Introduction
This executive summary briefly describes the overall goals and content of the report on safety considerations for intensity modulated radiation therapy (IMRT) and is intentionally limited in length and content. Please see the full report published electronically at www.practicalradonc.org. This abridged version is not intended to replace the full length report but rather to highlight key recommendations of the report. Background information for those less familiar with IMRT is limited to the full report.
Scope of this document on patient safety for IMRT
This report is part of a series of white papers addressing patient safety commissioned by the American Society for Radiation Oncology (ASTRO) Board of Directors as part of ASTRO's Target Safely Campaign. The full length document was approved by the ASTRO Board of Directors on February 14, 2011 and has been endorsed by the American Association of Physicists in Medicine, American Association of Medical Dosimetrists, and the American Society of Radiologic Technologists. The document has also been reviewed and accepted by the American College of Radiology’s Commission on Radiation Oncology. These organizations have a long history of supporting efforts toward improving patient safety in the United States.
This report is related to other reports of the ASTRO white paper series on patient safety, still in preparation, especially those on peer review and on image-guided radiation therapy, since both of these areas have implications on the practice of IMRT. There are sections of the report that defer to guidance that will be published by those groups in future reports. Because this is the first report in the safety series, some of the concerns included in this report are not limited to IMRT.
IMRT provides increased capability to conform isodose distributions to the shape of the target(s), thereby reducing dose to some adjacent critical structures. This promise of IMRT is one of the reasons for its widespread use. However, the promise of IMRT is counterbalanced by the complexity of the IMRT planning and delivery processes, and the associated risks.
The New York Times reported on serious accidents involving both IMRT and other radiation treatment modalities.1, 2 The full length report broadly addresses safe delivery of IMRT, with a primary focus on recommendations for human error prevention and methods to reduce the occurrence of errors or machine malfunctions that can lead to catastrophic failures or errors.
The full treatment team should be composed of individuals with proper credentials and training specific to radiation therapy for the simulation, treatment planning, quality assurance (QA), and delivery processes. Additional training specific to IMRT is important. See the full length report (section 1.2, available online only at www.practicalradonc.org) for a description of the responsibilities of IMRT team members, including radiation oncologists, medical physicists, dosimetrists (or treatment planners), radiation therapists, and administrative staff. Special attention should be paid to the roles of the physician and physicist; both board certified medical specialists who share responsibility for IMRT quality.
Safety concerns
Tools and techniques that can be used by individual clinics to reassess and strengthen the safety of their IMRT programs are presented in the full length document. Due to the complexity of IMRT delivery, we believe it is unsafe for IMRT to be delivered in emergent situations that would encourage staff to skip the needed QA steps. And yet, clinical pressures can make it difficult to ensure support for this approach.
Hazards within an IMRT program can be broadly categorized as environmental or technical. Environmental concerns that can affect all patient treatments include the lack of standard operating procedures, haste, habituation, incomplete understanding or misuse of procedures or equipment, an inadequate QA program, and a lack of continuing staff education. While these hazards are not unique to IMRT, their impact may be greater due to the complexity of IMRT. Technical concerns that affect safety can include inadequate commissioning of the clinical IMRT program, inadequate validation of the accuracy of treatment delivery parameters, improper use of one or more parts of the planning and delivery process, and an inadequate investigation of discrepancies between treatment plan parameters and QA results.
The responsibilities of members of the treatment team are defined in the report (see Table 2 in the full document; available online only at www.practicalradonc.org). Also, specific example process steps are presented for workflow (Appendix 1 in full document; available online only at www.practicalradonc.org) and checklists (Appendix 2; available online only at www.practicalradonc.org), which may address ways to prevent or detect catastrophic failures for IMRT. The 54 process steps and 15 hand-offs between the personnel in the example workflow illustrate the critical need for clearly defined roles, and unambiguous or robust hand-offs (and means of communication) between personnel.
Supporting a culture of safety for IMRT: environmental considerations
The departmental leadership establishes the foundation for patient safety and teamwork. While these elements are not unique to IMRT, we believe that they are crucial for ensuring a safe radiation therapy program, especially since IMRT requires additional equipment, personnel, and procedures for safety. The following considerations (discussed in detail in the full length document available online only at www.practicalradonc.org) are important for creation of a culture of safety:
-
•
Department members must trust each other3
-
•
Administration must provide strong support for safety
-
•
Event tracking, review, investigation, and follow-up for events and near misses
-
•
Appropriately qualified personnel and ongoing training
-
•
Use of standard operating procedures (SOPs)
-
•
Defined roles and responsibilities for team members
-
•
Strong communication among team members
-
•
American College of Radiology /ASTRO practice accreditation
-
•
Continuous quality improvements.
Each institution should customize procedures to reflect their own processes and resources but should have a basis founded in national or international guidance documents (section 4) to create a program that explicitly incorporates patient safety. SOPs should be written and should empower individuals to halt planning or treatment when a problem is encountered, allowing for proper investigation into the problem, and then a decision regarding the best course of action to maintain patient safety. In the midst of a situation where adequate time is not allowed for performing all of the necessary QA steps prior to treatment, time pressures may stand in the way of identifying and resolving problems. The SOP should not permit staff to skip QA steps.
Implementation of and adherence to detailed policies and procedures are necessary to avoid both quality errors and catastrophic failures. Details of what should be included in an IMRT SOP are in the full length document (available online only at www.practicalradonc.org).
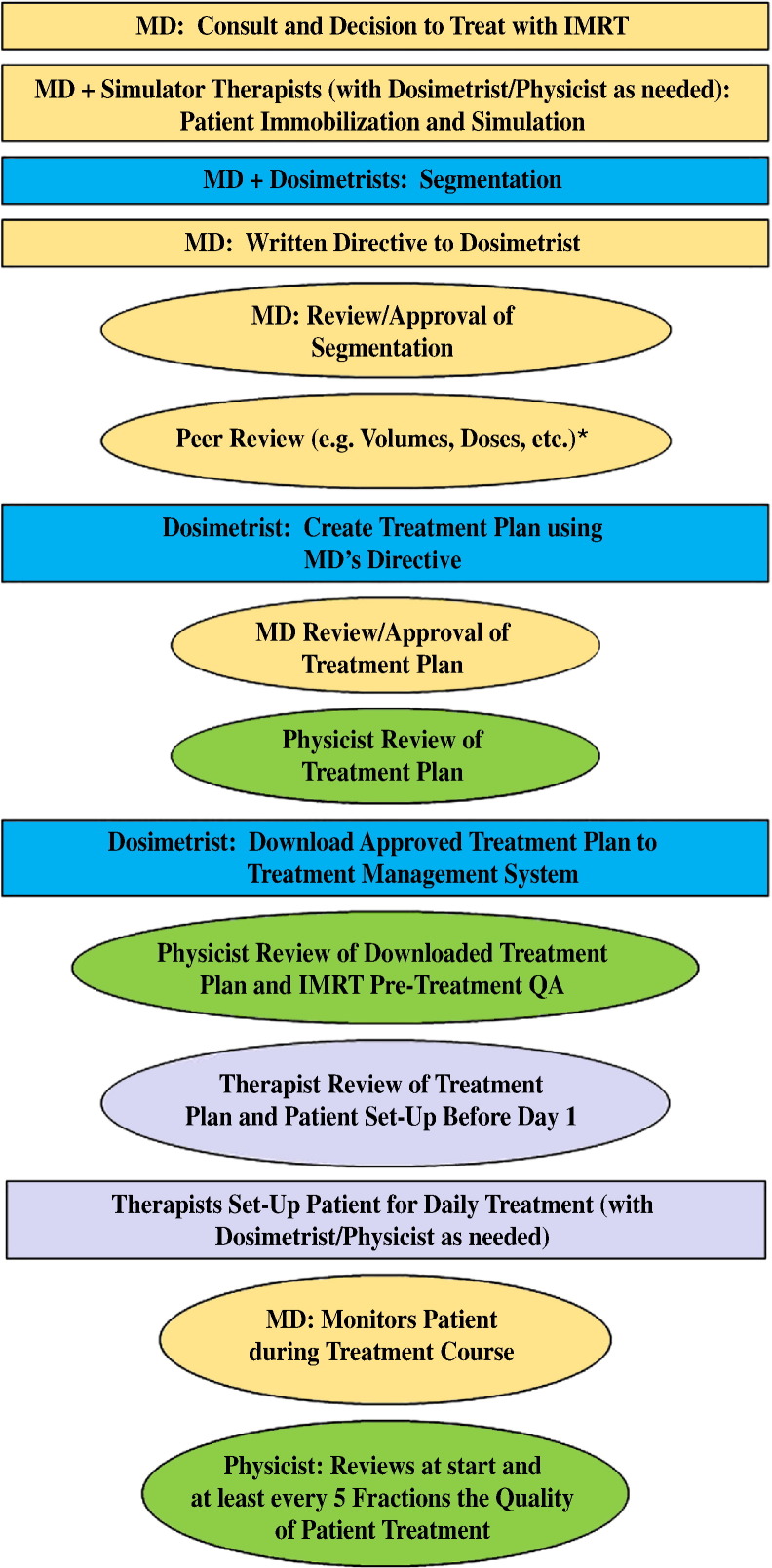
Figure 1 shows the complexity of the IMRT process as a series of process steps and review steps by members of the IMRT team. There are numerous situations (eg, a change in the patient geometry) that can lead to restarting the process from the beginning, which can cause pressures to skip or short-circuit QA procedures. Risks may also increase if inadequate time is allotted for, and in between, the various steps (eg, image segmentation, written directive, planning, patient-specific QA). The full length report discusses many of these time considerations (with respect to safety) in detail.
Figure 1.
An abbreviated diagram of the process (boxes) and review (ovals) steps for intensity modulated radiation therapy planning for an individual patient. Each color (or shade) represents member of the treatment team. *Peer review will be addressed in detail in a report of the white paper series on patient safety.
IMRT guidance for quality assurance experience: technical considerations
A summary of the existing guidance documents for IMRT is presented in the full length document (text and Table 5 available online only at www.practicalradonc.org). These earlier IMRT QA documents emphasized establishing a quality IMRT program and did not explicitly concentrate on the potential for catastrophic failures in IMRT delivery. In fact, several documents suggested that some QA efforts could be decreased or even eliminated after the accumulation of a stated amount of experience. In this work, we acknowledge that certain types of catastrophic failures resulting from human error and equipment (hardware or software) malfunction might not be predictable based on past experience. In some situations, periodic testing alone may be inadequate for identifying these types of problems.
The processes and tasks performed by the IMRT team are addressed with special attention to patient safety and to minimizing the potential for catastrophic failures. The full length document (available online only at www.practicalradonc.org) includes a detailed discussion of the following with respect to patient safety and quality:
4.2.1 Training
4.2.2 Commissioning an IMRT system
4.2.3 Establishing a QA program
4.2.4 Pretreatment IMRT QA program
4.2.5 Monitoring the IMRT program
Each institution should have clear criteria for a pass or fail of the IMRT patient-specific IMRT QA technique. There is interinstitutional variation in the content of pretreatment IMRT QA, as well as the equipment and software used. There is no formal consensus on the desired or required level of agreement between the planned or expected calculation and the measured data for patient-specific IMRT QA. Also, the impact of failing to meet a given set of criteria on these patient-specific measurements is often not explicitly addressed (eg, remeasure the fields, generate an alternate plan, estimate the clinical impact qualitatively, etc). Therefore, further guidance is needed from national organizations in each of these areas.
Until formal guidance is available, we recommend that users establish acceptance criteria that they have determined will identify plans that should fail the QA check. For example, users should deliberately create plans with known errors such as the incorrect fluence for regions of high or low dose across the irradiated volume or critical structures, plans with one field with a rotated collimator and/or an incorrect fluence distribution, and other discrepancies that should be identified by the QA method. The IMRT QA criteria should be established using tests of the most highly modulated fields that are seen in the local clinic.
The full length report includes a summary of the primary recommendations, tasks, and assigned personnel to guard against catastrophic failures for IMRT, primarily concentrating on multileaf collimator-based delivery systems since they are the most common (Table 4).
Table 4.
Recommendations to guard against catastrophic failures for IMRT
| Recommended tests and procedures | Person who performs task | Primary review responsibility | Second review |
|---|---|---|---|
| Halt a procedure if the operator is unclear about what is being done. | All | All | All |
| Verify the patient information, treatment site, and prescription. | All | All | All |
| Verify correct positioning of the high dose region of isodose plan relative to targets. | Dosimetrist | Physician | Physicist |
| Verify the recording of reference and shift information from the planning scan in patient chart (electronic or paper). | Dosimetrist | Physicist | Therapist |
| Assess pretreatment localization/portal images with respect to corresponding reference images before first treatment; physician determines frequency of IGRT techniques. | Dosimetrist exports reference images from treatment planning system | Physician | Therapist |
| Verify that the correct version of the patient's treatment plan is approved, sent to treatment management system, and used for patient-specific QA. | Dosimetrist exports from the treatment planning system | Physicist | Therapists confirm against prescription for each treatment; physician prescription should specify the physician approved plan |
| Before the first treatment or for any change in treatment, perform patient-specific QA to guarantee that data transfer between systems is correct before patient treatment begins. | Physicist, dosimetrist, therapist or physics assistant | Physicist | Therapists confirm that only fully approved plans are used for treatment |
| Perform a complete chart check including review of information in treatment management system prior to the start of any treatment and after any change in treatment before changes are used for treatment. Visually review field apertures in treatment management system. Perform a check of dose to verify TPS calculation (measurement or calculation using DICOM export of data from RTP system). |
Physicist | Therapist | |
| Perform a time out prior to treatment delivery. | Therapist | Second therapist | |
| Perform a check of treatment parameters before start of and during first treatment against a fixed version of the treatment plan. Includes visual verification of field apertures during first treatment and after any change in treatment. At each fraction, verify motion of leaves (if MLC delivery) and total monitor units. |
Dosimetrist exported from TPS; verified by physicist | Therapist | Second therapist |
| Perform end-to-end testing to guarantee transfer of data among all systems involved in imaging, planning and dose delivery (periodically and after any software or hardware changes). | Physicist, therapist, or physics assistant | Physicist | Second physicist to review |
| Perform a time out prior to treatment delivery. | Therapist | Second therapist | |
| Perform a check of treatment parameters before start of and during first treatment against a fixed version of the treatment plan. Includes visual verification of field apertures during first treatment and after any change in treatment. At each fraction, verify motion of leaves (if MLC delivery) and total monitor units. |
Dosimetrist exported from TPS; verified by physicist | Therapist | Second therapist |
| Perform end-to-end testing to guarantee transfer of data among all systems involved in imaging, planning and dose delivery (periodically and after any software or hardware changes). | Physicist, therapist, or physics assistant | Physicist | Second physicist to review |
IGRT, image-guided radiation therapy; IMRT, intensity modulated radiation therapy; MLC, multileaf collimator; QA, quality assurance; TPS, treatment planning system.
Collaboration between users and manufacturers to improve IMRT safety
Improvements in IMRT equipment and methods to enhance patient safety are needed and would be facilitated by collaborative efforts between manufacturers, users, and regulatory agencies such as the United States Food and Drug Administration. The full length report includes a detailed list of possible improvements, including the following:
-
A.
Methods to directly and independently verify or validate patient plan and treatment data on the treatment machine prior to, during, and after radiation delivery.
-
B.
Provision of safety measures in the IMRT workflow such as communication features, checklists, data integration and tracking.
-
C.
Integration of IMRT sub-systems and QA procedures
-
D.
Human interaction with equipment
Successful improvements to existing and future systems will require joint efforts by the users, vendors, and regulators. The prioritization, implementation, testing, and commercial release of any improvements should be a partnership between users, manufacturers, and regulators.
Summary
IMRT is time and resource intensive. Environmental and technical concerns need to be addressed to improve patient safety. Timely treatment is important, but undue pressure and real-time changes to the treatment plan can lead to errors. The report suggests use of a “forced time out” to assure adequate time to perform reviews and QA at key points in the process. Team members need to acknowledge that initiation of treatment may need to be delayed to allow time for necessary QA checks and subsequent investigations of problems.
The recommendations in the full length report are intended to provide guidance to aid clinics in avoiding catastrophic errors and to improve the safety and quality of care for patients receiving IMRT. It is expected that there will be further developments with respect to the evaluation of IMRT programs for accreditation, and that new guidance documents will continue to enhance the quality and safety of IMRT use.
Acknowledgments
This document was prepared by the Multidisciplinary Quality Assurance Subcommittee of the Clinical Affairs and Quality Committee of the American Society for Radiation Oncology as a part of ASTRO's Target Safely Campaign.
The IMRT white paper was reviewed by 8 experts from the field of intensity modulated radiation therapy. In December 2010, the IMRT white paper was posted for public comments for 4 weeks. We received comments from physicians, physicists, therapists, and representatives from radiation therapy manufacturers, including general and specific comments from the American Association of Physicists in Medicine. All the comments were reviewed and discussed by the entire writing group and appropriate revisions were incorporated into the paper with group consensus.
ASTRO white papers present scientific, health, and safety information, and may to some extent reflect scientific or medical opinion. They are made available to ASTRO members and to the public for educational and informational purposes only. Any commercial use of any content in this white paper without the prior written consent of ASTRO is strictly prohibited.
Adherence to this white paper will not ensure successful treatment in every situation. Furthermore, this white paper should not be deemed inclusive of all proper methods of care or exclusive of other methods of care reasonably directed to obtaining the same results. The ultimate judgment regarding the propriety of any specific therapy must be made by the physician and the patient in light of all circumstances presented by the individual patient. ASTRO assumes no liability for the information, conclusions, and findings contained in its white papers.
This white paper was prepared on the basis of information available at the time the writing group was conducting its research and discussions on this topic. There may be new developments that are not reflected in this white paper and that may, over time, be a basis for ASTRO to consider revisiting and updating the white paper.
We thank the reviewers who took on the challenge of providing a comprehensive review of an earlier draft of this document. These reviewers provided their responses within a limited time frame to support the timeliness of the report. Their comments and suggestions were invaluable. We respectfully acknowledge Gary Ezzell, PhD, Anne Greener, MS, Joseph Hanley, PhD, Daniel Low, PhD, Jeff Michalski, MD, Jatinder Palta, PhD, Arthur Pinkerton, Warren Suh, MD, and Michael Sharpe, PhD. We also thank the many reviewers who took the time to comment during the public comment period. All comments were reviewed and further changes were incorporated as determined to be appropriate. Finally, we thank Anushree Vichare, MBBS, MPH, of ASTRO, who supported our writing group throughout the process.
Footnotes
Supplementary material for this article (doi:10.1016/j.prro.2011.04.008) can be found at www.practicalradonc.org.
Conflicts of interest: Before initiation of this white paper all members of the White Paper Task Group were required to complete disclosure statements. These statements are maintained at the American Society for Radiation Oncology (ASTRO) Headquarters in Fairfax, VA and pertinent disclosures are published with the report. The ASTRO Conflict of Interests Disclosure Statement seeks to provide a broad disclosure of outside interests. Where a potential conflict is detected, remedial measures to address any potential conflict are taken and will be noted in the disclosure statement. Dr. Jean Moran has received a research grant, paid to University of Michigan, from Varian Medical Systems. Dr. Avraham Eisbruch is a Chair of an independent review committee assessing the complications of investigational protocol at Amgen. Dr. Geoffrey Ibbott has received a research grant, paid to the University of Texas M. D. Anderson Cancer Center, from Varian Medical Systems, and is a consultant with the Young Ricchiuti Caldwell and Heller Law Firm LLC. Dr. Benedick Fraass serves on the Varian Patient Safety Council. He receives no compensation or reimbursement for this work. The Writing Group Chair ensured that the white paper was built by consensus to deliberately minimize any potential conflicts of interest. The Chair of the Multidisciplinary Quality Assurance (QA) Subcommittee, as well as the Writing Group Chair, reviewed these disclosures and determined that they do not present a conflict with respect to these Writing Group members’ work on this white paper.
Supplementary data.
The full report, available as supplementary data associated with this article, can be found with the online version at doi:10.1016/j.prro.2011.04.008.
Full Length White Paper
References
- 1.Bogdanich W. Radiation offers new cures, and ways to do harm. New York Times. 2010:A1. [Google Scholar]
- 2.Bogdanich W. As technology surges, radiation safeguards lag. New York Times. 2010:A1. [Google Scholar]
- 3.Kohn L.T., Corrigan J., Donaldson M.S. National Academics Press; Washington, DC: 2000. To err is human: building a safer health system. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Full Length White Paper