Abstract
Prostate cancer is the most prevalent cancer in males in Western countries. The reported incidence in Asia is much lower than that in African Americans and European Caucasians. Although the lack of systematic prostate cancer screening system in Asian countries explains part of the difference, this alone cannot fully explain the lower incidence in Asian immigrants in the United States and west-European countries compared to the black and non-Hispanic white in those countries, nor the somewhat better prognosis in Asian immigrants with prostate cancer in the United States. Soy food consumption, more popular in Asian populations, is associated with a 25% to 30% reduced risk of prostate cancer. Prostate-specific antigen (PSA) is the only established and routinely implemented clinical biomarker for prostate cancer detection and disease status. Other biomarkers, such as urinary prostate cancer antigen 3 RNA, may increase accuracy of prostate cancer screening compared to PSA alone. Several susceptible loci have been identified in genetic linkage analyses in populations of countries in the West, and approximately 30 genetic polymorphisms have been reported to modestly increase the prostate cancer risk in genome-wide association studies. Most of the identified polymorphisms are reproducible regardless of ethnicity. Somatic mutations in the genomes of prostate tumors have been repeatedly reported to include deletion and gain of the 8p and 8q chromosomal regions, respectively; epigenetic gene silencing of glutathione S-transferase Pi (GSTP1); as well as mutations in androgen receptor gene. However, the molecular mechanisms underlying carcinogenesis, aggressiveness, and prognosis of prostate cancer remain largely unknown. Gene-gene and/or gene-environment interactions still need to be learned. In this review, the differences in PSA screening practice, reported incidence and prognosis of prostate cancer, and genetic factors between the populations in East and West factors are discussed.
Keywords: Prostate cancer, epidemiology, Asia, ethnic difference
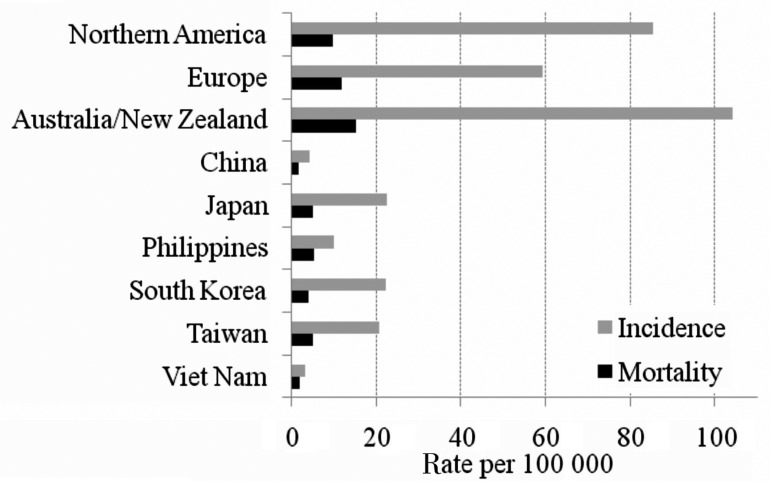
Prostate cancer is the most frequent cancer among males in economically developed countries[1]. A total of 903 500 prostate cancer patients were diagnosed in 2008, accounting for 14% of the total new cancer cases in the world. Prostate cancer was also the 6th leading cause of cancer deaths in males in 2008. The disease is well known to be more prevalent in Western countries, including Oceanian, North American, and European countries, than in Asian countries (Figure 1). However, there are many factors to consider when comparing the incidence and mortality of prostate cancer across countries. In this review, the factors that impact cross-country comparisons include prostate-specific antigen (PSA) screening practice and genetic background.
Figure 1. Age-standardized incidence and mortality of prostate cancer in selected countries.
Data were obtained from GLOBOCAN 2008 [http://globocan.iarc.fr/]. Incidence and mortality in all ages (0 to 75 years) were standardized using the world standard population.
Reported Incidence and Prostate-Specific Antigen Screening
Despite the limited specificity on detecting true cancer cases, prostate-specific antigen (PSA) is the only established and routinely implemented clinical biomarker. PSA level and its change from the baseline can be a signal of prostate cancer development, progression, recurrence, and efficacy measure of medical treatments. PSA-based cancer screening, however, still varies in practice by country. The European Randomized Study of Screening for Prostate Cancer (ERSPC) reported that PSA-based screening significantly reduced prostate cancer mortality[2]–[4]. Also, population-based studies in Tyrol showed PSA-based screening can reduce prostate cancer mortality[5],[6]. However, the results are still controversial[7]. In the future, cancer screening programs with better accuracy and cost-efficiency may be implemented more widely by combining PSA with urinary biomarker(s), e.g. prostate cancer antigen 3 (PCA3)[8],[9], but is currently not officially recommended and the cost of the test is not always reimbursed to all men worldwide.
The reported incidence would be lower in those countries without a systematic prostate cancer screening program. Knowledge and access to the PSA-based cancer screening would impact the detection rate of prostate cancer that might have otherwise not been diagnosed, resulting in an earlier stage at diagnosis[10]–[14]. Here, the relationship between the PSA screening practice and reported incidence in populations in mainland China, Japan, and Korea is discussed. In these representative Asian countries, prostate cancer and its screening were off the radar probably because of the relatively low reported incidence and slower progress compared to the other cancers. However, Asian immigrants in the United States, Canada, Australia, and west-European countries, where they could have better access to PSA screening, still show a lower incidence compared to the black and European Caucasian living in the same regions.
In China and Japan, the nationwide prostate cancer screening rate is unknown. Tianjin, the third largest city in China, is a member of the International Agency for Research on Cancer. The incidence of prostate cancer in Tianjin is significantly increasing[15] but is still low at 2.84 per 100 000 in 2004[16]. Considering the high mortality/incidence (M/I) ratio (0.68 in Qidong, Jiangsu province, China in 1978–2002[17] and 0.42 in GLOBOCAN 2008, each higher than the rate of 0.12 in Northern America, Figure 1), Chinese patients with prostate cancer in mainland China may still be diagnosed in relatively advanced stage[18]. In Japan, the estimated age-standardized incidence rates increased until 2003, when the National Cancer Center changed the estimation methods, after which rates became stable[19] (age-standardized rate using world population was 27.3 per 100 000 in 2003 and 27.1 per 100 000 in 2006[20],[21]). In regional cancer registries, the stage at diagnosis was not reported in 35% to 40% of prostate cancer cases, but distant metastasis at diagnosis was reported in 15% to 17% of the remaining cases[20],[22]–[24]. In Korea, Park et al.[25] mentioned as “unpublished data” that PSA screening is not common in Korea, and a telephone survey of 700 men older than 50 years in a small city revealed that approximately 15% had been screened for prostate cancer during the previous two years. However, the age-standardized incidence of prostate cancer in Korea has dramatically increased from 8.5 per 100 000 in 1999 to 23.1 per 100 000 in 2008[26].
Several studies reported that the incidence of prostate cancer in Asian immigrants living in North America[27]–[30] and European countries[31]–[35] was much higher than that in their countries of birth. Could this be because of the better access to the PSA screening in the Western countries?
Many European countries do not offer routine PSA screening; however, the incidence is much higher than that in Asian countries (Figure 1). In United Kingdom, all men are enabled to make an informed choice about PSA screening. In 2007, the screening rate and age-standardized incidence was estimated as 6.2% in men aged 45 to 89[36] and 100.5 per 100 000, respectively. It is estimated that if population-based PSA screening were introduced, prostate cancer diagnosis rates in men aged 50 to 69 years would increase more than 20-fold compared to the current rates[11].
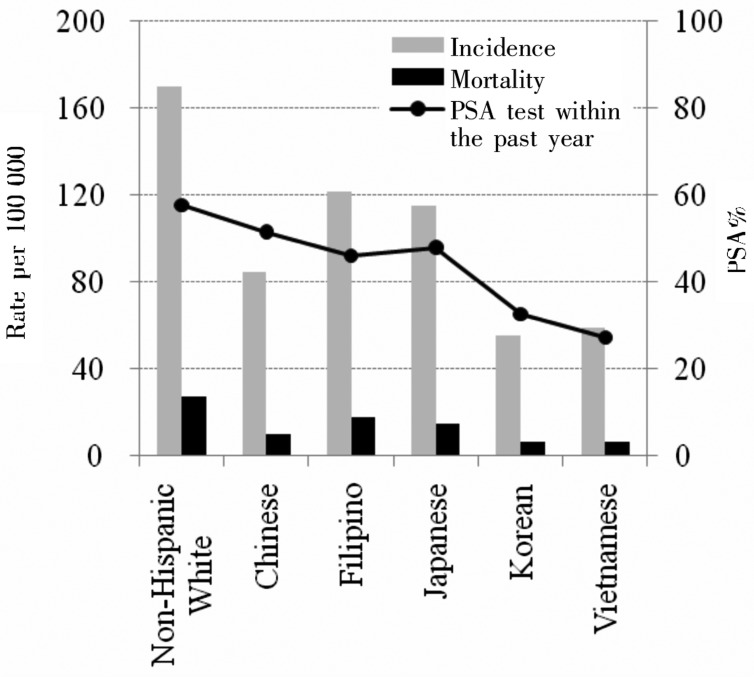
On the other hand, there are countries which have higher PSA screening rate as well as higher prostate cancer incidence rate. In the Unites States, all men over 50 years are recommended to have an annual PSA test. The Behavioral Risk Factor Surveillance System of 2008 showed that 54.8% (median of states' statistics) of men aged 40 years or older underwent PSA screening in the previous two years[37]. In Canada, 24.7% of men aged 40 years or older and 34.1% of men aged 50 years or older received PSA screening less than 1 year prior to the Canadian Community Health Survey of 2000–2001 despite PSA testing not being generally recommended. In Australia, the PSA screening rate was 21% to 25% in men aged 50–79 years in 2008–2009[38]. In these countries, the incidence of prostate cancer peaked before 1995 and then decreased, followed by a relatively stable incidence[39]. However, screening rates for visible minorities did not follow the same trend in these countries. In Canada, visible minorities (three largest groups are Chinese, South Asian, and black) had lower lifetime PSA screening rates compared to whites (30.4% vs. 44.7%)[40]. Australian immigrants from East Asia had significantly lower PSA screening rates than Australian-born men (odds ratio, 0.4; 95% confidence interval, 0.3–0.6)[41]. According to the California Health Interview Survey[28], the PSA screening rate within the past year in men aged 50 years or older was higher in non-Hispanic whites (57.7%) but not too different from the rates in Asian Americans: Chinese (51.6%), Filipino (46.1%), and Japanese (48.0%). However, the incidence and mortality of prostate cancer in Chinese, Filipino, and Japanese living in the US was 1/2 to 3/4 compared to those in non-Hispanic white based on Surveillance, Epidemiology, and End Results (SEER) data from 1998–2002[27] (Figure 2). Rates in Koreans (32.7%) and Vietnamese (27.3%) were low, as was the incidence in these populations. In the military-based Center for Prostate Disease Research (CPDR), where all men underwent mandatory annual screening with an equal access healthcare system, 5% of patients registered database were Asian despite the fact that only 3.4% of the military population is Asian[30], indicating American men of Asian descent might not have lower incidence of prostate cancer compared to Caucasians.
Figure 2. Age-standardized incidence and mortality of prostate cancer and PSA screening rates in Asians in the United States.
Incidence and mortality in the US population (non-Hispanic whites and Asian Americans) standardized by using the 2000 US Standard Population and 95% confidence interval were obtained from Miller et al.[27] based on Surveillance, Epidemiology, and End Results data from 1998–2002. The PSA screening rates in men aged 50 years and older who had heard of and underwent the PSA test was obtained from California Health Interview Survey of 2003[28].
In summary, as illustrated in Figure 1, the reported incidence and mortality is much lower in Asian countries compared to the countries in North America, Europe, and Oceania. Also, the incidence in Asian immigrants in Western countries had higher incidence of prostate cancer compared to those in their countries of birth. The PSA screening rate seems to be low in Asian countries and some of the Asian populations in Western countries, which may partially explain the low incidence in Asian populations. However, as illustrated in Figure 2, American Asians in California with comparable PSA screening rate still had a lower incidence compared to non-Hispanic white. Therefore, the low PSA screening rate is not the only reason of lower incidence in Asian. It is also possible that elderly migrants might leave to their countries of birth, which may lead to a relatively smaller proportion of elderly population in these countries[42], and/or patients with cancer might be more prone to leave on diagnosis of cancer. Therefore, it is challenging to generalize the relationship between the PSA screening rate and reported incidence and underlying reasons of the difference in populations between East and West.
Survival and Prognostic Differences between Asian and US Prostate Cancer Patients
The differences between East and West are not only in the PSA screening rate and the reported incidence of prostate cancer but also in clinical conditions at diagnosis and survival. Several studies suggested that Asian prostate cancer patients are prone to present with higher stages/worse grades at diagnosis but had similar or even better prognosis[43]–[49] (Table 1). Several background factors impact the outcomes, including age at diagnosis, clinical stage at diagnosis, Gleason scores, choice of therapy, and comorbidities. For example, Huang et al.[50] compared the clinical outcomes of Taiwanese men with localized prostate cancer who underwent radical prostatectomy in Taiwan with similar studies in the United States and the European Union and reported inferior outcomes for Taiwanese patients, largely because of delayed surgery at higher PSA level. The 5-year survival in Japanese prostate cancer patients diagnosed between 1993 and 1996 (observed and relative survival of 50.2% and 67.6%, respectively)[51] was much worse than that in Japanese Americans diagnosed between 1988 and 1994 (91.1%)[47]. Notably, the relative survival in Japanese patients with distant metastasis was low (35.2% and 39.6% in patients diagnosed between 1993 and 1996 and between 1997 and 1999, respectively)[52]. On the other hand, under an equal access healthcare system, Asian Americans were diagnosed at significantly younger age (mean of 66.4 years in Caucasians vs. 62.4 years in Asians), had lower clinical stage (but worse biopsy grade), and experienced improved overall survival rates (hazard ratio for Caucasians was 2.9, 95% CI 1.8–4.8, compared to Asians)[30].
Table 1. Racial/ethnic difference in outcomes of prostate cancer patients.
| Author (country) | Year of diagnosis | Patient population | Baseline difference (Asian vs. white) | Follow-up | Outcomes (Asian vs. white) |
| Man[43] (Canada) | 1994– | Radical radiotherapy, 63 Asian and 1,804 non-Asian | Greater % of Asian patients present with high risk CaP | Median 33 mo | No significant difference in time to first biochemical failure (P = 0.7 for log-rank test) and cause specific survival (P = 0.4 for log-rank test) after radiotherapy |
| Oakley-Girvan[44] (US, Canada) | 1987–1991 | Population-based cancer registry < 85 years: 484 White, 396 North America-born Asian, 157 Foreign-born Asian | Foreign-born Asian were more likely to be diagnosed with advance cancer | Till end of 1998 | 95% CI for death rate ratio crosses 1 with or without adjustment for age, SES, and comorbidity. |
| Robbins[45] (US, California) | 1995–2004 | Population-based cancer registry: 108 076 White, 8 840 Asian (Chinese, Filipino, Japanese, Korean, South Asian, Vietnamese) | Asian had risk profile at diagnosis for survival disadvantage | Till end of 2004 | Multivariate hazard ratios for death (and 95% CI) referent to white were: Chinese, 0.51 (0.43–0.62) Japanese, 0.59 (0.51–0.70) Filipino, 0.49 (0.37–0.65) Korean, 0.60 (0.37–0.98) |
| Cohen[46] (US) | 1986–1996 | SEER/Medicare, localized CaP aged 65–84; 23 353 white and 566 Asian | Asian presented with higher grade disease | Till end of 1998 | Multivariate hazard ratio for disease recurrence in Asian was 0.97 (95% CI, 0.68–1.38) |
| Holmes[48] (US) | 1992–1999 | SEER/Medicare, locoregional CaP ≥ 65 years: 53 764 Caucasians, 1 830 Asians | Higher % of Asian presented with worse biopsy grades | Till end of 2003 | Multivariable hazard ratio for overall survival was 37% lower in Asian |
| Lin[47] (US) | 1988–1994 | SEER; 93 767 white, 978 Chinese, 1 872 Japanese, and 1 417 Filipino | Filipino were more likely to be diagnosed with advanced stage | Till end of 1997 | Cause-specific 5-year survival and 95% CI were: White, 89.3% (89.1 %–89.6%) Chinese, 91.4% (89.3%–93.4%) Japanese, 91.1% (89.6%–92.5%) Filipino, 85.8% (83.8%–87.9%) |
| Raymundo[30] (US) | 1989–2007 | Military-based cancer registry; 8 335 Caucasians and 583 Asians | Asian American had lower clinical stage but worse biopsy grade | Till Nov 2008 | Multivariate hazard ratio for overall survival in white referent to Asian was 2.92 (1.78–4.79) |
| Fukagai[49](US, Hawaii) | 1992–2001 | 59 Caucasian and 105 Japanese American CaP with hormonal therapy at one center | No statistical difference but tended to higher PSA level and Gleason Scores in Japanese American | Till end of 2001 | Japanese American had significantly better overall (P = 0.001 for log-rank test) and cause-specific survival (P = 0.036 for log-rank test). |
CaP, prostate cancer patients; 95% CI, 95% confidence interval; SES, socioeconomic status defined as census education and census poverty; SEER, surveillance, epidemiology, and end results program; RP, radical prostatectomy.
Nutrition Factors and Genetic Susceptibility of Prostate Cancer in Asians and Caucasians
As indicated previously, the Asian immigrants in the Western countries had higher incidence of prostate cancer compared to those in their countries of birth. It might be because of the different medical systems, but diet could also be attributable. Generally, it is speculated that the westernized diet in Asian countries may be related to the elevated risk of prostate cancer, but it is challenging to separately discuss the impact of diet from the improvement of medical practice and detection methods. Soy foods are popular among Asian culture and it is interesting that the soy foods, especially nonfermented soy foods, have been consistently reported to be associated with a 25%–30% reduced risk of prostate cancer[53]–[55].
The etiology of prostate cancer remains largely unknown, but considering that the family history is one of the established risk factors for prostate cancer[56], and that gene and/or environmental factor should be involved in its etiology. An individual with a positive family history has a 2–3 times higher risk of having prostate cancer[57]–[59], and 10%–20% of prostate cancer cases are estimated to be such non-sporadic prostate cancer. Lee et al.[60] reported that 11.5% (25/218) of Korean patients with prostate cancer diagnosed and/or treated in a large hospital during a three-month study period had a positive family history. The International Consortium for Prostate Cancer Genetics conducted combined linkage analyses on a large number of families (mainly white) with prostate cancer[61],[62]. These studies showed a significant linkage at 22q12 and several other regions with “suggestive” linkage. There are few linkage studies in Asian populations. Matsui et al.[63] reported a nominal linkage at chromosome 8p23 and 1p36 in Japanese.
Considering the relatively late onset of the disease and low reported incidence rate 20–30 years ago in Asia, collecting familial genomic samples may be challenging. Case-control studies on candidate genes may have greater power compared to linkage analysis, but the results have been largely controversial[64]. One candidate gene is 2′-5′-oligoadenylate-dependent RNase L (RNASEL), which is located in the hereditary prostate cancer (HPC) 1 region (1q24-25). A meta-analysis showed that the Glu allele for the Asp541Glu polymorphism was associated with an increased risk in Caucasians[65], whereas a small Japanese study suggested a protective effect for Gln/Gln genotype[66]. Another meta-analysis on elaC homolog-2 (ELAC2) gene/HPC2 at 17p11 indicated that the Ser allele of Ser217Leu and the Ala allele of the Ala541Thr polymorphisms significantly increased prostate cancer risk in Asians but had only marginal impact in Caucasians[67]. The Gln allele of the Arg399Gln polymorphism of the X-ray repair cross-complementing group 1 (XRCC1) gene may be associated with a higher prostate cancer risk in Asians but not in Caucasians[68],[69]. A meta-analysis quantified (CAG)n and (GGN)n repeat polymorphisms in androgen receptor (AR) gene and concluded that although shorter repeats modestly associated with prostate cancer risk, the absolute difference was less than one repeat between cases and controls[70]. The polymorphisms on vitamin D receptor (VDR) gene[71], steroid 5-α -reductase type 2 (SRD5A2) gene[72],[73], and genes on folate-pathway (e.g. MTHFR)[74] were not significantly associated with prostate cancer risk in meta-analyses in Caucasians or in Asians. Interestingly, patients with diabetes mellitus have lower risk of prostate cancer compared with those without diabetes mellitus in European Americans (relative risk, 0.65; 95% CI, 0.50–0.84), as well as in Japanese Americans (RR, 0.80; 95% CI, 0.69–0.96)[75], probably based on somewhat protective effects of diabetes-susceptible SNPs[76].
Although a study of twins from Sweden, Denmark, and Finland suggested that an estimated 42% of prostate cancer risk can be explained by heritable factors[77], risk alleles may be rather common and weakly penetrant[78]. In order to differentiate high-risk men, several but not single candidate genetic polymorphisms may need to be combined with family history[79].
Many genome-wide association studies (GWAS) have been conducted, mainly in Caucasians. A meta-analysis of 21 studies found significant association between 31 single nucleotide polymorphisms (SNP) and prostate cancer[80]. Among 71 subgroups of studied population, only two were executed in Asians (Chinese Americans and Japanese Americans)[81],[82], and associations with some of the 31 SNPs disappeared in Asian subgroup analysis. However, a large Japanese GWAS study, which was not included in this meta-analysis, showed significant relationships between prostate cancer risk and most of those SNPs[83], including the ones on chromosome 8q24, an established prostate cancer susceptibility locus[84]. These genetic polymorphisms can work interactively with each other as well as with environmental factors; however, Lindstrom et al.[85] reported that based on the US National Cancer Institute Breast and Prostate Cancer cohort consortium data, these SNPs were, rather, independent risk factors and that there is little evidence of such interaction.
According to a combined analysis of comparative genomic hybridization (CGH) studies, chromosome 8p and 8q were the most commonly deleted and gained regions in the genome of prostate tumors, respectively[86]. Ethnic difference in CGH between Asians and Caucasians remains to be learned[87]. Gene silencing by CpG island hypermethylation in the GSTP1 promoter region occurs in over 90% of prostate cancers[88]. Also, other somatic mutations, including AR “activating” mutations[89], have been reported. However, none of these markers have yet been employed routinely in clinical practice. Ethnic sensitivities on mutation sites, frequencies, and clinical implications remain unclear.
Conclusions
The epidemiology of prostate cancer has changed dramatically since implementation of PSA-based screening in some Western countries[10]. The reported incidence of prostate cancer in Asian men is currently much lower than that in Asian immigrants, African Americans, and European Caucasians in Western countries, but it is increasing probably along with the change of medical practice, diet, and awareness of the disease. Many susceptible loci and genetic polymorphisms have been reported to modestly increase the risk of prostate cancer. No genetic and somatic biomarkers other than PSA have been established for segregating patient population according to disease aggressiveness, recurrence rate, responsiveness to treatments, or survival. Several observational studies suggested better prognosis and survival in Asian patients with prostate cancer for unknown reasons. This finding should be considered when planning multi-regional clinical trials including Asian countries.
References
- 1.Jemal A, Bray F, Center MM, et al. et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Schroeder FH, Hugosson J, Roobol MJ, et al. et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 3.Hugosson J, Carlsson S, Aus G, et al. et al. Mortality results from the Göteborg randomised population-based prostate-cancer screening trial. Lancet Oncol. 2011;11:725–732. doi: 10.1016/S1470-2045(10)70146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roobol MJ, Kerkhof M, Schroder FH, et al. et al. Prostate cancer mortality reduction by prostate-specific antigen-based screening adjusted for nonattendance and contamination in the European randomised study of screening for prostate cancer (ERSPC) Eur Urol. 2009;56:584–591. doi: 10.1016/j.eururo.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 5.Oberaigner W, Horninger W, Klocker H, et al. et al. Reduction of prostate cancer mortality in Tyrol, Austria, after introduction of prostate-specific antigen testing. Am J Epidemiol. 2006;164:376–384. doi: 10.1093/aje/kwj213. [DOI] [PubMed] [Google Scholar]
- 6.Oberaigner W, Siebert U, Horninger W, et al. et al. Prostate-specific antigen testing in Tyrol, Austria: prostate cancer mortality reduction was supported by an update with mortality data up to 2008. Int J Public Health. 2012;57:57–62. doi: 10.1007/s00038-011-0266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ilic D, O'Connor D, Green S, et al. et al. Screening for prostate cancer: an updated Cochrane systematic review. BJU Int. 2011;107:882–891. doi: 10.1111/j.1464-410X.2010.10032.x. [DOI] [PubMed] [Google Scholar]
- 8.Shen M, Chen W, Yu K, et al. et al. The diagnostic value of PCA3 gene-based analysis of urine sediments after digital rectal examination for prostate cancer in a Chinese population. Exp Mol Pathol. 2011;90:97–100. doi: 10.1016/j.yexmp.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Day JR, Jost M, Reynolds MA, et al. et al. PCA3: from basic molecular science to the clinical lab. Cancer Lett. 2011;301:1–6. doi: 10.1016/j.canlet.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 10.Stamey TA, Caldwell M, McNeal JE, et al. et al. The prostate specific antigen era in the United States is over for prostate cancer: what happened in the last 20 years? J Urol. 2004;172:1297–1301. doi: 10.1097/01.ju.0000139993.51181.5d. [DOI] [PubMed] [Google Scholar]
- 11.Moore AL, Dimitropoulou P, Lane A, et al. et al. Population-based prostate-specific antigen testing in the UK leads to a stage migration of prostate cancer. BJU Int. 2009;104:1592–1598. doi: 10.1111/j.1464-410X.2009.08652.x. [DOI] [PubMed] [Google Scholar]
- 12.Hua L, Qiao D, Xu B, et al. et al. Clinical and pathological characteristics of screen-detected versus clinically diagnosed prostate cancer in Nanjing, China. Med Oncol. 2011;28:357–364. doi: 10.1007/s12032-009-9409-3. [DOI] [PubMed] [Google Scholar]
- 13.Okihara K, Kitamura K, Okada K, et al. et al. Ten year trend in prostate cancer screening with high prostate-specific antigen exposure rate in Japan. Int J Urol. 2008;15:156–160. doi: 10.1111/j.1442-2042.2007.01957.x. [DOI] [PubMed] [Google Scholar]
- 14.Galper SL, Chen MH, Catalona WJ, et al. et al. Evidence to support a continued stage migration and decrease in prostate cancer specific mortality. J Urol. 2006;175:907–912. doi: 10.1016/S0022-5347(05)00419-2. [DOI] [PubMed] [Google Scholar]
- 15.Song F, He M, Li H, et al. et al. A cancer incidence survey in Tianjin: the third largest city in China—between 1981 and 2000. Cancer Causes Control. 2008;19:443–450. doi: 10.1007/s10552-007-9105-6. [DOI] [PubMed] [Google Scholar]
- 16.Song FJ, Zhang BL, He M, et al. et al. Trend analysis of the incidence of prostate cancer in Tianjin between 1981 and 2004. Zhonghua Yi Xue Za Zhi. 90:2811–2814. [in Chinese] [PubMed] [Google Scholar]
- 17.Chen JG, Zhu J, Parkin DM, et al. et al. Trends in the incidence of cancer in Qidong, China, 1978–2002. Int J Cancer. 2006;119:1447–1454. doi: 10.1002/ijc.21952. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Wu S, Guo LR, et al. et al. Diagnostic strategies and the incidence of prostate cancer: reasons for the low reported incidence of prostate cancer in China. Asian J Androl. 2009;11:9–13. doi: 10.1038/aja.2008.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuda T, Marugame T, Kamo K, et al. et al. Cancer incidence and incidence rates in Japan in 2005: based on data from 12 population-based cancer registries in the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2011;41:139–147. doi: 10.1093/jjco/hyq169. [DOI] [PubMed] [Google Scholar]
- 20.Center for Cancer Control and information Services, National Cancer Center, Japan Monitoring of cancer incidence in Japan 2006. 2011. http://ganjoho.jp/data/professional/statistics/odjrh3000000hwsa-att/mcij2006_report.pdf.
- 21.Center for Cancer Control and information Services, National Cancer Center, Japan Cancer incidence 1975–2005. 2010. http://ganjoho.jp/data/professional/statistics/odjrh3000000hwsa-att/cancer_incidence(1975-2005).xls.
- 22.Center for Cancer Control and information Services, National Cancer Center, Japan Monitoring of cancer incidence in Japan 2005. 2010. http://ganjoho.jp/data/professional/statistics/odjrh3000000hwsa-att/mcij2005_report_rev.pdf.
- 23.Center for Cancer Control and information Services, National Cancer Center, Japan Monitoring of cancer incidence in Japan 2004. 2009. http://ganjoho.jp/data/professional/statistics/odjrh3000000hwsa-att/mcij2004_report.pdf.
- 24.Center for Cancer Control and information Services, National Cancer Center, Japan Monitoring of cancer incidence in Japan 2003. 2009. http://ganjoho.jp/data/professional/statistics/odjrh3000000hwsa-att/mcij2003_report.pdf.
- 25.Park SK, Sakoda LC, Kang D, et al. et al. Rising prostate cancer rates in South Korea. Prostate. 2006;66:1285–1291. doi: 10.1002/pros.20419. [DOI] [PubMed] [Google Scholar]
- 26.Korea National Cancer Center National cancer registration and statistics 2008. 2010. http://www.apan.net/meetings/Hanoi2010/Session/Slides/Medical/h8.pdf.
- 27.Miller B, Chu K, Hankey B, et al. et al. Cancer incidence and mortality patterns among specific Asian and Pacific Islander populations in the U.S. Cancer Causes Control. 2008;19:227–256. doi: 10.1007/s10552-007-9088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCracken M, Olsen M, Chen MS, Jr, et al. et al. Cancer incidence, mortality, and associated risk factors among Asian Americans of Chinese, Filipino, Vietnamese, Korean, and Japanese ethnicities. CA Cancer J Clin. 2007;57:190–205. doi: 10.3322/canjclin.57.4.190. [DOI] [PubMed] [Google Scholar]
- 29.Luo W, Birkett N, Ugnat AM, et al. et al. Cancer incidence patterns among Chinese immigrant populations in Alberta. J Immigr Health. 2004;6:41–48. doi: 10.1023/B:JOIH.0000014641.68476.2d. [DOI] [PubMed] [Google Scholar]
- 30.Raymundo EM, Rice KR, Chen Y, et al. et al. Prostate cancer in Asian Americans: incidence, management and outcomes in an equal access healthcare system. BJU Int. 2010;107:1216–1222. doi: 10.1111/j.1464-410X.2010.09685.x. [DOI] [PubMed] [Google Scholar]
- 31.Arnold M, Razum O, Coebergh JW. Cancer risk diversity in non-western migrants to Europe: an overview of the literature. Eur J Cancer. 2010;46:2647–2659. doi: 10.1016/j.ejca.2010.07.050. [DOI] [PubMed] [Google Scholar]
- 32.Metcalfe C, Patel B, Evans S, et al. et al. The risk of prostate cancer amongst south Asian men in southern England: the process cohort study. BJU Int. 2008;102:1407–1412. doi: 10.1111/j.1464-410X.2008.07818.x. [DOI] [PubMed] [Google Scholar]
- 33.Bouchardy C, Parkin DM, Khlat M. Cancer mortality among Chinese and south-east Asian migrants in France. Int J Cancer. 1994;58:638–643. doi: 10.1002/ijc.2910580504. [DOI] [PubMed] [Google Scholar]
- 34.Wild SH, Fischbacher CM, Brock A, et al. et al. Mortality from all cancers and lung, colorectal, breast and prostate cancer by country of birth in England and Wales, 2001–2003. Br J Cancer. 2006;94:1079–1085. doi: 10.1038/sj.bjc.6603031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Visser O, van Leeuwen FE. Cancer risk in first generation migrants in North-Holland/Flevoland, The Netherlands, 1995–2004. Eur J Cancer. 2007;43:901–908. doi: 10.1016/j.ejca.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 36.Williams N, Hughes LJ, Turner EL, et al. et al. Prostate-specific antigen testing rates remain low in UK general practice: a cross-sectional study in six English cities. BJU Int. 2011;108:1402–1408. doi: 10.1111/j.1464-410X.2011.10163.x. [DOI] [PubMed] [Google Scholar]
- 37.Hughes E, Kilmer G, Li Y, et al. et al. Surveillance for certain health behaviors among states and selected local areas—United States, 2008. MMWR Surveil Summ. 2010;59:1–221. [PubMed] [Google Scholar]
- 38.Baade PD, Youlden DR, Coory MD, et al. et al. Urban-rural differences in prostate cancer outcomes in Australia: what has changed? Med J Aust. 2011;194:293–296. doi: 10.5694/j.1326-5377.2011.tb02977.x. [DOI] [PubMed] [Google Scholar]
- 39.Baade PD, Youlden DR, Krnjacki LJ. International epidemiology of prostate cancer: geographical distribution and secular trends. Mol Nutr Food Res. 2009;53:171–184. doi: 10.1002/mnfr.200700511. [DOI] [PubMed] [Google Scholar]
- 40.Quan H, Fong A, De Coster C, et al. et al. Variation in health services utilization among ethnic populations. CMAJ. 2006;174:787–791. doi: 10.1503/cmaj.050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weber MF, Banks E, Smith DP, et al. et al. Cancer screening among migrants in an Australian cohort; cross-sectional analyses from the 45 and up study. BMC Public Health. 2009;9:144. doi: 10.1186/1471-2458-9-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olson SH, Layne TM, Simon JA, et al. et al. Studying cancer in minorities. Cancer. 2011;117:2762–2769. doi: 10.1002/cncr.25871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Man ADA, Pickles TOM, Chi KN. Asian race and impact on outcomes after radical radiotherapy for localized prostate cancer. J Urol. 2003;170:901–904. doi: 10.1097/01.ju.0000081423.37043.b4. [DOI] [PubMed] [Google Scholar]
- 44.Oakley-Girvan I, Kolonel LN, Gallagher RP, et al. et al. Stage at diagnosis and survival in a multiethnic cohort of prostate cancer patients. Am J Public Health. 2003;93:1753–1759. doi: 10.2105/ajph.93.10.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robbins AS, Koppie TM, Gomez SL, et al. et al. Differences in prognostic factors and survival among white and Asian men with prostate cancer, California, 1995-2004. Cancer. 2007;110:1255–1263. doi: 10.1002/cncr.22872. [DOI] [PubMed] [Google Scholar]
- 46.Cohen J, Schoenbach V, Kaufman J, et al. et al. Racial differences in clinical progression among medicare recipients after treatment for localized prostate cancer (United States) Cancer Causes Control. 2006;17:803–811. doi: 10.1007/s10552-006-0017-7. [DOI] [PubMed] [Google Scholar]
- 47.Lin SS, Clarke CA, Prehn AW, et al. et al. Survival differences among Asian subpopulations in the United States after prostate, colorectal, breast, and cervical carcinomas. Cancer. 2002;94:1175–1182. [PubMed] [Google Scholar]
- 48.Holmes L, Jr, Chan W, Jiang Z, et al. et al. Impact of androgen deprivation therapy on racial/ethnic disparities in the survival of older men treated for locoregional prostate cancer. Cancer Control. 2009;16:176–185. doi: 10.1177/107327480901600210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fukagai T, Namiki TS, Carlile RG, et al. et al. Comparison of the clinical outcome after hormonal therapy for prostate cancer between Japanese and Caucasian men. BJU Int. 2006;97:1190–1193. doi: 10.1111/j.1464-410X.2006.06201.x. [DOI] [PubMed] [Google Scholar]
- 50.Huang SP, Huang CY, Liu CC, et al. et al. Clinical outcome of Taiwanese men with clinically localized prostate cancer post-radical prostatectomy: a comparison with other ethnic groups. The Aging Male. 2010;13:10–17. doi: 10.3109/13685530903294370. [DOI] [PubMed] [Google Scholar]
- 51.Tsukuma H, Ajiki W, Ioka A, et al. et al. Survival of cancer patients diagnosed between 1993 and 1996: a collaborative study of population-based cancer registries in Japan. Jpn J Clin Oncol. 2006;36:602–607. doi: 10.1093/jjco/hyl068. [DOI] [PubMed] [Google Scholar]
- 52.Matsuda T, Ajiki W, Marugame T, et al. et al. Population-based survival of cancer patients diagnosed between 1993 and 1999 in Japan: a chronological and international comparative study. Jpn J Clin Oncol. 2011;41:40–51. doi: 10.1093/jjco/hyq167. [DOI] [PubMed] [Google Scholar]
- 53.Yan L, Spitznagel EL. Soy consumption and prostate cancer risk in men: a revisit of a meta-analysis. Am J Clin Nutr. 2009;89:1155–1163. doi: 10.3945/ajcn.2008.27029. [DOI] [PubMed] [Google Scholar]
- 54.Kurahashi N, Iwasaki M, Sasazuki S, et al. et al. Soy product and isoflavone consumption in relation to prostate cancer in Japanese men. Cancer Epidemiol Biomarkers Prev. 2007;16:538–545. doi: 10.1158/1055-9965.EPI-06-0517. [DOI] [PubMed] [Google Scholar]
- 55.Hwang YW, Kim SY, Jee SH, et al. et al. Soy food consumption and risk of prostate cancer: a meta-analysis of observational studies. Nutr Cancer. 2009;61:598–606. doi: 10.1080/01635580902825639. [DOI] [PubMed] [Google Scholar]
- 56.Colloca G, Venturino A. The evolving role of familial history for prostate cancer. Acta Oncol. 2011;50:14–24. doi: 10.3109/0284186X.2010.521191. [DOI] [PubMed] [Google Scholar]
- 57.Zeegers MPA, Jellema A, Ostrer H. Empiric risk of prostate carcinoma for relatives of patients with prostate carcinoma. Cancer. 2003;97:1894–1903. doi: 10.1002/cncr.11262. [DOI] [PubMed] [Google Scholar]
- 58.Johns LE, Houlston RS. A systematic review and meta-analysis of familial prostate cancer risk. BJU Int. 2003;91:789–794. doi: 10.1046/j.1464-410x.2003.04232.x. [DOI] [PubMed] [Google Scholar]
- 59.Watkins Bruner D, Moore D, Parlanti A, et al. et al. Relative risk of prostate cancer for men with affected relatives: systematic review and meta-analysis. Int J Cancer. 2003;107:797–803. doi: 10.1002/ijc.11466. [DOI] [PubMed] [Google Scholar]
- 60.Lee SH, Park KK, Chung MS, et al. et al. Clinical features of familial or hereditary prostate cancer in Korean men: a pilot study [J] Korean J Urol. 2011;52:9–12. doi: 10.4111/kju.2011.52.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu J, Dimitrov L, Chang BL, et al. et al. A combined genomewide linkage scan of 1233 families for prostate cancer-susceptibility genes conducted by the international consortium for prostate cancer genetics. Am J Hum Genet. 2005;77:219–229. doi: 10.1086/432377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Christensen GB, Baffoe-Bonnie AB, George A, et al. et al. Genome-wide linkage analysis of 1 233 prostate cancer pedigrees from the international consortium for prostate cancer genetics using novel sumlink and sumlod analyses. Prostate. 2010;70:735–744. doi: 10.1002/pros.21106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matsui H, Suzuki K, Ohtake N, et al. et al. Genomewide linkage analysis of familial prostate cancer in the Japanese population. J Hum Genet. 2003;49:9–15. doi: 10.1007/s10038-003-0099-y. [DOI] [PubMed] [Google Scholar]
- 64.Schleutker J. Polymorphisms in androgen signaling pathway predisposing to prostate cancer. Mol Cell Endocrinol. 2012;360:25–37. doi: 10.1016/j.mce.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 65.Li H, Tai BC. RNASEL gene polymorphisms and the risk of prostate cancer: a meta-analysis. Clin Cancer Res. 2006;12:5713–5719. doi: 10.1158/1078-0432.CCR-05-2799. [DOI] [PubMed] [Google Scholar]
- 66.Nakazato H, Suzuki K, Matsui H, et al. et al. Role of genetic polymorphisms of the RNASEL gene on familial prostate cancer risk in a Japanese population. Br J Cancer. 2003;89:691–696. doi: 10.1038/sj.bjc.6601075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu B, Tong N, Li JM, et al. et al. Elac2 polymorphisms and prostate cancer risk: a meta-analysis based on 18 case-control studies. Prostate Cancer Prostatic Dis. 2010;13:270–277. doi: 10.1038/pcan.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wei B, Zhou Y, Xu Z, et al. et al. XRCC1 Arg399Gln and Arg194Trp polymorphisms in prostate cancer risk: a meta-analysis. Prostate Cancer Prostatic Dis. 2011;14:225–231. doi: 10.1038/pcan.2011.26. [DOI] [PubMed] [Google Scholar]
- 69.Geng J, Zhang Q, Zhu C, et al. et al. Xrcc1d genetic polymorphism Arg399Gln and prostate cancer risk: a meta-analysis. Urology. 2009;74:648–653. doi: 10.1016/j.urology.2009.02.046. [DOI] [PubMed] [Google Scholar]
- 70.Zeegers MP, Kiemeney LA, Nieder AM, et al. et al. How strong is the association between CAG and GGN repeat length polymorphisms in the androgen receptor gene and prostate cancer risk? Cancer Epidemiol Biomarkers Prev. 2004;13:1765–1771. [PubMed] [Google Scholar]
- 71.Ntais C, Polycarpou A, Ioannidis JP. Vitamin D receptor gene polymorphisms and risk of prostate cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2003;12:1395–1402. [PubMed] [Google Scholar]
- 72.Wang C, Tao W, Chen Q, et al. et al. SRD5A2 V89L polymorphism and prostate cancer risk: a meta-analysis. Prostate. 2010;70:170–178. doi: 10.1002/pros.21050. [DOI] [PubMed] [Google Scholar]
- 73.Li J, Coates RJ, Gwinn M, et al. et al. Steroid 5-{alpha)-reductase Type 2 (SRD5a2) gene polymorphisms and risk of prostate cancer: a HuGE review. Am J Epidemiol. 2010;171:1–13. doi: 10.1093/aje/kwp318. [DOI] [PubMed] [Google Scholar]
- 74.Collin SM, Metcalfe C, Zuccolo L, et al. et al. Association of folate- pathway gene polymorphisms with the risk of prostate cancer: a population-based nested case-control study, systematic review, and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2009;18:2528–2539. doi: 10.1158/1055-9965.EPI-09-0223. [DOI] [PubMed] [Google Scholar]
- 75.Waters KM, Henderson BE, Stram DO, et al. et al. Association of diabetes with prostate cancer risk in the multiethnic cohort. Am J Epidemiol. 2009;169:937–945. doi: 10.1093/aje/kwp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pierce BL, Ahsan H. Genetic susceptibility to type 2 diabetes is associated with reduced prostate cancer risk. Hum Hered. 2010;69:193–201. doi: 10.1159/000289594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lichtenstein P, Holm NV, Verkasalo PK, et al. et al. Environmental and heritable factors in the causation of cancer—analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 78.Pomerantz MM, Freedman ML. Genetics of prostate cancer risk. Mt Sinai J Med. 2010;77:643–654. doi: 10.1002/msj.20222. [DOI] [PubMed] [Google Scholar]
- 79.Sun J, Kader AK, Hsu FC, et al. et al. Inherited genetic markers discovered to date are able to identify a significant number of men at considerably elevated risk for prostate cancer. Prostate. 2011;71:421–430. doi: 10.1002/pros.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu H, Wang B, Han C. Meta-analysis of genome-wide and replication association studies on prostate cancer. Prostate. 2011;71:209–224. doi: 10.1002/pros.21235. [DOI] [PubMed] [Google Scholar]
- 81.Zheng SL, Hsing AW, Sun J, et al. et al. Association of 17 prostate cancer susceptibility loci with prostate cancer risk in Chinese men. Prostate. 2010;70:425–432. doi: 10.1002/pros.21076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Waters KM, Le Marchand L, Kolonel LN, et al. et al. Generalizability of associations from prostate cancer genome-wide association studies in multiple populations. Cancer Epidemiol Biomarkers Prev. 2009;18:1285–1289. doi: 10.1158/1055-9965.EPI-08-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takata R, Akamatsu S, Kubo M, et al. et al. Genome-wide association study identifies five new susceptibility loci for prostate cancer in the Japanese population. Nat Genet. 2010;42:751–754. doi: 10.1038/ng.635. [DOI] [PubMed] [Google Scholar]
- 84.Cheng I, Plummer SJ, Jorgenson E, et al. et al. 8q24 and prostate cancer: association with advanced disease and meta-analysis. Eur J Hum Genet. 2008;16:496–505. doi: 10.1038/sj.ejhg.5201959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lindstrom S, Schumacher F, Siddiq A, et al. et al. Characterizing associations and SNP-environment interactions for GWAS- identified prostate cancer risk markers results from BPC3. PLoS ONE. 2011;6:e17142. doi: 10.1371/journal.pone.0017142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sun J, Liu W, Adams TS, et al. et al. DNA copy number alterations in prostate cancers: a combined analysis of published CGH studies. Prostate. 2007;67:692–700. doi: 10.1002/pros.20543. [DOI] [PubMed] [Google Scholar]
- 87.Mao X, Yu Y, Boyd LK, et al. et al. Distinct genomic alterations in prostate cancers in Chinese and western populations suggest alternative pathways of prostate carcinogenesis. Cancer Res. 2010;70:5207–5212. doi: 10.1158/0008-5472.CAN-09-4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Meiers I, Shanks JH, Bostwick DG. Glutathione S-transferase pi (GSTP1) hypermethylation in prostate cancer: review 2007. Pathology. 2007;39:299–304. doi: 10.1080/00313020701329906. [DOI] [PubMed] [Google Scholar]
- 89.Gottlieb B, Beitel LK, Wu JH, et al. et al. The androgen receptor gene mutations database (ARDB): 2004 update. Hum Mutat. 2004;23:527–533. doi: 10.1002/humu.20044. [DOI] [PubMed] [Google Scholar]