of myopia between 3 months and 5 1/2 years in preterm infants with and without retinopathy of prematurity. Ophthalmology. 1998;105(7):1292–1300. doi: 10.1016/s0161-6420(98)97036-1. [DOI] [PubMed] [Google Scholar]
- Quinn GE, Dobson V, Repka MX, Reynolds J, Kivlin J, Davis B, Palmer EA, The Cryotherapy for Retinopathy of Prematurity Cooperative Group Development of myopia in infants with birth weights less than 1251 grams. Ophthalmology. 1992;99(3):329–340. doi: 10.1016/s0161-6420(92)31968-2. [DOI] [PubMed] [Google Scholar]
- Rabin J, Bradley A, Freeman RD. On the relation between aniseikonia and axial anisometropia. American Journal of Optometry & Physiological Optics. 1983;60(7):553–558. doi: 10.1097/00006324-198307000-00001. [DOI] [PubMed] [Google Scholar]
- Rabin J, Van Sluyters RC, Malach R. Emmetropization: a vision-dependent phenomenon. Investigative Ophthalmology & Visual Science. 1981;20(4):561–564. [PubMed] [Google Scholar]
- Raviola E, Wiesel TN. Effect of dark-rearing on experimental myopia in monkeys. Investigative Ophthalmology & Visual Science. 1978;17(6):485–488. [PubMed] [Google Scholar]
- Raviola E, Wiesel TN. An animal model of myopia. New England Journal of Medicine. 1985;312(25):1609–1615. doi: 10.1056/NEJM198506203122505. [DOI] [PubMed] [Google Scholar]
- Raviola E, Wiesel TN. Neural control of eye growth and experimental myopia in primates. Ciba Foundation Symposium. 1990;155:22–38. doi: 10.1002/9780470514023.ch3. discussion 39-44. [DOI] [PubMed] [Google Scholar]
- Reinecke RD. Stereoacuity assessment in amblyopia. Transactions of the Ophthalmological Societies of the United Kingdom. 1979;99(3):398–400. [PubMed] [Google Scholar]
- Repka MX, Kraker RT, Beck RW, Holmes JM, Cotter SA, Birch EE, Astle WF, Chandler DL, Felius J, Arnold RW, Tien DR, Glaser SR, Pediatric Eye Disease Investigator Group A randomized trial of atropine vs patching for treatment of moderate amblyopia: follow-up at age 10 years. Archives of Ophthalmology. 2008;126(8):1039–44. doi: 10.1001/archopht.126.8.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repka MX, Simons K, Kraker R. Laterality of Amblyopia. American Journal of Ophthalmology. 2010;150(2):270–274. doi: 10.1016/j.ajo.2010.01.040. doi: 10.1016/j.ajo.2010.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repka MX, Wallace DK, Beck RW, Kraker RT, Birch EE, Cotter SA, Donahue S, Everett DF, Hertle RW, Holmes JM, Quinn GE, Scheiman MM, Weakley DR, Pediatric Eye Disease Investigator Group Two-year follow-up of a 6-month randomized trial of atropine vs patching for treatment of moderate amblyopia in children. Archives of Ophthalmology. 2005;123(2):149–57. doi: 10.1001/archopht.123.2.149. [DOI] [PubMed] [Google Scholar]
- Rezvan F, Khabazkhoob M, Fotouhi A, Hashemi H, Ostadimoghaddam H, Heravian J, Yekta AA. Prevalence of refractive errors among school children in Northeastern Iran. Ophthalmic and Physiological Optics. 2012;32(1):25–30. doi: 10.1111/j.1475-1313.2011.00879.x. doi: 10.1111/j.1475-1313.2011.00879.x. [DOI] [PubMed] [Google Scholar]
- Robaei D, Rose K, Ojaimi E, Kifley A, Huynh S, Mitchell P. Visual acuity and the causes of visual loss in a population-based sample of 6-year-old Australian children. Ophthalmology. 2005;112(7):1275–1282. doi: 10.1016/j.ophtha.2005.01.052. [DOI] [PubMed] [Google Scholar]
- Robaei D, Rose KA, Ojaimi E, Kifley A, Martin FJ, Mitchell P. Causes and associations of amblyopia in a population-based sample of 6-year-old Australian children. Archives of Ophthalmology. 2006;124(6):878–884. doi: 10.1001/archopht.124.6.878. [DOI] [PubMed] [Google Scholar]
- Robb RM. Refractive errors associated with hemangiomas of the eyelids and orbit in infancy. American Journal of Ophthalmology. 1977;83(1):52–58. doi: 10.1016/0002-9394(77)90191-x. [DOI] [PubMed] [Google Scholar]
- Rosman M, Wong TY, Koh CL, Tan DT. Prevalence and causes of amblyopia in a population-based study of young adult men in Singapore. American Journal of Ophthalmology. 2005;140(3):551–552. doi: 10.1016/j.ajo.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Rutstein RP, Corliss D. Relationship between anisometropia, amblyopia, and binocularity. Optometry & Vision Science. 1999;76(4):229–233. doi: 10.1097/00006324-199904000-00026. see comment. [DOI] [PubMed] [Google Scholar]
- Rutstein RP, Corliss DA. Long-term changes in visual acuity and refractive error in amblyopes. Optometry & Vision Science. 2004;81(7):510–515. doi: 10.1097/00006324-200407000-00012. [DOI] [PubMed] [Google Scholar]
- Sachsenweger R. Problems of organic lesions in functional amblyopia. In: Arruga A, editor. International Strabismus Symposium (University of Giessen, 1966) S Karger AG; Basel and New York: 1968. p. 63. [Google Scholar]
- Salleras A, JC O. d. Z. Recessive sex-linked inheritance of external ophthalmoplegia and myopia coincident with other dysplasias. British Journal of Ophthalmology. 1950;34(11):662–667. doi: 10.1136/bjo.34.11.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders KJ, Westall CA. Comparison between near retinoscopy and cycloplegic retinoscopy in the refraction of infants and children. Optom Vis Sci. 1992;69(8):615–622. doi: 10.1097/00006324-199208000-00004. [DOI] [PubMed] [Google Scholar]
- Saunders KJ. Early refractive development in humans. Survey of Ophthalmology. 1995;40(3):207–216. doi: 10.1016/s0039-6257(95)80027-1. [DOI] [PubMed] [Google Scholar]
- Saunders KJ, Woodhouse JM, Westall CA. Emmetropisation in human infancy: rate of change is related to initial refractive error. Vision Research. 1995;35(9):1325–1328. doi: 10.1016/0042-6989(94)00222-8. [DOI] [PubMed] [Google Scholar]
- Saw SM. A synopsis of the prevalence rates and environmental risk factors for myopia. Clinical & Experimental Optometry. 2003;86(5):289–294. doi: 10.1111/j.1444-0938.2003.tb03124.x. [DOI] [PubMed] [Google Scholar]
- Saw SM, Chan YH, Wong WL, Shankar A, Sandar M, Aung T, Wong TY. Prevalence and risk factors for refractive errors in the Singapore Malay Eye Survey. Ophthalmology. 2008;115(10):1713–1719. doi: 10.1016/j.ophtha.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Saw SM, Gazzard G, Koh D, Farook M, Widjaja D, Lee J, Tan DT. Prevalence rates of refractive errors in Sumatra, Indonesia. Investigative Ophthalmology & Visual Science. 2002;43(10):3174–3180. [PubMed] [Google Scholar]
- Sawada A, Tomidokoro A, Araie M, Iwase A, Yamamoto T, Tajimi Study, G. Refractive errors in an elderly Japanese population: the Tajimi study. Ophthalmology. 2008;115(2):363–370. e363. doi: 10.1016/j.ophtha.2007.03.075. [DOI] [PubMed] [Google Scholar]
- Schaffer DB, Quinn GE, Johnson L. Sequelae of arrested mild retinopathy of prematurity. Archives of Ophthalmology. 1984;102(3):373–376. doi: 10.1001/archopht.1984.01040030291021. [DOI] [PubMed] [Google Scholar]
- Schapero M. Amblyopia. Chilton Co.; Philidelphia: 1971. [Google Scholar]
- Scheiman MM, Hertle RW, Kraker RT, Beck RW, Birch EE, Felius J, Tamkins SM. Patching vs atropine to treat amblyopia in children aged 7 to 12 years: a randomized trial. Archives of Ophthalmology. 2008;126(12):1634–42. doi: 10.1001/archophthalmol.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid KL, Wildsoet CF. The sensitivity of the chick eye to refractive defocus. Ophthalmic & Physiological Optics. 1997a;17(1):61–67. [PubMed] [Google Scholar]
- Schmid KL, Wildsoet CF. Natural and imposed astigmatism and their relation to emmetropization in the chick. Experimental Eye Research. 1997b;64(5):837–47. doi: 10.1006/exer.1997.0282. [DOI] [PubMed] [Google Scholar]
- Sen DK. Anisometropic amblyopia. J Pediatr Ophthalmol Strabismus. 1980;17(3):180–184. doi: 10.3928/0191-3913-19800501-13. [DOI] [PubMed] [Google Scholar]
- Shah R, Edgar DF, Rabbetts R, Harle DE, Evans BJ. Standardized patient methodology to assess refractive error reproducibility. Optometry & Vision Science. 2009;86(5):517–528. doi: 10.1097/OPX.0b013e31819fa590. [DOI] [PubMed] [Google Scholar]
- Shankar S, Bobier WR. Corneal and lenticular components of total astigmatism in a preschool sample. Optometry & Vision Science. 2004;81(7):536–542. doi: 10.1097/00006324-200407000-00015. [DOI] [PubMed] [Google Scholar]
- Shaw DE, Fielder AR, Minshull C, Rosenthal AR. Amblyopia--factors influencing age of presentation. Lancet. 1988;2(8604):207–209. doi: 10.1016/s0140-6736(88)92301-x. [DOI] [PubMed] [Google Scholar]
- Shih YF, Lin LL, Peng Y, Ko LS. Clinical observations on occlusion myopia. Taiwan i Hsueh Hui Tsa Chih - Journal of the Formosan Medical Association. 1989;88(2):164–168. [PubMed] [Google Scholar]
- Shotton K, Powell C, Voros G, Hatt SR. Interventions for unilateral refractive amblyopia. Cochrane Database of Systematic Reviews. 2008;(4):CD005137. doi: 10.1002/14651858.CD005137.pub2. [DOI] [PubMed] [Google Scholar]
- Simons K. Amblyopia characterization, treatment, and prophylaxis. Survey of Ophthalmology. 2005;50(2):123–166. doi: 10.1016/j.survophthal.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Simons K, Preslan M. Natural history of amblyopia untreated owing to lack of compliance. British Journal of Ophthalmology. 1999;83(5):582–587. doi: 10.1136/bjo.83.5.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sireteanu R, Thiel A, Fikus S, Iftime A. Patterns of spatial distortions in human amblyopia are invariant to stimulus duration and instruction modality. Vision Research. 2008;48(9):1150–1163. doi: 10.1016/j.visres.2008.01.028. [DOI] [PubMed] [Google Scholar]
- Smith EL, 3rd, Harwerth RS, Crawford ML. Spatial contrast sensitivity deficits in monkeys produced by optically induced anisometropia. Investigative Ophthalmology & Visual Science. 1985;26(3):330–342. [PubMed] [Google Scholar]
- Smith EL, 3rd, Huang J, Hung LF, Blasdel TL, Humbird TL, Bockhorst KH. Hemiretinal form deprivation: evidence for local control of eye growth and refractive development in infant monkeys. Investigative Ophthalmology & Visual Science. 2009;50(11):5057–5069. doi: 10.1167/iovs.08-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, 3rd, Hung LF. Form-deprivation myopia in monkeys is a graded phenomenon. Vision Research. 2000;40(4):371–381. doi: 10.1016/s0042-6989(99)00184-4. [DOI] [PubMed] [Google Scholar]
- Smith EL, 3rd, Hung LF, Harwerth RS. Effects of optically induced blur on the refractive status of young monkeys. Vision Research. 1994;34(3):293–301. doi: 10.1016/0042-6989(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Smith EL, 3rd, Hung LF. The role of optical defocus in regulating refractive development in infant monkeys. Vision Research. 1999;39(8):1415–1435. doi: 10.1016/s0042-6989(98)00229-6. [DOI] [PubMed] [Google Scholar]
- Smith EL, 3rd, Hung LF, Harwerth RS. Developmental visual system anomalies and the limits of emmetropization. Ophthalmic & Physiological Optics. 1999;19(2):90–102. [PubMed] [Google Scholar]
- Smith EL, 3rd, Hung LF, Huang J, Blasdel TL, Humbird TL, Bockhorst KH. Effects of optical defocus on refractive development in monkeys: evidence for local, regionally selective mechanisms. Investigative Ophthalmology & Visual Science. 2010;51(8):3864–3873. doi: 10.1167/iovs.09-4969. doi: 10.1167/iovs.09-4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, 3rd, Ramamirtham R, Qiao-Grider Y, Hung LF, Huang J, Kee CS, Paysse E. Effects of foveal ablation on emmetropization and form-deprivation myopia. Investigative Ophthalmology & Visual Science. 2007;48(9):3914–3922. doi: 10.1167/iovs.06-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LK, Thompson JR, Woodruff G, Hiscox F. Social deprivation and age at presentation in amblyopia. Journal of Public Health Medicine. 1994;16(3):348–351. [PubMed] [Google Scholar]
- Snir M, Nissenkorn I, Sherf I, Cohen S, Ben Sira I. Visual acuity, strabismus, and amblyopia in premature babies with and without retinopathy of prematurity. Annals of Ophthalmology. 1988;20(7):256–258. [PubMed] [Google Scholar]
- Sorsby A, Leary GA, Richards MJ. Correlation ametropia and component ametropia. Vision Research. 1962a;2:309–313. [Google Scholar]
- Sorsby A, Leary GA, Richards MJ. The optical components in anisometropia. Vision Research. 1962b;2:43–51. [Google Scholar]
- Sorsby A, Sheridan M, Leary GA. Vision, visual acuity and ocular refraction of young men. British Medical Journal. 1960;1:1394–1398. doi: 10.1136/bmj.1.5183.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorsby A, Sheridan M, Leary GA. Spec Res Ser no. 303 Medical Research Council. Her Majesty’s stationery office; 1963. Refraction and its components in twins; p. 43. [Google Scholar]
- Stanković-Babić G, Vujanović M, Cekić S. Identical twins with “mirror image” anisometropia and esotropia. Srp Arh Celok Lek. 2011;139(9-10):661–5. [PubMed] [Google Scholar]
- Stark N, Walther C. Refractive errors, amblyopia and strabismus in congenital ptosis. Klinische Monatsblatter fur Augenheilkunde. 1984;184(1):37–39. doi: 10.1055/s-2008-1054405. [DOI] [PubMed] [Google Scholar]
- Stewart CE, Moseley MJ, Stephens DA, Fielder AR. Treatment dose-response in amblyopia therapy: the Monitored Occlusion Treatment of Amblyopia Study (MOTAS) Investigative Ophthalmology & Visual Science. 45(9):3048–3054. doi: 10.1167/iovs.04-0250. [DOI] [PubMed] [Google Scholar]
- Stewart CE, Fielder AR, Stephens DA, Moseley MJ. Treatment of unilateral amblyopia: factors influencing visual outcome. Investigative Ophthalmology & Visual Science. 2005;46(9):3152–3160. doi: 10.1167/iovs.05-0357. doi: 10.1167/iovs.05-0357. [DOI] [PubMed] [Google Scholar]
- Stewart CE, Stephens DA, Fielder AR, Moseley MJ. Objectively monitored patching regimens for treatment of amblyopia: randomised trial. British Medical Journal. 2007;335(7622):707. doi: 10.1136/bmj.39301.460150.55. doi: 10.1136/bmj.39301.460150.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stigmar G, Crawford JS, Ward CM, Thomson HG. Ophthalmic sequelae of infantile hemangiomas of the eyelids and orbit. American Journal of Ophthalmology. 1978;85(6):806–813. doi: 10.1016/s0002-9394(14)78109-7. [DOI] [PubMed] [Google Scholar]
- Tan CS, Chan YH, Wong TY, Gazzard G, Niti M, Ng TP, Saw SM. Prevalence and risk factors for refractive errors and ocular biometry parameters in an elderly Asian population: the Singapore Longitudinal Aging Study (SLAS) Eye. 2011;25(10):1294–1301. doi: 10.1038/eye.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanlamai T, Goss DA. Prevalence of monocular amblyopia among anisometropes. American Journal of Optometry & Physiological Optics. 1979;56(11):704–715. doi: 10.1097/00006324-197911000-00006. [DOI] [PubMed] [Google Scholar]
- Tanner JM. Physical growth and development. In: Forfar JO, Arneil GC, editors. Textbook of Paediatrics. 2nd Ed. Churchill Livingstone; London: 1978. [Google Scholar]
- Tarczy-Hornoch K, Varma R, Cotter SA, McKean-Cowdin R, Lin JH, Borchert MS, Giordano L. Risk Factors for Decreased Visual Acuity in Preschool Children: The Multi-Ethnic Pediatric Eye Disease and Baltimore Pediatric Eye Disease Studies. Ophthalmology. 2011;118(11):2262–2273. doi: 10.1016/j.ophtha.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K, Powell C, Hatt SR, Stewart C. Interventions for unilateral and bilateral refractive amblyopia. Cochrane Database Syst Rev. 2012;18(4):CD005137. doi: 10.1002/14651858.CD005137.pub3. doi: 10.1002/14651858.CD005137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Pediatric Eye Disease Investigator Group (PEDIG) A randomised trial of atropine vs patching for treatment of moderate amblyopia in children. Archives of Ophthalmology. 2002;120:268–278. doi: 10.1001/archopht.120.3.268. [DOI] [PubMed] [Google Scholar]
- Thibos LN, Wheeler W, Horner D. Power vectors: an application of Fourier analysis to the description and statistical analysis of refractive error. Optometry & Vision Science. 1997;74(6):367–375. doi: 10.1097/00006324-199706000-00019. [DOI] [PubMed] [Google Scholar]
- Thiel A, Sireteanu R. Strabismic amblyopes show a bilateral rightward bias in a line bisection task: Evidence for a visual attention deficit. Vision Research. 2009;49(3):287–294. doi: 10.1016/j.visres.2008.08.005. doi: 10.1016/j.visres.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Thomas S, Schaeffel F. Developmental compensation of imposed astigmatism is not initiated by astigmatic accommodation in chickens. Vision Research. 2000;40(26):3553–8. doi: 10.1016/s0042-6989(00)00209-1. [DOI] [PubMed] [Google Scholar]
- Tian Y, Tarrant J, Wildsoet CF. Optical and biometric characteristics of anisomyopia in human adults. Ophthalmic & Physiological Optics. 2011;31(5):540–549. doi: 10.1111/j.1475-1313.2011.00858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigges M, Tigges J, Fernandes A, Eggers HM, Gammon JA. Postnatal axial eye elongation in normal and visually deprived rhesus monkeys. Investigative Ophthalmology & Visual Science. 1990;31(6):1035–1046. [PubMed] [Google Scholar]
- Tong L, Chan YH, Gazzard G, Tan D, Saw SM. Longitudinal study of anisometropia in Singaporean school children. Investigative Ophthalmology & Visual Science. 2006;47(8):3247–3252. doi: 10.1167/iovs.05-0906. [DOI] [PubMed] [Google Scholar]
- Tong L, Saw SM, Chia KS, Tan D. Anisometropia in Singapore school children. American Journal of Ophthalmology. 2004;137(3):474–479. doi: 10.1016/j.ajo.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Townshend AM, Holmes JM, Evans LS. Depth of anisometropic amblyopia and difference in refraction. American Journal of Ophthalmology. 1993;116(4):431–436. doi: 10.1016/s0002-9394(14)71400-x. see comment. [DOI] [PubMed] [Google Scholar]
- Townshend AM, Holmes JM, Evans LS. Depth of anisometropic amblyopia and difference in refraction - reply. American Journal of Ophthalmology. 1994;117(5):681–682. doi: 10.1016/s0002-9394(14)70087-x. [DOI] [PubMed] [Google Scholar]
- Troilo D, Judge SJ. Ocular development and visual deprivation myopia in the common marmoset (Callithrix jacchus) Vision Research. 1993;33(10):1311–1324. doi: 10.1016/0042-6989(93)90039-y. [DOI] [PubMed] [Google Scholar]
- Troilo D, Totonelly K, Harb E. Imposed anisometropia, accommodation, and regulation of refractive state. Optometry & Vision Science. 2009;86(1):E31–39. doi: 10.1097/OPX.0b013e318194072e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaegan, Taylor D. Critical period for deprivation amblyopia in children. Transactions of the Ophthalmological Societies of the United Kingdom. 1979;99(3):432–439. [PubMed] [Google Scholar]
- Vanathi M, Tandon R, Titiyal JS, Vajpayee RB. Case series of 12 children with progressive axial myopia following unilateral cataract extraction. Journal of AAPOS. 2002;6(4):228–232. doi: 10.1067/mpa.2002.123658. [DOI] [PubMed] [Google Scholar]
- Varghese RM, Sreenivas V, Puliyel JM, Varughese S. Refractive status at birth: its relation to newborn physical parameters at birth and gestational age. PLoS ONE [Electronic Resource] 2009;4(2):e4469. doi: 10.1371/journal.pone.0004469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent S, Collins M, Read S, Carney L, Yap M. Interocular symmetry in myopic anisometropia. Optometry & Vision Science. 2011;88(12):1454–1462. doi: 10.1097/OPX.0b013e318233ee5f. [DOI] [PubMed] [Google Scholar]
- von Noorden GK. Mechanisms of amblyopia. Adv Ophthalmol. 1977;34:93–115. [PubMed] [Google Scholar]
- von Noorden GK. Application of basic research data to clinical amblyopia. Ophthalmology. 1978;85(5):496–504. doi: 10.1016/s0161-6420(78)35652-9. [DOI] [PubMed] [Google Scholar]
- von Noorden GK. In: Binocular Vision and Ocular Motility Theory and Management of Strabismus. Campos EC, editor. 2002. [Google Scholar]
- von Noorden GK, Lewis RA. Ocular axial length in unilateral congenital cataracts and blepharoptosis. Investigative Ophthalmology & Visual Science. 1987;28(4):750–752. [PubMed] [Google Scholar]
- Voo I, Lee DA, Oelrich FO. Prevalences of ocular conditions among Hispanic, white, Asian, and black immigrant students examined by the UCLA Mobile Eye Clinic. Journal of the American Optometric Association. 1998;69(4):255–261. [PubMed] [Google Scholar]
- Wallace DK, Pediatric Eye Disease Investigator Group. Edwards AR, Cotter SA, Beck RW, Arnold RW, Weise KK. A randomized trial to evaluate 2 hours of daily patching for strabismic and anisometropic amblyopia in children. Ophthalmology. 2006;113(6):904–12. doi: 10.1016/j.ophtha.2006.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallman J, Gottlieb MD, Rajaram V, Fugate-Wentzek LA. Local retinal regions control local eye growth and myopia. Science. 1987;237(4810):73–77. doi: 10.1126/science.3603011. [DOI] [PubMed] [Google Scholar]
- Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43(4):447–468. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liang YB, Sun LP, Duan XR, Yuan RZ, Wong TY, Wang JJ. Prevalence and causes of amblyopia in a rural adult population of Chinese the Handan Eye Study. Ophthalmology. 2011;118(2):279–283. doi: 10.1016/j.ophtha.2010.05.026. doi: 10.1016/j.ophtha.2010.05.026. [DOI] [PubMed] [Google Scholar]
- Weakley DR. The association between anisometropia, amblyopia, and binocularity in the absence of strabismus. Transactions of the American Ophthalmological Society. 1999;97:987–1021. [PMC free article] [PubMed] [Google Scholar]
- Weakley DR., Jr. The association between nonstrabismic anisometropia, amblyopia, and subnormal binocularity. Ophthalmology. 2001;108(1):163–171. doi: 10.1016/s0161-6420(00)00425-5. see comment. [DOI] [PubMed] [Google Scholar]
- Weakley DR, Jr., Birch E. The role of anisometropia in the development of accommodative esotropia. Transactions of the American Ophthalmological Society. 2000;98:71–76. discussion 76-79. [PMC free article] [PubMed] [Google Scholar]
- Weakley DR, Jr., Birch E, Kip K. The role of anisometropia in the development of accommodative esotropia. Journal of AAPOS. 2001;5(3):153–157. doi: 10.1067/mpa.2001.114662. [DOI] [PubMed] [Google Scholar]
- Weale RA. On the age-related prevalence of anisometropia. Ophthalmic Research. 2002;34(6):389–392. doi: 10.1159/000067040. [DOI] [PubMed] [Google Scholar]
- Weale RA. Epidemiology of refractive errors and presbyopia. Survey of Ophthalmology. 2003;48(5):515–543. doi: 10.1016/s0039-6257(03)00086-9. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Single-Cell Responses in Striate Cortex of Kittens Deprived of Vision in One Eye. Journal of Neurophysiology. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. Journal of Neurophysiology. 1965;28(6):1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Raviola E. Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature. 1977;266(5597):66–68. doi: 10.1038/266066a0. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Raviola E. Increase in axial length of the macaque monkey eye after corneal opacification. Investigative Ophthalmology & Visual Science. 1979;18(12):1232–1236. [PubMed] [Google Scholar]
- Wildsoet C. Neural pathways subserving negative lens-induced emmetropization in chicks--insights from selective lesions of the optic nerve and ciliary nerve. Current Eye Research. 2003;27(6):371–385. doi: 10.1076/ceyr.27.6.371.18188. [DOI] [PubMed] [Google Scholar]
- Williams C, Harrad RA, Sparrow JM, Harvey I, Golding J. Future of preschool vision screening. Conclusions for or against services are invalid without appropriate research evidence. British Medical Journal. 1998;316(7135):937. doi: 10.1136/bmj.316.7135.937a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C, Northstone K, Harrad RA, Sparrow JM, Harvey I, Team AS. Amblyopia treatment outcomes after screening before or at age 3 years: follow up from randomised trial. British Medical Journal. 2002;324(7353):1549. doi: 10.1136/bmj.324.7353.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C, Northstone K, Howard M, Harvey I, Harrad RA, Sparrow JM. Prevalence and risk factors for common vision problems in children: data from the ALSPAC study. British Journal of Ophthalmology. 2008;92(7):959–964. doi: 10.1136/bjo.2007.134700. doi: 10.1136/bjo.2007.134700. [DOI] [PubMed] [Google Scholar]
- Williamson TH, Andrews R, Dutton GN, Murray G, Graham N. Assessment of an inner city visual screening programme for preschool children. British Journal of Ophthalmology. 1995;79(12):1068–1073. doi: 10.1136/bjo.79.12.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JR, Fernandes A, Chandler CV, Tigges M, Boothe RG, Gammon JA. Abnormal development of the axial length of aphakic monkey eyes. Investigative Ophthalmology & Visual Science. 1987;28(12):2096–2099. [PubMed] [Google Scholar]
- Wong TY, Foster PJ, Hee J, Ng TP, Tielsch JM, Chew SJ, Seah SK. Prevalence and risk factors for refractive errors in adult Chinese in Singapore. Investigative Ophthalmology & Visual Science. 2000;41(9) [PubMed] [Google Scholar]
- Wood IC, Hodi S, Morgan L. Longitudinal change of refractive error in infants during the first year of life. Eye (Lond) 1995;9(Pt 5):551–557. doi: 10.1038/eye.1995.138. doi: 10.1038/eye.1995.138. [DOI] [PubMed] [Google Scholar]
- Woodruff G, Hiscox F, Thompson JR, Smith LK. The presentation of children with amblyopia. Eye (Lond) 1994;8(Pt 6):623–626. doi: 10.1038/eye.1994.156. doi: 10.1038/eye.1994.156. [DOI] [PubMed] [Google Scholar]
- Woodruff ME, Samek MJ. A study of the prevalence of spherical equivalent refractive states and anisometropia in Amerind populations in Ontario. Can J Public Health. 1977;68(5):414–424. [PubMed] [Google Scholar]
- Wu HM, Casson RJ, Newland HS, Muecke J, Selva D, Aung T. Anisometropia in an adult population in rural myanmar: the Meiktila Eye Study. Ophthalmic Epidemiology. 2008;15(3) doi: 10.1080/09286580701843796. doi: 10.1080/09286580701843796. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Watanabe S, Ohba N. A longitudinal study of cycloplegic refraction in a cohort of 350 Japanese schoolchildren. Anisometropia. Ophthalmic & Physiological Optics. 1999;19(1):30–33. doi: 10.1046/j.1475-1313.1998.00407.x. [DOI] [PubMed] [Google Scholar]
- Yassur Y, Yassur S, Zaifrani S, Sachs U, Ben-Sira I. Amblyopia among African pupils in Rwanda. British Journal of Ophthalmology. 1972;56(4) doi: 10.1136/bjo.56.4.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yekta A, Fotouhi A, Hashemi H, Dehghani C, Ostadimoghaddam H, Heravian J, Khabazkhoob M. Prevalence of refractive errors among schoolchildren in Shiraz, Iran. Clinical & Experimental Ophthalmology. 2010;38(3):242–248. doi: 10.1111/j.1442-9071.2010.02247.x. [DOI] [PubMed] [Google Scholar]
- Yen MY, Cheng CY, Wang AG. Retinal nerve fiber layer thickness in unilateral amblyopia. Investigative Ophthalmology & Visual Scienc. 2004;45(7):2224–30. doi: 10.1167/iovs.03-0297. [DOI] [PubMed] [Google Scholar]
- Zaka-Ur-Rab S. Evaluation of relationship of ocular parameters and depth of anisometropic amblyopia with the degree of anisometropia. Indian Journal of Ophthalmology. 2006;54(2):99–103. doi: 10.4103/0301-4738.25830. [DOI] [PubMed] [Google Scholar]
- Zhang M-Z, Saw S-M, Hong R-Z, Fu Z-F, Yang H, Shui Y-B, Chew S-J. Refractive Errors in Singapore and Xiamen, China-A Comparative Study in School Children Aged 6 to 7 Years. Optometry & Vision Science. 2000;77(6):302–308. doi: 10.1097/00006324-200006000-00010. [DOI] [PubMed] [Google Scholar]
- Zhao J, Pan X, Sui R, Munoz SR, Sperduto RD, Ellwein LB. Refractive error study in children: results from Shunyi District, China. American Journal of Ophthalmology. 2000;129(4):427–435. doi: 10.1016/s0002-9394(99)00452-3. [DOI] [PubMed] [Google Scholar]
- Zhong X, Ge J, Nie H, Smith EL. Compensation for Experimentally Induced Hyperopic Anisometropia in Adolescent Monkeys. Investigative Ophthalmology & Visual Science. 2004;45(10):3373–3379. doi: 10.1167/iovs.04-0226. doi: 10.1167/iovs.04-0226. [DOI] [PubMed] [Google Scholar]
- Zonis S, Miller B. Refractions in Israeli newborn. Journal of Pediatric Ophthalmology & Strabismus. 1974;11(2):77–81. [Google Scholar]